Abstract
Background
Diagnosing urinary tract infections (UTIs) in nursing home residents is complex, as specific urinary symptoms are often absent and asymptomatic bacteriuria (ASB) is prevalent. The aim of this study was to assess the sensitivity of blood C-reactive protein (CRP) and procalcitonin (PCT), measured by point-of-care tests (PoCTs), to diagnose UTIs in this setting.
Methods
Elderly residents (≥65 years old) with a suspected UTI were recruited from psychogeriatric, somatic, or rehabilitation wards across 13 participating nursing homes. CRP and PCT were tested simultaneously in the same study participants. To assess the tests’ sensitivities, a stringent definition of “true” UTI was used that included the presence of symptoms, urinary leucocytes, a positive urine culture, and symptom resolution during antibiotic treatment covering isolated uropathogen(s). The original sample size was 440 suspected UTI episodes, in order to detect a clinically relevant sensitivity of at least 65% when calculated using the matched analysis approach to compare both PoCTs.
Results
After enrollment of 302 episodes (68.6% of the planned sample size), an unplanned and funder-mandated interim analysis was done, resulting in premature discontinuation of the study for futility. For 247 of 266 eligible episodes, all mandatory items required for the true UTI definition (92.9%) were available. In total, 49 episodes fulfilled our stringent UTI definition (19.8%). The sensitivities of CRP (cut-off, 6.5 mg/L) and PCT (cut-off, 0.025 ng/mL) were 52.3% (95% confidence interval [CI], 36.7–67.5%) and 37.0% (95% CI, 23.2–52.5%), respectively.
Conclusions
Our results indicate that CRP and PCT are not suitable tests for distinguishing UTI and ASB in nursing home residents.
Clinical Trials Registration
Netherlands Trial Registry NL6293.
Keywords: urinary tract infections, C-reactive protein, procalcitonin, point-of-care test, nursing home
Blood C-reactive protein and procalcitonin measured by point-of-care tests are not suitable to distinguish urinary tract infection and asymptomatic bacteriuria in nursing home residents.
Suspected urinary tract infections (UTIs) rank among the most common reasons for antibiotic use in nursing homes [1–3]. Diagnosing UTIs in this setting can be challenging. The expression of specific urinary symptoms (eg, dysuria or urgency) in elderly residents with cognitive impairments can be limited [4, 5]. UTI may present solely by nonspecific symptoms [6], which are frequently reported in elderly residents but can also be attributed to many other causes [7]. Also, asymptomatic bacteriuria (ASB) is common, which complicates the attribution of causality to bacteria in urine [8–10]. Therefore, current urine tests have limited value in diagnosing UTIs in this population, which leads to antibiotic overuse, fueling antimicrobial resistance in nursing homes [11, 12].
Point-of-care tests (PoCTs) measuring blood C-reactive protein (CRP) and procalcitonin (PCT) have the potential to improve UTI diagnoses and subsequent antibiotic use in elderly residents. CRP and PCT have proven to be useful in diagnosing and monitoring respiratory tract infections and sepsis in both hospital and primary care settings [13–16]. For the diagnosis of UTI, the use of CRP and PCT has not been extensively studied, particularly among elderly patients in nursing home settings. Previous studies comprised mainly severely ill patients requiring hospitalization or focused on differentiating upper UTIs from lower UTIs [17–22]. These studies are less applicable to nursing home residents, as suspected UTIs in this elderly population mainly involve lower UTIs (cystitis) [23].
Two previous studies in adults, including elderly adults, suggest that PCT can contribute to the reduction in antibiotic exposure by ruling out a UTI. A randomized controlled trial using a PCT-based algorithm at the emergency ward in adults with UTI found a 30% reduction in antibiotic exposure, including in elderly patients with lower UTIs [24]. A retrospective study in older adults with suspected lower UTIs showed a high negative predictive value of PCT (91% at 0.25 ng/mL) [25]. However, these studies did not establish the sensitivity of CRP or PCT to diagnose UTIs in an elderly population.
The objective of this study was to assess the sensitivity of blood CRP and PCT levels, measured by PoCTs, to diagnose UTIs in nursing home residents.
METHODS
Study Design
The initial aim of this study was to assess whether 1 or both inflammatory markers, measured by PoCTs, had a sensitivity of ≥65% in a matched study design [26]. Due to slow patient enrollment, an unplanned interim analysis was performed at the request of the funder. This led to premature discontinuation of the study and cancellation of the matched analyses. This manuscript reports the results of the interim analysis, including the sensitivity of both PoCTs separately, and a futility analysis. This manuscript is written using the STAndards for the Reporting of Diagnostic accuracy studies (STARD) reporting guidelines. [27]
Sample Size Calculation
The original sample size was 440 suspected UTI episodes, calculated for the matched analysis approach comparing both PoCTs. This interim analysis includes 186 participants with 266 episodes of a suspected UTI.
Participants
Elderly residents (≥65 years old) were eligible for enrollment any time a UTI was suspected based on the clinical judgement of the attending nurse or physician. The exclusion criteria were: (1) previous enrollment in the past 30 days; (2) a suspected respiratory tract infection; (3) any suspected other infection requiring antibiotic therapy; and (4) urine collected or blood drawn >24 hours after initiation of antibiotic therapy. Consecutive participants were identified in 13 nursing homes belonging to 3 nursing home organizations in the Netherlands, with a total number of 1.400 resident beds, of which 80% were in psychogeriatric wards and 10% each were in somatic and rehabilitation wards.
Study Procedures
The full study protocol was published previously [26]. Briefly, the attending physician or nurse informed the study staff about potentially eligible participants on weekdays. These professionals also registered demographics, signs and symptoms, antibiotic use, and symptom resolution during follow-up (10 days) on clinical report forms. The attending physician decided whether or not to prescribe antibiotics based on current clinical practice. Data were collected and encoded through an electronic data capture system using software from Open Data Kit [28]. The urine collection method was dependent on the condition of participant. Accepted specimens were spontaneously voided urine or urine from insert pans, indwelling urinary catheters, or incontinence pads. The feasibility of diaper-collected urine was previously assessed [29]. The study staff performed blood draws by venipuncture or the finger prick method. Participants, attending physicians, and nurses were not informed of PoCT, urine dipstick, or bacterial culture results.
Index Tests
Collected blood samples were used within 4 hours for point-of-care index tests. For CRP, the Afinion AS100 platform (Alere Health B.V.) was used, with upper (ULQ) and lower limits of quantification (LLQ) of 200 and 5 mg/L, respectively. For PCT, the Samsung LABGEO IB10 PoC platform (Avant Medical B.V.) was used in the first months of data collection, with a ULQ and LLQ of 10 and 0.08 ng/mL, respectively. After this period, the Afias1 PCT Plus (Avant Medical B.V.) became available, enabling finger prick blood sample collection instead of venipuncture, and was used instead (ULQ and LLQ: 50 and 0.02 ng/mL, respectively). All analyzers were used according to the manufacturers’ protocols. No prespecified test positivity cut-offs were defined, as the empirical cut-off was identified during the analysis to derive the sensitivities of both PoCTs.
Reference Test
As no reference test for the diagnosis of a UTI existed, a stringent post hoc definition for a “true” UTI was used to minimize the probability of misclassification while assessing the sensitivity of the index tests (CRP and PCT). A UTI was considered present when 5 criteria were met: (1) at least 2 urinary or nonspecific symptoms were present; (2) a urine leucocyte esterase test was positive; (3) uropathogens were present in bacterial culture at ≥104 colony-forming units/mL; (4) there were not more than 2 uropathogens; and (5) symptoms resolved in the course of adequate antibiotic treatment (ie, proven susceptibility of isolated uropathogens to the administered antibiotic). The research staff was blinded to the reference test while performing the index tests.
Analysis
Enrolled cases with missing index or reference test results were excluded from the analysis. We derived empirical cut-off values for PCT and CRP from the receiver-operator curve for the classification of study-defined UTIs. For dealing with multiple episodes in a single participant, we used resampling to derive sensitivity, specificity, and area under the receiver-operator curve (AUROC). Resampling entailed selecting a single episode from each participant through simple random sampling with replacement, and calculating the test characteristics. This was repeated 1.000 time, which provided the 95% confidence interval (CI) of the test characteristics.
PoCT values below the LLQ were set at 0. In a sensitivity analysis, values were set at the midpoint between 0 and the LLQ. Calculations were performed in STATA version 14 [30]. Violin plots were made in RStudio Version 1.2.1335 [31].
We present classification curves to depict the frequencies of true and false-positive classifications of study-defined UTIs over the range of marker values identified in the study [32].
Post Hoc Analysis
During the study, a new Dutch guideline on clinical management of UTIs in vulnerable elderly adults presented a clinical algorithm to define a UTI [33]. This algorithm was only based on signs and symptoms and the presence of leucocytes or nitrite in urine. In a post hoc analysis, we used this less stringent definition of a UTI to estimate the test characteristics of the 2 markers.
Patient and Public Involvement
Patients or the public were not involved in this study.
Ethical Considerations
The Medical Ethical Committee of Amsterdam Universitair Medische Centra (UMC) location Vrije Universiteit (VUmc) approved the study protocol (reference number 2017.350, National Central Committee on Research Involving Human Subjects reference number NL62067.029.17). A preemptive informed consent procedure was used. Participants, or representatives when patients were incapacitated, were asked for written informed consent a priori at admission, to participate once a UTI was suspected during the study period. Residents’ capacity to give informed consent was assessed by the attending physician or nurse. The study was conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
RESULTS
Informed Consent
Overall, 40% of nursing home residents or their representatives gave informed consent, while 20% did not (because of vulnerability of the resident, old age, comorbidities, or study burden) and 40% did not respond to the request sent by mail. This resulted in a limited number of eligible residents.
Participant Flow
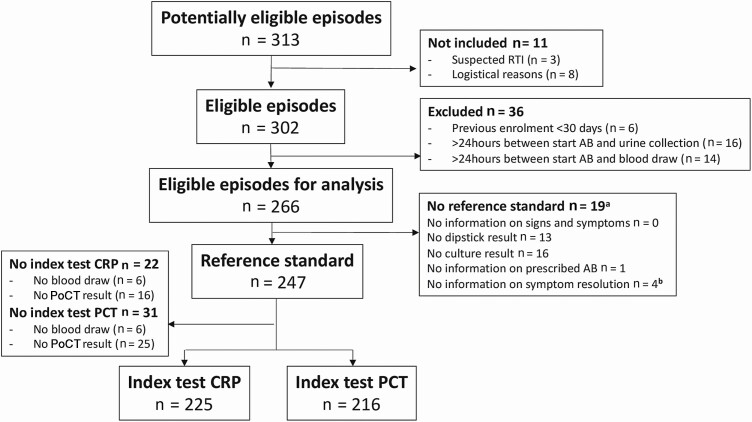
At the time of the interim analysis, the study had collected data on 313 episodes of a suspected UTI between November 2017 and August 2019 (Figure 1). Of these episodes, 39 met the exclusion criteria, while 8 were not included due to logistical reasons. For 247 of the resulting 266 eligible episodes, all mandatory items for the post hoc UTI definition (reference test) were available (92.9%). For CRP and PCT index PoCTs, results were available in 225 and 216 suspected UTI episodes, respectively. Missing PoCT results were due to failed blood draws, inadequate sample volumes, or PoCT errors.
Figure 1.
Flow of suspected UTI episodes in the Point-of-care diagnostics to guide appropriate antimicrobial therapy of urinary tract infections in nursing homes (PROGRESS) study. a Multiple missing items required for reference testing can occur within 1 episode. b No symptom resolution info at Days 5 and 10. Abbreviations: AB, antibiotic therapy; CRP, C-reactive protein; PCT, procalcitonin; PoCT, point-of-care test; RTI, respiratory tract infection; UTI, urinary tract infection.
Characteristics of Participants
The 266 eligible suspected UTI episodes were identified in 186 unique participants (30.1% recurrent suspected UTIs; Table 1). The majority of participants were female (n = 144; 77.4%) and the median age was 87 years (range, 66–107). Participants were enrolled mainly from psychogeriatric wards (n = 130; 70.0%).
Table 1.
Baseline Characteristics Participants Point-of-care diagnostics to guide appropriate antimicrobial therapy of urinary tract infections in nursing homes (PROGRESS) Study
| Baseline characteristics | |
|---|---|
| Number of unique patients | 186 (30.1% recurrent infections) |
| Female | 144 (77.4%) |
| Median age, years | 87 (range, 66–107) |
| Type of ward | |
| Psychogeriatric | 130 (70.0%) |
| Rehabilitation | 29 (15.6%) |
| Somatic | 27 (14.5%) |
Data are shown as n (%) unless otherwise indicated.
Frequency of “True” UTIs
Of the 247 suspected UTI episodes with a complete reference test, in 180 episodes at least 2 symptoms required for the stringent UTI definition were present (Table 2). Of these 180 episodes, urinary leucocytes were present in 146 episodes. In 79 of these 146 episodes, bacterial cultures were positive, and 65 of these 79 episodes were treated with antibiotics covering the isolated uropathogen(s). In 49 of these episodes the initial symptoms resolved during adequate antibiotic therapy, meaning that these 49 fulfilled our post hoc definition of a true UTI (19.8%). Ten suspected UTI episodes were related to an indwelling urinary catheter.
Table 2.
Presence of the 5 criteria required to fulfill the “true” urinary tract infection definition
| Presenting symptomsa | ||
|---|---|---|
| ≥2 symptoms present | 180 | 72.9% (180/247) |
| Dipstick results,b leucocytes positive | 146 | 81.1% (146/180) |
| Number of positive bacterial culturesc | 79 | 54.1% (79/146) |
| Number of prescribed antibiotics covering identified uropathogen(s)d | 65 | 82.3% (65/79) |
| Symptom resolutione | 49 | 75.4% (49/65) |
| Total number of “true” UTIs present based on stringent definition | 49 | 19.8% (49/247) |
Abbreviations: CFU, colony-forming units; UTI, urinary tract infection.
a Presence of at least 2 urinary or nonspecific symptoms.
b Positive urine leucocyte esterase test: ≥1 + leucocytes detected by Combur2 dipstick analysis.
c Positive bacterial culture was defined as the presence of 1 or 2 uropathogens at ≥104 CFU/mL.
d Proven susceptibility of isolated uropathogens to the administered antibiotic, in case of positive urine culture.
e Resolution of the initially presented symptoms during follow-up; unknown symptom resolution is no symptom resolution. In case of missing data on symptom resolution at Day 10, symptom resolution at Day 5 was used.
Primary Outcome
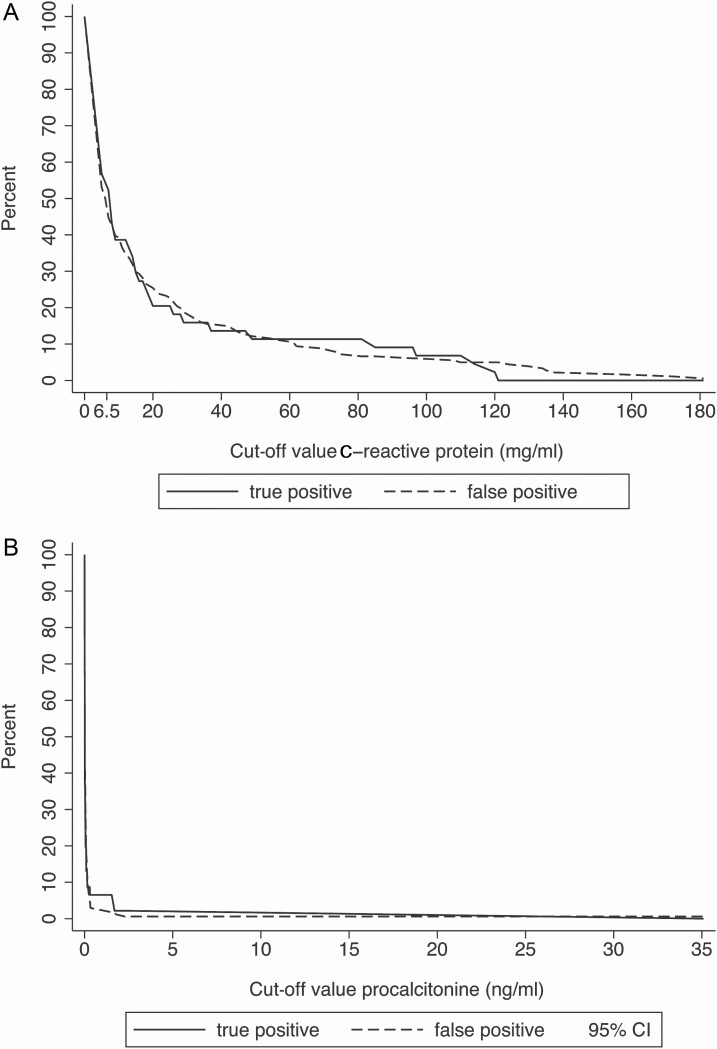
The empirical estimated cut-off level for CRP was 6.5 mg/L, which resulted in a sensitivity of 57.2% (95% CI, 48.9–65.4%) and a specificity of 54.4% (95% CI, 51.8–57.0%; Table 3). For PCT, the empirical estimated cut-off level was 0.025 ng/mL, with a sensitivity of 35.3% (95% CI, 27.0–43.6%) and a specificity of 57.8% (95% CI, 54.5–61.1%). The corresponding AUROCs were 54.4% (95% CI, 50.0–58.9%) for CRP and 46.5 (95% CI, 41.9–51.0) for PCT. The poor ability to discriminate true and false-positive classifications is illustrated in Figure 2.
Table 3.
Sensitivity of C-reactive protein and procalcitonin to detect urinary tract infection
| Sensitivity (%) | Specificity (%) | AUROC (%) | |||||
|---|---|---|---|---|---|---|---|
| Marker | Empirical cut-off | Est | 95% CI | Est | 95% CI | Est | 95% CI |
| Primary analysis using the stringent “true” UTI definition | |||||||
| CRP | 6.5 mg/L | 57.2 | 48.9–65.4 | 54.4 | 51.8–57.0 | 54.4 | 50.0–58.9 |
| PCT | .025 ng/ml | 35.3 | 27.0–43.6 | 57.8 | 54.5–61.1 | 46.5 | 41.9–51.0 |
| Post hoc analysis using the clinical algorithma UTI definition [33] | |||||||
| CRP | 7.5 mg/L | 54.8 | 50.6–59.1 | 62.3 | 58.7–65.9 | 59.1 | 55.9–62.2 |
| PCT | .035 ng/mL | 42.9 | 38.2–47.6 | 69.4 | 65.8–73.0 | 57.0 | 53.6–60.4 |
Data were calculated using ROC empirical cut-off estimation in primary and post hoc analyses. Abbreviations: AUROC, area under the receiver-operator curve; CI, confidence interval; CRP, C-reactive protein; Est, point estimate; PCT, procalcitonin; ROC, receiver-operator curve; UTI, urinary tract infection.
a In this post hoc analysis, the clinical algorithm described in the Dutch guideline on UTIs in the vulnerable elderly [33] was used as reference standard. This algorithm is based on signs and symptoms and the presence of leucocytes or nitrite in urine, irrespective of bacterial culture results or symptom resolution during adequate antibiotic treatment.
Figure 2.
Classification curves for study-defined UTIs for (A) C-reactive protein and (B) procalcitonin. The poor ability to discriminate true and false-positive classifications is illustrated by the lines of the percentages of true and false-positive classifications, which are not separated over the full range of the marker values observed in the study. Abbreviations: CI, confidence interval; UTI, urinary tract infection.
No differences in empirical cut-off, sensitivity, specificity, or AUROC results were found using either a value for the LLQ of 0 or a value midway between 0 and the LLQ for both CRP and PCT.
Index Test Results
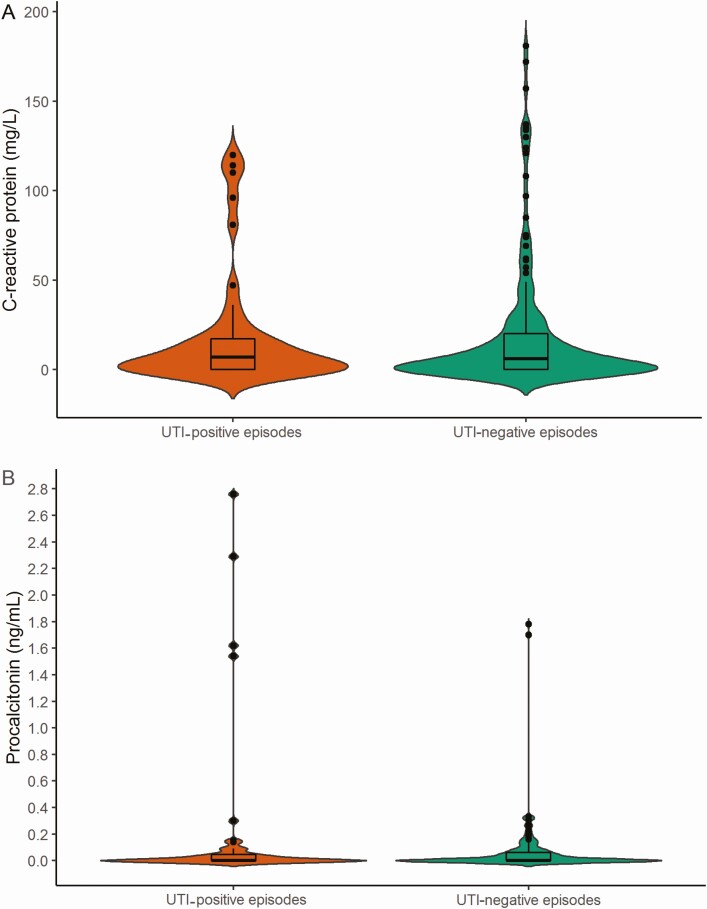
The concentrations of CRP and PCT were generally low, in terms of the number of episodes of suspected UTI that both met and did not meet the stringent UTI definition (Figure 3). The number of index test results below the LLQ were 46.2% and 58.8% for CRP and PCT, respectively. The median CRP concentrations in episodes with and without a study-defined UTI were 7.0 mg/L (interquartile range [IQR], 0–17.8 mg/L) and 6.0 mg/L (IQR, 0–20 mg/L), respectively. The median PCT concentrations in episodes with and without a true UTI were 0 ng/mL (IQR, 0–0.05 ng/mL) and 0 ng/mL (IQR, 0–0.06 ng/mL), respectively.
Figure 3.
Distribution of (A) C-reactive protein and (B) procalcitonin concentrations in UTI episodes fulfilling or not fulfilling the stringent “true” UTI definition. Violin plots with a box plot overlay showing (A) the distribution of C-reactive protein in UTI-positive (orange; n = 44) and in UTI-negative episodes (green; n = 181) and (B) the distribution of procalcitonin in UTI-positive (orange; n = 46) and in UTI-negative episodes (green; n = 169). The widths of the orange and green areas show the proportion of cases along the concentration of C-reactive protein or procalcitonin. The box plots show the medians, interquartile ranges, and outliers. There was 1 observation left out: procalcitonin 35 ng/mL, UTI negative. UTI-negative episodes were defined as episodes that did not fulfill the stringent “true” UTI definition; UTI-positive episodes were defined as episodes that did fulfill the stringent “true” UTI definition. Abbreviation: UTI, urinary tract infection.
Post Hoc Analysis
In our post hoc analysis, we included 252 of the eligible 266 episodes with full information on symptoms and dipstick results (see Supplementary File A). We identified 101 episodes (40.1%) fulfilling the latest Dutch UTI guideline definition [33]. Of the 252 episodes, 227 could be used in the CRP analysis and 219 could be used in the PCT analysis. The sensitivities of CRP (empirical cut-off 7.5 mg/L) and PCT (empirical cut-off 0.035 ng/mL) remained low at 54.8% (95% CI, 50.6–59.1%) and 42.9% (95% CI. 38.2–47.6%), respectively (Table 3; for violin plots, see Supplementary File B).
Futility Analysis
Based on this unplanned interim analysis, we assessed whether the study should be stopped for futility. We assumed an identical proportion of the remaining participants to be classified as having a UTI according to our study definition. We calculated that for CRP, the sensitivity in the remaining population would need to be 97% to achieve 65% sensitivity, the a priori−defined minimal value, for the full sample size. For PCT, this minimal sensitivity could not be reached. The study was therefore discontinued for futility.
DISCUSSION
In nursing home residents, sensitivities of CRP and PCT, measured by PoCTs to identify UTIs and fulfilling a stringent post hoc definition for true UTIs, were low. About half of all PoCT results were below the LLQ in both true UTI episodes and episodes not fulfilling the UTI definition. We hypothesize that UTIs in nursing home residents are mainly mild local infections (cystitis) that do not cause increased inflammatory markers in blood.
Strengths and Limitations of the Study
This is a prospective, multicenter study involving elderly nursing home residents, a frail, caregiver-dependent population that is understudied. Involving psychogeriatric residents in clinical research can be especially complex [34]. The lack of a reference test for diagnosing UTIs is a challenge. We aimed to circumvent this by using a stringent UTI definition for true UTIs, including a clinical response during adequate antibiotic treatment (based on drug-susceptibility results), to reduce misclassification of the outcome. Although this might have excluded some patients who did have a UTI, the post hoc analysis using less stringent criteria [33] showed very similar sensitivity results.
In our study, the very low concentrations of both CRP and PCT drove the poor performance of PoCTs to diagnose UTIs. With a very high frequency of test results being below the LLQ, a less stringent outcome definition (eg, the Dutch guidelines [33]) will likely not improve performance, as many “newly identified” UTIs will still have a test result below the LLQ. It is noteworthy that the definition of the Dutch guidelines is not exceptionally specific, but fits with the “everyday definition” in a primary care setting.
The study has some limitations. First, we enrolled a heterogeneous study population without performing subgroup analyses. The overall aim of the study was to assess the effect of a single marker on the diagnosis of UTIs in a routine setting of a nursing home. As such, we specifically did not want to divide the eligible population into subgroups, as this did does not align with the potential implementation of PoCTs, where most likely all nursing home residents would be eligible to be tested when there is clinical suspicion of a UTI on the part of the medical staff. In Supplemental File C, we calculated for both markers the number of participants with a test result below the LLQ, and compared these numbers between age groups, type of nursing home ward, and the likely presence of tissue involvement (as a proxy for distinguishing between upper and lower UTIs). We found no clear evidence that there are marked differences between these groups, which strengthens our resolve in the decision to perform the analysis on the total study population.
Second, we also did not take into account differences in urine collection methods. Collection of urine samples in the elderly can be challenging, mostly due to a high prevalence of urine incontinence. To enhance study feasibility, we accepted various urine collection methods, such as insert pans and incontinence pads, for dipstick urinalysis and bacterial cultures. Based on test results of urine collected by diapers [29], we do not expect the different collection methods to have notably comprised our reference test. In addition, we only enrolled 8 diaper samples during this study (3.2%), which further limited the risk of influencing our reference test.
Third, the clinical judgement to suspect a UTI might have differed among staff members, across different locations, or across time (due to the published new guideline), potentially causing clinical heterogeneity in the study population by influencing the pretest probability of the patient having a UTI. Yet, this clinical uncertainty is exactly the rationale of this study and reflects the setting where PoCTs, in cases of sufficient sensitivity, would have been implemented.
Lastly, PoCTs were performed off site because of logistical reasons. However, all PoCTs were performed within 4 hours after blood collection, and all analyzers were adequately validated for delayed testing by in-depth experiments before the start of the study.
Comparison With Other Studies
A retrospective study investigating PCT in lower UTIs in adults (mean age, 77.1 years) found a PCT sensitivity of 67% at a cut-off level of 0.25 ng/mL [25]. This cut-off is 10-fold higher than the cut-off found in our study. An explanation for the discrepancy may be the study location: at an emergency ward patients are likely to be severely ill, while UTIs in nursing home residents are mostly mild and in the lower tract [23]. Another difference with our study was that this study aimed to rule out UTIs (below a cut-off level of 0.25 ng/mL), instead of ruling in UTIs (above the determined PCT cut-off level). Finally, the definition of a UTI used was only based on typical UTI symptoms, which are often not present in elderly nursing home patients.
In another study, which aimed to reduce antibiotic use for UTIs in adults at an emergency ward, around half of the patients had a PCT value of ≥0.25 μg/L [24]. Here, two-thirds of the enrolled patients had a case of “febrile” UTI or pyelonephritis, while in our study systemic symptoms (fever, flank pain, and/or chills) were present in fewer than a quarter of the UTI episodes. Moreover, in the elderly patients (≥70 years) in the emergency ward, antibiotic therapy was initiated independent of PCT levels (which were not shown), since UTIs in this group were seen as complicated.
In sum, 2 earlier studies showed that PCT could contribute to diagnosing UTIs in elderly patients at the emergency ward, while we did not show this added value in our study population of nursing home residents. As far as we know, there are currently no comparable studies on the use of CRP in elderly or nursing home patients for diagnosing UTIs.
Conclusions, Policy Implications, and Questions for Future Research
CRP and PCT PoCTs do not contribute to diagnosing UTIs in elderly nursing home residents, possibly because these cases are mainly of relatively mild infections of the lower urinary tract without systemic inflammation. Diagnosing UTIs in this population remains complex due to the high prevalence of nonspecific symptoms and ASB. The necessity of a diagnostic test in this population is clear, and future research should focus on novel urinary markers to distinguish ASB from true UTIs to guide appropriate antimicrobial therapy.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
S. D. K. took part in data collection, analyzed the results, and wrote the manuscript. S. H. took part in data collection. F. v.L., J. C. F., J. H., C. M. P. M. H., J. M. P., M. D. d.J., and C. S. designed the study and secured funding for this project. C. S., F. v.L., and M. D. d.J. monitored the study and wrote the first draft of the manuscript. F. v.L. provided the statistical analysis designs and database design and analyzed the results. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All data for the Point-of-care diagnostics to guide appropriate antimicrobial therapy of urinary tract infections in nursing homes (PROGRESS) project will be made available in Figshare through a dedicated project page.
Acknowledgments. The authors thank all residents for participating in this study and all physicians and nurses of the participating nursing home wards who completed the clinical report forms and collected urine samples.
Disclaimer. The funder had no role in the collection, management, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
Financial support. This work was supported by The Netherlands Organization for Health Research and Development, ZonMW (grant number 541001003).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med 2005; 165:2514–20. [DOI] [PubMed] [Google Scholar]
- 2. Lutters M, Vogt-Ferrier NB. Antibiotic duration for treating uncomplicated, symptomatic lower urinary tract infections in elderly women. Cochrane Database Syst Rev 2008:CD001535. doi: 10.1002/14651858.CD001535.pub2 [DOI] [PubMed] [Google Scholar]
- 3. Resultaten van Wekelijkse Surveillance. Referentiecijfers 2011–2015. Rijksinstituut voor Volksgezondheid en Milieu Surveilance Netwerk Infectieziekten in Verpleeghuizen. Available at: https://www.rivm.nl/documenten/referentiecijfers-incidentie-sniv-2011-2015. Accessed 20 January 2020. [Google Scholar]
- 4. Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am 2014; 28:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sobel JD, Kaye D.. Urinary tract infections. In: John EB, Raphael D, Martin JB, eds. Principles and practice of infectious diseases. 8th ed, Philadelphia: Saunders, Elsevier Inc., 2015:896. [Google Scholar]
- 6. Arinzon Z, Shabat S, Peisakh A, Berner Y. Clinical presentation of urinary tract infection (UTI) differs with aging in women. Arch Gerontol Geriatr 2012; 55:145–7. [DOI] [PubMed] [Google Scholar]
- 7. High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:149–71. [DOI] [PubMed] [Google Scholar]
- 8. Hedin K, Petersson C, Widebäck K, Kahlmeter G, Mölstad S. Asymptomatic bacteriuria in a population of elderly in municipal institutional care. Scand J Prim Health Care 2002; 20:166–8. [DOI] [PubMed] [Google Scholar]
- 9. Eberle CM, Winsemius D, Garibaldi RA. Risk factors and consequences of bacteriuria in non-catheterized nursing home residents. J Gerontol 1993; 48:M266–71. [DOI] [PubMed] [Google Scholar]
- 10. Nicolle L. Symptomatic urinary tract infection or asymptomatic bacteriuria? Improving care for the elderly. Clin Microbiol Infect 2019; 25:779–81. [DOI] [PubMed] [Google Scholar]
- 11. D’Agata E, Loeb MB, Mitchell SL. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc 2013; 61:62–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Buul LW, Veenhuizen RB, Achterberg WP, et al. Antibiotic prescribing in Dutch nursing homes: how appropriate is it? J Am Med Dir Assoc 2015; 16:229–37. [DOI] [PubMed] [Google Scholar]
- 13. Aabenhus R, Jensen JU, Jorgensen KJ, Hrobjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014:CD010130. doi: 10.1002/14651858.CD010130.pub2 [DOI] [PubMed] [Google Scholar]
- 14. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017; 10:CD007498. doi: 10.1002/14651858.CD007498.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonine-Guided Antibiotic Therapy. Comparative effectiveness review. Vol. 78. Rockville: Agency for Health Care Research and Quality, 2012:1–16. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/related_files/procalcitonin_executive.pdf. Accessed 26 November 2020. [PubMed] [Google Scholar]
- 16. Do NT, Ta NT, Tran NT, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health 2016; 4:e633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dwolatzky T, Olshtain-Pops K, Yinnon AM, et al. Procalcitonin in the elderly: normal plasma concentrations and response to bacterial infections. Eur J Clin Microbiol Infect Dis 2005; 24:763–5. [DOI] [PubMed] [Google Scholar]
- 18. Liu A, Bui T, Van Nguyen H, Ong B, Shen Q, Kamalasena D. Serum C-reactive protein as a biomarker for early detection of bacterial infection in the older patient. Age Ageing 2010; 39:559–65. [DOI] [PubMed] [Google Scholar]
- 19. Lai CC, Chen SY, Wang CY, et al. Diagnostic value of procalcitonin for bacterial infection in elderly patients in the emergency department. J Am Geriatr Soc 2010; 58:518–22. [DOI] [PubMed] [Google Scholar]
- 20. Sugimoto K, Shimizu N, Matsumura N, et al. Procalcitonin as a useful marker to decide upon intervention for urinary tract infection. Infect Drug Resist 2013; 6:83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chuang YC, Tyagi V, Liu RT, Chancellor MB, Tyagi P. Urine and serum C-reactive protein levels as potential biomarkers of lower urinary tract symptoms. Urol Sci 2010; 21:132–6. [Google Scholar]
- 22. Shaikh N, Borrell JL, Evron J, Leeflang MM. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst Rev 2015; 1:CD009185. doi: 10.1002/14651858.CD009185.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nace DA, Perera SK, Hanlon JT, et al. The improving outcomes of UTI management in long-term care project (IOU) consensus guidelines for the diagnosis of uncomplicated cystitis in nursing home residents. J Am Med Dir Assoc 2018; 19:765–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drozdov D, Schwarz S, Kutz A, et al. Procalcitonin and pyuria-based algorithm reduces antibiotic use in urinary tract infections: a randomized controlled trial. BMC Med 2015; 13:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levine AR, Tran M, Shepherd J, Naut E. Utility of initial procalcitonin values to predict urinary tract infection. Am J Emerg Med 2018; 36:1993–7. [DOI] [PubMed] [Google Scholar]
- 26. Kuil SD, Hidad S, Fischer JC, et al. Sensitivity of point-of-care testing C reactive protein and procalcitonin to diagnose urinary tract infections in Dutch nursing homes: PROGRESS study protocol. BMJ Open 2019; 9:e031269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bossuyt PM, Reitsma JB, Bruns DE, et al. STAndards for the Reporting of Diagnostic accuracy studies Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351:5527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Open Data Kit. Available at: http://www.opendatakit.org/. Accessed 31 January 2020.
- 29. Kuil SD, Hidad S, van Leth F, et al. Diaper urine a reliable alternative for obtaining urine samples for UTI diagnosis in elderly suffering from urine incontinence. In: Program and abstracts of the European Congress of Clinical Microbiology and Infectious Diseases ( Madrid, Spain). 2018. [Google Scholar]
- 30. StataCorp. Stata 14 base reference manual. College Station, Texas: StataCorp, 2015. [Google Scholar]
- 31. RStudio Team. RStudio: integrated development for R. Boston, Massachusetts:RStudio P, 2020. Available at: http://www.rstudio.com/. Accessed 20 January 2020. [Google Scholar]
- 32. Verbakel JY, Steyerberg EW, Uno H, et al. ROC curves for clinical prediction models part 1. ROC plots showed no added value above the AUC when evaluating the performance of clinical prediction models. J Clin Epidemiol 2020; 126:207–16. [DOI] [PubMed] [Google Scholar]
- 33. Hertogh CMPM, . Haaijman J. Richtlijn Urineweginfecties bij kwetsbare ouderen. Verenso: Vereniging van specialisten ouderengeneeskunde, 2018. Available at: https://www.verenso.nl/richtlijnen-en-praktijkvoering/richtlijnendatabase/urineweginfecties. Accessed 20 January 2020. [Google Scholar]
- 34. Gustavson AM, Drake C, Lakin A, et al. ; Post-Acute Care Research and Team Science (PACRATS) Investigators. Conducting clinical research in post-acute and long-term nursing home care settings: regulatory challenges. J Am Med Dir Assoc 2019; 20:798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.