Abstract
Background
Despite the burden of varicella, there is no universal varicella vaccination (UVV) program in the United Kingdom (UK) due to concerns that it could increase herpes zoster (HZ) incidence. We assessed the cost-utility of a first-dose monovalent (varicella [V]) or quadrivalent (measles-mumps-rubella-varicella [MMRV]) followed by a second-dose MMRV UVV program. GSK and MSD varicella-containing vaccines (VCVs) were considered.
Methods
Dynamic transmission and cost-effectiveness models were adapted to the UK. Outcomes measured included varicella and HZ incidences and the incremental cost-utility ratio (ICURs) over a lifetime horizon. Payer and societal perspectives were evaluated.
Results
The impact of V-MMRV and MMRV-MMRV UVV programs on varicella incidence was comparable between both VCVs at equilibrium. HZ incidence increased by 1.6%–1.7% over 7 years after UVV start, regardless of the strategies, then decreased by >95% at equilibrium. ICURs ranged from £5665 (100 years) to £18 513 (20 years) per quality-adjusted life-year (QALY) gained with V-MMRV and from £9220 to £27 101 per QALY gained with MMRV-MMRV (payer perspective). MMRV-MMRV was cost-effective in the medium- and long-terms with GSK VCV and only cost-effective in the long term with MSD VCV at a £20 000 per QALY gained threshold. Without the exogenous boosting hypothesis, HZ incidence decreased through UVV implementation. ICURs were most sensitive to discount rates and MMRV price.
Conclusions
A 2-dose UVV was demonstrated to be a cost-effective alternative to no vaccination. With comparable effectiveness as MSD VCV at lower costs, GSK VCV may offer higher value for the money.
Keywords: cost-utility, vaccination strategies, varicella, United Kingdom
A 2-dose universal varicella vaccination was demonstrated to be a cost-effective alternative to no vaccination. With comparable effectiveness as MSD varicella-containing vaccines (VCVs) at lower costs, GSK VCV may offer higher value for the money.
Varicella (chicken pox) is a preventable disease predominant in childhood that is caused by the varicella-zoster virus (VZV). After a primary infection, VZV remains dormant in the dorsal root ganglion and can reactivate at older ages, causing herpes zoster (HZ) with post-herpetic neuralgia as a possible complication. Usually, varicella incidence is highest in children aged <5 years, with high primary care, hospitalization, and mortality burdens [1–3]. Annual hospitalization costs were recently estimated to be approximately £7 million and indirect costs associated with parental off-work time indicated to contribute considerably to the economic impact of varicella [3, 4].
Live-attenuated varicella vaccines have a clinically acceptable profile and are effective in reducing varicella burden. Two monovalent varicella (V) vaccines are commonly used: the Oka-recombinant immunotoxin Varilrix (GSK, Belgium) and the Oka/Merck Varivax (MSD, United States). Other varicella-containing vaccine (VCV) formulations include the quadrivalent measles-mumps-rubella-varicella (MMRV) vaccines Priorix-Tetra (GSK, Belgium) and ProQuad (MSD, United States).
Both Varilrix and Varivax are licensed in the United Kingdom. However, varicella vaccination is limited to specific high-risk groups (nonimmune healthcare workers, close contacts of immunosuppressed individuals) [5]. In 2010, the Joint Committee on Vaccination and Immunisation (JCVI) did not recommend implementation of 2-dose childhood universal varicella vaccination (UVV) [6]. This decision was notably driven by the model-predicted increase in HZ incidence in the first 30–50 years following UVV, which would make the program cost-ineffective [7]. Brisson et al also reported an increase in HZ incidence following 1-dose UVV, which would offset the benefits associated with vaccination [8]. The theoretical assumption behind these models is that UVV reduces circulating VZV, thereby limiting reexposure to exogenous virus from varicella-infected individuals. Consequently, cell-mediated immunity (CMI) is not boosted and maintained above a threshold, which would increase the risk of HZ [9, 10]. Real-world data have not confirmed these model predictions [11]. An increase in HZ incidence over the past decade was reported in countries without UVV, including the United Kingdom [12]. Unlike van Hoek et al [7] and Brisson et al [13], Poletti et al [14] predicted a continuous decrease in HZ incidence in line with the progress of 2-dose UVV in the United Kingdom. They concluded that HZ incidence following UVV appeared to depend on the presence or absence of factors that promote a strong boosting intensity that may (or may not) be heavily affected by changes in varicella circulation due to UVV.
The question of UVV implementation in the United Kingdom is becoming increasingly important, with Ogunjimi et al’s immunological study demonstrating the limited protective effect of reexposure to varicella, with boosting occurring in only 17%–25% of grandparents who were exposed to varicella and lasting less than 1 year. Ethical concerns have also been raised regarding withholding the beneficial impact on mortality and morbidity of varicella vaccination in children in order to protect adults from HZ, especially with the recent licensure of a highly efficacious recombinant HZ subunit vaccine [11, 15].
The implementation of a 2-dose UVV program in the United Kingdom raises key questions about the age (12 or 13 months) at first dose vaccination, and implicitly the use of a monovalent or a quadrivalent varicella vaccine as first dose, given the crowded vaccination schedule at 12 months of age. Therefore, a dynamic transmission model was first used to evaluate the impact of UVV on varicella epidemiology. Cost-benefit and cost-utility analyses were performed to determine whether UVV could be recommended for implementation in the United Kingdom. Vaccination strategies using GSK or MSD VCV were compared in order to evaluate their impact on the epidemiology and economic burden of varicella.
METHODS
Vaccination Strategies
Note that we use a hyphen to indicate separation of the first dose from the second dose vaccine. Given the crowded vaccination schedule at 12 months of age, the following strategies were considered: (1) V-MMRV: first dose monovalent at 13 months (at 87% coverage), based on the proportion of children who received a first and second dose of MMR by their fifth birthday [16]. MMRV is assumed to replace the second-dose MMR given at 3 years and 4 months at equivalent coverage. It is further hypothesized that a stand-alone varicella vaccine at 13 months would result in lower coverage than a scenario that uses the first-dose MMR vaccination platform. Consequently, the coverage of the first and second doses were conservatively assumed to be similar. (2) MMRV-MMRV: first and second dose quadrivalent varicella vaccines. With this scenario, it is assumed that MMRV will replace MMR vaccines at equivalent coverages; with first- and second-dose ages of 12 months (at 95% coverage) and 3 years and 4 months (at 87% coverage), respectively.
In additional scenario analysis, the exogenous boosting assumption was excluded from the MMRV-MMRV; defining the scenario MMRV-MMRV-no boosting. Vaccination strategies were compared with no varicella vaccination.
Models
Descriptions of the dynamic and cost-utility models and associated inputs are provided in Table 1, Appendix Tables 1–3, and Appendix Figure 1. A stationary population was assumed (Appendix Figure 2), and the basic reproduction number was computed using the next-generation matrix method (Appendix Table 4). Economic model outcomes included the incremental cost-utility ratio (ICUR) and the net monetary benefit (NMB). A willingness-to-pay (WTP) threshold of £20 000 per quality-adjusted life-year (QALY) gained was considered, and results are reported at 20, 40, and 100 years post-UVV.
Table 1.
Key Model Input Parameters
| Parameter | Value | Source and Comments |
|---|---|---|
| Epidemiological and vaccine parameters | ||
| Duration of boosting or cell-mediated immunity (ie, number of years before protection returns to previous levels and recovered varicella to become HZ susceptible), 1/δ | Values of δ tested in calibration: 1, 2, 5, 10, and 20 years | Calibration |
| Value included in the analyses: 2 years | ||
| % of effective varicella contacts that boost against HZ by age, g | 0–49 years: 75.0% | [13] |
| 50–69 years: 71.0% | ||
| 70–79 years: 57.0% | ||
| 80+ years: 32% | ||
| Varicella vaccine efficacy after the first dose, Tv | GSK: 67.2% (62.3%–71.5%) | GSK [20] |
| MSD: 78.0% (76.6%–79.4%) | MSD [21, 22] | |
| Varicella vaccine efficacy after the second dose, Tv2 | GSK: 95.4% (94.0%–96.4%) | GSK [20] |
| MSD: 98.3% (97.3%–99.0%) | MSD [21] | |
| % HZ cases with PHN | 0–14 years: 0.00% (0.00%–0.38%) | [25] |
| 15–44 years: 6.28% (5.18%–7.62%) | ||
| 45–64 years: 10.75% (8.76%–12.94%) | ||
| 65+ years: 18.80% (15.62%–22.10%) | ||
| Relative propensitya for BKT HZ cases with PHN | 0.01 (0.00–0.05) | [26] |
| Risk of death associated with WT varicella | 0–14 years: 4.20E-07 (2.33E-07–6.53E-07) | Calculated from [3] |
| 15–44 years: 1.60E-07 (1.00E-07–2.20E-07) | ||
| 45–64 years: 3.37E-07 (2.32E-07–4.40E-07) | ||
| 65+ years: 8.70E-07 (6.30E-07–1.10E-06) | ||
| Relative propensitya for BKT HZ to cause death | 0.01 (0.00–0.01) | Assumption |
| % of WT varicella cases hospitalized | 0–14 years: 0.39% (0.31%–0.46%) | Calculated from [7] |
| 15–44 years: 0.80% (0.64%–0.96%) | ||
| 45–64 years: 1.90% (1.52%–2.28%) | ||
| 65 + years: 7.00% (5.60%–8.40%) | ||
| Relative propensity for BKT hospitalized varicella | 0.25 (0.16–0.35) | [27, 28] |
| % of HZ cases hospitalized | 0–14 years: 4.47% (3.57%–5.36%) | Calculated from [7] |
| 15–44 years: 0.00% (0.00%–0.00%) | ||
| 45–64 years: 1.00% (0.80%–1.20%) | ||
| 65+ years: 2.52% (2.02%–3.02%) | ||
| Quality-adjusted life-years lost per case | ||
| WT varicella | 0–14 years: 0.0040 (0.0032–0.0048) | [8] |
| 15+ years: 0.0050 (0.0040–0.0060) | ||
| BKT varicella | 0.0010 (0.0008–0.0012) | [8] |
| WT or BKT HZ without PHN | 0–14 years: 0.0220 (0.0100–0.0715) | [29] |
| 15–44 years: 0.0220 (0.0100–0.0715) | ||
| 45–64 years: 0.0222 (0.0100–0.0715) | ||
| 65+ years: 0.0238 (0.0117–0.0715) | ||
| WT or BKT HZ with PHN | 0–14 years: 0.1892 (0.1060–0.3140) | [29] |
| 15–44 years: 0.1892 (0.1060–0.3140) | ||
| 45–64 years: 0.1897 (0.1060–0.3140) | ||
| 65+ years: 0.2367 (0.1489–0.3140) |
Extensive details on the model parameters are provided in Appendix Tables 1 and 2.
The numbers between parentheses represent the 95% confidence interval.
Abbreviations: BKT, breakthrough; CI, confidence interval; HZ, herpes zoster; MSD, Merck Sharp & Dohme; PHN, post-herpetic neuralgia; WT, wild-type or natural disease.
a Compared with WT disease.
Vaccine Parameters
Primary vaccine failure after 1 dose of varicella vaccine was based on similar seroconversion rates between GSK and MSD monovalent varicella vaccines [17–19].
With respect to vaccine efficacy, estimates of 67.2% and 95.4% after the first and second doses were considered for GSK VCV, respectively [20]. Similarly, Kuter et al reported values of 94.4% and 98.3% after the first and the second dose for MSD VCV, respectively [21]. However, vaccinees were aged 1 to 12 years, which contrasts with the average age at vaccination of 14 months in GSK studies [20]. Chan et al indicated a lower risk of VZV infection as age increases and calculated an efficacy of 78.0% in 1-dose Varivax recipients aged 18 months [22]. Consequently, MSD VCV first- and second-dose efficacies of 78.0% and 98.3% were considered. Sensitivity analyses on vaccine parameters were conducted (Appendix Tables 5–7).
Price information was not available for MMRV vaccines as currently they are not marketed in the United Kingdom. GSK and MSD MMRV prices of £56.4 and £62.6 per dose were assumed, respectively (see Appendix for details). Monovalent prices were £27.3 and £30.3 per dose for GSK and MSD VCVs, respectively [23].
Febrile seizure is a common adverse event of measles-containing vaccines. Based on Ma et al, we performed a random effects meta-analysis on the risk of febrile seizure associated with first-dose MMRV, considering a follow-up period of 42 days post-vaccination and excluding coadministration with other vaccines [24]. A first-dose pooled risk of 2.4‰ (95% confidence interval [CI], 1.2‰–3.6‰) and 2.7‰ (95% CI, 1.2‰–4.2‰) was estimated for GSK and MSD MMRV vaccines, respectively. GSK and MSD VCV-associated injection site adverse events were 19.5% (95% CI, 14.2%–25.7%) and 21.7% (95% CI, 16.2%–28.0%), respectively [30].
RESULTS
Impact on Varicella Incidence
Within 5 years following UVV implementation, the incidence of wild-type (WT) varicella was reduced by half: GSK VCV from 12 565 to 5944 and 5900 per million with V-MMRV and MMRV-MMRV, respectively, and from 12 565 to 5884 and 5862 per million for MSD VCV. At equilibrium, the total (ie, WT and breakthrough [BKT]) incidence of varicella was reduced by 95.6% under MMRV-MMRV and by 91.0% under V-MMRV with GSK VCV (Figure 1A). Similarly, with MSD VCV, the total incidence of varicella was reduced by 96.9% and 93.8% under MMRV-MMRV and V-MMRV, respectively (Figure 1B). BKT varicella contributed to 46.2% and 69.2% of the total varicella incidence with GSK VCV compared with 38.0% and 63.7% with MSD VCV in V-MMRV and MMRV-MMRV, respectively.
Figure 1.
Total incidence of varicella and HZ. Yearly total incidence of varicella using GSK (A) or MSD (B) VCV per million. Yearly total incidence of HZ using GSK (C) or MSD (D) VCV. In black is the monovalent-quadrivalent varicella vaccine (V-MMRV) strategy corresponding to a first dose of monovalent varicella vaccine administered at age 13 months (at 87% coverage) followed by a second dose of quadrivalent varicella vaccine administered at age 3 years and 4 months (at 87% coverage). In purple is the quadrivalent-quadrivalent varicella vaccine (MMRV-MMRV) strategy corresponding to a quadrivalent varicella vaccine administered as first and second dose at ages 12 months (at 95% coverage) and 3 years and 4 months (at 87% coverage). Abbreviations: HZ, herpes zoster; MMRV, measles-mumps-rubella-varicella; MSD, Merck Sharp & Dohme; V, varicella; VCV, varicella-containing vaccine.
Impact on HZ Incidence
The estimated prevaccination incidence of HZ was 7469 per million. At equilibrium, the total incidence of HZ was reduced by 98.2% and 95.1% under MMRV-MMRV and V-MMRV with GSK VCV, respectively (Figure 1C). Similarly, the total incidence of HZ using MSD VCV decreased by 98.5% and 96.1% under MMRV-MMRV and V-MMRV, respectively (Figure 1D). HZ in vaccinees represented 9.8% and 20.5% of all HZ cases in V-MMRV and MMRV-MMRV with GSK VCV. Comparatively, 7.0% and 16.2% of all HZ cases were predicted to occur in MSD VCV recipients under V-MMRV and MMRV-MMRV. With respect to the early effect of UVV on HZ, a maximum increase of 1.6% in the total incidence of HZ was predicted 4 and 3 years after UVV start with V-MMRV and MMRV-MMRV strategies using GSK VCV, respectively. Similarly, maximum increases of 1.6% and 1.7% in HZ incidence were predicted with V-MMRV and MMRV-MMRV 4 and 3 years after UVV start using MSD VCV, respectively. Overall, HZ incidence returned to levels below those of the prevaccination era as of the eighth year following UVV start.
Impact on Age at Varicella and HZ Infection
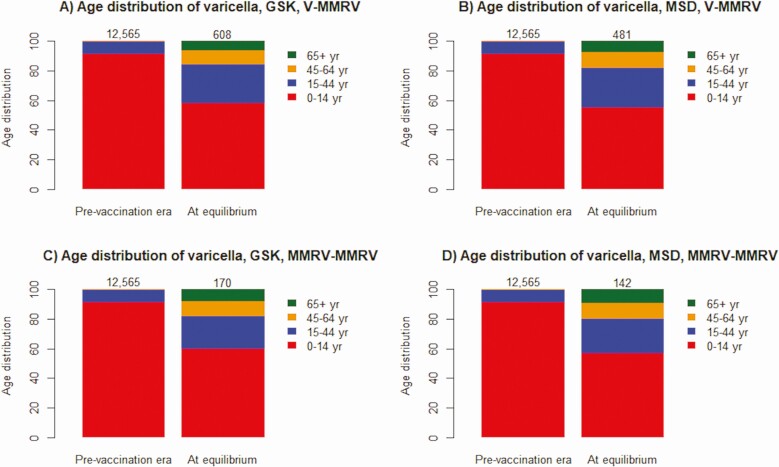
Acknowledging a reduction in varicella cases in all ages following UVV, Figure 2A and 2B show that 42.2% (respectively 45.2%) of varicella cases are predicted to occur among individuals aged >14 years at equilibrium compared with 9.0% in the same age group in the prevaccination era with GSK (respectively MSD) VCV under V-MMRV. Similarly, under MMRV-MMRV, 40.2% (respectively 43.2%) of varicella cases are predicted in those aged >14 years with GSK (respectively MSD) VCV at equilibrium (Figure 2C and 2D). Supplementary Figure 3 shows that the age distribution of HZ cases at equilibrium was almost comparable to that of the prevaccination era and at peak.
Figure 2.
Age distribution of wild-type (WT) varicella infection. Age distribution of varicella cases among individuals aged 0–14 (red), 15–44 (blue), 45–64 (orange), and 65 + (green) years as estimated for the monovalent-quadrivalent varicella vaccine (V-MMRV) strategy (top row) and the quadrivalent-quadrivalent varicella vaccine (MMRV-MMRV) strategy (bottom row) using GSK (left column) or MSD (right column) varicella-containing vaccines. Results are reported at the prevaccination era and at equilibrium (ie, 100 years following universal varicella vaccination implementation corresponding to a new steady state). The numbers above the bars indicate the WT annual incidence of varicella at the prevaccination era and at equilibrium. Abbreviations: MMRV, measles-mumps-rubella-varicella; MSD, Merck Sharp & Dohme; V, varicella.
Additional Scenario Analysis on Vaccination
Figure 3 shows that the MMRV-MMRV–no boosting scenario differed from MMRV-MMRV in predicting a continuous decrease in HZ incidence following UVV implementation. Additional analyses indicated that 1-dose UVV and targeted adolescent strategies were less effective in reducing varicella and HZ incidence compared with a 2-dose UVV, with the latter being the worst (eg, varicella incidence reduction of 55.3% (respectively 62.2%) and 5.2% (respectively 5.9%) with GSK (respectively MSD) monovalent vaccines for infant and adolescent strategies, respectively (see Supplementary Figure 4). These scenarios were excluded in subsequent economic analyses.
Figure 3.
Scenario analysis on the exogenous boosting hypothesis. Yearly total incidence of varicella using GSK (A) or MSD (B) VCV per million considering or not (no boosting) exogenous boosting. In green is the quadrivalent-quadrivalent varicella vaccines (MMRV-MMRV) strategy that included the exogenous boosting hypothesis with a duration of boosting or cell-mediated immunity of 2 years. In red is the equivalent varicella vaccination strategy, excluding the exogenous boosting hypothesis. At equilibrium, the incidence of HZ was reduced by 98.3% (respectively 98.2%) and 98.6% (respectively 98.5%) with GSK and MSD VCVs when no boosting (respectively boosting) was assumed, respectively. At the third year following universal varicella vaccination implementation, while the peak in HZ incidence was predicted under the exogenous boosting hypothesis, a 0.4% reduction in HZ incidence was predicted under the no boosting hypothesis for both GSK and MSD VCV. Abbreviations: HZ, herpes zoster; MMRV, measles-mumps-rubella-varicella; MSD, Merck Sharp & Dohme; VCV, varicella-containing vaccine.
Cost-Utility and Cost-Benefit Analyses
Table 2 summarizes cumulative discounted costs and QALYs, ICURs, and NMBs. The V-MMRV strategy using GSK or MSD VCV was cost-effective with ICURs less than £20 000 per QALY gained and positive NMBs increasing with the time horizon. The NMBs for strategies that used GSK VCV were consistently higher than for those that used MSD VCV, indicating greater value for the money independently of the vaccination strategy and time horizon. Of note, MMRV-MMRV was cost-effective with GSK VCV in the medium and long terms and only cost-effective in the long term with MSD VCV.
Table 2.
Cost-Utility and Cost-Benefit Results
| Vaccination Strategies | Total Direct Costs (£) | Total Indirect Costs (£) | QALYs Loss | ICUR (Total Direct, £ per QALY Gained) | ICUR (Total Direct and Indirect, £ per QALY Gained) | Net Monetary Benefits (Direct, £) |
|---|---|---|---|---|---|---|
| V-MMRV, short-term time horizon: 20 years | ||||||
| No vaccination | 2 290 273 101 | 1 639 488 089 | 414 965 | - | - | - |
| GSK VCV | 2 890 794 348 | 1 411 523 084 | 378 361 | 16 678 | 10 353 | 131 551 596 |
| MSD VCV | 2 964 774 791 | 1 416 879 723 | 377 941 | 18 513 | 12 174 | 65 819 225 |
| V-MMRV, medium-term time horizon: 40 years | ||||||
| No vaccination | 3 365 623 421 | 2 409 275 779 | 609 803 | - | - | - |
| GSK VCV | 4 249 272 900 | 1 944 223 034 | 536 160 | 12 181 | 5 769 | 589 216 311 |
| MSD VCV | 4 367 659 099 | 1 945 479 596 | 535 443 | 13 691 | 7 185 | 484 942 641 |
| V-MMRV, equilibrium: 100 years | ||||||
| No vaccination | 4 314 160 537 | 3 088 284 454 | 781 665 | - | - | - |
| GSK VCV | 5 192 494 305 | 2 160 120 734 | 624 542 | 5665 | Dominant | 2 264 126 949 |
| MSD VCV | 5 347 068 933 | 2 142 323 956 | 623 130 | 6608 | 454 | 2 137 496 944 |
| MMRV-MMRV, short-term time horizon: 20 years | ||||||
| No vaccination | 2 286 552 203 | 1 652 903 043 | 412 808 | - | - | - |
| GSK VCV | 3 191 078 916 | 1 415 800 543 | 374 975 | 24 301 | 17 932 | –147 857 617 |
| MSD VCV | 3 310 753 431 | 1 410 061 211 | 374 462 | 27 101 | 20 714 | –253 653 534 |
| MMRV-MMRV, medium-term time horizon: 40 years | ||||||
| No vaccination | 3 360 155 452 | 2 428 989 448 | 606 634 | - | - | - |
| GSK VCV | 4 708 266 138 | 1 937 708 532 | 530 617 | 18 021 | 11 455 | 172 232 041 |
| MSD VCV | 4 896 529 651 | 1 926 935 075 | 530 225 | 20 353 | 13 719 | –2 859 180 |
| MMRV-MMRV, equilibrium: 100 years | ||||||
| No vaccination | 4 307 151 525 | 3 113 554 046 | 777 602 | - | - | - |
| GSK VCV | 5 781 037 871 | 2 118 158 312 | 615 335 | 9220 | 2994 | 1 771 454 409 |
| MSD VCV | 6 027 578 648 | 2 097 842 784 | 614 787 | 10 642 | 4345 | 1 542 711 358 |
Cost-utility and cost-benefit results for the monovalent and quadrivalent varicella vaccine (V-MMRV) strategy and the quadrivalent and quadrivalent varicella vaccine (MMRV-MMRV) strategy considering the National Health Service and the societal perspectives. Direct costs consisted of general practitioner visits, hospitalization-associated costs, and vaccination costs. Indirect costs were determined by the number of days off from work secondary to varicella or herpes zoster multiplied by the mean income. Cumulative discounted costs and outcomes are reported. Cost-utility and cost-benefit results were expressed as ICUR and net monetary benefits estimates, respectively. Evaluations were performed at short-term (20 years), medium-term (40 years), and long-term (100 years) time horizons. Discount rates of 3.5% were used for both costs and QALYs. Vaccination vs no-vaccination strategies were run independently for GSK and MSD VCVs; values for the no-vaccination strategy were from the GSK VCV-based scenarios. Additionally, for every analysis, a new Monte Carlo simulation was performed, resulting in values for the no-vaccination strategy being slightly different for the same time horizon.
Abbreviations: ICUR, incremental cost utility ratio; MMRV, measles-mumps-rubella-varicella; MSD, Merck Sharp & Dohme; QALY, quality-adjusted life-year; V, varicella; VCV, varicella-containing vaccine.
From a societal perspective, V-MMRV was cost-effective at £20 000 per QALY gained in the short, medium, and long terms with any vaccine but dominant (ie, less costly and more effective than no vaccination) with GSK VCV in the long term. Similarly, MMRV-MMRV demonstrated to be consistently cost-effective but cost-ineffective with MSD VCV in the short-term.
The cost effectiveness acceptability curve shows the likelihood of UVV strategies to be cost-effective at a WTP threshold. Figure 4A indicates that the probability of V-MMRV being cost-effective at £20 000 per QALY gained with GSK VCV was 88.7% at 20 years and 100.0% at other time horizons. Corresponding values for MSD VCV were 73.0% at 20 years, 99.6% at 40 years, and 100.0% at 100 years. The probability of MMRV-MMRV being cost-effective at £20 000 per QALY gained with GSK and MSD VCVs was 9.6% and 2.5% at 20 years, 78.2% and 46.3% at 40 years, and 100% for both at 100 years, respectively (Figure 4B).
Figure 4.
CEACs for base-case immunizations. CEACs showing the probability of the monovalent-quadrivalent varicella vaccines (V-MMRV) strategy (left column) and the quadrivalent-quadrivalent varicella vaccines (MMRV-MMRV) strategy (right column) to be cost-effective using GSK (magenta) or MSD (brown) VCVs compared with no vaccination for a range of willingness-to-pay (WTP) thresholds. Results are reported at short- (20 years), medium- (40 years), and long-term (100 years) time horizons. The vertical line represents the WTP threshold of £20 000 per QALY gained. Abbreviations: CEAC, cost-effectiveness acceptability curve; MMRV, measles-mumps-rubella-varicella; MSD, Merck Sharp & Dohme; QALY, quality-adjusted life-year; V, varicella; VCV, varicella-containing vaccine.
Sensitivity Analyses on Cost-Utility Outcomes and MMRV Price
In 1-way sensitivity analysis, discount rates and MMRV costs were identified as parameters that ICURs were most sensitive to (Appendix Figures 5 and 6). Using a discount rate of 1.5% for costs and QALYs, absolute NMB values increased for V-MMRV and MMRV-MMRV (data not shown). On the other hand, cost-effectiveness results did not change, except that MSD VCV-based MMRV-MMRV turned to be cost-effective at 40 years. The direct medical breakeven price was £74.2 and £71.1 for GSK and MSD MMRV after 20 years of UVV, respectively. MMRV price parity analysis showed that the cost-effectiveness probability of V-MMRV with MSD VCV increased to 87.2%, 100.0%, and 100.0% at 20, 40, and 100 years at £20 000 per QALY gained, respectively (Appendix Figure 7A). The same analysis under MMRV-MMRV reported cost-effectiveness probabilities of 10.4%, 77.0%, and 100% at 20, 40, and 100 years at £20 000 per QALY gained, respectively (Appendix Figure 7B). This analysis further supports the comparable effectiveness of the vaccines and suggests that MMRV price would drive the cost-effective utilization of GSK or MSD VCV in a UVV program.
DISCUSSION
In this study, we evaluated the impact of 2-dose UVV on varicella epidemiology and associated cost-effectiveness estimates. The epidemiological model showed that although different in efficacy, GSK and MSD VCVs presented comparable impact on the total incidence of varicella and HZ. These results were further confirmed when MMRV price parity in sensitivity analyses was assumed and agreed with Marin et al’s meta-analysis [31]. Additionally, we showed that the greater the coverage, the greater the reduction in varicella incidence (95.6%–96.9% with MMRV-MMRV compared with 91.0%–93.8% with V-MMRV at equilibrium). Holl et al demonstrated that out of efficacy, number of doses, dosing intervals, and coverage, ensuring high coverage remains the critical success factor when implementing UVV [32]. Related to this outcome is the question of age at first-dose vaccination. Adding a standalone monovalent varicella vaccine at 13 months to the national immunization program would pose a logistical challenge with an additional injection and visit, potentially hampering acceptance and vaccination coverage. The rationale for MMRV vaccines is to reduce the number of injections and vaccination visits and to increase overall acceptance, compliance, and coverage of the varicella vaccine [33]. This argues for the implementation of first-dose MMRV at 12 months of age in agreement with the vaccine product information. However, an approximately 2-fold increase in the risk of febrile seizure for 5–12 days after vaccination was reported in children aged 10–24 months who received a first-dose MMRV compared with those who received a first-dose MMR vaccine with or without varicella monovalent vaccine [24]. The approximately 2-fold increased risk could be translated into 1 extra febrile seizure per 2300–2600 MMRV doses. Therefore, it will be important for healthcare providers to inform parents about the risk of fever and seizure associated with first-dose MMRV. In Europe, first-dose MMRV is at 13 months of age in Italy [34], with a second dose between age 23 months and 6 years depending on the country [35].
The 2010 JCVI recommendation to not implement UVV was largely motivated by a predicted increase in HZ in the first 30–50 years post-UVV due to loss of exogenous boosting. The recommendation is based on model predictions that assume that exposure to VZV boosts immunity against HZ for 20 years and that 100% of those susceptible to HZ become immune due to contact with varicella [7, 8]. Ogunjimi et al’s immunological study aligned with an individual-based model for VZV that estimated the duration of boosting (DoB) to last for 1–2 years [36, 37]. Building further on that, Rafferty et al estimated a 2- to 7-year DoB by simultaneously varying the DoB and the coefficient that determines the annual loss of protection based on VZV CMI to ensure the best fit with epidemiological data [38]. In our study, a 2-year DoB was determined to be optimal based on calibration of VZV reactivation rates to best fit HZ empirical incidence data (see Appendix Materials for further details on DoB selection and calibrations, Appendix Figure 8–12). Our estimated DoB aligns, therefore, with Ogunjimi et al’s immunological assay results [36]. Consequently, only a 1.6%–1.7% marginal increase in HZ incidence compared with the prevaccination era was predicted for about 7 years following UVV. These results contrast with van Hoek et al’s predictions of 20% increase over 40–60 years in England [39] or 30–50 years in the United Kingdom [7]. Last, but not least, real-world data are inconsistent with model predictions of an increase in HZ incidence following UVV implementation [11, 40]. Overall, these contrasting results further highlight the complex interplay between UVV and HZ incidence. As expected, when considering no exogenous boosting, HZ incidence decreased from the start of UVV.
With respect to the long-term effect of UVV on HZ, our results aligned with the literature and showed a continuous decrease in HZ incidence. As a greater proportion of the population is vaccinated, the naturally infected cohort is progressively replaced by the vaccinated cohort. As a result, and given the lower reactivation rate of vaccine strain compared with WT strain VZV [41], HZ incidence is expected to decrease. From the Humes et al study, this cohort effect starts being visible with a lower rate of HZ hospitalization in the 0–14 age group in the post-UVV era compared with the equivalent age group in the prevaccination era [42]. Weinmann et al also reported the benefit of UVV to prevent pediatric HZ, with a 72% reduction in the HZ incidence among vaccinees aged 0–17 years vs age-equivalent unvaccinated children aged >12 years [43]. This cohort effect, together with the predicted marginal increase in HZ cases, explains the age distribution of HZ cases in the long term. The increase in HZ cases could be explained by the sudden decline in the force of boosting caused by the introduction of the vaccine, which is accompanied by an increased flow from a varicella-recovered state to an HZ-susceptible state.
Congruent with the literature, UVV is further predicted to result in an age shift of varicella toward older age groups in the long term, with lower incidence rates across all age groups [14, 32, 44]. Most of the predicted varicella cases in older age groups are mild BKT cases.
From the National Health Service perspective, V-MMRV using GSK or MSD VCV was cost-effective in the short to long terms at £20 000 per QALY gained. For MMRV-MMRV, cost-ineffectiveness was demonstrated only in the short term with GSK VCV and in both short and medium terms with MSD VCV at £20 000 per QALY gained. These results indicate that the cost-effective benefits of a V-MMRV program would be visible in short to long horizons. For MMRV-MMRV, cost-effective benefits would be observed earlier with GSK than with MSD VCV. These differences between vaccines rest on vaccine prices. Under current MMRV price assumptions, GSK VCV appears to offer a higher value for the money, independent of the vaccination strategy.
Among the study limitations, demographic changes were not modeled to facilitate comparison with previous studies and because the focus was on the relative impact of vaccination vs no vaccination [45]. Additionally, the complex interplay between varicella and HZ is poorly understood, and model parameters for exogenous boosting are speculative and possibly oversimplified. For example, DoB is a determinant parameter of unknown value.
Next, the literature debates the range of seroprotection rates associated with MSD VCV [46–50]. To enable a straightforward comparison between GSK and MSD VCVs, seroconversion rates derived from clinical studies using the same laboratory assays and cutoffs have been used. Importantly, there is no correlate of protection against varicella.
In conclusion, we show that GSK and MSD VCVs have similar impact on the incidence of varicella and HZ. A high-coverage 2-dose UVV appears to be the most effective strategy to reduce the burden associated with varicella. The early impact of UVV on the incidence of HZ is also predicted to be marginal. Cost-utility analyses show that 2-dose UVV with either GSK or MSD VCV will be a cost-effective alternative to no vaccination, with MMRV price and discount rates being key drivers.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Christophe Sauboin for his contribution to model development, scientific advice on study conception, data sourcing, result interpretation, and manuscript review. The authors thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Benjamin Lemaire (Business & Decision Life Sciences) coordinated the manuscript development and editorial support.
Disclaimer. Varilrix and Priorix-Tetra are trademarks owned by or licensed to the GSK group of companies. Varivax and ProQuad are trademarks owned by or licensed to Merck Sharp & Dohme.
Financial support. GlaxoSmithKline Biologicals SA funded the study (HO-19-19880) and was involved in all stages of its conduct, including data analysis. GlaxoSmithKline Biologicals SA also covered all costs associated with manuscript development and publication.
Potential conflicts of interest. E. I. H. A., M. H., and G. C. are employees of the GSK group of companies and hold shares in the GSK group of companies. O. C. is a consultant for Creativ-Ceutical on behalf of GSK and received fees for performing project-related tasks. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bollaerts K, Riera-Montes M, Heininger U, et al. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect 2017; 145:2666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riera-Montes M, Bollaerts K, Heininger U, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis 2017; 17:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobbelen PH, Stowe J, Amirthalingam G, Miller L, van Hoek AJ. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013. J Infect 2016; 73:241–53. [DOI] [PubMed] [Google Scholar]
- 4. Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination—the global experience. Expert Rev Vaccines 2017; 16:833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Service NH. Chickenpox vaccine overview 2019. Available at: https://www.nhs.uk/conditions/vaccinations/chickenpox-vaccine/. Accessed 7 October 2020.
- 6. Immunisation JCoVa. Statement on varicella and herpes zoster vaccines 2010. Available at: https://webarchive.nationalarchives.gov.uk/20120907151317/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_133599.pdf. Accessed 7 October 2020.
- 7. van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine 2012; 30:1225–34. [DOI] [PubMed] [Google Scholar]
- 8. Brisson M, Edmunds WJ. Varicella vaccination in England and Wales: cost-utility analysis. Arch Dis Child 2003; 88:862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oxman MN. Herpes zoster pathogenesis and cell-mediated immunity and immunosenescence. J Am Osteopath Assoc 2009; 109:S13–7. [PubMed] [Google Scholar]
- 11. Wutzler P, Casabona G, Cnops J, Akpo EIH, Safadi MAP. Herpes zoster in the context of varicella vaccination—an equation with several variables. Vaccine 2018; 36:7072–82. [DOI] [PubMed] [Google Scholar]
- 12. Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 2010; 28:3385–97. [DOI] [PubMed] [Google Scholar]
- 14. Poletti P, Melegaro A, Ajelli M, et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PLoS One 2013; 8:e60732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finn A. The Hope-Simpson hypothesis and its implications regarding an effect of routine varicella vaccination on herpes zoster incidence. J Infect Dis 2018; 219:1681. [DOI] [PubMed] [Google Scholar]
- 16. Service NH. Childhood vaccination coverage statistics—England 2017–18. 2018. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-immunisationstatistics/england-2017-18. Accessed 7 October 2020.
- 17. Mufson MA, Diaz C, Leonardi M, et al. Safety and immunogenicity of human serum albumin-free MMR vaccine in US children aged 12-15 months. J Pediatric Infect Dis Soc 2015; 4:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faust SN, Le Roy M, Pancharoen C, et al. Safety and immunogenicity of a varicella vaccine without human serum albumin (HSA) versus a HSA-containing formulation administered in the second year of life: a phase III, double-blind, randomized study. BMC Pediatr 2019; 19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein NP, Abu-Elyazeed R, Povey M, et al. Immunogenicity and safety of a measles-mumps-rubella vaccine administered as a first dose to children aged 12 to 15 months: a phase III, randomized, noninferiority, lot-to-lot consistency study. J Pediatric Infect Dis Soc 2019; 9:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Povey M, Henry O, Riise Bergsaker MA, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect Dis 2019; 19:287–97. [DOI] [PubMed] [Google Scholar]
- 21. Kuter B, Matthews H, Shinefield H, et al. ; Study Group for Varivax . Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J 2004; 23:132–7. [DOI] [PubMed] [Google Scholar]
- 22. Chan IS, Li S, Matthews H, et al. Use of statistical models for evaluating antibody response as a correlate of protection against varicella. Stat Med 2002; 21:3411–30. [DOI] [PubMed] [Google Scholar]
- 23. Mokiou S, Standaert B, Li X, De Cock E. Measuring the cost of a pediatric vaccine administration in the UK. Vaccine 2018; 36:237–42. [DOI] [PubMed] [Google Scholar]
- 24. Ma SJ, Xiong YQ, Jiang LN, Chen Q. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: a systematic review and meta-analysis. Vaccine 2015; 33:3636–49. [DOI] [PubMed] [Google Scholar]
- 25. Van Oorschot DAM, Hunjan M, Bracke B, Lorenc S, Curran D, Starkie-Camejo H. Public health impact model estimating the impact of introducing an adjuvanted recombinant zoster vaccine into the UK universal mass vaccination programme (to be published). BMJ Open 2019; 9:e025553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melegaro A, Marziano V, Del Fava E, et al. The impact of demographic changes, exogenous boosting and new vaccination policies on varicella and herpes zoster in Italy: a modelling and cost-effectiveness study. BMC Med 2018; 16:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vázquez M, Shapiro ED. Varicella vaccine and infection with varicella-zoster virus. N Engl J Med 2005; 352:439–40. [DOI] [PubMed] [Google Scholar]
- 28. Thiry N, Beutels P, Van Damme P, Van Doorslaer E. Economic evaluations of varicella vaccination programmes: a review of the literature. Pharmacoeconomics 2003; 21:13–38. [DOI] [PubMed] [Google Scholar]
- 29. Hunjan M, van Oorschot DA, Starkie Camejo H, Bracke B, Lorenc S, Curran D. PRM100—weighted method for estimating incidence of herpes zoster in the general population. Value Health 2018; 21:S372–3. [Google Scholar]
- 30. Lau YL, Vessey SJ, Chan IS, et al. A comparison of safety, tolerability and immunogenicity of Oka/Merck varicella vaccine and VARILRIX in healthy children. Vaccine 2002; 20:2942–9. [DOI] [PubMed] [Google Scholar]
- 31. Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics 2016; 137:e20153741. [DOI] [PubMed] [Google Scholar]
- 32. Holl K, Sauboin C, Amodio E, Bonanni P, Gabutti G. Coverage, efficacy or dosing interval: which factor predominantly influences the impact of routine childhood vaccination for the prevention of varicella? A model-based study for Italy. BMC Public Health 2016; 16:1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kowalzik F, Faber J, Knuf M. MMR and MMRV vaccines. Vaccine 2018; 36:5402–7. [DOI] [PubMed] [Google Scholar]
- 34. Ministero della Salute - Vaccinazioni. Salute Md. Available at: http://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4814&area=vaccinazioni&menu=fasce. Accessed 23 November 2020.
- 35. Control ECfDPa. Vaccine schedules in all countries of the European Union. Available at: https://vaccine-schedule.ecdc.europa.eu/. Accessed 7 October 2020.
- 36. Ogunjimi B, Van den Bergh J, Meysman P, et al. Multidisciplinary study of the secondary immune response in grandparents re-exposed to chickenpox. Sci Rep 2017; 7:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogunjimi B, Willem L, Beutels P, Hens N. Integrating between-host transmission and within-host immunity to analyze the impact of varicella vaccination on zoster. eLife 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rafferty E, McDonald W, Qian W, Osgood ND, Doroshenko A. Evaluation of the effect of chickenpox vaccination on shingles epidemiology using agent-based modeling. PeerJ 2018; 6:e5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 2011; 29:2411–20. [DOI] [PubMed] [Google Scholar]
- 40. Harpaz R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Rev Vaccines 2019; 18:793–811. [DOI] [PubMed] [Google Scholar]
- 41. Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J 2009; 28:954–9. [DOI] [PubMed] [Google Scholar]
- 42. Humes EA, Weinberger DM, Kudish KS, Hadler JL. Trends in hospitalizations with primary varicella and herpes zoster during the prevaricella and initial postvaricella and herpes zoster vaccine eras, Connecticut, 1994–2012. Open Forum Infect Dis 2015; 2:ofv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weinmann S, Naleway AL, Koppolu P, et al. Incidence of herpes zoster among children: 2003–2014. 2019; 144:e20182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Horn J, Karch A, Damm O, et al. Current and future effects of varicella and herpes zoster vaccination in Germany—insights from a mathematical model in a country with universal varicella vaccination. Hum Vaccin Immunother 2016; 12:1766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Horn J, Damm O, Greiner W, et al. Influence of demographic changes on the impact of vaccination against varicella and herpes zoster in Germany—a mathematical modelling study. BMC Med 2018; 16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Michalik DE, Steinberg SP, Larussa PS, et al. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J Infect Dis 2008; 197:944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arvin A, Gershon A. Control of varicella: why is a two-dose schedule necessary? Pediatr Infect Dis J 2006; 25:475–6. [DOI] [PubMed] [Google Scholar]
- 48. Shinefield H, Black S, Williams WR, et al. ; Dose Selection Study Group for Proquad . Dose-response study of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J 2005; 24:670–5. [DOI] [PubMed] [Google Scholar]
- 49. Shinefield H, Black S, Digilio L, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J 2005; 24:665–9. [DOI] [PubMed] [Google Scholar]
- 50. Ma SJ, Li X, Xiong YQ, Yao AL, Chen Q. Combination measles-mumps-rubella-varicella vaccine in healthy children: a systematic review and meta-analysis of immunogenicity and safety. Medicine (Baltimore) 2015; 94:e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.