Abstract
Background
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involves severe fatigue, unrefreshing sleep, and cognitive impairment, leading to functional difficulties; prior studies have not evaluated risk factors with behavioral and immune data collected before developing ME/CFS. Up to 5% of university students develop infectious mononucleosis (IM) annually, and 9–12% meet criteria for ME/CFS 6 months later. We sought to determine predictors of ME/CFS.
Methods
We enrolled college students at the start of the school year (time 1), identified those who developed IM (time 2), and followed them for 6 months (time 3), identifying 3 groups: those who developed ME/CFS, severe ME/CFS (meeting >1 set of criteria), and who were asymptomatic. We conducted 8 behavioral and psychological surveys and analyzed cytokines at 3 time points.
Results
238 of the 4501 students (5.3%) developed IM; 6 months later, 55 of the 238 (23%) met criteria for ME/CFS and 157 (66%) were asymptomatic. 67 of the 157 asymptomatic students served as controls. Students with severe ME/CFS were compared with students who were asymptomatic at 3 time points. The former group was not different from the latter group at time 1 (prior to developing IM) in stress, coping, anxiety, or depression but were different in several behavioral measures and had significantly lower levels of IL-6 and IL-13. At time 2 (when they developed IM), the 2 ME/CFS groups tended to have more autonomic complaints and behavioral symptoms while the severe-ME/CFS group had higher levels of IL-12 and lower levels of IL-13 than the recovered group.
Conclusions
At baseline, those who developed ME/CFS had more physical symptoms and immune irregularities, but not more psychological symptoms, than those who recovered.
Keywords: infectious mononucleosis, chronic fatigue syndrome, myalgic encephalomyelitis
Of 4501 college students, 238 (5.3%) developed infectious mononucleosis; 55 (23%) met criteria for myalgic encephalomyelitis/chronic fatigue syndrome 6 months later. Those who recovered had significantly less fatigue and higher levels of cytokines at baseline, but not more psychological symptoms.
(See the Editorial Commentary by Feder and Wormser on pages e3747–9.)
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) involves severe fatigue, unrefreshing sleep, and cognitive impairment, leading to functional difficulties. Of university students, 1% to 5% develop infectious mononucleosis (IM) annually [1], and 9–12% of individuals meet criteria for ME/CFS 6 months later [2–5]. Severity of IM correlates with the development of ME/CFS [3, 6, 7].
Other studies have examined incomplete recovery from infection. In 1 study, of 26 individuals who subsequently developed influenza, 14 recovered within 2 weeks and 12 had symptoms for longer than 3 weeks (nonrecovered). Results of the Minnesota Multiphasic Personality Inventory, obtained prior to illness, showed a depressive propensity contributing to nonrecovery [8]. In another study, military recruits were assessed both psychologically and serologically for the development of IM. Approximately half became infected with Epstein-Barr virus, and approximately one-fourth of those developed symptomatic IM. High motivation and poor academic performance correlated with the development of symptomatic IM [9].
In a third study, we showed that autonomic symptoms approximately 2 months after the diagnosis of IM in 301 adolescents were significantly worse and the number of days in bed since IM significantly greater among those who went on to develop ME/CFS at 6 months [10]. At 24-month follow-up, levels of interleukin (IL)-2, IL-6, IL-8, and IL-23 could classify individuals as patients or controls with an accuracy of more than 80% [11].
Identification of risk factors predisposing to ME/CFS following IM may help uncover underlying mechanisms of illness. We report a longitudinal study of college students followed from pre-IM baseline to the development of IM, and then either for the development of ME/CFS or recovery. We analyzed behavioral, psychological, and immunological predictors of ME/CFS.
METHODS
E-mails were sent to all incoming students inviting them to participate. During the baseline pre-IM stage (time 1), we enrolled Northwestern University (NU) college students; no student baseline data were included if they developed IM within 6 weeks of enrollment. Infectious mononucleosis was diagnosed as previously described [7]. The NU Health Service and other medical providers diagnosed and tracked the IM. Briefly, any student with compatible symptoms was diagnosed with IM if they had a positive monospot or specific Epstein-Barr virus serologies (a positive viral capsid antigen [VCA] immunoglobulin [Ig] M or a positive VCA IgG with a negative Epstein-Barr nuclear antigen antibody). Students were compensated for their participation in each stage of the study.
After online consent, participants completed the DePaul Symptom Questionnaire (DSQ) [12], Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) [13], Compass 31 autonomic symptom questionnaire [14], Perceived Stress Scale (PSS) [15], Fatigue Severity Scale (FSS) [16], Coping Orientation to Problems Experienced (COPE) Scale [17], Beck Depression Inventory (BDI) [18], and the Beck Anxiety Inventory (BAI) [19]. These questionnaires were expected to take between 30 and 45 minutes to complete and could be completed in more than 1 sitting. Serum and plasma were stored.
Those students who went on to develop IM were enrolled (time 2) within 6 weeks of the diagnosis. Students were re-consented and completed the same questionnaires. Serum, plasma, and viable white blood cells (WBCs; for future anticipated functional analyses) were stored.
Five months after the IM diagnosis, participants were screened by phone to determine if they were recovered or experiencing ongoing symptoms. All students deemed not recovered and an equal number of recovered students (controls) matched by age, sex, and class status when IM developed were invited to participate in a time 3 assessment 6 months following the onset of IM. After again obtaining consent, in addition to a third round of questionnaires and another sample of serum, plasma, and viable WBCs, time 3 participants underwent a comprehensive medical and psychiatric examination to exclude medical causes of ME/CFS [4, 20]. The comprehensive psychiatric examination was used to exclude individuals with a psychiatric illness from being diagnosed with ME/CFS. Further analysis of these data is beyond the scope of this paper. This study was approved by all relevant institutional review boards before data collection began.
Questionnaires
Compass 31 assesses autonomic symptoms. It is validated, with good reliability [14].
The SF-36 measures the impact of participants’ health on physical and mental functioning, using 8 subscales: Physical Functioning, Role Physical, Bodily Pain, General Health, Social Functioning, Mental Health, Role Emotional, and Vitality. Higher subscale scores indicate less impairment. The SF-36 evidences good internal consistency and discriminant validity [13].
The DSQ assesses sociodemographic, medical, occupational, and social history and is a self-report measure of ME/CFS symptomatology and illness history [12] providing a standardized method for assessing various case definitions of ME/CFS, including Fukuda et al [20], Canadian Clinical [21], and Institute of Medicine (IOM) [22] criteria. Participants rate each symptom’s frequency and severity over the past 6 months on a 5-point Likert scale. Frequency and severity scores are multiplied by 25 to create 100-point scales, which are then averaged into 1 composite score. Higher scores indicate more problems. The DSQ has evidenced good test-retest reliability among patient and control groups [23]. An independent group [24] found that the DSQ demonstrated excellent internal reliability and good internal consistency and optimally differentiated patients and controls.
The FSS is a measure of fatigue that includes 9 items rated on 7-point scales [16], with a score of 4 or more on each item signifying more than moderate fatigue. The FSS can discriminate between individuals with ME/CFS, multiple sclerosis (MS), and primary depression [25] and was normed on a sample of individuals with MS, systemic lupus erythematosus, and healthy controls [16].
The COPE scale assesses how participants cope with stress [17] and has distinct problem-focused and emotion-focused scales. It is validated and has adequate reliability. There are 28 items from which 14 subscales are extracted. We summarized the 14 subscales into 3 second-order dimensions: emotion focused, problem focused, and dysfunctional coping [26].
The PSS is a 4-item measure of global perceived stress over the previous month, with higher scores indicating more stress [15]. The coefficient-ɑ reliability is 0.72.
The BDI-II measuring depressive symptomatology is a 21-item self-report with well-established psychometric properties [18]. Higher scores indicate more depression. It is one of the only depression rating scales tested and interpreted for depressed and nondepressed patients with ME/CFS [27, 28].
The BAI measuring anxiety is a 21-item self-report instrument with high internal consistency and adequate reliability [19]. Higher scores indicate more anxiety.
Biological Samples
We obtained peripheral blood for serum, plasma, and viable WBC storage. Serum and plasma were separated and stored at −80°C in multiple aliquots; whole blood was spun down over a Ficoll-Hypaque gradient and cells were viably frozen in aliquots of 2 × 106 in liquid nitrogen.
For cytokine analysis, plasma was used. The following cytokines were evaluated using a multiple analyte platform and commercially customized kits from Millipore (Billerica, MA): IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12(p70), IL-13, IL-15, IL-17α, IL-23, interferon-γ (IFN-γ), tumor necrosis factor (TNF) α, and TNF-β. Each plasma sample was run in duplicate. We used the same cytokine panel as in our previous study [11].
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Case Definitions
Fukuda et al [20] criteria require persistent or relapsing fatigue for a period of 6 or more months concurrent with at least 4 of 8 somatic symptoms that do not predate the fatigue, including sore throat, lymph node pain, muscle pain, joint pain, postexertional malaise (PEM), new or different headaches, memory/concentration difficulties, and unrefreshing sleep. Participants also needed to experience substantial reductions in occupational, educational, or personal activities [28], by scoring at or below at least 2 of the 3 following subscale cutoffs on the SF-36: Role Physical of 50 or less, Social Functioning of 62.5 or less, and Vitality of 35 or less.
Canadian criteria [21] require unexplained, persistent, or relapsing chronic fatigue over the past 6 months that is not the result of ongoing exertion and not substantially alleviated by rest; a substantial reduction in previous levels of educational, social, and personal activities, as measured using the SF-36 cutoffs described above; PEM; unrefreshing sleep; myofascial, joint, abdominal, and/or head pain; and 2 or more neurocognitive manifestations (eg, memory impairment). Additionally, 1 symptom from 2 of the following 3 categories is required: autonomic (eg, neurally mediated hypotension), neuroendocrine (eg, recurrent feelings of feverishness), or immunologic (eg, recurrent flu-like symptoms).
The IOM criteria [22] were operationalized by having participants meet the following 4 criteria: (1) substantial reductions in functioning, as described above; (2) PEM; (3) sleep dysfunction (including unrefreshing sleep); and (4) neurocognitive impairment (eg, difficulty paying attention) or orthostatic intolerance (eg, dizziness). Substantial reduction is again measured using the SF-36 cutoffs described. The symptoms needed to occur at least half of the time with at least moderate severity.
Participants’ 6-month medical examination and their DSQ and SF-36 results from times 1–3 were evaluated to determine whether they met 1 or more of the 3 case definitions of ME/CFS; participants who met more than 1 case definition were defined as having severe ME/CFS (S-ME/CFS) [7].
Statistical Analysis
For survey data, a mixed-model analysis of variance was conducted on the questionnaires between the 3 groups (ME/CFS, S-ME/CFS, Recovered) and time 1, time 2, and time 3. For each questionnaire we examined the effect of time (time 1, time 2, time 3) and group (ME/CFS, S-ME/CFS, Recovered) and the interaction of time and group. Post hoc comparisons were conducted using either Bonferroni or Games-Howell (for unequal variances), where appropriate. Because of multiple comparisons the cutoff criterion for P values was .01.
Cytokine concentrations were measured (pg/mL) and were analyzed after logarithmic transformation [11]. Because the data were not normally distributed, we used nonparametric Friedman tests to examine changes in cytokine expression over time, nonparametric Kruskal-Wallis tests to compare the overall difference in groupwise expression for each cytokine, and followed up with the Mann-Whitney test. Values below the detection limit were replaced with half the value of the lowest concentration for each cytokine. Of the 363 possible biological samples from students who participated through all 3 times, 20 (5.5%) were incomplete. One individual had cytokine values outside of the linear range and those data were excluded. Statistical significance was evaluated using a Monte Carlo exact method [29]. The resampling method calculated the parameter distributions and estimations at the 99% confidence interval. Because cytokine analyses involved fewer comparisons than survey data, and used less powered nonparametric statistics, we report on some cytokine data that approached significance (from P < .05 to >.01).
RESULTS
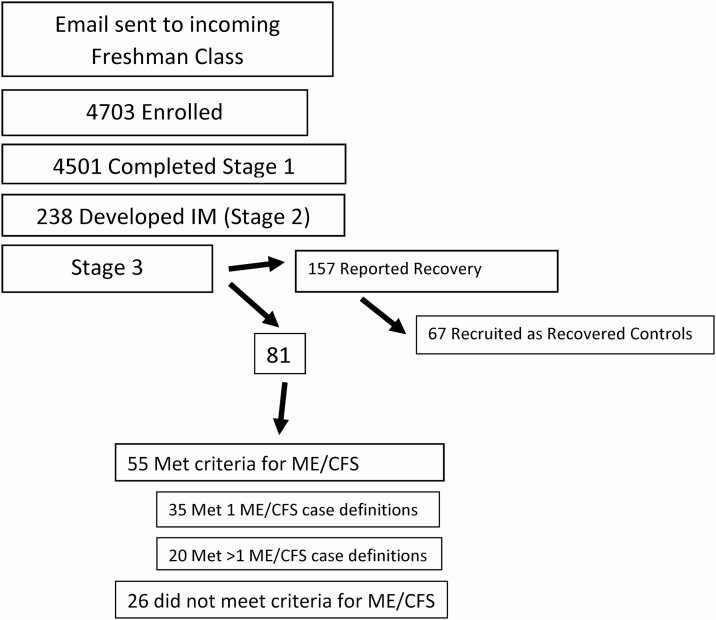
A total of 4703 NU students were enrolled between 5 March 2014 and 30 June 2018; 4501 completed the surveys and had blood taken at time 1, over 80% of whom were freshmen. About half of each incoming class enrolled in our study. Two hundred and thirty-eight (5.3%) developed IM by 30 June 2019, and were followed for a total of 338.92 person-years. Three additional students developed IM within 6 weeks of enrolling and their stage 1 data were not used. At time 3, 157 students were no longer symptomatic. Sixty-seven of the 157 asymptomatic students were recruited as matched, recovered controls. At time 3, 55 students (23%) met criteria for ME/CFS, 20 of whom (8%) met more than 1 case definition of ME/CFS and were termed S-ME/CFS. Students who did not fully recover but who did not meet criteria for ME/CFS (n = 26) were not analyzed (see Figure 1).
Figure 1.
Outline of study. Abbreviations: IM, infectious mononucleosis; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome.
For the behavioral and psychological survey measures, complete data were available for 109 participants at all 3 time points (18 S-ME/CFS, 31 ME/CFS, 60 Recovered). There were some data loss from the original sample; however, there were no sociodemographic differences between cases with missing data versus cases without missing data on gender, race, or received diagnosis (data not shown).
Statistically significant sociodemographic differences were not found between the 3 groups (Table 1). The percentage of females and ethnic/racial mix of participants roughly correlated with the percentages present in each class at NU.
Table 1.
Baseline Demographic Information
| Characteristics | S-ME/CFS (n = 18) | ME/CFS (n = 31) | Recovered (n = 60) |
|---|---|---|---|
| Age, mean (SD), years | 18.80 (.45) | 18.86 (.85) | 18.67 (2.56) |
| Gender, % (n) | |||
| Female | 61.1 (11) | 74.2 (23) | 55.0 (33) |
| Male | 38.9 (7) | 25.8 (8) | 45.0 (27) |
| Race, % (n) | |||
| White/Caucasian | 52.9 (9) | 71.0 (22) | 73.3 (44) |
| Latinx or Hispanic | 29.4 (5) | 16.1 (5) | 6.7 (4) |
| Black/African American | 11.8 (2) | 3.2 (1) | 10.0 (6) |
| Asian or Pacific Islander | 5.9 (1) | 6.5 (2) | 3.3 (2) |
| Middle Eastern | 0 (0) | 0 (0) | 1.7 (1) |
Abbreviations: ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; S-ME/CFS, severe myalgic encephalomyelitis/chronic fatigue syndrome.
Compass 31
There was a main effect of group, time, and their interaction in Compass 31 results (Table 2) (P < .001). At time 1, there were no significant differences between the 3 groups. At time 2, the Recovered group scored significantly better than both ME/CFS groups, and the S-ME/CFS group scored significantly worse than the ME/CFS group. At time 3, the Recovered group scored significantly better than both ME/CFS groups.
Table 2.
Summary and Domain Scores of Survey Measures Over Time
| Inventory | Time 1 | Time 2 | Time 3 |
|---|---|---|---|
| Compass 31 | |||
| S-ME/CFS | 19.18 (15.83) | 33.83 (10.08)a,b | 29.57 (15.42)d |
| ME/CFS | 15.73 (10.00) | 22.01 (13.84)a,c | 22.53 (11.45)e |
| Recovered | 11.03 (10.51) | 13.48 (11.03)b,c | 09.57 (09.27)d,e |
| SF-36 | |||
| Physical health | |||
| S-ME/CFS | 54.38 (06.87) | 39.62 (06.20) | 44.18 (08.49)a |
| ME/CFS | 53.75 (05.81) | 40.97 (09.39) | 49.04 (06.13)b |
| Recovered | 55.31 (05.42) | 44.08 (08.37) | 56.00 (04.27)a,b |
| Mental health | |||
| S-ME/CFS | 40.18 (11.25) | 29.50 (10.60)a | 30.42 (09.81)c |
| ME/CFS | 42.46 (11.08) | 36.99 (11.10)b | 35.03 (10.14)d |
| Recovered | 46.84 (08.69) | 45.14 (09.44)a,b | 48.31 (07.50)c,d |
| DSQ | |||
| S-ME/CFS | 21.15 (10.30)a | 36.13 (12.07)c,d | 37.30 (13.50)f,g |
| ME/CFS | 16.86 (08.72)b | 26.66 (09.73)c,e | 19.61 (08.78)f,h |
| Recovered | 12.19 (07.27)a,b | 17.22 (08.08)d,e | 08.93 (05.70)g,h |
| FSS | |||
| S-ME/CFS | 36.72 (09.42)a | 50.39 (10.15)b | 46.78 (10.07)c |
| ME/CFS | 32.61 (11.00) | 43.10 (12.54) | 38.87 (08.64)d |
| Recovered | 27.42 (10.39)a | 36.17 (11.94)b | 23.38 (09.94)c,d |
| COPE | |||
| Emotional focused | |||
| S-ME/CFS | 19.72 (07.89) | 20.17 (06.54) | 19.94 (07.12) |
| ME/CFS | 22.29 (05.02) | 22.58 (04.19) | 21.39 (05.43) |
| Recovered | 20.75 (04.98) | 21.17 (04.77) | 20.45 (05.35) |
| Problem focused | |||
| S-ME/CFS | 12.50 (04.16) | 13.33 (04.78) | 13.72 (04.53) |
| ME/CFS | 14.68 (03.99) | 14.74 (03.85) | 15.19 (05.55) |
| Recovered | 14.27 (03.95) | 14.03 (03.66) | 14.02 (04.69) |
| Dysfunctional | |||
| S-ME/CFS | 18.44 (05.78) | 20.89 (06.45) | 20.11 (05.44) |
| ME/CFS | 20.26 (04.70) | 21.71 (05.18)a | 20.52 (04.74) |
| Recovered | 18.45 (03.41) | 17.57 (03.29)a | 17.90 (03.96) |
| PSS | |||
| S-ME/CFS | 07.28 (03.08) | 08.89 (02.97)a | 08.56 (03.20)c |
| ME/CFS | 06.71 (03.21) | 08.68 (02.68)b | 07.97 (02.77)d |
| Recovered | 05.58 (02.74) | 05.80 (02.87)a,b | 05.23 (02.64)c,d |
| BDI-II | |||
| S-ME/CFS | 11.39 (07.88) | 22.78 (14.09)a | 20.89 (10.51)c |
| ME/CFS | 08.97 (07.59) | 16.61 (09.84)b | 13.48 (08.33)d |
| Recovered | 05.43 (04.50) | 07.72 (06.47)a,b | 03.83 (04.26)c,d |
| BAI | |||
| S-ME/CFS | 09.22 (08.63) | 17.17 (10.46)a | 16.50 (10.95)c |
| ME/CFS | 07.81 (06.76) | 13.77 (08.71)b | 09.23 (07.45)d |
| Recovered | 03.88 (04.57) | 06.03 (06.73)a,b | 03.13 (03.87)c,d |
Data are presented as means (SD). Similar letters in columns for each domain indicate significant differences.
Abbreviations: BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; COPE, Coping Orientation to Problems Experienced; DSQ, DePaul Symptom Questionnaire; FSS, Fatigue Severity Scale; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; PSS, Perceived Stress Scale; S-ME/CFS, severe myalgic encephalomyelitis/chronic fatigue syndrome.
SF-36
For the physical health domain, there was a main effect for group, time, and their interaction (P < .001). There were no significant physical health domain differences at times 1 or 2, but at time 3, the Recovered group had significantly better scores than both ME/CFS groups. For the mental health domain, there was a main effect of group, time, and their interaction (P < .001). There were no significant differences between the 3 groups at time 1. Recovered group scores were significantly better than both ME/CFS groups at times 2 and 3.
DSQ
There was a main effect of group, time, and their interaction (P < .001). The Recovered group scored significantly better than both ME/CFS groups at all 3 times. The S-ME/CFS group scored significantly worse than the ME/CFS group at times 2 and 3.
FSS
There was a main effect of group, time, and their interaction (P < .001). At times 1 and 2, the Recovered group evidenced significantly less fatigue than the S-ME/CFS group. At time 3, the Recovered group evidenced significantly less fatigue than both the S-ME/CFS and the ME/CFS groups.
COPE
There were no main effects for either emotion- or problem-focused coping strategies between the 3 groups. For dysfunctional coping, there was a main effect of group (P < .001) but not for time or interaction. There were no significant differences between the 3 groups at times 1 or 3. However, at time 2, the ME/CFS group scored significantly worse than the Recovered group.
PSS
There was a main effect of group and time (P < .001); however, there was no significant interaction. There were no significant differences between the groups at time 1. The Recovered group was significantly less stressed than both ME/CFS groups at times 2 and 3.
BDI-II
There was a main effect of group, time, and their interaction (P < .001). At time 1, there were no significant differences between the 3 groups. At times 2 and 3, the Recovered group evidenced significantly less depression than both ME/CFS groups.
BAI
There was a main effect of group, time, and their interaction (P < .001). At time 1, there were no significant differences. At times 2 and 3, the Recovered group evidenced significantly less anxiety than both ME/CFS groups.
Cytokines
Complete data were available for 105 participants at all 3 time points (18 S-ME/CFS, 29 ME/CFS, 58 Recovered) (see Table 3). There were significant time effects for IL-6 (P = .01), IFN-γ (P < .01), and TNF-α (P < .01), and close to significant differences for IL-13 (P = .015). There were significant group effects for IL-5 (P = .01), IL-12(p70) (P = .01), IL-13 (P < .01), and TNF-β (P < .01). At time 1, the S-ME/CFS group had a significantly lower mean rank of cytokine expression than the Recovered group for IL-6 (P = .01) and IL-13 (P =< .01) and a close to significant difference for IL-5 (P = .02). Additionally, the ME/CFS group had a directionally lower mean rank of cytokine expression for IL-5 than the Recovered group (P < .03). At time 2, the S-ME/CFS group had a significantly lower cytokine expression of IL-13 than the Recovered group (P < .01). At time 3, the S- ME/CFS group had a significantly greater cytokine expression of IL-12(p70) than either the Recovered (P = .01) or the ME/CFS (P = .01) groups; additionally, cytokine expression for IL-13 was lower for the S-ME/CFS group than for the Recovered group (P < .04).
Table 3.
Summary of Cytokine Levels (pg/mL) Over Time
| Cytokine | Time 1 | Time 2 | Time 3 |
|---|---|---|---|
| IL-1α | |||
| S-ME/CFS | 3.75 (1.15) | 3.89 (1.16) | 4.22 (1.35) |
| ME/CFS | 4.09 (1.56) | 3.61 (1.64) | 4.09 (1.72) |
| Recovered | 3.94 (1.50) | 3.65 (1.51) | 3.81 (1.33) |
| IL-1β | |||
| S-ME/CFS | 1.89 (0.69) | 1.82 (0.53) | 2.01 (0.83) |
| ME/CFS | 1.71 (0.66) | 1.72 (0.72) | 1.74 (0.74) |
| Recovered | 1.79 (0.67) | 1.78 (0.75) | 1.81 (0.69) |
| IL-2 | |||
| S-ME/CFS | 1.43 (0.70) | 0.84 (0.53) | 0.84 (0.78) |
| ME/CFS | 1.03 (0.80) | 0.83 (0.77) | 0.84 (0.87) |
| Recovered | 0.83 (0.65) | 0.84 (0.61) | 0.84 (0.72) |
| IL-4 | |||
| S-ME/CFS | 4.12 (0.80) | 3.90 (0.74) | 4.34 (0.87) |
| ME/CFS | 4.43 (0.99) | 3.95 (1.25) | 3.99 (1.43) |
| Recovered | 4.22 (1.28) | 4.24 (1.20) | 4.35 (1.25) |
| IL-5 | |||
| S-ME/CFS | 1.25 (1.05)a | 1.20 (0.82) | 0.92 (1.24) |
| ME/CFS | 1.44 (0.95)b | 1.36 (0.92) | 1.20 (0.89) |
| Recovered | 1.84 (1.09)a,b | 1.46 (1.15) | 1.66 (1.01) |
| IL-6 | |||
| S-ME/CFS | 0.85 (1.10)a | 0.79 (0.76) | 1.61 (0.86) |
| ME/CFS | 1.06 (1.00) | 0.92 (0.95) | 1.06 (0.99) |
| Recovered | 1.01 (1.14)a | 0.94 (1.02) | 1.06 (1.01) |
| IL-8 | |||
| S-ME/CFS | 0.80 (1.24) | 0.81 (1.09) | 0.88 (1.35) |
| ME/CFS | 0.86 (1.26) | 0.86 (1.22) | 1.57 (1.28) |
| Recovered | 1.81 (1.33) | 0.88 (1.27) | 1.47 (1.24) |
| IL-10 | |||
| S-ME/CFS | 2.77 (0.58) | 2.81 (0.97) | 2.94 (0.90) |
| ME/CFS | 2.50 (0.99) | 2.59 (0.98) | 2.46 (1.15) |
| Recovered | 2.83 (0.88) | 2.54 (0.79) | 2.64 (0.93) |
| IL-12(p70) | |||
| S-ME/CFS | 2.32 (0.96) | 2.40 (0.77) | 2.76 (0.88)a,b |
| ME/CFS | 2.04 (0.78) | 2.01 (0.71) | 2.10 (1.04)a |
| Recovered | 2.10 (0.84) | 2.06 (0.72) | 2.22 (0.71)b |
| IL-13 | |||
| S-ME/CFS | 0.91 (2.05)a | 0.88 (1.86)a | 0.93 (2.28)a |
| ME/CFS | 2.48 (2.03) | 1.02 (2.10) | 1.58 (2.01) |
| Recovered | 3.08 (2.21)a | 1.02 (2.15)a | 2.20 (2.04)a |
| IL-15 | |||
| S-ME/CFS | 1.89 (0.60) | 1.94 (0.73) | 2.06 (0.86) |
| ME/CFS | 1.96 (0.91) | 1.71 (0.93) | 1.66 (1.03) |
| Recovered | 2.13 (0.83) | 1.87 (0.81) | 1.88 (0.82) |
| IL-17α | |||
| S-ME/CFS | 2.03 (0.97) | 1.97 (0.63) | 2.24 (0.88) |
| ME/CFS | 1.84 (0.69) | 1.90 (0.69) | 2.07 (0.97) |
| Recovered | 2.00 (0.81) | 1.87 (0.77) | 2.03 (0.66) |
| IL-23 | |||
| S-ME/CFS | 7.36 (1.24) | 7.43 (0.83) | 7.50 (0.91) |
| ME/CFS | 7.06 (1.22) | 7.11 (1.43) | 7.11 (1.84) |
| Recovered | 7.42 (1.03) | 7.28 (1.07) | 7.26 (1.42) |
| IFN-γ | |||
| S-ME/CFS | 2.99 (1.06) | 2.92 (0.74) | 3.29 (0.80) |
| ME/CFS | 2.72 (0.77) | 3.01 (0.78) | 3.08 (1.06) |
| Recovered | 2.96 (0.70) | 2.81 (0.65) | 3.03 (0.72) |
| TNF-α | |||
| S-ME/CFS | 3.00 (0.57) | 3.20 (0.55) | 0.88 (1.35) |
| ME/CFS | 2.64 (0.37) | 2.95 (0.51) | 2.78 (0.55) |
| Recovered | 2.82 (0.53) | 3.04 (0.48) | 2.84 (0.49) |
| TNF-β | |||
| S-ME/CFS | 2.47 (1.68) | 2.24 (1.70) | 3.08 (0.56) |
| ME/CFS | 3.16 (2.01) | 1.77 (2.03) | 2.03 (1.98) |
| Recovered | 3.23 (1.93) | 2.90 (1.93) | 3.31 (1.80) |
Data are presented as medians (SD). Similar letters in columns for each cytokine indicate significant differences.
Abbreviations: IFN-γ, interferon-γ; IL, interleukin; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; S-ME/CFS, severe myalgic encephalomyelitis/chronic fatigue syndrome; TNF, tumor necrosis factor.
DISCUSSION
Our major finding was that students who developed ME/CFS following IM did not have significant baseline differences in stress, coping, anxiety, or depression, although their overall DSQ symptom score and several cytokines were significantly different. After the onset of IM, multiple measures differentiated those who went on to develop ME/CFS from those who recovered, and a number of these were maintained at 6 months.
In a previous study, we identified mononucleosis severity as a risk factor for S-ME/CFS [7]. That relationship was found in the present analysis as well (P = 0.04; data not shown), which might account for some of the symptomatic differences seen beginning at time 2.
Baseline deficiencies in IL-5, IL-6, and IL-13 in the S-ME/CFS group might suggest predisposing irregularities in immune response. IL-6 has both proinflammatory and anti-inflammatory roles in immune activation and is crucial in immune regulation [30, 31]. Both IL-5 and IL-13 are critical signaling proteins for eosinophil recruitment and production [32]. Deficiencies in production of these cytokines prior to contracting IM may influence immune response and immune dysregulation once the virus is contracted. Although these deficiencies are largely eliminated by time 3, and the differences were modest, a trend towards IL-13 remaining low and the elevated levels of IL-12(p70) in the S-ME/CFS group could indicate an inflammatory response that is still activated, as has been observed in autoimmune illnesses [32]. It is unclear whether these immunologic perturbations are direct results of infection or postinfectious.
Our immune findings differ from those of our previous study that examined participants 24 months following IM [11]. However, that study examined subjects 24 months following IM and did not have access to data prior to IM. As cytokines are highly localized, steep variations are expected in an illness in which symptoms fluctuate individually both in terms of frequency and severity.
At stage 2, all 3 groups evidenced more symptoms and reduced functioning after contracting IM. However, at stage 2, those who went on to develop S-ME/CFS had greater levels of autonomic dysfunction (confirming our previous results [10]) and had more stress, depression, and anxiety than those who ultimately recovered.
The DSQ indicated that the S-ME/CFS group was even more symptomatic at baseline than the ME/CFS group. Note that in most cases the ME/CFS group only met the Fukuda criteria [20], while the S-ME/CFS group always met the Fukuda criteria [20] and either the Canadian [21] or IOM [22] criteria. Past studies have found that patients meeting other ME/CFS criteria aside from Fukuda et al [20] often display more severe symptoms [31]. The consistent differences in symptom severity between these 2 groups along with the cytokine differences suggest that the 2 groups may differ biologically.
Previous studies have identified both biological and psychological factors contributing to ME/CFS [7–10]. In the current study, no significant differences between the 3 groups were found on autonomic or psychological measures prior to contracting IM; however, significant differences were found on baseline immune and symptom behavioral measures. In other words, at baseline, those participants who developed ME/CFS following IM had more physical complaints but not perceived stress, depression, anxiety, or abnormal coping. At the time of IM at stage 2, differences were found in autonomic symptoms and mental health items, extending previous findings [10]. This might imply that participants with certain underlying physical complaints (eg, fatigue) and immune irregularities, when affected by IM, are more likely to develop ME/CFS.
One limitation of our study was incomplete data on all participants. However, there were no significant differences between those few participants on whom we lacked complete data and those whom we analyzed.
Another difference between our study and previous reports [2–5] was the high rate (23%) of ME/CFS following IM; this may be related to very close surveillance in our confined population of college students or high levels of baseline fatigue seen in college students [33]. The identification of risk factors for ME/CFS will, it is hoped, translate to successful future prevention strategies.
Notes
Acknowledgments. The authors thank Drs Robert Palinkas and John Alexander, the current and past Directors of the Northwestern University Student Health Service; Brian Druley, Director for Administration and Information Technology; M. Susan Whiting, Clinical Practice Manager; and their staffs, especially Ann Lyman, for helping as we recruited and tested participants in this study. We thank the Comprehensive Metabolic Core of Northwestern University for expert technical assistance in performing the cytokine analyses. Everyone who contributed significantly to this work has been acknowledged.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number AI 105781).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Williams-Harmon YJ, Jason LA, Katz BZ. Incidence of infectious mononucleosis in universities and U.S. military settings. J Diag Tech Biomed Anal 2016; 3:1. Available at: https://www.scitechnol.com/peer-review/incidence-of-infectious-mononucleosis-in-universities-and-us-military-settings-y2QN.php?article_id=4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchwald DS, Rea TD, Katon WJ, Russo JE, Ashley RL. Acute infectious mononucleosis: characteristics of patients who report failure to recover. Am J Med 2000; 109:531–7. [DOI] [PubMed] [Google Scholar]
- 3. Hickie I, Davenport T, Wakefield D, et al. ; Dubbo Infection Outcomes Study Group . Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ 2006; 333:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katz BZ, Shiraishi Y, Mears CJ, Binns HJ, Taylor R. Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics 2009; 124:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White PD, Thomas JM, Amess J, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry 1998; 173:475–81. [DOI] [PubMed] [Google Scholar]
- 6. Cameron B, Bharadwaj M, Burrows J, et al. ; Dubbo Infection Outcomes Study . Prolonged illness after infectious mononucleosis is associated with altered immunity but not with increased viral load. J Infect Dis 2006; 193:664–71. [DOI] [PubMed] [Google Scholar]
- 7. Katz BZ, Reuter C, Lupovitch Y, et al. A validated scale for assessing the severity of acute infectious mononucleosis. J Pediatr 2019; 209:130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imboden JB, Canter A, Cluff LE. Convalescence from influenza: a study of the psychological and clinical determinants. Arch Intern Med 1961; 108:115–21. [DOI] [PubMed] [Google Scholar]
- 9. Kasl SV, Evans AS, Niederman JC. Psychosocial risk factors in the developmental of infectious mononucleosis. Psychosom Med 1979; 41:445–66. [DOI] [PubMed] [Google Scholar]
- 10. Jason LA, Katz BZ, Shiraishi Y, Mears CJ, Im Y, Taylor R. Predictors of post-infectious chronic fatigue syndrome in adolescents. Health Psychol Behav Med 2014; 2:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broderick G, Katz BZ, Fernandes H, et al. Cytokine expression profiles of immune imbalance in post-mononucleosis chronic fatigue. J Transl Med 2012; 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jason LA, Sunnquist M. The development of the DePaul Symptom Questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr 2018; 6:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994; 32:40–66. [DOI] [PubMed] [Google Scholar]
- 14. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. Compass 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc 2012; 87:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24:386–96. [PubMed] [Google Scholar]
- 16. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46:1121–3. [DOI] [PubMed] [Google Scholar]
- 17. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol 1989; 56:267–83. [DOI] [PubMed] [Google Scholar]
- 18. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
- 19. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56:893–7. [DOI] [PubMed] [Google Scholar]
- 20. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994; 121:953–9. [DOI] [PubMed] [Google Scholar]
- 21. Carruthers BM, Jain AK, De Meirleir KL. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols (Canadian case definition). J Chronic Fatigue Syndr 2003; 11:7–115. [Google Scholar]
- 22. Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC: The National Academies Press, 2015. [PubMed] [Google Scholar]
- 23. Jason LA, So S, Brown AA, Sunnquist M, Evans M. Test-retest reliability of the DePaul Symptom Questionnaire. Fatigue 2015; 3:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murdock KW, Wang XS, Shi Q, Cleeland CS, Fagundes CP, Vernon SD. The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome. Qual Life Res 2017; 26:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pepper CM, Krupp LB, Friedberg F, Doscher C, Coyle PK. A comparison of neuropsychiatric characteristics in chronic fatigue syndrome, multiple sclerosis, and major depression. J Neuropsychiatry Clin Neurosci 1993; 5:200–5. [DOI] [PubMed] [Google Scholar]
- 26. Brown M, Kaplan C, Jason L. Factor analysis of the Beck Depression Inventory-II with patients with chronic fatigue syndrome. J Health Psychol 2012; 17:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson SK, DeLuca J, Natelson BH. Depression in fatiguing illness: comparing patients with chronic fatigue syndrome, multiple sclerosis and depression. J Affect Disord 1996; 39:21–30. [DOI] [PubMed] [Google Scholar]
- 28. Jason L, Brown M, Evans M, et al. Measuring substantial reductions in functioning in patients with chronic fatigue syndrome. Disabil Rehabil 2011; 33:589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berry KJ, Mielke PW Jr. Exact and Monte Carlo resampling procedures for the Wilcoxon-Mann-Whitney and Kruskal-Wallis tests. Percept Mot Skills 2000; 91:749–54. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka T, Narazaki M, Masuda K, Kishimoto T. Regulation of IL-6 in immunity and diseases. Adv Exp Med Biol 2016:79–88. [DOI] [PubMed] [Google Scholar]
- 31. Lacy P. Eosinophil cytokines in allergy. In: Foti M and Locati M, eds. Cy tokine effector functions in tissues. London: Elsevier/Academic Press, 2017:173–218. [Google Scholar]
- 32. Jin J, Xie X, Xiao Y, et al. Epigenetic regulation of the expression of Il12 and Il23 and autoimmune inflammation by the deubiquitinase Trabid. Nat Immunol 2016; 17:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cotler J, Katz BZ, Torres CM, Jason LA. College student symptoms as assessed by a student health surv ey. J Am Coll Health. 2020. doi: 10.1080/07448481.2020.1845705 [DOI] [PMC free article] [PubMed] [Google Scholar]