Abstract
Background
Cell-mediated immunity is a specific target of several medications used to prevent or treat rejection in orthotopic heart transplantation. Low absolute lymphocyte count (ALC) has potential to be a useful and accessible clinical indicator of overall infection risk. Though some studies have demonstrated this association in other transplant populations, it has not been assessed in heart transplant recipients.
Methods
A single-center retrospective cohort study examined adult heart transplant recipients transplanted between 2000 and 2018. The exposure of interest was ALC ≤0.75 × 103 cells/µL at 1 month posttransplant, and the primary endpoint was a composite outcome of infection (including cytomegalovirus [CMV], herpes simplex I/II or varicella zoster virus [HSV/VZV], bloodstream infection [BSI], invasive fungal infection [IFI]) or death occurring after 1 month and before 1 year posttransplant. A multivariable Cox proportional hazards model was created to control for confounders identified using clinical judgment and statistical criteria.
Results
Of 375 subjects analyzed, 101 (27%) developed the composite outcome (61 CMV, 3 HSV/VZV, 19 BSI, 10 IFI, 8 deaths). Lymphopenia (ALC ≤0.75 × 103 cells/µL) at 1 month was associated with a >2-fold higher rate of the composite outcome (hazard ratio [HR], 2.26 [95% confidence interval {CI}, 1.47–3.46]; P < .001) compared to patients without lymphopenia at 1 month. After adjustment for confounding variables, the presence of lymphopenia remained statistically significantly associated with the composite outcome (HR, 1.72 [95% CI, 1.08–2.75]; P = .02).
Conclusions
ALC measured at 1 month after heart transplant is associated with an increased risk of infectious outcomes or death in the ensuing 11 months. This is a simple, accessible laboratory measure.
Keywords: lymphopenia, heart transplant, cytomegalovirus, invasive fungal infection, bloodstream infection
Low absolute lymphocyte count measured at 1 month after heart transplant is associated with increased risk of serious infection or death in the first posttransplant year. This clinical tool can help guide decision making to mitigate infection risk in this population.
Infection is one of the leading causes of morbidity and mortality following orthotopic heart transplantation. The challenge in predicting severe infection in an individual patient lies in quantifying the cumulative effect of several complex, idiosyncratic, interrelated factors including the individual’s native immune function and iatrogenic immunosuppression [1].
In other immunosuppressed disease states, models exist for serum markers of increased risk of infection, such as a low CD4 T-cell count in human immunodeficiency virus or presence of neutropenia in hematologic malignancy [2, 3]. In recent years, multiple assays have been developed that use sophisticated serum markers of inflammatory response to stratify infection risk in the transplant population [4–9]. However, these tests are expensive, often predict just one type of infection (typically cytomegalovirus [CMV]) and are of unproven benefit in the day-to-day clinical setting. Alternatively, evolving data suggest that lymphocyte count, a simple and easily accessible measure of cellular immunity, is associated with increased risk of CMV infection [10–15]. This association is not yet studied in the heart transplant population and the precise threshold for increased risk is not well-defined. A deeper understanding of this relationship might guide transplant providers in changing immunosuppression or tailoring screening strategies to better meet the needs of an individual patient.
The aim of this study was to evaluate if an independent association exists between low lymphocyte count in the early posttransplant period and infection (CMV infection, herpes simplex I/II or varicella zoster virus infections [HSV/VZV], bloodstream infection [BSI], invasive fungal infection [IFI]) or death within the first year following heart transplantation. A secondary aim was to explore the functional relationship between absolute lymphocyte count (ALC) and the composite outcome in order to characterize the transition point at which this risk increases.
METHODS
Study Population and Data Collection
All heart transplant recipients at Tufts Medical Center (TMC) from January 2000 through October 2018 who received follow-up care at TMC were eligible for inclusion. Patients who underwent dual heart-kidney transplant, died within 1 month of transplant, or had insufficient data in the medical record were excluded.
All data were collected retrospectively from the electronic medical record. The exposure of interest was low ALC (lymphopenia) at 1 month posttransplant. The primary endpoint was time to a composite outcome that included CMV infection, HSV/VZV, BSI, IFI, or death. These infections are known to have significant morbidity and mortality, can be reliably proven with microbiologic data, and are known to be associated either with poor cell-mediated immunity or with CMV in the setting of solid organ transplant [13, 16–18]. The follow-up period ranged from 1 month to 1 year posttransplantation. Determination of the outcome was based on clinical assessment by an infectious diseases physician directly caring for the patient. If there was any uncertainty based on information from the medical record, cases were reviewed independently by 3 transplant infectious disease physicians who were blinded to the subject’s lymphocyte count. Additional demographic and clinical data were collected for global characterization of the cohort and for control of confounding. Please see Supplementary Appendix for full details of all variables collected. This study was approved by the TMC Institutional Review Board.
Definitions
Lymphopenia
Lymphopenia was defined as ALC ≤0.75 × 103 cells/µL collected 4 weeks (± 1 week) after transplant. If multiple ALC measures were available from 3 to 5 weeks, the closest measure to the exact 1-month mark was selected. A cut-point of 0.75 × 103 cells/µL was chosen based on synthesis of prior literature [10–14]. A time-point of 1 month posttransplant was chosen because (1) patients are likely to have reached a steady state in renal function, gut absorption, and titration of the immunosuppressive regimen; (2) this is a valuable period for risk assessment and resultant management decisions; and (3) in the majority of cases, patients have been discharged from the hospital, shifting away from nosocomial, postoperative, and device-related infections, which may be driven more heavily by factors other than lymphopenia.
Standard definitions of CMV infection and disease, HSV and VZV infection, BSI, and IFI were used (Supplementary Appendix) [16, 19–21]. In addition to standard IFI definition, pulmonary or intracranial Nocardia infections were grouped in this category given the similar opportunistic nature of Nocardia. All infections included microbiologic or histopathologic evidence of the pathogen. Death was attributable to any cause.
Immunosuppression and Rejection
Patients did not typically receive induction immunosuppression with a cytolytic medication unless renal function was significantly impaired. In such cases, agents used for induction were antithymocyte globulin, muromonab-CD3 (OKT3), or basiliximab. On rare occasions, induction with rituximab was given to highly sensitized patients.
Standard maintenance immunosuppression included an antimetabolite, a calcineurin inhibitor, and prednisone. Over the period of study, there were 2 notable changes in practice. Around 2002, there was a shift from the use of azathioprine to mycophenolate, and in 2008, a shift from cyclosporine to tacrolimus. In the statistical model, maintenance immunosuppression was treated as a binary variable with either a standard contemporary regimen—which included both mycophenolate and tacrolimus—or otherwise. The regimen was assessed at a single time-point: at time of index discharge or at 1 month posttransplant, whichever came first.
Episodes of rejection were proven with endomyocardial biopsy and counted only if severe enough to be treated with corticosteroids, typically methylprednisolone 1 g daily for 3 days. Antithymocyte globulin was used in cases of severe or steroid-refractory cell-mediated rejection. Those with antibody-mediated rejection were treated with rituximab, plasmapheresis, intravenous immunoglobulin, bortezomib, or, occasionally, photopheresis.
Antimicrobial Prophylaxis
Patients were stratified into CMV risk groups according to consensus guidelines; high risk (donor seropositive, recipient seronegative) and intermediate risk (recipient seropositive) groups received 3 months of antiviral prophylaxis with either valganciclovir or ganciclovir [20]. They received the equivalent of valganciclovir 900 mg daily, adjusted for renal function, for 3 months following transplantation. The low-risk group (donor and recipient seronegative) instead received famciclovir 500 mg twice daily, adjusted for renal function. In 2008, antiviral prophylaxis in high-risk recipients was extended to a 6-month duration. Until 2015, patients receiving a heart from a seropositive donor also received prophylactic CMV immunoglobulin.
Trimethoprim-sulfamethoxazole (TMP-SMX), typically for 1 year, was given to all patients for prophylaxis against Pneumocystis jirovecii and toxoplasmosis (if donor or recipient seropositive). If poorly tolerated, dapsone or atovaquone was used instead.
Statistical Analysis
The primary analysis assessed the relationship between lymphopenia (binary) and time to composite outcome. Censoring occurred at loss-to-follow-up or at 1 year. Kaplan-Meier survival curves were constructed demonstrating time to composite outcome stratified by presence or absence of lymphopenia at 1 month, and were compared with the log-rank test.
A Cox proportional hazards model was used to characterize this relationship further. Univariate relationships were calculated relating the outcome to a set of a priori–specified variables that were potentially confounding based on prior literature, clinical experience, and biologic plausibility. Candidate variables (all treated as binary unless otherwise stated) included nonischemic etiology of heart failure, history of diabetes, history of chronic kidney disease, CMV risk group (categorical), use of induction immunosuppression, rejection episode within the first month, requirement for renal replacement therapy within the first month, inpatient status at 1 month, tacrolimus and mycophenolate-based immunosuppressive regimen, white blood cell count at 1 month (continuous), use of CMV immune globulin, use of TMP-SMX, and year of transplant (continuous). If the univariate P value was <.1, the variable was included in the multivariable model.
As a sensitivity analysis, 2 time-dependent covariates were added to the model described above: any treatment for rejection, and treatment for rejection specifically with cytolytic therapy. Both variables were treated as binary; if a patient experienced the exposure at any point after 1 month and during the patient’s follow-up period, they were considered “exposed” for the remainder of the follow-up period.
The components of the composite outcome (CMV, HSV/VZV, BSI, IFI, and death) were examined as individual outcomes, but only the outcome of CMV had enough events to perform hypothesis testing.
The nature of the relationship between ALC and the composite outcome was explored in 3 ways. First, the distribution of ALC was plotted and compared between those who did and did not experience the composite outcome. Second, additional Kaplan-Meier curves were constructed with stratification into 3 and 4 categories of lymphopenia. Last, ALC was plotted against likelihood of the composite outcome using a technique that allowed for a nonlinear representation (restricted cubic spline) [22].
All statistical analysis was completed using either RStudio version 3.6.1 or SAS 9.4. P values <.05 were considered statistically significant, unless otherwise indicated.
RESULTS
Four hundred thirty-one patients underwent orthotopic heart transplantation at TMC between January 2000 and October 2018; after applying exclusion criteria, 375 subjects were analyzed (Figure 1). Ten of these patients received initial follow-up care at TMC but transitioned care to an affiliated institution at 3 or 6 months.
Figure 1.
Cohort selection.
Baseline characteristics of the cohort stratified by presence (n = 201) or absence (n = 174) of lymphopenia are listed in Table 1. One hundred one subjects in the total cohort developed the composite outcome (61 CMV, 3 HSV/VZV, 19 BSI, 10 IFI, 8 deaths). Additional details regarding infection types and pathogens are shown in Supplementary Table 1.
Table 1.
Patient Characteristics
| Characteristic | Not Lymphopenic | Lymphopenic |
|---|---|---|
| (n = 174) | (n = 201) | |
| Age, y, mean (SD) | 48.7 (12.6) | 56.2 (9.6) |
| Male sex | 116 (66.7) | 155 (77.1) |
| Race | ||
| White | 139 (79.9) | 178 (88.6) |
| Black | 11 (6.3) | 10 (5) |
| Hispanic | 21 (12.1) | 10 (5) |
| Asian | 3 (1.7) | 3 (1.5) |
| Body mass index, kg/m2, mean (SD) | 27.4 (5.5) | 27.9 (5.3) |
| Etiology of heart failure | ||
| Ischemic cardiomyopathy | 57 (32.8) | 89 (44.3) |
| Nonischemic cardiomyopathy | 118 (67.8) | 114 (56.7) |
| Giant cell myocarditis | 2 (1.1) | 5 (3.5) |
| Comorbidity | ||
| Diabetes | 49 (28.2) | 78 (38.8) |
| Chronic kidney disease | 37 (21.3) | 70 (34.8) |
| History of solid tumor | 9 (5.2) | 12 (6.0) |
| History of hematologic malignancy | 11 (6.3) | 10 (5.0) |
| History of autoimmune disease | 10 (5.7) | 9 (4.5) |
| CMV serostatus risk group | ||
| Low | 40 (23.0) | 51 (25.4) |
| Intermediate | 86 (49.4) | 87 (43.3) |
| High | 48 (27.6) | 63 (31.3) |
| White blood cell count, ×103 cells/µL | ||
| Baseline, mean (SD) | 8.4 (3.3) | 7.7 (2.5) |
| 1 mo posttransplant, mean (SD) | 8.0 (3.1) | 7.5 (3.3) |
| Absolute neutrophil count, ×103 cells/µLa | ||
| Baseline, mean (SD) | 5.6 (3.0) | 5.5 (2.1) |
| 1 mo posttransplant, mean (SD) | 5.8 (2.6) | 6.4 (2.9) |
| Baseline ALC, ×103 cells/µLa, mean (SD) | 1.7 (0.6) | 1.1 (0.5) |
| Estimated GFR, mL/min/1.73 m2 | ||
| Pretransplant GFR, median (25th, 75th percentile) | 78.9 (59.4, 94.9) | 63.1 (49.0, 80.0) |
| 1 mo posttransplant GFR, median (25th, 75th percentile) | 76.9 (52.1, 96.3) | 54.2 (40.6, 76.8) |
| Required RRT following transplant | 8 (4.6) | 30 (14.9) |
| Induction therapy | ||
| None | 163 (95.9) | 161 (81.7) |
| Antithymocyte globulin | 0 (0.0) | 7 (3.6) |
| Basiliximab | 5 (2.9) | 21 (10.7) |
| Rituximab | 0 (0.0) | 3 (1.5) |
| Muromonab | 2 (1.2) | 5 (2.5) |
| Maintenance immunosuppressionb | ||
| Mycophenolate | 129 (91.4) | 176 (88.0) |
| Tacrolimus | 131 (75.3) | 130 (65.0) |
| Azathioprine | 9 (5.2) | 15 (7.4) |
| Cyclosporine | 43 (24.7) | 65 (32) |
| Sirolimus/everolimus | 3 (2) | 7 (3) |
| Antimicrobial prophylaxis | ||
| CMV immunoglobulin | 62 (35.6) | 68 (33.8) |
| Valganciclovir or ganciclovir | 132 (75.9) | 147 (73.9) |
| Trimethoprim-sulfamethoxazole | 161 (92.5) | 166 (83.0) |
| Dapsone | 3 (1.7) | 12 (6.0) |
| Rejection | ||
| Within first month | 23 (13.2) | 19 (9.5) |
| Within first year | 49 (28.2) | 54 (26.9) |
| After 1 mo, before 1 y, and prior to and outcome event | 8 (4.6) | 11 (5.5) |
| Inpatient at 1 mo | 20 (11.5) | 57 (28.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ALC, absolute lymphocyte count; CMV, cytomegalovirus; GFR, glomerular filtration rate estimated based on Chronic Kidney Disease Epidemiology Collaboration formula using serum creatinine; RRT, renal replacement therapy; SD, standard deviation [23].
aThere were 40 patients for whom a baseline blood count differential was not available within 2 weeks prior to surgery.
bAll but 2 patients in the cohort were also on a steroid in addition to the agents listed.
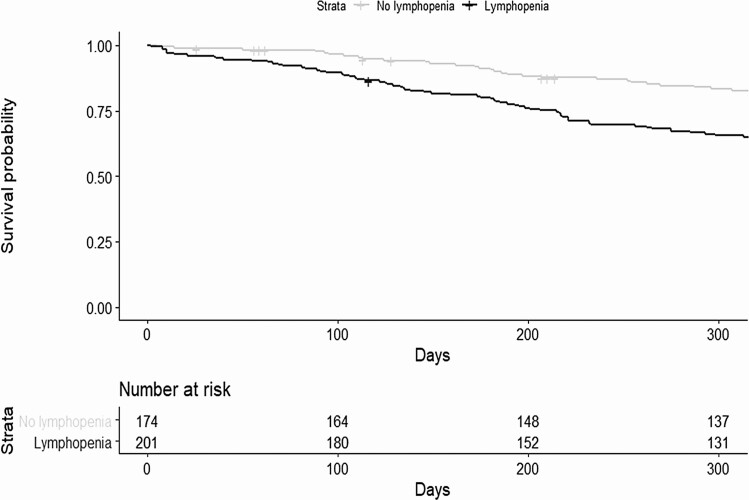
Kaplan-Meier curves comparing time to composite outcome between lymphopenic to nonlymphopenic subjects are displayed in Figure 2. The lymphopenic group had significantly greater infection and mortality (P < .001).
Figure 2.
Kaplan-Meier curves showing survival from composite outcome stratified by presence or absence of lymphopenia at 1 month. Day 0 is the first day of the follow-up period, 28 days after transplantation.
Table 2 shows the unadjusted relationship between lymphopenia and the composite outcome (hazard ratio [HR], 2.26 [95% confidence interval {CI}, 1.47–3.46]). After adjustment for confounding variables, the presence of lymphopenia at 1 month conferred a 1.72-fold (95% CI, 1.08–2.75) increased hazard of developing the composite outcome (P = .022). The variables ultimately included in the final model were nonischemic heart failure, history of diabetes, CMV risk group, use of induction immunosuppression, rejection episode within the first posttransplant month, requirement for renal replacement therapy within the first posttransplant month, inpatient status at 1 month, white blood cell count at 1 month, use of TMP-SMX, and transplant year. Finally, the 2 time-dependent variables were added to the model. The addition of these variables had minimal effect on the relationship, which remained statistically significant.
Table 2.
Summary of Unadjusted and Adjusted Models
| Model | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Unadjusted model | 2.26 | 1.47–3.46 | <.001 |
| Model adjusted for baseline covariatesa | 1.72 | 1.08–2.75 | .02 |
| Adjusted model plus time-dependent covariatesb | 1.68 | 1.05–2.70 | .03 |
aCovariates meeting criteria for inclusion in the multivariable model: nonischemic heart failure, history of diabetes, cytomegalovirus risk group, use of induction immunosuppression, rejection episode within the first posttransplant month, requirement for renal replacement therapy within the first posttransplant month, inpatient status at 1 month, white blood cell count at 1 month, use of trimethoprim-sulfamethoxazole, and transplant year.
bTime-dependent covariates were rejection occurring after the first month and rejection specifically treated with lymphocyte-depleting therapy after the first month.
CMV was the most common type of infection during the follow-up period (Supplementary Table 1 includes both primary and nonprimary events). Seven patients had asymptomatic CMV infection and 58 were categorized as having CMV disease (42 with gastrointestinal infection, 16 with CMV syndrome). Of those with CMV disease, 1 was in the low-risk category, 18 intermediate-risk, and 39 high-risk. Patients who experienced CMV disease, did so at a median of 219 days posttransplant. Those with lymphopenia at 1 month had 1.71 (95% CI, 1.00–2.93; P = .05) times the hazard of developing CMV disease in the first year when compared with those who did not have lymphopenia at 1 month.
BSI was the second most common type of infection (n = 22); a very high proportion of these events were in patients with lymphopenia (Supplementary Table 1). Although 15 of these patients remained hospitalized from their transplant at 1 month, only 5 of them experienced the BSI event during this index admission; the other 17 were admitted at a later date with bacteremia. The median time to BSI among those who experienced this type of infection was 94 days (range, 36–231 days; interquartile range, 65–136 days).
There were 21 patients who experienced >1 outcome during the 11-month follow-up period; only the first event was counted in the primary analysis. In this group, 17 (81%) had lymphopenia (compared with 52% of the remainder of the cohort). The most frequent combination was BSI and IFI (not necessarily occurring concomitantly), which occurred in 6 patients.
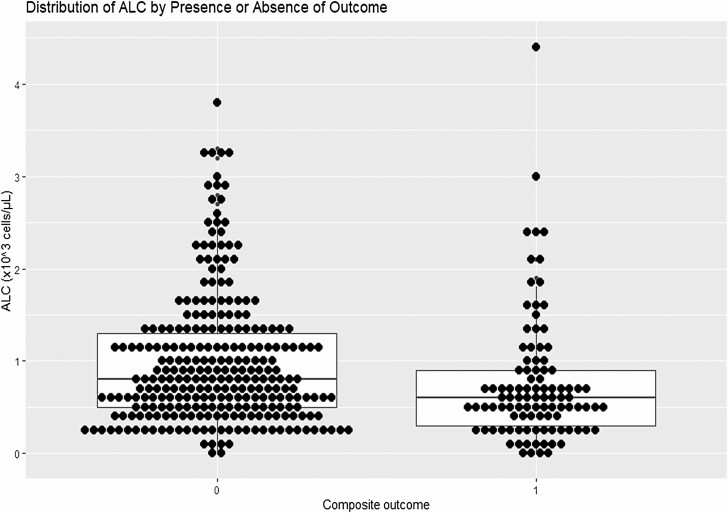
The exploratory analysis plotting the distribution of ALC for those who did and did not experience the composite outcome is shown in Figure 3. Patients who experienced the outcome had a lower median (0.6 × 103 cells/µL vs 0.8 × 103 cells/µL) and interquartile range (0.3–0.9 × 103 cells/µL vs 0.5–1.3 × 103 cells/µL) than those who did not. The exploratory Kaplan-Meier comparison plots looking at additional ALC strata are shown in Supplementary Figures 2 and 3. Overall, the lowest strata (≤0.6 × 103 cells/µL in both plots) demonstrated the greatest separation of the curves; other, higher ALC strata largely overlapped, providing little additional information. Finally, the nonlinear representation of probability of composite outcome according to ALC (Supplementary Figure 1) also supports this finding, with a stabilization of risk at an ALC of about 0.8 × 103 cells/µL.
Figure 3.
Distribution of absolute lymphocyte count (ALC) by presence or absence of outcome. Figure displays ALC distribution for those who did (n = 101) and did not (n = 274) experience the composite outcome. Each point represents an individual patient and is overlaid on a standard boxplot.
DISCUSSION
The results of this study build on a growing body of literature that show the practical utility of lymphocyte count as an independent predictor of overall infection risk in solid organ transplant recipients. Low ALC (≤0.75 × 103 cells/µL) at 1 month posttransplant was associated with an increased hazard of infection or death in the first year (HR, 1.72 [95% CI, 1.04–2.67]) after adjusting for a robust list of confounders. Adding the time-dependent variables of later rejection or cytolytic treatments did not substantially change the HR.
Although ALC is a dynamic metric, the time-point of 1 month was chosen with particular attention to clinical application. The 1-month mark, a period when patients have typically stabilized from surgery and transitioned home, provides an opportunity for evaluation of anticipated risk during the highest period of vulnerability for opportunistic infection; allowing for strategic adjustment of immunosuppression or surveillance. An examination of serial ALC measures with attention to trajectory has also been shown to be useful and would be a worthwhile area for future study in this population as it might guide management later in the posttransplant course [15].
While several prior studies have shown the association of lymphopenia to infection among multiple different solid organ transplant populations, no single threshold of “lymphopenia” has been established. Studies have used thresholds ranging from 0.5 to 1 based either on extrapolation from neutropenia categories or on ALC distribution in small data sets [10–15]. The exploratory portion of this study used several techniques to define the range where infection risk rose in our large cohort, which was around 0.6–0.8. Thus, the threshold of 0.75 × 103 cells/µL chosen a priori for dichotomization in the primary analysis was reasonable.
This study had some limitations. First, the data were collected retrospectively; certain variables, such as medications, lacked granular detail (dose, frequency, and duration of maintenance immunosuppression or antimicrobial prophylaxis). Second, there is potential for residual and unmeasured confounding due to secular trends in clinical care over the 18-year study period. Transplant year was used as a variable in the model as a strategy to adjust by proxy for such practice changes. Last, at this center, there was no uniform strategy for serial screening of CMV DNA until late 2017 when a protocol was implemented for weekly screening beginning after cessation of CMV prophylaxis (high and intermediate risk groups) and extending through 2 months; thus, some cases of asymptomatic CMV infection could have been gone undetected.
The strengths of this study are also important. It was performed on a large cohort of heart transplant recipients with excellent follow-up and high-quality data collection. It was designed with a practical time-point for measurement of the exposure and a clinically useful composite endpoint.
Now that we have demonstrated an independent association between lymphopenia and the composite outcome, and provided greater insight into a meaningful definition of lymphopenia, we consider next steps for incorporation into clinical practice. Lymphopenia could have the greatest impact if used in combination with other strong risk factors for infection; it would likely be a useful component of a clinical prediction model. Such a model would be a novel tool to synthesize various markers of known risk in these complex patients.
CONCLUSIONS
An ALC level ≤0.75 × 103 cells/µL, collected 1 month following heart transplantation, is independently associated with the composite endpoint of CMV, HSV/VZV, BSI, IFI, or death occurring in the first posttransplant year. This is a simple, inexpensive measure that does not require outsourcing to a specialty laboratory, complex interpretation, or standardization across various laboratories. The threshold for increased risk appears to be somewhere in the range of 0.6–0.8 × 103 cells/µL. This data should inform transplant providers as they manage immunosuppression with the goal of mitigating infection risk.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge the assistance of Julia Parker and Hayley Hauck in data collection.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health (grant number UL1TR002544). Additional support was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (grant number 5K12HD092535) (Tufts Building Interdisciplinary Research Careers in Women’s Health Career Development Award); the Francis P. Tally, MD, fellowship in Infectious Diseases; and by generous donations to the Tupper Research fund at Tufts Medical Center.
Potential conflicts of interest. D. R. S. reports consulting fees from Shire (Chairman of Endpoints Evaluation Committee for maribavir phase 3 trial), Merck (Advisory Board), Chimerix (Data and Safety Monitoring Board [DSMB] Chair), Takeda (DSMB), and Saol Therapeutics (Consultant). All other authors report no potential conflicts of interest.
The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998; 338:1741–51. [DOI] [PubMed] [Google Scholar]
- 2. Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. CC1-7, Table 1. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection/whats-new-guidelines. Accessed 15 January 2020.
- 3. Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 1966; 64:328–40. [DOI] [PubMed] [Google Scholar]
- 4. Rodrigo E, López-Hoyos M, Corral M, et al. ImmuKnow as a diagnostic tool for predicting infection and acute rejection in adult liver transplant recipients: a systematic review and meta-analysis. Liver Transpl 2012; 18:1245–53. [DOI] [PubMed] [Google Scholar]
- 5. Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 2019; 19:2505–16. [DOI] [PubMed] [Google Scholar]
- 6. Sood S, Testro AG. Immune monitoring post liver transplant. World J Transplant 2014; 4:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walker S, Fazou C, Crough T, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis 2007; 9:165–70. [DOI] [PubMed] [Google Scholar]
- 8. Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 2013; 56:817–24. [DOI] [PubMed] [Google Scholar]
- 9. Sester U, Gärtner BC, Wilkens H, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant 2005; 5:1483–9. [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Ruiz M, López-Medrano F, Romo EM, et al. Pretransplant lymphocyte count predicts the incidence of infection during the first two years after liver transplantation. Liver Transpl 2009; 15:1209–16. [DOI] [PubMed] [Google Scholar]
- 11. Fernández-Ruiz M, López-Medrano F, Allende LM, et al. Kinetics of peripheral blood lymphocyte subpopulations predicts the occurrence of opportunistic infection after kidney transplantation. Transpl Int 2014; 27:674–85. [DOI] [PubMed] [Google Scholar]
- 12. Gardiner BJ, Nierenberg NE, Chow JK, Ruthazer R, Kent DM, Snydman DR. Absolute lymphocyte count: a predictor of recurrent cytomegalovirus disease in solid organ transplant recipients. Clin Infect Dis 2018; 67:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nierenberg NE, Poutsiaka DD, Chow JK, et al. Pretransplant lymphopenia is a novel prognostic factor in cytomegalovirus and noncytomegalovirus invasive infections after liver transplantation. Liver Transpl 2014; 20:1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meesing A, Razonable RR. Absolute lymphocyte count thresholds: a simple, readily available tool to predict the risk of cytomegalovirus infection after transplantation. Open Forum Infect Dis 2018; 5:ofy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helou GE, Lahr B, Razonable R. Absolute lymphocyte count as marker of cytomegalovirus and allograft rejection: is there a “safe corridor” after kidney transplantation? Transpl Infect Dis 2020:e13489. doi: 10.1111/tid.13489. [DOI] [PubMed] [Google Scholar]
- 16. George MJ, Snydman DR, Werner BG, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med 1997; 103:106–13. [DOI] [PubMed] [Google Scholar]
- 17. Shoji K, Funaki T, Kasahara M. Risk factors for bloodstream infection after living-donor liver transplantation in children. Pediatr Infect Dis J 2015; 34:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodríguez C, Muñoz P, Rodríguez-Créixems M, Yañez J, Palomo J, Bouza E. Bloodstream infections among heart transplant recipients. Transplantation 2006; 81:384–91. [DOI] [PubMed] [Google Scholar]
- 19. Arbo MD, Snydman DR. Influence of blood culture results on antibiotic choice in the treatment of bacteremia. Arch Intern Med 1994; 154:2641–5. [DOI] [PubMed] [Google Scholar]
- 20. Kotton CN, Kumar D, Caliendo AM, et al. The Third International Consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 21. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 22. Croxford R; Institute for Clinical Evaluative Sciences. Restricted cubic spline regression: a brief introduction. Available at: https://support.sas.com/resources/papers/proceedings16/5621-2016.pdf. Accessed 10 February 2020.
- 23. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.