Abstract
Objectives
To review temporal changes in the proportions of different Enterococcus species recorded in two UK bacteraemia surveillance systems. Antibiotic resistance trends were also considered.
Methods
We reviewed data for enterococci from 2001 to 2019 in: (a) the BSAC Resistance Surveillance Programme, which collected up to 7–10 bloodstream enterococci every year from each of 23–39 hospitals in the UK and Ireland and tested these centrally; and (b) PHE bacteraemia surveillance, using routine results from NHS microbiology laboratories in England.
Results
BSAC surveillance, based upon 206–255 enterococci each year (4486 in total), indicated that the proportion of Enterococcus faecium rose from 31% (212/692) in the period 2001–3 to 51% (354/696) in the period 2017–19, balanced by corresponding falls in the proportion of Enterococcus faecalis. PHE surveillance provided a larger dataset, with >5000 enterococcus reports per year; although its identifications are less precise, it too indicated a rise in the proportion of E. faecium. BSAC surveillance for E. faecium indicated no consistent trends in resistance to ampicillin (≥86% in all years), vancomycin (annual rates 19%–40%) or high-level resistance to gentamicin (31%–59%). Resistance to vancomycin remained <4% in E. faecalis in all years, whilst high-level resistance to gentamicin fell, perhaps partly reflecting the decline of two initially prevalent gentamicin- and ciprofloxacin-resistant clones.
Conclusions
Both surveillance systems indicate a growing proportion of E. faecium in enterococcal bloodstream infections. This is important because fewer therapeutic options remain against this frequently multiresistant species than against E. faecalis.
Introduction
Enterococci rank among the top 10 pathogens causing bloodstream infections (BSI) worldwide.1Enterococcus faecalis has long been viewed as the predominant species, whereas Enterococcus faecium is more often antibiotic resistant, notably to penicillins and glycopeptides.2 Consequently, a change in the ratio of E. faecalis to E. faecium would be a concern, since infections caused by E. faecium are more difficult to treat and they are also associated with higher mortality, perhaps owing to this greater resistance, or to biological associations with a sicker patient demographic.3
Two surveillance systems have monitored trends in BSIs in the UK, including those involving enterococci, over long periods. First, PHE (now UK Health Security Agency, UKHSA), with a dataset covering England, has collected diagnostic laboratories’ susceptibility results for all bacteraemias since 1990. Data submission is voluntary but, by 2019, recorded an estimated 98% of all bacteraemias, based on cross-reference to mandatory surveillance of Escherichia coli BSI episodes. Secondly, since 2001, the BSAC Resistance Surveillance Programme has collected BSI isolates, including enterococci, from sentinel laboratories throughout the UK and Ireland. These were then re-identified centrally, and MICs were determined.
Here we review the temporal changes in the proportions of E. faecium and E. faecalis revealed over the 19 years (from 2001 to 2019) when both surveillance schemes functioned. Resistance trends within species are also considered.
Materials and methods
BSAC bacteraemia surveillance
The BSAC Resistance Surveillance Programme has been described previously.4 Consecutive enterococci (up to 7–10 isolates each year from 23–39 laboratories) causing clinically significant bacteraemia (as determined by the local Consultant Microbiologist) were collected from laboratories throughout the UK and Ireland from 2001–19. There was some year-to-year turnover of participating sites. Repeat isolates from the same patient within 14 days were excluded, being assumed to originate from the same infective episode. Isolates were re-identified centrally by PCR for ddl (encoding d-Ala-d-Ala ligase) until 2012,5 and thereafter by MALDI-TOF MS (Biotyper, Bruker Daltonics, Bremen, Germany).
The BSAC agar dilution method was used to determine MICs,4 which were reviewed against current EUCAST breakpoint criteria (v11.0, 2021).6 In the absence of EUCAST breakpoints, high-level ciprofloxacin resistance was defined as >16 mg/L.7 Vancomycin resistance genotypes for E. faecium and E. faecalis were inferred by interpretive reading of resistance phenotypes, on the basis that resistance to both teicoplanin and vancomycin predicts VanA, whereas resistance to vancomycin combined with susceptibility, or near-susceptibility, to teicoplanin predicts VanB.2
PHE bacteraemia surveillance
From the larger PHE bacteraemia dataset, data were analysed for all enterococcal BSIs reported to PHE from 2001–19 by NHS microbiology laboratories in England. Over time, data capture by PHE has migrated from paper to a first electronic system, LabBase2, used from 2002–14, then, since 2015, to the SGSS (Second Generation Surveillance System).8 Identification and susceptibility testing were by the laboratories’ own methods, using the breakpoints applicable at the time for categorization. Most susceptibility testing was by BSAC and, latterly, EUCAST disc methods, however some sites used automated systems. Species-level identification is not always reported and (based on high rates of ‘ampicillin-resistant E. faecalis’ in the years before MALDI-TOF was widely adopted) is not always accurate. To offset this limitation, the proportion of ampicillin resistance was also considered as a proxy measure for the proportion of E. faecium, since a very large majority of enterococcal isolates are either E. faecium or E. faecalis, and ampicillin resistance is exceptionally rare in E. faecalis, whereas ampicillin susceptibility is uncommon (<5%) in E. faecium.
If an individual patient had multiple positive blood cultures of the same Enterococcus species in a 14 day period, the episode was recorded at the date of the first isolate but with the antimicrobial result for the most resistant isolate. If an individual patient had multiple positive blood cultures of reportedly different species in the 14 day period, these were classified as different infection episodes.
Statistical methods
All analysis used Stata version 15.1 (StataCorp LLC, 2017; College Station, TX, USA). Trends in proportions over time were estimated as risk ratios (RR) per year using generalized linear regression (binomial family, log link) with cluster-robust errors to allow for clustering by collection centre. We looked for departures from constant trend by comparing segmented models with a single knot (allowing different RRs in two time periods) or no knot (constant RR) using the Akaike Information Criterion (AIC).
Results
Rise in E. faecium as a proportion of enterococci
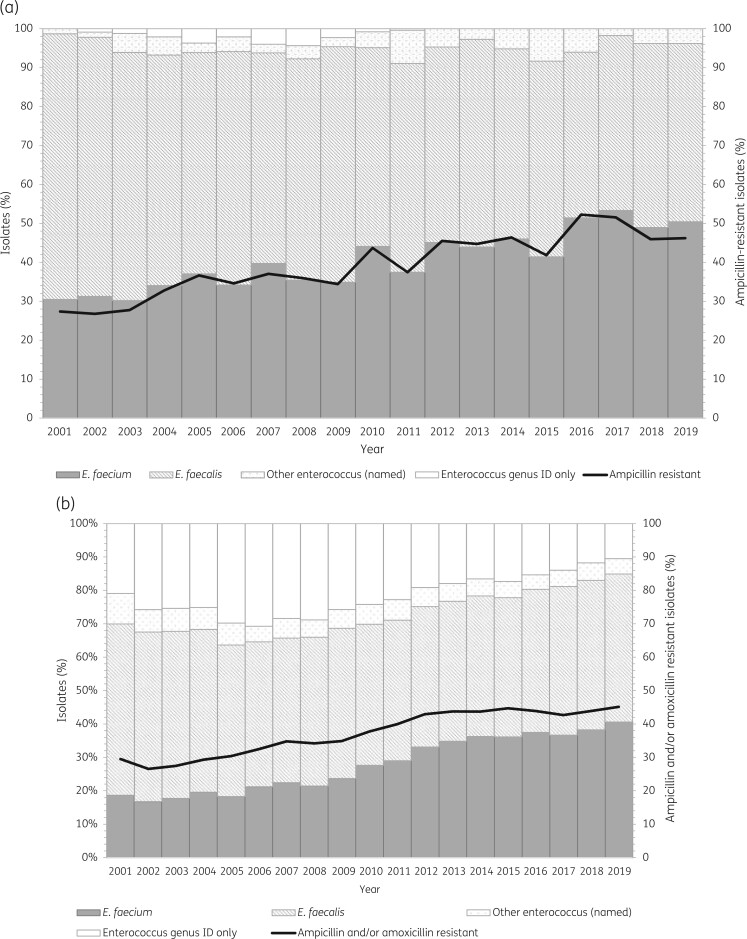
The BSAC bacteraemia surveillance included data for 4486 isolates comprising 2432 E. faecalis and 1823 E. faecium along with 231 isolates identified as another named species or as Enterococcus spp. only. The proportion of E. faecium increased progressively over time (Figure 1a), from 31% in the period 2001–3 to 51% in 2017–19, with an estimated risk ratio of 1.03 (95% CI 1.02–1.04) per year. The proportion of E. faecium (127/247, 51%) exceeded that of E. faecalis (105/247, 43%) for the first time in 2016, and E. faecium remained the predominant species thereafter (Figure 1a). In the BSAC surveillance, where all isolates were tested for ampicillin resistance as well as to identify species, the proportion of ampicillin-resistant isolates closely matched the proportion of E. faecium (Figure 1a).
Figure 1.
(a) Distribution of enterococci causing bloodstream infection in the UK and Ireland, 2001–19 (BSAC data). (b) Distribution of enterococci causing bloodstream infection, 2001–19, based on reported identification or proxy identification using ampicillin/amoxicillin resistance (PHE data). Key: Other enterococcus, isolates identified as an enterococcal species other than E. faecalis or E. faecium; Enterococcus genus ID only, isolates identified to Enterococcus genus level only; Ampicillin resistant, enterococci identified as resistant to ampicillin; Ampicillin/amoxicillin resistant, enterococci identified as resistant to ampicillin and/or amoxicillin among those enterococci that were tested against either of these agents.
PHE surveillance provided a larger dataset, with 146 862 reports in total, comprising 5420 to 11 471 per annum (mean 7730). A limitation was that 22% of isolates (n = 32 856) in the PHE dataset were not identified to species level (Figure 1b), with this proportion highest in 2006 (3457/11 331, 31%) and thereafter decreasing to 19% (1069/5641) in 2012 and 10% (828/7917) by 2019 (Figure 1b). There was also evidence of misidentification, with an appreciable percentage of resistance among isolates reported as E. faecalis and tested against ampicillin and/or amoxicillin: 10% (110/1119) in 2001, falling to 2% (54/2770) in 2019. It is likely that the quality of identification improved with increasing adoption of MALDI-TOF.
Both factors (misidentification and lack of species identification) would tend to cause underestimation of the true proportion of E. faecium in the PHE surveillance, and the proportion of reported E. faecium was consistently lower than the proportion of ampicillin/amoxicillin-resistant isolates (Figure 1b). Nonetheless, the proportion of E. faecium increased over time (Figure 1b) regardless of whether it was estimated by reported identification or by ampicillin resistance.
Trends in antimicrobial susceptibility
E. faecium
Resistance trends were primarily reviewed using the BSAC surveillance data, based on its more robust species identifications, standardized methodology, and the problem that data reported to PHE do not reliably discriminate inherent and high-level aminoglycoside resistances for enterococci.
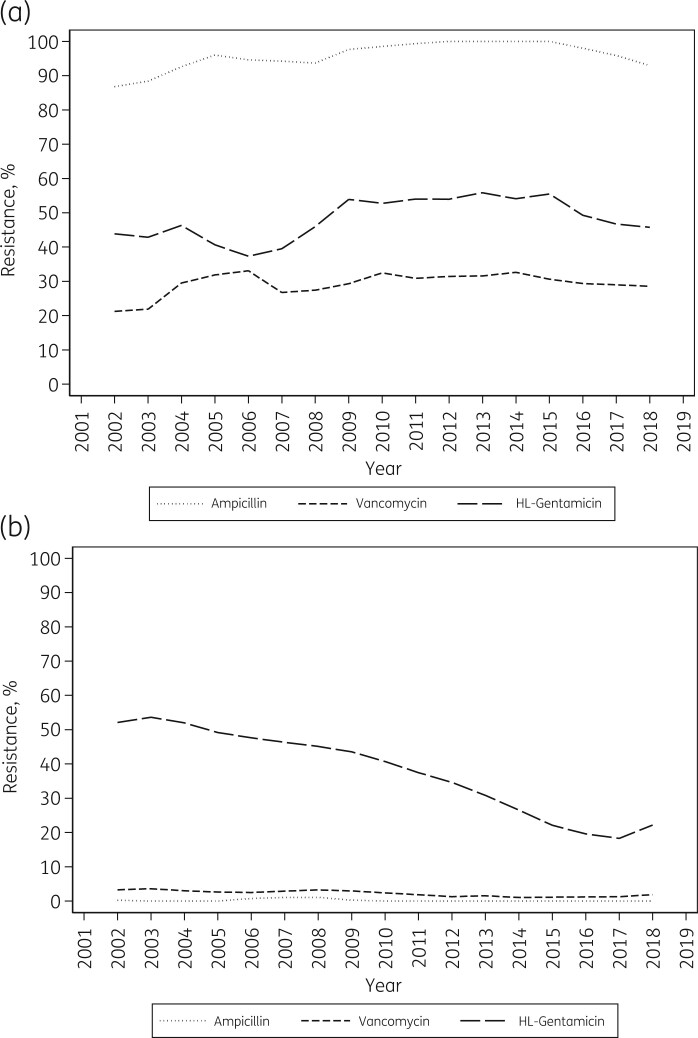
BSAC data showed no convincing temporal change in the proportion of E. faecium resistant to ampicillin, vancomycin, or high-level gentamicin. Resistance to ampicillin was consistently high, averaging 96% (1743/1823) with annual rates of 86%–100% (Figure 2a). Almost 30% of E. faecium (530/1823) were resistant to vancomycin, with annual rates ranging from 19% to 40%. Around 95% (502/530) of the resistant isolates had a VanA phenotype. The proportion of E. faecium with high-level resistance to gentamicin (MIC >128 mg/L) fluctuated between 31% (25/81, 2006) and 59% (75/127, 2016), perhaps reflecting local centre clustering and/or testing variability, but with no clear trend in resistance (Figure 2a). Ciprofloxacin was tested until 2015. Nearly half (49%; 651/1342) of E. faecium in this period had high-level gentamicin resistance, and most (>95%) of those were also highly resistant to ciprofloxacin (MIC >16 mg/L) (Figure 3a).
Figure 2.
Ampicillin, vancomycin, and high-level (HL) gentamicin resistance in Enterococcus faecium (a) and Enterococcus faecalis (b) from bloodstream infection, 2001–19 (BSAC data): 3 year weighted average. Averages are shown at the middle of each 3 year period, so are not available for the first and last years of the data series. High-level resistance to gentamicin is defined as MIC >128 mg/L.6
Figure 3.
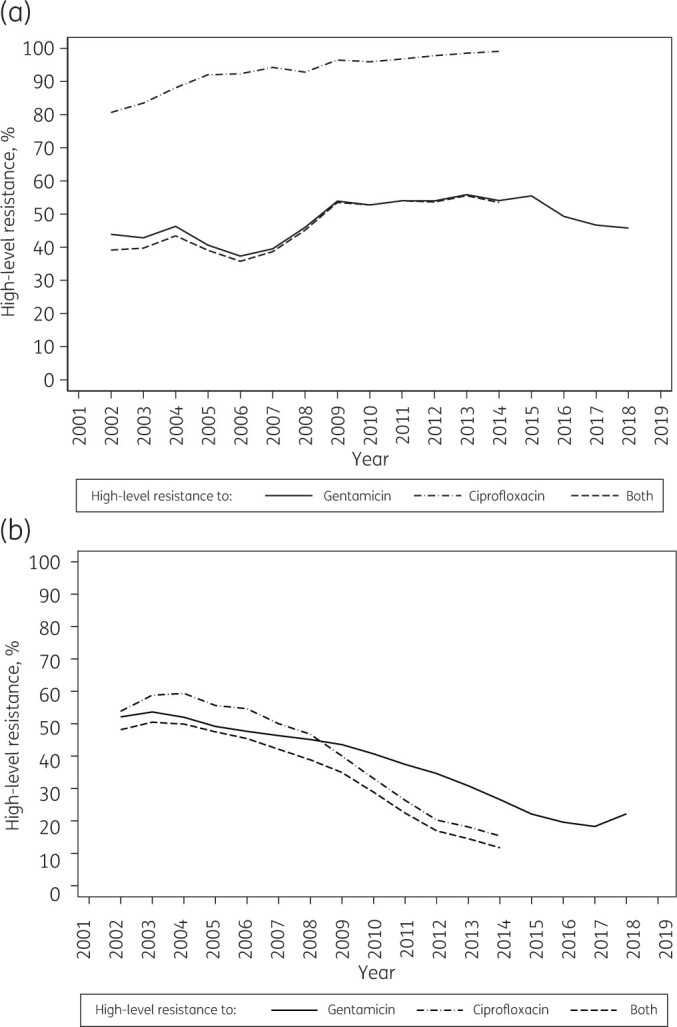
High-level resistance to gentamicin and ciprofloxacin in Enterococcus faecium (a) and Enterococcus faecalis (b) from bloodstream infection, 2001–19 (BSAC data): 3 year weighted averages. Ciprofloxacin was last tested in 2015. Averages are shown at the middle of each 3 year period, so are not available for the first and last years of the data series. High-level resistance to gentamicin is defined as MIC >128 mg/L;6 high-level resistance to ciprofloxacin is defined as MIC >16 mg/L.7
PHE data were reviewed for glycopeptide resistance, defined as resistance to vancomycin and/or teicoplanin. Among the 28 081 E. faecium isolates tested, annual resistance rates fluctuated between 15% and 26% (mean 21%), with no linear trend.
E. faecalis
Five out of 2432 E. faecalis isolates in the BSAC collection were recorded as resistant to ampicillin; these had been identified in years (2001, 2007 and 2008) prior to the use of MALDI-TOF. On further investigation, three of the five cultures were found to be mixed, being contaminated by species with ampicillin resistance (Streptococcus anginosus/E. faecium) whereas two were re-identified as E. faecium.
The proportion of E. faecalis resistant to vancomycin in the BSAC collection remained low, at <3% overall (56/2432), with annual rates ranging from 1% to 4% (Figure 2b); most (53/56, 95%) vancomycin-resistant isolates had a VanA phenotype. By contrast with this low prevalence, the proportion of E. faecalis with high-level gentamicin resistance (MIC >128 mg/L) was considerable and showed marked trends, falling slowly from 55% in 2002 (82/149) to 45% in 2009 (58/130) (RR for 2002–09: 0.965/year, 95% CI 0.941–0.989) then more swiftly to 19% in 2018 (21/111) (RR for 2009–19: 0.918/year, 95% CI 0.893–0.945) (Figure 2b). Outliers were the terminal years 2001 (69/152, 45%) and 2019 (32/108, 30%); given the lack of data for earlier and later years, we cannot say whether these represent random scatter or reversal points.
Until 2006, the great majority of E. faecalis with high-level gentamicin resistance were also highly resistant to ciprofloxacin (Figure 3b). Subsequently, the proportion of isolates with this double high-level resistance (to both gentamicin and ciprofloxacin) fell more swiftly than did the prevalence of isolates with high-level gentamicin resistance (Figure 3b). Overall, high-level ciprofloxacin resistance was seen in only 10% (118/1153) of E. faecalis lacking high-level aminoglycoside resistance, but in 81% (692/854) of those with high-level gentamicin resistance, falling from 92% (220/238) in 2001–03 to 44% (45/102) in 2013–15 (Figure 3b).
PHE data indicated low (2%–4%) annual rates of glycopeptide resistance in E. faecalis, without trend, based on reported identifications (data not shown).
Discussion
Both the BSAC and PHE surveillance systems revealed a major increase in the proportion of E. faecium among enterococci from BSI over the 19 years from 2001 to 2019.
The BSAC surveillance indicated that, by 2016, E. faecium had become the most prevalent Enterococcus species in the setting, whereas it accounted for only 31% of BSI enterococci collected in 2001–03.
The data presented here relate to the UK and Ireland or, in the case of the PHE series, specifically to England; however, the literature suggests that similar shifts are occurring internationally. During the early 2000s, hospitals in Denmark9 and The Netherlands10 demonstrated an increasing E. faecium shift in invasive enterococcal infections associated with replacement of E. faecalis by a multi-resistant lineage of E. faecium belonging to clonal complex 17 (CC17). The SENTRY antimicrobial surveillance programme reported a decline in ampicillin susceptibility among BSI enterococci in the Asia-Pacific region, Europe, Latin America and North America, between 1997 and 2016, similarly suggesting a rising proportion of E. faecium.11 A switch in the historic E. faecalis/E. faecium ratio has been reported in the USA too, with E. faecium becoming the more-prevalent species and, as here, the one more often associated with vancomycin resistance.12 In contrast, surveillance in Switzerland identified a substantial increase in E. faecalis BSI.13
The BSAC and PHE surveillance programmes do not indicate any consistent increase in the proportion of E. faecium resistant to vancomycin or teicoplanin. This is not unique to the UK; other countries in Europe report similarly stable rates of vancomycin resistance in E. faecium.14 Rather, the rise of this more-often-resistant species is responsible for an increased burden of glycopeptide-resistant enterococcal BSI.
A second notable finding was the marked decrease in the proportion of E. faecalis with high-level gentamicin and ciprofloxacin resistances. These falls are inferred to substantially reflect the decline of two gentamicin- and ciprofloxacin-resistant clonal lineages that were circulating in the UK in the early 2000s.7 In our earlier studies, relating to isolates from 2001, these lineages were characterized by PFGE.7 Subsequently, the genomic sequences of 94 E. faecalis isolates from the BSAC surveillance collection (2001–11) were determined, comprising all the vancomycin-resistant E. faecalis then available in the collection (n = 35), 35 vancomycin-susceptible E. faecalis matched by hospital and year of isolation, plus an additional 24 vancomycin-susceptible isolates.15 Among these 94, 50 had the combination of high-level ciprofloxacin and gentamicin resistance and this was found to map to eight multi-locus sequence types, with ST6 (30/50) and ST28 (10/50) accounting for 80% and ST103 (4/50) for a further 8%. As only a few of the available isolates were sequenced (94/1495), it is not possible to map the prevalent lineages throughout the 2001–11 period.
Reasons for the rising proportion of E. faecium are unclear. PHE reported its highest-ever population rate of bacteraemias due to Enterococcus spp. in England, Wales, and Northern Ireland in 2018, at 13.3 per 100 000 population.16 However this is partly accounted for by increasing reporting of bacteraemias in general. Perhaps the decline of the two formerly successful gentamicin- and ciprofloxacin-resistant clones of E. faecalis has driven the change in the E. faecalis/E. faecium ratio reported here, reducing the proportion of E. faecalis bacteraemias. Another possibility is changed antibiotic use: heavy cephalosporin use until the mid-2000s may have selected for all enterococci, as they are inherently cephalosporin resistant.17 Subsequently, cephalosporin use declined owing to concerns about selection of healthcare-associated infection with Clostridioides difficile,18,19 with prescribing becoming more dominated by penicillin/inhibitor combinations,20 which may be more selective for E. faecium, as a mostly penicillin-resistant species. Caution is, however, needed owing to trial evidence that piperacillin/tazobactam, in particular, does not select for gut colonization with vancomycin-resistant E. faecium.20,21 An alternative hypothesis is that increased use of oral vancomycin to treat C. difficile infection may have selected for vancomycin resistance and therefore for E. faecium, though this is rendered doubtful by the lack of any rise of vancomycin resistance in E. faecium as oral vancomycin substantially replaced metronidazole as anti-C. difficile therapy. Last, it is postulated that BSIs caused by E. faecalis and E. faecium should be treated as different clinical entities:3 those due to E. faecalis mostly have a urinary origin whereas those due to E. faecium more often have a gastrointestinal origin and predominantly affect patients with more severe underlying illness. Accordingly, changed patient types, UTI management, and demographic shifts over two decades may be the real drivers of change. What is clear is that there are fewer therapeutic options available for multiresistant E. faecium infection than for E. faecalis, and this may result in poorer outcomes and higher mortality.
In conclusion, our findings identify an important shift in enterococcal BSIs in favour of a more resistant species. This could have important consequences as healthcare provision contends with an ageing population vulnerable to enterococcal infection. We emphasize the value of longitudinal surveillance and the need to monitor antimicrobial resistance over long periods in order to track gradual changes.
Acknowledgements
These data were presented at the 28th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Madrid, 2018 (poster number P0455). The authors thank those companies that have sponsored the BSAC Resistance Surveillance Programme over the years; staff in the sentinel laboratories submitting isolates, and at the Central Testing Laboratory, PHE, London. Members of the BSAC Resistance Surveillance Standing Committee: M. Allen, D.F.J. Brown, A.P. Johnson, D.M. Livermore, C. Longshaw, A.P. MacGowan and N. Woodford.
Funding
The BSAC Resistance Surveillance Programme was wholly supported by the pharmaceutical industry. A list of companies that provided sponsorship throughout the surveillance programme is available at http://www.bsacsurv.org. R.R. received support from the BSAC and the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol.
Transparency declarations
M.A. is a Trustee of the BSAC Council and is employed by Merck Sharp & Dohme (UK) Limited, London, UK. C.L. is a Trustee of the BSAC Council and is employed by Shionogi B.V. D.M.L. declares: Advisory Boards or ad hoc consultancy for Accelerate, Antabio, Centauri, Entasis, Integra-Holdings, Meiji, Menarini, Mutabilis, Nordic, Paion, ParaPharm, Pfizer, QPEX, Shionogi, Summit, T.A.Z., VenatoRx, Wockhardt and Zambon; paid lectures for bioMérieux, Beckman Coulter, Cardiome, GSK, Hikma, Merck/MSD, Menarini, Nordic, Pfizer and Shionogi; relevant shareholdings or options: Dechra, GSK, Merck and Pfizer, amounting to less than 10% of portfolio value. D.M.L. also has nominated holdings in Arecor, Avacta, Diaceutics, Evgen, Genedrive, Polarean Imaging, Renalytics AI and Synairgen (all of which have research/products pertinent to medicines or diagnostics) through Enterprise Investment Schemes but has no authority to trade these shares directly. N.W. and S.M. are members of UKHSA's Antimicrobial Resistance and Healthcare Associated Infections Reference Unit, which has received financial support for conference attendance, lectures, research projects, or contracted evaluations from numerous sources, including Accelerate Diagnostics, Achaogen Inc., Allecra Therapeutics, Amplex, AstraZeneca UK Ltd, AusDiagnostics, Basilea Pharmaceutica, Becton Dickinson Diagnostics, bioMérieux, Bio-Rad Laboratories, BSAC, Cepheid, Check-Points B.V., Cubist Pharmaceuticals, Department of Health, Enigma Diagnostics, Food Standards Agency, GlaxoSmithKline Services Ltd, Helperby Therapeutics, Henry Stewart Talks, IHMA Ltd, Innovate UK, Integra holdings, Kalidex Pharmaceuticals, Melinta Therapeutics, Merck Sharpe & Dohme Corp, Meiji Seika Pharma Co. Ltd, Mobidiag, Momentum Biosciences Ltd, Neem Biotech, Nordic Pharma Ltd, Norgine Pharmaceuticals, Paratek Pharmaceuticals, Rempex Pharmaceuticals Ltd, Roche, Rokitan Ltd, Smith & Nephew UK Ltd, Shionogi & Co. Ltd, Trius Therapeutics, T.A.Z., VenatoRx Pharmaceuticals and Wockhardt Ltd. All other authors have none to declare.
References
- 1. Diekema DJ, Hsueh P-R, Mendes RE. et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2019; 63: e00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cetinkaya Y, Falk P, Mayhall CG.. Vancomycin-resistant enterococci. Clin Microbiol Rev 2000; 13: 686–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billington EO, Phang SH, Gregson DB. et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 2014; 26: 76–82. [DOI] [PubMed] [Google Scholar]
- 4. Reynolds R, Hope R, Williams L.. Survey, laboratory and statistical methods for the BSAC Resistance Surveillance Programmes. J Antimicrob Chemother 2008; 62: ii15–28. [DOI] [PubMed] [Google Scholar]
- 5. Dutka-Malen S, Evers S, Courvalin P.. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol 1995; 33: 24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anon. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Clinical breakpoints. Version 11.0. 2021. http://www.eucast.org/clinical_breakpoints/.
- 7. Woodford N, Reynolds R, Turton J. et al. Two widely disseminated strains of Enterococcus faecalis highly resistant to gentamicin and ciprofloxacin from bacteraemias in the UK and Ireland. J Antimicrob Chemother 2003; 52: 711–4. [DOI] [PubMed] [Google Scholar]
- 8. Public Health England. Laboratory reporting to Public Health England. A guide for diagnostic laboratories. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926838/PHE_Laboratory_reporting_guidelines_October-2020-v3.pdf.
- 9. Lester C, Sandvang D, Olsen S. et al. Emergence of ampicillin-resistant Enterococcus faecium in Danish hospitals. J Antimicrob Chemother 2008; 62: 1203–6. [DOI] [PubMed] [Google Scholar]
- 10. Top J, Willems R, Blok H. et al. Ecological replacement of Enterococcus faecalis by multiresistant clonal complex 17 Enterococcus faecium. Clin Microbiol Infect 2007; 13: 316–9. [DOI] [PubMed] [Google Scholar]
- 11. Pfaller MA, Cormican M, Flamm RK. et al. Temporal and geographic variation in antimicrobial susceptibility and resistance patterns of enterococci: results from the SENTRY antimicrobial surveillance program, 1997-2016. Open Forum Infect Dis 2019; 6: S54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Top J, Willems R, Bonten M.. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS Immunol Med Microbiol 2008; 52: 297–308. [DOI] [PubMed] [Google Scholar]
- 13. Piezzi V, Gasser M, Atkinson A. et al. Increasing proportion of vancomycin-resistance among enterococcal bacteraemias in Switzerland: a 6-year nation-wide surveillance, 2013 to 2018. Euro Surveill 2020; 25: pii=1900575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control (ECDC). Surveillance Atlas of Infectious Diseases. http://atlas.ecdc.europa.eu/public/index.aspx.
- 15. Raven KE, Reuter S, Gouliouris T. et al. Genome-based characterization of hospital-adapted Enterococcus faecalis lineages. Nat Microbiol 2016; 1: 15033. [DOI] [PubMed] [Google Scholar]
- 16. Public Health England. Laboratory surveillance of Enterococcus spp. bacteraemia in England, Wales and Northern Ireland: 2018. 2019. https://www.gov.uk/government/publications/enterococcus-spp-bacteraemia-voluntary-surveillance.
- 17. Dolk FCK, Pouwels KB, Smith DRM. et al. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother 2018; 73: ii2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health England. Updated guidance on the management and treatment of Clostridium difficile infection. 2013. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/321891/Clostridium_difficile_management_and_treatment.pdf.
- 19. National Institute for Clinical Excellence (NICE). Clostridium difficile infection: risk with broadspectrum antibiotics. Evidence Summary. 2015. https://www.nice.org.uk/advice/esmpb1/resources/clostridium-difficile-infection-risk-with-broadspectrum-antibiotics-pdf-1502609568697285.
- 20. Bradley SJ, Wilson ALT, Allen MC. et al. The control of hyperendemic glycopeptide-resistant Enterococcus spp. on a haematology unit by changing antibiotic usage. J Antimicrob Chemother 1999; 43: 261–6. [DOI] [PubMed] [Google Scholar]
- 21. Donskey CJ, Hanrahan JA, Hutton RA. et al. Effect of Parenteral Antibiotic Administration on the Establishment of Colonization with Vancomycin-Resistant Enterococcus faecium in the Mouse Gastrointestinal Tract. J Infect Dis 2000; 181: 1830–3. [DOI] [PubMed] [Google Scholar]