Abstract
We previously found normal polysomnographic (PSG) sleep efficiency, increased slow-wave sleep (SWS), and a blunted melatonin secretion in women with premenstrual dysphoric disorder (PMDD) compared to controls. Here, we investigated the effects of exogenous melatonin in five patients previously studied. They took 2 mg of slow-release melatonin 1 h before bedtime during their luteal phase (LP) for three menstrual cycles. At baseline, patients spent every third night throughout one menstrual cycle sleeping in the laboratory. Measures included morning urinary 6-sulfatoxymelatonin (aMt6), PSG sleep, nocturnal core body temperature (CBT), visual analog scale for mood (VAS-Mood), Prospective Record of the Impact and Severity of Menstrual Symptoms (PRISM), and ovarian plasma hormones. Participants also underwent two 24-hour intensive physiological monitoring (during the follicular phase and LP) in time-isolation/constant conditions to determine 24-hour plasma melatonin and CBT rhythms. The same measures were repeated during their third menstrual cycle of melatonin administration. In the intervention condition compared to baseline, we found increased urinary aMt6 (p < 0.001), reduced objective sleep onset latency (p = 0.01), reduced SWS (p < 0.001), and increased Stage 2 sleep (p < 0.001). Increased urinary aMt6 was correlated with reduced SWS (r = −0.51, p < 0.001). Circadian parameters derived from 24-hour plasma melatonin and CBT did not differ between conditions, except for an increased melatonin mesor in the intervention condition (p = 0.01). Ovarian hormones were comparable between the conditions (p ≥ 0.28). Symptoms improved in the intervention condition, as measured by the VAS-Mood (p = 0.02) and the PRISM (p < 0.001). These findings support a role for disturbed melatonergic system in PMDD that can be partially corrected by exogenous melatonin.
Keywords: premenstrual dysphoric disorder, menstrual cycle, melatonin, sleep, circadian rhythm, mood, body temperature
Statement of Significance.
Reduced melatonin secretion and increased slow-wave sleep were previously found in women with premenstrual dysphoric disorder (PMDD) and insomnia compared to controls. Using a within-subject design, we studied women with PMDD and insomnia across a full menstrual cycle before and after the administration of 2 mg of slow-release melatonin. We have shown a reduction in slow-wave sleep and in self-reported PMDD symptoms after administrating exogenous melatonin. These changes may relate to an abnormal MT1/MT2 ratio and appear independent from melatonin effects on circadian phase, temperature, or steroidogenesis. This study could pave the way for new treatments of PMDD. A large randomized-controlled trial for slow-release exogenous melatonin as an adjunct/treatment in PMDD is warranted.
Introduction
Around 2%–5% of women suffer from premenstrual dysphoric disorder (PMDD) [1], a constellation of mood and somatic symptoms causing clinically significant distress/functional interference during the luteal phase (LP) of their menstrual cycle. A majority of PMDD patients report sleep disturbances [2]. Previously our group conducted a study of the objective polysomnographic (PSG) sleep profile in women with PMDD and insomnia. We observed that despite no differences in sleep duration and efficiency, PMDD patients had increased slow-wave sleep (SWS) across the menstrual cycle compared to controls (large effect size, d = 1.74) [3]. Likewise, Baker et al. [4] found increased SWS during the follicular phase (FP) and LP in a group of women with severe premenstrual syndrome (PMS; a milder version of PMDD) compared to controls. The only other study comparing PMDD and controls found no differences between the groups [5].
PMDD is thought to result from the interaction between cyclic changes in ovarian steroids and the functioning of central neurotransmitters including serotonin and gamma-aminobutyric acid (GABA) [6–8]. Ovarian hormones also interact with the circadian system and PMDD patients responded favorably to chronotherapies including light therapy and sleep deprivation [9–14]. PMDD patients appear to have a disturbed circadian system (trait-marker) that is further dysregulated during the LP (state-marker), which might contribute to their symptomatology [15]. Across several studies, reduced melatonin levels were found in PMDD patients compared to controls, with reduced secretion during LP compared to FP [16–18].
To our knowledge, exogenous melatonin use has never been studied as a potential therapy in PMDD. In prior uses of “melatonin replacement” therapy in low melatonin secretors, it was recommended to administer 2 mg of prolonged-release melatonin 60 min before bedtime [19]. Here, we applied this approach to compare PMDD patients before and after taking melatonin during their LP. We hypothesized exogenous melatonin could improve sleep and reduce symptoms in PMDD. Primary outcomes included changes in PSG sleep, core body temperature (CBT), and melatonin circadian rhythms. Secondary outcomes included changes in PMDD symptoms and subjective sleep across the menstrual cycle. An N-of-1 approach was used due to the intensity and intrusiveness of the protocol required to assess these outcomes.
Methods
This study is the third part of a larger research project and more details on the protocol can be found in previous publications [3, 18].
Participants
The sample size was determined to detect variation of sleep across the menstrual cycle. An average sigma of 2.7% is attributed to the variation of rapid eye movement (REM) sleep observed throughout the menstrual cycle in women [20]. Therefore, to detect a mean difference of 4.5% at key phases of the menstrual cycle, a minimum of five subjects was necessary. We targeted the recruitment of nine subjects to detect an average within-group difference of 3% for REM sleep across phases of the menstrual cycle (beta = 0.1 and alpha = 0.05). Patients were recruited from January 20, 2001 to December 31, 2012. Five PMDD patients from the original research project were studied. The diagnosis was confirmed by two psychiatric assessments (at FP and late-LP, by P.L.) and supported by two validated scales completed daily for ≥2 consecutive menstrual cycles: (1) the Prospective Record of the Impact and Severity of Menstrual Symptoms (PRISM) and (2) a Visual Analog Scale (VAS) [3, 18]. The PRISM score includes 12 physical symptoms, 11 psychological symptoms, and lifestyle impact resulting from the symptoms. The 11-item VAS (100-mm bipolar scale, with 0 mm being “not at all” and 100 mm being “extreme symptoms”) was based on the four core symptoms for PMDD diagnosis (depressed mood, tension, affective lability, and irritability) and the seven secondary symptoms (anhedonia, difficulty concentrating, decreased energy, change in appetite, change in sleep, feeling out of control, and physical symptoms). The study was developed under DSM-IV but those criteria are in line with DSM-5 [21]. Eligibility criteria required the presence of ≥5 symptoms during late-LP, and an increase of ≥200% on one, or ≥100% on two core symptoms for the mean late-LP score compared to FP. Recruited PMDD patients indicated insomnia symptoms during LP, but not FP, and were screened for primary sleep disorders. Two indicated sleep-onset insomnia, two indicated sleep-maintenance insomnia, and one reported general insomnia (not specified). The severity of insomnia was not systematically documented by a scale. Participants were otherwise healthy, drug-free, and had no psychiatric comorbidity. All had a history of regular menstrual cycles (range: 24–29 ± 2 days), were ≥6 months postpartum and were non-lactating. Participants had no history of night-shift work or transmeridian travel within 3 months of study.
For ≥3 weeks before the experimental procedures, participants maintained a regular schedule of 8-hour sleep/darkness (no naps allowed) confirmed by sleep-wake log, calls to the laboratory, and wrist actigraphy (Actiwatch, Mini-Mitter, Bend, OR). Chronotype was assessed with the Horne and Ostberg Morningness-Eveningness Questionnaire [22]. The mean morningness-eveningness score was within the range of “moderately morning type” (mean ± SEM = 59.2 ± 3.6). Two participants were “neither type” and three were “moderately morning type” [22]. The Douglas Mental Health University Institute Research Ethics Board approved all procedures, which were in accordance with the Declaration of Helsinki. All participants provided informed written consent. The study was retrospectively registered as when study procedures were done, registering trials were not common (https://www.isrctn.com/ISRCTN96702191).
Design
At baseline, participants entered the laboratory for PSG recordings every third night of a complete menstrual cycle and left upon awakening. Data were allocated into one of eight menstrual phases: menses (ME), early follicular (EF), mid-follicular (MF), late follicular (LF), ovulation (OV), early luteal (EL), mid-luteal (ML), and late luteal (LL) phases. Menstrual cycle delineation is detailed in previous publications [3, 18]. The day of the first laboratory visit ranged from day one to three of the menstrual cycle (mean ± SD: 2.20 ± 0.63). Data from ME was excluded as it served as an adaptation and screening night. Wakefulness occurred in regular lighting (~150 lux) and 8-hour sleep episodes occurred in darkness (< 0.3 lux) following each participant’s regular sleep/wake schedule. Photoperiod duration on the day of laboratory admission was defined as dawn-dusk difference according to https://nrc.canada.ca/en/research-development/products-services/software-applications/sun-calculator/.
Participants underwent 24-hour intensive physiological monitoring under constant posture (CP) conditions during FP and LP. The CP included the requisite controls of a constant routine procedure [23] but allowed for an 8-hour sleep episode. Throughout the wake period of the CP, conditions remained constant, including semi-recumbent posture, time-cue-free environment, hourly iso-caloric snacks [24], and dim light levels (<10 lux).
During the intervention condition, the same participants were given 2-mg slow-release melatonin tablets (Rx Balance, Port Coquitlam, BC, Canada) to be taken 60 min prior to bedtime during the LP. On average, melatonin use per menstrual cycle was 13.5 ± 2 days (mean ± SEM). Melatonin administration lasted three consecutive menstrual cycles. During the third month, participants returned to the laboratory to repeat procedures but continued taking melatonin in LP. The order of conditions was not randomized.
Measures
PSG recordings, including central and occipital electroencephalogram, submental electromyogram, and electrooculogram, were made on a computerized system (Harmonie, Stellate Systems, Montreal, QC, Canada) at a sampling rate of 250 Hz and high- and low-pass filtered at 0.3 and 35 Hz, respectively. Sleep apnea/hypopnea and periodic limb movements in sleep were screened using established criteria [3]. Sleep was visually scored in 30-s epochs according to Rechtschaffen and Kales criteria [25] to be consistent with our prior publication [3]. Total sleep time (TST) was the sum of sleep Stages 1–4 plus REM sleep. Sleep stages are expressed as a percent of TST. Sleep efficiency (SE) was the percentage of TST in the period from lights-off to lights-on. Sleep onset latency (SOL) was the time from lights-off to the first appearance of at least two epochs of Stage 1 sleep or the first appearance of deeper sleep. SWS was the sum of sleep Stages 3–4.
CBT was continuously monitored (4×/min) in laboratory via a thermistor (Steri-Probe, Cincinatti Sub-Zero Products, Inc., Cincinnati, OH) inserted 10 cm into the rectum. Probe malfunctions or “slips,” identified visually and/or by an ad hoc program, were discarded. Mean 8-hour CBT, from lights-off to lights-on, was calculated.
Participants were requested to empty their bladder at bedtime and urine samples were collected at rising time. Urinary progesterone concentration was assessed using the Beckman Coulter DxI 800 system and Beckman reagents for chemiluminescence immunoassays (Beckman Coulter Inc., Brea, CA; coefficient of variation [CV]: 6.8%). 6-Sulfatoxymelatonin (aMt6) was measured in duplicate using a commercially available radioimmunoassay kit (Stockgrand Ltd., Surrey, UK). The sensitivity of the assay was 0.05 ng/mL, with a CV ranging from 11.3% to 12.4%.
Throughout the CPs, blood samples were collected 1×/hour via an indwelling catheter connected to an extension allowing sampling without disruption. The heparinized-saline infused (7.5 iu/cc at 30 cc/hour) to prevent clotting was cleared from the line before sampling. A morning sample was assayed for estradiol and progesterone concentration using Beckman reagents for chemiluminescence immunoassays (Beckman Coulter, Inc., Brea, CA; estradiol CV: 10.7%; progesterone CV: 6.8%). Hourly melatonin levels were determined by radioimmunoassay (125I-labeled melatonin) using the LDN Melatonin Direct Assay Kit (Medicorp, Montreal, QC, Canada). The sensitivity of the assay is 2 pg/mL. The intra-assay CV is 9.9%–12.3% for mean melatonin concentrations of 15–157 pg/mL, and the inter-assay CV is 9.6%–16.2% for 21–205 pg/mL.
PMDD symptoms were measured daily with the 11-item VAS. Mean scores per menstrual phase were calculated for each symptom. The four core symptoms were averaged together to yield a mean score called the VAS-Mood [26, 27]. Symptoms were also tracked using the PRISM calendar. Participants completed post-sleep questionnaires including a VAS (0–100 mm) for anxiety, subjective report of SOL, and 7-point Likert scales assessing sleep quality (1 = extremely bad, 7 = extremely good).
Circadian parameter assessment
Plasma melatonin data were log10-transformed due to the non-normal distribution. The area under the curve (AUC, spline method) was used to determine the total amount of circulating melatonin per 24-hour. Mesor (rhythm-adjusted mean), amplitude (mean-to-trough difference), and acrophase (peak time) were determined using a 3-harmonic regression model applied to individual melatonin curves [28]. To generate 24-hour melatonin curves, each hourly data point throughout the CP was assigned a time relative to the lights-on (time since lights on; TSLOn). Data were collapsed per participant into 2-hours bins and across participants per condition and menstrual phase.
Mesor, amplitude, nadir (minimum time), and phase angle (between waketime and nadir) were obtained from a dual-harmonic regression model applied to individual CBT curves (1-min bins) [28]. To generate 24-hour CBT curves, each hourly data point throughout the CP was assigned a time relative to TSLOn. Data were collapsed per participant into 1-hour bins, and across participants per condition and menstrual phase.
Statistics
Before analyses, SOL and urinary aMt6 levels were log10-transformed. Two-way repeated-measures ANOVAs (factors: baseline or intervention condition × menstrual phase) were used to analyze (1) sleep parameters, aMt6, mean 8-hour CBT, and questionnaires across seven menstrual phases and (2) circadian parameters derived from CPs and ovarian hormones across two menstrual phases. Tukey’s post hoc test was used to analyze significant effects. Effect sizes were calculated using partial eta-squared () with pooled standard deviation. Small, medium, and large effect sizes were 0.01, 0.06, and 0.14 [29]. Based on a priori hypotheses, Pearson’s correlations were computed between anxiety and reported sleep quality [4], and between aMt6 and SWS [3]. Analyses were performed using R Statistical Software [30]. Significance was set at p < 0.05. Data are presented as mean ± standard deviation (SD), except when specified otherwise.
Results
Participant characteristics
Five women with PMDD completed the study (33.6 ± 2.7 years old; Figure S1 in the Supplementary Appendix). Baseline laboratory visits for Subjects #1, #2 occurred in Winter, Subject #3 in Winter/Spring, Subject #4 in Summer, and Subject #5 in Fall. Intervention laboratory visits for Subjects #4, #5 occurred in Winter and Subjects #1, #2, #3 in Spring. On average, photoperiod duration on the day of laboratory admission was similar in the two conditions (baseline: 12.7 ± 2.4 h, intervention: 13.1 ± 2.3 h; Figure S2 in the Supplementary Appendix).
Ovarian hormones
Ovulation was confirmed for each participant and a main effect of menstrual phase was observed for urinary and plasma progesterone (p ≤ 0.005; Table 1) with increased levels in LP versus FP (p ≤ 0.001). Two-way ANOVA did not show an intervention condition × menstrual phase interaction or main effect of condition for progesterone or estradiol.
Table 1.
Ovarian hormones across the menstrual cycle at baseline and intervention conditions (mean ± SD)
| Follicular phase | Luteal phase | |||||
|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | ANOVA | ||
| Urinary progesterone (nmol/L) | 15.3 ± 6.4 | 13.1 ± 10.6 | 31.0 ± 23.3 | 24.1 ± 16.8 | C: F1,12 = 1.27, p = 0.28 MP: F1,12= 11.3, p = 0.005 C × MP: F1,12 = 0.34, p = 0.57 |
0.09 0.47 0.03 |
| Plasma progesterone (nmol/L) | 7.2 ± 1.4 | 9.1 ± 2.7 | 52.1 ± 22.4 | 51.6 ± 8.3 | C: F1,12 = 0.01, p = 0.91 MP: F1,12= 40.9, p < 0.001 C × MP: F1,12 = 0.03, p = 0.86 |
<0.01 0.77 <0.01 |
| Plasma estradiol (pmol/L) | 403.8 ± 175.4 | 536.3 ± 186.1 | 549.0 ± 264.2 | 648.4 ± 281.9 | C: F1,12 = 1.05, p = 0.32 MP: F1,12= 1.29, p = 0.27 C × MP: F1,12 = 0.02, p = 0.88 |
0.07 0.09 <0.01 |
C, condition; MP, menstrual phase; , partial eta-squared.
Polysomnographic sleep
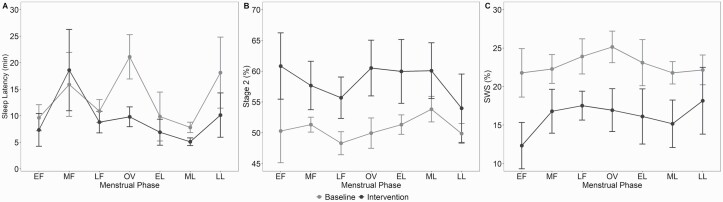
There was a main effect of menstrual phase and a main effect of intervention condition on SOL with a reduced SOL in intervention versus baseline condition (p = 0.01; Figure 1 and S3 in the Supplementary Appendix). There was a significant main effect of condition for Stage 2 sleep % and SWS % (p < 0.001; ≥ 0.30) with increased Stage 2 sleep and reduced SWS in intervention versus baseline condition (p ≤ 0.001). Two-way ANOVAs did not show any condition × menstrual phase interaction for TST, SE, WASO, Stage 1 sleep %, Stage 2 sleep %, SWS %, and REM sleep %. SWS was negatively associated with aMt6 (r = −0.53, p < 0.001).
Figure 1.
PSG sleep measures across the menstrual cycle at baseline and intervention conditions (mean ± SEM). (A): Sleep onset latency across the menstrual cycle in baseline and intervention conditions. Data reported in minutes, but analyses performed in log10. (B): Percentage of Stage 2 sleep across the menstrual cycle. (C): Percentage of slow-wave sleep across the menstrual cycle. EF, early follicular; MF, mid-follicular; LF, late follicular; OV, ovulation; EL, early luteal; ML, mid-luteal; and LL, late luteal.
Melatonin
Values across menstrual phases.
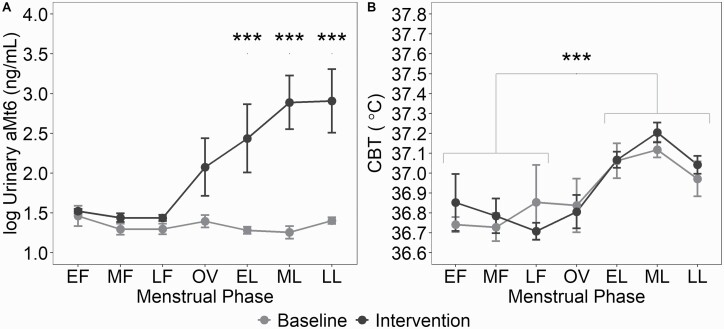
Two-way ANOVA revealed a condition × menstrual phase interaction for aMt6 (F6,46 = 5.42, p < 0.001, = 0.41; Figure 2). Post hoc comparisons revealed between-condition differences throughout the LP with increased levels aMt6 in the intervention versus baseline condition (p ≤ 0.01).
Figure 2.
Morning excretion of urinary aMt6 and CBT and across the menstrual cycle at baseline and intervention conditions (mean ± SEM).(A): Log10-transformed concentration of urinary aMt6 across the menstrual cycle in baseline and intervention conditions. *** indicates p < 0.001. (B): Nocturnal mean 8-hour CBT across the menstrual cycle in baseline and intervention conditions. aMt6, 6-sulfatoxymelatonin; CBT, core body temperature, EF, early follicular; MF, mid-follicular; LF, late follicular; OV, ovulation; EL, early luteal; ML, mid-luteal; and LL, late luteal.
Circadian profile.
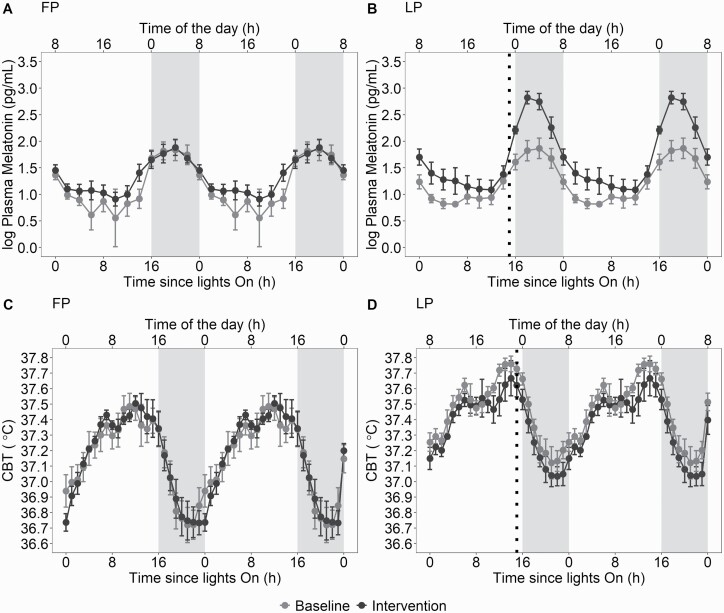
Melatonin curves from the CPs are depicted in Figure 3. There was a main effect of condition for the mesor (Table 2; F1,14 = 7.7, p = 0.01, = 0.36), with an increased mesor in the intervention versus baseline condition. Two-way ANOVAs did not show any condition × menstrual phase interaction.
Figure 3.
Circadian variation of plasma melatonin and CBT during the FP and LP during baseline and intervention conditions (mean ± SEM). (A): Circadian variation of plasma melatonin during the FP at baseline and intervention conditions. (B): Circadian variation of plasma melatonin during the LP at baseline and intervention conditions. (C): Circadian variation of CBT during the FP at baseline and intervention conditions. (D): Circadian variation of CBT during the LP at baseline and intervention conditions. Time of the day corresponds to clock time for a woman with a bedtime at midnight and waketime at 8:00. The dotted lines represent timing of slow-released melatonin ingestion. Gray shaded areas represent projected sleep. Detailed information about melatonin secretion and circadian parameters derived from 24-hour melatonin secretion can be found in a previous publication [18]. CBT, core body temperature; FP, follicular phase; LP, luteal phase.
Table 2.
Circadian melatonin and CBT profiles at baseline and intervention conditions (mean ± SD)
| Follicular phase | Luteal phase | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Intervention | Baseline | Intervention | ANOVA | |||
| MT | Mesor | 1.20 ± 0.28 | 1.34 ± 0.18 | 1.24 ± 0.23 | 1.71 ± 0.29 |
C:F
1,14
=7.7, p = 0.01
MP: F1,14 = 3.02, p = 0.10 C × MP: F1,14 = 2.10, p= 0.16 |
0.36
0.17 0.13 |
| Amplitude | 0.70 ± 0.42 | 0.55 ± 0.23 | 0.60 ± 0.24 | 0.92 ± 0.26 | C: F1,14= 0.64, p = 0.43 MP: F1,14 = 1.02, p = 0.32 C × MP: F1,14 = 3.02, p = 0.10 |
0.04 0.06 0.17 |
|
| Acrophase TOD |
2.77 ± 0.77 |
2.55 ± 0.79 |
2.89 ± 1.03 |
2.47 ± 1.36 |
C: F1,14 = 0.45, p = 0.51 MP: F1,14 = 0.01, p = 0.96 C × MP: F1,14 = 0.03, p = 0.85 |
0.03 <0.01 <0.01 |
|
| TSLOn | 19.71 ± 0.71 | 19.61 ± 0.71 | 19.84 ± 0.55 | 19.22 ± 0.92 | C: F1,14 = 1.24, p = 0.28 MP: F1,14 = 0.13, p = 0.72 C × MP: F1,14 = 0.54, p =0.47 |
0.08 <0.01 0.04 |
|
| AUC | 112133 ± 19100 |
113159 ± 27089 | 116249 ± 14417 |
147342 ± 25744 | C: F1,14 = 3.54, p = 0.08 MP: F1,14 = 2.08, p = 0.17 C × MP: F1,14 = 1.82, p = 0.19 |
0.20 0.13 0.12 |
|
| CBT | Mesor | 37.14 ± 0.15 | 37.16 ± 0.13 | 37.36 ± 0.09 | 37.45 ± 0.07 | C: F1,16 = 0.95, p = 0.34 MP:F1,16=24.6, p < 0.001 C × MP: F1,16 = 0.49, p = 0.49 |
0.06 0.61 0.03 |
| Amplitude | 0.4 ± 0.09 | 0.41 ± 0.07 | 0.32 ± 0.05 | 0.33 ± 0.09 | C: F1,16 = 0.07, p = 0.78 MP:F1,16=5.41, p = 0.03 C × MP: F1,16 = 0.01, p = 0.94 |
<0.01 0.25 <0.01 |
|
| Nadir TOD |
4.4 ± 1.18 |
4.82 ± 0.87 |
4.33 ± 0.9 |
4.57 ± 1 |
C: F1,16 = 0.55, p = 0.46 MP: F1,16 = 0.13, p = 0.71 C × MP: F1,16 = 0.04, p = 0.84 |
0.03 <0.01 <0.01 |
|
| TSLOn | 21.35 ± 1.24 | 21.57 ± 0.74 | 21.28 ± 0.58 | 21.32 ± 0.97 | C: F1,16 = 0.10, p = 0.75 MP: F1,16 = 0.15, p = 0.69 C × MP: F1,16 = 0.04, p = 0.82 |
<0.01 0.01 <0.01 |
|
| Phase angle | 2.65 ± 1.24 | 2.43 ± 0.74 | 2.72 ± 0.58 | 2.68 ± 0.97 | C: F1,16 = 0.10, p = 0.75 MP: F1,16 = 0.15, p = 0.69 C × MP: F1,16 = 0.04, p = 0.82 |
<0.01 0.01 <0.01 |
|
C, Condition; MP, Menstrual Phase; MT, melatonin; CBT, core body temperature; TOD; time of the day, TSLOn, time since lights on; AUC, area under the curve; , partial eta-squared.
Core body temperature
Values across menstrual phases.
Two-way ANOVA showed a main effect of menstrual phase for mean 8-hour sleep episode CBT (F6,41 = 17.67, p < 0.001, = 0.72; Figure 2) with increased CBT in EL, ML, and LL versus EF, MF, and LF (p < 0.001). Two-way ANOVA did not show a condition × menstrual phase interaction.
Circadian profile.
CBT curves from the CPs are depicted in Figure 3. There was a main effect of menstrual phase for the mesor (F1,16 = 24.6, p < 0.001, = 0.61; Table 2) and the amplitude (F1,16 = 5.41, p = 0.03, = 0.25) with an increased mesor and reduced amplitude in LP versus FP (p < 0.001). Two-way ANOVAs did not show any condition × menstrual phase interaction.
PMDD symptom scales
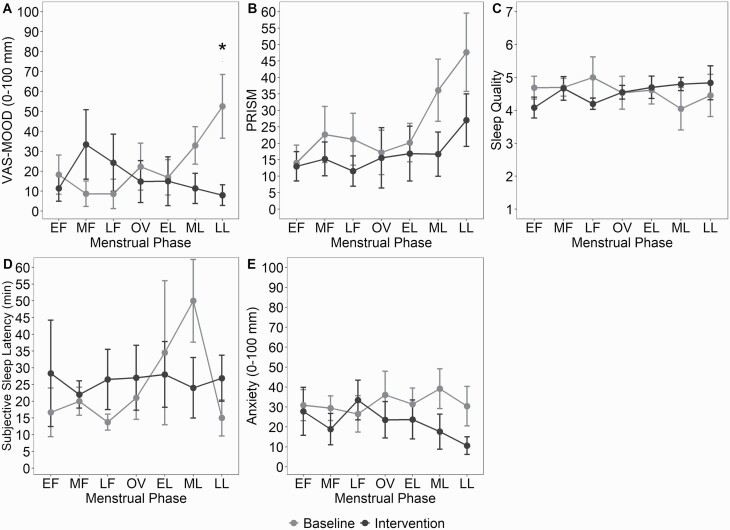
Two-way ANOVA showed a condition × menstrual phase interaction for the VAS-Mood (F6,43 = 2.87, p = 0.02, = 0.28, Figure 4). Post hoc comparisons revealed reduction of VAS-Mood in LL during intervention versus baseline (mean difference: −44.5 mm, p = 0.04).
Figure 4.
Morning questionnaires scores across the menstrual cycle at baseline and intervention conditions (mean ± SEM).(A): VAS-Mood across the menstrual cycle in baseline and intervention conditions. * indicates p < 0.05. (B): PRISM across the menstrual cycle in baseline and intervention conditions. Main between condition difference. (C): Sleep quality across the menstrual cycle in baseline and intervention conditions. (D): Subjective sleep onset latency across the menstrual cycle in baseline and intervention conditions. (E): Anxiety across the menstrual cycle in baseline and intervention conditions. Main between condition difference. ME, menses; EF, early follicular; MF, mid-follicular; LF, late follicular; OV, ovulation; EL, early luteal; ML, mid-luteal; LL, late luteal.
Two-way ANOVA revealed a main effect of menstrual phase (F6,52 = 5.90, p < 0.001, = 0.40) and a main effect of condition (F1,52 = 12.40, p < 0.001, = 0.19) for the PRISM score with a score reduction during intervention versus baseline (p < 0.001). Two-way ANOVA showed a main effect of condition (F1,52 = 21.65, p < 0.001, = 0.40) for the PRISM Lifestyle Impact with a score reduction during intervention versus baseline (p < 0.001). Two-way ANOVAs did not show a condition × menstrual phase interaction for the PRISM and the PRISM Lifestyle Impact. No harms or unintended effects were reported.
Post-sleep questionnaires
Two-way ANOVA showed a main effect of condition (F1,46 = 15.64, p < 0.001, = 0.25; Figure 4) for VAS-anxiety with decreased post-sleep anxiety scores in the intervention versus baseline (p < 0.001). Two-way ANOVAs did not show any condition × menstrual phase or main menstrual effect for VAS-anxiety. Two-way ANOVAs did not show any condition × menstrual phase interaction or main effects of menstrual phase or condition for subjective sleep quality and SOL. Anxiety in the morning was negatively associated with sleep quality (r = −0.44, p < 0.001).
Discussion
This study is the first, to our knowledge, to investigate the effects of exogenous melatonin on sleep, body temperature, and mood in women with PMDD.
One of our main findings is the large effect-size reduction of SWS in the intervention condition. The synchronized EEG pattern typical of SWS consists of multiple oscillation patterns, which are associated with the cortical slow oscillations at the cellular level in the thalamocortical network [31]. Neuroimaging studies in PMDD/PMS revealed abnormal thalamocortical pathways and clinical scales correlated with these findings supporting a role for the thalamocortical connectivity in the etiology of PMDD/PMS [32, 33]. Compared to controls, women with PMDD/PMS were found to have increased gray matter volume in the thalamus [33] and hyperconnectivity of the thalamus with cortical structures during FP and LP, suggesting the altered connectivity is a trait rather than a state marker of PMDD/PMS [32, 34]. Altered thalamocortical connectivity could contribute to the increased levels of SWS measured across the menstrual cycle in PMDD/PMS women versus healthy controls [3, 4].
In our prior analysis, increased SWS co-occurred with a reduced melatonin secretion across the menstrual cycle in PMDD patients compared to controls [3]. In the current study, melatonin replacement led to a reduction of SWS, and the relationship between SWS and melatonin is supported by a significant negative correlation between aMt6 and SWS. Recent evidence suggests that manipulation of circulating melatonin can modify brain connectivity in humans, although the neurophysiological processes underlying this remain to be clarified [35, 36]. Melatonin acts mostly through the G protein-coupled melatonin receptors termed MT1 and MT2, and structures involved in SWS, namely the reticular thalamus and the cortex, are rich in MT2 receptors [37, 38]. Animal models furthermore suggest MT2 receptors activation promote deep sleep, whereas MT1 receptors are thought to be involved in reducing neuron firing and regulating REM sleep [39, 40]. Interestingly, studies have shown that MT1 and MT2 receptors react differently to melatonin [39, 41]. Exposure of MT1 receptors to melatonin does not induce observable changes in melatonin-receptor density, affinity, or functional sensitivity [42]. Exposure of MT2 receptors to melatonin induced receptor desensitization and internalization [39, 41, 43]. Since PMDD patients have chronically low levels of melatonin, we hypothesize they might have overexpression of MT2 receptors leading to increased SWS, likely by contributing to the development/maintenance of abnormal thalamocortical connectivity. Exogenous melatonin could have led to an internalization of MT2 receptors [44] and concomitant reduction of SWS, possibly via alteration of thalamocortical connectivity. The persistence of SWS reduction through the entire menstrual cycle could be a carry-over effect from the previous intervention cycles and could represent the correction of a sleep trait-marker. Once internalized, the MT2 receptors depend on new protein synthesis for regeneration and this process is known to take more than 24 h after a single supraphysiological dose of melatonin [43, 45]. MT1 receptors (regulating REM sleep) are not involved in this model. Accordingly, %REM sleep is similar in PMDD/PMS and controls [3, 4], and exogenous melatonin did not alter REM sleep. In our study, the decrease of SWS came with increased Stage 2 sleep. Likewise, daytime administration of exogenous melatonin, when endogenous melatonin levels are low, was shown to reduce SWS and increase Stage 2 sleep without effect on Stage 1 or REM duration [46, 47]. In patients using beta-blockers (which are melatonin-suppressing), exogenous melatonin increased Stage 2 sleep with no effects on SWS [48].
Prolonged-release melatonin has otherwise minor effects on nocturnal PSG in healthy people [49] and modest effects on reducing objective and subjective SOL in patients with primary sleep disorders [50]. We found reduced objective, but not subjective SOL in the intervention condition compared to baseline, likely due to a lack of statistical power. Subjective SOL was overestimated compared with objective SOL at a similar extent to women with and without PMS [4]. A correlation between anxiety and subjective sleep quality was reported in women with severe PMS [4]. As morning anxiety levels are known to influence self-reported sleep quality [51], Baker et al. suggested that reducing mood symptoms in PMDD/PMS would improve subjective sleep quality [4]. We found a similar correlation between anxiety and subjective sleep quality but the significant (large effect-size) reduction of anxiety in the intervention condition did not improve subjective sleep quality.
Besides sleep regulation, melatonin receptors contribute to the regulation of circadian rhythms. MT1 and MT2 receptors were identified along the retinohypothalamic tract and could be involved in the photic entrainment of the suprachiasmatic nucleus (SCN) neuronal activity [37, 39, 52]. The abnormal response in the phase-shift of SCN activity in PMDD suggests an abnormal MT1/MT2 ratio. Parry and colleagues indeed demonstrated a blunted and directionally altered melatonin phase shift in response to morning bright light during LP, but not in FP [17, 53]. In our studies, CBT nadir and melatonin acrophase were similar in both conditions and compared to controls, which could be explained by the experimental stabilization of the sleep-wake cycle [18]. Melatonin is a weak circadian synchronizer compared to light, and a phase-shift was not expected due to the timing and dosage of the exogenous melatonin used [54]. Moreover, exogenous melatonin is not expected to change the strength of the circadian pacemaker, and accordingly, similar CBT amplitudes were observed between baseline and intervention. The increased mesor and reduced amplitude of the CBT rhythm during LP compared to FP are consistent with findings in women with [55–59] and without PMDD/PMS [58, 60], and with the thermogenic properties of progesterone [58, 61] which modulates thermosensitive neurons in the preoptic anterior hypothalamus [62]. The absence of CBT reduction despite exogenous melatonin use is consistent with the progesterone-induced resistance to the hypothermic properties of melatonin reported during the LP of healthy women [60, 63]. Cagnacci et al. also reported that the LP was one of the exceptions to which the inverse association between melatonin and CBT did not apply [64].
Another major finding is the large effect-size reduction in PMDD symptoms with exogenous melatonin. Importantly, the reduction of symptoms translated into improved reported functioning. A placebo effect cannot be excluded, but the long intervention duration and the high frequency of assessments at baseline might mitigate its extent [12, 65, 66]. Ovarian hormones are at the core of the PMDD symptomatology and ovarian hormones suppression can induce a remission of the symptoms [8, 67]. Whether exogenous melatonin can improve gonadotropin secretion in PMDD remains unclear [68]. Despite in vitro studies supporting the modulation of ovarian steroidogenesis by melatonin [69–71], in vivo studies in healthy women failed to reproduce these findings [72–74] and no modulation of steroidogenesis by exogenous melatonin was noted in our study. Mounting evidence shows that levels of ovarian hormones [75] and their neurometabolites (namely allopregnanolone) do not differ significantly between PMDD patients and healthy controls [76]. Alternative hypotheses, such as differences in the rate of change of ovarian hormones or alterations in GABA receptors sensitivity (hypo/hypersensitivity) have been proposed [8]. Our study design did not allow us to investigate the effects of melatonin on these parameters. The progesterone metabolite allopregnanolone potentiates GABA receptor-mediated inhibition of 5-HT neuronal activity [77]. The serotonergic system was postulated to be central to PMDD physiopathology and serotonin levels were found to be low in PMDD compared to controls [78]. Since melatonin is synthesized in the pineal gland from the precursor serotonin, the low melatonin levels observed in PMDD compared to controls could be explained by lower serotonin levels [18, 79]. The rate-limiting effects of noradrenergic mechanisms on melatonin synthesis might also help explain the lower melatonin secretion noted in PMDD compared to control. Markers of serotonergic and noradrenergic activity were not measured in this study which precludes us from making any definitive statements on the interaction between these systems in PMDD.
Selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment for PMDD and their rapid-onset efficacy suggests a mechanism of action different than in other depressive disorders [80]. SSRIs can provoke adverse effects resulting in poor compliance and treatment dropout in PMDD [81–83]. Exogenous melatonin is generally well tolerated [84] and the VAS-Mood reduction we found during the LL (when symptoms usually peak [8, 85]) is larger than what was reported in randomized controlled trials with paroxetine (12.5 or 25 mg) [86–88]. Although melatonin seems to have a regulatory role on serotonin based on in vitro and animal studies [89, 90], melatonin is generally not expected to have antidepressant properties in humans and its clinical efficacy is limited at best [91]. Nevertheless, the effects of exogenous melatonin on sleep noted in our study are unlikely to explain by themselves the reduction in PMDD symptoms, as several antidepressants (e.g. SSRIs) can improve mood without improving sleep. Thus, melatonin may have improved mood ratings in this study because of, in conjunction with, or despite its effect on SWS. Melatonin and SSRIs share some mechanisms of action, which might offer some insight into the improvement of the symptoms noted in our study. They can both alter the MT1/MT2 ratio which can potentially restore brain signaling and reduce affective symptoms [92, 93]. They can also modulate neuroplasticity and restore connectivity in key brain regions [94–96]. More studies are needed to understand the interaction between sex hormones, serotonin, melatonin receptors, brain connectivity, and symptoms in PMDD.
There were limitations in this study including the absence of placebo and the small sample size increasing the possibility of type II errors and reducing power (especially while investigating condition × menstrual phase interactions). Person-to-person variability in the bioavailability of melatonin should also be noted [97]. The inclusion of a sleep episode in the CPs may have influenced observed circadian rhythms. A strength of the study is that all patients reached the criteria for PMDD diagnosis, as opposed to commonly studied heterogeneous groups of PMS and PMDD. We also utilized a within-subject design, whereby all participants were studied at both FP and LP, at baseline and intervention which we believe adds to the statistical control of the study.
In conclusion, we have shown normalization of SWS and reduction in self-reported symptoms after administration of exogenous melatonin in women with PMDD and insomnia symptoms. These changes may relate to an abnormal MT1/MT2 ratio and appear independent from melatonin effects on circadian phase, temperature, or steroidogenesis. A large randomized-controlled trial for slow-release exogenous melatonin as an adjunct/treatment in PMDD is warranted.
Funding
This study was supported by an Operating grant from the Canadian Institutes of Health Research (CIHR, grant MOP-38064). D.B.B. was supported by salary support awards from the Fonds de la recherche en santé du Québec (FRQS) and CIHR.
Disclosure Statements
Financial Disclosure Statement
All authors declare no competing interests.
Nonfinancial Disclosure Statement
All authors declare no competing interests.
Authors’ Contributions
Conceived, designed, and supervised the experiments: DBB. Performed the experiments: DBB’s team. Sleep scoring and data entry: DBB's team. Statistical analyses: CM and PB. Wrote the paper: CM and DBB. Conducted medical and psychiatric evaluations of patients for inclusion in the study: DB and PL. Revised manuscript critically for important intellectual content: CM, PB, AS, PL, and DBB. Provided final approval of the submitted manuscript: CM, PB, AS, PL, and DBB.
Supplementary Material
References
- 1. Nevatte T, et al. ; Consensus Group of the International Society for Premenstrual Disorders. ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health. 2013;16(4):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker LJ, et al. Potential strategies to avoid progestogen-induced premenstrual disorders. Menopause Int. 2012;18(2):73–76. [DOI] [PubMed] [Google Scholar]
- 3. Shechter A, et al. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. 2012;13(8):1071–1078. [DOI] [PubMed] [Google Scholar]
- 4. Baker FC, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parry BL, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. 1999;85(2):127–143. [DOI] [PubMed] [Google Scholar]
- 6. Pearlstein T, et al. Premenstrual dysphoric disorder: burden of illness and treatment update. Focus. 2012;10(1):90–101. [PMC free article] [PubMed] [Google Scholar]
- 7. Dubol M, et al. Neuroimaging premenstrual dysphoric disorder: a systematic and critical review. Front Neuroendocrinol. 2020;57:100838. [DOI] [PubMed] [Google Scholar]
- 8. Sundström-Poromaa I, et al. Progesterone – Friend or foe? Front Neuroendocrinol. 2020;59:100856. [DOI] [PubMed] [Google Scholar]
- 9. Shechter A, et al. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parry BL, et al. Morning versus evening bright light treatment of late luteal phase dysphoric disorder. Am J Psychiatry. 1989;146(9):1215–1217. [DOI] [PubMed] [Google Scholar]
- 11. Parry BL, et al. Light therapy of late luteal phase dysphoric disorder: an extended study. Am J Psychiatry. 1993;150(9):1417–1419. [DOI] [PubMed] [Google Scholar]
- 12. Lam RW, et al. A controlled study of light therapy in women with late luteal phase dysphoric disorder. Psychiatry Res. 1999;86(3):185–192. [DOI] [PubMed] [Google Scholar]
- 13. Parry BL, et al. Therapeutic effect of sleep deprivation in patients with premenstrual syndrome. Am J Psychiatry. 1987;144(6):808–810. [DOI] [PubMed] [Google Scholar]
- 14. Parry BL, et al. Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder and normal comparison subjects. Am J Psychiatry. 1995;152(3):404–412. [DOI] [PubMed] [Google Scholar]
- 15. Parry BL, et al. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102–S108. [DOI] [PubMed] [Google Scholar]
- 16. Parry BL, et al. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12(1):47–64. [DOI] [PubMed] [Google Scholar]
- 17. Parry BL, et al. Reduced phase-advance of plasma melatonin after bright morning light in the luteal, but not follicular, menstrual cycle phase in premenstrual dysphoric disorder: an extended study. Chronobiol Int. 2011;28(5):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shechter A, et al. Pilot investigation of the circadian plasma melatonin rhythm across the menstrual cycle in a small group of women with premenstrual dysphoric disorder. PLoS One. 2012;7(12):e51929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson S, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol. 2019;33(8):923–947. [DOI] [PubMed] [Google Scholar]
- 20. Driver HS, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. [DOI] [PubMed] [Google Scholar]
- 21. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 22. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 23. Duffy JF, et al. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17(1):4–13. [DOI] [PubMed] [Google Scholar]
- 24. Harris JA, et al. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4(12):370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kales A, et al. ; University of California, Los Angeles. Brain Information Service, NINDB Neurological Information Network (U.S.). A Manual for Standardized Terminology, Techniques and Scoring System for Sleep Stages in Human Subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 26. Steiner M, et al. The measurement of premenstrual mood symptoms. J Affect Disord. 1999;53(3):269–273. [DOI] [PubMed] [Google Scholar]
- 27. Steinberg S, et al. A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria. Biol Psychiatry. 1999;45(3):313–320. [DOI] [PubMed] [Google Scholar]
- 28. Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988;13. [Google Scholar]
- 30. R: A Language and Environment for Statistical Computing [Computer Program] . Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 31. Steriade M, et al. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37(4):563–576. [DOI] [PubMed] [Google Scholar]
- 32. Liu P, et al. Thalamocortical dysconnectivity in premenstrual syndrome. Brain Imaging Behav. 2019;13(3):717–724. [DOI] [PubMed] [Google Scholar]
- 33. Liu P, et al. Altered brain structure in women with premenstrual syndrome. J Affect Disord. 2018;229:239–246. [DOI] [PubMed] [Google Scholar]
- 34. Dan R, et al. Trait-related changes in brain network topology in premenstrual dysphoric disorder. Horm Behav. 2020;124:104782. [DOI] [PubMed] [Google Scholar]
- 35. Zhang F, et al. The effect of jet lag on the human brain: a neuroimaging study. Hum Brain Mapp. 2020;41(9):2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iyer KK, et al. Neural correlates of sleep recovery following melatonin treatment for pediatric concussion: a randomized controlled trial. J Neurotrauma. 2020;37(24):2647–2655. [DOI] [PubMed] [Google Scholar]
- 37. Ng KY, et al. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct. 2017;222(7):2921–2939. [DOI] [PubMed] [Google Scholar]
- 38. Klosen P, et al. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J Pineal Res. 2019;67(1):e12575. [DOI] [PubMed] [Google Scholar]
- 39. Gobbi G, et al. Differential function of melatonin MT1 and MT2 receptors in REM and NREM sleep. Front Endocrinol (Lausanne). 2019;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gobbi G, et al. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. J Pineal Res. 2019;66(3):e12544. [DOI] [PubMed] [Google Scholar]
- 41. Gerdin MJ, et al. Short-term exposure to melatonin differentially affects the functional sensitivity and trafficking of the hMT1 and hMT2 melatonin receptors. J Pharmacol Exp Ther. 2003;304(3):931–939. [DOI] [PubMed] [Google Scholar]
- 42. Gerdin MJ, et al. Melatonin-mediated regulation of human MT(1) melatonin receptors expressed in mammalian cells. Biochem Pharmacol. 2004;67(11):2023–2030. [DOI] [PubMed] [Google Scholar]
- 43. Gerdin MJ, et al. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18(14):1646–1656. [DOI] [PubMed] [Google Scholar]
- 44. Dubocovich ML, et al. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101–110. [DOI] [PubMed] [Google Scholar]
- 45. Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. [DOI] [PubMed] [Google Scholar]
- 46. Hughes RJ, et al. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20(2):124–131. doi: 10.1093/sleep/20.2.124. [DOI] [PubMed] [Google Scholar]
- 47. Rajaratnam SM, et al. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561(Pt 1):339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scheer FA, et al. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: a randomized controlled trial. Sleep. 2012;35(10):1395–1402. doi: 10.5665/sleep.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arbon EL, et al. Randomised clinical trial of the effects of prolonged-release melatonin, temazepam and zolpidem on slow-wave activity during sleep in healthy people. J Psychopharmacol. 2015;29(7):764–776. [DOI] [PubMed] [Google Scholar]
- 50. Ferracioli-Oda E, et al. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8(5):e63773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harvey AG, et al. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep. 2008;31(3):383–393. doi: 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pfeffer M, et al. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol Int. 2012;29(4):415–429. [DOI] [PubMed] [Google Scholar]
- 53. Parry BL, et al. Blunted phase-shift responses to morning bright light in premenstrual dysphoric disorder. J Biol Rhythms. 1997;12(5):443–456. [DOI] [PubMed] [Google Scholar]
- 54. Burgess HJ, et al. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586(2):639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. J Biol Rhythms. 1988;3(3):255–263. [Google Scholar]
- 56. Severino SK, et al. High nocturnal body temperature in premenstrual syndrome and late luteal phase dysphoric disorder. Am J Psychiatry. 1991;148(10):1329–1335. [DOI] [PubMed] [Google Scholar]
- 57. Parry BL, et al. Temperature circadian rhythms during the menstrual cycle and sleep deprivation in premenstrual dysphoric disorder and normal comparison subjects. J Biol Rhythms. 1997;12(1):34–46. [DOI] [PubMed] [Google Scholar]
- 58. Cagnacci A, et al. Regulation of the 24h body temperature rhythm of women in luteal phase: role of gonadal steroids and prostaglandins. Chronobiol Int. 2002;19(4):721–730. [DOI] [PubMed] [Google Scholar]
- 59. Shechter A, et al. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cagnacci A, et al. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol (1985). 1996;80(1):25–29. [DOI] [PubMed] [Google Scholar]
- 61. Baker FC, et al. Oral contraceptives alter sleep and raise body temperature in young women. Pflügers Arch. 2001;442(5):729–737. [DOI] [PubMed] [Google Scholar]
- 62. Nakayama T, et al. Action of progesterone on preoptic thermosensitive neurones. Nature. 1975;258(5530):80. [DOI] [PubMed] [Google Scholar]
- 63. Zhang S, et al. Changes in sleeping energy metabolism and thermoregulation during menstrual cycle. Physiol Rep. 2020;8(2):e14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cagnacci A, et al. Homeostatic versus circadian effects of melatonin on core body temperature in humans. J Biol Rhythms. 1997;12(6):509–517. [DOI] [PubMed] [Google Scholar]
- 65. Rubinow DR, et al. The treatment of premenstrual syndrome—forward into the past. N Engl J Med. 1995;332(23):1574–1575. [DOI] [PubMed] [Google Scholar]
- 66. Posternak MA, et al. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry. 2007;190:287–292. [DOI] [PubMed] [Google Scholar]
- 67. Comasco E, et al. Ulipristal acetate for treatment of premenstrual dysphoric disorder: a proof-of-concept randomized controlled trial. Am J Psychiatry. 2021;178(3):256–265. [DOI] [PubMed] [Google Scholar]
- 68. Meers JM, et al. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43–49. [DOI] [PubMed] [Google Scholar]
- 69. Webley GE, et al. Melatonin directly stimulates the secretion of progesterone by human and bovine granulosa cells in vitro. J Reprod Fertil. 1986;78(2):711–717. [DOI] [PubMed] [Google Scholar]
- 70. Fang L, et al. Melatonin induces progesterone production in human granulosa-lutein cells through upregulation of StAR expression. Aging (Albany NY). 2019;11(20):9013–9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Olcese JM. Melatonin and female reproduction: an expanding universe. Front Endocrinol (Lausanne). 2020;11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Siegrist C, et al. Lack of changes in serum prolactin, FSH, TSH, and estradiol after melatonin treatment in doses that improve sleep and reduce benzodiazepine consumption in sleep-disturbed, middle-aged, and elderly patients. J Pineal Res. 2001;30(1):34–42. [DOI] [PubMed] [Google Scholar]
- 73. Woo MM, et al. Direct action of melatonin in human granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86(10):4789–4797. [DOI] [PubMed] [Google Scholar]
- 74. Schernhammer ES, et al. Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res. 2006;40(2):116–124. [DOI] [PubMed] [Google Scholar]
- 75. Schiller CE, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nguyen TV, et al. The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: effects of estradiol and progesterone addback. Transl Psychiatry. 2017;7(8):e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kaura V, et al. The progesterone metabolite allopregnanolone potentiates GABA(A) receptor-mediated inhibition of 5-HT neuronal activity. Eur Neuropsychopharmacol. 2007;17(2):108–115. [DOI] [PubMed] [Google Scholar]
- 78. Parry BL. The role of central serotonergic dysfunction in the aetiology of premenstrual dysphoric disorder: therapeutic implications. CNS Drugs. 2001;15(4):277–285. [DOI] [PubMed] [Google Scholar]
- 79. Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974;184(4144):1341–1348. [DOI] [PubMed] [Google Scholar]
- 80. Marjoribanks J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;2013(6):Cd001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lundin C, et al. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143. [DOI] [PubMed] [Google Scholar]
- 82. Segebladh B, et al. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2009;201(2):139.e1–139.e8. [DOI] [PubMed] [Google Scholar]
- 83. Sundström-Poromaa I, et al. Compliance to antidepressant drug therapy for treatment of premenstrual syndrome. J Psychosom Obstet Gynaecol. 2000;21(4):205–211. [DOI] [PubMed] [Google Scholar]
- 84. Besag FMC, et al. Adverse events associated with melatonin for the treatment of primary or secondary sleep disorders: a systematic review. CNS Drugs. 2019;33(12):1167–1186. [DOI] [PubMed] [Google Scholar]
- 85. Hartlage SA, et al. Criteria for premenstrual dysphoric disorder: secondary analyses of relevant data sets. Arch Gen Psychiatry. 2012;69(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cohen LS, et al. Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial. Psychosom Med. 2004;66(5):707–713. [DOI] [PubMed] [Google Scholar]
- 87. Pearlstein TB, et al. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry. 2005;7(2):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Steiner M, et al. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193(2):352–360. [DOI] [PubMed] [Google Scholar]
- 89. Míguez JM, et al. Evidence for a regulatory role of melatonin on serotonin release and uptake in the pineal gland. J Neuroendocrinol. 1995;7(12):949–956. [DOI] [PubMed] [Google Scholar]
- 90. Aldegunde M, et al. Effects of pinealectomy on regional brain serotonin metabolism. Int J Neurosci. 1985;26(1–2):9–13. [DOI] [PubMed] [Google Scholar]
- 91. Hansen MV, et al. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24(11):1719–1728. [DOI] [PubMed] [Google Scholar]
- 92. Wu YH, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148(2-3):357–367. [DOI] [PubMed] [Google Scholar]
- 93. Klosen P, et al. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J Pineal Res. 2019;67(1):e12575. [DOI] [PubMed] [Google Scholar]
- 94. Valdés-Tovar M, et al. Circadian modulation of neuroplasticity by melatonin: a target in the treatment of depression. Br J Pharmacol. 2018;175(16):3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Imbesi M, et al. Drug- and region-specific effects of protracted antidepressant and cocaine treatment on the content of melatonin MT(1) and MT(2) receptor mRNA in the mouse brain. Int J Neuroprot Neuroregener. 2006;2:185–189. [PMC free article] [PubMed] [Google Scholar]
- 96. Hirsch-Rodriguez E, et al. The pattern of melatonin receptor expression in the brain may influence antidepressant treatment. Med Hypotheses. 2007;69(1):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Di WL, et al. Variable bioavailability of oral melatonin. N Engl J Med. 1997;336(14):1028–1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.