Abstract
Study Objectives
Total sleep deprivation is known to have significant detrimental effects on cognitive and socio-emotional functioning. Nonetheless, the mechanisms by which total sleep loss disturbs decision-making in social contexts are poorly understood. Here, we investigated the impact of total sleep deprivation on approach/avoidance decisions when faced with threatening individuals, as well as the potential moderating role of sleep-related mood changes.
Methods
Participants (n = 34) made spontaneous approach/avoidance decisions in the presence of task-irrelevant angry or fearful individuals, while rested or totally sleep deprived (27 h of continuous wakefulness). Sleep-related changes in mood and sustained attention were assessed using the Positive and Negative Affective Scale and the psychomotor vigilance task, respectively.
Results
Rested participants avoided both fearful and angry individuals, with stronger avoidance for angry individuals, in line with previous results. On the contrary, totally sleep deprived participants favored neither approach nor avoidance of fearful individuals, while they still comparably avoided angry individuals. Drift-diffusion models showed that this effect was accounted for by the fact that total sleep deprivation reduced value-based evidence accumulation toward avoidance during decision making. Finally, the reduction of positive mood after total sleep deprivation positively correlated with the reduction of fearful display avoidance. Importantly, this correlation was not mediated by a sleep-related reduction in sustained attention.
Conclusions
All together, these findings support the underestimated role of positive mood-state alterations caused by total sleep loss on approach/avoidance decisions when facing ambiguous socio-emotional displays, such as fear.
Keywords: total sleep deprivation, approach/avoidance decisions, emotional expressions, positive mood, fear, anger, drift-diffusion models
Statement of Significance.
Sleep deprivation has significant detrimental effects on cognitive and socio-emotional functioning. Here, we assessed whether mood-state alterations caused by a total lack of sleep is a potential pathway through which total sleep deprivation impacts decisions in social contexts. Our results suggest that while sleep deprived individuals conserve the ability to respond adaptively to direct and clear social threat (anger), they do not perform as well when facing decisions based on ambiguous social cues (fear). Moreover, following total sleep deprivation, it is diminished positive affect, rather than decreased vigilance or sustained attention, which correlates with difficulties in ambiguous threat-related decisions. This result is of relevance, as there are clear links between sleep loss, depression and impaired social decisions.
Introduction
Total sleep deprivation has significant detrimental effects on everyday human functioning. It is known to affect a whole range of cognitive functions including alertness, sustained attention (e.g. [1–4]), memory, response inhibition and decision-making (e.g. [1,5–7]). Moreover, emerging evidence suggests that a total lack of sleep has a strong impact on mood [8] and profoundly disrupts affective and socio-emotional functioning (e.g. [9–13]), interfering with an individual’s ability to interact with their social world (e.g. [14–17]). Yet, the mechanisms by which sleep loss disturbs decisions in social situations are poorly understood. Here, we propose that mood-state alterations caused by a total lack of sleep is one mechanism through which total sleep deprivation impacts social decisions.
Mood typically refers to slow fluctuations in general affect, which, differently from emotions, are not tied to specific events. Studies in healthy individuals using both acute total sleep deprivation and chronic sleep restriction protocols have reported mood-state alterations caused by disrupted sleep (e.g. [11]), with larger effects on mood than on either cognitive or motor responses [8]. However, sleep loss may not equally impact negative and positive mood states (e.g. [18–20]). Several studies have observed a significant decrease in positive affect (PA), but no change in negative affect (NA) [18, 20–26], after total sleep deprivation, suggesting that PA may be more sensitive to the effects of sleep deprivation than NA. Knowing that high PA is a good predictor of adapted social functioning in both healthy children and adults (e.g. [27]), the above-described results suggest that mood-state alterations caused by a total lack of or restricted sleep might impact social functioning. Moreover, there is empirical support for a bidirectional relationship between sleep and mood disorders, notably depression (e.g. [28,29]). Low PA, in particular the diminished ability to experience pleasure and joy (anhedonia), is considered a risk marker for depressive disorders [28, 30] and is a cardinal symptom of depression [29, 31], known to be associated with significant and pervasive impairments in social functioning (see reviews [32, 33]).
These results raise the question as to whether and how mood-state alterations, caused by total sleep deprivation, contribute to the impairments observed in social functioning. One of the mechanisms regularly reported to be altered by mood fluctuations is decision making (e.g. [34]). Recent evidence pertaining to economic decisions indicates that mood changes impact at least two key processes of decision making: (a) the valuation of the expected outcome associated with each available action, and (b) the subsequent evaluation of the obtained action outcomes [35, 36]. For instance, Vinckier et al. [37] showed that mood fluctuations modulate the relative weight of expected gain and loss prospects assigned to different choice options. Similar effects on value-based decisions were also observed using total sleep deprivation protocols: total lack of sleep impacted the valuation of expected gains and losses [38, 39], of expected effort associated with action possibilities [40] and/or the ability to learn from previous outcomes [41–44]. Additionally, a diminished ability to learn from reward revealed in sleep restricted healthy subjects was suggested to be mediated by changes in PA [24]. Finally, anhedonia in clinical depression has also been associated with a blunted expected value of reward at the time of decision [45] as well as decreased motivation to act for reward [46, 47]. Together, these results suggest that mood-state alterations caused by a total lack of or restricted sleep might mediate the impact of sleep deprivation on decisions, possibly by altering valuation between option alternatives.
While the above-described studies focused on economic decisions, it is worth mentioning that other studies have investigated the effects of total sleep deprivation on aspects more related to human social cognition. Several studies exist concerning the impact of sleep deprivation on the perceptual processing of socio-emotional information (see [10, 14, 48]), as well as its effects on complex social behavior (e.g. moral dilemmas [49]; prosocial decisions using the Ultimatum, Dictator game or Trust games [50]). Nonetheless, to our knowledge, no study has focused on whether sleep loss influences simple action decisions in response to socio-emotional signals. Moreover, as clear relationships appear between sleep loss, depression (anhedonia) and impaired social functioning [17], determining whether difficulties in social decisions observed under sleep loss are linked to mood-state alterations, rather than decreased vigilance or sustained attention, is clearly relevant.
The present study aimed at investigating the impact of total sleep deprivation on action-related decisions in a social context and the potential moderating role of mood-state alterations caused by sleep deprivation. To do so, we used a novel social decision task developed by Vilarem et al. [51] which has been shown to assess the impact of task-irrelevant threat-signaling expressions (anger and fear) on action decisions (approach-avoidance) between two alternative targets of action. Angry and fearful displays are of negative valence, yet they differ in their social meaning and therefore in their action request to the perceiver [52]. Angry expressions are clear signals of impending verbal or physical assault [53], which in most contexts leads to avoidance. Fearful expressions, in contrast, signal both the presence of a potential danger [54] and a need for affiliation [55] and are therefore more ambiguous in terms of avoidance and approach responses. Contrasting these two emotional expressions therefore allows us to determine whether total sleep deprivation differentially impacts decisions based on clear, ambiguous and subtler social cues. Understanding how such social decisions are made under total sleep deprivation is of great relevance for individuals submitted to sleep deprivation related to their working conditions such as nurses, doctors or policemen.
To assess the impact of total sleep deprivation, participants performed the above-described free choice task while sleep rested (Rested Baseline: RB) or totally sleep deprived (SD - 27 hours of continuous wakefulness). In addition to model-free analyses, we used Drift Diffusion Models (DDMs) [56] that allowed us to translate the raw proportion of choice and the distribution of response times into parameters representing the contribution of different cognitive processes. We evaluated two alternative hypotheses: The first is related to past evidence that total SD deteriorates inhibitory efficiency [57, 58], increases reliance on automatic behaviors [59] as well as impulsivity and sensitivity to negative, especially threat-related, stimuli [9, 17, 60]. In this scenario, total SD and the associated sensitivity to threat-related stimuli would strongly prompt avoidance action tendencies. This stimulus-evoked, pre-decisional bias would influence the final action choice. The second hypothesis arises from past studies suggesting an impact of total SD [38] as well as mood [35, 36] on the valuation process at the time of choice during economic decisions. In the present context, total SD would alter the decisional process itself, i.e. the valuation process between option alternatives.
We therefore compared DDMs where impact of sleep (RB and SD) on behavior emerged from a modulation of (1) the pre-decisional bias, i.e. the starting point of the accumulation process, which would reduce the amount of stimulus evidence needed to produce the response (e.g. avoidance); (2) the decisional process itself, i.e. the rate of evidence accumulated in favor of each option allowing for the rapid arbitration between action alternatives; or (3) both of the above. Finally, to assess the potential moderating role of alterations in mood-state caused by total sleep deprivation, we investigated whether changes between Rested Baseline (RB) and total Sleep Deprivation (SD) in choice behavior/model parameters were related to changes in positive and/or negative mood. We also evaluated whether disruptions of sustained attention caused by total SD could mediate the effect of alterations in mood-state on changes in choice behavior.
Methods
Participants
All participants involved in this study reported no history of neurological or psychiatric disorders. The experimental protocol was approved by the Cochin – CPP Ile de France IV (Paris) Ethics Committee and by the French National Agency for Medicines and Health Products Safety (ANSM) (ID-RCB: 2017-A02793-50), and was carried out in accordance with the Declaration of Helsinki. The participants provided informed written consent and were compensated for their participation. This protocol is part of a project declared on the clinical trial database (NCT03859882, February 25, 2019).
As we consistently replicated the main effect of Emotion (anger vs. fear) in our previous studies [51], we calculated the a-priori required sample size for detecting a significant effect of Emotion on the proportion of choice for the present experiment (alpha = .05; power = .80). We used the raw data of the study presented in the supplementary material of Vilarem and colleagues’ paper, which had a larger sample size, i.e. 40 subjects. We recalculated the ANOVA on the proportion of away responses, obtaining an η 2p = .268 for the effect of Emotion, corresponding to a required sample size of 25 participants. We aimed at enrolling 42 participants into the study, to compensate for potential dropouts (two sessions), habituation (as each participant were performing the task four times) and technical problems. Five participants did not show up for the second session of testing and three of them were excluded based on their behavioral data (see below). The final sample (N = 34, 18 women) had a mean age of 32.6 ± 7.4 years (age range = 22–52). The mean BMI was 22.8 ± 2.5 (mean ± SD; 23.3 ± 2.4 for men; 22.4 ± 2.6 for women). Using a French version of a self-assessment questionnaire for chronotype [61], we determined that most of our participants were either middle (56%) or moderately morning type (29%) and to a lesser extent extremely morning type (12%) or moderately evening type (3%).
Total sleep deprivation procedure and monitoring of participants
This study has been conducted in the sleep laboratory of the French armed forces biomedical research institute (IRBA) at Brétigny sur Orge in France. Participants took part in two experimental sessions for three consecutive days, spaced 2–6 weeks apart. Each experimental session included (1) an habituation/training day (D0) during which participants came to the sleep laboratory in the late afternoon. They underwent an habituation period (training, Supplementary Figure S1) for the behavioral tests and were then given 9 h of sleep opportunity (baseline night: 10 pm–7 am), (2) a Rested Baseline (RB or D1) day beginning at 07:15 am until 12:00 pm; (3) the total Sleep Deprivation (SD or D2) day beginning at 00:00 am until 21:00 pm, and finally (4) a recovery night, i.e. 10 h of sleep opportunity (9 pm–7 am) before leaving the laboratory (D3, Supplementary Figure S1). The order of Rested Baseline (D1), day and Totally Sleep Deprived day (D2) was not counterbalanced between participants (Supplementary Figure S1).
Participants were maintained in an individual temperature-controlled (22 ± 1.5°C) room. Restroom and bathroom facilities were collectively available in the sleep-laboratory flat that contains a main living room (34 m2). Illumination was maintained between 150 and 300 lux during the entire experimental period (lights off during sleep periods). Meals and caloric intake were standardized for all subjects (2600 kcal/day). Participants were not allowed to practice exercise, taking tobacco, alcohol, or other psychoactive substances during the study.
The morning sessions were devoted to mental tasks such as the social free-choice task, whereas the afternoon sessions were mostly devoted to physical tasks (e.g. jump task test and force–velocity test). These physical tasks sessions were composed of two kind of physical tasks that is a jump task (six jumps) and a force-velocity task (on a bike). These two physical tasks lasted around 15 min (for one subject) and were assessed from 4 to 5 pm.
When not engaged in any specific testing or meals, participants followed a standardized activity program (reading, watching videos, playing games and speaking with others subjects or staff members). Investigators were systematically present in the laboratory with at least one of them staying with subjects. When participants were about to fall asleep (eyes closed, head down), they were gently and immediately woken up (i.e. no period of sleep > 30 s). During cognitive testing periods of RB and SD days (Supplementary Figure S1), participants were individually monitored by an experimenter.
Caffeine protocol
While part of the overall project aimed at addressing the impact of caffeine on several cognitive tasks assessed when sleep-deprived (see [4]), here we did not have any specific hypotheses regarding the effect of caffeine on action-decisions in social context, and therefore only checked for potential effects on both mood states and performances during our task. We still provide the procedure used in this double-blind placebo-controlled and crossover study. Participants were randomly assigned to either Caffeine or Placebo condition using the order of inscription to the study by an independent member of the staff following a random list. The randomized plan has been made in order to have two subjects with Caffeine and two subjects with Placebo in each session. Caffeine or Placebo was administrated in decaffeinated beverage, at 08:30 and 14:30 on RB and SD days. During Caffeine condition, caffeine powder was pre-measured by the project supervisor to amount to 2.5 mg/kg body mass for each participant and then mixed with decaffeinated beverage. Participant and staff members were blind to treatments. This amount of caffeine powder was chosen because a range from 0.2 to 5.5 mg/kg has been found to enhance response time in total sleep-deprived conditions [62]. The mean daily total sleep time for the baseline night (N0, see Supplementary Table S1) was 7.62 ± 0.25 h (7.66 ± 0.14 h for the Placebo session and 7.59 ± 0.11 h for the Caffeine session). Overall, subjects’ average habitual daily caffeine consumption (prior to participation in this experiment) was 233 ± 188 mg (caffeine range = 0–600) and subjects’ Epworth sleepiness score, determined at the time of subjects’ inclusion (2 weeks prior to participation in this experiment) was 6.9 ± 3.5.
Positive and Negative Affective Scale
Changes in mood were assessed at 9:30 am on the experimental mornings, in both RB and total SD conditions, using the Positive and Negative Affective Scale [63]. The PANAS consists of two 10-items mood scales, assessing either positive or negative mood, consisting of words (e.g. interested, excited, distressed, upset). Participants are asked to rate how much each of the words describes them right now on a 5-item scale from “very slightly” or “not at all” to “extremely”. As alterations in mood commonly co-occur with sleep disruption [64], these measures provide an important test of whether changes in behaviors were or were not related to changes in mood.
The psychomotor vigilance task (PVT)
Throughout the two days of testing, as well as during the SD night, participants regularly (09:15, 15:15, 21:15, and 03:15) completed a computer-based version of the 10-min psychomotor vigilance task (PVT [65]), a sustained-attention task that measures the speed with which subjects respond to a visual stimulus. We used the PVT task completed at 9:15 am, which was the closest in time from the PANAS and the social free-choice task (Supplementary Figure S1). Participants were instructed to respond, by clicking the left mouse button, as soon as a visual stimulus appeared (incrementing millisecond counter) without making false starts. The inter-stimulus interval was randomized between 2 and 10 s. The response times (RTs) were quantified in milliseconds and were regarded valid if RT was ≥150 ms.
The social free-choice task
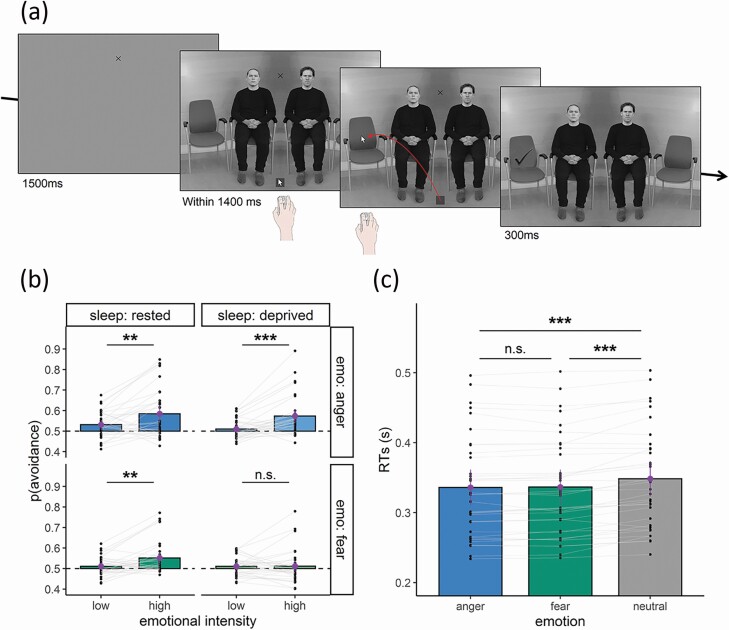
The social free-choice task (Figure 1, a) was adapted from a previous study [51] and administrated at the same time (at 10 am) during the RB (D1) and the SD (D2) day (Supplementary Figure S1).
Figure 1.
(a) The social free-choice task. Time course of a trial where participants were asked to indicate where they would like to sit. The identities displayed were not used in the actual experiment and were selected for illustration purposes only, following the guidelines of the Redboud Faces Database. (b) Choices. Violet dots and vertical lines represent the mean and the confidence interval of the mean proportion of away responses. Smaller gray dots represent the mean for each subject (smaller grey dots). (c) Response time (RTs). Violet dots and vertical lines represent the mean and the confidence interval of each subject’s median RTs for Anger, Fear and Neutral scenes, irrespective of sleep conditions. Smaller grey dots represent the median RTs for each subject. In both graphs, thinner grey lines connect the values in the “low” and “high” emotional intensity conditions within subjects. ***p < 0.001; **p < 0.01; *p < 0.05; n.s. = p > 0.05. ***p < 0.001; **p < 0.01; *p < 0.05; n.s. = p > 0.05.
Stimuli.
Subjects were presented with a scene representing a waiting room with four seats, where the two middle seats were occupied by two individuals and the two outer seats were empty (see Figure 1, a). Each scene was the composite of one template female or male hemi-scene (photograph depicting either one female or one male sitting next to an empty seat) juxtaposed to its mirrored version, on which faces were superimposed. Ten (five females) fixed pairs of identities (RadBoud Faces Database [66]) that were matched for gender as well as perceived trustworthiness and threat traits (for details, see [51]) were used. One actor of the pair always displayed a neutral expression while the other displayed either a neutral, angry or fearful expression. Faces varied in emotion (neutral, angry, or fearful expressions) and in intensity (four levels of morphs for anger and fear, created from the neutral to the emotional expression using a simple linear morphing transformation), and were equalized in perceived emotional intensities [67]. The identities, as well as the side of the actor expressing emotions, were fully counterbalanced. This resulted in 480 trials: 10 pairs × (2 emotional expressions × 4 levels of morphs + 1 neutral expression × 4 repetitions) × 2 emotional actor’s identity × 2 emotional actor’s side.
Social free-choice task procedure.
Participants were seated such that the distance from their eyes to the screen was 60 cm so that the eccentricity to the central fixation cross was 4.5 degrees for the center of the faces, and of 8 degrees for the center of the seats. The experiment was run using Psychtoolbox on Matlab R2012b. Each trial began with a grey screen, presented for 1000 ms, then a fixation cross was superimposed upon the grey screen for a duration varying between 500 and 750 ms. After the fixation, the scene appeared and remained on the screen until a correct response was registered, or until a maximum time of 1400 ms in the case of no response. Participants were asked to choose which seat they would like to occupy in the scene, while maintaining fixation on the cross displayed between the faces throughout the trial. To indicate their choice, participants had to left-click on the mouse, move the cursor from the bottom center of the scene and release the click on the chosen seat. The cursor was automatically re-centered at every new trial. Participants were requested to make spontaneous free choices and were instructed that there were no correct choices in this task. Nevertheless, they were instructed that their movements needed to be correctly performed for their responses to be registered. A correct movement was defined by the release of the button on one of the seats within 1400 ms after scene onset, and was signaled to the participants by the appearance of tick symbol superimposed on the scene at the release location, for 300 ms, providing a feedback that the choice had been correctly registered. If the button release was not made on the seat, or did not occur within 1400 ms, the trial was considered incorrect.
Participants underwent training until their accuracy (proportion of correct movements) in the task reached 60% and then completed the experiment. They were informed of their percentage of correct responses at the end of each block and were asked to do their best to maximize it.
Statistical analyses
We rejected invalid trials including non-responses, invalid responses (trials where the movement was not correctly performed), click times (RTs) faster than 100 ms to eliminate response anticipations, as well as trials where subjects had moved the mouse outside the bottom center of the scene before clicking. After cleaning the data, and in order to be sure to have enough trials in each condition, subjects with a proportion of valid trials below 65% were excluded (n = 3).
Responses were coded as follows: if the subject sat on the chair far from the individual displaying threat-related facial expressions, the response was coded “away.” On the other hand, if the subject sat on the chair close to the individual displaying threat-related facial expressions, the response was coded “toward.” The Intensity factor was re-coded on two levels (i.e. low-intensity = level1 + level 2 and high-intensity = level 3 + level 4).
Repeated ANOVAs.
First, repeated-measures ANOVAs were fitted on the variables of interest using the “EzANOVA” function of the “Ez” package [68] in RStudio (v 1.3.1073). For the positive and negative PANAS, we first ran separate repeated-measures ANOVAs on the scores with Session (First, Second) and Sleep (Rested, Deprived) as within-subject factors. We then assessed the potential effect of caffeine by running separate repeated-measures ANOVAs on the scores with Caffeine (Caffeine, Placebo) and Sleep (Rested, Deprived) as within-subject factors.
For the proportion of choice, we first assessed whether there was an effect of session by running a repeated-measures ANOVA on the mean proportion of away responses, with Session (First, Second), Sleep (Rested, Deprived), Emotion (Anger, Fear) and Intensity (High, Low) as within-subject factors. We then assessed whether there was an effect of caffeine by running a repeated-measures ANOVA on the mean proportion of away responses, with Caffeine (Caffeine or Placebo), Sleep (Rested, Deprived), Emotion (Anger, Fear) and Intensity (High, Low) as within-subject factors. As we did not observe any effect of Session (all ps > 0.064) or of Caffeine (all ps > 0.26) on choices (see Supplementary Tables S2 and S3), we pursued our analyses without these factors. To follow-up on a three-way interaction, we fitted separated ANOVAs on Anger and Fear trials respectively, with Sleep (Rested, Deprived) and Intensity (High, Low) as within-subject factors.
Second, for RTs, a repeated-measures ANOVA on the median of RTs for each subject was fitted with Sleep (Rested, Deprived), Emotion (Anger, Fear), Intensity (High, Low) and Decision (Approach, Avoidance) as within-subject factors. A second repeated-measures ANOVA on the median RTs for each subject was fitted, with Sleep (Rested, Deprived) and Emotion (Anger, Fear, Neutral) as within-subject factors. The same ANOVAs were fitted on the median movement times.
A Huynh-Feldt correction was applied when the assumption of sphericity was not met. When appropriate, post-hoc analyses with paired Student’s t-tests were computed and all p-values were adjusted for multiple comparisons (Holm correction). All data are expressed as mean (m) and 95% confidence intervals (CIs). Generalized eta-squared measure of effect size (η2G) is reported as the effect size of the F statistics and Cohen’s d (d) as the effect size of the t statistics.
Drift-diffusion models.
We employed the fast-dm software (version 30.2) [69–71] to fit DDMs on the subjects’ choices and RTs. DDMs are used to infer the cognitive processes involved in binary decision tasks: the decision process itself, defined as the rate (v) at which evidence for one of the choices is accumulated; the threshold (a) that represents the amount of information which separates the two alternative choices; the pre-decisional bias which is mapped on the starting point (z)—the closer the starting point to a threshold, the less information is needed to decide for that option; and finally the non-decision time (t0) that captures stimulus encoding and response execution, which respectively precedes and follows the decision process [71].
On the basis of our previous experiment [72], four models were run to understand the influence of Sleep on the decision: (1) a null model, where none of the parameters varied as a function of our factors of interest (Sleep, Emotion, and Intensity); (2) a model where only the starting point (z) was allowed to vary as a function of our factors of interest; (3) a model where only the drift rate (v) was allowed to vary as a function of our factors of interest; (4) a model where both z and v were allowed to vary as a function of our factors of interest. Due to the moderate number of trials per condition, we simplified the models as much as possible, in several ways: the thresholds of the model were associated with away (upper threshold) and toward responses (lower threshold); furthermore, the inter-trial variabilities of drift rate and threshold (but not of the non-decision time) were fixed to zero, since a proper fit of these parameters is particularly challenging, especially with small to medium-sized trials number [73].
Minimum-Norm optimization was used for parameter’s estimation. The AIC was extracted for each subject and the mean AIC for each model was computed to permit model comparison. In addition to the mean AIC, which could be affected by subject heterogeneity, we relied on a hierarchical Bayesian model selection criterion, in which models are random variables [74, 75]. The model estimates the parameters of a Dirichlet distribution which describes model probabilities, which in turn define a multinomial distribution over model space, allowing for the calculation of the exceedance probability of the winning model being more likely than the others. Finally, parameter estimates from the winning drift diffusion model were tested using repeated-measures ANOVAs.
Correlations with PANAS scores.
We then assessed whether changes in choice behavior between Rested Baseline (RB) and totally Sleep Deprived (SD) were related to changes in positive and negative mood between RB and SD. To obtain individual subject measures of change in choice behavior per emotion (Anger and Fear), we calculated the difference in the proportion of away responses for High intensity expressions between Sleep Deprived and Rested Baseline (e.g. High Anger SD – High Anger RB). To obtain individual subject measures of change in mood, for PA and NA separately, we calculated the difference in PANAS scores between Sleep Deprived and Rested Baseline, e.g. Positive Affective score SD – Positive Affective score RB). We then assessed whether those two measures of change (choice and mood) were correlated (Spearman correlations) using RStudio (v 1.1.453).
Measuring the potential impact of disruption of sustained attention on observed effects.
To determine whether sleep-related disruption of sustained attention could mediate the effect of sleep-related changes in positive PANAS on sleep-related changes in choice behavior for fearful stimuli (see above correlation), we ran a mediation analysis using reaction times (PVT-rt) from the classical PVT test as the mediator variable. We conducted structural equation modeling (sem) using the ‘lavaan’ package [76], sem function in RStudio (v 1.1.453). We assessed the relationship between the change in positive PANAS scores (X) and the change in the proportion of away responses to fearful stimuli (Y), using PVT-rt as the mediator (M). Analyses were performed using Maximum Likelihood (ML) as the estimator. Indirect effects, using 95% bias-corrected bootstrap confidence intervals based on 10,000 samples, were estimated. To strengthen our results, we ran a second mediation analysis using the change between Rested Baseline (RB) and totally Sleep Deprived (SD) conditions in the number of non-responses during the social decision task. Missed responses during our task can be considered a complementary measure of sustained attention, which has the advantage of providing information about sustained attention during the task of interest. Therefore, we expected this measure to correlate with PVT-rts and to provide similar results in the mediation analysis.
Results
Impact of total sleep deprivation on PANAS positive and negative scores
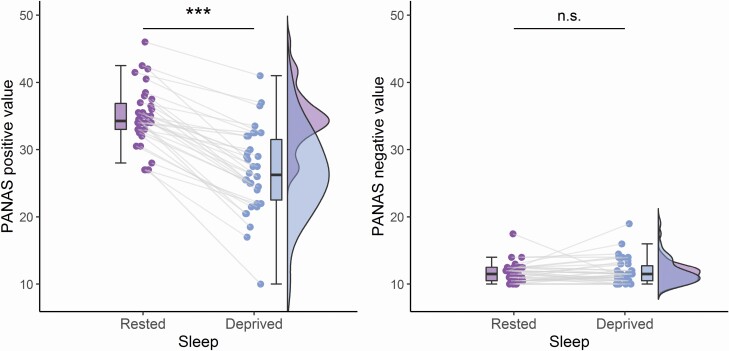
The repeated measures ANOVA on positive PANAS scores highlighted a strong main effect of Sleep [F(1,33) = 144.92, p < 0.001, η2G = .30], but no main effect of Session [F(1,33) = 1.05, p = 0.313, η2G = .01], nor any interaction between Sleep and Session [F(1,33) = 0.17, p = 0.685, η2G < .001], indicating that the scores on the positive PANAS decreased between RB and SD. In contrast, the repeated measures ANOVA on negative PANAS scores only highlighted a small main effect of Session [F(1,33) = 5.51, p = 0.025, η2G = 0.043], but no main effect of Sleep [F(1,33) = 0.32, p = 0.577, η2G = 0.001], nor any interaction between Sleep and Session [F(1,33) = 0.91, p = 0.347, η2G = 0.005] (Figure 2, Supplementary Table S4).
Figure 2.
PANAS. Changes in positive (left) and negative (right) PANAS scores between Sleep Rested and totally Sleep Deprived conditions, respectively. Distributions are represented using an adaptation of raincloud plots (Allen et al. [77]).
Regarding Caffeine, the repeated measures ANOVA on positive PANAS scores highlighted a strong main effect of Sleep [F(1,33) = 144.92, p < 0.001, η2G = .30], a main effect of Caffeine [F(1,33) = 6.82, p = 0.013, η2G = .031], but no interaction between Sleep and Caffeine [F(1,33) = 0.42, p = 0.523, η2G < .001], indicating that the scores on the positive PANAS decreased between RB and SD, and that Caffeine was associated with higher positive PANAS scores as compared to Placebo. In contrast, the repeated measures ANOVA on negative PANAS scores showed no main effect of Caffeine [F(1,33) = 3.04, p = 0.090, η2G = .025], no main effect of Sleep [F(1,33) = 0.32, p = 0.577, η2G = .001], nor any interaction between Caffeine and Sleep [F(1,33) = 2.19, p = 0.148, η2G = .011] (Supplementary Table S5).
Impact of total sleep deprivation on the proportion of choice
The repeated measures ANOVA on the proportion of choice (Figure 1, b, Supplementary Tables S6, and S7) highlighted a main effect of Sleep [F(1,33) = 6.38, p = 0.017, η2G = .011], a main effect of Emotion [F(1,33) = 15.93, p < 0.001, η2G = .027], as well as a main effect of Intensity [F(1,33) = 13.83, p = 0.001, η2G = .048], respectively indicating that the proportion of “away” responses was greater in the RB than in the SD condition, greater for anger than for fear, and for high vs. low emotional intensity. These main effects were further characterized by an interaction between Emotion and Intensity factors [F(1,33) = 11.31, p = 0.002, η2G = .011], and between Sleep, Emotion and Intensity factors [F(1,33) = 8.41, p = 0.007, η2G = .005].
To understand the triple interaction, we fitted a repeated measures ANOVA on Anger trials only, which revealed a main effect of Intensity [F(1,33) = 19.02, p < 0.001, η2G = .105], while the ANOVA on Fear trial only revealed a main effect of Sleep [F(1,33) = 8.33, p = 0.007, η2G =.024], as well as an interaction between Sleep and Intensity [F(1,33) = 11.31, p = 0.002, η2G = 0.023]. Paired t-tests and Cohen’s d effect sizes showed that the difference between high vs. low emotional intensity was medium for anger trials both when sleep rested [t(33) = 3.22, p = 0.006, d = .488], as well as when sleep deprived [t(33) = 4.84, p < 0.001, d = .721], whereas for fear trials, the difference between high vs. low emotional intensity was only significant when sleep rested [t(33) = 3.45, p = 0.005, d = .579], and not when sleep deprived [t(33) = 0.068, p = 0.946, d = .012].
Impact of total sleep deprivation on Response times
ANOVA Sleep, Emotion, Intensity and Decision.
This ANOVA on RTs revealed a main effect of Intensity [F(1,33) = 4.61, p = 0.039, η2G < .001], and a main effect of Decision [F(1,33) = 11.59, p = 0.002, η2G = .002], further characterized by an interaction between Intensity and Decision [F(1,33) = 7.38, p = 0.010, η2G = .001] (Supplementary Tables S8 and S9). The difference in RTs between trials where participants decided to avoid vs. approach low emotional intensity was not significant [t(33) = 1.62, p = 0.114, d = .045], however, the difference was significant for high emotional intensity [t(33) = 3.62, p = 0.002, d = .136].
ANOVA Sleep and Emotion (Anger, Fear and Neutral).
This ANOVA on RTs (Figure 1, c – right, Supplementary Table S10) revealed a main effect of Emotion [F(1,47) = 32.49, p < 0.001, η2G = .006], characterized by the fact that participants responded with similar speed in the presence of an angry or a fearful individual in the scene [t(33) = −0.552, p = 0.584, d = −.007], but quicker in the presence of an angry [t(33) = −6.599, p < 0.001, d = −.171], or a fearful individual [t(33) = −5.942, p < 0.001, d = −.166], as compared to two neutral individuals. No main effect of Sleep [F(1,33) = 2.23, p = 0.139, η2G = .005], nor an interaction between Emotion and Sleep [F(1,33) = 2.74, p = 0.072, η2G < .001], was observed.
Impact of total sleep deprivation on movement times
ANOVA Sleep, Emotion, Intensity, and Side.
No significant main effects or interactions were observed for this ANOVA on movement times (all ps > 0.79, Supplementary Tables S11 and S12).
ANOVA Sleep and Emotion (Anger, Fear, and Neutral).
This ANOVA on movement times (Supplementary Table S13) revealed a main effect of Emotion [F(1,53) = 13.15, p < 0.001, η2G = .001], as well as an interaction between Emotion and Sleep [F(2,63) = 4.00, p = 0.024, η2G < .001]. This interaction was characterized by the fact that, when rested, participants took the same time to reach the chair of their choice in the presence of an angry or a fearful individual in the scene [t(33) = 0.12, p = 0.902, d = .002], but took longer to reach the chair in the presence of an angry [t(33) = 4.62, p < 0.001, d = .097], or a fearful individual [t(33) = 4.32, p < 0.001, d = .090], as compared to two neutral individuals. However, when totally SD, participants continued to take the same time to reach the chair of their choice in the presence of an angry or a fearful individual in the scene [t(33) = 1.89, p = 0.158, d = .032], and longer in the presence of an angry [t(33) = 2.85, p = 0.029, d = .070] as compared to two neutral individuals while the difference between fearful scenes and neutral scenes was no longer significant [t(33) = 2.00, p = 0.158, d = .039].
Drift diffusion models
The mean AIC indicated that, overall, the model where only the drift rate (v) varied over Sleep, Emotion and Intensity factors (model 3, mean AIC = −710.32) fitted the data better, compared to the model where only the pre-decisional bias (starting point z) varied over Sleep, Emotion, and Intensity factors (model 2, mean AIC = −702.80). Importantly, this model also fitted the data better, compared to the model were both starting point and drift rate varied over Sleep, Emotion, and Intensity factors (model 4, mean AIC = −704.50) as well as compared to the null model (model 1, mean AIC = −701.37). Critically, the exceedance probability of model 3 was of 0.9987 compared to model 4, of 0.9839 compared to model 2 and of 0.9437 compared to model 1. Overall, there is strong support for the hypothesis that model 3 better explains the data compared to the other models tested. Finally, the fit of the winning model (model 3) was also assessed visually, ensuring that it could reproduce the main features of the data (see Supplementary Figure S2).
Repeated-measures ANOVA on the drift rate parameter extracted from the winning model 3 (Supplementary Table S14) highlighted a significant main effect of Sleep [F(1,33) = 4.64, p = 0.039, η2G = .005], indicating that evidence accumulation for away vs. toward responses was higher when sleep rested than when totally sleep deprived. The main effect of Emotion [F(1,33) = 14.67, p < 0.001, η2G = .014], indicated that evidence accumulation for away vs. toward responses was higher for anger than for fear trials. The main effect of Intensity [F(1,33) = 13.31, p < 0.001, η2G = .028] indicated that evidence accumulation for away vs. toward responses increased with emotional intensity. Both the interaction between Emotion and Intensity [F(1,33) = 13.09, p < 0.001, η2G = .007] and interaction between Sleep, Emotion, and Intensity [F(1,33) = 11.09, p = 0.002, η2G = .003] emerged.
Running the ANOVA separately for Anger and Fear revealed that the Sleep by Intensity interaction was only significant for Fear [F(1,33) = 12.52, p = 0.001, η2G = .009], but not for Anger [F(1,33) = 1.27, p = 0.268, η2G < .001]. Paired t-tests and Cohen’s d effect sizes showed that, when sleep rested, the difference between high vs. low emotional intensity was significant for both anger trials [t(33) = 3.11, p = 0.007, d = .375], and fear trials [t(33) = 3.39, p = 0.005, d = .358]. However, when totally Sleep Deprived, the difference between high vs. low emotional intensity was only significant for anger trials [t(33) = 4.86, p < 0.001, d = .500], and not for fear trials [t(33) = −0.09, p = 0.924, d = −.009], suggesting that the effect of Intensity on evidence accumulation was only present for anger and not for fear.
Relation between changes in PANAS and in proportion of choice
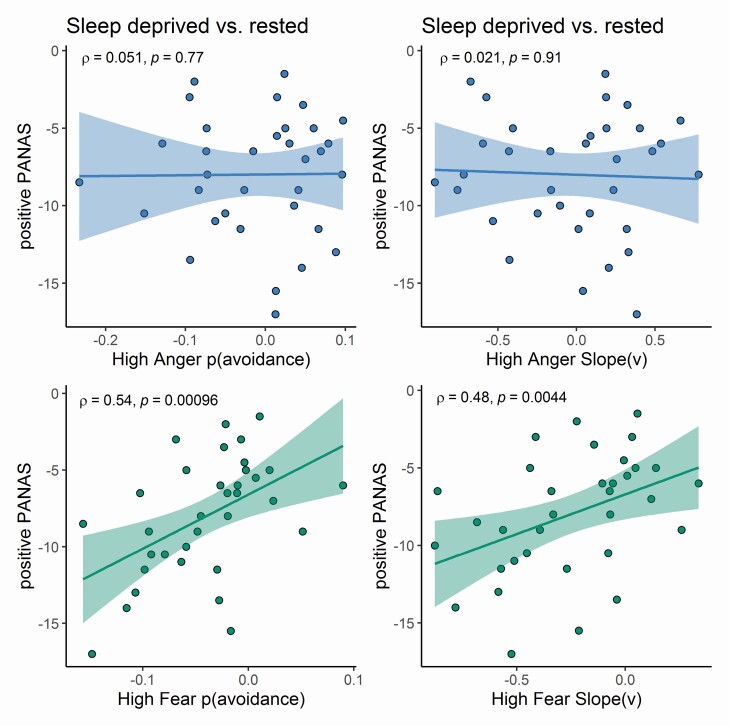
The change in positive PANAS between Sleep Deprived (SD) and Rested Baseline (RB) was positively correlated with the difference in the proportion of away responses for High intensity between SD and RB for Fear [r(32) = 0.54, p < 0.001], but not for Anger [r(32) = 0.051, p = 0.77]. The change in negative PANAS between SD and RB was not correlated with the difference in the proportion of away responses for High intensity between SD and RB for Fear [r(32) = −0.26, p = 0.13], nor for Anger [r(32) = −0.066, p = 0.71]. Hence, the more participants’ positive mood was affected by total Sleep Deprivation (i.e. decreased their positive score between RB and SD), the lower their tendency to avoid High Fear when Sleep Deprived as compared to Rested (see Figure 3).
Figure 3.
Relation between PANAS positive scores and behaviors. Left panel: Spearman correlations between the changes in Positive PANAS scores between totally Sleep Deprived and Sleep Rested conditions and the changes in the proportion of away responses between Sleep Deprived and Sleep Rested conditions for High Anger (top) and High Fear (bottom) respectively. Right panel: Spearman correlations between the changes in Positive PANAS scores between Sleep Deprived and Sleep Rested conditions and the changes in drift-rate (slope v) between Sleep Deprived and Sleep Rested conditions for High Anger (top) and High Fear (bottom) respectively. ***p < 0.001; **p < 0.01; *p < 0.05; n.s. = p > 0.05.
Relation between changes in PANAS and in drift-rate
The change in positive PANAS between SD and RB was positively correlated with the difference in drift-rate (slope v) for high intensity between SD and RB for Fear [r(32) = 0.48, p = 0.004], but not for Anger, [r(32) = 0.021, p = 0.91]. The change in negative PANAS between SD and RB was not correlated with the difference in drift-rate (slope v) for high intensity between SD and RB for Fear, [r(32) = −0.16, p = 0.36], nor for Anger [r(32) = 0.01, p = 0.95]. Hence, the more participants’ positive mood was affected when totally Sleep Deprived (i.e. decreased their positive score between RB and SD), the less the effect of High Intensity of Fear on evidence accumulation when Sleep Deprived as compared to Rested (see Figure 3).
Mediation analysis using PVT response time as a mediator
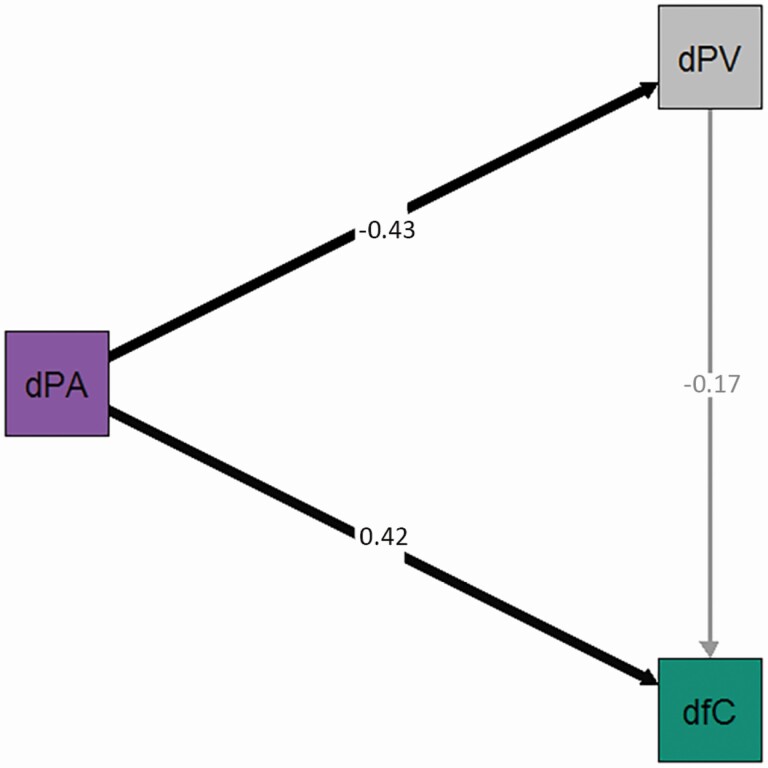
We first observed that the change in PVT response times (PVTrt) between totally SD and RB correlated with the change in the proportion of away response to fearful faces (Choice & PVT-rt: r(32) = −0.34, p = 0.047) as well as with the change in positive PANAS (PANAS & PVT-rt: r(32) = −0.49, p = 0.0029). We therefore ran a mediation analysis to assess whether the change in PVTrt between Sleep Deprived and Rested Baseline could mediate the influence of the change in Positive PANAS on the change in the proportion of away response to fearful faces. We found that the effect of the change in Positive PANAS on the proportion of away response to fearful faces was not mediated via a change in PVTrt between totally SD and RB. Indeed, the results from the mediation analysis indicated that of the estimated 0.007 (Total Effect (direct + indirect), p = 0.001) change in proportion of away responses due to change in Positive PANAS, an estimated 0.001 (average causal mediation effects, p = 0.458) is from the change in PVTrt and the remaining 0.006 (average direct effects, p = 0.028) is from the change in Positive PANAS (see Figure 4, Supplementary Tables S15 and S16). The second mediation analysis, using the change between Rested Baseline (RB) and totally Sleep Deprived (SD) in the number of non-responses, another indicator of attention capacity more specific to the social decision task, resulted in similar findings (see Supplementary Tables S15 and S16).
Figure 4.
Mediation analysis to assess the relationship between the change in positive PANAS scores (dPA) and the change in the proportion of away responses to fearful stimuli (dfC), using PVT-rt as the mediator (dPV). The numbers on each path are the standardized regression coefficients between variables.
Discussion
The present study aimed at investigating the impact of total sleep deprivation on spontaneous approach/avoidance decisions in a social context and the potential moderating role of alterations in mood-state on such sleep deprivation effects. Our results are threefold: First, sleep rested (RB) participants choose to avoid angry, more often than fearful, individuals and this effect was stronger for intense expressions. When totally sleep deprived (SD), we observed that, while avoidance was still favored in the presence of angry faces, it was reduced for fearful faces. Second, drift-diffusion model analyses revealed that total SD alters the decisional process itself, i.e. the valuation process between option alternatives, rather than the pre-decisional bias, and this specifically for decisions in the presence of fearful faces. Finally, we replicated previous findings showing that SD caused positive but not negative mood-state alterations (e.g. [21–24,78]). Importantly here, positive mood-state alterations correlated with changes in choices and drift rates to fearful displays, without being mediated by sleep-related disruption of sustained attention. All together, these findings provide convincing support for the hitherto underestimated role of positive mood-state alterations induced by total sleep loss on social decisions.
Participants’ approach/avoidance decisions were influenced by the presence of facial displays of emotion, as a function of their implied threat, i.e. their behavioral relevance to them [53]. Replicating previous findings from experiments using the same protocol, avoidance was more common when faced with angry compared to fearful individuals, and this effect was stronger for intense expressions in sleep rested participants [51, 72]. Although angry and fearful displays are both of negative valence, they differ in their social meaning and therefore in their action request directed toward the perceiver [52]. Angry expressions are clear signals of impending verbal or physical assault [53], which in most contexts leads to avoidance. In contrast, fearful expressions signal both the presence of a potential danger [54] and a need for affiliation [55] and are therefore more ambiguous in terms of avoidance and approach responses. We suggest that the above-described influence of threat-related expressions on participant’s approach-avoidance decisions was mediated by a change in the expected value of each available action option [51]. Decisions between competing targets for action rest upon the valuation and comparison of available options to generate a choice [79, 80]; a mechanism characterized as evidence accumulation. Here, replicating Mennella et al.’s findings [72], drift-diffusion model analyses revealed a higher rate of evidence accumulation (i.e. higher value estimates) when sleep-rested participants decided to avoid (not approach) individuals displaying angry (compared to fearful) expressions, especially at high emotional intensity. This replication confirms our suggestion that the choice of seating oneself next to or far from an emotional individual is a highly motivational outcome per se. Anger, and to a lesser extent fear, increases the value of the action leading to the most desirable outcome, i.e. avoiding individuals displaying threat-signaling expressions.
How does total SD impact these spontaneous approach/avoidance decisions? Here, using drift-diffusion models, we were able to adjudicate between two contrasting hypotheses: The first is related to past evidence that total SD deteriorates inhibitory efficiency [57, 58], increasing reliance on automatic behaviors [59] as well as impulsivity and sensitivity to negative, notably threat-related, stimuli [9, 17, 60]. In this scenario, total SD would strongly prompt avoidant action tendencies and this stimulus-evoked pre-decisional bias would influence the final action choice. The second hypothesis arises from past studies suggesting an impact of total SD [38], as well as of mood [35, 36], on the valuation process at the time of choice during economic decisions. In the present context, the perception of threat-signaling displays would influence the decisional process itself, i.e. the rate of evidence accumulation that allows rapid arbitration between action alternatives. Our results favor the second hypothesis: the model wherein the drift rate varied over the Sleep, Emotion and Intensity factors fitted participants’ choice behavior and RTs better, compared to models wherein only the pre-decisional bias or both pre-decisional bias and drift rate varied.
Total sleep deprivation however, had a differential impact on decisions in the presence of anger or fearful displays. While the rate of evidence accumulation (value estimates) for avoidance responses remained high and comparable between RB and SD conditions for anger of high intensity, we observed a significant decrease between RB and SD conditions in the avoidance value estimates for high fear. These results are in line with recent findings from Johnson et al. [81] that showed, in a two-forced choices version of a simulated shooting task, that total SD has a selective impact on participants’ ability to extract evidence from stimuli (drift rate), and in particular in less threatening and more ambiguous contexts. Here, we further demonstrate that the same mechanism (drift rate) is involved in a free choice task, and more importantly, that positive mood-state alterations caused by total sleep deprivation correlate with both changes in choices and drift rates to fearful displays only. How can we explain the differential impact between anger and fear? We discuss two possible interpretations: The first rests upon motivational mechanisms that drive action decisions and the second on perceptual mechanisms. Both interpretations take into account the ambiguity of fearful displays, that simultaneously signal both the presence of potential danger and a need for affiliation [82], in contrast with anger displays that communicate an unambiguous aggressive intent toward the observer [83].
The first possible interpretation is related to the impact of total SD on the motivation to engage in physical and social activities [84], and, by extrapolation, on the motivation to avoid individuals displaying ambiguous (fear) expressions. Sleep rested participants appeared to be highly motivated (high value estimates) to seat themselves far from angry, and, to a lesser extent, fearful individuals. When deciding between option alternatives, the valuation process consists in a trade-off between the expected benefit (value) of each potential action and its associated expected cost (effort) [85]. Of interest, the decrease in intrinsic motivation, and associated low allocation of task-related effort induced by sleep deprivation, can be partially countered by high incentives (e.g. money). In contrast, the absence of clear extrinsic incentives fails to generate sufficient motivation in sleep deprived individuals to maintain their level of performance [86, 87]. Moreover, mood fluctuations were shown to modulate the expected outcome value assigned to the options of choice at the time of decision [35–37], while anhedonia decreased the motivation to act (make an effort) for reward [46, 47] and diminished the expected reward value [45]. Together, these results suggest that, in the present task, while being seated far from an angry individual remained a highly motivational outcome, even for totally sleep deprived participants, this was no longer true in the presence of fearful individuals. This reflects a decrease in motivation mediated by positive mood deterioration. Nevertheless, it might be asked whether our findings reflect a decrease in the motivation to avoid individuals displaying ambiguous (fear) expressions leading to stochastic choices, or to an increase in the motivation to approach fearful individuals. In previous experiments using the same protocol [51], we suggested that, as fearful displays simultaneously signal the presence of potential danger and a need for affiliation [82], some participants may be prone to approach fearful individuals to either comfort them (prosocial motivation, e.g. [88]) or to alleviate their own fear (self-preservative motivation, e.g. [89]). However, we did not find strong arguments in support of an increase in motivation to approach fearful individuals (see Supplementary analyses) and therefore believe our findings favor the interpretation that sleep deprivation leads to stochastic choices in response to individuals displaying ambiguous (fear) expressions. Nonetheless, it would be interesting to replicate the present study using emotions like happiness and sadness, which are clear signals of affiliation and distress respectively, as sleep deprivation has also been shown to impair recognition of these prosocial emotional expressions, to a greater extent than those more relevant to our immediate survival such as anger and fear (e.g. [90,91]).
An alternative explanation may be related to the fact that total SD reduced the ability to accurately perceive morphed facial expressions, particularly when facial cues were ambiguous (e.g. [92,93]). For instance, Goldstein-Piekarski et al. [91] observed impaired discrimination of subtle threatening from affiliative facial cues. Moreover, low positive affect in healthy subjects is associated with decreased voluntary processing of and/or sustained attention to rewarding stimuli [94, 95] and threat stimuli [95]. Therefore, total sleep deprivation, and its associated low positive affect, may be considered to weaken an individual’s ability to attend to and discern fearful from neutral faces leading to more random choices. However, the fact that sleep rested and sleep deprived participants were quicker to initiate their actions in response to both anger and fear, as compared to neutral, displays [51, 72] suggests that these displays were processed to a similar extent in both conditions. Furthermore, the link between alterations in positive mood-state and changes in choices to fearful displays was not mediated by downstream disruption of sustained attention processes related to total sleep deprivation. We therefore believe that the observed influence of total sleep loss on approach and avoidance decisions to fearful displays was not due to differences in the processing of or attention directed toward anger and fear displays.
The conclusions reached in this study ought to be interpreted in light of some limitations. Notably, a sequence effect could potentially confound our results. Effectively, both the fact that participants performed the task several times and that the total sleep deprivation condition always followed a sleep rested condition may have altered the way participants responded. Regarding the former, even if we cannot completely rule out the possibility of a short-term effect of task repetition within a session, we found that there was no significant effect of session (1st session, 2nd session of testing) on participants’ choices. Regarding the latter, the fact that anger and fear displays were processed to a similar extent in sleep rested and sleep deprivation conditions was reassuring. Nevertheless, a completely counterbalanced version of the present study is needed before more definitive conclusions on the present findings can be drawn.
Overall, the present study strongly suggests that individuals who are frequently exposed to total sleep deprivation, such as nurses, doctors, policemen and military personnel, may continue to respond adaptively to direct and clear social threat. They may nonetheless perform less well when facing decisions based on ambiguous and subtler social cues. Moreover, difficulties in ambiguous social decision making, due to total sleep deprivation, may be tightly coupled to diminished positive affect rather than to a decrease in vigilance or sustained attention. This should to be taken into serious consideration, as there are clear links between sleep loss, depression (anhedonia) and impaired social decisions [17]. Finally, from a broader perspective, by highlighting how a sleepy brain integrates environmental social signals (here, threat related angry and fearful facial displays) in decision making, our results support the recent hypothesis that sleepiness is a dynamic motivational drive that competes with other needs (i.e. social interactions) and incentives [84].
Supplementary Material
Acknowledgments
J.G. and R.M. were supported by Fondation pour la Recherche Médicale (FRM) DEQ20160334878; INSERM; Ecole Normale Supérieure (ENS) and the French National Research Agency under Grants ANR-20-CE28-0003, ANR-10-LABX-0087 IEC and ANR-17-EURE-0017 FrontCogn. M.E., M.Q., M.G., P.V.B., F.S. and A.R. were supported by grants from General Directorate for Armament (DGA; Contract No. PDH-1-SMO-2-0509). We would like to thank Michèle Chadwick for proofreading our manuscript and the referees for their valuable and constructive comments.
Disclosure Statement
Financial disclosure: None.
Non-financial disclosure: None.
References
- 1. Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. PMID: 21075236. [DOI] [PubMed] [Google Scholar]
- 2. Lim J, et al. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–322. [DOI] [PubMed] [Google Scholar]
- 3. Lim J, et al. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136(3):375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erblang M, et al. Genetic determinants of neurobehavioral responses to caffeine administration during sleep deprivation: a randomized, cross over study. Genes. 2021;12(4):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krause AJ, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18(7):404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison Y, et al. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236–249. [DOI] [PubMed] [Google Scholar]
- 7. Slama H, et al. Sleep deprivation triggers cognitive control impairments in task-goal switching. Sleep. 2018;41(2). doi: 10.1093/sleep/zsx200 [DOI] [PubMed] [Google Scholar]
- 8. Pilcher JJ, et al. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–326. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein AN, et al. The role of sleep in emotional brain function. Annu Rev Clin Psychol. 2014;10:679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beattie L, et al. Social interactions, emotion and sleep: a systematic review and research agenda. Sleep Med Rev. 2015;24:83–100. [DOI] [PubMed] [Google Scholar]
- 11. Watling J, et al. Sleep loss and affective functioning: more than just mood. Behav Sleep Med. 2017;15(5):394–409. [DOI] [PubMed] [Google Scholar]
- 12. Palmer CA, et al. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. [DOI] [PubMed] [Google Scholar]
- 13. Tempesta D, et al. Sleep and emotional processing. Sleep Med Rev. 2018;40:183–195. [DOI] [PubMed] [Google Scholar]
- 14. Cote KA. Sleep on it: everything will look better in the morning. Sleep Med Rev. 2017;31:3–5. [DOI] [PubMed] [Google Scholar]
- 15. Satterfield BC, et al. Sleep loss, executive function, and decision-making. In: Sleep and Health. Elsevier; 2019:339–358. [Google Scholar]
- 16. Gordon AM, et al. Sleep and social processes. In: Križan Z, ed. Sleep, Personality, and Social Behavior. Cham: Springer International Publishing; 2019:3–12. [Google Scholar]
- 17. Ben Simon E, et al. Sleep loss and the socio-emotional brain. Trends Cogn Sci. 2020;24(6):435–450. [DOI] [PubMed] [Google Scholar]
- 18. Lo JC, et al. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen L, et al. Positive and negative emotions: differential associations with sleep duration and quality in adolescents. J Youth Adolesc. 2018;47(12):2584–2595. [DOI] [PubMed] [Google Scholar]
- 20. Talbot LS, et al. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10(6):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franzen PL, et al. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Finan PH, et al. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38(11):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finan PH, et al. Partial sleep deprivation attenuates the positive affective system: effects across multiple measurement modalities. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finan PH, et al. Experimental sleep disruption and reward learning: moderating role of positive affect responses. Sleep. 2019;42(5):zsz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Peer JM, et al. Effects of threat and sleep deprivation on action tendencies and response inhibition. Emotion. 2019;19(8):1425–1436. [DOI] [PubMed] [Google Scholar]
- 26. Saksvik-Lehouillier I, et al. Mild to moderate partial sleep deprivation is associated with increased impulsivity and decreased positive affect in young adults. Sleep. 2020;43(10):zsaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanmartín R, et al. Positive and negative affect as predictors of social functioning in Spanish children. PLoS One. 2018;13(8):e0201698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kendall AD, et al. Prospective associations of low positive emotionality with first onsets of depressive and anxiety disorders: results from a 10-wave latent trait-state modeling study. J Abnorm Psychol. 2015;124(4):933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clark LA, et al. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100(3):316–336. [DOI] [PubMed] [Google Scholar]
- 30. Messerotti Benvenuti S, et al. Appetitive and aversive motivation in depression: the temporal dynamics of task-elicited asymmetries in alpha oscillations. Sci Rep. 2019;9(1):17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirschfeld RM, et al. Social functioning in depression: a review. J Clin Psychiatry. 2000;61(4):268–275. [DOI] [PubMed] [Google Scholar]
- 33. Kupferberg A, et al. Social functioning in major depressive disorder. Neurosci Biobehav Rev. 2016;69:313–332. [DOI] [PubMed] [Google Scholar]
- 34. Nettle D, et al. The evolutionary origins of mood and its disorders. Curr Biol. 2012;22(17):R712–R721. [DOI] [PubMed] [Google Scholar]
- 35. Eldar E, et al. Interaction between emotional state and learning underlies mood instability. Nat Commun. 2015;6:6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eldar E, et al. Mood as representation of momentum. Trends Cogn Sci. 2016;20(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinckier F, et al. Neuro-computational account of how mood fluctuations arise and affect decision making. Nat Commun. 2018;9(1):1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Libedinsky C, et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front Behav Neurosci. 2011;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venkatraman V, et al. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31(10):3712–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Massar SAA, Lim J, Huettel SA. Sleep deprivation, effort allocation and performance. In: Progress in Brain Research. Vol. 246. Elsevier; 2019:1–26. [DOI] [PubMed] [Google Scholar]
- 41. Killgore WD, et al. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15(1):7–13. [DOI] [PubMed] [Google Scholar]
- 42. Chen J, et al. Sleep deprivation promotes habitual control over goal-directed control: behavioral and neuroimaging evidence. J Neurosci. 2017;37(49):11979–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sundelin T, et al. Framing effect, probability distortion, and gambling tendency without feedback are resistant to two nights of experimental sleep restriction. Sci Rep. 2019;9(1):8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brunet JF, et al. The association between REM sleep and decision-making: Supporting evidences. Physiol Behav. 2020;225:113109. [DOI] [PubMed] [Google Scholar]
- 45. Huys QJ, et al. Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord. 2013;3(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dillon DG, et al. Peril and pleasure: an RDOC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety. 2014;31(3):233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Husain M, et al. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19(8):470–484. [DOI] [PubMed] [Google Scholar]
- 48. Dorrian J, et al. Self-regulation and social behavior during sleep deprivation. In: Progress in Brain Research. Vol. 246. Elsevier; 2019:73–110. [DOI] [PubMed] [Google Scholar]
- 49. Killgore WDS, et al. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30(3):345–352. [DOI] [PubMed] [Google Scholar]
- 50. Dickinson DL, McElroy T. Sleep restriction and circadian effects on social decisions. Eur Econ Rev. 2017;97:57–71. [Google Scholar]
- 51. Vilarem E, et al. Action opportunities modulate attention allocation under social threat. Emotion. 2020;20(5):890–903. [DOI] [PubMed] [Google Scholar]
- 52. Horstmann G. What do facial expressions convey: feeling states, behavioral intentions, or action requests? Emotion. 2003;3(2):150–166. [DOI] [PubMed] [Google Scholar]
- 53. Sander D, et al. Interaction effects of perceived gaze direction and dynamic facial expression: Evidence for appraisal theories of emotion. Eur J Cogn Psychol. 2007;19(3):470–480. [Google Scholar]
- 54. Paulus A, et al. It depends: Approach and avoidance reactions to emotional expressions are influenced by the contrast emotions presented in the task. J Exp Psychol Hum Percept Perform. 2016;42(2):197–212. [DOI] [PubMed] [Google Scholar]
- 55. Marsh AA, et al. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion. 2005;5(1):119–124. [DOI] [PubMed] [Google Scholar]
- 56. Ratcliff R, et al. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20(4):873–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chuah YM, et al. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26(27):7156–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rabat A, et al. Limited benefit of sleep extension on cognitive deficits during total sleep deprivation: illustration with two executive processes. Front Neurosci. 2019;13:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dickinson DL, et al. The effects of total sleep deprivation on Bayesian updating. Judgm Decis Mak. 2008;3(2):11. [Google Scholar]
- 60. Anderson C, et al. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217(2):463–466. [DOI] [PubMed] [Google Scholar]
- 61. Taillard J, et al. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J Biol Rhythms. 2004;19(1):76–86. [DOI] [PubMed] [Google Scholar]
- 62. McLellan TM, et al. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312. [DOI] [PubMed] [Google Scholar]
- 63. Watson D, et al. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 64. Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol. 2011;7:297–319. [DOI] [PubMed] [Google Scholar]
- 65. Khitrov MY, et al. PC-PVT: a platform for psychomotor vigilance task testing, analysis, and prediction. Behav Res Methods. 2014;46(1):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Langner O, et al. Presentation and validation of the Radboud Faces Database. Cogn Emot. 2010;24(8):1377–1388. [Google Scholar]
- 67. El Zein M, et al. Anxiety dissociates the adaptive functions of sensory and motor response enhancements to social threats. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lawrence MA. ez: Easy analysis and visualization of factorial experiments. 2016. cran.rproject.org/package=ez. [Google Scholar]
- 69. Voss A, et al. A fast numerical algorithm for the estimation of diffusion model parameters. J Math Psychol. 2008;52(1):1–9. [Google Scholar]
- 70. Voss A, et al. Fast-dm: a free program for efficient diffusion model analysis. Behav Res Methods. 2007;39(4):767–775. [DOI] [PubMed] [Google Scholar]
- 71. Voss A, et al. Assessing cognitive processes with diffusion model analyses: A tutorial based on fast-dm-30. Front Psychol. 2015;6(MAR):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mennella R, et al. Rapid approach-avoidance responses to emotional displays reflect value-based decisions: Neural evidence from an EEG study. Neuroimage. 2020; 222:117253. [DOI] [PubMed] [Google Scholar]
- 73. Lerche V, et al. Model complexity in diffusion modeling: benefits of making the model more parsimonious. Front Psychol. 2016;7. doi: 10.3389/fpsyg.2016.01324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rigoux L, et al. Bayesian model selection for group studies - revisited. Neuroimage. 2014;84:971–985. [DOI] [PubMed] [Google Scholar]
- 75. Stephan KE, et al. Bayesian model selection for group studies. Neuroimage. 2009;46(4):1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosseel Y. Package ‘lavaan’. https://cran.r-project.org/web/packages/lavaan/lavaan.pdf.
- 77. Allen M, et al. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 2021;4:63. doi: 10.12688/wellcomeopenres.15191.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu L, et al. Effect of 72 h of sleep deprivation on the iowa gambling task. Noro Psikiyatr Ars. 2016;53(4):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wunderlich K, et al. Neural computations underlying action-based decision making in the human brain. Proc Natl Acad Sci U S A. 2009;106(40):17199–17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xie J, et al. Neuronal remapping and circuit persistence in economic decisions. Nat Neurosci. 2016;19(6):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Johnson DJ, et al. Sleep deprivation and racial bias in the decision to shoot: a diffusion model analysis. Soc Psychol Personal Sci. July 2020;12(5):638–647. [Google Scholar]
- 82. Marsh A, et al. The influence of the fear facial expression on prosocial responding. Cogn Emot. 2007;21:225–247. [Google Scholar]
- 83. Sell A, Cosmides L, Tooby J. The human anger face evolved to enhance cues of strength. Evol Hum Behav. 2014;35(5):425–429. [Google Scholar]
- 84. Axelsson J, et al. Sleepiness as motivation: a potential mechanism for how sleep deprivation affects behavior. Sleep. 2020;43(6). doi: 10.1093/sleep/zsz291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pessiglione M, et al. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain. 2018;141(3):629–650. [DOI] [PubMed] [Google Scholar]
- 86. Sasmita K, et al. Reward motivation normalises temporal attention after sleep deprivation. J Sleep Res. 2019;28(4):e12796. [DOI] [PubMed] [Google Scholar]
- 87. Massar SAA, et al. Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia. 2019;123:169–177. [DOI] [PubMed] [Google Scholar]
- 88. Kaltwasser L, et al. On the relationship of emotional abilities and prosocial behavior. Evol Hum Behav. 2017;38(3):298–308. [Google Scholar]
- 89. Isbell LA. Predation on primates: Ecological patterns and evolutionary consequences. Evol Anthropol Issues News Rev. 1994;3(2):61–71. [Google Scholar]
- 90. Killgore WDS, et al. Sleep deprivation impairs recognition of specific emotions. Neurobiol Sleep Circadian Rhythms. 2017;3:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goldstein-Piekarski AN, et al. Sleep deprivation impairs the human central and peripheral nervous system discrimination of social threat. J Neurosci. 2015;35(28):10135–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huck NO, et al. The effects of modafinil, caffeine, and dextroamphetamine on judgments of simple versus complex emotional expressions following sleep deprivation. Int J Neurosci. 2008;118(4):487–502. [DOI] [PubMed] [Google Scholar]
- 93. Cote KA, et al. Impact of total sleep deprivation on behavioural neural processing of emotionally expressive faces. Exp Brain Res. 2014;232(5):1429–1442. [DOI] [PubMed] [Google Scholar]
- 94. Kessel EM, et al. Behavioral observations of positive and negative valence systems in early childhood predict physiological measures of emotional processing three years later. J Affect Disord. 2017;216:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weinberg A, et al. Distinct associations between low positive affect, panic, and neural responses to reward and threat during late stages of affective picture processing. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(1):59–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.