Abstract
Fistula laser closure (FiLaC) is a relatively new sphincter-sparing technique in fistula surgery that was initially reported in 2011. It involves the radial dissipation of laser energy in the fistula tract and, through a combination of coagulation and shrinkage of the tract, is proposed to result in progressive sealing of fistulas. Early studies have suggested minimal impact on continence and touted the advantage of minimal morbidity with potential of repeat procedures if the technique fails initially. Despite early promising results, ten years on, questions remain on the technique, patient selection and long-term outcomes. This narrative review assesses the evidence reported to-date of radially emitting laser fistula surgery in the treatment of perianal fistulas.
Keywords: fistula, surgery, FiLaC, fistula laser closure, perianal fistula, perianal Crohn’s disease
Introduction
The modern surgical approach to anal fistulas includes several sphincter-saving procedures, which offer an attempt at cure whilst minimising morbidity, especially the impact on continence. Coloproctologists now have an armamentarium of options, which includes the closure of the fistula tract with plugs, fibrin glue, or collagen paste without fistulotomy (ie, laying open) or by means of fistulectomy (ie, core-out technique). Further sphincter-saving options include advancement flaps,1 LIFT (ligation of the intersphincteric fistula tract),2 VAAFT (video-assisted anal fistula treatment)3 and laser ablation procedures.4 Varying success rates and lack of long-term data mean that there is no one universally agreed gold standard and thus treatments are assessed by a combination of patient and surgeon factors.5
In 2011 Wilhelm reported on the use of a novel diode laser source and radial emitting laser probe to obliterate the fistula tract throughout its length from within.4 Its conceptual development was based on varicose vein laser treatment and follows the same principle of limiting penetration and uniformly distributing photothermal energy to ensure homogeneity. The thermal energy generated from the laser is dissipated radially, which is different to previous lasers used in coloproctology such as the neodymium yttrium aluminium garnet (YAG) laser6 or the CO2 laser7 where linear energy was used. The aim is the destruction of the granulation tissue and the epithelial cells through a combination of coagulation and shrinkage of the tract. The thermal energy acts on proteins within the tissue, disrupting the structure and supposedly aids the sealing effect.8 It is also thought that better accuracy of the laser (in comparison to electrocautery) decreases the risk of damage to surrounding structures (ie, anal sphincters).
In this paper we review the evidence available on this novel technique and assess its emerging role in fistula surgery.
Methods
All articles published in the English literature in peer-reviewed journals on FiLaC™ in patients with anorectal fistula were considered. A systematic search using MEDLINE and Embase databases was performed through to 18 March 2021, using the MeSH terms “fistula”, “laser”, “surgery” and “fistula tract laser closure” and including relevant sub-classifications. The obtained studies were supplemented with searches of reference lists and bibliographies of selected articles in order to ensure that no relevant articles were missed during the original searches. All relevant studies on FiLaC describing the patient population were included in this review. Any discrepancies were discussed between the independent assessors to reach agreeable consensus. Collected data were expressed in spreadsheet format (using Microsoft Excel, Microsoft, Redmond, WA) and analysed to ascertain any possible conclusions from their collective information. Exclusion criteria were case reports, systematic reviews, commentaries (without new data) and studies published with overlapping data from centres/authors at multiple time points (to avoid duplicity).
Data collected for each study included number of patients, age range/mean, gender distribution, success of operation (ie, healing of fistula), duration of follow-up and endpoints, including complications. These were expressed as totals, percentages and in descriptive terms, as applicable.
Results
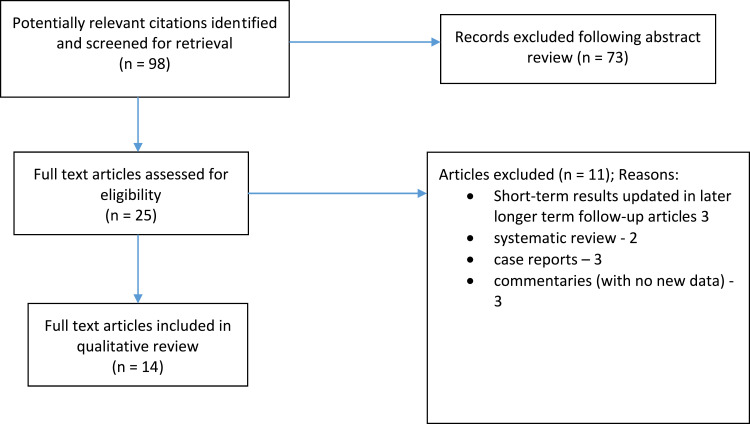
The literature search revealed 14 published studies9–22 on FiLaC after excluding some studies; amongst these were two systematic reviews, both published in 202023,24 (see Figure 1 for search strategy). Since these reviews, however, there have been more recent studies9,10,12,13,17–20 including larger series (n>80),9,13,18 as well as a study assessing FiLaC in solely Crohn’s-related perianal fistulas.20 Table 1 illustrates the studies assessed and their characteristics when evaluating FiLaC for treatment of perianal fistulas. Study numbers varied from 10–117, with follow up ranging from 2–87 months. All published data were retrospective case series, albeit with largely prospectively collected data.
Figure 1.
Flow diagram of search strategy.
Table 1.
Demographics for FiLaC in the 14 Retrospective Studies
| Study | Country | Number | Number of Males (%) | Age in Years Median (Range) | Crohn’s Disease (%) | Median Duration of Follow-Up in Mths (Range) |
|---|---|---|---|---|---|---|
| Nordholm-Carstensen et al (2021) 12 | Denmark | 66 | 28 (42) | 40* | 11 (16) | 19 (12–26) |
| Isik et al (2020)13 | Turkey | 100 | 72 (72) | 42 (21–83) | 0 (0) | 48 (6–56) |
| Wolicki et al (2020)18 | Germany | 83 | 64 (77.1) | 50.01 (14.59)* | 2 (2.4) | 41.99 (4–87)* |
| de Bonnechose et al (2020)9 | France | 100 | 65 (65) | 43 (22–88) | 10 (10) | 13.6 (6–23) |
| Serin et al (2020)10 | Turkey | 35 | 24 (69) | 43.9 (12.9)* | 0 (0) | 11 (6–17.6) |
| Alam et al (2019)20 | France | 20 | 10 (50) | 32 (9.61)* | 20 (100) | 7.1 (2–22.5) |
| De Hous et al (2019)19 | Belgium | 10 | NS | 50 (30–63) | 2 (20) | 9 (4–26) |
| Stijns et al (2019)17 | Netherlands | 20 | 4(20) | 45 (27–78)* | 0 (0) | 10 (7.3)** |
| Marref et al (2019)14 | France | 69 | 34 (49) | 40 (33–53) | 6 (8.7) | 6.3 (4.2–9.3) |
| Terzi et al (2018)22 | Turkey | 103 | 82 (80) | 43 (18–78) | 0 (0) | 28 (2–50) |
| Lauretta et al (2018)11 | Italy | 30 | 16 (53.3) | 52 (26–72) | 0 (0) | 11.3 (6–24) |
| Donmez et al (2017)16 | Turkey | 27 | 23 (85.2) | 35.6 (10.32)* | NS | 22 (17–26) |
| Wilhelm et al (2017)15 | Germany | 117 | 82 (70.1%) | 46 (17–82) | 13(11.1) | 25.4 (6–60) |
| Giamundo et al (2015)21 | Italy | 45 | 21 (47) | 46 (18–78) | 2 (4) | 30 (6–46) |
| 825 |
Notes: *Mean, standard deviation in parenthesis where available; **IQR, inter-quartile range.
Abbreviation: NS, not specified.
Patient Selection
Predominantly male patients were included in the studies and reported median/mean ages were fairly similar (ie, 35–50 year olds), although there was a wide range of those included (17–88 year olds). The majority of studies involved patients with previous attempted fistula surgeries, with only one study reporting on patients (n = 20) without prior fistula surgery.17 Six studies9,11,12,15,17,18 reported a significant majority (>75%) of treated patients having a seton drainage prior to FiLaC, although the duration was not always specified. The technique has been demonstrated in simple, complex, and recurrent fistula. Fistula assessment in the majority of studies was based on a combination of proctoscopy, endoanal ultrasound and manometry (in selected cases), in the pre-operative period. One study reported routine preoperative magnetic resonance imaging (MRI) in all patients undergoing the procedure,17 and Isik et al13 reported that, by performing an MRI prior to surgery and inserting a seton in all cases with an abscess, they could reduce their failure rate from 25% to 6.6%.13,25 The majority of fistulas were complex and these were largely transsphincteric, although the height of the fistula or proportion of sphincter involved was not always reported13,23,26,27 (Table 2). Four studies reported treatments in patients with secondary extensions,9,13,19,20 and the remainder either excluded patients with this fistula characteristic or did not specify. Eight studies9,12,14,15,18–21 reported outcomes in patients with Crohn’s-related fistula. One of these studies assessed sole treatment of Crohn’s perianal fistulas20 in a pilot study of 20 patients; the remaining studies either excluded these patients or included only a few of them (maximum n = 13/117, 11%15).
Table 2.
Reported Results for FiLaC Studies
| Study | Median Duration of Follow-Up Mths (Range) | Numbers | Type of Fistula | Secondary Extensions | Previous Fistula Surgery | Prior Seton | Closure of Internal Opening | Success |
|---|---|---|---|---|---|---|---|---|
| Nordholm-Carstensen et al (2021)12 | 19 (12–26) | 66 | High IS – 2 | Not reported | 22 (32%) | 66 (100%) | Suture | 30 (44.1%) |
| Low TS – 20 | ||||||||
| High TS – 41 | ||||||||
| SS – 5 | ||||||||
| Wolicki et al (2020)18 | 41.99 (4–87)* | 83 | TS/IS – numbers not specified | Not reported | 28 (33.7%) | 65 (78.3%) | Z-stitch suture closure | 62 (74.7%) |
| de Bonnechose et al (2020)9 | 13.6 (6–23) | 100 | TS low – 8 | 13 (13%) | NS (mean = 2.4 prior operations for entire study population) | 83 (100%) | None | 41 (44.6%)** |
| TS high – 79 | ||||||||
| SS - 13 | ||||||||
| Isik et al (2020)13 | 48 (6–56) | 100 | IS – 10 | Not reported | Not reported | Not reported | None | 62 (62%) |
| TS low – 28 | ||||||||
| TS high – 54 | ||||||||
| SS - 8 | ||||||||
| Serin et al (2020)10 | 11 (6–17.6) | 35 | IS – 21 | Not reported | 12 (34%) | 10 (28.5%) | Purse-string closure | 15 (42.9%) |
| TS – 12 | ||||||||
| SS/ES – 2 | ||||||||
| Alam et al (2019)20 | 7.1 (2–22.5) | 20 | IS – 1 | 6 (30%) | NS (mean - 2.45 prior fistula operations for study population) | “Selective” (number not specified) | None | 11 (55%) |
| TS low – 3 | ||||||||
| TS high – 14 | ||||||||
| SS – 1 | ||||||||
| ES – 1 | ||||||||
| De Hous et al (2019)19 | 9 (4–26) | 10 | TS – 12 | 4 (40%) | 10 (100%) | Not reported | Suture | 7(70%) |
| IS – 3 (NB. some patients had multiple fistulas) | ||||||||
| Stijns et al (2019)17 | 10 (7.3)*** | 20 | IS – 6 | 0 | – | 16 (80%) | None | 4 (20%) |
| TS – 14 | ||||||||
| Marref et al (2019)14 | 6.3 (4.2–9.3) | 69**** | IS – 2 | 22 (32%) | 37 (54.4%) | 33 (48.5%) | None | 31 (45.6%) |
| Low TS – 10 | ||||||||
| High TS – 45 | ||||||||
| SS – 11 | ||||||||
| Terzi et al (2018)22 | 28 (2–50) | 103 | Sup – 7 | Not reported | 34 (33%) | 19 (18.4%) | None | 41 (39.8%) |
| IS – 56 | ||||||||
| TS – 29 | ||||||||
| SS - 11 | ||||||||
| Lauretta et al (2018)11 | 11.3 (6–24) | 30 | TS - 30 | 0 | 22 (73.3%) | 26 (86.6%) | None | 10 (33%) |
| Donmez et al (2017)16 | 22 (17–26) | 27 | IS – 14 | N | Not reported | 5 (18.5%) | None | 24(89%) |
| TS – 7 | ||||||||
| SS – 5 | ||||||||
| ES – 1 | ||||||||
| Wilhelm et al (2017)15 | 25.4 (6–60) | 117 | IS – 8 | Not reported | 16 (14%) | 99 (84.6%) | Yes – suture/flap closure | 75 (64.1%) |
| TS – 90 | ||||||||
| SS – 13 | ||||||||
| ES – 6 | ||||||||
| Giamundo et al (2015)21 | 30 (6–46) | 45 | IS – 7 | 0 | 35 (78%) | 24 (53%) | None | 32 (71%) |
| Low TS – 7 | ||||||||
| Mid TS – 19 | ||||||||
| High TS – 10 | ||||||||
| SS – 2 |
Notes: *Mean/SD. **41/92 patients followed up. ***IQR, inter-quartile range. ****1 patient lost to follow-up (so analysis included 68 patients).
Abbreviations: Sup, superficial; IS, intersphincteric; TS, transsphincteric; SS, suprasphincteric; ES, extrasphincteric.
Technique Variations
The majority of the studies performed the FiLaCTM procedure using disposable laser fibres and either Ceralas® or, more recently, a Leonardo® DUAL 45 diode laser device form Bio-litec® (Biomedical Technology GmbH, Jena, Germany). Other devices used were the CORONATM fistula probe coupled with a neoVTM diode laser system) manufactured by neoLaser® (Endotherapeutics, Australia)17 and a EUFOTON® diode laser system (Eufoton, Trieste, Italy) coupled with the disposable probe (HF Ring Fiber).11 The laser energy used in the studies varied between 10 and 15 Watts and all (except one21) of the studies used a 1470nm wavelength diode laser device. The speed of withdrawal of the fistula probe varied between 1mm/s and 3mm/s.
The internal opening was not closed in the majority of studies.9,11,13,14,16,17,20–22 Four studies10,12,18,19 closed the internal opening with sutures and one study reported closure with a combination advancement flap and sutures.15
Outcomes
Nordholm-Carstensen et al12 followed up all patients with a clinical examination including MRI or endo-anal ultrasound (EAUS) one year after the procedure. A few studies also used EAUS,11,15 with some restricting imaging to assessing cases of failure or recurrence.11,21 In most studies, however, healing was assessed clinically, without radiological confirmation.
Isik et al recently reported the longest median follow-up of 48 months (range 6–56 months) in a cohort of 100 patients treated, whilst Wolicki et al reported a mean follow-up of 42 months (± SD 21.34; range 4.8–87.6 months).
Success rates, ie primary healing (mostly assessed clinically), varied from 20% (4/20)17 at a median follow-up of 10 months to 89% (24/27)16 reported by Donmez et al at a median follow-up of 22 months (Table 2). Seven of the studies reported success rates (fistula healing) in fewer than 50% of patients,9–12,14,17,22 whereas the remaining seven studies reported healing rates in more than 55% of the patients treated (Table 2). The four largest studies (including more than 100 patients) reported primary healing rates of 44% (41/92 patients followed up at a median of 13.6 months);9 40% (41/103 patients followed up at a median of 28 months);22 62% (62/100 patients followed up at a median of 48 months) and 64% (75/117 patients followed up at a median of 25.4 months).
Analysis of those studies reporting healing rates for FiLaC in “high” transsphincteric fistulas revealed a success rate of 54% (118/219) in cryptoglandular fistulas and one study reported healing in 71% (10/14) in Crohn’s perianal fistula.
Crohn’s Disease
Alam et al20 reported a success rate of 55%, with fistula healing noted in 11/20 patients with Crohn’s perianal fistulas at a median follow-up period of 7.1 months. Overall, a very small minority of all patients in the studies considered had Crohn’s perianal fistulas (66/825; 8%). Of the 66 patients, 39 (59.1%) were reported to have undergone fistula healing.
Complications/Adverse Effects
Few complications were reported following laser treatment of perianal fistulas in the studies assessed. There were no reports of any deterioration in faecal continence. Eight studies reported the absence of any morbidity following the procedure.9,10,12–14,16,19,20,22 Giamundo et al21 reported eight (8/43, 18%) patients with temporary pain and anismus postoperatively and three (3/43, 7%) patients with moderate bleeding after FiLaC treatment. All of these resolved without intervention. Similarly, Lauretta et al11 reported four patients with minor postoperative complications (two with fever, one with severe pain and one with moderate bleeding) that resolved spontaneously. Stijns et al17 reported four patients (20%, 4/20) who developed perianal abscess (one within 30 days, one at six weeks postoperatively and the remaining two more than six months after the procedure). Stijns et al also reported a postoperative deterioration in patient reported anorectal function in seven patients, when measured using the Faecal Incontinence Severity Index (FISI). Wolicki et al18 reported minor complications: 11 patients (11/83, 13%) developed pain and 7 (7/83, 8%) developed bleeding postoperatively.
Discussion
Lay-open remains the optimum means of curing a fistula. However, when the fistula involves the muscle, the benefit of cure must be balanced by the risk to the patient’s continence. There remains no optimum technique to negate this risk of incontinence, thus stimulating an array of surgical techniques to address this issue. However, none of these procedures guarantees a good result, and for this reason new techniques have been introduced. FiLaC has now been reported in over 600 patients and its feasibility and safety have been demonstrated in these studies. The efficacy from the literature is somewhat variable, ranging from 20% to 89% rates of fistula healing. There are no reports of faecal incontinence and the ability to repeat the procedure in the face of minimal morbidity makes it an attractive prospect in the context of sphincter-sparing fistula surgery.
Some of the success rates reported in the FiLaC studies16,18,19,26 compare satisfactorily with those of other sphincter-sparing techniques.5,28–30 Recent systematic reviews reveal pooled healing rates of approximately 70% for the LIFT procedure in almost 500 patients with perianal fistula.28,30 In advancement flaps, a pooled success rate of approximately 75% has been reported in analysis of close to 800 patients.28 It remains to be seen whether these initially high success rates reported by some of the FiLaC procedure results will stand the test of time in the upcoming years. There have already been some studies suggesting much more moderate/doubtful benefits.11,31
There were some notable differences between the reported studies of FiLaC, particularly the addition of the closure of the internal opening (by suture or advancement flap) that was employed in some cohorts.10,12,15,18,19 This combination has been questioned in prior literature,17,31 with the suggestion that the combination of two treatment strategies limits the ability to critique the impact of FiLaC alone on the outcome of the treatment. Furthermore, some studies reporting on FiLaC in the absence of treatment of the internal opening have reported better outcomes,13,16,26 thus questioning the value of the additional closure. The target patient cohort that would derive benefit from this procedure is difficult to discern in view of the heterogeneity in the studies and indeed the outcomes. The majority of patients had transsphincteric fistulas, but their height and complexity were not always clearly reported. Indeed this variability in type, length and size of the fistulas has been proposed as potentially contributing to the different outcomes.32 Further work is being undertaken to highlight the heterogeneity of outcome reporting and develop a core outcome set in this context.33,34
Lauretta et al11 reported in their case series (n = 30) that the only significant prognostic factor identified by statistical analysis was fistula length. The mean fistula length in the group of patients who were cured by fistula laser closure was 28.5 mm (10–41 mm; SD 12.4), while it was 46 mm (25–67 mm; SD 20.6) in those not cured. Fistula tracts shorter than 30 mm were associated with a primary healing rate of 58.3% while tracts longer than 30 mm were cured in only 16.6% of cases (p < 0.02). However, this is not consistent with other studies that have not corroborated this finding9,12,14,32 and longer tracts have been suggested to improve the shrinkage effect elicited by laser energy.32 There have been suggestions that prior seton drainage may be beneficial21,32 by aiding fistula “maturation” towards an optimum width, but again this was not unanimously employed and has not been consistently shown to be associated with better outcomes. There is no data about the optimal time for the seton to be in place or whether an advantage is conferred at all.
Strengths and Limitations of FiLaC and Studies
The FiLaC technique is minimally invasive and has been reported to be performed in outpatient settings in some series.11,21,22 There have been promising reports of success (in terms of primary fistula healing) and it can be performed multiple times with seemingly minimal impact on continence. It does, however, come with a significant cost implication when compared with other techniques. Anatomy of the fistula tract may present problems, and presence of secondary extensions may not always be accessible by the laser fistula probe. Also, very few studies adopt routine pre- and postoperative MRI to confirm tract anatomy and subsequent healing. This may affect success of the technique as hidden tracts and unidentified extensions are a common cause of failure for all fistula surgery. Furthermore, clinical healing may not always constitute radiological healing and this has been described often in the context of Crohn’s perianal fistulas, whereby radiological healing may lag behind for up to a year.35 In more complex fistula anatomy, it may be necessary for FiLaC to be used as a complement to other techniques, and indeed its combination with video-assisted anal fistula surgery (VAAFT) has recently been described.27 Further limitations of studies reporting on FiLaC are their retrospective nature, with single-centre data including heterogeneous study populations, which limits the external validity and reproducibility of the results.
Conclusion
The success and continence risks associated with laying open simple fistula mean that newer techniques need to be explored and assessed for superiority, both in terms of patient outcomes and cost benefit. The laser procedure is demonstrably feasible and appears a relatively easy to learn technique and has been demonstrated to be safe with no reports of faecal incontinence. The target patient population that would derive most benefit appears to be those with more complex fistulas or recurrent fistulas where a lay-open cannot be considered due to sphincter involvement and risks of incontinence. The impact of prior seton drainage or specific closure of the internal opening may play a role in the healing rate, although this is not proven. Further studies are still warranted to discern the role of laser therapy in the armamentarium of fistula surgery and comparisons with surgeons’ preferences (similar to the FIAT study36) will aid in this pursuit.
Funding Statement
There is no funding to report.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mizrahi N, Wexner SD, Zmora O, et al. Endorectal advancement flap: are there predictors of failure? Dis Colon Rectum. 2002;45(12):1616–1621. doi: 10.1097/01.DCR.0000037654.01119.CD [DOI] [PubMed] [Google Scholar]
- 2.Rojanasakul A. LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol. 2009;13(3):237–240. doi: 10.1007/s10151-009-0522-2 [DOI] [PubMed] [Google Scholar]
- 3.Meinero P, Mori L. Video-assisted anal fistula treatment (VAAFT): a novel sphincter-saving procedure for treating complex anal fistulas. Tech Coloproctol. 2011;15(4):417–422. doi: 10.1007/s10151-011-0769-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilhelm A. A new technique for sphincter-preserving anal fistula repair using a novel radial emitting laser probe. Tech Coloproctol. 2011;15(4):445–449. doi: 10.1007/s10151-011-0726-0 [DOI] [PubMed] [Google Scholar]
- 5.Adegbola SO, Sahnan K, Pellino G, et al. Short-term efficacy and safety of three novel sphincter-sparing techniques for anal fistulae: a systematic review. Tech Coloproctol. 2017;21(10):775–782. doi: 10.1007/s10151-017-1699-4 [DOI] [PubMed] [Google Scholar]
- 6.Ellison GW, Bellah JR, Stubbs WP, Van Gilder J. Treatment of perianal fistulas with ND:YAG laser–results in twenty cases. Vet Surg. 1995;24(2):140–147. doi: 10.1111/j.1532-950x.1995.tb01308.x [DOI] [PubMed] [Google Scholar]
- 7.Bodzin JH. Laser ablation of complex perianal fistulas preserves continence and is a rectum-sparing alternative in Crohn’s disease patients. Am Surg. 1998;64(7):627–632. [PubMed] [Google Scholar]
- 8.Bass LS, Treat MR, Dzakonski C, Trokel SL. Sutureless microvascular anastomosis using the THC:YAG laser: a preliminary report. Microsurgery. 1989;10(3):189–193. doi: 10.1002/micr.1920100309 [DOI] [PubMed] [Google Scholar]
- 9.de Bonnechose G, Lefevre JH, Aubert M, et al. Laser ablation of fistula tract (LAFT) and complex fistula-in-ano: “the ideal indication” is becoming clearer…. Tech Coloproctol. 2020;24(7):695–701. doi: 10.1007/s10151-020-02203-y [DOI] [PubMed] [Google Scholar]
- 10.Serin KR, Hacim NA, Karabay O, Terzi MC. Retrospective analysis of primary suturing of the internal orifice of perianal fistula during FiLaC procedure. Surg Laparosc Endosc Percutan Tech. 2020;30(3):266–269. doi: 10.1097/SLE.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 11.Lauretta A, Falco N, Stocco E, Bellomo R, Infantino A. Anal fistula laser closure: the length of fistula is the achilles’ heel. Tech Coloproctol. 2018;22(12):933–939. doi: 10.1007/s10151-018-1885-z [DOI] [PubMed] [Google Scholar]
- 12.Nordholm-Carstensen A, Perregaard H, Hagen KB, Krarup P-M. Fistula Laser Closure (FiLaCTM) for fistula-in-ano—yet another technique with 50% healing rates? Int J Colorectal Dis. 2021. doi: 10.1007/s00384-021-03932-8 [DOI] [PubMed] [Google Scholar]
- 13.Isik O, Gulcu B, Ozturk E. Long-term outcomes of laser ablation of fistula tract for fistula-in-ano: a considerable option in sphincter preservation. Dis Colon Rectum. 2020;6:831–836. doi: 10.1097/DCR.0000000000001628 [DOI] [PubMed] [Google Scholar]
- 14.Marref I, Spindler L, Aubert M, et al. The optimal indication for FiLaC® is high trans-sphincteric fistula-in-ano: a prospective cohort of 69 consecutive patients. Tech Coloproctol. 2019;23(9):893–897. doi: 10.1007/s10151-019-02077-9 [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm A, Fiebig A, Krawczak M. Five years of experience with the FiLaCTM laser for fistula-in-ano management: long-term follow-up from a single institution. Tech Coloproctol. 2017;21(4):269–276. doi: 10.1007/s10151-017-1599-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dönmez T, Hatipoğlu E. Closure of fistula tract with filacTM laser as a sphincter preserving method in anal fistula treatment. Turk J Color Dis. 2018. doi: 10.4274/tjcd.06025 [DOI] [Google Scholar]
- 17.Stijns J, van Loon YT, Clermonts SHEM, Gӧttgens KW, Wasowicz DK, Zimmerman DDE. Implementation of laser ablation of fistula tract (LAFT) for perianal fistulas: do the results warrant continued application of this technique? Tech Coloproctol. 2019;23(12):1127–1132. doi: 10.1007/s10151-019-02112-9 [DOI] [PubMed] [Google Scholar]
- 18.Wolicki A, Jäger P, Deska T, Senkal M. Sphincter-saving therapy for fistula-in-ano: long-term follow-up after FiLaC®. Tech Coloproctol. 2020;25(2):177–184. doi: 10.1007/s10151-020-02332-4 [DOI] [PubMed] [Google Scholar]
- 19.De Hous N, de Gheldere C, Van den Broeck S, Komen N. FiLaCTM as a last, sphincter-preserving resort for complex perianal fistula. Tech Coloproctol. 2019;23(9):937–938. doi: 10.1007/s10151-019-02070-2 [DOI] [PubMed] [Google Scholar]
- 20.Alam A, Lin F, Fathallah N, et al. FiLaC® and Crohn’s disease perianal fistulas: a pilot study of 20 consecutive patients. Tech Coloproctol. 2020;24(1):75–78. doi: 10.1007/s10151-019-02134-3 [DOI] [PubMed] [Google Scholar]
- 21.Giamundo P, Esercizio L, Geraci M, Tibaldi L, Valente M. Fistula-tract Laser Closure (FiLaCTM): long-term results and new operative strategies. Tech Coloproctol. 2015;19(8):449–453. doi: 10.1007/s10151-015-1282-9 [DOI] [PubMed] [Google Scholar]
- 22.Terzi MC, Agalar C, Habip S, Canda AE, Arslan NC, Obuz F. Closing perianal fistulas using a laser: long-term results in 103 patients. Dis Colon Rectum. 2018;61(5):599–603. doi: 10.1097/DCR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 23.Frountzas M, Stergios K, Nikolaou C, et al. Could FiLaCTM be effective in the treatment of anal fistulas? A systematic review of observational studies and proportional meta-analysis. Color Dis. 2020;22(12):1874–1884. doi: 10.1111/codi.15148 [DOI] [PubMed] [Google Scholar]
- 24.Elfeki H, Shalaby M, Emile SH, Sakr A, Mikael M, Lundby L. A systematic review and meta-analysis of the safety and efficacy of fistula laser closure. Tech Coloproctol. 2020;24(4):265–274. doi: 10.1007/s10151-020-02165-1 [DOI] [PubMed] [Google Scholar]
- 25.Öztürk E, Gülcü B. Laser ablation of fistula tract. Dis Colon Rectum. 2014;57(3):360–364. doi: 10.1097/DCR.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 26.Giamundo P, Geraci M, Tibaldi L, Valente M. Closure of fistula-in-ano with laser–FiLaCTM: an effective novel sphincter-saving procedure for complex disease. Colorectal Dis. 2014;16(2):110–115. doi: 10.1111/codi.12440 [DOI] [PubMed] [Google Scholar]
- 27.Yao YB, Xiao CF, Wang QT, et al. VAAFT plus FiLaCTM: a combined procedure for complex anal fistula. Tech Coloproctol. 2021;0123456789:1–3. doi: 10.1007/s10151-021-02411-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stellingwerf ME, van Praag EM, Tozer PJ, Bemelman WA, Buskens CJ. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and crohn’s high perianal fistulas. BJS Open. 2019;3(3):231–241. doi: 10.1002/bjs5.50129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alasari S, Kim NK. Overview of anal fistula and systematic review of ligation of the intersphincteric fistula tract (LIFT). Tech Coloproctol. 2014;18(1):13–22. doi: 10.1007/s10151-013-1050-7 [DOI] [PubMed] [Google Scholar]
- 30.Yassin NA, Hammond TM, Lunniss PJ, Phillips RKS. Ligation of the intersphincteric fistula tract in the management of anal fistula. A systematic review. Color Dis. 2013;15(5):527–535. doi: 10.1111/codi.12224 [DOI] [PubMed] [Google Scholar]
- 31.Stijns J, Wasowicz DK, Zimmerman DDE. Does laser fistuloplasty (FiLaCTM) offer any benefit over surgical closure of the internal orifice? Tech Coloproctol. 2017;21(6):489–490. doi: 10.1007/s10151-017-1626-8 [DOI] [PubMed] [Google Scholar]
- 32.Giamundo P. Laser treatment for anal fistulas: what are the pitfalls? Tech Coloproctol. 2020;24(7):663–665. doi: 10.1007/s10151-020-02225-6 [DOI] [PubMed] [Google Scholar]
- 33.Machielsen AJHM, Iqbal N, Kimman ML, et al. Heterogeneity in Outcome Selection, Definition and Measurement in Studies Assessing the Treatment of Cryptoglandular Anal Fistula: Findings from a Systematic Review. Vol. 25. Springer International Publishing; 2021. doi: 10.1007/s10151-021-02452-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machielsen AJHM, Iqbal N, Kimman ML, et al. The development of a cryptoglandular Anal Fistula Core Outcome Set (AFCOS): an international Delphi study protocol. United Eur Gastroenterol J. 2020;8(2):220–226. doi: 10.1177/2050640620907570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tozer P, Ng SC, Siddiqui MR, et al. Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn’s perianal fistulas. Inflamm Bowel Dis. 2012;18(10):1825–1834. doi: 10.1002/ibd.21940 [DOI] [PubMed] [Google Scholar]
- 36.Jayne DG, Scholefield J, Tolan D, et al. Anal fistula plug versus surgeon’s preference for surgery for trans-sphincteric anal fistula: the FIAT RCT. Health Technol Assess. 2019;23(21):1–76. doi: 10.3310/hta23210 [DOI] [PMC free article] [PubMed] [Google Scholar]