Abstract
Objectives
To estimate the risk of hospitalization among reported cases of the Delta variant of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) compared with the Alpha variant in Norway, and the risk of hospitalization by vaccination status.
Methods
A cohort study was conducted on laboratory-confirmed cases of SARS-CoV-2 in Norway, diagnosed between 3 May and 15 August 2021. Adjusted risk ratios (aRR) with 95% confidence intervals (CI) were calculated using multi-variable log-binomial regression, accounting for variant, vaccination status, demographic characteristics, week of sampling and underlying comorbidities.
Results
In total, 7977 cases of the Delta variant and 12,078 cases of the Alpha variant were included in this study. Overall, 347 (1.7%) cases were hospitalized. The aRR of hospitalization for the Delta variant compared with the Alpha variant was 0.97 (95% CI 0.76–1.23). Partially vaccinated cases had a 72% reduced risk of hospitalization (95% CI 59–82%), and fully vaccinated cases had a 76% reduced risk of hospitalization (95% CI 61–85%) compared with unvaccinated cases.
Conclusions
No difference was found between the risk of hospitalization for Delta cases and Alpha cases in Norway. The results of this study support the notion that partially and fully vaccinated cases are highly protected against hospitalization with coronavirus disease 2019.
Keywords: Norway, SARS-CoV-2, Hospitalization, Variants of concern, Delta, Alpha
1. Background
Multiple variants of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), have been observed worldwide. Some of these variants have been designated as variants of concern (VOCs). VOCs are defined by the World Health Organization as variants associated with increased transmissibility; increased disease severity or change in clinical disease presentation; and/or decreased effectiveness of public health and social measures or available diagnostics, vaccines and therapeutics (World Health Organization, 2021a). VOCs include the Alpha variant [phylogenetic assignment of named global outbreak (Pango) lineage designation B.1.1.7; earliest documented samples from the UK in September 2020] and the Delta variant (Pango lineage B.1.617.2; earliest documented samples from India in October 2020) (World Health Organization, 2021a). Since their emergence, both variants have spread worldwide (World Health Organization, 2021b).
In Norway (population 5.4 million), testing activity for COVID-19 is high, with 3–5% of the population consistently tested weekly (defined as one or more tests per person within a 7-day period) between March and August 2021. Mathematical modelling estimated that consistently >50% of all cases weekly were detected in this period (Norwegian Institute of Public Health, 2021a). Sequencing capacity in Norwegian laboratories was scaled up rapidly from early December 2020, and the capacity to screen for variants or perform whole-genome sequencing (WGS) was further increased following reports of widespread transmission of the Alpha variant in the UK. From early April 2021 until mid-August 2021, >70% of cases diagnosed had data available on the variant of SARS-CoV-2 that caused their infection.
The Alpha variant has been shown to be transmitted more easily than non-VOC variants (Davies et al., 2021), and was the dominant circulating SARS-CoV-2 variant in Norway during the third wave of infections in the winter and spring of 2021. It was also associated with a 1.9-fold increased risk of hospitalization compared with non-VOC variants (Veneti et al., 2021). Similar associations were observed in other European countries (Bager et al., 2021a; Funk et al., 2021; Grint et al., 2021). Local and national non-pharmaceutical interventions and increasing vaccination coverage gradually decreased transmission, and Norway started its national reopening plan in the spring of 2021 (Norwegian Institute of Public Health, 2021a).
The first case of the Delta variant was diagnosed in Norway in April 2021, and local transmission was first evident at the beginning of May 2021. Delta superseded Alpha as the dominant circulating variant in early July 2021, accounting for >90% of new infections by the end of that month. This coincided with the start of the fourth wave of SARS-CoV-2 infections, and a subsequent increase in the number of new hospitalizations (Norwegian Institute of Public Health, 2021a). There is evidence of increased transmissibility (Campbell et al., 2021; Dhar et al., 2021; Public Health England, 2021a) and lower vaccine effectiveness against infection (Lopez Bernal et al., 2021; Seppälä et al., 2021; Sheikh et al., 2021) for the Delta variant compared with the Alpha variant. In addition, studies from Scotland (Sheikh et al., 2021), England (Twohig et al., 2021), Denmark (Bager et al., 2021b) and Ontario, Canada (Fisman and Tuite, 2021) have suggested that infection with the Delta variant increased the risk of hospitalization 1.5–2.8-fold in those settings.
In order to understand the impact of the Delta variant on the burden of COVID-19 in Norway, and support preparedness planning in the hospital sector, linked individual-level data were used to estimate the risk of hospitalization among reported cases of the Delta variant compared with reported cases of the Alpha variant, accounting for demographic characteristics, vaccination status and underlying comorbidities. In addition, the risk of hospitalization by vaccination status was estimated.
2. Methods
2.1. Data sources and study design
Data were obtained from the Norwegian National Preparedness Registry for COVID-19 (Norwegian Institute of Public Health, 2021b). The Preparedness Registry contains individual-level data from central health registries, national clinical registries and other national administrative registries. It covers all residents in Norway, and includes data on all laboratory-confirmed cases of COVID-19 in Norway, all hospitalizations among cases, and COVID-19 vaccinations (see Section 1, online supplementary material).
A cohort study was conducted, including cases who first tested positive for SARS-CoV-2 infection between 3 May (Week 18) and 15 August (Week 32) 2021, who had a national identity number registered, and who had been infected with the Alpha or Delta variant. Re-infections (>6 months since previous positive test and/or determined to be a re-infection by the national reference laboratory based on sequence data) were included. During the study period, SARS-CoV-2 tests were available free of charge for everyone, including those with mild or no symptoms, close contacts and individuals in quarantine. All positive and negative tests were registered in the national laboratory database. Variants were identified based on WGS using Illumina or Nanopore technology, partial sequencing by Sanger sequencing, or polymerase chain reaction screening for selected targets (Norwegian Institute of Public Health, 2021c). Data were extracted up to 30 August 2021, ensuring at least 15 days of follow-up since the last date of sampling.
2.2. Definitions
2.2.1. Hospitalization
Hospitalization was defined as hospital admission following a positive SARS-CoV-2 test, where COVID-19 was reported as the main cause of admission. Cases hospitalized with other or unknown main cause of admission were excluded from the study population in order to avoid bias. All admissions to hospital, regardless of length of stay, were included.
2.2.2. Vaccination status
In Norway, Comirnaty (BioNTech-Pfizer, Mainz, Germany/New York, NY, USA) and Spikevax (mRNA-1273, Moderna, Cambridge, MA, USA) were the two most frequently administered vaccines in the study period, using a two-dose schedule (Norwegian Institute of Public Health, 2021d). SARS-CoV-2 cases were defined according to their vaccination status:
-
•
Those unvaccinated with a COVID-19 vaccine before a positive test.
-
•
Those vaccinated with one dose of a COVID-19 vaccine <21 days before a positive test.
-
•
Partially vaccinated – those who tested positive ≥21 days after their first dose of a COVID-19 vaccine, and <7 days after the second dose.
-
•
Fully vaccinated – those who tested positive ≥7 days after their second dose (Andrews et al., 2021; Nasreen et al., 2021) with at least the recommended minimum interval between doses depending on the type of vaccine (Norwegian Institute of Public Health, 2021e), or 7 days after their first dose if they had previously been diagnosed with SARS-CoV-2 infection ≥21 days before vaccination. Cases who received the Janssen vaccine were considered fully vaccinated 21 days after one dose.
2.3. Underlying comorbidities with increased risk of severe COVID-19
Some people have underlying comorbidities that cause them to have moderate or high risk of severe COVID-19, regardless of age. These individuals were prioritized for vaccination in Norway (Norwegian Institute of Public Health, 2021f). Cases were categorized into three groups: no underlying comorbidities, medium-risk comorbidity and high-risk comorbidity, as detailed in Section 1 of the online supplementary material.
2.4. Data analysis
Cases were described in terms of variant, vaccination status, demographic characteristics, underlying comorbidities and hospitalization. In addition, cases were described in terms of admission to an intensive care unit (ICU) and COVID-19-related death (Norwegian Institute of Public Health, 2021g).
2.5. Statistical analysis
Adjusted risk ratios (aRR) with 95% confidence intervals (CI) were calculated using multi-variable log-binomial regression. The outcome of interest was hospitalization. Variables considered as possible confounders in the analysis were variant (Alpha or Delta), vaccination status (four levels), age (four age groups), sex, country of birth (three levels), period of sampling (biweekly as categorical variable and week as continuous variable), county of residence (12 levels) and underlying comorbidities (three levels). Model selection for the multi-variable binomial regression was conducted using the likelihood ratio test and the Akaike Information Criterion. The variables ‘variant’ (due to the main aim of the study) and ‘sex’ [demographic characteristic associated with risk of hospitalization in previous analyses in Norway (Veneti et al., 2021)] were kept in the multi-variable analysis, even if they were not significant. The authors checked for interactions between the co-variates by including interaction terms in the models. The main analysis was conducted separately for some of the groups of variables (subgroup analysis) to ensure that the associations remained robust for variant and vaccination status.
In addition to the main analysis, a number of sensitivity analyses were conducted by extending or restricting the study population (e.g. including only those cases who had WGS results), by adjusting the outcome definitions (e.g. including all cases who were hospitalized regardless of main cause of admission) and by changing the analysis method (e.g. using Cox regression) to further explore if the main results were robust (see Section 2.1 in the online supplementary material).
The power of this study to detect a range of potential effect sizes for the risk of hospitalization with the Delta variant compared with the Alpha variant was assessed (see Section 2.2 in the online supplementary material).
Statistical analysis was performed using Stata Version 16 (Stata Corp., College Station, TX, USA) and R Version 4.1.0.
2.6. Ethics
Ethical approval for this study was granted by Regional Committees for Medical Research Ethics - South East Norway (Ref. No. 249509). The need for informed consent was waived by the ethics committee.
3. Results
3.1. Description of cohort
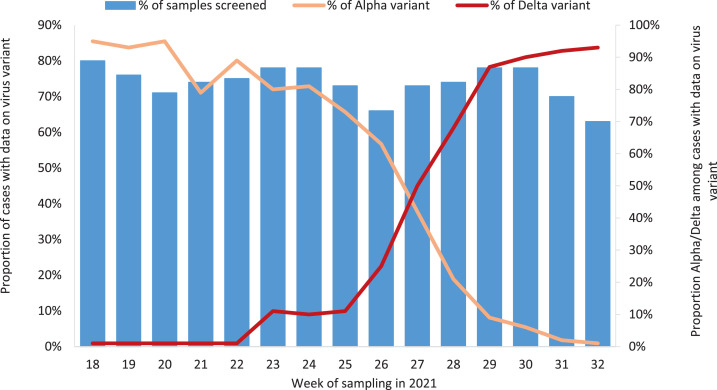
In total, 30,386 cases of COVID-19 were diagnosed between 3 May and 15 August 2021 in Norway. Of these, 644 cases who did not have a national identity number were excluded. Of the remaining 29,742 cases, 21,794 (73%) had data on virus variant. The proportion of cases with data on virus variant varied from 63% to 80% per week during the study period. In Section 2.3 of the online supplementary material, characteristics of cases with data on virus variant compared with all notified cases are presented.
Of the 21,794 cases, 8002 (37%) were the Delta variant and 12,118 (56%) were the Alpha vaiant. Delta superseded Alpha as the predominant circulating variant in Week 27 (Figure 1 ). By August 2021, only sporadic cases of the Alpha variant were detected. There were also seven cases of the Beta variant (B.1.351), four cases of the Gamma variant (P.1) and 389 cases of non-VOCs, while 1274 cases could not clearly be categorized as one of the VOCs or a non-VOC based on the information available. Of the Delta and Alpha cases, 62 cases hospitalized with another main cause of hospitalization than COVID-19 and three cases with an unknown main cause of hospitalization were excluded. The remaining 7977 Delta cases and 12,078 Alpha cases made up the study cohort. Characteristics of the study cohort are presented in Table 1 . The median age was 26 years [interquartile range (IQR) 18–37] for Delta cases and 24 years (IQR 18–39) for Alpha cases. WGS was used to determine the variant in 3040 (38%) Delta cases and 6557 (54%) Alpha cases. At the time of diagnosis, 15,140 (75%) cases were not vaccinated, 1722 (9%) cases had been vaccinated with one dose <21 days before a positive test, 2386 (12%) cases were partially vaccinated and 807 (4%) cases were fully vaccinated. More details on the distribution of cases by vaccination status, and vaccine types among partially and fully vaccinated cases by variant are presented in Section 2.4 of the online supplementary material.
Figure 1.
Proportion of cases of severe acute respiratory syndrome coronavirus-2 with data on virus variant, and proportion with Alpha and Delta variant, by week of sampling, Norway, 3 May–15 August 2021.
Table 1.
Distribution of cases of severe acute respiratory syndrome coronavirus-2 in study cohort by detected variants for different characteristics and proportion hospitalized, Norway, 3 May–15 August 2021.
| Characteristics | Study cohort | Variant type (% by characteristic) |
Hospitalized cases (% of diagnosed cases) |
||||
|---|---|---|---|---|---|---|---|
| Alpha | Delta | Alpha | Delta | Total | |||
| Total | 20,055 (100%) | 12,078 (60%) | 7977 (40%) | 240 (2.0%) | 107 (1.3%) | 347 (1.7%) | |
| Sex | Female | 9433 (47%) | 5750 (48%) | 3683 (46%) | 104 (1.8%) | 53 (1.4%) | 157 (1.7%) |
| Male | 10,622 (53%) | 6328 (52%) | 4294 (54%) | 136 (2.2%) | 54 (1.3%) | 190 (1.8%) | |
| Age group (years) | 0–24 | 9986 (50%) | 6292 (52%) | 3964 (46%) | 11 (0.2%) | 13 (0.4%) | 24 (0.2%) |
| 25–44 | 6559 (33%) | 3523 (29%) | 3036 (38%) | 69 (2.0%) | 44 (1.5%) | 113 (1.7%) | |
| 45–64 | 3060 (15%) | 2003 (17%) | 1057 (13%) | 120 (6.0%) | 35 (3.3%) | 155 (5.1%) | |
| ≥65 | 450 (2.2%) | 260 (2.2%) | 190 (2.4%) | 40 (15%) | 15 (7.9%) | 55 (12%) | |
| Norwegian born | Yes | 14,106 (70%) | 8743 (72%) | 5363 (67%) | 131 (1.5%) | 49 (0.9%) | 180 (1.3%) |
| No | 5802 (29%) | 3241 (27%) | 2561 (32%) | 101 (3.1%) | 55 (2.2%) | 156 (2.7%) | |
| Unknown | 147 (0.7%) | 94 (0.8%) | 53 (0.7%) | 8 (8.5%) | 3 (5.7%) | 11 (7.5%) | |
| Risk for severe COVID-19a | No underlying comorbidities | 18,291 (91%) | 10,962 (91%) | 7329 (92%) | 173 (1.6%) | 86 (1.2%) | 259 (1.4) |
| Medium-risk comorbidity | 1637 (8.2%) | 1040 (8.6%) | 597 (7.5%) | 57 (5.5%) | 16 (2.7%) | 73 (4.5%) | |
| High-risk comorbidity | 127 (0.6%) | 76 (0.6%) | 51 (0.6%) | 10 (13%) | 5 (9.8%) | 15 (12%) | |
| Vaccination status at date of positive test | Not vaccinated | 15,140 (75%) | 10,713 (89%) | 4427 (56%) | 184 (1.7%) | 73 (1.6%) | 257 (1.7%) |
| Vaccinated with one dose <21 days before positive test | 1722 (8.5%) | 726 (6.0%) | 996 (12%) | 35 (4.8%) | 7 (0.7%) | 42 (2.4%) | |
| Partially vaccinated | 2386 (12%) | 461 (3.8%) | 1925 (24%) | 12 (2.6%) | 14 (0.7%) | 26 (1.1%) | |
| Fully vaccinated | 807 (4.0%) | 178 (1.5%) | 629 (7.9%) | 9 (5.1%) | 13 (2.1%) | 22 (2.7%) | |
COVID-19, coronavirus disease 2019.
Risk for severe disease based on underlying comorbidities that are associated with moderate or high risk of serious illness regardless of age. Details on the definitions of medium- and high-risk categories are provided in Section 1 of the online supplementary material.
3.2. Risk of hospitalization
During the study period, 347 (1.7%) cases were hospitalized with COVID-19 as the main cause of hospitalization. Among the Delta cases, 107 (1.3%) were hospitalized, compared with 240 (2.0%) Alpha cases (Table 1). Time from positive test to hospitalization was ≤15 days for 344/347 hospitalized cases. Three cases were hospitalized 17–27 days after a positive test. No additional hospitalizations >15 days following a positive test were observed in subsequent data extractions with updated data. The median time from testing to hospitalization was slightly shorter for Delta cases (5 days, IQR 1–7) than Alpha cases (6 days, IQR 3–8.5; Wilcoxon rank-sum P-value=0.016).
In the univariate analysis, the crude RR of hospitalization among those infected with the Delta variant compared with the Alpha variant was 0.68 (95%CI 0.54–0.85), suggesting a lower risk of hospitalization among Delta cases. In the multi-variable model, after adjusting for sex, age group, country of birth, vaccination status and underlying comorbidities, no difference was found in the risk of hospitalization between Delta and Alpha cases, with an aRR of hospitalization of 0.97 (95% CI 0.76–1.23) (Table 2 ). Week of sampling and county of residence were not significant predictors in the multi-variable model, and were excluded from the final model presented here (a sensitivity analysis including these two variables is presented in Section 2.1 of the online supplementary material). On checking for interactions, only an interaction between age group and vaccination status was detected. In order to simplify the main results presented here, the decision was made not to include the interaction term in the main model. The association between vaccination status and hospitalization in the subgroup analysis by age group and other variables is presented in Section 2.5 of the online supplementary material. No interaction was found in the main multi-variable analysis between variant and vaccination status. Table 3 shows the subgroup aRR estimates for the Delta variant compared with the Alpha variant, which confirm the findings in the main analysis. The aRR of hospitalization among unvaccinated cases for the Delta variant compared with the Alpha variant was 1.10 (95% CI 0.84–1.45). The results were robust in all sensitivity analyses (see Section 2.1 of the online supplementary material).
Table 2.
Risk ratios for hospitalization with coronavirus disease 2019 (COVID-19) as main cause of admission from univariate and multi-variable binomial regression adjusted for variant, sex, age group, country of birth, underlying comorbidities, and vaccination status at date of positive test, Norway, 3 May–15 August 2021.
| Hospitalization |
|||||
|---|---|---|---|---|---|
| No | Yes (%) | Crude risk ratio(95% CI) | Adjusted risk ratio(95% CI) | ||
| Variant | Alpha | 11,838 | 240 (2.0%) | Ref | Ref |
| Delta | 7870 | 107 (1.4%) | 0.68 (0.54–0.85) | 0.97 (0.76-1.23) | |
| Sex | Female | 9276 | 157 (1.7%) | Ref | Ref |
| Male | 10,432 | 190 (1.8%) | 1.07 (0.87–1.33) | 1.05 (0.85-1.28) | |
| Age group (years) | 25–44 | 6446 | 113 (1.8%) | Ref | Ref |
| 0–24 | 9962 | 24 (0.2%) | 0.14 (0.09–0.22) | 0.14 (0.09-0.22) | |
| 45–64 | 2905 | 155 (5.3%) | 2.94 (2.32–3.73) | 3.18 (2.48-4.08) | |
| ≥65 | 395 | 55 (14%) | 7.09 (5.21–9.65) | 10.27 (7.06-14.95) | |
| Norwegian born | Yes | 13,926 | 180 (1.3%) | Ref | Ref |
| No | 5646 | 156 (2.8%) | 2.11 (1.70–2.61) | 1.38 (1.12-1.70) | |
| Unknown | 136 | 11 (8.1%) | 5.86 (3.26–10.54) | 1.23 (0.66-2.30) | |
| Risk for severe COVID-19a | No underlying comorbidities | 18,032 | 259 (1.4) | Ref | Ref |
| Medium-risk comorbidity | 1564 | 73 (4.7%) | 3.15 (2.44–4.06) | 1.75 (1.33-2.30) | |
| High-risk comorbidity | 112 | 15 (13%) | 8.34 (5.11–13.62) | 2.94 (1.74-4.99) | |
| Vaccination status at date of positive test | Not vaccinated | 14,883 | 257 (1.7%) | Ref | Ref |
| Vaccinated with one dose <21 days before positive test | 1680 | 42 (2.5%) | 1.44 (1.04–1.98) | 0.78 (0.56-1.08) | |
| Partially vaccinated | 2360 | 26 (1.1%) | 0.64 (0.43–0.96) | 0.28 (0.18-0.41) | |
| Fully vaccinated | 785 | 22 (2.8%) | 1.61 (1.05–2.47) | 0.24 (0.15-0.39) | |
CI, confidence interval.
Week of sampling and county of residence were not significant predictors in the multi-variable model and were not included in the final model.
Risk of severe disease based on underlying comorbidities that are associated with moderate or high risk of serious illness regardless of age. Details on the definitions of medium- and high-risk categories are provided in Section 1 of the online supplementary material.
Table 3.
Association between hospitalization and infection with the Delta variant compared with the Alpha variant of severe acute respiratory syndrome coronavirus-2 (multi-variable binomial regression adjusted for demographic characteristics and underlying comorbidities), overall and stratified (subgroup analysis) by sex, age group, country of birth, underlying comorbidities and vaccination status at date of positive test, Norway, 3 May–15 August 2021.
| Hospitalization | ||||
|---|---|---|---|---|
| Alpha cases (n=12,078) | Delta cases (n=7,977) | Delta vs Alpha, adjusted risk ratio (95% CI) | ||
| Overall | 240 (2.0 %) | 107 (1.3 %) | 0.97 (0.76–1.23) | |
| Subgroup analysis by: | ||||
| Sex | Female | 104 (1.8%) | 53 (1.4%) | 1.12 (0.79–1.58) |
| Male | 136 (2.2%) | 54 (1.3%) | 0.85 (0.61–1.17) | |
| Age group (years) | 0–24 | 11 (0.2%) | 13 (0.4%) | 1.96 (0.83–4.64) |
| 25-44 | 69 (2.0%) | 44 (1.5%) | 1.00 (0.68–1.48) | |
| 45–64 | 120 (6.0%) | 35 (3.3%) | 1.10 (0.74–1.65) | |
| >65 | 40 (15%) | 15 (7.9%) | 0.60 (0.34–1.05) | |
| Country of birth (Norwegian born) | Yes | 131 (1.5%) | 49 (0.9%) | 1.01 (0.72–1.41) |
| No | 101 (3.1%) | 55 (2.2%) | 1.03 (0.74–1.43) | |
| Unknown | 8 (8.5%) | 3 (5.7%) | 1.49 (0.40–5.49) | |
| Risk for severe COVID-19a | No underlying comorbidities | 173 (1.6%) | 86 (1.2%) | 1.06 (0.81–1.39) |
| Medium-risk comorbidity | 57 (5.5%) | 16 (2.7%) | 0.61 (0.35–1.09) | |
| High-risk comorbidity | 10 (13%) | 5 (9.8%) | 1.51 (0.69–3.29) | |
| Vaccination status at date of positive test | Not vaccinated | 184 (1.7%) | 73 (1.6%) | 1.10 (0.84–1.45) |
| Vaccinated with one dose <21 days before positive test | 35 (4.8%) | 7 (0.7%) | 0.43 (0.17–1.11) | |
| Partially vaccinated | 12 (2.6%) | 14 (0.7%) | 1.45 (0.51–4.15) | |
| Fully vaccinated | 9 (5.1%) | 13 (2.1%) | 0.70 (0.29–1.67) | |
COVID-19, coronavirus disease 2019; CI, confidence interval.
Week of sampling and county of residence were not significant predictors in the multi-variable model, and not included in the final model.
Risk for severe disease based on underlying comorbidities that are associated with moderate or high risk of serious illness regardless of age. Details on the definitions of medium- and high-risk categories are provided in Section 1 of the online supplementary material.
The crude RR for hospitalization among fully vaccinated cases compared with unvaccinated cases changed following adjustment for other factors due to confounding. In the multi-variable model, following adjustment for sex, age group, country of birth, variant and underlying comorbidities, partially vaccinated cases had a 72% reduced risk of hospitalization (95% CI 59–82%) and fully vaccinated cases had a 76% reduced risk of hospitalization (95% CI 61–85%) compared with unvaccinated cases (Table 2). Section 2.5 of the online supplementary material presents the subgroup analysis for the risk of hospitalization by vaccination status, which seemed to be robust with the main findings. In the subgroup analysis by variant type, partially vaccinated cases had a 77% (95% CI 58–87%) and 72% (95% CI 50–85%) reduced risk of hospitalization, and fully vaccinated cases had a 79% (95% CI 59–89%) and 70% (95%CI 39%–85%) reduced risk of hospitalization compared with unvaccinated cases infected with the Alpha and Delta variants, respectively.
3.3. Admission to ICU and COVID-19 related deaths
Among the 107 patients hospitalized with the Delta variant, 16 (15%) were admitted to ICU compared with 40 (17%) of 240 patients hospitalized with the Alpha varant. Among the 56 cases admitted to ICU, 40 were unvaccinated and 14 had been vaccinated <21 days before a positive test. In total, there were 24 deaths among the study cohort, of which 20 were reported as COVID-19 related (Norwegian Institute of Public Health, 2021g). Of these 20 COVID-19-related deaths, five were Delta cases and 15 were Alpha cases. Additional analyses were not performed on these outcomes due to small numbers.
4. Discussion
This study analysed individual-level data on laboratory-confirmed cases of COVID-19 in Norway and hospitalizations among cases within the study period, as well as demographic characteristics, vaccination status and underlying comorbidities. Earlier analyses of other VOCs in Norway showed increased risk of hospitalization for the Alpha and Beta variants compared with non-VOCs (Veneti et al., 2021), in line with other studies (Bager et al., 2021a; Funk et al., 2021; Grint et al., 2021; Fisman and Tuite, 2021). The findings of the present study indicate no difference in the risk of hospitalization for SARS-CoV-2 cases infected with the Delta variant compared with the Alpha variant in Norway, in contrast to published estimates from other countries. An analysis from Scotland suggested an adjusted hazard ratio for hospitalization of 1.85 (95% CI 1.39–2.47) for Delta cases compared with Alpha cases (Sheikh et al., 2021). In England, a similar association was observed (hazard ratio 2.26, 95% CI 1.32–3.89) (Twohig et al., 2021). The study from England (hazard ratio 2.32, 95% CI 1.29–4.16) and a study from Denmark (aRR 3.01, 95% CI 2.02–4.50) (Bager et al., 2021b) suggested increased risk of hospitalization in an unvaccinated cohort, which the present study does not support. In addition, the study from Ontario, Canada estimated an adjusted odds ratio for hospitalization of 1.45 (95% CI 1.27–1.64) for the Delta variant compared with N501Y-positive VOCs (Alpha, Beta or Gamma), as well as 2.01-fold higher risk of ICU admission (95% CI 1.60–2.47) and 1.69-fold higher risk of death (95% CI 1.16–2.35) (Fisman and Tuite, 2021).
In comparing estimates, the study settings need to be considered, with each conducted in a different population, time period and healthcare system. For example, any differences in SARS-CoV-2 testing criteria and activity, and capacity to screen for variants may lead to differences in the subset of Alpha and Delta cases diagnosed between the studies. Outcome definitions and analysis methods could also have played a role; however, it is unlikely that these factors have impacted the results presented in the different studies to an extent that would explain the different associations observed. The statistical methodology would have been sufficient to detect a comparable increase in risk as the other studies if it existed, and the sensitivity analyses gave robust results using different outcome definitions.
These results highlight the importance of taking local epidemiological characteristics into account when endeavouring to understand the effect that different variants have on the COVID-19 epidemic in different settings. The results are representative of a young cohort of SARS-CoV-2 cases in a country with broad testing criteria, and high testing activity and capacity to screen for variants. In the study period, the health system operated well within capacity, criteria for hospital admission were consistent, and hospital treatment was available to all those who would benefit. Aside from local restrictions in the event of outbreaks, there were no notable lockdowns. There was high vaccination coverage among populations at greater risk of severe COVID-19, and vaccination coverage was increasing steadily as Delta superseded Alpha as the dominant variant (Norwegian Institute of Public Health, 2021a).
This study also underlines the need for more research to further understand the association between the Delta variant and severe disease. The authors did not have access to data on clinical disease severity among cases, and the number of ICU admissions and COVID-19-related deaths were low in both groups. Therefore, the results cannot conclude directly whether there was a difference in virulence for the Delta variant compared with the Alpha variant. What the results do suggest is that other factors, such as age, country of birth, underlying comorbidities and vaccination status, are associated with the risk of hospitalization among SARS-CoV-2 cases in Norway. However, even if the risk of hospitalization among Delta and Alpha cases in Norway is similar, risk of infection with Delta is higher given evidence of increased transmissibility (Campbell et al., 2021; Dhar et al., 2021; Public Health England, 2021a) and lower vaccine effectiveness against infection (Lopez Bernal et al., 2021; Seppälä et al., 2021; Sheikh et al., 2021). This must be considered as prevention and control measures are weighed up in view of the burden of disease in society, capacity in the healthcare system and progress of vaccination programmes. In Norway, vaccine effectiveness against laboratory-confirmed infection with the Delta variant has been estimated to be 22% among partially vaccinated individuals and 65% among fully vaccinated individuals (Seppälä et al., 2021). The present results suggest that partially and fully vaccinated cases infected with the Delta variant are highly protected against hospitalization, in line with published estimates from elsewhere (Fisman and Tuite, 2021; Public Health England, 2021b; Sheikh et al., 2021; Statens Serum Institut, 2021). This highlights the importance of ensuring high vaccination uptake. The vast majority of vaccinated cases received the mRNA vaccine Comirnaty, which did not enable the authors to investigate whether vaccine type had an additional impact on the risk of hospitalization (see Section 2.4 of online supplementary material).
Sampling effects can bias the estimate of risk when using surveillance data. For example, if a larger proportion of milder cases were diagnosed in the Delta cohort, this could underestimate the risk of hospitalization for Delta cases compared with Alpha cases. As the authors did not have data on relevant parameters that would have helped to explore this further, such as clinical disease severity or viral loads, this bias cannot be ruled out. However, such bias should be considered in light of the consistent COVID-19 testing strategy in Norway during the study period. In addition, it has been suggested that, when comparing two variants for a post-infection outcome at a time when one variant is in the process of supplanting the other, a larger proportion of severe cases of the new variant (in this case, Delta) could be diagnosed (Seaman et al., 2021). This underlines one of several challenges with using surveillance data to determine the relative disease severity of new variants in an evolving epidemic setting (Bager et al., 2021b).
This study had some limitations. While the sample size was marginally larger than the study from Scotland (Sheikh et al., 2021), both in terms of number of cases overall and number of Delta cases, the power calculations indicated that this study may be underpowered if the Delta variant was associated with a small increased risk of hospitalization compared with the Alpha variant (see Section 2.2. of online supplementary material). In addition, the method used to determine underlying comorbidities will likely underestimate the true prevalence, as only individuals that have been in contact with health services are identified. Data on medications used and procedure codes are currently not taken into account, which would improve the definitions and detect more individuals with underlying comorbidities.
The present findings indicate no difference in the risk of hospitalization for cases infected with the Delta variant of SARS-CoV-2 compared with the Alpha variant in Norway. This is a more encouraging finding than previous studies for the ongoing response to the COVID-19 pandemic in settings where the Delta variant is circulating, although evidence of increased transmissibility and lower vaccine effectiveness against infection for the Delta variant must also be considered. These results highlight the importance of taking local epidemiological characteristics into account, when endeavouring to understand the effect that different variants have on the COVID-19 epidemic in different settings. Data on protection against severe disease are crucial to guide future vaccination strategy, and the results from this study support the notion that partially and fully vaccinated cases are highly protected against hospitalization with COVID-19.
Acknowledgements
First and foremost, the authors wish to thank all those who have helped report data to the National Emergency Preparedness Registry at the Norwegian Institute of Public Health (NIPH) throughout the pandemic. The authors also acknowledge the efforts that regional laboratories have put into establishing a routine variant screening procedure or WGS at short notice, and registration of all analyses in national registries for surveillance. The authors also wish to thank the staff at the Virology and Bacteriology Departments at NIPH involved in national variant identification and whole-genome analysis of SARS-CoV-2 viruses. The authors also acknowledge the efforts of staff at hospitals around Norway to ensure the reporting of timely and complete data to the Norwegian Intensive Care and Pandemic Registry, as well as colleagues at the register itself. The authors wish to thank Anja Elsrud Schou Lindman, Project Director for the National Preparedness Registry, and all those who have enabled data transfer to this registry, especially Gutorm Høgåsen at NIPH who has been in charge of the establishment and administration of the registry. Finally, the authors wish to thank Hanne Gulseth and ‘Team risk group’ at NIPH, who developed the data cleaning procedure for underlying comorbidities in the Preparedness Registry, and Trude Marie Lyngstad, Anders Skyrud Danielsen, Nora Dotterud and Evy Dvergsdal at NIPH for their assistance in cleaning the data from different registries.
Conflict of interest statement
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Data sharing statement
The dataset analysed in the study contains individual-level linked data from various central health registries, national clinical registries and other national administrative registries in Norway. The researchers had access to the data through the National Emergency Preparedness Registry for COVID-19 (Beredt C19), housed at the Norwegian Institute of Public Health. In Beredt C19, only fully anonymized data (i.e. data that are neither directly nor potentially indirectly identifiable) are permitted to be shared publicly. Legal restrictions therefore prevent the researchers from publicly sharing the dataset used in the study that would enable others to replicate the study findings. However, external researchers are freely able to request access to linked data from the same registries from outside the structure of Beredt C19, as per normal procedure for conducting health research on registry data in Norway. Further information on Beredt C19, including contact information for the Beredt C19 project manager, and information on access to data from each individual data source, is available at https://www.fhi.no/en/id/infectious-diseases/coronavirus/emergency-preparedness-register-for-covid-19/.
Author contributions
All co-authors were involved in the conceptualization of the study. RW drafted the study protocol and coordinated the study. MLS, KB, OH, RK and EAB contributed directly to the acquisition of data. LaVe, BVS, ES, JS, MLS, KB, OH, HB and RW contributed to data cleaning, verification and preparation. LaVe, BVS, ES, JS, HB and RW had access to the final linked dataset. LaVe conducted the statistical analysis with support from BVS, JS and RW. BVS conducted the power calculation. All co-authors contributed to the interpretation of the results. LaVe and RW drafted the manuscript. All co-authors contributed to the revision of the manuscript and approved the final version for submission.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.12.321.
Appendix. Supplementary materials
References
- Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. MedRxiv 2021. https://www.medrxiv.org/content/10.1101/2021.09.15.21263583v2
- Bager P, Wohlfahrt J, Fonager J, Rasmussen M, Albertsen M, Yssing Michaelsen T, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 lineage B.1.1.7 in Denmark: an observational cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bager P, Wohlfahrt J, Rasmussen M, Albertsen M, Krause TG. Hospitalisation associated with SARS-CoV-2 Delta variant in Denmark. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. pii=2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar MS, Marwal R, Radhakrishnan VS, Ponnusamy K, Jolly B, Bhoyar RC, et al. Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. MedRxiv 2021. https://www.medrxiv.org/content/10.1101/2021.06.02.21258076v1 [DOI] [PMC free article] [PubMed]
- Fisman DN, Tuite AR. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario. Canada. CMAJ. 2021 doi: 10.1503/cmaj.211248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.16.2100348. pii=2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grint DJ, Wing K, Houlihan C, Gibbs HP, Evans SWJ, Williamson E, et al. Severity of SARS-CoV-2 alpha variant (B.1.1.7) in England. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, et al. Effectiveness of mRNA and ChAdOx1 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. MedRxiv 2021. https://www.medrxiv.org/content/10.1101/2021.06.28.21259420v3 [DOI] [PubMed]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Ukerapporter om koronavirus og covid-19.https://www.fhi.no/publ/2020/koronavirus-ukerapporter/ Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Emergency preparedness register for COVID-19 (Beredt C19)https://www.fhi.no/en/id/infectious-diseases/coronavirus/emergency-preparedness-register-for-covid-19/ Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Påvisning og overvåkning av SARS-CoV 2-virusvarianter.https://www.fhi.no/nettpub/coronavirus/testing/pavisning-og-overvakning-av-sars-cov-2-virusvarianter/ Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Coronavirus vaccine – information for the public.https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/ Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Vanlige problemstillinger om koronasertifikat.https://www.fhi.no/om/koronasertifikat/til-helsepersonell-vanlige-problemstillinger-om-koronasertifikat/#oversikt-over-intervall-mellom-koronavaksiner Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Who will get the coronavirus vaccine?https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/who-will-get-coronavirus-vaccine-first/ Available at: (accessed 11 October 2021) [Google Scholar]
- Norwegian Institute of Public Health . Norwegian Institute of Public Health; Oslo: 2021. Spørsmål og svar om koronastatistikken og de interaktive diagrammene.https://www.fhi.no/sv/smittsomme-sykdommer/corona/dags–og-ukerapporter/sporsmal-og-svar-om-koronaovervaking-og-statistikk/ Available at: (accessed 11 October 2021) [Google Scholar]
- Public Health England . Public Health England; London: 2021. SARS-CoV-2 variants of concern and variants under investigation in England.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/993879/Variants_of_Concern_VOC_Technical_Briefing_15.pdf Technical briefing 15Available at: (accessed 11 October 2021) [Google Scholar]
- Public Health England . Public Health England; London: 2021. COVID-19 vaccine surveillance report Week 34.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1013553/Vaccine_surveillance_report_-_week_34.pdf Available at: (accessed 11 October 2021) [Google Scholar]
- Seaman SR, Nyberg T, Overton CE, Pascall D, Presanis AM, De Angelis D. Adjusting for time of infection or positive test when estimating the risk of a post-infection outcome in an epidemic. MedRxiv 2021. Available at: https://www.medrxiv.org/content/10.1101/2021.08.13.21262014v1 (accessed 11 October 2021). [DOI] [PMC free article] [PubMed]
- Seppälä E, Veneti L, Starrfelt J, Danielsen AS, Bragstad K, Hungnes O, et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.35.2100793. pii=2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021 doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statens Serum Institut . Statens Serum Institut; Copenhagen: 2021. Vaccineeffektivitet for covid-19 vaccinerne Comirnaty, Spikevax og Vaxzevria i perioden, hvor alfa og delta varianterne har cirkuleret i Danmark.https://www.ssi.dk/-/media/arkiv/dk/aktuelt/nyheder/2021/notat—vaccineeffektivitet-for-covid-19-vaccinerne.pdf?la=da Available at: (accessed 11 October 2021) [Google Scholar]
- Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti L, Seppälä E, Larsdatter Storm M, Valcarcel Salamanca B, Alnes Buanes E, Aasand N, et al. Increased risk of hospitalisation and intensive care admission associated with infection with SARS-CoV-2 variants B.1.1.7 and B.1.351 in Norway, December 2020–May 2021. PLoS One. 2021 doi: 10.1371/journal.pone.0258513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2021. Tracking SARS-CoV-2 variants.https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ Available at: (accessed 11 October 2021) [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2021. Weekly epidemiological update on COVID-19 –17 August 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—17-august-2021 Available at: (accessed 11 October 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.