Abstract
On April 13, 2021, U.S. authorities announced an investigation into potential adverse events associated with the Johnson & Johnson (Janssen, J&J) COVID-19 vaccine and recommended “a pause in the use of this vaccine out of an abundance of caution.” We examined whether public attitudes toward COVID-19 vaccination shifted after this recommended suspension using an interrupted time series with data from the Census Bureau’s Household Pulse Survey, which was fielded bi-weekly between January 6 and April 26, 2021. We found no significant changes in trends of the proportion of the U.S. adult population hesitant about getting a COVID-19 vaccine, but a significant increase in concerns about safety and efficacy of COVID-19 vaccines among the already hesitant population.
Keywords: Vaccine hesitancy, COVID-19, Vaccine safety, USA
1. Introduction
In early April 2021, COVID-19 cases were high, with a rolling 7-day average of over 60,000 [1]. In an effort to vaccinate as many people as possible given an initially limited supply, states began providing three vaccines produced by Moderna, Pfizer, and Johnson & Johnson (Janssen, J&J) to their residents, roughly in accordance with the priority groups established by the Centers for Disease Control and Prevention (CDC) [2]. On April 13, 2021, U.S. authorities announced an investigation into potential adverse events associated with the J&J COVID-19 vaccine and recommended “a pause in the use of this vaccine out of an abundance of caution.” [3] While evaluating potential adverse events is a crucial part of the vaccine safety system, public health and medical experts worried that this recommendation could have also heightened public concerns about COVID-19 vaccine safety in general and added to vaccine hesitancy, potentially reducing uptake. On the other hand, because there were other vaccines available, news about J&J may simply have shifted public preference for Moderna and Pfizer vaccines without changing overall hesitancy or uptake. Moreover, the suspension was short-lived as authorities recommended resuming using the J&J vaccine on April 23.
We assessed whether hesitancy toward COVID-19 vaccination, and stated reasons for hesitancy, shifted among U.S. adults after the announcement.
2. Methods
We used microlevel data (n = 459,235) from the Census Bureau’s Household Pulse Survey (HPS) collected before the suspension, between January 6–March 29, 2021, as well as for the post-suspension period, April 14–26, 2021. The HPS utilizes an online questionnaire to collect data on a bi-weekly basis from a random sample of the U.S. adult population (aged years old) living in households included in the Census Bureau’s Master Address File. While the HPS response rate ranges from 6.4 to 7.5% in our study period [4], the Census’ methods for data collection and survey weighting have consistently produced good results [5], [6]. The detailed design and procedure of data collection is described in the technical documentation of the Household Pulse Survey [4].
The HPS asks individuals whether they have received the COVID-19 vaccine and, if not, whether they intend to get it. Respondents who have not or do not plan to receive all doses and those who did not express that they “definitely” would get the vaccine are then asked about reasons. The earliest data related to COVID-19 vaccine and vaccine hesitancy were collected on January 6, 2021.
We considered two primary outcomes. We defined hesitancy as the percentage of those who “probably will”, are “unsure about”, “probably will not” or “definitely will not” get a COVID-19 vaccine (i.e., all those who have not received a vaccine and did not express they “definitely will” get it), among respondents who have or have not received a COVID-19 vaccine. We defined strong hesitancy as those who said they “definitely will not” get it. As secondary outcomes we examined the 11 reasons for hesitancy captured by the HPS among those who have not or do not plan to receive all doses.
We examined whether the announcement of the J&J vaccine suspension affected hesitancy using an interrupted time series design [7]. For each outcome, we used a logistic model to regress individual responses to the survey question on time (six survey rounds) and used the model to estimate the expected post-suspension outcomes based on the observed pre-suspension trends. This provides a counterfactual: the outcome we would expect if the J&J vaccine had not been suspended. We then compared the post-suspension outcomes predicted by our model with actual responses in the post-suspension round of the HPS. The HPS sampling weights were used to obtain estimates representative of the US adult population. The 95% prediction intervals (PI) of the point estimates were generated based on asymptotic normality of the maximum likelihood estimates of the logistic model. The Appendix describes handling of non-responses and changes in the survey instrument over time.
3. Results
Before the J&J suspension, the estimated proportion of US adults with hesitancy decreased from 43.7% (95% PI: 43.2–44.2%) in early January to 28.4% (95% PI: 28.0–28.8) in late March. The proportion with strong hesitancy decreased from 8.8% (95% PI: 8.5–9.1) to 7.7% (95% PI: 7.5–8.0). After the J&J suspension, 22.5% and 7.02% expressed hesitancy and strong hesitancy, respectively, within or very close to the range of the expected values for hesitancy (23.3%, 95% PI: 22.8–23.9%) and strong hesitancy (7.3%, 95% PI: 7.0–7.7%) (Fig. 1 ).
Fig. 1.
The estimated percentages of the US adult population who expressed vaccine hesitancy (light blue) and strong hesitancy (dark blue). Black dots represent the estimated means from the pre-suspension data; ribbons represent the 95% prediction interval; and the red dots are the observed proportions from the post-suspension data. The dashed line indicates the CDC and FDA announcement of the J&J vaccine suspension recommendation on April 13, 2021. Source: author analysis of HPS data. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
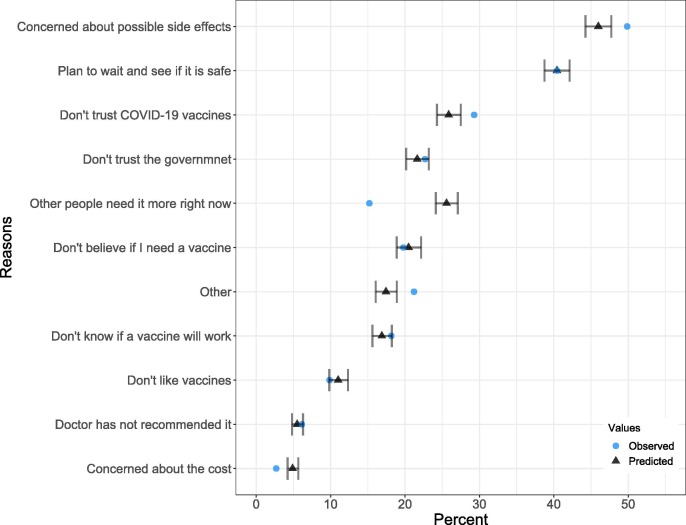
After the suspension, among those with vaccine hesitancy, the proportion concerned about possible COVID-19 vaccine side effects (49.9%) was significantly higher than the expected proportion (46.0%, 95% PI: 44.3–47.7%) if the J&J suspension did not happen. The proportion expressing distrust towards COVID-19 vaccines was significantly higher as well (29.3% versus 25.9%, 95 %PI: 24.3–27.5%). Meanwhile, the proportion of people concerned about COVID-19 vaccine costs (2.7%) between April 14–29 was significantly lower than the expected values (4.9%, 95 %PI: 4.2–5.7%), so was the proportion of those who expressed that other people may need the vaccine (15.2% versus 25.6%, 95 %PI: 24.1–27.1%). There was no significant difference in the observed and expected outcomes among other listed reasons for vaccine hesitancy after the J&J vaccine suspension (Fig. 2 ).
Fig. 2.
Pre-suspension survey data were used to predict the secular trends (from January 9 to April 29) of the proportion of those with vaccine hesitancy who selected each of the 11 reasons to explain why they were not definite on receiving the vaccine, assuming the J&J vaccine suspension did not happen. The black dots represent the predicted point estimates for April 14–29, and the error bars represent the associated 95% prediction intervals. The blue dots are the observed values after the J&J vaccine suspension occurred. Source: author analysis of HPS data. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
As vaccine availability increases and willing adults get vaccinated, achieving herd immunity increasingly requires understanding the attitudes of those who are hesitant and the factors that shift their attitudes and behaviors - even inadvertently. Our analysis suggests that the CDC and Food and Drug Administration (FDA) recommendation on the J&J vaccine suspension was not associated with an increase in hesitancy overall, but could have had an impact on how individuals rationalize their vaccine hesitancy. Specifically, among those expressing vaccine hesitancy, we observed a significant increase in the likelihood of people being concerned about the side effects of COVID-19 vaccines.
Our findings align with an ongoing Kaiser Family Foundation (KFF) survey tracking the public's attitudes and experiences with COVID-19 vaccinations, which recently found a decrease in vaccine hesitancy among the US adult population over time, as well as a decreasing and yet relatively stable trend for strong hesitancy [8]. Similarly, their results also suggest that news on blood-clots possibly related to the J&J vaccine did not significantly slow the vaccine uptake but may have increased the proportion of those concerned about side effects among those who were not vaccinated, especially among women.
Our analysis has several limitations. First, we only had one round of post-suspension data and thus cannot evaluate potential long-term impacts on hesitancy and uptake. Second, we did not have data to examine vaccine attitudes towards the different types of vaccines in the US: J&J, Moderna, and Pfizer. The third caveat relates to a change in the HPS survey instrument in the pre- and post-suspension periods. Prior to the suspension, survey respondents were given four options (“definitely yes”, “probably yes”, “probably no”, “definitely no” when prompted whether they would receive the vaccine. An additional category “unsure” was added to the instrument used after the J&J suspension. While this did not affect our two primary measures of hesitancy and strong hesitancy, we were not able to explore more subtle shifts of different levels of vaccine hesitancy among those who were in the middle of the hesitancy spectrum.
Our findings shed light on the potential impact of recommendations from US health authorities on vaccine hesitancy, which have several implications for effective policy and related communications. As shown in our results, the suspension may have played a role in increasing concerns about COVID-19 vaccine safety among the already hesitant population. This emphasizes the importance of using trusted channels to disseminate messages that address safety-specific concerns among those hesitant to receive the vaccine. At the same time, it is encouraging to see that precautionary communication from the US health authorities on vaccine side effects did not increase the size of the vaccine hesitant population, in aggregate. It is important to note that there were other vaccines available in the US when the CDC and FDA issued the recommendation for J&J vaccine suspension. This decision and the news about the J&J vaccine may simply have shifted public preference for Moderna and Pfizer vaccines without decreasing the overall demand of COVID-19 vaccine. The aforementioned KFF survey also showed that after the J&J suspension, people were less confident of the safety of the J&J vaccine compared to the Pfizer and Moderna vaccines [8], and the possibility that this affected brand preference should be investigated with additional data in future studies. It is unclear how the suspension would have affected the overall COVID-19 vaccine rollout if the J&J vaccine was the only vaccine available, but plausibly there may have been a more direct negative effect. This suggests there may be a benefit to having multiple vaccines available from a hesitancy perspective, as well as an operational one.
5. Conclusion
Understanding the impact of pauses like the J&J vaccine suspension is important for the US and globally, as such pauses are an important piece of the safety system in place for vaccine roll-out. Our results suggest that developing anticipatory communication strategies that focus on overall vaccine safety, and supporting vaccine research and development to have more than one vaccine options in face of an ongoing pandemic may be especially important for effective vaccine roll-out.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr SteelFisher's partner is a partial owner of a company that does consulting for Eli Lilly, a pharmaceutical company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.11.085.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.CDC. COVID data tracker – trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory [Internet]. Centers for Disease Control and Prevention; 2021 [cited 2021 Nov 20]. Available from: https://covid.cdc.gov/covid-data-tracker.
- 2.KFF. How are states prioritizing who will get the COVID-19 vaccine first? [Internet]; 2020 [cited 2021 Nov 20]. Available from: https://www.kff.org/policy-watch/how-are-states-prioritizing-who-will-get-the-covid-19-vaccine-first/.
- 3.FDA, CDC. Joint CDC and FDA statement on Johnson & Johnson COVID-19 vaccine (04/13/2021) [Internet]. FDA; 2021. Available from: https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine.
- 4.Census. Source of the data and accuracy of the estimates for the household pulse survey [Internet]. US Census Bureau; 2021. Available from: https://www2.census.gov/programs-surveys/demo/technical-documentation/hhp/Phase3_Source_and_Accuracy_Week_27.pdf.
- 5.Pew Research Center. Assessing the representativeness of public opinion surveys. Pew Research Center; 2012 [Pew Research Center Report].
- 6.Keeter S, Hatley N, Kennedy C, Lau A. What low response rates mean for telephone surveys. Pew Research Center; 2017 [Pew Research Center Report].
- 7.Bernal J.L., Cummins S., Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KFF. KFF COVID-19 vaccine monitor [Internet]. Kaiser Family Foundation; 2021. Available from: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-april-2021/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.