Abstract
Background
We evaluated clinical outcomes, functional burden, and complications 1 month after coronavirus disease 2019 (COVID-19) infection in a prospective US Military Health System (MHS) cohort of active duty, retiree, and dependent populations using serial patient-reported outcome surveys and electronic medical record (EMR) review.
Methods
MHS beneficiaries presenting at 9 sites across the United States with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test, a COVID-19-like illness, or a high-risk SARS-CoV-2 exposure were eligible for enrollment. Medical history and clinical outcomes were collected through structured interviews and International Classification of Diseases–based EMR review. Risk factors associated with hospitalization were determined by multivariate logistic regression.
Results
A total of 1202 participants were enrolled. There were 1070 laboratory-confirmed SARS-CoV-2 cases and 132 SARS-CoV-2-negative participants. In the first month post–symptom onset among the SARS-CoV-2-positive cases, there were 212 hospitalizations, 80% requiring oxygen, 20 ICU admissions, and 10 deaths. Risk factors for COVID-19-associated hospitalization included race (increased for Asian, Black, and Hispanic compared with non-Hispanic White), age (age 45–64 and 65+ compared with <45), and obesity (BMI≥30 compared with BMI<30). Over 2% of survey respondents reported the need for supplemental oxygen, and 31% had not returned to normal daily activities at 1 month post–symptom onset.
Conclusions
Older age, reporting Asian, Black, or Hispanic race/ethnicity, and obesity are associated with SARS-CoV-2 hospitalization. A proportion of acute SARS-CoV-2 infections require long-term oxygen therapy; the impact of SARS-CoV-2 infection on short-term functional status was substantial. A significant number of MHS beneficiaries had not yet returned to normal activities by 1 month.
Keywords: COVID-19, risk, predictive symptoms, burden, outcomes
The clinical manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection range from mild, self-limited respiratory illness [1–3] to hospitalization with multi-organ involvement and death [4–6]. Observational studies often use hospitalization as a measure of SARS-CoV-2 infection severity and lack longer-term follow-up beyond acute presentations, including nonpulmonary complications. This is a limited measure of the burden of coronavirus disease 2019 (COVID-19), especially in younger populations.
The EPICC study is an adaptive observational cohort study of the epidemiology, immunology, and clinical characteristics of pandemic infectious diseases among adult and pediatric Military Health System (MHS) beneficiaries, including US military active duty service members, retirees, and their family members. Here we describe a multisite prospective cohort within the MHS to identify the clinical outcomes, functional outcomes, complications, and correlates of COVID-19 severity in active duty, retiree, and dependent populations through 1 month postinfection. The geographically distributed, centralized US military health care system affords a unique opportunity for the evaluation of sociodemographic characteristics, comorbidities, and clinical practice patterns associated with COVID-19, as well as incidence of longer-term sequelae, relatively unconfounded by issues related to access to care.
Through the comprehensive capture of MHS beneficiaries diagnosed with COVID-19, EPICC was designed to inform the development and evaluation of clinical practice policies, diagnostic tools, and strategies for the treatment and prevention of COVID-19. In this manuscript, we describe (i) factors associated with severe COVID-19, (ii) pulmonary and extrapulmonary complications of infections, and (iii) the functional impact of SARS-CoV-2 infection among EPICC enrollees. In addition, the parallel enrollment of those testing negative for SARS-CoV-2 enabled us to describe severity correlates unique to SARS-CoV-2 infection vs other respiratory infections.
METHODS
Population and Setting
Participants were enrolled at 10 military treatment facilities (MTFs) in the United States (Supplementary Figure 1); data are reported from the 9 MTFs (Brooke Army Medical Center, Darnall Army Medical Center, Fort Belvoir Community Hospital, Madigan Army Medical Center, Naval Medical Center Portsmouth, Naval Medical Center San Diego, Tripler Army Medical Center, Walter Reed National Military Medical Center, and William Beaumont Army Medical Center) with enrollments from March 20 through December 31, 2020.
Eligible participants either (a) presented with a positive SARS-CoV-2 test, (b) presented with a COVID-19-like illness, or (c) had a high-risk SARS-CoV-2 exposure. Recruitment at MTFs is accomplished through advertising and provider contact, as well as review of inpatient rosters and daily SARS-CoV-2 testing/positive lists under an approved partial Health Insurance Portability and Accountability Act (HIPAA) waiver. Among those approached, 33% agreed to participate in the study. Participants were compensated for filling out surveys and providing samples. Following consent, demographic, comorbidity, and illness data are collected from all subjects, either through structured in-person interviews and a review of the participant’s electronic medical record (EMR) or using participant completed surveys, which were implemented in November 2020 (visit schedule in Supplementary Table 1). In terms of demographics, “other” race refers to participants who either reported multiple races or who reported being Native American or marked “other.” A patient-reported outcome survey (FLU-PRO [7]), with 2 additional questions about loss of sense of smell and taste (FLU-PRO plus), was used to collect information about symptom severity for 14 days.
Patient Consent
The EPICC cohort study was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Written or verbal informed consent was provided by all study participants. This study was approved by the Uniformed Services University Institutional Review Board (IDCRP-085).
Biospecimen Collection
Study personnel obtained respiratory (eg, nasal, throat, and/or oral swab) and venous blood specimens for virologic and immunologic outcomes, respectively, according to the study schedule (Supplementary Table 1). Since July 2020, participants were also offered the option for at-home self-collection of nasal swabs and blood (Mitra kit; Neoteryx LLC, Torrance, CA, USA) at select time points to allow for collection of specimens while in isolation and return of specimens to the study team using prepaid, pre-addressed mailers.
Quantitative Polymerase Chain Reaction Assay
In addition to a baseline clinical polymerase chain reaction (PCR) test, quantitative PCR (qPCR) was performed on research specimens (specimen and time point as per Supplementary Table 1). Briefly, we utilized the SARS-CoV-2 (2019-nCoV) Centers for Disease Control and Prevention (CDC) qPCR Probe Assay research use–only kits (Cat. #10006770) manufactured by Integrated DNA Technologies, Inc. (Coralville, IA, USA), and consistent with the most recent revision of the Emergency Use Authorization (EUA) issued to the CDC on December 1, 2020 [8]. The assay targeted 2 regions of the nucleocapsid (N) gene with an additional primer/probe set to detect the RNase P gene (RP) in specimens. Additionally, target-specific viral loads of each specimen were calculated from plate-specific standard curves using 3 dilutions of known SARS-CoV-2 gene copy numbers.
SARS-CoV-2 Infection Definition
Participants were identified as SARS-CoV-2-positive based on at least 1 of the following measures: (1) positive clinical SARS-CoV-2 PCR test identified in the subject’s EMR between a range of 7 days before symptom onset if symptomatic, or enrollment in asymptomatic cases, and 21 days post-symptom onset, or (2) positive follow-up research SARS-CoV-2 qPCR test within 17 days post-symptom onset (day 14 viral sample +3-day compliance window). SARS-CoV-2-negative participants had only negative tests in the EMR and research records. Individuals who tested positive outside of the predefined windows were dropped from this analysis.
Severity Classification
All data collected for the participants were reviewed to determine the maximum severity of their illness in the first month post–symptom onset. Participants were deemed asymptomatic if they did not have symptoms reported in the surveys or FLU-PRO plus and did not have a symptom onset date or symptoms recorded in the staff-collected forms. Outpatients with limited activity were identified if they reported that they missed work or were unable to do daily activities because of their symptoms on a survey, or if they reported interference with usual activities on the FLU-PRO plus survey. Hospitalization was considered a severity outcome in SARS-CoV-2-positive participants only if the hospitalization was related to their SARS-CoV-2 infection (eg, if they were hospitalized for a traumatic musculoskeletal injury and during that hospitalization they were found to be SARS-CoV-2 positive, they did not count as hospitalized for their maximum severity). Type of oxygen treatment was used to further categorize hospitalization severity.
Surveys
Surveys collecting information on functional outcomes (such as time off work, time with symptoms, or home oxygen treatments) were implemented in EPICC in November 2020; therefore, individuals enrolled before November did not have enrollment surveys and were sent “catch-up” surveys to obtain this information. For the comparison of functional outcomes, individuals were required to have either an enrollment and a follow-up survey or a catch-up survey. If an individual reported symptoms >28 days after symptom onset, they were categorized as having ongoing symptoms; those with resolution within 28 days were considered resolved.
Medical Encounter Information
Study personnel reviewed the medical records for hospitalized participants and recorded details about their hospitalizations such as oxygen requirement. In addition, health record data were extracted from the central MHS database for all participants, including laboratory test results and International Classification of Diseases, Tenth Revision (ICD-10), codes resulting from health care encounters. ICD-10 diagnoses were categorized into 8 major diagnosis categories (cardiovascular, endocrine, liver, mental health, neurology, pulmonology, renal, and other) and 35 subcategories (ICD-10 codes detailed in Supplementary Table 2). Diagnoses were classified as new if they were recorded in participants after symptom onset (or after enrollment if no onset date was identified) but not identified previously in the medical record during the preceding 12 months. Participants were excluded from this part of the analysis if they did not have a full year of MHS data before symptom onset (or enrollment). The MHS database was also used to ascertain death.
Statistical Analyses
Statistical analyses were performed in R, version 4.0 (R Core Team). Age was categorized into 4 groups, <18, 18–44, 45–64, and ≥65 years; following initial description of characteristics and testing, children <18 years were excluded from the analyses because of limited numbers (n=36). Multivariate logistic regression models were used to compare the odds of hospitalization by demographic characteristics and comorbidities, controlling for possible heterogeneity by study site. As a means of variable selection, univariable models were initially run for each comorbidity; those comorbidities with a P value <.15 were then tested in a multivariable model that included sex, race, and age, and those comorbidities that improved the Akaike Information Criterion were retained in the final model. The model was developed in the SARS-CoV-2-positive participants, and the same model was then run in the SARS-CoV-2-negative participants.
RESULTS
SARS-CoV-2 Infection Outcomes in MHS Beneficiaries Include Hospitalization and ICU Admission
We restricted this analysis to the first 1202 participants with known SARS-CoV-2 infection status enrolled from 9 sites (Table 1). The largest groups of participants were 18–44 years of age and were male, active duty, and/or White. Participants who reported symptom onset dates were enrolled a median of 10 days after onset of symptoms, due in part to restriction at MTFs of in-person visits for COVID-19 patients. Among the 1070 SARS-CoV-2-positive subjects, there were 212 (20%) hospitalizations, 20 (2%) ICU admissions, and 10 (0.9%) deaths in the first month post–symptom onset. The most common cause of death was acute respiratory distress syndrome (5/10, 50%) followed by cardiac arrest (2/10, 20%). No specific cause of death (other than COVID-19) was available for 3 SARS-CoV-2-positive participants. Among the 132 SARS-CoV-2-negative subjects, there were 30 (23%) hospitalizations, 2 (2%) ICU admissions, and 1 death during the period of observation. One hundred six (80%) of the SARS-CoV-2-negative participants sought health care in the month after their reported symptom onset, and the most common diagnoses were hypertension (25%), pneumonia (from unspecified organism; 13%), and anxiety (12%) (details in Supplementary Table 3), and there are no recorded positive tests for SARS-CoV-2 or any other respiratory viruses in the medical health records for the negative participants. Sixty-seven participants (62 of whom were SARS-CoV-2 positive and 5 of whom never tested positive) had their first dose of SARS-CoV-2 vaccine in December 2020.
Table 1.
Characteristics of Participants Enrolled in the EPICC MTF Cohort Study
| Inpatient, SARS-CoV-2 Negative (n=30), No. (%) | Inpatient, SARS-CoV-2 Positive (n=212), No. (%) | Outpatient, SARS-CoV-2 Negative (n=102), No. (%) | Outpatient, SARS-CoV-2 Positive (n=858), No. (%) | |
|---|---|---|---|---|
| Age group | ||||
| <18 y | 1 (3.3) | 2 (0.9) | 9 (8.8) | 23 (2.7) |
| 18–44 y | 9 (30.0) | 43 (20.3) | 56 (54.9) | 593 (69.1) |
| 45–64 y | 14 (46.7) | 104 (49.1) | 27 (26.5) | 193 (22.5) |
| 65+ y | 6 (20.0) | 63 (29.7) | 10 (9.8) | 49 (5.7) |
| Male | 17 (56.7) | 142 (67.0) | 47 (46.1) | 530 (61.8) |
| Race/ethnicity | ||||
| Asian/Native Hawaiian | 2 (6.7) | 19 (9.0) | 2 (2.0) | 39 (4.5) |
| Black | 5 (16.7) | 44 (20.8) | 18 (17.6) | 130 (15.2) |
| Hispanic | 2 (6.7) | 63 (29.7) | 21 (20.6) | 229 (26.7) |
| Other | 2 (6.7) | 12 (5.7) | 7 (6.9) | 55 (6.4) |
| White | 19 (63.3) | 74 (34.9) | 54 (52.9) | 405 (47.2) |
| Military status | ||||
| Active duty | 6 (20.0) | 38 (17.9) | 35 (34.3) | 492 (57.3) |
| Dependent | 14 (46.7) | 61 (28.8) | 43 (42.2) | 226 (26.3) |
| Retired military | 10 (33.3) | 113 (53.3) | 24 (23.5) | 140 (16.3) |
| DoD affiliation | ||||
| Air Force | 8 (26.7) | 58 (27.4) | 22 (21.6) | 110 (12.8) |
| Army | 9 (30.0) | 69 (32.5) | 41 (40.2) | 375 (43.7) |
| Coast Guard | 0 (0.0) | 2 (0.9) | 0 (0.0) | 14 (1.6) |
| Marines | 4 (13.3) | 17 (8.0) | 7 (6.9) | 63 (7.3) |
| Navy | 9 (30.0) | 62 (29.2) | 29 (28.4) | 275 (32.1) |
| Othera | 0 (0.0) | 4 (1.9) | 3 (2.9) | 21 (2.4) |
| Days from symptom onset to enrollment | ||||
| Median (Q1, Q3) | 7.0 (4.0, 12.5) | 10.5 (8.0, 15.0) | 5.0 (2.0, 18.0) | 11.0 (6.0, 17.0) |
| No. | 30 | 212 | 88 | 838 |
| Maximum severity | ||||
| Asymptomatic | 0 (0.0) | 0 (0.0) | 13 (12.7) | 17 (2.0) |
| Outpatient | 0 (0.0) | 0 (0.0) | 47 (46.1) | 308 (35.9) |
| Outpatient, limited activity | 0 (0.0) | 0 (0.0) | 42 (41.2) | 533 (62.1) |
| Hospitalized, no O2 | 14 (46.7) | 42 (19.8) | 0 (0.0) | 0 (0.0) |
| Hospitalized, conventional O2 | 10 (33.3) | 91 (42.9) | 0 (0.0) | 0 (0.0) |
| Hospitalized, high-flow O2 | 5 (16.7) | 50 (23.6) | 0 (0.0) | 0 (0.0) |
| Hospitalized, invasive O2 | 0 (0.0) | 19 (9.0) | 0 (0.0) | 0 (0.0) |
| Death | 1 (3.3) | 10 (4.7) | 0 (0.0) | 0 (0.0) |
Abbreviations: DoD,Department of Defense; EPICC, observational cohort study of the epidemiology, immunology, and clinical characteristics of pandemic infectious diseases; MTF, military treatment facility; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Other DoD affiliation includes National Guard, National Oceanic and Atmospheric Administration, US Public Health Service, and missing affiliations.
Surveys were completed by 572 (48%) participants, in which they reported the functional outcomes of their illness (Table 2). Overall, 76% of the participants who filled out the surveys missed work or were unable to participate in their daily activities in the first month post–symptom onset, and 30% of those who reported missing work had not yet returned to work or their normal daily activities within the first month. Among those who reported an impact on their ability to work or complete normal activities, the median time reported off work was 2 weeks. A new requirement for oxygen therapy within 1 month post–symptom onset was reported by 3% of the participants. Survey data were also collected on duration of symptoms; more than half reported recovering from symptoms within 28 days, with a median time to recovery (interquartile range) of 10 (5–14) days.
Table 2.
Functional Outcomes Reported by SARS-CoV-2-Positive Participantsa at 28 Days Post–Symptom Onset/Enrollment
| In, + (n=106) | Out, + (n=466) | Total (n=572) | |
|---|---|---|---|
| New requirement for O2, No. (%) | 15 (14.2) | 2 (0.4) | 17 (3.0) |
| Missed work or unable to do daily activities, No. (%) | 85 (80.2) | 351 (75.3) | 436 (76.2) |
| Returned to activities/duty, No. (%) | 36 (34.0) | 268 (57.5) | 304 (53.1) |
| Days unable to conduct activities/return to duty | |||
| Median (Q1, Q3) | 15.5 (10.0, 21.0) | 14.0 (10.0, 14.0) | 14.0 (10.0, 15.0) |
| No. | 36 | 268 | 304 |
| Symptom category, No. (%) | |||
| Recovered (<29 d) | 50 (47.2) | 277 (59.4) | 327 (57.2) |
| Ongoing symptoms | 51 (48.1) | 142 (30.5) | 193 (33.7) |
| Missing information | 5 (4.7) | 47 (10.1) | 52 (9.1) |
| Time to recoveryb | |||
| Median (Q1, Q3) | 11.0 (7.0, 18.0) | 9.0 (5.0, 14.0) | 10.0 (5.0, 14.0) |
| No. | 50 | 277 | 327 |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Participants filled out a combination of enrollment, catch-up, and/or follow-up surveys.
Among those who reported that they had recovered.
Age, Race, Obesity, and Chronic Pulmonary Disease Are Associated With Hospitalization Risk
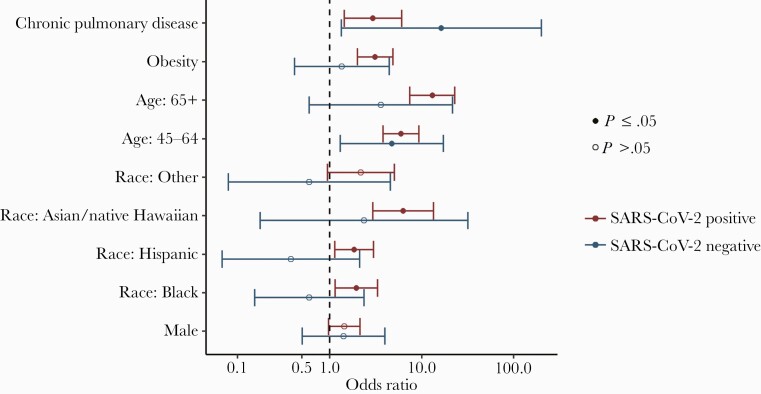
Overall, less than half (46%) of participants reported a preexisting comorbidity; however, among SARS-CoV-2-positive inpatients, 78% had at least 1 comorbidity, and 56% had multiple comorbidities (Supplementary Table 4), with hypertension (47%), obesity (38%), and diabetes mellitus (30%) being the most frequently reported. Adjusted odds of hospitalization among SARS-CoV-2-negative participants were not statistically significantly different by sex, race, or obesity status (Figure 1). However, among SARS-CoV-2-positive participants, those who reported being Asian/Native Hawaiian (aOR, 6.3; 95% CI, 2.9–13.5), Black (aOR, 2.0; 95% CI, 1.2–3.3), or Hispanic (aOR, 1.9; 95% CI, 1.1–3.0) had higher odds of hospitalization than non-Hispanic White participants. Obese subjects, defined as BMI≥30.0 (aOR, 3.1; 95% CI, 2.0–4.9), had higher odds of hospitalization compared with nonobese subjects. Older age (both 45–64 and ≥65 compared with <45) was associated with hospitalization in SARS-CoV-2-positive individuals (aOR45-64years, 5.9; 95% CI, 3.8–9.3; aOR≥65 years, 13.1; 95% CI, 7.4–23.1), but was only statistically significantly associated with hospitalization in the 45–64-year-old age group in SARS-CoV-2-negative participants (aOR, 4.8; 95% CI, 1.3–17.4). Chronic pulmonary disease was associated with higher odds of hospitalization in both SARS-CoV-2-positive and -negative participants. Other comorbidities were tested individually (eg, hypertension, asthma, diabetes) but were not found to significantly contribute to the model fit and were therefore dropped. Odds of hospitalization were similar in men and women.
Figure 1.
Odds of hospitalization associated with risk factors in a multivariate logistic model, run separately for SARS-CoV-2-positive and -negative participants. To adjust for possible heterogeneity across by site, study site (military treatment facility covariate) was included in the model. Odds ratios were adjusted for the covariates shown in the figure plus study site. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Acute Complications of COVID-19 Include a Spectrum of Pulmonary and Extrapulmonary Organ Impairment
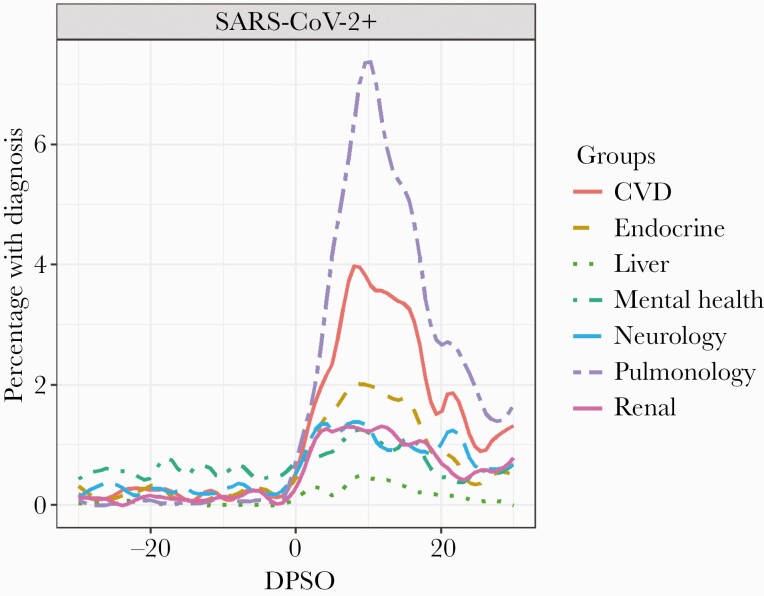
New diagnoses in the 30 days post–symptom onset period (or 30 days postenrollment) by organ system are described in Table 3, and all diagnoses resulting from health care encounters are visualized in Figure 2. Pulmonary diagnoses and complications were the most frequent and included viral pneumonia, acute respiratory distress syndrome (ARDS), bacterial pneumonia, and pneumothorax, as well as new-onset obstructive and restrictive lung disease. Neurological complications were the second most frequent category, and this ranged from loss of sense of smell and taste to postviral fatigue syndromes. Cardiac complications made up the third most common category and included atrial fibrillation, cardiomyopathy, and presentations of ischemic heart disease. Renal, liver, endocrine, and mental health were less frequent categories but nevertheless reflected a substantive breadth of extrapulmonary complications. As visualized in Figure 2, health care encounters increased substantially after SARS-CoV-2 infection in almost all categories of diagnoses, and this was primarily due to inpatient encounters.
Table 3.
New Diagnoses Identified in Participants Through Their Medical Records Within 30 Days Post–Symptom Onset (or Enrollment if No Symptom Onset Date Was Identified)
| Inpatient, SARS-CoV-2 Negative (n=30), No. (%) | Inpatient, SARS-CoV-2 Positive (n=212), No. (%) | Outpatient, SARS-CoV-2 Negative (n=88), No. (%) | Outpatient, SARS-CoV-2 Positive (n=838), No. (%) | |
|---|---|---|---|---|
| Pulmonary | ||||
| Viral pneumonia | 4 (13.3) | 148 (69.8) | 2 (2.3) | 23 (2.7) |
| Unspecified pneumonia | 9 (30.0) | 53 (25.0) | 3 (3.4) | 14 (1.7) |
| ARDS | 0 (0.0) | 19 (9.0) | 0 (0.0) | 0 (0.0) |
| Bacterial pneumonia | 2 (6.7) | 14 (6.6) | 0 (0.0) | 0 (0.0) |
| Asthma | 5 (16.7) | 5 (2.4) | 0 (0.0) | 3 (0.4) |
| Obstructive lung disease | 1 (3.3) | 5 (2.4) | 1 (1.1) | 2 (0.2) |
| Restrictive lung disease | 1 (3.3) | 7 (3.3) | 0 (0.0) | 1 (0.1) |
| Pneumothorax | 0 (0.0) | 3 (1.4) | 0 (0.0) | 0 (0.0) |
| Cardiovascular | ||||
| Hypertension | 6 (20.0) | 26 (12.3) | 2 (2.3) | 10 (1.2) |
| Pulmonary embolism/DVT | 1 (3.3) | 14 (6.6) | 0 (0.0) | 1 (0.1) |
| Ischemic heart disease | 3 (10.0) | 13 (6.1) | 1 (1.1) | 5 (0.6) |
| Cardiac arrest | 0 (0.0) | 6 (2.8) | 0 (0.0) | 0 (0.0) |
| Heart failure | 2 (6.7) | 5 (2.4) | 0 (0.0) | 1 (0.1) |
| Myocarditis | 1 (3.3) | 5 (2.4) | 0 (0.0) | 1 (0.1) |
| Atrial fibrillation | 2 (6.7) | 5 (2.4) | 0 (0.0) | 0 (0.0) |
| Cardiomyopathy | 0 (0.0) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Cerebrovascular disease | 0 (0.0) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Neurology | ||||
| Headache | 1 (3.3) | 12 (5.7) | 3 (3.4) | 6 (0.7) |
| Pain syndrome | 0 (0.0) | 10 (4.7) | 1 (1.1) | 1 (0.1) |
| Sleep disorders | 2 (6.7) | 10 (4.7) | 0 (0.0) | 4 (0.5) |
| Postviral fatigue/encephalopathy | 0 (0.0) | 7 (3.3) | 0 (0.0) | 0 (0.0) |
| Neuropathies | 1 (3.3) | 3 (1.4) | 0 (0.0) | 1 (0.1) |
| Meningitis/encephalitis | 0 (0.0) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Movement disorders | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Other acute central nervous system signs and symptoms | 1 (3.3) | 15 (7.1) | 1 (1.1) | 28 (3.3) |
| Mental health | ||||
| Anxiety somatoform disorders | 3 (10.0) | 15 (7.1) | 0 (0.0) | 9 (1.1) |
| Depression | 1 (3.3) | 11 (5.2) | 0 (0.0) | 4 (0.5) |
| Endocrine | ||||
| Diabetes | 1 (3.3) | 11 (5.2) | 2 (2.3) | 3 (0.4) |
| Liver | ||||
| Chronic liver disease | 3 (10.0) | 15 (7.1) | 0 (0.0) | 1 (0.1) |
| Renal | ||||
| Acute kidney injury | 7 (23.3) | 32 (15.1) | 0 (0.0) | 3 (0.4) |
| Chronic kidney disease | 2 (6.7) | 10 (4.7) | 1 (1.1) | 2 (0.2) |
| Other | ||||
| Bacteremia | 4 (13.3) | 5 (2.4) | 0 (0.0) | 1 (0.1) |
| DIC/coagulopathy | 1 (3.3) | 5 (2.4) | 0 (0.0) | 0 (0.0) |
Abbreviations: ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
Percentage of SARS-CoV-2-positive participants with diagnoses in different categories in the 30 days before and after SARS-CoV-2 symptom onset in the EPICC cohort. Abbreviations: CVD, cardiovascular disease; DPSO, Days post-symptom onset; EPICC, observational cohort study of the epidemiology, immunology, and clinical characteristics of pandemic infectious diseases; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
COVID-19 has imposed a burden on the health and readiness of the US Department of Defense, with >196 000 confirmed cases recorded as of June 2021 [9]. This prospective cohort study offers insights into the functional and clinical outcomes experienced by SARS-CoV-2-infected individuals, including extrapulmonary complications and return-to-health up to 1 month, as well as correlates of COVID-19 severity contrasted against a SARS-CoV-2-negative group in MHS beneficiaries.
The findings from this longitudinal cohort of US military members and beneficiaries are consistent with other studies that have found that individuals from certain racial/ethnicity groups [10, 11] and obese individuals [12–14] are disproportionately affected by severe COVID-19. In the MHS, in which all individuals have open access to health care and medications, Asian, Black, and Hispanic individuals had higher odds of hospitalization when compared with non-Hispanic White individuals after controlling for age, obesity, and other comorbidities. This association with race was not observed among the SARS-CoV-2-negative study participants. The basis of the relationship between race and severe COVID-19 has been debated [10, 11, 15–17]. Obesity has previously been described as a risk factor for SARS-CoV-2 and other severe respiratory infections, including influenza [18], and these results further support the role of obesity in COVID-19 hospitalization in the MHS beneficiary population. Chronic pulmonary disease was associated with hospitalization in both the SARS-CoV-2-positive and -negative participants in our cohort, and these findings align with previous publications [19–21]. Older age was a risk factor for hospitalization in both the SARS-CoV-2-positive and -negative groups (although only statistically significant for the positive participants, perhaps due to sample size limitations). We did not see differences in hospitalization between men and women.
Our study noted a spectrum of COVID-19 complications, including those in extrapulmonary organ systems (Table 3, Figure 2). Acute lung complications correlated with findings in other studies, including ARDS, pulmonary emboli, and pneumothoraces [22–25]. As with other studies, bacterial coinfections or secondary bacterial infections were generally infrequent [26]. New diagnoses of restrictive and obstructive lung disease highlight the potential for SARS-CoV-2 to be associated with longer-term pulmonary compromise, including in young adults. Indeed, our follow-up surveys indicated that >2% of respondents reported a need for supplemental oxygen therapy at 28 days post–illness onset (Table 2). Additional work is under way looking at longer-term complications associated with SARS-CoV-2 infection.
Our findings also highlight the neurological impact of COVID-19, with neurological diagnoses making up the second most common category of ICD diagnoses and including a broad range of pathologies including loss of sense of taste and smell, encephalopathy, neuropathy, and postviral fatigue. Cardiac complications made up the third most common category and included arrhythmia, cardiomyopathy, and ischemic heart disease. These findings correlate with a growing body of literature on the neurologic and cardiac sequelae of SARS-CoV-2 infection [27–29]. Despite enrolling many young, active duty participants, almost three-quarters of our study sample was unable to perform daily activities, with 31% unable to perform their usual activities 1 month after symptom onset. The impact of SARS-CoV-2 on long-term heart, lung, and brain health is the current focus of the EPICC study, and our ICD and patient-reported outcome assessments indicate a longer-term impact of COVID-19 that will be explored further.
This study has several strengths. Our use of EMR data enabled observation of acute and subacute complications of COVID-19 infection in both pulmonary and extrapulmonary organ systems. Longitudinal follow-up with serial surveys allowed for the collection of patient-reported outcomes up to 1 month post–symptom onset. In addition, the study included a SARS-CoV-2-negative comparator group used to distinguish risk factors for hospitalization specific to SARS-CoV-2-positive participants.
There are some limitations to this study. This is a heterogenous convenience sample of participants enrolled at different military sites and may not be generalizable to the broader MHS or US population. In addition, the participants who chose to respond to the surveys may be more likely to reflect those with longer-term symptoms due to SARS-CoV-2 infection. Finally, as with any EMR-based study, the sensitivity and specificity of ICD coding are a limitation [30]. The low frequency of “viral pneumonia” diagnoses (despite a substantive proportion of hospitalized cases) is one example of this.
The number of SARS-CoV-2-negative participants was small, reflecting changes in test positivity rates by time and location over the study period, with preference for enrollment of positive cases when staffing was a limiting factor, and it may be possible that some of the people who tested negative for SARS-CoV-2 had been positive but were tested too late in the illness or falsely tested negative for another reason. The impact of this misclassification of SARS-CoV-2 status would lead the groups to be more similar; however, we note differences in the relationship between race and hospitalization in that SARS-CoV-2-positive participants who reported being Black, Asian, or Hispanic were 2.0, 6.3, and 1.9 times more likely to be hospitalized than participants who were non-Hispanic White, and this relationship was not observed in the SARS-CoV-2-negative participants. Similarly, obesity was identified as a risk factor for hospitalization in the SARS-CoV-2 participants, but not in the SARS-CoV-2-negative participants.
Taken together, we show that COVID-19 has a substantial impact on the short-term health of MHS beneficiaries, including young active duty subjects. We show a range of complications across multiple organ systems, with evidence of substantial pulmonary compromise in some out to 1 month. These findings stress the importance of vaccination as well as improved acute therapeutic options and prompt further study into how such countermeasures may mitigate the risk of longer-term disease morbidity and functional impairment.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We appreciate the EPICC participants for their central role in this study. Many thanks to the IDCRP team at the clinical research sites—physician/clinical investigators, site managers, regulatory staff, clinical research coordinators, and laboratory personnel—for their support of this study and contributions to its success under very challenging circumstances. The authors would like to thank Camille Estupigan for her editorial assistance.
We thank the members of the EPICC COVID-19 Cohort Study Group for their many contributions in conducting the study and ensuring effective protocol operations. The following members were all closely involved with the design, implementation, and oversight of the study: Brooke Army Medical Center, Fort Sam Houston, TX: Col J. Cowden; Maj D. Lindholm; A. Markelz; K. Mende; T. Merritt; LTC R. Walter; CPT T. Wellington. Carl R. Darnall Army Medical Center, Fort Hood, TX: MAJ S. Bazan. Fort Belvoir Community Hospital, Fort Belvoir, VA: N. Dimascio-Johnson; MAJ E. Ewers; LCDR K. Gallagher; LCDR D. Larson; MAJ M. Odom; A. Rutt. Henry M. Jackson Foundation, Inc., Bethesda, MD: P. Blair; J.G. Chenoweth; D. Clark. Madigan Army Medical Center, Joint Base Lewis McChord, WA: S. Chambers; LTC C. Colombo; R. Colombo; CPT C. Conlon; CPT K. Everson; LTC P. Faestel; COL T. Ferguson; MAJ L. Gordon; LTC S. Grogan; CPT S. Lis; COL C. Mount; LTC D. Musfeldt; CPT D. Odineal; MAJ R. Sainato; C. Schofield; COL C. Skinner; M. Stein; MAJ M. Switzer; MAJ M. Timlin; MAJ S. Wood. Naval Medical Center Portsmouth, Portsmouth, VA: R. Carpenter; L. Kim; CAPT K. Kronmann; T. Lalani; LCDR T. Lee; LCDR A. Smith; CDR T. Warkentien. Naval Medical Center San Diego, San Diego, CA: CAPT J. Arnold; CDR C. Berjohn; S. Cammarata; LCDR S. Husain; LCDR A. Lane; CAPT R. Maves; J. Parrish; G. Utz. Tripler Army Medical Center, Honolulu, HI: S. Chi; MAJ E. Filan; K. Fong; CPT T. Horseman; MAJ M. Jones; COL A. Kanis; LTC A. Kayatani; MAJ W. Londeree; LTC C. Madar; MAJ J. Masel; MAJ M. McMahon; G. Murphy; COL V. Ngauy; P. Schmidt; MAJ E. Schoenman; C. Uyehara; LTC R. Villacorta Lyew. Uniformed Services University of the Health Sciences, Bethesda, MD: B. Agan; C. Broder; CAPT T. Burgess; C. Byrne; C. Coles; C. English; COL P. Hickey; E. Laing; LTC J. Livezey; A. Malloy; COL T. Oliver; E. Parmelee; S. Pollett; M. Rajnik; S. Richard; J. Rozman; M. Sanchez; A. Scher; CDR M. Simons; A. L. Snow; D. Tribble. United States Air Force School of Medicine, Dayton, OH: A. Fries. Womack Army Medical Center, Fort Bragg, NC: MAJ A. Farmer; B. Barton; LTC D. Hostler; C. Maldonado; MAJ T. Musich; MAJ R. Radcliffe; M. Swain. William Beaumont Army Medical Center, El Paso, TX: CPT M. Banda; CPT B. Davis; MAJ T. Hunter; CPT O. Ikpekpe-Magege; CPT S. Kemp; R. Mody; COL M. Wiggins. Walter Reed National Military Medical Center, Bethesda, MD: A. Ganesan; D. Gunasekera; MAJ N. Huprikar.
Disclaimer. The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS); the Department of Defense (DoD); the Departments of the Army, Navy, or Air Force; Brooke Army Medical Center; Walter Reed National Military Medical Center; Naval Medical Center San Diego; Madigan Army Medical Center; Naval Medical Center Portsmouth; Tripler Army Medical Center; Walter Reed Army Institute of Research; United States Air Force School of Aerospace Medicine; Fort Belvoir Community Hospital; or the Henry M. Jackson Foundation for the Advancement of Military Medicine. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46. Some of the authors are service members or employees of the US Government. This work was prepared as part of their official duties. Title 17 USC. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 USC. §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Financial support. This work was supported by awards from the Defense Health Program and the National Institute of Allergy and Infectious Disease (HU00011920111). The protocol was executed by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed by the Uniformed Services University of the Health Sciences (USUHS) through a cooperative agreement by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, under an interagency agreement (Y1-AI-5072).
Potential conflicts of interest. S. D. P., T. H. B, and D.R.T. report that the Uniformed Services University (USU) Infectious Diseases Clinical Research Program (IDCRP), a US Department of Defense institution, and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc (HJF) were funded under a Cooperative Research and Development Agreement to conduct an unrelated phase III COVID-19 monoclonal antibody immunoprophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense to augment the conduct of an unrelated phase III vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response. Neither is related to the work presented here. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
EPICC COVID-19 Cohort Study Group:
J Cowden, D Lindholm, A Markelz, K Mende, T Merritt, R Walter, T Wellington, S Bazan, N Dimascio-Johnson, E Ewers, K Gallagher, D Larson, M Odom, A Rutt, P Blair, J G Chenoweth, D Clark, S Chambers, C Colombo, R Colombo, C Conlon, K Everson, P Faestel, T Ferguson, L Gordon, S Grogan, S Lis, C Mount, D Musfeldt, D Odineal, R Sainato, C Schofield, C Skinner, M Stein, M Switzer, M Timlin, S Wood, R Carpenter, L Kim, K Kronmann, T Lalani, T Lee, A Smith, T Warkentien, J Arnold, C Berjohn, S Cammarata, S Husain, A Lane, R Maves, J Parrish, G Utz, S Chi, E Filan, K Fong, T Horseman, M Jones, A Kanis, A Kayatani, W Londeree, C Madar, J Masel, M McMahon, G Murphy, V Ngauy, P Schmidt, E Schoenman, C Uyehara, R Villacorta Lyew, B Agan, C Broder, T Burgess, C Byrne, C Coles, C English, P Hickey, E Laing, J Livezey, A Malloy, T Oliver, E Parmelee, S Pollett, M Rajnik, S Richard, J Rozman, M Sanchez, A Scher, M Simons, A L Snow, D Tribble, A Fries, A Farmer, B Barton, D Hostler, C Maldonado, T Musich, R Radcliffe, M Swain, M Banda, B Davis, T Hunter, O Ikpekpe-Magege, S Kemp, R Mody, M Wiggins, A Ganesan, D Gunasekera, and N Huprikar
References
- 1. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020; 63:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nepal G, Rehrig JH, Shrestha GS, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care 2020; 24:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020; 75:2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powers JH, Guerrero ML, Leidy NK, et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. 2020. Available at: https://www.fda.gov/media/134922/download. Accessed 1 December 2020.
- 9. US Department of Defense. Coronavirus: DoD response. Available at: https://www.defense.gov/explore/spotlight/coronavirus/. Accessed 6 June 2021.
- 10. Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA 2020; 323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price-Haywood EG, Burton J, Fort D, Seoane L.. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med 2020; 382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis 2020; 71:896–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonnet A, Chetboun M, Poissy J, et al. ; LICORN and theLille COVID-19andObesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020; 28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabarriti R, Brodin NP, Maron MI, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open 2020; 3:e2019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open 2020; 3:e2022310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open 2020; 3:e2026881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green WD, Beck MA.. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc 2017; 14:406–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kebisek J, Forrest LJ, Maule AL, et al. Special report: prevalence of selected underlying health conditions among active component Army service members with coronavirus disease 2019, 11 February-6 April 2020. MSMR 2020; 27:50–4. [PubMed] [Google Scholar]
- 20. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibson PG, Qin L, Puah SH.. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust 2020; 213:54–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenan-Barrett J, Perera A.. COVID-19 and pulmonary emboli: a case series and literature review. Clin Pract Cases Emerg Med 2020; 4:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ekanem E, Podder S, Donthi N, et al. Spontaneous pneumothorax: an emerging complication of COVID-19 pneumonia. Heart Lung 2021; 50:437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belletti A, Palumbo D, Zangrillo A, et al. Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth 2021; 35:3642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musuuza JS, Watson L, Parmasad V, et al. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One 2021; 16:e0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varatharaj A, Thomas N, Ellul MA, et al. ; CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry 2020; 7:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chou SH, Beghi E, Helbok R, et al. ; GCS-NeuroCOVID Consortium and ENERGY Consortium. Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open 2021; 4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vehar S, Boushra M, Ntiamoah P, Biehl M.. Post-acute sequelae of SARS-CoV-2 infection: caring for the ‘long-haulers.’ Cleve Clin J Med 2021; 88:267–72. [DOI] [PubMed] [Google Scholar]
- 30. Quan H, Li B, Saunders LD, et al. ; IMECCHI Investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008; 43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.