Abstract
Candida endophthalmitis is a serious complication of candidemia. Diagnosis requires identification of ocular lesions on dilated fundoscopy, aided by isolation of the organism from blood and/or vitreous humor. However, the initial ophthalmological examination may be negative in some cases. Experience with isavuconazole for the treatment of Candida endophthalmitis is limited. We present a case of a 65-year-old woman with metastatic breast cancer on chemotherapy who developed Candida dubliniensis endophthalmitis with initial negative ophthalmological examination. She was treated with vitrectomy and 6 weeks of oral fluconazole. Despite vitrectomy and culture-directed antifungal treatment, management was complicated by lack of response to fluconazole and intolerance to other antifungals, necessitating the use of isavuconazole, which proved efficacious.
Keywords: Candida endophthalmitis, candidemia, candidiasis, vitritis
Because candidemia first seeds the highly vascular choroid, the initial manifestation is chorioretinitis. If left untreated, chorioretinitis progresses to vitritis. Candida endophthalmitis is defined as ocular candidiasis with vitreal involvement. The true prevalence of ocular candidiasis in patients with candidemia is debated due to challenges in diagnosis. In a study of 370 patients with candidemia, possible or probable ocular involvement occurred in 60 patients (16%), only 6 of which had endophthalmitis [1]. A systematic review in 2019 found that less than 1% of 7472 patients with candidemia who underwent routine ophthalmological screening had endophthalmitis [2]. Presence of a central venous catheter (odds ratio 8.35), intravenous drug use (odds ratio 4.76), immunosuppression (odds ratio 2.40), and receipt of total parenteral nutrition (odds ratio 2.28) were risk factors for developing Candida endophthalmitis [3], the most common species of which is Candida albicans [1, 4]. We present a case of Candida dubliniensis endophthalmitis in a patient receiving chemotherapy for metastatic breast cancer to highlight a few challenges in the management of Candida endophthalmitis.
CASE REPORT
The patient, a 65-year-old Chinese woman, was found to have breast cancer with extensive metastases to lymph nodes and liver when she presented with abdominal distension and jaundice. After diagnosis, palliative chemotherapy (paclitaxel/trastuzumab/pertuzumab) was initiated. Two weeks after chemotherapy, she presented with fever, and blood cultures grew C dubliniensis and Candida tropicalis. She never developed neutropenia from the chemotherapy. Concern for central line-related blood stream infection prompted removal of her peripherally inserted central catheter whose tip culture grew C tropicalis, although the presence of mucositis suggested that the candidemia might be due to gastrointestinal translocation. The fluconazole and anidulafungin minimum inhibitory concentrations (MICs) for the C dubliniensis were 0.25 µg/mL and 0.12 µg/mL, respectively. The fluconazole and anidulafungin MICs for the C tropicalis were 4 µg/mL and 0.12 µg/mL, respectively (Tables 1 and 2). Because of the fluconazole MIC of 4 µg/mL, which was interpreted as susceptible dose dependent (SDD), she was treated with a 100-mg intravenous (IV) dose of anidulafungin every 24 hours. Blood cultures drawn on day 3 of anidulafungin were negative. A transthoracic echocardiogram found no vegetation. Although she did not report any visual symptoms, she was referred to an ophthalmologist to rule out ocular candidiasis. Dilated fundoscopy on day 3 and a routine second fundoscopy on day 17 from date of documented candidemia showed no ocular candidiasis. She completed 2 weeks of treatment with anidulafungin.
Table 1.
Results of Antifungal Susceptibility Testing of Candida dubliniensis in Blood Culturea
| Drug | MIC (µg/mL) | Interpretation |
|---|---|---|
| Fluconazole | 0.25 | WT |
| Voriconazole | ≤0.008 | NA |
| Isavuconazole | 0.016 | NA |
| Anidulafungin | 0.12 | WT |
| Amphotericin B | 0.5 | WT |
Abbreviations: MIC, minimum inhibitory concentration; NA,no breakpoints/epidemiological cutoff value (ECV) available; WT,wild-type.
Antifungal susceptibility testing was performed using Sensititre YeastOne susceptibility plates (Thermo Scientific) [23] for fluconazole, voriconazole, anidulafungin, and amphotericin B, and gradient diffusion test strip (Liofilchem) [24] for isavuconazole. The results are interpreted using Clinical and Laboratory Standards Institute [25] breakpoints or ECVs [26], where available.
Table 2.
Results of Antifungal Susceptibility Testing of Candida tropicalis in Blood Culturea
| Drug | MIC (µg/mL) | Interpretation |
|---|---|---|
| Fluconazole | 4 | SDD |
| Voriconazole | 0.25 | I |
| Isavuconazole | 0.012 | NA |
| Anidulafungin | 0.12 | S |
| Amphotericin B | 1 | WT |
Abbreviations: MIC, minimum inhibitory concentration; I,intermediate; NA,no breakpoints/epidemiological cutoff value (ECV) available; S,susceptible; SDD,susceptible dose dependent; WT,wild-type.
Antifungal susceptibility testing was performed using Sensititre YeastOne susceptibility plates (Thermo Scientific) [23] for fluconazole, voriconazole, anidulafungin, and amphotericin B, and gradient diffusion test strip (Liofilchem) [24] for isavuconazole. The results are interpreted using Clinical and Laboratory Standards Institute [25] breakpoints or ECVs [26], where available.
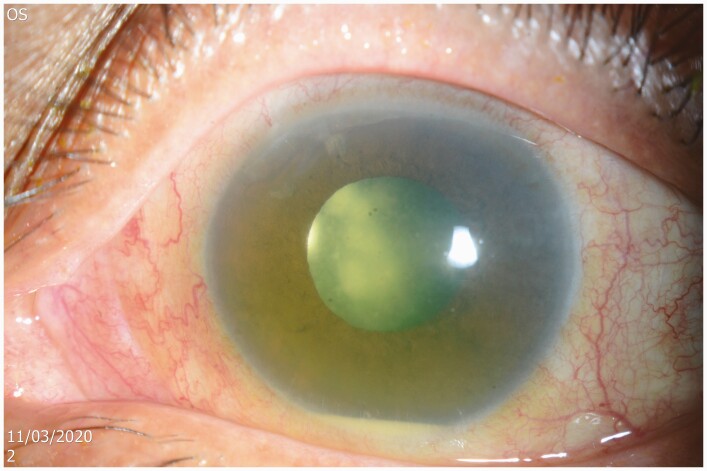
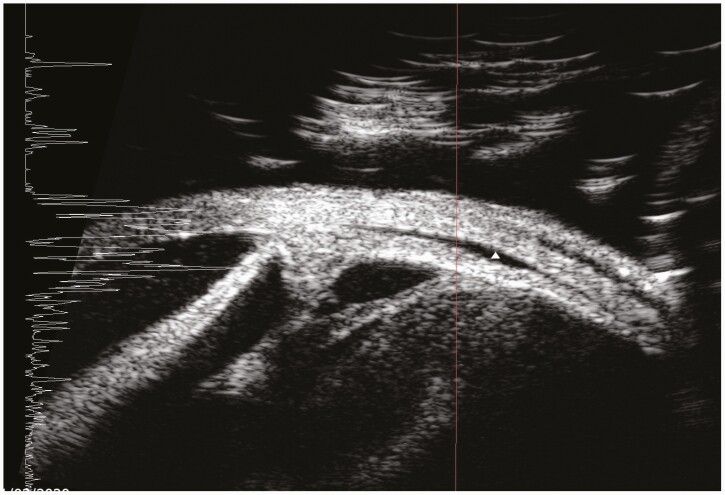
Three months later, the patient returned with progressive blurring of vision in the left eye of 1-month duration. Visual acuity in this pseudophakic eye was hand movements. The anterior chamber had moderate flare and cells that accumulated to form a 1-mm hypopyon. Dilated fundoscopy revealed white “snow ball”-like clumps in the anterior vitreous (Figure 1), which precluded view of the retina. B-scan ultrasonography did not reveal any choroidal nor subretinal abscess, and the retina was attached. Ultrasound biomicroscopy demonstrated organized vitreous opacities and choroidal effusion (Figure 2), suggesting chronicity. The right eye was quiescent. There was no growth in multiple blood cultures. Nonetheless, Candida endophthalmitis was suspected based on the fundoscopic examination. She received intravitreal amphotericin B and underwent trans-pars plana vitrectomy, fluid/silicone-oil exchange, and removal of intraocular lens of the left eye. Intraoperatively, a subretinal abscess near the superior temporal arcade was noted. She was started on IV liposomal amphotericin B at 5mg/kg and amphotericin B eye drop every hour. Eventually, vitreous cultures grew C dubliniensis. Susceptibility testing showed fluconazole MIC of 0.25 µg/mL, anidulafungin MIC of 0.12 µg/mL, and amphotericin B MIC of 0.5 µg/mL (Table 3)—values identical to those for the previous C dubliniensis isolate from the blood culture 3 months prior. Based on the susceptibility results, IV liposomal amphotericin B was changed to oral fluconazole 400mg daily (6mg/kg).
Figure 1.
This slit-lamp photograph of the left pseudophakic eye shows mild conjunctival injection and a 1-mm white hypopyon in the anterior chamber. The mid-dilated pupil reveals multiple large white snowball opacities in the retrolental space admixed with numerous smaller snowball opacities typically seen in Candida endogenous endophthalmitis.
Figure 2.
This ultrasound biomicroscopy image shows organized vitreous opacities in the retrolental area and membrane formation extending from the pars plana region towards the ciliary body and intraocular lens implant. In addition, a small choroidal effusion is seen (white triangle).
Table 3.
Results of Antifungal Susceptibility Testing of Candida dubliniensis in Vitreous Culture
| Drug | MIC (µg/mL) | Interpretation |
|---|---|---|
| Fluconazole | 0.25 | WT |
| Voriconazole | ≤0.008 | NA |
| Isavuconazole | 0.023 | NA |
| Anidulafungin | 0.12 | WT |
| Amphotericin B | 0.5 | WT |
| Flucytosine | ≤0.06 | NA |
Abbreviations: MIC, minimum inhibitory concentration; NA,no breakpoints/epidemiological cutoff value available; WT,wild type.
Despite 6 weeks of oral fluconazole, the patient’s endophthalmitis failed to improve. The vision in her left eye remained hand movements. She developed progressive choroidal effusion and hypotony so intravitreal injection could not be administered. Oral fluconazole was ceased, and she was started on IV conventional amphotericin B at 0.5mg per kg every 24 hours and oral flucytosine at 100mg per kg every 24 hours. After 2 weeks of treatment, she developed acute kidney injury. Due to a prolonged QT interval of 509 milliseconds, she was initiated on isavuconazole at a loading dose of 200mg every 8 hours for 48 hours, followed up by 200mg once daily. After 2 weeks of oral isavuconazole, the vision in her left eye improved. Examination showed gradual resolution of inflammation in her left eye. Due to the severe prolonged infection, the hypotony persisted and she did not recover useful vision. She had received 10 weeks of antifungal treatment in total.
DISCUSSION
Candida endophthalmitis is a serious condition. Visual outcome is usually poor in patients who present with poor visual acuity or central lesions [5]. Because vision loss may develop days to weeks after candidemia and pain is often minimal until the infection is advanced, the Infectious Diseases Society of America recommends that all patients with candidemia, including those without visual symptoms, undergo a dilated ophthalmological examination within the first week of therapy in nonneutropenic patients [6]. For neutropenic patients, it is recommended to delay the examination until neutrophil recovery [6]. However, this practice of routine screening for Candida endophthalmitis is being challenged.
The American Academy of Ophthalmology (AAO) recently stated that routine ophthalmologic consultation in candidemia seems to be a low-value practice because risk of endophthalmitis in candidemia is low [2], and medical management is not different when eye disease is present [7]. They stressed that mild endophthalmitis or chorioretinitis may resolve with systemic treatment only [7], and they highlighted the CANDIPOP study, which found that ocular involvement was not more frequent in patients who received initial therapy with an echinocandin for candidemia [8]. Hence, the AAO does not recommend routine ophthalmological consultation in all candidemia and suggests ophthalmological consultation only in patients with signs or symptoms suggestive of an ocular infection [7].
Echinocandins achieve good levels in the vascular choroid but penetrate poorly into the vitreous humor [9]. In the CANDIPOP study, routine dilated fundoscopy was performed in 46% of the patients [8]. When ocular candidiasis was diagnosed, patients who were receiving an echinocandin as initial therapy had their treatment switched to fluconazole [8]. Accordingly, no patient with ocular candidiasis was definitively treated with an echinocandin only [8]. As such, one cannot conclude from the CANDIPOP study that mild endophthalmitis or chorioretinitis may resolve with systemic treatment only, especially when routine dilated fundoscopy is not performed to exclude ocular candidiasis and echinocandin is the preferred treatment. Case reports of patients with isolated chorioretinitis who were treated with caspofungin had inconsistent treatment outcomes [10, 11]. Based on available data, it would be prudent to avoid echinocandins for the treatment of ocular candidiasis [12]. Because echinocandins have emerged as the drug of choice for initial treatment of candidemia [6], we believe that routine ophthalmological examination remains important. The consequence of missing ocular candidiasis far outweighs the cost of an ophthalmological consultation. If Candida endophthalmitis is present, an antifungal agent with good vitreous penetration such as fluconazole should be administered for 4 to 6 weeks [6], in contrast to a standard 2-week course for candidemia.
In our patient, seeding of the choroid likely occurred during the initial episode of candidemia because the MICs of the tested antifungals for the C dubliniensis isolates in blood and vitreous cultures were almost identical (Tables 1 and 2), although confirmatory molecular studies were not performed. Furthermore, when our patient presented with visual symptoms 3 months later, there was already vitritis with tractional membranes at the ciliary body, choroidal effusion, and a subretinal abscess, suggesting that the onset of infection was not recent.
Dilated fundoscopy on days 3 and 17 from date of documented candidemia did not detect ocular candidiasis in our patient. Although most ocular lesions are detected at the baseline examination, patients may have a normal initial ophthalmological examination. In a study of 370 patients with candidemia, 11 of 60 (18%) patients with ocular candidiasis had normal baseline examination [1]. In these cases, hematogenous inoculation likely occurred early during candidemia but the ocular lesions required some time to become visible [1]. Although fluffy lesions with extensions into vitreous are characteristic of Candida endophthalmitis, chorioretinitis may manifest as nonspecific retinal lesions, which makes initial diagnosis challenging. Oude Lashof et al [1] recommended that dilated fundoscopy be performed at least 1 week after the onset of therapy in candidemic patients without visual symptoms, to increase its sensitivity to detect ocular candidiasis.
Successful treatment of Candida endophthalmitis is difficult. With macular involvement, intravitreal injection is usually required, in addition to systemic antifungals. When there is vitritis, vitrectomy is crucial for source control [6, 12]. The choice of the antifungal agent depends on the susceptibility profile of the Candida species. In instances in which the Candida is susceptible to fluconazole, fluconazole is the drug of choice because concentrations of fluconazole in the vitreous are approximately 70% of those in the serum [13]. Voriconazole achieves 40% of serum concentrations in the vitreous [14] and has been used successfully in Candida endophthalmitis [15]. Amphotericin B does not achieve adequate concentration in the posterior chamber of the eye, but ocular penetration is enhanced by inflammation [16].
Isavuconazole has been shown to be noninferior to voriconazole for primary treatment of invasive mould disease [17] but did not prove itself noninferior to caspofungin in invasive candidiasis [18]. Data on the ocular penetration of isavuconazole are limited. Isavuconazole demonstrated clinical activity in a retrospective analysis of 36 patients with invasive fungal infection (Mucorales 30.6%, Aspergillus species 22.2%) with central nervous system involvement [19]. Animal models have demonstrated good drug levels in the brain and uveal tract [20]. Compared with voriconazole, isavuconazole is associated with a lower risk of liver dysfunction [17] and shortens QT interval [21]. Because our patient developed nephrotoxicity to amphotericin B and had prolonged QT interval, isavuconazole was considered. Because there was lack of clinical data for isavuconazole use in endophthalmitis, patient and family were counseled on the risk-benefit ratio.
There are a few possible reasons why a switch to isavuconazole led to improvement when fluconazole was unsuccessful. First, although C dubliniensis grew in the vitreous cultures and was tested to be wild type to fluconazole, there was concern for an occult coinfection by C tropicalis because this latter organism had also been found in blood cultures from several months prior. This prompted the switch to amphotericin B and then isavuconazole to which both the C dubliniensis and C tropicalis were susceptible. Second, although there is no Clinical and Laboratory Standards Institute breakpoint or epidemiological cutoff value for C dubliniensis to isavuconazole, given the low isavuconazole MIC of 0.023 µg/mL, we postulated that an effective intravitreal concentration of isavuconazole could have been more readily achieved compared with fluconazole (MIC 0.25 µg/mL). Antifungal therapeutic drug monitoring was not conducted and thus adequate fluconazole levels could not be ascertained. Third, because the endophthalmitis was already advanced by the time she presented, a longer-than-conventional course of systemic antifungal treatment may be required before treatment response can be observed. Nonetheless, a switch to isavuconazole did not lead to worsening of the endophthalmitis but was instead associated with improvement in the left eye.
In the treatment of patients with endophthalmitis caused by Candida species that are SDD or resistant to fluconazole, isavuconazole seems to be able to overcome the blood-ocular barrier, and it may be considered when higher doses of fluconazole or voriconazole cannot be tolerated. In patients who have significant prior azole exposure, susceptibility testing before the switch to isavuconazole should be performed due to risk of cross-resistance [22].
CONCLUSIONS
Candida endophthalmitis may occur even after 2 negative ophthalmological examinations and become symptomatic 2 months after the episode of candidemia. There should be a high index of suspicion for Candida endophthalmitis in patients who present with visual symptoms after recent candidemia. Source control with vitrectomy, in combination with systemic and intravitreal antifungal treatment, is crucial to achieve treatment success. When first-line treatment is contraindicated, isavuconazole may be an option for Candida endophthalmitis.
Acknowledgments
Author contributions. We declare that all authors have seen and approved the manuscript and contributed significantly to the work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Patient Consent. In accordance with Singhealth Centralised Institutional Review Board, informed consent was waived for publication of this case report.
References
- 1. Oude Lashof AM, Rothova A, Sobel JD, et al. . Ocular manifestations of candidemia. Clin Infect Dis 2011; 53:262–8. [DOI] [PubMed] [Google Scholar]
- 2. Breazzano MP, Day HR Jr, Bloch KC, et al. . Utility of ophthalmologic screening for patients with Candida bloodstream infections: a systematic review. JAMA Ophthalmol 2019; 137:698–710. [DOI] [PubMed] [Google Scholar]
- 3. Seidelman J, Fleece M, Bloom A, et al. . Endogenous Candida endophthalmitis: who is really at risk? J Infect 2021; 82:276–81. [DOI] [PubMed] [Google Scholar]
- 4. Lingappan A, Wykoff CC, Albini TA, et al. . Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012; 153:162–6.e1. [DOI] [PubMed] [Google Scholar]
- 5. Sallam A, Taylor SR, Khan A, et al. . Factors determining visual outcome in endogenous Candida endophthalmitis. Retina 2012; 32:1129–34. [DOI] [PubMed] [Google Scholar]
- 6. Pappas PG, Kauffman CA, Andes DR, et al. . Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breazzano MP, et al. . American Academy of Ophthalmology recommendations on screening for endogenous Candida endophthalmitis. Ophthalmology 2021. doi: 10.1016/j.ophtha.2021.07.015. [DOI] [PubMed] [Google Scholar]
- 8. Muñoz P, Vena A, Padilla B, et al. ; CANDIPOP Project, GEIH-GEMICOMED (SEIMC), and REIPI. No evidence of increased ocular involvement in candidemic patients initially treated with echinocandins. Diagn Microbiol Infect Dis 2017; 88:141–4. [DOI] [PubMed] [Google Scholar]
- 9. Felton T, Troke PF, Hope WW.. Tissue penetration of antifungal agents. Clin Microbiol Rev 2014; 27:68–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gauthier GM, Nork TM, Prince R, Andes D.. Subtherapeutic ocular penetration of caspofungin and associated treatment failure in Candida albicans endophthalmitis. Clin Infect Dis 2005; 41:e27–8. [DOI] [PubMed] [Google Scholar]
- 11. Sarria JC, Bradley JC, Habash R, et al. . Candida glabrata endophthalmitis treated successfully with caspofungin. Clin Infect Dis 2005; 40:e46–8. [DOI] [PubMed] [Google Scholar]
- 12. Riddell J 4th, Comer GM, Kauffman CA.. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin Infect Dis 2011; 52:648–53. [DOI] [PubMed] [Google Scholar]
- 13. Tod M, Lortholary O, Padoin And C, Chaine G.. Intravitreous penetration of fluconazole during endophthalmitis. Clin Microbiol Infect 1997; 3:379A. [DOI] [PubMed] [Google Scholar]
- 14. Hariprasad SM, Mieler WF, Holz ER, et al. . Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol 2004; 122:42–7. [DOI] [PubMed] [Google Scholar]
- 15. Hariprasad SM, Mieler WF, Lin TK, et al. . Voriconazole in the treatment of fungal eye infections: a review of current literature. Br J Ophthalmol 2008; 92:871–8. [DOI] [PubMed] [Google Scholar]
- 16. Goldblum D, Rohrer K, Frueh BE, et al. . Ocular distribution of intravenously administered lipid formulations of amphotericin B in a rabbit model. Antimicrob Agents Chemother 2002; 46:3719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maertens JA, Raad II, Marr KA, et al. . Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016; 387:760–9. [DOI] [PubMed] [Google Scholar]
- 18. Kullberg BJ, Viscoli C, Pappas PG, et al. . Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: The ACTIVE Trial. Clin Infect Dis 2019; 68:1981–9. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz S, Cornely OA, Hamed K, et al. . Isavuconazole for the treatment of patients with invasive fungal diseases involving the central nervous system. Med Mycol 2020; 58:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmitt-Hoffmann A-H, et al. . Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother 2017; 61:e01292-17. doi: 10.1128/AAC.01292-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mellinghoff SC, Bassetti M, Dörfel D, et al. . Isavuconazole shortens the QTc interval. Mycoses 2018; 61:256–60. [DOI] [PubMed] [Google Scholar]
- 22. Ellsworth M, Ostrosky-Zeichner L.. Isavuconazole: mechanism of action, clinical efficacy, and resistance. J Fungi (Basel) 2020; 6:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sensititre YeastOne Susceptibility Plates [product information] [029-Yeast-ROW IVD CID10305]. Waltham, MA: Thermo Scientific; 2019. [Google Scholar]
- 24. MIC Test Strip Technical Sheet Yeast [product information] [MTS24, Rev. 5.2]. Abruzzi TE, Italy: Liofilchem; 2016. [Google Scholar]
- 25. CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts. CLSI document M60. Wayne, PA; Clinical and Laboratory Standards Institute; 2020. [Google Scholar]
- 26. CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing. CLSI document M59. Wayne, PA; Clinical and Laboratory Standards Institute; 2020. [Google Scholar]