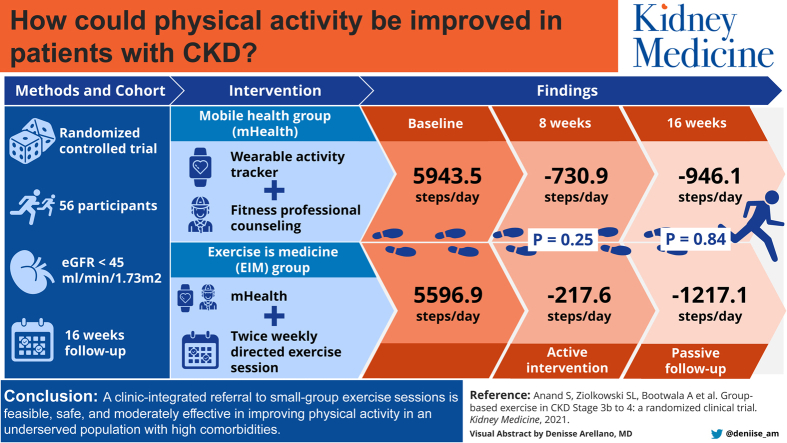
Abstract
Rationale & Objective
We aimed to test interventions to improve physical activity in persons with advanced chronic kidney disease not yet receiving dialysis.
Study Design
Randomized controlled trial with parallel-group design.
Setting & Participants
We embedded a pragmatic referral to exercise programming in high-volume kidney clinics servicing diverse populations in San Jose, CA, and Atlanta, GA. We recruited 56 participants with estimated glomerular filtration rates < 45 mL/min/1.73 m2.
Interventions
We randomly assigned participants to a mobile health (mHealth) group—wearable activity trackers and fitness professional counseling, or an Exercise is Medicine intervention framework (EIM) group—mHealth components plus twice-weekly small-group directed exercise sessions customized to persons with kidney disease. We performed assessments at baseline, 8 weeks at the end of active intervention, and 16 weeks after passive follow-up and used multilevel mixed models to assess between-group differences.
Outcomes
Activity tracker total daily step count.
Results
Of 56 participants, 86% belonged to a racial/ethnic minority group; randomly assigned groups were well balanced on baseline step count. In intention-to-treat analyses, the EIM and mHealth groups both experienced declines in daily step counts, but there was an attenuated reduction in light intensity physical activity (standard error 0.2 [5.8] vs −8.5 [5.4] min/d; P = 0.08) in the EIM compared with the mHealth group at 8 weeks. In as-treated analyses, total daily step count, distance covered, and light and moderate-vigorous activity minutes per day improved in the EIM group and declined in the mHealth group at 8 weeks (standard error +335 [506] vs −884 [340] steps per day; P = 0.05; P < 0.05 for secondary measures), but group differences faded at 16 weeks. There were no differences in quality-of-life and mental health measures during the study.
Limitations
Small sample size, limited duration of study, assessment of intermediate outcomes (steps per day).
Conclusions
A clinic-integrated referral to small-group exercise sessions is feasible, safe, and moderately effective in improving physical activity in an underserved population with high comorbid conditions.
Funding
Normon S Coplon Applied Pragmatic Clinical Research program.
Trial Registration
Index Words: Chronic kidney disease, exercise physical activity, mobile health, underserved populations
Graphical abstract
Plain-Language Summary.
Exercise is associated with longer life spans in patients with advanced kidney disease, but minimal programming exists to support patients even as they are dealing with complex medical transitions to dialysis and often experiencing loss in physical function in the process. We wanted to create a sustainable academic-community partnership in clinics servicing diverse populations to provide technology-enabled group exercise programming for patients with advanced kidney disease. Despite challenges in recruitment, we were able to create such an infrastructure, and among the people who engaged with the customized programming, we saw improvements in physical activity as measured by step counts. Implementing research projects such as ours will be key to increasing the reach and scale of much-needed exercise programming for patients with kidney disease.
Physical activity is associated with improved cardiovascular health, physical function, quality of life, transplant outcomes, and survival in persons with chronic kidney disease (CKD).1, 2, 3, 4, 5, 6 However, at the time of dialysis initiation, 44% cannot walk 1 block and 56% cannot climb 12 stairs.7 Further, after starting dialysis, only 13% of elderly persons maintain functional status in the first year.8
Exercise interventions in persons with CKD have been primarily limited to the subpopulation who are receiving dialysis. In a meta-analysis of 41 exercise intervention trials targeted to patients with kidney disease, 6 enrolled patients with CKD not yet receiving dialysis.9 An intervention at earlier stages of CKD could mitigate the near-doubling of risk for mortality seen in the first 120 days of initiation10, 11, 12 and engage patients before frequent medical interactions with centers and hospitals.13
Given the dearth of studies in patients with advanced CKD, the type of programming most effective and acceptable to this population—and thus amenable to wider implementation at scale—is unclear. Group-based exercise training has a track record in multiple chronic conditions.14,15 In the most prominent and now widely scaled example, lifestyle coaching paired with exercise sessions in the Diabetes Prevention Program reduced diabetes incidence by 58% in high-risk persons.16 Wearable technology-enabled interventions are also gaining prominence; 1 systematic review of 28 studies noted a 24% increase in daily step count and 27% increase in moderate to vigorous physical activity with wearable activity tracker–related interventions.17 However, a study of persons with peripheral vascular disease investigating the effect of telephone coaching and wearable technology found no improvement over 9 months compared with usual care, suggesting the importance of additional on-site interventions.18
We sought to test the feasibility, acceptability, and effectiveness of integrating, through clinical referral, a technology-enabled group-based exercise program in persons with advanced CKD not yet receiving dialysis. Working in 2 diverse clinical populations and using the American College of Sports Medicine Exercise Is Medicine (EIM) intervention framework,19,20 we randomly assigned persons to a wearable activity tracker and physical activity counseling (mobile health [mHealth] group) versus the more intensive intervention of mHealth augmented by in-person small-group training sessions led by an EIM fitness professional (EIM group). In addition to assessing recruitment, adherence, and safety, we evaluated the preliminary effectiveness of the interventions on: (1) objectively measured steps (through wearable activity tracker), (2) physical function measures (6-minute walk test and handgrip strength), and (3) self-reported mental health.
Methods
The study protocol is published21 and registered on clinicaltrials.gov (NCT03311763). Briefly, we recruited participants from 2 high-volume nephrology clinics at Emory University (Atlanta, GA; 1,200 patient encounters per month) and Stanford University/Santa Clara Valley Medical Center (San Jose, CA; 950 patient encounters per month). The 2 study sites serve largely minority populations (African American in Atlanta and Asian and Hispanic in San Jose). Clinics administered a screening questionnaire to assess patients’ interest in improving physical activity in the waiting room, and thereafter, study research coordinators assessed eligibility based on prespecified inclusion/exclusion criteria (Table S1). The study received institutional review board (IRB) approval from all participating centers (Stanford IRB approval number: 43198) and Emory University (IRB number: IRB00099894).
Upon obtaining informed consent, we used a random number generator to a priori assign treatment group to 28 participant identification numbers at each site. We assigned participant identification numbers sequentially in the order by which study coordinators obtained consent. After we obtained consent, we first completed baseline assessment and then disclosed the assigned treatment to the participant.
Intervention
At baseline, we provided all participants with the wearable Garmin Vivofit 3 activity tracker, a tutorial on device use, and a free custom smartphone application for data syncing (IOS or Android). The application, developed as part of the EIM framework, allows for participants to track their step count and enables the study staff to remotely monitor participants’ physical activity.
Trained fitness professionals counseled participants randomly assigned to the mHealth group in a 30-minute face-to-face session.22 The counseling session used brief-action planning, a self-management support technique grounded in motivational interviewing and behavior change strategies (goal setting, identify preferences and barriers, problem solving, stages of change, and self-monitoring).23 Fitness professionals encouraged integration of moderate physical activity throughout the week to achieve 100 to 150 minutes per week, primarily through walking for leisure and transportation or other preferred activities. The fitness professional performed weekly short (5-minute) interactions by telephone or text messaging to support, check on progress, and answer questions as needed for the 8-week intervention period.
Participants randomly assigned to the EIM group underwent the same counseling sessions and additionally were enrolled in an 8-week exercise program with twice-weekly 1-hour sessions led by the EIM fitness professionals and offered at community centers or facility grounds available near (<5 km) the nephrology clinic. Sessions were held for up to 8 participants and included a progressive training plan including aerobic conditioning, resistance, flexibility, stability, and balance training, slowly progressing toward 50 minutes of light to moderate physical activity per session. Blood pressure cuffs and glucometers were available for participants who experienced symptoms related to hypotension or hypoglycemia before, during, or after the class. After 8 weeks of intervention, the group-based exercise sessions were discontinued and participants were encouraged to accumulate moderate physical activity throughout the week on their own during the 8-week passive follow-up period.
Study Measures
We undertook study assessments at 3 time points: baseline, 8 weeks (at the end of exercise sessions for the EIM group), and 16 weeks. We obtained the following questionnaires at each study visit: Center for Epidemiological Studies-Depression questionnaire,24 12-Item Short-Form Health Survey,25 Exercise Confidence Survey,26,27 Brief Resilience Scale,28 and International Physical Activity Questionnaire.29 We additionally obtained body composition and functional fitness at each study visit using a standardized protocol30; height, weight, Quételet (body mass) index, waist circumference, blood pressure at rest, 6-minute walk test,31 and grip strength with a digital dynamometer (Takei 5401; Takei Scientific Instruments, Inc).
We also obtained an exit survey, and for participants in the EIM group, we additionally documented hospitalizations and session-related adverse events. The wearable activity tracker captured daily step count (primary outcome), distance traveled, and minutes of light and moderate to vigorous physical activity per day. This device was highly ranked in terms of validity, behavior change features, and data integration feasibility in a recent review of consumer-oriented wearable activity measurement devices for use in health care settings.32,33 Furthermore, that this device has a 1-year battery life and is waterproof greatly enhances the likelihood of adequate wear time (see Item S1 for details on wearable activity tracker assessments).
Statistical Analysis
To compare the distribution of baseline characteristics after randomization to the mHealth and EIM groups, we used χ2 test, Fisher exact test, 2-sample t test, and Wilcoxon rank sum test, as appropriate. We used multilevel mixed models with the restricted maximum likelihood approach to analyze the outcomes measured at 8 and 16 weeks and handle missing data. For each outcome measure, the model accounted for the intervention, time since intervention (baseline, 8 weeks, and 16 weeks), study site, and intervention-by-time interaction. The intervention-by-time interaction, which reflects the relative difference in change in the parameters over time, was the primary parameter for testing of difference between groups. We modeled dependent variables using fixed effects and incorporated individual level as the random effects to account for the correlation of outcomes measured over time. We report adjusted means and standard errors for each outcome. We conducted intention-to-treat and as-treated analyses, for which the "as-treated" EIM group was defined as participants attending at least 1 EIM session, and participants who did not attend any EIM sessions were assigned to the mHealth group.
Analyses were conducted using SAS, version 9.4 (SAS Institute). Results presented fulfill the Consolidated Standards of Reporting Trials (CONSORT) guidelines for randomized-controlled trials.34
Results
Participant Enrollment and Characteristics
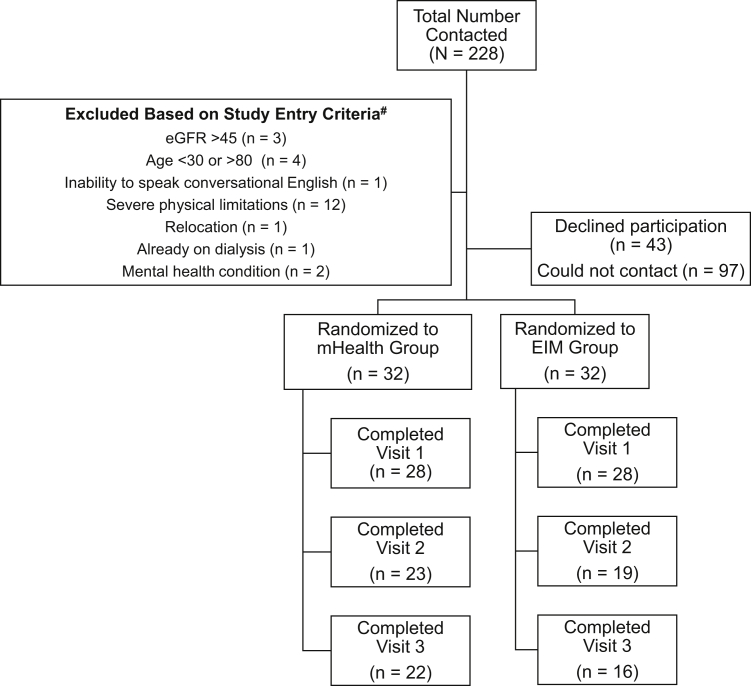
Figure 1 outlines participant enrollment; 56 participants enrolled in the study (28 at Stanford and 28 at Emory). Table 1 demonstrates participant characteristics by study group, which did not differ by age, sex, race, ethnicity, smoking status, comorbid conditions, cause of CKD, and baseline daily step total. Most participants (86%) belonged to a racial or ethnic minority group and 30% had diabetes as cause of CKD. Randomization was well balanced by characteristics listed in Table 1. The groups were well balanced at baseline by physical function measures of the 6-minute walk test and handgrip strength (Table S2).
Figure 1.
Study participant enrollment flowchart. Abbreviations: eGFR, estimated glomerular filtration rate; EIM, Exercise Is Medicine; mHealth, mobile health.
Table 1.
Trial Participant Characteristics at Baseline
| Intervention (N = 28) | Control (N = 28) | |
|---|---|---|
| Age, y | 56.2 (12.3) | 58.1 (9.9) |
| Female sex | 16 (57.1%) | 15 (53.6%) |
| Hispanic | 4 (14.3%) | 5 (17.9%) |
| Race | ||
| Asian | 5 (17.9%) | 5 (17.9%) |
| Black | 14 (50.0%) | 13 (46.4%) |
| White | 4 (14.3%) | 4 (14.3%) |
| Other | 5 (17.9%) | 6 (21.4%) |
| Smoking status | ||
| Current smoker | 2 (7.1%) | 0 (0.0%) |
| Never smoker | 15 (53.6%) | 13 (46.4%) |
| Smoked in past | 11 (39.3%) | 15 (53.6%) |
| Cause of chronic kidney disease | ||
| Diabetes | 10 (35.7%) | 7 (25.0%) |
| Glomerulonephritis | 2 (7.1%) | 6 (21.4%) |
| Hypertension or vascular disease | 6 (21.4%) | 5 (17.9%) |
| Other/unknown | 10 (35.7%) | 10 (35.7%) |
| Diabetes | 15 (53.6%) | 14 (50.0%) |
| Congestive heart failure | 4 (14.3%) | 5 (17.9%) |
| Cancer | 3 (10.7%) | 3 (10.7%) |
| Cardiovascular disease | 8 (28.6%) | 4 (14.3%) |
| Referred to dialysis education | 8 (28.6%) | 7 (25.0%) |
| Referred for dialysis access placement | 3 (10.7%) | 5 (17.9%) |
| Dialysis access placed | 2 (7.1%) | 4 (14.3%) |
| No. of home medications | 11.2 (4.9) | 10.4 (4.4) |
| eGFR, mL/min/1.73 m2 | 30.4 (9.8) | 31.1 (12.3) |
Note: Data presented as mean (standard deviation) and number (percent)
Abbreviation: eGFR, estimated glomerular filtration rate.
Feasibility Assessment: Adherence and Safety
A larger number of participants in the EIM group (16 [57%]) compared with the mHealth group (7 [25%]) missed at least 1 study assessment session. Of the 16 EIM group participants missing a measurement, 11 (69%) also did not come to any exercise sessions. Adherence to wearable activity tracker use was similar in both groups: 3 participants (11%) in the EIM and 4 (14%) in the mHealth group did not log any wearable activity tracker data.
Table 2 compares participants who did and did not attend offered sessions. Participants who did not attend exercise sessions (N = 11 [39%]) were more likely to have been hospitalized during the study (46% vs 24% of participants, respectively). Among participants who attended at least 1 session, median for attendance was 10 (25th percentile, 7; 75th percentile, 12) sessions. During a single exercise session, 1 participant had elevated blood pressure at the time of arrival, which precluded their participation in that session. No other study-related adverse events were recorded.
Table 2.
Comparison of Persons Randomly Assigned to EIM Group on Attendance to Group Exercise Classes
| Attended Exercise Session (N = 17) | Did Not Attend Exercise Session (N = 11) | |
|---|---|---|
| Age, y | 59 (53, 61) | 64 (48, 70) |
| Female sex | 9 (52.9%) | 7 (63.6%) |
| Hispanic | 3 (17.7%) | 1 (9.1%) |
| Race | ||
| Asian | 3 (17.7%) | 2 (18.2%) |
| Black | 8 (47.1%) | 6 (54.6%) |
| White | 2 (11.8%) | 2 (18.2%) |
| Other | 4 (23.5%) | 1 (9.1%) |
| No. of comorbid conditions | 1 (1, 1) | 1 (0, 2) |
| No. of medications | 11 (8, 14) | 12 (5, 16) |
| Hospitalized during study | 4 (23.5%) | 5 (45.5%) |
| Initiated dialysis during study | 2 (11.8%) | 1 (9.1%) |
| Mean eGFR, mL/min/1.73 m2 | 30.6 (7.9) | 30.1 (12.7) |
| Exercise confidence: sticking to it | 4.2 (3.7, 4.9) | 4.8 (3.8, 5.0) |
| Exercise confidence: making time for exercise | 4.5 (4.0, 5.0) | 4.8 (3.8, 5.0) |
| SF-12 Physical Component Summary score | 40.9 (37.7, 45.4) | 42.2 (38.0, 43.4) |
| SF-12 Mental Component Summary score | 47.6 (38.1, 50.2) | 47.7 (43.2, 52.6) |
| Distance from exercise session, miles | 11 (6, 19) | 13 (7, 21) |
Note: Data presented as median (25th, 75th percentile), or N (%) due to small sample sizes, unless otherwise noted to be mean (SD).
Abbreviations: eGFR, estimated glomerular filtration rate; EIM, Exercise Is Medicine; SF-12, 12-Item Short-Form Health Survey.
Effectiveness Assessment: Physical Activity
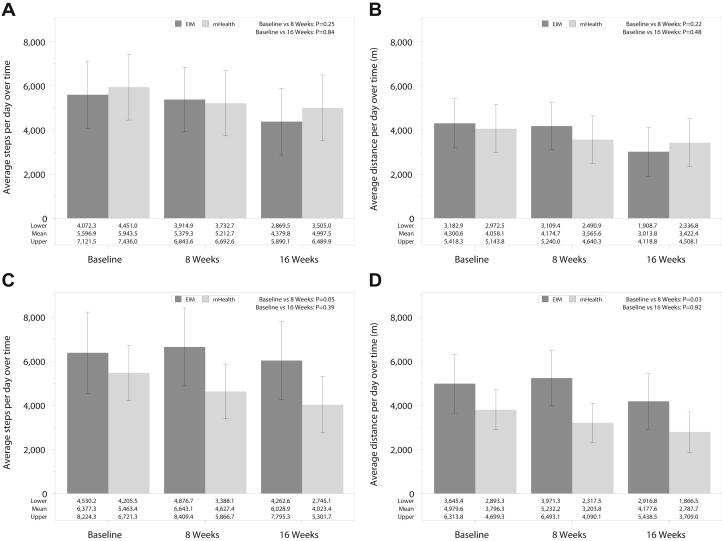
In the intention-to-treat analyses, adjusted mean step counts and distance traveled decreased in both the EIM group and mHealth group at 8 and 16 weeks, without differences in mean change in step count in the groups over time (Standard Error [SE] −217.6 [610.4] vs −730.9 [568.1] steps per day; P = 0.25; and −1,217.1 [624.4] vs −946.1 [576.9] steps per day; P = 0.84) comparing 8 and 16 weeks with baseline in the EIM versus mHealth groups, respectively (Fig 2A and B). In as-treated analyses, the EIM group experienced an improvement at 8 weeks and had a smaller decrease at 16 weeks compared with the mHealth group, which continued to decline over time (SE +334.6 [506.0] vs −883.8 [339.6] steps per day; P = 0.05, and +381.8 [923.7] vs −1,362.9 [644.3] steps per day; P = 0.39, comparing 8 and 16 weeks with baseline in the EIM vs mHealth groups, respectively). A similar pattern emerged for distance traveled (Fig 2C and D).
Figure 2.
Overall physical activity assessment over time in the intervention.∗ (A) Adjusted means for step counts per day over time in intention-to-treat analyses (N = 28 for Exercise Is Medicine [EIM] and N = 28 for mobile health [mHealth]). Differences in EIM versus mHealth as follows: −217.6 (standard errors [SE] 610.4) versus −730.9 (SE 568.1) steps comparing 8 weeks with baseline, P = 0.25, and −1,217.1 (SE 624.4) versus −946.1 [SE 576.9] steps, P = 0.84, comparing 16 weeks to baseline. (B) Adjusted means for distance (meters) per day over time in intention-to-treat analyses. Differences in EIM versus mHealth as follows: −126.0 (SE 490.5) versus −492.6 (SE 457.8) m comparing 8 weeks with baseline, P = 0.22, and −1,286.9 (SE 502.2) versus −635.7 (SE 464.7) m, P = 0.48 comparing 16 weeks with baseline. (C) Adjusted means for step counts per day over time in as-treated analyses (N = 16 for EIM and N = 30 for mHealth). Differences in EIM versus mHealth as follows: +334.6 (SE 506.0) versus −883.8 (SE 339.6) steps, P = 0.05 comparing 8 weeks with baseline, and +381.8 (SE 923.7) versus −1,362.9 (SE 644.3) steps, P = 0.39, comparing 16 weeks with baseline. (D) Adjusted means for distance (meters) per day over time in in as-treated analyses. Differences in EIM versus mHealth as follows: +374.5 (SE 358.6) versus −619.6 (SE 240.7) m, P = 0.03, comparing 8 weeks with baseline, and −865.5 (SE 734.4) versus 957.8 (SE 513.3) m, P = 0.92, comparing 16 weeks with baseline. ∗In the intention-to-treat analysis, sample sizes were 20, 24, and 21 in the EIM group and 23, 24, and 23 in the mHealth group at the baseline, 8-week, and 16-week assessments. In the as-treated analysis, sample sizes were 13, 16, and 16 in the EIM group and 30, 32, and 28 in the mHealth group at the baseline, 8-week, and 16-week assessments, respectively.
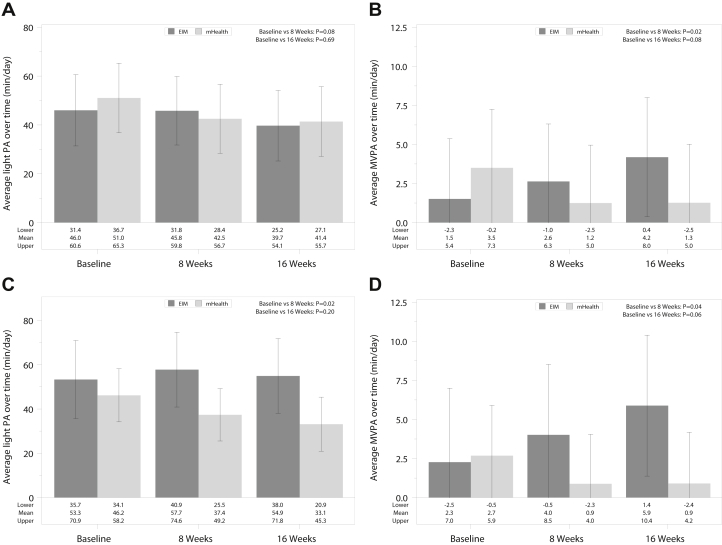
For intensity of physical activity, in the intention-to-treat analyses, light physical activity levels declined over time in both groups, but to a lesser degree in the EIM group at 8 weeks (Fig 3; 0.2 [5.8] vs −8.5 [5.4]; P = 0.08, and ≤6.3 [6.0] vs ≤9.7 [5.5] minutes per day; P = 0.69 comparing 8 and 16 weeks with baseline in the EIM vs mHealth groups, respectively). In the as-treated analyses, mirroring the step count data, light physical activity levels improved at 8 weeks and had a smaller decrease at 16 weeks in the EIM compared with the mHealth group, which experienced a continuous decline (4.5 [7.0] vs ≤8.8 [4.7]; P = 0.02, and 1.6 [7.0] vs ≤13.0 [4.9] minutes per day; P = 0.20 comparing 8 and 16 weeks to baseline in the EIM vs mHealth groups, respectively). Moderate to vigorous physical activity levels improved slightly in the EIM group compared with the mHealth group both at 8 and 16 weeks in the intention-to-treat and as-treated analyses.
Figure 3.
Intensity of physical activity assessment over time in the intervention.∗ (A) Minutes of light physical activity (PA) per day over time in intention-to-treat analyses (N = 28 for Exercise Is Medicine [EIM] and N = 28 for mobile health [mHealth]). Differences in EIM versus mHealth as follows: −0.2 (standard errors [SE] 5.8) versus −8.5 (SE 5.4) minutes per day comparing 8 weeks with baseline, P = 0.08, and −6.3 (SE 6.0) versus −9.7 (SE 5.5) minutes per day, P = 0.69, comparing 16 weeks with baseline. (B) Minutes of moderate to vigorous PA (MVPA) per day over time in intention-to-treat analyses. Differences in EIM versus mHealth as follows: 1.1 (SE 1.6) versus −2.3 (SE 1.5) minutes per day comparing 8 weeks with baseline, P = 0.02, and 2.7 (SE 1.7) versus -2.2 (SE 1.6) minutes per day, P = 0.08 comparing 16 weeks with baseline. (C) Minutes of light PA per day over time in as-treated analyses (N = 16 for EIM and N = 30 for mHealth). Differences in EIM versus mHealth as follows: 4.5 (SE 7.0) versus −8.8 (SE 4.7) minutes per day, P = 0.02, comparing 8 weeks with baseline, and 1.6 (SE 7.0) versus −13.0 (SE 4.9) minutes per day, P = 0.20, comparing 16 weeks with baseline. (D) Minutes of MVPA per day over time in as-treated analyses. Differences in EIM versus mHealth: 1.7 (SE 2.0) versus −1.8 (SE 1.4) minutes per day comparing 8 weeks with baseline, P = 0.04, and 3.6 (SE 2.0) versus −1.8 (SE 1.4) minutes per day, P = 0.06, comparing 16 weeks with baseline. ∗In the intention-to-treat analysis, sample sizes were 20, 24, and 21 in the EIM group and 23, 24, and 23 in the mHealth group at the baseline, 8-week, and 16-week assessments, respectively. In the as-treated analysis, sample sizes were 13, 16, and 16 in the EIM group and 30, 32, and 28 in the mHealth group at the baseline, 8-week, and 16-week assessments, respectively.
Effectiveness Assessment: Physical Function Measures, Blood Pressure, and Anthropometrics
Table S2 delineates physical function, blood pressure, and anthropometric measurements in the 2 groups. Mean overall results for all study participants at baseline are as follows: 405.8 (standard deviation [SD], 117.4) m in the 6-minute walk test, 25.4 (SD, 11.7) kg handgrip strength, 41.4 (SD, 5.8) Physical Component Summary scores on the 12-Item Short-Form Health Survey, 37.5 (SD, 64.7) minutes per day in moderate and vigorous physical activity, 17.2 (SD, 20.3) minutes per day in walking time, and 6.9 (SD, 3.4) hours per day in sedentary time measured by International Physical Activity Questionnaire. In as-treated analyses, systolic blood pressure decreased modestly in the EIM compared with the mHealth group at 8 weeks (P = 0.06). None of these assessments differed by group at baseline or 8 or 16 weeks.
Effectiveness Assessment: Self-reported Mental Health and Exercise Self-Efficacy
Table 3 delineates the results of mental health and exercise self-efficacy questionnaires of study participants in the 2 study groups. Similar to physical function measures, the measures did not differ by group at baseline or over the study period.
Table 3.
Mental Health Measures of Study Participants During the 16-Week Study Period
| Intention to Treat |
As-Treated |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EIM |
mHealth |
Mean Diff | 95% CI | EIM |
mHealth |
Mean Diff | 95% CI | |||||
| N | Adjusted Mean (SEM) | N | Adjusted Mean (SEM) | N | Adjusted Mean (SEM) | N | Adjusted Mean (SEM) | |||||
| CES-D | ||||||||||||
| Baseline | 28 | 14.1 (1.9) | 28 | 11.8 (1.9) | 2.3 | −3.1 to 7.8 | 17 | 16.2 (2.4) | 39 | 11.5 (1.6) | 4.7 | −1.1 to 10.4 |
| 8 wk | 17 | 12.3 (2.2) | 24 | 12.7 (2.0) | −0.4 | −6.4 to 5.5 | 15 | 13.9 (2.6) | 26 | 12.4 (1.7) | 1.5 | −4.7 to 7.7 |
| 16 wk | 12 | 15.9 (2.8) | 21 | 12.6 (2.1) | 3.3 | −3.6 to 10.3 | 10 | 16.8 (3.1) | 23 | 12.6 (1.9) | 4.2 | −3.1 to 11.6 |
| Brief Resilience Scale | ||||||||||||
| Baseline | 28 | 3.7 (0.2) | 28 | 3.6 (0.2) | 0.1 | −0.3 to 0.6 | 17 | 3.6 (0.2) | 39 | 3.7 (0.1) | −0.1 | −0.6 to 0.4 |
| 8 wk | 17 | 3.7 (0.2) | 24 | 3.5 (0.2) | 0.2 | −0.3 to 0.6 | 15 | 3.5 (0.2) | 26 | 3.6 (0.1) | −0.1 | −0.6 to 0.4 |
| 16 wk | 12 | 3.9 (0.2) | 21 | 3.5 (0.2) | 0.4 | −0.1 to 0.9 | 10 | 3.8 (0.2) | 23 | 3.6 (0.2) | 0.1 | −0.5 to 0.7 |
| Exercise Confidence Survey | ||||||||||||
| Sticking to it | ||||||||||||
| Baseline | 28 | 4.2 (0.2) | 28 | 4.1 (0.2) | 0.1 | −0.4 to 0.6 | 17 | 4.1 (0.2) | 39 | 4.2 (0.2) | −0.1 | −0.6 to 0.4 |
| 8 wk | 17 | 3.9 (0.2) | 24 | 3.7 (0.2) | 0.2 | −0.3 to 0.8 | 15 | 3.9 (0.2) | 26 | 3.7 (0.2) | 0.1 | −0.4 to 0.7 |
| 16 wk | 12 | 3.9 (0.3) | 21 | 3.6 (0.2) | 0.3 | −0.4 to 0.9 | 10 | 4.1 (0.3) | 23 | 3.6 (0.2) | 0.5 | −0.2 to 1.2 |
| Making time for exercise | ||||||||||||
| Baseline | 28 | 4.3 (0.2) | 28 | 4.0 (0.2) | 0.2 | −0.3 to 0.8 | 17 | 4.3 (0.2) | 39 | 4.1 (0.2) | 0.2 | −0.4 to 0.7 |
| 8 wk | 17 | 3.9 (0.2) | 24 | 3.9 (0.2) | 0 | −0.6 to 0.6 | 15 | 3.9 (0.2) | 26 | 4.0 (0.2) | 0 | −0.6 to 0.6 |
| 16 wk | 12 | 4.0 (0.3) | 21 | 3.7 (0.2) | 0.3 | −0.4 to 0.9 | 10 | 4.3 (0.3) | 23 | 3.7 (0.2) | 0.6 | −0.1 to 1.3 |
| SF-12 | ||||||||||||
| Physical Component Summary score | ||||||||||||
| Baseline | 28 | 41.3 (1.1) | 28 | 41.2 (1.2) | 0.2 | −3.1 to 3.4 | 17 | 41.3 (1.4) | 39 | 41.2 (1.0) | 0.1 | −3.3 to 3.6 |
| 8 wk | 17 | 42.5 (1.3) | 24 | 39.3 (1.3) | 3.2 | −0.5 to 6.8 | 15 | 42.6 (1.5) | 26 | 39.5 (1.2) | 3.1 | −0.7 to 6.9 |
| 16 wk | 12 | 40.9 (1.5) | 21 | 39.4 (1.3) | 1.5 | −2.5 to 5.6 | 10 | 40.8 (1.7) | 23 | 39.6 (1.2) | 1.2 | −3.0 to 5.4 |
| Mental Component Summary score | ||||||||||||
| Baseline | 28 | 45.8 (1.6) | 28 | 47.1 (1.7) | −1.2 | −6.0 to 3.5 | 17 | 44.6 (2.0) | 39 | 47.4 (1.4) | −2.8 | −7.8 to 2.2 |
| 8 wk | 17 | 46.1 (2.0) | 24 | 45.8 (1.8) | 0.3 | −5.1 to 5.7 | 15 | 45.7 (2.1) | 26 | 45.9 (1.7) | −0.2 | −5.8 to 5.3 |
| 16 wk | 12 | 42.2 (2.3) | 21 | 45.6 (1.9) | −3.3 | −9.4 to 2.7 | 10 | 43.2 (2.6) | 23 | 44.6 (1.8) | −1.4 | −7.7 to 4.8 |
Note: SF-12 mental health component scores were lower than the mean for individuals without serious mental or physical at baseline and throughout the course of the study conditions (Ware et al, mean ± SD Physical Component Summary scores: 47.42 ± 0.4; Mental Component scores: 53.82 ± 0.3).25 CES-D scores for both study groups were on average <15, suggesting subclinical or absence of depression.24 The overall mean Brief Resilience Scale score for study participants throughout the study is scored as “normal resilience” (3.00-4.30).28 The Exercise Confidence Survey is scored from 0 to 5, with higher scores indicating higher confidence.27
Abbreviations: CES-D, Center for Epidemiological Studies-Depression; EIM, Exercise Is Medicine; mHealth, mobile health; SEM, standard error of mean; SF-12, 12-Item Short-Form Health Survey.
Intervention Feedback
A total of 26 participants returned exit surveys on the components of the program. All agreed that fitness professionals communicated simply and empathetically; 1 participant found the wearable activity tracker difficult to use and unhelpful. Most (92%) found the consent and recruitment to be well integrated within the clinical setting. Although 81% of participants wished for more engagement from their clinical team in exercise intervention, 42% reported that their clinical team commented on participation during visits. Dieticians were the most common recommendation to be added to the next iteration of the program. Participants who came to more than 1 class listed anticipated or experienced improvement in other areas on life, overall health and well-being, blood glucose control, and mental health as motivators for continued participation. Item S2 provides videos of patient testimonials from 2 EIM group participants.
Discussion
We successfully integrated recruitment, physical activity assessment, and group-based exercise interventions into diverse clinical settings servicing largely patients of racial and ethnic minority descent with advanced CKD. At baseline, participants were generally inactive with poor aerobic capacity and strength. The intervention was safe, and retention in group programming was high among patients who came to at least 1 session. We observed modest improvements or an attenuated reduction in physical activity levels when fitness counseling and wearable device provision were coupled with CKD-customized small-group exercise sessions in persons who participated in at least 1 session, compared with participants receiving counseling and wearable activity trackers alone.
We delivered our intervention in high-volume clinical settings servicing minority patients. However, after screening 228 participants who all expressed initial interest in increasing physical activity levels, only a quarter reached the first study visit, indicating that a small select subset of this patient population is able to commit to participation in formal exercise programming. Among other CKD exercise intervention studies of equal duration and intensity, between 10% and 30% of patients who were screened proceeded to randomization.35, 36, 37 Our low-cost low-intensity recruitment may nonetheless provide benefits justifying its retention. Integrating physical activity screening—known as the "physical activity vital sign"—in clinical visits improves physical activity awareness and counseling in patients and providers respectively.33,38, 39, 40
However, our data demonstrating safety of the intervention and weak signal for any variation in formal physical function assessments over time can inform a further even more pragmatic iteration of our protocols. For example, modified verbal consent documented digitally—increasingly accepted in pragmatic clinical trials41,42—and mail delivery of the wearable activity tracker could decrease the burden of in-person study-related interactions and facilitate a focus on the exercise programming.
Unlike in other group-exercise programming studies, in which adherence typically decreases over time, we observed an "all-in" or "none" phenomenon.13,43, 44, 45 Participants randomly assigned to but not arriving at a single offered session had similar demographic and clinical characteristics and exercise self-efficacy scores, but higher rates of hospitalizations, compared with regular attendees. Numerous postdischarge exercise rehabilitation programs exist for patients with heart failure,46,47 acute myocardial infarction,48 and chronic obstructive pulmonary disease49 to serve as secondary prevention measures. No such tailored programs exist to support possible physical setbacks and re-focus on lifestyle interventions for persons with CKD but are justified in the context of nationwide data reporting high rates of hospitalizations.50 A third of our participants were hospitalized, further supporting the need for future programming to specifically accommodate postdischarge exercise rehabilitation.51 In addition, we observed lower drop out in the mHealth group, implying that a lower intensity intervention may be more acceptable to patients receiving dialysis.
Despite the challenges in recruitment and attendance of the exercise sessions, small sample size, and highly comorbid patient population, we observed a modest effect of our intervention. In a similar intervention delivered to patients in earlier stages of CKD and with a larger sample size, Rossi et al35 demonstrated improvements in both self-reported physical activity levels and physical function measures such as 6-minute walk test at the end of twice-weekly exercise sessions delivered for 12 weeks. Other exercise intervention studies in persons with CKD have described improvements in self-reported physical activity levels13,52 but to our knowledge, none included objectively measured activity trackers.
In our study at baseline, participants performed poorly on assessments of physical function, walking an average of 406 (SD, 117) m on the 6-minute walk test compared with 571 (SD, 90) m observed in the general population.53 Forty-six percent of all participants were considered to have weak handgrip strength compared with the general population53 and walked a mean of approximately 5,000 steps per day, between about the 10th and 20th percentile of US adults.54 Because our study design did not have a run-in period, it is likely that the "baseline" physical activity level reflects a boost after initial recruitment, assessment, 1-on-1 physical activity counseling, and provision of a wearable activity tracker. Furthermore, although the mHealth alone group had lesser benefit as compared with the EIM group, the effect of no intervention at all was not studied; therefore, some potential benefit may have accrued in this group as well.55
However, we observed an additional benefit of the small-group sessions, with as-treated analyses demonstrating modest but consistent improvements in daily steps, distance traveled, and both light and vigorous physical activities at 8 weeks compared with the mHealth group. The “fading out” of the effect after the discontinuation of the intervention at 8 weeks is consistent with exercise intervention literature52,55,56 and highlights the critical need for a sustainable intervention of longer duration and of interventions integrating novel behavioral modification strategies, including modelling and incentivization.57
Although we did not expect major changes in anthropometrics, physical function, or mental assessments due to the small size and short duration of this study, the lack of variation in these objective measurements over time has been seen in many but not all35 other studies of both persons with CKD and end-stage kidney disease.9 Participants can experience disappointment and frustration with exercise programs when focused mainly on weight loss and functional measures due to embarrassment and other exercise-related physical and emotional barriers that can diminish the overall perceived benefit of exercise. The importance of improving fatigue and life participation, defined as the ability to participate in meaningful life activities such as work, school, and recreational activities, is emerging as a critically important outcome among persons receiving dialysis58, 59, 60 and kidney transplant recipients.61 Although improvements in patient-reported outcomes have been reported in other CKD exercise intervention studies,13,35 these have not been the primary outcome of interest. Physical function measures obtained throughout the course of this study did not reflect the modest changes in physical activity or the subjective patient-reported benefit provided to investigators in testimonials. Due to high participant and clinician interest, the exercise intervention was successfully converted to a CKD-tailored program available at one of the clinical sites (San Jose, CA), supported financially by the local chapter of the National Kidney Foundation. Thus, further research is needed to ensure that exercise programs are being designed with a focus on patient-centered outcomes, implementation, and scalability.
Limitations of our study include its small sample size, high rate of drop out, and unblinded design without a run-in period to obtain unbiased baseline measures of physical activity. Although we worked in diverse settings, due to resource-limitations, we restricted recruitment to persons with conversational English capabilities. We used wearable activity tracker data, rather than accelerometers, to measure daily physical activity, although similar device-based step count data have been correlated with outcomes in the National Health and Nutrition Examination Survey54 and are used in various clinical settings.33,62
To our knowledge, this is the first study to implement a technology-enabled group-based exercise intervention in persons with advanced CKD. During this first phase, in which we had a diverse sample and well-balanced randomization, we learned that a pragmatic approach with integration into the clinical setting is feasible and safe but that increased support around hospitalizations may be required to augment retention in group exercise sessions. We observed high patient acceptability and satisfaction and modest benefits in physical activity levels that faded over time, indicating crucial need for focus on patient-reported outcomes and sustainability of programming beyond 8 weeks. Our work will inform future larger scale implementation of CKD-tailored exercise programming in underserved populations using remote access and simplified accessibility.
Article Information
Authors’ Full Names and Academic Degrees
Shuchi Anand, MD, MS, Susan L. Ziolkowski, MD, MS, Ahad Bootwala, MPH, Jianheng Li, PhD, Nhat Pham, MD, Jason Cobb, MD, and Felipe Lobelo, MD, PhD.
Authors’ Contributions
Study design and secured funding: SA, FL; Participant recruitment, follow-up, and measurements: SA, SLZ, AB, NP, JC. fitness professional training: FL; data analysis: JL. SA and SLZ contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This project was funded by the Satellite Healthcare's Normon S Coplon Applied Pragmatic Research Award. Dr Anand was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK101826. Dr Ziolkowski was supported by NIDDK F32 and Division of Nephrology funds. The funders had no role in study design or data collection, analysis or interpretation.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We are grateful to our research participants and their nephrologists, our friendly and warm research coordinator Ms Lani Demchak, and our dedicated fitness professionals CJ Jones, Robert Chapman, Garion Williams, and Benicia Malone. We also thank Emory University and Timpany Center for the exercise facilities.
Peer Review
Received January 4, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor and the Editor-in-Chief. Accepted in revised form April 25, 2021.
Data Sharing
Authors will provide deidentified data after reviewing data request.
Footnotes
Complete author and article information provided before references.
Item S1: Description of wearable activity tracker used in study
Item S2: Video testimonials from 2 EIM-CKD participants
Table S1: Exercise Is Medicine in CKD Study Inclusion and Exclusion Criteria
Table S2: Physical Function Measures of Study Participants During the 16-Week Study Period
Supplementary Material
Items S1-S2; Tables S1-S2 .
References
- 1.Zelle D.M., Corpeleijn E., Stolk R.P., et al. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(4):898–905. doi: 10.2215/CJN.03340410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwood S.A., Castle E., Lindup H., et al. Mortality and morbidity following exercise-based renal rehabilitation in patients with chronic kidney disease: the effect of programme completion and change in exercise capacity. Nephrol Dial Transplant. 2019;34(4):618–625. doi: 10.1093/ndt/gfy351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke A.B., Ta V., Iqbal S., et al. The impact of intradialytic pedaling exercise on arterial stiffness: a pilot randomized controlled trial in a hemodialysis population. Am J Hypertens. 2018;31(4):458–466. doi: 10.1093/ajh/hpx191. [DOI] [PubMed] [Google Scholar]
- 4.Baggetta R., D'Arrigo G., Torino C., et al. Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr. 2018;18(1):248. doi: 10.1186/s12877-018-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manfredini F., Mallamaci F., D'Arrigo G., et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28(4):1259–1268. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beddhu S., Baird B.C., Zitterkoph J., Neilson J., Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4(12):1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen K.L., Chertow G.M., Ng A.V., et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6) doi: 10.1046/j.1523-1755.2000.00116.x. 2564-2370. [DOI] [PubMed] [Google Scholar]
- 8.Kurella Tamura M., Covinsky K.E., Chertow G.M., Yaffe K., Landefeld C.S., McCulloch C.E. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64(3):383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Chan K.E., Maddux F.W., Tolkoff-Rubin N., Karumanchi S.A., Thadhani R., Hakim R.M. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson B.M., Zhang J., Morgenstern H., et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soucie J.M., McClellan W.M. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996;7(10):2169–2175. doi: 10.1681/ASN.V7102169. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood S.A., Lindup H., Taylor K., et al. Evaluation of a pragmatic exercise rehabilitation programme in chronic kidney disease. Nephrol Dial Transplant. 2012;27(suppl 3):iii126–iii134. doi: 10.1093/ndt/gfs272. [DOI] [PubMed] [Google Scholar]
- 14.Effing T., Zielhuis G., Kerstjens H., van der Valk P., van der Palen J. Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med. 2011;105(3):418–426. doi: 10.1016/j.rmed.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Flynn K.E., Piña I.L., Whellan D.J., et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickwood K.J., Watson G., O'Brien J., Williams A.D. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7(4) doi: 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott M.M., Spring B., Berger J.S., et al. Effect of a home-based exercise intervention of wearable technology and telephone coaching on walking performance in peripheral artery disease: the HONOR randomized clinical trial. JAMA. 2018;319(16):1665–1676. doi: 10.1001/jama.2018.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. Health care providers-making a difference in your health care setting. http://exerciseismedicine.org/ Accessed July 21, 2020.
- 20.Lobelo F., Stoutenberg M., Hutber A. The Exercise is Medicine Global Health Initiative: a 2014 update. Br J Sports Med. 2014;48(22):1627–1633. doi: 10.1136/bjsports-2013-093080. [DOI] [PubMed] [Google Scholar]
- 21.Jagannathan R., Ziolkowski S.L., Weber M.B., et al. Physical activity promotion for patients transitioning to dialysis using the "Exercise is Medicine" framework: a multi-center randomized pragmatic trial (EIM-CKD trial) protocol. BMC Nephrol. 2018;19(1):230. doi: 10.1186/s12882-018-1032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin B.A., Thompson P.D., Al-Zaiti S.S., et al. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation. 2020;141(13):e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 23.Dixon D., Johnston M. The Scottish Government; 2010. Health Behaviours Change Competency Framework: Competencies to Deliver Interventions to Change Lifestyle Behaviours That Affect Health. [Google Scholar]
- 24.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25.Ware J., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Sallis J.F., Grossman R.M., Pinski R.B., Patterson T.L., Nader P.R. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 27.James F.S. Self-efficacy for Exercise Behaviors: Survey and Scoring. https://drjimsallis.org/measure_selfefficacy.html Accessed July 21, 2020.
- 28.Smith B.W., Dalen J., Wiggins K., Tooley E., Christopher P., Bernard J. The Brief Resilience Scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. doi: 10.1080/10705500802222972. [DOI] [PubMed] [Google Scholar]
- 29.Craig C.L., Marshall A.L., Sjöström M., et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 30.National Health and Nutrition Examination Survey (NHANES) Body Composition Procedures Manual. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf Date last accessed May 10, 2019.
- 31.American Thoracic Society Statement guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 32.Murakami H., Kawakami R., Nakae S., et al. Accuracy of wearable devices for estimating total energy expenditure: comparison with metabolic chamber and doubly labeled water method. JAMA Intern Med. 2016;176(5):702–703. doi: 10.1001/jamainternmed.2016.0152. [DOI] [PubMed] [Google Scholar]
- 33.Lobelo F., Rohm Young D., Sallis R., et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137(18):e495–e522. doi: 10.1161/CIR.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 34.Eldridge S.M., Chan C.L., Campbell M.J., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi A.P., Burris D.D., Lucas F.L., Crocker G.A., Wasserman J.C. Effects of a renal rehabilitation exercise program in patients with CKD: a randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9(12):2052–2058. doi: 10.2215/CJN.11791113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barcellos F.C., Del Vecchio F.B., Reges A., et al. Exercise in patients with hypertension and chronic kidney disease: a randomized controlled trial. J Hum Hypertens. 2018;32(6):397–407. doi: 10.1038/s41371-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 37.Watson E.L., Greening N.J., Viana J.L., et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis. 2015;66(2):249–257. doi: 10.1053/j.ajkd.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45(6):473–474. doi: 10.1136/bjsm.2010.083469. [DOI] [PubMed] [Google Scholar]
- 39.Golightly Y.M., Allen K.D., Ambrose K.R., et al. Physical activity as a vital sign: a systematic review. Prev Chronic Dis. 2017;14:E123. doi: 10.5888/pcd14.170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant R.W., Schmittdiel J.A., Neugebauer R.S., Uratsu C.S., Sternfeld B. Exercise as a vital sign: a quasi-experimental analysis of a health system intervention to collect patient-reported exercise levels. J Gen Intern Med. 2014;29(2):341–348. doi: 10.1007/s11606-013-2693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grady C. Informed consent. N Engl J Med. 2017;376(20):e43. doi: 10.1056/NEJMc1704010. [DOI] [PubMed] [Google Scholar]
- 42.Kim S.Y., Miller F.G. Informed consent for pragmatic trials--the integrated consent model. N Engl J Med. 2014;370(8):769–772. doi: 10.1056/NEJMhle1312508. [DOI] [PubMed] [Google Scholar]
- 43.Cox K.L., Gorely T.J., Puddey I.B., Burke V., Beilin L.J. Exercise behaviour change in 40 to 65-year-old women: the SWEAT Study (Sedentary Women Exercise Adherence Trial) Br J Health Psychol. 2003;8(pt 4):477–495. doi: 10.1348/135910703770238329. [DOI] [PubMed] [Google Scholar]
- 44.Caserta M.S., Gillett P.A. Older women's feelings about exercise and their adherence to an aerobic regimen over time. Gerontologist. 1998;38(5):602–609. doi: 10.1093/geront/38.5.602. [DOI] [PubMed] [Google Scholar]
- 45.Arikawa A.Y., O'Dougherty M., Kaufman B.C., Schmitz K.H., Kurzer M.S. Attrition and adherence of young women to aerobic exercise: lessons from the WISER study. Contemp Clin Trials. 2012;33(2):298–301. doi: 10.1016/j.cct.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piña I.L., Apstein C.S., Balady G.J., et al. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 47.Taylor R.S., Long L., Mordi I.R., et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, meta-analysis, and trial sequential analysis. JACC Heart Fail. 2019;7(8):691–705. doi: 10.1016/j.jchf.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 48.Lawler P.R., Filion K.B., Eisenberg M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162(4):571–584.e2. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Lindenauer P.K., Stefan M.S., Pekow P.S., et al. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among Medicare beneficiaries. JAMA. 2020;323(18):1813–1823. doi: 10.1001/jama.2020.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mix T.C., St Peter W.L., Ebben J., et al. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis. 2003;42(5):972–981. doi: 10.1016/j.ajkd.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Le Berre M., Maimon G., Sourial N., Guériton M., Vedel I. Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc. 2017;65(7):1597–1608. doi: 10.1111/jgs.14828. [DOI] [PubMed] [Google Scholar]
- 52.Howden E.J., Leano R., Petchey W., Coombes J.S., Isbel N.M., Marwick T.H. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8(9):1494–1501. doi: 10.2215/CJN.10141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casanova C., Celli B.R., Barria P., et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 54.Saint-Maurice P.F., Troiano R.P., Bassett D.R., et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323(12):1151–1160. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellberg M., Höglund P., Svensson P., Clyne N. Randomized controlled trial of exercise in CKD-the RENEXC Study. Kidney Int Rep. 2019;4(7):963–976. doi: 10.1016/j.ekir.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knowler W.C., Fowler S.E., Hamman R.F., et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evangelidis N., Craig J., Bauman A., Manera K., Saglimbene V., Tong A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2019-031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manera K.E., Johnson D.W., Craig J.C., et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology-Peritoneal Dialysis) Consensus Workshop. Am J Kidney Dis. 2020;75(3):404–412. doi: 10.1053/j.ajkd.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Ju A., Unruh M., Davison S., et al. Establishing a core outcome measure for fatigue in patients on hemodialysis: a Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop Report. Am J Kidney Dis. 2018;72(1):104–112. doi: 10.1053/j.ajkd.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 60.Evangelidis N., Tong A., Manns B., et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am J Kidney Dis. 2017;70(4):464–475. doi: 10.1053/j.ajkd.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 61.Ju A., Josephson M.A., Butt Z., et al. Establishing a core outcome measure for life participation: a Standardized Outcomes in Nephrology-kidney Transplantation Consensus Workshop Report. Transplantation. 2019;103(6):1199–1205. doi: 10.1097/TP.0000000000002476. [DOI] [PubMed] [Google Scholar]
- 62.Lobelo F., Kelli H.M., Tejedor S.C., et al. The Wild Wild West: a framework to integrate mHealth software applications and wearables to support physical activity assessment, counseling and interventions for cardiovascular disease risk reduction. Prog Cardiovasc Dis. 2016;58(6):584–594. doi: 10.1016/j.pcad.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Items S1-S2; Tables S1-S2 .