Abstract
To evaluate the effect of an Escherichia coli lipopolysaccharide (LPS) challenge on the digestible lysine (Lys) requirement for growing pigs, a nitrogen (N) balance assay was performed. Seventy-two castrated male pigs (19 ± 1.49 kg body weight [BW]) were allocated in a 2 × 6 factorial design composed of two immune activation states (control and LPS-challenged) and six dietary treatments with N levels of 0.94, 1.69, 2.09, 3.04, 3.23, and 3.97% N, as fed, where Lys was limiting, with six replicates and one pig per unit. The challenge consisted of an initial LPS dose of 30 μg/kg BW via intramuscular (IM) injection and a subsequent dose of 33.6 μg/kg BW after 48 h. The experimental period lasted 11 d and was composed of a 7-d adaptation and a subsequent 4-d sampling period in which N intake (NI), N excretion (NEX), and N deposition (ND) were evaluated. Inflammatory mediators and rectal temperature were assessed during the 4-d collection period. A three-way interaction (N levels × LPS challenge × time, P < 0.05) for IgG was observed. Additionally, two-way interactions (challenge × time, P < 0.05) were verified for IgA, ceruloplasmin, transferrin, haptoglobin, α-1-acid glycoprotein, total protein, and rectal temperature; and (N levels × time, P < 0.05) for transferrin, albumin, haptoglobin, total protein, and rectal temperature. LPS-challenged pigs showed lower (P < 0.05) feed intake. A two-way interaction (N levels × LPS challenge, P < 0.05) was observed for NI, NEX, and ND, with a clear dose–response (P < 0.05). LPS-challenged pigs showed lower NI and ND at 2.09% N and 1.69 to 3.97% N (P < 0.05), respectively, and higher NEX at 3.23% N (P < 0.05). The parameters obtained by a nonlinear model (N maintenance requirement, NMR and theoretical maximum N deposition, NDmaxT) were 152.9 and 197.1 mg/BWkg0.75/d for NMR, and 3,524.7 and 2,077.8 mg/BWkg0.75/d for NDmaxT, for control and LPS-challenged pigs, respectively. The estimated digestible Lys requirements were 1,994.83 and 949.16 mg/BWkg0.75/d for control and LPS-challenged pigs, respectively. The daily digestible Lys intakes required to achieve 0.68 and 0.54 times the NRmaxT value were 18.12 and 8.62 g/d, respectively, and the optimal dietary digestible Lys concentration may change depending on the feed intake levels. Based on the derived model parameters obtained in the N balance trial with lower cost and time, it was possible to differentiate the digestible Lys requirement for swine under challenging conditions.
Keywords: digestible lysine requirement, exponential model, growing pigs, inflammatory response, nitrogen balance
Introduction
Dietary amino acid requirements depend on the metabolic priorities of the animals and therefore can be modified by systemic changes in metabolism due to immune system activation and the incidence of infectious disease. In such contexts, systemic inflammatory responses elicit adaptive reductions in feed intake and body weight (BW) gain, concomitant with a loss of muscle mass in pigs (Le Floc’h et al., 2004; Kampman, 2015). Understanding the causes and consequences of immune system activation and its effects on the animals’ metabolic and energy status are essential to mitigate the negative effects of immune activation (Kvidera and Kay, 2017).
Dietary lysine (Lys) is typically the first limiting amino acid in pig diets (Htoo et al., 2016) due to the central role of this amino acid in muscle protein deposition. The relationship between the use of Lys and protein deposition capacity can be affected by genetics, sex, age, and animal health status (Ceron et al., 2013). For instance, a genetic propensity for growth is a major driver of amino acid requirements (Hauschild et al., 2015), but dramatic shifts in metabolic priorities due to immune activation must also be considered. Immune system stimulation results in increased metabolic use of body proteins and amino acids, which negatively affects protein deposition by animals (Williams et al., 1997a, b).
In this sense, determining the nutritional requirements of pigs under challenging conditions is of utmost importance for the development of feeding strategies to achieve the maximum growth rate and efficient use of nutrients (Ceron et al., 2013). Evaluation of the nutrient retention capacity during times of immune activation has distinct advantages when estimating dietary amino acid requirements. To this end, nonlinear models associated with nitrogen balance assays have been proposed to determine amino acid requirements by estimating the maximum protein deposition and the efficiency of the use of amino acids for growing animals (Samadi and Liebert, 2006a, 2006b). Given this context, the objective of this study was to modeling digestible lysine (lys) requirements via nitrogen balance assay in growing pigs challenged with E. coli lipopolysaccharide (LPS). We hypothesized that the dietary Lys requirement estimated for LPS-challenged pigs would be decreased relative to control pigs due to shifts in metabolic demands for body protein (i.e., N) and this can be determined through balance nitrogen assay that allows determine the model parameters for maximum nitrogen retention, nitrogen maintenance requirement, and the efficiency of lysine utilization.
Materials and Methods
Animal care and handling were in accordance with Brazilian Legislation on Animal Experimentation and Welfare, and the experimental protocol was approved by the Ethics Committee on the Use of Farm Animals (CEUAP-UFV) of the Universidade Federal de Viçosa (protocol 113/2018).
Animals, housing, and experimental design
A nitrogen balance assay was conducted at the Swine Extension, Research and Teaching Unit of the Department of Animal Science, Universidade Federal de Viçosa, Viçosa, Brazil. Seventy-two castrated male pigs (Sire line 337 × Dam line Camborough, PIC) with an initial body weight (BW) of 19 ± 1.49 kg were allocated in a 2 × 6 factorial design with two immune activation states (control and LPS-challenged) and six dietary treatments with increasing nitrogen (N) levels (0.94, 1.69, 2.09, 3.04, 3.23, and 3.97% N, as fed basis), with six replicates and one animal per experimental unit.
Pigs were individually housed in adjustable metabolism cages (1.27 m × 0.56 m × 0.75 m) with individual stainless feeders, that was used to provide water before and after meals.
The trial was performed in experimental period with a total 11 d, with 7 d of adaptation to the metabolic cages and experimental diets, and a subsequent 4-d sampling period in which total feces and urine collection were performed to determine the N intake (NI), N excretion (NEX), and N deposition (ND). During the adaptation phase, average daily feed consumption was quantified, and the lowest feed intake per metabolic weight (BW0.75) in this phase was considered the standard for calculating feed intake for all animals during the collection period (Sakomura and Rostagno, 2016). The mash diets were provided twice a day (0700 h and 1600 h) and water was provided ad libitum.
The stimulus of the inflammatory response consisted of two intramuscular (IM) injections of Escherichia coli LPS (serotype O55: B5; Sigma-Aldrich); the first injection administered on the first day of the collection period was 30 μg/kg BW, and the second 48 h later was 33.6 μg/kg BW, following the protocol of Mc Gilvray et al. (2018). The pigs assigned to the unchallenged (i.e., control) group received two IM injections of sterile saline solution (0.9%) at the same time as the LPS-challenged pigs.
Experimental diets
The principles of the diet dilution technique were applied as described by Fisher and Morris (1970). Initially, two diets were formulated: a concentrated diet (N6) with a higher N level based on corn, soybean meal, and corn gluten, with 27.0% crude protein (CP), 1.59% digestible Lys, and 3230 kcal/kg of metabolizable energy, following the ideal protein ratio recommended by Rostagno et al. (2017) (Table 1), and a protein-free diet (N0) defined as a dilution diet formulated to meet the nutritional requirements, except for protein and amino acids (AA). According to the diet dilution technique, graded mixing of proportions of diet N6 and diet N0 yielded the final diets, with N ranging between 0.94% (N1) and 3.97% (N6). The ratios between the N6 and N0 diets were N1 = 23.03:76.97; N2 = 38.42:61.58; N3 = 53.82:46.18; N4 = 69.21:30.79; N5 = 84.61:15.39; and N6 = 100:0 to obtain diets with CP concentrations of 6.22, 10.37, 14.53, 18.69, 22.84, and 27%, respectively, and 0.36, 0.61, 0.85, 1.10,1.34, and 1.59% digestible Lys, respectively. The Lys concentration was the limiting factor in the composition of the experimental diets, and the relationship of the other AAs to Lys was maintained (Table 2). The diets were formulated to be isoenergetic and were supplemented with minerals and vitamins according to the recommendations of Rostagno et al. (2017).
Table 1.
Composition of the concentrated diet (3.97% N) and the PFD, as fed-basis
| Ingredients | 27.0% CP | PFD- Dilution diet1 |
|---|---|---|
| Corn, 7.88% | 40.73 | - |
| Soybean meal, 45% | 47.38 | - |
| Corn gluten, 60% | 3.00 | - |
| Sugar | 2.00 | 3.00 |
| Starch | - | 82.15 |
| Soy oil | 1.909 | 2.500 |
| Dicalcium phosphate | 1.257 | 1.962 |
| Calcitic limestone | 0.791 | 0.661 |
| Salt | 0.389 | 0.434 |
| Sodium bicarbonate | - | 0.103 |
| Potassium carbonate | - | 1.767 |
| DL-Methionine, 99% | 0.163 | - |
| L-Lysine HCl, 79% | 0.397 | - |
| L-Threonine, 98% | 0.155 | - |
| L-Valine, 96.5% | 0.009 | - |
| Choline chloride, 60 | 0.100 | 0.100 |
| Vitamin supplement2 | 0.125 | 0.125 |
| Mineral supplement3 | 0.125 | 0.125 |
| BHT4 | 0.010 | 0.010 |
| Inert | 1.453 | 7.053 |
| Total | 100.00 | 100.00 |
| Calculated Composition | ||
| Net Energy, kcal/kg | 2,344 | 2,662 |
| Crude protein, % | 27.00 | - |
| Available phosphorus, % | 0.363 | 0.363 |
| Calcium, % | 0.733 | 0.733 |
| Chlorine, % | 0.259 | 0.259 |
| Sodium, % | 0.200 | 0.200 |
| Potassium, % | 0.999 | 0.999 |
| dEB5, meq/kg | 270.0 | 270.0 |
| Digestible amino acids, % | ||
| Lys | 1.585 | - |
| Met | 0.533 | - |
| Met+Cys | 0.888 | - |
| Thr | 0.999 | - |
| Trp | 0.300 | - |
| Arg | 1.694 | - |
| Val | 1.094 | - |
| Ile | 1.060 | - |
| Leu | 2.146 | - |
| Phe | 1.232 | - |
| Phe+Tyr | 2.058 | - |
| His | 0.641 | - |
1PFD, Protein-Free Diet.
2Vitamin supplement containing per kg of feed: Vit.A, 6875 U.I.; Vit. D3, 1500 U.I.; Vit. E, 40.0 U.I.; Vit. B1, 1.00 mg; Vit. B2, 3.13 mg; Vit. B6, 2.00 mg; Vit. B12, 0.020 mg; Pantothenic Acid, 15.0 g; Biotin, 0.100 mg; Vit.K3, 3.00 mg; Folic Acid, 0.300 mg; Nicotinic acid, 30.0 mg.
3Mineral supplement containing per kg of feed, Iron - 80.0 mg; Copper - 12.0 mg; Manganese - 40.0 mg; Zinc - 110 mg; Iodine - 1.00 mg; Selenium - 0.36 mg.
4BHT, butylated hydroxytoluene
5dEB, dietary electrolyte balance
Table 2.
Total amino acid content of the diets (N1 to N6), as fed-basis
| Diets1 | ||||||
|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N4 | N5 | N6 | |
| Amino acids2, % | ||||||
| Aspartic acid | 0.50 | 0.71 | 1.59 | 1.82 | 2.49 | 2.96 |
| Glutamic acid | 1.06 | 1.62 | 2.64 | 3.26 | 4.18 | 4.80 |
| Serine | 0.30 | 0.47 | 0.68 | 0.90 | 1.10 | 1.28 |
| Glycine | 0.28 | 0.43 | 0.64 | 0.81 | 1.01 | 1.16 |
| Histidine | 0.15 | 0.28 | 0.40 | 0.49 | 0.63 | 0.71 |
| Arginine | 0.42 | 0.69 | 1.00 | 1.25 | 1.55 | 1.78 |
| Threonine | 0.26 | 0.36 | 0.53 | 0.78 | 0.94 | 1.10 |
| Alanine | 0.31 | 0.49 | 0.71 | 0.90 | 1.13 | 1.29 |
| Proline | 0.33 | 0.61 | 0.86 | 1.10 | 1.35 | 1.58 |
| Tyrosine | 0.20 | 0.36 | 0.53 | 0.69 | 0.81 | 0.93 |
| Valine | 0.30 | 0.50 | 0.71 | 0.88 | 1.11 | 1.24 |
| Methionine | 0.12 | 0.20 | 0.29 | 0.35 | 0.38 | 0.45 |
| Cystine | 0.09 | 0.19 | 0.28 | 0.47 | 0.41 | 0.45 |
| Isoleucine | 0.28 | 0.47 | 0.67 | 0.84 | 1.06 | 1.18 |
| Leucine | 0.57 | 0.94 | 1.31 | 1.68 | 1.98 | 2.24 |
| Phenylalanine | 0.32 | 0.54 | 0.79 | 1.00 | 1.24 | 1.39 |
| Lysine | 0.46 | 0.69 | 0.98 | 1.30 | 1.54 | 1.78 |
| Tryptophan | 0.07 | 0.14 | 0.26 | 0.25 | 0.32 | 0.32 |
| Sum of amino acids | 6.02 | 9.69 | 14.87 | 18.77 | 23.23 | 26.62 |
1Nitrogen levels: N1, 0.94; N2, 1.69; N3, 2.09; N4, 3.04; N5, 3.23 and N6, 3.97 % N, as fed basis.
2Analyzed composition of the amino acid in the experimental diets.
Sample collection, analysis, and calculations
Pigs were weighed at the beginning of the adaptation period and the beginning of the total collection period. Samples from each experimental diet (N1 to N6) were collected randomly during feed manufacturing, homogenized, and stored at −20 °C for nutrient composition analysis. Blood samples were collected via sinus orbital puncture at 0700 h on the first day of collection (day 1), as well as 3 h and 96 h after the start of the challenge, and were collected in tubes without anticoagulant, immediately placed on ice and centrifuged at 1,900 × g at 4 °C (centrifuge R/5702, Copyright Eppendorf AG, Germany) to obtain serum samples. Rectal temperature was measured using a G-TESH digital thermometer (digital thermometer, TH186, Onbo Eletronics - Shenzhen, China) during the collection period on day 1, 3, and 5 at 0700 h (hereafter referred to as LPS-d1, LPS-d3, and LPS-d5) and on day 1 and 3 at 1500 h, which was 3 h after the initial LPS injection (LPS-d1 + 3h, LPS-d3 + 3h).
The N balance study was carried out according to the methodology of the total collection of feces and urine (Sakomura and Rostagno, 2016), reducing the collection period by one day. This change was performed so that the samples were obtained under similar conditions, considering the challenge protocol with an interval between LPS injections of 48 h. Fecal collection was carried out once a day for four consecutive days, with samples being weighed daily and stored in plastic bags at −20 °C. At the end of the collection period, all fecal samples were thawed and homogenized, and subsamples were lyophilized and stored for further analysis. Urine collection began at the time of IM injection, and the total urine volume per pig was collected over 4 d in buckets containing 7.5 to 10 mL of HCl and positioned below the funnel coupled to each cage. After each 24-h period of urine collection, samples of 10% of the urine volume were taken and stored at −20 °C to capture a homogenous and representative urine sample per pig over the 4-d collection period.
The diets, feces, and urine subsamples were analyzed to determine the chemical composition at the Animal Nutrition Laboratory (Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil). Before chemical analysis, feces and diet samples were ground in a ball mill (Micro spray mill, R-TE 350, TECNAL - São Paulo, Brazil). The samples were weighed (Analytical balance - AR2140, Adventurer PRO Analytical - OHAUS Corp., USA) to quantify the dry matter content. The N content of the diets, feces and urine was analyzed in a N distiller (N distiller, TE-036/1, TECNAL - São Paulo, Brazil) using the Kjeldahl method (determination of nitrogen content and calculation of crude protein content, method 988.05, AOAC, 2006). A factor of 6.25 was used to convert the nitrogen content to CP. Additionally, homogenous subsamples of the diets were submitted to the CBO Laboratory (Valinos, SP, Brazil) for quantification of the total amino acid content using high-performance liquid chromatography (CBM 20A, Shimadzu – São Paulo, Brazil) based on White et al. (1986), and tryptophan was measured (Spectrophotometer, 600 S, FEMTO – São Paulo, Brazil) based on Lucas and Sotelo (1980). The digestible AA data were calculated based on the tables of Rostagno et al. (2017).
Triplicate analysis was performed to determine the dry matter (DM) content in the diets and feces (dry matter content method 930.15, AOAC, 2006). N determination in the diets, feces, and urine samples was analyzed in duplicate; repetitions were performed for a sample coefficient of variation above 5%.
The N deposition (mg/BWkg0.75/d) was calculated as a result of the difference between the N ingested (mg/BWkg0.75/d) (i.e., determined by the N of the feed provided minus the N of the leftover feed) and the N excreted (Nfeces [mg/BWkg0.75/d] + Nurine [mg/BWkg0.75/d]).
Total serum protein determination was performed by the Biuret method with the aid of a set of reagents (Bioclin, Total Protein Monoreagent K031, colorimetric test) through an automatic biochemical analyzer (Mindray BS-200E, Shenzhen Mindray Bio-Medical Electronics Co., Shenzhen, China) at the Animal Physiology and Reproduction Laboratory (Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil).
Electrophoresis (SDS–PAGE)
Serum protein analysis was performed at the Department of Clinical and Veterinary Surgery (Universidade Estadual de São Paulo, Jaboticabal, São Paulo, Brazil). The serum proteins were separated by polyacrylamide gel electrophoresis with a matrix containing sodium dodecyl sulfate (SDS–PAGE). Electrophoresis was performed according to the modified technique described by Laemmli (1970) using the vertical electrophoresis system. The molecular weight and protein fraction concentrations were determined by computer densitometry (SHIMADZU 9301 PC, Shimadzu Corp, Kyoto, Japan) using a simple scanner. For protein identification, biomarkers were used (SIGMA MARKER, Sigma-Aldrich Biotechnology LP, Germany). For densitometric evaluation of protein bands, reference curves were made from the reading of the standard marker. From electrophoresis (SDS–PAGE), the proteins albumin, ceruloplasmin, haptoglobin, immunoglobulins (IgA and IgG), transferrin, α-1-acid glycoprotein (α1AGp), and PM 20,000 were quantified at 3 h and 96 h after the first application of LPS. These proteins are considered biomarkers of the inflammatory response, enabling the identification and monitoring of animal health.
Statistical analysis
All data were analyzed by analysis of variance (ANOVA) using a randomized complete block design with a 2 × 6 factorial arrangement with repeated measures over time to estimate the effects of N levels, immune activation, and collection time as fixed effects, along with all interactions (PROC MIXED, SAS Inst. Inc., version 9.4). Individual pigs served as the experimental unit for all outcomes. Interactive effects were further evaluated using orthogonal contrasts for linear and quadratic responses to dietary Lys concentration. Differences between averages were determined using the “slice” function of “LSmeans” and Tukey’s test. For all outcomes, significance was accepted at P < 0.05.
Modeling
Modeling was performed to estimate amino acid requirements using parameters including N maintenance requirements (NMR), N retention (NR), and theoretical maximum N retention (NRmaxT). Determining the NMR is part of the total N retention and indicates the amount of N to be deposited to replace endogenous losses (feces and urine). A regression analysis between N intake and excretion was applied to estimate NMR following an exponential function, as performed in previous studies with birds and pigs (Samadi and Liebert, 2007, 2008; Wecke and Liebert, 2009; Pastor et al., 2013; Khan et al., 2015; Liebert, 2015, Dorigam et al., 2017):
| (1) |
where NMR is the N maintenance requirement (mg/BWkg0.75/d), NI is the N intake (mg/BWkg0.75/d), NEX is the N excretion (mg/BWkg0.75/d), b is the slope of the exponential function, and exp is the base number of the natural logarithm (ln). NMR was estimated by calculating the curve intersection on the y-axis (NEX) when NI = 0. The exponential model (Equation 1) was adjusted to the N excretion data using the nonlinear optimization technique (Levenberg-Marquardt) with SAS (Statistical Analysis System, version 9.4). Once the NMR is estimated, it is possible to calculate NR. The ND was calculated as the difference between NI and NEX. N retention represents the total nitrogen utilization calculated as NR = ND + NMR (Wecke and Liebert, 2009).
The theoretical maximum N retention (NRmaxT) is represented by the exponential function asymptote and is used to classify NR performance data. A regression analysis between NI and NR was performed to fit another exponential model:
| (2) |
where NR is the N retention (mg/BWkg0.75/d), NRmaxT is the theoretical maximum N retention (mg/BWkg0.75/d), NI is the N intake (mg/BWkg0.75/d), b is the slope of the NR curve that expresses the quality of the protein in the diet, and exp is the base number of the natural logarithm (ln). The exponential model (Equation 2) was adjusted to the NR data using the nonlinear optimization technique (Levenberg-Marquardt) in SAS (Statistical Analysis System, version 9.4).
The NRmaxT is the asymptotic value in the exponential function. It was estimated by a statistical procedure, following several steps of the Levenberg-Marquardt algorithm, until the sum of the residual squares was minimized. Therefore, the adjective “theoretical” is assigned to this parameter because the estimated value is not attainable under production conditions, even if animals are raised under ideal conditions. However, this is an essential parameter in the modeling procedure to derive AA requirements considering gradual levels defined to make use of the theoretical maximum within the practical data scope.
The requirement for digestible Lys was calculated after a logarithmic transformation of Equation 2, according to previous studies (Samadi and Liebert, 2007, 2008; Wecke and Liebert, 2009; Pastor et al., 2013; Khan et al., 2015; Liebert, 2015, Dorigam et al., 2017).
| (3) |
where LAAI is the required daily intake of limiting AA (mg/BWKg0.75/d), NR is the N retention (mg/BWKg0.75/d), NRmaxT is the theoretical maximum retention of N (mg/BWKg0.75/d), c is the limiting AA concentration in dietary protein (g/16 g N), b is the slope of the NR curve that expresses the quality of the protein in the diet, and bc− 1 is the efficiency parameter of using limiting AA in the diet (inclination between b and c). The number 16 results from the limitation of the AA concentration in the dietary protein (g/16 g N). Lys was adjusted as the limiting AA in the experimental diets. Consequently, the Lys requirement was derived according to Equation 3, and the Lys in the diet (%) was calculated as the Lys requirement (g/d) divided by the feed intake (g) multiplied by 100. The Lys percentage in the diet was multiplied by the DM content ratio and divided by 100 and then multiplied by the Lys digestibility factor. The feed consumption used in the simulation was calculated based on the recommendations of Rostagno et al. (2017) for growing pigs with 20 kg of BW. In addition, the difference in feed intake observed in the LPS-challenged group and the 0.10-fold increase or decrease to simulate the feed intake in these three scenarios for control and LPS-challenged animals was considered.
The N balance data were analyzed statistically by one-way ANOVA using the GLM procedure, and these data were adjusted to exponential models using the PROC NLIN procedure in SAS (Statistical Analysis System, version 9.4).
Results
Blood parameters
The effects of the dietary N concentration, immune system stimulation, and time on blood parameters and their interactions are shown in Figures 1 and 2 and Table 3. A three-way interaction (N levels × LPS challenge × time, P < 0.05) was observed for IgG, with a linear effect for the control group at 3 h and 96 h after LPS administration (Figure 1). A two-way interaction (LPS challenge × time, P <0.05) was observed for IgA, ceruloplasmin, transferrin, haptoglobin, α1AGp, PM 20,000, and total protein. A difference between the control and LPS-challenged groups was observed at 3 h after LPS administration for IgA, transferrin, haptoglobin, and total protein and after 96 h for IgA, ceruloplasmin, haptoglobin, APG, and PM 20,000 (Table 3).
Figure 1.
Interactive effects of nitrogen levels × challenge status × time (hours after first challenge) for serum IgG concentrations from growing pigs challenged with E. coli lipopolysaccharide (LPS). 1Nitrogen levels (0.94 to 3.97), % N as fed. 2Polynomial contrasts: Linear effect for the control group at 3 h (P = 0.0006), y = −0.4686x + 7.15, R2 = 86% and 96 h (P = 0.0059), y = −0.3646x + 7.466, R2 = 88%. 3LPS was used to induce an inflammatory response in the challenged groups and consisted of an initial dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM). A,BDifferent superscript capital letters indicate a difference between times (3 h × 96 h) using the Tukey test (α = 5%). a,bDifferent superscript letters indicate differences for challenge status (control × LPS-challenged) by the Tukey test (α = 5%).
Figure 2.
Interactive effects of nitrogen levels × time (hours after first challenge) for serum albumin, haptoglobin, transferrin and total protein concentrations from growing pigs challenged with E. coli lipopolysaccharide (LPS). 1Nitrogen levels (0.94 to 3.97), % N as fed. 2LPS was used to induce an inflammatory response in the challenged groups and consisted of an initial dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM). 3Polynomial contrasts: (A) Linear effect for albumin at 96 h (P < 0.0001), y = 0.7882x + 29.518, R2 = 67%; (B) Linear effect for haptoglobin at 3 h (P = 0.0467), y = 0.03x + 0.4147, R2 = 45% and 96 h (P = 0.0002), y = 0.0588x + 0.523, R2 = 87%; (C) Linear effect for total protein at 3 h (P = 0.0248), y = 0.4849x + 45.221, R2 = 53% and 96 h (P < 0.0001), y = 1.008x + 46.592, R2 = 78%; (D) Quadratic effect for transferrin at 3 h (P < 0.0001), y = 0.0228x2 - 0.0102x + 3.5785, R2 = 91%.a,bDifferent superscript letters indicate differences between nitrogen levels by the Tukey test (α = 5%).
Table 3.
Interactive effects of challenge status and time for serum proteins from growing pigs challenged with E. coli lipopolysaccharide1
| Interactions | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-value | |||||||||||||||
| Means, mg/mL | Challenge Status | Time | |||||||||||||
| Control | LPS-Challenged | CON x LPS |
CON x LPS |
CON x LPS |
CON | LPS | CON | LPS | LPS x Time |
||||||
| Item2 | Time (hours) | ||||||||||||||
| 0 | 3 | 96 | 0 | 3 | 96 | SEM | 0 | 3 | 96 | 3 × 96 | 3 × 96 | 0 × 3 × 96 | 0 × 3 × 96 | ||
| IgA | - | 1.11 | 0.97 | - | 1.28 | 1.41 | 0.077 | - | 0.011 | <0.01 | 0.077 | 0.077 | - | - | 0.001 |
| Cer | - | 0.48 | 0.47 | - | 0.51 | 1.04 | 0.029 | - | 0.535 | <0.01 | 0.820 | <0.01 | - | - | <0.01 |
| Trans | - | 4.05 | 4.27 | - | 3.73 | 4.21 | 0.132 | - | 0.001 | 0.598 | 0.016 | <0.01 | - | - | 0.006 |
| Hp | - | 0.47 | 0.55 | - | 0.57 | 0.91 | 0.046 | - | 0.039 | <0.01 | 0.004 | <0.01 | - | - | <0.01 |
| α1AGp | - | 0.04 | 0.04 | - | 0.05 | 0.03 | 0.002 | - | 0.125 | 0.024 | 0.512 | 0.001 | - | - | 0.006 |
| PM20000 | - | 1.60 | 1.84 | - | 1.52 | 1.38 | 0.085 | - | 0.331 | <0.01 | <0.01 | 0.012 | - | - | <0.01 |
| TP | 48.1A | 47.7Ba | 50.3A | 48.4A | 46.2Bb | 49.9A | 0.710 | 0.695 | 0.036 | 0.569 | - | - | <0.01 | <0.01 | 0.045 |
1 E. coli lipopolysaccharide (LPS) was used to induce inflammatory response in challenged group. The challenge consisted of an initial LPS dose of 30 μg/ kg IM and subsequent dose of 33.6 μg/ kg IM after 48 h. The control group received saline solution (0.9%, IM).
2Serum proteins Immunoglobulin A (IgA), Ceruloplasmin (Cer), Transferrin (Trans), Haptoglobin (Hp), α-1-acid glycoprotein (α1AGp), PM 20000 and Total Protein (TP) was measured using SDS-PAGE.
A,BDifferent superscripts capital letters indicate a difference between time (3 h × 96 h after first LPS challenge) using the Tukey test (α = 5%).
a,bDifferent superscripts letters indicate differences between thechallenge status(control × LPS-challenged) by the Tukey test (α = 5%).
A two-way interaction (N level × time, P < 0.05) was observed for transferrin, albumin, haptoglobin, and total protein. An effect of dietary N levels over time was observed for transferrin and albumin (2.09 to 3.97% of N), total protein (0.94 to 3.97% of N), and haptoglobin (0.94 to 2.09, 3.29, and 3.97% of N). Transferrin and haptoglobin protein concentrations exhibited linear increases and U-shaped quadratic responses, respectively, 3 h after LPS administration. Similarly, albumin, haptoglobin, and total protein all exhibited linear responses 96 h after LPS administration (Figure 2).
Rectal temperature
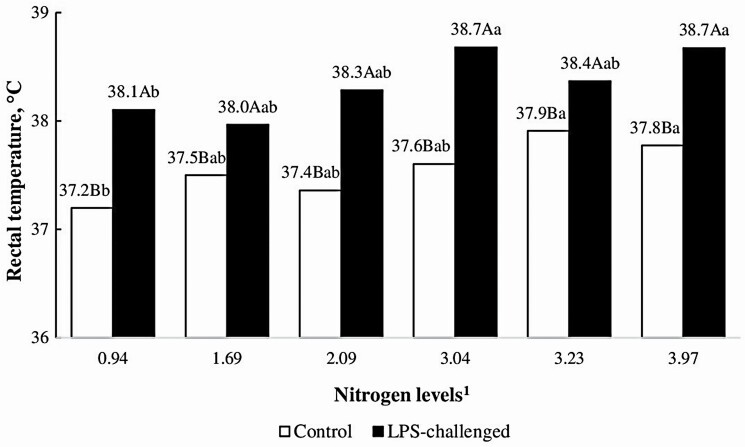
The effects of N levels, immune challenge and time, and their interactions on rectal temperature are shown in Figures 3 and 4. A two-way interaction (LPS challenge × time, P < 0.001) was observed. LPS challenge increased the rectal temperature in LPS-d1 + 3 h and LPS-d3 + 3 h. A measurement time effect was observed within each group, with an elevation of the rectal temperature after LPS-d1 + 3 h and LPS-d3 + 3 h for the LPS-challenged group.
Figure 3.
Interactive effects of challenge status × rectal temperature (°C) for growing pigs challenged with E. coli lipopolysaccharide (LPS). 1Measurements were carried out at 7:00 am (LPS-d1, LPS-d3, and LPS-d5) before the challenge and at 3:00 pm (LPS-d1 + 3 h and LPS-d3 + 3 h), 3 h after LPS injections. 2LPS was used to induce an inflammatory response in the challenged groups and consisted of an initial dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM). A,BDifferent superscript capital letters can differentiate the challenge status (control and LPS-challenged) between the times (3 h and 96 h after the first LPS challenge) using the Tukey test (α = 5%). a,b Different superscript letters indicate differences between nitrogen levels within each challenge status by the Tukey test (α = 5%).
Figure 4.
Interactive effects of nitrogen levels × rectal temperature (°C) for growing pigs challenged with E. coli lipopolysaccharide (LPS). 1Nitrogen levels (0.94 to 3.97), % N as fed. 2LPS was used to induce an inflammatory response in the challenged group. The challenge consisted of an initial LPS dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM). 3Polynomial contrasts: A linear effect of N levels on the control group was observed (P < 0.0001), y = 0.1243x + 37.122, R2 = 79%. A,BDifferent superscript capital letters indicate differences between the control and challenged groups within a nitrogen level (0.94 to 3.97% N, as fed) by the Tukey test (α = 5%). a,bDifferent superscript letters indicate differences between nitrogen levels for each group (control and LPS-challenged) by the Tukey test (α = 5%).
A two-way interaction (N levels × LPS challenge, P < 0.05) was observed. The LPS challenge raised the rectal temperature with all levels of N compared to the control group. The effect of the N level was greater for the control groups and LPS-challenged groups as the N content in the diet increased.
Nitrogen balance
Differences between the control and LPS-challenged groups were observed for feed consumption (P < 0.05) (Table 4), with a reduction in feed intake in the LPS-challenged group. A two-way interaction (N levels × LPS challenge) was observed (P < 0.05) for N intake, excretion, and deposition (mg/BWkg0.75/d), and the equations differed (P < 0.05) between groups. The linear regression for the N deposition in the control group is given by 127.86 + 246.95 N level (mg/BWkg0.75/d), and for the LPS-challenged group by 150.66 + 155.4 level of N (mg/BWkg0.75/d). LPS challenge negatively affected the capacity of N retention for growing pigs, where for each one-point increase in the level of N in the diet, the ND was reduced by 37%.
Table 4.
Average of feed intake (g/d) body weight (kg), nitrogen intake (mg/BWkg0.75/d), nitrogen excretion (mg/BWkg0.75/d), and nitrogen deposition (mg/BWkg0.75/d) obtained in nitrogen balance trials for growing pigs challenged with E. coli lipopolysaccharide1
| Nitrogen levels, % N as fed basis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables measured | 0.94 | 1.69 | 2.09 | 3.04 | 3.23 | 3.97 | Average | SEM | Linear | Quadratic |
| Feed Intake1 | ||||||||||
| Control | 411.9 | 417.2 | 419.1 | 418.8 | 413.3 | 426.6 | 417.8 | 9.70 | 0.532 | 0.917 |
| Challenged | 373.5 | 375.0 | 384.5 | 370.6 | 362.7 | 371.6 | 373.0 | 9.70 | 0.533 | 0.754 |
| Body Weight 2 | ||||||||||
| Control | 19.6 | 19.3 | 18.7 | 18.7 | 18.4 | 19.2 | 19.0 | 0.66 | 0.409 | 0.272 |
| Challenged | 19.4 | 19.0 | 18.8 | 18.7 | 18.7 | 19.1 | 18.9 | 0.66 | 0.272 | 0.661 |
| Nitrogen Intake 3,4 | ||||||||||
| Control | 519.7 | 958.5 | 1,216.0 | 1,773.3 | 1,883.0 | 2,309.7 | 1,443.3 | 24.82 | <0.001 | 0.029 |
| Challenged | 473.2 | 871.2 | 1,111.0 | 1,569.7 | 1,628.5 | 1,976.0 | 1,271.6 | 24.82 | <0.001 | 0.001 |
| P-value | 0.306 | 0.057 | 0.023 | <0.001 | <0.001 | <0.001 | ||||
| Nitrogen Excretion 3,4 | ||||||||||
| Control | 184.1 | 309.3 | 407.3 | 545.9 | 483.9 | 783.2 | 452.3 | 36.23 | <0.001 | 0.683 |
| Challenged | 246.0 | 369.5 | 478.2 | 626.7 | 848.6 | 884.9 | 575.6 | 36.23 | <0.001 | 0.474 |
| P-value | 0.249 | 0.262 | 0.188 | 0.135 | <0.001 | 0.061 | ||||
| Nitrogen Deposition 3,4 | ||||||||||
| Control | 335.6 | 649.2 | 808.6 | 1,227.3 | 1,405.9 | 1,526.5 | 992.2 | 42.13 | <0.001 | 0.028 |
| Challenged | 227.2 | 501.7 | 632.8 | 943.1 | 771.4 | 1091.1 | 694.6 | 42.13 | <0.001 | <0.001 |
| P_value | 0.079 | 0.018 | 0.005 | <0.001 | <0.001 | <0.001 |
1Effect of challenge (P < 0.01), E. coli lipopolysaccharide (LPS) was used to induce inflammatory response in challenged group. The challenge consisted of an initial LPS dose of 30 μg/kg IM and subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM).
2Body weightbefore samplingperiod.
3Effect of N levels × challenge status interaction (P < 0.05).
4Polynomial contrasts: Linear effect of N levels (P < 0.01): NI, control NI = 215.13 + 350.91 NL and challenged NI = 246.87 + 292.80 NL (mg/BWkg0.75/d); NEX, control NEX = 86.27 + 104.54 NL and challenged NEX = 97.27 + 136.69 NL (mg/BWkg0.75/d); ND, control ND = 127.86 + 246.95 NL and challenged ND = 150.66 + 155.40 NL (mg/BWkg0.75/d).
The effect of N levels was observed (P < 0.05) on the N intake, N excreted, and N deposited (mg/BWkg0.75/d) between the groups. There was a reduction (P < 0.05) in the N intake from 1.69% N and in the N deposited from 2.09% N for the LPS-challenged group; however, the excreted N was higher with 3.29% N.
Model parameters
The nonlinear models, used to determined NMR (Equation 1) and NRmaxT (Equation 2), are shown in Figures 5 and 6. In response to LPS challenge, there was a 22.4% increase in NMR. The results of the nonlinear regression fitting between NI and NR demonstrated that NRmaxT decreased by 41.1% in the LPS-challenged group compared with control (non-LPS-challenged) pigs. The estimated requirements for digestible Lys are shown in Table 5 and are dependent on the rate of body protein deposition, the efficiency of Lys use, and the immune activation status of pigs. Based on the derived model parameters and the response of the pigs in the control and LPS-challenged groups required to achieve 0.68 and 0.54 times the NRmaxT value, the estimated digestible lysine requirements were 1,994.83 and 949.16 mg/BWkg0.75/d, respectively, for control and LPS-challenged pigs. The criterion for selecting the percentage for the NRmaxT value was taken from the mean ND value for each group. The daily digestible Lys intake required was 18.12 and 8.62 g/d for the control and LPS-challenged groups, respectively, and the optimal dietary Lys concentration may change depending on the feed intake levels. LPS-challenged animals were projected to have a lower estimated digestible Lys requirement than nonchallenged pigs.
Figure 5.
Estimation of the nitrogen requirements for maintenance (NMR) by fitting an exponential function between the nitrogen intake (NI) and nitrogen excretion (NEX) during a gradual increase in supplied protein limited in lysine for growing pigs (Sire line 337 x Dam line Camborough, PIC) challenged with E. coli lipopolysaccharide (LPS). Observed (♦) and predicted (—) values for the control group. Observed (□) and predicted (-----) values for the challenged group. 1Nitrogen intake (NI) and nitrogen excretion (NEX) were obtained in nitrogen balance trials of 11 d. The nitrogen balance data were used in the nonlinear model established by the University of Goettingen. 2LPS was used to induce an inflammatory response in the challenged group. The challenge consisted of an initial LPS dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM).
Figure 6.
Estimation of the theoretical potential for nitrogen deposition (NDmaxT) in growing pigs (Sire line 337 × Dam line, Camborough, PIC) challenged with E. coli lipopolysaccharide (LPS), based on the ratio of nitrogen intake (NI) and nitrogen deposition (ND). Observed (♦) and predicted (—) values for the control group. Observed (□) and predicted (-----) values for the challenged group. 1Nitrogen intake (NI) and nitrogen excretion (NEX) were obtained in nitrogen balance trials of 11 d. The ND was calculated as a result of the difference between N intake and N excretion, ND = NI – NEX (mg/BWkg0.75/d). The nitrogen balance data were used in the nonlinear model established by the University of Goettingen. 2LPS was used to induce an inflammatory response in the challenged group. The challenge consisted of an initial LPS dose of 30 μg/kg IM and a subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM).
Table 5.
Model calculation of the lysine requirement (Lys) for growing pigs (Sire line 337 × Dan line Camborough, PIC) challenged with E. coli lipopolysaccharide2, depending on the determined efficiency of lysine utilization and different predictions for feed intake
| Item | Control | Challenged | ||
|---|---|---|---|---|
| NDmaxT1, mg/ BWkg0.75/ d | 3,524.7 | 2,077.8 | ||
| NDmaxT, % | 68 | 54 | ||
| Protein deposition, g/d | 136 | 64 | ||
| Lys efficiency, bc-1 | 0.00003703 | 0.00005713 | ||
| Lys requirement, mg/ BWkg0.75/d | 1,994.83 | 949.16 | ||
| Lys requirement, g/d | 18.12 | 8.62 | ||
| Optimal dietary lysine concentration | ||||
| Feed intake3 g/d | Digestible Lys, % | Feed intake3 g/d | Digestible Lys, % | |
| 1,100 | 1.29 | 981 | 0.69 | |
| 1,000 | 1.42 | 893 | 0.76 | |
| 900 | 1.58 | 803 | 0.84 | |
| 800 | 1.77 | 714 | 0.94 |
1NDmaxT is the theoretical maximum nitrogen deposition.
2 E. coli lipopolysaccharide (LPS) was used to induce inflammatory response in challenged group. The challenge consisted of an initial LPS dose of 30 μg/ kg IM and subsequent dose of 33.6 μg/kg IM after 48 h. The control group received saline solution (0.9%, IM).
3The feed intake was calculated based on the recommendations of Rostagno et al. (2017) for pigs of 20 kg. Feed intake was adjusted for the challenged group, considering the 10.73% reduction in feed intake.
Discussion
This study aimed to modeling digestible lysine requirements via nitrogen balance assay in growing pigs challenged with E. coli LPS and established the key parameters of the nonlinear model (NMR, NRmaxT, efficiency of lysine utilization) that is need to model. It was possible to estimate the requirement for Lys according to the rate of protein deposition, the efficiency of the use of Lys, and the inflammatory response through these parameters. An established model to induce an inflammatory response through repeated doses of E. coli LPS was used, inducing a controlled and relatively moderate inflammatory response, thereby allowing the direct investigation of nutrient utilization (Ridder et al., 2012; Litvak et al., 2013; Rakhshandeh et al., 2014; Mc Gilvray et al., 2018). The effect of this model of stimulation was evidenced 3 h after the start of the initial LPS administration, which was marked by an increase in rectal temperature and serum concentrations of acute and chronic response proteins and a reduction in feed intake. Collectively, these clinical signs are compatible with those reported in the literature for LPS-challenged pigs (Campos et al., 2014; Mc Gilvray et al., 2018).
Acute-phase proteins represent nonspecific markers of the different inflammatory etiologies as part of routine monitoring of animal health (Petersen et al., 2004). Among them, haptoglobin has been listed as the main acute-phase protein in pigs (Alava et al., 1997; Petersen et al. 2004). As expected, haptoglobin demonstrated higher sensitivity and stability than other acute-phase proteins, with pigs exhibiting a significant increase in haptoglobin within 3 h of the initial LPS administration and maintaining this inflammatory profile through 96 h after injection (Campos et al., 2014). The difference in the response pattern of the different proteins evaluated is related to individual characteristics (Gruys et al., 1994; Kaneko, 1997, Eckersall, 2008), as α1AGp, ceruloplasmin, and haptoglobin are considered acute-phase positive proteins since their concentration in the blood increases in response to the challenge and the proteins albumin and transferrin have a reduction in their levels and are considered proteins of the acute negative phase (Gruys et al., 1994; Kaneko, 1997, Eckersall, 2008), as observed in this study.
In general, the interaction between challenge and response time demonstrates that the concentration of acute-phase proteins is rapidly elevated in the blood compared to specific (late-response) immunoglobulins. Despite the increase in IgA and IgG concentrations within 3 h of LPS administration, the response was accentuated at the 96-h time point. This response pattern allows the monitoring of the progression of the inflammatory response through the concentration of immunoglobulins, such as IgA and IgG, indicating in this study that the challenge was consistent throughout the collection period.
In addition to the interaction between LPS challenge and response time, the diet also influenced the inflammatory response, as observed by the effect of experimental diets on acute- and chronic-phase proteins. As the concentration of crude protein in the diet increased, the inflammatory response was more exaggerated, especially in the LPS-challenged group (Le Floc´h et al., 2004; Kampman, 2015). According to the literature, the impact of the challenge on protein (and consequently amino acid) metabolism can generate specific nutritional requirements, for aromatic amino acids, threonine, glutamine, and arginine (Le Floc´h et al., 2004). Under this condition, due to changes in specific metabolic pathways, the amino acids resulting from muscle catabolism are different from those required for the maintenance of the inflammatory and immune response, leading to the relative excess of nonlimiting amino acids. In contrast, other amino acids become limiting for the immune response as body proteins are repartitioned while the animal experiences an inflammatory challenge (Reeds and Jahoor, 2001). In this context, the reduction in nitrogen (from 2.09% to 0.94% N) may have limited the expression of the inflammatory response, prioritizing some acute-phase proteins to the detriment of others according to the importance of each one. This hypothesis highlights the relevance of haptoglobin as an essential biomarker of the inflammatory response of pigs. It also indicates that the ratio of amino acids in the ideal protein paradigm needs to be changed for pigs experiencing an inflammatory challenge.
The interaction found between N levels and rectal temperature measurements for the control and LPS-challenged groups can be attributed to different factors mediated by inflammation and nutrient metabolism. The increase in rectal temperature in animals 3 h after LPS challenge (LPS-d1 + 3h; LPS-d3 + 3h) compared with the control group was expected since the increase in rectal temperature is a consequence of proinflammatory cytokine signaling (Johnson, 1998; Petry et al., 2017). A similar response of rectal temperature to inflammatory challenge was observed by Campos et al. (2014), where the rectal temperatures throughout the LPS challenge were higher relative to baseline, and the rectal temperatures were higher at the first injection of LPS than at the second 48 h later. The effect of diets was also related to the increased metabolism of animals in this study, with increased protein metabolism due to excess N supplementation, leading to the rise in rectal temperature at the highest levels. The increase in metabolism may also be associated with body temperature, as homeothermic temperature regulation is achieved through a balance between the production of heat by metabolism and its loss (Henken et al., 1993; Pedersen and Sallvik, 2002). The second is related to the caloric increase considering the pre- and postprandial state. Due to the metabolic changes presented under challenging conditions, the ratio of protein synthesis to degradation is shifted to a catabolic state, potentiating turnover (Le Floc´h et al., 2004).
Under these conditions, the association between increased N excretion per unit of N intake is common, resulting in less N retention (Rakhshandeh and de Lange, 2011). The same relationship was found in this study, as evidenced by the interaction between LPS challenge and N intake, excretion, and deposition. According to McGilvray et al. (2019), the challenge not only reduces protein retention by changing the protein synthesis and degradation ratio but also by lowering the deposition efficiency.
Through the regression equations involving N deposition as a function of N intake, we observed that the LPS challenge negatively affected the N retention capacity of growing pigs, where each one-point increase in the dietary N level reduced the amount of N deposited by 37% (mg/BWkg0.75/d). The influence of the challenge on N retention was similar to that reported in the literature, where the stimulation of the immune system through repeated doses of E. coli LPS influenced the reduction in protein deposition from 3% to 20% in pigs weighing approximately 20 kg (Ridder et al., 2012; Litvak et al., 2013).
The nitrogen balance data were used in the nonlinear model. The effects of the different interactions between the inflammatory response and the nitrogen balance are reflected in the NMR since endogenous basal losses in feces and urine and protein turnover impact it (Moughan, 2008). The determination of NMR is described by Equation 1 and represents the inevitable metabolic losses of nitrogen (Wecke and Liebert, 2009). Within the model, NMR is obtained by mathematical extrapolation; however, the 22.4% increase in LPS-challenged animals demonstrates alteration in basal metabolism, characterized by higher protein mobilization to maintain the inflammatory response and altered nitrogen balance. Simultaneously, the impact of the challenge on protein metabolism compromised the ability to deposit muscle protein, consequently leading to a 41.1% reduction in NRmaxT in the LPS-challenged group. In this study, the model was responsive to the change in protein metabolism caused by LPS challenge and allowed the estimation of dietary Lys requirements for challenged pigs, associating the practicality of the nitrogen balance and the segmentation of maintenance and production requirements observed by the factorial model. The data obtained through nitrogen balance (NMR, NRmaxT) are essential for supplying a database for later application of models, including modeling for estimating the amino acid requirement based on daily protein deposition and efficiency of use of the limiting amino acid in the diet (Samadi and Liebert, 2006a). Perhaps, the LPS challenge did cause an increase in NMR and a concomitant decrease in NRmaxT that was responsible for reducing the digestible Lys requirement in this group.
The body mainly uses Lys to synthesize body proteins, representing approximately 80% of its use in young animals (Klasing, 2009). Given this characteristic, the high variation in its requirement in the literature can be associated with the difference in the potential for gaining lean mass due to the different genetics available on the market. In comparison, weaned pigs (5–20 kg) in the 1990s had an average daily gain (ADG) of 350 g/d. With the advancement of genetic improvement, the current literature shows the evolution of the protein deposition capacity for the same category, with gains close to 500 g/d (NRC, 1998; Dean et al., 2007), reaching 620 g/d for pigs weighing 15 to 30 kg (Rostagno et al., 2017). The constant evolution of animal breeding reflects the need to update swine nutritional requirements.
The estimate of the digestible Lys requirement of 18.12 g/d and the optimal dietary lysine concentration of 1.42% (considering 1000 g of feed intake) is in agreement with Graham et al. (2017) and Fruge et al. (2017). In these studies, the minimum requirement for ADG was 1.25%, reaching 1.40% for feed conversion. These results indicate that the nonlinear model was able to adjust the Lys requirements of healthy pigs, providing support for the estimation in challenged pigs.
As expected, we observed a reduction in the Lys requirement for LPS-challenged pigs. This result was similar to Williams et al. (1997a, b), where pigs with low levels of chronic immune system activation required higher dietary Lys concentrations and daily Lys intakes for body growth and protein accretion than pigs with high levels of chronic immune system activation. Pigs from 6 to 112 kg in low-level chronic immune system activation required.15 to.30 percentage units greater dietary Lys concentrations and 2 to 5 g higher daily Lys intakes for each 14-kg BW increment (Williams et al., 1997b). This behavior is directly related to the use of Lys for the synthesis of muscle protein, which, due to the lower protein deposition capacity, becomes less demanding (Littiere et al., 2017).
In face of a challenging condition, the requirement for Lys decreases in relation to other amino acids (Kampman, 2015; Mc Gilvray et al., 2018) due to its lesser involvement in the maintenance of the immune response. In this study, the estimated requirement for digestible Lys was 8.62 g/d, and the optimal dietary lysine concentration of 0.76% was based on 0.54 times NRmaxT due to the reduction in consumption (893 g of feed intake) and N retention due to the challenge induced by LPS.
The nonlinear model we employed made it possible to quantify the effect of the immune challenge on the Lys requirement for protein deposition, considering the inflammatory response in growing pigs. However, the estimated requirement for challenged pigs is not intended to achieve the same performance as control animals. Although our results for control group are in accordance with the literature (Vier et al., 2016; Graham et al., 2017; Fruge et al., 2017; Kahindi et al., 2017), it is necessary to consider that the recommendation of the digestible Lys requirement also depends on other factors, such as genotype, environment, feed intake levels, the ratio of the amino acids supplied, and the intensity and type of health challenges, that can be studied in future models.
In conclusion, stimulation of the inflammatory response through repeated injections of E. coli LPS altered protein metabolism, thereby influencing the reduction in N intake and deposition and increasing N excretion. Inflammatory mediator responses, especially haptoglobin, are useful outcomes to identify and monitor the progression of the immune response. The nonlinear model adjustment associated with the nitrogen balance allowed estimation of model parameters (NMR, NRmaxT) for growing pigs challenged with E. coli LPS, indicating an increase in NMR by 22.4% and a reduction in NRmaxT of 41.1%. Additionally, the modeling procedure including control and LPS-challenged pigs provided different estimates of the maximum genetic potential for nitrogen retention, namely, 0.68 and 0.54 times that of NRmaxT, respectively, and the digestible Lys requirements were estimated to be 1,994.83 and 949.16 mg/BWkg0.75/d, respectively, for control and LPS-challenged pigs. The daily digestible Lys intake required was 18.12 and 8.62 g/d for the control and LPS-challenged groups, respectively, and the optimal dietary Lys concentration may change depending on the feed intake levels.
In general, the differences in the nutritional requirements of the animals due to factors such as immunological challenge must be considered for the determination of swine feeding programs, and the applied modeling approach allows the calculation of the requirements of the first limiting amino acid for pigs according to the conditions of the immune challenge.
Acknowledgments
We appreciate the financial support from FAPEMIG, CAPES, and INCt for students and the Animal Science Department and PIC partner for supporting animal breeding at UEPE Swine, UFV.
Glossary
Abbreviations
- AA
amino acid
- ADG
average daily gain
- ANOVA
analysis of variance
- bc-1
lysine efficiency
- BW
body weight
- BW0.75
metabolic weight
- CP
crude protein
- DM
dry matter
- HCl
hydrochloric acid
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IM
intramuscular
- LPS
E. coli lipopolysaccharide
- ND
nitrogen deposition
- NDmaxT
theoretical maximum nitrogen deposition
- NEX
nitrogen excretion
- NMR
nitrogen maintenance requirement
- NR
nitrogen retention
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- α1AGp
α-1-acid glycoprotein
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Alava, M. A., González-Ramón N., Heegaard P., Guzylack S., Toussaint M. J. M., Lipperheide C. M., Gruys F., Echersall E., P. D., Lampreave, . et al. 1997. Pig-MAP, porcine acute phase proteins and standardization of assays in Europe. Comp. Haematol. Int. 7:208–213. doi: 10.1007/BF02658691 [DOI] [Google Scholar]
- Association of official analytical chemists—AOAC . 2006. Official methods of analysis. 18th ed. Gaithersburg (MD): AOAC International. [Google Scholar]
- Campos, P. H., Merlot E., Damon M., Noblet J., and Le Floc’h N.. . 2014. High ambient temperature alleviates the inflammatory response and growth depression in pigs challenged with Escherichia coli lipopolysaccharide. Vet. J. 200:404–409. doi: 10.1016/j.tvjl.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Ceron, M. S., Oliveira V., Lovatto P. A., and Vale M. M.. . 2013. Maintenance requirement and deposition efficiency of lysine in pigs. Pesq. Agropec. Bras. 48:1269–1274. doi: 10.1590/S0100-2013000900011 [DOI] [Google Scholar]
- Dean, D. W., Southern L. L., Kerr B. J., Bidner T. D.. . 2007. The lysine and total sulfur amino acid requirements of six to twelve - kilogram pigs. J. Anim. Sci. 23:527–535. doi: 10.1532/S1080-7446(15)31015-9 [DOI] [Google Scholar]
- Dorigam, J. C. P., Sakomura N. K., Soares L., Fernandes J. B. K., Sünder A., and Liebert F.. . 2017. Modelling of lysine requirement in broiler breeder hens based on daily nitrogen retention and efficiency of dietary lysine utilization. Anim. Feed Sci. Technol. 226:29–38. doi: 10.1016/j.anifeedsci.2016.12.003 [DOI] [Google Scholar]
- Eckersall, P. D. 2008. Proteins, proteomics and the dysproteinemias. In: Kaneko J. J., Harvey J. W., Bruss M. L., editors, Clinical biochemistry of domestic animals. 6th ed. Burlington: Academic Press. p. 117–155. [Google Scholar]
- Fisher, C., and Morris T. R.. . 1970. The determination of the methionine requirement of laying pullets by a diet dilution technique. Br. Poult. Sci. 11: 67–82. doi: 10.1080/00071667008415793 [DOI] [Google Scholar]
- Fruge, E. D., Gerhart A. J., Hansen E. L., Hansen S. A., and Coble K. F.. . 2017. Effects of increasing standardized ileal digestible (SID) lysine on performance of 11 to 23 kg nursery pigs. J. Anim. Sci. 95:102–103. (Abstr.) doi: 10.2527/asasmw.2017.12.213 [DOI] [Google Scholar]
- Graham, A., Knopf B., Greiner L., Goncalves M. A. D., Orlando U. A. D., and Connor J.. . 2017. Evaluation of the lysine requirement of eleven- to twenty-three-kilogram nursery pigs. J. Anim. Sci. 95:146–147 (Abstr.). doi: 10.2527/asasmw.2017.301 [DOI] [Google Scholar]
- Gruys, E., Obwolo M. J., and Tousaint M. J. M.. . 1994. Diagnostic significance of the major acute phase proteins in veterinary clinical chemistry: a review. Vet. Bull. Farnham Royal. 64:1009–1018. [Google Scholar]
- Hauschild, L., Sakomura N. K., and Silva E. P.. . 2015. Avinesp Model: predicting poultry growth, energy and amino acid requirements. In: Sakomura N. K., Gous R. M., Kyriazakis I., Hauschild L., editors. Nutritional modelling for pigs and poultry. Wallingford: CAB International. p. 188–207. doi: 10.1079/9781780644110.0188 [DOI] [Google Scholar]
- Henken, A. M., Brandsma H. A., van der Hel W., and Verstegen M. W.. . 1993. Circadian rhythm in heat production of limit-fed growing pigs of several breeds kept at and below thermal neutrality. J. Anim. Sci. 71:1434–1440. doi: 10.2527/1993.7161434x [DOI] [PubMed] [Google Scholar]
- Htoo, J. K., Oliveira J. P., Albino L. F. T., Hannas M. I., Barbosa N. A. A., and Rostagno H. S.. . 2016. Bioavailability of L-lysine HCl and L-lysine sulfate as lysine sources for growing pigs. J. Anim. Sci. 94:253–256. doi: 10.2527/jas.2015-9797 [DOI] [Google Scholar]
- Johnson, R. W. 1998. Immune and endocrine regulation of food intake in sick animals. Domest. Anim. Endocrinol. 15:309–319. doi: 10.1016/s0739-7240(98)00031-9 [DOI] [PubMed] [Google Scholar]
- Kahindi, R. K., Htoo J. K., and Nyachoti C. M.. . 2017. Dietary lysine requirement for 7-16 kg pigs fed wheat-corn-soybean meal-based diets. J. Anim. Physiol. Anim. Nutr. (Berl). 101:22–29. doi: 10.1111/jpn.12491 [DOI] [PubMed] [Google Scholar]
- Kampman-Van De Hoek, E. 2015. Impact of health status on amino acid requirements of growing pigs: towards feeding strategies for farms differing in health status. PhD Diss., Wageningen University, Wageningen, the Netherlands. [Google Scholar]
- Kaneko, J. J. 1997. Serum proteins and the dysproteinemias. In: Kaneko J. J., Harvey J. W., Bruss M.L., editors. Clinical biochemistry of domestic animals. San Diego (CA): Academic Press. p. 117–138. [Google Scholar]
- Khan, D. R., Wecke C., Sharifi A. R., and Liebert F.. . 2015. Evaluating the age-dependent potential for protein deposition in naked neck meat type chicken. Animals (Basel). 5:56–70. doi: 10.3390/ani5010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing, K. C. 2009. Minimizing amino acid catabolism decreases amino acid requirements. J. Nutr. 139:11–12. doi: 10.3945/jn.108.099341 [DOI] [PubMed] [Google Scholar]
- Kvidera, S., and Kay S.. . 2017. Causes and consequences of immune activation and its effect on metabolic and energetic status in production animals. Graduate Theses and Dissertations. Iowa State University. p. 15344. [Google Scholar]
- Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. doi: 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Le Floc’h, N., Melchiora D., and Oble C.. . 2004. Modifications of protein and amino acid metabolism during inflammation and immune system activation. Livest Prod Sci. 87:37–45. doi: 10.1016/j.livprodsci.2003.09.005 [DOI] [Google Scholar]
- Liebert, F. 2015. Basics and applications of an exponential nitrogen utilization model (‘Goettingen approach’) for assessing amino acid requirements in growing pigs and meat type chickens based on dietary amino acid efficiency. In: Sakomura N.K., Gous R.M., Kyriazakis I., Hauschild L., editors, Nutritional modelling for pigs and poultry. Wallingford: CAB International. p. 73–87. doi: 10.1079/9781780644110.0073 [DOI] [Google Scholar]
- Littiere, T. O., Campos P. H. R. F., Merlot E., Renaudeau D., Noblet J., and Le Floc’h N.. . 2017. Lysine, threonine and tryptophan postprandial metabolism in LPS challenged growing pigs. IN: 54ª. Reunião Anual da Sociedade Brasileira de Zootecnia. Anais da 54ª. Reunião Anual da Sociedade Brasileira de Zootecnia; | Foz do Iguaçu. [Google Scholar]
- Litvak, N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. . 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Lucas, B., and Sotelo A.. . 1980. Effect of different alkalies, temperature, and hydrolysis times on tryptophan determination of pure proteins and of foods. Anal. Biochem. 109:192–197. doi: 10.1016/0003-2697(80)90028-7 [DOI] [PubMed] [Google Scholar]
- Mc Gilvray, W. D., Johnson B., Wooten H., Rakhshandeh A. R., and Rakhshandeh A.. . 2019. Immune system stimulation reduces the effiency of whole-body protein deposition and alters muscle fiber characteristics in growing pigs. Animals 9:323. doi: 10.3390/ani9060323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Gilvray, W. D., Klein D., Wooten H., Dawson J. A., Hewitt D., Rakhshandeh A. R., de Lange C. F. M., and Rakhshandeh A.. . 2018. Immune system stimulation induced by Escherichia coli lipopolysaccharide alters plasma free amino acid flux and dietary nitrogen utilization in growing pigs. J. Anim. Sci. 97:315–326. doi: 10.1093/jas/sky401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughan, P. J. 2008. Efficiency of amino acid utilization in simple-stomached animals and humans—a modeling approach. In: France J., Kebreab E., Editors. Mathematical modelling in animal nutrition. Palmerston North: Massey Univesity, London. p. 241–253. doi: 10.1079/9781845933548.0241 [DOI] [Google Scholar]
- NRC. 1998. Nutrient requirements of swine. 10th edn. Washington (DC): National Academic Press. [Google Scholar]
- Pastor, A., Wecke C., and Liebert F.. . 2013. Assessing the age-dependent optimal dietary branched-chain amino acid ratio in growing chicken by application of a nonlinear modeling procedure. Poult. Sci. 92:3184–3195. doi: 10.3382/ps.2013-03340 [DOI] [PubMed] [Google Scholar]
- Pedersen, S., and Sallvik K.. . 2002. 4th report of working group on climatization of animal houses. Heat and moisture production at animal and house levels. International Commission of Agricultural Engineering, Section II. Research Centre Bygholm, Danish Institute of Agricultural Sciences. Available from www.agrisci.dk/jbt/spe/CIGReport.
- Petersen, H. H., Nielsen J. P., and Heegaard P. M.. . 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. doi: 10.1051/vetres:2004002 [DOI] [PubMed] [Google Scholar]
- Petry, A., McGilvray W., Rakhshandeh A. R., and Rakhshandeh A.. . 2017. Technical note: assessment of an alternative technique for measuring body temperature in pigs. J. Anim. Sci. 95: 3270–3274. doi: 10.2527/jas.2017.1566 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh, A., and De Lange C. F. M.. . 2011. Immune system stimulation in the pig: effect on performance and implications for amino acid nutrition. In: Van Barnevled R. J., editor. Manipulating pig production XIII. Werribee, Victoria, Australia: Australasian Pig Science Association Incorporation. p. 31–46. [Google Scholar]
- Rakhshandeh, A., Htoo J. K., Karrow N., Miller S. P., De Lange C. F. M.. . 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilization of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955 [DOI] [PubMed] [Google Scholar]
- Reeds, P. J., and Jahoor F.. . 2001. The amino acid requirement of disease. Clin. Nutr. 20:15–22. doi: 10.1054/clnu.2001.0402 [DOI] [Google Scholar]
- de Ridder, K., Levesque C. L., Htoo J. K., and de Lange C. F.. . 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830 [DOI] [PubMed] [Google Scholar]
- Rostagno, H. S., Albino L. F. T., and Donzele J. L., . et al. 2017. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. 4rd ed. UFV, Viçosa. p. 488. [Google Scholar]
- Sakomura, N. K., and Rostagno H. S.. . 2016. Métodos de pesquisa em nutrição de monogástricos. 2 ed. Editor FUNEP, Jaboticabal. [Google Scholar]
- Samadi, F., and Liebert F.. . 2006a. Estimation of nitrogen maintenance requirements and potential for nitrogen deposition in fast-growing chickens depending on age and sex. Poult. Sci. 85:1421–1429. doi: 10.1093/ps/85.8.1421 [DOI] [PubMed] [Google Scholar]
- Samadi, F., and Liebert, F. 2006b. Modeling of threonine requirement in fast- growing chickens, depending on age, sex, protein deposition, and dietary threonine efficiency. Poultry Sci. 85:1961–1968. [DOI] [PubMed] [Google Scholar]
- Samadi, F., and Liebert, F. 2007. Lysine requirement of fast growing chickens - effect of age, sex, level of protein deposition and dietary lysine efficiency. J. Poult. Sci. 44:63–72. doi: 10.1093/ps/86.6.1140 [DOI] [PubMed] [Google Scholar]
- Samadi, F., and Liebert, F. 2008. Modelling the optimal lysine to threonine ratio in growing chickens depending on age and efficiency of dietary amino acidutilization. Br. Poult. Sci. 49:45–54. doi: 10.1080/00071660701821667 [DOI] [PubMed] [Google Scholar]
- SAS—Statistical analyses system. Statistical analysis system user’s guide. Version 9.4. 2013 Cary: Statistical Analysis System Institute. [Google Scholar]
- Vier, C. M., De Souza I. B., De Jong J. A., Goncalves M. A. D., Jones A. M., Goodband R. D., Tokach M. D., Derouchey J. M., Woodworth J. C., and Dritz S. S.. . 2016. Determining the standardized ileal digestible lysine requirement of 6.8 to 15.9 kg pigs. J. Anim. Sci. 94:191 (Abstr). doi: 10.2527/msasas2016-408 [DOI] [Google Scholar]
- Wecke, C., and Liebert F.. . 2009. Lysine requirement studies in modern genotype barrows dependent on age, protein deposition and dietary lysine efficiency. J. Anim. Physiol. Anim. Nutr. (Berl). 93:295–304. doi: 10.1111/j.1439-0396.2009.00923.x [DOI] [PubMed] [Google Scholar]
- White, J. A., Hart R. J., and Fry J. C.. . 1986. An evaluation of the Waters Pico-Tag system for the amino-acid analysis of food materials. J. Automat. Chem. 8:170–177. doi: 10.1155/S1463924686000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, N. H., Stahly T. S., and Zimmerman D. R.. . 1997a. Effect of chronic immune system activation on the rate, efficiency, and composition of growth and lysine needs of pigs fed from 6 to 27 kg. J. Anim. Sci. 75:2463–2471. doi: 10.2527/1997.7592463x [DOI] [PubMed] [Google Scholar]
- Williams, N. H., Stahly T. S., and Zimmerman D. R.. . 1997b. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J. Anim. Sci. 75:2481–2496. doi: 10.2527/1997.7592481x [DOI] [PubMed] [Google Scholar]