Abstract
Three penetrating captive bolt (PCB) placements were tested on cadaver heads from swine with estimated body weight (BW) >200 kg (sows = 232.9 ± 4.1 kg; boars = 229.3 ± 2.6 kg). The objectives were to determine tissue depth, cross-sectional brain area, visible brain damage (BD), regions of BD, and bolt–brain contact; and determine relationships between external head dimensions and tissue depth at each placement. A Jarvis PAS-Type P 0.25R PCB with a Long Stunning Rod Nosepiece Assembly and 3.5 g power loads was used at the following placements on heads from 111 sows and 46 boars after storage at 2 to 4 °C for ~62 h before treatment: FRONTAL (F)—3.5 cm superior to the optic orbits at midline, TEMPORAL (T)—at the depression posterior to the lateral canthus of the eye within the plane between the lateral canthus and the base of the ear, or BEHIND EAR (BE)—directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye. For sows, the bolt path was in the plane of the brain for 42/42 (100%, 95% confidence interval [CI]: 91.6% to 100.0%) F heads, 39/40 (97.5%, 95% CI: 86.8% to 99.9%) T heads, and 34/39 (87.5%, 95% CI: 72.6% to 95.7%) BE heads; for the heads that could reliably be assessed for BD damage was detected in 25/26 (96.2%, 95% CI: 80.4% to 99.9%) F heads, 24/35 (68.6%, 95% CI: 50.7% to 83.2%) T heads, and 5/40 (12.5%, 95% CI: 4.2% to 26.8%) BE heads. For boars, the bolt path was in the plane of the brain for 17/17 (100.0%, 95% CI: 80.5% to 100.0%) F heads, 18/18 (100.0%, 95% CI: 81.5% to 100.0%) T heads, and 14/14 (100.0%, 95% CI: 76.8% to 100.0%) BE heads; damage was detected in 11/12 (91.7%, 95% CI: 61.5% to 99.8%) F heads, 2/15 (13.3%, 95% CI: 1.7% to 40.5%) T heads, and 7/14 (50.0%, 95% CI: 23.0% to 77.0%) BE heads. Tissue depth was reported as mean ± standard error followed by 95% one-sided upper reference limit (URL). For sows, total tissue thickness was different (P < 0.05) between placements (F: 52.7 ± 1.0 mm, URL: 64.1 mm; T: 69.8 ± 1.4 mm, URL: 83.9 mm; BE: 89.3 ± 1.5 mm, URL: 103.4 mm). In boars, total tissue thickness was different (P < 0.05) between placements (F: 41.2 ± 2.1 mm, URL: 56.3 mm; T: 73.2 ± 1.5 mm, URL: 83.4 mm; BE: 90.9 ± 3.5 mm, URL: 113.5 mm). For swine > 200 kg BW, F placement may be more effective than T or BE due to less soft tissue thickness, which may reduce concussive force. The brain was within the plane of bolt travel for 100% of F heads with BD for 96.2% and 91.7% of F sow and boar heads, respectively.
Keywords: boar, captive bolt, euthanasia, sow, swine, welfare
Introduction
Penetrating captive bolt (PCB) is a method of euthanasia approved for use in nonsuckling swine, including sows and boars, by the American Veterinary Medical Association (AVMA, 2013; AVMA, 2020) and jointly approved by the National Pork Board and American Association of Swine Veterinarians (NPB and AASV, 2016). Although the method is approved, and commonly used, it has not been scientifically validated as effective for sows and boars estimated to be over 200 kg (Woods, 2012). Specifically, Woods (2012) found that the common frontal application was not 100% effective in sows and boars that were visually estimated to be over 200 kg in trials with anesthetized animals and field trials on production sites. The most common PCB placement for pigs is a frontal placement (AVMA, 2013; NPB and AASV, 2016; AVMA, 2020); however, two alternative placements have been identified: temporal and behind the ear toward the opposite eye (AVMA, 2013). The temporal and behind the ear placements were recently described for gunshot only (AVMA, 2020). Validation studies for the temporal placement did not exist in the published literature at the time of this study and the only published data to validate the behind the ear placement was focused on market weight pigs (Anderson et al., 2019). Our study was designed to serve as a first step in scientifically validating the behind the ear and temporal PCB placement for sows and boars larger than 200 kg body weight (BW).
By definition, the objective of euthanasia is to ensure a good death, which includes the “rapid loss of consciousness followed by cardiac or respiratory arrest and, ultimately, a loss of brain function” (AVMA, 2020). However, the magnitude of distress experienced by an animal at the time of death may be highly variable depending on the selected euthanasia method (OIE, 2016; AVMA, 2020). Thus, how an animal dies, including the potential for distress, needs to be considered when selecting euthanasia methods.
Additionally, the Humane Slaughter Association (HSA) described pigs as one of the most difficult species to effectively stun with a PCB, in part due to the smaller target area of the brain compared with other species and in part due to the location of the brain beyond the sinus cavities in pigs (HSA, 2013). In mature sows and boars, a PCB may fail to penetrate the brain cavity because of the more developed and expansive sinus cavities and a bony ridge along the front of the skull (Woods et al., 2010; HSA, 2013). Additionally, ensuring correct aim and direction of PCB placement may be difficult for mature sows and boars as a result of the variety of forehead face shapes in these animals (EFSA, 2020). The NPB and AASV recognized that PCB may only be sufficient to stun, and require a second step to effectively euthanize, pigs as they mature due to the increased cranial thickness, along with the developed sinus cavities and bony ridge previously described (NPB and AASV, 2016).
The objectives of this study were (a) to determine the tissue depth, cross-sectional brain area, regions of brain damage (BD), overall BD, and bolt–brain contact associated with the frontal, temporal, and behind the ear shot placements for the euthanasia of swine via PCB on sow and boar heads, and (b) to determine the relationship between external dimensional head measurements and tissue depth, cross-sectional brain area, regions of BD, and bolt–brain contact associated with the frontal, temporal, and behind the ear PCB placements. Our hypothesis was that differences in soft tissue thickness, cranial thickness, total tissue thickness, cross-sectional brain area, overall BD, and regions of BD would be detected between PCB placements.
Materials and Methods
Animal use protocol
It was not necessary to submit an animal use protocol to the University of Wisconsin—River Falls Institutional Animal Care and Use Committee (IACUC) because live animals were not directly manipulated in this study. The pigs from which the heads were obtained were slaughtered at a commercial slaughter establishment under inspection by the United States Department of Agriculture Food Safety and Inspection Service (USDA FSIS) in accordance with regulations in 9 CFR 313 (United States Electronic Code of Federal Regulations 2021). The exemption from IACUC approval followed the precedent established by Anderson et al. (2019, 2021).
Description of cadaver heads
Cadaver heads were selected because they serve as a replacement for the use of live animals to achieve the objectives of this research. Cadaver heads were obtained on eight collection days from a total of 111 sows (average BW = 232.9 ± 4.1 kg, mean ± SD) and 46 boars (average BW = 229.3 ± 2.6 kg, mean ± SD) that were electrically stunned and slaughtered at a regional commercial processing facility under federal inspection. Estimated live weight was determined for each source sow or boar retrospectively, using the average dressing percentage from the processing facility (58%) and the hot carcass weight (HCW) of the skinned carcasses. Additionally, 4.545 kg were added to the weight of each pig to account for the neck tissue remaining on the heads (formula: estimated BW = HCW (kg)/0.58 + 4.535 kg). Heads were not scalded and had the skin on and intact jowls, with ~6 cm of neck tissue left behind the ear to prevent soft tissue distortion at the PCB application site. All heads were placed in large storage totes with secured lids (Sterilite 1466—27 Gallon Industrial Tote, Sterilite Corp., Townsend, MA) with two heads per tote, and stored at ambient temperature for ~3 h postmortem prior to unrefrigerated transport (distance traveled: 188 km; duration: ~2 h) to the University of Wisconsin–River Falls Meat Science Laboratory. Heads were stored in the storage totes with the lids slightly offset inside a walk-in cooler for ~62 h at 2 to 4 °C prior to PCB application and head processing to improve the rigidity of brain tissue. The mean temperature of the exposed internal cranial surface after postapplication head processing was 7.70 ± 1.83 °C (mean ± SD).
Description of captive bolt tool, placement, and treatment assignment
The PCB device used in this study was a Jarvis Model PAS-Type P 0.25R Caliber Captive Bolt Pistol (Order #: 4144035, Jarvis Corp., Middletown, CT) equipped with a Long Stunning Rod Nosepiece Assembly (Order #: 3116605, Jarvis Corp.). Jarvis Orange Powder Cartridges 0.25R Caliber, 3.5 GR (Order #: 1176019, Jarvis Corp.) were used for all PCB applications. Orange cartridges were selected for use based on manufacturer recommendation for mature sows and boars. Additionally, the Orange cartridge was the highest power cartridge that was approved by the manufacturer for repeated use in the pistol PAS type PCB used in this study. The bolt travel distance predicted by the manufacturer was 76.2 mm, with the stunning rod and cartridge combination used in our study. The bolt travel distance was calculated by determining the force generated by the detonation of the cartridge and then applying that force to the bolt to compress the buffers. The distance between the tip of the bolt and the tip of the muzzle was then measured and then reported as the bolt travel distance (M. Abdul, Jarvis Corp., Middletown, CT, personal communication). Three PCB placement treatments (Figure 1) were applied in this study: FRONTAL—shot placed 3.5 cm superior to the optic orbits at midline (Woods et al., 2010), TEMPORAL—shot placed at the depression caudal to the lateral canthus of the eye within the plane between the lateral canthus and the base of the ear, or BEHIND EAR—shot placed directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye (AVMA, 2013; Anderson et al., 2019). For all PCB placements, the muzzle of the PCB was placed firmly against the head prior to activation. Additionally, each head was placed in a custom-fabricated benchtop-mounted brace of stainless steel bent to form a corner so that each head could be braced against the corner and firmly secured for each of the three placements (Figure 1). The brace was designed to hook around the edge of the table on which it was located to prevent shifting during head positioning and PCB application. The PCB applicator was the same for all heads and was trained in the safe and accurate use of the PCB equipment by an industry consultant and coauthor on this study prior to use.
Figure 1.
Penetrating captive bolt (PCB) placement placements. (A) FRONTAL—shot placed 3.5 cm superior to the optic orbits at the midline; (B) TEMPORAL—shot placed at the depression posterior to the lateral canthus of the eye within the plane between the lateral canthus and the base of the ear; (C) BEHIND EAR—shot placed directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye. All heads were secured within a custom-fabricated stainless steel brace for PCB application, shown here for each of the PCB placements. The brace in this image was designed to hook around the edge of the table on which it was located to prevent shifting during head positioning and PCB application.
Heads were assigned to their respective placements by sorting each day’s sample group by HCW, then randomly allocating the three placements to each cluster of three heads working from heaviest to lightest HCW using a random number generator in Excel version 2107 (Microsoft Corp., Spokane, WA); each head received a single PCB application in one of the three placements evaluated in this study.
On sampling days, the PCB was tested before use at the beginning of sampling and prior to cleaning at the end of sampling using a Jarvis PAS Stunning System Tester (Order #: 4116001, Jarvis Corp., Middletown, CT). The average bolt speed for all sampling days was 48.42 ± 2.11 m/s (mean ± SD). The expected bolt speed from the manufacturer for the PCB device and cartridge combination used in our study was 51.82 ± 3.05 m/s (mean ± SD) (M. Abdul, Jarvis Corp., personal communication).
Postapplication head processing
A total of 36 heads were obtained for each head processing date; however, only animals with a BW of 200 kg or greater were enrolled in the present study. Thus, between 12 and 28 heads were included in this study for each head processing date. After the PCB application, each head was cut along the bolt path with a Hobart 6801 Vertical Meat Band Saw (Hobart, Troy, OH) equipped with a blade that was 0.06 mm thick, 360.68 cm long, with 4 teeth/2.54 cm, and a hook angle of 3 degrees (Product #: C78529545, Bunzl Processor Division, Riverside, MO). Following each cut, digital images were collected from both sides of each intracranial surface and both with and without the brain. A painted wooden dowel was included in a duplicate set of images to indicate the bolt path. Thermal images (Model E8, FLIR Systems, Boston, MA) were also collected from both sides of each exposed intracranial surface for temperature assessment. Thermal images were collected both with and without the brain for each head. Brain temperature was determined using thermal images collected with the brain. All images were taken with the thermal camera and digital camera positioned 50.8 cm directly above and perpendicular to the exposed cut surface.
Tissue and cranial measurements
Measurements of soft tissue thickness (mm), cranial thickness (mm), and cross-sectional brain area (mm2) were determined from images collected at the time of head processing following procedures adapted from Anderson et al. (2021). All images included a 15.0-cm ruler used as a reference for an online irregular area calculator (SketchandCalc, ICalc, Inc., Palm Coast, FL) which was utilized between January and September of 2020 (updates to the program were made in this time period). Soft tissue thickness referred to the tissue from the application site to the exterior surface of the cranium. This measurement was collected on both sides of the exposed bolt path. Cranial thickness referred to the thickness from the exterior surface of the cranium to the interior surface of the cranium along the bolt path. This measurement was also collected on both sides of the exposed bolt path. When the actual distance of bolt travel did not reach the brain cavity, measurements were collected in line with the existing bolt path. Total tissue thickness (mm), which referred to the total soft tissue and cranial thickness from the site of application to the interior surface of the cranium, was determined from the summation of the soft tissue and cranial thickness for each cadaver head. This measurement was collected on both sides of the exposed bolt path. Soft tissue thickness, cranial thickness, and total tissue thickness were determined by averaging the measurements from the two halves of each head on both sides of the exposed bolt path. Cross-sectional brain area (mm2) referred to the cross-sectional surface area of the exposed brain within the plane of bolt travel as described by Anderson et al. (2019). All cross-sectional brain area measurements were performed by one observer. Measurements of cross-sectional brain area were calculated from both halves of each head along the paths of bolt travel and averaged prior to statistical analysis. Tissue depth parameters and cross-sectional brain area for FRONTAL heads are depicted in Figure 2, for TEMPORAL heads are depicted in Figure 3, and for BEHIND EAR heads are depicted in Figure 4.
Figure 2.
Frontal tissue measurements. Soft tissue thickness—the tissue from the application site to the exterior surface of the cranium. Cranial thickness—the tissue from the exterior surface of the cranium along the bolt path. Brain area—the cross-sectional surface area of the exposed brain within the plane of bolt travel.
Figure 3.
Temporal tissue measurements. Soft tissue thickness—the tissue from the application site to the exterior surface of the cranium. Cranial thickness—the tissue from the exterior surface of the cranium along the bolt path. Brain area—the cross-sectional surface area of the exposed brain within the plane of bolt travel.
Figure 4.
Behind ear tissue measurements. Soft tissue thickness—the tissue from the application site to the exterior surface of the cranium. Cranial thickness—the tissue from the exterior surface of the cranium along the bolt path. Brain area—the cross-sectional surface area of the exposed brain within the plane of bolt travel.
Tissue thickness observer training
Soft tissue thickness and cranial thickness were measured by one of three trained observers. Each observer received a training package including video and written standard operating procedures followed by a synchronous training session for each of the measurements that included two heads per PCB placement. Interobserver reliability included the comparison of measurements on a subset of 36 heads across the three trained observers. These measurements were then repeated by each observer to assess intraobserver reliability, which included the comparison of measurements on the subset of 36 heads that were collected by the same individual. Across all measurements, the mean interobserver percent coefficient of variation was 12.4%. Across all duplicate measurements, the mean intraobserver percent coefficient of variation was 5.0%.
Brain damage assessment
Brain damage (BD) and regions of BD were determined using digital images of the brain that were collected at the time of head processing. Damage to the frontal lobe, parietal lobe, temporal lobe, occipital lobe, corpus callosum, diencephalon, mesencephalon, brainstem (the region including the pons and medulla), and cerebellum (adapted from Wagner et al., 2019) was assessed on a yes/no basis where yes meant there was visually detectable damage to the brain and no meant there was no visually detectable damage to at least one region of the brain. Following the initiation of this study, we realized that bolt–brain contact could not be reliably discerned from BD associated with bone fragment movement during passage of the bolt. As a result, we assessed the occurrence of visible BD and refer to BD instead of bolt–brain contact in this research.
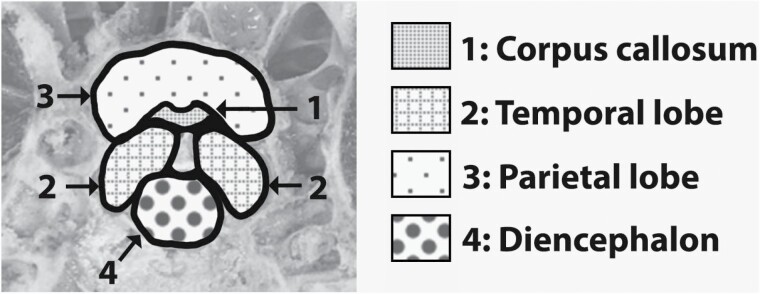
Brain maps that depicted regions of the brain were developed for each PCB placement (FRONTAL, TEMPORAL, and BEHIND EAR) to guide the assessment of BD. These brain maps were adapted from images of rostrocaudal slices of preserved porcine brain (Wouterlood and van de Berg, 2010). For the FRONTAL placement, the following regions and structures were included on the brain map (Figure 5) and assessed for damage: parietal lobe, frontal lobe, occipital lobe, corpus callosum, mesencephalon, diencephalon, brainstem, and cerebellum. For the TEMPORAL placement, two brain maps were developed: one for heads where only the parietal lobe was visible (Figure 6) and another for heads where the parietal lobe, occipital lobe, corpus callosum, and diencephalon were visible (Figure 7). For the BEHIND EAR placement, the following regions and structures were included on the brain map (Figure 8) and assessed for damage: parietal lobe, temporal lobe, occipital lobe, corpus callosum, diencephalon, and mesencephalon. Brain regions or structures that were not included in the brain map for each placement were not included in analyses.
Figure 5.
Brain map that was developed and utilized for the BD assessment of FRONTAL heads.
Figure 6.
Brain map that was developed and used for the BD assessment of TEMPORAL heads where the exposed region of the brain included only the parietal lobe.
Figure 7.
Brain map that was developed and used for the BD assessment of TEMPORAL heads when multiple regions of the brain were exposed within the plane of bolt travel.
Figure 8.
Brain map that was developed and used for the BD assessment of BEHIND EAR heads.
In addition to visually detectable damage to the brain, images were also assessed to determine whether the bolt path was in the plane of the brain; this assessment was referred to as brain contact plane. The bolt path was considered to have been in the plane of the brain when the bolt reached the brain or would have reached the brain if the bolt had been long enough to pass through the soft tissue and the cranium. On the contrary, the bolt path was considered to not be in the plane of the brain if it did not or would not have hit the brain, even with sufficient bolt length.
External head measurements
On a subset of 56 sows from two sampling days, the following external head measurements were collected: snout to poll distance (cm), distance between optic orbits (cm), and maximum deflection distance (cm). Snout to poll distance referred to the distance from the tip of the snout to the first point of contact between a taught measurement tape at the crest of the head. Distance between optic orbits referred to the distance between the medial aspects of the optic orbits. Maximum deflection distance referred to the maximum distance from a straight edge that was placed between the tip of the snout and the poll or the first point where the straight edge touched the head when placed from the tip of the snout. All measurements except head weight were collected in duplicate by two trained data collection technicians using a flexible measuring tape (Singer 218 60 in, The Singer Company Ltd., Boston, MA) and were averaged prior to statistical analysis. The mean interobserver percent coefficient of variation for snout to poll distance was 3.3%. The mean interobserver percent coefficient of variation for distance between optic orbits was 4.6%. The mean interobserver percent coefficient of variation for maximum deflection distance was 8.2%. Head weight (kg) was collected for all heads in the study using a benchtop scale that was calibrated prior to use on each sampling day.
Statistical analyses
Continuous data outcomes (soft tissue thickness, cranial thickness, total tissue thickness, and cross-sectional brain area) for PCB placement treatment (FRONTAL, TEMPORAL, and BEHIND EAR) effects were analyzed using models constructed within the MIXED procedure, of SAS 9.4 (Statistical Analysis System Institute, Inc., Cary, NC). A repeated statement with grouping by treatment and the Satterthwaite method for degrees of freedom was used to account for unequal variances by treatment. Models included treatment effects (FRONTAL, TEMPORAL, BEHIND EAR) only. The random effect of sampling date was included in the initial model and removed because it was not statistically significant (P > 0.05). Comparisons between sex were not conducted because of unknown sex-related morphological changes as swine mature and these changes could not be accurately factored into the model. Our focus was on describing differences between placement within sex. Mean separation was determined by using Student’s T-tests, protected by the Bonferroni–-Holm adjustment. Differences between means were recognized as significant when P < 0.05.
Additionally, to estimate the bolt length needed to effectively reach the brain for the majority of sows and boars greater than 200 kg BW, a 95% one-sided reference range was computed using a t-based prediction interval for normally distributed data with unknown mean and standard deviation (Horn, 1988), along with a 90% confidence interval for the upper reference limit (URL), for soft tissue thickness, cranial thickness, and total tissue thickness for the FRONTAL, TEMPORAL, and BEHIND EAR placements. All reference interval calculations were performed for sows and boars separately using R version 4.0.2 (R Core Team, 2020).
The occurrence of BD was calculated and 95% confidence intervals were determined using the Clopper–Pearson exact method in R version 4.0.2 (R Core Team, 2020). Statistical comparisons were not made between placements for BD and regions of BD due to different planes of bolt travel that resulted in varying regions of the brain that were accessible to the bolt and therefore assessed for damage across the three placements.
The relationship between external head measurements (snout to poll distance, distance between ocular orbits, and maximum deflection distance of the frontal aspect of the head from a straight line from snout to poll) were assessed for correlations with soft tissue thickness, cranial thickness, and total tissue thickness of a subset of 19 heads in the FRONTAL placement using the Regression procedure in SAS 9.4 (Statistical Analysis System Institute, Inc., Cary, NC). The relationship between head weight was assessed for correlations with soft tissue thickness, cranial thickness, and total tissue thickness for all heads in the FRONTAL, TEMPORAL, and BEHIND EAR placements for sows and boars using the Regression procedure in SAS 9.4 (Statistical Analysis System Institute, Inc.).
Results and Discussion
Tissue and cranial measurements
Tissue measurements collected in this study for sows and boars can be observed in Table 1 (means and standard errors) and Table 2 (95% reference interval upper limit [URL] and the 90% confidence interval for the URL [URL 90% CI]). All tissue depth measurements (soft tissue thickness, cranial thickness, and total tissue thickness) are reported as mean ± SE; URL; URL 90% CI. Thus, the URL indicates the value at which we estimate 95% of sow or boar heads will have less tissue depth. The URL 90% CI indicates the 90% confidence interval around the URL estimate. Cross-sectional brain areas collected in this study for sows and boars can also be observed in Table 1.
Table 1.
Soft tissue thickness, cranial thickness, total tissue thickness, and cross-sectional brain area of cadaver heads from mature sows and boars1 (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements2 and sectioned by band saw following the plane of bolt entry
| Sex | Dependent variable | PCB placement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FRONTAL | TEMPORAL | BEHIND EAR | ||||||||
| n | LS means | SE | n | LS means | SE | n | LS means | SE | ||
| SOWS | ||||||||||
| Soft tissue thickness, mm | 42 | 5.6b | 0.2 | 34 | 52.2a | 2.1 | 29 | 59.1a | 2.0 | |
| Cranial thickness, mm | 42 | 47.1a | 1.0 | 34 | 17.6c | 1.7 | 29 | 30.2b | 1.6 | |
| Total tissue thickness, mm | 42 | 52.7c | 1.0 | 34 | 69.8b | 1.4 | 29 | 89.3a | 1.5 | |
| Cross-sectional brain area, mm2 | 27 | 4,330.5a | 86.9 | 34 | 1,612.0c | 130.1 | 37 | 2,769.8b | 78.5 | |
| BOARS | ||||||||||
| Soft tissue thickness, mm | 16 | 6.4c | 0.4 | 14 | 51.1b | 3.1 | 12 | 59.3a | 2.3 | |
| Cranial thickness, mm | 16 | 34.8a | 2.1 | 14 | 22.1b | 3.1 | 12 | 31.6ab | 4.4 | |
| Total tissue thickness, mm | 16 | 41.2c | 2.1 | 14 | 73.2b | 1.5 | 12 | 90.9a | 3.5 | |
| Cross-sectional brain area, mm2 | 12 | 4,058.4a | 144.6 | 15 | 1,216.0c | 109.3 | 14 | 2,467.5b | 140.5 |
1Comparisons between sexes were not conducted because it is unknown how morphological changes in head conformation change as sows and boars continue to grow heavier than the sows and boars that provided the heads for this study.
2Placements: FRONTAL—Medial bolt entry ~3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear; BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
a–cSuperscripts that differ within a row identify significant differences between means within dependent variables across placements (P ≤ 0.05).
Table 2.
Upper 95% reference interval limits (URL) and associated 90% confidence intervals (URL 90% CI) for tissue depth parameters of cadaver heads from mature sows and boars1 (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements2 and sectioned by band saw following the plane of bolt entry
| Sex | Dependent variable | PCB placement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FRONTAL | TEMPORAL | BEHIND EAR | ||||||||
| n | URL | URL 90% CI | n | URL | URL 90% CI | n | URL | URL 90% CI | ||
| SOWS | ||||||||||
| Soft tissue thickness, mm | 42 | 7.8 | 7.3 to 8.3 | 34 | 73.6 | 68.2 to 79.0 | 29 | 77.4 | 72.4 to 82.4 | |
| Cranial thickness, mm | 42 | 58.1 | 55.6 to 60.6 | 34 | 34.4 | 30.2 to 38.7 | 29 | 45.4 | 41.3 to 49.5 | |
| Total tissue thickness, mm | 42 | 64.1 | 61.5 to 66.7 | 34 | 83.9 | 80.3 to 87.4 | 29 | 103.4 | 99.6 to 107.2 | |
| BOARS | ||||||||||
| Soft tissue thickness, mm | 16 | 8.9 | 8.1 to 9.8 | 14 | 72.3 | 64.5 to 80.0 | 12 | 74.3 | 68.4 to 80.1 | |
| Cranial thickness, mm | 16 | 50.0 | 44.7 to 55.3 | 14 | 43.7 | 35.7 to 51.6 | 12 | 60.3 | 49.2 to 71.5 | |
| Total tissue thickness, mm | 16 | 56.3 | 51.1 to 61.6 | 14 | 83.4 | 79.6 to 87.1 | 12 | 113.5 | 104.7 to 122.2 |
1Comparisons between sexes were not conducted because it is unknown how morphological changes in head conformation change as sows and boars continue to grow heavier than the sows and boars that provided the heads for this study.
2Placements: FRONTAL—Medial bolt entry ~3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear. BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
For sows, cranial thickness, total tissue thickness, and cross-sectional brain area were significantly different (P < 0.05) between the FRONTAL, TEMPORAL, and BEHIND EAR placements. The least soft tissue thickness was observed in the FRONTAL placement (mean ± SE: 5.6 ± 0.2 mm; URL: 7.8 mm; URL 90% CI: 7.3 to 8.3 mm). Soft tissue thickness was significantly (P < 0.05) greater in the TEMPORAL (52.2 ± 2.1 mm; URL: 73.6 mm; 90% URL 90% CI: 68.2 to 79.0 mm) and BEHIND EAR (59.1 ± 2.0 mm; URL: 77.4; URL 90% CI: 72.4 to 82.4) placements; however, there was no evidence to support a significant difference (P > 0.05) in soft tissue thickness for the TEMPORAL and BEHIND EAR placements. Cranial thickness was greatest (P < 0.05) for FRONTAL heads (47.1 ± 1.0 mm; URL: 58.1 mm; URL 90% CI: 55.6 to 60.6 mm), followed by BEHIND EAR heads (30.2 ± 1.6 mm; URL: 45.4 mm; URL 90% CI: 41.3 to 49.5 mm), and was least for TEMPORAL heads (17.6 ± 1.7 mm; URL 34.4 mm; URL 90% CI: 30.2 to 38.7 mm). Total tissue thickness was greatest (P < 0.05) for BEHIND EAR heads (89.3 ± 1.5 mm; URL: 103.4; URL 90%: 99.6 to 107.2 mm), followed by TEMPORAL heads (69.8 ± 1.4 mm; URL: 83.9 mm; URL 90% CI: 80.3 to 87.4 mm), and least for FRONTAL heads (52.7 ± 1.0 mm; URL: 64.1 mm; URL 90% CI: 61.5 to 66.7 mm). Cross-sectional brain area was greatest (P < 0.05) for FRONTAL heads (4330.5 ± 86.9 mm2), followed by BEHIND EAR heads (2769.8 ± 78.5 mm2), and least for TEMPORAL heads (1612.0 ± 130.1 mm2).
For boars, soft tissue thickness, total tissue thickness, and cross-sectional brain area were significantly different (P < 0.05) between the FRONTAL, TEMPORAL, and BEHIND EAR placements. Soft tissue thickness greatest (P < 0.05) for BEHIND EAR heads (59.3 ± 2.3 mm; URL: 74.3 mm; URL 90% CI: 68.4 to 80.1 mm), followed by TEMPORAL heads (51.1 ± 3.1 mm; URL: 72.3 mm; URL 90% CI: 64.5 to 80.0 mm), and least for FRONTAL heads (6.4 ± 0.4 mm; URL: 8.9 mm; URL 90% CI: 8.1 to 9.8 mm). Cranial thickness for boars only was significantly different (P < 0.05) between only the FRONTAL (34.8 ± 2.1 mm; URL: 50.0; URL 90% CI: 44.7 to 55.3 mm) and TEMPORAL (22.1 ± 3.1 mm; URL: 43.7 mm; URL 90% CI: 35.7 to 51.6 mm) treatments, but there was no evidence to support a significant difference for either from the BEHIND EAR (31.6 ± 4.4 mm; URL: 60.3 mm; URL 90% CI: 49.2 to 71.5 mm) (P > 0.05) placement. Total tissue thickness greatest (P < 0.05) for BEHIND EAR heads (90.9 ± 3.5 mm; URL: 113.5 mm; URL 90% CI: 104.7 to 122.2 mm), followed by TEMPORAL heads (73.2 ± 1.5 mm; URL: 83.4 mm; URL 90% CI: 79.6 to 87.1 mm), and least for FRONTAL heads (41.2 ± 2.1 mm; URL: 56.3 mm; URL 90% CI: 51.1 to 61.6 mm). Cross-sectional brain area was greatest (P < 0.05) for FRONTAL heads (4,058.4 ± 144.6 mm2), followed by BEHIND EAR heads (2,467.5 ± 140.5 mm2), and least for TEMPORAL heads (1,216.0 ± 109.3 mm2).
The URL values reported in this study are particularly valuable for manufacturers of PCB devices and the individuals responsible for selecting PCB devices for on-farm or in-plant use. These values can be used as a metric to predict whether a PCB device with a known penetration depth would have the capacity to reach the brain in the majority (95%) of sows or boars in the FRONTAL, TEMPORAL, or BEHIND EAR placements. Based on our findings, it is suggested that a penetration depth of 64.1 mm would be required to reach the brain at the FRONTAL placement for 95% of sows, a penetration depth of 83.9 mm would be required to reach the brain at the TEMPORAL placement for 95% of sows, and a penetration depth of 103.4 mm would be required to reach the brain at the BEHIND EAR placement for 95% of sows. Similarly, it is suggested that a penetration depth of 56.3 mm would be required to reach the brain at the FRONTAL placement for 95% of boars, a penetration depth of 83.4 mm would be required to reach the brain at the TEMPORAL placement for 95% of boars, and a penetration depth of 113.5 mm would be required to reach the brain at the BEHIND EAR placement for 95% of boars.
All URL values, the URL 90% CIs, and the sample distributions for each tissue depth measurement for each PCB placement can be viewed in Figure 9. Ideally, a larger sample size would be required to have confidence in the normality of the data or to perform the recommended non-parametric reference intervals (Friedrichs et al., 2012). However, this is an important first estimate because the mean tissue depth measurements alone do not provide sufficient information to determine the bolt length needed at each of the three PCB placements. A bolt length equivalent to the mean total tissue thickness at a given placement would result in the use of a bolt that was not long enough half of the time—provided the data was symmetric. In our study, a false positive is preferred to a false negative; it is better to have overestimated the total tissue thickness and subsequently the needed bolt length than to have underestimated. As such, the t-based method was utilized, rather than the normal-based method recommended by Friedrichs et al. (2012), as the upper bound will tend to be higher.
Figure 9.
Upper 95% reference interval limits (URL, black dot) and associated 90% confidence intervals (URL 90% CI, black bars), along with data distribution for tissue depth parameters of cadaver heads from mature sows and boars (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements and sectioned by band saw following the plane of bolt entry.
These results align with Anderson et al. (2019) who reported that the frontal PCB placement was more reliable and had fewer risks than the behind the ear placement for the euthanasia of market weight pigs, as indicated by a greater incidence of the bolt contacting the brain.
Brain damage assessment
Overall BD observations can be observed in Table 3. BD was detected in 25/26 (96.2%, 95% CI: 80.4% to 99.9%) sow heads with the FRONTAL placement, 24/35 (68.6%, 95% CI: 50.7% to 83.2%) sow heads with the TEMPORAL placement, and 5/40 (12.5%, 95% CI: 4.2% to 26.8%) sow heads with the BEHIND EAR placement. BD was detected in 11/12 (91.7%, 95% CI: 6.15% to 99.8%) boar heads with the FRONTAL placement, 2/15 (13.3%, 95% CI: 1.7% to 40.5%) boar heads with the TEMPORAL placement, and 7/14 (50.0%, 95% CI: 23.0% to 77.0%) boar heads with the BEHIND EAR placement.
Table 3.
Occurrence of visible BD in cadaver heads from mature sows and boars (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements1 and sectioned by band saw following the plane of bolt entry
| Sex | PCB placement | Overall BD | ||
|---|---|---|---|---|
| Proportion (observed/total) | Percentage2 | 95% CI | ||
| SOWS | ||||
| FRONTAL | 25/26 | 96.2 | 80.4 to 99.9% | |
| TEMPORAL | 24/35 | 68.6 | 50.7 to 83.2% | |
| BEHIND EAR | 5/40 | 12.5 | 4.2 to 26.8% | |
| BOARS | ||||
| FRONTAL | 11/12 | 91.7 | 61.5 to 99.8% | |
| TEMPORAL | 2/15 | 13.3 | 1.7 to 40.5% | |
| BEHIND EAR | 7/14 | 50.0 | 23.0 to 77.0% |
1Placements: FRONTAL—Medial bolt entry ~3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear; BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
While it is important to assess overall BD, the identification of specific regions and structures of the brain where damage can be visually detected provides valuable additional information. An assessment of overall BD provides limited information as to whether or not there was any damage to the brain from the bolt path, while an assessment of damage to the individual regions and structures exposed within the plane of the bolt path can provide more information regarding the magnitude of damage. Additionally, assessing individual regions and structures of the brain allows for the potential to identify whether the bolt path resulted in damage to structures most critical for a loss of sensibility, such as the diencephalon, which contains the thalamus (Redinbaugh et al., 2020), and those structures which play a key role in the cessation of respiration and heartbeat, such as the brainstem (AVMA, 2020). In a study by Wagner et al. (2019) that utilized a pneumatic PCB that was more powerful than the PCB used in our study, there was no observed damage to the structures which comprise the brainstem, but all cattle were stunned effectively. This information suggested that physical disruption to the brainstem is not a requirement for a loss of sensibility and is further supported by the findings of Redinbaugh et al. (2020) that the central lateral thalamus is the most responsible for maintaining sensibility. Further, the ascending reticular activating system was found to be responsible for achieving sensibility in humans by Yeo et al. (2013); this structure begins in the brainstem—specifically the pontine reticular formation—and terminates in the thalamus.
Damage to specific regions and structures of the brain for heads from sows can be observed in Table 4. Damage to the frontal lobe was detected in 8/26 (30.8%, 95% CI: 14.3% to 51.8%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements. Damage to the parietal lobe was detected in 25/26 (96.2%, 95% CI: 80.4% to 99.9%) heads shot in the FRONTAL placement, 25/35 (71.4%, 95% CI: 53.7% to 85.4%) heads shot in the TEMPORAL placement, and 2/40 (5.0%, 95% CI: 0.6% to 16.9%) heads shot in the BEHIND EAR placement. Damage to the temporal lobe was not assessed for FRONTAL heads, but was detected in 8/13 (61.5%, 95% CI: 31.6% to 88.1%) heads shot in the TEMPORAL placement and 4/40 (10.0%, 95% CI: 2.8% to 23.7%) heads shot in the BEHIND EAR placement. Damage to the occipital lobe was detected in 1/26 (3.9%, 95% CI: 0.1% to 19.6%) heads shot in the FRONTAL placement, 3/40 (7.5%, 95% CI: 1.6% to 20.4%) heads shot in the BEHIND EAR placement, and was not assessed for the TEMPORAL placement. Damage to the corpus callosum was detected in 21/26 (80.8%, 95% CI: 60.7% to 92.5%) heads shot in the FRONTAL placement, 5/13 (38.5%, 95% CI: 13.9% to 68.4%) heads shot in the TEMPORAL placement, and 0/40 (0.0%, 95% CI: 0.0% to 8.8%) heads shot in the BEHIND EAR treatment. Damage to the diencephalon was detected in 13/26 (50.0%, 95% CI: 29.9% to 70.1%) heads shot in the FRONTAL placement, 0/13 (0.0%, 95% CI: 0.0% to 24.7%) of heads shot in the TEMPORAL placement, and 1/40 (2.5%, 95% CI: 0.1% to 13.2%) heads shot in the BEHIND EAR placement. Damage to the mesencephalon was detected in 3/26 (11.5%, 95% CI: 2.5% to 30.2%) heads shot in the FRONTAL placement, 0/40 (0.0%, 95% CI: 0.0% to 8.8%) heads shot in the BEHIND EAR placement, and was not assessed for the TEMPORAL placement. Damage to the brainstem was detected in 0/26 (0.0%, 95% CI: 0.0% to 13.2%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements. Damage to the cerebellum was detected in 1/26 (3.9%, 95% CI: 0.1% to 19.6%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements.
Table 4.
Occurrence of damage to specific brain regions within cadaver heads from mature sows (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements1 and sectioned by band saw following the plane of bolt entry
| PCB placement | Brain structures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frontal lobe | Parietal lobe | Temporal lobe | Occipital lobe | Corpus callosum | Diencephalon | Mesencephalon | Brainstem (pons and medulla) | Cerebellum | ||
| FRONTAL | ||||||||||
| Proportion | 8/26 | 25/26 | — | 1/26 | 21/26 | 13/26 | 3/26 | 0/26 | 1/26 | |
| Percentage2 | 30.8 | 96.2 | — | 3.9 | 80.8 | 50.0 | 11.5 | 0.0 | 3.9 | |
| 95% CI3 | 14.3 to 51.8% | 80.4 to 99.9% | to — | 0.1 to 19.6% | 60.7 to 92.5% | 29.9 to 70.1% | 2.5 to 30.2% | 0.0 to 13.2% | 0.1 to 19.6% | |
| TEMPORAL | ||||||||||
| Proportion | — | 25/35 | 8/13 | — | 5/13 | 0/13 | — | — | — | |
| Percentage2 | — | 71.4 | 61.5 | — | 38.5 | 0.0 | — | — | — | |
| 95% CI3 | 53.7 to 85.4% | 31.6 to 88.1% | to — | 13.9 to 68.4% | 0.0 to 24.7% | — | — | — | ||
| BEHIND EAR | ||||||||||
| Proportion | — | 2/40 | 4/40 | 3/40 | 0/40 | 1/40 | 0/40 | — | — | |
| Percentage2 | — | 5.0 | 10.0 | 7.5 | 0.0 | 2.5 | 0.0 | — | — | |
| 95% CI3 | — | 0.6 to 16.9% | 2.8 to 23.7% | 1.6 to 20.4% | 0.0 to 8.8% | 0.1 to 13.2% | 0.0 to 8.8% | — | — |
1Placements: FRONTAL—Medial bolt entry approximately 3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear; BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
2Statistical models to compare captive bolt placement effects would not converge due to the different regions of the brain which were accessible to the bolt path and exposed on the split head between PCB placements. As a result, 95% CIs were calculated and reported.
395% confidence interval for the percentage of BD.
Damage to specific regions and structures of the brain for boars can be observed in Table 5. Damage to the frontal lobe was detected in 5/12 (41.7%, 95% CI: 15.2% to 72.3%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements. Damage to the parietal lobe was detected in 11/12 (91.7%, 95% CI: 61.5% to 99.8%) heads shot in the FRONTAL placement, 2/15 (13.3%, 95% CI: 1.7% to 40.5%) heads shot in the TEMPORAL placement, and 2/14 (14.3%, 95% CI: 1.8% to 42.8%) heads shot in the BEHIND EAR placement. Damage to the temporal lobe was detected in 0/4 (0.0%, 95% CI: 0.0% to 60.2%) of heads shot in the TEMPORAL placement, 4/14 (28.6%, 95% CI: 8.4% to 58.1%) heads shot in the BEHIND EAR placement, and was not assessed for heads shot in the FRONTAL placement. Damage to the occipital lobe was detected in 4/12 (33.3%, 95% CI: 9.9% to 65.1%) heads shot in the FRONTAL placement, 7/14 (50.0%, 95% CI: 23.0% to 77.0%) heads shot in the BEHIND EAR placement, and was not assessed for heads shot in the TEMPORAL placement. Damage to the corpus callosum was detected in 11/12 (91.7%, 95% CI: 61.5% to 99.8%) heads shot in the FRONTAL placement, 0/4 (0.0%, 95% CI: 0.0% to 60.2%) of heads shot in the TEMPORAL placement, and 1/14 (7.1%, 95% CI: 0.2% to 33.9%) heads shot in the BEHIND EAR placement. Damage to the diencephalon was detected in 6/12 (50.0%, 95% CI: 21.1% to 78.9%) heads shot in the FRONTAL placement, 0/4 (0.0%, 95% CI: 0.0-60.2%) of heads shot in the TEMPORAL placement, and 2/14 (14.3%, 95% CI: 1.8% to 42.8%) heads shot in the BEHIND EAR placement. Damage to the mesencephalon was detected in 2/12 (16.7%, 95% CI: 2.1% to 48.5%) heads shot in the FRONTAL placement, 0/14 (0.0%, 95% CI: 0.0% to 8.8%) heads shot in the BEHIND EAR placement, and was not assessed for heads shot in the TEMPORAL placement. Damage to the brainstem was detected in 0/12 (0.0%, 95% CI: 0.0% to 26.5%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements. Damage to the cerebellum was detected in 0/12 (0.0%, 95% CI: 0.0% to 26.5%) heads shot in the FRONTAL placement and was not assessed for heads shot in the TEMPORAL or BEHIND EAR placements.
Table 5.
Occurrence of damage to specific brain regions within cadaver heads from mature boars (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements1 and sectioned by band saw following the plane of bolt entry
| PCB placement | Brain structures | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frontal lobe | Parietal lobe | Temporal lobe | Occipital lobe | Corpus callosum | Diencephalon | Mesencephalon | Brainstem (pons and medulla) | Cerebellum | ||
| FRONTAL | ||||||||||
| Proportion | 5/12 | 11/12 | — | 4/12 | 11/12 | 6/12 | 2/12 | 0/12 | 0/12 | |
| Percentage2 | 41.7 | 91.7 | — | 33.3 | 91.7 | 50.0 | 16.7 | 0.0 | 0.0 | |
| 95% CI3 | 15.2 to 72.3% | 61.5 to 99.8% | — | 9.9 to 65.1% | 61.5 to 99.8% | 21.1 to 78.9% | 2.1 to 48.4% | 0.0 to 26.5% | 0.0 to 26.5% | |
| TEMPORAL | ||||||||||
| Proportion | — | 2/15 | 0/4 | — | 0/4 | 0/4 | — | — | — | |
| Percentage2 | — | 13.3 | 0.0 | — | 0.0 | 0.0 | — | — | — | |
| 95% CI3 | — | 1.7 to 40.5% | 0.0 to 60.2% | — | 0.0 to 60.2% | 0.0 to 60.2% | — | — | ||
| BEHIND EAR | ||||||||||
| Proportion | — | 2/14 | 4/14 | 7/14 | 1/14 | 2/14 | 0/14 | — | — | |
| Percentage2 | — | 14.3 | 28.6 | 50.0 | 7.1 | 14.3 | 0.0 | — | — | |
| 95% CI3 | — | 1.8 to 42.8% | 8.4 to 58.1% | 23.0 to 77.0% | 0.2 to 33.9% | 1.8 to 42.8% | 0.0 to 8.8% | — | — |
1Placements: FRONTAL—Medial bolt entry approximately 3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear; BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
2Statistical models to compare captive bolt placement effects would not converge due to the different regions of the brain which were accessible to the bolt path and exposed on the split head between PCB placements. As a result, 95% CIs were calculated and reported.
395% confidence interval for the percentage of BD.
Brain contact plane for sows and boars can be observed in Table 6. For sow heads shot in the FRONTAL placement the bolt path was in the plane of the brain for 42/42 (100%, 95% CI: 91.6% to 100%) heads. For sow heads shot in the TEMPORAL placement, the bolt path was in the plane of the brain for 39/40 (97.5%, 95% CI; 86.8% to 99.9%) heads. For sow heads shot in the BEHIND EAR placement, the bolt path was within the plane of the brain for 34/39 (87.2%; 95% CI: 72.6% to 95.7%) heads. For boars shot in the FRONTAL placement, the bolt path was within the plane of the brain for 17/17 (100.0%, 95% CI: 80.5% to100.0%) heads. For boars shot in the TEMPORAL placement, the bolt path was in the plane of the brain for 18/18 (100.0%, 95% CI: 81.5% to 100.0%) heads. For boar heads shot in the BEHIND EAR placement, the bolt path was within the plane of the brain for 14/14 (100.0%, 95% CI: 76.8% to 100.0%) heads.
Table 6.
Percentage of cadaver heads from mature sows and boars (BW > 200 kg) assigned to three penetrating captive bolt (PCB) placements1 in which the brain was located within the plane of bolt travel
| Sex | PCB placement | Brain within plane of bolt travel | ||
|---|---|---|---|---|
| Proportion (observed/total) | Percentage2 | 95% CI2 | ||
| SOWS | ||||
| FRONTAL | 42/42 | 100.0 | 91.6 to 100.0% | |
| TEMPORAL | 39/40 | 97.5 | 86.8 to 99.9% | |
| BEHIND EAR | 34/39 | 87.2 | 72.6 to 95.7% | |
| BOARS | ||||
| FRONTAL | 17/17 | 100.0 | 80.5 to 100.0% | |
| TEMPORAL | 18/18 | 100.0 | 81.5 to 100.0% | |
| BEHIND EAR | 14/14 | 100.0 | 76.8 to 100.0% |
1Placements: FRONTAL—Medial bolt entry ~3.5 cm superior to the optic orbits at midline perpendicular with the external surface of the head; TEMPORAL—Bolt entry at the depression located between the right eye and right ear; BEHIND EAR—Bolt entry directly caudal to the pinna of the ear on the same plane as the eyes and targeting the middle of the opposite eye.
295% confidence interval for the percentage of heads with the brain in the plane of bolt travel.
External head measurements
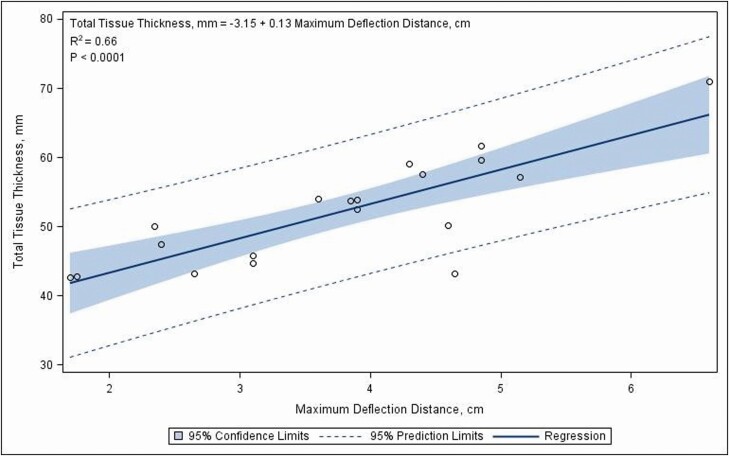
Linear relationships were identified for maximum deflection distance with the FRONTAL cranial thickness (Figure 10) and frontal total tissue thickness (Figure 11) via simple linear regressions. These simple linear regressions were calculated to predict FRONTAL cranial thickness and total tissue thickness based upon maximum deflection distance. For each centimeter of maximum deflection distance, the expected cranial thickness increased 0.14 ± 0.81 mm (INTERCEPT = −2.72 ± 3.22, R2 = 0.70, P < 0.0001) and the expected total tissue thickness increased 0.13 ± 0.02 mm (INTERCEPT = −3.15 ± 1.21, R2 = 0.66, P < 0.0001). Woods (2012) investigated the impact of the frontal plate of the head with a description of “plank” vs. “dish” profiles. Our results support the concept that the frontal profile of the head and the underlying tissue thickness may be related.
Figure 10.
Relationship of frontal cranial thickness (mm) and maximum deflection distance (cm) between a straight line from snout to poll and the frontal surface of cadaver heads from sows >200 kg body weight (n = 19).
Figure 11.
Relationship of total frontal tissue thickness (mm) and maximum deflection distance (cm) between a straight line from snout to poll and the frontal surface of cadaver heads from sows >200 kg body weight (n = 19).
For sows, linear relationships were also identified for head weight with FRONTAL cranial thickness (Figure 12), FRONTAL total tissue thickness (Figure 13), TEMPORAL soft tissue thickness (Figure 14), TEMPORAL total tissue thickness (Figure 15), and BEHIND EAR total tissue thickness (Figure 16). For each kilogram of head weight, the expected FRONTAL cranial thickness increased 1.44 ± 0.25 mm (intercept = 19.66 ± 4.76, R2 = 0.4605, P < 0.0001) and the expected FRONTAL total tissue thickness increased 1.52 ± 0.25 mm (intercept = 23.68 ± 4.92, R2 = 0.4715, P < 0.0001). Similarly, for each kilogram of head weight, the expected TEMPORAL soft tissue thickness increased 1.51 ± 0.64 mm (intercept = 24.48 ± 11.85, R2 = 0.1501, P = 0.0236) and the expected TEMPORAL total tissue thickness increased 1.83 ± 0.32 mm (intercept = 36.20 ± 5.93, R2 = 0.5080, P < 0.0001). Finally, for each kilogram of head weight, the expected BEHIND EAR total tissue thickness increased 1.03 ± 0.50 mm (intercept = 69.79 ± 9.56, R2 = 0.1364, P = 0.0486).
Figure 12.
Relationship of frontal cranial thickness (mm) and head weight (kg) of cadaver heads from sows > 200 kg body weight (n = 42).
Figure 13.
Relationship of frontal total tissue thickness (mm) and head weight (kg) of cadaver heads from sows > 200 kg body weight (n = 42).
Figure 14.
Relationship of temporal soft tissue thickness (mm) and head weight (kg) of cadaver heads from sows > 200 kg body weight (n = 34).
Figure 15.
Relationship of temporal total tissue thickness (mm) and head weight (kg) of cadaver heads from sows > 200 kg body weight (n = 34).
Figure 16.
Relationship of behind ear total tissue thickness (mm) and head weight (kg) of cadaver heads from sows > 200 kg body weight (n = 29).
For boars, linear relationships were identified for head weight with FRONTAL cranial thickness (Figure 17), FRONTAL total tissue thickness (Figure 18), TEMPORAL total tissue thickness (Figure 19), BEHIND EAR cranial thickness (Figure 20), and BEHIND EAR total tissue thickness (Figure 21). For each kilogram of head weight, the expected FRONTAL cranial thickness increased 1.90 ± 0.26 mm (intercept = 4.66 ± 4.32, R2 = 0.7863, P < 0.0001) and the expected FRONTAL total tissue thickness increased 1.84 ± 0.29 mm (intercept = 11.99 ± 4.69, R2 = 0.7462, P < 0.0001). For each kilogram of head weight, the expected TEMPORAL total tissue thickness increased 0.60 ± 0.24 mm (intercept = 62.23 ± 4.59, R2 = 0.3411, P = 0.0283). For each kilogram of head weight, the expected BEHIND EAR cranial thickness increased 2.45 ± 0.40 mm (intercept = −13.09 ± 7.62, R2 = 0.7893, P < 0.0001) and the expected BEHIND EAR total tissue thickness increased 1.81 ± 0.38 mm (intercept = 58.01 ± 7.19, R2 = 0.6947, P = 0.0008).
Figure 17.
Relationship of frontal cranial thickness (mm) and head weight (kg) of cadaver heads from boars > 200 kg body weight (n = 16).
Figure 18.
Relationship of frontal total tissue thickness (mm) and head weight (kg) of cadaver heads from boars > 200 kg body weight (n = 16).
Figure 19.
Relationship of temporal total tissue thickness (mm) and head weight (kg) of cadaver heads from boars > 200 kg body weight (n = 14).
Figure 20.
Relationship of behind ear cranial thickness (mm) and head weight (kg) of cadaver heads from boars > 200 kg body weight (n = 12).
Figure 21.
Relationship of behind ear total tissue thickness (mm) and head weight (kg) of cadaver heads from boars > 200 kg body weight (n = 12).
In an evaluation of the efficacy of a pistol type captive bolt kit that included penetrating and nonpenetrating assemblies for varying species and animal sizes in the frontal placement for the euthanasia of swine, Woods (2012) concluded that PCB was not consistently reliable or effective as a single-step euthanasia method for swine estimated to be larger than 200 kg; however, the method was shown to be effective as a single-step method of euthanasia for pigs weighing 2 to 120 kg. The European Food Safety Authority (EFSA) reported that PCB may not effectively cause a loss of consciousness in mature sows and boars and identified a need for the development and evaluation of a PCB device capable of effectively stunning, and ultimately killing, mature sows and boars (EFSA, 2004). Chevillon et al. (2004) reported that captive bolt is the recommended method of euthanasia for piglets over 8 kg, growing pigs, and breeding animals due to the efficacy, feasibility, and costs compared with other methods. Likewise, PCB is listed as a recommended method of euthanasia for sows and boars by the AVMA due to the method’s efficacy and low costs, in addition to safety advantages over firearms (AVMA, 2020). However, the method has not yet been validated as an effective single-step method of euthanasia for these animals.
Our results may differ from those reported in other studies because the recommended location for PCB placement is inconsistent across guidelines. More specifically, euthanasia guidelines do not provide a consistent description of the FRONTAL placement, despite the inclusion of this placement in all euthanasia guidelines. A placement of the PCB in the center of the forehead slightly above a line drawn between the eyes, with the bolt directed toward the spinal canal is described by the AVMA (2013, 2020). The NPB and AASV described the PCB placement as 1.27 cm above eye level and even with the eyebrows, so the PCB is aimed toward the brain and directed toward the tail (NPB and AASV, 2016). The placement described by the HSA is 2.0 cm above eye level, at the midline and aiming toward the tail (HSA, 2016). The EFSA described a PCB placement of 1 to 2 cm above the eyes with the PCB aimed toward the tail, with the recommendation for a higher placement of 3 to 4 cm with an off-center placement for boars and large sows (EFSA, 2020). Further, Woods et al. (2010) described a placement of ~2.5 cm above the eyes at the midline for market weight pigs; 3 to 4 cm above the eyes, just to the side of the ridge on the skull for sows and boars.
Kramer et al. (2021) evaluated the frontal, temporal, and behind ear PCB placements for sows and boars greater than 200 kg BW with both a pistol type PCB and an inline type PCB. The study was done in two stages, first on live-anesthetized animals, then on live-conscious animals for placement-device combinations, where death was achieved for 3/3 animals in the first stage. The model of PCB used by Kramer et al. (2021) was redesigned by the manufacturer to accommodate a higher power cartridge (personal communication: M. Abdul, Jarvis Corporation, Middletown, CT). In Kramer et al. (2021), additional PCB applications were required for live-conscious animals to be rendered insensible in three instances. Those instances included the following placement-device combinations behind ear-inline, temporal-inline, and behind ear-pistol; these additional PCB applications were required due to human error in PCB placement during the initial application (personal communication, S. Moeller, The Ohio State University, Colombus, OH). The need for a second PCB application in the behind ear and temporal placements in the work done by Kramer et al. (2021) highlights the need for an increased emphasis on understanding tissue depths and determining appropriate PCB placement and angle for the alternative TEMPORAL and BEHIND EAR placements, which is in alignment with our findings.
Our results suggest that the FRONTAL PCB placement may provide a bolt path with less tissue to travel through than either the TEMPORAL or BEHIND EAR PCB placements for sows and boars over 200 kg. It must be noted that cadaver heads were used in this study, so conclusions related directly to the efficacy of a single PCB placement cannot be made; however, this information should be used to guide future research related to PCB placement and PCB device selection for mature sows and boars. In addition, all cadaver heads utilized in this study were observed to have expansive sinus cavities typical of mature sows and boars (Woods et al., 2010; HSA, 2013; NPB and AASV, 2016).
Aspects of our current study that require follow-up with future work include the limited weight range of source animals, the type of PCB device used, and a series of limitations which result from the use of cadaver heads. Our study did not explore the tissue parameters of sows and boars with BW less than 200 kg. Specifically, at the time of this publication, minimal published data existed regarding the population of mature swine with BW between 120 and 200 kg. It is also noteworthy that only one type of PCB device, with only one bolt length and powder cartridge combination was used in our study. There are multiple commercially available PCB devices of both the pistol and inline varieties, which have different features that result in different bolt travel distances and therefore the tool’s ability to reach and consistently cause damage to the brain.
Additionally, overall BD was determined by the presence of visually detectable damage to one or more regions of the brain and was not assessed for hemorrhaging or traumatic brain injury (TBI) as a means of quantifying damage. Brain hemorrhage and tissue damage resultant of concussive force could not be assessed because heads were sourced from fully exsanguinated animals and there was no available blood supply or cerebrospinal fluid. Because whole, intact brains were not available for assessment of BD, the TBI scoring system developed by Millar and Mills (2000) that was adapted by Woods (2012) and Kramer et al. (2021) could not be applied. Further, because BD was assessed from cross-sections of the brain, it was not possible to make direct comparisons between brain regions and structures among PCB placements.
Implications
This study was intended to serve as an initial step in the scientific validation of the TEMPORAL and BEHIND EAR PCB placements as alternatives to the common FRONTAL placement based upon tissue thicknesses and cross-sectional brain area. Of the three PCB placements evaluated in this study, the FRONTAL placement appeared to be the most reliable with the pistol type PCB tool that we used. The reliability of the FRONTAL placement over the TEMPORAL and BEHIND EAR placements was indicated by the least tissue for the bolt to travel through, the greatest potential target area, and the greatest observed prevalence of BD. Future studies related to the TEMPORAL and BEHIND EAR alternative placements are warranted, as are studies related to the capacity of various PCB devices to reach and disrupt brain tissue. Our results confirmed that some PCB tools may not be able to consistently reach the brain depending on bolt travel distance. The FRONTAL placement may present the least risk of failure for the PCB euthanasia of sows and boars weighing 200 kg or more with the PCB used in this study at this time.
Acknowledgments
The authors thank the commercial slaughter establishment that provided the cadaver heads for this research. The authors greatly appreciated the data collection assistance of S. Albers, J. Bignell, K. Bishop, E. Boyd, J. Buerck, B. Brown, J. Haines, C. Hanna-Stokes, K. Hefter, R. Hoff, E. Holst, O. Horsman, C. Huber, P. Isensee, S. Isensee, K. Nelson, A. Kirk, V. Rakoczy, T. Rauenhorst, and R. Rehnelt (all affiliated with the University of Wisconsin–River Falls at the time of this study). The authors also appreciated the guidance of D. Renter for statistical analysis. The provision of captive bolt stunning equipment by Jarvis Corporation and technical support by Bunzl Processor Division was greatly appreciated. The authors would also like to thank the commercial meat processing company that provided the bandsaw used for this research. Funding was provided in part by National Pork Checkoff (Grant #19-227).
Glossary
Abbreviations
- BD
brain damage
- BW
body weight
- CI
confidence interval
- HCW
hot carcass weight
- PCB
penetrating captive bolt
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Anderson, K. N., Albers S. E., Allen K. J., Bishop K. D., Greco B. J., Huber C. M., Kirk A. A., Olsen H., and Vogel K. D.. . 2021. Quantification of cooling effects on basic tissue measurements and exposed cross-sectional brain area of cadaver heads from market pigs. Transl. Anim. Sci. 5:1–6. doi: 10.1093/tas/txab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, K., Ries E., Backes J., Bishop K., Boll M., Brantner E., Hinrichs B., Kirk A., Olsen H., Risius B., . et al. 2019. Relationship of captive bolt stunning location with basic tissue measurements and exposed cross-sectional brain area in cadaver heads from market pigs. Transl. Anim. Sci. 3:1405–1409. doi: 10.1093/tas/txz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVMA. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition.https://www.avma.org/sites/deault/files/resources/euthanasia.pdf (accessed 2 February 2021).
- AVMA. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition.https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf (accessed 2 February 2021).
- Chevillon, P., Mircovich C., Dubroca S., and Fleho J.. . 2004. Comparison of different pig euthanasia methods available to the farmers. In: Proc. Int. Soc. Anim. Hyg., St-Malo, France; p. 45–46. [Google Scholar]
- EFSA. 2004. Opinion of the scientific panel on animal health and welfare on a request from the commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. EFSA J. 45:1–29. doi: 10.2903/j.efsa.2004.45 [DOI] [Google Scholar]
- EFSA. 2020. Welfare of pigs during killing for purposes other than slaughter. EFSA. J. 18:6195. doi:10.2903.j.efsa.2020.6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs, K. R., Harr K. E., Freeman K. P., Szladovits B., Walton R. M., Barnhart K. F., and Blanco-Chavez J.; American Society for Veterinary Clinical Pathology. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 41:441–453. doi: 10.1111/vcp.12006 [DOI] [PubMed] [Google Scholar]
- Horn, P. 1988. A biweight prediction interval for random samples. J. Am. Stat. Assoc. 83:249–256. doi: 10.2307/2288947 [DOI] [Google Scholar]
- HSA. 2013. Captive bolt stunning of livestock. https://www.hsa.org.uk/downloads/publications/captive-bolt-stunning-of-livestock-updated-logo-2016.pdf (accessed 2 February 2021).
- Kramer, S. A., Wagner B. K., Robles I., Moeller S. J., Bowman A. S., Kieffer J. D., Arruda A. G., Cressmen M. D., and Pairis-Garcia M. D.. . 2021. Validating the effectiveness of alternative euthanasia techniques using penetrating captive bolt guns in mature swine (Sus scrofa domesticus). J. Anim. Sci. doi: 10.1093/jas/skab052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, G. I. and Mills D. S.. . 2000. Observation on the trajectory of the bullet in 15 horses euthanized by free bullet. Vet. Rec. 146:754–757. doi: 10.1136/vr.146.26.754 [DOI] [PubMed] [Google Scholar]
- NPB and AASV. 2016. On-farm euthanasia of swine: recommendations for the producer. Tech. Bull. No. 04970-11/16. Des Moines (IA): National Pork Board. [Google Scholar]
- OIE. 2016. Terrestrial animal health code: killing of animals for disease control purposes.https://www.oie.int/fileadmin/Home/eng/Health_standards_tahc/current/chaptire_aw_killing.pdf (accessed 2 February 2021).
- R Core Team. 2020. R: a language environment for statistical computing. https://www.R-project.org/ (accessed 3 June 2021).
- Redinbaugh, M. J., Phillips J. M., Kambi N. A., Mohanta S., Andryk S., Dooley G. L., Afrasiabi M., Raz A., and Saalmann Y. B.. . 2020. Thalamus modulates consciousness via layer-specific control of cortex. Neuron 106:66–75.e12. doi: 10.1016/j.neuron.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Electonic Code of Federal Regulations. 2021.. https://www.ecfr.gov/cgi-bin/text-idx?node=pt9.2.313&rgn=div5 (accessed 23 February 2021).
- Wagner, D. R., Kline H. C., Martin M. S., Alexander L. R., Grandin T., and Edwards-Callaway L. N.. . 2019. The effects of bolt length on penetration hole characteristics, brain damage and specified-risk material dispersal in finished cattle stunned with a penetrating captive bolt stunner. Meat Sci. 155:109–114. doi: 10.1016/j.meatsci.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Woods, J. 2012. Analysis of the use of the “CASH” Dispatch Kit captive bolt gun as a single stage euthanasia process for pigs [MS thesis]. Ames (IA): Iowa State University. [Google Scholar]
- Woods, J., Shearer J. K., and Hill J.. . 2010. Recommended on-farm euthanasia practices. In: Grandin T., editor, Improving animal welfare: a practical approach. Oxfordshire (UK): CAB International; p. 195–201. [Google Scholar]
- Wouterlood, F. and van de Berg W.. . 2010. The brain of Sus scrofa.http://www.anatomie-amsterdam.nl/sub_sites/pig_brain_atlas/start.htm.
- Yeo, S. S., Chang P. H., and Jang S. H.. . 2013. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front. Hum. Neurosci. 7:416. doi: 10.3389/fnhum.2013.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]