Abstract
While aldehydes represent a classic class of electrophilic synthons, the corresponding acyl radicals are inherently nucleophilic, which exhibits umpolung reactivity. Generation of acyl radicals typically requires noble metal catalysts or excess oxidants to be added. Herein, we report a convenient and green approach to access acyl radicals, capitalizing on neutral eosin Y-enabled hydrogen atom transfer (HAT) photocatalysis with aldehydes. The generated acyl radicals underwent SOMOphilic substitutions with various functionalized sulfones (X–SO2R’) to deliver value-added acyl products. The merger of eosin Y photocatalysis and sulfone-based SOMOphiles provides a versatile platform for a wide array of aldehydic C–H functionalizations, including fluoromethylthiolation, arylthiolation, alkynylation, alkenylation and azidation. The present protocol features green characteristics, such as being free of metals, harmful oxidants and additives; step-economic; redox-neutral; and amenable to scale-up assisted by continuous-flow technology.
Subject terms: Synthetic chemistry methodology, Photocatalysis
Acyl radicals represent a reactive species that allow for aldehyde subunits to be nucleophilic instead of their typical electrophilic behavior; however, these species are difficult to access in mild conditions. Here the authors show a method to generate acyl radicals using only an organic photocatalyst and light, and these species are shown as competent nucleophiles in a variety of couplings.
Introduction
Acyl radicals are versatile synthetic intermediates in C–C bond-forming reactions, such as Giese addition1,2 and Minisci acylation3, as well as transition metal-mediated cross-coupling reactions4–6. The exploration of acyl radical chemistry greatly expanded the scope of accessible carbonyl-containing functional molecules7. However, conventional approaches to accessing acyl radicals normally require harsh conditions such as high temperature, ultraviolet irradiation, or the use of hazardous reagents. Emerging and rapidly expanding photocatalysis has offered enormous opportunities to access acyl radicals in a green and sustainable fashion from a variety of precursors, including aldehydes, carboxylic acids, acid derivatives, and acyl silanes8–13. Among them, the use of aldehydes for acyl radical generation represents the most straightforward and atom- and step-economical pathway.
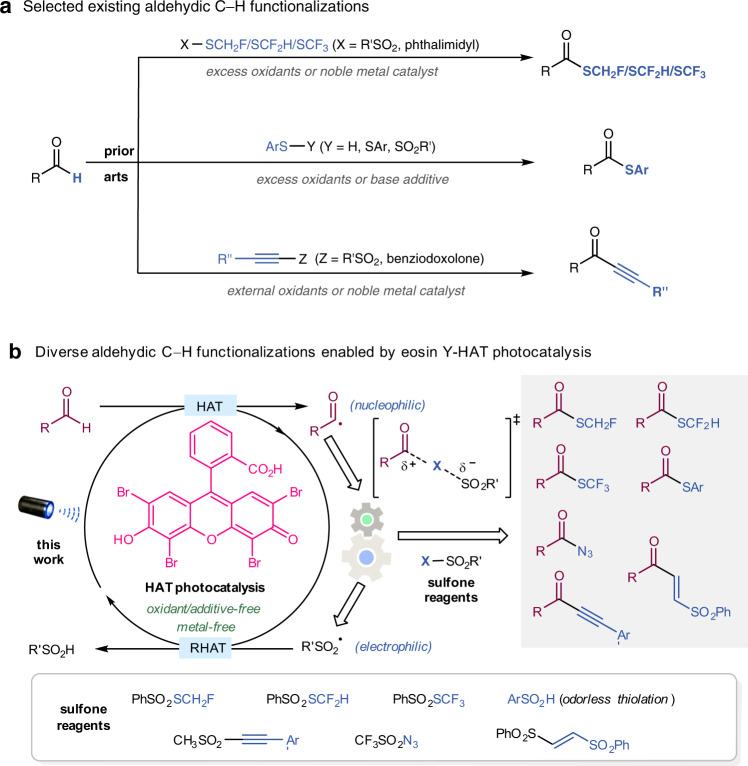
By taking advantage of aldehyde feedstocks as acyl radical precursors14–16, a plethora of acyl–C bond-forming reactions have been developed. This bond formation is normally realized by acyl radical addition to unsaturated alkenes or (hetero)aromatics, leading to acyl–C(sp3) or acyl–C(sp2) bond formation. In stark contrast, the construction of acyl–X (X = S, N, D) and acyl–C(sp) bonds from aldehydes is largely underexplored (Fig. 1a). Excess oxidants17,18, additives19,20, or noble metal catalysts21–24 are usually required to achieve such transformations.
Fig. 1. Diverse aldehydic C–H functionalizations.
a Selected existing aldehydic C–H functionalizations. b This work: diverse aldehydic C–H functionalizations by HAT photocatalysis using sulfone regents.
Direct photocatalyzed hydrogen atom transfer (HAT) has enabled a remarkable breakthrough in C–H functionalizations25,26. Capitalizing on HAT activity of C–O biradical of excited ketones or quinones, a series of HAT photocatalysts were developed27–31. Although anionic eosin Y was commonly used as a photoredox catalyst32–34, a previous study by our group established neutral eosin Y as an excellent direct HAT photocatalyst that can activate a wide range of C–H bonds to access the corresponding carbon radicals under simple and mild conditions35. Eosin Y was capable of providing access to acyl radicals from aldehyde feedstocks, which have been employed to react with alkenes and alkynes for asymmetric 1,4-addition36 and a radical Smiles rearrangement37, respectively. With the cumulative insights gained from the eosin Y-HAT photocatalytic system38, we envisioned that its merger with sulfone SOMOphiles39,40 can provide a general platform for aldehydic C–H functionalization to access various types of functional acyl compounds. Acyl radicals generated by photoinduced HAT undergo nucleophilic substitution with X–SO2R′, which will benefit from polarity matching41–43, delivering diverse functionalized acyl compounds accompanied by electrophilic sulfonyl radical species. The sulfonyl radical then participates in a reversed HAT (RHAT) process with eosin Y-H to complete the catalytic cycle.
In this work, by using different SOMOphilic sulfone reagents as acyl radical traps in HAT photocatalysis, we achieve aldehydic C–H fluoromethylthiolation, arylthiolation, alkynylation, alkenylation, and azidation (Fig. 1b). Notably, arylsulfinic acid is utilized as an odorless thiolation reagent for thioester generation. Compared to existing protocols for aldehydic C–H functionalizations, eosin Y-HAT photocatalysis features operationally simple, inexpensive, and metal-, oxidant-, and additive-free green attributes.
Results
Development of aldehydic C–H fluoromethylthiolation
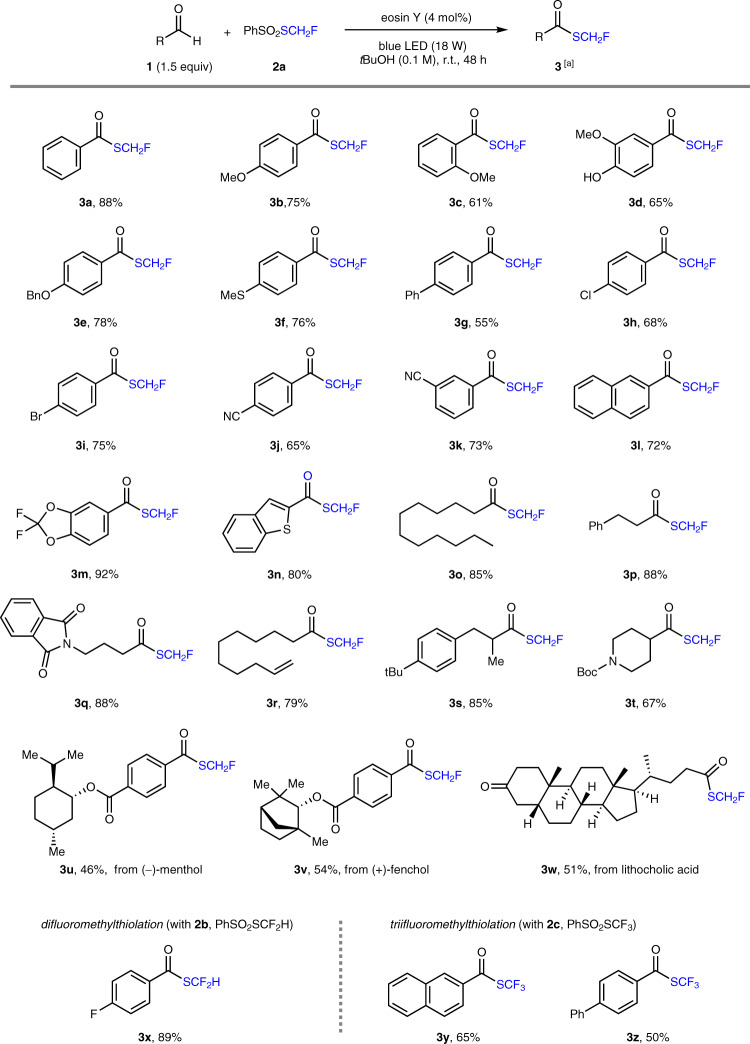
The monofluoromethylthio moiety (SCH2F) widely exists in a variety of biologically active compounds (Supplementary Fig. 1)44,45. Previous reports to achieve aldehydic C–H monofluoromethylthiolation relied on the use of stoichiometric oxidants such as 2,2′-azodi(2-methylbutyronitrile) (AMBN)46 or PhI(O2CCF3)2/NaN317. We envisioned that eosin Y-based HAT photocatalysis may enable this transformation in a redox-neutral fashion. After extensive condition optimization using benzaldehyde 1a and S-(fluoromethyl) benzenesulfonothioate (PhSO2–SCH2F) 2a as the model substrates (Supplementary Table 1), we found that neutral eosin Y (4 mol%) in tert-butanol (tBuOH) under blue light (18 W, 470 nm LED) irradiation at ambient temperature afforded desired product 3a in optimal yield (88%). Notably, no product was generated using anionic eosin Y as the photocatalyst, while other photocatalytic systems for HAT4,22,24,27,47 gave inferior product yields (Supplementary Table 4), highlighting the effectiveness of eosin Y catalysis. Light irradiation was essential, as no product was detected when the reaction was performed in darkness.
With the optimized conditions, the scope of aldehydes amenable to monofluoromethylthiolation was investigated (Fig. 2). Electron-rich (3b–f) and electron-deficient (3g–k) arene derivatives possessing ortho-, meta-, or para-substituents all provided the corresponding monofluoromethyl thioester products in 55–78% yields. A wide range of functionalities, including ether (3b–e), phenol (3d), thioether (3f), halide (3h, 3i), and cyanide (3j), were well tolerated. Naphthalene- or heterocycle (such as benzodioxole and benzothiophene)-substituted aldehydes smoothly participated in the transformation to afford products 3l–n in good yields (72–92%). The scope with respect to aliphatic aldehydes was evaluated next. Both linear (3o–r) and branched (3s, 3t) alkyl aldehydes afforded the desired products in good yields (67–88%). The incorporation of amide (3q), terminal alkene (3r) and piperidine (3t) substrates was compatible with our conditions. However, tertiary aldehydes such as pivalaldehyde failed to give the corresponding product (not shown), probably due to the facile decarbonylation of the unstable tert-alkyl acyl radical48. Moreover, the protocol can be applied to late-stage functionalization of natural product derivatives. Useful yields (46–54%) of monofluoromethylthiolation products were obtained with complex molecules derived from (−)-menthol (3u), (+)-fenchol (3v), and lithocholic acid (3w). Importantly, by simply changing fluoromethylthio-sulfone reagents 2a to 2b (PhSO2–SCF2H) and 2c (PhSO2–SCF3), this protocol could be successfully extended to aldehydic C–H difluoromethylthiolation (3x) and trifluoromethylthiolation (3y, 3z), respectively, representing a general method to access diverse fluoromethylthioesters in a simple and green manner.
Fig. 2. Aldehydes scope for C–H fluoromethylthiolation.
[a] Reaction conditions unless otherwise noted: 1 (0.3 mmol), 2 (0.2 mmol), eosin Y (4 mol%), and tert-butanol (2.0 mL) in an argon-filled Schlenk tube (20 mL) at room temperature (~27 °C) under 470 nm light (18 W LED) irradiation.
Development of aldehydic C–H thiolation using arylsulfinic acid as an odorless sulfur reagent
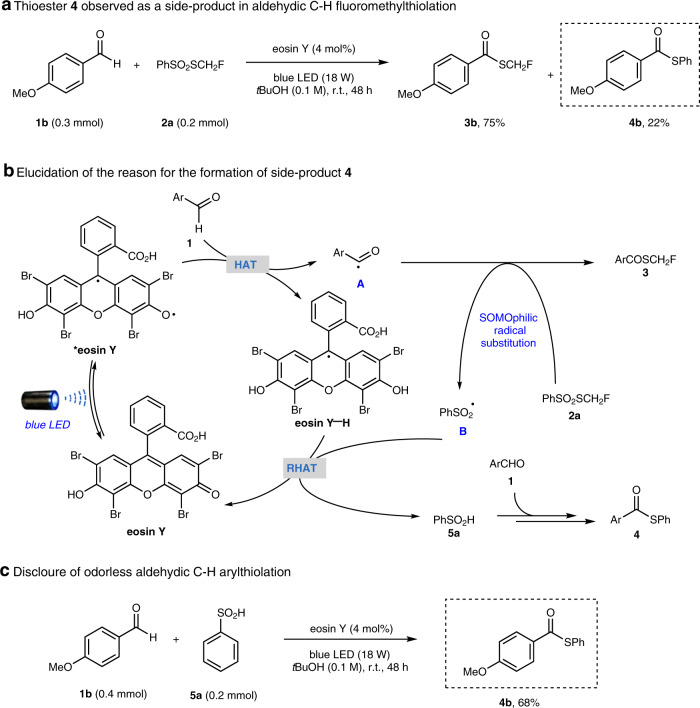
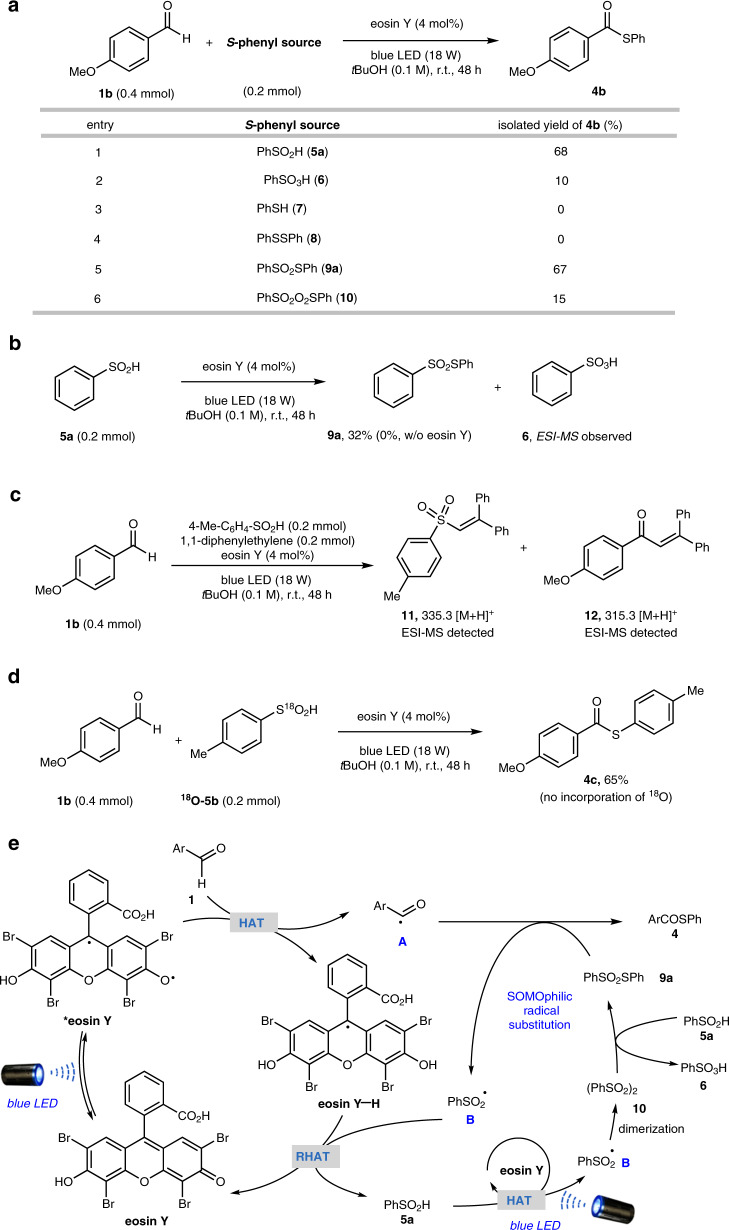
During the study of aldehydic C–H fluoromethylthiolation, we found that the eosin Y-photocatalyzed reaction between 4-methoxybenzaldehyde 1b and 2a gave major product 3b accompanied by S-phenyl thioester 4b in >20% yield (Fig. 3a). In light of our previous study on HAT photocatalysis34 and related reports on photocascade catalysis49–51, we speculated that S-phenyl thioester 4b might be derived from benzenesulfinic acid 5a generated in situ (Fig. 3b). The photo-generated acyl radical A underwent radical substitution with 2a to deliver monofluorothiolation product 3 and benzenesulfonyl radical B simultaneously. RHAT with eosin Y-H would convert benzenesulfonyl radical B to benzenesulfinic acid 5a, which accumulated in the reaction mixture and served as the sulfur reagent for thiolation. This hypothesis was verified by treatment of 1b with 5a under eosin Y photocatalysis conditions, which delivered thioester 4b in 68% yield (Fig. 3c).
Fig. 3. Discovery of aldehydic C–H thiolation using arylsulfinic acid as an odorless sulfur reagent.
a Thioester 4 observed as a side-product in aldehydic C–H fluoromethylthiolation. b Possible rationale for the formation of side-product 4. c Development of odorless aldehydic C–H thiolation.
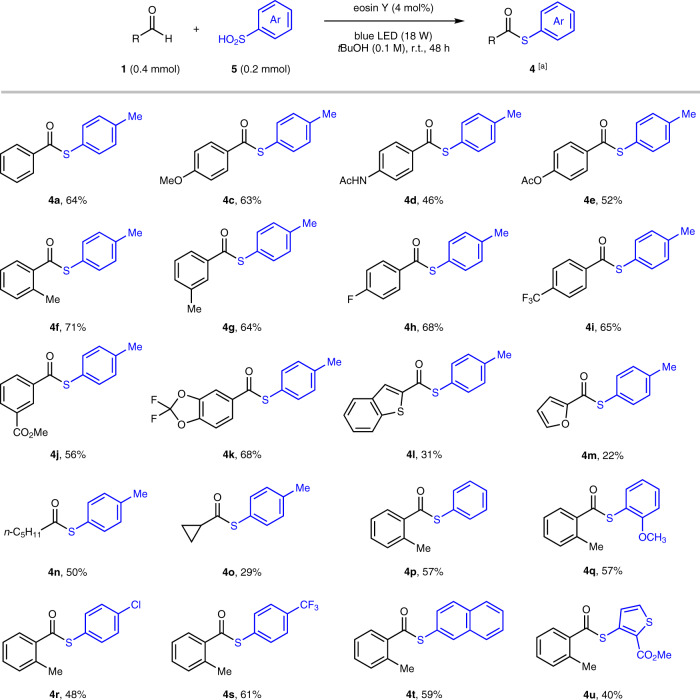
Given the synthetic value of thioesters52 and the appealing property of arylsulfinic acid as an odorless and readily available sulfur reagent, we attempted to examine the arylthiolation scope of this operationally simple method (Fig. 4). A diverse set of aromatic aldehydes took part in this transformation to give moderate to good yields (46–71%) of thioester products (4a–k), tolerating a variety of functional moieties such as amide (4d), ester (4e, 4j), halide (4 h), trifluoromethyl (4i), and 2,2-difluorobenzodioxole (4k) groups. Aromatic aldehydes with para-, meta-, or ortho-substituents smoothly participated in the transformation. Heteroaryl (4l, 4m) and alkyl (4n, 4o) aldehydes were also amenable to thiolation, but with lower yields (22–50%), where a substantial amount of sulfonic acids was observed as the side-products. Furthermore, variation with respect to arylsulfinic acids was evaluated, illustrating that a broad scope of (hetero)arylsulfinic acids afforded the corresponding thioesters (4p–u) in useful yields (40–61%).
Fig. 4. Scope of the odorless aldehydic C–H thiolation.
[a] Reaction conditions unless otherwise noted: 1 (0.4 mmol), 2 (0.2 mmol), eosin Y (4 mol%), and tert-butanol (2.0 mL) in an argon-filled Schlenk tube (20 mL) at room temperature (~27 °C) under 470 nm light (18 W LED) irradiation.
To gain a mechanistic understanding of the aldehydic C–H arylthiolation, a range of control experiments were performed to elucidate the reaction intermediates. Various S-phenyl sources were examined (Fig. 5a). Both benzenesulfinic acid 5a and S-phenyl benzenesulfonothioate 9a could afford thioester 4b in good yields, while other S-aryl sources such as benzenesulfonic acid 6, thiol 7, disulfide 8, and disulfone 10 were not effective at all or delivered 4b in very low yields. These results indicated that arylthiosulfonate 9 may act as a key intermediate in the reaction. This was further supported by the fact that benzenesulfinic acid 5a could be converted to S-phenyl benzenethiosulfonate 9a under eosin Y photocatalytic conditions, but 9a could not be generated in the absence of eosin Y (Fig. 5b). The reaction became sluggish with the addition of radical scavengers such as 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) and butylated hydroxytoluene (BHT), supporting a radical-based mechanism. When 1,1-diphenylethylene was added as an additive, adducts 11 and 12 were detected by electrospray ionization mass spectrometry (ESI-MS), indicating the presence of arylsulfonyl radicals and acyl radicals, respectively (Fig. 5c). Moreover, 18O incorporation was not observed when 18O-labeled 4-methylbenzenesulfinic acid 18O-5b53 was employed, which suggested that the carbonyl oxygen in the thioester product was derived from the aldehyde (Fig. 5d).
Fig. 5. Proposed mechanism of aldehydic C–H arylthiolation with supporting evidence.
a Evaluation of different S-phenyl sources. b Eosin Y photocatalytic formation of S-phenyl benzenethiosulfonate 9a from benzenesulfinic acid 5a. c Radical trapping experiments. d 18O-Labeling experiments. e Proposed plausible mechanisms.
In light of all the experimental data and related literature, a tentative mechanistic pathway for aldehydic C–H arylthiolation is proposed (Fig. 5e). Photo-excited *eosin Y undergoes HAT with benzenesulfinic acid 5a to generate benzenesulfonyl radical B54, which dimerizes to give disulfone species 10. 10 is reduced by benzenesulfinic acid 5a to give thiosulfonate 9a55,56. 9a then participates in radical substitution with acyl radical A, which is formed via eosin Y-HAT photocatalysis with aldehyde 1. Arythioester product 4 is obtained together with arylsulfonyl radical B, which closes the photocatalytic cycle and regenerates benzenesulfinic acid 5a (see Supplementary Fig. 12 for more discussion).
Development of Aldehydic C–H Alkynylation
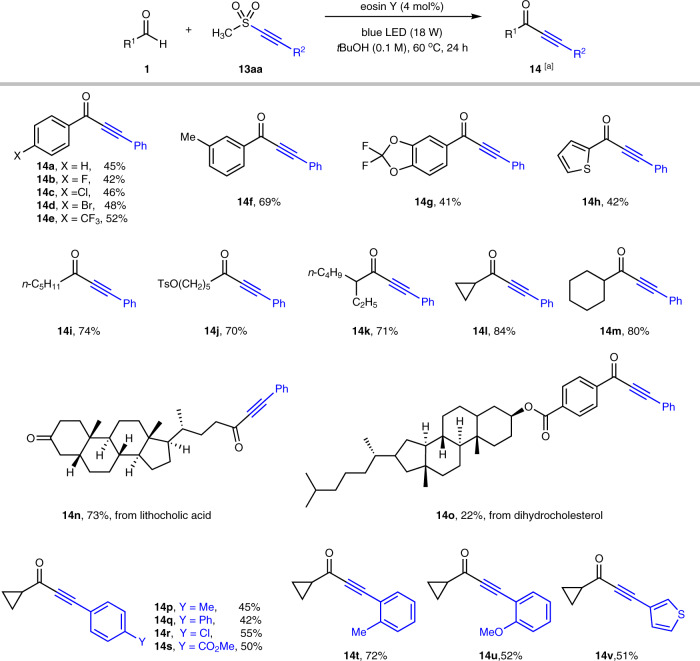
To further demonstrate HAT photocatalysis by eosin Y with sulfone reagents as a versatile platform for aldehydic C–H functionalizations, we found that methanesulfonyl alkyne 13aa was a suitable alkynylation reagent to deliver synthetically valuable ynone compounds (Fig. 6). A series of alkynyl sulfones bearing different R′SO2 group were examined and methanesulfonyl alkyne 13aa was identified as the optimal reagent, probably due to the small steric hinderance (see Supplementary Tables 2 and 3 for optimization study). Diverse substituents (e.g., F, Cl, Br, CF3) could be incorporated in arylaldehydes to produce ynones 14a–g in moderate yields (41–69%). Aldehydes possessing heteroaromatics such as thiophene (14h) were suitable substrates as well. Aliphatic aldehydes including both linear (14i, 14j) and branched (14k–m) alkyl substituents participated in the alkynylation more efficiently to afford ynones in good yields (70–84%). Moreover, aldehydes derived from lithocholic acid and dihydrocholesterol underwent the transformations smoothly, leading to the facile formation of 14n (73%) and 14o (22%), respectively. Next, the scope regarding alkynyl sulfone 13 was explored. The protocol was feasible for both arylalkynyl sulfones (14p–u) bearing a variety of functionalities (e.g., Cl, CO2Me, OMe) and heteroarylalkynyl sulfones (14v) to produce the corresponding ynones with moderate to good yields (42–72%).
Fig. 6. Substrate scope of aldehydic C–H alkynylation.
[a] Reaction conditions: 1 (0.4 mmol), 13 (0.2 mmol), eosin Y (4 mol%), and tert-butanol (2.0 mL) in an argon-filled Schlenk tube (20 mL) at 60 °C under 470 nm light (18 W LED) irradiation.
Preliminary study on aldehydic C–H azidation and alkenylation and scale-up in continuous-flow reactors
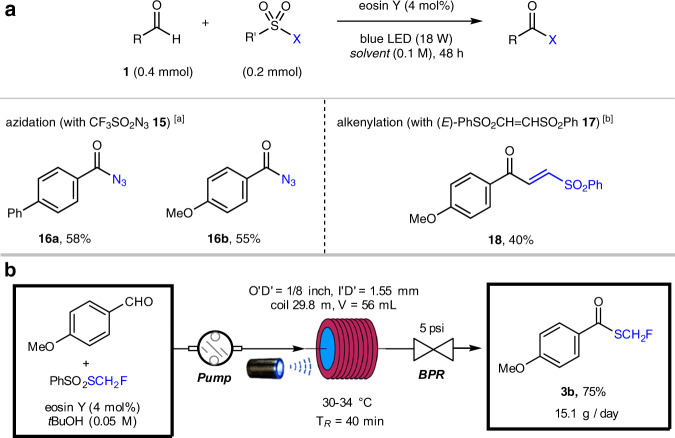
To further showcase the versatility of eosin Y-HAT photocatalysis with sulfone reagents, aldehydic C–H azidation and alkenylation were investigated (Fig. 7a). Our preliminary study illustrated that triflic azide (CF3SO2N3) 15 could be applied to forge C–N bonds, delivering acyl azides (16a, 16b) in moderate yields (55–58%). This protocol provides a mild alternative to previous aldehydic C–H azidation reactions, which require either excess oxidants57,58 or inconvenient reagents59. Aldehydic C–H alkenylation was also feasible using (E)-1,2-bis(phenylsulfonyl)ethene 17 as the alkenylation reagent, delivering enone 18 in 40% yield. Finally, the aldehydic C–H monofluoromethylation reaction was smoothly transferred to an operationally simple continuous-flow reactor to achieve >15 g per day production, indicating the potential of our strategy for large-scale synthesis (Fig. 7b).
Fig. 7. Aldehydic C–H diversification and scale-up synthesis in flow.
a Aldehydic C–H azidation and vinylation. Reaction conditions unless otherwise noted: 1 (0.4 mmol), sulfone (0.2 mmol), eosin Y (4 mol%), and solvent (2.0 mL) in an argon-filled Schlenk tube (20 mL) under 470 nm light (18 W LED) irradiation. [a] Reaction was performed in CH3CN at room temperature. [b] Reaction was performed in ethyl acetate at 80 °C. b Reaction scale-up in continuous-flow microtubing reactors. Psi pound per square inch.
Discussion
In summary, we have demonstrated a versatile platform for aldehydic C–H functionalization by merging neutral eosin Y-HAT photocatalysis with a variety of sulfone-based SOMOphiles to directly construct acyl–S, acyl–C and acyl–N bonds. The eosin Y-sulfone system will serve as a more green and sustainable method with easier handling for aldehydic functionalization compared to existing catalytic/stoichiometric systems and has several practically or mechanistically notable features. First, PhSO2–SCHxFy 2 proved particularly effective for metal-, additive- and oxidant-free fluoromethythiolation with diverse aldehydes, including complex natural product derivatives. The fluoromethythiolation reaction was performed under continuous-flow conditions to achieve a productivity of 15 g per day. Second, arylsulfinic acid (ArSO2H), as an odorless and easily accessible reagent, was employed for arylthiolation of aldehydes. A preliminary mechanistic study supported that an in situ-generated arylthiosulfonate (ArSO2–SAr) species participated in the subsequent radical substitution step. Third, the acyl–C(sp) bond was successfully forged via acyl radical addition-sulfonyl radical elimination with a methanesulfonyl alkyne. Finally, exploiting the versatility of radical addition-elimination, the present strategy was further extended to aldehydic C–H azidation and alkenylation. Extension of the C–H substrate scope to abundant alkanes and expanding the chemical space of sulfone SOMOphilic reagents to new reaction patterns are currently under investigation in our laboratory.
Methods
General procedure of neutral-eosin Y-photocatalyzed aldehydic C–H fluoromethylthiolation
A 20 mL Schlenk tube equipped with a magnetic stir bar was charged with eosin Y (0.008 mmol, 5.2 mg), aldehyde 1 (0.3 mmol), and fluoromethylthiolation reagents 2 (0.2 mmol). Then, 2.0 mL of anhydrous tert-butanol was added. The Schlenk tube was connected to Schlenk line and freeze–pump–thaw was performed for three times to completely remove air inside the reaction mixture. Eventually the Schlenk tube was refilled with an atmosphere of argon at room temperature and sealed. The reaction vessel was surrounded by a coil of blue LED strip (2 m, 18 W). Then the reaction was running at ambient temperature (~27 oC) using a fan to cool down the reaction mixture and stopped after 48 h. The solvent was removed under reduced pressure and the crude mixture was purified by silica gel column chromatography or prepared TLC (eluent: hexane/diethyl ether or hexane/ethyl acetate; 10/1–3/1) to give the corresponding product 3. Note that the workup procedure was performed under weak vacuum (~50 mbar) and low temperature (~30 oC) due to volatility of the corresponding product 3.
General procedure of neutral-eosin Y-photocatalyzed aldehydic C–H arylthiolation
A 20 mL Schlenk tube equipped with a magnetic stir bar was charged with eosin Y (0.008 mmol, 5.2 mg), aldehyde 1 (0.4 mmol), and arylsulfinic acid 5 (0.2 mmol). Then, 2.0 mL of anhydrous tert-butanol was added. The Schlenk tube was connected to Schlenk line and freeze–pump–thaw was performed for three times to completely remove air inside the reaction mixture. Eventually the Schlenk tube was refilled with an atmosphere of argon at room temperature and sealed. The reaction vessel was surrounded by a coil of blue LED strip (2 m, 18 W). Then the reaction was running at ambient temperature (~27 oC) using a fan to cool down the reaction mixture and stopped after 48 h. The solvent was removed under reduced pressure and the crude mixture was purified by silica gel column chromatography or prepared TLC (eluent: hexane/diethyl ether or hexane/ethyl acetate; 10/1–3/1) to give the corresponding product 4.
General procedure of neutral-eosin Y-photocatalyzed aldehydic C–H alkynylation
A 20 mL Schlenk tube equipped with a magnetic stir bar was charged with eosin Y (0.008 mmol, 5.2 mg), aldehyde 1 (0.4 mmol), acetylenic sulfone reagents 13 (0.2 mmol). Then, 2.0 mL of anhydrous tert-butanol was added. The Schlenk tube was connected to Schlenk line and freeze–pump–thaw was performed for three times to completely remove air inside the reaction mixture. Eventually the Schlenk tube was refilled with an atmosphere of argon at room temperature and sealed. The reaction vessel was surrounded by a coil of blue LED strip (2 m, 18 W). Then the reaction tubes were placed in a water bath covered by top oil layer (to prevent evaporation of water bath). The reaction was running at 60 oC and stopped after 24 h. The solvent was removed under reduced pressure and the crude mixture was purified by silica gel column chromatography or prepared TLC (eluent: hexane/diethyl ether or hexane/ethyl acetate; 10/1–3/1) to give the corresponding product 14.
Computational details
Density functional theory calculations were performed to shed light on the mechanism of eosin Y regeneration (Supplementary Fig. 17). RHAT (red line) is the favored pathway, which features a barrier 2.1 kcal/mol lower than an alternative single electron transfer (SET, black line). The geometries optimization in this study was performed at the uB3LYP density functional with a standard def2-SVP basis set. The nature of the stationary points (minima with no imaginary frequency or transition states with one imaginary frequency) were confirmed. The free energies of the optimized geometries were calculated at the same level of theory, considering the solvent effect of acetone using an SMD continuum solvation model. Unless specified otherwise, the Gibbs free energy was used throughout. For transition state, intrinsic reaction coordinate calculations were performed to verify whether it connected with correct reactants and products or intermediates. All calculations were performed using the Gaussian 16 Rev. A.03 software suite60.
Supplementary information
Acknowledgements
We are grateful for the financial support provided by Pfizer, the National University of Singapore (R-143-000-B60-114), National University of Singapore Flagship Green Energy Program (R-279-000-553-646 and R-279-000-553-731), the National Natural Science Foundation of China (Grant No. 22071170), and the National University of Singapore (Suzhou) Research Institute. We thank Prof. Qilong Shen (SIOC) for the gifted samples of 2a and 2b. The authors would like to thank Dr. Juan Colberg, Dr. Patrick O’Neill, Dr. Srinivas Reddy Dubbaka, and Dr. Zhihui Peng (Pfizer) for helpful discussion.
Source data
Author contributions
J.Y. discovered and developed the reaction. J.Y., J.L.P., S.D., and J.W. conceived and designed the investigations. X.S. conducted DFT calculations. J.Y., H.T., E.J.R.K., and C.L. performed the experiments. J.Y., H.T., M.Z., and J.W. wrote the manuscript.
Peer review information
Nature Communications thanks Takashi Koike and the anonymous reviewer(s) for their contribution to the peer review of this work.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jianming Yan, Haidi Tang.
Contributor Information
Muliang Zhang, Email: muliang0206@foxmail.com.
Shengquan Duan, Email: shengquan.duan@pfizer.com.
Jie Wu, Email: chmjie@nus.edu.sg.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-27550-8.
References
- 1.Giese B. Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. Engl. 1983;22:753–764. [Google Scholar]
- 2.Boger DL, Mathvink RJ. Acyl radicals: intermolecular and intramolecular alkene addition reactions. J. Org. Chem. 1992;57:1429–1443. [Google Scholar]
- 3.Proctor RSJ, Phipps RJ. Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed. 2019;58:13666–13699. doi: 10.1002/anie.201900977. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, MacMillan DWC. Direct aldehyde C−H arylation and alkylation via the combination of nickel, hydrogen atom transfer, and photoredox catalysis. J. Am. Chem. Soc. 2017;139:11353–11356. doi: 10.1021/jacs.7b07078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu L, Lipshultz JM, Macmillan DWC. Merging photoredox and nickel catalysis: the direct synthesis of ketones by the decarboxylative arylation of α-oxo acids. Angew. Chem. Int. Ed. 2015;54:7929–7933. doi: 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, et al. Direct C-H arylation of aldehydes by merging photocatalyzed hydrogen atom transfer with palladium catalysis. ACS Catal. 2020;10:7543–7551. [Google Scholar]
- 7.Chatgilialoglu C, Crich D, Komatsu M, Ryu I. Chemistry of acyl radicals. Chem. Rev. 1999;99:1991–2070. doi: 10.1021/cr9601425. [DOI] [PubMed] [Google Scholar]
- 8.Raviola C, Protti S, Ravelli D, Fagnoni M. Photogenerated acyl/alkoxycarbonyl/carbamoyl radicals for sustainable synthesis. Green. Chem. 2019;21:748–764. [Google Scholar]
- 9.Banerjee A, Lei Z, Ngai M-Y. Acyl radical chemistry via visible-light photoredox catalysis. Synthesis. 2019;51:303–333. doi: 10.1055/s-0037-1610329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penteado F, et al. α-Keto acids: acylating agents in organic synthesis. Chem. Rev. 2019;119:7113–7278. doi: 10.1021/acs.chemrev.8b00782. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. Visible-light-mediated decarboxylation/oxidative amidation of α-keto acids with amines under mild reaction conditions using O2. Angew. Chem. Int. Ed. 2014;53:502–506. doi: 10.1002/anie.201308614. [DOI] [PubMed] [Google Scholar]
- 12.De Pedro Beato E, Mazzarella D, Balletti M, Melchiorre P. Photochemical generation of acyl and carbamoyl radicals using a nucleophilic organic catalyst: applications and mechanism thereof. Chem. Sci. 2020;11:6312–6324. doi: 10.1039/d0sc02313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capaldo L, Riccardi R, Ravelli D, Fagnoni M. Acyl radicals from acylsilanes: photoredox-catalyzed synthesis of unsymmetrical ketones. ACS Catal. 2018;8:304–309. [Google Scholar]
- 14.Kumar P, et al. Aldehydes: magnificent acyl equivalents for direct acylation. Org. Biomol. Chem. 2020;18:7987–8033. doi: 10.1039/d0ob01458c. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y-L, Ouyang Y-J, Zheng H, Liu H, Wei W-T. Recent advances in acyl radical enabled reactions between aldehydes and alkenes. Chem. Commun. 2021;57:6111–6120. doi: 10.1039/d1cc02112e. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu C, Yuan J, Lei A. Copper-catalyzed oxidative coupling of alkenes with aldehydes: direct access to α,β-unsaturated ketones. Angew. Chem. Int. Ed. 2013;52:2256–2259. doi: 10.1002/anie.201208920. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, et al. Radical fluoroalkylthiolation of aldehydes with PhSO2SRf (Rf = CF3, C2F5, CF2H or CH2F): a general protocol for the preparation of fluoroalkylthioesters. Org. Chem. Front. 2018;5:2163–2166. [Google Scholar]
- 18.Guo S-H, et al. Synthesis of difluoromethylthioesters from aldehydes. Angew. Chem. Int. Ed. 2018;57:1663–1667. doi: 10.1002/anie.201710731. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. Organocatalytic transformation of aldehydes to thioesters with visible light. Chem. Eur. J. 2019;25:8225–8228. doi: 10.1002/chem.201900932. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ji P, Dong Y, Wei Y, Wang W. Deuteration of formyl groups via a catalytic radical H/D exchange approach. ACS Catal. 2020;10:2226–2230. doi: 10.1021/acscatal.9b05300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J, et al. Light-mediated difluoromethylthiolation of aldehydes with a hydrogen atom transfer photocatalyst. Org. Lett. 2020;22:8272–8277. doi: 10.1021/acs.orglett.0c02902. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Patra T, Glorius F. Cooperative catalysis: a strategy to synthesize trifluoromethyl-thioesters from aldehydes. ACS Catal. 2018;8:5842–5846. [Google Scholar]
- 23.Capaldo L, Ravelli D. Decatungstate as direct hydrogen atom transfer photocatalyst for SOMOphilic alkynylation. Org. Lett. 2021;23:2243–2247. doi: 10.1021/acs.orglett.1c00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S, Garza-Sanchez RA, Tlahuext-Aca A, Glorius F. Alkynylation of Csp2(O)−H bonds enabled by photoredox-mediated hydrogen-atom transfer. Angew. Chem. Int. Ed. 2017;56:14723–14726. doi: 10.1002/anie.201708037. [DOI] [PubMed] [Google Scholar]
- 25.Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C−H bonds elaboration. Chem. Rev. 121, 10.1021/acs.chemrev.1c00263 (2021). [DOI] [PMC free article] [PubMed]
- 26.Cao H, Tang X, Tang H, Yuan Y, Wu J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 2021;1:523–598. [Google Scholar]
- 27.Shen Y, Gu Y, Martin R. sp3 C–H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc. 2018;140:12200–12209. doi: 10.1021/jacs.8b07405. [DOI] [PubMed] [Google Scholar]
- 28.Kamijo S, Kamijo K, Maruoka K, Murafuji T. Aryl ketone catalyzed radical allylation of C(sp3)−H bonds under photoirradiation. Org. Lett. 2016;18:6516–6519. doi: 10.1021/acs.orglett.6b03586. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Lei M, Gong L. Photocatalytic regio- and stereoselective C(sp3)−H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2019;2:1016–1026. [Google Scholar]
- 30.Cao S, Hong W, Ye Z, Gong L. Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)−H functionalization. Nat. Commun. 2021;12:2377. doi: 10.1038/s41467-021-22690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen Y, Dai Z-Y, Zhang C, Wang P-S. Palladium-catalyzed allylic alkylation via photocatalytic nucleophile generation. ACS Catal. 2021;11:6757–6762. [Google Scholar]
- 32.Hari DP, König B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014;50:6688–6699. doi: 10.1039/c4cc00751d. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava V, Singh PP. Eosin Y catalysed photoredox synthesis: a review. RSC Adv. 2017;7:31377–31392. doi: 10.1039/d2ra90108k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan D-M, Chen J-R, Xiao W-J. New roles for photoexcited eosin Y in photochemical reactions. Angew. Chem. Int. Ed. 2019;58:378–380. doi: 10.1002/anie.201811102. [DOI] [PubMed] [Google Scholar]
- 35.Fan X-Z, et al. Eosin Y as a direct hydrogen-atom transfer photocatalyst for the functionalization of C−H bonds. Angew. Chem. Int. Ed. 2018;57:8514–8518. doi: 10.1002/anie.201803220. [DOI] [PubMed] [Google Scholar]
- 36.Kuang Y, et al. Asymmetric synthesis of 1,4-dicarbonyl compounds from aldehydes by hydrogen atom transfer photocatalysis and chiral Lewis acid catalysis. Angew. Chem. Int. Ed. 2019;58:16859–16863. doi: 10.1002/anie.201910414. [DOI] [PubMed] [Google Scholar]
- 37.Yan J, et al. A radical smiles rearrangement promoted by neutral eosin Y as a direct hydrogen atom transfer photocatalyst. J. Am. Chem. Soc. 2020;142:11357–11362. doi: 10.1021/jacs.0c02052. [DOI] [PubMed] [Google Scholar]
- 38.Inoa J, Dominici G, Eldabagh R, Foley JJ, IV, Xing Y. Synthetic applications and computational perspectives on eosin Y induced direct HAT process. Synthesis. 2021;53:2183–2197. [Google Scholar]
- 39.Xia Y, Wang L, Studer A. Site-selective remote radical C−H functionalization of unactivated C−H bonds in amides using sulfone reagents. Angew. Chem. Int. Ed. 2018;57:12940–12944. doi: 10.1002/anie.201807455. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Xia Y, Bergander K, Studer A. Remote site-specific radical alkynylation of unactivated C−H bonds. Org. Lett. 2018;20:5817–5820. doi: 10.1021/acs.orglett.8b02514. [DOI] [PubMed] [Google Scholar]
- 41.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- 42.Panferova LI, Zubkov MO, Kokorekin VA, Levin VV, Dilman AD. Using the thiyl radical for aliphatic hydrogen-atom transfer: thiolation of unactivated C–H bonds. Angew. Chem. Int. Ed. 2021;60:2849–2854. doi: 10.1002/anie.202011400. [DOI] [PubMed] [Google Scholar]
- 43.Constantin T, et al. Aminoalkyl radicals as halogen-atom transfer agents for activation of alkyl and aryl halides. Science. 2020;367:1021–1026. doi: 10.1126/science.aba2419. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Q, Lu L, Shen Q. Direct monofluoromethylthiolation with S-(fluoromethyl) benzenesulfonothioate. Angew. Chem. Int. Ed. 2017;56:11575–11578. doi: 10.1002/anie.201705633. [DOI] [PubMed] [Google Scholar]
- 45.Liu F, Jiang L, Qiu H, Yi W. Bunte salt CH2FSSO3Na: an efficient and odorless reagent for monofluoromethylthiolation. Org. Lett. 2018;20:6270–6273. doi: 10.1021/acs.orglett.8b02753. [DOI] [PubMed] [Google Scholar]
- 46.Guo S-H, et al. Synthesis of monofluoromethylthioesters from aldehydes. Adv. Synth. Catal. 2018;360:1861–1869. [Google Scholar]
- 47.Deng H-P, Zhou Q, Wu J. Microtubing-reactor-assisted aliphatic C−H functionalization with HCl as a hydrogen-atom-transfer catalyst precursor in conjunction with an organic photoredox catalyst. Angew. Chem. Int. Ed. 2018;57:12661–12665. doi: 10.1002/anie.201804844. [DOI] [PubMed] [Google Scholar]
- 48.Morack T, Mück-Lichtenfeld C, Gilmour R. Bioinspired radical Stetter reaction: radical umpolung enabled by ion-pair photocatalysis. Angew. Chem., Int. Ed. 2019;58:1208–1212. doi: 10.1002/anie.201809601. [DOI] [PubMed] [Google Scholar]
- 49.Capaldo L, Quadri LL, Ravelli D. Photocatalytic hydrogen atom transfer: the philosopher’s stone for late-stage functionalization? Green. Chem. 2020;22:3376–3396. [Google Scholar]
- 50.Capaldo L, Ravelli D. Hydrogen atom transfer (HAT): a versatile strategy for substrate activation in photocatalyzed organic synthesis. Eur. J. Org. Chem. 2017;2017:2056–2071. doi: 10.1002/ejoc.201601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J-R, Yan D-M, Wei Q, Xiao W-J. Photocascade catalysis: a new strategy for cascade reactions. ChemPhotoChem. 2017;1:148–158. [Google Scholar]
- 52.Luo J, et al. Formation of thioesters by dehydrogenative coupling of thiols and alcohols with H2 evolution. Nat. Catal. 2020;3:887–892. [Google Scholar]
- 53.Reilly SW, Bennett F, Fier PS, Ren SM, Strotman NA. Late-stage 18O labeling of primary sulfonamides via a degradation-reconstruction pathway. Chem. Eur. J. 2020;26:4251–4255. doi: 10.1002/chem.202000484. [DOI] [PubMed] [Google Scholar]
- 54.Griesser M, Chauvin JPR, Pratt DA. The hydrogen atom transfer reactivity of sulfinic acids. Chem. Sci. 2018;9:7218–7229. doi: 10.1039/c8sc02400f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kice JL, Bowers WK. The mechanism of the disproportionation of sulfinic acids. J. Am. Chem. Soc. 1962;84:605–610. [Google Scholar]
- 56.Cao L, et al. Disproportionate coupling reaction of sodium sulfinates mediated by BF3·OEt2: an approach to symmetrical/unsymmetrical thiosulfonates. Org. Lett. 2018;20:4754–4758. doi: 10.1021/acs.orglett.8b01808. [DOI] [PubMed] [Google Scholar]
- 57.De Sarkar S, Studer A. Oxidative amidation and azidation of aldehydes by NHC catalysis. Org. Lett. 2010;12:1992–1995. doi: 10.1021/ol1004643. [DOI] [PubMed] [Google Scholar]
- 58.Yadav VK, Srivastava VP, Yadav LDS. Visible light induced azidation of aldehydic C–H with carbon tetrabromide and sodium azide. Tetrahedron Lett. 2016;57:2502–2505. [Google Scholar]
- 59.Shinomoto Y, et al. Tetra-n-butylammonium iodide catalyzed C−H azidation of aldehydes with thermally stable azidobenziodoxolone. Org. Lett. 2015;17:5212–5215. doi: 10.1021/acs.orglett.5b02543. [DOI] [PubMed] [Google Scholar]
- 60.Frisch, M. J. et al. Gaussian 16, revision C.01 (Gaussian, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon request. Source data are provided with this paper.