Abstract
Organoids—cellular aggregates derived from stem or progenitor cells that recapitulate organ function in miniature—are of growing interest in developmental biology and medicine. Organoids have been developed for organs and tissues such as the liver, gut, brain, and pancreas; they are used as organ surrogates to study a wide range of questions in basic and developmental biology, genetic disorders, and therapies. However, many organoids reported to date have been cultured in Matrigel, which is prepared from the secretion of Engelbreth-Holm-Swarm mouse sarcoma cells; Matrigel is complex and poorly defined. This complexity makes it difficult to elucidate Matrigel-specific factors governing organoid development. In this review, we discuss promising Matrigel-free methods for the generation and maintenance of organoids that use decellularized extracellular matrix (ECM), synthetic hydrogels, or gel-forming recombinant proteins.
Subject terms: Biomaterials, Stem-cell differentiation, Biological models
Kozlowski, Crook, and Ku review current state-of-the-art techniques in culturing and manipulating organoids without the use of EHS-tumor-derived matrix, the most popular of which is Matrigel by Corning. They cover organoid-based applications of different matrix types, ranging from natural sources (decellularized ECM, purified ECM proteins, polysaccharides), synthetic sources (e.g. PEG-based), and recombinant peptide-based systems.
Introduction
Organoids are multicellular structures derived from stem and progenitor cells that mimic the function and spatial organization of organs1. Organoids recapitulate important organ functions in vitro while remaining small in size and often free of interfering cell types such as vascular, nerve, or other undesired epithelial cells. For these reasons, organoids are used to study organ development2 and model various diseases such as cancers3, neural disorders4, and autism5; they are also used as pharmaceutical testing platforms6, model systems for CRISPR-CAS9-mediated treatment of genetic diseases7, and replacement organs for transplantation8,9. It is possible to culture organoids from induced pluripotent stem cells (iPSCs)10 or adult stem cells from patient tissues11, which may lead to personalized medicine. The wide range of clinical applications of organoids is the subject of a recent review by Drost and Clevers12. Many excellent reviews have been published about different organoid types, such as heart13,14, brain15–17, liver18,19, kidney20–22, pancreas23–25, and female reproductive tract26.
Many organoids have been cultured in Matrigel, a material derived from the secretion of Engelbreth–Holm–Swarm mouse sarcoma cells and enriched for extracellular matrix (ECM) proteins27. In an early report of organoid culture, Sato and colleagues grew murine intestinal Lgr5+ stem cells in high concentrations of Matrigel supplemented with the growth factors WNT, Noggin, R-spondin, and EGF28. This culture system has been widely adapted for other organs such as the colon, stomach, and liver29–33. Related methods have been used to construct simulated versions of the inner ear34 and pancreas35–39, and the similarities between pancreatic and liver-organoid-generating cells suggest that methods used for making liver organoids may be applicable to the pancreas40. The many organoid-specific applications of Matrigel-based culture methods have been thoroughly discussed elsewhere41–43.
Despite its versatility and affordability, Matrigel is extremely complex; proteomic analysis shows that it contains more than 1800 unique proteins44. The undefined nature of Matrigel makes it difficult to identify the signals necessary for organoid structure and function; this difficulty is compounded by lot-to-lot variations of Matrigel45–48. Furthermore, Matrigel may not contain all of the necessary components for proper organoid formation; gut organoids cultured in Matrigel lack the characteristic architecture of mammalian intestines, which could be due to a sub-optimal amount of laminin-511 and the absence of other cell types such as mesenchymal cells49,50. Finally, it has become increasingly clear that the mechanical properties in three-dimensional (3D) culture systems can have large effects on cell51, organoid52, tissue53, and organ development54,55. The mechanical properties, such as elastic modulus, pore size, stress relaxation, and creep56–59, cannot be easily separated from the chemical cues in the Matrigel-based culture systems. Furthermore, the mechanical properties of Matrigel samples are heterogeneous; local regions of such gels have been found to exhibit elastic moduli several times higher than the average modulus of the sample60,61. Finally, the fact that Matrigel is originated from mouse cells hampers its use in human clinical transplantation due to potential immunogenicity18.
Given these limitations, there is an emerging need to develop Matrigel-independent organoid culture methods. In this review, we discuss recently-developed Matrigel-free techniques for the culture of organoids. We will review undefined matrices, focusing on ECM derived from decellularized tissues and collagen, and defined matrices, including synthetic polymer hydrogels and engineered ECM proteins (Fig. 1). Table 1 summarizes the advantages and disadvantages of each category of material. Table 2 summarizes studies that discuss the effects of elastic modulus on the organoid formation of various tissues.
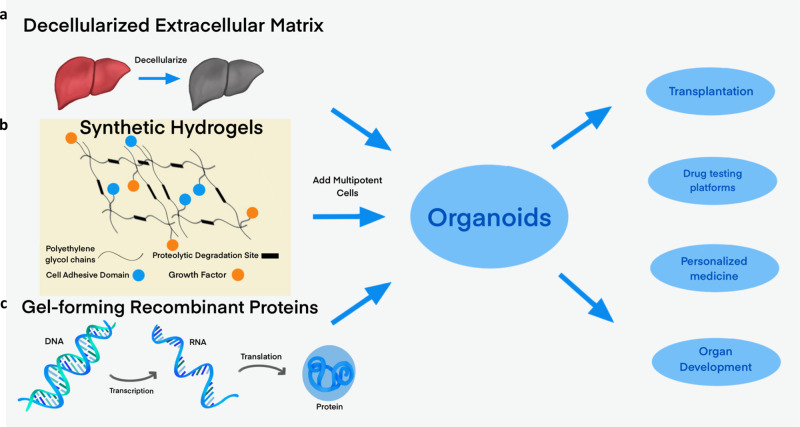
Fig. 1. Methods of making organoids without Matrigel.
Replacing the undefined medium of Matrigel is a major goal of organoid culture. We will discuss three main alternative media: (a) decellularized extracellular matrix and other derived proteins, (b) synthetic hydrogels, which generally incorporate cell-adhesive domains or proteolytic degradation sites, and (c) gel-forming recombinant peptides. Adding multipotent cells to these matrices enables the growth of organoids, which are potentially applicable as transplants, drug-testing platforms, personalized medicine, and means to understand organ development.
Table 1.
Different types of materials for the generation of organoids from various tissues in three-dimensional culture.
| Materials | Advantages | Disadvantages | Organoids made using this type of material | References |
|---|---|---|---|---|
| Matrigel | Inexpensive and commercially available, extensively used with well-developed protocols | Undefined culture system, subject to lot-to-lot variation, poor control of mechanical properties, may not contain all chemical cues necessary for differentiation, immunogenicity | Gut, heart, brain, liver, kidney, pancreas, female reproductive tract, inter alia. | See references12–27 for reviews |
| Decellularized tissue | Preserves native chemical cues and mechanical properties, resulting organoids can be large | Preparation is difficult, limited by donor availability, lack of definition | Liver, intestine, heart, lung, kidney, pancreas, testicular, stomach | Liver:73–77,80 Intestine:79,80 Heart:69,70 Lung:70,71,121 Kidney:67,68,70 Pancreas:80–82 Testes:72 Stomach80: |
| Collagen and other biomacromolecules derived from natural sources | Low cost, wide availability | No structural information preserved, not all necessary chemical cues present, often requires feeder cells, lot-to-lot variation | Liver, intestine, pancreas, epithelium, brain, lung, vascular, stomach, kidney | Liver:98 Intestine:84–86,89–91,102,103 Pancreas:104 Epithelium:86,88,89 Brain:101,111–116 Lung:99,100 Vascular:105 Stomach:85,102 Kidney:87 |
| Synthetic polymers | Excellent control of mechanical and chemical properties, repeatability, tunable degradation rate | Requires functionalization with cell-binding peptides or presence of feeder cells, possible cytotoxicity concerns | Brain, liver, intestine, pancreas, salivary glands | Neural and Brain:156,157,161,165,169,174 Liver:158–160,179 Intestine:162–164 Pancreas:175 Salivary Glands:183 |
| Recombinant proteins and peptides | Precise placement of chemical cues, tunable mechanical properties, tunable degradation rate, easy to include cell-binding domains | Possible endotoxin contamination, higher cost, possible immunogenicity | Pancreas, brain, intestine, heart | Pancreas:192–195 Brain:196,198–202 Intestine:191 Heart:189,190 |
Table 2.
Ideal elastic moduli for generating organoids from different organs.
Organoid culture in decellularized ECM and other naturally-derived proteins
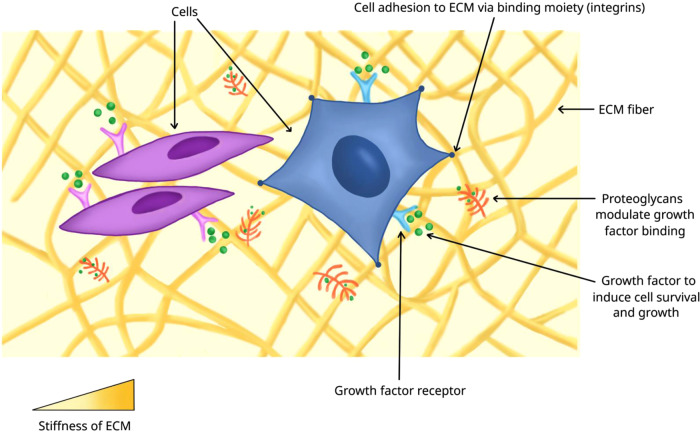
In organ development, ECM proteins provide signaling cues, serve as an adhesive substrate, and sequester growth factors (Fig. 2)62. In order to accurately recapitulate the composition, structure, and vascularization of native ECM in organ development, some organoids have been grown in decellularized ECM from human or animal donors. The methods of decellularization used are dependent on the target tissue and not readily generalizable; a number of these methods have been reviewed elsewhere63. While xenogeneic ECM has the potential to cause immune responses, this risk can be greatly reduced by using proper preparation techniques64; similar ECM scaffolds derived from animals are FDA-approved for clinical applications such as heart valve replacement, facial reconstruction, and osteopathic implants62,65. Decellularized ECM may also provide additional cues that promote regeneration of damaged tissue, ultimately supporting the organoid transplant and promoting its function66. Decellularization approaches have been demonstrated for human kidney67, murine kidney67,68, murine heart69,70, human and porcine lung71, and porcine testicular72 tissues, with each type posing unique challenges. To illustrate some of these challenges and methods, we will focus on the decellularization of the liver, gut, and pancreas.
Fig. 2. Microenvironment of cells.
Cells in an organ or organoid are surrounded by other cells, extracellular matrix (ECM) proteins, and growth factors sequestered in the proteoglycan-modified ECM proteins. Cells bind to ECM proteins via adhesion molecules, such as integrin receptors, which provide signaling cues to exert biological functions. The stiffness of ECM experienced by the cells also affects their biological activities.
Liver organoids grown in decellularized ECM
Liver-specific ECM can be obtained from a surgically resected portion of a patient’s damaged liver, or from livers unsuitable for transplantation. Lin and colleagues reported that liver tissue decellularization supported growth and maintenance of rat hepatocytes; however, this method relied on mechanical disruption of resected tissue, which resulted in the loss of organ architecture and vascular networks73. In contrast, Baptista and colleagues perfused Triton X-100 and ammonium hydroxide through a ferret hepatic vascular network to remove cells. This method preserved the underlying ECM and vasculature while retaining most of the glycosaminoglycans, collagens, and elastins. The decellularized material could be colonized by human fetal liver and endothelial cells to produce a functioning organoid74. An illustration of the method of Baptista and coworkers is shown in Fig. 3. The liver can also be decellularized, ground into powder, and redissolved. Lee and colleagues used this approach with rat liver ECM to promote the differentiation of human adipose-derived stem cells into functional hepatocytes75. More recently, Saheli and colleagues seeded sheep liver ECM gel with a combination of human hepatocarcinoma cells, mesenchymal stem cells, and umbilical cord stem cells; the resulting tumor organoids had greater hepatocyte function than tumor organoids grown in comparable collagen I-based culture76. Lewis and colleagues observed that growing murine small cholangiocytes (a committed progenitor cell type) in porcine liver ECM gel resulted in the formation of complex, branching structures similar to biliary ducts, and these cells also secreted small amounts of bile. In contrast, cholangiocytes cultured in Matrigel formed cysts while those in collagen I proliferated and spread in all directions without spontaneously forming structures77. Thus, Matrigel alone does not provide all of the needed factors for small cholangiocyte differentiation, whereas a decellularized liver ECM gel appears to be a better alternative.
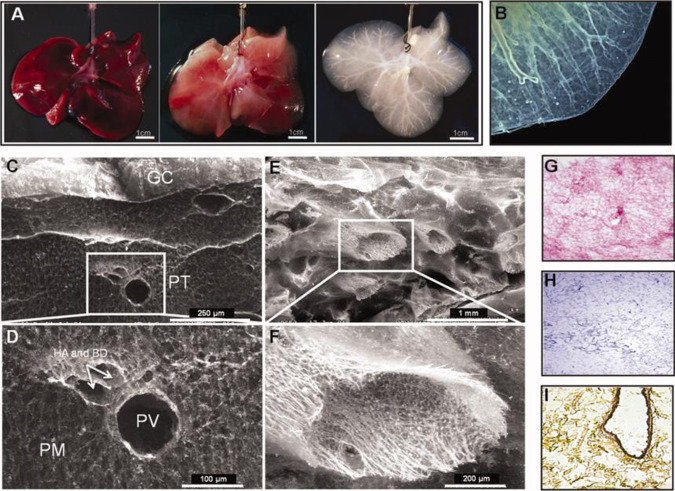
Fig. 3. An example of whole-organ ferret liver decellularization with excellent retention of structural information, for use as an organoid scaffold.
Figure (a) shows the liver at the start of treatment, then after 20 and 120 min of decellularization. A micrograph of the decellularized liver, (b), shows that the liver capsule and vasculature remain intact after cell removal. Scanning electron microscope images show that the structure of the liver is remarkably well-conserved, with an intact Glisson’s capsule (GC) visible in (c), and an intact hepatic artery (HA), hepatic portal vein (PV), and biliary duct (BD) visible in (d). Other blood vessels are structurally intact, despite cell removal (e), with the structural details apparent even at high magnification (f). H&E staining (g) shows that all cells have been removed, with the pink stain showing protein-containing extracellular matrix; this absence of cellular material is further confirmed by Mason’s Trichrome staining (h). Movat-Pentachrome staining (i) shows the presence of collagen in yellow, and a dark stain shows elastin around an artery. Decellularization can proceed gently enough to retain structural information, yielding scaffolds that can be colonized by pluripotent cells which then differentiate into mature organoids. The image is from ref. 74 and is reproduced with permission.
Gut organoids grown in decellularized ECM
Decellularized matrices have also been used for the growth of human intestinal organoids (HIOs) derived from pluripotent stem cells (PSCs), as well as enteroids derived from adult crypt stem cells78. Finkbeiner and colleagues found that undifferentiated human embryonic stem cells (ESCs) could not directly differentiate into HIOs in a decellularized porcine intestinal ECM. However, pre-differentiated HIOs were able to seed onto decellularized porcine intestinal ECM and form correct spatial orientation-mimicking intestine79. Giobbe and colleagues developed a method for decellularizing the porcine small intestine to form an intestinal ECM gel similar to the liver ECM gels discussed in the previous section. The porcine intestinal ECM gel was able to support the formation of enteroids from murine Lgr5+ crypt cells, and from human pediatric stomach and intestinal crypts. The authors were also able to achieve mechanical control of the ECM gel by incorporating poly-acrylamide to achieve different stiffness for two-dimensional (2D) culture of human and mouse enteroids, which may be important for future research. This intestinal ECM gel is also applicable to grow organoids from the liver, stomach, and pancreas80.
Decellularized ECM from the pancreas
Decellularized ECM has been prepared from various pancreatic cell sources, such as adult human pancreas81 and porcine pancreas82. Using mass spectrometry, Bi and colleagues found major differences in protein compositions comparing decellularized rat pancreatic extracellular matrix to Matrigel; Matrigel contains lower levels of collagen V than are normally present in pancreatic ECM. By coating plates with Matrigel plus commercially available collagen V, the authors were able to enhance endocrine differentiation of human iPSCs in 2D culture, compared to Matrigel alone83. One key limitation of this study, however, is that the proteomic profile of the rat pancreas may be very different from that of the human pancreas. The human pancreas presents a challenge in decellularization as it has a higher lipid content than animal models. With specific preparation methods that remove lipids, Sackett et al. found that decellularized human ECM is capable of supporting the survival of undifferentiated hPSCs and their pancreatic lineage derivatives, including insulin-expressing beta-like cells, in vitro81.
Tissue organoids grown in other naturally-derived proteins and biomacromolecules
A complementary approach to the use of decellularized ECM is naturally-derived proteins, such as collagen I derived from porcine tendon, porcine skin, or bovine lens capsules. Collagen I has been used to form human colorectal carcinoma model organoids in a 3D culture of rabbit colons84 and as a support for the culture of human and murine intestinal, stomach, and colonic organoids85. Other examples are vitrified collagen I for human intestinal organoids86 and murine renal organoids87, as well as fibrin supplemented with laminin-111 capable of supporting various murine and human epithelial organoids88.
The Tokyo Medical and Dental University (TMDU) method uses collagen I for intestinal enteroid culture89. Yui and colleagues showed that embedding intact murine colonic crypts and isolated Lgr5+ progenitor cells in collagen I with hepatocyte growth factor, R-spondin 1, EGF, and Noggin generated organoids that were able to engraft onto damaged mouse intestinal epithelia upon transplantation. In contrast to the TMDU method, the Ootani method uses collagen I gel in which small and large intestinal cells are kept suspended at an air-liquid interface; this method improves oxygenation of the organoid, allows viable murine organoids to be maintained in culture for up to 350 days, and preserves the mesenchymal niche in the organoids90.
Two factors have been shown to affect the differentiation of progenitor cells into organoids in collagen-based matrices: the source of seeded cells, and the spatial arrangement of collagen types around the cells. Isshiki and colleagues reported that the choice between the TMDU and Ootani methods should be governed by the source of cells used to generate the intestinal organoid. More consistent results are obtained for growing intestinal organoids from seeded isolated rat intestinal crypts using the TMDU method, whereas the Ootani method is better for growing rat colon organoid cultures of homogenized tissue91.
In addition to judicious selection of cell type, cell fate is intimately tied to interactions between cell surface integrins and biochemical cues in the ECM92. Collagen I has a high affinity for α2β1 integrin, whereas collagen IV binds more strongly to α1β1 integrin93. Collagen IV tends to occur exclusively in basement membranes93. β4 integrin expressed by intestinal organoids is distributed only at the basal surface94, while β1 integrin is required for proper apical-basal polarization95–97. A combination of collagen I and fibronectin compared to collagen I alone functionalized within a PEG gel enhanced hepatic differentiation from human mesenchymal stem cells98. Clearly, the culture of organoids must take the 3D spatial positioning of the relevant materials into account.
In addition to organoids grown in naturally-derived proteins, several laboratories have grown a wide range of organoids in polysaccharides such as alginate or alginate-chitosan mixtures. Organoid types grown in alginate include human lung99,100, human brain101, murine intestinal102, human intestinal102,103, human pancreatic104, and human and murine vascular105. Capeling and colleagues grew HIOs on an alginate substrate and found that differentiation of human pluripotent cells into HIOs could be supported without the alginate providing chemical cues to the cells. The authors hypothesized that cells create their own niche within the alginate hydrogel by secreting basement membrane proteins and forming mesenchyme, allowing cellular survival and differentiation into HIOs103. Rossen and colleagues demonstrated the development of murine and human vascular organoids in a non-functionalized alginate setting105. Alginate has a number of advantages that make it attractive as a material for further study; it is inexpensive, relatively easy to modify and functionalize, biocompatible, and has been used in a wide range of biological and materials applications106–108. The mechanical properties of alginate, such as elastic modulus, extensibility, and characteristic relaxation time, can also be easily tuned109. For these reasons, alginate is a promising material for further exploration. However, because alginate is biologically derived, its mechanical properties are still subject to lot-to-lot variability110. Similarly, hyaluronic acid and mixtures of hyaluronic acid and chitosan have been extensively used in the growth and construction of neural organoids111–116.
Advantages and disadvantages of decellularized ECM and other naturally-derived proteins or biomacromolecules
Decellularized ECM-based methods can quickly recapitulate organ function. Many or all of the chemical cues required for the formation of a spatially-defined organ, including difficult-to-introduce glycoproteins, are already present, minimizing the need for additional chemical modification of the ECM. Decellularized ECM retains the compositional differences observed between basal and apical regions. Collagen- and alginate-based materials have been approved by the FDA for a wide variety of applications117, which allows for rapid clinical translation.
Decellularized ECM does have disadvantages. Most importantly, the quantity of ECM that is available for study is limited by the availability of donor animals or humans, and the quality of ECM can be affected by the health of a donor. For example, emphysematous or fibrotic lung tissue has hardened and undergone alterations in its architecture. These alterations can lead to cells failing to survive beyond one week of culture118 or broad changes in the phenotype of seeded cells that do survive119. Contrarily, myocardial infarct is known to trigger remodeling events that stiffen the ECM and change its chemical composition; yet when mesenchymal stem cells are seeded on infarcted tissue, the cells secrete higher levels of pro-survival and immunomodulatory growth factors120. While myocardial infarct appears to enhance the survival of seeded cells, the negative effects of other diseased tissue on organoid development should not be discounted.
Even with healthy donor tissue, batch-to-batch variability remains. The physical properties of decellularized ECM are difficult to control or modify, which limits the experiments that can be conducted. Decellularized ECM is also chemically undefined; the factors driving differentiation are often unknown. Surface proteoglycans that are necessary for successful organoid formation may be removed by harsh decellularization121. A related difficulty is that not all decellularization protocols are equally effective at removing cells or other immunogenic species, which can cause varying host immune responses and failure of implants in clinical trials122. Finally, the occasional need for PSC differentiation into organ-specific progenitor cells that are then introduced into the decellularized matrix requires an additional step.
Collagen-based culture methods are not limited by donor tissue availability; biomedical-grade collagen can be harvested on an industrial scale from cows and pigs. However, some collagen-based culture methods rely on coculture with supporting cells98, which introduces undefined components into the organoid culture. Furthermore, it is difficult to modify the mechanical properties of these culture systems without altering chemical concentrations. To elucidate the effects of mechanical properties on organoid development, researchers have turned to synthetic hydrogels that have been functionalized with cell-binding cues.
Organoid culture in synthetic hydrogels
Native ECM is complex; it contains over 300 different proteins, each of which has a different biological function and stiffness123. This large number of proteins means that many variables cannot be easily dissected to study the influences of ECM on organoid behavior and development. Synthetic hydrogels are attractive because their mechanical properties, functionality, and erosion rate can be controlled. The matrix metalloprotease (MMP) family of enzymes affects cellular and organoid development by degrading ECM proteins124. By including MMP recognition sites on synthetic hydrogels, it is possible to tune the rate of the hydrogel’s erosion. Manipulating synthetic hydrogels using methods such as electrospinning125, photopatterning126,127, spraying of microspheres128, inkjet and 3D printing129, or microfluidic channels130 further enables control over the shape and size of the organoids. The ability to exert local control over chemical and mechanical properties allows researchers to duplicate the heterogeneity in stiffness and composition found in organs, generate interfaces between materials similar to those found in the ECM, and duplicate essential elements of material microstructure; each of these controls has implications for organ function and disease131,132. Synthetic hydrogels can also be made responsive to external stimuli. For example, a thermoreversible hyaluronic acid- poly(N-isopropylacrylamide) (PNIPAAm) based hydrogel that solidifies at 37 oC and re-liquefies upon cooling enabled culture and recovery of human pluripotent stem cells without enzymatically digesting the matrix133. Light-sensitive polyvinyl alcohol matrices have recently been developed for cell culture; these matrices allow for control over the spacing of biochemical cues and the material environment134. Both of these materials may be useful for future organoid studies.
The use of synthetic hydrogels may also open up new avenues by altering the porosity of the scaffold on which the cells are grown. Dye and coworkers found that human lung organoids transplanted in mice could merge together to form airway structures. However, this was only possible if the scaffold was able to degrade, which increased the material’s pore size135. Choi and coworkers reviewed the pore sizes typically used in making tissue-engineering scaffolds, ranging from 5–15 microns for fibroblasts to 200–400 microns for osteoblasts, and developed a method for creating an artificial kidney scaffold using microstereolithography136. However, these studies observed the effects of pore size on mature cells rather than organoid development from multipotent cells. Broguiere and coworkers found that a Matrigel culture system had a pore size smaller than 200 nm, or the resolution limit of their confocal microscope, but that a fibrin-based material had a pore size closer to 4 microns; these materials had comparable elastic moduli and colony-forming efficiency88. To our knowledge, studies showing an explicit connection between pore size and organoid differentiation have not yet been reported.
Synthetic hydrogels also have readily tunable viscoelastic properties, such as loss modulus and characteristic relaxation time. Many relevant tissues are viscoelastic, with brain tissue in particular not only having a strong dissipative component (i.e., high loss modulus) in its response to stress137 but also having slight differences in viscoelastic properties between white matter and gray matter138. The viscoelastic properties of a material affect matrix remodeling, cell spreading, migration, differentiation, and consequently, organoid fate139–141. The effects of viscoelastic properties on cell culture and behavior are complex but have been thoroughly reviewed elsewhere140,142. The effects of materials properties other than stiffness have only recently begun to be explored, and tunable hydrogels will enable more sophisticated experiments to be conducted.
The role of chemical cues in organoid differentiation
Synthetic polymer-based culture allows organoid formation conditions to be evaluated using high-throughput methods143–145. Synthetic hydrogels can be functionalized with biologically active moieties that permit the growth and spread of cells146–151; concentration and spacing of these cues can be changed independently152–155. A striking demonstration of the utility of high-throughput approaches was provided by Ranga and colleagues, who prepared PEG-based gels in 1536-well plates and studied murine ESCs (mESCs) expressing an Oct4-GFP reporter. The authors analyzed 1000 variations of matrix elastic modulus, cell-binding peptides, and matrix susceptibility to MMP degradation and their effects on murine ESC fate156. The optimal conditions (elastic moduli ranged from 2–4 kPa and scaffolds with MMP insensitivity) produce murine neural tube organoids that are more homogenous in colony size and morphology, as well as more polarized, than those grown in Matrigel. The percentage of cells containing an actomyosin contractile ring is used as a metric for the polarity of the cells157.
Cell-binding cues from collagen, fibronectin, or laminin have frequently been added to synthetic hydrogels to allow for organoid growth and differentiation. Ng and colleagues created functional human liver organoids derived from human iPSCs in a colloidal crystal of PEG functionalized with collagen I, fibronectin, or laminin-521158,159. Attachment of human iPSCs was successful in assemblies functionalized with collagen I and laminin-521 but not with fibronectin. This result builds on previous work by the authors, in which basic human liver function was recapitulated by a PEG-based scaffold160. To promote iPSC differentiation into human neuronal progenitor cells, Ovadia and colleagues compared photo-crosslinked PEG-based gels that contained chemical cues such as the laminin-derived cell-binding sequences YIGSR and IKVAV, the fibronectin-derived sequences PHSRNG10RGDS and RGDS, and the vitronectin-derived sequence KKQRFRHRNRKG161. The authors found that PEG gels functionalized with YIGSR and PHSRNG10RGDS were permissive for human iPSC survival and differentiation into neural progenitor cells in 3D culture.
The role of stiffness in organoid differentiation
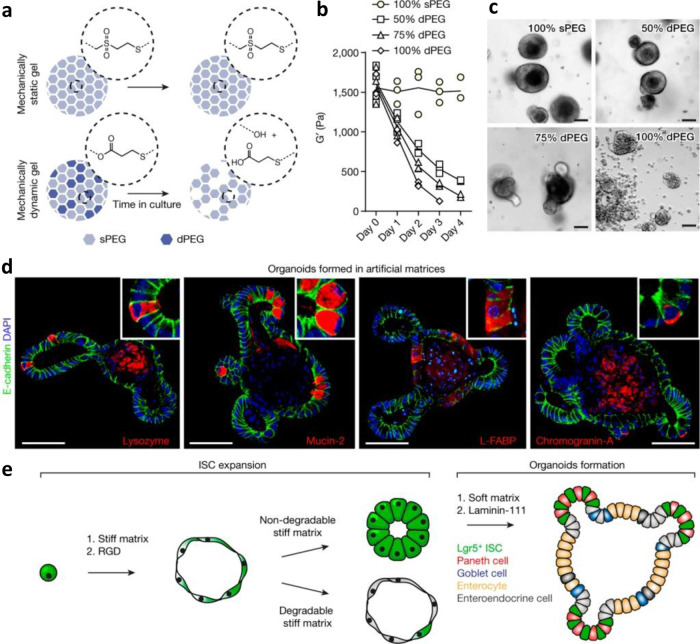
The stiffness of synthetic hydrogels can be controlled and has an effect on organoid formation. Gjorevski and colleagues reported a synthetic matrix for the intestinal organoid culture of murine and human Lgr5+ progenitor cells derived from the intestinal crypt. The material consisted of a PEG gel functionalized with either an RGD fibronectin-derived peptide or a laminin-111-derived peptide162. A stiffer matrix containing an RGD fibronectin-derived peptide promoted survival and proliferation of undifferentiated progenitor cells. In contrast, the softer matrix containing a laminin-111-derived peptide promoted the differentiation of progenitor cells into functional murine and human organoids. The authors found that organoid formation in minimal nutrient conditions was permissible only within a narrow range of matrix stiffness; the optimal elastic modulus was 190 Pa. Making the PEG gels more susceptible to MMP degradation resulted in depolarized organoids with irregular shapes. Major findings of this study are illustrated in Fig. 4, which also demonstrates the effect of matrix stiffness and degradability on the formation of one class of organoids.
Fig. 4. Growth of intestinal organoids on synthetic hydrogels, and effects of matrix stiffness and degradability on their formation.
Gjorevski and colleagues demonstrated defined PEG-based intestinal organoid culture. The stiffness and degradability has a major effect on the ability of induced pluripotent cells to differentiate into intestinal organoids. By varying the ratio of hydrolytically labile functional groups (dPEG) to stable functional groups (sPEG), the rate of degradation of the gel can be controlled (a, b). Higher ratios of sPEG are associated with the expansion of intestinal stem cells, whereas the degradable gels lead to the formation of organoids containing differentiated cells (c). In fact, organoid formation is observed only in gels that have a stiffness of ~190 Pa: cells expressing lysozyme (Paneth cells), mucin-2 (Goblet cells), and Chromogranin-A (enteroendocrine cells) are in different compartments, indicating that specialized cells are spatially separated (d). In short, a stiff matrix leads to intestinal stem cell proliferation and expansion, but a soft matrix and functionalization with laminin-111 promotes differentiation (e). The image is from ref. 162 and is reproduced with permission.
Cruz-Acuna and colleagues also found that PEG-based materials may need to be soft and degradable in order to support differentiated intestinal organoids. The authors used a 4-arm PEG maleimide to encapsulate and culture Matrigel-derived HIOs. Organoid viability was reduced at high PEG density and matrix stiffness; RGD- or AG73 (CGGRKRLQVQLSIRT)-functionalized PEG matrices promoted greater organoid viability than laminin-derived IKVAV- or type I collagen-derived GFOGER peptides163,164. After successful engraftment, HIOs generated from 4-arm PEG maleimide promoted the healing of mucosal wounds in a mouse colon injury model. Intriguingly, when PEG was crosslinked with dithiothreitol to inhibit matrix degradation, organoid viability at seven days was poor as measured by live-dead staining. This demonstrates a requirement of a degradable matrix for prolonged survival.
Synthetic hydrogels in action: Modeling difficult tissues such as the brain and pancreas
Neural organoids present special challenges including a lack of reproducibility, batch-to-batch variation in transcriptional profiles, and susceptibility to small microenvironmental changes that may have a considerable effect on organoid fate165.
Essential elements of the complexity of the brain must be recapitulated to enable clinical applications of neural organoids. For example, toxicity in brain tissue has many potential causes involving multiple cell types166–168. Therefore, the most useful organoids for toxicity models should include multiple populations. Schwartz and colleagues used a PEG-based gel functionalized with pendant RGD cell-binding domains and crosslinked by MMP-degradable peptides to generate neural organoids169. In this study, cells were introduced in three sequential stages: neural cells were introduced at day 0, vascular and mesenchymal stem cells at day 9, and microglia and macrophage precursors at day 13. The organoids were then exposed to a library of known toxic and nontoxic compounds, and the resulting RNA-seq data of the organoid response were used to build a machine-learning algorithm to assess the neurotoxicity of known and unknown compounds. In a blinded test, nine out of ten tested chemicals were correctly identified as toxic or nontoxic; in contrast, the true positive rate of chemical identification in animal models is between 41 and 71%170.
Brain organoids cultured in hyaluronic acid hydrogels were used to model Down syndrome by Wu and colleagues114. The authors found that the differentiation of both normal and Down syndrome iPSCs into neurons was dependent on matrix stiffness; cells could be grown at a softer elastic modulus of ca. 500 Pa but not ca. 1500 Pa, as indicated by higher expression of β-2 tubulin and microtubule-associated protein 2. However, Down syndrome patient-derived iPSCs that were differentiated in the softer gel showed no discernable neurite outgrowth, suggesting a block in the maturation of the differentiated neurons.
Hyaluronic acid hydrogels can also be functionalized with various peptides to examine brain organoid differentiation. Lam and colleagues found that the concentration of laminin-derived IKVAV, with 300 μM being the optimal concentration, was critical to neural organoid survival; however, this concentration did not enhance neuronal differentiation115. Bejoy and colleagues showed that functionalizing hyaluronic acid with heparin affects neuronal patterning; the addition of heparin favored differentiation of human progenitors into neurons with a hindbrain fate, whereas non-functionalized hyaluronic acid favored a forebrain fate. The authors also established that the stiffness of this hybrid material is relevant to cell fate determination; lower elastic moduli, ca. 300 Pa, led to forebrain development, whereas higher elastic moduli, ca. 1000 Pa, led to hindbrain development116.
Neural organoids have also been cultured in Matrigel-free conditions using microfluidic approaches such as microwell printing. A number of groups were able to generate spheroids in chip-based devices171–173, but the spheroids lack spatial complexity and cell types compared to fully-developed organoids. Without having to functionalize the well substrates, Chen and coworkers used a 3D printed mold to cast polydimethylsiloxane (PDMS) microwells to generate human embryoid bodies that have the potential to differentiate into brain organoids in a suspension culture174. They found that a critical factor affecting differentiation was the ridges of the culture vessel, unlike previous studies using smooth wells. This study represents a new direction towards the generation of organoids, where the shape of the culture vessel might be tuned in order to change cellular phenotype, growth, and differentiation.
Pancreatic organoids have proven difficult to prepare without resorting to Matrigel-based culture. To our knowledge, Candiello and colleagues were the first group to use a synthetic hydrogel, amikacin hydrate crosslinked with poly(ethylene glycol) diglycidyl ether known as Amikagel, to culture hESC-derived islet organoids175. Amikagels with elastic moduli ranging from 37 to 320 kPa were created, but no chemical signaling peptides were incorporated into the gels. The authors found that stiffer gels drove pancreatic progenitor cells to aggregate, leading to increased differentiation and maturation into beta-like cells; this may have been mediated through paracrine signaling enhanced by cellular proximity. Compared to cells grown in Matrigel, beta-like cells grown in Amikagel produced higher levels of functional beta-cell markers PDX1 and NKX6.1 and were more responsive to a glucose challenge. Their finding challenges the conventional wisdom that cell-binding domains are required for effective organoid formation. The stiffness of the gel is also very high, exceeding the elastic modulus of materials typically used in the culture of bone51. Mechanistically, the authors propose that the culture system is forcing the formation of a compressed organ, rather than serving as a mimetic. A potential disadvantage of this approach is limited control of cellular aggregate sizes and attendant consequences of cell viability of no more than five days. The smallest cell aggregates reported by the authors were ca. 200 microns in diameter; the typical human islet has a diameter of ca. 130 microns176. Larger islets have been known to form necrotic centers because of a lack of oxygen and nutrient diffusion; other studies have found that islets best maintain cellular identity and function when they have a size of ca. 100–150 microns177. Further work should be done in this system to establish long-term viability.
Advantages and disadvantages of synthetic polymeric matrices
A major advantage of using synthetic polymers for organoid culture is that they are amenable to systematic variation in structure and properties and can be used to explore the effects of mechanical and chemical cues on cellular fate178,179. Moreover, many such materials, including PEG and PLGA, have been approved by the FDA for use in human therapeutics. Nguyen and colleagues recently assessed more than 1200 synthetic polymer formulations for toxicity and abilities to promote implant vascularization and endothelial cell network formation180. This work provides a valuable resource for organoid researchers, particularly those concerned with vascularization of organoids postimplantation.
There are several disadvantages of synthetic hydrogels. First, many synthetic hydrogels require the incorporation of biochemical cues such as cell-binding peptides. In the absence of biochemical cues cells may not attach to the hydrogel, leading to anoikis (a type of programmed cell death)181 instead of organoid formation162. Improper spacing of biochemical cues can also lead to cell death182. While the backbone materials of synthetic hydrogels are cheap and can be produced on an industrial scale, functionalization of these materials with precisely-placed, custom-made peptides significantly increases cost and requires expertise in materials science, making these materials less attractive to cell biology labs. There have been a number of studies showing that organoids can be grown on unmodified surfaces such as alginate, but this requires more research103,105,110,175,183. Further, synthetic hydrogels may degrade into cytotoxic by-products184 or require cytotoxic initiators185, limiting the types of polymers that can be used in cell culture186. Synthetic hydrogels may contain pendant or other unreacted groups, which may be toxic to cells (such as neurotoxic maleimides)187. Finally, synthetic hydrogels used as medical implants can trigger foreign body reactions188; similar effects may be seen in immunogen-containing organoids. For these reasons, it may be advantageous to engineer recombinant protein gels.
Organoid culture in peptide and recombinant protein matrices
Recombinant proteins made by genetically engineered organisms have found wide applications in medicine, food processing, and catalysis. Engineered recombinant protein gels possess major advantages compared to other culture methods: chemical cues can be added with exact definition; chemical and mechanical properties of the gel can be altered independently; polydispersity is low; and degradation rates can be programmed by including appropriate recognition sites for MMP degradative enzymes.
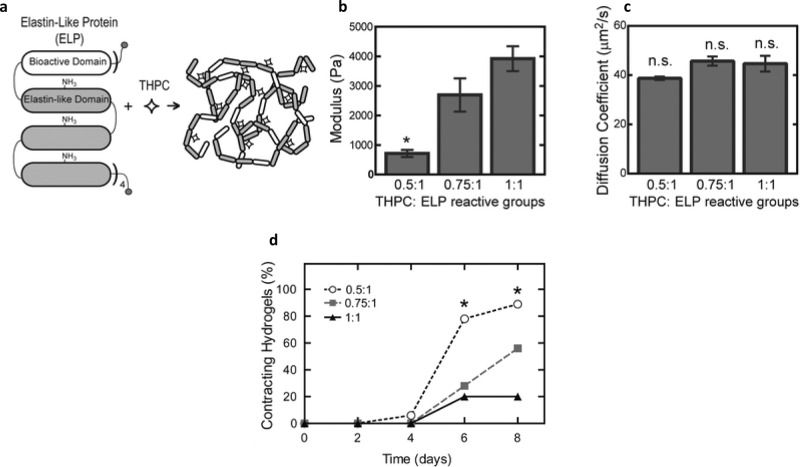
Chung and colleagues generated a hydrogel using an elastin-like polypeptide matrix containing a fibronectin-derived RGD cell-binding domain with tetrakis(hydroxymethyl)phosphonium chloride (THPC) as an amine-reactive crosslinker; this material transiently inhibited contractility of murine ESC-derived cardiomyocytes and enhanced survival of dorsal root ganglia cells from chick embryos189. In a follow-up study, murine cardiomyocyte differentiation (measured by α-myosin expression, cell contractility, and metabolic activity) was found to be dependent on the stoichiometric ratio of THPC to protein, which tuned the stiffness of the hydrogel. Among the elastic moduli studied (700, 3000, and 4000 Pa), the softest material favored the proliferation of embryoid bodies that contain mesodermal progenitor cells and promoted rapid cardiomyocyte differentiation (Fig. 5). Embryoid bodies cultured in the 700 Pa matrix displayed the highest level of MMP secretion. Inhibition of MMP secretion was deleterious to proliferation and differentiation, suggesting that remodeling of the matrix is essential in cardiomyocyte differentiation190.
Fig. 5. Growth of cardiomyocytes on recombinant proteins, and effects of elastic modulus on cardiomyocyte differentiation.
The elastin-like proteins (ELPs) used by Chung and colleagues (a) consist of a bioactive domain translationally fused to one or more elastin-like domains; these domains contain lysine groups to facilitate crosslinking by tetrakis hydroxymethyl phosphonium chloride (THPC). By varying the ratio of THPC to ELP reactive groups, it is possible to tune the elastic modulus of the resulting culture matrix (b) without significantly altering the diffusion of nutrients or other vital factors through the gel (c). Embryoid bodies embedded in the matrix undergo differentiation into cardiomyocytes most favorably in the gels with the lowest elastic modulus (d); the cells show the greatest contractility when grown in protein crosslinked with a 0.5:1 ratio of THPC:ELP reactive groups. The image is from ref. 190 and is reproduced with permission.
In another follow-up study, a very soft matrix with an elastic modulus of 180 Pa promoted intestinal organoid-forming efficiencies comparable to those observed in collagen I-based matrices. Organoid-forming efficiency was higher when the engineered ECM proteins contained 3.2 mM RGD peptide, compared to no RGD. Interestingly, MMP activity was significantly higher in the stiffer matrices. Inhibition of MMP activity reduced organoid-forming efficiency in the stiffer engineered hydrogel matrices, suggesting that secretion of degradative enzymes in adult intestinal organoids may be a response to overly stiff conditions191.
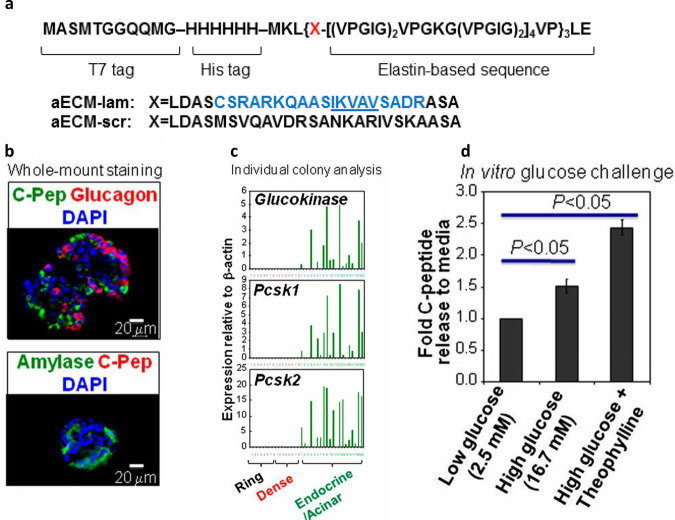
Recombinant ECM protein has been investigated for pancreatic organoid culture. The Tirrell and Ku groups have jointly developed and studied an artificial elastin-like polypeptide that incorporates an 18-amino acid sequence derived from α1 laminin; this polypeptide has been named artificial (a) ECM-lam (Fig. 6). aECM-lam was used to supplement a methylcellulose-based 3D pancreatic organoid culture that was devoid of Matrigel. Adult murine Sox9/EGFP+ ductal progenitor cells were first proliferated in Matrigel, then transferred to a culture containing aECM-lam but not Matrigel. After 2 weeks, endocrine-acinar organoids were observed, demonstrating that aECM-lam was capable of inducing differentiation of ductal progenitor cells into endocrine and acinar lineages192. A follow-up study established that when Matrigel was added, endocrine and acinar cell development was inhibited while ductal cell formation was promoted193, demonstrating the importance of the ECM microenvironment in pancreatic organoid differentiation. Using aECM-lam, other morphologically-distinct organoids were formed from murine postnatal pancreas194 and sorted adult ductal progenitor cells195. Finally, the exact population of adult progenitor cells capable of giving rise to endocrine/acinar cells in aECM-lam was determined to be ductal cells, which have high levels of CD133 but low levels of CD71 expression195. Collectively, these studies demonstrate the utility of aECM-lam in promoting endocrine and acinar cell differentiation in pancreatic organoid culture and identifying the responsible progenitor population.
Fig. 6. Generating pancreatic organoids with a recombinant ECM protein.
An artificial elastin-like polypeptide functionalized with a sequence from laminin can be used to generate organoids from pancreatic ductal progenitor cells from adult mice. (a) The recombinant protein (named aECM-lam) incorporates an IKVAV-containing 18-amino acid sequence derived from α1 laminin. The aECM-scr is a scrambled sequence control for aECM-lam. (b) aECM-lam permits the differentiation of endocrine (expressing C-peptide and glucagon) and acinar cell lineages (expressing amylase). (c) Individual organoids (Endocrine/Acinar) grown in aECM-lam express beta-cell maturation markers glucokinase, Pcsk1, and Pcsk2. (d) Organoids grown in aECM-lam are capable of secreting insulin in vitro when challenged by high concentrations of d-glucose or a combination of d-glucose and cAMP activator theophylline. The image is from ref. 192 and is reproduced with permission.
Peptide-based hydrogels have recently been employed to model Alzheimer’s disease. Zhang and colleagues used the self-assembling peptide RADA-16 to culture human neuronal cells treated with exogenous amyloid-β oligomers, known contributors to Alzheimer’s disease. A 3D culture in RADA-16 resulted in activation of a p21-activated kinase in response to amyloid-β oligomers. Both the activation and localization patterns of the p21-activated kinase are characteristic of neurons in an Alzheimer’s disease state. In contrast, the corresponding 2D culture did not show this activation and localization, suggesting that the 3D organoid culture of neurons is critical for modeling Alzheimer’s disease196.
The HYDROSAP self-assembling peptide hydrogel is a system recently developed by Pugliese, Marchini, and colleagues. In this system, multi-functionalized and branched self-assembling peptides (SAPs) can generate hydrogels with controllable elastic moduli197. The authors used HYDROSAP peptide 3D hydrogels with elastic moduli of ~800 Pa (similar to the stiffness of human brain tissue) to culture human fetal neural stem cells198, which were able to differentiate into various lineages including astrocytes, oligodendrocytes, and neurons.
Related work has been performed by Edelbrock and colleagues, with peptide amphiphiles capable of forming long, self-assembled nanostructures within a hydrogel. The peptides contain brain-derived neurotrophic factor (BDNF), which enables the formation of mature neurons via activation of the TrkB pathway. Display of BDNF on the peptide amphiphile is necessary for this effect to be observed199. Similar work has shown promise in stem cell differentiation and neural regeneration following spinal injury in vivo200–202, further demonstrating the utility of peptide-based materials in cell culture.
Advantages and disadvantages of recombinant protein matrices
Self-assembling peptides and recombinant proteins offer important advantages in organoid culture. Recombinant proteins are molecularly well-defined and can be tuned independently for stiffness, viscoelastic behavior, and chemical functionality203–205. They can be programmed to degrade and remodel at controlled rates by including protease recognition sites206 or changing crosslinking chemistry207. Protein-based hydrogels can be outfitted with a broad range of chemical functionalities by introducing noncanonical amino acids208,209; they can also be readily tailored to a wide variety of biomedical contexts210,211 and made thermally responsive212,213. The programmability of recombinant proteins has prompted increasing interest in the design of protein-based hydrogels as matrices for organoid culture.
Protein-based materials have several disadvantages. First, not all proteins can be recombinantly expressed and ensuring re-folding and functionality of these proteins can be challenging. Certain recombinant proteins and self-assembling peptides are immunogenic214–218. Ensuring that the recombinant protein is of human origin does not guarantee non-immunogenicity219. Care must be taken to avoid introducing other immunogenic factors, such as bacterial endotoxin. Therefore, proteins for clinical use would preferably be expressed in mammalian expression systems (e.g., Chinese Hamster Ovary) or in yeasts (e.g., Pichia pastoris).
Outlook and conclusions
Although several Matrigel-free techniques have been developed, they have been used in a narrow range of target tissues; expanding the number of tissue types will increase the acceptance of these alternative techniques. The ideal material for organoid culture should allow independent changes in the chemical and mechanical properties so that the effects on organoid growth, development, or morphology can be correlated. It should also functionalize biologically-relevant cell-binding proteins or peptides with ease. Finally, the ideal material should mimic the dynamic nature of the ECM in terms of erosion rate, viscoelasticity, and susceptibility to degradation. Because of these requirements, synthetic materials and programmable recombinant proteins represent fruitful areas of future research.
This review has proceeded with the assumption that a matrix is required to culture organoids, but matrix-free culture systems have also been developed. For instance, Pagliuca and colleagues kept human embryonic stem cells suspended in liquid culture at 70 rpm, added specific growth factors to encourage differentiation, and found that the resulting beta-like cells behaved similarly to mature beta cells220. A similar process was used by Nair and colleagues using mechanical agitation to keep human cells suspended in culture, resulting in functional beta-like clusters221. Control over the mechanical environment in such a system could be exerted by changing the speed of agitation. This method has the advantage of allowing easy harvesting of cells, which are simply allowed to settle to the bottom of a tube.
Using the methods discussed above, we anticipate a gradual shift away from the use of Matrigel in organoid culture and towards methods that enable exact control of the cell’s mechanical and chemical environments with a more precise definition.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank Elena C. Chen for assisting in graphic illustration. We also thank Prof. David A. Tirrell for helpful discussions and editing assistance. M.T.K. was supported by the Department of Defense through the National Defense Science & Engineering Graduate (NDSEG) Fellowship Program, and H.T.K. was supported by National Institutes of Health Grant R01DK099734. Support from The Wanek Family Project for Type 1 Diabetes to H.T.K. is also gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
Conception: M.T.K. and H.T.K.; Writing: M.T.K., H.T.K., and C.J.C.
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Anam Akhtar. Peer reviewer reports are available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02910-8.
References
- 1.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345:10. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 2.Grapin-Botton A. Three-dimensional pancreas organogenesis models. Diabetes Obes. Metab. 2016;18:33–40. doi: 10.1111/dom.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boj SF, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian XY, Nguyen HN, Jacob F, Song HJ, Ming GL. Using brain organoids to understand Zika virus-induced microcephaly. Development. 2017;144:952–957. doi: 10.1242/dev.140707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi H, Song J, Park G, Kim J. Modeling of autism using organoid technology. Mol. Neurobiol. 2017;54:7789–7795. doi: 10.1007/s12035-016-0274-8. [DOI] [PubMed] [Google Scholar]
- 6.van de Wetering M, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwank G, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Takebe T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–48. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 9.Takebe T, et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 10.Dekkers JF, et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 2016;8:12. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee S, Basak P, Buchel E, Murphy LC, Raouf A. A robust cell culture system for large scale feeder cell-free expansion of human breast epithelial progenitors. Stem Cell Res. Ther. 2018;9:264–264. doi: 10.1186/s13287-018-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drost J, Clevers H. Translational applications of adult stem cell-derived organoids. Development. 2017;144:968–975. doi: 10.1242/dev.140566. [DOI] [PubMed] [Google Scholar]
- 13.Nugraha B, Buono MF, von Boehmer L, Hoerstrup SP, Emmert MY. Human cardiac organoids for disease modeling. Clin. Pharmacol. Therap. 2019;105:79–85. doi: 10.1002/cpt.1286. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto, M., Nam, L., Kannan, S. & Kwon, C. Heart organoids and tissue models for modeling development and disease. Sem. Cell Develop. Biol.10.1016/j.semcdb.2021.03.011 (2021). [DOI] [PMC free article] [PubMed]
- 15.Sidhaye J, Knoblich JA. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell death Differ. 2021;28:52–67. doi: 10.1038/s41418-020-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang, H. Modeling neurological diseases with human brain organoids. Front. Synaptic Neurosci.10.3389/fnsyn.2018.00015 (2018). [DOI] [PMC free article] [PubMed]
- 17.Qian, X., Song, H. & Ming, G. L. Brain organoids: advances, applications and challenges. Development10.1242/dev.166074 (2019). [DOI] [PMC free article] [PubMed]
- 18.Schneeberger K, et al. Converging biofabrication and organoid technologies: the next frontier in hepatic and intestinal tissue engineering? Biofabrication. 2017;9:013001–013001. doi: 10.1088/1758-5090/aa6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogoke O, Maloy M, Parashurama N. The science and engineering of stem cell-derived organoids-examples from hepatic, biliary, and pancreatic tissues. Biol. Rev. Camb. Philos. Soc. 2021;96:179–204. doi: 10.1111/brv.12650. [DOI] [PubMed] [Google Scholar]
- 20.Yousef Yengej, F. A., Jansen, J., Rookmaaker, M. B., Verhaar, M. C. & Clevers, H. Kidney Organoids and Tubuloids. Cells10.3390/cells9061326 (2020). [DOI] [PMC free article] [PubMed]
- 21.Little MH, Combes AN. Kidney organoids: accurate models or fortunate accidents. Genes Dev. 2019;33:1319–1345. doi: 10.1101/gad.329573.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu T, Yamagata K, Osafune K. Kidney organoids: research in developmental biology and emerging applications. Dev. Growth Differ. 2021;63:166–177. doi: 10.1111/dgd.12714. [DOI] [PubMed] [Google Scholar]
- 23.Balak JRA, Juksar J, Carlotti F, Lo Nigro A, de Koning EJP. Organoids from the human fetal and adult pancreas. Curr. Diabetes Rep. 2019;19:160. doi: 10.1007/s11892-019-1261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreira L, et al. Pancreas 3D organoids: current and future aspects as a research platform for personalized medicine in pancreatic cancer. Cell. Mol. Gastroenterol. Hepatol. 2018;5:289–298. doi: 10.1016/j.jcmgh.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driehuis E, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc. Natl Acad. Sci. USA. 2019;116:26580. doi: 10.1073/pnas.1911273116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chumduri C, Turco MY. Organoids of the female reproductive tract. J. Mol. Med. 2021;99:531–553. doi: 10.1007/s00109-020-02028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–U147. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 29.Sato T, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Stange DE, et al. Differentiated Troy(+) chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Dekkers JF, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013;19:939–93. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 33.Huch M, et al. In vitro expansion of single Lgr5(+) liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie J, Koehler KR, Hashino E. Directed differentiation of mouse embryonic stem cells into inner ear sensory epithelia in 3D culture. Methods Mol. Biol. 2017;1597:67–83. doi: 10.1007/978-1-4939-6949-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huch M, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiriac H, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai S, et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018;18:13. doi: 10.1186/s12885-018-4238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seino T, et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell. 2018;22:454–45. doi: 10.1016/j.stem.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Wang WW, Jin S, Ye KM. Development of islet organoids from H9 human embryonic stem cells in biomimetic 3D scaffolds. Stem Cells Dev. 2017;26:394–404. doi: 10.1089/scd.2016.0115. [DOI] [PubMed] [Google Scholar]
- 40.Dorrell C, et al. The organoid-initiating cells in mouse pancreas and liver are phenotypically and functionally similar. Stem Cell Res. 2014;13:275–283. doi: 10.1016/j.scr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 42.Yiangou L, Ross ADB, Goh KJ, Vallier L. Human pluripotent stem cell-derived endoderm for modeling development and clinical applications. Cell Stem Cell. 2018;22:485–499. doi: 10.1016/j.stem.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 10.1038/s41580-020-0259-3 (2020). [DOI] [PMC free article] [PubMed]
- 44.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein AS, et al. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat. Protoc. 2011;6:656–667. doi: 10.1038/nprot.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vukicevic S, et al. Identification of multiple active growth-factors in basement-membrane matrigel suggests caution in interpretation of cellular-activity related to extracellular-matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 47.Spence JR. Taming the wild west of organoids, enteroids, and mini-guts. Cell. Mol. Gastroenterol. Hepatol. 2018;5:159–160. doi: 10.1016/j.jcmgh.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huch M, Knoblich JA, Lutolf MP, Martinez-Arias A. The hope and the hype of organoid research. Development. 2017;144:938–941. doi: 10.1242/dev.150201. [DOI] [PubMed] [Google Scholar]
- 49.Mahoney ZX, Stappenbeck TS, Miner JH. Laminin alpha 5 influences the architecture of the mouse small intestine mucosa. J. Cell Sci. 2008;121:2493–2502. doi: 10.1242/jcs.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gjorevski N, Ranga A, Lutolf MP. Bioengineering approaches to guide stem cell-based organogenesis. Development. 2014;141:1794–1804. doi: 10.1242/dev.101048. [DOI] [PubMed] [Google Scholar]
- 51.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 52.Dahl-Jensen S, Grapin-Botton A. The physics of organoids: a biophysical approach to understanding organogenesis. Development. 2017;144:946–951. doi: 10.1242/dev.143693. [DOI] [PubMed] [Google Scholar]
- 53.Nelson, C. M. & Gleghorn, J. P. in Annual Review of Biomedical Engineering Vol. 14 (ed. Yarmush, M. L.) (Annual Reviews, 2012).
- 54.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat. Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudhuri O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017;5:1480–1490. doi: 10.1039/c7bm00261k. [DOI] [PubMed] [Google Scholar]
- 57.Slater, K., Partridge, J. & Nandivada, H. Tuning the Elastic Moduli of Corning® Matrigel® and Collagen I 3D Matrices by Varying the Protein Concentration: Application Notehttps://www.corning.com/catalog/cls/documents/application-notes/CLS-AC-AN-449.pdf (2018).
- 58.Nemir S, West JL. Synthetic materials in the study of cell response to substrate rigidity. Ann. Biomed. Eng. 2010;38:2–20. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- 59.Miroshnikova YA, et al. Engineering strategies to recapitulate epithelial morphogenesis within synthetic three-dimensional extracellular matrix with tunable mechanical properties. Phys. Biol. 2011;8:13. doi: 10.1088/1478-3975/8/2/026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. The elastic modulus of matrigel (TM) as determined by atomic force microscopy. J. Struct. Biol. 2009;167:216–219. doi: 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reed J, Walczak WJ, Petzold ON, Gimzewski JK. In situ mechanical interferometry of matrigel films. Langmuir. 2009;25:36–39. doi: 10.1021/la8033098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussey GSD, J.L.; Badylak SF. Extracellular matrix- based materials for regenerative medicine. Nat. Rev. Mater. 2018;3:159–173. [Google Scholar]
- 63.Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34. doi: 10.1016/j.ymeth.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Allman AJ, et al. Xenogeneic extracellular matrix grafts elicit a Th2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 65.Parmaksiz M, Dogan A, Odabas S, Elcin AE, Elcin YM. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016;11:14. doi: 10.1088/1748-6041/11/2/022003. [DOI] [PubMed] [Google Scholar]
- 66.Yu, Y., Alkhawaji, A., Ding, Y. & Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget10.18632/oncotarget.10945 (2016). [DOI] [PMC free article] [PubMed]
- 67.Orlando G, et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials. 2013;34:5915–5925. doi: 10.1016/j.biomaterials.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Batchelder CA, Martinez ML, Tarantal AF. Natural scaffolds for renal differentiation of human embryonic stem cells for kidney tissue engineering. PLoS ONE. 2015;10:18. doi: 10.1371/journal.pone.0143849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong X, et al. Skeletal extracellular matrix supports cardiac differentiation of embryonic stem cells: a potential scaffold for engineered cardiac tissue. Cell. Physiol. Biochem. 2018;45:319–331. doi: 10.1159/000486813. [DOI] [PubMed] [Google Scholar]
- 70.Guyette JP, et al. Perfusion decellularization of whole organs. Nat. Protoc. 2014;9:1451–1468. doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- 71.Gilpin SE, et al. Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J. Heart Lung Transplant. 2014;33:298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 72.Vermeulen MDV, et al. Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int. J. Mol. Sci. 2019;20:5476. doi: 10.3390/ijms20215476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin P, Chan WCW, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 74.Baptista PM, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 75.Lee JS, et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules. 2014;15:206–218. doi: 10.1021/bm4015039. [DOI] [PubMed] [Google Scholar]
- 76.Saheli M, et al. Three-dimensional liver-derived extracellular matrix hydrogel promotes liver organoids function. J. Cell Biochem. 2018;119:4320–4333. doi: 10.1002/jcb.26622. [DOI] [PubMed] [Google Scholar]
- 77.Lewis PL, et al. Complex bile duct network formation within liver decellularized extracellular matrix hydrogels. Sci. Rep. 2018;8:12220. doi: 10.1038/s41598-018-30433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zachos NC, et al. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J. Biol. Chem. 2016;291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finkbeiner SR, et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol. Open. 2015;4:1462–1472. doi: 10.1242/bio.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giobbe GG, et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019;10:5658. doi: 10.1038/s41467-019-13605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sackett SD, et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018;8:16. doi: 10.1038/s41598-018-28857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaimov D, et al. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. J. Controlled Release. 2017;257:91–101. doi: 10.1016/j.jconrel.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 83.Bi H, Ye K, Jin S. Proteomic analysis of decellularized pancreatic matrix identifies collagen V as a critical regulator for islet organogenesis from human pluripotent stem cells. Biomaterials. 2020;233:119673. doi: 10.1016/j.biomaterials.2019.119673. [DOI] [PubMed] [Google Scholar]
- 84.Devarasetty M, Skardal A, Cowdrick K, Marini F, Soker S. Bioengineered submucosal organoids for in vitro modeling of colorectal cancer. Tissue Eng. Part A. 2017;23:1026–1041. doi: 10.1089/ten.tea.2017.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jee JH, et al. Development of collagen-based 3D matrix for gastrointestinal tract-derived organoid culture. Stem Cells Int. 2019;2019:8472712–8472712. doi: 10.1155/2019/8472712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takezawa T, Ozaki K, Nitani A, Takabayashi C, Shimo-Oka T. Collagen vitrigel: a novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004;13:463–473. doi: 10.3727/000000004783983882. [DOI] [PubMed] [Google Scholar]
- 87.Wang PC, Takezawa T. Reconstruction of renal glomerular tissue using collagen vitrigel scaffold. J. Biosci. Bioeng. 2005;99:529–540. doi: 10.1263/jbb.99.529. [DOI] [PubMed] [Google Scholar]
- 88.Broguiere N, et al. Growth of epithelial organoids in a defined hydrogel. Adv. Mater. 2018;30:1801621. doi: 10.1002/adma.201801621. [DOI] [PubMed] [Google Scholar]
- 89.Yui SR, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 90.Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15:1–U140. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isshiki H, et al. Establishment of a refined culture method for rat colon organoids. Biochem. Biophys. Res. Commun. 2017;489:305–311. doi: 10.1016/j.bbrc.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 92.Streuli CH. Integrins and cell-fate determination. J. Cell Sci. 2009;122:171. doi: 10.1242/jcs.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc. Res. Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fatehullah A, Appleton PL, Nathke IS. Cell and tissue polarity in the intestinal tract during tumourigenesis: cells still know the right way up, but tissue organization is lost. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 2013;368:20130014. doi: 10.1098/rstb.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee JL, Streuli CH. Integrins and epithelial cell polarity. J. Cell Sci. 2014;127:3217–3225. doi: 10.1242/jcs.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Myllymäki SM, Teräväinen TP, Manninen A. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PLoS ONE. 2011;6:e19453. doi: 10.1371/journal.pone.0019453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Co JY, et al. Controlling epithelial polarity: a human enteroid model for host-pathogen interactions. Cell Rep. 2019;26:2509–2520.e2504. doi: 10.1016/j.celrep.2019.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y, et al. Extracellular matrix functionalization and Huh-7.5 cell coculture promote the hepatic differentiation of human adipose-derived mesenchymal stem cells in a 3D ICC hydrogel scaffold. ACS Biomater. Sci. Eng. 2016;2:2255–2265. doi: 10.1021/acsbiomaterials.6b00487. [DOI] [PubMed] [Google Scholar]
- 99.Wilkinson DC, et al. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl. Med. 2017;6:622–633. doi: 10.5966/sctm.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilkinson DC, et al. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Curr. Protoc. Stem Cell Biol. 2018;46:e56. doi: 10.1002/cpsc.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu YJ, et al. A hollow fiber system for simple generation of human brain organoids. Integr. Biol. 2017;9:774–781. doi: 10.1039/c7ib00080d. [DOI] [PubMed] [Google Scholar]
- 102.Lu Y-C, et al. Scalable production and cryostorage of organoids using core-shell decoupled hydrogel capsules. Adv. Biosyst. 2017;1:1700165. doi: 10.1002/adbi.201700165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Capeling MM, et al. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 2019;12:381–394. doi: 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu HT, et al. A droplet microfluidic system to fabricate hybrid capsules enabling stem cell organoid engineering. Adv. Sci. 2020;7:9. doi: 10.1002/advs.201903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossen NS, et al. Injectable therapeutic organoids using sacrificial hydrogels. iScience. 2020;23:101052. doi: 10.1016/j.isci.2020.101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen X, Zhao X, Wang G. Review on marine carbohydrate-based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr. Polym. 2020;244:116311. doi: 10.1016/j.carbpol.2020.116311. [DOI] [PubMed] [Google Scholar]
- 107.Fernando IPS, Lee W, Han EJ, Ahn G. Alginate-based nanomaterials: fabrication techniques, properties, and applications. Chem. Eng. J. 2020;391:13. [Google Scholar]
- 108.Cattelan G, et al. Alginate formulations: current developments in the race for hydrogel-based cardiac regeneration. Front. Bioeng. Biotechnol. 2020;8:16. doi: 10.3389/fbioe.2020.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kong HJ, Wong E, Mooney DJ. Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules. 2003;36:4582–4588. [Google Scholar]
- 110.Fu S, et al. Rheological evaluation of inter-grade and inter-batch variability of sodium alginate. AAPS PharmSciTech. 2010;11:1662–1674. doi: 10.1208/s12249-010-9547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Führmann T, et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 112.Lindborg BA, et al. A chitosan-hyaluronan-based hydrogel-hydrocolloid supports in vitro culture and differentiation of human mesenchymal stem/stromal cells. Tissue Eng. Part A. 2015;21:1952–1962. doi: 10.1089/ten.TEA.2014.0335. [DOI] [PubMed] [Google Scholar]
- 113.Lindborg BA, et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl. Med. 2016;5:970–979. doi: 10.5966/sctm.2015-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu SH, Xu RJ, Duan B, Jiang P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mat. Chem. B. 2017;5:3870–3878. doi: 10.1039/C7TB00721C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam J, Carmichael ST, Lowry WE, Segura T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv. Healthc. Mater. 2015;4:534–539. doi: 10.1002/adhm.201400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bejoy J, et al. Differential effects of heparin and hyaluronic acid on neural patterning of human induced pluripotent stem cells. ACS Biomater. Sci. Eng. 2018;4:4354–4366. doi: 10.1021/acsbiomaterials.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matarasso SL. The use of injectable collagens for aesthetic rejuvenation. Semin. Cutan. Med. Surg. 2006;25:151–157. doi: 10.1016/j.sder.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 118.Wagner DE, et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials. 2014;35:3281–3297. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Booth AJ, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD. Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res. Ther. 2014;5:15. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shojaie S, et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep. 2015;4:419–430. doi: 10.1016/j.stemcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 123.Hynes RO, Naba A. Overview of the matrisome−an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012;4:a004903–a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Qi DJ, et al. Establishment of a human iPSC- and nanofiber-based microphysiological blood-brain barrier system. ACS Appl. Mater. Interfaces. 2018;10:21825–21835. doi: 10.1021/acsami.8b03962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012;3:9. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 128.Qayyum AS, et al. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication. 2017;9:16. doi: 10.1088/1758-5090/aa703c. [DOI] [PubMed] [Google Scholar]
- 129.Skardal, A. et al. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink. J. Vis. Exp. 10.3791/53606 (2016). [DOI] [PMC free article] [PubMed]
- 130.Tabata Y, Lutolf MP. Multiscale microenvironmental perturbation of pluripotent stem cell fate and self-organization. Sci. Rep. 2017;7:11. doi: 10.1038/srep44711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malandrino A, Mak M, Kamm RD, Moeendarbary E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extrem. Mech. Lett. 2018;21:25–34. doi: 10.1016/j.eml.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu JR, Liang L, Jiao Y, Liu LY, Allianc, U. S.-C. P. S.-O. Enhanced invasion of metastatic cancer cells via extracellular matrix interface. PLoS ONE. 2015;10:17. doi: 10.1371/journal.pone.0118058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ekerdt BL, et al. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv. Healthc. Mater. 2018;7:12. doi: 10.1002/adhm.201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qin XH, Wang XP, Rottmar M, Nelson BJ, Maniura-Weber K. Near-infrared light-sensitive polyvinyl alcohol hydrogel photoresist for spatiotemporal control of cell-instructive 3D microenvironments. Adv. Mater. 2018;30:7. doi: 10.1002/adma.201705564. [DOI] [PubMed] [Google Scholar]
- 135.Dye BR, et al. Human lung organoids develop into adult airway-like structures directed by physico-chemical biomaterial properties. Biomaterials. 2020;234:119757. doi: 10.1016/j.biomaterials.2020.119757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi J-W, et al. Fabrication of 3D biocompatible/biodegradable micro-scaffolds using dynamic mask projection microstereolithography. J. Mater. Process. Technol. 2009;209:5494–5503. [Google Scholar]
- 137.Li W, Shepherd DET, Espino DM. Frequency dependent viscoelastic properties of porcine brain tissue. J. Mech. Behav. Biomed. Mater. 2020;102:103460. doi: 10.1016/j.jmbbm.2019.103460. [DOI] [PubMed] [Google Scholar]
- 138.Budday S, Sommer G, Holzapfel GA, Steinmann P, Kuhl E. Viscoelastic parameter identification of human brain tissue. J. Mech. Behav. Biomed. Mater. 2017;74:463–476. doi: 10.1016/j.jmbbm.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 139.Chaudhuri O, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature. 2020;584:535–546. doi: 10.1038/s41586-020-2612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crispim JF, Ito K. De novo neo-hyaline-cartilage from bovine organoids in viscoelastic hydrogels. Acta Biomater. 2021;128:236–249. doi: 10.1016/j.actbio.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Hofer M, Lutolf MP. Engineering organoids. Nat. Rev. Mater. 2021;6:402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ranga A, Lutolf MP. High-throughput approaches for the analysis of extrinsic regulators of stem cell fate. Curr. Opin. Cell Biol. 2012;24:236–244. doi: 10.1016/j.ceb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 144.Gobaa S, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat. Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 145.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 146.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 148.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr. Opin. Chem. Biol. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tibbitt MW, Anseth KS. Dynamic microenvironments: the fourth dimension. Sci. Transl. Med. 2012;4:4. doi: 10.1126/scitranslmed.3004804. [DOI] [PubMed] [Google Scholar]
- 150.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 151.Lutolf MR, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 152.Wylie RG, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 153.DeForest CA, Anseth KS. Photoreversible patterning of biomolecules within click-based hydrogels. Angew. Chem. Int. Ed. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mosiewicz KA, et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013;12:1071–1077. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 155.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 156.Ranga A, et al. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 2014;5:10. doi: 10.1038/ncomms5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ranga A, et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl Acad. Sci. USA. 2016;113:E6831–E6839. doi: 10.1073/pnas.1603529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ng SS, et al. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311. doi: 10.1016/j.biomaterials.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shirahama, H. et al. Fabrication of inverted colloidal crystal poly(ethylene glycol) scaffold: a three-dimensional cell culture platform for liver tissue engineering. J. Vis. Exp. 10.3791/54331 (2016). [DOI] [PMC free article] [PubMed]
- 160.Ng SS, et al. Long-term culture of human liver tissue with advanced hepatic functions. JCI Insight. 2017;2:11. doi: 10.1172/jci.insight.90853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ovadia EM, Colby DW, Kloxin AM. Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells. Biomater. Sci. 2018;6:1358–1370. doi: 10.1039/c8bm00099a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–56. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 163.Cruz-Acuna R, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017;19:1326–132. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cruz-Acuna R, et al. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018;13:2102–2119. doi: 10.1038/s41596-018-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017;18:573. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 167.de Groot MWGDM, Westerink RHS, Dingemans MML. Don’t judge a neuron only by its cover: neuronal function in in vitro developmental neurotoxicity testing. Toxicol. Sci. 2012;132:1–7. doi: 10.1093/toxsci/kfs269. [DOI] [PubMed] [Google Scholar]
- 168.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126:5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 169.Schwartz MP, et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl Acad. Sci. USA. 2015;112:12516–12521. doi: 10.1073/pnas.1516645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Olson H, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 171.Patra B, et al. A microfluidic device for uniform-sized cell spheroids formation, culture, harvesting and flow cytometry analysis. Biomicrofluidics. 2013;7:054114. doi: 10.1063/1.4824480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee GH, et al. Networked concave microwell arrays for constructing 3D cell spheroids. Biofabrication. 2017;10:015001. doi: 10.1088/1758-5090/aa9876. [DOI] [PubMed] [Google Scholar]
- 173.Yoon S-J, et al. Reliability of human cortical organoid generation. Nat. Methods. 2019;16:75–78. doi: 10.1038/s41592-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen C, Rengarajan V, Kjar A, Huang Y. A matrigel-free method to generate matured human cerebral organoids using 3D-Printed microwell arrays. Bioact. Mater. 2021;6:1130–1139. doi: 10.1016/j.bioactmat.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Candiello J, et al. 3D heterogeneous islet organoid generation from human embryonic stem cells using a novel engineered hydrogel platform. Biomaterials. 2018;177:27–39. doi: 10.1016/j.biomaterials.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 176.Rorsman, P. & Braun, M. in Annual Review of Physiology Vol. 75 (ed. Julius, D) (Annual Reviews, 2013).
- 177.Hilderink J, et al. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J. Cell. Mol. Med. 2015;19:1836–1846. doi: 10.1111/jcmm.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li CY, et al. Micropatterned cell-cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Eng. Part A. 2014;20:2200–2212. doi: 10.1089/ten.tea.2013.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee HJ, et al. Elasticity-based development of functionally enhanced multicellular 3D liver encapsulated in hybrid hydrogel. Acta Biomater. 2017;64:67–79. doi: 10.1016/j.actbio.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 180.Nguyen EH, et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 2017;1:14. doi: 10.1038/s41551-017-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hagbard L, et al. Developing defined substrates for stem cell culture and differentiation. Philos. Trans. R. Soc. B-Biol. Sci. 2018;373:9. doi: 10.1098/rstb.2017.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hof KS, Bastings MMC. Programmable control in extracellular matrix-mimicking polymer hydrogels. Chimia. 2017;71:342–348. doi: 10.2533/chimia.2017.342. [DOI] [PubMed] [Google Scholar]
- 183.Hosseini ZF, et al. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018;131:jcs208728. doi: 10.1242/jcs.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam) Biomaterials. 2005;26:3055–3064. doi: 10.1016/j.biomaterials.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 185.Liu VA, Bhatia SN. Three-dimensional photopatterning of hydrogels containing living Ccells. Biomed. Microdevices. 2002;4:257–266. [Google Scholar]
- 186.Kharkar PM, Kiick KL, Kloxin AM. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013;42:7335–7372. doi: 10.1039/c3cs60040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010;1:17–36. [Google Scholar]
- 188.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chung C, Lampe KJ, Heilshorn SC. Tetrakis(hydroxymethyl) phosphonium chloride as a covalent cross-linking agent for cell encapsulation within protein-based hydrogels. Biomacromolecules. 2012;13:3912–3916. doi: 10.1021/bm3015279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chung C, Pruitt BL, Heilshorn SC. Spontaneous cardiomyocyte differentiation of mouse embryoid bodies regulated by hydrogel crosslink density. Biomater. Sci. 2013;1:1082–1090. doi: 10.1039/C3BM60139K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.DiMarco RL, Dewi RE, Bernal G, Kuoc C, Heilshorn SC. Protein-engineered scaffolds for in vitro 3D culture of primary adult intestinal organoids. Biomater. Sci. 2015;3:1376–1385. doi: 10.1039/c5bm00108k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jin L, et al. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc. Natl Acad. Sci. USA. 2013;110:3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ghazalli N, et al. Postnatal pancreas of mice contains tripotent progenitors capable of giving rise to duct, acinar, and endocrine cells in vitro. Stem Cells Dev. 2015;24:1995–2008. doi: 10.1089/scd.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jin L, et al. Colony-forming progenitor cells in the postnatal mouse liver and pancreas give rise to morphologically distinct insulin-expressing colonies in 3D cultures. Rev. Diabet. Stud. 2014;11:35–50. doi: 10.1900/RDS.2014.11.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Jin L, et al. Cells with surface expression of CD133highCD71low are enriched for tripotent colony-forming progenitor cells in the adult murine pancreas. Stem Cell Res. 2016;16:40–53. doi: 10.1016/j.scr.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang DW, et al. A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials. 2014;35:1420–1428. doi: 10.1016/j.biomaterials.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 197.Pugliese R, Fontana F, Marchini A, Gelain F. Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels. Acta Biomater. 2018;66:258–271. doi: 10.1016/j.actbio.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 198.Marchini A, et al. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl Acad. Sci. USA. 2019;116:7483–7492. doi: 10.1073/pnas.1818392116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edelbrock AN, et al. Supramolecular nanostructure activates TrkB receptor signaling of neuronal cells by mimicking brain-derived neurotrophic factor. Nano Lett. 2018;18:6237–6247. doi: 10.1021/acs.nanolett.8b02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stephanopoulos N, et al. Bioactive DNA-peptide nanotubes enhance the differentiation of neural stem cells into neurons. Nano Lett. 2015;15:603–609. doi: 10.1021/nl504079q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee SS, et al. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv. Health. Mater. 2015;4:131–141. doi: 10.1002/adhm.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Berns EJ, et al. Aligned neurite outgrowth and directed cell migration in self-assembled monodomain gels. Biomaterials. 2014;35:185–195. doi: 10.1016/j.biomaterials.2013.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Madl CM, Katz LM, Heilshorn SC. Bio-orthogonally crosslinked, engineered protein hydrogels with tunable mechanics and biochemistry for cell encapsulation. Adv. Funct. Mater. 2016;26:3612–3620. doi: 10.1002/adfm.201505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu X, et al. Versatile engineered protein hydrogels enabling decoupled mechanical and biochemical tuning for cell adhesion and neurite growth. ACS Appl. Nano Mater. 2018;1:1579–1585. [Google Scholar]
- 205.Dooling LJ, Tirrell DA. Engineering the dynamic properties of protein networks through sequence variation. ACS Cent. Sci. 2016;2:812–819. doi: 10.1021/acscentsci.6b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD. Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J. Am. Chem. Soc. 2010;132:3217–3223. doi: 10.1021/ja910481t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shen W, Zhang KC, Kornfield JA, Tirrell DA. Tuning the erosion rate of artificial protein hydrogels through control of network topology. Nat. Mater. 2006;5:153–158. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 208.Link AJ, Mock ML, Tirrell DA. Non-canonical amino acids in protein engineering. Curr. Opin. Biotechnol. 2003;14:603–609. doi: 10.1016/j.copbio.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 209.Connor RE, Tirrell DA. Non‐canonical amino acids in protein polymer design. Polym. Rev. 2007;47:9–28. [Google Scholar]
- 210.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 211.Fong E, Tirrell DA. Collective cell migration on artificial extracellular matrix proteins containing full-length fibronectin domains. Adv. Mater. 2010;22:5271–5275. doi: 10.1002/adma.201002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li NK, Quiroz FG, Hall CK, Chilkoti A, Yingling YG. Molecular description of the LCST behavior of an elastin-like polypeptide. Biomacromolecules. 2014;15:3522–3530. doi: 10.1021/bm500658w. [DOI] [PubMed] [Google Scholar]
- 213.MacEwan SR, Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 214.Vigneswaran Y, et al. This paper is the winner of an SFB award in the hospital intern, residency category: peptide biomaterials raising adaptive immune responses in wound healing contexts. J. Biomed. Mater. Res. Part A. 2016;104:1853–1862. doi: 10.1002/jbm.a.35767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Collier JH, Rudra JS, Gasiorowski JZ, Jung JP. Multi-component extracellular matrices based on peptide self-assembly. Chem. Soc. Rev. 2010;39:3413–3424. doi: 10.1039/b914337h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rudra JS, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6:1557–1564. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc. Natl Acad. Sci. USA. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self/nonself. 2010;1:314–322. doi: 10.4161/self.1.4.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pagliuca FeliciaW, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nair GG, et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 2019;21:263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.