Abstract
We performed a pilot study in anticipation of using long-aged precut formalin fixed paraffin embedded (FFPE) tissue sections stored in real-world conditions for translational biomarker studies of TOP2A, Ki67, and HER2 in endometrial cancer. FFPE tissue blocks or unstained slides or both from GOG-0177 were collected centrally (1999-2000) and stored at room temperature. During 2004-2011 specimens were stored at 4°C. Matched pairs of stored slides and freshly cut slides from stored blocks were analyzed for TOP2A (KiS1), Ki67 (MIB1) and HER2 (Herceptest™) proteins. To assess DNA stability (HER2 PathVision), FISH was repeated on stored slides from 21 cases previously shown to be HER2-amplified. IHC staining intensity and extent, mean FISH copies/cell, and copy number ratios were compared using the kappa statistic for concordance or signed rank test for differences in old cut versus new cut slides. IHC results reflected some protein degradation in stored slides. The proportion of cells with TOP2A staining was lower on average by 12% in older sections (p=.03). The proportion of Ki67 positive cells was lower in stored slides by an average of 10% (p<.01). Too few cases in the IHC cohort were FISH positive for any conclusions. HER2 amplification by FISH was unaffected by slide storage. We conclude that use of aged stored slides for proliferation markers TOP2A and Ki67 is feasible but may modestly underestimate true values in endometrial cancer. Pilot studies for particular storage conditions/durations/antigens to be used in translational studies are warranted.
Keywords: Slide storage, Immunohistochemistry, Protein degradation, Epitope preservation, FFPE specimens
Introduction
Multi-institutional clinical trials commonly include biomarker analysis of formalin fixed paraffin embedded (FFPE) samples performed en masse at the end of the trial. As blocks may not always be released by institutions for clinical trial use, or may be recalled, central repositories often store pre-cut FFPE sections mounted on glass slides at room temperature. There are more than 60 pre-analytical variables that are capable of impacting immunohistochemistry (IHC) during tissue fixation and processing 1,2. If significant loss of immunoreactivity is a consequence of storage of sections on glass slides 3,4, the use of aged cut sections may contribute to spurious conclusions. Prior to performing a larger correlative study assessing the predictive value of Topoisomerase 2A (TOP2A, which has been reported to have predictive benefit for use of anthracyclines in breast cancer)5, Ki67, and Human Epidermal Growth Factor Receptor 2 (HER2) on the efficacy of anthracycline-based therapy in the therapy of metastatic endometrial cancer for women enrolled on GOG 177 (which compared doxorubicin/cisplatin to paclitaxel/doxorubicin/cisplatin), we performed a pilot study to compare IHC staining on years-old stored unstained slides with IHC staining on new cut slides from stored blocks of endometrial tumor specimens. Nearly half of available samples from the GOG-0177 cohort were in the form of pre-cut unstained slides. We also evaluated the effect of slide storage on quantitation of HER2 gene copies by Fluorescent in situ hybridization (FISH).
Materials and methods
Tumor samples and storage conditions
This study was approved by the GOG/NRG Oncology Group (GOG protocol 8013) and the University of Chicago Institutional Review Board and conducted on FFPE tumor samples from research subjects enrolled on GOG protocol 0177 6 (Fig 1). Informed consent was obtained from all patients on GOG-0177 before sample submission. Multiple institutions participated in this study; information related to pre-analytical conditions of sample processing is unknown.
Fig. 1.
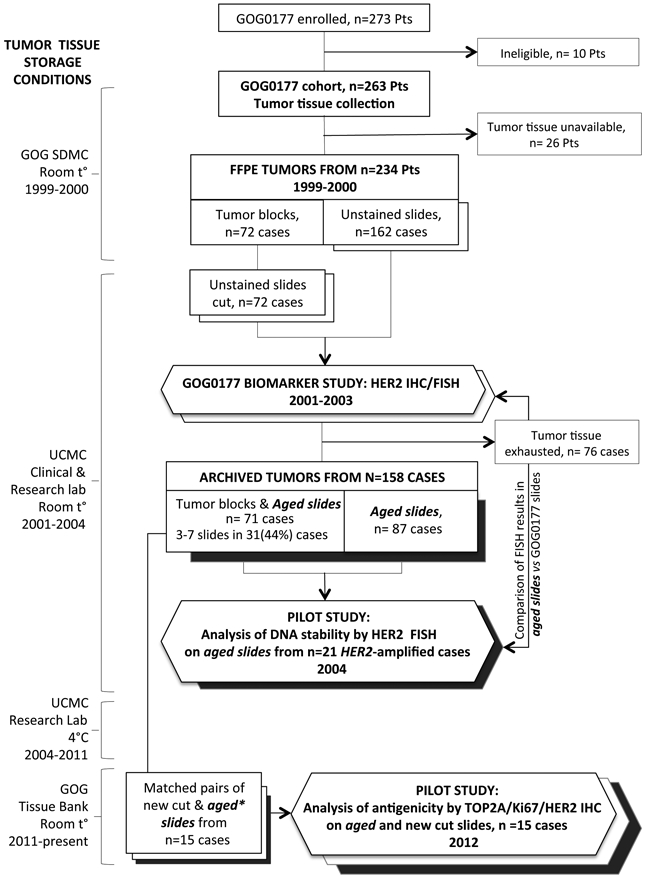
Flow chart depicting the tumor samples collection, storage and use in pilot study assays. Abbreviations: GOG SDMC, Gynecologic Oncology Group Statistics and Data Management Center; UCMC, University of Chicago Medical Center. Sent backward rectangles and hexagons: open indicate new cut slides; filled in indicate aged (old cut slides).
For IHC evaluation, 15 cases with specimens from primary endometrial tumors for which both tumor blocks and ≥ 3 precut slides were available were analyzed. Selection of number of cases was based on the desire to evaluate assays in stored precut tissue yet preserve as much tissue as possible for future correlative studies. Matched pairs of stored (>10 years) and new cut (2-3-weeks old) tumor tissues were prepared.
For detection of DNA stability, we used slides from a separate group of GOG-0177 cases that had previously (from slides cut in 2001) been determined to be HER2 amplified 7. Left-over tissue sections from this set of 21 HER2 amplified cases had been stored at room temperature and the analysis of HER2 amplification status by FISH was repeated on these old cut slides in 2004 (22 months old). Histology on these cases was as follows: clear cell (n=3), serous (n=6), mixed, grade 3 (n=4), endometrioid (G1:1, G2: 1, G3: 5), other (n=1).
Immunohistochemistry (IHC) and Fluorescence in situ hybridization (FISH) assays
IHC was performed on 5 μm-thick FFPE tissue sections mounted on positively charged slides. All tissues were stained simultaneously to ensure consistency of the procedure with the given antibody. The antibodies (Ab) used were anti-TOP2A (KiS1, DAKO Cytomation, Denmark, dilution 1:400), anti-Ki67 (MIB1, DAKO, Carpinteria, CA, dilution 1:100) and anti-HER2 (CerbB2, HercepTest Kit™, K5204, DAKO, Carpinteria, CA, ready to use). Stroma and inflammatory cells were negative internal controls for all biomarkers. Isotype staining with corresponding immunoglobulin instead of Ab was used as the negative control for antibody specificity. All IHC procedures were performed centrally at the University of Chicago Human Tissue Resource Center (HTRC) IHC Core Facility using standardized antigen retrieval protocols and appropriate controls. Tissue sections from normal testis and tonsil were used as positive IHC controls for TOP2A and Ki67, respectively, and were provided by the HTRC. HER2 IHC control slides were included in the HercepTest® Kit.
For this portion of the study 15 tumors were used, 3 endometrioid gr1, 2 endometrioid gr 2, 6 endometrioid gr 3, 1 clear cell, 2 mixed and 1 undifferentiated carcinoma. Histology was confirmed by two gynecologic pathologists.
Sections were initially deparaffinized in xylene and rehydrated via graded ethanol and then immunostained for the marker using the EN Vision + (DAKO) assay with an automated staining system (I 6000, BioGenex, USA) according to the manufacturer’s instructions and as described previously 8. To minimize loss of antigenicity, microwave heating (MWH) antigen retrieval was used 3,9. For TOP2A and Ki67 immunostaining, pretreatment included 20 min in a steamer and the use of antigen retrieval buffer (S1699, DAKO); for HER2 immunostaining, pretreatment included 40 min water bath or MWH and the use of epitope retrieval solution (HercepTest Kit™, DAKO). Thereafter, sections were incubated with the primary Ab for 30 minutes at room temperature and then incubated with polymer for 30 minutes. The antigen-antibody reaction was visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB) as a chromogen substrate and counterstained with hematoxylin and eosin (H&E). New-cut and old-cut H&E-stained slides were paired with corresponding new-cut and old-cut IHC -stained slides. Image acquisition was performed using a Leica DMLB microscope with DFC450 camera and LASX software (Leica Microsystems).
Each entire slide was scored manually in a blinded fashion without knowledge of slide pairing or slide age by two pathologists (TM and MA) jointly in a semi quantitative manner using conventional bright-field microscopy (x10, x20, and x40 objectives). Scoring disagreements were resolved at the time of reading. For TOP2A, the nuclear pattern of intensity and percentage of positive cells were recorded; positivity was interpreted using ImmunoReactive Scoring system (IRS) as described by Faggad and colleagues 10:
0 (negative), 1+ (weak), 2+ (moderate) and 3+ (strong). The percentage of immunostained cells was captured at each intensity level and scored as following: 0 (0% staining), 1 (staining in 1-10% of tumor cells), 2 (11–50%), 3 (51-80%) and 4 (> 80%). The intensity of staining multiplied by percentage of positive cells resulted in a combined score with a value between 0 and 12. Scores of 0-3 were designated as negative or low expression, whereas scores of 4-12 were designated as positive (high) expression.
Ki67 expression was scored by recording the percentage of cells with nuclear staining. High (above the cutoff point) versus low (below the cutoff point) expression was determined based on a 10% cut off 11-13. Other cut off points of 13% and 1% 12,14,15 were also explored.
HER2 expression was detected using the same antibody and scoring system as in GOG-0177 and according to manufacturer and FDA recommendations 7. HER2 IHC results were correlated with previously published HER2 FISH data from these cases 7.
FISH assay and analysis were performed as described previously 7,16, using the Vysis HER2/CEP17 DNA Probe Mixture according to manufacturer recommendations (Vysis/Abbott Molecular, Des Plaines, IL). Control FFPE breast cancer cell lines were provided by the manufacturer. The pretreatment step was adjusted for use in archival material 16. Analysis was performed using a Zeiss AxioImagerZ2 fluorescence microscope with Axiocam MRm camera and Axiovision software (Carl Zeiss MicroImaging). The mean copy number per cell and the ratio of HER2 to chromosome 17 centromere enumeration probe (CEP17) were compared. Cells with a gene to chromosome signal ratio ≥ 2 were considered amplified. FISH results in old slides (stained in 2004) were analyzed blindly without knowledge of previous HER2 FISH scores or scores in new cut slides from the same cases (stained in 2001-2002) 7.
Statistical evaluation
The kappa statistic for marker expression concordance (0.75-1.0 considered good to perfect; 0.40-0.75 considered fair to good; and below 0.40 considered poor agreement)17 and signed rank test for differences in percentage cells staining positive in old-cut versus new-cut slides were applied. The differences in percentages of cells stained positive between new and old sections (vertical axis) were mapped against average values of percentages of positive cells from pairs of new and old sections (horizontal axis) in Bland-Altman plots.
RESULTS
TOP2A assessment
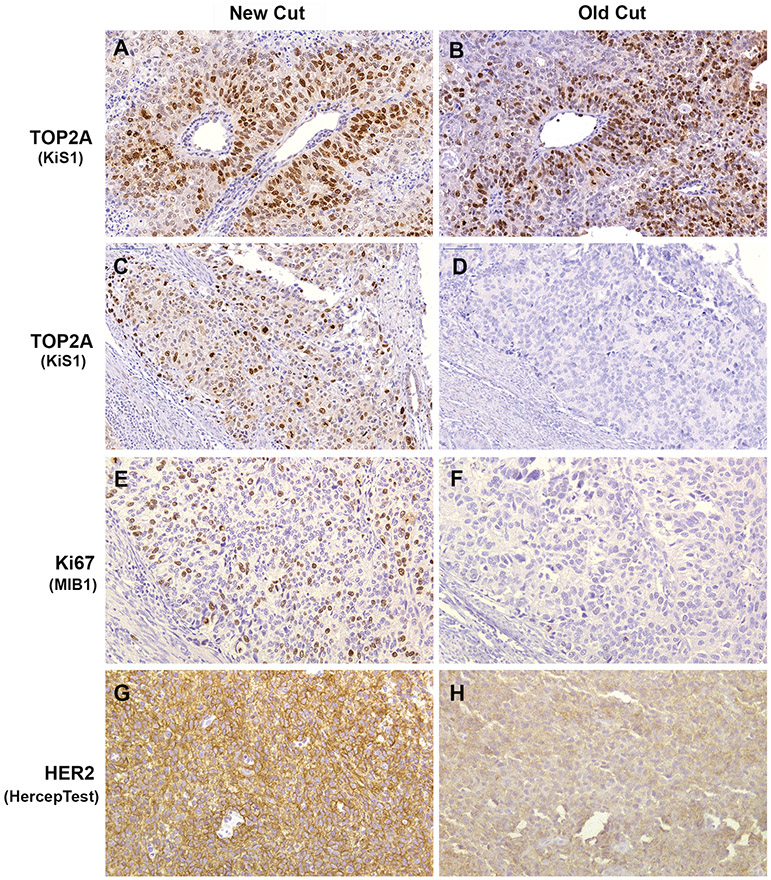
TOP2A nuclear staining ranged from weak (1+) to strong (3+) with the exception of one case showing no staining (0), and this was an old cut slide (Fig. 2D). The levels of intensity between old- and new-cut slides were concordant in 53% of cases (Fig. 2A-B; Table I).
Fig. 2.
Photomicrographs of comparative TOP2A, Ki67 and HER2 IHC staining of new cut (3 weeks old) and stored (old cut, >10 years old) slides sectioned from the same FFPE tumor blocks of endometrial cancers. (A & B) Example of endometrial cancer demonstrating no effect of slide storage on TOP2A IHC interpretation. Strong (3+) positive nuclear staining was observed in both sections. (C – F) Staining patterns of TOP2A (C & D) and Ki67 (E & F) illustrating complete loss of immunoreactivity in old cut slides of the same case. In new cut sections of this tumor, the percentages of positively stained cells were 50% for TOP2A and 30% for Ki67. (G & H) Tumor showing change in HER2 intensity staining from strongly positive (3+; G) to weak (1+; H). Stroma and inflammatory cells were negative internal controls for all biomarkers. Original magnification x200.
Table I.
TOP2A and HER2 staining intensity in new cut versus old cut slides
| Old cut Slides |
New Cut Slides | |||
|---|---|---|---|---|
| 0, 1+ | 2+ | 3+ | Total | |
| TOP2A | ||||
| 0, 1+ | 1 | 2 | 0 | 3 |
| 2+ | 0 | 4 | 4 | 8 |
| 3+ | 0 | 2 | 2 | 4 |
| Total | 1 | 8 | 6 | 15 |
| Concordance 87% (in 13 of 15 pairs) by groups (0,1+) versus (2+, 3+) | ||||
| HER2 | ||||
| 0, 1+ | 13 | 0 | 1 | 14 |
| 2+ | 0 | 1 | 0 | 1 |
| 3+ | 0 | 0 | 0 | 0 |
| Total | 13 | 1 | 1 | 15 |
| Concordance 93% (in 14 of 15 pairs) by groups (0,1+) versus (2+) versus (3+) | ||||
NOTE: Staining intensity: (0,1+), no staining/weak; (2+), moderate; (3+), strong
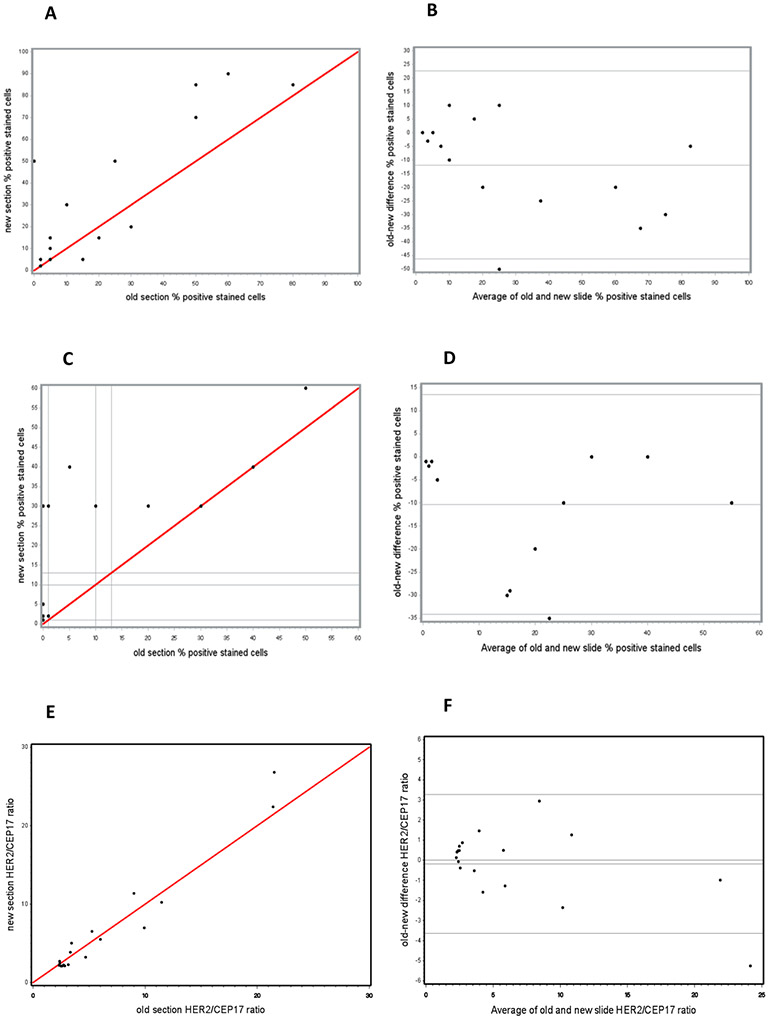
When intensity levels were grouped as no/weak versus moderate/strong, the concordance reached 87%. Figure 3A shows the percentages of positive cells in old sections plotted against those in new sections. The proportion of cells with TOP2A immunostaining was lower by 12% on average in the older sections and differences ranged between 50% lower and 10% higher (Bland-Altman Plot Figure 3B); this reduction was statistically significant (p=.03).
Fig. 3.
Graphical presentation of percentages of IHC positive cells for TOP2A (A & B) and Ki67 (C & D) and FISH HER2/CEP17 ratios (E & F) in new and old cut sections for each case of endometrial cancer. (A, C & E) Scatter Plots of identity: red lines mark agreement. (B, D & F) Bland-Altman Plots of difference. Solid grey lines mark the average difference and the limits of agreement specified as an average difference ± 1.96 x Standard Deviation. (A & C) Percentages of stained cells in new slides were mapped against percentages of stained cells in old slides from the same cases for TOP2A and Ki67, respectively. (B & D) Differences in percentages of cells stained positive between new and old sections were mapped against average values of percentages of positive cells from pairs of new and old sections for TOP2A (B) and Ki67 (D). In old slides, the difference from values in new slides ranged between 50% lower and 10% higher for TOP2A and between 0% and 35% lower for Ki67. Statistically significant loss of staining in stored slides by an estimated 12% for TOP2A and 10% for Ki67 was detected. (E) HER2/CEP17 ratios in new slides were mapped against HER2/CEP17 ratios in old slides from the same cases. (F) Differences in HER2/CEP17 ratios between new and old sections were mapped against average values of HER2/CEP17 ratios from pairs of new and old sections. In old slides, the variability from new slides ranged between 5.2 lower and 3.0 higher with an average estimated HER2/CEP17 ratio lover by 0.1 showing no difference from new sections.
Using the IRS scoring system [TOP2A was classified as either no to low expression (no/low, negative) or high (positive) expression (Table 2)], expression was concordant in 12 of 15 pairs. No or low expression of TOP2A in the older slide with high expression in the newer slide was observed in two of three discordant cases (2 of 15, 13%), (Fig. 2C-D). In the third case the opposite was observed: high expression in the older slide and low expression in the newer slide. The kappa statistic for TOP2A expression concordance was 0.57 (fair to good).
Table 2.
TOP2A and Ki67 IHC interpretation in new cut versus old cut slides
| Old cut Slides |
New Cut Slides | |||
|---|---|---|---|---|
| No/Low | High | Total | Kappa 95% CI |
|
| TOP2Aa | ||||
| No/Low | 4 | 2 | 6 | 0.57 (0.14, 1.00) |
| High | 1 | 8 | 9 | |
| Total | 5 | 10 | 15 | |
| Concordance 80% (in 12 of 15 pairs) | ||||
| Ki67b | ||||
| No/Low | 7 | 3 | 10 | 0.61 (0.24, 0.97) |
| High | 0 | 5 | 5 | |
| Total | 7 | 8 | 15 | |
| Concordance 80% (in 12 of 15 pairs) | ||||
Expression categories defined by combined score of IRS system
Expression categories defined by cutoff point of 10% positive cells
Ki67 assessment
The Scatter Plot reveals a clear shift toward higher percentages of Ki67 positive cells in new cut slides (Fig. 3C). The percentage of positive cells varied between 1% and 60% in new slides and between 1% and 50% in 8 of 15 old slides; the other seven (47%) old slides demonstrated loss of staining (<1%). In the seven corresponding new cut slides staining was detected in 1-5% of cells; the seven other new cut slides had 30-40% cells staining positive and one additional case had 60% of cells staining positive. The Bland-Altman Plot (Fig. 3D) showed consistently lower expression of Ki67 in stored slides by an average of 10% (p<.01), ranging between 0% and 35% lower in stored slides.
Categorization of Ki67 using a cut-off of 10% to define no/low proliferation (negative) versus high proliferation (positive) was associated with moderate agreement between old and new cut slides (kappa =0.61; Table 2). There were 5 pairs of specimens that had over 10% positive cells. Three of those pairs had over 25% positive cells in both old and new cut slides. The other two pairs had moderate staining (10%-20%) in the old slide and a high level of staining (≥ 30%) in the new slide. Slide interpretation was concordant in 80% of cases (Table 2). Importantly, complete or partial loss of immunoreactivity was observed for both Ki67 and TOP2A in the old slides of the same cases (Fig. 2C-F).
The use of a more stringent cut off point of 13% resulted in lowered concordance between old and new slides of 73% and a lower kappa value of 0.48. When three-group (no proliferation vs low proliferation vs high proliferation) comparison was performed, concordance was even lower; 40% and 33% for cut off points of 0 and 10% and 0 and 13%, respectively. The lowest cut off point of 1% had a concordance of 53% (Table S1 shows the effect on concordance of using 13% and 1% cut off points in new cut versus old cut slides).
HER2 assessment
In the set of slides for which IHC staining was performed, one concordant pair of slides with moderately stained tumor cells and one discordant pair with a strongly stained new cut slide and a faintly stained old cut slide were found. All other slide pairs showed either no or faint staining in both new and old slides for a concordance of 93% (Table 1). Percentages of cells staining positive for HER2 between old and new slides were not significantly different (p=1.0). The interpretation of HER2 score confirmed concordance in all but one pair: the new slide was positive (score 3+) and old slide was negative (score 1+; Fig. 2G-H). Overall, the overexpression of HER2 protein in this set was detected in 2 (13%) of 15 cases and correlated well with HER2 gene amplification (Table S2).
The DNA stability was studied by HER2 FISH on cases shown in Figure 1 (see also Table S3). In old slides, the number of HER2 signals per cell ranged between 2.53 and 48.07 compared to between 2.69 and 62.66 in new slides. The baseline values of HER2/CEP17 ratios were 2.32 and 2.1 and the maximal values reached 21.52 and 26.78 in old slides and new slides, respectively. The average amplification ratio in tumor cells of stored slides was comparable to the average amplification ratio in tumor cells of new cut slides (Fig 3 E-F).
Discussion
Although the antigen loss with immunohistochemical analysis of aged slides is a known phenomenon, particularly for membrane and nuclear antigens, and a number of studies have reported on potential issues with stored paraffin sections used for biomarker research, many of these studies were conducted on breast cancer specimens and the majority were under controlled experimental conditions18,19. Little has been published about this phenomenon in tissues from other organs including the uterus, or in real-world settings with prolonged storage. Moreover, to our knowledge, the effect of slide storage duration on TOP2A antigenicity has not been reported previously.
We found that even after many years of storage there was only modestly lower protein expression in old cut versus new cut sections of endometrial cancer, with an average decrease in the percentages of stained cells for TOP2A and Ki67 in the range of 10% to12% for old cut slides.
The Ki67 antigen instability found in our study is supported by a number of reports on breast cancer samples stained with the same MIB1 clone and stored at ambient temperature 19-28. The mean decrease in the proportion of positively stained cells was 10% after 4.5 years in a study by Combs et al 21 and 10-25% after 10 years in a study by Ramos-Vara and colleagues 26 of slide storage followed by a plateau. Ki67 decay has been noted to occur either within first several weeks or months to one year 20,23,24. Even at −80°C, prolonged storage (average 12.8 years), while it resulted in little loss of staining for HER2, showed only an agreement of 0.67 for Ki67 (using a cutoff of 20%)4. Data on susceptibility of HER2 to degradation are conflicting 21,27,29-32. In our study, too few cases were HER2 positive to draw firm conclusions about changes in HER2 staining.
Unlike the situation in breast cancer where heterogeneity of HER2 expression is uncommon, significant heterogeneity of HER2 expression has been reported in endometrial cancer, with a recently published proposal that would define a IHC 2+ score (which should be followed by FISH testing) as “intense complete or basolateral/lateral membrane staining in ≤ 30%, or weak to moderate in ≥ 10% of tumor cells”.33 Such a cutoff might be importantly affected by minor degrees of loss in staining.
Interestingly, in our study the obvious degradation of two different nuclear proteins (TOP2A and Ki67) was concordantly observed in old slides relative to matched new slides of the same cases. This suggests that archival effect on protein decay is nonrandom and may be enhanced by suboptimal tissue processing before sectioning. Overall, results of our study contribute to those in the literature showing that for most molecular markers, a moderate loss of immunoreactivity in stored tissue section is apparent 1,20,21,25-27, and provide an estimate of potential extent of such loss.
There is no consensus in the literature on the mechanism of loss of immunoreactivity over time. Exposure to oxygen during sectioning and exposure to light, high humidity and elevated temperature during storage may be involved 1,19,21,25,26,28,34. While the efficacy of antigen preservation at −20°C, 4°C, or ambient temperature varies among reports, slide storage at 4°C (in sealed boxes) has been reported to be superior to storage at ambient temperature 1,3,9,35, and storage at −80°C was not been found to be superior to storage at −20°C.36
Our study confirmed earlier reports in breast cancer that DNA targets (HER2 FISH assay) seem to be unaffected by slide storage. Differences between old and new slides in this study were non-significant. FISH was conducted in slides with almost two-year interval between repeats. This is one of the longest archival times studied to date in the analysis of DNA stability by HER2 FISH 24,32.
In summary, FISH assay results for HER2 were unaffected by slide storage for approximately two years. Protein expression by IHC for TOP2A and Ki67 was modestly decreased after many years slide storage, and results may still be informative. In cases where presence of protein (or gene) in borderline or small quantities is scientifically important, or when cut-points are used for endpoint determination, the degradation of signal is particularly relevant, and use of older pre-cut tissue sections may produce a false-negative result 25,26,37.
Supplementary Material
Ki67 IHC interpretation in new cut versus old cut slides. Table that show the effect of using 13% and 1% cut off points on the defining proliferation in new cut versus old cut slides.
Association of HER2 IHC and FISH data. Table that show percentages of HER2-positive cells and HER2 IHC score in association with HER2 FISH results in new cut versus old cut slides from each case.
HER2 FISH results in old cut versus new cut slides. Table that show mean copies of HER2 per cell and mean ratios of HER2 to CEP17 and average values in old cut versus new cut slides for 18 cases.
Acknowledgments
The authors are grateful to Terri Li (UCMC IHC Core Facility) for help with experimental procedures, Lise Sveen, Mariann Coyle and Niu Qun for general support; Cheryl Landini (University of Chicago Cancer Clinical Trials Office) and Leah Madden (GOG Administrative Office) for project coordination. We are very grateful to all of the patients who contributed specimens to this study.
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Community Clinical Oncology Program, Penn State Milton S. Hershey Medical Center, Wayne State University/Karmanos Cancer Institute, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Tufts-New England Medical Center, Women’s Cancer Center of Nevada, University of Alabama at Birmingham, Abington Memorial Hospital, University of Minnesota Medical Center-Fairview, Fred Hutchinson Cancer Research Center, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center, Rush University Medical Center, Cleveland Clinic Foundation, Stony Brook University Medical Center, Washington University School of Medicine, Cooper Hospital University Medical Center, Fox Chase Cancer Center, University of Virginia, and Moffitt Cancer Center and Research Institute
Funding
This study was supported by National Cancer Institute grants K12CA139160 and CTSA-ITM (CS UL1 RR024999) to TG, CA 27469 to the Gynecologic Oncology Group (GOG) Administrative Office, CA 37517 to the GOG Statistical Office, 1U10 CA180822 to NRG Oncology, U10CA180868 to NRG Oncology Operations, U10 CA27469, U24 CA114793, U10 CA180868 to the Gynecologic GOG Tissue Bank, and P30 CA14599 to the University of Chicago Cancer Center and IHC core facility. O.I.O was supported by EIF NWCRA.
COI
Filiaci: grants from NIH during the conduct of this study and additional funding from GOG Foundation, Inc. outside the submitted work for other gynecologic clinical trials.
Seward: Speakers Burea for GSK (Zejula), AstraZeneca (Olaparib), and Merk (Keytruda+lenvatanib).
Grushko: current employee of Abbott
Method: current employee of Lilly and own Lilly stock
Fleming: Ad board for GSK, institutional PI for industry trials of Roche, Syros, GSK, Iovance, Sanofi, Sermonix, Incyte, Compugen, Abbvie, Eisai, Celldex, Astra Zeneca, Corcept, Merck, Plexxicon
All others report no conflicts
Abbreviations
- ASCO
American Society for Clinical Oncology
- CEP17
chromosome 17 centromere enumeration probe
- CAP
College of American Pathologists
- DAB
3,3-diaminobenzidine tetrahydrochloride
- FISH
fluorescence in situ hybridization
- FFPE
formalin fixed paraffin embedded
- GOG
Gynecologic Oncology Group
- H&E
hematoxylin and eosin
- HER2
human epidermal growth factor receptor 2
- HTRC
Human Tissue Resource Center
- IHC
immunohistochemistry
- IRS system
ImmunoReactive Scoring system
- Ki67
Proliferation marker protein Ki-67 encoded by MKI67 gene
- MWH
microwave heating
- NRG Oncology
National Surgical Adjuvant Breast and Bowel Project (NSABP), the Radiation Therapy Oncology Group (RTOG), and the Gynecologic Oncology Group (GOG)
- SDMC
Statistics and Data Management Center
- TOP2A
topoisomerase II alpha
- UCMC
The University of Chicago Medical Center
Footnotes
Preliminary presentation at the Society of Gynecologic Oncology (SGO) 45th Annual Meeting on Women’s Cancer (Tampa, FL, March 22 –25, 2014)
Translational Trial Registration:
8013, NCI-2011-02245, CDR0000681556, NCT01164735.
References
- 1.Engel KB, Moore HM: Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med 135:537–43, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Libard S, Cerjan D, Alafuzoff I: Characteristics of the tissue section that influence the staining outcome in immunohistochemistry. Histochem Cell Biol 151:91–96, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs TW, Prioleau JE, Stillman IE, et al. : Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 88:1054–9, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Forse CL, Pinnaduwage D, Bull SB, et al. : Fresh Cut Versus Stored Cut Paraffin-embedded Tissue: Effect on Immunohistochemical Staining for Common Breast Cancer Markers. Appl Immunohistochem Mol Morphol 27:231–237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin M, Romero A, Cheang MC, et al. : Genomic predictors of response to doxorubicin versus docetaxel in primary breast cancer. Breast Cancer Res Treat 128:127–36, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Fleming GF, Filiaci VL, Bentley RC, et al. : Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol 15:1173–8, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Grushko TA, Filiaci VL, Mundt AJ, et al. : An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 108:3–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsiambas E, Alexopoulou D, Lambropoulou S, et al. : Targeting topoisomerase IIa in endometrial adenocarcinoma: a combined chromogenic in situ hybridization and immunohistochemistry study based on tissue microarrays. Int J Gynecol Cancer 16:1424–31, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Xie R, Chung JY, Ylaya K, et al. : Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 59:356–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faggad A, Darb-Esfahani S, Wirtz R, et al. : Topoisomerase IIalpha mRNA and protein expression in ovarian carcinoma: correlation with clinicopathological factors and prognosis. Mod Pathol 22:579–88, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Konstantinos K, Marios S, Anna M, et al. : Expression of Ki-67 as proliferation biomarker in imprint smears of endometrial carcinoma. Diagn Cytopathol 41:212–7, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Yerushalmi R, Woods R, Ravdin PM, et al. : Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–83, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Sehdev AS, Kurman RJ, Kuhn E, et al. : Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Mod Pathol 23:844–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faratian D, Munro A, Twelves C, et al. : Membranous and cytoplasmic staining of Ki67 is associated with HER2 and ER status in invasive breast carcinoma. Histopathology 54:254–7, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Jonat W, Arnold N: Is the Ki-67 labelling index ready for clinical use? Ann Oncol 22:500–2, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Grushko TA, Blackwood MA, Schumm PL, et al. : Molecular-cytogenetic analysis of HER-2/neu gene in BRCA1-associated breast cancers. Cancer Res 62:1481–8, 2002 [PubMed] [Google Scholar]
- 17.Mandrekar JN: Measures of interrater agreement. J Thorac Oncol 6:6–7, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Omilian AR, Zirpoli GR, Cheng TD, et al. : Storage Conditions and Immunoreactivity of Breast Cancer Subtyping Markers in Tissue Microarray Sections. Appl Immunohistochem Mol Morphol 28:267–273, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grillo F, Pigozzi S, Ceriolo P, et al. : Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol 144:93–9, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Blows FM, Ali HR, Dawson SJ, et al. : Decline in Antigenicity of Tumor Markers by Storage Time Using Pathology Sections Cut From Tissue Microarrays. Appl Immunohistochem Mol Morphol 24:221–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combs SE, Han G, Mani N, et al. : Loss of antigenicity with tissue age in breast cancer. Lab Invest 96:264–9, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Divito KA, Berger AJ, Camp RL, et al. : Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res 64:8773–7, 2004 [DOI] [PubMed] [Google Scholar]
- 23.DiVito KA, Charette LA, Rimm DL, et al. : Long-term preservation of antigenicity on tissue microarrays. Lab Invest 84:1071–8, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Karlsson C, Karlsson MG: Effects of long-term storage on the detection of proteins, DNA, and mRNA in tissue microarray slides. J Histochem Cytochem 59:1113–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos-Vara JA, Miller MA: When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet Pathol 51:42–87, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Vara JA, Webster JD, DuSold D, et al. : Immunohistochemical evaluation of the effects of paraffin section storage on biomarker stability. Vet Pathol 51:102–9, 2014 [DOI] [PubMed] [Google Scholar]
- 27.van den Broek LJ, van de Vijver MJ: Assessment of problems in diagnostic and research immunohistochemistry associated with epitope instability in stored paraffin sections. Appl Immunohistochem Mol Morphol 8:316–21, 2000 [PubMed] [Google Scholar]
- 28.Wester K, Wahlund E, Sundstrom C, et al. : Paraffin section storage and immunohistochemistry. Effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol 8:61–70, 2000 [PubMed] [Google Scholar]
- 29.Blind C, Koepenik A, Pacyna-Gengelbach M, et al. : Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol 61:79–83, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Fergenbaum JH, Garcia-Closas M, Hewitt SM, et al. : Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev 13:667–72, 2004 [PubMed] [Google Scholar]
- 31.Mirlacher M, Kasper M, Storz M, et al. : Influence of slide aging on results of translational research studies using immunohistochemistry. Mod Pathol 17:1414–20, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Risio M, De Rosa G, Sarotto I, et al. : HER2 testing in gastric cancer: molecular morphology and storage time-related changes in archival samples. Int J Oncol 23:1381–7, 2003 [PubMed] [Google Scholar]
- 33.Buza N: HER2 Testing in Endometrial Serous Carcinoma. Arch Pathol Lab Med, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Economou M, Schoni L, Hammer C, et al. : Proper paraffin slide storage is crucial for translational research projects involving immunohistochemistry stains. Clin Transl Med 3:4, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JH, Mepham BL, Wright DH: Tissue preparation for immunocytochemistry. J Clin Pathol 50:422–8, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andeen NK, Bowman R, Baullinger T, et al. : Epitope Preservation Methods for Tissue Microarrays: Longitudinal Prospective Study. Am J Clin Pathol 148:380–389, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balgley BM, Guo T, Zhao K, et al. : Evaluation of archival time on shotgun proteomics of formalin-fixed and paraffin-embedded tissues. J Proteome Res 8:917–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ki67 IHC interpretation in new cut versus old cut slides. Table that show the effect of using 13% and 1% cut off points on the defining proliferation in new cut versus old cut slides.
Association of HER2 IHC and FISH data. Table that show percentages of HER2-positive cells and HER2 IHC score in association with HER2 FISH results in new cut versus old cut slides from each case.
HER2 FISH results in old cut versus new cut slides. Table that show mean copies of HER2 per cell and mean ratios of HER2 to CEP17 and average values in old cut versus new cut slides for 18 cases.