Abstract
Background:
Insomnia symptoms may be an important etiological factor for substance use disorders; however, whether improving sleep leads to reductions in problematic substance use among at-risk populations remains unclear.
Method:
As such, the current pilot study used a randomized controlled design to test the effects of Brief Behavioral Treatment for Insomnia (BBTI) against a waitlist control among a sample of trauma-exposed young adults with elevated insomnia symptoms who regularly use cannabis (N=56).
Results:
Intent-to-treat multilevel modeling analyses indicated that BBTI may be more efficacious than waitlist control in reducing self-reported insomnia symptoms, with large effects three months post-treatment (d = 1.34). Further, our initial evidence suggested that BBTI resulted in reductions in cannabis-related problems with medium to large effects at three months post-treatment (d=.75). The current pilot analyses indicated BBTI also reduced cravings to use cannabis to reduce negative emotions in response to trauma cues with a large effect size.
Conclusion:
This pilot study suggests BBTI may be efficacious not only in improving insomnia symptoms among cannabis users but also in reducing cannabis-related problems and cravings over three months. Future research should replicate these results in a larger, fully powered sample with improved follow-up rates designed to test temporal mediation using multimethod assessments of insomnia symptoms and problematic cannabis use. Overall, BBTI may be a promising intervention for trauma-exposed cannabis users to improve sleep and reduce cannabis-related problems.
Keywords: Cannabis, Substance use disorder, Insomnia, Trauma, Post-traumatic stress
1. Introduction
Substance use disorders (SUDs) are a leading public health burden in the United States, resulting in an estimated cost of up to $200 billion annually, and affecting more than 20 million Americans (Substance Abuse and Mental Health Services Administration, 2015). SUDs are characterized by recurrent use of substances despite negative consequences, leading to clinically significant impairment. Unfortunately, the negative impact of SUDs is far-reaching and extends to many aspect of sufferers’ lives, including physical and mental health, occupational and/or academic functioning, and interpersonal relationships (Substance Abuse and Mental Health Services Administration, 2015).
Trauma-exposed individuals are at high risk for SUD and in need of preventative interventions. Trauma-exposed individuals report earlier substance use onset, increased use frequency, greater maladaptive coping motives for use (associated with risk for SUD; Cooper, Kuntsche, Levitt, Barber, & Wolf, 2016), and a higher prevalence of SUD compared to those who are not trauma exposed (Khoury, Tang, Bradley, Cubells, & Ressler, 2010). Furthermore, SUD among individuals with post-traumatic stress disorder (PTSD) symptoms is notably impairing and difficult to treat (Roberts, Roberts, Jones, & Bisson, 2015), as both PTSD and SUD are more severe and less amenable to treatment when they are comorbid. Although the mechanism for the association between trauma, PTSD symptoms, and SUD is not fully understood, it is likely accounted for by factors such as shared vulnerability for both trauma and SUD, as well as the motivation to use substances to cope with PTSD symptoms (Roberts et al., 2015). Considering the high risk of SUD in this population, a need exists to identify and intervene upon modifiable risk factors for SUD.
Insomnia symptoms are a common sequela of trauma (Kessler et al., 2005). Insomnia symptoms are common and occur in up to 30% of the population (Mai & Buysse, 2008), and up to 90% of those with PTSD (Neylan et al., 1998). For some, these symptoms are clinically significant and meet criteria for insomnia disorder. Insomnia disorder is defined as difficulties with initiating or maintaining sleep combined with daytime dysfunction (e.g., fatigue, problems with mood or concentration), and occurs in up to 10% of the population (Mai & Buysse, 2008). Regardless of whether they meet formal diagnostic criteria for insomnia disorder, elevated insomnia symptoms may be a risk factor for SUD as they predict earlier substance use onset, maladaptive use motives, development of SUD, and interfere with substance use cessation (Babson, Boden, & Bonn-Miller, 2013; Wong, Brower, Fitzgerald, & Zucker, 2004). In particular, insomnia symptoms are closely linked with cannabis use disorder (CUD), coping-oriented cannabis use, earlier onset of cannabis use, and relapse after cannabis cessation attempts (Babson et al., 2013; Babson & Bonn-Miller, 2014; Wong et al., 2004).
There are a variety of possible mechanisms accounting for why insomnia symptoms lead to CUD. For example, many cannabis users perceive that cannabis may facilitate sleep onset, though evidence for this notion is mixed (Drazdowski, Kliewer, & Marzell, 2019; Goodhines, Gellis, Ansell, & Park, 2019). For those with PTSD symptoms, however, insomnia symptoms are likely to be associated with a variety of difficulties in affective functioning that may lead individuals to use cannabis to cope with distress despite long-term consequences. Specifically, research has linked sleep deprivation and/or restriction with reduced thresholds for perceiving situations as stressful (Zohar, Tzischinsky, Epstein, & Lavie, 2005) and increased self-reported, physiological, and neurobiological reactivity to stress (Minkel et al., 2012; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). Similarly, research has linked poor sleep quality with reduced capability to down-regulate negative emotional reactivity (Mauss, Troy, & LeBourgeois, 2013; Yoo et al., 2007), diminished distress tolerance and increased avoidance in response to stress (Short et al., 2016; Short & Schmidt, 2017), and sleep deprivation with reduced capacity to inhibit impulses for reward (Gujar, Yoo, Hu, & Walker, 2011), particularly in the context of negative affect. These problems may be exacerbated among individuals with PTSD symptoms who already experience heightened stress and craving reactivity to trauma cues (Kwako et al., 2015; Orr & Roth, 2000), with evidence that sleep deprivation may increase reactivity to such cues (Babson, Badour, Feldner, & Bunaciu, 2012). In the context of PTSD, insomnia symptoms may increase cravings and use of cannabis to cope with stress and negative affect, particularly in response to trauma cues (Chakravorty et al., 2010; Freeman & Gottfredson, 2018).
Fortunately, insomnia symptoms can be treated effectively. Specifically, Brief Behavioral Treatment for Insomnia (BBTI) is an efficacious treatment for insomnia disorder that improves sleep, reduces depression, anxiety, and PTSD symptoms (Buysse et al., 2011; Germain et al., 2006; Germain, Shear, Hall, & Buysse, 2007; Troxel, Germain, & Buysse, 2012). In addition, preliminary evidence from small or uncontrolled trials indicates that improving sleep reduces problematic substance use (Babson, Ramo, Baldini, Vandrey, & Bonn-Miller, 2015; Bootzin & Stevens, 2005; Chakravorty, Vandrey, He, & Stein, 2018). Specifically, an open trial testing a group sleep treatment indicated that improving sleep led to reductions in substance abuse problems among adolescents one year post-treatment (Bootzin & Stevens, 2005). Further, in a small pilot RCT, veterans with CUD and sleep problems who received a Cognitive Behavioral Therapy for Insomnia (CBT-I) app reported decreased cannabis use and improved sleep quality compared to those who received a control app (Babson et al., 2015). Although research has not empirically examined the mechanisms underlying these effects, BBTI may improve sleep, thereby improving affective functioning (Cunningham & Shapiro, 2018), and, specifically, stress reactivity (Babson et al., 2012; Minkel et al., 2012) and cravings or substance use (Freeman & Gottfredson, 2018) to cope with distress. Finally, sleep interventions could be an opportunity to bring individuals at risk for SUD into treatment: sleep problems may motivate treatment-resistant individuals to engage in treatment, and young adults at risk for SUD are interested in sleep-related interventions (Fucito et al., 2015).
Considering the potential for leveraging behavioral sleep medicine as a preventative strategy for SUD and gaps in the extant literature, the current pilot study tested whether BBTI vs. waitlist control led to reductions in SUD risk among a relatively small sample of trauma-exposed young adults with elevated insomnia symptoms. We chose to focus on cannabis because of its connection with poor sleep (Babson & Bonn-Miller, 2014) and its widespread use (Substance Abuse and Mental Health Services Administration, 2015), and utilized a waitlist control as, to our knowledge, this is the first study to examine BBTI in an active cannabis-using sample. Our goal was to conduct a pilot study to detect whether BBTI had any effects on insomnia symptoms in this group, and to assess preliminary evidence of whether improving sleep may impact cannabis-related problems. Specific hypotheses were as follows and tested using intent-to-treat (ITT) multilevel modeling analyses: 1) Consistent with other populations, BBTI would be more efficacious than waitlist control in reducing insomnia symptoms and sleep efficiency (SE) assessed through retrospective self-report and prospective sleep diary among trauma-exposed cannabis users over three months post-treatment; 2) Consistent with the potential role of insomnia symptoms as a risk factor for CUD, BBTI will result in reductions in cannabis-related problems and use compared to a waitlist control over three months post-treatment; and 3) As an initial test of mechanisms of treatment action (i.e., improving sleep would reduce stress and craving reactivity to stressors), BBTI would result in reduced cannabis cravings related to emotionality in response to idiographic trauma script-driven imagery (Orr, Pitman, Lasko, & Herz, 1993) from baseline to post-treatment. We hypothesized effects would persist regardless of whether individuals had a diagnosis of PTSD.
2. Method
2.1. Participants
Based on results of a power analysis to detect the large effects of BBTI on insomnia symptoms at power of 0.80 and balancing the expected small to medium effects on substance use with the nature of this pilot study (Buysse et al., 2011; Germain et al., 2006), we recruited 56 adults aged 18–30 with trauma exposure according to the Posttraumatic Diagnostic Scale (PDS) checklist (Foa, Cashman, Jaycox, & Perry, 1996) who reported poor sleep (scoring >8 on the Insomnia Severity Index [ISI]; Morin, Belleville, Bélanger, & Ivers, 2011) and used cannabis at least weekly (Timeline Followback; Sobell & Sobell, 1992). Exclusion criteria included currently receiving sleep/SUD treatment, high risk for sleep apnea according to the STOP-BANG (Chung, Abdullah, & Liao, 2016), or instability on psychiatric medications (defined as any new onset or change of psychiatric medications in the six weeks prior to enrollment reported during their telephone screen). Recruitment and follow-ups occurred from August 2017 to October 2018.
Participants included a slight majority of women (58.9%) aged 18 to 30 (M=20.69, SD=3.93; Table 1). The majority identified as White (73.2%). The study recruited about half from the community (49.2%), and the student participant pool (50.8%).1 Participants endorsed an index trauma of sexual assault (26.7%), non-sexual assault (17.9%), accident (16.1%), life-threatening illness (7.1%), disaster (3.6%), combat (3.6%), and other (e.g., near drowning, witnessing violence; 17.9%). Based on the Structured Clinical Interview for DSM-5 (First, Williams, Karg, & Spitzer, 2015), a minority of participants (16.1%) had PTSD. More than half (58.9%) met criteria for insomnia disorder. Finally, 92.9% met criteria for >1 diagnosis, most commonly CUD (26.8%), followed by anxiety (25.0%), depressive disorders (10.7%), and PTSD (10.7%).
Table 1.
Demographic and clinical characteristics by condition
| Active (n=28) | Control (n=28) | |
|---|---|---|
| % (n) | % (n) | |
| Age M (SD) | 21.00 (4.85) | 20.39 (2.79) |
| Women | 57.1 (16) | 60.7 (11) |
| Race | ||
| White | 78.6 (22) | 67.9 (19) |
| Black/African American | 17.9 (5) | 25.0 (7) |
| Asian | 0.0 (0) | 3.6 (1) |
| Other | 3.6 (1) | 3.6 (1) |
| Hispanic or Latino/a | 25.0 (7) | 25.0 (7) |
| Recruited through community | 50.0 (14) | 42.9 (12) |
| Daily TLFB average cannabis use | 3.48 (2.74) | 2.87 (2.52) |
| Uses cannabis to help sleep | 96.4 (27) | 92.3 (26) |
| Alcohol use frequency | ||
| Monthly or less | 17.9 (5) | 28.6 (8) |
| 2–4 time/month | 39.3 (11) | 35.7 (10) |
| 2–3 times/week | 35.7 (10) | 25.0 (7) |
| 4+ times/week | 7.1 (2) | 10.7 (3) |
| Any other past 30 day drug use | 46.4 (13) | 21.4 (6) |
| Current cigarette/vape smoker | 25.0 (7) | 14.3 (4) |
| Cannabis use disorder diagnosis | 67.9 (19) | 78.6 (22) |
| PTSD diagnosis | 17.9 (5) | 14.3 (4) |
| Insomnia diagnosis | 53.6 (15) | 64.3 (18) |
Note. PTSD=posttraumatic stress disorder. Other past 30 day drug use excludes cannabis. TLFB=Timeline Followback. This measure is scaled so that 0=no use, 1=a hit, 4=joint, 8=a blunt, thus participants reported using nearly a joint of cannabis daily in the current study. An item regarding using cannabis to help one sleep was created for the current study in which participants rated on a four-point Likert scale (1=Never/Almost Never, 4=Always/Almost Always) how often they use cannabis “To help me fall asleep easier.” The percentage of individuals who reported doing so at least sometimes is reported.
2.2. Procedure
The study recruited participants from the community using fliers and online postings advertised to cannabis users interested in improving their sleep. Interested individuals completed a phone screen with a trained postbaccalaureate research assistant who scheduled participants for appointments at our research clinic. The study recruited undergraduates via the university’s participant pool by completing a screening survey and invited them to participate and schedule online if they met criteria. Students received course credit and monetary compensation, while community participants received monetary compensation. See Figure 1 for study flow. All participants provided written, informed consent, and procedures were approved by the university’s Institutional Review Board approved the procedures, which we pre-registered (NCT03226132).
Figure 1. CONSORT Diagram.
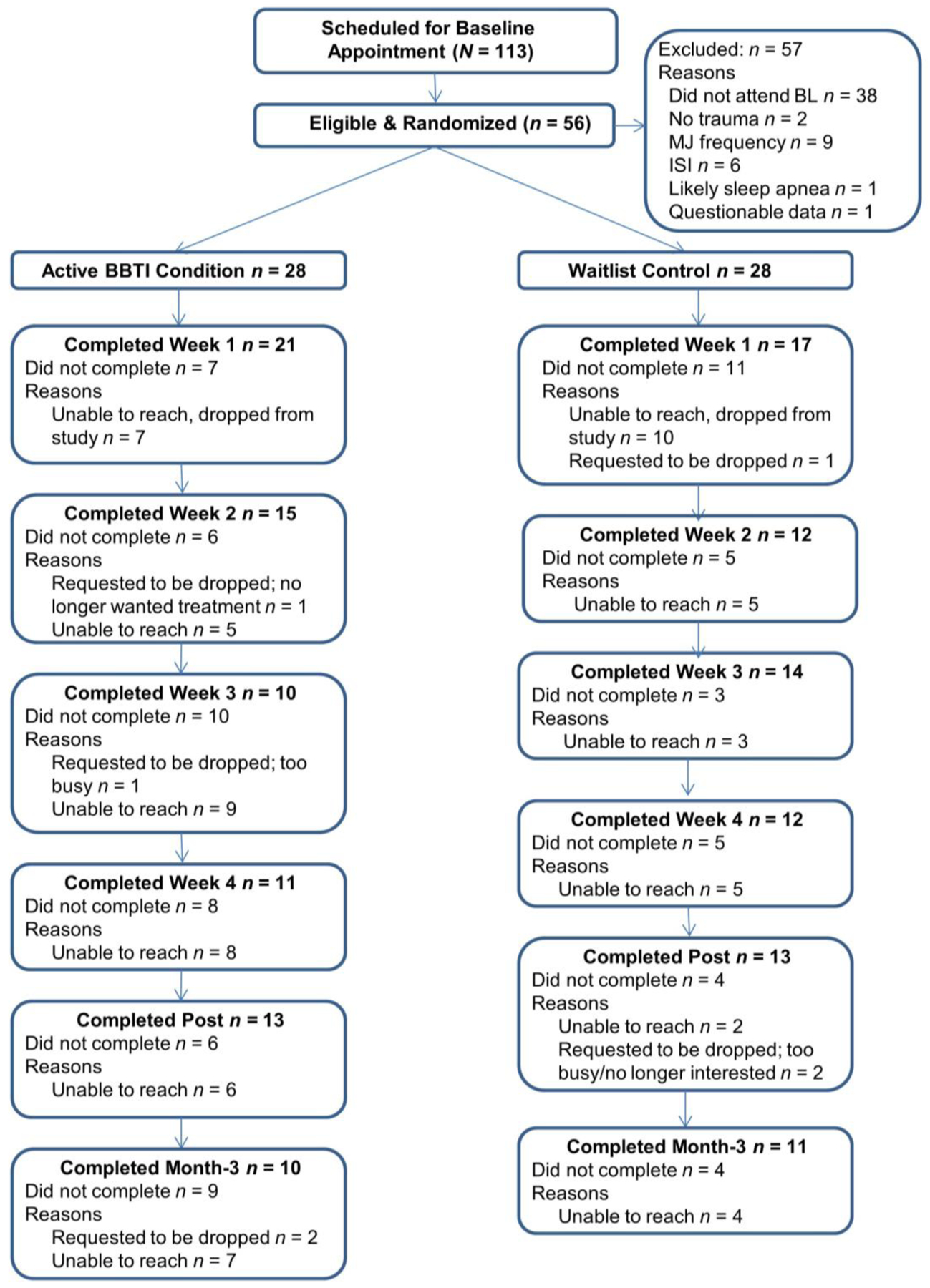
Note. BL=Baseline; MJ=Marijuana; MJ Frequency=self-reported cannabis use less than once per week; ISI=Insomnia Severity Index – this indicates participants did not meet the required cut-score of 8; Likely sleep apnea is based on the STOP-BANG; Questionable Data refers to data with mismatch between interview and self-report; BBTI=Brief Behavioral Treatment for Insomnia. Weeks 1 and 3 were in-person, Weeks 2 and 4 were conducted via phone.
2.2.1. Baseline.
First, participants provided consent and ined research assistants confirmed eligibility. Next, participants completed self-report measures and trauma script-driven imagery (Orr et al., 1993). Specifically, participants reported trauma details, including physiological and emotional reactions to the trauma, using a preparation form. The study used these details to create a script in second-person, present-tense, which a trained research assistant then read aloud and audiotaped. The research assistant asked participants to listen to the script and imagine it as vividly as possible. Before and after, participants self-reported negative affect and cravings to use cannabis. Finally, the research assistants randomized participants using a random numbers table evenly split between conditions, and scheduled participants and provided them with a sleep diary.
2.3. Experimental conditions
2.3.1. Brief Behavioral Treatment for Insomnia (BBTI).
BBTI consists of 4 sessions. In the current study, an advanced doctoral student, with limited prior insomnia treatment experience, delivered following the BBTI treatment manual and receiving supervision from a licensed psychologist. In session 1 (60 minutes), participants learned psychoeducation about sleep and the rationale for sleep restriction and stimulus control, “rules” about sleep, and set a prescribed schedule for sleep restriction (Buysse et al., 2011; Troxel et al., 2012). For the remaining sessions (20–30 minutes each), the doctoral student reviewed sleep diaries with participants, appropriately titrated time in bed based on SOL and wake after sleep onset, and discussed any challenges with following the recommendations. The second and fourth session took place over the phone. No references to PTSD or cannabis use were made. Total treatment time ranged from 2 hours and 20 minutes to 3 hours (including completing self-report assessments). If participants asked, study staff told them that the treatment did not make recommendations as to whether they should use cannabis for sleep.
2.3.2. Waitlist control.
Study staff informed participants that they were on a waitlist and assessed at the same time points as the active group. Total time in the control condition was only the brief self-report measures at each session (~20 minutes total). After study completion, we offered them BBTI.
2.4. Intervention and follow-up
2.4.1. Intervention.
Throughout the four weeks of intervention, participants completed self-report measures either in-person (weeks 1 and 3) or via the Internet (weeks 2 and 4).
2.4.2. Post-treatment.
Participants completed self-report measures and trauma script-driven imagery (with the same procedures at baseline) one week after completing their assigned intervention. Research assistants were blind to participants’ conditions.
2.4.3. Month 3 follow-up.
Participants completed self-report measures three months after treatment.
2.5. Measures
2.5.1. Structured Clinical Interview for DSM-5 (SCID).
The SCID is a widely used interview assessing DSM-5 diagnoses (First et al., 2015) completed by trained clinical psychology doctoral students or research assistants who achieved high interrater reliability in our lab (i.e., kappa of .86; Schmidt, Norr, Allan, Raines, & Capron, 2017).
2.5.2. Marijuana Problems Scale (MPS).
The MPS assesses problems related to cannabis use using a 19-item self-report questionnaire (Stephens, Roffman, & Curtin, 2000). The study asked participants whether cannabis use has caused them a variety of problems (e.g., problems in your family, miss days at work or miss class, medical problems, withdrawal, financial difficulties, procrastinate, lose self-confidence) and respond by rating whether it was no problem (0), a minor problem (1), or a serious problem (2). The MPS has good psychometric properties (Buckner, Silgado, & Schmidt, 2011) and is sensitive to change (Stephens et al., 2000). The study used the MPS as an outcome measure to evaluate whether BBTI results in reductions in cannabis problems compared to the waitlist control. The MPS demonstrated good reliability in the current study (α = .87) at baseline and at follow-ups (αs =.82–.95).
2.5.3. Insomnia Severity Index (ISI).
The ISI is a psychometrically sound 7-item self-report measure of insomnia symptom severity (Morin et al., 2011). Instructions for the ISI asked participants to assess their symptoms in the last week for weekly sessions and the past two weeks, as is consistent with the standard instructions, for the month 3 follow-up. The study staff gave the ISI at each session and had adequate internal consistency (αs=.72–.92).
2.5.4. The Consensus Sleep Diary (CSD).
The CSD is completed daily prior to bedtime and after awakening (Carney et al., 2012; Monk et al., 1994). Participants report on various items resulting in assessments of sleep onset latency, wake after sleep onset, sleep duration, sleep quality, sleep medication use, and SE. The current study computed SE by dividing total sleep time by time in bed (hours between self-reported bedtime and wake time).
2.5.5. Credibility/Evaluation Questionnaire (CEQ).
The CEQ is a 6-item self-report measure assessing whether clients believe a particular therapy will help in reducing their symptoms (Devilly & Borkovec, 2000). The CEQ comprises two components: credibility (how much participants think the therapy will be helpful) and expectancy (how much do participants feel the therapy will be helpful). Participants evaluate the treatment on a 9-point Likert scale (e.g., how logical does the therapy offered to you seem?) and also respond on a 10-point percentage scale how much they think the therapy will reduce their symptoms (0–100%). The CEQ has demonstrated good psychometric properties and associations with therapeutic outcomes (Devilly & Borkovec, 2000). In the current study, the CEQ demonstrated excellent internal consistency (α = .93).
2.5.6. The Marijuana Craving Questionnaire (MCQ).
The MCQ is a 12-item self-report measure of state cannabis cravings designed to be used in conjunction with cues that may elicit cannabis use cravings (Heishman et al., 2009). Participants respond to a list of items (e.g., If I smoked marijuana right now, I would feel less tense; I would feel less anxious if I smoked marijuana right now) on a 7-point scale (strongly disagree to strongly agree). Participants completed the emotionality domain assessing cravings to use cannabis to reduce negative emotions before and after trauma imagery. The MCQ had excellent internal consistency (αs =.92–.95).
2.5.7. Positive and Negative Affect Schedule – Negative Affect (PANAS-NA).
The study used the state PANAS-NA (Watson & Clark, 1994) before and after trauma imagery. The PANAS-NA is a well-validated measure of negative emotions, including ten items rated on a 5-point Likert scale. The PANAS-NA had good internal consistency (αs =.87–.90).
2.5.8. Timeline Followback (TLFB).
The TLFB is a well-validated assessment of daily substance use completed via brief interview (Sobell & Sobell, 1992). A calendar and memory aids assist participants in reporting the quantity of their daily cannabis use on a 0–8 scale (0=none; 1=a hit; 4=a joint; 8=a blunt; Bonn-Miller et al., 2015). Trained clinical psychology doctoral students or postbaccalaureate research assistants administered TLFBs. The current study used TLFBs to quantify average daily levels of cannabis use for the sample.
2.5.9. Post-traumatic Diagnostic Scale (PDS).
The PDS is a self-report measure of trauma exposure and PTSD symptoms. Specifically, the PDS includes a 12-item checklist of traumatic event exposure. In the current study, this checklist assessed exposure to a DSM-5 criterion A trauma, and thus eligibility, and we used it to characterize trauma types (Foa, Cashman, Jaycox, & Perry, 1997).
2.6. Data analysis
The study team used ITT analyses to test all hypotheses. To maximize power, we used multilevel modeling to take into account each assessment time point in one model while handling missing data at some time points, rather than traditional ANOVA or regression analyses. The study assessed condition as a predictor of changes in insomnia symptoms and cannabis-related problems using random intercept and slope multilevel models using robust maximum likelihood (MLR) estimation. We first assessed unconditional models to determine the best fitting model (e.g., linear vs. quadratic change). We assessed the effect of treatment on changes in insomnia symptoms, SE, cannabis-related problems, and average daily cannabis use by including condition and condition by session as predictors. The study team included PTSD diagnoses (presence or absence) as a covariate in adjusted analyses. We report unstandardized regression coefficients, estimated within-group changes from baseline to month 3 and estimated between group differences. The study calculated effect sizes as additional parameters in the model by computing the regression coefficients of the impact of condition on the relevant outcome variable (e.g., insomnia symptoms) standardized to the baseline standard deviation of the relevant outcome (e.g., insomnia symptoms) in the full sample (Feingold, 2009). Because we assessed cravings a total of only four times (pre- and post-trauma imagery at baseline and post-treatment), multilevel models were not appropriate and we instead used path analysis in Mplus with condition as a predictor of residualized change in cravings from pre- to post-trauma imagery at post follow-up, covarying for residualized change in cravings at baseline, and PTSD diagnostic status. The study included participants in these analyses regardless of whether the imagery procedure evoked negative affect or cravings for that particular participant. We considered analyses statistically significant at the p<.05 level.
3. Results
3.1. Descriptive statistics
Initial analysis included means and standard deviations (Tables 1 and 2), and we examined zero-order correlations. Mean ISI scores were above the clinical cut-off of 15 (Morin et al., 2011). No differences occurred between conditions in demographic or clinical variables (ps>.494), suggesting that randomization was successful. At baseline, insomnia symptoms were significantly correlated with cannabis-related problems (r=.41, p=.002). In terms of missing data, no significant differences occurred between attenders vs. nonattenders in age, race, condition, insomnia symptoms, cannabis-related problems, PTSD symptom severity, or diagnostic status (ps>.089), with the exception of those with PTSD diagnoses being less likely to attend month 3 (p=.034). Thus, the study used PTSD diagnosis (presence or absence) as a covariate in relevant analyses.
Table 2.
Raw symptom measure scores over time by treatment condition
| BBTI (n=28) | Control (n=28) | |||||
|---|---|---|---|---|---|---|
| n | M (SD) | M 95% CI | n | M (SD) | M 95% CI | |
| Insomnia symptoms | ||||||
| Baseline | 28 | 16.86 (4.04) | 15.43, 18.43 | 28 | 16.07 (4.49) | 14.50, 17.64 |
| Post | 13 | 5.31 (4.70) | 1.89, 8.56 | 13 | 11.31 (6.47) | 7.80, 15.62 |
| Month 3 | 12 | 5.70 (4.19) | 2.03, 7.00 | 11 | 9.73 (6.11) | 7.22, 13.90 |
| Cannabis-related problems | ||||||
| Baseline | 28 | 5.79 (4.61) | 3.12, 6.72 | 28 | 5.61 (5.39) | 3.30, 11.38 |
| Post | 13 | 2.80 (2.55) | 1.23, 3.16 | 13 | 6.77 (7.57) | 3.05, 12.00 |
| Month 3 | 12 | 2.82 (3.82) | 1.73, 4.70 | 11 | 6.18 (9.70) | 2.25, 12.81 |
| Sleep efficiency | ||||||
| Session 1 M | 17 | .73 (.18) | .65, .81 | 16 | .77 (.13) | .70, .83 |
| Mid-Treatment (Session 3) M | 11 | .85 (.21) | .72, .94 | 9 | .84 (.10) | .77, .90 |
| Post M | 5 | .87 (.19) | .69, .99 | 4 | .83 (.03) | .82, .86 |
| SOL (minutes) | ||||||
| Session 1 M | 17 | 23.39 (19.41) | 16.01, 31.43 | 16 | 18.13 (11.33) | 12.42, 24.25 |
| Mid-Treatment (Session 3) M | 11 | 17.47 (19.18) | 7.82, 26.54 | 9 | 25.00 (25.55) | 11.42, 42.92 |
| Post M | 5 | 17.79 (28.19) | 7.82, 26.54 | 4 | 23.31 (13.12) | 11.42, 42.92 |
| WASO (minutes) | ||||||
| Session 1 M | 17 | 11.59 (13.74) | 7.02, 17.03 | 16 | 22.50 (20.49) | 11.80, 34.41 |
| Mid-Treatment (Session 3) M | 11 | 13.59 (18.77) | 4.74, 25.14 | 9 | 19.45 (14.15) | 11.75, 28.19 |
| Post M | 5 | 4.39 (3.03) | 2.14, 6.43 | 4 | 29.57 (26.73) | 13.86, 45.40 |
| Total Sleep Time (hours) | ||||||
| Session 1 M | 17 | 3.88 (1.75) | 3.15, 4.64 | 16 | 4.28 (1.78) | 3.15, 4.64 |
| Mid-Treatment (Session 3) M | 11 | 6.37 (1.43) | 5.57, 7.06 | 9 | 6.71 (5.39) | 4.78, 5.97 |
| Post M | 5 | 5.78 (2.66) | 3.02, 7.51 | 4 | 6.81 (1.03) | 6.22, 7.58 |
| Time in Bed (hours) | ||||||
| Session 1 M | 17 | 7.44 (1.40) | 6.84, 8.02 | 16 | 7.82 (1.11) | 7.23, 8.30 |
| Mid-Treatment (Session 3) M | 11 | 6.59 (1.80) | 5.42, 7.67 | 9 | 6.99 (1.18) | 5.90, 7.82 |
| Post M | 5 | 7.32 (.89) | 6.73, 7.96 | 4 | 6.96 (2.67) | 4.54, 8.55 |
| Average Daily Cannabis Use | ||||||
| Baseline | 22 | 3.90 (2.85) | 2.77, 5.14 | 24 | 3.02 (2.69) | 2.05, 4.10 |
| Post | 12 | 2.71 (2.11) | 1.62, 3.88 | 12 | 1.66 (1.80) | .69, 2.71 |
| Month 3 | 5 | 3.05 (.79) | 2.44, 3.67 | 11 | 1.79 (1.47) | 1.04, 2.58 |
Note. BBTI=Brief Behavioral Treatment for Insomnia, SOL=Sleep Onset Latency, WASO=Wake after sleep onset. Average daily cannabis use was measured by the Timeline Followback on a 0–8 point scale (0=no use, 1=a hit, 4=joint, 8=a blunt)
3.1.1. Manipulation check
To determine whether the trauma script-driven imagery task was successful in eliciting stress and cravings, we performed t-tests comparing pre- to post-levels of negative affect and cannabis cravings. Negative affect significantly increased from pre- to post-trauma imagery (t (55)=5.17, p<.001), as did emotionality-related cannabis cravings (t (55)=3.41, p<.001).
3.2. Treatment participation and dose
On average, participants in BBTI attended 2.04/4 treatment sessions; however, this value was influenced by 7 participants who were randomized to BBTI but did not attend any treatment sessions. Those who attended >1 session attended an average of 2.70/4 sessions, with the largest proportion (n=7) attending 3 sessions (36.8%), 6 (31.6%) attending all 4 sessions, 4 (21.1%) attending 2 sessions, and 2 (10.5%) attending only one session. For participants who did not complete all 4 treatment sessions, mean ISI scores during their final attended session ranged from 9.00 for those who dropped out after session 3 to 13.00 for those who dropped out after session 1. No association occurred between number of sessions attended and reductions in ISI from baseline to post (r=.12, p=.533).
3.3. Treatment credibility
Mean CEQ scores were higher in the BBTI group (M = 40.95, SD=7.29) than the control group (M=30.47, SD=14.52; t=−2.71, p=.013).
3.4. Main hypotheses
3.4.1. Insomnia symptoms.
Unconditional models revealed that linear change best fit the data. In ITT analyses, insomnia symptoms improved somewhat in both groups (Figure 2), but significantly more in the BBTI group (Mwithin-group Δ= −11.89, 95% CI −15.07, −8.70; Tables 2 and 3), even when the study included PTSD status at baseline as a covariate (Table 4). At month 3, those in the BBTI group scored about 4 points less on the ISI vs. the control (Cohen’s d=1.34, p=.001).
Figures 2a–d.
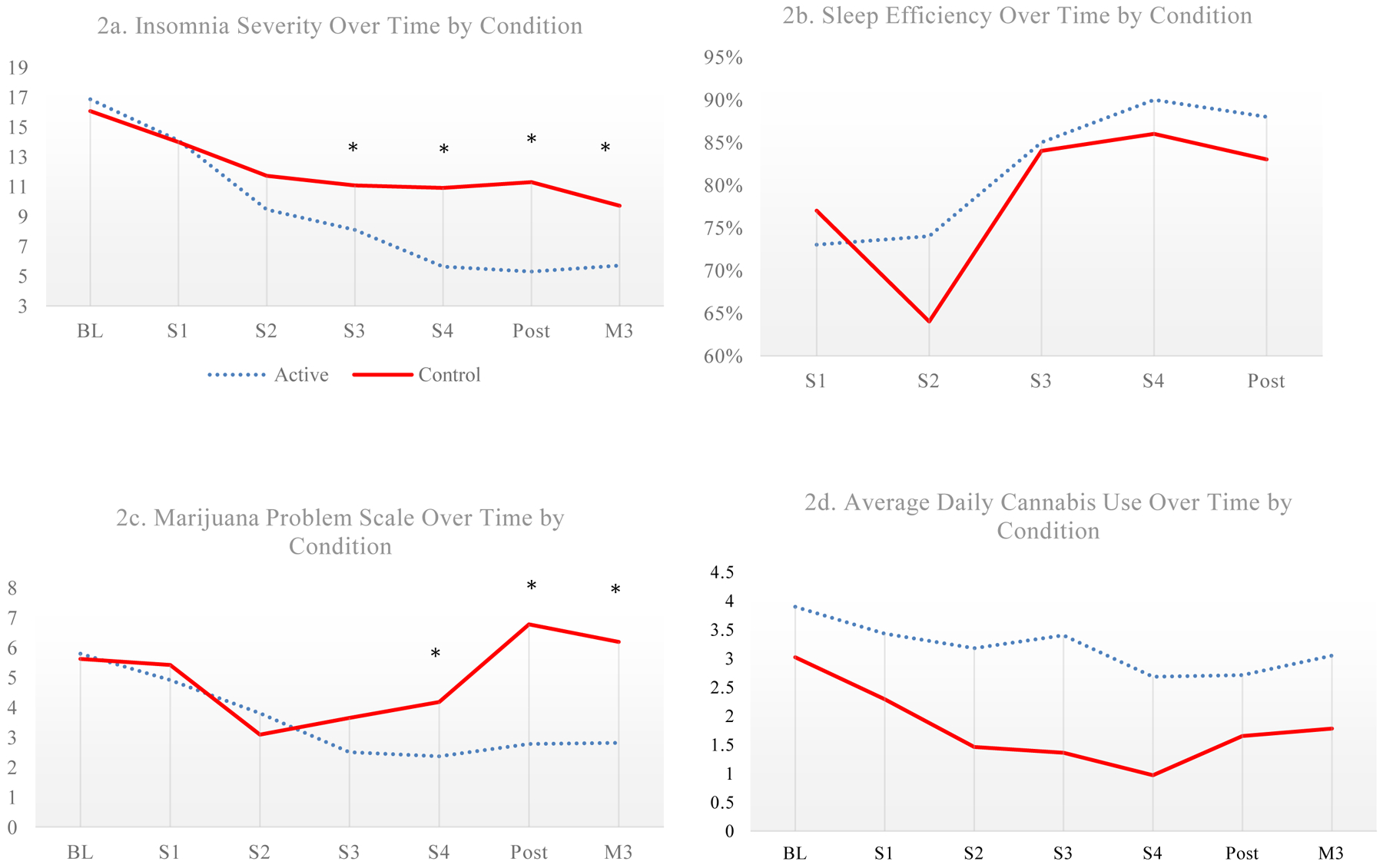
Insomnia severity, sleep efficiency, and marijuana problems scale over time by condition. Note. BL=Baseline, S1-4=Sessions 1–4; M3=Month 3. * = p < .05 in intent-to-treat multilevel models
Table 3.
Unadjusted intent-to-treat multilevel models of clinical outcomes by condition
| Insomnia Symptoms | Sleep Efficiency | Cannabis-Related Problems | Average Daily Cannabis Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | SE | B | 95% CI | SE | B | 95% CI | SE | B | 95% CI | SE | |
| Intercept (Baseline) | 15.99*** | 14.47, 17.52 | .78 | .72*** | .65, .78 | .04 | 5.53*** | 3.87, 7.19 | .85 | 3.45*** | 2.51, 2.66 | .48 |
| Condition | −.95 | −2.99, 1.10 | 1.04 | .01 | −.09, .11 | .05 | −.68 | −2.93, 1.56 | 1.15 | −.92 | −2.19, .36 | .65 |
| Session | −.16 | −2.51, −1.45 | .90 | .00 | −.01, .01 | .01 | .08 | −1.46, 1.63 | .79 | −.33 | −.77, .12 | .23 |
| Condition by session | 1.10** | .47, 1.74 | .32 | −.03 | −.07, .01 | .02 | .65* | .14, 1.15 | .26 | .07 | −.08, .22 | .08 |
Note.
p<.05;
p<.01;
p<.001.
Treatment condition (0=Brief Behavioral Treatment for Insomnia, 1=Waitlist Control).
SE=standard error.
Table 4.
Adjusted intent-to-treat multilevel models of clinical outcomes by condition
| Insomnia Symptoms | Sleep Efficiency | Cannabis-Related Problems | Average Daily Cannabis Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | SE | B | 95% CI | SE | B | 95% CI | SE | B | 95% CI | SE | |
| Intercept (Baseline) | 15.51*** | 14.00, 17.02 | .77 | .73*** | .67, .79 | .03 | 5.53*** | 3.82, 7.29 | .89 | 3.35*** | 2.41, 4.29 | .48 |
| PTSD diagnosis | 3.27* | .17, 6.37 | 1.58 | −.10 | −.24, .04 | .07 | −.18 | −3.20, 2.85 | 1.54 | .85 | −1.03, 2.73 | .96 |
| Condition | −1.03 | −2.99, .93 | 1.00 | .02 | −.08, .12 | .05 | −.68 | −2.91, 1.55 | 1.14 | −.96 | −2.22, .29 | .64 |
| Session | −.17 | −1.87, 1.54 | 1.10 | .00 | −.01, .01 | .00 | .08 | −1.46, 1.62 | .79 | −.30 | −.72, .12 | .22 |
| Condition by session | 1.12*** | .50, 1.74 | .32 | −.03 | −.07, .01 | .02 | .65* | .13, 1.15 | .26 | .08 | −07, .23 | .08 |
Note.
p<.05;
p<.01;
p<.001.
Treatment condition (0=Brief Behavioral Treatment for Insomnia, 1=Waitlist Control).
SE=standard error. Models are adjusted for Baseline posttraumatic stress disorder (PTSD) diagnostic status.
3.4.2. Sleep efficiency.
SE improved in both the BBTI (Mwithin-group Δ=.30, 95% CI 4.01, .00) and control groups, but no significant differences by condition occurred (Tables 3 and 4).
3.4.3. Cannabis-related problems.
Cannabis problems reduced for BBTI but worsened in the control. Those completing BBTI had reduced cannabis-related problems over time, even after adjusting for PTSD diagnoses (Mwithin-group Δ= −3.71, 95% CI −5.11, −2.30; Tables 3 and 4). At month 3, BBTI participants reported approximately 4 points less on the MPS (Cohen’s d=.75, p=.045) vs. the control.
3.4.4. Cannabis use frequency.
Average daily cannabis use did not differ between the active and control groups. Both groups’ cannabis use decreased, but not significantly (p=.152).2
3.4.4. Cravings.
Those completing BBTI had reduced emotionality craving reactivity to trauma script imagery from baseline to post vs. control (B=2.96, SE=.89, p=.001), including after covarying for PTSD (B=.59, SE=.12, p<.001), with large effects (Cohen’s d=1.02).
4. Discussion
Findings provide tentative evidence that BBTI may be efficacious among trauma-exposed cannabis users with elevated insomnia symptoms in reducing not only insomnia symptoms and cannabis-related problems over three months post-treatment, but also cannabis cravings one week post-treatment. First, as expected, BBTI had high levels of self-reported acceptability, which were higher than the waitlist control and prior depression treatment studies (De Graaf et al., 2009). ITT multilevel modeling analyses indicated that BBTI was more efficacious than waitlist control in reducing insomnia symptoms with large effects that participants maintained three months post-treatment at levels corresponding to no clinically significant insomnia symptoms. This is consistent with prior research (Buysse et al., 2011; Germain et al., 2007), but, to our knowledge, this study is the first to provide preliminary evidence that BBTI is potentially efficacious in an actively substance-using sample. This finding is noteworthy because research often assumes that behavioral therapy is not as effective among substance active users, or that SUD must be addressed prior to other treatments (Bujarski et al., 2016). Participants also rated BBTI as credible, thus it may fit within patient preferences (Fucito et al., 2015). However, results must be replicated in larger and more diverse samples with improved follow-up rates prior to drawing firm conclusions.
Second, we observed that BBTI was associated with reduced cannabis-related problems, with medium to large effects maintained three months after treatment. This finding is consistent with one uncontrolled trial (Bootzin & Stevens, 2005) and one small RCT (Babson et al., 2015). Further research should continue to explore whether treating insomnia symptoms may improve emotional processes and potentially lead to reductions in cannabis-related problems using well-powered designs that enable temporal mediation analyses. However, results from this initial RCT are promising and suggest the value of future research in this area, as there are few efficacious interventions for CUD, and many individuals with CUD may be receptive to insomnia treatment (Fucito et al., 2015).
Third, our preliminary findings provided initial evidence that individuals in the BBTI condition reduced emotionality-related cravings in response to trauma imagery. This finding is consistent with research suggesting that poor sleep may result in increased cravings (Freeman & Gottfredson, 2018). Further, these cravings may be motivated by a desire to reduce negative emotions associated with trauma cues. This idea is in line with research suggesting that poor sleep interferes with emotional functioning in response to environmental challenges (Minkel et al., 2012). Thus, pending replication in a large sample, particularly one using mechanistic mediation models, improving sleep may confer resilience to distress and cravings that trauma cues evoke, and potentially reduce cannabis use to cope with PTSD symptoms.
Contrary to our hypothesis, we found no effect of BBTI on SE in this study. This finding could be due to power as 1) there were fewer sleep diary time points (i.e., 5 sleep diary time points vs. 7 self-report time points because sleep diaries were not available at baseline or month 3), and 2) relatively fewer participants completed sleep diaries vs. self-report measures in-session (Table 2). Thus, our sample size may not have allowed us to detect the often relatively smaller effects of insomnia treatment on SE vs. global retrospective perceptions of sleep (Germain et al., 2006). Future research should test this in an adequately powered sample. Similarly, the study found no effect of BBTI on cannabis use frequency. Both groups reduced cannabis use, though not significantly, and with no between-group differences. BBTI may not directly impact use per se, but rather use-related problems, as poor sleep may be associated with risky or problematic use vs. any use. Also, any effects of BBTI on cannabis use frequency could possibly be mediated by insomnia symptoms vs. having direct effects, or that use patterns may be more complex than can be detected by only daily use (e.g., trajectories of use, use by time of day, or use related to negative affect, but not overall use). Future research should continue to examine whether BBTI impacts cannabis use frequency. Ecological momentary assessment designs may be particularly valuable to collect more accurate prospective data on use, timing and type of use, and whether use is related to previous or same day’s sleep (Shiffman, 2009).
The current preliminary results are in line with the idea that insomnia symptoms are an etiological factor in CUD (Babson & Bonn-Miller, 2014), and this study provides initial evidence of the potentially influential role of insomnia symptoms in cannabis-related problems, and cravings to use cannabis to regulate PTSD symptoms. However, results must be replicated with a well-powered RCT, including a temporal mediational design. Findings are consistent with the role of poor sleep in increased negative emotional reactivity to stressors (Minkel et al., 2012), reduced distress tolerance, and increased avoidance of distress (Short et al., 2016), all of which may contribute to using substances to regulate one’s negative emotions. With the support of future clinical trials, the current results may also have clinical implications. Pending replication, BBTI may be efficacious for reducing insomnia symptoms among cannabis users. Clinicians should assess insomnia symptoms among cannabis users, and potentially consider BBTI as part of a comprehensive approach for improving quality of life and reducing problematic substance use.
Findings must be viewed in the context of study limitations and the nature of this pilot study. First, the study had attrition, potentially due to the sample’s nature, as active substance users can be difficult to retain (Bootzin & Stevens, 2005), and PTSD symptoms may lead to avoidance of trauma-related appointments. Indeed, those with PTSD were less likely to attend month 3. We found this attrition in the treatment group as well, meaning BBTI had poor adherence, and not all participants in the active condition received a full dose of BBTI. However, even one or two sessions of BBTI may be sufficient for improvements as individuals may learn the critical skills during session 1. The majority of attrition occurred prior to treatment initiation, thus attrition does not necessarily speak to treatment acceptability and tolerability. Further, our rate of treatment attendance was in line with a prior study of adolescent substance use outpatients who completed a sleep treatment in which ~40% completed 4/6 sessions of 7 (Bootzin & Stevens, 2005).
Future research may consider only recruiting individuals with a full diagnosis of insomnia disorder, as this population may be more motivated to engage in sleep treatment, randomizing participants after they have confirmed interest and arrived for treatment sessions. Encouragingly, all analyses were ITT, accounting for dropout, and results were still encouraging. Second, the sample was unique and comprised undergraduates and volunteers, thus results may not generalize to all cannabis users. It remains to be seen whether such an approach would be effective for other individuals with SUD. Third, many theoretical pathways exist through which improving sleep may reduce problematic use that we did not test, and future research should incorporate these alternate mechanisms. For example, a large proportion of the sample reported cannabis use to help sleep, which could be a mechanism through which improving sleep would reduce cannabis-related problems. Fourth, the current pilot study used a waitlist control. Future research should use an active comparator to rule out expectancy effects. Fifth, this is a small pilot study and results should be replicated in a larger study utilizing a temporal mediational design. Sixth, although the CSD is the gold-standard self-report prospective insomnia measure (Carney et al., 2012), participants completed it on paper; thus, we are unable to confirm if they were completed upon awakening as directed, or retrospectively. Similarly, future research should ensure the ability to conduct a multimodal assessment of common sleep disturbances in PTSD, such as nightmares (Neylan et al., 1998), to determine their specific roles in cannabis-related problems and whether they are addressed by BBTI.
In summary, the current study provides preliminary evidence that BBTI is a potentially efficacious and credible intervention for reducing insomnia symptoms and cannabis-related problems among exposed cannabis users. Future studies should replicate the efficacy of BBTI in a larger sample and further explore mechanisms of how improving sleep may lead to reductions in problematic cannabis use.
Highlights.
The current pilot RCT tested Brief Behavioral Therapy for Insomnia (BBTI)
BBTI was tested among 56 trauma-exposed cannabis using young adults
BBTI may be efficacious in reducing insomnia symptoms compared to waitlist control
BBTI potentially reduces cannabis-related problems and cravings
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA, Grant no. F31 DA044689-01). The authors would like to thank the research assistants involved in the current study: Alexa Raudales, Emmalina Mozier, Allysa Quick, and Savannah Woller.
Declaration of Interest:
This work was supported by the National Institute on Drug Abuse (NIDA, Grant no. F31 DA044689-01). NIDA had no role in the study design, collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication. There are no other conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There were no significant differences using t-tests between undergraduate and community participants in terms of condition (p = .975), clinical characteristics (ps > .180), demographic characteristics (ps > 280), with the exception of age (undergraduates being younger age on average; p = .006).
Average daily cannabis use was calculated at each relevant timepoint to match other analyses. However, we also conducted analyses without averaging use and instead using each day as a timepoint during days 1–35 post-treatment in which daily cannabis use was continuously collected. This analysis resulted in consistent results as the results presented using daily average cannabis use at each timepoint.
References
- Babson KA, Badour CL, Feldner MT, & Bunaciu L (2012). The relationship of sleep quality and PTSD to anxious reactivity from idiographic traumatic event script-driven imagery. Journal of Traumatic Stress, 25(5), 503–510. [DOI] [PubMed] [Google Scholar]
- Babson KA, Boden MT, & Bonn-Miller MO (2013). The impact of perceived sleep quality and sleep efficiency/duration on cannabis use during a self-guided quit attempt. Addictive behaviors, 38(11), 2707–2713. [DOI] [PubMed] [Google Scholar]
- Babson KA, & Bonn-Miller MO (2014). Sleep disturbances: Implications for cannabis use, cannabis use cessation, and cannabis use treatment. Current Addiction Reports, 1(2), 109–114. [Google Scholar]
- Babson KA, Ramo DE, Baldini L, Vandrey R, & Bonn-Miller MO (2015). Mobile app-delivered cognitive behavioral therapy for insomnia: feasibility and initial efficacy among veterans with cannabis use disorders. JMIR research protocols, 4(3), e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Moos RH, Boden MT, Long WR, Kimerling R, & Trafton JA (2015). The impact of posttraumatic stress disorder on cannabis quit success. The American journal of drug and alcohol abuse, 41(4), 339–344. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, & Stevens SJ (2005). Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review, 25(5), 629–644. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Silgado J, & Schmidt NB (2011). Marijuana craving during a public speaking challenge: Understanding marijuana use vulnerability among women and those with social anxiety disorder. Journal of Behavior Therapy and Experimental Psychiatry, 42(1), 104–110. doi: 10.1016/j.jbtep.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski SJ, Galang JN, Short NA, Trafton JA, Gifford EV, Kimerling R, … Bonn-Miller MO (2016). Cannabis use disorder treatment barriers and facilitators among veterans with PTSD. Psychology of Addictive Behaviors, 30(1), 73–81. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, … Reynolds CF (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of internal medicine, 171(10), 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep, 35(2), 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Kuna ST, Zaharakis N, O’Brien CP, Kampman KM, & Oslin D (2010). Covariates of craving in actively drinking alcoholics. The American Journal on Addictions, 19(5), 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Vandrey RG, He S, & Stein MD (2018). Sleep management among patients with substance use disorders. Medical Clinics, 102(4), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F, Abdullah HR, & Liao P (2016). STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest, 149(3), 631–638. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Kuntsche E, Levitt A, Barber LL, & Wolf S (2016). Motivational models of substance use: A review of theory and research on motives for using alcohol, marijuana, and tobacco. In Sher KJ (Ed.), The Oxford Handbook of Substance Use and Substance Use Disorders: Two-Volume Set (Vol. 1, pp. 375–420). Chicago, IL: Oxford University Press. [Google Scholar]
- Cunningham JE, & Shapiro CM (2018). Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: A systematic review. Journal of Psychosomatic Research, 106, 1–12. [DOI] [PubMed] [Google Scholar]
- De Graaf L, Gerhards S, Arntz A, Riper H, Metsemakers J, Evers S, … Huibers M (2009). Clinical effectiveness of online computerised cognitive–behavioural therapy without support for depression in primary care: randomised trial. The British Journal of Psychiatry, 195(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31(2), 73–86. doi: 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- Drazdowski TK, Kliewer WL, & Marzell M (2019). College students’ using marijuana to sleep relates to frequency, problematic use, and sleep problems. Journal of American College Health, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods, 14(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Foa E, Cashman L, Jaycox L, & Perry K (1996). The validation of a self-report measure of PTSD: The Posttraumatic Diagnostic Scale (PDS). Philadelphia: Medical College of Pennsylvania & Hahnemann University. [Google Scholar]
- Foa EB, Cashman L, Jaycox L, & Perry K (1997). The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological assessment, 9(4), 445–451. [Google Scholar]
- Freeman LK, & Gottfredson NC (2018). Using ecological momentary assessment to assess the temporal relationship between sleep quality and cravings in individuals recovering from substance use disorders. Addictive Behaviors, 83, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, DeMartini KS, Hanrahan TH, Whittemore R, Yaggi HK, & Redeker NS (2015). Perceptions of heavy-drinking college students about a sleep and alcohol health intervention. Behavioral Sleep Medicine, 13(5), 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, Monk TH, & Buysse DJ (2006). Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine, 2(4), 403–406. [PubMed] [Google Scholar]
- Germain A, Shear MK, Hall M, & Buysse DJ (2007). Effects of a brief behavioral treatment for PTSD-related sleep disturbances: a pilot study. Behaviour Research and Therapy, 45(3), 627–632. [DOI] [PubMed] [Google Scholar]
- Goodhines PA, Gellis LA, Ansell EB, & Park A (2019). Cannabis and alcohol use for sleep aid: A daily diary investigation. Health Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo S-S, Hu P, & Walker MP (2011). Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. Journal of Neuroscience, 31(12), 4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, & Gorelick DA (2009). Reliability and Validity of a Short Form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence, 102, 35–40. doi: 10.1016/j.drugalcdep.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, & Ressler KJ (2010). Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depression and Anxiety, 27(12), 1077–1086. doi: 10.1002/da.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, … Heilig M (2015). Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: Behavioral and physiological outcomes. Addiction Biology, 20(4), 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai E, & Buysse DJ (2008). Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Medicine Clinics, 3(2), 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, & LeBourgeois MK (2013). Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion, 27(3), 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, … Dinges DF (2012). Sleep deprivation and stressors: Evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion, 12(5), 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, … Ritenour AM (1994). The Pittsburgh sleep diary. Journal of Sleep Research, 3(2), 111–120. [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, & Ivers H (2011). The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Marmar CR, Metzler TJ, Weiss DS, Zatzick DF, Delucchi KL, & Schoenfeld FB (1998). Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Sleep(7). [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, & Herz LR (1993). Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology, 102(1), 152. [DOI] [PubMed] [Google Scholar]
- Orr SP, & Roth WT (2000). Psychophysiological assessment: Clinical applications for PTSD. Journal of Affective Disorders, 61(3), 225–240. [DOI] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, & Bisson JI (2015). Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: a systematic review and meta-analysis. Clinical psychology review, 38, 25–38. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Norr AM, Allan NP, Raines AM, & Capron DW (2017). A randomized clinical trial targeting anxiety sensitivity for patients with suicidal ideation. Journal of Consulting and Clinical Psychology, 85(6), 596. [DOI] [PubMed] [Google Scholar]
- Shiffman S (2009). Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment, 21(4), 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NA, Babson KA, Schmidt NB, Knight CB, Johnson J, & Bonn-Miller MO (2016). Sleep and affective functioning: Examining the association between sleep quality and distress tolerance among veterans. Personality and Individual Differences, 90, 247–253. [Google Scholar]
- Short NA, & Schmidt NB (2017). A Multimethod Examination of the Effect of Insomnia Symptoms on Anxious Responding to a Social Stressor. Behavior Therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back Measuring alcohol consumption (pp. 41–72). Chicago, IL: Humana Press. [Google Scholar]
- Stephens RS, Roffman RA, & Curtin L (2000). Comparison of extended versus brief treatments for marijuana use. Journal of Consulting and Clinical Psychology, 68(5), 898–908. doi: 10.1037/0022-006X.68.5.898 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2015). Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD. [Google Scholar]
- Troxel WM, Germain A, & Buysse DJ (2012). Clinical management of insomnia with Brief Behavioral Treatment (BBTI). Behavioral Sleep Medicine, 10(4), 266–279. doi: 10.1080/15402002.2011.607200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1994). The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. The University of Iowa: Ames. [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, & Zucker RA (2004). Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research, 28(4), 578–587. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, & Walker MP (2007). The human emotional brain without sleep--a prefrontal amygdala disconnect. Current Biology, 17(20), R877–878. doi: 10.1016/j.cub.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, & Lavie P (2005). The effects of sleep loss on medical residents’ emotional reactions to work events: a cognitive-energy model. Sleep, 28(1), 47–54. [DOI] [PubMed] [Google Scholar]


