Abstract
Background:
Androgen deprivation therapy (ADT) may impact cognitive function in men with prostate cancer (PCa). This study examined whether insomnia symptoms mediate the relationship between ADT and perceived cognitive function and whether depressive symptoms, fatigue severity, and physical activity moderate the strength of this relationship.
Methods:
This was a prospective study compared in ADT recipients (n=83) who were matched to control patients with PCa not on ADT (n=92) and controls with no history of cancer (n=112) over a 2-year follow-up period. Perceived cognitive function and satisfaction was assessed using the Everyday Cognition Scale. Insomnia was assessed using the Insomnia Severity Index. Multilevel mediation analyses were conducted to estimate the indirect effect of ADT on perceived cognitive function through insomnia symptoms. Exploratory moderated mediation analyses assessed whether the indirect effect of ADT on perceived cognitive function through insomnia symptoms was dependent on levels of fatigue, depression, or physical activity.
Results:
Insomnia symptoms significantly mediated the relationship between receipt of ADT on perceived cognitive function (p < .001) and satisfaction with cognition (p < .001), after controlling for comorbidities. Men with greater fatigue had a more pronounced association of ADT with insomnia severity. Men with greater depressive symptoms had a stronger association between insomnia severity and worse perceived cognitive function. Physical activity was not a significant moderator of the relationship between ADT and perceived cognitive function.
Conclusions:
Insomnia influenced the relationship between ADT and perceived cognitive abilities. Interventions to address insomnia, fatigue, and depression may improve perceived cognitive function.
Keywords: prostate cancer, insomnia symptoms, androgen deprivation therapy, cognitive function, depression, fatigue, physical activity
Precis:
Insomnia symptoms significantly mediated the relationship between receipt of ADT and subjective cognition, after controlling for comorbidities, which was moderated by fatigue and depression. Interventions to improve sleep, fatigue, and/or depression may also have a secondary effect of improving self-appraisal of cognitive function.
Background
There is conflicting research on the impact of androgen deprivation therapy (ADT) on cognitive impairment in men with prostate cancer. Prospective research reported that men treated with ADT were 1.7 and 2.4 times more likely to demonstrate impaired cognitive performance at 6 and 12 months after initiating ADT than controls.1 Impairment was also objectively demonstrated in a prospective study of 12 months of continuous treatment with ADT.2 Retrospective studies suggest that ADT recipients are at an increased risk of Alzheimer’s disease3 and all-cause dementia.4 However, other studies have demonstrated no subjective or objective impairment after 6, 12, or 36 months of ADT.5,6 These discrepancies may be due in part to differences in measurement and study methodology, but the lack of consensus does not help oncologists educate their patients about detecting and managing those side effects.
While the evidence supports a possible link between ADT and cognitive impairments, it is not entirely clear what factors may influence this relationship. Once potentially important factor is insomnia symptoms. ADT is known to raise risk of insomnia for PCa patients.7 One study documented symptoms of insomnia in 25–39% of PCa patients on ADT.8 In a cross-sectional study assessed sleep and daily functioning in 60 PCa patients on ADT using self-report and objective sleep measures,9 men took > 30 minutes to fall asleep and were awake for roughly 30 minutes during the night, resulting in only ~6 hours of sleep per night. Similarly, a prospective study comparing ADT recipients and controls not treated with ADT found higher rates of subjectively- and objectively-assessed sleep disturbance among ADT recipients.10
Neuropsychological testing has been criticized for having low ecological validity given that performance on objective tests usually falls within the range of normal to mild cognitive deficits and does not correlate well with the patients’ report of a much greater impact of cancer on cognitive functioning.11 Accordingly, Savard and Ganz have argued compellingly that subjective measures of cognitive functioning should be considered the primary indicator of cognitive impairments in cancer patients.12 For this reason, we were more interested in the relationships between perception of cognitive function, as opposed to performance on cognitive testing, and reported insomnia severity.
We previously reported that ADT recipients had worse objective cognitive function than controls.1 The objective of these secondary analyses was to examine whether the relationship between ADT and perceived cognitive function could be partially attributed to greater insomnia symptoms in a prospective sample. We hypothesized that insomnia symptoms would mediate the relationship between ADT and perceived cognitive function. Given the associations with insomnia and cognition, we also explored whether depressive symptoms, fatigue, and physical activity would moderate the strength of this relationship.
Methods
Participants
This study reports secondary analyses of data from a longitudinal study of QoL in men receiving ADT for PCa.1 In addition to men treated with ADT for PCa, two matched control groups were recruited: men with PCa who were treated with surgery only and men with no history of PCa. All participants were required to: be ≥ 18 years of age, able to speak and read English, have a ≥ 6th grade education, have no history of stroke, have no demonstrated impaired mental status, have no visual, auditory, or psychiatric conditions that would impede participation.
Men treated with ADT for PCa (ADT+ group) were also required to: be diagnosed with non-metastatic or asymptomatic metastatic PCa, be scheduled to start ADT (or have started ADT within the past month) for ≥ 6 months, have no history of brain cancer or cranial irradiation, and have no ADT treatment in the previous 12 months or anti-androgen in the previous 6 months. Controls with PCa not treated with ADT (ADT− group) were required to: be diagnosed with non-metastatic PCa, have undergone prostatectomy but no other treatment for PCa, have no history of recurrence, and not be receiving testosterone supplementation. Controls with no history of PCa (CA− group) were required to: have no history of cancer except non-melanoma skin cancer and not be receiving testosterone supplementation. PCa patients not treated with ADT were matched to ADT recipients on time since diagnosis. Because of associations between age and education with cognitive function, men in both control groups were matched to ADT recipients on age (within 5 years) and educational level (≤ 12 years, 13 – 16 years, ≥ 17 years).
Procedure
ADT recipients completed baseline self-report questionnaires before or within one month of starting ADT as well as 6, 12, and 24 months later. Matched controls completed assessments at similar intervals. Participants were recruited between September 2008 and October 2013. Written informed consent was obtained from all participants, and participants were paid $80 at each assessment. This study was approved by the University of South Florida Institutional Review Board.
Measures
Age, race, and ethnicity were assessed at baseline via self-report. A self-report version of the Charlson Comorbidities Index13 was used to assess comorbidities at baseline. Baseline Gleason score, height, and weight were assessed via medical chart review. Perceived cognitive function was assessed using the Everyday Cognition Scale,14 which provides an assessment of the change in one’s overall subjective cognitive function relative to 10 years ago on a scale of 1 (better or no change) to 4 (consistently much worse), with higher scores representing worse perceived cognitive function. This scale also provides a measure of satisfaction with one’s current cognitive function on a scale of 1 (completely dissatisfied) to 4 (completely satisfied), with higher scores representing better satisfaction with cognitive function. The Everyday Cognition Scale was chosen in part because it is widely used in the literature of subjective cognitive impairment15 does not exhibit ceiling effects among individuals with mild cognitive impairment. This enables the measure to be sensitive to change over time among individuals with mild cognitive decline.16
Insomnia was assessed using the Insomnia Severity Index,17 with higher scores reflecting greater insomnia severity. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale,18 with higher scores indicating greater depressive symptoms. Fatigue severity was assessed using the four-item severity scale Fatigue Severity Inventory,19 with higher scores reflecting worse fatigue. Self-reported leisure-time physical activity was assessed using the Godin’s Leisure-Time Exercise Questionnaire.20 Activity during a typical was reported as the number of times strenuous, moderate, or light activity was completed for more than 15 minutes. Higher scores reflect greater intensity and time spent in physical activity.
Statistical Analyses
Descriptive statistics were first calculated for demographic and clinical factors. T-tests, χ2 tests, and Fisher’s exact tests were conducted, as appropriate, to identify demographic and clinical factors that differed between groups. We then used linear mixed model analyses to determine whether the two control groups differed at baseline and in change over time on cognitive function and satisfaction with cognitive function. Next, multilevel mediation analyses were conducted to test the theoretical model shown in Figure 1. This model includes some factors that do not change over time (e.g., ADT recipient group) and others that do change over time (e.g., sleep disturbance, cognitive function). Thus, we used multilevel mediation that appropriately treats these unchanging factors as level-2 factors and time-varying factors as level-1 factors. We used the MLmed macro21–23 in SPSS.24 Results provided an estimate of the indirect effect of receipt of ADT on perceived cognitive function through its influence on insomnia symptoms with Monte Carlo confidence intervals. Because ADT is a level-2 (i.e., between-person) variable, it can only explain between-person variability. Thus, all indirect effects of ADT presented are between-person indirect effects. The indirect effect estimates with 95% confidence intervals that do not include zero were deemed statistically significant at α = .05. Lastly, exploratory multilevel moderated mediation analyses were conducted to determine whether the indirect effect of receipt of ADT on perceived cognitive function through insomnia symptoms were dependent on levels of depression, fatigue, or physical activity. The moderated mediation analyses yield a between-person index of moderated mediation23 that, as with the estimate of the indirect effect, is accompanied by a 95% confidence interval. Indices of moderated mediation with 95% confidence intervals that do not include zero were deemed statistically significant at α = .05. All multilevel mediation models were re-run after log-transforming mediator and outcome variables to account for skewed variables. Lastly, we standardized the outcome variables (i.e., perceived cognitive function and satisfaction with cognitive function) to obtain standardized indirect effect estimates.
Figure 1.

Conceptual model showing ADT influencing cognitive function indirectly through insomnia symptoms as well as three potential moderators of this indirect effect.
Results
Demographic and Clinical Characteristics
Participant flow is presented in Supplementary Figures 1–3. Sociodemographic and clinical characteristics of the sample at baseline are presented in Table 1. ADT recipients reported more comorbidities than PCa patients not treated with ADT (p = .002). Groups did not differ on age, BMI, time since diagnosis, education, race, or ethnicity (ps ≥ .06). Thus, in subsequent analyses comparing groups, we controlled for comorbidities only. As expected, medical record reviews demonstrated that ADT recipients had higher Gleason scores and were less likely to have been treated with surgery than PCa patients not treated with ADT (p < .0001). Because ADT is typically prescribed for patients with more advanced disease, Gleason score and treatment with surgery were not included as covariates. Descriptive means and SDs for symptoms of insomnia, depression, fatigue, and physical activity are included in Figure 2.
Table 1.
Demographic and Clinical Characteristics of the Sample
| ADT+ n=83 | ADT− n=92 | CA− n=112 | ADT+ vs. ADT− | ADT+ vs. CA− | ADT+ vs. All Controls | |
|---|---|---|---|---|---|---|
| Characteristic | Mean (SD) | Mean (SD) | Mean (SD) | p | p | p |
| Age, years | 67.92 (8.72) | 67.82 (7.46) | 68.41 (8.26) | .93 | .69 | .84 |
| Comorbidity index score | 2.87 (1.09) | 2.40 (0.83) | 2.72 (1.05) | .002 | .35 | .04 |
| Body Mass Index | 28.84 (4.74) | 29.39 (4.13) | 29.55 (5.16) | .47 | .39 | .36 |
| ≥ 17 | 10 (12%) | 17 (18%) | 15 (13%) | |||
| Missing | 1 (1%) | 0 (0%) | 0 (0%) | |||
| Missing | 1 (1%) | 0 (0%) | 0 (0%) | |||
| Missing | 4 (5%) | 8 (9%) | - | |||
| Treated with surgery (n%) | 20 (24%) | 66 (72%) | - | <.0001 | - | - |
| Years since diagnosis | 3.77 (4.97) | 4.60 (4.47) | - | .26 | - | - |
Note: ADT+: prostate cancer patients treated with androgen deprivation therapy. ADT−: control prostate cancer patients not treated with androgen deprivation therapy. CA−: cancer-free controls.
Figure 2.

Changes over time in (A) cognitive function, (B) satisfaction with cognitive function, (C–E) proposed moderators, and (F) insomnia.
Note: ADT = prostate cancer patients treated with androgen deprivation therapy. Standard error bars are shown.
Comparing Control Groups
Consistent with previous studies,1,25–28 we first compared the two groups of patients not treated with ADT on perceived cognitive function and cognitive satisfaction. Supplementary Figure 4 displays change over time in cognitive function and proposed moderators. The two matched control groups did not differ on baseline perceived cognitive function (d = .13, p = .41) or change over time in perceived cognitive function (d = .15, p = .26). Similarly, the two control groups did not differ on baseline satisfaction with cognitive function (d = .14, p = .31) or change over time in satisfaction with cognitive function (d = .19, p = .07). The two control groups were therefore combined into a single control group in order to reduce the number of statistical tests performed and to improve statistical power.
Indirect Effect of Insomnia Severity
Figure 1 shows the study conceptual model. Analyses using log-transformed and untransformed were very similar. We present analyses with raw values below to better facilitate interpretation of results.
We began by examining whether the impact of ADT on perceived cognitive function is partly attributable to insomnia severity. Figure 3 shows the between-person components of multilevel mediation models tested to determine whether insomnia severity mediated the relationship between ADT and worse perceived cognitive function (panel A) and between receipt of ADT and lower satisfaction with cognitive function (panel B). The association between receipt of ADT and worse perceived cognitive function was partly attributable to insomnia severity, as demonstrated by a significant indirect effect (0.0747, 95% CI = 0.0432 to 0.1115). This indirect effect value indicates that ADT recipients had perceived cognitive function scores 0.07 units higher as a function of the association between ADT and worse insomnia as well as the association between worse insomnia and lower perceived cognitive function. The standardized indirect effect estimate indicated that perceived cognitive function scores of ADT recipients were 0.15 standard deviation units lower (i.e., worse) than scores of controls by way of insomnia severity. In assessments when participants had particularly severe insomnia symptoms (relative to their other assessments) they also tended to have worse perceived cognitive function (relative to their other assessments), as demonstrated by a significant within-person effect of insomnia severity on perceived cognitive function (0.0054, SE = 0.0021).
Figure 3.
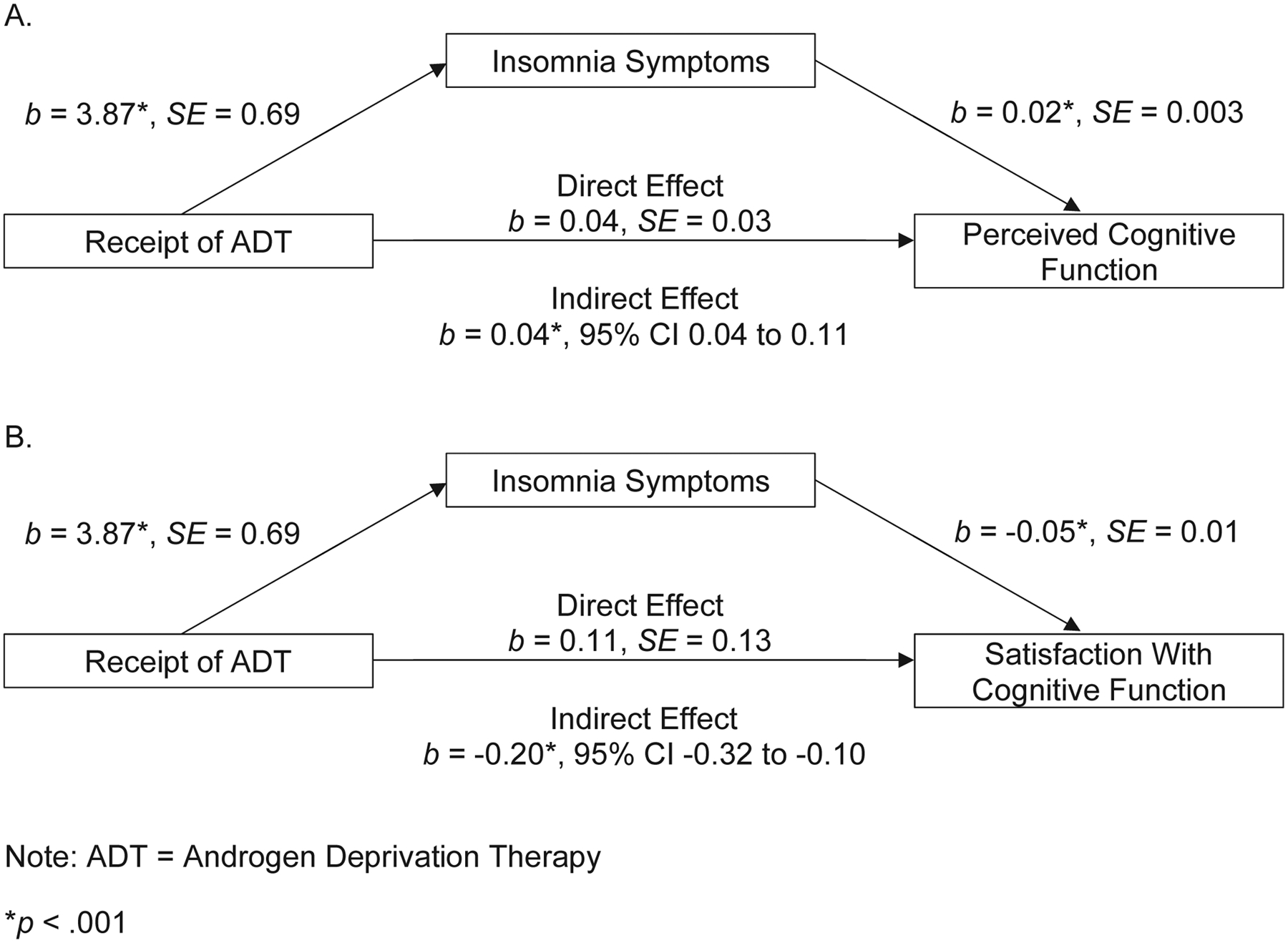
Between-person components of multilevel mediation models testing the effects of ADT on perceived cognitive function (A) and satisfaction with cognitive function (B) through its influence on insomnia symptoms.
Note: The direct effects of ADT on perceived cognitive function and satisfaction with cognitive function are no longer significant when insomnia symptoms are added as a mediator.
The association between receipt of ADT and worse cognitive satisfaction was also partly attributable to insomnia severity, as demonstrated by a significant indirect effect (−0.1995, 95% CI = −0.3203 to −0.0994). The standardized indirect effect estimate indicated that cognitive satisfaction scores of ADT recipients were 0.15 standard deviation units lower (i.e., worse) than scores of controls by way of insomnia severity. No significant within-person effect of insomnia severity on cognitive satisfaction was found (0.0067, SE = 0.0142).
Moderators of the Indirect Effect of Insomnia Severity
Moderator analyses examined whether the role of insomnia severity in explaining the impact of ADT on perceived cognitive function was influenced by other factors. We examined whether this relationship differed as a function of participants’ depressive symptoms, fatigue, and physical activity. These models, displayed conceptually in Figure 1 and reported in Table 2, examined each potential moderator’s influence on the relationship between ADT and insomnia severity (i.e., first-stage moderation) and on the relationship between insomnia severity and perceived cognitive function (i.e., second-stage moderation).
Table 2.
Influence of receiving ADT on cognitive function through insomnia severity
| Indirect Effect through Insomnia Severity | Moderation of Indirect Effect (First-Stage) | Moderation of Indirect Effect (Second-Stage) | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Perceived Cognitive Function | 0.0747 | 0.0432 to 0.1115 | - | - | ||
| Moderator: Depressive symptoms | - | 0.0015 | −0.0001 to 0.0033 | 0.0031 | 0.0015 to 0.0050 | |
| Moderator: Fatigue | - | 0.0163 | 0.0084 to 0.0254 | −0.0025 | −0.0089 to 0.0034 | |
| Moderator: Physical activity | - | −0.0003 | −0.0007 to 0.0001 | 0.0004 | 0.0000 to 0.0009 | |
| Satisfaction with Cognitive Function | −0.1995 | −0.3203 to −0.0994 | - | - | ||
| Moderator: Depressive symptoms | - | −0.0041 | −0.0092 to 0.0002 | 0.0003 | −0.0065 to 0.0071 | |
| Moderator: Fatigue | - | −0.0436 | −0.0723 to −0.0202 | 0.0254 | −0.0056 to 0.0598 | |
| Moderator: Physical activity | - | 0.0008 | −0.0002 to 0.0020 | −0.0010 | −0.0034 to 0.0012 | |
Note: First-stage moderation estimate describes the influence of the moderator (e.g., fatigue) on the relationship between receipt of ADT and insomnia severity. Second-stage moderation estimate describes the influence of the moderator on the relationship between insomnia severity and the outcome variable (e.g., perceived cognitive function, satisfaction with cognitive function. CI: confidence interval.
These analyses showed that those with greater fatigue had a more pronounced impact of ADT on insomnia severity, resulting in a stronger indirect effect of ADT on perceived cognitive function through insomnia severity (0.0163, 95% CI = 0.0084 to 0.0254). The stronger association between ADT and insomnia severity among men with greater fatigue also resulted in a stronger indirect effect of ADT on satisfaction with perceived cognitive function through insomnia severity (−0.0436, 95% CI = −0.0723 to −0.0202). Fatigue was not a significant moderator through its influence on the relationship between either insomnia severity and perceived cognitive function (−0.0025; 95% CI = −0.0089 to 0.0034) or satisfaction with cognitive function (0.0254; 95% CI = −0.0056 to 0.0598).
Men with greater depressive symptoms had a stronger association between insomnia severity and cognitive function, resulting in a stronger indirect effect of ADT on cognitive function through insomnia severity (0.0031, 95% CI = 0.002 to 0.005). Depression was not a significant moderator through its influence in the relationship between receipt of ADT and insomnia severity in a model predicting perceived cognitive function (0.0015; 95% CI = −0.0001 to 0.0033). Depression was not a significant moderator of the indirect of ADT on satisfaction with cognitive function through insomnia severity, either as a first-stage moderator (−0.0041; 95% CI = −0.0092 to 0.0002) or second-stage moderator (0.0003; 95% CI = −0.0065 to 0.0071).
Self-reported physical activity was not a significant first-stage moderator of the indirect effect of ADT on perceived cognitive function (−0.0003; 95% CI = −0.0007 to 0.0001) or satisfaction with cognitive function (0.0008; 95% CI = −0.0002 to 0.0020). Similarly, physical activity was not a significant second-stage moderator of the indirect effect of ADT on perceived cognitive function (0.0004; 95% CI = 0.0000 to 0.0009) or satisfaction with cognitive function (−0.0010; 95% CI = −0.0034 to 0.0012).
Discussion
In this prospective study, insomnia symptoms significantly mediated the relationship between receipt of ADT on perceived cognitive function and satisfaction with cognition, after controlling for comorbidities. This supports recent evidence placing sleep into the spotlight as a potential contributor to cognitive impairments in cancer patients.29 In a sample of 67 women with breast cancer, those with insomnia performed significantly worse on neuropsychological tests of episodic memory and executive function than those without insomnia.30 There is some prospective evidence suggesting that patients with insomnia are more likely to develop cognitive impairment.31 Further, the relationship we observed between ADT, insomnia, and perceived cognitive function, was significantly moderated depending on coexisting levels of fatigue and depression. Men with greater fatigue had a more pronounced association of ADT with insomnia severity, whereas men with greater depressive symptoms had a stronger association between insomnia severity and perceived cognitive function. This suggests that interventions to improve sleep, fatigue, and/or depression may also have a secondary effect of improving self-appraisal of cognitive function.
In order to provide interventions effectively, it is first necessary to identify those in greatest need. Guidelines recommend that symptoms of insomnia be screened for at regular intervals or at key transition in cancer care.32,33 Evidence suggests that existing screening tools including the Edmonton Symptom Assessment System34 can be modified to assess sleep concerns. Once identified, Cognitive Behavior Therapy for insomnia (CBT-I) is currently recommended as the first line treatment by the American College of Physicians35 and has strong evidence of efficacy in cancer survivors and the general population,36 and offers concomitant improvements in fatigue, anxiety, and depression,37 all of which have been associated with cognitive impairment. A recent systematic review and meta-analysis reported small to moderate effects of CBT-I on subjective measures of cognitive functioning,38 but larger studies with more robust methodology is needed to confirm these initial findings.
Physical activity was not a significant moderator of the relationship between ADT and perceived cognitive function. This is in contrast to research documenting the protective effects of physical activity on cognitive function in the general population.39 The inconsistency in findings on the impact of physical activity on cognitive function in men on ADT may have to do with the subjective way physical activity data were captured and/or the heterogeneity in research regarding how to appropriately classify cognitive impairment. Research examining the effect of physical activity on cognition in men with prostate cancer has yet to include a measure of sleep so it is unknown what role, if any, this would have played in the outcome.
Our findings should be considered in the context of several limitations. First, our study relied on self-report for our measure of cognitive function; however, there is evidence that subjective measures of cognitive function are more clinically useful than objective measures.12 Although we controlled for baseline comorbidities, we did not assess or control for change over time in comorbidities. We were unable to identify minimal clinically important difference scores for the outcome measures, limiting our ability to examine the clinical significance of these findings. Further, this was a secondary analysis of data collected as part of a longitudinal study of associations between ADT and cognitive function. This may have reduced our power to assess relationships among various moderators. The sample also had a limited representation from racially and ethnically diverse groups and the majority of the sample was college-educated. Lastly, we used self-reported leisure-time physical activity rather than more reliable objective measures of physical activity.
Conclusions
This is the first study to assess perceived cognitive function and insomnia symptoms in a cohort of men on ADT compared to healthy controls and prospectively observe change up to 2 years. The results suggest that symptoms of insomnia significantly impact perception of, and satisfaction with, cognitive function, a relationship that was differentially impacted by levels depression and fatigue. There are two obvious future directions given these findings. First, it seems relevant to examine whether early interventions to improve sleep, fatigue, and/or depression can prevent the development of perceived cognitive function. Second, it is also possible that interventions to improve sleep, fatigue, and/or depression may be effective treatments for cognitive impairment once it has developed.
Supplementary Material
Supplementary Figure 1. Participant flow among recipients of androgen deprivation therapy (ADT) for prostate cancer (ADT+ group).
Supplementary Figure 2. Participant flow among patients with prostate cancer not treated with androgen deprivation therapy (ADT; ADT− group).
Supplementary Figure 3. Participant flow among individuals with no history of cancer (CA− group).
Supplementary Figure 4. Change over time in cognitive function (A), insomnia (B), and proposed moderators (C, D, E) among only control group participants. Standard error bars are shown.
Funding:
Dr. Garland is supported by a New Investigator Award from the Beatrice Hunter Cancer Research Institute. This work was supported by National Cancer Institute grants K01 CA211789 (PI: Gonzalez), R01 CA132803 (PI: Jacobsen), and in part by the Tissue Core and the Participant Research, Interventions, and Measurement (PRISM) Core at Moffitt Cancer Center (P30-CA076292; PI: Cleveland).
Footnotes
Conflicts of Interest: BG: SureMed Compliance; KemPharm; Elly Health, Inc.
This study was presented in part at the 21st World Congress of Psychosocial Oncology as an oral presentation in September 2019.
References
- 1.Gonzalez BD, Jim HS, Booth-Jones M, et al. : Course and Predictors of Cognitive Function in Patients With Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison. J Clin Oncol 33:2021–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunlusoy B, Ceylan Y, Koskderelioglu A, et al. : Cognitive Effects of Androgen Deprivation Therapy in Men With Advanced Prostate Cancer. Urology 103:167–172, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Nead KT, Gaskin G, Chester C, et al. : Androgen deprivation therapy and future Alzheimer’s disease risk. Journal of Clinical Oncology 34:566, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayadevappa R, Chhatre S, Malkowicz SB, et al. : Association Between Androgen Deprivation Therapy Use and Diagnosis of Dementia in Men With Prostate Cancer. JAMA Netw Open 2:e196562, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alibhai SM, Timilshina N, Duff-Canning S, et al. : Effects of long-term androgen deprivation therapy on cognitive function over 36 months in men with prostate cancer. Cancer 123:237–244, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Marzouk S, Naglie G, Tomlinson G, et al. : Impact of Androgen Deprivation Therapy on Self-Reported Cognitive Function in Men with Prostate Cancer. J Urol 200:327–334, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Savard J, Hervouet S, Ivers H: Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology 22:1381–8, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Savard J, Ivers H, Villa J, et al. : Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 29:3580–6, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Hanisch LJ, Gooneratne NS, Soin K, et al. : Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur J Cancer Care (Engl) 20:549–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez BD, Small BJ, Cases MG, et al. : Sleep Disturbance in Men Receiving Androgen Deprivation Therapy for Prostate Cancer: The Role of Hot Flashes and Nocturia. Cancer 124:499–506, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henneghan AM, Van Dyk K, Kaufmann T, et al. : Measuring Self-Reported Cancer-Related Cognitive Impairment: Recommendations from the Cancer Neuroscience Initiative Working Group. JNCI: Journal of the National Cancer Institute, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savard J, Ganz PA: Subjective or Objective Measures of Cognitive Functioning-What’s More Important? JAMA Oncol 2:1263–1264, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, et al. : Can comorbidity be measured by questionnaire rather than medical record review? Medical care 34:73–84, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Farias ST, Mungas D, Reed BR, et al. : The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 22:531, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen F, Amariglio RE, Buckley RF, et al. : The characterisation of subjective cognitive decline. Lancet Neurol 19:271–278, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farias ST, Chou E, Harvey DJ, et al. : Longitudinal trajectories of everyday function by diagnostic status. Psychol Aging 28:1070–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin CM, Benca RM: Insomnia nature, diagnosis, and treatment. Handb Clin Neurol 99:723–46, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS: The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement 1:385–401, 1977 [Google Scholar]
- 19.Hann D, Jacobsen P, Azzarello L, et al. : Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Quality of Life research 7:301–310, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Godin G, Shephard RJ: Godin Leisure-Time Exercise Questionnaire. Medicine and Science in Sports and Exercise 29:36–38, 1997 [Google Scholar]
- 21.Rockwood N: MLMED User Guide. Beta Version, 2017 [Google Scholar]
- 22.Rockwood NJ, Hayes AF: MLmed: An SPSS macro for multilevel mediation and conditional process analysis, Poster presented at the annual meeting of the Association of Psychological Science (APS). Boston, MA, 2017 [Google Scholar]
- 23.Hayes AF, Rockwood NJ: Conditional process analysis: Concepts, computation, and advances in the modeling of the contingencies of mechanisms. American Behavioral Scientist 64:19–54, 2020 [Google Scholar]
- 24.Valla M, Engstrom MJ, Ytterhus B, et al. : FGD5 amplification in breast cancer patients is associated with tumour proliferation and a poorer prognosis. Breast Cancer Res Treat 162:243–253, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez BD, Jim HS, Donovan KA, et al. : Course and moderators of hot flash interference during androgen deprivation therapy for prostate cancer: A matched comparison. The Journal of Urology 194:690–695, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez BD, Jim HS, Small BJ, et al. : Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Supportive Care in Cancer 24:2201–2207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Jim HS, Fishman M, et al. : Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psycho‐Oncology 24:472–477, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson AM, Gonzalez BD, Jim HS, et al. : Characteristics and predictors of fatigue among men receiving androgen deprivation therapy for prostate cancer: A controlled comparison. Supportive Care in Cancer 24:4159–4166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S, Thompson W, Ancoli-Israel S, et al. : Cognition, quality-of-life, and symptom clusters in breast cancer: Using Bayesian networks to elucidate complex relationships. Psychooncology 27:802–809, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caplette-Gingras A, Savard J, Savard MH, et al. : Is insomnia associated with cognitive impairments in breast cancer patients? Behav Sleep Med 11:239–57, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Savard J, Ivers I: Insomnia and subjective cognitive impairments in cancer patients: A prospective analysis. Presented at the International Cognition and Cancer Taskforce Conference, Seattle, WA, February 10–13, 2014 [Google Scholar]
- 32.Denlinger CS, Ligibel JA, Are M, et al. : Survivorship: sleep disorders, version 1.2014. Journal of the National Comprehensive Cancer Network 12:630–642, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell D, Oliver TK, Keller-Olaman S, et al. : A Pan-Canadian practice guideline: prevention, screening, assessment, and treatment of sleep disturbances in adults with cancer. Supportive Care in Cancer 21:2695–2706, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Savard J, Ivers H: Screening for clinical insomnia in cancer patients with the Edmonton Symptom Assessment System-Revised: a specific sleep item is needed. Supportive Care in Cancer 27:3777–3783, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Qaseem A, Kansagara D, Forciea MA, et al. : Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med 165:125–33, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Johnson JA, Rash JA, Campbell TS, et al. : A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev 27:20–8, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Fleming L, Randell K, Harvey CJ, et al. : Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology 23:679–684, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Herbert V, Kyle SD, Pratt D: Does cognitive behavioural therapy for insomnia improve cognitive performance? A systematic review and narrative synthesis. Sleep medicine reviews 39:37–51, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Engeroff T, Ingmann T, Banzer W: Physical Activity Throughout the Adult Life Span and Domain-Specific Cognitive Function in Old Age: A Systematic Review of Cross-Sectional and Longitudinal Data. Sports Med 48:1405–1436, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Participant flow among recipients of androgen deprivation therapy (ADT) for prostate cancer (ADT+ group).
Supplementary Figure 2. Participant flow among patients with prostate cancer not treated with androgen deprivation therapy (ADT; ADT− group).
Supplementary Figure 3. Participant flow among individuals with no history of cancer (CA− group).
Supplementary Figure 4. Change over time in cognitive function (A), insomnia (B), and proposed moderators (C, D, E) among only control group participants. Standard error bars are shown.


