Abstract
Objective:
Medically treated opioid overdoses identify a population at high risk of subsequent mortality and need for treatment. This study reports on medically treated opioid overdose trends in a state with rapid fentanyl spread.
Methods:
We conducted stratified trend analysis of medically treated overdose due to heroin, synthetic opioids, methadone, or other natural opioids among New Jersey Medicaid beneficiaries aged 12–64 years (2014–2019); evaluated associations with demographics and co-occurring conditions; and examined trends in fentanyl penetration in suspected heroin seizures from New Jersey State Police data.
Results:
Overdose risk more than tripled from 2014 to 2019, from 120.5 to 426.8 per 100,000 person-years, respectively. Increases primarily involved heroin and synthetic opioids and were associated with co-occurring alcohol and other non–opioid drug disorders, major depressive disorder, and hepatitis C. Concurrent changes in the drug exposure environment (2015–2019) included an increase in fentanyl penetration (proportion of suspected heroin seizures that included fentanyls) from 2% to 80%, and a decrease in the proportion of Medicaid beneficiaries who received opioid analgesic prescriptions from 23% to 13%.
Conclusion:
Results document a rapid increase in overdose risk among individuals with opioid use disorder in an environment in which fentanyl is highly prevalent, and highlight the need for intensified services and engagement of non–treatment seekers, and integrated models to address multiple co-occurring conditions and risk factors.
Keywords: Overdose, Medicaid, Fentanyl, Opioid, Policy, Comorbidity
1. Introduction
Public and research attention to opioid overdose has focused predominantly on fatalities, yet the great majority of opioid-involved overdoses do not result in death. Medically treated overdoses are far more common and are strongly associated with increased risk of permanent disability, subsequent overdose, and subsequent mortality. During the year following a medically treated non–fatal overdose, the rate of fatal opioid overdose has been found in one study of Medicaid beneficiaries to be 1,154 per 100,000 person-years, and the overall mortality rate to be 7,783 per 100,000 person-years with a standardized mortality rate ratio (compared to the demographically matched general population) of 24.2 for all-cause mortality, 132.1 for drug use-associated diseases, 45.9 for HIV, 41.1 for chronic respiratory diseases, 30.9 for viral hepatitis, and 25.9 for suicide (Olfson et al., 2018).
Efforts to reduce the burden of nonfatal overdose—particularly in Medicaid populations, which have elevated rates of opioid use disorder (OUD)—need evidence on patterns and trends in medically treated opioid overdose events (CDC, 2018; O’Brien et al, 2021). Medically treated overdose data complement mortality data and help us to understand trends in the epidemic and the complex health and behavioral health care needs of individuals experiencing these events. Although several prior studies have examined rates of medically treated overdose (Dilokthornsakul et al., 2016; Samples et al., 2020; Tedesco et al., 2017; Unick & Ciccarone, 2017), insufficient information is available on comorbidity patterns and changing risks in the evolving opioid epidemic, particularly in geographical areas where adulteration of heroin with synthetic opioids such as fentanyl and related analogs has become widespread (Spencer et al., 2019).
Since expanding eligibility under the Affordable Care Act in 2014, New Jersey’s Medicaid population includes a broader range of low-income individuals (up to 138% FPL for adults) as well as the traditional categorically eligible population of parents, their children, and persons with disability. The state has also experienced a rapid spread of fentanyl in illicit drug markets (NFLIS, 2020), a development that increasingly threatens other areas of the country (Pardo et al., 2019). New Jersey moved from 33rd among states in opioid overdose fatalities in 2014 (rate: 8.2 per 100,000) to 5th in 2018 (rate: 29.7 per 100,000) (CDC, 2021a).
Using New Jersey Medicaid data for all beneficiaries aged 12–64 years, this study aims to: 1) characterize the changing demographic profile of individuals with overdoses; 2) assess comorbidity among individuals with overdose; and 3) identify changes in the risk factors for overdose. We hypothesized that over the study period of 2014–2019, risk of overdose would increase, especially for younger males and non-White beneficiaries, reflecting the evolution of the opioid epidemic and growing presence of fentanyl in the illicit drug supply.
2. Methods
2.1. Medicaid data and sample
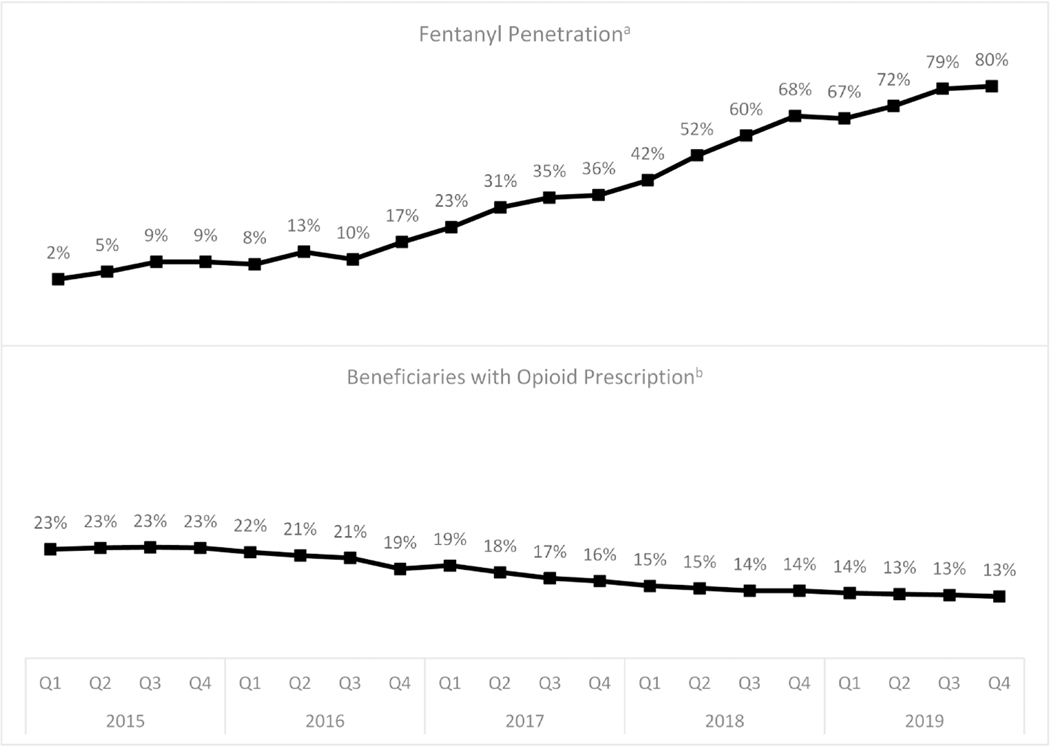
Analyses used de-identified New Jersey Medicaid claims data for overdoses occurring from 2014 to 2019, a period during which Medicaid enrollment stabilized following expansion. These data include outpatient, inpatient, and emergency department (ED) services, filled prescriptions, diagnoses associated with each claim, and demographic characteristics. In this repeated cross-sectional analysis, we included patients aged 12–64 years with at least 11 months of Medicaid eligibility during the index year and continuous enrollment for the last 6 months of the prior year, and excluded those dually eligible for Medicare, as Medicaid data may be incomplete for those who are dual-eligible. The analyses included individuals identified with medically treated opioid overdoses resulting in an acute care visit (ED visit or inpatient stay) during the index year using the International Classification of Diseases, 9th and 10th revision (ICD-9-CM and ICD-10-CM) codes for opioid poisoning (Table A.1) (CDC, 2015; CDC, 2021b; World Health Organization, 2015). The study categorized overdose visits into one of two groups: 1) heroin or synthetic opioid (e.g., fentanyl) overdose visit, regardless of whether the visit had diagnosis codes for other types of opioid overdoses; and 2) overdoses including only codes for natural, semi-synthetic, methadone, or other opioid overdoses (e.g., oxycodone or other prescription opioids). Analyses included the year of index overdose, coded as 0–5, representing years 2014–2019. For the period January 1, 2014, to September 2015, the “heroin or synthetic opioid” category included only poisonings attributed to heroin since no diagnosis code existed for synthetic opioids in ICD-9-CM (before the switch to ICD-10-CM in late 2015); however, fentanyl was minimally present in street drug supplies in New Jersey during most of this period (Figure 1; Ciccarone, 2019).
Fig. 1.
Quarterly trends in opioid prescribinga and fentanyl penetrationb, 2015–2019.
a Values are the percentages of suspected heroin seizures containing fentanyl by quarter, 2015–2019. Data are from the New Jersey State Police Drug Monitoring Initiative.
b Values are the percentages of NJ Medicaid beneficiaries prescribed an opioid (excluding medication assisted treatment for opioid use disorder ) by quarter 2015–2019.
Diagnosis codes from all claims in the 6 months prior to the year of index overdose identified comorbid conditions (index overdoses in 2014 used data from 2013 for the lookback period). The study considered patients to have a comorbid condition if they had at least one claim with that diagnosis during the lookback period. Substance use disorder (SUD) diagnoses include disorders related to alcohol, cannabis, benzodiazepines, and opioids. Mental health diagnoses include schizophrenia, bipolar disorder, and major depression. General medical diagnoses include HIV, hepatitis C, diabetes, heart failure, cerebrovascular diseases, pain disorders, and conditions associated with respiratory dysfunction known to increase overdose risk (i.e., asthma, chronic obstructive pulmonary disease, pneumonia, sleep apnea). Table A.2 lists ICD-9/ICD-10 codes for comorbid conditions. The study identified demographics (age during index year, sex, racial/ethnic group) using the Medicaid eligibility file, which included mutually exclusive categories for non-Hispanic Black, non-Hispanic White, Hispanic, and non-Hispanic other/unknown race.
2.2. Opioid prescribing and drug seizure data
To provide context for overdose trends, we calculated the proportion of Medicaid beneficiaries aged 12–64 years prescribed any opioid on a quarterly basis by identifying beneficiaries with a pharmacy claim containing National Drug Codes for opioids (Table A.3), excluding medications formulated to treat OUD (e.g., oral buprenorphine or methadone). Using aggregate data on fentanyl seizures from the New Jersey State Police (NJSP) Regional Operations Intelligence Center, which uses law enforcement and health care data to address the opioid epidemic through its Drug Monitoring Initiative, we identified the drug environment context during the study period. The NJSP receives and compiles forensic drug analysis data on drug seizures from its Office of Forensic Sciences labs and the five independent county forensic labs in New Jersey. NJSP provided quarterly percentages of suspected heroin seizures containing fentanyl throughout New Jersey from 2015 to 2019.
2.3. Statistical analysis
Descriptive analyses identified demographic and clinical characteristics of beneficiaries with one or more medically treated opioid overdoses resulting in acute care in 2014 and 2019. Stratified trend analyses then identified demographic and clinical groups at increasing overdose risk, by calculating overdose rates in each year within each demographic and clinical subgroup. Next, we calculated rate ratios conditioned on each characteristic using unadjusted Poisson regression to compare 2014 and 2019 rates for any opioid overdose; heroin/synthetic opioid overdose; and other (i.e., prescription) opioid overdose. Finally, Poisson regression estimated rate ratios for covariates predicting opioid overdose, with person-year as the unit of analysis with the 2014–2019 data. Entering explanatory variables in the models in blocks helped to distinguish associations with fixed demographic characteristics from those that may be on the causal pathway to overdose, and identify the potential role of clinical variables in explaining demographic differences in overdose risk and change over time. Model 1 includes demographic characteristics (age, sex, race/ethnicity); calendar year; and a year by race/ethnicity interaction to examine differential trends within racial/ethnic groups. Model 2 adds substance use, psychiatric, and medical comorbidities (with the exception of OUD). Model 3 includes OUD given its strong association with opioid overdose. All models used generalized estimating equations with an exchangeable correlation matrix to account for nonindependence of observations of individuals across multiple years. The study team conducted analyses using SAS Enterprise Guide version 7.1.
3. Results
3.1. Characteristics of Medicaid beneficiaries with opioid overdose
In the overall Medicaid population, the rate of medically treated overdose per 100,000 person-years increased from 120.5 in 2014 to 426.8 in 2019 (a 3.54-fold increase). Table 1 shows demographic and clinical characteristics of beneficiaries with overdose in 2014 and 2019, and the overdose rate per 100,000 person-years within each subgroup. The first four columns compare demographic and clinical profiles of the overall Medicaid population in 2014 and 2019 to those with at least one overdose resulting in acute care. Those with overdose had higher proportions of diagnosed mental health disorders, other SUDs, infectious disease (hepatitis C and HIV), and comorbid medical conditions. Among individuals with overdose in 2019, 30.4% were diagnosed with chronic pain conditions, 50.9% with major depression, 30.0% with hepatitis C, 5.6% with HIV, 28.0% with bipolar disorder, 11.5% with schizophrenia, 39.0% with alcohol use disorder, 26.5% with cannabis use disorder, and 21.4% with sedative/hypnotic use disorder. Compared to the total age-eligible Medicaid population, those with overdose also were disproportionately diagnosed with pulmonary conditions that may reduce respiratory reserve, including chronic obstructive pulmonary disease (14.7% vs. 2.4%), pneumonia (16.0% vs. 1.8%), and asthma (12.3% vs. 6.4%).
Table 1 –
Characteristics of Medicaid population, Medicaid population with opioid overdose, and prevalence of overdose in Medicaid population.
| Medicaid Population, Totala (%) | Individuals with Opioid Overdose (%) | Within Group, Opioid Overdose Rate per 100,000 Person-Years | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariate | 2014 N = 630,009 | 2019 N = 717,585 | 2014 N = 661 | 2019 N = 2,269 | 2014 N = 630,009 | 2019 N = 717,585 |
|
| ||||||
| Overall | 120.5 | 426.8 | ||||
|
| ||||||
| Age | ||||||
| 12–24 | 42.8 | 42.2 | 11.2 | 5.3 | 31.2 | 60.4 |
| 25–39 | 25.9 | 24.7 | 39.3 | 41.8 | 178.4 | 712.3 |
| 40–55 | 22.5 | 21.3 | 36.6 | 37.3 | 195.2 | 723.5 |
| 56–64 | 8.8 | 11.7 | 12.9 | 15.6 | 192.4 | 605.7 |
|
| ||||||
| Racial/Ethnic Group | ||||||
| White | 37.1 | 37.7 | 59.6 | 55.8 | 191.0 | 628.4 |
| Black | 26.3 | 24.4 | 22.7 | 27.9 | 104.8 | 501.4 |
| Hispanic | 16.4 | 23.3 | 10.7 | 11.2 | 61.1 | 198.7 |
| Other/Unknown | 20.2 | 14.7 | 7.0 | 5.2 | 59.1 | 147.4 |
|
| ||||||
| Gender | ||||||
| Female | 58.2 | 56.9 | 44.6 | 38.3 | 90.0 | 283.2 |
| Male | 41.8 | 43.1 | 55.4 | 61.7 | 162.9 | 616.8 |
|
| ||||||
| SUD Comorbidities | ||||||
| Alcohol Use Disorder | 2.5 | 3.4 | 33.9 | 39.0 | 1821.1 | 5340.8 |
| Cannabis Use | 1.5 | 2.6 | 15.9 | 26.5 | 1346.9 | 4774.4 |
| Benzodiazepine Use Disorder | 0.3 | 0.6 | 14.5 | 21.4 | 7396.3 | 17603.9 |
| Opioid Use Disorder | 2.4 | 5.1 | 71.0 | 86.2 | 3634.8 | 7451.6 |
|
| ||||||
| Psychiatric | ||||||
| Schizophrenia | 1.7 | 2.1 | 12.0 | 11.5 | 875.4 | 2435.6 |
| Bipolar Disorder | 3.1 | 3.8 | 30.6 | 28.0 | 1218.5 | 3290.5 |
| Major Depression | 3.7 | 10.5 | 29.3 | 50.9 | 997.6 | 2101.2 |
|
| ||||||
| Medical Comorbidities | ||||||
| HIV | 1.5 | 1.1 | 5.1 | 5.6 | 600.5 | 2256.1 |
| Hepatitis C | 1.4 | 2.1 | 23.9 | 30.0 | 2212.3 | 6503.1 |
| Diabetes | 11.6 | 15.6 | 20.7 | 30.1 | 237.0 | 926.4 |
| Heart Failure | 1.4 | 1.5 | 13.9 | 8.6 | 1245.6 | 2598.4 |
| Cerebrovascular Disease | 1.8 | 2.0 | 9.8 | 7.6 | 835.7 | 1725.7 |
| Chronic Pain Diagnosis | 11.6 | 12.6 | 31.6 | 30.4 | 352.5 | 1119.2 |
| Asthma | 10.4 | 6.4 | 29.0 | 12.3 | 334.2 | 880.2 |
| COPD | 0.9 | 2.4 | 8.8 | 14.7 | 1337.1 | 2790.9 |
| Pneumonia | 1.5 | 1.8 | 19.5 | 16.0 | 1720.3 | 4350.5 |
| Sleep Apnea | 2.3 | 3.3 | 4.7 | 3.4 | 250.3 | 429.6 |
Includes the full Medicaid population meeting study inclusion criteria.
Co-occurring diagnoses with increasing prevalence during the study period in both the general Medicaid population and the group with overdose included major depressive disorder, hepatitis C, and non–opioid SUDs. In both years, just less than one-third of individuals with overdoses were diagnosed with conditions associated with chronic pain (31.6% in 2014 and 30.4% in 2019; see Table A.2 for criteria).
3.2. Change in overdose risk within demographic and clinical subgroups
Within all clinical and demographic groups, the risk of overdose was greater in 2019 than in 2014 (Table 1). The increase was especially notable among Black beneficiaries; males; individuals ages 25–55; those with hepatitis C, HIV, and diabetes diagnoses; and individuals diagnosed with alcohol use disorder and cannabis use disorder.
The first column of Table 2 shows the rate ratio (RR) of overdose for each subgroup in 2019 compared to 2014. Although the absolute risk remained higher for whites than other racial/ethnic groups, the relative increase was higher for Black (RR = 4.81, 95% CI = 3.90, 5.93) than white (RR = 3.27, 95% CI = 2.89, 3.69) beneficiaries. Overdoses were more common among male than female beneficiaries in both 2014 and 2019, with an increase (rate ratio) for males of 3.81 (95% CI = 3.35, 4.34) compared to 3.18 (95% CI = 2.74, 3.68) for females. Among those diagnosed with SUDs, the proportionate increase in risk of opioid overdose was greatest for individuals diagnosed with cannabis use disorder (RR = 3.60, 95% CI = 2.86, 4.54) and alcohol use disorder (RR = 2.94, 95% CI = 2.48, 3.47). Medical comorbidities associated with the greatest increase in risk of opioid overdose included HIV (RR = 3.76, 95% CI = 2.32, 6.10), diabetes (RR = 3.91, 95% CI = 3.13, 4.88), and chronic pain diagnoses (RR = 3.08, 95% CI = 2.58, 3.69).
Table 2 –
Trends in opioid overdose risk of Medicaid beneficiaries 2014 to 2019, by opioid type and beneficiary characteristics.
| Any Opioid Overdose | Heroin/Synthetic Opioid Overdosea | Prescription Opioid Overdoseb | |
|---|---|---|---|
|
| |||
| Characteristics | Rate Ratio (95% CI)c | Rate Ratio (95% CI)c | Rate Ratio (95% CI)c |
|
| |||
| Overall | 3.56 (3.23, 3.93) | 4.39 (3.89, 4.96) | 2.03 (1.76, 2.35) |
|
| |||
| Age | |||
| 12–24 | 1.94 (1.40, 2.68) | 2.11 (1.41, 3.16) | 1.32 (0.81, 2.16) |
| 25–39 | 3.87 (3.35, 4.47) | 4.10 (3.45, 4.87) | 2.62 (2.05, 3.34) |
| 40–55 | 3.72 (3.17, 4.36) | 4.99 (4.04, 6.16) | 2.13 (1.70, 2.66) |
| 56–64 | 3.15 (2.35, 4.22) | 7.18 (4.73, 0.90) | 1.32 (0.92, 1.89) |
|
| |||
| Racial/Ethnic Group | |||
| White | 3.27 (2.89, 3.69) | 3.89 (3.34, 4.53) | 1.97 (1.63, 2.39) |
| Black | 4.81 (3.90, 5.93) | 7.70 (5.86, 10.11) | 2.30 (1.73, 3.06) |
| Hispanic | 3.19 (2.19, 4.64) | 3.65 (2.30, 5.81) | 1.98 (1.09, 3.59) |
| Other/Unknown | 2.51 (1.84, 3.43) | 2.68 (1.78, 4.02) | 1.96 (1.42, 2.72) |
|
| |||
| Sex | |||
| Female | 3.18 (2.74, 3.68) | 4.20 (3.47, 5.09) | 1.81 (1.47, 2.23) |
| Male | 3.81 (3.35, 4.34) | 4.42 (3.77, 5.18) | 2.21 (1.79, 2.71) |
|
| |||
| SUD Comorbidities | |||
| Alcohol Use Disorder | 2.94 (2.48, 3.47) | 4.18 (3.39, 5.14) | 1.64 (1.29, 2.10) |
| Cannabis Use Disorder | 3.60 (2.86, 4.54) | 5.10 (3.76, 6.92) | 1.96 (1.42, 2.72) |
| Benzodiazepine Use Disorder | 2.42 (1.89, 3.10) | 3.13 (2.29, 4.29) | 1.62 (1.12, 2.35) |
| Opioid Use Disorder | 2.10 (1.87, 2.35) | 2.64 (2.30, 3.03) | 1.96 (1.42, 2.72) |
|
| |||
| Psychiatric | |||
| Schizophrenia | 2.75 (2.10, 3.62) | 3.15 (2.19, 4.53) | 1.96 (1.42, 2.72) |
| Bipolar Disorder | 2.71 (2.26, 3.24) | 3.72 (2.95, 4.70) | 1.67 (1.28, 2.17) |
| Major Depression | 2.13 (1.79, 2.53) | 3.09 (2.44, 3.93) | 1.24 (0.99, 1.55) |
|
| |||
| Medical Comorbidities | |||
| HIV | 3.76 (2.32, 6.10) | 6.81 (3.62, 2.82) | 2.12 (1.12, 4.03) |
| Hepatitis C | 2.96 (2.41, 3.64) | 3.44 (2.73, 4.35) | 2.24 (1.56, 3.22) |
| Diabetes | 3.91 (3.13, 4.88) | 8.43 (5.98, 1.87) | 1.57 (1.19, 2.08) |
| Heart Failure | 2.04 (1.55, 2.69) | 3.16 (2.19, 4.57) | 1.26 (0.86, 1.87) |
| Cerebrovascular Disease | 2.06 (1.45, 2.95) | 3.33 (2.05, 5.40) | 1.31 (0.83, 2.05) |
| Chronic Pain Diagnosis | 3.08 (2.58, 3.69) | 4.93 (3.88, 6.26) | 1.81 (1.43, 2.28) |
| Asthma | 2.64 (2.13, 3.26) | 3.92 (2.96, 5.20) | 1.61 (1.22, 2.14) |
| COPD | 2.11 (1.53, 2.92) | 3.23 (1.98, 5.26) | 1.34 (0.92, 1.96) |
| Pneumonia | 2.53 (2.01, 3.18) | 4.26 (3.04, 5.98) | 1.96 (1.42, 2.72) |
| Sleep Apnea | 1.77 (1.11, 2.80) | 3.71 (1.72, 8.02) | 1.24 (0.69, 2.20) |
ICD-9 codes for synthetic opioids did not exist in 2014 (under ICD-9) and were captured as other opioids. However, our analyses of drug seizures in NJ show that there were only 1.7 fentanyl seizures per 100 heroin seizures in 2014, indicating that heroin adulteration with fentanyl was relatively uncommon.
Prescription opioids include methadone or other natural/semisynthetic opioids. Individuals with overdoses involving both heroin/synthetic opioid and methadone/other natural opioid during the year were assigned to the heroin/synthetic category. Beneficiaries in this column may have also had overdose caused by methadone or other natural/semi-synthetic opioids.
Rate ratios compare risk of opioid overdose in 2019 compared to 2014.
3.3. The increasing role of heroin and synthetic-opioid overdoses
The second and third columns of Table 2 show the change in overdose risk from 2014 to 2019 by type of opioid involved in the overdose, expressed as unadjusted rate ratios. Risk of overdose involving heroin or synthetic opioids increased fourfold from 2014 to 2019 (RR=4.39, 95% CI = 3.89, 4.96). Risk of overdose involving only prescription opioids also increased from 2014 to 2019, though the increase was smaller than for heroin and synthetic opioid overdoses (RR=2.03, 95% CI = 1.76, 2.35). Increase in risk of heroin or synthetic opioid overdoses was particularly marked among Black beneficiaries (RR=7.70, 95% CI = 5.86, 10.11).
3.4. Multivariate analysis of overdose risk
Table 3 shows results of Poisson regression analyses of overdose risk over the 2014–2019 period, including a covariate for year to estimate annual rate of change. In model 1, including demographic characteristics only, overall adjusted risk of overdose increased by 20% per year. Over the six-year period, male sex was associated with 168% higher risk of overdose than female sex. Risk of overdose for Black beneficiaries was less than half that of non-Hispanic Whites over the study period but increased more quickly than for Whites, as reflected in a significant year by race/ethnicity interaction term.
Table 3 –
Predictors of opioid overdose among New Jersey Medicaid beneficiaries, 2014–2019.
| Covariate | Model 1 ARR (95% CI) | Model 2 ARR (95% CI) | Model 3 ARR (95% CI) |
|---|---|---|---|
|
| |||
| Year | 1.20 (1.19, 1.22) | 1.12 (1.11, 1.14) | 1.07 (1.06, 1.09) |
|
| |||
| Age | |||
| 12–24 | 0.16 (0.14, 0.18) | 0.42 (0.37, 0.47) | 0.93 (0.82, 1.05) |
| 25–39 | 1.50 (1.39, 1.62) | 2.09 (1.92, 2.28) | 1.69 (1.55, 1.83) |
| 40–55 | 1.29 (1.19, 1.39) | 1.42 (1.31, 1.54) | 1.15 (1.07, 1.25) |
| 56–64 (Ref) | 1 | 1 | 1 |
|
| |||
| Racial/Ethnic Group | |||
| Black | 0.45 (0.41, 0.51) | 0.49 (0.44, 0.55) | 0.55 (0.49, 0.62) |
| Hispanic | 0.31 (0.26, 0.37) | 0.42 (0.35, 0.50) | 0.59 (0.49, 0.70) |
| Other/Unknown | 0.19 (0.17, 0.23) | 0.33 (0.29, 0.39) | 0.54 (0.46, 0.64) |
| White (Ref) | 1 | 1 | 1 |
|
| |||
| Year x Racial/Ethnic Group | |||
| Year x Black | 1.10 (1.07, 1.14) | 1.13 (1.10, 1.17) | 1.12 (1.08, 1.15) |
| Year x Hispanic | 1.03 (0.98, 1.08) | 1.06 (1.01, 1.11) | 1.06 (1.01, 1.11) |
| Year x Other/Unknown | 1.02 (0.98, 1.07) | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) |
| Year x White (Ref) | 1 | 1 | 1 |
|
| |||
| Gender | |||
| Male | 2.68 (2.54, 2.83) | 2.23 (2.11, 2.35) | 1.80 (1.71, 1.90) |
| Female (Ref) | 1 | 1 | 1 |
|
| |||
| SUD Comorbiditiesa | |||
| Alcohol Use Disorder | --- | 2.94 (2.72, 3.17) | 1.88 (1.77, 1.99) |
| Cannabis Use Disorder | --- | 1.87 (1.73, 2.02) | 1.35 (1.27, 1.44) |
| Benzodiazepine Use Disorder | --- | 3.36 (3.07, 3.68) | 1.93 (1.80, 2.06) |
| Opioid Use Disorder | --- | --- | 42.64 (39.47, 46.07) |
|
| |||
| Psychiatric Comorbiditiesa | |||
| Schizophrenia | --- | 0.90 (0.82, 0.99) | 1.01 (0.92, 1.10) |
| Bipolar Disorder | --- | 1.46 (1.35, 1.57) | 1.16 (1.09, 1.24) |
| Major Depression | --- | 2.35 (2.19, 2.51) | 1.47 (1.39, 1.55) |
|
| |||
| Medical Comorbiditiesa | |||
| HIV | --- | 1.07 (0.94, 1.21) | 0.95 (0.85, 1.06) |
| Hepatitis C | --- | 3.34 (3.09, 3.60) | 1.71 (1.61, 1.81) |
| Diabetes | --- | 1.32 (1.24, 1.41) | 1.38 (1.29, 1.46) |
| Heart Failure | --- | 1.56 (1.41, 1.73) | 1.54 (1.40, 1.69) |
| Cerebrovascular Disease | --- | 1.09 (0.98, 1.21) | 1.06 (0.97, 1.17) |
| Chronic Pain Diagnosis | --- | 1.38 (1.30, 1.46) | 1.11 (1.05, 1.17) |
| Asthma | --- | 1.28 (1.19, 1.38) | 1.09 (1.01, 1.17) |
| COPD | --- | 1.28 (1.17, 1.40) | 1.10 (1.01, 1.20) |
| Pneumonia | --- | 2.32 (2.13, 2.51) | 2.15 (2.00, 2.30) |
| Sleep Apnea | --- | 0.59 (0.52, 0.67) | 0.65 (0.57, 0.75) |
Note. N = 4,364,424. ARR = adjusted rate ratio; CI = confidence interval; COPD = chronic obstructive pulmonary disease. SUD = substance use disorder. Adjusted rate ratios were estimated using GEE Poisson regression.
Reference for each comorbid condition is the absence of the condition.
Model 2 adds a range of substance use, psychiatric, and medical diagnoses. Strong associations existed between opioid overdose and comorbid SUDs, with both alcohol use disorder (RR=2.94, 95% CI = 2.72, 3.17) and benzodiazepine use disorder (RR=3.36, 95% CI = 3.07, 3.68) associated with approximately tripled the risk of opioid overdose. Diagnoses of major depressive disorder (RR=2.35, 95% CI = 2.19, 2.51) and bipolar disorder (RR=1.46, 95% CI = 1.35, 1.57) were associated with increased risk of overdose, as were diagnoses of hepatitis C (RR=3.34, 95% CI = 3.09, 3.60), chronic pain conditions (RR=1.38, 95% CI = 1.30, 1.46), diabetes (RR=1.32, 95% CI = 1.24, 1.41), heart failure (RR=1.56, 95% CI = 1.41, 1.73), asthma (RR=1.28, 95% CI = 1.19, 1.38), COPD (RR=1.28, 95% CI = 1.17, 1.40), and pneumonia (RR=2.32, 95% CI = 2.13, 2.51).
Model 3 additionally controlled for presence of a diagnosis of OUD on a claim in the lookback period. This addition attenuated associations of demographic and clinical characteristics with opioid overdose, but many remained significant. Accounting for diagnosed OUD, risk of overdose remained substantially elevated for chronic pain diagnosis, hepatitis C, diabetes, heart failure, respiratory disorders (except for sleep apnea), bipolar disorder, major depressive disorder, and non–opioid SUD diagnoses.
3.5. Trends in fentanyl penetration and opioid prescribing
To contextualize the findings reported above with information on the changing drug environment, Figure 1 displays trends in state-level fentanyl penetration, available from 2015 to 2019, and changes in rates of opioid prescribing among Medicaid beneficiaries during the same period. During this period, the percentage of suspected heroin seizures containing fentanyl increased from 2% to 80%, while the percentage of Medicaid beneficiaries with opioid prescriptions for pain decreased from 23% in early 2015 to 13% in late 2019.
4. Discussion
4.1. Challenges for Intervention in a high-fentanyl environment
Substantial changes in the opioid epidemic and illicit drug supply have considerable import for health system responses. While high rates of opioid prescribing likely contributed to earlier increases in OUD, actions to further limit such prescribing alone may do little to reduce opioid overdose in the current environment. High rates of medically treated overdoses in an increasingly dangerous environment for illicit drug users challenge the health care system to implement initiatives for individuals who survive overdoses. Such initiatives should be assertive and integrate care to engage and retain high-risk individuals in medication for opioid use disorder (MOUD) treatment while addressing the substantial burden of co-occurring conditions that complicates their disease course. These conditions include a high burden of major depressive disorder; other mental health conditions; medical risk factors such as pulmonary conditions; and polysubstance use. The increase in multiple substance use disorders in New Jersey is consistent with national studies that show that at least one additional substance is involved in approximately 70% of fatal heroin or fentanyl overdoses (Hedegaard et al., 2018).
Results of the current analysis show that during 2014–2019, the rate of medically treated opioid overdoses more than tripled, largely driven by increases in overdoses involving heroin or synthetic opioids such as fentanyl. These changes took place during a period in which opioid prescribing in the Medicaid population declined by almost half, while heroin was increasingly adulterated with (or replaced by) fentanyl compounds statewide. Taken together, these trends suggest that in the most recent wave of the opioid epidemic, opioid overdose in the state may have become increasingly decoupled from opioid prescribing activity and increasingly connected to developments in illicit drug markets.
Policies also need to be attentive to the possibility that, if not well managed, reductions in access to prescribed opioids could lead some individuals with pain conditions and other complications, including OUD, to turn to heroin and other illicit drugs, in an increasingly dangerous environment (Beheshti, 2019; Kim, 2021;). The high degree of risk faced by heroin users in an environment of extensive fentanyl penetration is illustrated by our observation that in 2019, nearly 7 percent of individuals with diagnosed hepatitis C infection, a rough marker for injection drug use history, were treated for opioid overdose. These observations highlight the increasingly high risk of overdose that injection drug users face as fentanyl has penetrated the heroin supply, and the critical importance of risk reduction initiatives for this population.
4.2. Initiatives to address opioid harms in New Jersey
New Jersey has launched a number of recent initiatives to address opioid harms through more cautious opioid prescribing, increased availability of OUD treatment, and greater outreach to at-risk individuals in need of treatment. In 2017, state legislation limited initial opioid prescriptions to five days and imposed other requirements for prescribing and managing opioids. The state also increased access to naloxone for overdose reversal efforts. In 2019, the state took the important step of eliminating Medicaid prior authorization requirements for MOUD; launched the Office Based Addiction Treatment program, creating new reimbursement incentives for primary care providers to provide MOUD; and created Centers of Excellence for opioid treatment to support MOUD providers (New Jersey Office of the Governor, 2019). Further recent innovative initiatives include stationing navigators in emergency departments to link overdose survivors with treatment through the Opioid Overdose Recovery Program; buprenorphine initiation in some emergency departments and for prisoners approaching release; and navigator-based programs to support re-entry for released prisoners with histories of OUD. The state implemented many of these initiatives after the time period of the current study. State data indicate that fatal overdose, after increasing by 127% from 2015 through 2017, increased by a much lower rate (14%) from 2017 to 2018, and experienced a slight decline (–3%) from 2018 to 2019 (NJOAG, 2020), suggesting potential progress in reducing the lethality of overdose, although suspected overdoses continued to claim just over 3,000 lives per year in the state in 2019. Nevertheless, the substantial extent of fentanyl penetration in the state continues to challenge the capacity of health system responses and has created, in recent years, an increasingly risky environment for persons with OUD.
4.3. Implications for national policy in a changing opioid environment
While particularly dramatic in New Jersey, the shift from predominantly natural heroin to an illicit drug supply that is largely adulterated with, or replaced by, fentanyl and other synthetic opioids has occurred in many states, and may affect more states as fentanyl spreads in the illicit drug supply (Pardo et al., 2019). As such, New Jersey’s experience may be a leading indicator of developments occurring or likely to be faced in many other states, including those in regions where fentanyl penetration is at an early stage. Research has described the opioid crisis as a four-wave epidemic, with overdose deaths attributed to prescription opioids, heroin, fentanyl, and most recently, polysubstance use of fentanyl in combination with stimulants, notably methamphetamine (Ciccarone, 2019; Jones et al., 2020). New Jersey exemplifies a state with rapid progression through these stages. As the epidemic evolves, the profile of overdose risk has also shifted, requiring the health system to continuously adapt to the changing epidemiology of overdoses, varying across regions, states, and time. Health systems across the nation should continue to monitor these changes and continually adjust their responses. Medicaid claims data can play an important role in monitoring evolving overdose patterns, as well as in engaging high-risk populations in evidence-based treatment. Treatment programs should develop comprehensive treatment models that assertively engage these patients in low-barrier, evidence-based treatment. The urgency of developing these interventions increases as fentanyl penetration and other challenges evolve.
4.4. Prevalence of comorbidity and implications for the treatment system
The high level of behavioral health and medical comorbidity that we identified among individuals with overdoses has important implications for interventions in a system in which substance use treatment, mental health care, and primary medical care are often siloed. Interventions for conditions such as alcohol use disorder (diagnosed in 39.0% of those with overdoses in 2019), sedative-hypnotic use disorder (21.4%), and chronic obstructive pulmonary disease (14.7%) could reduce overdose risk (Maciejewski et al., 2021). High rates of mental health comorbidity among this population, including major depression (50.9%, bipolar disorder (28.0%) and schizophrenia (11.5%), also highlight the need for concomitant mental health treatment (Scherrer et al., 2021), which can be facilitated by organizational and payment system designs that support integrated, multidisciplinary, co-located care. Health systems must effectively manage co-occurring pain conditions, diagnosed in 30.4%, as these conditions may contribute to illicit drug use if not effectively managed. These results point to the importance of integrated care models that addresses the underlying pain conditions comprehensively, with optimal use of non–pharmacological strategies. Studies also suggest that for many patients with both OUD and pain conditions, treatment with buprenorphine may be optimal and yet is greatly underutilized across practice settings (Chen et al., 2014; Peck et al., 2021; Roux et al., 2013).
Treatment for general medical conditions, such as respiratory disorders, and mental health conditions typically requires accessing multiple service sectors that are often poorly integrated in the current health care system (Crowley et al., 2015). Drug use treatment programs are often inadequately linked with other providers of medical and mental health care and may lack the capacity to support recovery for patients with complex comorbidities. Many program, although a declining percentage, do not offer MOUD, which constrains their capacity to provide optimal evidence-based support for recovery. This is true despite evidence that counseling and peer-support approaches alone are likely to be insufficient in this population (Connery, 2015; National Academies of Sciences, Engineering, and Medicine, 2019; Sofuoglu et al., 2019; Wakeman et al., 2020;). Some may provide detoxification without transition to maintenance MOUD, a practice that can increase overdose risk in high-risk individuals through loss of opioid tolerance (Morgan et al., 2020; Strang, 2015). Unfortunately, in many jurisdictions, separate licensing is required for substance use and for mental health services while dual licensing is relatively uncommon and sometimes outright disallowed (Jacobi et al., 2016; Stewart et al., 2019). Data from the National Survey of Substance Abuse Treatment Services indicate that while 89% of licensed drug use treatment programs provide mental health services, only 53% offer programs specifically tailored for clients with co-occurring mental and SUDs (SAMHSA, 2020). Conversely, many mental health treatment programs still lack the capability to provide substance use services (McGovern et al., 2014). Both sectors are typically poorly integrated with the provision of general medical services, particularly with management of pain conditions, which are common in populations with high rates of overdose.
Some states have worked to address these care silos through licensing requirements. In New Jersey, for example, addiction treatment facilities with state fee-for-service contracts must be approved by the Office of Licensing to provide co-occurring services, and demonstrate readiness to provide integrated care for individuals with co-occurring mental health disorders, such as medication management and counseling services provided in-house or through referral to external providers (NJDMHAS, 2020).
While much research has studied service delivery models that integrate care across general medical, substance use, and mental health sectors, few programs truly integrate all three sectors. Much of the development of integrated care has involved incorporation of mental health services into primary medical care settings. A few models, such as the Comprehensive Community Behavioral Health Center (CCBHC; SAMHSA, 2016) model that has been piloted in a number of states including New Jersey, focus on integration across all three sectors. However, the number of sites offering such programs is limited, and programs may not have sufficient capacity to address the need for individuals diagnosed with multiple conditions. Many federally qualified health centers provide both primary care and mental health services on-site, and some have also initiated programs to provide OUD services on site, but these initiatives are often still in developmental stages and may lack the capacity to meet a growing need. The United States Preventive Services Task Force recently released updated guidance now encouraging universal screening of adults in primary care settings for illicit drug use (USPTF, 2020). While screening may help to identify high-risk patients, it will be insufficient if not coupled with access to evidence-based care (Mauro et al, 2020).
In addition to expanding integrated care models, alternative payment strategies need to be developed to support the substantial and complex care needs of this population. New Jersey’s Office Based Addiction Treatment (OBAT) program represents a significant step in this direction, providing enhanced reimbursement for supportive services, including navigator and peer support components as well as for comprehensive evaluation. Other payment models such as addiction treatment health homes have shown promise in multiple states for providing access to and coordination of physical and behavioral health services to improve health outcomes, particularly in Medicaid populations (Clemans-Cope et al., 2017; Moses & Klebonis, 2015).
4.5. Limitations
The study limited the sample to continuously enrolled Medicaid beneficiaries and, therefore, the sample is not representative of New Jersey residents who were commercially insured, uninsured, enrolled in Medicaid only intermittently, or covered by Medicare. These findings represent a Medicaid expansion state and might not generalize to states with different eligibility criteria, service availability, and population and environmental characteristics that may affect overdose and related comorbidity. The data may have misclassified poisonings with fentanyl or other synthetic opioids as other opioid poisonings in 2014 and much of 2015 since no diagnosis code existed for synthetic opioids in ICD-9-CM (before the switch to ICD-10-CM in late 2015), though fentanyl poisonings only began to increase in 2014 (Ciccarone, 2019). This study included only overdose events that resulted in acute medical care.
5. Conclusions
The increase in medically treated overdoses in the New Jersey Medicaid population, concurrent with substantial declines in opioid prescribing, highlights the impact that the spread of fentanyl has had as well as the limits that reducing opioid prescribing alone has had in addressing the current opioid crisis. While reductions in opioid prescribing may reduce the flow of individuals into long-term opioid dependence, research and treatment providers need different measures to address the high prevalence of established OUD. Providers need to increase access to MOUD in communities of color, given our finding that risk of overdose increased fastest among Black and Hispanic beneficiaries during the 2014–2019 period, as fentanyl spread through illicit drug markets in all parts of the state.
The increasing overdose risks that individuals using illicit drugs face in a period of increasing fentanyl penetration highlight the urgent need to engage and retain these individuals on MOUD. Those who are medically treated for overdose are at especially high risk and should be especially high priorities for intervention. Along with OUD treatment, comprehensive care strategies should address the complex patterns of mental health, substance use, and medical comorbidity that characterize those who survive overdoses. The advent of the COVID-19 pandemic made meeting the needs of this vulnerable population more complex, with its high burden of co-existing conditions that likely increases risk of adverse outcomes related to infectious disease as well as substance use. Many treatment programs were deploying proactive strategies during COVID-19 to maintain services to vulnerable populations with OUD, including regulatory flexibilities in the provision of MOUD that offer promise, if made permanent, to reduce barriers to treatment for individuals with OUD (Stringer et al., 2021). These and other innovations to improve MOUD access and pro-actively engage at-risk individuals in treatment may be particularly important for improving outcomes for the especially high-risk group of individuals who survive opioid overdoses.
Supplementary Material
Highlights.
Risk of medically-treated overdose more than tripled over 5 years amidst rapid fentanyl penetration.
Increase not mitigated by declining opioid analgesic prescribing.
Overdoses increased fastest among Black and Hispanic beneficiaries.
Mental health and substance use comorbidity substantially increased risk.
Assertive integrated-care interventions suggested to be key for this complex high-risk population.
Acknowledgments
Funding
This work was supported by the National Institute on Drug Abuse (grant number 1R01DA047347-01), with additional support from the Foundation for Opioid Response Efforts, National Center for Advancing Translational Sciences (grant number UL1TR003017), and National Institute on Drug Abuse grant number 1K01DA049950.
Footnotes
Ethical Approval
This study was approved by the Rutgers University Institutional Review Board (IRB # Pro2018002878).
Stephen Crystal: conceptualization, methodology, writing – original draft, writing – review & editing, supervision, funding acquisition; Molly Nowels: methodology, software, formal analysis, data curation, writing – original draft, writing – review & editing, visualization; Mark Olfson: conceptualization, methodology, writing – review & editing; Hillary Samples: conceptualization, methodology, writing – review & editing; Arthur Robin Williams: methodology, writing – review & editing; Peter Treitler: writing – original draft, writing – review & editing, visualization.
Declaration of Competing Interest
Dr. Samples has received consulting fees from the American Society of Addiction Medicine. Dr. Williams receives consulting fees and equity from Ophelia Health, Inc. a telehealth buprenorphine provider.
The other authors have no competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beheshti D. (2019). Adverse health effects of abuse-deterrent opioids: Evidence from the reformulation of OxyContin. Health Economics, 28(12), 1449–1461. 10.1002/hec.3944 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2015). ICD-9-CM addenda, conversion table, and guidelines. https://www.cdc.gov/nchs/icd/icd9cm_addenda_guidelines.htm. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2018). 2018 annual surveillance report of drug-related risks and outcomes — United States. https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2021a). CDC Wonder. https://wonder.cdc.gov/ [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2021b). 2019 Release of ICD-10-CM. https://www.cdc.gov/nchs/icd/icd10cm.htm [Google Scholar]
- Chen KY, Chen L, & Mao J. (2014). Buprenorphine-naloxone therapy in pain management. Anesthesiology, 120(5), 1262–1274. 10.1097/ALN.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone D. (2019). The triple wave epidemic: Supply and demand drivers of the US opioid overdose crisis. The International Journal on Drug Policy, 71, 183–188. 10.1016/j.drugpo.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemans-Cope L, Wishner JB, Allen EH, Lallemand N, Epstein M, & Spillman BC (2017). Experiences of three states implementing the Medicaid health home model to address opioid use disorder - Case studies in Maryland, Rhode Island, and Vermont. Journal of Substance Abuse Treatment, 83, 27–35. 10.1016/j.jsat.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Connery HS (2015). Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Review of Psychiatry, 23(2), 63–75. 10.1097/HRP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- Crowley RA, Kirschner N, & Moyer DV (2016). The integration of care for mental health, substance abuse, and other behavioral health conditions into primary care. Annals of Internal Medicine, 164(6), 447–448. 10.7326/L15-0524 [DOI] [PubMed] [Google Scholar]
- Dilokthornsakul P, Moore G, Campbell JD, Lodge R, Traugott C, Zerzan J, Allen R, & Page RL, 2nd (2016). Risk factors of prescription opioid overdose among Colorado Medicaid beneficiaries. The Journal of Pain, 17(4), 436–443. 10.1016/j.jpain.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Bastian BA, Trinidad JP, Spencer M, & Warner M. (2018). Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. National Vital Statistics Reports, 67(9), 1–14. https://stacks.cdc.gov/view/cdc/61381 [PubMed] [Google Scholar]
- Jacobi JV, Ragone TA, Greenwood K. (2016). Integration of behavioral and physical health care: Licensing and reimbursement barriers and opportunities in New Jersey. Seton Hall University School of Law, Center for Health & Pharmaceutical Law & Policy. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2802830 [Google Scholar]
- Jones CM, Bekheet F, Park JN, & Alexander GC (2020). The evolving overdose epidemic: Synthetic opioids and rising stimulant-related harms, Epidemiologic Reviews, mxaa011. 10.1093/epirev/mxaa011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. (2021). Must-access prescription drug monitoring programs and the opioid overdose epidemic: The unintended consequences. Journal of Health Economics, 75, 102408. 10.1016/j.jhealeco.2020.102408 [DOI] [PubMed] [Google Scholar]
- Maciejewski ML, Zepel L, Hale SL, Wang V, Diamantidis CJ, Blaz JW, Olin S, Wilson-Frederick SM, James CV, & Smith VA (2021). Opioid prescribing in the 2016 Medicare fee-for-service population. Journal of the American Geriatrics Society, 69(2), 485–493. 10.1111/jgs.16911 [DOI] [PubMed] [Google Scholar]
- Mauro P, Samples H, Klein K, & Martins SS (2020). Discussing drug use with health care providers is associated with perceived need and receipt of drug treatment among adults in the United States. Medical Care, 58(7), 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern MP, Lambert-Harris C, Gotham HJ, Claus RE, & Xie H. (2014). Dual diagnosis capability in mental health and addiction treatment services: An assessment of programs across multiple state systems. Administration and Policy in Mental Health, 41(2), 205–214. 10.1007/s10488-012-0449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Wang J, Barocas JA, Jaeger JL, Durham NN, Babakhanlou-Chase H, Bharel M, Walley AY, & Linas BP (2020). Opioid overdose and inpatient care for substance use disorder care in Massachusetts. Journal of Substance Abuse Treatment, 112, 42–48. 10.1016/j.jsat.2020.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K, & Klebonis J. (2015). Designing Medicaid health homes for individuals with opioid dependency: Considerations for states. Center for Medicare and Medicaid Services Health Home Information Resource Center. https://www.chcs.org/resource/designing-medicaidhealth-homes-individuals-opioid-dependency-considerations-states/ [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2019). Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press. 10.17226/25310. [DOI] [PubMed] [Google Scholar]
- National Forensic Laboratory Information System (NFLIS). (2020). Drug Enforcement Agency’s National Forensic Laboratory Information System. https://www.nflis.deadiversion.usdoj.gov/ [Google Scholar]
- New Jersey Office of the Governor. (2019). Governor Murphy announces new initiatives to combat the opioid epidemic. https://www.state.nj.us/humanservices/news/press/2019/approved/20190401.html [Google Scholar]
- New Jersey Division of Mental Health and Addiction Services (NJDMHAS). (2020). NJSAMS contract/FFS documents, annex A boiler plate standard SFY 2019–2020. https://njsams.rutgers.edu/njsams/Documents.aspx. [Google Scholar]
- NJ Office of the Attorney General (NJOAG). (2020). NJCARES dashboard of opioid-related data and information. https://www.njcares.gov [Google Scholar]
- O’Brien P, Henke RM, Schaefer MB, Lin J, & Creedon TB. (2021). Adverse events among adult Medicaid enrollees with opioid use disorder and co-occurring substance use disorders. Drug and Alcohol Dependence, 221, 108555. 10.1016/j.drugalcdep.2021.108555 [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Wall M, Wang S, Liu SM, & Blanco C. (2018). Causes of death after nonfatal opioid overdose. JAMA Psychiatry, 75(8), 820–827. 10.1001/jamapsychiatry.2018.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Taylor J, Caulkins JP, Kilmer B, Reuter P. & Stein BD (2019). The future of fentanyl and other synthetic opioids. RAND Corporation. https://www.rand.org/pubs/research_reports/RR3117.html [Google Scholar]
- Peck KR, Ochalek TA, Streck JM, Badger GJ, & Sigmon SC (2021). Impact of current pain status on low-barrier buprenorphine treatment response among patients with opioid use disorder. Pain Medicine, pnab058. Advance online publication. 10.1093/pm/pnab058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Sullivan MA, Cohen J, Fugon L, Jones JD, Vosburg SK, Cooper ZD, Manubay JM, Mogali S, & Comer SD (2013). Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: Reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain, 154(8), 1442–1448. 10.1016/j.pain.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples H, Williams AR, Crystal S, & Olfson M. (2020). Impact of long-term buprenorphine treatment on adverse health care outcomes in Medicaid. Health Affairs (Project Hope), 39(5), 747–755. 10.1377/hlthaff.2019.01085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Salas J, Grucza R, Sullivan MD, Lustman PJ, Copeland LA, & Ballantyne JC (2021). Depression and buprenorphine treatment in patients with non-cancer pain and prescription opioid dependence without comorbid substance use disorders. Journal of Affective Disorders, 278, 563–569. 10.1016/j.jad.2020.09.089 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, & Carroll KM (2019). Pharmacological and behavioral treatment of opioid use disorder. Psychiatric Research and Clinical Practice, 1(1), 4–15. 10.1176/appi.prcp.20180006 [DOI] [Google Scholar]
- Spencer MR, Warner M, Bastian BA, Trinidad JP, & Hedegaard H. (2019). Drug overdose deaths involving fentanyl, 2011–2016. National Vital Statistics Reports, 68(3), 1–19. https://stacks.cdc.gov/view/cdc/77832. Accessed November 29, 2020. [PubMed] [Google Scholar]
- Strang J. (2015). Death matters: Understanding heroin/opiate overdose risk and testing potential to prevent deaths. Addiction, 110(Suppl 2), 27–35. 10.1111/add.12904 [DOI] [PubMed] [Google Scholar]
- Stewart MT, O’Brien PL, Shields MC, White MC, & Mulvaney-Day N. (2019). State residential treatment for behavioral health conditions: Regulation and policy environmental scan. U.S. Department of Health and Human Services, Assistant Secretary for Planning and Evaluation. https://aspe.hhs.gov/basic-report/state-residential-treatment-behavioral-healthconditions-regulation-and-policy-environmental-scan [Google Scholar]
- Stringer KL, Langdon KJ, McKenzie M, Brockmann B, & Marotta P. (2021). Leveraging COVID-19 to sustain regulatory flexibility in the treatment of opioid use disorder. Journal of Substance Abuse Treatment, 123, 108263. 10.1016/j.jsat.2020.108263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2018). Section 223 demonstration program for certified community behavioral health clinics. https://www.samhsa.gov/section-223 [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2020). National Survey of Substance Abuse Treatment Services (N-SSATS): 2019. https://wwwdasis.samhsa.gov/dasis2/nssats.htm [Google Scholar]
- Tedesco D, Asch SM, Curtin C, Hah J, McDonald KM, Fantini MP, & Hernandez-Boussard T. (2017). Opioid abuse and poisoning: Trends in inpatient and emergency department discharges. Health Affairs (Project Hope), 36(10), 1748–1753. 10.1377/hlthaff.2017.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, & Ciccarone D. (2017). US regional and demographic differences in prescription opioid and heroin-related overdose hospitalizations. The International Journal on Drug Policy, 46, 112–119. 10.1016/j.drugpo.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force (USPTF). (2020). Screening for unhealthy drug use: US Preventive Services Task Force recommendation statement. JAMA, 323(22), 2301–2309. 10.1001/jama.2020.8020 [DOI] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, & Sanghavi DM (2020). Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open, 3(2), e1920622. 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2015). ICD-10: International classification of diseases and related health problems, 10th revision. World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.