Abstract
Background:
The 12-lead ECG plays an important role in triaging patients with symptomatic coronary artery disease, making automated ECG interpretation statements of “Acute MI” or “Acute Ischemia” crucial, especially during prehospital transport when access to physician interpretation of the ECG is limited. However, it remains unknown how automated interpretation statements correspond to adjudicated clinical outcomes during hospitalization. We sought to evaluate the diagnostic performance of prehospital automated interpretation statements to four well-defined clinical outcomes of interest: confirmed ST- segment elevation myocardial infarction (STEMI); presence of actionable coronary culprit lesions, myocardial necrosis, or any acute coronary syndrome (ACS).
Methods:
An observational cohort study that enrolled consecutive patients with non-traumatic chest pain transported via ambulance. Prehospital ECGs were obtained with the Philips MRX monitor from the medical command center and re-processed using manufacturer-specific diagnostic algorithms to denote the likelihood of >>>Acute MI<<< or >>>Acute Ischemia<<<. Two independent reviewers retrospectively adjudicated the study outcomes and disagreements were resolved by a third reviewer.
Results:
Our study included 2400 patients (age 59 ± 16, 47% females, 41% Black), with 190 (8%) patients with documented automated diagnostic statements of acute MI or acute ischemia. The sensitivity / specificity of the automated algorithm for detecting confirmed STEMI (n=143, 6%); presence of actionable coronary culprit lesions (n=258, 11%), myocardial necrosis (n=291, 12%), or any ACS (n=378, 16%) were 62.9% / 95.6%; 37.2% / 95.6%; 38.5% / 96.4%; and 30.7% / 96.3%, respectively.
Conclusion:
Although being very specific, automated interpretation statements of acute MI / acute ischemia on prehospital ECGs are not satisfactorily sensitive to exclude symptomatic coronary disease. Patients without these automated interpretation statements should be considered further for significant underlying coronary disease based on the clinical context.
Keywords: ACS, STEMI, Acute MI, Automated Interpretation, Diagnostic Statements
INTRODUCTION
Automated ECG interpretation algorithms were introduced in the 1960s,1 with vast improvements in their performance throughout the years cementing their role as a routine tool in cardiac care. Further advancement of interpretation capabilities provided an impetus for algorithms to shift from merely providing measurements of ECG metrics to identifying possible patterns of diagnosis. However, the wide array of possible diagnoses, accompanied by the fact that each ECG manufacturer developed their own set of diagnostic statements, deemed it necessary to standardize automated ECG interpretation reports. Thus, the International Society of Computerized Electrocardiology endorsed a recommendation to standardize ECG interpretation, including a comprehensive classification with numerous standardized diagnostic statements.2 This includes 117 primary diagnostic statements under 14 categories, with the aim of being clinically useful statements devoid of ambiguity and overlap.
Among the most important primary statements is the classification of myocardial infarction (MI) and its likely territory (i.e., Anterior MI, Lateral MI). The primary statement is further supported by a modifier, denoting the acuity of the event (i.e. Acute, Recent). Hence, the statement label of an “acute MI” indicates a top emergency, requiring the execution of specific actions across the continuum of care to expeditiously address the condition. Particularly, less experienced ECG readers rely on these defined statements since expert interpreters are not always available for an overread. The importance of an accurate and precise acute MI statement is further magnified as approximately 10 million Americans visit the emergency department (ED) per year with symptomatic coronary artery disease (CAD), making this population the leading cause for medical malpractice lawsuits.3
Previous efforts have been made to provide uniform guidelines for diagnostic statements on “acute MI”, however this has proven to be a challenging task as the target clinical population significantly varies.2 ECG manufacturers often report high performance measures for “acute MI” statements, however an absolute definition of the target population used for accuracy validation of such statements remains nebulous, and physicians remain unaware of their implications4. It is well established that acute cardiac ischemia presents as acute coronary syndrome (ACS), which entails a spectrum of conditions, which further adds to the complexity.5 ECG manufacturers historically tailored their interpretation to target ST-elevation (STE) ACS findings due to necessity of time-critical interventions.6 However, a significant proportion of patients with ACS present with an acute coronary occlusion (ACO) and no apparent STE, which equally requires urgent intervention.7–9 Patients with ACO and no STE findings remain the most important cause for missed ACS diagnoses, making them a true diagnostic challenge.10
Clinicians hold a high index of suspicion for all ECGs stamped with an “acute MI” or “acute ischemia” statement, particularly when documented prior to hospital arrival (i.e., emergency medical service [EMS] or outside hospital). It remains unknown how often the automated ECG computer algorithm misclassifies presence or absence of an acute MI, and more importantly how these documented statements correspond to clinical outcomes. To shed light on how these automated interpretation statements perform against various clinical outcomes of interest across the spectrums of ACS, we sought to evaluate the diagnostic performance of prehospital automated interpretation statements to four well-defined clinical outcomes of interest in a real-life cohort of patients with chest pain transported by EMS.
METHODS
Design, Sample, and Setting
This was a secondary analysis using data from ECG Methods for the Prompt Identification of Coronary Events (EMPIRE study).11,12 Briefly, EMPIRE was an observational cohort study that enrolled consecutive patients >18 years of age with a chief complaint of non-traumatic chest pain who were transported via ambulance by Pittsburgh EMS to one of three participating UPMC-affiliated tertiary care centers. The parent study was approved by the University of Pittsburgh Institutional Review Board.
Data Collection
Independent reviewers manually abstracted in-hospital data elements from the electronic health record (EHR) using recommendations by the American College of Cardiology (ACC) for measuring the management and outcomes of patients with ACS (demographics, past medical history, home medications, clinical presentation and course of hospitalization, laboratory tests, imaging studies, cardiac catheterization, treatments, and in-hospital complications).13 The presence of an acute MI statement was based on an automated interpretation of “>>>Acute MI<<<” or “>>>Acute Ischemia<<<” according to the most recent manufacturer algorithm. Two independent reviewers blinded to the study outcomes manually over-read the prehospital ECG and the subsequent serial ECGs performed in the ED. The presence of STE and ST Depression (STD) was based on the ECG criteria determined by the fourth universal definition of MI,5 while territorial ECG changes were documented as regions based on the recommendations for the standardization interpretation of the ECG,14 The documentation of a low-quality ECG was based on the presence of noise and baseline wander at the discretion of the reviewer.
ECG Methods
For this analysis, we used the prehospital 12-lead ECG obtained during first medical contact. The ECGs were acquired using Mason-Likar electrode positioning. Raw ECG data were acquired using the HeartStart MRX monitor-defibrillator at 500 samples/second (Philips Healthcare). Standard ECG signal pre-processing was completed at the Philips Healthcare Advanced Algorithm Research Center (Andover, MA). The raw digital ECG signal was re-analyzed using the Philips DXL diagnostic algorithm to denote which ECGs met criteria for the diagnostic statements of >>>Acute MI<<< or >>>Acute Ischemia<<<. The diagnostic algorithm does not adjust for electrode positioning. To ensure such diagnostic statements are up to date per the proprietary algorithm, the dataset was processed on 5/26/2020. Annotations documented on the ECG paper header during time of initial ECG retrieval by paramedics were discarded.
Clinical Outcomes
Two independent reviewers retrospectively adjudicated the clinical outcomes of interest during the indexed admission and up to 30-days after discharge. All disagreements were resolved by a third reviewer. The reviewers were provided full access to patient indexed admission data, discharge and follow up records. Reviewers used Cerner and EPIC, the UPMC EHRs of in-hospital and outpatient medical charts respectively, to identify all relevant subsequent medical visits within 30 days of the indexed admission. All Troponin tests were measured using Troponin I assay (Access AccuTnI assay, Beckman Coulter Inc.; 99th percentile cut-off at 0.04 ng/mL). Patients were adjudicated to meeting the criteria of one of the following progressively overlapping clinical outcomes of interest (not mutually exclusive):
confirmed STEMI, defined as documented STE ECG criteria based on the universal definition of MI any time throughout the course of prehospital care or indexed admission, and corresponding confirmatory troponin assay and/or catheterization lab findings.5
actionable coronary culprit lesion, defined according to ACC guidelines to define and measure the degree of coronary artery occlusion.13 Actionable culprit lesions were based on a newly placed stent due to a critical coronary occlusion as per interventional cardiologist discretion, who usually refers to TIMI flow criteria (not simply the degree of occlusion). Culprit lesions were categorized according to the coronary artery involved, including left main coronary artery (LMCA), Left Anterior Descending Artery (LAD), Left Circumflex (LCX), or Right Coronary Artery (RCA) or one of their main branches (first Obtuse Marginal [OM1], first Diagonal [D1], or Right Posterior Descending Artery [RPDA]).
myocardial necrosis, defined as elevation of cardiac troponin (i.e., > 99th percentile) and the presence of focal myocardial ischemia on cardiac imaging (i.e., echocardiogram, nuclear imaging, or angiographic evidence). We set the imaging criteria to exclude other non-ACS causes of ischemia that leads to troponin leak such as pericarditis, pulmonary embolism, demand ischemia, tachycardia, HF exacerbation. Specifically, there were 471 patients with elevated troponin in whom only 304 were attributed to ACS ischemia and 167 were adjudicated as non-ACS related in our study, with all ACS ischemia cases confirmed by some sort of imaging.
any ACS event (Myocardial necrosis + unstable angina), defined as any of the previous categories plus unstable angina (UA). UA was identified based on symptoms, ECG findings, cardiologist disposition, and provocative testing and catheterization lab findings if applicable. It is worth noting that patients with UA in this study (n=74, 3%) were included under this umbrella category because it would be clinically unreasonable to consider UA as a separate diagnostic category. The latter would split the sample into patients with UA versus all others (including those with acute MI), which would misleadingly translate into a very low sensitivity. Thus, we included UA under this umbrella category; an approach that most accurately resembles clinical reasoning during chest pain diagnostics.
Finally, we adjudicated for 30-day major adverse cardiac events (MACE), which was based on the composite outcome of an event of cardiac arrest, ventricular arrythmia, acute heart failure, cardiogenic shock, mechanical ventilation, reinfarction, revascularization, or any cause death within 30 days of the indexed admission.
Statistical Analysis
SPSS Statistics software version 27 (IBM Corporation, Armonk, NY) was used for statistical analysis. Continuous variables were reported as means ± SD or median [25th – 75th percentiles], categorical variables were reported as n (%). Comparison between groups was made using chi square for categorical variables. The performance of the automated algorithm in each subpopulation was assessed by examining the accuracy, sensitivity, specificity, positive predictive value, and negative predictive value.15 The performance in each group was compared using Delong’s test to assess for differences in performance measures. The level of statistical significance was set at P < 0.05 for two-sided hypothesis testing.
RESULTS
Our study included 2400 patients (age 59 ± 16, 47% females, 41% Black), with 190 (8%) patients with documented automated interpretation statement of “acute MI” (n=134) or “acute ischemia” (n=56) on their prehospital ECGs. There were 378 (16%) cases of ACS, including 291 (12%) cases of acute MI, 258 (11%) with culprit lesions (127 LAD culprits, 55 LCX culprits, 117 RCA culprits), and 143 (6%) patients with STEMI.
Table 1 shows the diagnostic performance of automated ECG interpretation statements against the four clinical outcomes of interest. The highest accuracy was seen for confirmed STEMI at 93.7% (95% CI 92.6% - 94.6%); decreasing as the clinical outcome of interest broadens to capture any ACS event to 86.0% (95% CI 84.6% - 87.4%). This drastic decrease in performance is highly attributable to loss in sensitivity rather than specificity, which dropped from 62.9% to 30.7%. The specificity was consistent stable at ~96%. When examining “acute MI” statements alone, the positive predictive value improved slightly for STEMI (47.6% → 63.4%), actionable culprit lesions (50.5% → 61.2%), and myocardial necrosis (59.3% → 69.4%).
Table 1.
Performance of the automated interpretation statements in detecting in-hospital outcomes of interest
| Confirmed STEMI (n=143) | Actionable Culprits Lesion (n=258) | Myocardial necrosis (n=291) | Any ACS event (n=378) | |
|---|---|---|---|---|
| Accuracy | 93.7 (92.6–94.6) | 89.3 (88.0–90.5) | 89.3 (88.0–90.5) | 86.0 (84.6–87.4) |
| Sensitivity | 62.9 (54.5–70.9) | 37.2 (31.3–43.4) | 38.5 (32.9–44.3) | 30.7 (26.1–35.6) |
| Specificity | 95.6 (94.7–96.4) | 95.6 (94.7–96.4) | 96.4 (95.5–97.1) | 96.3 (95.4–97.1) |
| PPV | 47.6 (41.9–53.4) | 50.5 (44.2–56.8) | 59.3 (52.8–65.4) | 61.1 (54.5–67.3) |
| NPV | 97.6 (97.1–98.1) | 92.7 (92.0–93.3) | 91.9 (91.2–92.6) | 88.1 (87.4–88.8) |
Abbreviations- STEMI: ST Segment Elevation Myocardial Infarction; MI: Myocardial Infarction; ACS: Acute Coronary Syndrome; PPV: Positive Predictive Value; NPV: Negative Predictive Value.
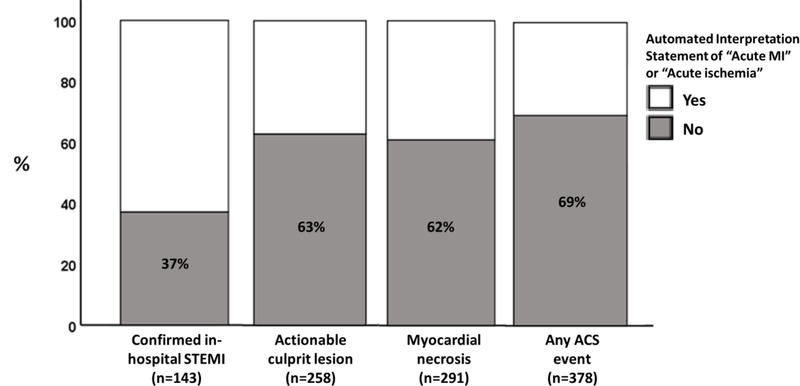
To appreciate the loss of sensitivity as the main driver of diagnostic performance of the automated interpretation algorithms, Figure 1 shows the proportion of missed patients at each of the four clinical outcomes of interest. The proportion of confirmed STEMI cases with no “acute MI” or “acute ischemia” on their prehospital ECG was 37%, increasing up to 69% in those with any ACS event (p < 0.001). Notably, ~62% of patients with either actionable culprit lesions or myocardial necrosis also had no “acute MI” or “acute ischemia” on their prehospital ECG. Such low sensitivity was also evident when we used an “acute MI” statement alone, with a sensitivity of 59% for STEMI, 32% for culprit lesions, and 25% for ACS. Table 2 describes the demographic and clinical characteristics of these patients with missed diagnostic statements on their prehospital ECG (false negatives). Importantly, older patients with more comorbidities seem to be more likely to be missed as the outcome of interest broadens beyond STEMI detection.
Figure 1. Diagnostic accuracy of automated ECG interpretation statements across various clinical outcomes of interest.
The percentages on the bar chart reflect the proportion of patients with the clinical outcome of interest but were missed by the automated interpretation software.
Table 2.
Characteristics of missed cases with no automated statement of “acute MI” or “acute ischemia” on their prehospital ECG
| Outcome of Interest | ||||
|---|---|---|---|---|
|
| ||||
| Confirmed STEMI (n=53/143, 37%) | Actionable Culprit Lesion (n=162/258, 63%) | Myocardial necrosis (n=179/291,62%) | Any ACS event (n=262/378, 69%) | |
|
| ||||
| Age | 63 ± 13 | 65 ± 13 | 66 ± 15 | 67 ± 14 |
|
| ||||
| Female Sex | 26% | 40% | 40% | 42% |
|
| ||||
| Black Race | 23% | 27% | 32% | 34% |
|
| ||||
| Comorbidities | ||||
| Hypertension | 72% | 80% | 78% | 82% |
| Diabetes | 25% | 21% | 34% | 35% |
| Hyperlipidemia | 47% | 54% | 52% | 56% |
| Smoking History | 47% | 58% | 59% | 60% |
| Heart Failure | 9% | 19% | 18% | 21% |
| CAD | 38% | 60% | 50% | 60% |
| Prior PCI/CABG | 38% | 59% | 46% | 55% |
|
| ||||
| Physician over-read of prehospital ECG | ||||
| ECG of low quality | 33% | 27% | 40% | 34% |
| Documented STE | 41% | 15% | 16% | 12% |
| Documented STD | 47% | 32% | 34% | 30% |
|
| ||||
| Physician Over-read of serial ECGs | ||||
| Documented STE | 60% | 25% | 24% | 19% |
| Documented STD | 57% | 38% | 45% | 38% |
|
| ||||
| Treatment | ||||
| PCI/CABG | 87% | 84% | 62% | 55% |
|
| ||||
| 30-day MACE | 49% | 39% | 45% | 37% |
Values are mean ± SD; or n (%). Percentages in heading indicates the proportion missed by the automated interpretation from total in corresponding ACS subgroup. Abbreviations- CAD: Coronary Artery Disease; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Grafting; PH: Prehospital; ED: Emergency Department STE: ST-segment elevation; STD: ST-segment depression; MACE: Major Adverse Cardiac Events. PH manual Interpretation indicates interpretation of the prehospital ECG by independent reviewers. ED manual interpretation indicates interpretation of the first ED ECG by independent reviewers.
DISCUSSION
In this study, we sought to evaluate the diagnostic performance of automated ECG interpretation statements in patients with symptomatic CAD. We found that the highest accuracy corresponds to identifying cases of STEMI, yet the accuracy sharply declines when moving to other clinical outcomes of interest. Most notably, as the number of false negatives increases, accuracy declines. This is reflected in the sharp reduction in sensitivity from approximately 60% in STEMI cases, to approximately 30% when examining other ACS subtypes.
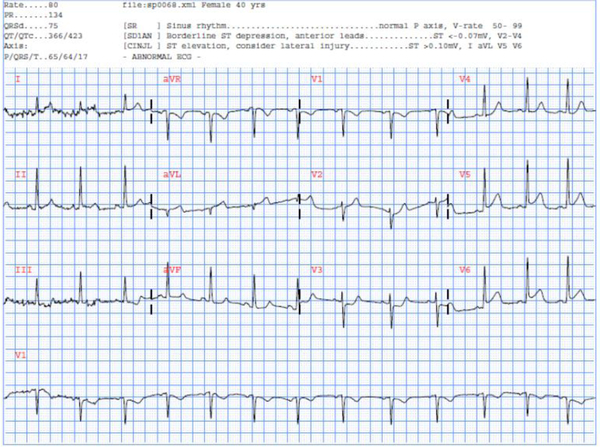
Computerized ECG interpretation algorithms have been primarily focused on identifying patients in need of critical intervention, particularly STEMI. Our findings show that the automated interpretation falls short in identifying all patients in this subgroup, with many false negatives. It is well established that automated interpretation has high false negative rates for STEMI, and very high variability across algorithms.16 Among the problems cited due to this variability is the lack of gold standard population characterization for true validation of the algorithms. Defining the validation population is central to the American Heart Association’s (AHA) recommendation for avoiding reliance on automated interpterion for recognition and diagnosis of STEMI.17 Moreover, it has been shown that artifacts, among other confounders, heavily influence automated interpretations, further leading to misinterpreation.18 Figure 2 shows an ECG from our study with a discharge diagnosis of lateral STEMI and documented total occlusion in the LAD. Our independent ECG reviewers documented the presence of an STE in the lateral leads. However, while the automated algorithm did note the ischemic changes, it did not declare a diagnostic statement of “acute MI” or “acute ischemia” of which many prehospital providers rely. This is possibly due to borderline STE and the ST/T ratio not meeting the software algorithm threshold, both significant causes of false negatives.18
Figure 2: Selected example of a confirmed STEMI case but not automated interpretation statement of “acute MI” or “acute ischemia”.
This was a 40-year-old female patient who had a peak troponin of 9 and was admitted to the ICU due to concomitant acute heart failure. The independent ECG reviewers documented the presence of an STE in the lateral leads. The automated algorithm did note the changes, however, did not provide an acute MI statement.
Our findings further highlight that the performance of computerized interpretations sharply declines when the condition of interest is documented as an actionable culprit lesion. Patients with total occlusion are increasingly identified as a high-risk population requiring urgent intervention, independent of their ST segment ECG findings.7,19 Aslanger et al. recently called for a shift from a STEMI only paradigm for identifying patients requiring critical intervention, citing clinicians’ ability to recognize those with total occlusion when comprehensively assessing the ECG.19,20 The capabilities of automated interpretations to identify those requiring urgent intervention by accounting for findings beyond the classical cut-offs for STE segment changes are unknown, especially since adjustments require nuance to balance sensitivity and specificity. 21 To the best of our knowledge, we are the only study to investigate the performance of computerized interpretation to identify occlusion MI (i.e., actionable culprit lesions inclusive of both STEMI and NSTEMI patients). Garvey et al. reported the performance of three automated interpretations to identify the presence of culprit lesions as the primary outcome, noting universally low sensitivity, however, all patients had some form of STE on their prehospital ECGs.6
The computerized interpretation performance was the poorest in our ‘any ACS’ subgroup, missing approximately 70% of this population. This raises a concern regarding the ability of algorithms to aid in identifying which patients need to be transferred to a specialized center for care, regardless of their need for urgent intervention or catheterization lab activation. A recent study comparing the performance of seven ECG algorithms in identifying ACS noted a high rate of false negatives, with all algorithms reporting a false negative rate above 50%.22 Notably, the aforementioned study compared performance of the automated algorithms relative to the ECG interpretation to expert cardiologist majority votes, reporting low agreement. Among the challenges cited in the study include the lack of uniform ACS diagnostic statements across ECG manufactures, hence they devised a repository of statements labeled “critical result statements” to eliminate variability. Similarly, ter Haar et al. investigated the performance of an automated algorithm on prehospital ECGs in identifying any myocardial ischemia, with a reported sensitivity of 67%, with the authors further highlighting the difficulty of establishing an objective measure for myocardial ischemia.23. Our results add to the shortcoming highlighted in these previous studies, with the lack of elucidation of what population an acute MI/acute ischemia truly targets, particularly in light our findings across different ACS subclassifications. Albeit some ACS/acute MI do not require intervention, it is well known that patients who develop NSTEMI/UA are usually sicker with higher co-morbidity burden, which means any delay in transport to the proper facility could lead to dire outcomes, independent of their need for catherization lab activation and immediate intervention.
It is worth mentioning that our findings report the results of a single algorithm in a high-risk population with known diagnostic challenges. It is well known that confounders and artifacts highly influence the performance of automated algorithms, which is applicable in our study sample.18 Moreover, the automated algorithm used in this study does not adjust the algorithm according to Mason-Likar ECG lead configuration, which could influence performance.24 A third limitation to this work is that the degree of ischemia in patients with UA fluctuates, suggesting that it is possible the prehospital ECG was acquired after ischemia subsided. In this case (normal prehospital ECG), these ECGs could have exaggerated the rate of false negatives (and low sensitivity) seen when evaluating the algorithm performance against “any ACS” event.
Our results have important clinical implications with potential immediate impact. First, we highlight that without a clear definition of the validation population, wide variability in performance measures may occur. This should provide an impetus to have a well-defined and standardized criterion for the population recruited for algorithm validation. While having standardized reporting statements does aid in the utility of automated interpretations, validation of these statements in widely heterogenous diagnostic entities undermine the root purpose of their existence. Second, as the paradigm of care is shifting from using STE as the sole criterion for identifying patients requiring urgent intervention,20 automated interpretations seem to be ill equipped to identify populations beyond those with clear STE findings. If automated interpretations are aiming to be the cornerstone for catherization lab activation, future work should aim to identity all patients who require urgent intervention, beyond only those with STEMI. Finally, considering the capabilities of modern machine learning algorithms to provide versatile ECG-based diagnostic assessments, future work on automated interpretations should include input from the reader regarding the setting they are acquiring the ECG and their condition of interest. General screening for a physician office visit could utilize difference electrode placement when acquiring ECG, and inherently requires a different analysis than a chest pain patient calling EMS, hence a 12-lead ECG analysis based on user input for the that specific patient could possibly provide better utility and improved performance.
CONCLUSION
Automated ECG interpretation statements on prehospital ECGs show the highest accuracy in identifying cases of STEMI, yet the accuracy sharply declines when moving to other clinical outcomes of interest across the wide spectrum of coronary syndromes. The accuracy declines as a manifestation of the increase in number of false negatives, which is reflected in the sharp decline of sensitivity when moving from STEMI cases to other ACS subtypes. A clear and mutual definition of clinical outcomes of interest for which automated ECG interpretation algorithms are targeted is needed.
Acknowledgment
The study team would like to thank the paramedics and leadership of the City of Pittsburgh Bureau of EMS for their partnership in completing this research, and for the acquisition and transmission of 12-lead ECGs for the study cohort as part of clinical care.
Funding:
National Institute of Health grant # R01HL137761
Footnotes
Conflict of Interest: US Patent # 10820822
Trial Registration: ClinicalTrials.gov # NCT04237688
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pipberger HV, Arms RJ, Stallmann FW. Automatic screening of normal and abnormal electrocardiograms by means of digital electronic computer. Proc Soc Exp Biol Med. 1961;106:130–132. [DOI] [PubMed] [Google Scholar]
- 2.Mason JW, Hancock EW, Gettes LS, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part II: electrocardiography diagnostic statement list a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2007;49(10):1128–1135. [DOI] [PubMed] [Google Scholar]
- 3.Storrow AB, Gibler WB. Chest pain centers: diagnosis of acute coronary syndromes. Ann Emerg Med. 2000;35(5):449–461. [PubMed] [Google Scholar]
- 4.Estes NA 3rd. Computerized interpretation of ECGs: supplement not a substitute. Circ Arrhythm Electrophysiol. 2013;6(1):2–4. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618–e651. [DOI] [PubMed] [Google Scholar]
- 6.Garvey JL, Zegre-Hemsey J, Gregg R, Studnek JR. Electrocardiographic diagnosis of ST segment elevation myocardial infarction: An evaluation of three automated interpretation algorithms. J Electrocardiol. 2016;49(5):728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan AR, Golwala H, Tripathi A, et al. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017;38(41):3082–3089. [DOI] [PubMed] [Google Scholar]
- 8.Wang TY, Zhang M, Fu Y, et al. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non–ST-elevation acute coronary syndromes undergoing diagnostic angiography. American Heart Journal. 2009;157(4):716–723. [DOI] [PubMed] [Google Scholar]
- 9.IJkema BB, Bonnier JJ, Schoors D, Schalij MJ, Swenne CA. Role of the ECG in initial acute coronary syndrome triage: primary PCI regardless presence of ST elevation or of non-ST elevation. Neth Heart J. 2014;22(11):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–1170. [DOI] [PubMed] [Google Scholar]
- 11.Al-Zaiti S, Besomi L, Bouzid Z, et al. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat Commun. 2020;11(1):3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Zaiti SS, Martin-Gill C, Sejdic E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the Electrocardiographic Methods for the Prehospital Identification of Non-ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48(6):921–926. [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Brindis RG, Chaitman BR, et al. 2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease. J Am Coll Cardiol. 2013;61(9):992–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner GS, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram: Part VI: Acute Ischemia/Infarction A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology. 2009;53(11):1003–1011. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11):e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlapfer J, Wellens HJ. Computer-Interpreted Electrocardiograms: Benefits and Limitations. J Am Coll Cardiol. 2017;70(9):1183–1192. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S787–817. [DOI] [PubMed] [Google Scholar]
- 18.Bosson N, Sanko S, Stickney RE, et al. Causes of Prehospital Misinterpretations of ST Elevation Myocardial Infarction. Prehosp Emerg Care. 2017;21(3):283–290. [DOI] [PubMed] [Google Scholar]
- 19.Pendell Meyers H, Bracey A, Lee D, et al. Accuracy of OMI ECG findings versus STEMI criteria for diagnosis of acute coronary occlusion myocardial infarction. Int J Cardiol Heart Vasc. 2021;33:100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslanger EK, Meyers PH, Smith SW. STEMI: A transitional fossil in MI classification? J Electrocardiol. 2021;65:163–169. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Pahlm O, Warren JW, Sapp JL, Horacek BM. Criteria for ECG detection of acute myocardial ischemia: Sensitivity versus specificity. J Electrocardiol. 2018;51(6S):S12–S17. [DOI] [PubMed] [Google Scholar]
- 22.De Bie J, Martignani C, Massaro G, Diemberger I. Performance of seven ECG interpretation programs in identifying arrhythmia and acute cardiovascular syndrome. J Electrocardiol. 2020;58:143–149. [DOI] [PubMed] [Google Scholar]
- 23.Ter Haar CC, Peters RJG, Bosch J, et al. An initial exploration of subtraction electrocardiography to detect myocardial ischemia in the prehospital setting. Ann Noninvasive Electrocardiol. 2020;25(3):e12722–e12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevilla DC, Dohrmann ML, Somelofski CA, Wawrzynski RP, Wagner NB, Wagner GS. Invalidation of the resting electrocardiogram obtained via exercise electrode sites as a standard 12-lead recording. Am J Cardiol. 1989;63(1):35–39. [DOI] [PubMed] [Google Scholar]