Abstract
Primary aldosteronism is a common, yet highly under-diagnosed, cause of hypertension that leads to disproportionately high rates of cardiovascular disease. Hypertension plus hypokalemia is a guideline-recommended indication to screen for primary aldosteronism, yet the uptake of this recommendation at the population level remains unknown. We performed a population-based retrospective cohort study of adults ≥18 years-old in Ontario, Canada with hypertension plus hypokalemia (potassium <3.5 mEq/L) from 2009–2015 with follow-up through 2017. We measured the proportion of individuals who underwent primary aldosteronism screening via the aldosterone-to-renin ratio based upon hypokalemia frequency and severity along with concurrent antihypertensive medication use. We assessed clinical predictors associated with screening via Cox regression. The cohort included 26,533 adults of which only 422 (1.6%) underwent primary aldosteronism screening. When assessed by number of instances of hypokalemia over a 2-year time window, the proportion of eligible patients who were screened increased only modestly from 1.0% (158/15,983) with one instance to 4.8% (71/1,494) with ≥5 instances. Among individuals with severe hypokalemia (potassium <3.0 mEq/L), only 3.9% (58/1,422) were screened. Among older adults prescribed ≥4 antihypertensive medications, only 1.0% was screened. Subspecialty care with endocrinology (HR 1.52 [95%CI 1.10–2.09]), nephrology (HR 1.43 [95%CI 1.07–1.91]), and cardiology (HR 1.39 [95%CI 1.14–1.70]) were associated with an increased likelihood of screening while age (HR 0.95 [95%CI 0.94–0.96]) and diabetes (HR 0.66 [95%CI 0.50–0.89]) were inversely associated with screening. In conclusion, population-level uptake of guideline recommendations for primary aldosteronism screening is exceedingly low. Increased education and awareness are critical to bridge this gap.
Keywords: Primary aldosteronism, hyperaldosteronism, hypokalemia, hypertension, screening
Summary
Population-level uptake of guideline recommendations for primary aldosteronism screening is exceedingly low. Increased education and awareness are critical to bridge this gap.
INTRODUCTION
Primary aldosteronism (PA), characterized by renin-independent aldosterone secretion from one or both adrenal glands, is increasingly recognized as a common cause of hypertension. Modern-day prevalence studies estimate PA to account for 5–20% of all cases of hypertension.1–5 Compared with essential hypertension, PA results in substantially higher rates of cardiovascular disease.6–8 As much of the excess cardiovascular risk associated with PA occurs independent of blood pressure,6 early diagnosis and implementation of targeted therapies, such as mineralocorticoid receptor (MR) antagonists or surgical adrenalectomy, are vital to improve health outcomes for individuals with PA.
The recommended diagnostic approach to PA begins with measuring the aldosterone-to-renin ratio (ARR), a test easily measured in the ambulatory setting typically without any additional preparation beyond holding MR antagonists.9–11 An elevated ARR along with renin suppression reflects renin-independent aldosteronism and, when combined with an overt clinical phenotype and/or dynamic confirmatory testing, helps to establish a formal diagnosis of PA.11 Current international PA guidelines9, 10 advise ARR screening for high-risk populations including hypertension plus hypokalemia, hypertension plus an adrenal adenoma, and severe or resistant hypertension. Hypertension plus hypokalemia is one of the classic clinical scenarios in which physicians are trained to consider the diagnosis of primary aldosteronism. In fact, up to 30% of patients with hypertension plus unexplained hypokalemia may have primary aldosteronism.2
Single-center studies assessing ARR testing among individuals who meet guideline-recommended clinical criteria for PA screening have demonstrated alarmingly low screening rates of 1–3%.12–14 Two recent studies extended these findings to the population-level by demonstrating exceedingly low rates of screening among adults with hypertension in Alberta, Canada (0.7%)15 and among United States veterans with resistant hypertension (1.6%).16 However, no similar population-level study has been performed to specifically explore, in an in-depth fashion, PA screening rates among individuals with hypertension plus hypokalemia. Herein, we conducted a large population-level study of adults with hypertension plus hypokalemia in Ontario, Canada to determine PA screening rates and investigate how screening varied as a function of factors such as severity of hypokalemia, frequency of hypokalemia, and concurrent medication use.
METHODS
The de-identified, patient-level data used in this study will not be made publically available.
Study Design and Setting
We conducted a population-level, retrospective cohort study of adults ≥18 years of age with hypertension plus hypokalemia from October 1, 2009 through September 30, 2015 with follow-up through September 30, 2017 in Ontario, Canada using linked databases held at the Institute for Clinical Evaluative Sciences (ICES). Ontario is Canada’s largest province with >14 million residents.17 ICES captures data on all Ontario residents who undergo a healthcare encounter including healthcare visits, laboratory tests, hospitalizations, and vital statistics. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. The reporting of this study follows guidelines for observational studies (Table S1).18, 19
Data Sources
We ascertained baseline characteristics, medication data, and outcome data from de-identified, linked databases housed at ICES. Demographic and vital status information was obtained from the Ontario Registered Persons Database. Diagnostic and procedural information from all hospitalizations were determined using the Canadian Institute for Health Information Discharge Abstract Database. Diagnostic information from emergency room and day surgery visits was determined using the National Ambulatory Care Reporting System. Information was also obtained from the Ontario Health Insurance Plan (OHIP) database, which contains all health claims for inpatient and outpatient physician services. Whenever possible, we defined baseline characteristics and outcomes using validated codes (Table S2). Laboratory information is contained in the Ontario Laboratory Information System which captures laboratory tests for individuals in Ontario. Medication information was obtained for individuals ≥65 years of age (the ages at which all Ontario residents have access to universal public healthcare including drug coverage) from the Ontario Drug Benefit Claims database. This database contains highly accurate records of all outpatient prescriptions dispensed to individuals ≥65 years of age, with an error rate of <1%.20 Medication data was not available for individuals <65 years of age. The databases were complete for all other variables used except for rural residence and income, which were missing in <0.5% of individuals. The only reason for lost follow-up was emigration from the province which occurs in <0.5% of Ontario residents annually.21
Cohort Definition
All Ontario residents ≥18 years of age with a diagnosis of incident hypertension plus outpatient hypokalemia (K+ <3.5 mEq/L) between October 1, 2009 and September 30, 2015 were included (Figure 1). Hypertension was defined by either: a) a hospital admission with an International Statistical Classification of Diseases and Related Health Problems (ICD)-9 or ICD-10 code for hypertension or b) an OHIP claim with a hypertension diagnosis followed by either an additional OHIP claim or a hospital admission with a hypertension diagnosis within two years -- see Table S2 for the full list of ICD and OHIP diagnosis codes included. This definition of hypertension using administrative data has been shown to have a sensitivity of 75%, specificity of 94%, positive predictive value of 81%, and negative predictive value of 92%.22, 23 Only outpatient hypokalemia measurements were included in an attempt to avoid hypokalemia related to acute illness. The date upon which an individual met the guideline-recommended screening criteria9 for PA by having both hypertension and hypokalemia (i.e., the latter of the two dates between when an individual was diagnosed with hypertension and when they were found to be hypokalemic) served as the study index date, date of cohort entry, and beginning of follow-up. Individuals were excluded if they were prescribed an MR antagonist at or within two years prior to index, if they had an adrenalectomy prior to index, if they had no healthcare visits or laboratory data within two years prior to index, if they had an ARR measurement performed prior to index, or if they had an eGFR <30 mL/min/1.73m2 prior to index. The maximum follow-up date was September 30, 2017.
Figure 1. Flow chart for cohort assembly.
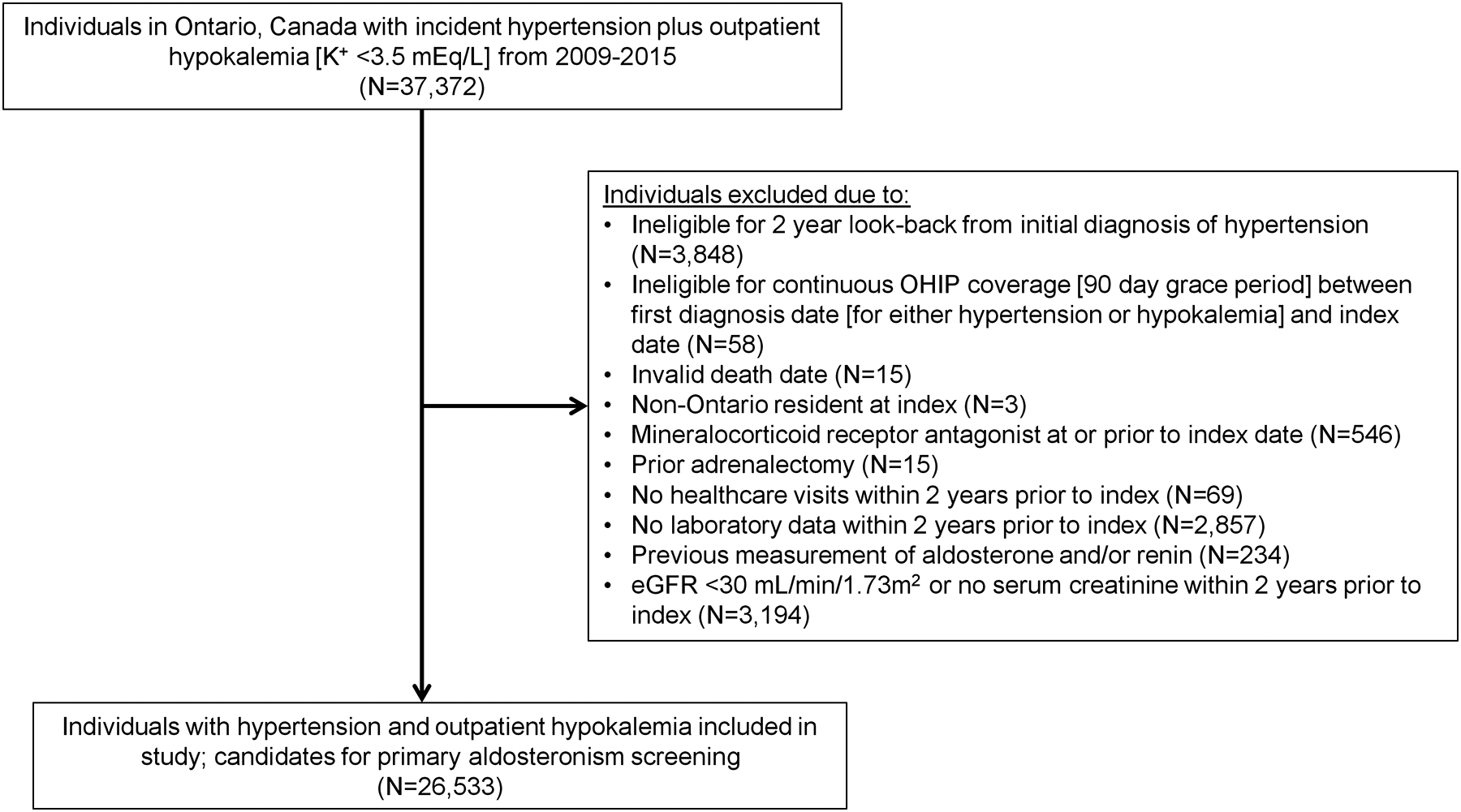
Abbreviations: K+, potassium; eGFR, estimated glomerular filtration rate; N, number; OHIP, Ontario Health Insurance Plan.
Exposure
The study exposure was the presence of hypertension plus outpatient hypokalemia as reflective of guideline-recommended indications to screen for PA.9 Our primary analysis defined hypokalemia as a serum K+ <3.5 mEq/L. In an additional analysis, we examined a lower threshold for hypokalemia as a serum K+ <3.0 mEq/L.
Outcomes
The outcome of interest was an outpatient measurement of aldosterone and/or renin (used to determine the ARR) as a screen for PA. We examined two- and five-year periods for ARR screening to be performed after the index date.
Statistical Analysis
We compared baseline characteristics of individuals with hypertension plus hypokalemia who underwent ARR screening and those who did not. Continuous variables were reported as mean (standard deviation [SD]) if normally distributed and median (25th-75th percentile interquartile range [IQR]) if non-normally distributed. Categorical variables were reported as overall number (%). We then calculated crude proportions of ARR screening by i) year of index, ii) absolute number of instances of outpatient hypokalemia [separated by a minimum of 14 days], iii) the more stringent definition of hypokalemia as a serum K+ <3.0 mEq/L, and iv) extension of the follow-up period from two years to five years. Further, we calculated the proportion of individuals with hypertension plus hypokalemia limited to those with medication information availability (≥65 years of age) by the number of antihypertensive medications prescribed and the use of potassium-wasting diuretics. Finally, we used Cox proportional hazards modeling to examine predictors associated with ARR screening. Our models included the following variables selected a priori based upon clinical knowledge and previous literature: age, sex, rural residence, diabetes mellitus, cardiovascular disease (defined as a composite of coronary artery disease, myocardial infarction, coronary artery bypass graft, congestive heart failure, and stroke), and subspecialty care including general internal medicine, endocrinology, nephrology, and cardiology care.2, 6, 8, 9, 15, 16, 24, 25 We conducted all analyses using SAS v7.15 (SAS Institute Inc., Cary, NC, USA). 95% confidence intervals (CI) that did not overlap with 1.0 and two-sided p-values <0.05 were treated as statistically significant.
RESULTS
Baseline Characteristics
From a total of 37,372 eligible Ontario residents with hypertension plus outpatient hypokalemia within the accrual period, 26,533 met our inclusion criteria (Figure 1). Among this population, 422 (1.6%) underwent ARR screening while 26,111 (98.4%) did not. Table 1 displays the baseline characteristics among the overall cohort, the individuals who underwent ARR screening, and the individuals who did not undergo ARR screening. Individuals who underwent ARR screening were younger, of higher socioeconomic status, had higher eGFR, and had generally less comorbidities.
Table 1:
Baseline characteristics among individuals with hypertension and hypokalemia by primary aldosteronism screening status.
| Characteristic | Overall Cohort | No ARR Screening | ARR Screening |
|---|---|---|---|
| Total (N) | 26533 | 26111 | 422 |
| Age, Mean (SD) | 58 (14) | 58 (14) | 48 (13) |
| Female Sex, N (%) | 16372 (62) | 16112 (62) | 260 (62) |
| Income Quintile, N (%) * | |||
| Quintile 1 | 6389 (24) | 6311 (24) | 78 (18) |
| Quintile 2 | 5681 (21) | 5605 (21) | 76 (18) |
| Quintile 3 | 5186 (20) | 5094 (20) | 92 (22) |
| Quintile 4 | 4861 (18) | 4771 (18) | 90 (21) |
| Quintile 5 | 4333 (16) | 4249 (16) | 84 (20) |
| Rural, N (%) † | 2163 (8) | 2133 (8) | 30 (7) |
| Laboratory Data, Mean (SD) | |||
| Serum Potassium, mEq/L | 3.3 (0.2) | 3.3 (0.2) | 3.2 (0.3) |
| eGFR, mL/min/1.73m2 | 88 (20) | 88 (20) | 95 (20) |
| # of Prescribed Antihypertensive Medications, Mean (SD) ‡ | 1.6 (1.1) | 1.6 (1.1) | 1.7 (1.2) |
| Comorbidities, N (%) § | |||
| Coronary Artery Disease | 3937 (15) | 3894 (15) | 43 (10) |
| Myocardial Infarction | 431 (2) | 426–430 (2) | ≤5 (1)|| |
| Coronary Artery Bypass Graft | 141 (1) | 141 (1) | 0 (0) |
| Congestive Heart Failure | 1939 (7) | 1925 (7) | 14 (3) |
| Atrial Fibrillation | 907 (3) | 902–906 (3) | ≤5 (1)|| |
| Arrhythmia | 1393 (5) | 1381 (5) | 12 (3) |
| Stroke | 575 (2) | 568 (2) | 7 (2) |
| Diabetes Mellitus | 5207 (20) | 5149 (20) | 58 (14) |
| Specialty Care, N (%) # | |||
| General Internal Medicine | 9588 (36) | 9428 (36) | 160 (38) |
| Cardiology | 12678 (48) | 12455 (48) | 223 (53) |
| Endocrinology | 2057 (8) | 2011 (8) | 46 (11) |
| Nephrology | 2247 (8) | 2191 (8) | 56 (13) |
Income quintile missing in 83 individuals (0.3% of cohort).
Rural defined as residing in a location with population <10,000, missing in 32 individuals (0.1% of cohort).
Medication data only available for individuals ≥65 years of age (N=8216, 31% of cohort).
Comorbidities were ascertained in the 2 years prior to cohort entry.
In accordance with ICES privacy policies, cell sizes less than or equal to five cannot be reported.
Specialty care was ascertained in the one year prior to cohort entry.
Abbreviations: ARR, aldosterone-to-renin ratio; N, number; SD, standard deviation.
ARR Screening by Year of Index
When stratified by year, ARR screening among individuals with hypertension plus hypokalemia ranged from 1.2% to 2.7% (Figure 2). There was no clear increase or decrease in the rates of screening over the time period of this study.
Figure 2. Primary aldosterone screening via the aldosterone-to-renin ratio among individuals with hypertension plus hypokalemia (serum K+ <3.5 mEq/L) by year.

Abbreviations: ARR, aldosterone-to-renin ratio; K+, potassium.
ARR Screening by Number of Hypokalemia Measurements
Among individuals with repeat hypokalemia measurements within the 2-year time frame post-index, the proportion of ARR screening increased from 158/16141 (1.0%) with one instance of hypokalemia to 88/5658 (1.6%) with two instances of hypokalemia to 63/2199 (2.9%) with three instances of hypokalemia to 42/1041 (4.0%) with four instances of hypokalemia to 71/1494 (4.8%) with five or more instances (Figure 3A). Notably, the overall proportion of ARR screening across each of these categories of multiple hypokalemia measurements remained low at <5%.
Figure 3. Primary aldosterone screening via the aldosterone-to-renin ratio among individuals with hypertension plus hypokalemia based on number of hypokalemia measurements, definition of hypokalemia threshold, length of follow-up time, and concurrent antihypertensive medication use.
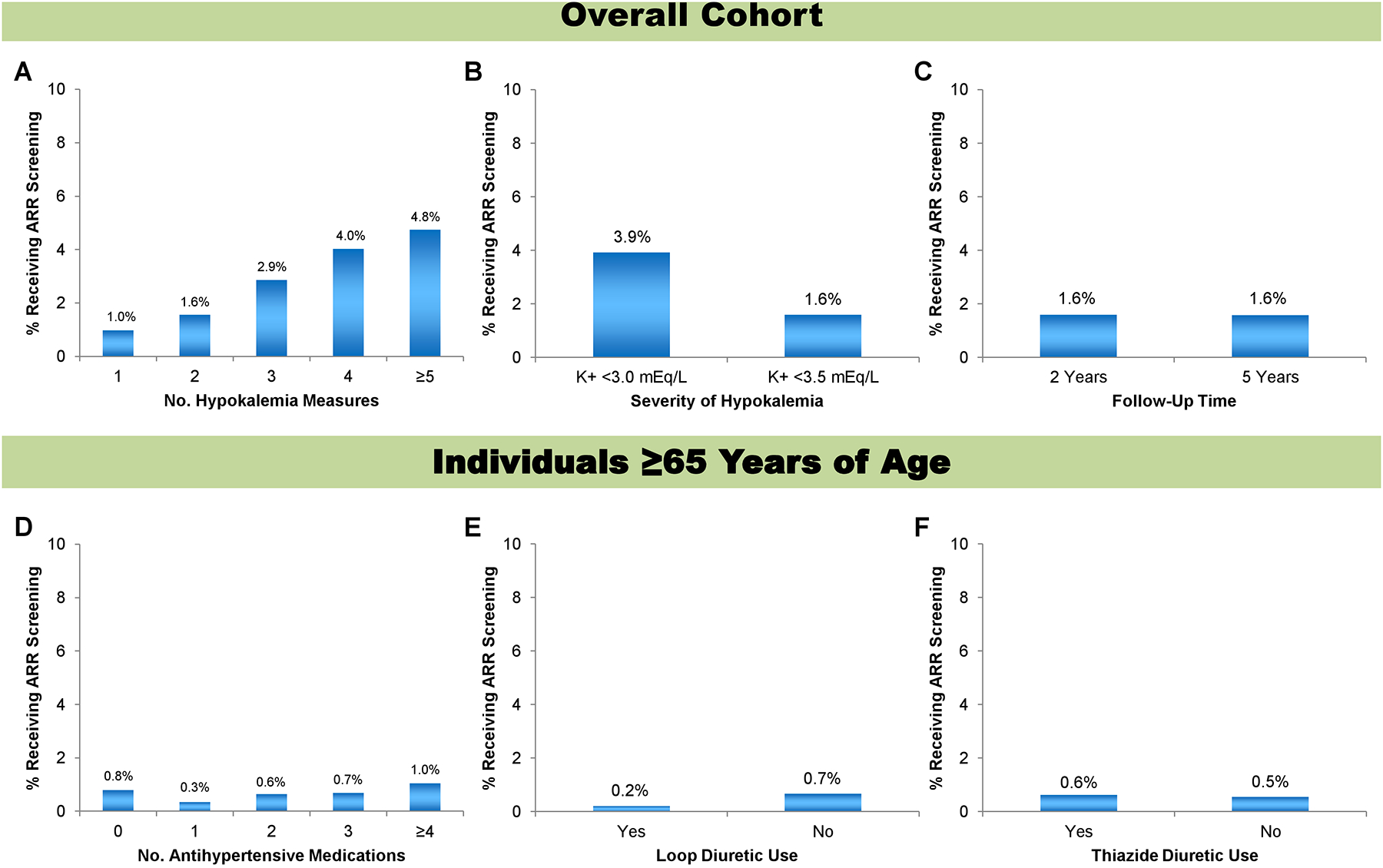
A-C include the overall study cohort while D-F only include individuals ≥65 years of age. A) Number of instances of hypokalemia within a 2-year time window and separated by at least 14 days. B) Hypokalemia definition redefined from serum K+ <3.5 mEq/L [primary analysis] to serum K+ <3.0 mEq/L. C) Length of follow-up modified from two years [primary analysis] to five years. D) Number of antihypertensive medications prescribed. E) Loop diuretic prescription. F) Thiazide diuretic prescription.
Abbreviations: ARR, aldosterone-to-renin ratio; K+, potassium.
ARR Screening with Stricter Hypokalemia Definition (K+ <3.0 mEq/L)
When we examined a stricter definition of hypokalemia as a serum K+ <3.0 mEq/L, the proportion of ARR screening increased from 422/26533 (1.6%) to 58/1480 (3.9%) but overall remained low (Figure 3B).
ARR Screening Over a Longer Follow-Up Period
When we expanded the follow-up time period over which ARR screening could be performed post-index, the proportion of ARR screening among individuals with hypertension plus hypokalemia remained similar at 422/26533 (1.6%) and 94/5965 (1.6%) using 2-year and 5-year follow-up time frames, respectively (Figure 3C).
ARR Screening Based on Antihypertensive Medication Use
This analysis was restricted to individuals ≥65 years old with hypertension plus hypokalemia as medication data was only available for this age group. Proportions of ARR screening were 10/1276 (0.8%), 10/2915 (0.3%), 16/2467 (0.6%), 8/1177 (0.7%), and 1.0% for individuals prescribed zero, one, two, three, or four or more antihypertensive medications, respectively (Figure 3D). The proportion of ARR screening among individuals prescribed four or more antihypertensive medications could not be reported in accordance with ICES privacy policies as the event number was ≤5. Notably, the prescription of ≥4 antihypertensive medications is a separate guideline-recommended criterion for PA screening.9 Therefore, in older individuals meeting two separate criteria for PA screening (≥4 antihypertensive medications and hypertension plus hypokalemia), only 1.0% had an ARR measured. We also found screening rates of <1% among older adults regardless of whether they were prescribed a loop diuretic (0.2%) or not (45/6793 [0.7%]) or whether they were prescribed a thiazide diuretic (23/3636 [0.6%]) or not (25/4532 [0.5%]) (Figure 3E and 3F). The proportion of ARR screening among individuals prescribed a loop diuretic could not be reported in accordance with ICES privacy policies as the event number was ≤5.
Predictors Associated with ARR Screening
We found that older age (hazard ratio [HR] 0.95 [95% CI 0.94, 0.96]) and diabetes mellitus (HR 0.66 [95% CI 0.50, 0.89]) were associated with a lower likelihood of ARR screening among individuals with hypertension plus hypokalemia (Figure 4). We found that subspecialty care from endocrinology (HR 1.52 [95% CI 1.10, 2.09]), nephrology (HR 1.43 [95% CI 1.07, 1.91]), and cardiology (HR 1.39 [95% CI 1.14, 1.70]) were associated with an increased likelihood of ARR screening. We found no significant difference in the likelihood of ARR screening based upon female sex (HR 0.94 [95% CI 0.77, 1.15]), rural residence (HR 1.08 [95% CI 0.74, 1.57]), pre-existing cardiovascular disease (HR 0.81 [95% CI 0.60, 1.09]), internal medicine subspecialty care (HR 1.14 [95% CI 0.93, 1.40]), or income quintile (quintile 1 HR 0.74 [95% CI 0.46, 1.20]; quintile 2 HR 0.79 [95% CI 0.53, 1.18]; quintile 3 HR 0.99 [95% CI 0.70, 1.40]; quintile 4 HR 0.99 [95% CI 0.72, 1.35]; quintile 5 referent).
Figure 4. Risk factors associated with aldosterone-to-renin ratio screening among individuals with hypertension plus hypokalemia.
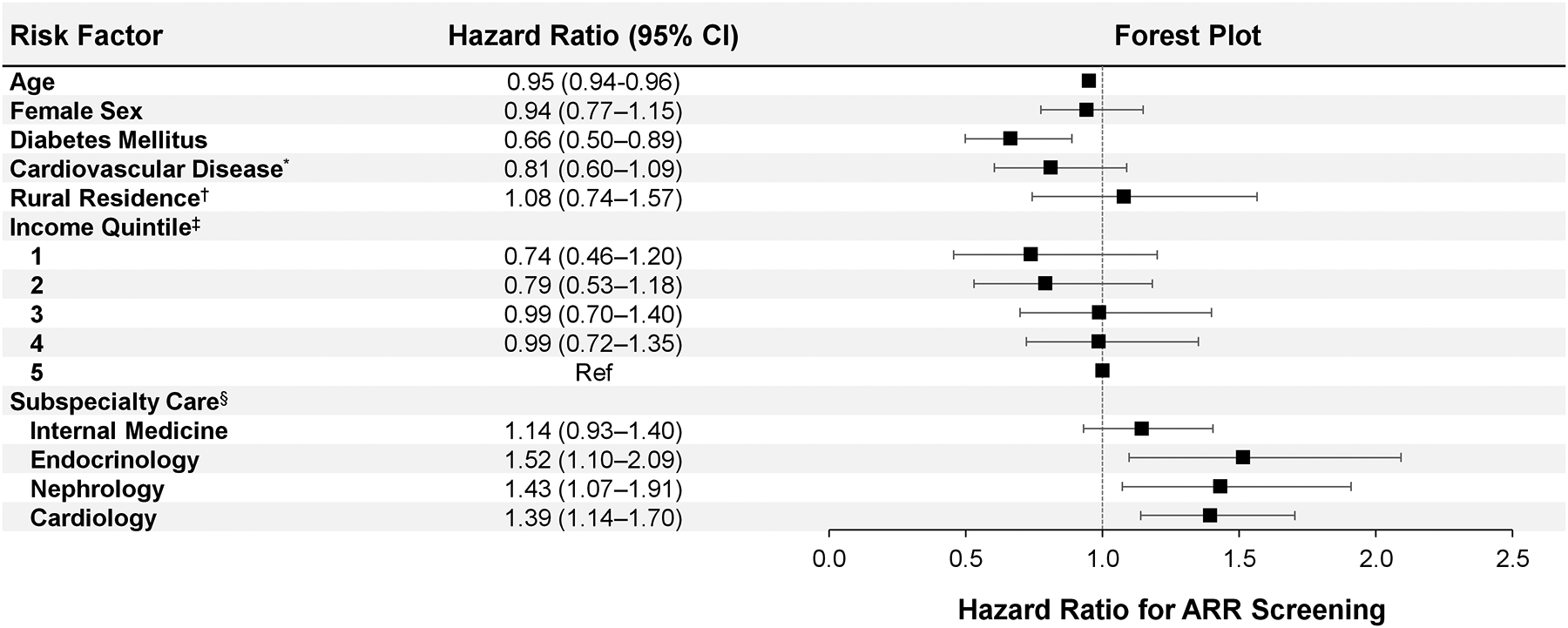
Risk factors are captured upon the index date. Error bars represent 95% confidence intervals.
* Cardiovascular disease defined as a composite of history of coronary artery disease, myocardial infarction, coronary artery bypass graft, congestive heart failure, or stroke.
† Rural residence defined as residing in a location with population <10,000.
‡ Income quintile is a measure of relative household income, adjusted for household size and cost of living, and is determined based upon after-tax income by postal code.
§ Subspecialty care defined as a clinical visit with the specialist within one year prior to index.
Abbreviations: CI, confidence interval.
DISCUSSION
In this population-based cohort study of adults with hypertension plus hypokalemia in the most populous province of Canada, we found that only 1.6% of eligible individuals overall underwent ARR testing as recommended by expert guidelines. Strikingly, screening remained consistently less than 5% even among individuals with up to five or more hypokalemia measurements within a 2-year time window, when the hypokalemia was severe (serum K+ <3.0 mEq/L), or when they also were prescribed four or more antihypertensive medications. When we looked at predictors associated with screening using the ARR, we found that older age and diabetes mellitus were associated with a lower likelihood of receiving guideline-recommended screening whereas subspecialty care with an endocrinologist, nephrologist, or cardiologist was associated with a higher likelihood of screening.
Our results expand upon prior studies demonstrating an alarmingly low population-level uptake of guideline recommendations for PA screening. Cohen et al assessed PA screening in the United States Veterans Affairs Health System among veterans with resistant hypertension and found that only 1.6% of such individuals went on to receive guideline-recommended screening.16 The authors did report that a history of hypokalemia approximately doubled the likelihood that an individual with resistant hypertension would receive ARR screening, though the overall proportion remained very low. Liu et al assessed PA screening across all individuals with hypertension in Alberta, Canada and found that only 0.7% of hypertensive individuals went on to receive screening.15 Among the subset of hypertensive individuals who also had a history of hypokalemia, PA screening increased only slightly to 1.0%.
We would like to highlight several unique strengths with our current study as compared to prior work on ARR screening among individuals with hypertension plus hypokalemia. Rather than a single-center design, our study was population-based and covered the full heterogeneous landscape of healthcare settings that comprise the province of Ontario, Canada. Also, we only included outpatient hypokalemia measurements. We chose not to include in-hospital or emergency room hypokalemia measures so as to avoid common acute medical issues that can result in hypokalemia (e.g., vomiting, diarrhea, excessive sweating/volume depletion, intravenous diuretics, etc.) and which could have led to an under-estimation of ARR screening with prior studies on this subject. Moreover, we were able to go into greater detail regarding the frequency and severity of hypokalemia given the comprehensive data available through ICES. We now demonstrate that even with repeated hypokalemia over a relatively short time period and with severe hypokalemia, individuals with hypertension plus hypokalemia continue to be screened at an extremely low level.
Why is the uptake of PA screening guideline recommendations so poor? The low levels of screening may reflect that many providers do not recognize that the health impact of untreated PA goes far beyond just blood pressure alone. Recent evidence shows that PA causes disproportionately higher rates of cardiovascular disease compared with essential hypertension, independent of blood pressure.6–8 The consistently low level of PA screening among populations that clearly should be screened in order to mitigate these risks suggest that this evidence may not have trickled down beyond the fields of hypertension, endocrinology, and cardiology to the medical field as a whole. This may explain why we found that subspecialists in these fields were significantly more likely to request ARR screening for individuals with hypertension plus hypokalemia. Moreover, many providers may find the complexity of the current diagnostic algorithm for PA (which often includes dynamic confirmatory testing and lateralization testing to determine which adrenal gland is the problem) too cumbersome.26 Perhaps, rather than putting in the amount of time and effort required to proceed down that pathway, many providers instead settle for the simpler approach of treating blood pressure alone – though as discussed above, many of the negative effects of PA occur independent of blood pressure.
Our results must be interpreted within the context of the study design. First, blood pressure measurement data was not available within our datasets. Instead, we utilized a definition for the diagnosis of hypertension based on billing and diagnostic codes that has been well-validated in administrative datasets.22 Second, comprehensive medication data in Ontario is only available for individuals ≥65 years of age. While we did explore medication-level data in this older age group (e.g., potassium-wasting diuretic prescription, number of antihypertensive medications prescribed), we were unable to determine these associations for individuals <65 years. Notably, however, ARR screening is guideline recommended for individuals with hypertension plus hypokalemia regardless of whether the hypokalemia is spontaneous or diuretic-induced.9 Third, given the extremely low number of individuals who underwent ARR screening and were ≥65 years of age with medication data available, we were under-powered to assess whether ARR screening ultimately resulted in greater prescription of MR antagonists. Fourth, while we were able to explore a number of risk factors associated with ARR screening (e.g., age, sex, rural residence, income, subspecialty care), there are a number of other potential risk factors that we were unable to assess given limitations in the databases used (e.g., education level, language barriers, healthcare setting, healthcare center/size). Finally, our inclusion and exclusion criteria reduced the population size we were able to study which may limit the generalizability of our findings.
Supplementary Material
PERSPECTIVES.
This study adds to the mounting evidence that the number of patients who undergo guideline-recommended screening for PA is almost zero, underscoring a lack of awareness and testing as the primary reason for why PA is severely unrecognized. The low level of screening occurs even among individuals with recurrent hypokalemia, in those with severe hypokalemia, and in those with hypertension requiring four or more antihypertensive medications. These findings are particularly alarming as we now clearly recognize that without targeted treatment, PA results in disproportionately high rates of cardiovascular disease compared with essential hypertension independent of blood pressure.6–8 Therefore, our healthcare systems are missing a major opportunity for cardiovascular disease risk mitigation among a large proportion of our hypertensive populations. Increased education and awareness are critical to bridge this gap.
NOVELTY AND SIGNIFICANCE.
What is New?
We examined the population-level uptake of guideline recommendations to screen patients with hypertension plus hypokalemia for primary aldosteronism in Ontario, Canada.
What is Relevant?
Only 1.6% of patients with hypertension plus hypokalemia were screened for primary aldosteronism.
Even among patients with ≥5 instances of hypokalemia, severe hypokalemia, or concurrent resistant hypertension, screening rates remained alarmingly low (<5%).
Subspecialty care with endocrinology, nephrology, or cardiology was associated with an increased likelihood of screening whereas older age and diabetes were associated with a decreased likelihood of screening.
ACKNOWLEDGEMENTS
This study was supported by the ICES Ottawa site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Ottawa facility. The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed in the material are those of the authors, and not necessarily those of the Canadian Institute for Health Information. We would like to thank IMS Brogan Inc. for use of their Drug Information Database.
SOURCES OF FUNDING
Dr. Hundemer is supported by the Canadian Institutes of Health Research Institute of Nutrition, Metabolism and Diabetes (Grant # PJT-175027) and the Kidney Research Scientist Core Education and National Training (KRESCENT) Program New Investigator Award (Grant # 2019KP-NIA626990). Dr. Vaidya is supported by the National Institutes of Health (Grant #s: R01 DK115392, R01 HL153004, and R01 DK16618). Dr. Sood is supported by the Jindal Research Chair for the Prevention of Kidney Disease.
Footnotes
DISCLOSURES
Dr. Sood received speaker fees from AstraZeneca. All other authors have no potential conflicts of interest to report.
REFERENCES
- 1.Brown JM, Siddiqui M, Calhoun DA, Carey RM, Hopkins PN, Williams GH, Vaidya A. The unrecognized prevalence of primary aldosteronism: A cross-sectional study. Ann Intern Med. 2020;173:10–20: 10.7326/M20-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820: 10.1016/j.jacc.2017.01.052 [DOI] [PubMed] [Google Scholar]
- 3.Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, Huete A, Gederlini A, Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161–165: 10.1161/01.HYP.0000079505.25750.11 [DOI] [PubMed] [Google Scholar]
- 4.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in japan. Hypertens Res. 2004;27:193–202 [DOI] [PubMed] [Google Scholar]
- 5.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300: 10.1016/j.jacc.2006.07.059 [DOI] [PubMed] [Google Scholar]
- 6.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50: 10.1016/S2213-8587(17)30319-4 [DOI] [PubMed] [Google Scholar]
- 7.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3:768–774: 10.1001/jamacardio.2018.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59: 10.1016/S2213-8587(17)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 11.Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: Updating the approach. J Clin Endocrinol Metab. 2020;105: 10.1210/clinem/dgaa606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffe G, Gray Z, Krishnan G, Stedman M, Zheng Y, Han J, Chertow GM, Leppert JT, Bhalla V. Screening rates for primary aldosteronism in resistant hypertension: A cohort study. Hypertension. 2020;75:650–659: 10.1161/HYPERTENSIONAHA.119.14359 [DOI] [PubMed] [Google Scholar]
- 13.Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: Deficiencies in screening at-risk hypertensives. Surgery. 2019;165:221–227: 10.1016/j.surg.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 14.Sivarajah M, Beninato T, Fahey TJ 3rd. Adherence to consensus guidelines for screening of primary aldosteronism in an urban healthcare system. Surgery. 2020;167:211–215: 10.1016/j.surg.2019.05.087 [DOI] [PubMed] [Google Scholar]
- 15.Liu YY, King J, Kline GA, Padwal RS, Pasieka JL, Chen G, So B, Harvey A, Chin A, Leung AA. Outcomes of a specialized clinic on rates of investigation and treatment of primary aldosteronism. JAMA Surg. 2021: 10.1001/jamasurg.2021.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen JB, Cohen DL, Herman DS, Leppert JT, Byrd JB, Bhalla V. Testing for primary aldosteronism and mineralocorticoid receptor antagonist use among u.S. Veterans : A retrospective cohort study. Ann Intern Med. 2021;174:289–297: 10.7326/M20-4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statistics Canada. Table 17-10-0005-01 population estimates on july 1st, by age and sex. Accessed April 19, 2021
- 18.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM, Committee RW. The reporting of studies conducted using observational routinely-collected health data (record) statement. PLoS Med. 2015;12:e1001885: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Prev Med. 2007;45:247–251: 10.1016/j.ypmed.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 20.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the ontario drug benefit database. Can J Clin Pharmacol. 2003;10:67–71 [PubMed] [Google Scholar]
- 21.Statistics Canada. Table 17-10-0022-01 estimates of interprovincial migrants by province or territory of origin and destination, annual. Accessed April 29, 2019
- 22.Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA, Hypertension O, Surveillance Team of the Canadian Hypertension Education P. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428: 10.1161/HYPERTENSIONAHA.109.139279 [DOI] [PubMed] [Google Scholar]
- 23.Tu K, Chen Z, Lipscombe LL, Canadian Hypertension Education Program Outcomes Research T. Prevalence and incidence of hypertension from 1995 to 2005: A population-based study. CMAJ. 2008;178:1429–1435: 10.1503/cmaj.071283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: Implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39:1057–1088: 10.1210/er.2018-00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833: 10.1210/jc.2013-2805 [DOI] [PubMed] [Google Scholar]
- 26.Kline GA. Primary aldosteronism: Unnecessary complexity in definition and diagnosis as a barrier to wider clinical care. Clin Endocrinol (Oxf). 2015;82:779–784: 10.1111/cen.12798 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


