Abstract
Background
Adolescent and young adult (AYA) cancer patients have not seen the same improvements in survival compared to younger (pediatric) and older (adults≥40 years) patients. This may be related to their lower participation in clinical trials.
Methods
We examined AYA patient accrual to SWOG Cancer Research Network phase I–III clinical treatment trials for 29 cancers over 25-years (January, 1996-December, 2020). Trial enrollments for AYA patients (15–39 years) were compared to older (40+ years) patients in SWOG and to U.S. AYA cancer population rates derived from U.S. Census and NCI-SEER data.
Results
In total, 84,219 patients were enrolled to SWOG treatment trials, including n=7,109 (8.4%) AYA patients, compared to 3.8% in the U.S. cancer population. By histology, the highest proportion of AYA patients were in Hodgkin’s disease (825/1220, 67.6%) and acute lymphocytic leukemia (350/678, 51.6%) trials, while the greatest number of AYA patients were from breast cancer trials (3,032/32,693, 9.3%). SWOG AYA patients were more often female (68.8% vs. 58.7%, p<.001), Black (10.1% vs. 8.2%, p<.001), and Hispanic (10.6% vs. 5.6%, p<.001) than SWOG patients 40 or older, and were more often female (68.8% vs. 65.1%, p<.001) but less often Black (10.1% vs. 11.8%, p<.001) or Hispanic (10.6% vs. 12.8%, p<.001) then AYA patients in the U.S. cancer population.
Conclusions
AYA cancer patients were well represented in SWOG clinical trials compared to U.S. cancer population patients with the same cancers. The SWOG AYA population was more racially/ethnically diverse than older SWOG patients, though less diverse than the U.S. AYA cancer population.
Keywords: Cancer clinical trials, Enrollment, Adolescent and young adults, Representativeness, Disparities
Precis:
AYA cancer patients were well represented in SWOG clinical trials compared to U.S. cancer population patients with the same cancers. The SWOG AYA population was more diverse than older SWOG patients, though less racially/ethnically diverse than the U.S. AYA cancer population.
Lay Summary:
Adolescent and young adult (AYA) cancer patients (ages 15–39 years) have not seen the same improvements in survival compared to younger (pediatric) and older (adults≥40 years) patients. This may be related to their lower participation in clinical trials. We evaluated the extent to which AYA patients are enrolled to a large, NCI-sponsored network group over 25 years (1996 through 2020). Overall, 8.4% (7,109/84,219) enrolled patients were AYA, twice the corresponding rate of 3.8% in the U.S. cancer population. AYA patients were also more racially/ethnically diverse than older trial patients, though less racially/ethnically diverse than the U.S. AYA cancer population.
Introduction
Adolescent and young adult (AYA) oncology concerns the demography, biology, treatment, and longitudinal outcomes of patients affected by cancer between the ages of 15 and 39 years old.1 Researchers and clinical investigators have deepened their understanding of these areas and aimed to improve the healthcare of AYA patients through varied efforts, including therapeutic trials. Currently, AYAs with cancer have 5-year survival rate of 85%.2 However, a gap in the rate of survival improvements between AYA patients compared to their younger (pediatric) and older (adults 40 years or older) counterparts with cancer has been repeatedly demonstrated.3,4 Relative survival estimates over a 40-year period (1975–2014) increased approximately 0.9% per year for adults 40 or older and children <15 years, whereas the corresponding estimate in AYA patients was just 0.5% per year.5 These limited improvements in relative survival over time for AYA patients have been attributed to factors such as differences in disease presentation, socioeconomic disparities, and the disproportionate impact of the HIV/AIDS epidemic on AYAs.1,3–6 Further, evidence suggests that low participation in cancer clinical trials for AYA patients could be limiting treatment advancements and, consequently, survival gains.7–10
Cancer research in the United States is conducted through both private and public mechanisms. While pharmaceutical companies aim to discover and market new cancer treatments, clinical research sponsored by the National Cancer Institute (NCI) has a broader mandate to serve the community of cancer patients. Clinical trials sponsored by the NCI’s National Clinical Trials Network (NCTN) address complex clinical research questions related to combinations of treatments and treatment modalities in patients receiving care in both the community and academic setting.11 The SWOG Cancer Research Network is one of the four adult NCTN network groups. Founded in 1956, SWOG has over one thousand member institutions and 12,000 participating investigators across all regions of the U.S.12 SWOG has a decades-long history of conducting trials for adults with all major cancers, including AYA patients, and has been in the vanguard of studying and furthering the care of patients within the AYA demographic.
The objective of this analysis was to examine accrual of AYA patients to SWOG clinical treatment trials over a 25-year period. Specifically, we examined patterns of accrual over time, as well as by demographic factors and cancer types, compared to older SWOG patients and compared to AYA patients in the cancer population. Our goal was to provide a better understanding of the representativeness and the potential research opportunities afforded AYA patients through the NCI’s network groups.
Methods
The data were from the SWOG Cancer Research Network, which conducts treatment trials for adults with cancer in a wide variety of malignancies. AYA patients were defined as those 15 to 39 years old. We examined accrual over a 25-year period, from January 1, 1996 through December 31, 2020. We included enrollments to phase I–III therapeutic trials for 29 different cancers for which SWOG enrolled 100 or more patients during the period. For patients enrolled to multiple trials, only the first enrollment was included; thus, the denominator represents unique patient enrollments. Enrollments were from trials previously approved by an institutional review board (IRB), or by the NCI’s Central IRB (for trials activated in 2014 or after);13 written informed consent was previously obtained for all patients.
Accrual patterns – by cancer type and demographic factors – were compared between AYA patients to patients 40 or older in SWOG and, separately, to rates for AYA patients (also age 15–39 years old) among all patients 15 or older in the U.S. cancer population. To derive U.S. cancer population rates, we used NCI Surveillance, Epidemiology, and End Results (SEER) data in combination with U.S. Census data.14,15 The use of Census data allowed us to adjust rates identified within SEER to better reflect the U.S. population in general with respect to age, sex, and race, based on a previously developed approach.16,17 Comparisons of rates of AYA representation between SWOG trials and the U.S. cancer population were conducted only for the cancer types included in the SWOG database. For analyses by sex, we examined all cancers combined, and separately, only the non-sex-specific cancers. To compare sample estimates between SWOG AYA patients and SWOG patients 40 or older, Chi-square tests were used. To compare estimates between SWOG AYA patients and AYA patients in the U.S. cancer population, rates for the U.S. cancer population were considered fixed, and one-sample binomial tests were used. Race (Black vs. Asian/Pacific Islander vs. Native American vs. White vs. multiple) and ethnicity (Hispanic vs. non-Hispanic) status were self-reported. The proportion of patients reporting any category of Black, Asian/Pacific Islander, Native American, or Hispanic (defined as “any minority”) was calculated among those with known race and/or ethnicity data. Patients characteristics between AYA patients <30 vs. 30–39 were also compared.
To examine enrollment patterns over time, SWOG rates by year were plotted and local regression curves were superimposed to highlight general trends (loess function in R, version 4.02). Patterns were compared to average 5-year interval rates for the U.S. AYA cancer population.
We also assessed the extent to which variations in the types of available trials may have influenced overall rates of AYA enrollment over time. SWOG studies were defined as having the potential for higher AYA enrollment if AYA proportional enrollment for the cancer type in the U.S. cancer population exceeded 10%. Patterns of trial activations and duration were described. Further, we devised a test statistic to assess whether the “density” of active trial time for cancers with the potential for higher AYA proportional enrollment actually predicted AYA enrollment. For each of the 25 individual years in the analysis, the percentage of active trial time for studies conducted in cancers with >10% AYA patients in the U.S. cancer population was calculated. In a weighted least squares regression (weighted by the annual enrollment denominator), we examined whether this rate predicted percent AYA enrollment by testing whether the regression coefficient statistically significantly differed from zero. The Pearson correlation coefficient was also estimated.
Analyses were conducted in SAS version 9.4 (SAS Institute) and R version 4.0.2 (R Project for Statistical Computing). To adjust for multiple comparisons, only two-tailed P-values of 0.01 or less (rather than the standard 0.05) were considered to indicate statistical significance.
Results
From 1996–2020, n=84,219 patients from 444 different trials conducted in 29 different disease categories were enrolled, of whom n=7,109 (8.4%) were AYA patients. Overall, 1,893 (2.2%) of patients were 15–29 years and 5,178 (6.2%) of patients were 30–39 years. Therefore most AYA patients were 30 or older (73.2%; Table 1). AYA patients were more commonly female than patients 40 or older (68.8% vs. 58.7%, p<.001). The AYA cohort was more diverse, with higher proportions of Black (10.1% vs. 8.2%), Asian/Pacific Islander (4.7% vs. 3.3%), and Hispanic (10.6% vs. 5.6%) patients (p<.001). Overall, 25.0% of AYA patients were of any racial/ethnic minority group compared to 17.2% for patients 40 or older (p<.001).
Table 1:
Patient Characteristics
| AYA Patients (n=7,071) |
Patients 40 or Older (N=77,110) |
||||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Age | |||||
| <30 | 1,893 | 26.7 | NA | NA | NA |
| 30–39 | 5,178 | 73.2 | NA | NA | |
| Sex (all cancers) | <.001 | ||||
| Female | 4,862 | 68.8 | 45,281 | 58.7 | |
| Male | 2,209 | 31.2 | 31,829 | 41.3 | |
| Sex (non-sex specific cancers1) | <.001 | ||||
| Female | 1,731 | 44.1 | 14,193 | 37.9 | |
| Male | 2,190 | 55.9 | 23,259 | 62.1 | |
| Race2 | <.001 | ||||
| Black | 684 | 10.1 | 6,020 | 8.2 | |
| Asian/PI | 316 | 4.7 | 2,453 | 3.3 | |
| Native American | 45 | 0.7 | 335 | 0.5 | |
| White | 5,687 | 84.2 | 64,618 | 87.8 | |
| Multiple | 26 | 0.4 | 150 | 0.2 | |
| Unknown | 313 | 3,534 | |||
| Ethnicity2 | <.001 | ||||
| Hispanic | 701 | 10.6 | 4,042 | 5.6 | |
| Not Hispanic | 5,928 | 89.4 | 67,681 | 94.4 | |
| Unknown | 442 | 5,386 | |||
| Racial/Ethnic Minority3 | <.001 | ||||
| Any Minority | 1,740 | 25.0 | 12,851 | 17.2 | |
| Not Minority | 5,211 | 75.0 | 61,815 | 82.8 | |
| Unknown | 120 | 2,444 | |||
Excludes breast, cervical, endometrial, ovarian, and prostate.
Percentages calculated among those with known data.
Any racial/ethnic minority defined as self-report of any category of Black, Asian/PI, Native American, or Hispanic.
Among the subset of AYA patients, patients 30–39 years (n=5,178) were more likely to be female than patients <30 years (n=1,893) for all cancers (74.5% vs. 53.1%, p<.001), likely due to the large number of patients 30–39 years enrolled to breast cancer trials (Table 2). In contrast, in the non-sex specific cancers, there was no difference between patients 30–39 vs. <30 years in the proportion of female patients (43.8% vs. 44.6%, p=.62). In total, 27.2% of patients <30 years were of any racial/ethnic minority group compared to 24.3% for patients 30–39 years (p=.01).
Table 2:
Patient Characteristics among AYA Patients
| <30 Years Old (n=1,893) |
30–39 Years Old (N=5,178) |
||||
|---|---|---|---|---|---|
| N | % | N | % | p-value | |
| Sex (all cancers) | <.001 | ||||
| Female | 1,006 | 53.1 | 3,856 | 74.5 | |
| Male | 887 | 46.9 | 1,322 | 25.5 | |
| Sex (non-sex specific cancers1) | .62 | ||||
| Female | 710 | 44.6 | 1,021 | 43.8 | |
| Male | 881 | 55.4 | 1,309 | 56.2 | |
| Race2 | .05 | ||||
| Black | 201 | 11.2 | 483 | 9.7 | |
| Asian/PI | 69 | 3.8 | 247 | 5.0 | |
| Native American | 15 | 0.8 | 30 | 0.6 | |
| White | 1,504 | 83.6 | 4,183 | 84.4 | |
| Multiple | 10 | 0.6 | 16 | 0.3 | |
| Unknown | 94 | 219 | |||
| Ethnicity2 | |||||
| Hispanic | 218 | 12.4 | 483 | 9.9 | .003 |
| Not Hispanic | 1,534 | 87.6 | 4,394 | 90.1 | |
| Unknown | 141 | 301 | |||
| Racial/Ethnic Minority3 | .01 | ||||
| Any Minority | 503 | 27.2 | 1,237 | 24.3 | |
| Not Minority | 1,348 | 72.8 | 3,863 | 75.7 | |
| Unknown | 42 | 78 | |||
Excludes breast, cervical, endometrial, ovarian, and prostate.
Percentages calculated among those with known data.
Any racial/ethnic minority defined as self-report of any category of Black, Asian/PI, Native American, or Hispanic.
AYA Enrollment over Time
Over the 25-year period from 1996–2020, the estimated proportion of AYA patients in the U.S. cancer population aged 15 or higher was 3.8%; thus, representation of AYA patients on SWOG clinical trials (8.4%) was more than double the rate in the U.S. cancer population over the period (Figure 1). The proportion of SWOG cancer patients in the AYA category was highest in the earliest 5-year period (10.5% from 1996–2000). For the 29 cancers represented in this analysis, over the entire time period, AYA proportional enrollment in the U.S. cancer population dropped from 4.1% (1996–2000) down to 3.6% (2011–2015) and 3.7% for 2016–2020.
Figure 1:
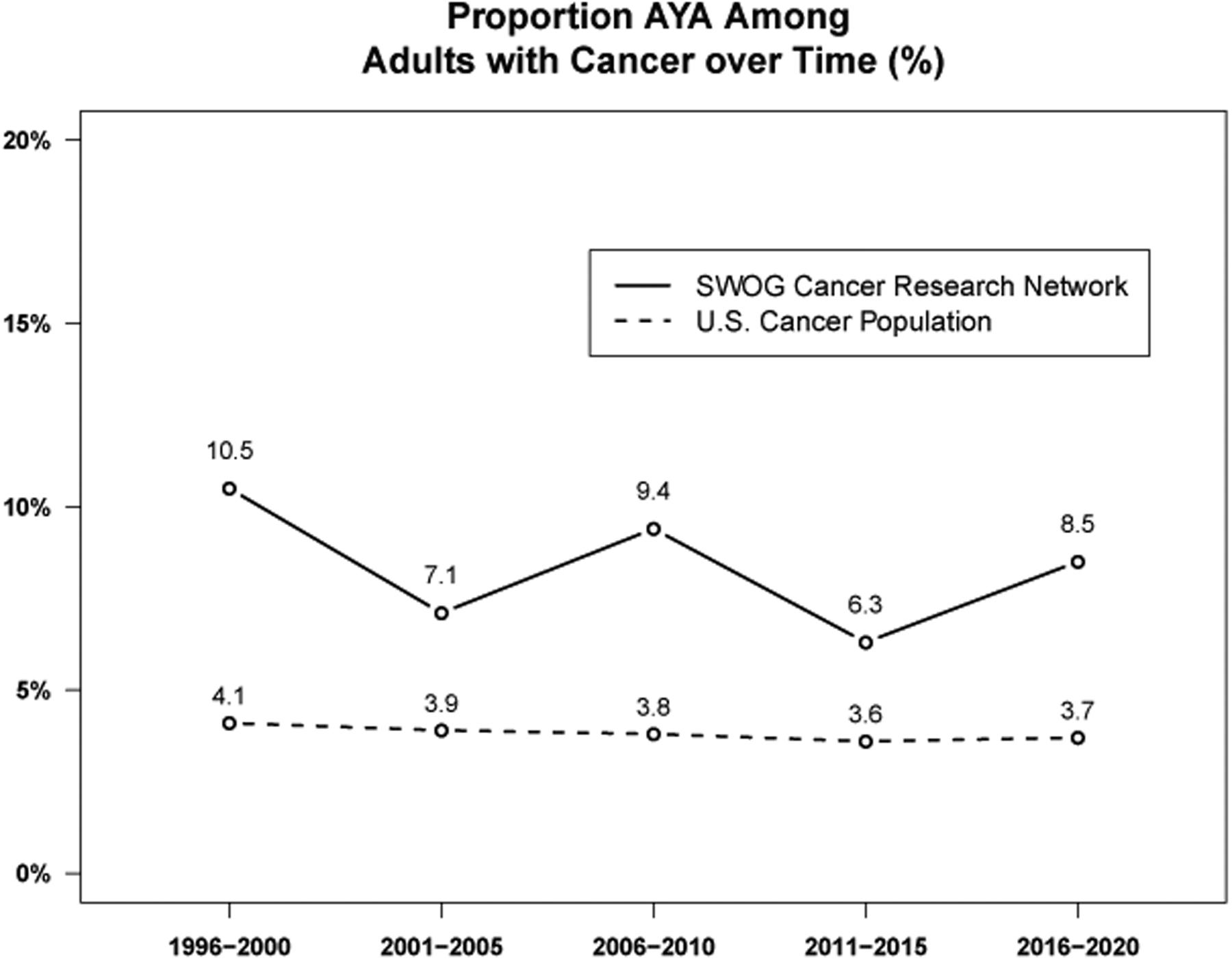
Proportion AYA among adults with cancer over time by five-year increments. Over the entire 25 year period, 8.4% of enrollments to SWOG treatment trials were AYA patients, compared to 3.8% for similar cancers in the U.S. cancer population.
Representation of AYA patients on SWOG trials was consistently higher than the U.S. cancer population for both younger AYA patients (15–29 years, 2.2% vs. 1.2%, respectively) and older AYA patients (30–39 years, 6.2% vs. 2.6%, respectively).
AYA Enrollment by Cancer Type
Figure 2 shows accrual by the 29 cancer types, alongside cancer-specific rates of AYA patients in the U.S. cancer population for those 15 or older. Trials were most commonly conducted in lung cancer (73), breast cancer (37), Non-Hodgkin’s lymphoma (32), melanoma (27), and prostate cancer (26). Consistent with proportional incidence rates in the U.S. cancer population, the cancers with the highest proportions of AYA patients in SWOG (versus the U.S. cancer population) were Hodgkin’s disease (67.6% vs. 49.5%), acute lymphocytic leukemia (51.6% vs. 38.7%), and acute promyelocytic leukemia (37.1% vs. 29.2%). The cancers enrolling the greatest numbers of AYA patients in SWOG were breast (3,032; 42.9% of total AYA enrollments in SWOG), Hodgkin’s disease (825; 11.7% of total), melanoma (613; 8.7% of total), and acute myeloid leukemia (470; 6.6% of total); combined, these 4 cancers represented 69.9% of the total AYA patients enrolled to SWOG trials over the period. The rate of AYA patients in breast cancer trials was more than twice the corresponding cancer-specific rate in the U.S. AYA cancer population (9.3% vs. 4.5%).
Figure 2:
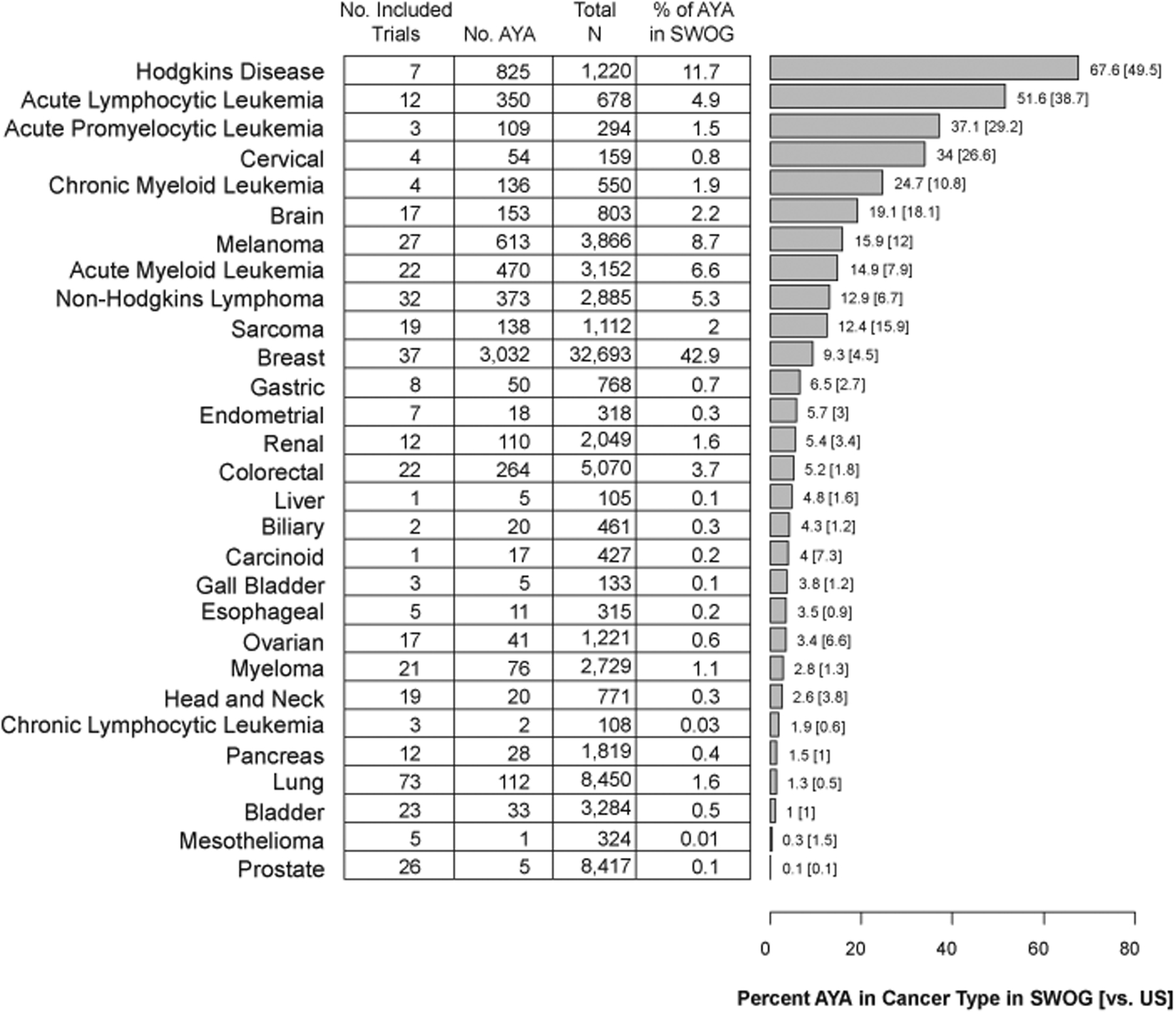
Number and proportion of AYA patients in SWOG trials from 1996–2020 by cancer type. The horizontal bar chart indicates the proportion of patients for a given cancer who are AYA. The corresponding rate in the U.S. AYA cancer population is indicated in square brackets. Cancers are ordered in descending order of the proportion of patients for a given cancer in SWOG trials who are AYA.
AYA Enrollments by Demographic Factors
Figure 3 shows rates of enrollment for different demographic groups for SWOG AYA patients compared to SWOG patients 40 or older and to AYA patients in the U.S. cancer population. The rate of SWOG AYA patients who were 30–39 years old was higher compared to the U.S. AYA cancer population (72.8% vs. 68.5%, p<.001). Females represented 68.8% of all SWOG AYA cancer patients, significantly higher than the rate among older SWOG cancer patients (58.7%, p<.001) and in the U.S. AYA cancer population (65.1%, p<.001). In the non-sex specific cancers, females represented a significantly higher proportion of patients (44.1%) in SWOG AYA cancer patients compared to SWOG patients 40 or older (37.9%, p<.001), but a lower proportion compared to the U.S AYA cancer population (48.1%, p<.001). SWOG AYA cancer patients were more racially and ethnically diverse than older SWOG patients; a significantly higher proportion of SWOG AYA patients were Black (10.1% vs. 8.2%, p<.001), Asian/Pacific Islander (4.7% vs. 3.3%, p<.001), and Hispanic (10.6% vs. 5.6%, p<.001). SWOG AYA cancer patients were less likely to be Black (10.1% vs. 11.8%, p<.001) or Hispanic (10.6% vs. 12.8%, p<.001) than patients in the U.S. AYA cancer population. In contrast, enrollments of Asian/Pacific Islanders and Native Americans were representative of the U.S. AYA cancer population. SWOG AYA patients were much more likely to be in any minority group (25.0%) than SWOG patients 40 or older (17.2%), whereas patients of any minority group comprised an estimated 29.2% of the U.S. AYA cancer population.
Figure 3:
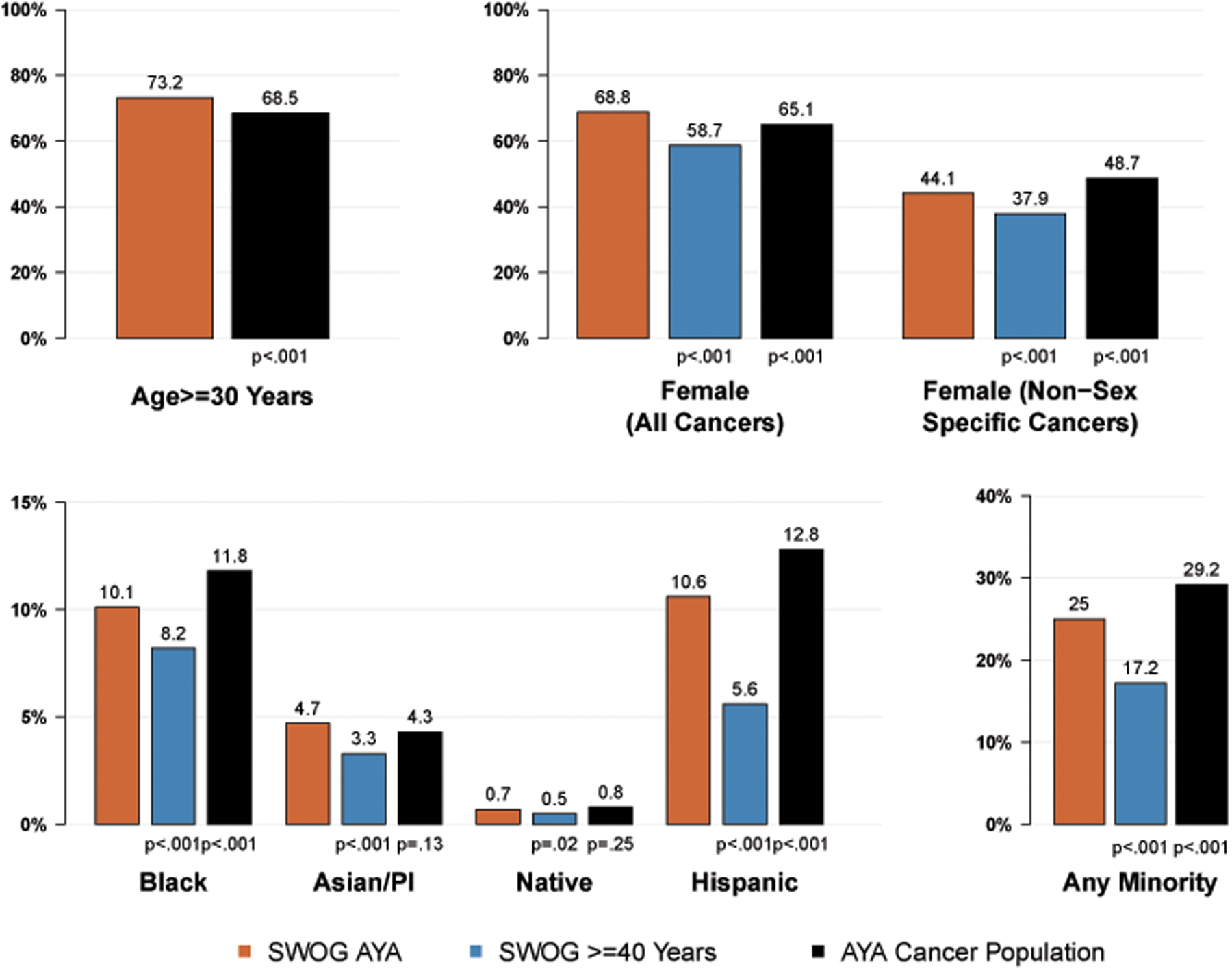
Representation by age, sex, race, and ethnicity groups among AYA patients.
Enrollment Trends by Demographic Factors over Time
Figure 4 shows trends in enrollment by demographic factors over time among SWOG AYA cancer patients compared to both SWOG non-AYA patients and to the U.S. cancer population of AYA patients. The proportion of AYA patients 30 or older mostly varied between 70%−80% over the period. The proportion of SWOG AYA patients who are female exceeded the average U.S. AYA cancer population rates – and the rates for SWOG patients 40 or older – for most years. Representation of Black patients among SWOG AYA cancer patients mostly exceeded the rate among SWOG patients 40 or older. Proportional Black enrollment among SWOG AYA patients and SWOG patients 40 or older was notably lower between 2015–2019; rates for both groups returned to about 10% in 2020. Enrollment of Hispanic patients among SWOG AYA patients has trended higher over time and exceeded estimates for SWOG patients 40 or older nearly every year. The proportion of patients in any minority group were generally consistent over time for each cohort, and were highest for the U.S. AYA cancer population, followed by the SWOG AYA cohort and then the SWOG cohort of patients 40 or older.
Figure 4:
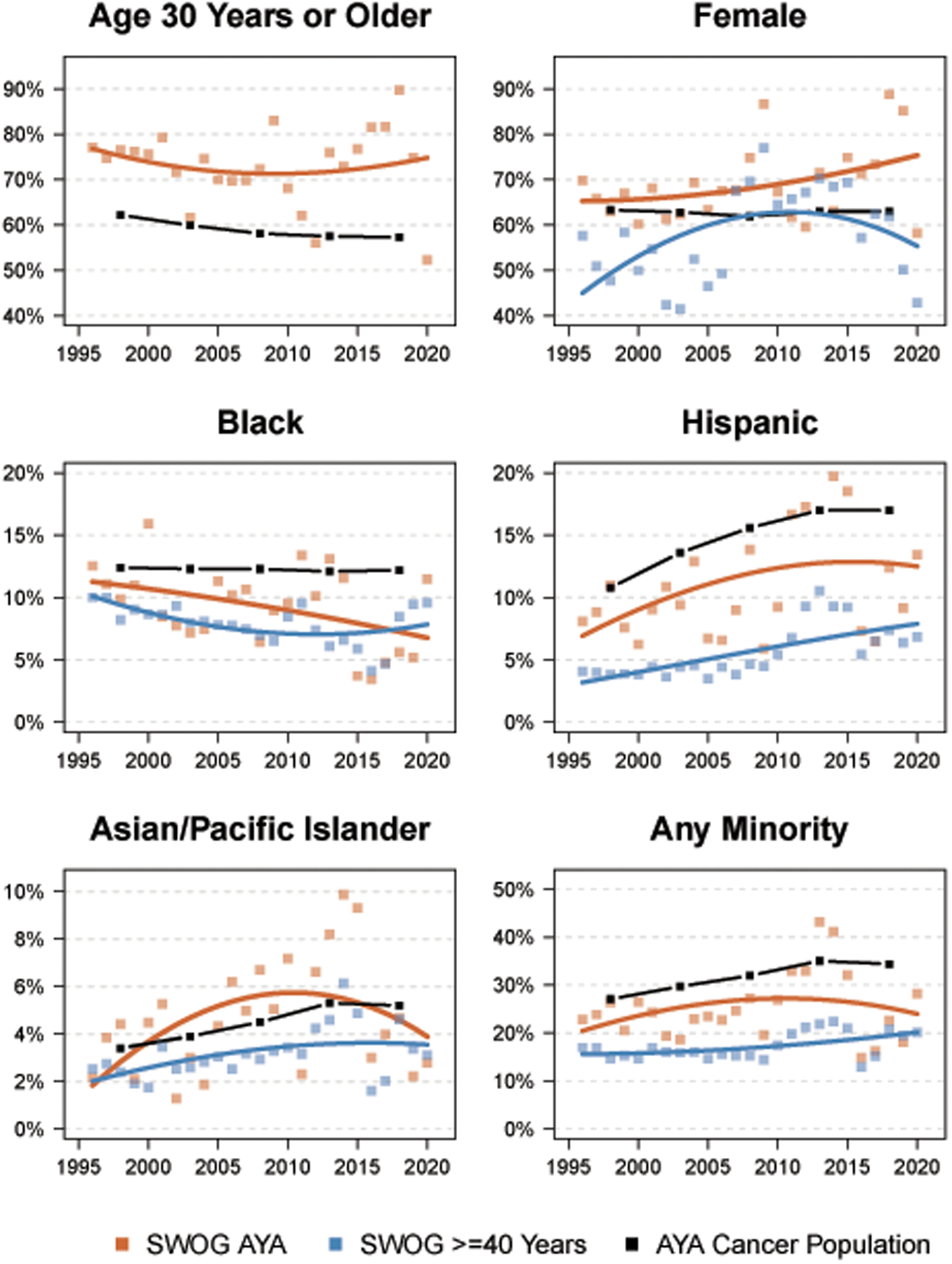
Representation by age, sex, race, and ethnicity groups among AYA patients over time by year. The squares represent observed rates for SWOG AYA patients (orange) and SWOG patients >=40 years of age (blue). Loess regression lines were plotted to indicate temporal trends. Observed rates in 5-year intervals for the U.S. cancer population of AYA patients are represented by the black boxes, connected by lines.
AYA Enrollment in Relation to Trial Availability over Time
As noted in Figure 2, the cancers with proportional enrollment of AYA patients >10% in the U.S. cancer population were Hodgkin’s disease, acute lymphocytic leukemia, acute promyelocytic leukemia, cervical cancer, chronic myeloid leukemia, brain cancer, melanoma, and sarcoma. As shown in Figure 5 – which shows the timing of trial activations over time and their duration for all of the 444 studies included in the analysis – these studies (shown in red) were represented across the 25-year spectrum. However, the proportion of active trial time attributable to these studies differed over time, ranging from 7.8% to 31.8% (Figure 5, inset). In weighted least squares regression, there was strong evidence that the proportion of active trial time for trials conducted in cancers with high proportion enrollment predicted AYA trial enrollment (regression coefficient=0.22, 95% CI, 0.06–0.38, p=.01). The Pearson correlation coefficient was 0.40, suggesting that approxiately 40% of the magnitude and variation in AYA enrollment was a function of the types of trials active over any period.
Figure 5:
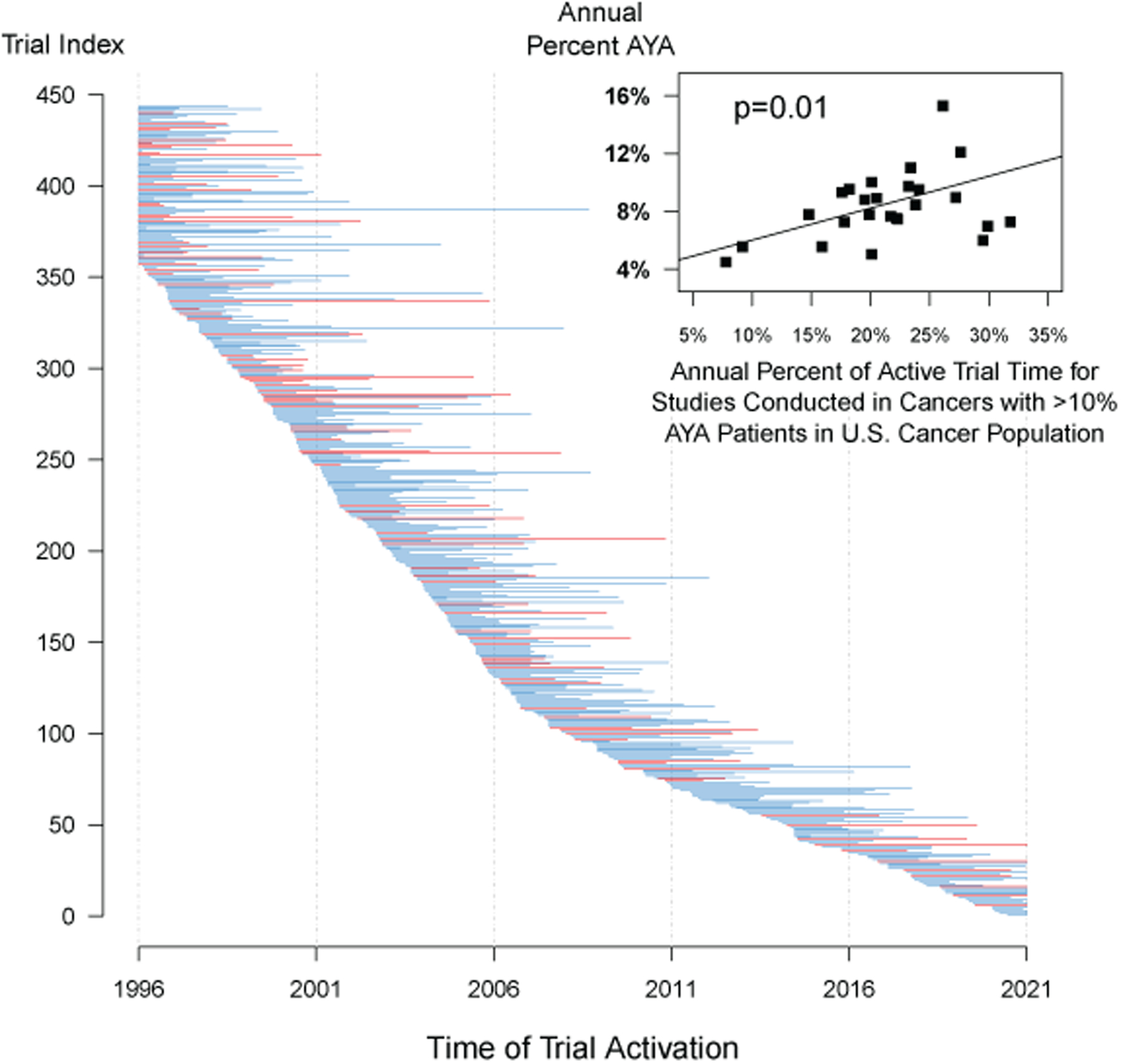
Trial activations and their duration over time for all n=444 studies included in the analysis. Trials in cancers with the potential for higher AYA enrollment (defined as AYA proportional enrollment for the cancer type in the U.S. cancer population exceeding 10%, including Hodgkin’s disease, acute lymphocytic leukemia, acute promyelocytic leukemia, cervical cancer, chronic myeloid leukemia, brain cancer, melanoma, and sarcoma) are shown in red; other studies are shown in blue. The lines are initiated at the trial activation time (or January 1, 1996, the start of the observation period, whichever comes last) and ended at the trial completion time (or December 31, 2020, the end of the observation period, whichever comes first). Additionally, for each of the 25 individual years in the analysis, the inset panel shows a scatterplot of the relationship between the percentage of active trial time for studies conducted in cancers with >10% AYA patients in the U.S. cancer population and the percentage of AYA enrollment within the given year. The association between these factors was strongly statistically significant (p=.01).
Discussion
AYA cancer patients were well represented in SWOG clinical trials compared to patients in the U.S. cancer population with the same cancers. The rate of SWOG patients who were AYA was more than twice the rate in the general U.S. cancer population from 1996–2020. This finding reflects the fact that trial patients are generally younger overall, and stands in stark contrast to enrollment patterns for older cancer patients, especially those 65 or older, who have been shown to be substantially under-represented in cancer clinical trials across numerous studies over time, including in SWOG.16–20 SWOG AYA patients were also more likely to be in the older (30–39 years) AYA category compared to the U.S. AYA cancer population (73.2% vs. 68.5%); however, both younger (15–29 years) and older (30–39 years) AYA patients were well-represented in SWOG trials compared to the U.S. cancer population. Consistent with population trends more broadly, SWOG AYA patients were more racially and ethnically diverse than SWOG patients 40 or older. This variety among study subjects could allow for greater generalizability of trial findings to the larger community of cancer patients and could also help advance understanding of endogenous patient differences that may then affect clinical outcomes.
AYA patients were well represented on SWOG trials compared to the U.S. AYA cancer population overall, although the proportion of SWOG patients who are AYA has varied over time, with some suggestion that rates have dropped over time. As we demonstrated, this is at least partly due to the types of trials that have been active over time. The Children’s Oncology Group (COG) has also observed variable AYA treatment trial participation over time compared to younger pediatric patients, with proportional AYA enrollment (ages 15–39) dropping from 33.5% from 2004–2008 to 30.7% from 2009–2013, even as the number of AYA-enrolling studies increased.21 A recent study showed that proportional SWOG AYA enrollments have been decreasing for all categories of institutions, including for NCI-designated cancer centers, where rates of AYA enrollment were greatest.22 Proportionally fewer AYA enrollments to network group trials may reflect in part increased competition at the local level for AYA patients from investigator-led or pharmaceutical-company sponsored studies. Regardless, observed reductions suggest fewer opportunities to enroll AYA patients to NCI-sponsored network group trials designed to examine treatments targeted towards the AYA population.
These patterns highlight the importance of collaboration among the NCI’s network groups to conduct studies in AYA patients.23 A national study activated in 2019 for advanced stage Hodgkin lymphoma in AYA patients (SWOG S1826; ClinicalTrials.gov Identifier, NCT03907488) represents a collaboration among all the NCI-sponsored network groups, with combined scientific leadership from both SWOG and COG.24 The COG previously partnered with NRG Oncology to complete a study examining the addition of pazopanib to neoadjuvant chemoradiotherapy for sarcoma patients, illustrating the success of such collaborations to enable enrollment of patients from across the age spectrum (age 2 or older).25 Broader design initiatives, including the lowering of age limits among adult cancer groups from 18 down to 12 and increasing trial collaborations with the pediatric oncology community, should also help boost enrollment of AYA patients.26 The importance of studies specifically targeting AYA patients is highlighted by evidence of potentially complex biologic signatures among AYA cancer patients that may not be exclusively familiar to either pediatric or adult hematologists and oncologists.1,27–29
The racial, ethnic, and gender diversity among SWOG AYA patients is encouraging and may reflect efforts among the NCI network groups to focus on diversifying accrual across the lifespan.30 The greater diversity in the AYA population compared to older SWOG patients is also consistent with the increasing diversity in the US population over many decades.31,32 Adult cancer clinical trials typically underrepresent sociodemographically vulnerable populations including Black patients and patients with lower income.33,34 A recent study of pivotal oncology trials leading to new drug approvals by the U.S. Food and Drug Administration showed that only about 3% of patients in these trials were Black;33 however, such studies are primarily sponsored by pharmaceutical companies.11 Clinical trials sponsored by the National Cancer Institute have broader outreach to community and minority-based sites; consequently, the corresponding enrollment rate of Black patients in these trials was notably higher (9%), though still lower than the portion of Black patients in the U.S. cancer population (12%).35 Our findings also indicate that enrollment trends for AYA demographic patient subgroups have varied widely over time. This variability is likely a byproduct of both the availability of trials that enroll different demographic groups of patients, and the relatively smaller number of AYA enrollments to trials overall. Nonetheless, if the overall observed trends towards greater diversity in younger patients over the past few decades represents a greater willingness on the part of diverse populations to participate in clinical trials, this holds promise for current initiatives to improve diversity in cancer study cohorts in future years.30
The greater diversity among AYA patients may derive from a greater potential towards mobility among young AYA patients, and thus a greater potential to access clinical trials by navigating transportation and relocation more easily than their older counterparts. Studies have shown that a plurality of AYA patients are treated at NCI-designated cancer centers.22,36 Future efforts should seek to increase availability and awareness of clinical trials in the community setting, such as through the NCI’s Community Oncology Research Program (NCORP), under which dedicated infrastructure to cancer clinical trials has already been established.37 Particularly among older AYA patients, who are more likely to receive care at a community site, compared to younger AYA patients, who more often receive care at a pediatric oncology academic institution, there is an urgent need to address system, provider, and patient-level barriers to clinical trials specifically for AYAs in the community. A combination of more ready access to large centers that specialize in AYA cancer research and improved access to AYA trials in the community setting is critical to advancing research into AYA-specific cancer treatments, with the long term aim to improve survival gains for AYA patients.6,38
As recently chronicled by Bleyer and colleagues, lymphomas and leukemias are among the cancers with the highest prevalence in AYAs,38 and indeed we found that proportional enrollment of AYA patients was highest among trials for these cancers. In contrast, fewer than 5% of breast cancers occur in AYA patients in the U.S. Yet due to the high prevalence of breast cancer in the U.S. population – and, accordingly, the prominent conduct of, and enrollment to, breast cancer clinical trials – nearly half of all AYA patients in SWOG were breast cancer patients. Further, breast cancer causes 12% of all cancer-related deaths in AYAs, more than any other malignancy.39 Thus breast cancer is a solid tumor with great relevance to the AYA population, necessitating the vital research into treatments and survivorship issues (e.g., fertility) for these patients.40,41
To our knowledge, no prior study has characterized the multi-decade history and diversity of AYA patients enrolled in NCI-sponsored cooperative group clinical trials. However, the study has limitations. Since the study included enrollments from only one of the NCI’s adult network groups, its generalizability may be limited. The Children’s Oncology Group reported a similar trend in decreasing proportional accrual overtime; however this study did not report on demographic factors of individual enrollees.21 These decreases in proportional accrual may be related to the availability of clinical trials, specifically large adjuvant treatment trials. Our study included a detailed assessment of the nature of actively-enrolling NCI network group clinical trials over time to aide in interpretation of aggregate AYA enrollment trends. However, given the granularity of the potential associations among cancers and demographic types, an examination of individual cancer trial activations in relation to demographic trends was beyond the scope of this analysis. In general, understanding the landscape of available trials and comparative enrollment patterns will further inform future targeted interventions to improve underrepresentation by specific populations but also address low accrual generally. Additionally, although the inspection of temporal trends in enrollment to specific subgroups is broadly informative, it was limited by heterogeneity across years, likely due to the small number of AYA patients overall. Thus the emphasis on interpretation of overall rates across the entire 25 year time period may be more reliable.
The U.S. Food and Drug Administration, by the direction of the U.S. Congress, has indicated that one of its institutional goals is to ensure that “people of different ages, races, ethnic groups, and genders are included in clinical trials,” such that drugs are tested in all the sub-populations they are ultimately intended to help.42 In our view, this principle should also extend to the study of AYA patients, especially given the possibility of biologic heterogeneity between AYAs and older adults in their natural histories, treatment responsiveness, and toxicity profiles. This landscape evaluation of AYA enrollment over a 25-year period to one of the NCI’s largest network groups can inform efforts to study this demographic group prospectively, in the hope of improving management of a population of patients who have been at-risk for healthcare disparities and inferior outcomes compared to other age-defined cohorts.
Funding information:
Research reported in this publication was supported in part by the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health under grant award 5UG1CA189974 (Dr. Hershman) and 5T32CA094061 (Dr. Beauchemin); in part by the Hope Foundation for Cancer Research in support of infrastructure data analysis and trial design within SWOG, at the SWOG Statistical and Data Management Center (Dr. Unger); and in part by a Research Professorship grant by the American Cancer Society (Dr. Hershman).
Footnotes
Publisher's Disclaimer: Disclaimer: The funder did not have any influence over the content of this manuscript or the decision to submit for publication.
Disclosures: The authors report no conflicts of interest.
References
- 1.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8(4):288–298. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). https://seer.cancer.gov/statfacts/html/aya.html#:~:text=84.6%25%20of%20AYAs%20diagnosed%20with,for%205%20years%20after%20diagnosis.&text=Cancers%20diagnosed%20among%20AYAs%2C%20ages%2015%E2%80%9339%2C%205.0%25. Accessed April 12, 2021. [Google Scholar]
- 3.Bleyer A Latest Estimates of Survival Rates of the 24 Most Common Cancers in Adolescent and Young Adult Americans. J Adolesc Young Adult Oncol. 2011;1(1):37–42. [DOI] [PubMed] [Google Scholar]
- 4.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 5.Coccia PF. Overview of Adolescent and Young Adult Oncology. J Oncol Pract. 2019;15(5):235–237. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Moke DJ, Tsai KY, et al. A Reappraisal of Sex-Specific Cancer Survival Trends Among Adolescents and Young Adults in the United States. J Natl Cancer Inst. 2019;111(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freyer DR, Seibel NL. The Clinical Trials Gap for Adolescents and Young Adults with Cancer: Recent Progress and Conceptual Framework for Continued Research. Curr Pediatr Rep. 2015;3(2):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger JM, Cook E, Tai E, Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book. 2016;35:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isenalumhe LL, Fridgen O, Beaupin LK, Quinn GP, Reed DR. Disparities in Adolescents and Young Adults With Cancer. Cancer Control. 2016;23(4):424–433. [DOI] [PubMed] [Google Scholar]
- 10.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29(30):4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine. National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press, 2010. [PubMed] [Google Scholar]
- 12.SWOG Cancer Research Network. History & Impact. Available at: https://www.swog.org/about/history-impact. Accessed August 10, 2020. .
- 13.Massett HA, Hampp SL, Goldberg JL, et al. Meeting the Challenge: The National Cancer Institute’s Central Institutional Review Board for Multi-Site Research. J Clin Oncol. 2018;36(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2014) National Cancer Institute, DCCPS, Surveillance Research Program; released April 2017 based on the November 2016 submission. 2017.
- 15.American Community Survey (ACS) Data. [cited 2018 March 15]; Available from: https://www.census.gov/programs-surveys/acs/data.html.
- 16.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr., Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 17.Unger JM, Coltman CA Jr., Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24(1):141–144. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–1389. [DOI] [PubMed] [Google Scholar]
- 19.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Jama. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 20.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–4631. [DOI] [PubMed] [Google Scholar]
- 21.Roth ME, O’Mara AM, Seibel NL, et al. Low Enrollment of Adolescents and Young Adults Onto Cancer Trials: Insights From the Community Clinical Oncology Program. J Oncol Pract. 2016;12(4):e388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth ME, Unger JM, O’Mara AM, et al. Enrollment of adolescents and young adults onto SWOG cancer research network clinical trials: A comparative analysis by treatment site and era. Cancer Med. 2020;9(6):2146–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110(11):2385–2393. [DOI] [PubMed] [Google Scholar]
- 24.Castellino SM. An intergroup collaboration for advanced stage classical Hodgkin lymphoma (cHL) in adolescents and young adults (AYA): SWOG S1826. 2020; ASCO Virtual Scientific Program. [Google Scholar]
- 25.Weiss AR, Chen YL, Scharschmidt TJ, et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21(8):1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim ES, Bruinooge SS, Roberts S, et al. Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. J Clin Oncol. 2017;35(33):3737–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coccia PF, Pappo AS, Beaupin L, et al. Adolescent and Young Adult Oncology, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(1):66–97. [DOI] [PubMed] [Google Scholar]
- 28.Ostrom QT, Gittleman H, de Blank PM, et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18 Suppl 1(Suppl 1):i1–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst. 2011;103(8):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute. Division of Cancer Prevention. NCI Community Oncology Research Program (NCORP) Expands to More States. Available at: https://prevention.cancer.gov/news-and-events/news/nci-community-oncology-1. Accessed August 12, 2020. . [Google Scholar]
- 31.United States Census Bureau. Current Population Survey (CPS). https://www.census.gov/programs-surveys/cps.html. Accessed June 14, 2020. .
- 32.Frey WH. Diversity explosion: How new racial demographics are remaking America. Brookings Institution Press; 2018. [Google Scholar]
- 33.Loree JM, Anand S, Dasari A, et al. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. 2019;5(10):e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA Oncol. 2016;2(1):137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger JM, Hershman DL, Osarogiagbon RU, et al. Representativeness of Black Patients in Cancer Clinical Trials Sponsored by the National Cancer Institute Compared With Pharmaceutical Companies. JNCI Cancer Spectr. 2020;4(4):pkaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muffly L, Alvarez E, Lichtensztajn D, Abrahão R, Gomez SL, Keegan T. Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv. 2018;2(8):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institutes of Health. National Cancer Institute Community Oncology Research Program (NCORP). Available at: https://ncorp.cancer.gov/. Accessed August 12, 2020. .
- 38.Bleyer A, Tai E, Siegel S. Role of clinical trials in survival progress of American adolescents and young adults with cancer-and lack thereof. Pediatr Blood Cancer. 2018;65(8):e27074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Institute. Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). https://seer.cancer.gov/statfacts/html/aya.html. Accessed February 16, 2021.
- 40.US Department of Health and Human Services. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. Report of the Adolescent and Young Adult Oncology Progress Review Group. NIH Publication No. 06–6067. Washington, DC: US Department of Health and Human Services; 2006., 2006. [Google Scholar]
- 41.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.U.S. Food and Drug Administration. Diversity in Clinical Trial Participation. Available at: https://www.fda.gov/patients/clinical-trials-what-patients-need-know/diversity-clinical-trial-participation. Accessed August 12, 2020. .


