Abstract
Objective:
To determine whether cART initiation alters the trajectory of cognitive performance in HIV+ men, and whether cognition prior to cART predicts post-cART function.
Design:
Longitudinal cohort study. Multicenter AIDS Cohort Study.
Methods:
From an initial set of 3701 men with complete neuropsychological data, men with HIV infection were initially matched with men without infection on cognitive status, race, age, and timeline (T0 defined as cART initiation). Propensity score matching was then used to match pairs on depressive symptoms at T0, education, T0 cognitive scores, and recruitment cohort. There were 506 matched pairs of infected and uninfected men in the final analysis. Mixed effect models were constructed to analyze the trajectories of cognitive functions and to test the effect of cART and HIV on cognitive functions over time.
Results:
Performance in each cognitive domain did not change following the initiation of cART among HIV-infected men with prior impairment and was comparable to the performance of their matched uninfected men. However, among the infected men who were unimpaired prior to cART, motor function declined significantly faster than it did for uninfected controls.
Conclusions:
Cognitive dysfunction is persistent in HIV-infected men and cART does not alter the trajectory of cognitive decline in men who were impaired prior to effective therapy. This suggests that current cognitive impairment in HIV+ men results from a legacy effect, and from factors other than the HIV itself. Furthermore, motor skills may be uniquely vulnerable to the virus, cART, or age-related co-morbidities.
Keywords: HIV, Cognition, cART, Legacy Effect, MACS
Introduction
HIV penetrates the central nervous system early after infection[1, 2] and if viral replication remains unchecked, neurological symptoms can be noted relatively early in the course of disease[3, 4]. Chronic infection leads to inflammatory and neurotoxic cascades that contribute to cognitive impairment in upwards of 50% of HIV+ individuals [5–7]. Combination antiretroviral therapy (cART) effectively suppresses HIV replication, slows the progression of disease [8] and extends the life expectancy of individuals with HIV infection (e.g., [9, 10]) to the point where what had been an acute and terminal illness can be viewed as a chronic condition. In addition, the incidence of dementia has fallen [11], white matter pallor [1, 12] has virtually disappeared, and the prevalence of HIV encephalitis has declined [9].
However, milder forms of cognitive dysfunction remain part of the clinical picture [5, 11, 13–15], and one factor that could explain this is a “legacy effect” [16]. That is, a residual mild neuropsychological dysfunction despite successful viral suppression, that is presumably due to prior CNS damage caused directly or indirectly by HIV prior to effective therapy [17, 18]. Alternatively, this persistent mild neuropsychological impairment may be due to incomplete cART CNS penetration, cART side effects, or/and non-HIV related comorbidities which might have a disproportionate effect on individuals with sustained CNS damage prior to cART [19].
Our goal here was to examine cognitive trajectories of HIV+ men to determine whether cART initiation altered this trajectory. We used longitudinal data from the Multicenter AIDS Cohort Study (MACS), a study of homosexual and bisexual men at risk for or infected with HIV. To examine whether the changing pattern of trajectories, if any, is related to the HIV effect and the potential interaction between HIV and cognitive impairment, we matched HIV-infected men with uninfected participants, separately for men with and without cognitive impairment prior to cART. We hypothesized that cART would result in improved cognition over time for HIV-infected participants (see Sacktor, and colleagues [11, 14]), but that cART would not overcome any legacy effect of pre-cART cognitive impairment.
Methods
Study Population and Design:
MACS participants were recruited from sites in Baltimore, Chicago, Los Angeles, and Pittsburgh. A subgroup of participants enrolled in the neuropsychology (NP) substudy beginning in 1988 (N=5470) (See Becker and Miller and colleagues [20, 21] for details). Recruitment occurred in three waves: 3687 men were enrolled in 1984–1985, 474 were enrolled in 1987–1991, and 1309 were enrolled between 2001 and 2003.
Participants in the NP study underwent a neuropsychological (NP) evaluation that assessed six cognitive domains, including motor function, executive function, learning, speed of information processing, memory, and attention/working memory [20]. Domain scores were derived at each visit by computing a T-score adjusted for age, race and education, standardized to have a mean of 50 and a standard deviation of 10. Tests were given at the time of regular semi-annual MACS visits originally. In 2005, this schedule changed, and participants were evaluated biannually, or semi-annually if they were cognitively impaired. Data used in this study were collected before October 2017.
The current analysis included only those visits where all NP domain scores were available: 22,900 visits from 3701 men. HIV-infected men who were using cART at the time of study enrollment were excluded (1497 men with 6300 visits). Included in the analysis were 537 HIV-infected participants with 6300 visits and 1667 uninfected participants with 10300 visits (see Supplemental Figure 1). A comparison between HIV-infected men that were included and HIV-infected participants who were excluded is shown in Supplemental table e-1.
Cognitive Function Assessment/Cognitive Impairment Classification
The Multivariate Normative Comparison (MNC) method was used to classify cognitive impairment. MNC has the advantage of controlling the false discovery rate by taking into account the intercorrelation of cognitive functions [22]. Because the likelihood that an individual is impaired in any single cognitive domain is a function of their performance in other domains, there is an inflated risk of classifying an individual as impaired, when in fact they are not; the MNC method addresses this problem. The MNC provides an overall metric of distance between each of an individual’s multiple domain scores and the corresponding norms of healthy controls and this adjusts the family-wise error (and false positive errors) in a way that the Frascati and Gisslen methods cannot [23–25]. We have used this cross-sectional method in the MACS cohort [22], and also developed a longitudinal application of the MNC [26] with the same effectiveness at controlling the Family-Wise Error Rate. In this paper we use MNC method to determine neuropsychological impairment and set the family-wise error rate at 0.05.
We used as a reference group for the MNC all 1667 uninfected men (10,300 MACS visits) to build the normative mean and covariance matrix of the six NP domain scores. For each participant, a single distance across all domains was calculated between his domain scores at one visit and the normative mean of the reference group, fitted along an F distribution. We consider a participant as cognitively impaired if this distance was above the 95th percentile of an F distribution and the average domain score fell below the normative means. Individuals were coded as having prior cognitive impairment if they were impaired at any visit prior to T0, where T0 was defined as initiation of cART for HIV-infected men. For uninfected men, T0 was defined during the matching steps (see below).
Initially, we also classified cognitive impairment using the Gisslen criteria [23] and examined the trajectories of domain scores in each group. The results were consistent with what we found using the MNC method, thus we used the latter method because it controlled the false discovery rate.
Matching
Our goal was to investigate how the trajectories of domain scores behaved after the initiation of cART, by comparing the slopes of trajectories before and after T0. To better appreciate changes due to treatment, we compared the trajectories of HIV-infected men with those of matched uninfected men with similar cognitive performance at T0. Therefore, we matched uninfected men with HIV-infected men in a two-step procedure to arrive at 4 groups based on HIV status (HIV-infected vs. uninfected) and cognitive impairment status prior to T0 (impaired vs. unimpaired). We first needed to define a T0 for each uninfected man; during the first step, we selected several potential uninfected match candidates for each HIV-infected man by temporarily setting T0 for these uninfected candidates as their MACS visit that was closest to the T0 for the infected participant. The participants were selected based on: 1) race, 2) prior cognitive impairment status, 3) aligned T0 timepoints at most 10 years apart, and 4) age (at most 10 years apart) at T0. These four criteria were chosen for the initial match due to their importance to predicting cognitive functions, resulting in 533 HIV-infected patients matched with 619 unique uninfected candidates. We used the 10-year interval so that the matched participants would have close visits and similar ages at T0. Had we used shorter intervals (e.g., 5 or 8 years) we would have had fewer matched pairs due to infrequent visits.
The second matching step used propensity score matching for each of the 533 HIV-infected men. The propensity score was calculated using a logistic regression model based on age at T0, depression symptoms at T0, education level, the six NP domain scores at T0, and recruitment cohort, using the data from the matched 533 HIV-infected patients and the 619 unique uninfected candidates identified in Step 1 (above).
Statistics
Demographic data, depression scores and domain scores for matched pairs were compared using paired t-tests and McNemar’s test. Effect sizes were calculated using standardized differences for both continuous and binary variables. We also compared HIV-infected participants that were included in our analysis and those excluded using two sample t-tests and two-proportion tests (Supplemental Tables e-1 and e-2). Effect sizes were calculated using Cohen’s d for continuous variables and Odds Ratio for categorical variables. The final propensity score matching yielded one-to-one matches between infected and uninfected men.
Trajectories
We plotted the local estimated scatterplot smoothing curves [27] of each domain score for each group of participants as a function of time. We used 0.95 as the smoothing span, which was selected by 10-fold cross-validation. Time ranged from T−5 to T15. Ninety five percent pointwise confidence intervals of the trajectories were obtained from 1000 bootstrap samples. Trajectories between pair of groups with the same infection status or impairment status were compared for each domain. To quantitatively measure how trajectories have changed after the initiation of cART, we fitted mixed effect models for domain scores with random intercepts to accommodate the paired design. We used an indicator of whether the observation was obtained after T0 (0 for before T0 and 1 for after T0) to allow change of slope of trajectories after the initiation of cART. The following terms were included in the model: HIV status (0 for uninfected and 1 for HIV-infected), time, interaction between HIV status and time, interaction between T0 indicator and time, and interaction of HIV status, T0 indicator and time. Models were fitted for each domain score. Separate models were fitted for participants with and without prior impairment. Several contrasts were conducted in each fitted model to estimate: 1) the slope of HIV-infected participants before T0, 2) the slope of HIV-infected participants after T0, 3) change of slope of HIV-infected participants’ cognitive function trajectories before and after T0 and 4) the comparison of slope after T0 between HIV-infected and uninfected participants. To control the false discovery rate, we used the Benjamini-Yekutieli procedure [28] with 24 tests in total (four tests for each of the six domains) to modify the threshold of rejection. Details of the mathematical model can be found in the Supplemental Materials.
Standard Protocol Approvals, Registrations, and Patient Consents
Informed consent for research was obtained from all participants. All study protocols were approved by an ethical standards committee at each participating institution and the coordinating center.
Results
Matching
Due to the overlap of potential candidates and the goal of one-to-one matching, 25 HIV-infected participants could not be matched. Their uninfected candidates were matched with other HIV-infected participants who had closer propensity scores. When we checked the discrepancy of propensity scores between matched pairs, two had discrepancies greater than 0.25σ of the pooled propensity scores and were excluded from the analyses. As a result, 506 matched pairs were used after matching (See Supplemental Figure 1).
The process resulted in four groups of participants based on their infection (infected vs. uninfected) and prior impairment status (impaired vs. unimpaired). HIV-infected men in matched pairs with prior impairment had, on average, 12 visits and 15.6 years of follow-up time; HIV-infected men without prior impairment had 11 visits and 19.0 years of follow-up time. The average number of visits was 12 and the average years of follow-up was 17.7 for uninfected men with prior impairment. For uninfected men without prior impairment, the two averages were 11 and 19.0.
Demographics, depression scales and cognitive functions at T0 are summarized in Tables 1 and 2. HIV-infected patients and matched uninfected participants with prior impairment had comparable age, depression scores, education level and cognitive functions at T0. Tests showed that matched pairs without prior impairment differed in age and education level, yet the effect size was small, and we considered the unimpaired pairs to be comparable. We also checked how patients with prior impairments differed from patients without prior impairment other than cognitive functions and observed that the matched pairs with prior impairment were significantly older.
Table 1:
Characteristics at T0 for 83 pairs of cognitively impaired participants by HIV status
| HIV Infected | HIV Uninfected | p-value | Effect size 1 | |
|---|---|---|---|---|
| Age | 47.97±8.7 | 46.87±8.6 | 0.07 | 0.20 |
| CESD | 13.37±10.5 | 13.18±12.4 | 0.89 | 0.01 |
| Caucasian | 72.29% | 72.29% | 1.00 | 0.00 |
| >16 yrs ed | 44.58% | 51.85% | 0.29 | 0.14 |
| Executive | 44.88±11.5 | 44.80±11.3 | 0.96 | 0.00 |
| Learning | 45.63±11.2 | 44.01±11.2 | 0.35 | 0.10 |
| Memory | 45.10±11.1 | 44.54±10.3 | 0.73 | 0.04 |
| Motor | 41.64±15.0 | 41.33±15.5 | 0.90 | 0.01 |
| Speed | 43.16±10.0 | 44.53±10.1 | 0.31 | 0.11 |
| Working Memory | 47.26±11.0 | 48.77±10.6 | 0.34 | 0.11 |
| Enrollment Cohort (2002) | 31.33% | 31.33% | 1.00 | 0.00 |
Effect sizes were calculated using standardized difference in comparison between matched pairs.
CESD = Center for Epidemiologic Studies Depression Scale. All cognitive domains are reported as T-scores
Table 2:
Characteristics at T0 for 423 pairs of unimpaired participants by HIV status.
| HIV Infected | HIV Uninfected | p-value | Effect size1 | |
|---|---|---|---|---|
| Age | 43.86±7.8 | 44.69±8.2 | 0.00 | 0.14 |
| CESD | 11.63±10.8 | 10.80±10.5 | 0.18 | 0.06 |
| Caucasian | 80.14% | 80.14% | 1.00 | 0.00 |
| >16 yrs ed | 51.30% | 57.92% | 0.01 | 0.13 |
| Executive | 52.62±8.7 | 53.00±8.5 | 0.48 | 0.03 |
| Learning | 51.69±8.7 | 51.77±7.9 | 0.90 | 0.00 |
| Memory | 51.51±8.6 | 51.83±7.8 | 0.58 | 0.03 |
| Motor | 49.35±8.6 | 49.90±8.6 | 0.36 | 0.04 |
| Speed | 51.74±8.9 | 52.27±9.0 | 0.36 | 0.04 |
| Working Memory | 51.92±10.1 | 51.90±9.2 | 0.97 | 0.00 |
| Enrollment Cohort (2002) | 24.59% | 22.22% | 0.04 | 0.11 |
Effect sizes were calculated using standardized difference in comparison between matched pairs.
CESD = Center for Epidemiologic Studies Depression Scale. All cognitive domains are reported as T-scores.
We compared the HIV-infected participants who were initially considered for matching (537 participants) with those who were excluded because of missing domain scores or not being naïve to cART (2246 participants) (See Supplemental table e-1). The HIV-infected participants who were excluded had less education and were younger at T0 than the included men. Excluded HIV-infected participants also had significantly lower scores in all NP domains at T0. Supplementary table e-2 presents the comparisons between those 506 matched HIV-infected participants and the remaining 31 unmatched HIV-infected participants. Participants who were not matched were younger at T0, more likely to be non-Caucasian, were recruited from the earlier cohort, but were comparable in all NP domain scores at T0.
Trajectories Comparison
We first compared the trajectories of the impaired and unimpaired (i.e., prior to T0) HIV-infected participants (Figure 1; red curves). The trajectories of executive, speed, learning, working memory, and memory scores of the impaired men (dashed lines) are consistently below those from unimpaired men (solid lines) throughout 15 years of follow-up after T0. Moreover, the 95% bootstrap confidence intervals (CI) of these two groups do not overlap for each year for the first 12 years. This indicates that cognitive impairment status prior to cART was persistent for 12 years following treatment initiation. The CIs begin to overlap after 12 years, which may have a biological basis, or may be a consequence of the smaller sample size and the relatively unstable estimation of the trajectories over the boundary; only 27.7% participants remained at 12 years in impaired HIV-infected group and 45.4% participants remained in unimpaired HIV-infected group (see Supplementary table e-3).
Figure 1.
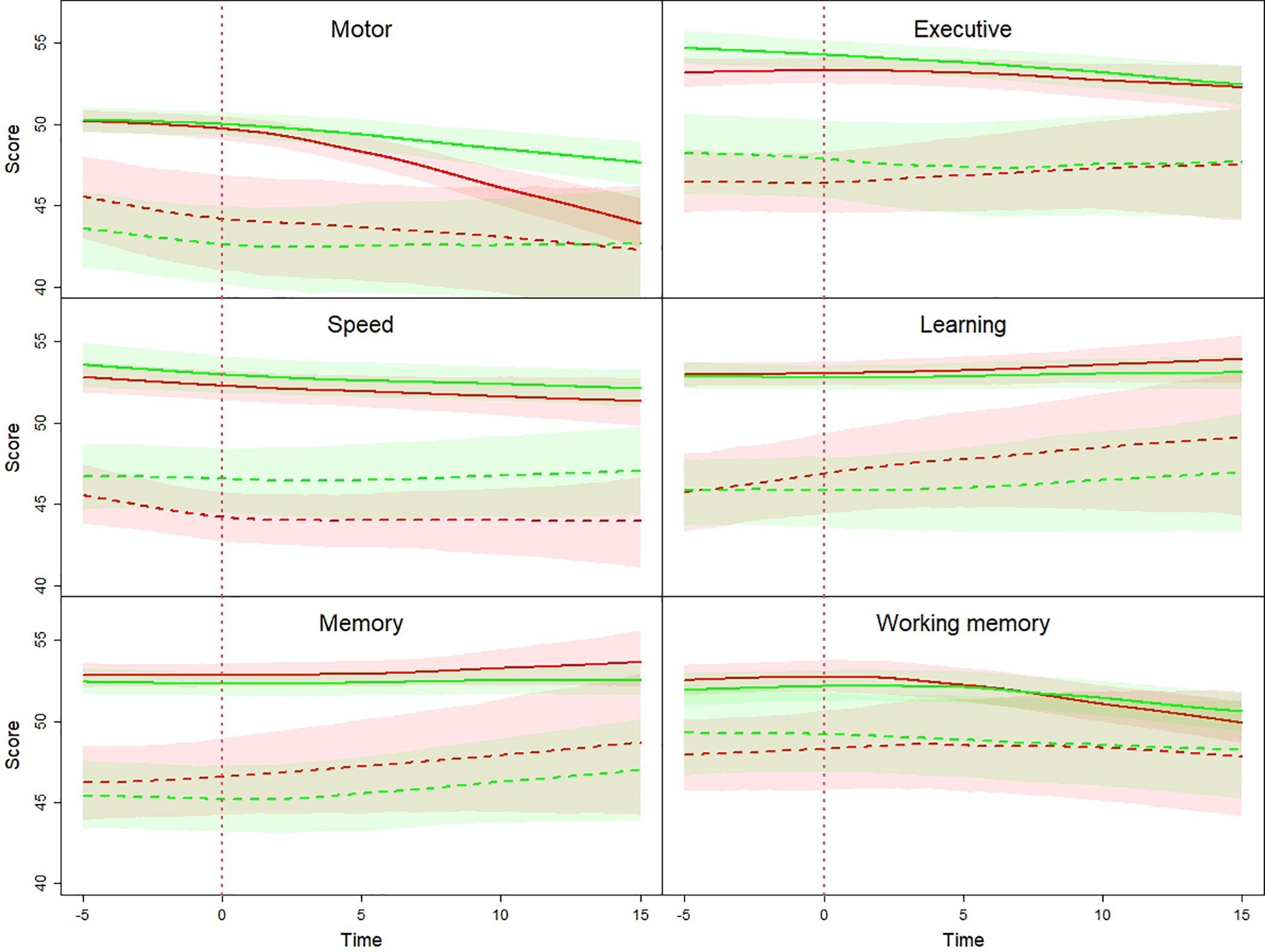
For the uninfected participants (Figure 1; green curves), we again observed that the trajectories of all six domain scores from those men with prior impairment (dashed lines) were consistently lower than the trajectories (solid lines) of unimpaired men across all time points. This again indicates persistence of cognitive trajectory over the course of follow-up, this time for uninfected men.
Mixed effect models provided further insights into how trajectories changed over time. Changes in the slopes of the trajectories before and after T0 were estimated as the slopes after T0 minus the slopes before T0. Comparisons of slopes after T0 between HIV-infected and uninfected men were given by slopes of the HIV-infected group minus that of the uninfected group.
First, we modeled cognitive functions for matched participants with prior impairment. The results are shown in Table 3. We used the Benjamini-Yekutieli procedure to adjust for multiple comparisons and significant results were denoted by asterisks. There were no significant changes in slopes before and after T0 in any domain for HIV-infected participants. After T0, cognitive functions of HIV-infected men remained stable, with no significant declines or improvements in domain scores, which is similar to their uninfected counterparts.
Table 3:
Test statistics from the mixed effect model for impaired participants.
| Change of slope for HIV+ 1 | Comparison of slope after T0 2 | |||||
| Estimate | Std.Error | P-value | Estimate | Std. Error | P-value | |
| Executive | −0.15 | 0.27 | 0.58 | 0.22 | 0.13 | 0.08 |
| Learning | −0.51 | 0.29 | 0.08 | −0.05 | 0.13 | 0.71 |
| Memory | −0.16 | 0.29 | 0.58 | −0.05 | 0.13 | 0.70 |
| Motor | −0.45 | 0.37 | 0.22 | −0.23 | 0.17 | 0.18 |
| Speed | 0.24 | 0.24 | 0.32 | −0.03 | 0.11 | 0.78 |
| Working Memory | −0.10 | 0.29 | 0.74 | 0.31 | 0.13 | 0.02 |
| Slope before T0 for HIV+ | Slope after T0 for HIV+ | |||||
| Estimate | Std. Error | P-value | Estimate | Std. Error | P-value | |
| Executive | 0.28 | 0.22 | 0.19 | 0.13 | 0.09 | 0.16 |
| Learning | 0.52 | 0.23 | 0.02 | 0.01 | 0.10 | 0.92 |
| Memory | 0.21 | 0.23 | 0.35 | 0.05 | 0.10 | 0.60 |
| Motor | 0.11 | 0.29 | 0.71 | −0.35 | 0.13 | 0.01 |
| Speed | −0.17 | 0.19 | 0.35 | 0.06 | 0.08 | 0.44 |
| Working Memory | 0.16 | 0.23 | 0.47 | 0.07 | 0.10 | 0.51 |
Change of slope is given by slope after T0 minus slope before T0
Comparison of slope is given by slope of HIV+ minus slope of HIV−
for rejection after multiple testing adjustment.
We did the same analysis for matched participants without prior impairment and summarized the results in Table 4. Changes of slopes before and after T0 were observed in motor and working memory scores for HIV-infected men in that HIV-infected men had a significant decline in motor score after T0. On average, their motor scores decreased 0.45 unit every year, and the rate of decrease was faster than the matched uninfected participants. We also saw a decline in working memory scores after T0 among HIV-infected men, but at a comparable rate of decrease as their uninfected counterparts.
Table 4:
Test statistics from the mixed effect model for unimpaired participants.
| Change of slope for HIV+ 1 | Comparison of slope after T02 | |||||
| Estimate | Std. Error | P-value | Estimate | Std. Error | P-value | |
| Executive | −0.23 | 0.10 | 0.03 | 0.04 | 0.04 | 0.36 |
| Learning | −0.30 | 0.11 | 0.01 | 0.01 | 0.04 | 0.86 |
| Memory | −0.22 | 0.11 | 0.04 | 0.01 | 0.04 | 0.80 |
| Motor | −0.67 | 0.12 | <0.0001* | −0.18 | 0.05 | 0.0003* |
| Speed | 0.04 | 0.11 | 0.73 | 0.06 | 0.04 | 0.18 |
| Working Memory | −0.55 | 0.11 | <0.0001* | −0.04 | 0.05 | 0.33 |
| Slope before T0 for HIV+ | Slope after T0 for HIV+ | |||||
| Estimate | Std. Error | P-value | Estimate | Std. Error | P-value | |
| Executive | 0.17 | 0.08 | 0.05 | −0.06 | 0.03 | 0.04 |
| Learning | 0.32 | 0.09 | 0.0003* | 0.02 | 0.03 | 0.52 |
| Memory | 0.23 | 0.09 | 0.01 | 0.01 | 0.03 | 0.80 |
| Motor | 0.22 | 0.10 | 0.03 | −0.45 | 0.04 | <0.0001* |
| Speed | −0.03 | 0.09 | 0.71 | 0.00 | 0.03 | 0.91 |
| Working Memory | 0.34 | 0.09 | 0.0002* | −0.22 | 0.03 | <0.0001* |
Change of slope is given by slope after T0 minus slope before T0
Comparison of slope is given by slope of HIV+ minus slope of HIV−
for rejection after multiple testing adjustment.
Conclusions
After HIV-infected men with prior impairment started cART, their cognitive functions did not change; they continued to have poor neuropsychological test performance at a level similar to their uninfected counterparts. HIV-infected men without prior impairment had comparable performance as their matched uninfected participants - with one exception. Motor functions declined faster among the unimpaired HIV-infected men compared to uninfected men. Thus, the cognitive impairment prior to cART is persistent, which forces us to reject our initial hypothesis regarding improved cognition following cART, and to consider that a legacy effect exists in these men in terms of cognitive functions (Alford & Vera [17], pg. 58f). The fact that HIV-infected men and uninfected individuals had similar trajectories after starting cART – with the exception of motor skills – indicate that a low level of HIV virus may not further impact cognitive functions.
These findings are important for both clinicians dealing with HIV-infected patients, and with NeuroHIV researchers. For the former, these data reinforce the need to examine multiple factors that may have an impact on cognition, including personal risk factors, age-related conditions, new infections, and possible medication interactions. For investigators of the impact of HIV on the CNS these data suggest that a better understanding of the altered milieu of the CNS as a consequence of long-term, poorly controlled infection may be important to understand the current clinical symptoms. None of these data mean that HIV is not neurotrophic or that it is irrelevant in the context of cART – quite the contrary. It is only that we need to consider the entire natural and treated history of HIV infection when diagnosing or treating CNS complications, or when modelling the pathological cascades that might initiate the symptoms.
Our data are consistent with other studies that find persistent cognitive impairment despite adequate treatment [29–31]. For example, infected women followed for an average of 6 years with consistent viral suppression had persistent cognitive impairment, including in motor skills, as compared to HIV-negative women [31]. Several other studies have found very low rates of cognitive impairment in HIV+ individuals that have adequate therapeutic treatment and are healthy overall [32]. Here we used a large sample that was followed for more than 15 years. The length of this follow-up period provides the opportunity to examine potential long-term effects of HIV infection and strengthens the trajectory analysis which shows that cognitive status was maintained for up to 20 years.
If HIV itself is not the direct mechanism of cognitive dysfunction in HIV+ individuals, this leaves open the question of other mechanisms of action. The CHARTER study [5] found that a history of more severe immunosuppression (assessed via CD4 nadir) and presence of confounding comorbidities were associated with the greatest risk for cognitive impairment, even after successful cART-related immune recovery [33]. However, when longitudinal performance was analyzed in the same cohort, cognitive decline was uncommon in individuals with undetectable HIV viral RNA and CD4 nadir did not predict decline [34]. Instead, kidney function (via glomerular filtration rate) was the greatest predictor of cognitive decline, with lower education, longer duration of HIV Disease, and CSF protein levels adding predictive value. Taken together with the findings of the current study, these data indicate that in addition to an HIV-legacy effect, factors other than the infection, including co-occurring physiological dysfunction or health behaviors, may be important contributors of the continuing, mild cognitive dysfunction in HIV-infected individuals. This is also true of the uninfected men in the present study, as HIV and related issues could not be the cause of their impairments.
There are several issues that remain unaddressed. For example, our data do not identify a reason for the significant decline of motor function in HIV-infected group without prior impairment. Motor dysfunction is common among individuals in the National NeuroAIDS Tissue Consortium [35]. In that sample, motor impairment was predicted by cerebrovascular disease, AIDS-related CNS diseases, as well as HIV-associated neuropsychological impairment. Thus, it may be the case that as these cognitively unimpaired individuals aged, the impact of subclinical CVD may have been sufficient to account for the decline [34, 36]. On the other hand, it may be the case that the motor systems are more sensitive to continued cellular inflammation or cART-induced peripheral neuropathy [37]. However, inflammation does not account for all cognitive pathologies in HIV [38], and there is a potential role for other cognitive pathologies including subcortical brain atrophy [39, 40], or other comorbid conditions.
We did not adjust for survivor biases (see also, McIntosh, and colleagues [41], pg., 375). If the factors that resulted in an individual failing to provide data (e.g., death, illness, drop-out) are related to neuropsychological test performance (i.e., competing risk), it will be critical to deal with this issue in future studies. However, the death rates among the 506 HIV-infected men with (24.10% (20 of 83)) and without prior impairment (17.02% (72 of 423)) did not differ (Odds ratio = 1.55, 95% CI = 0.88 – 2.72). Finally, we analyzed data only from men; with the creation of the MACS/WIHS Combined Cohort Study (https://mwccs.org/), an analysis similar to this one may be possible in the future that will include HIV-infected women. Also, we did not include medical comorbidities in our matching protocol. While that would have added further information on the role of medical conditions on cognitive trajectory, this would have made the matching procedure even more difficult, with a final sample size that would reduce our statistical power.
These data provide evidence that with the exception of fine motor coordination the trajectories of neuropsychological test performance are independent of HIV infection. cART did not alter the trajectory of cognitive decline in HIV+ men, which means that mild cognitive dysfunction after cART initiation, at least, is a consequence of a legacy effect. Furthermore, cognitive trajectories over the course of 15 years did not differ between seropositive and seronegative participants matched for cognitive status prior to T0. This suggests that the cognitive sequalae currently seen in HIV+ individuals may result from pathways independent of viral penetration in the CNS. More studies examining the role of inflammation, vascular health, comorbid disorders, and health behaviors are needed to understand the mechanisms of mild neuropsychological dysfunction in HIV-infected individuals.
Supplementary Material
References
- 1.Navia BA, Cho E-S, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol 1986; 19:525–535. [DOI] [PubMed] [Google Scholar]
- 2.Hellmuth J, Fletcher JL, Valcour V, Kroon E, Ananworanich J, Intasan J, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology 2016; 87(2):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur J Neurologic manifestations of AIDS. Medicine 1987; 66:407–437. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carne CA, Smith A, Elkington SG. Acute encephalopathy coincident with seroconversion for anti-HTLV-III. Lancet 1985; 1:1206–1208. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LG, Sharer LR, Cho ES. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res 1984; 1:447–454. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson LP, Li R, Phair J, Margolick JB, Rinaldo CR, Detels R, et al. Evaluation of the effectiveness of highly active antiretroviral therapy in persons with human immunodeficiency virus using biomarker-based equivalence of disease progression. Am J Epidemiol 2002; 155(8):760–770. [DOI] [PubMed] [Google Scholar]
- 9.Silva AC, Rodrigues BS, Micheletti AM, Tostes S Jr., Meneses AC, Silva-Vergara ML, et al. Neuropathology of AIDS: An Autopsy Review of 284 Cases from Brazil Comparing the Findings Pre- and Post-HAART (Highly Active Antiretroviral Therapy) and Pre- and Postmortem Correlation. AIDS Res Treat 2012; 2012:186850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007; 146(2):87–95. [DOI] [PubMed] [Google Scholar]
- 11.Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001; 56:257–260. [DOI] [PubMed] [Google Scholar]
- 12.Petito CK, Cho E-S, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol and Exper Neurol 1986; 45:635–646. [DOI] [PubMed] [Google Scholar]
- 13.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. Journal of NeuroVirology 2002; 8:136–142. [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010; 50(5):773–778. [DOI] [PubMed] [Google Scholar]
- 17.Alford K, Vera JH. Cognitive Impairment in people living with HIV in the ART era: A Review. Br Med Bull 2018; 127(1):55–68. [DOI] [PubMed] [Google Scholar]
- 18.Calcagno A, Barco A, Trunfio M, Bonora S. CNS-Targeted Antiretroviral Strategies: When Are They Needed and What to Choose. Curr HIV/AIDS Rep 2018; 15(1):84–91. [DOI] [PubMed] [Google Scholar]
- 19.Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 2014; 13(11):1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, et al. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller EN, Selnes OA, McArthur MB. Neuropsychological test performance in HIV1-infected homosexual men: The Multicenter AIDS Cohort Study (MACS). Neurology 1990; 40:197–203. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Molsberry SA, Cheng Y, Kingsley L, Levine AJ, Martin E, et al. Cross-sectional analysis of cognitive function using multivariate normative comparisons in men with HIV disease. AIDS 2019; 33(14):2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gisslen M, Price RW, Nilsson S. The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 2011; 11:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su T, Schouten J, Geurtsen GJ, Wit FW, Stolte IG, Prins M, et al. Multivariate normative comparison, a novel method for more reliably detecting cognitive impairment in HIV infection. AIDS 2015; 29(5):547–557. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Cheng Y, Seaberg EC, Rubin LH, Levine AJ, Becker JT, et al. Longitudinal multivariate normative comparisons. Stat Med 2021; 40(6):1440–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association 1979; 74(368):829–836. [Google Scholar]
- 28.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001; 125(1–2):279–284. [DOI] [PubMed] [Google Scholar]
- 29.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007; 45(2):174–182. [DOI] [PubMed] [Google Scholar]
- 30.Janssen MAM, Koopmans PP, Kessels RPC. Cognitive Decline in Relation to Psychological Wellbeing and HIV Disease- and Treatment Characteristics in HIV-Infected Patients on cART: A One-Year Follow-Up Study. AIDS Behav 2017; 21(6):1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 2017; 89(15):1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials 2011; 12(6):333–338. [DOI] [PubMed] [Google Scholar]
- 33.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25(14):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen T, Brouillette MJ, Fellows LK, Ellis RJ, Letendre S, Heaton R, et al. Personalized Risk Index for Neurocognitive Decline Among People With Well-Controlled HIV Infection. J Acquir Immune Defic Syndr 2017; 76(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson-Papp J, Gensler G, Navis A, Sherman S, Ellis RJ, Gelman BB, et al. Characteristics of Motor Dysfunction in Longstanding Human Immunodeficiency Virus. Clin Infect Dis 2020; 71(6):1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montoya JL, Iudicello J, Fazeli PL, Hong S, Potter M, Ellis RJ, et al. Elevated Markers of Vascular Remodeling and Arterial Stiffness Are Associated With Neurocognitive Function in Older HIV+ Adults on Suppressive Antiretroviral Therapy. J Acquir Immune Defic Syndr 2017; 74(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montoya JL, Campbell LM, Paolillo EW, Ellis RJ, Letendre SL, Jeste DV, et al. Inflammation Relates to Poorer Complex Motor Performance Among Adults Living With HIV on Suppressive Antiretroviral Therapy. J Acquir Immune Defic Syndr 2019; 80(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McArthur JC, Brew BA, Nath A. Neurological complications of HIV infection. Lancet Neurol 2005; 4(9):543–555. [DOI] [PubMed] [Google Scholar]
- 39.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011; 5(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor EE, Zeffiro T, Lopez OL, Becker JT, Zeffiro T. HIV infection and age effects on striatal structure are additive. J Neurovirol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh EC, Tureson K, Rotblatt LJ, Singer EJ, Thames AD. HIV, Vascular Risk Factors, and Cognition in the Combination Antiretroviral Therapy Era: A Systematic Review and Meta-Analysis. J Int Neuropsychol Soc 2021; 27(4):365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


