Abstract
The multi-drug resistance of Pseudomonas aeruginosa is an overwhelming cause of terminal and persistent lung infections in cystic fibrosis (CF) patients. Antimicrobial synergy has been shown for colistin and ivacaftor, and our study designed a relatively high drug-loading dry powder inhaler formulation containing nanoparticles of ivacaftor and colistin. The ivacaftor-colistin nanosuspensions (Iva-Col-NPs) were prepared by the anti-solvent method with different stabilizers. Based on the aggregation data, the formulation 7 (F7) with DSPG-PEG-OMe as the stabilizer was selected for further studies. The F7 consisted of ivacaftor, colistin and DSPG-PEG-OMe with a mass ratio of 1:1:1. The F7 powder formulation was developed using the ultrasonic spray-freeze-drying method and exhibited a rough surface with relatively high fine particle fraction values of 61.4 ± 3.4 % for ivacaftor and 63.3 ± 3.3 % for colistin, as well as superior emitted dose of 97.8 ± 0.3 % for ivacaftor and 97.6 ± 0.5 % for colistin. The F7 showed very significant dissolution improvement for poorly water soluble ivacaftor than the physical mixture. Incorporating two drugs in a single microparticle with synchronized dissolution and superior aerosol performance will maximize the synergy and bioactivity of those two drugs. Minimal cytotoxicity in Calu-3 human lung epithelial cells and enhanced antimicrobial activity against colistin-resistant P. aeruginosa suggested that our formulation has potential to improve the treatment of CF patients with lung infections.
Keywords: colistin, ivacaftor, cystic fibrosis, Pseudomonas aeruginosa, dry powder inhaler, in vitro dissolution
1. Introduction
A mutation in the CTFR-encoding gene causes cystic fibrosis (CF). CTFR, or cystic fibrosis transmembrane conductance regulator (CFTR), is an anion channel at the epithelial cell surface that allows secretion of chloride and bicarbonate (Nick et al., 2020; Talamo Guevara and McColley, 2017; Turcios, 2020). Defects in CFTR affect numerous organs, causing repeated lung infections, loss of exocrine pancreatic function, impaired intestines, reproductive dysfunction, and a high sweat salt level (Bell et al., 2020; Rowe et al., 2017). Most CF patients eventually experience progressive lung disease and mortality (Chalmers, 2020; Deeks, 2016; Heltshe et al., 2015). Ivacaftor (Iva, also known as VX-770, Fig. 1a), is a CFTR potentiator that improves chloride transport and boosts lung function in patients (Bell et al., 2020; Carter et al., 2015). The Food and Drug Administration (FDA) approved ivacaftor to treat the mutant CFTR protein (Paterson et al., 2020; Zhu et al., 2020). Meanwhile, ivacaftor can inhibit the viability of Pseudomonas aeruginosa (PA) due to its quinoline ring (Cho et al., 2019; Schneider et al., 2016; Zhu et al., 2020).
Figure 1.
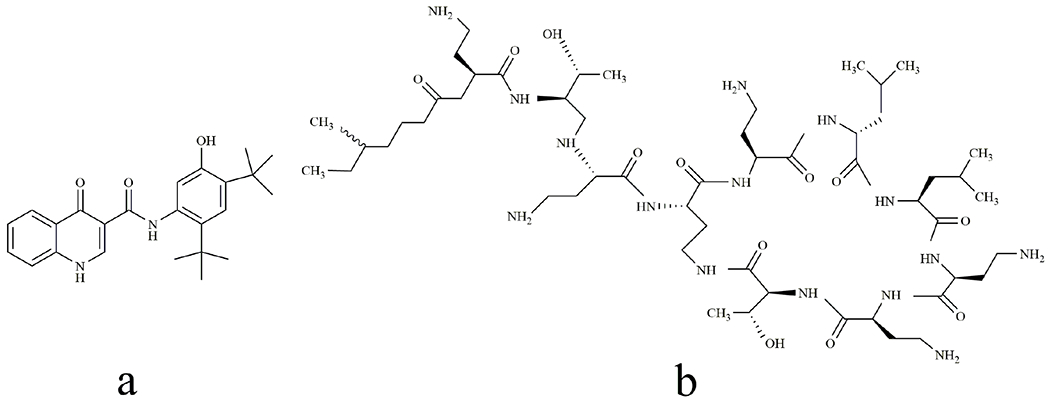
Chemical structures of ivacaftor(a) and colistin (b).
P. aeruginosa is the most ubiquitous cause of lower airway infections in CF patients (Gherardi et al., 2019; Law et al., 2019). Such infections significantly affect mortality and are extremely difficult to eradicate since they form biofilms that promote resistance development (Milczewska et al., 2020; Molchanova et al., 2019). Colistin (Col, Fig. 1b) is a potent cationic and lipopeptide antibiotic against Gram-negative pathogens such as P. aeruginosa (Bergen et al., 2006; Yu et al., 2020b). Unfortunately, parenteral colistin has been found to have high incidences of dose-limiting nephrotoxicity and its clinical application has been limited (Falagas et al., 2005; Gomez-Junyent et al., 2019). However, as all first-line antibiotics increasingly encounter drug resistance from Gram-negative bacteria, “old” polymyxin antibiotics such as the colistin and polymyxin B are garnering increasing interest (Abdelsalam et al., 2018; Lin et al., 2019; Nang et al., 2021).
Pulmonary drug delivery has become a popular option for treating lower respiratory infections (Hickey et al., 2016; Zhou et al., 2015). Dry powder inhalers (DPIs) are portable, exhibit superior stability (both physical and chemical) as well as greater delivery efficiency compared to the traditional devices (Brunaugh and Smyth, 2018; Hassan et al., 2020; Islam et al., 2012; Mangal et al., 2019; Shetty et al., 2020). The co-delivery of ivacaftor and colistin combinations may have the potential to treat colistin-resistant lung infections as earlier study showed enhanced antimicrobial activity for combining ivacaftor and polymyxin against polymyxin-resistant P. aeruginosa. (Schneider et al., 2016). However, ivacaftor has poor water-solubility and its aqueous solubility has limited its application (Zhu et al., 2020). The aqueous solubility of ivacaftor needs be improved to provide more effective inhalation therapy.
One of strategies to increase the solubility and dissolution of drugs with poor water-solubility is to formulate them into nano-sized drug particles (Hashem et al., 2020; Kuk et al., 2019). Recently, nanosuspensions have attracted attention owing to improved dissolution rate, saturation solubility and bioavailability of poorly water-soluble drugs (Bartos et al., 2019; Bohr et al., 2015; Qiao et al., 2020). Since nanoparticles tend to form strong agglomerates or are exhaled during inhalation, they are often formulated into composite microparticles by spray-drying or spray-freeze-drying techniques (Ali et al., 2014; Kwok et al., 2011; Liu et al., 2018; Yang et al., 2008). Our recent study has reported that the water-soluble colistin can act as a matrix material to bind nanoparticles of ivacaftor into inhalable microparticles (Chen et al., 2021; Zhu et al., 2020). Meanwhile, the amphipathic structure of colistin facilitated the stabilization of ivacaftor-colistin co-loaded nanosuspension. However, the drug loading may be limited when a large amount of bovine serum albumin (Islamiah et al.) is used as the carrier and stabilizer to form ivacaftor nanoparticles (Zhu et al., 2020).
To achieve higher drug-loading than the earlier BSA-based nanoparticles, we applied the anti-solvent method to prepare ivacaftor-colistin co-loaded nanosuspension that can effectively increase the solubility of the insoluble drug and impede the aggregation of particles. The effect of different stabilizers including PVP K30, F68, F388 and DSPE-PEG-OMe on particle size and nanosuspension stability were studied. Physico-chemical properties (e.g. particle size and crystallinity), in vitro aerosolization behavior, and morphology of the produced particles were also determined.
2. Materials and methods
2.1. Materials
Ivacaftor and colistin were purchased from AOKChem (Shanghai, China) and Betapharma Co. Ltd. (Jiangsu, China), respectively. N-(methylpolyyoxyethylene oxycarbonyl)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (DSPE-PEG-OMe, abbreviated as DSPE-PEG in the text, Mw: 2908) was purchased from NOF America Corporation (White Plains, NY, USA). N, N-Dimethylformamide (DMF), sodium dodecyl sulfate (SDS), poloxamer 188, poloxamer 388, PVP K30 and PVP K90 were obtained from Sigma-Aldrich (St. Louis, MO, USA). CyQUANT LDH Cytotoxicity Assay was purchased from Thermofisher Scientific (Waltham, MA, USA).
2.2. Preparation of nanosuspension (Iva-Col-NP)
Iva-Col-NPs were produced by modifying the antisolvent precipitation-ultrasonication method (Abdelbary et al., 2015). Briefly, 0.75 mL of 67 mg/mL ivacaftor DMF solution was slowly added to 10 mL aqueous solution at a constant rate of 1.0 mL/min under an ice bath and ultrasonic dispersion at 930 W (Ultrasonic Bath 5.7L, Thermo Fisher Scientific Inc.). The concentrations of ivacaftor, stabilizers and colistin are listed in the Table 1. Fig. 2 shows the manufacturing process of Iva-Col-NPs.
Table 1.
The particle size, polydispersity index, and stability of Iva-Col-NPs prepared in various formulations (n = 3).
| Formulation code | Ivacaftor | Colistin | Stabilizers | Drugs-to-stabilizer ratio | DMF (mL) | Water (mL) | Particle size (nm) | PDI | Stability |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 50 | - | - | - | 0.75 | 10 | - | - | < 0.1 h |
| F2 | 50 | 50 | - | - | 0.75 | 10 | 185.9 ± 7.2 | 0.140 ± 0.056 | < 2 h |
| F3 | 50 | 50 | Poloxamer 188 |
1:1:1 | 0.75 | 10 | 198.0 ± 5.0 | 0.174 ± 0.020 | < 72 h |
| F4 | 50 | 50 | Poloxamer 407 |
1:1:1 | 0.75 | 10 | 170.6 ± 4.3 | 0.157 ± 0.053 | < 24 h |
| F5 | 50 | 50 | Poloxamer 338 |
1:1:1 | 0.75 | 10 | 251.9 ± 3.1 | 0.139 ± 0.020 | < 1 h |
| F6 | 50 | 50 | PVP K90 | 1:1:1 | 0.75 | 10 | 228.1 ± 1.4 | 0.130 ± 0.023 | < 12 h |
| F7 | 50 | 50 | DSPE-PEG | 1:1:1 | 0.75 | 10 | 199.3 ± 4.3 | 0.100 ± 0.076 | > 120 h |
Figure 2.
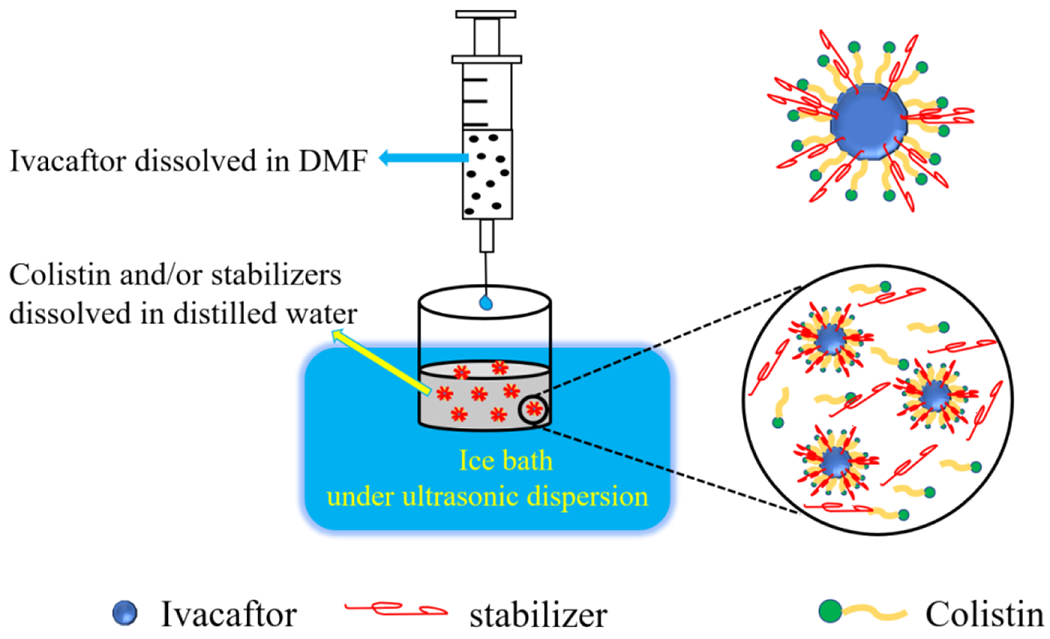
Schematic diagram of the anti-solvent precipitation method to produce nanosuspensions.
2.3. Particle size analysis of nanosuspension
Particle size analysis was conducted with a Malvern Zetasizer Nano ZS90 (Malvern Instruments Inc., Malvern, UK). The nanosuspension was diluted 100-fold with distilled water before the measurement. The polydispersity index (PI) ranges from 0 for a particle population with perfect mono-dispersion to 1.0 for a distribution that is very broad. Every measurement was taken in triplicate.
2.4. Preparation of inhalable powder formulation
The ultrasonic spray-freeze-drying (USFD) method could produce low-density porous particles with improved aerosolization performance. This process includes three steps: (1) atomizing: the feed solution is atomized using the ultrasonic nozzle; (2) freezing: the atomized fine mists are rapidly frozen in liquid nitrogen; (3) freeze drying: sublimation takes place at low temperature and pressure (Isleroglu et al., 2018). The particles, producing by this process, usually have a porous structure (Itatani et al., 2000). SFD is relatively new technology to the pharmaceutical sector and its capability for large-scale manufacturing deserves further study by the industry; nevertheless, it has attracted increasing interest to engineering pharmaceutical particles for inhalation (Cun et al., 2021; Lin et al., 2015). We used the ultrasonic spray-freeze-drying (USFD) method to create the dry powder as schematically illustrated in a previous paper (Rogers et al., 2003; Yu et al., 2020a; Yu et al., 2020b). Iva-Col-NPs were pumped (1B.1003-R/65, Petro Gas ausrüstungen Berlin GmbH, Berlin, Germany) at 2.5 mL/min through an ultrasonic nozzle (BÜCHI Labortechnik AG, Flawil, Switzerland) at 3.5 watt. The nanosuspension was atomized into the fine mists and the mists were sprayed into the liquid nitrogen in a glass beaker (250 mL). The nozzle was located at about 10 cm above the liquid nitrogen. After the spraying operation was completed, the excess liquid nitrogen was evaporated in the ambient condition and the frozen droplets were lyophilized in a freeze dryer (Labconco FreeZone, Kansas, MO, USA) for 48 hrs. The freeze dryer was set at −52 °C with a vacuum level of 0.014 mbar. The resultant spray-freeze-dried particles were gathered and kept in a vial that was sealed with desiccants at room temperature (Liao et al., 2019; Okuda et al., 2018).
For the physical mixture (PM) (Heltshe et al.), a blend of ivacaftor and colistin was co-milled using a jet-mill (Piconizer spiral jet-mill, Hosokawa Alpine AG, Augsburg, Germany). The feeding pressure, grind pressure and feeding rate were 6 bar, 5 bar and 50 mg/min, respectively.
2.5. Particle morphology
We used scanning electron microscopy (SEM, NOVA nanoSEM, FEI Company, Hillsboro, OR, USA) at 5.0 kV to characterize the morphology of the Iva-Col-NPs dry powder formulation and the physical mixture. Each sample was placed onto an SEM stub with double-sided tape and covered with a gold film at 40 mA for 120 s (Bhujbal et al., 2018; Yu et al., 2020a).
2.6. Crystallinity
Powder X-ray diffraction (PXRD) patterns were collected (SmartLab diffractometer; Rigaku Americas, Austin, USA) using a Cu Kα rays with a voltage of 40 kV and a current of 44 mA. We spread each powder formulation onto a glass slide and performed scanning at 5-60° 2θ at 2°/min and a step size of 0.02° (Shah et al., 2019; Shetty et al., 2018).
2.7. Assay of ivacaftor and colistin
Ivacaftor and colistin were analyzed by LC-MS/MS for dispersion and dissolution studies (Chai et al., 2019; Yu et al., 2020b). Chromatographic separation was achieved using a Kinetex C18 column (2.6 μm, 100 Å, 50 × 3 mm; Phenomenex, Torrance, CA, USA) with a mobile phase consisting of acetonitrile: water with 0.1 % v/v formic acid. The flow rate was 0.4 mL/min with an injection volume of 10 μL. The gradient elution procedure was set as follows: 0-0.5 min, 10 % acetonitrile; 1.0-1.5 min, 60 % acetonitrile; 2.5 min, 90 % acetonitrile; 3.0 min, 90 % acetonitrile; 3.5 min, 10 % acetonitrile; 6 min, stop.
Each component was measured by multiple reaction monitoring (MRM) (Table 2). Analytes were detected in the positive ionization mode at the following conditions: gas flow at 9 L/min, gas temperature at 350 °C, nebulizer pressure at 35 psi, sheath gas flow at 9 L/min, sheath gas temperature at 300 °C, capillary voltage at 4000 V and nozzle voltage at 1000 V.
Table 2.
MS conditions for ivacaftor and colistin (polymyxin B is an internal standard for colistin).
| Compound Name | Quantitative Transition (m/z) | Qualitative Transition (m/z) | Retention Time (min) | Fragmentor (V) | Collision Energy (V) |
|---|---|---|---|---|---|
| Polymyxin B1 | 602.3 - 101.1 | 602.3 - 241.1 | 0.631 | 135 | 20 |
| Polymyxin B2 | 595.4 - 101.1 | 595.4 - 227.2 | 0.629 | 135 | 20 |
| Colistin A | 585.5 - 101.1 | 585.5 - 241.1 | 0.630 | 135 | 20 |
| Colistin B | 578.5 - 101.1 | 578.5 - 227.2 | 0.627 | 135 | 20 |
| Ivacaftor | 393.2 - 337.2 | 393.2 - 172.1 | 4.728 | 80 | 8 |
2.8. In vitro aerosol performance
In vitro aerosolization capability was analyzed using a next-generation impactor (NGI, Copley, Nottingham, UK) with a USP induction port (USP throat). Each impactor stage was covered with silicone grease prior to each test to reduce particle bouncing. The sample containing 10.0 ± 0.5 mg of the powder formulation was weighed into the size 3 hydroxypropyl methylcellulose capsules (Qualicaps, Whitsett, NC, USA) and three capsules were dispersed through a low-resistant RS01 inhaler (Plastiape S.p.A., Osnago, Italy). A standard USP dispersion procedure was conducted by passing 4 L of air through the inhaler at an airflow of 100 L/min for 2.4 s with an air pressure of approximately 4 kPa. Before each run, the silicon grease was sprayed into each stage to minimize particle bounce. Drug particles left on capsule, inhaler device, USP throat and each stage of NGI were gathered using 10 mL of a co-solvent (acetonitrile/water, 1: 1) (Liao et al., 2020; Mangal et al., 2018). Emitted dose (ED) was calculated as the dose released from the capsule and inhaler device. Fine particle fraction (FPF) was defined as the percentage mass of drug particles less than 5 μm in aerodynamic diameter relative to the recovered drug. Triplicate measurements were taken for every sample.
2.9. In vitro dissolution
The in vitro dissolution studies were carried out using a Franz cell (V6B, PermeGear Inc., Hellertown, PA, USA). This method has been widely accepted for analyzing dissolution behavior of inhalable formulations (Chan et al., 2013; Salama et al., 2008). The experiments were conducted at 37 °C in 20 mL degassed phosphate buffer saline (PBS, pH 7.4) with 0.5 % SDS (Zhu et al., 2020). The stirring speed of Franz cell was 600 rpm. Powders (2 ± 0.1 mg) were aerosolized into the NGI as discussed in the previous section and gathered onto a filter paper (Whatman® Grade 2, pore size 5 μm, GE Healthcare, Buckinghamshire, UK) under the dispersion jets of NGI stage 3. The filter paper with aerosol powder was set on top of the Franz cell reservoir without any air bubbles under the filter paper. At selected intervals of 5, 10, 20, 30, 60, 120, 180, and 360 min, a 0.2 mL portion of the sample was removed and fresh medium in equal volume was added (Mangal et al., 2018; Wang et al., 2016).
2.10. Cell viability assay
Calu-3 cells were grown in 96 well plate in DMEM media supplemented with 10 % FBS, 1 % MEM and 1 % Penicillin and streptomycin at a density of 5×104 cells/well. A range of concentrations of 2-25 μg/mL of DSPE-PEG, ivacaftor, colistin and F7 formulation (the optimized formulation) have been used to assess the cell viability (Chen et al., 2021). Sterile double distilled water was used as a vehicle control (negative) and 10× cell lysis buffer was used as a positive control. After 24 hrs of treatment cytotoxicity was measured by CyQuant LDH cytotoxicity assay.
2.11. Antimicrobial activity
Static time-kill studies were conducted to examine the antimicrobial activity using Cation-adjusted Mueller Hinton Broth (CAMHB; Mg2+ at 12.2 mg/L and Ca2+ at 23.0 mg/L [Oxoid, Hampshire, England]) (Wang et al., 2018). A clinical isolate of P. aeruginosa (FADDI-070) was employed in this study. All experiments were performed with an initial inoculum of ~106 CFU/mL in 20 mL of CAMHB in 50 mL pyrogen-free and sterile polypropylene tubes. Samples (50 μL) were collected at 0, 1, 3, 6 and 24 hrs, centrifuged at 10000 × g for 10 min and cell pellets were resuspended in 0.9 % saline and diluted accordingly for viable counting on nutrient agar plates. The limit of detection was 100 CFU/mL. A ProtoCOL automated colony counter (Synbiosis, Cambridge, United Kingdom) was used to quantify bacteria after 24 hrs of incubation at 37 °C.
2.12. Statistical analysis
Statistical analysis was made with a One-way ANOVA and Dunnett’s multiple comparison test. The significance level was set at p < 0.05.
3. Results and discussion
3.1. Optimization of Iva-Col-NPs
Seven formulations of ivacaftor were evaluated on their ability to stabilize ivacaftor in the nanosuspension at ambient conditions (20 ± 3 °C, RH < 30 %). The parameters for evaluating the stability of formulations included particle size, PDI and aggregation rate. If the nanoparticle aggregated within 72 hrs, then the formulation had a poor stability. The formulation characteristics are listed in Table 1. The nanosuspensions displayed similar particle sizes (all are below 250 nm) and relatively uniform distribution (PDI < 0.2). Among all tested formulations, F7 was selected as the formulation for further studies due to its relatively superior physical stability.
3.2. Morphology of the powder formulation
Fig. 3 shows morphology of the physical mixture (PM, Fig. 3A) and F7 (Fig. 3B) particles. The PM particles had smooth surfaces and irregular shapes. The F7 has a porous structure with some nanoparticles embedded in the microparticle.
Figure 3.

SEM micrographs of the physical mixture powder (A) and USFD F7 powder (B). Red arrows point to some nanoparticles embedded in the microparticle. Scale bars represent 1 μm.
3.3. PXRD
The PXRD patterns of samples can be seen in Fig. 4. The diffractogram of the raw colistin indicates an amorphous form.
Figure 4.
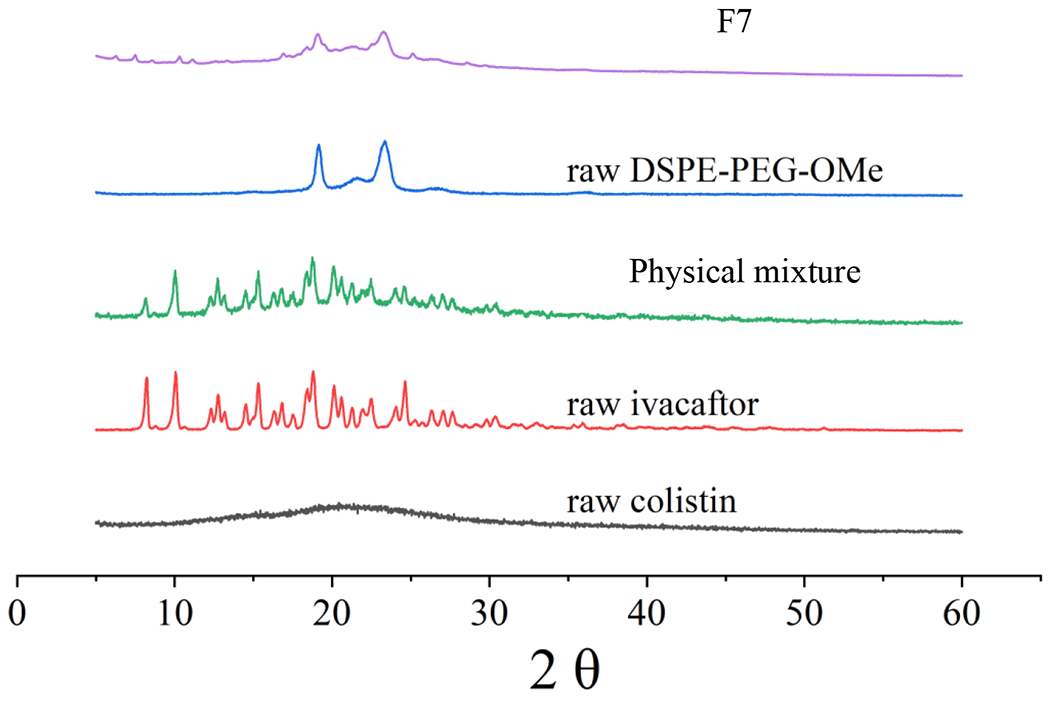
PXRD patterns of raw colistin, raw ivacaftor, raw DSPE-PEG-OMe, physical mixture and F7.
The F7 was composed of ivacaftor, colistin and DSPE-PEG in a ratio of 1:1:1. The PM was a physical mixture of ivacaftor and colistin in a ratio of 1:1. PXRD patterns for the Iva-Col PM was similar to the raw ivacaftor. The spray-freeze-drying powder F7 showed two sharp diffraction peaks between 2θ of 19.1° and 23.4° (attributed to DSPE-PEG-OMe) with some very minor peaks belonging to crystalline ivacaftor, suggesting ivacaftor was largely in the amorphous form in F7.
3.4. In vitro dissolution study
Ivacaftor has very low aqueous solubility (crystalline solubility was 80.1 ± 3.7 ng/mL), which is the main obstacle to drug release (Chen et al., 2011; Merisko-Liversidge and Liversidge, 2008). The F7 formulation showed a significant increase in the speed and extent of ivacaftor dissolution, greater than the PM powder, with the drug release being improved from 25.15 ± 4.90 % to 82.76 ± 9.50 % at 6 h (Fig. 5). In contrast, the release of colistin from spray-freeze-drying powder was similar to that of the PM powder with approximately 80 % of colistin released at 6 h.
Figure 5.
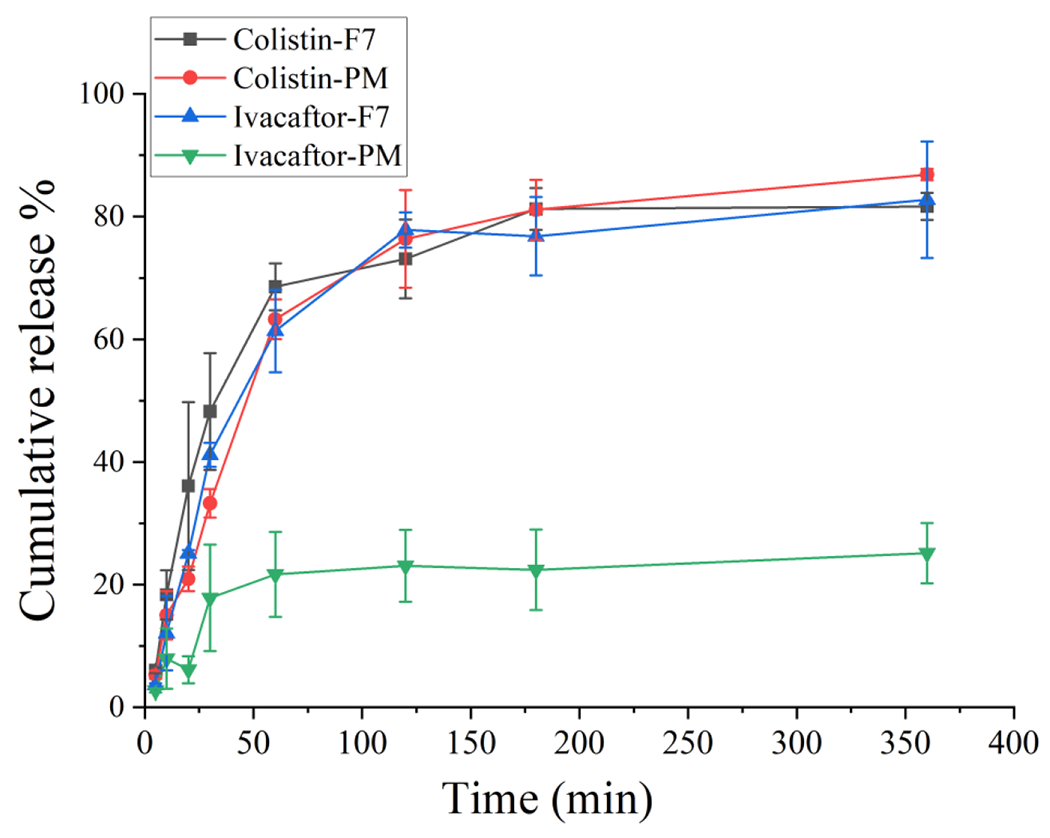
Dissolution profiles of Iva-Col-DSPE-PEG (F7) formulation and the jet-milled physical mixture (PM) (mean ± SD, n = 3).
3.5. In vitro aerosol performance
Aerosol performance of the PM was not tested due to its poor dissolution behavior. The F7 has relatively satisfactory aerosol performance and two drugs had equivalent deposition profiles and aerosol performance (FPF 61.4 ± 3.4 % and ED 97.8 ± 0.3 % for ivacaftor; FPF 63.3 ± 3.3 %; and ED 97.6 ± 0.5 % for colistin).
3.6. Calu-3 lung epithelial cell viability
DSPE-PEG, colistin, ivacaftor and F7 did not show any apparent cytotoxicity on Calu-3 lung epithelial cells at the concentrations used (2-25 μg/mL; cell viability≥ 90 %). There is a slight reduction in cell viability for the F7 but there is no statistical significance with other groups (p > 0.05).
3.7. Antimicrobial activity against P. aeruginosa
Ivacaftor, colistin and DSPE-PEG alone did not show antimicrobial effect on P. aeruginosa FADDI-070 (a colistin-resistant clinical isolate) at the concentration of 8 mg/L. However, the combinations of ivacaftor and colistin showed significantly enhanced antimicrobial activity against P. aeruginosa FADDI-070 in both the jet-milled (PM) formulation (p < 0.01) and the F7 p < 0.01) than the control, colistin alone and ivacaftor alone.
4. Discussion
The biggest source of health complications and death in cystic fibrosis patients is lung infections, most commonly caused by resistant P. aeruginosa. Therefore, innovative combination therapies are urgently needed for treating pulmonary infections in CF patients with resistant P. aeruginosa. The synergistic use of antibiotic-nonantibiotic combinations could be an instrumental and affordable path towards increasing clinical efficacy of antibiotics already available against multidrug resistant bacterial pathogens (Schneider et al., 2016). In this study, we designed a dry powder inhaler nanoparticles formulation containing ivacaftor (Iva) and colistin (Col) for improving synergistic treatment effect. This formulation improved dissolution of ivacaftor and synchronized the release of two drugs. This enhanced dissolution is attributed to the small particle size and partial amorphous form (Patravale et al., 2004). Moreover, the Iva-Col-NPs developed here achieve much higher drug loading (33 % w/w) than the Iva-BSA-NPs (3.2 % w/w) developed earlier (Zhu et al., 2020).
Stabilizers are crucial in the formulation of nanoparticles. The main function of stabilizers is to wet the surface of compounds with poor water-solubility and prevent agglomeration of nanoparticles in the medium. In our study, DSPE-PEG stabilizes the nanosuspension by electrostatic repulsion because it is an amphoteric surfactant, and poloxamer and PVP K90 as a non-ionic surfactant stabilize suspensions sterically (Patel et al., 2020; Patravale et al., 2004). The properties of the surfactants such as diffusion velocity, molecular weight, hydration, and affinity to the particle surface are vital for the physical stability of nanosuspensions (Jacob et al., 2020; Müller and Jacobs, 2002). Meanwhile, though the poloxamer contains hydrophobic and hydrophilic groups, its hydrophobic section was between two hydrophilic chains. The PPO chain was responsible for hydrophobic interactions of poloxamer with the drug by firming anchoring on the particle surface. In such a structure, the steric repulsive force of two PEO chains does not favor the formation of a well-oriented adsorption layer around the particles (Zong et al., 2017). Therefore, DSPE-PEG had a better stabilizing effect in our formulation.
According to Noyes-Whitney equation and literatures, nanosizing of drugs can increase the surface area of the drug particles and thereby dissolution rate would by enhanced for poorly water-soluble drugs. Additionally, reduction of particle size reduces the diffusion layer thickness surrounding the drug particles and thereby increases the dissolution rate (Li et al., 2020; Marzan et al., 2018; Pandey et al., 2018). Drug-stabilizer interaction and hydrophilic nature of the stabilizer may also improve the dissolution performance of nanosuspension for poorly water soluble ivacaftor (Baghel et al., 2016). In addition, improved dissolution rate can also be attributed to reduction in crystallinity as shown by substantially smaller ivacaftor peaks in PXRD for the F7 compared to the PM (Koradia and Parikh, 2012).
The F7 has relatively high aerosol performance, owing to its porous structure and rough surfaces. Our earlier study has shown a clear correlation between porosity/density and aerosol performance of the spray-freeze-dried particles (Zhu et al., 2020). SEM images show that the produced particles here have rough surfaces, which promote aerosolization/deagglomeration by decreasing the contact area between particles. Higher roughness of the powder is shown to reduce the particle-particle contact area and cohesiveness leading to better aerosol performance (Shetty et al., 2018) (Zhou et al., 2013; Zhu et al., 2020). Other techniques such as nano-spray drying may also produce rough microparticles containing nanoparticles of water insoluble drugs (Baba and Nishida, 2013; Schafroth et al., 2012). One of the challenges for antimicrobial activity against P. aeruginosa in the lungs is the thick mucus layer on the lung surface of CF patients, which may reduce the drug penetration (Sanders et al., 2000). Earlier study has shown that dense poly(ethylene glycol) coated polystyrene nanoparticles (PS-PEG NPs) have improved mucus penetration properties for colistin microparticles (Chai et al., 2020). Further investigations on mucus penetration of our formulation are warranted.
In addition, our formulation (F7) showed minimal cytotoxicity in Calu-3 human lung epithelial cells and enhanced antimicrobial activity against colistin-resistant P. aeruginosa than the individual drug of colistin or ivacaftor. Future in vivo studies in our mouse lung infection model are warranted to determine antimicrobial efficacy (Lin et al., 2017; Lin et al., 2018).
5. Conclusions
Composite microparticles containing ivacaftor-colistin co-loaded nanoparticles with relatively high drug-loading were developed and characterized in this study. The optimized powder formulation (F7) exhibited a rough surface with satisfactory FPF values of 61.4 ± 3.4 % for Iva and 63.3 ± 3.3 % for Col, and superior ED values of 97.8 ± 0.3 % for ivacaftor and 97.6 ± 0.5 % for colistin. The F7 also showed a significantly enhanced dissolution of poorly water soluble ivacaftor than the physical mixture. Minimal cytotoxicity in Calu-3 human lung epithelial cells and enhanced antimicrobial activity against colistin-resistant P. aeruginosa suggested that our formulation has potential to improve the treatment of CF patients with lung infections.
Figure 6.
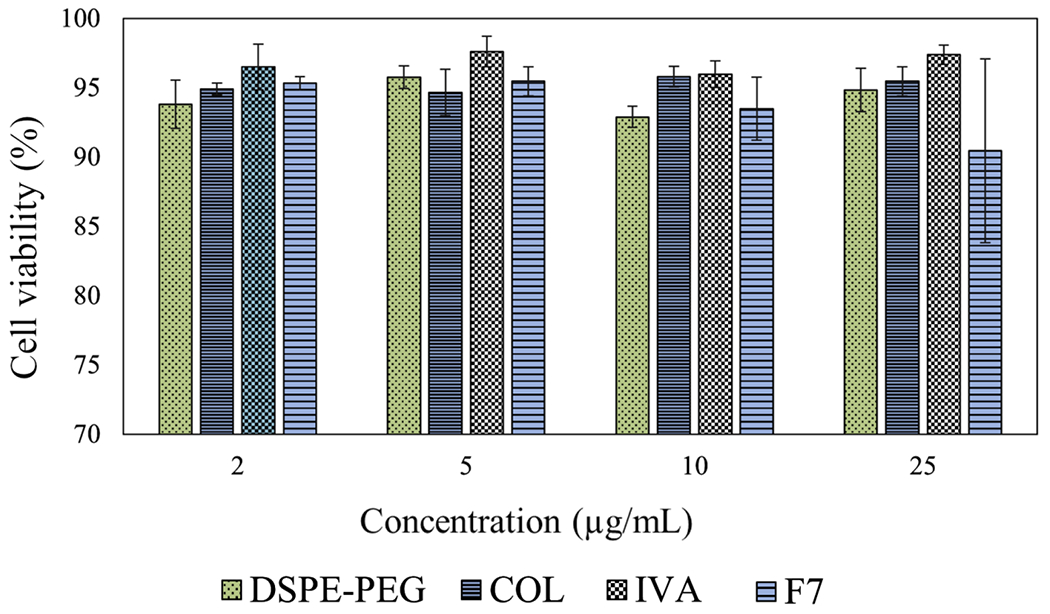
Viability of Calu-3 cells after 24 hrs exposure with DSPE-PEG, colistin (COL), ivacaftor (IVA) and formulation (F7). Data presented as mean ± SD (n=3).
Figure 7.
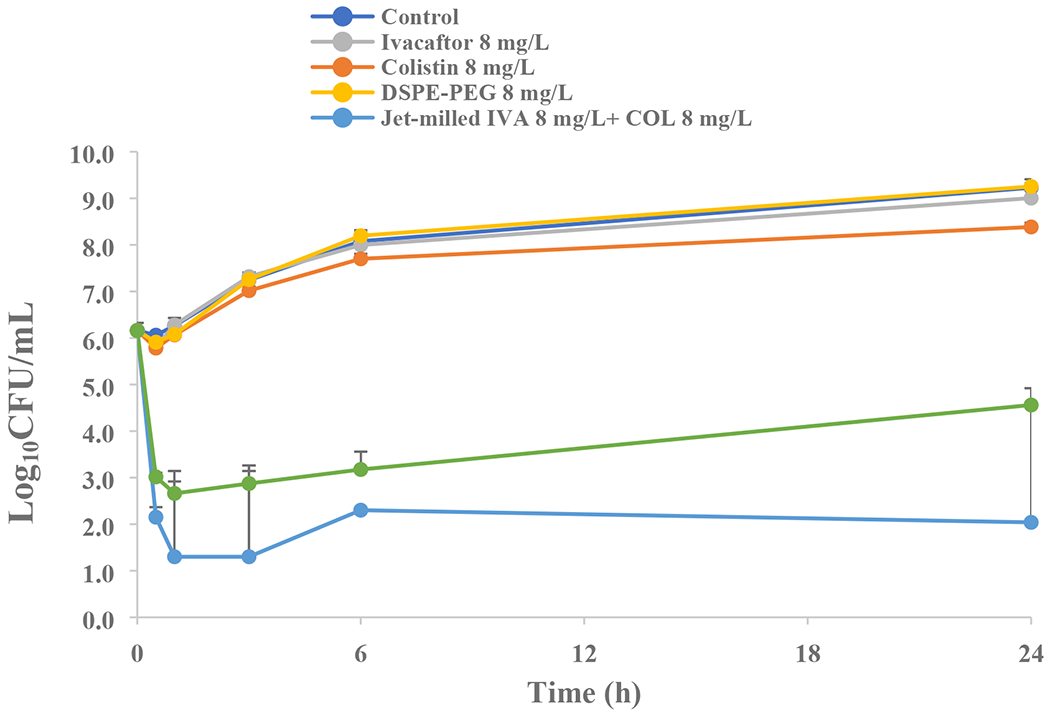
Static time-kill kinetics against the clinical isolate of P. aeruginosa FADDI-070 (mean ± SD, n=3).
Acknowledgement
This study was supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health (NIH) under Award Number R01AI132681. QTZ and JL are also supported by the NIH award R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdelbary AA, Al-mahallawi AM, Abdelrahim ME, Ali AM, 2015. Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int J Nanomedicine 10, 6339–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsalam MFA, Abdalla MS, El-Abhar HSE, 2018. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin-meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J Glob Antimicrob Resist 15, 127–135. [DOI] [PubMed] [Google Scholar]
- Ali ME, Lamprecht A.J.E.J.o.P., Biopharmaceutics, 2014. Spray freeze drying for dry powder inhalation of nanoparticles. 87, 510–517. [DOI] [PubMed] [Google Scholar]
- Baba K, Nishida K, 2013. Steroid nanocrystals prepared using the nano spray dryer B-90. Pharmaceutics 5, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghel S, Cathcart H, O’Reilly NJ, 2016. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J Pharm Sci 105, 2527–2544. [DOI] [PubMed] [Google Scholar]
- Bartos C, Ambrus R, Katona G, Sovany T, Gaspar R, Marki A, Ducza E, Ivanov A, Tomosi F, Janaky T, Szabo-Revesz P, 2019. Transformation of Meloxicam Containing Nanosuspension into Surfactant-Free Solid Compositions to Increase the Product Stability and Drug Bioavailability for Rapid Analgesia. Drug Des Devel Ther 13, 4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, Burgel P-R, Tullis E, Castaños C, Castellani C, Byrnes CA, Cathcart F, Chotirmall SH, Cosgriff R, Eichler I, Fajac I, Goss CH, Drevinek P, Farrell PM, Gravelle AM, Havermans T, Mayer-Hamblett N, Kashirskaya N, Kerem E, Mathew JL, McKone EF, Naehrlich L, Nasr SZ, Oates GR, O’Neill C, Pypops U, Raraigh KS, Rowe SM, Southern KW, Sivam S, Stephenson AL, Zampoli M, Ratjen F, 2020. The future of cystic fibrosis care: a global perspective. The Lancet Respiratory Medicine 8, 65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen PJ, Li J, Rayner CR, Nation RL, 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy 50, 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhujbal SV, Zemlyanov DY, Cavallaro A, Mangal S, Taylor LS, Zhou QT, 2018. Qualitative and Quantitative Characterization of Composition Heterogeneity on the Surface of Spray Dried Amorphous Solid Dispersion Particles by an Advanced Surface Analysis Platform with High Surface Sensitivity and Superior Spatial Resolution. Mol Pharm 15, 2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr A, Water J, Beck-Broichsitter M, Yang M, 2015. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Current pharmaceutical design 21, 5829–5844. [DOI] [PubMed] [Google Scholar]
- Brunaugh AD, Smyth H.D.J.I.J.o.P., 2018. Formulation techniques for high dose dry powders. 547, 489–498. [DOI] [PubMed] [Google Scholar]
- Carter S, Kelly S, Caples E, Grogan B, Doyle J, Gallagher CG, McKone EF, 2015. Ivacaftor as salvage therapy in a patient with cystic fibrosis genotype F508del/R117H/IVS8-5T. J Cyst Fibros 14, e4–5. [DOI] [PubMed] [Google Scholar]
- Chai G, Hassan A, Meng T, Lou L, Ma J, Simmers R, Zhou L, Rubin BK, Zhou QT, Longest PWJNN, Biology, Medicine, 2020. Dry powder aerosol containing muco-inert particles for excipient enhanced growth pulmonary drug delivery. 29, 102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai G, Park H, Yu S, Zhou F, Li J, Xu Q, Zhou QT, 2019. Evaluation of co-delivery of colistin and ciprofloxacin in liposomes using an in vitro human lung epithelial cell model. Int J Pharm 569, 118616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JD, 2020. Cystic fibrosis lung disease and bronchiectasis. The Lancet Respiratory Medicine 8, 12–14. [DOI] [PubMed] [Google Scholar]
- Chan JG, Chan HK, Prestidge CA, Denman JA, Young PM, Traini D, 2013. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur J Pharm Biopharm 83, 285–292. [DOI] [PubMed] [Google Scholar]
- Chen H, Khemtong C, Yang X, Chang X, Gao J, 2011. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today 16, 354–360. [DOI] [PubMed] [Google Scholar]
- Chen J, Ahmed MU, Zhu C, Yu S, Pan W, Velkov T, Li J, Zhou Q.T.J.I.J.o.P., 2021. In vitro evaluation of drug delivery behavior for inhalable amorphous nanoparticle formulations in a human lung epithelial cell model. 596, 120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DY, Lim DJ, Mackey C, Skinner D, Zhang S, McCormick J, Woodworth BA, 2019. Ivacaftor, a Cystic Fibrosis Transmembrane Conductance Regulator Potentiator, Enhances Ciprofloxacin Activity Against Pseudomonas aeruginosa. Am J Rhinol Allergy 33, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cun D, Zhang C, Bera H, Yang MJADDR, 2021. Particle engineering principles and technologies for pharmaceutical biologics. 174, 140–167. [DOI] [PubMed] [Google Scholar]
- Deeks ED, 2016. Lumacaftor/Ivacaftor: A Review in Cystic Fibrosis. Drugs 76, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Kasiakou SK, Saravolatz LD, 2005. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clinical Infectious Diseases 40, 1333–1341. [DOI] [PubMed] [Google Scholar]
- Gherardi G, Linardos G, Pompilio A, Fiscarelli E, Di Bonaventura G, 2019. Evaluation of in vitro activity of ceftolozane-tazobactam compared to other antimicrobial agents against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Diagn Microbiol Infect Dis 94, 297–303. [DOI] [PubMed] [Google Scholar]
- Gomez-Junyent J, Benavent E, Sierra Y, El Haj C, Soldevila L, Torrejon B, Rigo-Bonnin R, Tubau F, Ariza J, Murillo O, 2019. Efficacy of ceftolozane/tazobactam, alone and in combination with colistin, against multidrug-resistant Pseudomonas aeruginosa in an in vitro biofilm pharmacodynamic model. Int J Antimicrob Agents 53, 612–619. [DOI] [PubMed] [Google Scholar]
- Hashem FM, Abd Allah FI, Abdel-Rashid RS, Hassan AAA, 2020. Glibenclamide nanosuspension inhaler: development, in vitro and in vivo assessment. Drug Dev Ind Pharm 46, 762–774. [DOI] [PubMed] [Google Scholar]
- Hassan A, Farkas D, Longest W, Hindle M, 2020. Characterization of excipient enhanced growth (EEG) tobramycin dry powder aerosol formulations. International Journal of Pharmaceutics 591, 120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, Rowe SM, Network G.I.o.t.C.F.F.T.D., 2015. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 60, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey A, Durham P, Dharmadhikari A, Nardell E.J.J.o.C.R., 2016. Inhaled drug treatment for tuberculosis: Past progress and future prospects. 240, 127–134. [DOI] [PubMed] [Google Scholar]
- Islam N, Cleary M.J.J.M.e., physics, 2012. Developing an efficient and reliable dry powder inhaler for pulmonary drug delivery—A review for multidisciplinary researchers. 34, 409–427. [DOI] [PubMed] [Google Scholar]
- Islamiah M, Ismail N, Mohamad H, Sung YY, Muhammad T.S.T.J.I.J.o.R.i.P.S., 2018. Induction of apoptosis by Aaptos sp., fractions in human breast cancer cell line, MCF-7. 9. [Google Scholar]
- Isleroglu H, Turker I, Tokatli M, Koc B, 2018. Ultrasonic spray-freeze drying of partially purified microbial transglutaminase. Food and Bioproducts Processing 111, 153–164. [Google Scholar]
- Itatani K, Iwafune K, Howell FS, Aizawa MJMRB, 2000. Preparation of various calcium-phosphate powders by ultrasonic spray freeze-drying technique. 35, 575–585. [Google Scholar]
- Jacob S, Nair AB, Shah J, 2020. Emerging role of nanosuspensions in drug delivery systems. Biomater Res 24, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koradia DK, Parikh HR, 2012. Dissolution enhancement of albendazole through nanocrystal formulation. J Pharm Bioallied Sci 4, S62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk DH, Ha ES, Ha DH, Sim WY, Lee SK, Jeong JS, Kim JS, Baek IH, Park H, Choi DH, Yoo JW, Jeong SH, Hwang SJ, Kim MS, 2019. Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach. Pharmaceutics 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok PCL, Tunsirikongkon A, Glover W, Chan H.-K.J.P.r., 2011. Formation of protein nano-matrix particles with controlled surface architecture for respiratory drug delivery. 28, 788–796. [DOI] [PubMed] [Google Scholar]
- Law N, Logan C, Yung G, Furr CL, Lehman SM, Morales S, Rosas F, Gaidamaka A, Bilinsky I, Grint P, Schooley RT, Aslam S, 2019. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 47, 665–668. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang J, Fang Y, Yi J, Lu Z, Chen Y, Guo B, 2020. Enhancing Betulinic Acid Dissolution Rate and Improving Antitumor Activity via Nanosuspension Constructed by Anti-Solvent Technique. Drug Des Devel Ther 14, 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q, Lam ICH, Lin HHS, Wan LTL, Lo JCK, Tai W, Kwok PCL, Lam JKW, 2020. Effect of formulation and inhaler parameters on the dispersion of spray freeze dried voriconazole particles. Int J Pharm 584, 119444. [DOI] [PubMed] [Google Scholar]
- Liao Q, Yip L, Chow MYT, Chow SF, Chan HK, Kwok PCL, Lam JKW, 2019. Porous and highly dispersible voriconazole dry powders produced by spray freeze drying for pulmonary delivery with efficient lung deposition. Int J Pharm 560, 144–154. [DOI] [PubMed] [Google Scholar]
- Lin J, Xu C, Fang R, Cao J, Zhou TJAA, Chemotherapy, 2019. Resistance and Heteroresistance to Colistin in Pseudomonas aeruginosa Isolates from Wenzhou, China. 63, e00556–00519-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-W, Wong J, Qu L, Chan H-K, Zhou Q.T.J.C.p.d., 2015. Powder production and particle engineering for dry powder inhaler formulations. 21, 3902–3916. [DOI] [PubMed] [Google Scholar]
- Lin YW, Zhou AQ, Onufrak BNJ, Wirth CV, Chen DKJAA, Chemotherapy, 2017. Aerosolized Polymyxin B for Treatment of Respiratory Tract Infections: Determination of Pharmacokinetic/Pharmacodynamic Indices for Aerosolized Polymyxin B against Pseudomonas aeruginosa in a Mouse Lung Infection Model. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Zhou QT, Han ML, Chen K, Onufrak NJ, Wang J, Turnidge JD, Howden BP, Forrest A, Chan HK, Li J, 2018. Elucidating the Pharmacokinetics/Pharmacodynamics of Aerosolized Colistin against Multidrug-Resistant Acinetobacter baumannii and Klebsiella pneumoniae in a Mouse Lung Infection Model. Antimicrobial agents and chemotherapy 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Han M, Tian F, Cun D, Rantanen J, Yang M.J.C.p., 2018. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation. 181, 1143–1152. [DOI] [PubMed] [Google Scholar]
- Mangal S, Park H, Nour R, Shetty N, Cavallaro A, Zemlyanov D, Thalberg K, Puri V, Nicholas M, Narang AS, Zhou QT, 2019. Correlations between surface composition and aerosolization of jet-milled dry powder inhaler formulations with pharmaceutical lubricants. Int J Pharm 568, 118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangal S, Xu R, Park H, Zemlyanov D, Shetty N, Lin YW, Morton D, Chan HK, Li J, Zhou QT, 2018. Understanding the Impacts of Surface Compositions on the In-Vitro Dissolution and Aerosolization of Co-Spray-Dried Composite Powder Formulations for Inhalation. Pharm Res 36, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzan AL, Tabassum R, Jahan B, Asif MH, Reza HM, Kazi M, Alshehri SM, de Matas M, Shariare MH, 2018. Preparation and Characterization of Stable Nanosuspension for Dissolution Rate Enhancement of Furosemide: A Quality by Design (QbD) Approach. Curr Drug Deliv 15, 672–685. [DOI] [PubMed] [Google Scholar]
- Merisko-Liversidge EM, Liversidge GG, 2008. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol 36, 43–48. [DOI] [PubMed] [Google Scholar]
- Milczewska J, Wolkowicz T, Zacharczuk K, Mierzejewska E, Kwiatkowska M, Walicka-Serzysko K, Sands D, 2020. Clinical outcomes for cystic fibrosis patients with Pseudomonas aeruginosa cross-infections. Pediatr Pulmonol 55, 161–168. [DOI] [PubMed] [Google Scholar]
- Molchanova N, Wang H, Hansen PR, Hoiby N, Nielsen HM, Franzyk H, 2019. Antimicrobial Activity of alpha-Peptide/beta-Peptoid Lysine-Based Peptidomimetics Against Colistin-Resistant Pseudomonas aeruginosa Isolated From Cystic Fibrosis Patients. Front Microbiol 10, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R, Jacobs C.J.I.J.o.P., 2002. Buparvaquone mucoadhesive nanosuspension: preparation, optimization and long-range stability. Int J Pharm. 237, 151–161. [DOI] [PubMed] [Google Scholar]
- Nang SC, Azad MA, Velkov T, Zhou QT, Li JJPR, 2021. Rescuing the Last-Line Polymyxins: Achievements and Challenges. 73, 679–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick JA, St Clair C, Jones MC, Lan L, Higgins M, Team VXS, 2020. Ivacaftor in cystic fibrosis with residual function: Lung function results from an N-of-1 study. J Cyst Fibros 19, 91–98. [DOI] [PubMed] [Google Scholar]
- Okuda T, Morishita M, Mizutani K, Shibayama A, Okazaki M, Okamoto H, 2018. Development of spray-freeze-dried siRNA/PEI powder for inhalation with high aerosol performance and strong pulmonary gene silencing activity. J Control Release 279, 99–113. [DOI] [PubMed] [Google Scholar]
- Pandey P, Purohit D, Dureja H, 2018. Nanosponges -A Promising Novel Drug Delivery System. Recent Pat Nanotechnol 12, 180–191. [DOI] [PubMed] [Google Scholar]
- Patel D, Zode SS, Bansal AK, 2020. Formulation aspects of intravenous nanosuspensions. Int J Pharm 586, 119555. [DOI] [PubMed] [Google Scholar]
- Paterson SL, Barry PJ, Horsley AR, 2020. Tezacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Rev Respir Med 14, 15–30. [DOI] [PubMed] [Google Scholar]
- Patravale VB, Date AA, Kulkarni RM, 2004. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol 56, 827–840. [DOI] [PubMed] [Google Scholar]
- Qiao F, Zhao Y, Mai Y, Guo J, Dong L, Zhang W, Yang J, 2020. Isoliquiritigenin Nanosuspension Enhances Cytostatic Effects in A549 Lung Cancer Cells. Planta Med 86, 538–547. [DOI] [PubMed] [Google Scholar]
- Rogers TL, Nelsen AC, Sarkari M, Young TJ, Johnston KP, Williams R.OJ.P.r., 2003. Enhanced aqueous dissolution of a poorly water soluble drug by novel particle engineering technology: spray-freezing into liquid with atmospheric freeze-drying. 20, 485–493. [DOI] [PubMed] [Google Scholar]
- Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, McKee C, Lekstrom-Himes J, Davies JC, 2017. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N Engl J Med 377, 2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama R, Traini D, Chan H, Young P, 2008. Preparation and characterisation of controlled release co-spray dried drug—polymer microparticles for inhalation 2: Evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. European Journal of Pharmaceutics and Biopharmaceutics 70, 145–152. [DOI] [PubMed] [Google Scholar]
- Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J.J.A.j.o.r., medicine, c.c, 2000. Cystic fibrosis sputum: a barrier to the transport of nanospheres. 162, 1905–1911. [DOI] [PubMed] [Google Scholar]
- Schafroth N, Arpagaus C, Jadhav UY, Makne S, Douroumis D, 2012. Nano and microparticle engineering of water insoluble drugs using a novel spray-drying process. Colloids Surf B Biointerfaces 90, 8–15. [DOI] [PubMed] [Google Scholar]
- Schneider EK, Azad MA, Han ML, Tony Zhou Q, Wang J, Huang JX, Cooper MA, Doi Y, Baker MA, Bergen PJ, Muller MT, Li J, Velkov T, 2016. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect Dis 2, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NK, Wang Z, Gupta SK, Le Campion A, Meenach SA, 2019. Sustained release of a model water-soluble compound via dry powder aerosolizable acetalated dextran microparticles. Pharm Dev Technol 24, 1133–1143. [DOI] [PubMed] [Google Scholar]
- Shetty N, Cipolla D, Park H, Zhou QT, 2020. Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv 17, 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty N, Zeng L, Mangal S, Nie H, Rowles MR, Guo R, Han Y, Park JH, Zhou QT, 2018. Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations. Pharm Res 35, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo Guevara M, McColley SA, 2017. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Opin Drug Saf 16, 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcios NL, 2020. Cystic Fibrosis Lung Disease: An Overview. Respir Care 65, 233–251. [DOI] [PubMed] [Google Scholar]
- Wang S, Yu S, Lin Y, Zou P, Chai G, Heidi HY, Wickremasinghe H, Shetty N, Ling J, Li J.J.P.r., 2018. Co-delivery of ciprofloxacin and colistin in liposomal formulations with enhanced in vitro antimicrobial activities against multidrug resistant Pseudomonas aeruginosa. 35, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhou QT, Sun SP, Denman JA, Gengenbach TR, Barraud N, Rice SA, Li J, Yang M, Chan HK, 2016. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. AAPS J 18, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Peters JI, Williams R.O.J.T.j.o.p. III, 2008. Inhaled nanoparticles—a current review. 356, 239–247. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang S, Zou P, Chai G, Lin YW, Velkov T, Li J, Pan W, Zhou QT, 2020a. Inhalable liposomal powder formulations for co-delivery of synergistic ciprofloxacin and colistin against multi-drug resistant gram-negative lung infections. Int J Pharm 575, 118915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Yuan H, Chai G, Peng K, Zou P, Li X, Li J, Zhou F, Chan HK, Zhou QT, 2020b. Optimization of inhalable liposomal powder formulations and evaluation of their in vitro drug delivery behavior in Calu-3 human lung epithelial cells. Int J Pharm 586, 119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK, 2015. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev 85, 83–99. [DOI] [PubMed] [Google Scholar]
- Zhou QT, Morton DA, Yu HH, Jacob J, Wang J, Li J, Chan HK, 2013. Colistin powders with high aerosolisation efficiency for respiratory infection: preparation and in vitro evaluation. J Pharm Sci 102, 3736–3747. [DOI] [PubMed] [Google Scholar]
- Zhu C, Chen J, Yu S, Que C, Taylor LS, Tan W, Wu C, Zhou QT, 2020. Inhalable Nanocomposite Microparticles with Enhanced Dissolution and Superior Aerosol Performance. Mol Pharm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong L, Li X, Wang H, Cao Y, Yin L, Li M, Wei Z, Chen D, Pu X, Han J, 2017. Formulation and characterization of biocompatible and stable I.V. itraconazole nanosuspensions stabilized by a new stabilizer polyethylene glycol-poly(beta-Benzyl-l-aspartate) (PEG-PBLA). Int J Pharm 531, 108–117. [DOI] [PubMed] [Google Scholar]


