Abstract
Background.
In men with nongonococcal urethritis (NGU), clinicians and patients rely on clinical cure to guide the need for additional testing/treatment and when to resume sex, respectively; however, discordant clinical and microbiological cure outcomes do occur. How accurately clinical cure reflects microbiological cure in specific sexually transmitted infections (STI) is unclear.
Methods.
Men with NGU were tested for Neisseria gonorrhoeae, Chlamydia trachomatis (CT), Mycoplasma genitalium (MG), Trichomonas vaginalis, urethrotropic Neisseria meningitidis ST11 clade strains, and Ureaplasma urealyticum (UU). Men received azithromycin 1 g and returned for a 1-month test-of-cure visit. In MG infections, we evaluated for the presence of macrolide resistance-mediating mutations (MRM) and investigated alternate hypotheses for microbiological treatment failure using in situ shotgun metagenomic sequencing, phylogenetic analysis, multiple locus typing analyses, and quantitative PCR.
Results.
Of 280 men with NGU, 121 were included in this analysis. In the monoinfection group, 52 had CT, 16 had MG, 7 had UU, 10 had mixed infection, and 36 men had idiopathic NGU. Clinical cure rates were 85% for CT, 100% for UU, 50% for MG, and 67% for idiopathic NGU. Clinical cure accurately predicted microbiological cure for all STI, except MG. Discordant results were significantly associated with MG-NGU and predominantly reflected microbiological failure in men with clinical cure. MG MRM, but not MG load or strain, were strongly associated with microbiological failure.
Conclusions.
In azithromycin-treated NGU, clinical cure predicts microbiological cure for all STI, except MG. NGU management should include MG testing and confirmation of microbiological cure in azithromycin-treated MG-NGU when MRM testing is unavailable.
Keywords: Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma genitalium, cure, urethritis
SUMMARY
Resolution of nongonococcal urethritis signs and symptoms does not always correlate with microbiological cure; discordant cures occur in one third of men. Clinical cure accurately correlates with Gram stain and microbiological cure for NGU bacterial pathogens, except Mycoplasma genitalium.
INTRODUCTION
Nongonococcal urethritis (NGU) is the most common sexually transmitted infection (STI) syndrome in men, with approximately 50% of cases due to infection with Chlamydia trachomatis (CT) or Mycoplasma genitalium (MG).1 Other STI pathogens associated with NGU include the urethrotropic Neisseria meningitidis ST-11 clade strain (US_NmUC), Trichomonas vaginalis (TV), Haemophilus influenzae, Mycoplasma penetrans, and viruses, like HSV and adenovirus.2–4 Ureaplasma urealyticum (UU) is increasingly not considered an NGU pathogen, except perhaps when present at high organism load,5, 6 as increasing evidence suggests it is not associated with NGU and is likely a urethral commensal given its frequent detection with other NGU pathogens, like MG and CT.7, 8 Up to 50% of NGU cases have no identifiable etiology and have been termed idiopathic urethritis (IU).4, 7, 9 Some STI pathogens that elicit male urethritis can cause epididymo-orchitis, and rarely prostatitis, and can be transmitted to sexual partners, increasing the risk for complications like pelvic inflammatory disease and infertility.10, 11
Diagnosing NGU requires excluding Neisseria gonorrhoeae (NG) and objective confirmation of urethritis (i.e., symptoms alone are insufficient).12, 13 In the U.S., evidence of urethritis includes the presence of urethral discharge on genital examination or increased polymorphonuclear leukocytes (PMNs) in urine or urethral secretions.12 Treatment for NGU with doxycycline or azithromycin is currently recommended by the U.S. Centers for Disease Control and Prevention (CDC).12 Although an azithromycin 1 g single-dose regimen is preferred for NGU treatment by both U.S. clinicians and patients,14 azithromycin has been shown to select for macrolide resistance in MG15, 16 and efficacy rates vary by etiology and are decreasing over time.17, 18 Thus, UK, European and Australian STD treatment guidelines now recommend doxycycline as the first-line NGU treatment and the 2021 update to the U.S. guidelines is likely to follow.13, 19, 20
In most clinical settings, pathogen-guided treatment at the time of initial urethritis evaluation remains unavailable since point-of-care STI tests are still under development and, in the U.S., MG testing is not recommended in men for an initial urethritis episode (only persistent/recurrent urethritis).12 As a result, acute NGU treatment remains empiric and STI testing is typically limited to NG and CT. Further, current U.S. guidelines do not recommend confirmation of NGU pathogen clearance by test-of-cure (TOC) outside of high-risk populations due to historical high cure rates of first-line regimens and the belief that resolution of urethritis signs and symptoms (i.e., clinical cure) indicates STI clearance (i.e., microbiological cure).12, 13 Both clinicians and patients rely on clinical cure to guide the need for additional testing/treatment and when to resume sexual activity, respectively. However, discordant outcomes do occur (i.e., persistent symptoms despite microbiological cure and/or clinical cure without microbiological cure).13, 21–25 In men with NGU, understanding how accurately clinical cure reflects microbiological cure and resolution of urethritis on Gram stain (i.e., “Gram stain cure” could be a potential surrogate microbiological cure in resource-limited settings) is important for identifying risk factors for microbiological treatment failure.
The STI that is most strongly associated with post-azithromycin persistent NGU is MG.12,13,19,26 In the U.S., MG has been detected in up to 29% of NGU and may be increasing due to macrolide resistance.7, 27, 28 Macrolide resistance occurs through selection of single-nucleotide polymorphisms at positions A2058 and A2059 (E. coli numbering) in domain V of the MG 23S rRNA gene, termed macrolide resistance-mediating mutations (MRM); a recent meta-analysis showed a rapid increase in MRM prevalence from <10% before 2010 to >50% by 2017.28 In MRM-positive MG infections, because the efficacy of doxycycline alone against MG is low (<50%),18 moxifloxacin is recommended. However, quinolone resistance is also increasing.27
No recent studies have evaluated the relationship between clinical cure, Gram stain outcome, and microbiological cure in men with NGU. Prior studies combined clinical and Gram stain results into the definition of clinical cure,17, 29 but not all clinics perform urethral Gram stains and, instead, rely on patient-reported symptoms and evaluation of discharge on genital examination to infer NGU cure. Limitations in prior NGU studies may have confounded calculation of both clinical and microbiological cure rates due to variation in the timing of TOC visits or reinfection risk due to interval unprotected sex.17, 30, 31s Our primary objective was to define the relationship between clinical cure and the outcomes of Gram stain cure and microbiological cure by STI in men receiving azithromycin for acute NGU using strict criteria. A secondary objective was to determine if MG load, MRM, and/or a distinct subset of MG strains were associated with MG microbiological failure.
MATERIALS AND METHODS
Study Population and Procedures.
This is a subanalysis of cisgender men with acute NGU who enrolled in the Idiopathic Urethritis Men’s Project (IUMP) at the Bell Flower Clinic in Indianapolis, Indiana between September 12th, 2016, and January 8th, 2020, as described previously.7, 32s, 33s Briefly, after providing written consent, we collected participant demographic, clinical, and sexual behavioral data by computer-assisted self-interviewing (CASI). Men were evaluated for NGU, treated with azithromycin 1 g orally, and returned for a 1-month TOC visit (window period 21−35 days). At both visits, men provided a first-catch urine (FCU), a urethral Gram stain, and underwent a genital examination. Participants reported information on interval antibiotic use, partner treatment status, and interval sexual behaviors, including oral, vaginal, and anal sex and condom use. Queried consistency with condom use included: (1) condom use for all sexual activity; (2) condom use throughout the entire sexual encounter; and (3) any occurrences of condom failure. Participants were excluded if they reported interval sex with an untreated partner, had unprotected sex with a new partner, or had inconsistent condom use. Participants were diagnosed with NGU if they had ≥5 PMN/HPF with symptoms of discharge or dysuria and and/or urethral discharge on physical exam. Clinical cure was defined as resolution of urethritis symptoms without evidence of discharge or meatitis on genital examination. Gram stain cure was defined as a urethral swab Gram stain with ≤1 PMN/HPF. Microbiological cure was defined as NAAT-negative at the TOC visit. This study was approved by the Indiana University-Purdue University-Indianapolis (IUPUI) Institutional Review Board and Marion County Public Health Department.
NAAT, MG organism load, MG-MRM testing, Metagenomic shotgun sequencing and MG phylogenetic analysis and sequence typing.
FCU was tested for NG, US_NmUC, CT, TV, MG, and UU by NAAT as described.7, 34s Men with NG, US_NmUC, or TV were excluded as they received additional antibiotics. MG loads were determined on a LightCycler 2.0 (software v4.1) as described.35s MRM were evaluated by PCR and Sanger sequencing. Dual-indexed sequencing libraries were constructed with the NexteraXT library prep kit (Illumina Inc.), pooled into 12 samples per lane, and paired-end sequenced (2 × 150bp) on a HiSeq4000 sequencer at the Indiana University Center for Medical Genomics. MicroGMT (version 1.4)36s was used to identify single nucleotide polymorphisms (SNPs) with the following three types of data: (i) complete genome sequences for five MG strains available at NCBI (G37 [NC_000908.2], M2321 [NC_018495.1], M6282 [NC_018496.1], M6320 [NC_018497.1] and M2288 [NC_018498.1]), of which G37 was selected as the reference genome; the ART-illumina program37s was used to simulate paired-end sequences for the other four MG genome sequences (the simulation mimics HiSeq 2500, with read length of 150 bps, mean fragment size of 200 bps with standard deviation of 10 bps, fold coverage of 200, and minimum base quality of 30), (ii) raw shot-gun sequence reads for 28 MG isolates available at European Nucleotide Archive (M2282 [ERS390293], M2300 [ERS390285], M2341 [ERS390286], M30 [ERS390284], M6090 [ERS390289], M6151 [ERS390287], M6257 [ERS390300], M6270 [ERS390298], M6280 [ERS390288], M6283 [ERS390292], M6284 [ERS390294], M6285 [ERS390290], M6286 [ERS390297], M6303 [ERS390299], M6312 [ERS390295], M6327 [ERS390281], M6328 [ERS390291], M6475 [ERS390282], M6489 [ERS390301], M6593 [ERS390279], M6604 [ERS390303], M6711 [ERS390304], M6713 [ERS390280], R32G [ERS390307], TW10–5G [ERS390309], TW10–6G [ERS390308], TW48–5G [ERS390306], and UTMB-10G [ERS390305]), and (iii) 18 metagenomic samples from this project (005–1, 005–2, 026–1, 026–2, 030–1, 030–2, 033–1, 033–2, 034–1, 034–2, 049–1, 050–1, 083–1, 085–1, 085–2, 100–1, 100–2, and 158–1). The metagenomic reads from those 18 samples were aligned to each MG complete genome sequence from NCBI using to Burrows-Wheeler Aligner (BWA, version 0.7.17).38s Only the aligned reads were considered as MG reads for the input to MicroGMT. A custom R script was used to filter out SNP positions with read coverage <2. Based on the identified SNPs, the phylogenetic analysis was performed in MEGA X software,39s using Maximal likelihood method and Tamura-Nei model.40s Bootstrapping 100 times was used to construct the consensus tree. There were a total of 13771 informative positions in all 51 MG sequences in the final dataset.
Statistical analysis.
Analyses were conducted with R v4.0.3 and SAS v9.4. Significance was declared at α=0.05. Group comparisons of demographic characteristics and clinical outcomes were evaluated using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. Outcome-type concordance of MG, UU, IU (when applicable) and mixed infections were compared with concordance of CT using Fisher’s exact test. The Bonferroni correction was used to adjust the p-values for multiple comparisons. Area-proportional Euler diagrams were used to visualize cure type outcome proportions by STI. Association of clinical cure with Gram stain cure and microbiological cure in each STI group were evaluated using Fisher’s exact test. Association between MRM and microbiological failure was evaluated by Fisher’s exact test. Comparisons of MG organism loads between groups were evaluated by Mann-Whitney U test or Wilcoxon signed-rank test (paired data). Univariate and multivariable logistic regression models were used to identify risk factors for MG microbiological failure. Univariate logistic regression models were built for age, race/ethnicity, sexual orientation, age of sexual debut, prior NGU, sexual behaviors, MG load, MRM, clinical cure, and microscopic cure. The multivariable logistic regression model was built using stepwise variable selection.
RESULTS
Of 280 men who enrolled in IUMP, 121 were included in this analysis. (Figure 1). Exclusion criteria included a positive NAAT for NG, US_NmUC, or TV (N = 18), indeterminate/incomplete NAAT or Gram stain results (N = 17), did not return for a TOC visit (N = 59), had interval use of a beta-lactam or fluoroquinolone antibiotic (N = 12), acquired a new infection at the TOC visit (N = 15), had possible re-infection (N = 36), or had a study protocol deviation (N = 2). The algorithm used to exclude men who had interval unprotected sex or sex with an untreated partner (N = 36) is shown in Figure 2. Participant characteristics are shown in Table 1. In men with monomicrobial NGU, 52 (43%) were infected with CT, 16 (13%) with MG, and 7 (6%) with UU. Ten (8%) men had mixed infections identified: 8 had MG co-infections with either UU (N = 5) or CT (N = 3) and 2 had CT and UU coinfections. Thirty-six (30%) men were NAAT-negative for all six STI and were classified as having IU.
Figure 1.
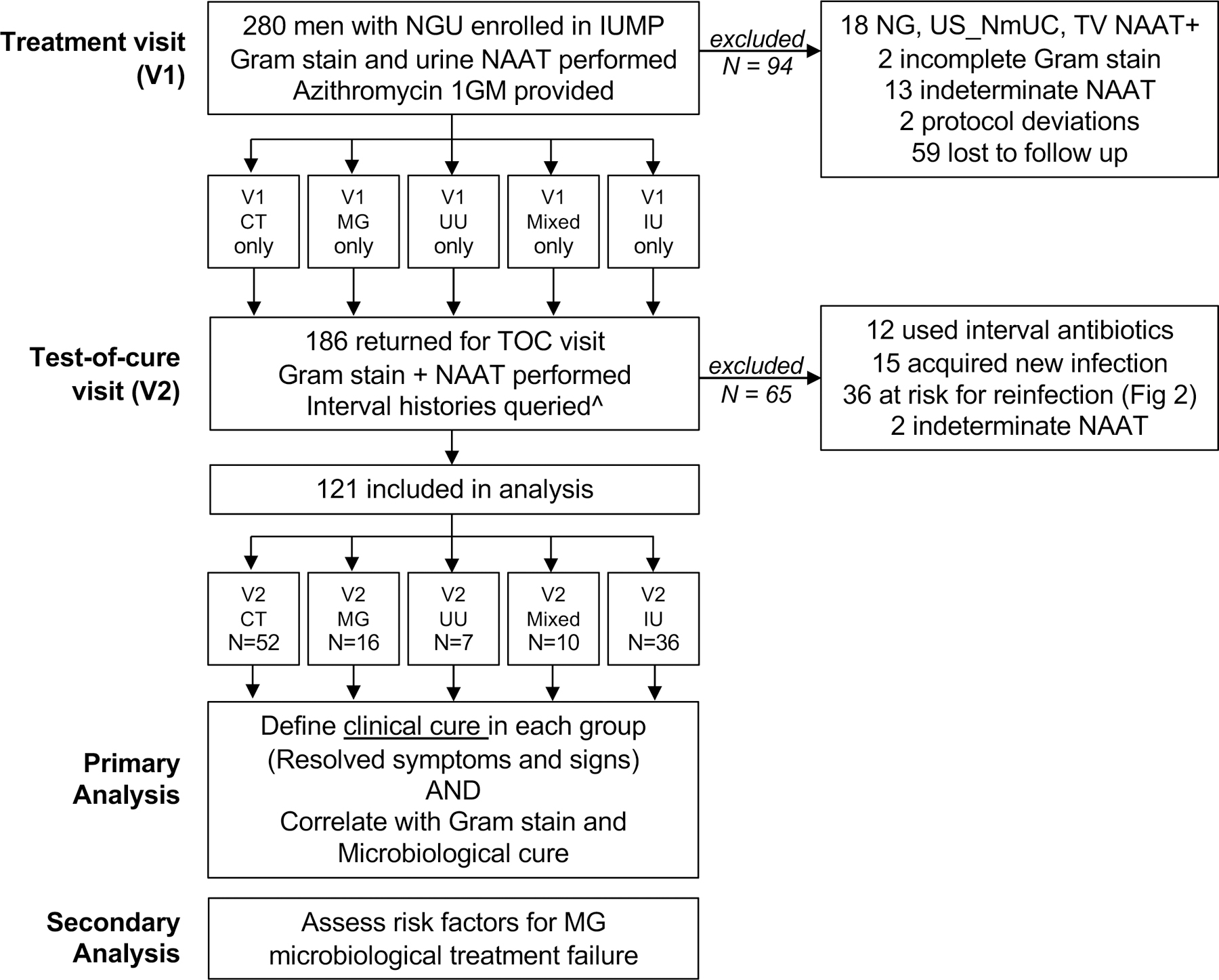
Study Participant Inclusion and Exclusion Flowchart
Figure 2. Flow chart of algorithm used to exclude interval STI reinfection.
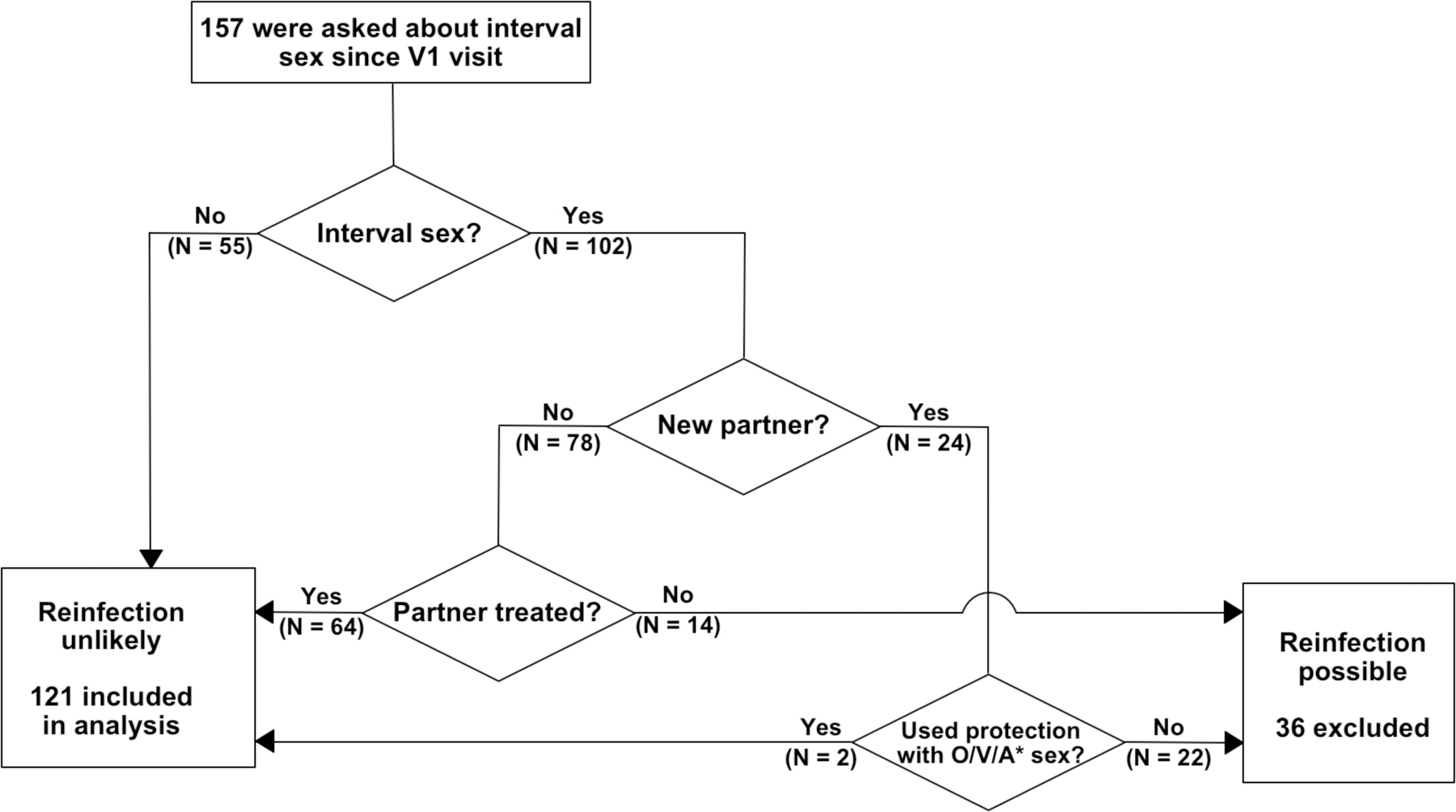
Participants were excluded from this analysis if they reported interval sex with an untreated partner, had unprotected sex with a new partner, or had inconsistent condom use. Criteria used to define consistent barrier protection included: (1) condom use for all sexual activity; (2) condom use throughout the entire sexual encounter; and (3) any occurrences of condom failure (breakage, slippage, or inconsistent use). Participants with missing responses were excluded. *O/V/A, receptive oral, vaginal, insertive anal sex.
Table 1.
Study Participant Characteristics
| Characteristics | Total | CT only | MG only | UU only | Mixed Infections | IU only | P-value |
|---|---|---|---|---|---|---|---|
| N (% of total) | 121 | 52 (43%) | 16 (13%) | 7 (6%) |
10 (8%) |
36 (30%) |
|
| Age median (IQR Range) | 28.6 (24.2–34.5) |
26.2 (22.8–30.7) |
28.6 (24.2–32.6) |
31.5 (26.7–36.1) |
34.0 (24.1–43.5) |
30.9 (26.6–38.2) |
0.0082 |
| Race | 0.0663 | ||||||
| Black/African American |
85 (70%) |
34 (65%) |
11 (69%) |
2 (29%) |
10 (100%) |
28 (78%) |
|
| White | 22 (18%) |
10 (19%) |
2 (13%) |
3 (43%) |
0 | 7 (19%) |
|
| Other | 14 (12%) |
8 (15%) |
3 (19%) |
2 (29%) |
0 | 1 (3%) |
|
| Ethnicity | 0.2719 | ||||||
| Non-Hispanic | 104 (91%) | 43 (91%) |
13 (87%) | 5 (71%) | 9 (100%) |
34 (94%) | |
| Hispanic | 10 (9%) |
4 (9%) |
2 (13%) | 2 (29%) |
0 | 2 (6%) |
|
| Self-reported sexual orientation | 0.0809 | ||||||
| Heterosexual | 107 (88%) |
46 (88%) |
16 (100%) |
7 (100%) |
10 (100%) |
28 (78%) |
|
| MSM | 9 (7%) |
2 (4%) |
0 | 0 | 0 | 7 (19%) |
|
| Other | 5 (4%) |
4 (8%) |
0 | 0 | 0 | 1 (3%) |
|
| Prior self-reported history of STI | |||||||
| Chlamydia | 65 (56%) | 29 (57%) |
9 (56%) | 1 (17%) |
7 (70%) |
19 (56%) |
0.3355 |
| Gonorrhea | 41 (36%) |
15 (30%) |
4 (29%) |
1 (17%) |
6 (60%) |
15 (44%) |
0.2341 |
| Trichomonas | 15 (13%) |
6 (12%) |
2 (13%) |
1 (14%) |
0 |
6 (18%) |
0.7065 |
| Herpes | 9 (8%) |
3 (6%) |
1 (8%) |
0 | 0 | 5 (16%) |
0.4137 |
| Syphilis | 6 (6%) |
1 (2%) |
1 (8%) |
0 | 1 (11%) |
3 (9%) |
0.5538 |
| NGU | 52 (44%) |
17 (33%) |
10 (63%) |
2 (33%) |
3 (30%) |
20 (57%) |
0.088 |
| Genital warts | 10 (9%) |
3 (6%) |
1 (8%) |
0 | 0 | 6 (19%) |
|
| Types of reported sex (last 60 days) | |||||||
| Vaginal sex | 97 (81%) |
42 (82%) |
14 (88%) |
7 (100%) |
10 (100%) |
24 (67%) |
0.0556 |
| Received oral sex | 97 (82%) |
38 (76%) |
12 (75%) |
6 (86%) |
9 (100%) |
32 (89%) |
0.233 |
| Insertive anal sex | 22 (18%) |
7 (14%) |
1 (6%) |
2 (29%) |
1 (10%) |
11 (31%) |
0.1815 |
| Reason for visit | 0.8567 | ||||||
| Genital symptoms | 104 (86%) |
43 (83%) |
15 (94%) |
7 (100%) |
8 (80%) |
31 (86%) |
|
| Worried about STI | 12 (10%) |
6 (12%) |
0 | 0 | 2 (20%) |
4 (11%) |
|
| Partner of someone diagnosed with STI | 3 (2%) |
2 (4%) |
1 (6%) |
0 | 0 | 0 | |
| General check up | 2 (2%) |
1 (2%) |
0 | 0 | 0 | 1 (3%) |
|
| Visits interval, median days (IQR) | 23.0 (21.0–28.0) |
23.5 (21.0–28.0) |
25.5 (21.8–28.0) |
21.0 (21.0–21.5) |
21.0 (21.0–24.3) |
23.5 (21.0–28.0) |
0.163 |
Abbreviations: IQR, interquartile range; STI, sexually transmitted infection. MSM, men who have sex with men.
Overall, rates of clinical, Gram stain, and microbiological cure were 74%, 69%, and 75%, respectively (Table 2). Excluding men with IU, clinical failure occurred in 20 men, of which 9 (45%) had microbiological cure; 4 (44%) of these 9 men had Gram stain cure. In men with persistent IU, Gram stain cure occurred in 50% of men. In men with monomicrobial NGU, clinical cure rates were the highest for UU (100%) and CT (85%) and lowest for MG (50%); mixed infections were 60% and IU was 67%. Gram stain cure rates were similar between STI and IU groups at approximately 50–75%, with the lowest cure rates for MG (50%). Microbiological cure rates varied across STIs from 31–100%, with the highest cure rates for UU and CT at 100% and 92%, respectively, and the lowest for MG and predominantly MG mixed infections at 31% and 40%, respectively. All microbiological failures in the mixed infection group were due to persistent MG infection (N = 6).
Table 2.
Characterization of post-azithromycin clinical, Gram stain, and microbiological cure outcomes in men with NGU
| Clinical Cure % (n/N) |
Gram stain Cure % (n/N) |
Microbiological Cure % (n/N) |
|
|---|---|---|---|
|
| |||
| NGU Pathogen | |||
|
| |||
| CT only | 85% (44/52) | 73% (38/52) | 92% (48/52) |
| MG only | 50% (8/16) | 50% (8/16) | 31% (5/16) |
| UU only | 100% (7/7) | 71% (5/7) | 100% (7/7) |
| IU | 67% (24/36) | 75% (27/36) | N/A |
| Mixed | 60% (6/10) | 60% (6/10) | 40% (4/10) |
| Total | 74% (89/121) | 69% (84/121) | 75% (64/85) |
Concordance rates were evaluated between cure outcomes by STI (Figure 3A). Comparing clinical vs Gram stain cure, clinical vs microbiological cure, and Gram stain vs microbiological cure, discordant cures varied from 0–44% of participants. By STI, the highest frequency of discordant outcomes occurred either in MG-NGU or mixed-infections, which were predominantly due to MG-coinfection. For example, in men with MG-NGU, discordant outcomes occurred in 38% of men comparing clinical and Gram stain cure, 44% of men comparing clinical and microbiological cure, and 31% of men comparing Gram stain and microbiological cure. In IU, 25% of men were discordant comparing clinical and Gram stain cure. Comparing clinical vs microbiological cure in MG- and CT-NGU, there was an increased frequency of discordant outcomes in men with MG-NGU (44% vs 15%, p = 0.102); a similar pattern occurred in mixed infections. This difference became significant when comparing the specific outcome of clinical cure and microbiological failure between CT-NGU (4%) and MG-NGU (31%, p = 0.0042) or mixed-NGU (30%, p = 0.013) (Figure 3B); no discordant outcomes occurred clinical and microbiological cure in UU-NGU. Sterile persistent urethritis (clinical failure with microbiological cure) occurred in approximately 10% of men with CT-, MG-, and mixed-NGU. To further explore the proportion of specific discordant outcomes in men with CT- and MG-NGU, we constructed area-proportional Euler plots to visually represent the contribution of each outcome in men with ≥1 cure type(Figure 3C). In men with CT-NGU, significant overlap occurred between the cure types. For example, 63% of participants were cured by all three outcomes and 90% by 2 or more outcomes. In contrast, men with MG-NGU had much less overlap between the cure types; only 25% were cured by all three outcomes. Only 50% of men had cure by ≥2 outcomes, compared to 90% in men with CT-NGU. Further, half of men with MG-NGU (50%) had only 1 cure, of which the most common was clinical cure (25%). No cure occurred in 2 men with CT and 4 men with MG-NGU.
Figure 3. Comparison of cure types in men with NGU treated with azithromycin.
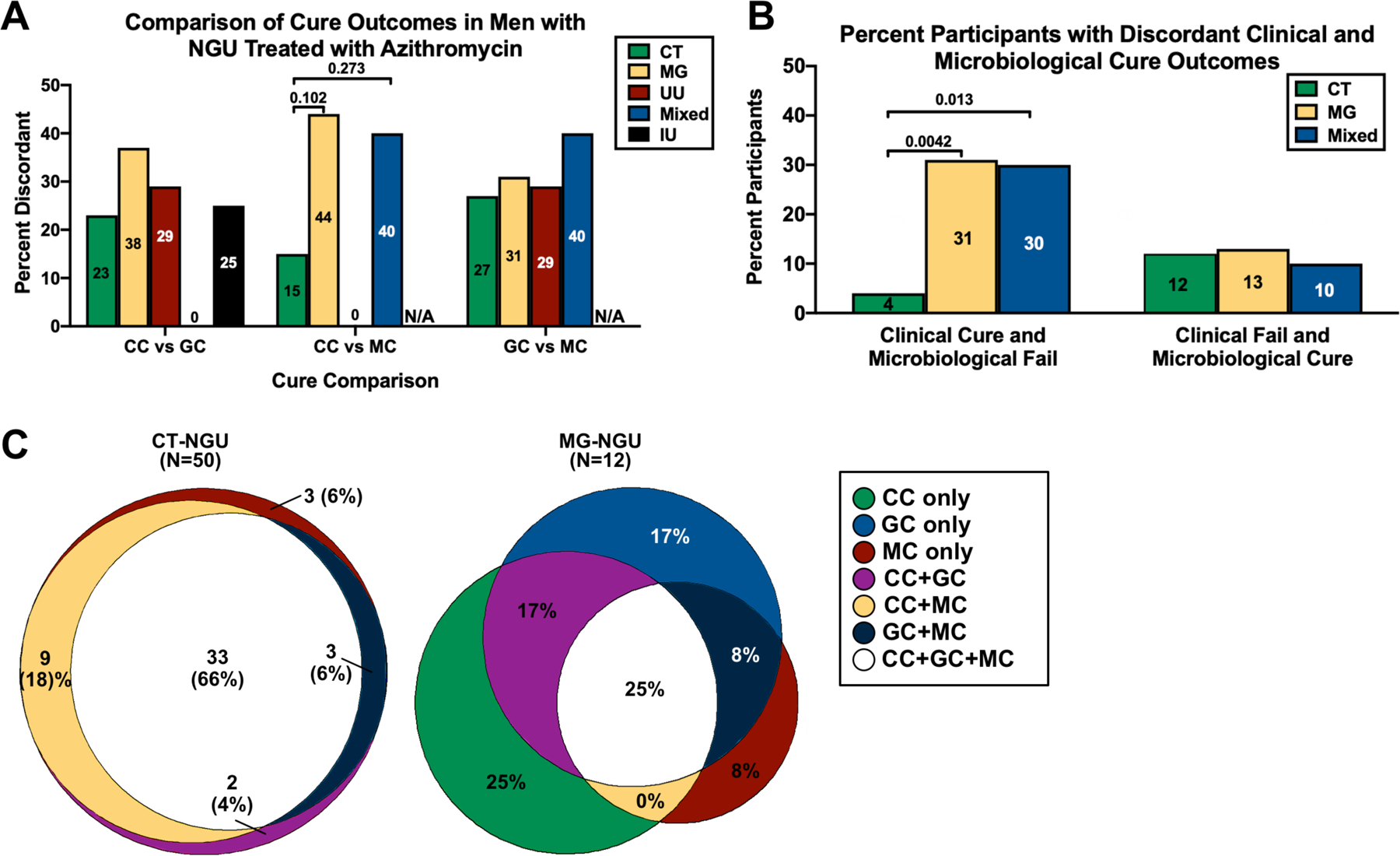
(A) Percentage of men with discordant cure, stratified by monomicrobial- or mixed-pathogen NGU or IU. Cure comparisons were clinical (CC) vs Gram stain (GC), clinical vs microbiological (MC), and Gram stain vs microbiological. (B) Percentage of men who had either clinical cure with microbiological failure or clinical failure with microbiological cure. (C) Area-proportional Euler graphs for CT- (N = 50) and MG-NGU (N = 12) in men with ≥1 cure type. Significance was evaluated by Fisher’s exact test. P-values were adjusted by the Bonferroni correction. Abbreviations: CC, clinical cure; GC, Gram stain cure; MC, microbiological cure; N/A, not applicable. Non-significant comparisons not shown.
Given the increase in discordant cures in men with MG-NGU, we hypothesized that clinical cure may be an unreliable indicator of either Gram stain or microbiological cure for MG-NGU, compared to the other STI studied. Clinical cure was significantly associated with Gram stain cure in men with CT (OR 6.5, 95% CI 1.3–32, p = 0.025) and mixed infections (OR 117, 95% CI 1.9–7060, p = 0.005), but not MG (OR 2.8, 95% CI 0.4–21, p = 0.619) (Table 3). Similarly, clinical cure trended towards being associated with microbiological cure in men with CT (OR 7.0, 95% CI 0.8–59.4, p = 0.107), but less so with MG (OR 1.8, 95% CI 0.2–15.4, p = 1) or mixed-NGU (OR 3.0, 95% CI 0.2–48.0, p = 0.571); UU had perfect concordance between clinical and microbiological cure.
Table 3.
Association of clinical cure with microbiological and Gram stain cure in men with NGU treated with azithromycin
| Gram stain evaluation | Microbiological evaluation | |||||||
|---|---|---|---|---|---|---|---|---|
| Cured % (n/N) |
Failed % (n/N) |
crude OR (95% CI) |
P-value* | Cured % (n/N) |
Failed % (n/N) |
crude OR (95% CI) |
P-value* | |
| CT only | ||||||||
| Clinical cure | 79.5% (35/44) | 20.5% (9/44) | 6.5 (1.3–32.4) | 0.025 | 96% (42/44) | 4% (2/44) | 7.0 (0.8–59.4) | 0.107 |
| Persistent signs/sx | 37.5% (3/8) | 62.5% (5/8) | 75% (6/8) | 25% (2/8) | ||||
| MG only | ||||||||
| Clinical cure | 62.5% (5/8) | 37.5% (3/8) | 2.8 (0.4–21.0) | 0.619 | 62.5% (5/8) | 37.5% (3/8) | 1.8 (0.2–15.4) | 1 |
| Persistent signs/sx | 37.5% (3/8) | 62.5% (5/8) | 37.5% (3/8) | 62.5% (5/8) | ||||
| UU only | ||||||||
| Clinical cure | 71.4% (5/7) | 28.6% (2/7) | N/A | N/A | 100% (7/7) | 0 | N/A | N/A |
| Persistent signs/sx | 0 | 0 | 0 | 0 | ||||
| Mixed infections ^ | ||||||||
| Clinical cure | 100% (6/6) | 0% (0/6) | 117 (1.9–7060) | 0.005 | 50% (3/6) | 50% (3/6) | 3.0 (0.2–48.0) | 0.571 |
| Persistent signs/sx | 0% (0/4) | 100% (4/4) | 25% (1/4) | 75% (3/4) | ||||
| IU only | ||||||||
| Clinical cure | 87.5% (21/24) | 12.5% (3/24) | 7.0 (1.3–36.7) | 0.036 | N/A | N/A | N/A | N/A |
| Persistent signs/sx | 50% (6/12) | 50% (6/12) | N/A | N/A | ||||
Abbreviations: sx, urethritis symptoms
Fishers exact test was used to compute p-values.
Haldane-Anscombe correction was used for computing the OR where applicable.
We next evaluated if microbiological failure in MG-NGU was due to macrolide resistance, high MG organism load, or a distinct clade of MG strains. MRMs were detected in 71% of the MG positive treatment visit specimens (N = 24, Figure 4A) and included A2058G/T (G = 7, T = 1) and A2059G (N=9). Although MG was eradicated by the TOC visit in 6/7 men (86%) who had wildtype MG at the treatment visit, azithromycin only cleared MG in 1/17 men (6%) who had MG containing an MRM (p = 0.0003, Figure 4B). However, Grain stain cure or clinical cure still occurred in 41% of these MRM MG-infected men, and 24% of men had both cure outcomes (data not shown).
Figure 4. MG characteristics in men with MG-NGU.
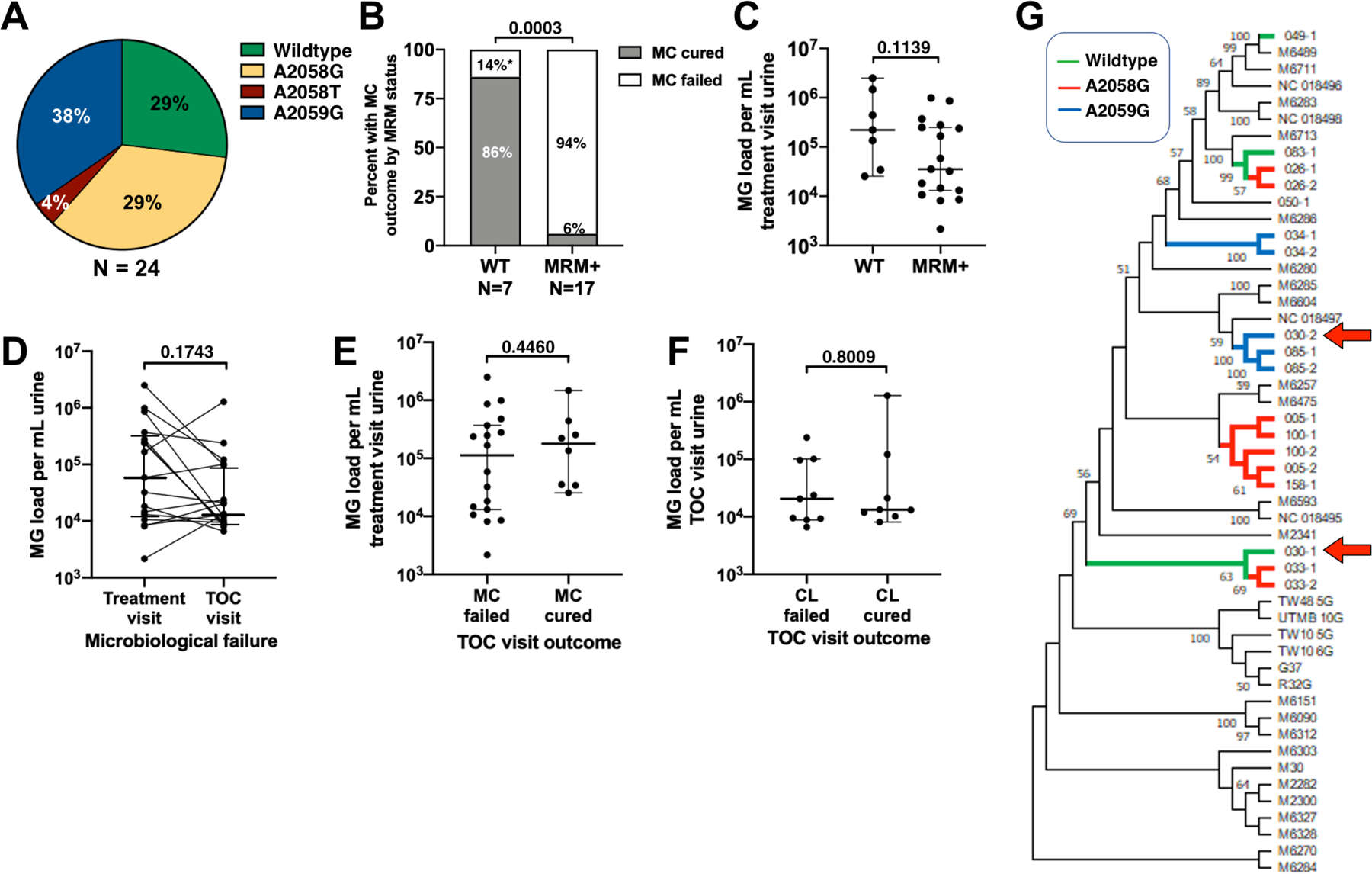
(A) Distribution of wildtype (WT) and MRM-containing MG alleles in mono- and mixed-infection MG-NGU at the treatment visit. MRM were A2058G, A2058T, and A2059G. (B) Percentage of men with WT or MRM-MG who had microbiological cure (MC). (C) Treatment visit urine MG organism load in men with WT or MRM MG-NGU. (D) Urine MG organisms load in treatment and TOC visits from men with MC failure. (E) Treatment visit urine MG organism load in men with/without MC cure. (F) TOC visit urine MG load in men with/without clinical (CC) cure. The y-axis in panels C-E is Log10. Horizontal bars denote the median and the whiskers denote 95% CI. Significance was evaluated by Fisher’s Exact (B) Mann-Whitney U (C, E, F) or Wilcoxon sign-rank test (D). (G) Phylogenetic Analysis of MG infections (N = 18) from treatment and/or TOC visits. Reference strains are denoted by the black lines. MG infections are denoted as study number followed by visit number (1 = treatment, 2 = TOC visit). Red arrows denote participant 30, the only participant with a wildtype MG infection who had microbiological failure; branch separation suggests the treatment and TOC MG infections are distinct genotypes.
We then tested if differences in urine MG loads could explain microbiological failure. Comparing treatment visit MG loads between WT and MRM+ MG, there was a trend towards MG loads being higher in WT MG (median 221,000 vs 35,400 copies per mL, p = 0.1123, Figure 4C). Also, there was a decrease in MG organism load between the treatment visit and the TOC visit in those with microbiological failure (58,500 vs 13,300 copies per mL, p = 0.1743, Figure 4D). This suggests that differences in organism load did not explain differences in microbiological cure (Figure 4E) or clinical cure (Figure 4F) outcomes.
Strain-specific virulence factors can facilitate MG transmission,41s so we hypothesized that different treatment outcomes could be associated with specific MG genotypes. Shotgun metagenomic sequencing and MEGA analysis were used to infer phylogeny of the MG strains in 18 study specimens that contained sufficient DNA for in situ deep sequencing (4 specimens from participants with microbiological cure and 7 paired specimens from participants with microbiological failure). The MRM-positive strains were interspersed among the MRM-negative strains in the cladogram, consistent with the hypothesis that macrolide pressure can select for the emergence of identical MRMs in otherwise genetically diverse MG lineages (Figure 4G).42s Phylogenetic analysis suggested that the only participant with wildtype MG-NGU who had a microbiological failure, had different strains in his treatment and TOC visits. To confirm this, we PCR-amplified and sequenced two highly discriminatory MG alleles (MG191-SNPs and MG309-STRs)43s in the paired specimens (treatment and TOC visits) from the seven microbiological failures. Identical MG191 and MG309-STR alleles were detected in all participants, except for participant 30. Consistent with the phylogenetic analysis, the discordant allele types in participant 30 suggest that the occurrence of MG at the TOC visit was the result of a new infection with a heterologous MRM strain, and not microbiological failure due to MRM acquisition of a previously WT infection (data not shown). No associations between specific MG clades and microbiological or clinical outcomes were evident, suggesting that discordant microbiological outcomes were not associated with a specific clade of MG strains.
Since phylogenetic analysis failed to provide an explanation for discordant cure outcomes, we performed multivariable logistic regression with stepwise selection to identify risk factors for MG microbiological failure, including age, race/ethnicity, sexual orientation, age of sexual debut, prior NGU, sexual behaviors, MG load, MRM, clinical cure, and Gram stain cure. Only MRM was associated with microbiological failure (OR 45.0, 95% CI 3.4–594, p = 0.0105, supplemental table). Similarly, we also performed univariate and multivariable logistic regression with the same variables to identify risk factors for MRM. No association was found (data not shown).
DISCUSSION
Clinicians and patients rely on resolution of NGU signs and symptoms to guide the need for additional testing/treatment and when to resume sexual activity, respectively. Whether symptom resolution accurately reflects microbiological cure for a specific STI in men with acute NGU has been unclear. We now show that clinical cure does reflect microbiological and Gram stain cure for CT and UU, but not MG. Discordant clinical and microbiological cure outcomes were relatively common for MG, occurring in almost half of men (44%) with MG-NGU, and were three times higher than CT-NGU among the same cohort. Most of these discordant outcomes were microbiological failures in the setting of clinical cure, suggesting that almost one third of all men who receive azithromycin for MG-NGU are at risk of resuming sexual activity and unknowingly transmitting MG infection. Romano et al. reported a high frequency of persistent asymptomatic MG infection in men treated with azithromycin, but reinfection couldn’t be excluded as all men either reported interval unprotected sex, most with ≥2 partners, or provided no response.24 The association of MG-NGU with high discordant cure rates reveals that clinical cure alone should not be used to infer cure in men treated with azithromycin in whom MRM status is unknown, and confirmation of microbiological cure is needed. We also found that approximately 10% of men with either CT, MG, or mixed infections had persistent symptoms despite microbiological cure, likely reflecting delayed resolution of the inflammatory response, despite STI clearance, as has been reported by Horner et al.44s Hence, in men with persistent symptoms but lacking risk factors for re-infection, delaying re-treatment while evaluating microbiological failure may be warranted.
Another key finding was that MRM, but not MG organism load, was associated with microbiological failure. This confirms that MRM are associated with high-level macrolide resistance.31s, 45s–48s Our findings strengthen this association as we excluded any participant who engaged in unprotected sexual activity and thus would be at risk for recurrent infection. Additionally, most of the MRM-containing MG infections were not clonally related, suggesting that MRM may have repeatedly arisen de novo within independent MG lineages; implying that MRM are being driven by pressure from azithromycin use. Therefore, it is likely that natural selection gives rise to MRM-MG strains and limiting azithromycin use for special populations (e.g., pregnancy) and using extended azithromycin therapy could slow the spread of MRM-MG.16, 49s, 50s, 51s
Our findings have implications for NGU management in the U.S. and align with recent UK, European and Australian guidelines.19, 51s, 52s First, symptomatic men with NGU should be routinely tested for both MG and MRM. Several MG diagnostics assays are now FDA-approved and MG remains the second most prevalent cause of NGU.7 We advocate for routine MG testing in NGU due to its prevalence and the risk for persistent infection in the setting of clinical cure. Second, given the high prevalence of discordant outcomes and lack of association between clinical cure and microbiological cure, we agree with the UK, European, and Australian guidelines and recommend that a TOC should be routinely performed in men who were treated with azithromycin for MG-NGU in whom MRM status is unknown regardless of symptoms; and perhaps all patients with MG-NGU.19, 51s, 52s Third, patients should be educated that some STIs may persist despite symptom resolution and that, in certain circumstances (i.e., azithromycin use without MRM testing), they may be instructed to continue abstinence until MG clearance is confirmed by TOC. Finally, our data suggests that Gram stain is not an appropriate surrogate for a NAAT test to evaluate for microbiological cure, even for IU.
A strength of our study was our comprehensive approach to minimize treatment failure misclassification by using a narrow follow-up window period, exclusion of men with interval antibiotic use, acquiring a new STI, or sexual behavior that could cause reinfection. We defined “unprotected sex” as sex with an untreated partner or any sex that did not include consistent condom use for all receptive oral, vaginal, and insertive anal sexual behaviors. Our approach likely over-estimated the number of men who were at risk for reinfection but was necessary to achieve our goal of minimizing reinfection risk.
Our study has limitations. This was a single-site study at an STD clinic and may not be generalizable to other populations. We also used a Gram stain threshold of ≥5 PMN/HPF to define NGU and whether our study findings also extend to men with a lower level inflammation cutoff of ≥2/HPF is unknown.12 The exclusion criterion of interval antibiotic use relied on antibiotic recall and participants who could not remember the antibiotic name or who identified azithromycin were not excluded. This may have resulted in some false negative TOC visit results due to interval antibiotic use. Our UU and MG sample sizes were small and may have lacked statistical power to detect small differences. Finally, as NAAT cannot distinguish between nonviable and viable organisms, whether men with persistent MG infection who lack symptoms and/or inflammation remain infectious is unclear.
In conclusion, azithromycin use in MG-NGU frequently leads to discordant outcomes, where MG infection persists despite apparent clinical cure. Most of these discordant outcomes were associated with MRM MG strains that were evolutionarily distinct, suggesting that the discordant outcomes were not linked to a specific MG virulence factor, but could be due to unidentified host factors or anti-inflammatory effects of azithromycin. Thus, empiric azithromycin use in men with MG-NGU may mask persisting infection and lead to premature resumption of sexual activity in asymptomatic, yet potentially infectious, individuals. We recommend incorporating routine MG testing, with a reflex to MRM testing, in symptomatic NGU, and men with MG-NGU continue to practice abstinence until a TOC is performed when MRM status is unknown, to decrease risk of MG transmission.
Supplementary Material
ACKNOWLEDGMENTS:
The authors are grateful to Lora Fortenberry, Virginia Caine, Janet Arno, Byron Batteiger, Netsanet Gebregziabher, Aaron Ermel, and Stanley Spinola.
FUNDING AND DISCLOSURES:
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [R01AI116706 to D.E.N.]. T.A.B., L.A.C., M.L., J.R., X.G., Y.X., and Q.D., have no conflicts of interest. S.J.J. and W.M.G. have received consulting fees from Hologic. E.T., J.A.W., and. D.E.N. retain the patent for the US_NmUC assay used in this manuscript.
REFERENCES
- 1.Martin D Urethritis in males. In: Holmes KKSP, Stamm WE, ed. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill; 2008:1107–26. [Google Scholar]
- 2.Bachmann LH, Manhart LE, Martin DH, et al. Advances in the understanding and treatment of male urethritis. Clin Infect Dis. 2015;61 Suppl 8:S763–9. [DOI] [PubMed] [Google Scholar]
- 3.Toh E, Gangaiah D, Batteiger BE, et al. Neisseria meningitidis ST11 complex isolates associated with nongonococcal urethritis, Indiana, USA, 2015–2016. Emerg Infect Dis. 2017;23(2):336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan S, Chambers LC, Tapia KA, et al. Urethral microbiota in men: association of Haemophilus influenzae and Mycoplasma penetrans with nongonococcal urethritis. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 5.Shimada Y, Ito S, Mizutani K, et al. Bacterial loads of Ureaplasma urealyticum contribute to development of urethritis in men. Int J STD AIDS. 2014;25(4):294–8. [DOI] [PubMed] [Google Scholar]
- 6.Frolund M, Lidbrink P, Wikstrom A, Cowan S, Ahrens P, Jensen JS. Urethritis-associated pathogens in urine from men with non-gonococcal urethritis: a case-control study. Acta Derm Venereol. 2016;96(5):689–94. [DOI] [PubMed] [Google Scholar]
- 7.Jordan SJ, Toh E, Williams JA, et al. Aetiology and prevalence of mixed-infections and mono-infections in non-gonococcal urethritis in men: a case-control study. Sex Transm Infect. 2020;96(4):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women? - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32(11):1845–51. [DOI] [PubMed] [Google Scholar]
- 9.Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis. 2011;38(3):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin DH. Nongonococcal urethritis: new views through the prism of modern molecular microbiology. Curr Infect Dis Rep. 2008;10(2):128–32. [DOI] [PubMed] [Google Scholar]
- 11.Lewis J, Horner PJ, White PJ. Incidence of pelvic inflammatory disease associated with Mycoplasma genitalium infection: evidence synthesis of cohort study data. Clin Infect Dis. 2020;71(10):2719–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Workowski KA, Bolan GA, Papp JR. Sexually transmitted diseases treatment guidelines, 2015.; 2015:1–137. [PMC free article] [PubMed] [Google Scholar]
- 13.Horner PJ, Blee K, Falk L, van der Meijden W, Moi H. 2016 European guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(11):928–37. [DOI] [PubMed] [Google Scholar]
- 14.Carlin EM, Barton SE. Azithromycin as the first-line treatment of non-gonococcal urethritis (NGU): a study of follow-up rates, contact attendance and patients’ treatment preference. Int J STD AIDS. 1996;7(3):185–9. [DOI] [PubMed] [Google Scholar]
- 15.Horner P, Ingle SM, Garrett F, et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Transm Infect. 2018;94(1):14–20. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Shimada Y, Yamaguchi Y, et al. Selection of Mycoplasma genitalium strains harbouring macrolide resistance-associated 23S rRNA mutations by treatment with a single 1 g dose of azithromycin. Sex Transm Infect. 2011;87(5):412–4. [DOI] [PubMed] [Google Scholar]
- 17.Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens--a randomized clinical trial. Clin Infect Dis. 2011;52(2):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manhart LE, Jensen JS, Bradshaw CS, Golden MR, Martin DH. Efficacy of antimicrobial therapy for Mycoplasma genitalium infections. Clin Infect Dis. 2015;61 Suppl 8:S802–17. [DOI] [PubMed] [Google Scholar]
- 19.Mycoplasma genitalium - Australasian STI Management Guidelines. http://www.sti.guidelines.org.au/sexually-transmissible-infections/mycoplasma-genitalium. Accessed January 13, 2021.
- 20.Update to the 2015 BASHH UK National Guidelines on the management of non-gonococcal urethritis. https://www.bashhguidelines.org/media/1199/ngu-bashh-update-2018.pdf. Accessed June 24, 2021.
- 21.Sena AC, Lensing S, Rompalo A, et al. Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis infections in men with nongonococcal urethritis: predictors and persistence after therapy. J Infect Dis. 2012;206(3):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32(7):995–1003. [DOI] [PubMed] [Google Scholar]
- 23.Stamm WE, Batteiger BE, McCormack WM, Totten PA, Sternlicht A, Kivel NM. A randomized, double-blind study comparing single-dose rifalazil with single-dose azithromycin for the empirical treatment of nongonococcal urethritis in men. Sex Transm Dis. 2007;34(8):545–52. [DOI] [PubMed] [Google Scholar]
- 24.Romano SS, Jensen JS, Lowens MS, et al. Long Duration of Asymptomatic Mycoplasma genitalium Infection After Syndromic Treatment for Nongonococcal Urethritis. Clin Infect Dis. 2019;69(1):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner P, Blee K, O’Mahony C, Muir P, Evans C, Radcliffe K. 2015 UK National Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(2):85–96. [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw CS, Chen MY, Fairley CK. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One. 2008;3(11):e3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann LH, Kirkcaldy RD, Geisler WM, et al. Prevalence of Mycoplasma genitalium infection, antimicrobial resistance mutations and symptom resolution following treatment of urethritis. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 28.Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(11):1302–14. [DOI] [PubMed] [Google Scholar]
- 29.Stamm WE, Hicks CB, Martin DH, et al. Azithromycin for empirical treatment of the nongonococcal urethritis syndrome in men. A randomized double-blind study. Jama. 1995;274(7):545–9. [PubMed] [Google Scholar]
- 30.Read TR, Fairley CK, Tabrizi SN, et al. Azithromycin 1.5g over 5 days compared to 1g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis. 2017;64(3):250–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


