Abstract
Background:
Individuals with schizophrenia exhibit deficits in visual contrast processing, though less is known about how these deficits impact neurocognition and functional outcomes. This study investigated effects of contrast sensitivity (CS) on cognition and capacity for independent living in schizophrenia.
Methods:
Participants were 58 patients with schizophrenia (n = 49) and schizoaffective disorder (n = 9). Patients completed a psychophysical paradigm to obtain CS with stimuli consisting of grating patterns of low (0.5 and 1 cycles/degree) and high spatial frequencies (4, 7, 21 cycles/degree). Patients completed the MATRICS Consensus Cognitive Battery and Wechsler Adult Intelligence Scales, Third Edition to assess cognition, and the problem-solving factor of the Independent Living Scales to assess functional capacity. We computed bivariate correlation coefficients for all pairs of variables and tested mediation models with CS to low (CS–LSF) and high spatial frequencies (CS–HSF) as predictors, cognitive measures as mediators, and capacity for independent living as an outcome.
Results:
Cognition mediated the relationship between CS and independent living with CS–LSF a stronger predictor than CS–HSF. Mediation effects were strongest for perceptual organization and memory-related domains. In an expanded moderated mediation model, CS–HSF was found to be a significant predictor of independent living through perceptual organization as a mediator and CS–LSF as a moderator of this relationship.
Conclusion:
CS relates to functional capacity in schizophrenia through neurocognition. These relationships may inform novel visual remediation interventions.
Keywords: vision, cognition, visual pathways, visual perception, independent living, mediation analysis
1. Introduction
Individuals with schizophrenia exhibit deficits in visual contrast processing compared to healthy controls in a number of psychophysical and neurophysiological studies (Butler et al., 2009; Butler and Javitt, 2005; Butler et al., 2007; Butler et al., 2001; Cadenhead et al., 2013; Calderone et al., 2013b; Cimmer et al., 2006; Fernandes et al., 2019; Keri et al., 2002; O’Donnell et al., 2006; Slaghuis, 1998, 2004; Zemon et al., 2021). Contrast, a basic property of visual perception, is fundamental for viewing the natural world and is involved in all visual input to the brain, leading to dynamic effects on cortical circuitry (Zemon and Gordon, 2006). Altered properties of visual cortical neurons associated with deficits in contrast processing in schizophrenia may lead to deficits in higher-level cortical processes, given that projections from visual cortex to these higher cortical areas are known to be involved in cognitive and behavioral functioning (e.g., attention, perceptual organization, working memory, and decision-making) (Butler and Javitt, 2005; Butler et al., 2008; Elston, 2003; Felleman and Van Essen, 1991; Javitt, 2009; Roelfsema and Lange, 2016; Silverstein and Keane, 2011).
Initial investigations of links between contrast processing deficits and higher-level cognitive and functional deficits in schizophrenia have been carried out through correlational analyses. Lower psychophysical contrast sensitivity (CS) (e.g., poorer ability to detect contrast) was associated with deficits in intelligence quotient (IQ), attention, working memory, reading, facial emotion recognition, and functional outcome in schizophrenia (Butler et al., 2009; Butler et al., 2005; Martinez et al., 2012; Revheim et al., 2006a; Revheim et al., 2014). Lower CS as measured by a contour integration task was associated with decreased independent functioning in schizophrenia (Keane et al., 2014). Electrophysiological assessments of contrast processes have shown similar findings, with smaller visual evoked potential responses to contrast modulation being related to poorer performance on a facial emotion recognition task (Butler et al., 2009), as well as on measures of functional outcome in people with schizophrenia (Butler et al., 2005).
Studies also show links between other visual functions and cognition, symptoms, and functional outcomes in schizophrenia. Motion perception and motion recognition have been associated with cognition (Brenner et al., 2002), and perceptual organization has been linked to disorganized symptoms (Silverstein and Keane, 2011). Green and colleagues utilized structural equation modeling and found significant pathways from visual masking performance to functional outcomes through factors such as social cognition, beliefs, and negative symptoms (Green et al., 2012; Rassovsky et al., 2011; Sergi et al., 2006). Given that neurocognition is well known to predict functional outcomes in schizophrenia (Brekke et al., 2007; Green et al., 2004; Nuechterlein et al., 2011), there may be a significant pathway from visual processing to functional status through neurocognition. While CS in schizophrenia has been well investigated (Zemon et al., 2021), its relationships to neurocognition and functional outcomes require more extensive exploration. These examinations may further our understanding of the neural origins of deficits in cognition and functional outcomes in schizophrenia, as well as provide insight into novel treatments that target low-level perceptual processing. For instance, visual remediation shows promise for improving CS as well as higher level cognitive and behavioral functioning (Butler et al., 2017; Silverstein et al., 2020).
CS to low spatial frequency (CS–LSF) and high spatial frequency (CS–HSF) stimuli have yielded differential contributions to object and face processing (Bar, 2003; Calderone et al., 2013a; Keane et al., 2014; Silverstein et al., 2014; Vakhrusheva et al., 2014), and are thought to be processed by different mechanisms. LSF (coarse) information appears to be processed preferentially by a transient mechanism, while HSF (fine detail) information appears to be processed preferentially by a sustained mechanism (Breitmeyer and Ganz, 1977; Legge, 1978; Merigan et al., 1991a; Merigan and Eskin, 1986; Merigan et al., 1991b; Zemon et al., 2021). A principal component analysis of CS to grating patterns presented across a range of spatial frequencies demonstrated that, indeed, CS–LSF and CS–HSF loaded onto separate principal components (Zemon et al., 2021).
The goal of the current study was to investigate pathways between CS–LSF, CS–HSF, cognitive abilities, and functional capacity in schizophrenia. The Problem Solving Factor of the Independent Living Scales (ILS-PB) was selected as a measure of functional capacity given that it is based on necessary cognitive skills for independent community living, and has been shown to be of value as a global measure of functional outcome in schizophrenia (Loeb, 1996; Revheim and Medalia, 2004a; Revheim and Medalia, 2004b; Revheim et al., 2006b). Here, it was hypothesized that cognitive abilities would mediate the relationships between CS–LSF/CS–HSF and capacity for independent living among individuals with schizophrenia. To our knowledge, this is the first study to assess the pathways from the low-level visual property of CS to functional capacity in schizophrenia.
2. Methods
2.1. Participants
Participants were 58 patients with schizophrenia (n = 49) and schizoaffective disorder (n = 9). This is a subsample of participants extracted from a larger dataset (Zemon et al., 2021) of patients who completed the CS task, cognitive measures, and Problem-Solving Factor of the Independent Living Scales (ILS-PB). Number of participants per analysis is noted when there are missing neurocognitive data. Participants were recruited from the Nathan Kline Institute for Psychiatric Research and all participants provided informed consent in accordance with the Declaration of Helsinki. Diagnoses were obtained using the Structured Clinical Interview for DSM-IV (SCID) and available clinical information. Exclusion criteria included the following: (a) met criteria for alcohol or substance dependence within the last six months, or abuse within the last month (b) had a neurological or ophthalmological disorder, including head injury that resulted in loss of consciousness for ≥ 30 minutes or loss of consciousness with any neurological sequelae, that might affect visual acuity or CS performance. Participants had a corrected visual acuity of 0.2 logMAR or better at 4 m based on the Logarithmic Visual Acuity Chart (Precision Vision, La Salle, IL) with decimal equivalent values reported here. This study was approved by the Nathan Kline Institute for Psychiatric Research/Rockland County Psychiatric Center Institutional Review Board.
Table 1 displays demographic and clinical characteristics of the sample. Note that there were more males than females in this sample. Chlorpromazine equivalents were calculated using known conversion factors (Hyman et al., 1991; Jibson and Tandon, 1998; Peuskens and Link, 1997; Woods, 2003). Patients’ mean total score on the PANSS (Kay et al., 1987) suggests a markedly ill Clinical Global Impressions classification (Leucht et al., 2005).
Table 1.
Demographic and clinical characteristics of patients with schizophrenia and schizoaffective disorder (N = 58)
| Age | 38.9 ± 10.0 |
| Gender (M/F) | 46/12 |
| Diagnosis | |
| Schizophrenia | 49 |
| Schizoaffective Disorder | 9 |
| Acuity | 0.92 ± 0.20 |
| Participant SESa (n = 55) | 25.4 ± 12.1 |
| Parent SESa (n = 49) | 48.3 ± 25.4 |
| PANSSb total score (n = 54) | 73.0 ± 12.7 |
| Positive Scale | 19.8 ± 6.2 |
| Negative Scale | 17.9 ± 4.2 |
| General Psychopathology Scale | 35.3 ± 6.5 |
| Duration of illness (years) (n = 48) | 16.3 ± 9.1 |
| Chlorpromazine daily equivalent, mg | 876.6 ± 689.7 |
| Antipsychotics | |
| Atypical | 40 |
| Typical | 5 |
| Combination | 12 |
| Not on antipsychotic | 1 |
| CS–LSFc | 1.9 ± 0.2 |
| CS–HSFd | 1.4 ± 0.3 |
| WAIS Perceptual Organization Indexe (n = 53) | 88.1 ± 16.6 |
| WAIS Processing Speed Indexe (n = 55) | 79.4 ± 13.7 |
| WAIS Working Memory Index (n = 53) | 88.5 ± 13.6 |
| MCCB Speed of processingf (n = 35) | 25.7 ± 12.6 |
| MCCB Attention/vigilancef (n = 55) | 30.2 ± 11.3 |
| MCCB Working memoryf (n= 35) | 30.9 ± 12.2 |
| MCCB Visual learningf (n = 35) | 33.0 ± 14.4 |
| MCCB Verbal learningf (n = 35) | 32.4 ± 7.5 |
| MCCB Reasoning and problem-solvingf (n = 35) | 35.6 ± 9.4 |
| ILS-PBg | 38.6 ± 11.5 |
Values are M ± SD with the exception of gender and antipsychotics in which they are counts. Number of participants is noted given missing data.
socioeconomic status measured by Hollingshead Four-Factor Index of Social Status (Hollingshead, 1975).
Positive and Negative Syndrome Scale (Kay et al., 1987).
mean log contrast sensitivity to low spatial frequencies (0.5 and 1 cycles/degree).
mean log contrast sensitivity to high spatial frequencies (4, 7, 21 cycles/degree).
Wechsler Adult Intelligence Scale-III.
MATRICS Consensus Cognitive Battery (MCCB) domains.
Problem-solving factor of the Independent Living Scales.
2.2. Measures and Procedures
2.2.1. Psychophysical CS task
A two-alternative spatial forced-choice paradigm was used to obtain contrast thresholds. Stimuli were displayed on a cathode ray tube monitor with 90 frames/s viewed at 190 cm. The stimulus display subtended 6 × 6 degrees of visual angle with space-average luminance of approximately 100 cd/m2. Stimuli were horizontal sine-wave gratings at spatial frequencies of 0.5, 1, 4, and 7 cycles/degree (cpd), and a square-wave grating of 21 cpd (VENUS system, NeuroScientific Corp.). Stimuli were presented at durations of 33 and 500 ms. The 33 ms condition was designed to bias processing toward a transient mechanism (phasic response, enhancing CS–LSF), and the 500 ms condition was designed to bias processing toward a sustained mechanism (tonic response, enhancing CS–HSF), respectively (Legge, 1978).
Gratings of the various spatial frequencies were presented randomly to the left or right side of the display, and the participant indicated which side of the display contained the grating by raising the right or left hand. The experimenter recorded the observer’s response. An up-down transformed response rule was applied to track contrast thresholds based on correct identification of the location of the stimulus on 70.7% of the trials. The tracking procedure was interleaved for the five spatial frequencies. Contrast threshold was estimated by taking the mean of 10 contrast reversals (five peaks and five troughs) in the tracking procedure. The reciprocal of contrast threshold for each spatial frequency was calculated to obtain CS. Greater CS indicates better performance. For additional details on this method, see (Zemon et al., 2021).
2.2.2. Cognitive measures
Patients completed The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) (Kern et al., 2008; Nuechterlein et al., 2008) and the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) (Wechsler, 1997) to assess cognitive abilities. T-scores were obtained for each of the MCCB domains (Speed of processing, Attention/vigilance, Working memory, Verbal learning, Visual learning, and Reasoning and problem-solving) and normalized scores were obtained for the Perceptual Organization Index (POI), Processing Speed Index (PSI), and Working Memory Index (WMI) of the WAIS-III.
2.2.3. Functional capacity
Patients completed the Problem-Solving Factor of the Independent Living Scales (ILS-PB) (Loeb, 1996) as a measure of functional capacity. The total ILS has five subscales including memory-orientation, managing money, managing home and transportation, health and safety, and social adjustment. Factor analysis yielded two constructs, a performance-information factor which reflects actual knowledge or skills to perform tasks and the ILS-PB. ILS-PB contains 33 items related to money, home management, health and safety issues, and social adjustment that measure the likelihood of successful independent community living by evaluating cognitive skills, such as abstract reasoning and judgment, required for daily activities (Loeb, 1996). This factor of the ILS was shown to be a highly predictive measure of cognitive determinants of community status, and reliably discriminates among various levels of care in schizophrenia (Revheim and Medalia, 2004a; Revheim and Medalia, 2004b). Standardized scores range from 20 to 63: scores ranging from 20 to 39 suggest maximum (full-time) supervision for daily living, scores ranging from 40 to 49 suggest moderate supervision, and scores ranging from 50 to 63 suggest minimum supervision, or independent living (Loeb, 1996).
2.3. Analyses
CS was converted to log10CS for analyses; logCS to low spatial frequencies and logCS to high spatial frequencies were considered separately in analyses. The arithmetic mean of logCS to 0.5 and 1 cpd gratings for both stimulus durations (33 ms and 500 ms) was taken as an LSF variable (CS–LSF) and the arithmetic mean of logCS to 4, 7, and 21 cpd gratings for both stimulus durations (33 ms and 500 ms) was taken as an HSF variable (CS–HSF). This is based on previous work demonstrating CS–LSF and CS–HSF as two distinct components (Zemon et al., 2021).
Zeroth-order Pearson correlations were computed among CS, individual cognitive domains/indices, and independent living scores.
Mediation analysis conducted in SPSS by means of Andrew Hayes’ PROCESS macro v3.5 tested a mediating relationship with CS as the predictor, cognitive measures as the mediator, and ILS-PB as the outcome measure (Hayes’ Model 4, simple mediation). Models were tested separately for CS–LSF and CS–HSF and separately for each cognitive measure. Fritz and MacKinnon (2007) conducted computer simulations to determine the sample size required for a specified mediation effect. To detect a medium size indirect effect, given an alpha level of .05 with desired power of .8, a sample size of 78 is needed using a percentile bootstrap method; for a large effect, a sample size of 36 is required. With a total sample of 58 we sought to identify large effects that would be meaningful for clinical relevance. Moderated mediation modeling (Hayes’ Model 8) was applied in follow-up analyses to simple mediation modeling. Models were estimated using ordinary least squares (OLS) regression path analyses. Bootstrapping for indirect effects was set at 5,000 samples. For moderated mediation modeling, heteroscedasticity-consistent standard error (HC3) estimation was used, and percentile 95% confidence intervals were used for all models. For the 18 simple mediation models tested, R2 values are reported along with unadjusted p values and adjusted p values based on controlling the false discovery rate as developed by Benjamini and Hochberg (Benjamini and Hochberg, 1995), calculated using an online tool (https://tools.carbocation.com/FDR). This type of adjustment is preferred because of the greatly inflated type II error that is associated with conventional adjustments for the familywise error rate (e.g., Bonferroni correction) (Greenland, 2021), but it is more conservative than the subsequently proposed adaptive false discovery rate controlling procedure (Benjamini et al., 2006). Number of participants varied per model given missing data; this sample was extracted from a larger dataset and thus some participants are missing neurocognitive data.
3. Results
3.1. Univariate descriptive statistics
The means and standard deviations of the variables are reported in Table 1. Age- and gender-corrected T scores are reported for MCCB domains (normative mean = 50; standard deviation = 10). On average, patients showed greatest impairment for processing speed and least impairment for reasoning/problem solving, consistent with previously reported data for patients with schizophrenia (Kern et al., 2011). The WAIS-III index scores indicate that patients scored in the low average range overall, though abilities ranged from extremely low to very superior. On the ILS-PB, patients’ independent living scores ranged from 20 (maximum, full-time supervision) to 58 (minimum supervision or independent living), with the average being maximum to moderate supervision.
3.2. Correlations
Correlation coefficients of contrast sensitivity, ILS-PB, and neuropsychological measures, along with corresponding density plots and bivariate scatterplots are presented in Figure 1. CS–LSF correlated significantly with all cognitive domains with the exception of reasoning/problem solving. CS–HSF correlated significantly with attention/vigilance, processing speed index, and working memory index. Cognitive domains correlated significantly with ILS-PB scores, with the exception of attention/vigilance. CS–LSF and CS–HSF did not correlate significantly with ILS-PB. These relationships informed the mediation models.
Figure 1.
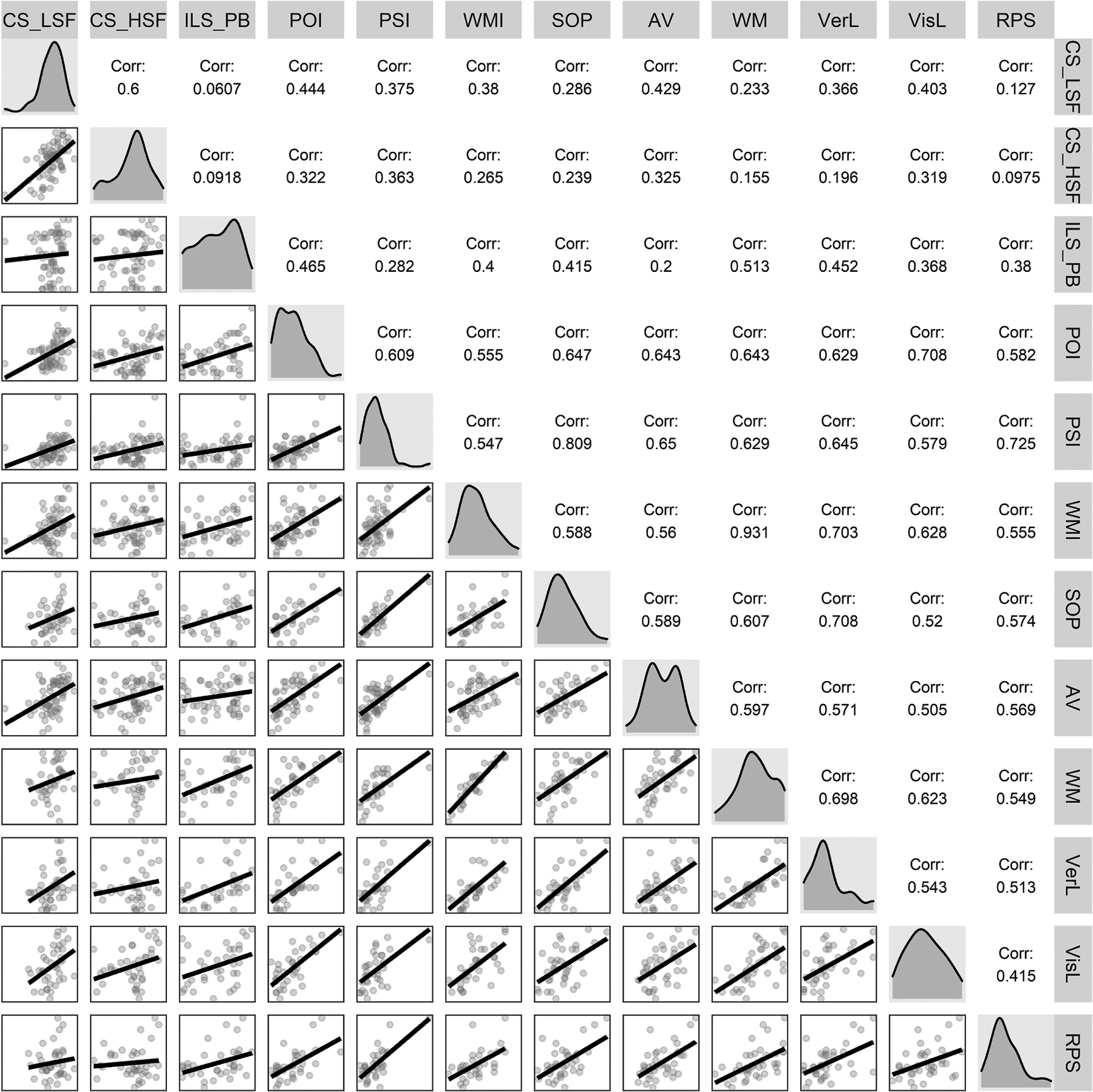
Intercorrelations among contrast sensitivity, independent living, and neuropsychological measures (n per correlation coefficient ranges from 32 to 58 given missing data: CS–LSF ≡ mean log contrast sensitivity at low spatial frequencies (0.5 and 1 cycles/degree); CS–HSF ≡ mean log contrast sensitivity at high spatial frequencies (4, 7, 21 cycles/degree); ILS-PB ≡ Problem Solving Factor of the Independent Living Scales; POI ≡ Perceptual Organization Index; PSI ≡ Processing Speed Index; WMI ≡ Working Memory Index; SOP ≡ Speed of processing; AV ≡ Attention/Vigilance; WM ≡ Working memory; VerL ≡ Verbal learning; VisL ≡ Visual learning; RPS ≡ Reasoning and problem-solving.
3.3. Mediation Modeling
Mediation modeling was used to test the indirect (mediation) effects of each cognitive domain on the relationship between CS and independent living in patients. Figure 2 shows an example conceptual model for visual learning mediating the relationship between CS–LSF and ILS-PB in patients. Table 2 shows the indirect effect (ab, product of path coefficients for the mediating pathway) for all simple mediation models tested, along with each model’s R2 value and Benjamini-Hochberg-adjusted p value (Benjamini and Hochberg, 1995). There were significant mediation effects based on the percentile bootstrapped 95% confidence intervals for all cognitive domains of the WAIS and MCCB, with the exception of Attention/vigilance, on the relation between CS–LSF and ILS-PB. Not all of these mediation models, however, achieved Benjamini-Hochberg-adjusted (B-H) p values below a .05 criterion. The models that achieved significance in terms of both indirect effects and B-H-adjusted p values were perceptual organization and working memory (WAIS and MCCB) given either CS–LSF or CS–HSF predictors, and also verbal learning for just CS–LSF. The c paths (total effects of CS–LSF and CS–HSF on ILS-PB in the absence of a mediator) and c’ paths (direct effects of CS–LSF and CS–HSF on ILS-PB in the presence of a mediator) were not significant for any model, indicating dominance of the mediating pathway. We also tested the models using CS to a single spatial frequency of 0.5 cycles/degree instead of the CS–LSF composite variable, and similar results were obtained with identical conclusions.
Figure 2.
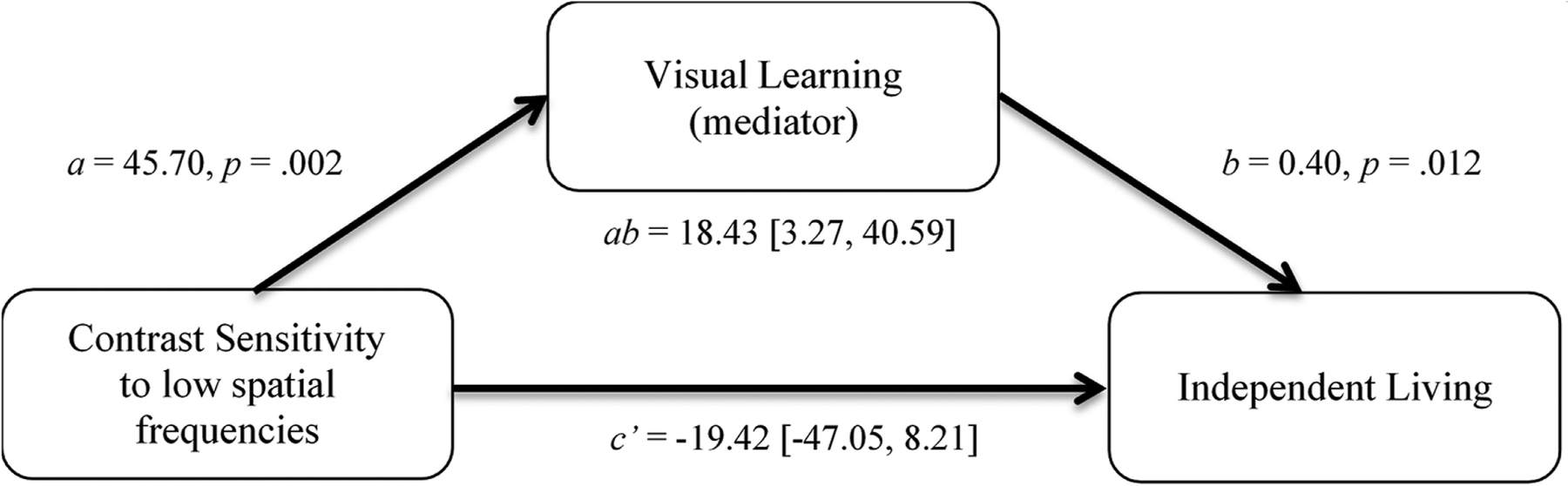
Mediation model showing the indirect effect (ab, product of regression coefficients in the mediating pathway) of contrast sensitivity at low spatial frequencies (0.5 and 1 cycles/degree) on independent living (Problem-Solving Factor of the Independent Living Scales, ILS-PB) through visual learning in patients. There is no significant direct effect (c’) of contrast sensitivity on independent living in the presence of the mediator, indicating dominance of the mediating pathway.
Table 2.
Indirect (mediation) effects of cognitive functions on the relations between CS at low and high spatial frequencies and independent living in patients with schizophrenia and schizoaffective disorder
| Cognitive Measure | n | CS-LSFa Indirect Effect ab [95% CI] | R 2 | p | B-H p value | CS–HSFb Indirect Effect ab [95% CI] | R 2 | p | B-H p value |
|---|---|---|---|---|---|---|---|---|---|
| WAIS-IIIc | |||||||||
| Perceptual Organization Index | 53 | 13.87 [4.91, 29.23] | .235 | .001 | .018 | 4.61 [0.55, 9.58] | .217 | .002 | .013 |
| Processing Speed Index | 55 | 7.38 [0.60, 18.81] | .086 | .097 | .116 | 3.52 [−0.09, 8.24] | .079 | .116 | .131 |
| Working Memory Index | 53 | 12.80 [4.13, 26.66] | .193 | .005 | .018 | 4.22 [0.43, 9.66] | .161 | .013 | .029 |
| MCCBd | |||||||||
| Visual learning | 35 | 18.43 [3.27, 40.59] | .187 | .036 | .059 | 5.24 [−0.10, 13.76] | .177 | .044 | .066 |
| Speed of processing | 35 | 12.91 [1.63, 28.31] | .203 | .027 | .053 | 3.72 [−2.04, 12.69] | .201 | .027 | .049 |
| Attention/vigilance | 55 | 5.97 [−3.30, 18.41] | .041 | .341 | .361 | 2.48 [−1.11, 8.86] | .041 | .341 | .341 |
| Reasoning & problem-solving | 35 | 8.97 [0.55, 20.57] | .161 | .060 | .077 | 3.14 [−2.34, 9.92] | .169 | .052 | .072 |
| Verbal learning | 35 | 19.07 [7.93, 34.73] | .264 | .007 | .021 | 4.61 [−0.14, 11.62] | .242 | .012 | .031 |
| Working memory | 35 | 18.31 [5.56, 38.44] | .321 | .002 | .019 | 5.73 [0.92, 12.57] | .312 | .003 | .014 |
The outcome measure was the Problem-solving factor of the Independent Living Scales (ILS-PB).
mean log contrast sensitivity at low spatial frequencies (0.5 and 1 cycles/degree), CS–HSF.
mean log contrast sensitivity at high spatial frequencies (4, 7, and 21 cycles/degree), CS–HSF.
Wechsler Adult Intelligence Scale-III indices.
MATRICS Consensus Cognitive Battery (MCCB) domains.
ab ≡ indirect (mediation) effect.
95% CI ≡ 95% confidence interval (percentile) based on 5,000 bootstrapped samples.
B-H p value ≡ Benjamini-Hochberg-adjusted p value based on false discovery rate control.
R2, p (unadjusted), and B-H p value for mediation model.
In addition, we explored moderated mediation models with CS–HSF as the predictor, ILS-PB as the outcome, and CS–LSF as a moderator of paths a and c’, with perceptual organization and both working memory measures (the cognitive measures that yielded significant indirect, mediation effects on ILS-PB in the simple mediation models) as mediating variables. Only the model with perceptual organization as a mediator (Figure 3a) yielded a significant conditional indirect effect of CS–HSF on ILS-PB (index of moderation = 27.52 95% CI [7.42, 57.33]). The indirect effect of the relation between CS–HSF and ILS-PB through perceptual organization changes from negative to positive as CS–LSF increases; the slope of this function is the index of moderation (Figure 3b). The indirect effect was significant when CS–LSF was 1.76 (−1 SD below the mean value of 1.95). Pairwise comparisons among the three levels of indirect effect conditioned on the three levels of CS–LSF were all significant based on 95% confidence intervals. In this model, 32% of the variance in perceptual organization is explained by CS–LSF, CS–HSF, and the interaction CS–LSF × CS–HSF (F(3,49) = 5.38, p = .003). The overall model explains 25.0% of the variance in ILS-PB (F(4,48) = 5.68, p < .001).
Figure 3.
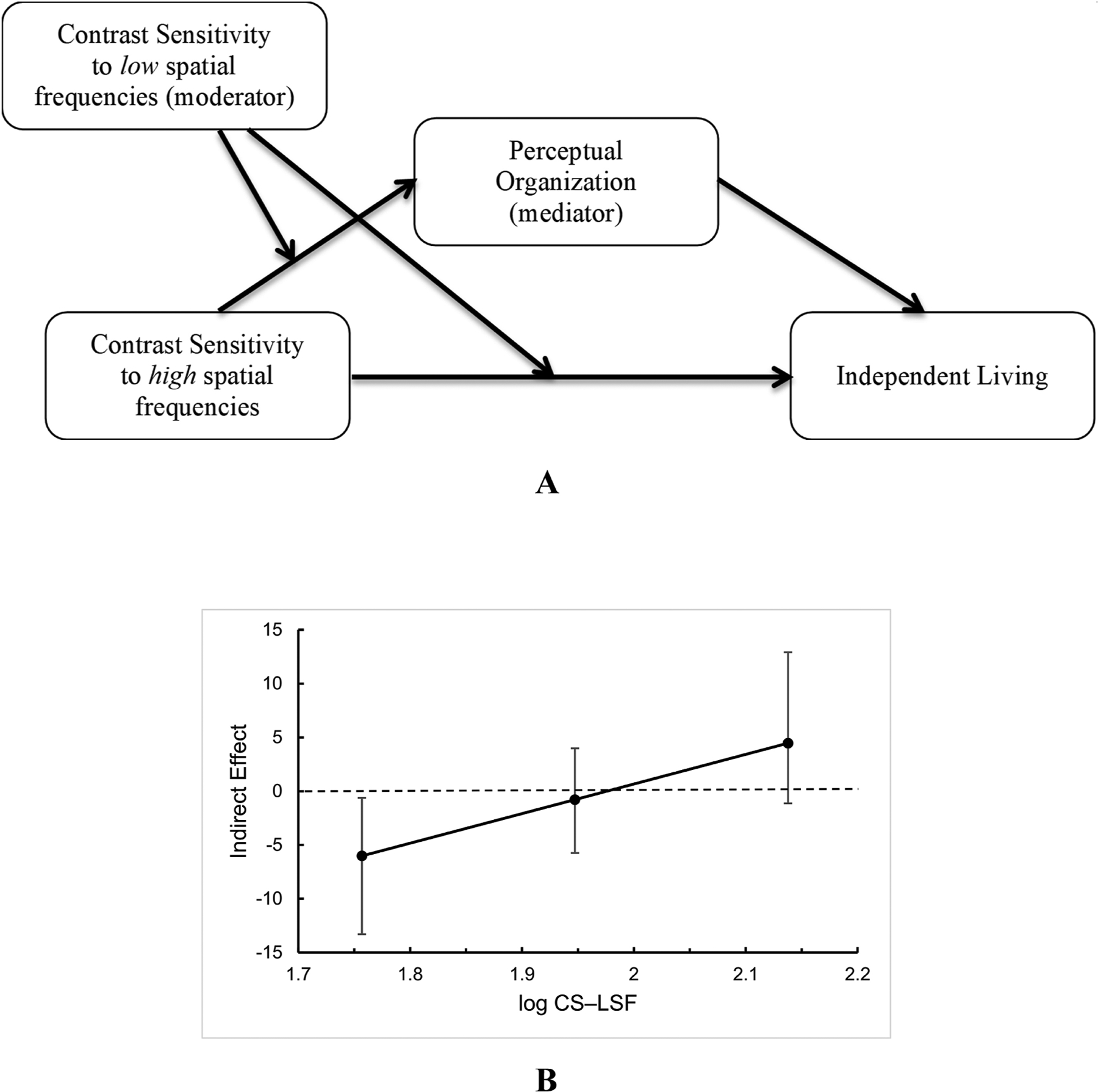
a) Moderated-mediation model showing a significant conditional indirect effect of CS–HSF on ILS-PB through the mediator, perceptual organization. As CS–LSF increases, there is an increasing indirect effect of the relation between CS–HSF and ILS-PB when the moderator CS–LSF is low. b) Plot of indirect effect of CS–HSF on ILS-PB through the mediator, perceptual organization, as a function of the moderator, CS–LSF.
4. Discussion
A number of basic visual deficits in schizophrenia have been linked to higher-level problems in cognition and functional outcomes (Brenner et al., 2002; Butler et al., 2009; Butler et al., 2005; Dias et al., 2011; Green et al., 2012; Martinez et al., 2012; Rassovsky et al., 2011; Sehatpour et al., 2010; Sergi et al., 2006). Contrast sensitivity deficits are well documented in this population (Butler and Javitt, 2005; Fernandes et al., 2019; Keri et al., 2002; Silverstein, 2016; Slaghuis, 1998; Zemon et al., 2021) and have been shown to be related positively to higher level functions such as facial processing/emotion recognition (Butler et al., 2009; Kim et al., 2015; Laprevote et al., 2010; Lee et al., 2011; McBain et al., 2010; Silverstein et al., 2010; Silverstein et al., 2014; Vakhrusheva et al., 2014), reading (Revheim et al., 2006a), visual learning (Dias et al., 2011), and object recognition (Calderone et al., 2013a; Laprevote et al., 2013).
The current study is the first to our knowledge to demonstrate that neurocognition mediates the relationship between CS and a measure of functional outcome in people with schizophrenia. Specifically, CS to low spatial frequencies (CS–LSF) was related to a measure of independent living through several neurocognitive domains tested. These findings support an effect of early visual processing on higher-level cognitive and functional outcomes in schizophrenia.
The indirect effects from CS to independent living skills through various cognitive abilities were stronger for CS–LSF compared to CS–HSF. Only the two measures of working memory and perceptual organization reached significance as mediators between CS–HSF and independent living, though other cognitive mediators (e.g., visual learning) demonstrated marginal effects. Previous studies showed CS deficits to both LSFs and HSFs in schizophrenia (Fernandes et al., 2019; Keri et al., 2002; Slaghuis, 1998; Zemon et al., 2021), however, the current results indicate that LSF information might be more critical for the cognitive functions investigated here.
The stronger mediating relationships involving CS–LSF compared to CS–HSF in the current study may be due, in part, to a need to process visual information rapidly during a number of cognitive tests. One theory of object recognition proposes that LSF information in an object is processed earlier than HSF information, and it reflects a ‘frame and fill’ process (Chen et al., 2006) consistent with a model proposed by Bar (Bar, 2003). CS–LSF was also found to moderate the mediating effect of perceptual organization in the relation between CS–HSF and ILS-PB. Thus, it appears that CS–LSF is the dominant visual factor and its explanatory power covers much of that explained by CS–HSF. The finding of a negative mediating effect of CS–HSF on ILS-PB through perceptual organization when CS–LSF is low, but a positive mediating effect when CS–LSF is high, might indicate that high spatial frequency information interferes with the relationship between perceptual organization and capacity for independent living when there is not sufficient low spatial frequency information to provide an adequate ‘frame’ for global perception. When this ‘frame’ is established with strong low spatial frequency information, the positive effect of high spatial frequency information might reflect details (‘fill’) added to the ‘frame’ with commensurate enhanced perceptual organization.
Perceptual organization and memory-related cognitive domains (i.e., working memory, verbal learning) demonstrated the strongest mediating effects in this study. On the MCCB, the memory-related domains include tests such as word recall and letter-number strings that need to be reordered. The finding of significance for a nonvisual task (i.e., verbal learning) may be surprising, however, visual processing involves about 50% of the neocortex (Van Essen, 2004) and deficits in visual processing can alter the dynamics of cortical processing globally (e.g., (Musacchia and Schroeder, 2009; Schroeder et al., 2008)). Specifically, changes in contrast of visual stimuli alter the temporal integration and gain control properties of visual cortical neurons and consequently influence those properties of neurons in higher cortical areas (Butler et al., 2008; Zemon and Gordon, 2006). The current findings are consistent with research suggesting that perceptual organization and working memory deficits are core features of schizophrenia (Green et al., 2009; Lee and Park, 2005; Silverstein, 2016; Silverstein and Keane, 2011) and may underlie other cognitive deficits (Goldman-Rakic, 1994; Javitt, 2009; Kim et al., 2006; Silverstein, 2016).
Cognitive remediation has been shown to improve cognition and functional outcomes in people with schizophrenia (Biagianti et al., 2016; Medalia et al., 2019; Revell et al., 2015; Wykes et al., 2011), and interventions that target low-level visual processes may also have benefits (Demmin et al., 2019). There is preliminary evidence that visual remediation improves CS and spatial frequency processing (Butler et al., 2017; Silverstein et al., 2020). Other studies have shown that in patients with schizophrenia visual remediation improved visual motion discrimination (Norton et al., 2011), visual backward masking training improved performance on a visuospatial memory test (Surti and Wexler, 2012), and pairing visual remediation with cognitive remediation improved cognition and psychosocial functioning (Contreras et al., 2017). The pathways from early stages of visual processing to functional outcomes discovered in the current study provide support for further investigations into the effects of visual remediation interventions on these pathways.
Limitations of this study included a modest sample size with greater number of males than females. Sample size was larger for the WAIS measures than for the MCCB measures with the exception of the Attention/vigilance domain of the MCCB in which case it was the same size. Notably, the Attention/vigilance domain yielded the highest p values in the mediation modeling with both LSF and HSF CS measures. It should be noted, however, that small samples might yield spuriously large effect sizes (Button et al., 2013), and therefore, additional studies are required to replicate these findings. The sample consisted of patients with chronic illness, some of whom are receiving typical antipsychotics. Longer illness duration and typical antipsychotics have been associated with poorer CS (Almeida et al., 2019; Fernandes et al., 2019). Studies with a larger sample are recommended to investigate more complex models (e.g., parallel mediation) with greater power (Fritz and Mackinnon, 2007; Hayes, 2013) and yield higher precision of parameter estimates (i.e., narrower confidence intervals).
Future studies should consider other mediating/moderating factors in these models, such as negative symptoms and social cognition (Green et al., 2012; Rassovsky et al., 2011; Sergi et al., 2006), in the relationship between CS and functional outcomes in schizophrenia. This study chose the ILS-PB as a measure of functional capacity given that it relies strongly on cognitive skills (Loeb 1966; Revheim et al., 2006). Additional work should include another, frequently used measure of functional capacity, the University of California, San Diego (UCSD) Performance-Based Skills Assessment (UPSA) (Patterson et al., 2001), as well as measures of social and role functioning.
In conclusion, this study provides evidence that early visual processing deficits may be a precursor to cognitive and functional deficits in schizophrenia. These results are encouraging for the development of novel treatments for schizophrenia, such as remediation of contrast detection.
Highlights.
Patients with schizophrenia (sz) show deficits in visual contrast detection.
First study to assess pathways from contrast sensitivity to functioning in sz.
Contrast sensitivity impacts cognition and functional outcomes in sz.
Novel treatments such as remediation of contrast detection may be helpful.
Acknowledgements
The authors wish to thank all study participants. This work was supported by the National Institutes of Mental Health (Grant numbers MH084848, MH083364 to PDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
None.
References
- Almeida NL, Fernandes TP, Lima EH, Sales HF, Santos NA, 2019. Combined influence of illness duration and medication type on visual sensitivity in schizophrenia. Braz J Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, 2003. A cortical mechanism for triggering top-down facilitation in visual object recognition. Journal of Cognitive Neuroscience 15 (4), 600–609. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling The False Discovery Rate - A Practical And Powerful Approach To Multiple Testing. 57, 289–300. [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D, 2006. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93 (3), 491–507. [Google Scholar]
- Biagianti B, Fisher M, Neilands TB, Loewy R, Vinogradov S, 2016. Engagement with the auditory processing system during targeted auditory cognitive training mediates changes in cognitive outcomes in individuals with schizophrenia. Neuropsychology 30 (8), 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer BG, Ganz L, 1977. Temporal studies with flashed gratings: inferences about human transient and sustained channels. Vision Research 17 (7), 861–865. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Hoe M, Long J, Green MF, 2007. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophrenia Bulletin 33 (5), 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner CA, Lysaker PH, Wilt MA, O’Donnell BF, 2002. Visual processing and neuropsychological function in schizophrenia and schizoaffective disorder. Psychiatry Research 111 (2–3), 125–136. [DOI] [PubMed] [Google Scholar]
- Butler PD, Abeles IY, Weiskopf NG, Tambini A, Jalbrzikowski M, Legatt ME, Zemon V, Loughead J, Gur RC, Javitt DC, 2009. Sensory contributions to impaired emotion processing in schizophrenia. Schizophrenia Bulletin 35 (6), 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Javitt DC, 2005. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry 18 (2), 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC, 2007. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain 130 (Pt 2), 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC, 2001. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry 158 (7), 1126–1133. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC, 2008. Visual perception and its impairment in schizophrenia. Biol Psychiatry 64 (1), 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Thompson JL, Seitz AR, Deveau J, Silverstein SM, 2017. Visual perceptual remediation for individuals with schizophrenia: Rationale, method, and three case studies. Psychiatr Rehabil J 40 (1), 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC, 2005. Early-stage visual processing and cortical amplification deficits in schizophrenia. Archives of General Psychiatry 62 (5), 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience 14 (5), 365–376. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Dobkins K, McGovern J, Shafer K, 2013. Schizophrenia spectrum participants have reduced visual contrast sensitivity to chromatic (red/green) and luminance (light/dark) stimuli: new insights into information processing, visual channel function, and antipsychotic effects. Front Psychol 4, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone DJ, Hoptman MJ, Martinez A, Nair-Collins S, Mauro CJ, Bar M, Javitt DC, Butler PD, 2013a. Contributions of low and high spatial frequency processing to impaired object recognition circuitry in schizophrenia. Cerebral Cortex 23 (8), 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone DJ, Martinez A, Zemon V, Hoptman MJ, Hu G, Watkins JE, Javitt DC, Butler PD, 2013b. Comparison of psychophysical, electrophysiological, and fMRI assessment of visual contrast responses in patients with schizophrenia. Neuroimage 67, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-M, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE, 2006. Functional Anatomy and Interaction of Fast and Slow Visual Pathways in Macaque Monkeys. Cerebral Cortex 17 (7), 1561–1569. [DOI] [PubMed] [Google Scholar]
- Cimmer C, Szendi I, Csifcsak G, Szekeres G, Ambrus Kovacs Z, Somogyi I, Benedek G, Janka Z, Keri S, 2006. Abnormal neurological signs, visual contrast sensitivity, and the deficit syndrome of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 30 (7), 1225–1230. [DOI] [PubMed] [Google Scholar]
- Contreras NA, Tan EJ, Lee SJ, Castle DJ, Rossell SL, 2017. Using visual processing training to enhance standard cognitive remediation outcomes in schizophrenia: A pilot study. Psychiatry Research, Advance online publication. doi: 10.1016/j.psychres.2017.1009.1031. [DOI] [PubMed] [Google Scholar]
- Demmin DL, Fradkin SI, Silverstein SM, 2019. Remediation of Visual Processing Impairments in Schizophrenia: Where We Are and Where We Need to Be. Current Behavioral Neuroscience Reports 6 (2), 13–20. [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC, 2011. Early sensory contributions to contextual encoding deficits in schizophrenia. Archives of General Psychiatry 68 (7), 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN, 2003. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13 (11), 1124–1138. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC, 1991. Distributed hierarchical processing in the primate cerebral cortex. Cerebral cortex (New York, N.Y. : 1991) 1 (1), 1–47. [DOI] [PubMed] [Google Scholar]
- Fernandes TP, Shaqiri A, Brand A, Nogueira RL, Herzog MH, Roinishvili M, Santos NA, Chkonia E, 2019. Schizophrenia patients using atypical medication perform better in visual tasks than patients using typical medication. Psychiatry Research 275, 31–38. [DOI] [PubMed] [Google Scholar]
- Fritz MS, Mackinnon DP, 2007. Required sample size to detect the mediated effect. Psychological Science 18 (3), 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, 1994. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences 6 (4), 348–357. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V, 2009. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophrenia Bulletin 35 (1), 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK, 2012. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry 69 (12), 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK, 2004. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research 72 (1), 41–51. [DOI] [PubMed] [Google Scholar]
- Greenland S, 2021. Analysis goals, error-cost sensitivity, and analysis hacking: Essential considerations in hypothesis testing and multiple comparisons. Paediatric and Perinatal Epidemiology 35 (1), 8–23. [DOI] [PubMed] [Google Scholar]
- Hayes AF, 2013. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. The Guilford Press, New York, NY. [Google Scholar]
- Hollingshead AB, 1975. Four Factor Index of Social Status, New Haven, CT. [Google Scholar]
- Hyman S, Arana G, Rosenbaum J, 1991. Antidepressant drugs. Handbook of Psychiatric Drug Therapy, 43–92. [Google Scholar]
- Javitt DC, 2009. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol 5, 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibson MD, Tandon R, 1998. New atypical antipsychotic medications. Journal of Psychiatric Research 32 (3–4), 215–228. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 13 (2), 261–276. [DOI] [PubMed] [Google Scholar]
- Keane BP, Erlikhman G, Kastner S, Paterno D, Silverstein SM, 2014. Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency? Neuropsychologia 65, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Antal A, Szekeres G, Benedek G, Janka Z, 2002. Spatiotemporal visual processing in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences 14 (2), 190–196. [DOI] [PubMed] [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR, 2011. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophrenia Research 126 (1–3), 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Mintz J, Seidman LJ, Stover E, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. American Journal of Psychiatry 165 (2), 214–220. [DOI] [PubMed] [Google Scholar]
- Kim DW, Shim M, Song MJ, Im CH, Lee SH, 2015. Early visual processing deficits in patients with schizophrenia during spatial frequency-dependent facial affect processing. Schizophrenia Research 161 (2–3), 314–321. [DOI] [PubMed] [Google Scholar]
- Kim J, Park S, Shin YW, Jin Lee K, Kwon JS, 2006. Self-initiated encoding facilitates object working memory in schizophrenia: implications for the etiology of working memory deficit. Schizophrenia Research 82 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- Laprevote V, Oliva A, Delerue C, Thomas P, Boucart M, 2010. Patients with schizophrenia are biased toward low spatial frequency to decode facial expression at a glance. Neuropsychologia 48 (14), 4164–4168. [DOI] [PubMed] [Google Scholar]
- Laprevote V, Oliva A, Ternois AS, Schwan R, Thomas P, Boucart M, 2013. Low Spatial Frequency Bias in Schizophrenia is Not Face Specific: When the Integration of Coarse and Fine Information Fails. Frontiers in Psychology 4, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Gosselin F, Wynn JK, Green MF, 2011. How do schizophrenia patients use visual information to decode facial emotion? Schizophrenia Bulletin 37 (5), 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S, 2005. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114 (4), 599–611. [DOI] [PubMed] [Google Scholar]
- Legge GE, 1978. Sustained and transient mechanisms in human vision: temporal and spatial properties. Vision Research 18 (1), 69–81. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR, 2005. What does the PANSS mean? Schizophrenia Research 79 (2–3), 231–238. [DOI] [PubMed] [Google Scholar]
- Loeb PA, 1996. Independent living scales (ILS) manual. Psychological Corp., San Antonio. [Google Scholar]
- Martinez A, Revheim N, Butler PD, Guilfoyle DN, Dias EC, Javitt DC, 2012. Impaired magnocellular/dorsal stream activation predicts impaired reading ability in schizophrenia. Neuroimage Clin 2, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain R, Norton D, Chen Y, 2010. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophrenia Research 122 (1–3), 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Saperstein AM, Qian M, Javitt DC, 2019. Impact of baseline early auditory processing on response to cognitive remediation for schizophrenia. Schizophr Res 208, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Byrne CE, Maunsell JH, 1991a. Does primate motion perception depend on the magnocellular pathway? Journal of Neuroscience 11 (11), 3422–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Eskin TA, 1986. Spatio-temporal vision of macaques with severe loss of P beta retinal ganglion cells. Vision Research 26 (11), 1751–1761. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Katz LM, Maunsell JH, 1991b. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. Journal of Neuroscience 11 (4), 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchia G, Schroeder CE, 2009. Neuronal mechanisms, response dynamics and perceptual functions of multisensory interactions in auditory cortex. Hear Res 258 (1–2), 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton DJ, McBain RK, Ongur D, Chen Y, 2011. Perceptual training strongly improves visual motion perception in schizophrenia. Brain and Cognition 77 (2), 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry 165 (2), 203–213. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Subotnik KL, Green MF, Ventura J, Asarnow RF, Gitlin MJ, Yee CM, Gretchen-Doorly D, Mintz J, 2011. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophrenia Bulletin 37 Suppl 2, S33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Bismark A, Hetrick WP, Bodkins M, Vohs JL, Shekhar A, 2006. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr Res 86 (1–3), 89–98. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV, 2001. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin 27 (2), 235–245. [DOI] [PubMed] [Google Scholar]
- Peuskens J, Link CG, 1997. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatrica Scandinavica 96 (4), 265–273. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Horan WP, Lee J, Sergi MJ, Green MF, 2011. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med 41 (3), 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, Drake RJ, 2015. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res 168 (1–2), 213–222. [DOI] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC, 2006a. Reading impairment and visual processing deficits in schizophrenia. Schizophrenia Research 87 (1–3), 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Corcoran CM, Dias E, Hellmann E, Martinez A, Butler PD, Lehrfeld JM, DiCostanzo J, Albert J, Javitt DC, 2014. Reading deficits in schizophrenia and individuals at high clinical risk: relationship to sensory function, course of illness, and psychosocial outcome. American Journal of Psychiatry 171 (9), 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revheim N, Medalia A, 2004a. The independent living scales as a measure of functional outcome for schizophrenia. Psychiatric Services 55 (9), 1052–1054. [DOI] [PubMed] [Google Scholar]
- Revheim N, Medalia A, 2004b. Verbal memory, problem-solving skills and community status in schizophrenia. Schizophrenia Research 68 (2), 149–158. [DOI] [PubMed] [Google Scholar]
- Revheim N, Schechter I, Kim D, Silipo G, Allingham B, Butler P, Javitt DC, 2006b. Neurocognitive and symptom correlates of daily problem-solving skills in schizophrenia. Schizophr Res 83 (2–3), 237–245. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lange F.P.d., 2016. Early Visual Cortex as a Multiscale Cognitive Blackboard. Annual Review of Vision Science 2 (1), 131–151. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A, 2008. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci 12 (3), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC, 2010. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Archives of General Psychiatry 67 (8), 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF, 2006. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. American Journal of Psychiatry 163 (3), 448–454. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, 2016. Visual Perception Disturbances in Schizophrenia: A Unified Model. Nebr Symp Motiv 63, 77–132. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, All SD, Kasi R, Berten S, Essex B, Lathrop KL, Little DM, 2010. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychological Medicine 40 (7), 1159–1169. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, 2011. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull 37 (4), 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, Papathomas TV, Lathrop KL, Kourtev H, Feigenson K, Roche MW, Wang Y, Mikkilineni D, Paterno D, 2014. Processing of spatial-frequency altered faces in schizophrenia: effects of illness phase and duration. PloS One 9 (12), e114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Seitz AR, Ahmed AO, Thompson JL, Zemon V, Gara M, Butler PD, 2020. Development and Evaluation of a Visual Remediation Intervention for People with Schizophrenia. J Psychiatr Brain Sci 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaghuis WL, 1998. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. Journal of Abnormal Psychology 107 (1), 49–62. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, 2004. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res 156 (2), 196–211. [DOI] [PubMed] [Google Scholar]
- Surti TS, Wexler BE, 2012. A pilot and feasibility study of computer-based training for visual processing deficits in schizophrenia. Schizophrenia Research 142 (1–3), 248–249. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva J, Zemon V, Bar M, Weiskopf NG, Tremeau F, Petkova E, Su Z, Abeles IY, Butler PD, 2014. Forming first impressions of others in schizophrenia: impairments in fast processing and in use of spatial frequency information. Schizophrenia Research 160 (1–3), 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, 2004. Organization of visual areas in macaque and human cerebral cortex, The visual neurosciences. MIT Press, Cambridge, Massachusetts, pp. 507–521. [Google Scholar]
- Wechsler D, 1997. WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio, TX. [Google Scholar]
- Woods SW, 2003. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry 64 (6), 663–667. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P, 2011. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 168 (5), 472–485. [DOI] [PubMed] [Google Scholar]
- Zemon V, Gordon J, 2006. Luminance-contrast mechanisms in humans: visual evoked potentials and a nonlinear model. Vision Research 46 (24), 4163–4180. [DOI] [PubMed] [Google Scholar]
- Zemon V, Herrera S, Gordon J, Revheim N, Silipo G, Butler PD, 2021. Contrast sensitivity deficits in schizophrenia: A psychophysical investigation. European Journal of Neuroscience 53 (4), 1155–1170. [DOI] [PubMed] [Google Scholar]


