Abstract
Background.
An efficacious pharmacotherapy for cannabis use disorder (CUD) has yet to be established. This study preliminarily evaluated the safety and efficacy of varenicline for CUD in a proof-of-concept clinical trial.
Methods.
Participants in this 6-week randomized, placebo-controlled pilot trial received either varenicline (n=35) or placebo (n=37), added to a brief motivational enhancement therapy intervention. Outcomes included cannabis withdrawal, cannabis abstinence, urine cannabinoid levels, percent cannabis use days, and cannabis sessions per day.
Results.
Both treatment groups noted significant decreases in self-reported cannabis withdrawal, percentage of days used, and use sessions per day during treatment compared to baseline. While this pilot trial was not powered to detect statistically significant between-group differences, participants randomized to varenicline evidenced numerically greater rates of self-reported abstinence at the final study visit [Week 6 intent-to-treat (ITT): Varenicline: 17.1% vs. Placebo: 5.4%; RR=3.2 (95% CI: 0.7,14.7)]. End-of-treatment urine creatinine corrected cannabinoid levels were numerically lower in the varenicline group and higher in the placebo group compared to baseline [Change from baseline: Varenicline −1.7 ng/mg (95% CI: −4.1,0.8) vs. Placebo: 1.9 ng/mg (95% CI: −0.4,4.3); Δ=3.5 (95% CI: 0.1,6.9)]. Adverse events related to study treatment did not reveal new safety signals.
Conclusions.
Findings support the feasibility of conducting clinical trials of varenicline as a candidate pharmacotherapy for CUD, and indicate that a full-scale efficacy trial, powered based on effect sizes and variability yielded in this study, is warranted.
Keywords: cannabis, marijuana, treatment, pharmacotherapy, varenicline, addiction
1. Introduction
The prevalence of past year cannabis use in the United States more than doubled between 2001 and 2013, from 4.1% to 9.5% of the adult population (Hasin et al., 2015). In 2019, nearly one million Americans received treatment for cannabis related problems (SAMHSA, 2020). Although a high demand for effective interventions exists, few specific treatments have been developed for cannabis use disorder (CUD). Further, current evidence-based treatments have limited efficacy, with few individuals achieving abstinence (Compton & Pringle, 2004; Kadden et al., 2007; Nordstrom & Levin, 2007; Sherman & McRae-Clark, 2016). As such, there is significant interest in exploring new strategies to improve treatment outcomes. In particular, the role that medications may play in the treatment of cannabis use disorder (CUD) has become an active area of research (Vandrey & Haney, 2009).
Varenicline, a selective nicotinic acetylcholine receptor (nACHr) partial agonist of the α4β2 subtype and a full agonist of the α7 subtype (Mihalak et al., 2006), is arguably the most effective first line pharmacotherapy for promoting tobacco cessation (Aubin et al., 2008; Eisenberg et al., 2008; Gonzales et al., 2006; Jorenby et al., 2006; Nides et al., 2006). Given its partial agonist profile, varenicline likely exerts its effects via dual mechanisms. First, it partially activates α4β2 receptors in the ventral tegmental area (VTA), resulting in increased dopamine levels and a reduction in withdrawal symptoms and craving (Rollema et al., 2007; Reperant et al., 2010) as well as striatal dopamine receptor binding (Crunelle et al., 2009; Crunelle et al., 2011). Further, through its antagonist properties, varenicline also blocks the ability of nicotine to further stimulate dopamine release, thereby attenuating nicotine’s reinforcing effects during smoking (Coe et al., 2005). Varenicline also reliably reduces reactivity to smoking-related cues among tobacco users, via its effects on reward and cognitive circuitry (Brandon et al., 2011; Franklin et al., 2011; Hartwell et al., 2013).
Given that the mesolimbic dopamine system is a key element in the brain reward pathways and that increased dopaminergic transmission in these pathways is important for the reinforcing effects of multiple drugs of abuse (Taylor & Robbins, 1984; Koob & LeMoal, 1997; Tanda et al, 1997; Volkow et al., 2016), varenicline has been identified as a prime candidate medication for evaluation in other substance use disorders (Crunelle et al., 2010). Positive findings have been reported in regard to varenicline reducing alcohol cue reactivity (Schacht et al., 2014), reducing alcohol self-administration among heavy drinking smokers (McKee et al., 2009), improving drinking outcomes in preliminary clinical trials (Fucito et al., 2011; Mitchell et al., 2012), and reducing alcohol use in a large, placebo-controlled trial (Litten et al., 2013). Two recent meta-analyses of varenicline’s impact on alcohol consumption have had mixed results, with one finding reduction in alcohol consumption but not heavy drinking days (Oon-arom et al., 2019) and another reporting a reduction in alcohol craving but not in drinking-related outcomes (Gandhi et al., 2020). A laboratory study by Herrmann et al. (2018) demonstrated that varenicline reduced tobacco use, craving, and negative affect in tobacco/cannabis co-users, though it had no effect on cannabis relapse. However, a case series reported reductions in amount of enjoyment of cannabis and self-report of cannabis use among cannabis- and nicotine-dependent individuals receiving varenicline (Newcombe et al., 2015). In addition, a small pilot trial reported reduced cannabis craving, cannabis use, and tobacco use when varenicline was added to standard care among a sample of individuals with opioid use disorder (Adams et al., 2018).
Importantly, α4β2 nACHRs in corticothalamic circuitry, which are saturated with varenicline dosing (Lotfipour et al., 2012), have also been heavily implicated in prefrontally mediated attentional and inhibitory control (Sarter & Paolone, 2011) and working memory (Vandesquille et al., 2013). In addition, α7 nACHRs are involved in hippocampal-dependent memory function (Levin et al., 2006). nACHr agonists improve frontally mediated executive function among nicotine-naïve animals (Levin et al., 2006) and humans (Froeliger et al., 2009). Varenicline has been shown to improve multiple forms of attention (Rhodes et al., 2012) including inhibitory control (Austin et al., 2014) among treatment-seeking tobacco users and in nicotine-naïve animal models (Rollema et al., 2009). Given that cannabinoid agonists inhibit cholinergic transmission (Varvel et al, 2001), the cholinergic system in particular may play an important role in cannabis-induced cognitive dysfunction. As such, varenicline, as a cholinergic modulator in prefrontal circuitry, is a promising candidate to ameliorate frontal-executive dysfunction (Sofuoglu et al., 2010).
Gonzales et al (2006) and Jorenby et al (2006) found that varenicline was superior to placebo in reducing tobacco withdrawal symptoms. Specifically, treatment with varenicline, compared to placebo, was associated with less withdrawal-related negative affect; a meta-analysis found that negative affect during tobacco cessation attempts modulates treatment efficacy (Foulds et al., 2013). These findings appear highly relevant to CUD, as cannabis withdrawal has been identified as a potentially high-yield behavioral target for CUD pharmacotherapy development (Brezing and Levin, 2018).
Although a strong theoretical framework supports the utility of varenicline for CUD, to date, varenicline has not been evaluated in a randomized clinical trial for treatment in this population. As such, the purpose of this study was to conduct a proof-of-concept pilot trial to preliminarily assess safety and initial efficacy of varenicline in cannabis using individuals.
2. Materials and methods
2.1. Design
This study was a 6-week, double-blind, 1:1, parallel group, placebo-controlled trial (NCT02892110) of varenicline (up to 2mg/day). Pre- and post-functional magnetic resonance imaging (fMRI) was completed on a subset of participants and will be reported separately. Participants were primarily recruited through media and internet advertisements. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received approval from the Medical University of South Carolina Institutional Review Board. All participants gave written, informed consent prior to study participation.
2.2. Participants
A total of 72 participants meeting DSM-5 criteria for CUD, aged 18 to 65 and using cannabis at least 3 days per week, were recruited from February 2017 to November 2018. Additional inclusion criteria included consent to random assignment, ability to read and provide informed consent, having a body mass index between 18 and 35 kg/m2, and interest in CUD treatment. Exclusion criteria included women who were pregnant, nursing, or planning to become pregnant during the course of the study; having a lifetime history of DSM-5 bipolar I or II disorder, schizophrenia or other psychotic disorder; suicidal ideation or behavior within the past six months; concomitant use of psychotropic medications, with the exception of stable doses (defined as no dosing adjustments in the past two months) of non-MAO-I antidepressants, non-benzodiazepine anxiolytics, and attention-deficit/hyperactivity disorder medications; contraindication to fMRI for individuals completing those procedures; and meeting criteria for any moderate or severe non-cannabis substance use disorder within the past 60 days with the exception of tobacco use disorder.
2.3. Assessments
The MINI International Neuropsychiatric Interview (MINI) was used to assess psychiatric and substance use diagnoses (Sheehan et al, 1998). A medical history, physical exam, laboratory assessment (comprehensive metabolic panel, complete blood count, and urine pregnancy test if indicated) was also completed. Self-report cannabis use for the 90 days prior to study entry was estimated using the Time-Line Follow-Back (TLFB) (Sobell et al., 1992). Cannabis use was recorded as times or “sessions” used per day, with each session being defined as cannabis use separated by an hour of no cannabis. We used previously utilized methods to standardize for different types of cannabis use (joints, bowls, blunts, etc.), as well as determine overall amount used per day (McRae-Clark et al., 2015; Gray et al., 2017). Tobacco, alcohol, and other substance use was also assessed. Cannabis withdrawal symptoms were assessed at screening and weekly using the Cannabis Withdrawal Scale (CWS; Allsop et al., 2011). The Columbia-Suicide Severity Rating Scale (Posner et al., 2011) was also completed weekly. Urine drug tests were administered twice weekly to qualitatively screen for the presence of opioids, cocaine, amphetamines, and benzodiazepines. In addition, a semi-quantitative urine cannabinoid tests [11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH)] was performed using the AXSSYM® system from Abbott Laboratories with a minimum detection cut-off value of 30.00 ng/ml (Abbott AXSYM® System package insert). Urine creatinine was also obtained, as creatinine normalization has been proposed as a method to differentiate new cannabis use from residual drug excretion (CN-THCCOOH; Huestis and Cone, 1998; Schwilke et al, 2011).
Adverse events were evaluated weekly by a clinician by asking the participant open-ended questions such as “Have you had any problems or side effects since we saw you last (such as cold, flu, nausea, headache, or any other problem)?” The type of adverse event, severity of adverse event, relationship to study medication, action taken, and outcome were recorded. Adverse events were coded on a weekly basis using Medical Dictionary for Regulatory Activities (MedDRA) rules.
Medication adherence was measured using smartphone video recording (Tomko et al., 2019). Participants recorded themselves taking their morning and evening medication doses with a smartphone and then submitted these videos to research staff via a REDCap survey. Validity of the REDCap data was verified by concurrent data collection with MEMS® caps (medication bottle caps containing an embedded computer chip which digitally records when pill bottles are opened) and participant self-report.
2.4. Interventions
Matching varenicline and placebo tablets were provided by Pfizer, at the standard recommended dose approved for tobacco cessation of 0.5mg daily for three days, then 0.5mg twice daily for four days, and then 1mg twice daily for the remainder of the six-week treatment period. When necessary, medication dose was reduced to 0.5 mg twice daily for tolerability.
All participants received brief motivational enhancement therapy consisting of three individual sessions. The first session occurred during the first week of medication administration, and the second session occurred approximately one week later. Sessions incorporated use of a personalized feedback report summarizing the participant’s problems related to use, reasons for quitting, and high-risk situations for use. The major goals of the first session were to build rapport, identify issues related to health behavior change, and goal setting. The second session focused on assessment/review of goals and barriers to goal achievement. The third session occurred at approximately Week 4 and was used to follow-up on action plans. We have successfully used a similar intervention in previous cannabis treatment studies (McRae-Clark et al, 2009; McRae-Clark et al, 2010; McRae-Clark et al. 2015) to provide an evidence-based treatment platform for all participants.
Participants received compensation for completion of study tasks, including completion of study assessments, imaging procedures, and uploading medication adherence videos, up to a possible total of $1375. Fishbowl contingency management was also utilized to enhance study retention, in which participants earned chances to draw a plastic chip from a prize bowl, with chips either having a motivational message (“Good job”) or prizes of monetary value (range $1 to $100 per draw).
2.5. Statistics
2.5.a. Study Outcomes and Randomization
Study outcome measures were assessed at baseline and weekly during the final 3 weeks of active treatment, following the initial 2-week medication titration and the targeted quit date. CUD symptom measures included cannabis withdrawal (CWS total score), as well as CWS item scores deemed clinically related to both negative affect and cannabis craving. Negative affect scores included CWS items 5: “I felt nervous”, 6: “I had some angry outbursts”, 7: “I had mood swings”, 8: “I felt depressed”, 9: “I was easily irritated”, 15: “Life seemed an uphill struggle”, and 18: “I felt physically tense”. Craving scores included items 1: “The only thing I could think about was smoking some cannabis” and 10: “I had been imagining being stoned”. In addition to the CWS total score, negative affect and craving scores were averaged across all items at each of the final 3 study visits. Additional cannabis use outcomes included a) cannabis abstinence; b) cannabis reduction, measured as changes in creatinine corrected urine cannabinoids taken at each weekly visit (CN-THCCOOH); and c) self-reported changes in cannabis use frequency and intensity, noted as the percentage of weekly use days (frequency) and average reported use sessions per day (intensity) from the TLFB. As abstinence was measured from 1 to 4 weeks following study medication titration, urine THCCOOH levels were not likely to reach the 50 ng/ml threshold and thus alternative markers of new-onset abstinence based on Baker et al. (2018) were included; specifically, creatinine adjusted cannabinoid decrease of 25% or greater from study baseline and urine THCCOOH levels < 200 ng/ml. Medication adherence was assessed weekly from the start of study mediation through the end of study treatment (weeks 1–6).
Participants were randomized in a 1:1 manner utilizing stratified random block design. Randomization was stratified on participant gender and cigarette smoking status. Randomization and dispensing were performed by the MUSC Investigational Drug Service, a centralized research pharmacy that compounds and manages clinical trial medications.
2.5.b. Sample Size Determination
The primary focus of this study was to assess whether varenicline, compared to placebo, would evidence greater reductions in cannabis related withdrawal and negative affect during treatment. Assuming a strong correlation between withdrawal and negative affect measures taken weekly within each subject (rho=0.8), a sample of n=68 participants (34 in each treatment group) was deemed necessary for adequate power (80%) to detect a clinically relevant effect size of d=0.60 between the two groups. With the stated sample size, similar differences (d=0.60) in weekly cannabis use quantity were deemed detectible between groups. For the secondary abstinence analysis, the sample size necessary to estimate 50% of a fully powered Phase 3 clinical trial for the abstinence endpoint was determined. To show that treatment with varenicline would yield an abstinence rate at least 20% greater than placebo at the end of study treatment under the most conservative conditions, at a 15% placebo abstinence rate, a sample size of n=72 participants in each treatment assignment (N=144 total) was deemed necessary to provide 80% power with a type 1 error of 5% to detect this difference at the end of a fully powered study. The a priori sample size for this pilot trial was therefore n=36 per treatment condition (N=72 total).
2.5.c. Statistical Methods
Baseline demographics and clinical characteristics were tabulated for study participants in the overall cohort as well as stratified by randomized treatment assignment (see Table 1). Additionally, these variables were independently assessed for association with cannabis use outcomes (i.e., abstinence and reduction). Variables that indicated association with outcomes were retained for model development (p<.05).
Table 1.
Demographics and Cannabis Use Characteristics.
| Demographics and Cannabis Use Characteristics | Treatment Assignment | ||
|---|---|---|---|
| Cohort N=72 | Placebo n=37 | Varenicline n=35 | |
| Age (yrs) | 30.2 (10.1) | 29.7 (8.3) | 30.8 (11.8) |
| Male n (%) | 49 (68.1) | 27 (73.0) | 22 (62.9) |
| Caucasian n (%) | 42 (58.3) | 23 (62.2) | 19 (54.3) |
| Smoker n (%) | 31 (43.1) | 19 (51.4) | 12 (34.3) |
| CPD (smokers only) | 9.0 (11.3) | 8.6 (11.8) | 9.7 (10.8) |
| Baseline Cannabis Use Characteristic | |||
| Percent of days using (90 day TLFB) | 92.9 (13.5) | 95.3 (10.4) | 90.4 (15.9) |
| Cannabis Use Session/day (90 day TLFB) | 3.1 (2.3) | 3.4 (2.5) | 2.8 (2.0) |
| Cannabinoids (ng/ml) | 457 (738) | 365 (436) | 555 (958) |
| Cannabis Withdrawal Scale | 34.9 (26.8) | 36.9 (29.5) | 32.7 (23.7) |
| Psychiatric Comorbidities | |||
| Alcohol Use Disorder, current | 3 (4.2) | 1 (2.7) | 2 (5.7) |
| Alcohol Use Disorder, past | 6 (8.3) | 5 (13.1) | 1 (2.9) |
| Other Substance Use Disorder, past | 4 (5.6) | 2 (5.4) | 2 (5.7) |
| Major Depressive Disorder, past | 10 (13.9) | 4 (10.8) | 6 (17.1) |
| Generalized Anxiety Disorder, current | 1 (1.4) | 1 (2.7) | 0 (0.0) |
| Other Axis 1 disorder, current | 4 (5.6) | 3 (8.1) | 1 (2.9) |
Data are shown as Means (standard deviations) unless otherwise noted. Continuous characteristics are compared across treatment assignments using Wilcoxon Rank Sum test and categorical characteristics are compared across treatment assignment using a Pearson chi square test statistic CPD=Cigarettes per day, TLFB=Timeline-follow-back.
p<0.05
The primary hypothesis that participants receiving varenicline would have superior reductions in cannabis withdrawal as compared to placebo participants during treatment was assessed using a generalized linear mixed effects framework. Cannabis withdrawal was operationalized utilizing the CWS total score as well as subsets of items deemed clinically related to negative affect and craving, analogous to those responsive to varenicline in tobacco cessation trials (Gonzales et al., 2006; Jorenby et al., 2006). Initial models were fit including the main effects of study treatment, visit, and baseline measures. Residual normality was assessed using QQ-plot. Model based group differences and associated 95% confidence intervals (CI) were computed for all estimates
The hypothesis that participants receiving varenicline would have a higher probability of weekly abstinence and lower creatinine corrected cannabinoid levels compared to placebo participants during treatment was assessed using a generalized linear mixed effects framework. A logistic regression model with a sandwich variance estimate was used to assess the efficacy of treatment with varenicline on weekly point prevalence abstinence. To assess the potential impact of missing outcome data on abstinence parameter estimates, sensitivity analyses were completed (a) with missing data imputed to not abstinent [intent-to-treat (ITT) sample] and (b) with all available data. Results are noted as the percent of abstinent participants at each weekly visit and the overall percent of abstinent visits across the 3 final weeks of treatment.
The hypotheses that varenicline participants would have greater reductions in CN-THCCOOH, percent of days using cannabis and craving during study treatment were assessed using a generalized linear mixed effects framework similar to that developed for the analysis of cannabis withdrawal. Initial models were fit including the main effects of study treatment, visit, and baseline measures of each outcome. Correlations between changes in CN-THCCOOH and cannabis use (percent of days using and sessions of use) were assessed using a spearman rank order correlation coefficient. Additionally, cigarette smoking status at study entry was included as a predictor in all cannabis use outcome models. Further, effect modification (model interactions) and stratified analysis of the smoking subgroup was analyzed to determine, what, if any effect smoking status had on use patterns and abstinence across study groups.
The proportion of expected medication doses taken was collected daily and tabulated each week. Medication adherence was determined as those who took at least 80% of expected doses during the study week and was calculated for all actively enrolled participants. A logistic regression model with a sandwich variance estimate was used to assess differences in medication adherence between randomized treatment groups over time. Results are presented as the overall percentage adherent, as well as the percentage adherent within each treatment group.
This was a pilot trial and was not powered a priori to detect statistically significant differences; however, statistically significant p values are noted in results tables along-side group level and between group difference estimates (and 95% confidence intervals). For continuous outcomes, effect sizes from weekly group differences are calculated from model-based means and pooled standard deviations and noted as Cohen’s d while overall treatment effect sizes are noted as partial eta square values (Ƞ2p). For binary abstinence, effect sizes are noted as relative risks and 95% confidence intervals. All models additionally control for baseline measures of each outcome. All statistical analysis was conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Significance is noted at a level of α=0.05 and no adjustments for multiple comparisons were made.
3. Results
3.1. Study Participants
Participant demographic and baseline characteristics are presented in Table 1 and progression through study procedures is summarized in Figure 1. Of 136 individuals screened, 82 (60.3%) were eligible to participate and 72 (52.9%) were randomized, 35 to varenicline and 37 to placebo. The end of study treatment visit was attended by 65% of participants (n=47; 22 varenicline and 25 placebo). Study participants averaged 30 years of age (SD=10) and were predominately male (68%; n=49) and Caucasian (58%; n=42). Thirty-one (43%) of the participants were cigarettes smokers and averaged 9 cigarettes per day (SD=11). Study participants reported using cannabis an average of 83 of the 90 days prior to study entry (93%; SD=14%) and noted an average of 3.1 (SD=2.3) cannabis use sessions per day. There were no statistically significant differences for any baseline variables between treatment groups. During study treatment, 93% (67/72) participants remained at the prescribed dose while 7% (5/72) had dosages decreased (varenicline 11.4%; 4/35 vs. placebo 2.7%; 1/37; p=0.14); all four dose reductions in the active treatment group were due to nausea and the single dose reduction in the placebo group was due to muscle spasms. 65% of participants completed all three motivational enhancement therapy sessions (varenicline 63%; 22/35 vs. placebo 68%; 25/37; p=0.85). Median fishbowl contingency management payment over all sessions was $104.50 (IQR: $50.00, $163.00) and was not different between randomized treatment assignments [varenicline $84.00 ($37.00, $195.00) vs. placebo $111.50 ($66.50, $153.50); p=0.63].
Figure 1.
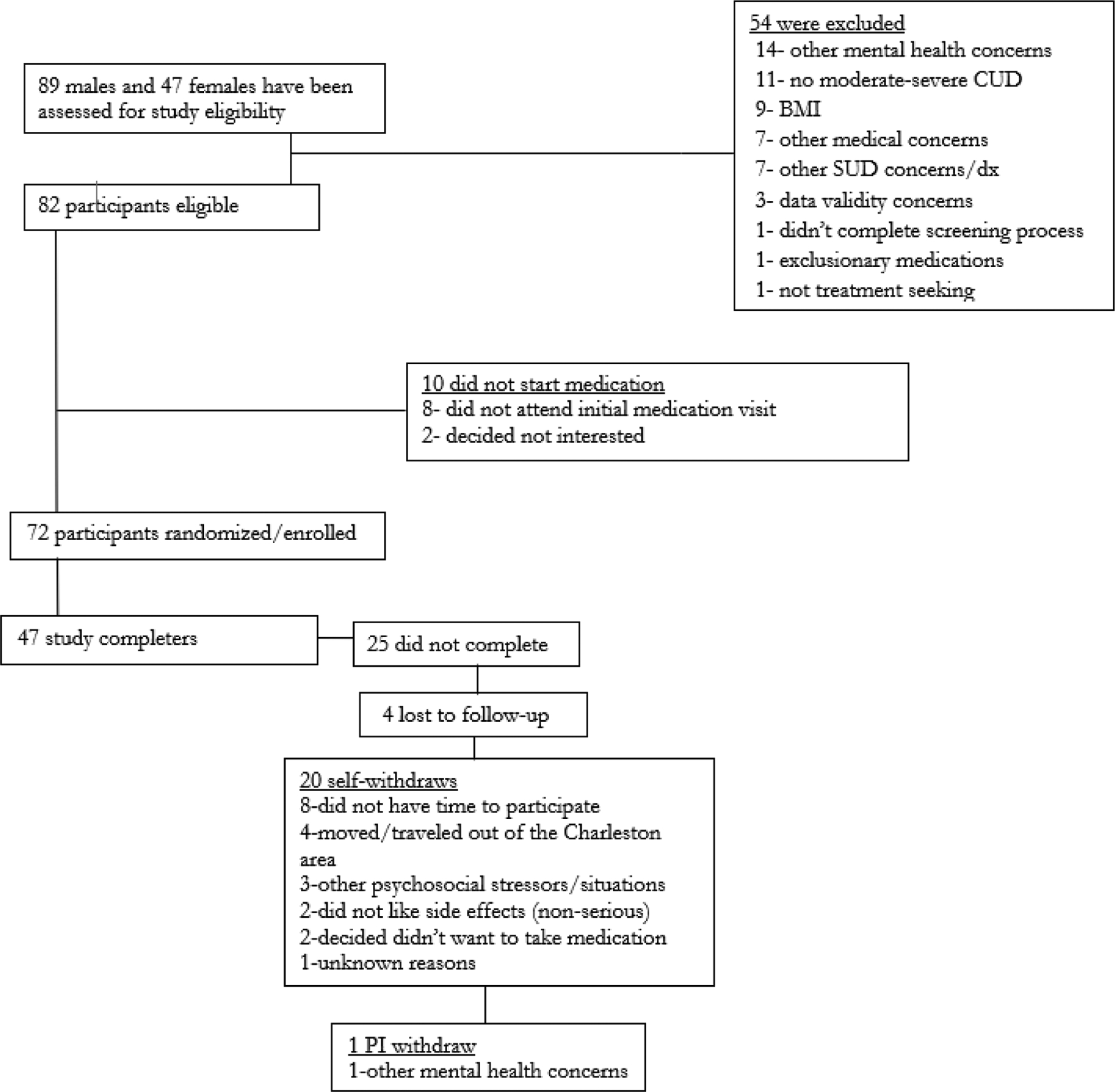
Study CONSORT flow chart
3.2. Cannabis Withdrawal
CWS total scores measured during the active phase of study treatment are shown in table 2. Participants randomized to receive varenicline had an average CWS total score decrease of 18.3 (95% CI: 12.1,24.5) and those randomized to placebo had an average decrease of 15.0 (95% CI: 9.2,20.9); between group differences in CWS total scores were not observed [Δ=3.3 (95% CI: −5.2,11.8); Cohen’s d=0.12]. Similarly, the average decrease in CWS negative affect score was similar in varenicline participants [1.0 (95% CI: −0.6,1.0)] and the placebo participants [0.6 (95% CI: −0.2,1.0)] with no between group difference [Δ=0.4 (95% CI: −0.2,1.0); Cohen’s d=0.16]. The average decrease in CWS craving items was significant in both varenicline 2.4 (95% CI: 2.0,2.9) and placebo 2.6 (95% CI: 2.2,3.1) participants but no difference was noted between groups [Δ=−0.2 (95% CI: −0.8,0.4)); Cohen’s d=0.12].
Table 2.
Summary data of Cannabis Withdrawal Scale.
| Study Visit | Cannabis Withdrawal | Effect Size ǂ | |||
|---|---|---|---|---|---|
| Placebo | Varenicline | Difference | Cohen’s d | Ƞ2p | |
| BL | 39.7 (26.3,53.0) | 31.7 (20.8,42.7) | -- | -- | |
| 4 | 21.4 (14.9,28.0)* | 20.1 (13.2,27.0)* | 1.4 (−8.2,10.9) | 0.06 | -- |
| 5 | 20.9 (14.3,27.6)* | 17.4 (10.5,24.2)* | 3.6 (−6.0,13.1) | 0.15 | -- |
| 6 | 18.8 (12.3,25.4)* | 13.8 (6.8,20.9)* | 5.0 (−4.7,14.6) | 0.21 | -- |
| Overallƚ | 20.4 (14.5,26.2)* | 17.1 (10.9,23.3)* | 3.3 (−5.2,11.8) | 0.14 | 0.001 |
Values are noted as model-based means and associated 95% Confidence intervals adjusted for baseline values of CWS.
Overall treatment difference noted as the model based mean treatment effect during weeks 4–6 adjusted for baseline values.
Effect sizes are noted as Cohen’s d and are calculated using adjusted model based mean differences and pooled standard deviations. Partial eta squared values are presented for the overall main effect of treatment adjusted for baseline differences.
Notes significant within group changes from study baseline levels (p<.05).
3.3. Cannabis Abstinence and Use Reduction
Self-reported cannabis abstinence during the active phase of study treatment is shown in Table 3. Participants randomized to receive varenicline noted numerically greater rates of overall weekly self-reported abstinence [ITT sample, varenicline: 14.3% vs. placebo: 6.3%; RR=2.3 (95% CI: 0.6,8.1)]. Although abstinence differences between groups were consistent over study visits, the differences were greatest at the end of study treatment [week 6: ITT sample, varenicline: 17.1% vs. placebo: 5.4%; RR=3.2 (95% CI: 0.7,14.7)]. When only available study visit data was included, relative risk ratios were consistent with those reported for the analysis of ITT data (Data shown in Table 2). Additionally, the creatinine corrected urine cannabinoid tests results appear consistent with self-reported use and are shown in Table 2. Within subject changes in measured CN-THCCOOH from study baseline in the varenicline group were significantly lower as at the end of study treatment visit (visit 6) and higher in the placebo group [Change in CNTHCCOOH from baseline: varenicline −1.7 ng/mg (95% CI: −4.1,0.8) vs. placebo: 1.9 ng/mg (95% CI: −0.4,4.3); Δ=3.5 (95% CI: 0.1,6.9)]. The mean within subject change from baseline CN-THCCOOH taken during the last three weeks of treatment was significantly lower in the varenicline group as compared to placebo [change in CN-THCCOOH from baseline: varenicline: −1.7 ng/mg (95% CI: −3.3, −0.1) vs. placebo: 0.9 ng/mg (95% CI: −0.6,2.5); Δ=2.6 (95% CI: 0.4,4.8)]. In the overall sample, during study weeks where participants reported abstinence, there was a median decrease in CN-THCCOOH from baseline of 71% (IQR: 33.4%, 82.1% decrease) while reported non-abstinent weeks had a median increase in CN-THCCOOH of 21% from baseline (IQR: 61% decrease, 128% increase). These numbers and ranges were similar across treatment assignments. When including biological confirmation with self-reported abstinence, participants randomized to receive varenicline noted numerically greater rates of overall weekly [ITT data: varenicline: 12.4% vs. placebo: 6.3%; RR=2.0 (95% CI: 0.5,7.2)] and end of study treatment abstinence [ITT data: varenicline: 14.3% vs. placebo: 5.4%; RR=2.6 (95% CI: 0.5,12.7)].
Table 3.
Summary of Self-Reported Cannabis Abstinence and creatinine corrected cannabinoids (ng/mg).
| Study Visit | ITT Data | Available Data | ||||
|---|---|---|---|---|---|---|
| Placebo | Varenicli ne | RR (95% CI) | Placebo | Varenicli ne | RR (95% CI) | |
| 4 | 8.1% (3) | 14.3% (5) | 1.8 (0.5,6.8) | 12.0% (3) | 22.7% (5) | 2.0 (0.5,7.3) |
| 5 | 5.4% (2) | 11.4% (4) | 2.1 (0.4,10.8) | 8.3% (2) | 17.4% (4) | 1.7 (0.4,7.2) |
| 6 | 5.4% (2) | 17.1% (6) | 3.2 (0.7,14.7) | 8.0% (2) | 27.3% (6) | 2.7 (0.7,10.0) |
| Overallƚ | 6.3% (7) | 14.3% (15) | 2.3 (0.6,8.1) | 9.5% (7) | 22.4% (15) | 2.1 (0.7,6.8) |
| Creatinine Corrected Cannabinoids (ng/mg) | Effect Size ǂ | ||||
|---|---|---|---|---|---|
| Placebo | Varenicline | Difference | Cohen’s d | Ƞ2p | |
| BL | 4.9 (2.2,7.7) | 4.8 (2.2,7.4) | 0.1 (−3.6,3.9) | -- | -- |
| 4 | 4.3 (2.0,6.7) | 2.7 (0.2,5.1) | 1.7 (−1.7,5.1) | 0.33 | -- |
| 5 | 6.2 (3.7,8.7) | 3.6 (1.2,6.0) | 2.6 (−0.9,6.1) | 0.38 | - |
| 6 | 6.7 (4.4,9.0) | 3.2 (0.7,5.6) | 3.5 (0.1,6.9)** | 0.55 | -- |
| Overallƚ | 5.8 (4.2,7.3) | 3.1 (1.6,4.7) | 2.6 (0.4,4.8)** | 0.42 | 0.026 |
Abstinence noted as self-reported abstinence from cannabis use since the last weekly visit % (n)
Creatinine corrected cannabinoid values are noted as model-based means and associated 95% Confidence intervals adjusted for baseline values.
overall treatment difference noted as the model based mean treatment effect during weeks 4–6 adjusted for baseline values.
Effect sizes are noted as Cohen’s d and are calculated using adjusted model based mean differences and pooled standard deviations. Partial eta squared values are presented for the overall main effect of treatment adjusted for baseline differences.
Notes significant within group changes from study baseline levels (p<.001).
Notes significant between group differences in changes from study baseline levels (p<.05).
3.4. Cannabis Use Frequency and Intensity
Weekly measures of percent using days and reported use sessions from the TLFB are noted in Table 4. Participants in both the varenicline and placebo treated group noted statistically significant decreases in both percentage of days used and use sessions per day during study treatment as compared to baseline. Participants randomized to receive varenicline reported numerically greater overall changes in percentage of study days using as compared to placebo [change in % days using from baseline: varenicline: −41.7% (95% CI: −26.3, −57.0) vs. placebo: −27.4% (95% CI: −13.0, −41.8); Δ=14.3% (95% CI: −7.1,35.7)]. Similarly, participants randomized to receive varenicline reported numerically greater overall changes in cannabis use sessions per day as compared to placebo [change in sessions per day from baseline: varenicline: −2.1 (95% CI: −1.7, −2.5) vs. placebo: −1.8 (95% CI: −1.4, −2.1); Δ=0.3 (95% CI: −0.2,0.9)]. Decreases in CNTHCCOOH from baseline were significantly and positively correlated with decreases in both percent use days (rho=0.33; p=0.001) and weekly use sessions (rho=0.26; p=0.002).
Table 4.
Summary data of cannabis use frequency and intensity.
| Study Visit | % Days Use | Effect Size ǂ | |||
|---|---|---|---|---|---|
| Placebo | Varenicline | Difference | Cohen’s d | Ƞ2p | |
| BL | 95.3 (90.1,100) | 88.6 (83.0–94.1) | 6.8 (−0.8,14.33) | -- | -- |
| 4 | 66.4 (51.3,81.4)* | 52.5 (36.4,68.6)* | 13.8 (−8.5,36.2) | 0.36 | -- |
| 5 | 64.8 (49.6,79.9)* | 47.5 (31.5,63.5)* | 17.3 (−5.1,39.6) | 0.49 | -- |
| 6 | 65.0 (49.8,80.2)* | 53.2 (37.2,69.3)* | 11.8 (−10.7,34.2) | 0.32 | -- |
| Overallƚ | 65.4 (51.0,79.8)* | 51.1 (35.7,66.4)* | 14.3 (−7.1,35.7) | 0.39 | 0.007 |
| Use Sessions per day | Cohen’s d | Ƞ2p | |||
| Placebo | Varenicline | Difference | |||
| BL | 3.22 (1.80,3.20) | 2.76 (1.47,2.74) | 0.46 (−0.80,1.71) | -- | -- |
| 4 | 1.33 (0.96,1.70)* | 0.93 (0.54,1.33)* | 0.40 (−0.14,0.94) | 0.40 | -- |
| 5 | 1.19 (0.82,1.56)* | 0.89 (0.50,1.28)* | 0.30 (−0.24,0.84) | 0.40 | -- |
| 6 | 1.21 (0.84,1.58)* | 0.89 (0.50,1.29)* | 0.32 (−0.22,0.86) | 0.33 | -- |
| Overallƚ | 1.24 (0.89,1.60)* | 0.91 (0.53,1.28)* | 0.34 (−0.18,0.86) | 0.36 | 0.009 |
Values are noted as model-based means and associated 95% Confidence intervals adjusted for baseline values of percent of days used or use sessions reported per day.
Overall treatment difference noted as the model based mean treatment effect during weeks 4–6 adjusted for baseline values.
Effect sizes are noted as Cohen’s d and are calculated using adjusted model based mean differences and pooled standard deviations. Partial eta squared values are presented for the overall main effect of treatment adjusted for baseline differences.
Notes significant within group changes from study baseline levels (p<.001).
Notes significant between group differences in changes from study baseline levels (p<.001).
3.5. Nicotine Co-Use
Participants that self-identified as cigarette smokers and had a positive qualitative urine cotinine test prior to study entry represented 43% of the randomized sample and reported relatively low smoking rates at study baseline [mean=9 cigarettes per day (CPD), range=1,40]. Twenty of the 31 cigarette smokers (64.5%) reported smoking less than 10 CPD in the 90 days prior to study entry. In the intent to treat analysis, smokers and non-smokers did not differ statistically in likelihood of achieving weekly cannabis abstinence [ITT sample, smokers: 8.6% vs. non-smokers: 11.4%; RR=0.8 (95% CI: 0.2,2.8)]. Similarly, changes from baseline in CNTHCCOOH [smokers 0.5 ng/mg (95% CI: −1.2,2.2) vs. non-smokers: 0.2 ng/mg (95% CI: −1.3,2.2); Δ=0.3 (95% CI: −2.6,2.1)] and percent cannabis using days [smokers: −33.1% (95% CI: −16.9, −49.4) vs. non-smokers: −35.6% (95% CI: −21.1, −50.1); Δ=−2.5% (95% CI: −24.9,20.0)] did not differ between smokers and non-smokers. Among cigarette smokers, treatment with varenicline did not affect reported CPD as compared to placebo [varenicline: 7.8 (95% CI: 5.6,10.1) vs. placebo: 7.6 (95% CI: 5.8,9.3); Δ=0.3 (95% CI: −3.1,2.6)]. Further, weekly cannabis use rates (sessions/day) were not significantly associated with co-occurring cigarette use (CPD; β=0.0004; SEM=0.038).
3.6. Adverse Events
Adverse events were tabulated at weekly visits in all randomized participants and relatedness to study treatment established. Adverse events deemed definitely, probably or possibly related to study treatment at the time of report were included in the analysis. At the close of study treatment, 43 (60%) of the 72 participants reported at least one study-related adverse event [20 (54%) in the placebo group and 23 (66%) in the varenicline group (p=0.31)]. Participants reported a total of 106 adverse events during treatment; 47 events were reported in the placebo group and 59 in the varenicline group. The most commonly reported adverse event was nausea (total sample: 23/106, 22%; varenicline 19/59, 32%; placebo 4/47, 9%) followed by dream disturbances (total sample: 16/106, 16%; varenicline 10/59, 17%; placebo 6/47, 13%) and insomnia (total sample: 12/106, 11%; varenicline 6/59, 10%; placebo 6/47, 13%). The majority of reported adverse events were mild/moderate (105/106; 99%). The noted severe adverse event considered related to study treatment occurred in the varenicline treated group (nausea).
3.7. Medication Adherence
Medication adherence was assessed using self-report, pill counts, MEMs Caps, and video diary at each of the six study weeks and reported as the total percent of doses taken. Reported medication adherence was numerically higher using self-report (87%) and pill count (86%) as compared to MEMs Cap (81%) and video diaries (72%). There were no between group differences in self-reported adherence (placebo=89% vs. varenicline=86%; p=0.58), pill count adherence (placebo=87% vs. varenicline=87%; p=.98), MEMs Cap adherence (placebo=81% vs. varenicline=80%; p=0.75) or video diary adherence (placebo=70% vs. varenicline=73%; p=0.89).
4. Discussion
Although not powered a priori to find statistically significant differences, this pilot trial provides preliminary data to support further evaluation of varenicline as a treatment for CUD; however, it is noted that overall cannabis abstinence in both treatment groups was low. Significant between group differences in creatinine-corrected urine cannabinoids from study baseline levels were noted, with greater reductions occurring in participants receiving varenicline. Although the study was not powered to detect group differences in self-reported cannabis use, the relative ratio for abstinence favoring the varenicline group at treatment end was similar to the odds ratio of the only positive pharmacotherapy trial abstinence outcome for CUD to date (Gray et al., 2012). However, it is important to note that different criteria for abstinence were utilized in this study due to the shorter duration than what was used by Gray and colleagues.
All participants reported reductions in cannabis withdrawal symptoms over the course of the study, with no significant between group differences noted and effect sizes (Cohen’s d) ranged from negligible to small. A confirmatory factor analysis has been performed on the Minnesota Nicotine Withdrawal Scale to allow measurement of specific withdrawal symptom clusters in tobacco trials (Toll et al., 2007). To date, however, there has not been a factor analysis of a cannabis withdrawal assessment instrument; as such, CWS items mapping on to negative affect and craving in the present trial were selected based on clinical judgment. Given this limitation, a more nuanced evaluation of cannabis withdrawal symptom subscales, as has been conducted in tobacco trials, may have utility in cannabis treatment research.
Overall, the incidence of participants reporting adverse events did not differ between study groups, although it is noted that nausea and dream disturbances, well-documented adverse effects of varenicline, occurred more commonly in varenicline than placebo treated individuals. In addition, retention was similar to that observed in other recent CUD pharmacotherapy trials (Gray et al., 2017; Levin et al., 2016). Multiple measures of adherence were utilized in this trial, and no between group differences in adherence were noted. Objectively measured adherence (video monitoring and MEMS cap) were higher than objective measurements (riboflavin) reported in previous CUD medication trials (McRae-Clark et al., 2015; Gray et al., 2017). However, it should be noted that this trial was only of six-weeks duration, as opposed to the 12-week study duration commonly utilized in CUD investigations. Previous work in this population has shown that medication adherence declines with length of treatment (McRae-Clark et al., 2015); as such, it will be important to evaluate if similar rates of adherence are seen with a full 12-week course of varenicline treatment.
Limitations of this study include the small sample size and truncated treatment duration, given its goal of feasibility testing and evaluation of preliminary efficacy to determine varenicline’s suitability for a fully powered trial for CUD. Sex and gender may impact treatment response in cannabis trials (McRae-Clark et al., 2017), and women have been shown to have better response to varenicline than other smoking cessation treatments in tobacco trials (Smith et al., 2017). Due to the small sample size we were not able to conduct sex or gender analyses. Across cannabis clinical trials, challenges exist in outcome measurement due to limitations in biological and self-report measurements (Loflin et al., 2020). Finally, the video uploads of medication taking and payment to attend study appointments may not easily translate to clinical settings and smaller effect sizes may be observed in trials not utilizing such monitoring. Strengths of the trial were use of validated assessments and multiple measures of cannabis outcomes and medication adherence. Although preliminary, these findings suggest future research is warranted to determine if varenicline improves cannabis use outcomes.
HIGHLIGHTS.
Varenicline was evaluated for cannabis use disorder (CUD) in a placebo-controlled, pilot trial.
Greater reductions in urinary cannabinoids were observed with varenicline vs placebo.
Additional research is warranted to determine if varenicline improves cannabis use outcomes.
ROLE OF FUNDING SOURCE
Funding for this study was provided by NIDA Grant UG3DA043231 (McRae-Clark/Gray, MPIs). NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Funding source:
This work was supported by the National Institutes of Health (UG3DA043231). Varenicline and matching placebo were provided by Pfizer Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Authors McRae-Clark and Gray designed the study and wrote the protocol. Authors McRae-Clark, Gray, Sherman, Squeglia, Wagner, and Tomko participated in the conduct of the study. Author Baker undertook the statistical analysis. All authors contributed to and have approved the final manuscript.
REFERENCES
- Adams TR, Arnsten JH, Ning Y, Nahvi S 2018. Feasibility and preliminary effectiveness of varenicline for treating co-occurring cannabis and tobacco use. J Psychoactive Drugs 50, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ, 2011. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend 119, 123–129. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB Jr., Gong J, Williams KE, Reeves KR, 2008. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax 63, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin AJ, Duka T, Rusted J, Jackson A 2014. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl) 231, 3771–3785. [DOI] [PubMed] [Google Scholar]
- Baker NL, Gray KM, Sherman BJ, Morella KL, Sahlem GL, Wagner AM, McRae-Clark AL 2018. Biological correlates of self-reported new and continuous abstinence in cannabis cessation treatment clinical trials. Drug Alcohol Depend 187, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ, 2011. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 218, 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA, Levin FR 2018. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology 43, 173–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetlino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley D, O’Neill BT, 2005. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem 48, 3474–3477. [DOI] [PubMed] [Google Scholar]
- Compton WM, Pringle B, 2004. Services research on adolescent drug treatment. Commentary on “The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials”. J. Subst. Abuse Treat 27, 195–196. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Miller ML, Booij J, van den Brink W, 2010. The nicotinic acetylcholine receptor partial agonist varenicline and the treatment of drug dependence: a review. Eur Neuropsychopharmacol 20, 69–79. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Schulz S, de Bruin K, Miller ML, van den Brink W, Booij J, 2011. Dose-dependent and sustained effects of varenicline on dopamine D2/3 receptor availability in rats. Eur Neuropsychopharmacol 21, 205–10. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Miller ML, de Bruin K, van den Brink W, Booij J 2009. Varenicline increases striatal dopamine D(2/3) receptor binding in rats. Addict Biol 14, 500–2. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, Gervais A, O’Laughlin J, Paradis G, Rinfret S, Pilote L, 2008. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ 179, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Russ C, Yu CR, Zou KH, Galaznik A, Franzon M, Berg A, Hughes JR, 2013. Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nicotine Tob. Res 15, 1849–1857. [DOI] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR 2011. Effects of varenicline on smoking cue-triggered neural and craving responses. Arch. Gen. Psychiatry 68, 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Gilbert DG, McClernon FJ 2009. Effects of nicotine on novelty detection and memory recognition performance: double-blind, placebo-controlled studies of smokers and nonsmokers. Psychopharmacology (Berl) 205, 625–633. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS, 2011. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 215, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi KD, Mansukhani MP, Karpyak VM, Schneekloth TD, Wang Z, Kolla BP 2020. The impact of varenicline on alcohol consumption in subjects with alcohol use disorders: Systematic review and meta-Analyses. J Clin Psychiatry 81, 19r12924. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study Group, 2006. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 296, 47–55. [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT, 2012. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am. J. Psychiatry 169, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghiza UE, Matthews AG, McRae-Clark AL, Carroll KM, Potter JS, Wiest K, Mooney LJ, Hasson A, Walsh SL, Lofwall MR, Babalonis S, Lindblad RW, Sparenborg S, Wahle A, King JS, Baker NL, Tomko RL, Haynes LF, Vandrey RG, Levin FR, 2017. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend 177, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Lematty T, McRae-Clark AL, Gray KM, George MS, Brady KT, 2013. Resisting the urge to smoke and craving during a smoking quit attempt on varenicline: results from a pilot fMRI study. Am. J. Drug Alcohol Abuse 39, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou P, Zhang H, Jung J, Pickering RP, Ruan J, Smith SM, Huang B, Grant BF, 2015. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, Foltin RW, Haney M, 2019. Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse. Addict. Biol 24, 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ 1998. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J. Anal. Toxicol 22, 445–454. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study Group, 2006. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296, 56–63. [DOI] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM, 2007. Abstinence rates following behavioral treatments for marijuana dependence. Addict, Behav 32, 1220–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 1997. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH 2006. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184, 523–539. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, Choi JC, 2016. Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 159, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R, 2013. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J. Addict. Med 7, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin MJE, Kiluk B, Huestis MA, Aklin WA, Budney AJ, Carroll KM, D’Souza DC, Dworkin RH, Gray KM, Hasin DS, Lee DC, Le Foll B, Levin FR, Lile JA, Mason BJ, McRae-Clark AL, Montoya I, Peters EN, Ramey T, Turk DC, Vandrey RC, Weiss RD, Strain EC 2020. The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda. Drug Alcohol Depend 212, 107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Mandelkern M, Alvarez-Estrada M, Brody AL 2012. A single administration of low-dose varenicline saturates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neuropsychopharmacology 37, 1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E, 2009. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, DeVane CL, Norton J, 2015. Buspirone treatment of cannabis dependence: A randomized, placebo-controlled trial. Drug Alcohol Depend 156, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Sonne SC, DeVane CL, Wagner A, Norton J, 2015. Concordance of Direct and Indirect Measures of Medication Adherence in A Treatment Trial for Cannabis Dependence. J Subst, Abuse Treat, 57, 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT, 2009. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend, 105, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT, 2010. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am. J. Addict 19, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW, 2006. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol. Pharmacol 70, 801–805. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL, 2012. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 223, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe DAL, Walker N, Sheridan J, Galea S, 2015. The effect of varenicline administration on cannabis and tobacco use in cannabis and nicotine dependent individuals: a case series. J. Addict. Res. Ther 6, 222. [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR, 2006. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch. Intern. Med 166, 1561–1568. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR 2007. Treatment of cannabis use disorders: a review of the literature. Am. J. Addict 16, 331–342. [DOI] [PubMed] [Google Scholar]
- Oon-Arom A Likhitsathain S, Srisurapanont M 2019. Efficacy and acceptability of varenicline for alcoholism: A systematic review and meta-analysis of randomized-controlled trials. Drug Alcohol Depend 205, 107631. [DOI] [PubMed] [Google Scholar]
- Reperant C, Pons S, Dufour E, Rollema H, Gardier AM, Maskos U, 2010. Effect of the alpha4beta2* nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area. Neuropharmacology 58, 346–350. [DOI] [PubMed] [Google Scholar]
- Rhodes JD, Hawk LW Jr., Ashare RL, Schlienz NJ, Mahoney MC 2012. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl) 223, 131–138. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE, 2007. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol, Sci, 28, 316–325. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffman WE, Johnson DE, McLean S, Freeman J, Williams KE 2009. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol 78, 813–824. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2020. National Survey on Drug Use and Health
- Sarter M, Paolone G 2011. Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci 125, 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H, 2014. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology (Berl) 231, 3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, Pope HG, Huestis MA, 2011. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction 106, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33. [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of Cannabis Use Disorder: Current Science and Future Outlook. Pharmacotherapy 36, 511–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA, 2017. Sex differences in smoking cessation pharmacotherapy comparative efficacy: A network meta-analysis. Nicotine Tob. Res 19, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Toneatto T, Sobell MB, Leo GI, Johnson L, 1992. Alcohol abusers’ perceptions of the accuracy of their self-reports of drinking: implications for treatment. Addict. Behav 17, 507–511. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, Carroll KM 2010. Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol, 18, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G, 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW, 1984. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 84, 405–412. [DOI] [PubMed] [Google Scholar]
- Toll BA, O’Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. 2007. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychol. Addict. Behav 21, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesquille M, Baudonnat M, Decorte L, Louis C, Lestage P, Beracochea D 2013. Working memory deficits and related disinhibition of the cAMP/PKA/CREB are alleviated by prefrontal alpha4beta2*-nAChRs stimulation in aged mice. Neurobiol Aging 34, 1599–1609. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Haney M, 2009. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs 23, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH 2001. Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 157, 142–150. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med 374, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]


