Abstract
Objectives:
To evaluate the effects of different polyphenols and solvents on dentin collagen’s crosslinking interactions and biostabilization against MMPs and collagenase degradation.
Methods:
Two polyphenols [proanthocyanidin (PA) and quercetin (QC)] with different water solubility were prepared as treatment solutions using ethanol (EtOH) or dimethyl sulfoxide (DMSO) as solvents. 6-um-thick dentin films were microtomed from dentin slabs of third molars. Following demineralization, films or slabs were subject to 60-s treatment (PA or QC) or no treatment (control) with subsequent extended-rinse with original solvent (EtOH or DMSO) or distilled water (DW). Collagen crosslinking interactions were assessed by FTIR. Biostability was assessed through endogenous MMPs activity via confocal laser scanning microscopy, and exogenous collagenase degradation via weight loss, hydroxyproline release and SEM. Finally, direct collagenase inactivation was also evaluated. Data were analyzed by three-way ANOVA and post-hoc tests (α=0.05%).
Results:
Distinct effects of two polyphenols and solvents on collagen crosslinking and biostabilization were observed. Higher crosslinking and biostability efficacy occurred with PA than QC (p < 0.001) that demonstrated negligible collagen interactions. With DMSO solvent, efficacy results were significantly reduced with both polyphenols (p < 0.05). DMSO-rinse further weakened interactions of PA with collagen, diminishing biostability (p < 0.05). Low biostability was detected with QC and DW-rinse, suggesting direct enzymatic inhibition due to physical presence in collagen.
Significance:
Collagen crosslinking interactions and biostability depend on polyphenol chemical characteristics. Treatment-solution solvents may affect interactions between polyphenols and collagen, specifically, DMSO showed detrimental effects on collagen crosslinking and biostability and should be used with caution.
Keywords: collagen crosslinking, dentin, polyphenols, proanthocyanidin, quercetin, dimethyl sulfoxide
1. INTRODUCTION
Type I collagen is the most abundant protein in human body, and is essential constituent for support and stability of body tissues such as vessels, skin, tendons, bone and dentin in teeth [1]. In dentin, collagen accounts for 90% of organic matrix and presents the particular interest in dentistry because it is the base for interactions between organic matrix and restorative resin materials. Moreover, use of collagen-associated biomaterials is also a trend in regenerative medicine. Despite of excellent biological properties, collagen is susceptible to degradation by collagenolytic proteases and biomechanical stress, limiting its strengths and applications. Dentin collagen degradation is the key reason for breakage of the resin-dentin interface, leading to failed composite restorations and progression of secondary caries [2]. Recently, it was reported that replacement of restorations still accounts for more than 50% of the restorations placed by dentists [3]. Also, a recent systematic review demonstrates that secondary caries is the major reason for failure after 3 years of composite restorations placed [4], meaning high costs for oral health systems. There is a strong need to reinforce collagen, which not only benefits dentistry, but also regenerative medicine.
The basis for collagen biostability, strength and function is derived from crosslinks at molecular and microfibrillar levels, which are mediated by enzymatic reactions between lysyl oxidase on lysine and hydroxylysine amino acids in the specific telopeptides regions of collagen molecules [1, 5]. However, the types and amount of crosslinks in collagen vary according to physiological and pathological conditions, leading tissues susceptible to degradation. In light of this, approaches to mimicking crosslinks have been widely investigated to increase collagen biostability for tissue repair and regeneration. Polyphenols have been highlighted in this task due to their biocompatibility and potential to interact with proteins [6, 7]. Besides enhancing mechanical properties via mineralization of dental tissues [6], increasing collagen biostability against enzymatic degradation has been one of main approaches to attest crosslinking ability of polyphenols [8–10].
Although several natural polyphenols showed outstanding in vitro results, low bioavailability in vivo is still a limiting factor for clinical application due to their poor water solubility [11, 12]. Thus, the use of a solvent is required for collagen treatment involving polyphenols. Most often used solvents include ethanol (EtOH), acetone and dimethyl sulfoxide (DMSO), either applied alone or in combination with water or phosphate-buffered saline (PBS). Besides the increase of mixture entropy and interaction between solute and solvent, some studies have recently demonstrated a synergistic effect promoted by the organic solvents in combination with polyphenols [13, 14]. For examples, the combinations of baicalein [13, 15], resveratrol [16], and epigallocatechin-3-gallate (EGCG) [17] with EtOH, most recently, EGCG with DMSO [14], were reported as a promising strategy to reduce degradation of dentin bond strength through increased collagen biostability against enzymatic activity.
Despite a real need for solvents in treatment solutions, solvent effect on crosslinking interactions between polyphenols and collagen has not been explored. In a treatment solution, the solvent is the medium where interactions take place, which could influence the polyphenols’ interactions with collagen [7]. Furthermore, another potential issue, often found in many studies of crosslinking efficacy of polyphenols via enzymatic degradation resistance, is that treated collagen is not rinsed with the original treatment solution solvent or only rinsed with water. This approach becomes more problematic when polyphenols with poor water solubility are used. Without using a solvent rinse or using water rinse only, a water-insoluble polyphenol could improve collagen biostability against degradation due to its physical presence in collagen matrix, and inappropriately be categorized as “an efficient crosslinker”. However, a true polyphenol collagen crosslinker should withstand the solvent rinse and show the ability to form strong stable interactions between the polyphenol and collagen even after the rinse step.
Therefore, in this study two polyphenols with different molecular characteristics and water solubility [proanthocyanidin (PA) and quercetin (QC)] were dissolved in different solvents (EtOH and DMSO). To distinguish collagen biostabilization via crosslinking as compared to just the physical presence, treated collagen was thoroughly rinsed with either the original solvent of treatment solution or distilled water (DW). Collagen crosslinking interactions were assessed by Fourier-transform infrared (FTIR) spectroscopy. Collagen biostability against endogenous MMPs activity was evaluated by confocal laser scanning microscopy (CLSM), and biostability against exogenous collagenase degradation was evaluated by weight loss (WL) and hydroxyproline release (HYP) of dentin films as well as by scanning electronic microscopy (SEM) of dentin slabs. A measurement of direct inactivation of collagenase by PA and QC was also performed. The research hypotheses in this study were that the crosslinking interactions, biostability against endogenous MMPs and exogenous collagenase degradation of dentin collagen would be affected by 1) polyphenols; 2) solvents in treatment solutions; or 3) the rinse solutions used.
2. MATERIAL AND METHODS
2.1. Reagents
QC (quercetin, ≥95%), DMSO (dimethyl sulfoxide, ≥99%), ethanol (absolute) and collagenase type I (from Clostridium histolyticum, 125 U/ mg) were purchase from Sigma-Aldrich (St. Louis, MO, USA). PA (Proanthocyanidin, ≥ 90%, from grape seed) was donated by the manufacturer (Polyphenolics, Madera, CA, USA). 0.96% phosphate buffered saline (PBS, pH = 7.4) was prepared using Sigma Life Science – Dulbeccos PBS packet, 0.002% sodium azide was added to prevent fungi or bacteria. TESCA buffer was prepared by dissolving 5.75 g of TES (Fischer BioReagents, Pittsburgh, PA, USA), and 26.5 mg of CaCl2 (Fischer Scientific, Waltham, MA, USA) in 500 mL of distilled water, and the pH was adjusted to 7.4 using NaOH.
2.2. Preparation of dentin collagen films
Twenty-five non-carious human third molars were collected with no associated patient identifiers, collection protocol determined as not human subject research (NHSR 12–50) as per the University Adult Heath Sciences Institutional Review Board. The teeth were stored in PBS containing 0.002% sodium azide at 4 °C for 2-week before removal of the occlusal enamel and roots using a water-cooled diamond saw (Buehler, Lake Bluff, IL, USA).
A total of ten teeth were sectioned into dentin blocks (6 × 6 × 5 mm), which were sectioned into 733 ultrathin dentin films (6-μm thick) with a tungsten carbide knife mounted on SM2500S microtome (Leica, Deerfield, IL, USA). The films were then randomly assigned into 10 groups (n = 73/group) according to the following combined factors: polyphenols (PA and QC), type of solvents in treatment solutions (EtOH and DMSO), and solutions used to rinse [original solvent (EtOH or DMSO) and DW]. Two groups treated with only the solvents and rinsed with DW were used as the controls. The experimental groups are detailed in the schematic representation (Fig. 1). All the treatment solutions were prepared at 1% of polyphenols for each solvent (w/w). The dentin films were first demineralized with 10% phosphoric acid for 30 min (3 times x 10 min, changing acid solution each time), rinsed with water for 30 min (3 times x 10 min), and spread on plastic cover slips (Fisher Scientific, Pittsburgh, PA, USA) using a paintbrush. After removal of excess water, the dentin films were treated with 30 μL of selected treatment solution for 60 s, then rinsed for 30 min (3 times x 10 min under agitation) with either original solvent or DW according to the experimental groups. The films subjected to FTIR, WL and HYP analyses were dried for 48 h under vacuum before use, while the films used for MMPs activity were freshly submitted to the preparation for CLSM analysis. The remaining 3 dentin films, which were demineralized and rinsed with water as described above, were used as negative control for CLSM analysis without any treatment.
Fig. 1.
Schematic design of experimental groups in this study.
2.3. Dentin collagen crosslinking interactions by FTIR
A total of ten treated and dried dentin collagen films from each group were submitted to FTIR analysis (Spectrum One, Perkin-Elmer, Waltham, MA, USA) on a BaF2 plate. The spectra of PA and QC powders were also obtained to identify the possible bands involved in interaction with collagen. The spectral range was set to 650 – 4000 cm−1 at a resolution of 4 cm−1 and 64 scans. In order to reveal crosslinking interactions, after baseline adjustment the respective control spectra were subtracted from spectra of treated collagen films, and the resulting difference spectra were compared with the powders’ spectra. Meanwhile, the integration areas of the band at 1608 cm−1 (associated with aromatic rings of polyphenols) of the difference spectra were quantified, while the band area at 1236 cm−1 (amide III) from untreated collagen was used as an internal reference band using previously published method [18]. Thus, a band ratio (1608 cm−1 / 1236 cm−1) was calculated for each of the treatment groups to show the amount of each polyphenol incorporated into collagen.
2.4. Biostability against endogenous MMPs activity by CLSM and in situ zymography
Three dentin collagen films from each group, as well as three untreated films (negative control) were utilized for analysis of MMPs 2 and 9 within the dentin collagen. A fluorescein-conjugated gelatin of EnzChek™ assay kit (E-12055, Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) was prepared immediately before use according to the manufacturer’s protocols. Right after each treatment, the collagen films were spread onto microscope glass slides, covered with a drop of the gelatin (3 μL), and then incubated in a humidified chamber which was protected from light for 24 h at 37°C. Each microscope slide containing the films was covered by coverslips, and visualized with a confocal laser scanning microscope (CLSM) (TCS SP5 II, Leica Microsystems, Buffalo Grove, IL, USA) in a fluorescence mode (40 × objective lens of 0.95 NA) at 488 nm of excitation and 530 nm of emission. Three images obtained from the same z layer were randomly captured for each collagen film. All images (n = 9 images for each group) were analyzed and quantified in terms of relative intensities of green fluorescence, indication of the activity of the endogenous gelatinolytic enzymes, using a NIH Image J1.8.0 software (Bethesda, MD, USA) [19].
2.5. Biostability against exogenous collagenase degradation by WL and HYP
The remaining 60 treated dentin collagen films from each experimental group were randomly assigned into 6 specimens containing 10 films per specimen. The films were immersed in 300 μL of 0.1% bacterial collagenase solution in TESCA at 37 °C for 1 h. The WL percentage was determined by measuring dry weight change before (W0) and after (W1) collagenase digestion for each specimen using an analytic balance (d = 0.01 mg, Mettler Toledo AG285, Zurich, Switzerland), and calculated using the following equation: WL%=(W0-W1)/W0×100%.
For the HYP, the digestion solution was collected and hydrolyzed in 6M HCl at 110 °C for 24 h. The dry residue (free hydroxyproline and other amino acids) of each specimen was pretreated by neutralization, oxidation and subjected to 5% Ehrlich’s reagent to develop the color. The absorbance was measured at 555 nm with a microplate reader (Biotek Instruments, Winooski, VT, USA). The trans-4-hydroxy-L-proline (analytical standard, Sigma-Aldrich) was used as the standard to make the working curves for quantifying the hydroxyproline released (n = 6) from each micro gram of demineralized dentin films during the digestion.
2.6. Morphology of demineralized dentin layer by SEM
Fifteen non-carious human third molars were processed into dentin slabs as follows. After removing the occlusal one-third of the crown, a uniform smear layer was created on the exposed dentin surface using wet 600-grit silicon carbide sandpaper (Buehler) for 60 s. In each tooth, four longitudinal sections (1-mm width) were made, followed by a cut approximately 1.5 mm parallel to the surface to obtain dentin slabs. A total of 60 dentin slabs were obtained and distributed into the 10 experimental groups (n=6/group). Each of the slabs was notched in the middle position on the bottom side for subsequent fracturing. The polished dentin surfaces were etched for 15 s with 32% phosphoric acid gel (Scotchbond Universal Etchant, 3M ESPE, St. Paul, MN, USA) and rinsed with water for 30 s, which then treated for 60 s followed by rinse according to the experimental conditions (Figure 1). For each group, the dentin slabs were evaluated with (n=3) or without (n=3) collagenase digestion (0.1% bacterial collagenase solution in TESCA for 1 h at 37°C). All the dentin slabs were fixed in 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate for 1 h, dehydrated in graded solutions of ethanol (33%, 67%, 85%, 95% and 100%) for 2 h each and dried overnight in a vacuum desiccator. The slabs were fractured, mounted on aluminum stubs, and coated with carbon with the fractured cross-sections examined in a FEI/Philips XL30 field-emission environmental SEM (Philips, Eindhoven, Netherlands) at 5000x magnification, either using secondary-electron (SE, 5 kV, 60 μA)) or backscattered-electron (BSE, 12kV, 60 μA) modes.
2.7. Direct inactivation of collagenase by treatment solutions of polyphenols
The measurement of direct inactivation of collagenase activity was determined using the EnzChek™ collagenase assay kit (E-12055, Invitrogen, ThermoFisher Scientific). Assays were performed in 96-well microplates with 0.2 U/mL Clostridium histolyticum collagenase incubated with the treatment solutions for up to 24 h according to the manufacturer’s instructions. A group without any treatment was included as a positive control. The fluorescence of kinetic of inactivation was monitored at 1, 2, 4, 16 and 24 h of incubation at 490 nm/520 nm (Biotek Instruments, Winooski, VT, USA). The extent of the collagenase activity at a different time point was determined, which was expressed as a percentage of fluorescence intensity radiated as compared to the no-treatment control (100%). The results of 1 and 24 h after incubation were evaluated for statistical analysis.
2.8. Statistical analysis
After the normality of distribution and the homogeneity of variances (Shapiro Wilk and Levene’ tests, respectively) were assessed, the averages of FTIR band ratio and MMPs activity were analyzed by three-way analysis of variance (ANOVA) and Games-Howell test (5%). The WL and HYP were analyzed by three-way ANOVA, while direct inactivation of collagenase by one-way ANOVA, all of which using Tukey post hoc test (5%).
3. RESULTS
3.1. FTIR characterization of PA and QC
Representative FTIR spectra of polyphenols PA (red line) and QC (green line) as well as their chemical characteristics including molecular weight, number of phenolic hydroxyl groups, water solubility are shown in Fig. 2. There are a few vibrational bands that are detected for both polyphenols since they are built on the similar flavan-3-ol unit containing three typical rings (A-C-B). These bands include phenolic OH groups (~3280 cm−1), aromatic rings (C=C stretching at ~1608 cm−1 and ~1520 cm−1, C-C at ~1161 cm−1), ester C-O stretching (~1279 cm−1), and ether C-O-C stretching (~1091 cm−1). There are differences in the degree of polymerization and the pattern or content of hydroxylation of the constituent flavanol unit between oligomeric PA and monomeric QC, which may induce different interactions with collagen. For example, the band ratio of 3280/1608 is 22.6 for PA, 5.8 for QC, respectively, indicating higher OH content in PA than QC.
Fig. 2.
Representative FTIR spectra of polyphenols proanthocyanidin (PA, red line) and quercetin (QC, green line) as well as their chemical characteristics including molecular weight, number of phenolic hydroxyl groups, water solubility*
*Data provided by the manufacturer - Polyphenolics, Madera, CA, USA, and complemented by National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 5280343, Quercetin. Retrieved January 14, 2021 from https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin [43], and Cadena et al. 2013 [44].
3.2. Dentin collagen crosslinking interactions by FTIR
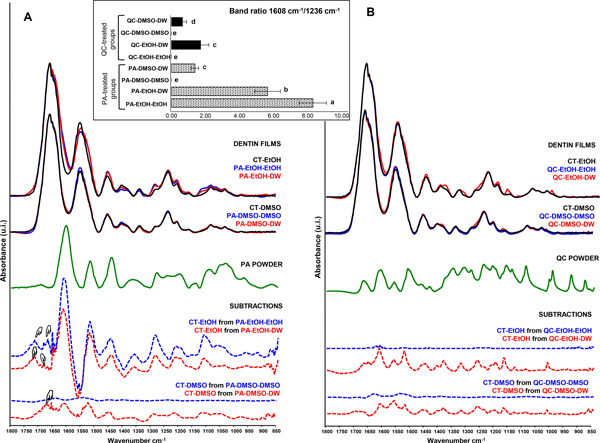
Representative spectra of dentin collagen films treated with PA or QC following the experimental procedures (outlined in Fig.1) are depicted in Fig. 3A or 3B, respectively, with the top spectra related to treated collagen, middle spectra to polyphenols and bottom spectra (dashed line) showing difference spectra after subtraction. The characteristic bands associated with collagen are more prominent in the spectra, which include amides I (~1660 cm−1), II (~1550 cm−1) and III (~1236 cm−1). Although some bands from polyphenols were overlapped with those of collagen, after a closer look more pronounced spectral changes were generally detected in most of the PA-treated collagen than QC-treated one after comparison with the controls (CT). The changes include amide I (broadening), amide II (decrease and shoulder formation) and a decrease at ~1400 cm−1 δ(CH2) bending. However, when DMSO as a solvent in PA, these spectral changes were reduced dramatically, especially after DMSO rinse. For QC in either EtOH or DMSO, almost no spectral change was observed after rinse with the respective solvent, only slight spectral change was detected when rinsed with DW.
Fig. 3.
Representative spectra of dentin collagen films treated with proanthocyanidin (PA, 3A) or quercetin (QC, 3B) following the experimental procedures (outlined in Fig.1). The top spectra (solid lines) are related to treated collagen, the middle spectra (green lines) are related to polyphenols, and the bottom spectra (dashed lines) show difference spectra after subtraction of control collagen from treated collagen. Inset: The band ratio (1608/1236) results. Means with different letters are significantly different (p < 0.05).
Subtraction of the CT collagen spectrum from those treated ones further revealed the potential collagen crosslinking interactions with polyphenols. For PA, most of the bands in the difference spectra could find matching counterparts in the spectrum of PA, except for new bands at ~1716 cm−1 and ~1668 cm−1, which may indicate the formation of new interactions with collagen. Interestingly, for the group of PA-DMSO-DMSO, the difference spectrum showed a nearly flat line, indicating potential absence of interactions between PA and collagen under this condition. While for QC, the subtraction produced virtually flat lines for treated collagen after solvent rinse, only generated the weak spectra when rinsed with DW, which matched with the spectrum of QC. In agreement with the above spectral changes, the band ratio (1608/1236) results (Fig. 3 inset) confirmed higher extent of PA incorporated into collagen than that of QC (p < 0.001), especially when EtOH as a solvent. When DMSO used as a solvent, a significant reduction in the amount of PA incorporated was detected in comparison with EtOH as a solvent (p < 0.05); moreover, negligible or no amount of PA remained in collagen after rinse with DMSO (p < 0.01). For QC, only small amount of QC could be detected in collagen after rinse with DW in comparison with minimal amount after the solvent rinse (p < 0.05).
3.3. Endogenous MMPs activity in dentin collagen
The endogenous MMPs activities within the collagen films were assessed using confocal laser scanning microscopy and in situ zymography. Representative images (top) and relative green fluorescence intensity representing endogenous MMPs activity or inhibition (bottom) of the controls and treated collagen films are shown in Fig. 4. The lowest MMPs activity was detected in most of the PA-treated groups exhibiting almost full inhibition (< 10%; p < 0.001). Only partial inhibition was promoted in QC-treated groups (QC-EtOH-EtOH 53.3%; QC-EtOH-DW 54.6%; QC-DMSO-DW 37.7%); however, not enough to designate a significant difference when compared with their respective solvent controls (p > 0.05). For both polyphenols, rinse with DMSO reduced their inhibition effect, especially for PA (p < 0.05).
Fig. 4.
Representative images (top) of confocal laser scanning microscopy (CLSM) and relative green fluorescence intensity representing endogenous MMPs 2 and 9 activity or inhibition (bottom graphs) of the controls and treated collagen films after 24 h of incubation in quenched fluorescein-labeled gelatin. For each experimental group, the upper image is obtained in green channel showing fluorescence attributed to MMPs activity, and the image below is created by merging differential interference contrast image with the image acquired in green channel. Means with different letters are significantly different (p < 0.05).
3.4. Biostability against exogenous collagenase degradation by WL and HYP
Results of collagen biostability against exogenous collagenase degradation are shown in Fig. 5. In Fig. 5A, the lowest WL was detected in the PA-treated groups when EtOH used as a solvent (PA-EtOH-EtOH 14.8% and PA-EtOH-DW 17.7%) (p < 0.001), indicating the highest biostabilization of collagen. A significant drop of biostabilization was detected when DMSO used as a solvent, with increased WL (PA-DMSO-DW 44.2% and PA-DMSO-DMSO 78.8%) (p < 0.001). A very low level of collagen biostabilization was detected in the QC-treated groups that rinse with DW (QC-EtOH-DW 80.6% and QC-DMSO-DW 84.2%) as compared to CTs (p < 0.001). However, complete collagen digestion was observed for the groups which rinse with original solvent (QC-EtOH-EtOH and QC-DMSO-DMSO), comparable to CTs (100%) (p > 0.05). Similarly, the HYP measurements (Fig. 5B) showed the lowest HYP release for PA-treated groups (PA-EtOH-EtOH 12.0 μg/mg; PA-EtOH-DW 25.7 μg/mg; PA-DMSO-DW 24.6 μg/mg), except for the group (PA-DMSO-DMSO, 59.0 μg/mg) which rinsed with DMSO (p < 0.05). No significant difference in HYP release was observed between the QC-treated groups and their respective solvent CTs (p > 0.05), except for the group QC-DMSO-DW, which showed a slight reduced HYP as compared with its DMSO solvent CT (p < 0.05).
Fig. 5.
(A) Percent weight loss of solvent controls and polyphenols (PA and QC) treated (for 60 s) demineralized dentin films after digestion with 0.1% collagenase solution for 1 hr. Means with different letters are significantly different (p < 0.05). (B) Hydroxyproline (HYP) release content (μg/mg film) of solvent controls and polyphenols (PA and QC) treated (for 60 s) demineralized dentin films after digestion with 0.1% collagenase solution for 1 hr. Means with different letters are significantly different (p < 0.05).
3.5. SEM morphology of demineralized dentin layer
Before collagenase digestion, similar morphology of demineralized dentin (DD) layer was observed for all the groups, which displayed collagen fibrils under secondary electron (SE) mode and a dark layer under backscattered electron (BSE) mode (Fig. 6). After digestion, the DD treated with PA-EtOH remained intact, regardless of the rinse solution (EtOH or DW). However, partial degradation of the DD layer was observed when treated with PA-DMSO followed by either DW or DMSO rinse, with the later showing more manifest degradation with a virtually imperceptible DD layer. On the other hand, comparable to the CTs, the DD layer did not survive for all the QC-treated groups, indicating collagen was totally digested by exogenous collagenase.
Fig. 6.
Representative photomicrographs (5000 x) of demineralized dentin slabs for all the controls and treatment groups before and after collagenase digestion obtained from SEM in secondary-electron (SE, left) and backscattered-electron (BSE, right) modes. For the PA-EtOH groups regardless of rinse, intact DD is observed after collagenase degradation, while noticeable reduction in DD thickness is observed for PA-DMSO groups, especially after DMSO rinse (PA-DMSO-DMSO). No DD is survived after degradation for all QC-treated groups, comparable to the controls. DD: demineralized dentin; ID: intact dentin.
3.6. Direct inactivation of collagenase by treatment solutions of polyphenols
The kinetics of direct inactivation of collagenase by the treatment solutions as a function of incubation time are shown in Fig. 7. The statistical results at 1 h and 24 h of incubation are also listed for comparison. A significant inactivation by both polyphenols was shown regardless of solvent, as compared to no treatment or solvent only treatments at the first hour of incubation (p < 0.001); however, a significantly reduced inactivation was promoted by PA in DMSO (PA-DMSO) when compared to PA in EtOH (PA-EtOH; p < 0.01) and QC solutions (p < 0.001). At 24 h, all the treatments promoted significant inactivation, even with the solvents (p < 0.001); higher inactivation was detected by polyphenols in EtOH, followed by EtOH, and then DMSO or polyphenols in DMSO (p < 0.05).
Fig. 7.
Kinetics of direct inactivation of collagenase by the treatment solutions of polyphenols (PA and QC) and solvents (EtOH and DMSO) as a function of incubation time (top), which are expressed by percentage as compared with untreated control. The statistical results (means ± standard deviation) at 1 h and 24 h of incubation are also listed for comparison (bottom). Means with different letters are significantly different, Tukey’s test (p < 0.0001).
4. DISCUSSION
The present study was designed to determine the effectiveness of polyphenols in collagen biostability imparted exclusively by crosslinking interactions as well as the potential solvent effect on polyphenols’ interactions with collagen. Two polyphenols (PA and QC) with different chemical characteristics and water solubility as well as two popular solvents (EtOH and DMSO) with different properties were used. The distinct effects of polyphenols and solvents on collagen crosslinking and biostability were observed, which would lead to deep understanding of factors involved in the crosslinking process, therefore, better selection of polyphenols and solvents for an effective collagen crosslinking treatment.
The first factor evaluated was the effect of polyphenols on collagen crosslinking ability and biostability against MMPs or collagenase. FTIR results showed more pronounced spectral changes of collagen treated by PA as compared with QC, indicating stronger PA-collagen crosslinking interactions over QC-collagen. The crosslinking between PA and collagen was identified through different mechanisms including hydrogen bonding, hydrophobic interactions and covalent bonds. Hydrogen bonding is confirmed by the spectral changes in amides I (broadening) and II (decrease and shoulder formation), which may be attributed to phenolic hydroxyl groups of PA as hydrogen donors forming hydrogen bonds with carbonyls in collagen as hydrogen receptors, or free amino groups in collagen as hydrogen donors with phenolic hydroxyls of PA as hydrogen receptors [20]. Hydrophobic interactions can be related to the dehydration effect on collagen demonstrated by decline of the band at 1400 cm−1, which involves the replacement of bound water in polypeptide Gly chains due to binding of PA with collagen [21, 22]. Furthermore, covalent bonds could be supported by the appearance of new bands at ~1716 cm−1 and ~1668 cm−1 after subtraction of untreated CT collagen spectra from PA-treated. Phenolic hydroxyl groups of PA can be oxidized into ortho quinones, which further react with amino groups of collagen to form C=N imine (~1668 cm−1) and C=O carbonyl (~1716 cm−1) through Schiff base reaction [23]. In contrast, the absence of the above spectral changes and nearly flat lines of the difference spectra after subtracting collagen control spectra were shown in most of QC-treated groups, indicating no/weak interactions between QC and collagen. The above results were confirmed by the band ratio (1608/1236) analysis indicating higher amounts of PA incorporated into collagen than QC (p<0.001).
Difference in collagen biostability against endogenous MMPs and exogenous collagenase degradation between PA and QC was further assessed. Endogenous MMPs are involved in the early digestion process of the dentin matrix, cleaving collagen at specific spots such as Gly-Ile in each α1 chain and Gly-Leu in each α2 chain [24], while exogenous bacterial collagenase cleaves collagen at multiple sites [25]. Therefore, two evaluation methods provided information on different levels of collagen protection. In comparison to QC, superior collagen biostability by PA was evident as demonstrated by either lower MMPs activity or lower WL and HYP values, indicating positive association with the stronger PA’s collagen crosslinking ability occurring at several levels as revealed by FTIR. An effective crosslinking effect could enhance the biostability of collagen by strengthening the triple helix, inhibiting unwinding of collagen fibrils, and sterically blocking the access of MMPs/collagenase to the cleavage sites. In addition, the increase of new hydrogen bonds and hydrophobicity of collagen could further hinder the enzymes that come in full contact with collagen molecules and hamper collagen hydrolysis [23]. To further test collagen crosslinking and biostability under a clinically relevant situation, dentin slabs were etched with a commercial phosphoric acid gel for 15 s to create demineralized dentin (DD) layers, which were subsequently treated according to the experimental procedures. The ability of polyphenols to penetrate into DD layers and induce collagen crosslinking in clinically relevant time (60 s) was evaluated by SEM. In situ observations of DD layers showed much better protection by PA over QC, which were consistent with the above quantitative biostability results.
The chemical characteristics of two polyphenols are directly related to their difference in collagen crosslinking and biostability. PA contains constituent flavanol units which are predominantly oligomeric (70–80%; varying from 2 to 7 units), 10% monomeric and 10–20% polymeric (more than 7 units) while QC is mainly monomeric (Fig. 2). In comparison, there are more phenolic hydroxyl groups (n = 12 at least) in PA, consequently higher complexity and molecular weight (~930 g/mol), to the contrary of QC which contains only 5 phenolic hydroxyl groups and a lower molecular weight (~302 g/mol). It is reported that phenolic hydroxyl groups are highly reactive with collagen, and the increase in molecular complexity and weight of polyphenols greatly contributes to crosslinking ability [7, 23, 26–28]. In the presence of a multitude of phenolic hydroxyl groups, more hydrogen bond donors and acceptors are available to interact with amino and carboxyl groups in collagen. In addition, the configuration of phenolic hydroxyl groups plays important role in crosslinking. The ortho-dihydroxyl (catechol) configuration in B-ring is considered as a determinant for delocalization of electrons [29, 30], which is fundamental driving force toward collagen. In the presence of several ortho-dihydroxyl groups, polyphenols can be more easily oxidized into ortho-quinones and covalently bind to amino groups in collagen. On average, there are several ortho-dihydroxyl groups in PA while only one in QC. Therefore, the multitude and configuration of phenolic hydroxyl groups in PA impacted on its capacity and mechanisms of interactions with collagen, consequently, its remarkable crosslinking ability and biostability over QC. All the above findings support the first research hypothesis.
The effect of solvents as another factor on collagen crosslinking was also evaluated. The results showed that collagen crosslinking interactions and biostability were affected differently by the solvents in polyphenol treatment solutions (p < 0.05). Both DMSO and EtOH have been extensively used to enhance dentin bonding through alteration of natural hydration state of collagen [31] [32–35], even both showing different physical/chemical characteristics. As an aprotic solvent and hydrogen bonding acceptor, DMSO can break down hydrogen bonds between collagen and bound water that maintain the hydration of triple helix [36], and causes water molecules to orient themselves by means of hydrogen bonding with oxygen and hydrophobic orientation towards methyl groups in DMSO [37]. Based on these characteristics, DMSO has the potential to expand the collagen matrix in demineralized dentin, allowing improved adhesive infiltration and durability of dentin bonding [35, 38]. Some studies also showed relative enzymatic inhibition promoted by DMSO [34, 35]. Due to these benefits, DMSO has been used in treatment solutions for enhanced collagen interactions with catechol containing polyphenols [14, 39]. However, it was discovered in this study that when used as a solvent in treatment solutions, DMSO dramatically weakened the crosslinking interactions between polyphenols and collagen, reducing the amount of polyphenols incorporated into collagen. In the treatment solutions, DMSO may compete with polyphenols for hydrogen bond donor sites in free amino groups of collagen. The methyl groups in DMSO may also strongly bind to collagen [40], reducing the available sites for interactions between polyphenols and collagen. Furthermore, DMSO could directly interact with hydroxyl groups of polyphenols, reducing the amount of this main reactive group to interact with collagen. For polyphenols exerting stronger collagen crosslinking interactions, such as PA, the negative effect of DMSO is more evident, hence, greater reduction in biostability is seen. In contrast to DMSO, EtOH is a protic solvent that increases the level of interpeptide hydrogen bonding between adjacent collagen fibrils, and disrupts collagen structure by substituting water with the solvent molecule [41]. It could enhance the diffusion of polyphenols into collagen matrix and increase their contact or interactions with collagen. Thus, this study showed that the solvents used in treatment solutions differently affected all the evaluated properties, supporting the second research hypothesis.
Effect of rinse solutions as the third factor on collagen crosslinking and biostability was evaluated. To assess at what extent collagen biostability is achieved via a crosslinking effect rather than physical presence, treated collagen was thoroughly rinsed with either the original solvents or DW, especially for polyphenols with poor water solubility like QC. The extended rinse (10 min x 3 times) using a good solvent (EtOH or DMSO) completely removes any unbound polyphenols, excluding possible protection effects caused by the physical presence of polyphenols in collagen. To facilitate the rinsing process, micrometric dentin films or DD layers were used for treatment of 60 s in this study. Unlike commonly used dentin beams in the thick, millimeter scale, use of ultrathin films/layers not only allows homogenous penetration of the treatment solution into the whole specimens in the short treatment time but also facilitates the rapid and thorough rinse-off of the lightly bound polyphenols from collagen matrix, affirming reliable comparison between different polyphenols.
For QC with poor water solubility, after thorough rinse with a good solvent no evidence of crosslinking interaction was detected between QC and collagen, showing a flat line after spectral subtraction and nearly zero amount of QC incorporated into collagen according to the band ratios. Slight spectral change was detected for QC-treated groups when rinsed with DW. The subtraction produced the weak difference spectra, which matched well with the spectrum of QC. This could be attributed to the physical presence of QC due to its very poor solubility in water (0.003 mg/mL). This explained why QC was able to increase collagen biostability (WL and HYP) after rinse with DW, but not after rinse with EtOH/or DMSO. The results of direct inactivation of collagenase by polyphenols (Fig. 7) indicate that QC is a very efficient inhibitor, comparable to or even better than PA. Therefore, when collagen is treated by QC without subsequent rinse or rinsed only with water as found in many studies, the QC remnant in collagen is available to interact directly with enzymes. In this case, collagen biostability is promoted mainly by the physical presence of QC not necessarily via crosslinking.
For PA with high solubility in water (>200 mg/mL), the rinse with either EtOH or DW did not affect all the evaluated properties, re-affirming PA’s high collagen crosslinking ability. However, there was difference in collagen crosslinking and biostability for PA-DMSO between rinse with DMSO and DW. As described above, DMSO solvent as a hydrogen bonding disruptor can break down new bonds between PA and collagen. The extended rinse with DMSO would further reduce collagen crosslinking interactions with PA and destabilize structure of collagen itself [42] [36], which explains the substantial reduction in collagen biostability. On the other hand, the reduction in crosslinking interactions and biostability was much lessened or vanished if the treated collagen was followed by DW rinse. For example, collagen stability efficacy was nearly doubled when comparing after DW rinse (WL 44.2%; HYP 24.6 μg/mg) with DMSO rinse (WL 78.8%; HYP 59.0 μg/mg) (p < 0.05). This could be explained as follows: since DMSO is a very hygroscopic liquid, freely miscible with water, it’s quickly removed from collagen with DW during rinse. At the same time, without the interference of DMSO PA-collagen interactions and biostability are re-established due to their instantaneous nature of crosslinking reactions [26]. The above results on rinse solutions support the third research hypothesis.
5. CONCLUSIONS
Within the limitations of this study, the following conclusions can be drawn:
Collagen crosslinking interactions and biostability imparted by polyphenols depend on their chemical and structural characteristics, with PA showing much higher collagen crosslinking and biostabilization efficacy than QC.
Solvents used in treatment solutions may affect interactions between polyphenols and collagen, specifically, DMSO shows detrimental effects on the collagen interactions with PA.
Collagen biostability can be achieved by exogenous crosslinking with effective crosslinkers such as PA; however, some biostability could be imparted by polyphenols with poor crosslinking ability such as QC when physically attached to collagen. Rinsing treated collagen with an appropriate solvent is a strategic way to remove unbound polyphenols and distinguish the actual crosslinking protection from direct enzymatic inhibition promoted by the physical presence of polyphenols in collagen.
ACKNOWLEDGMENTS
This study was supported by Research Grant R01-DE027049 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bertassoni LE, Orgel JPR, Antipova O, Swain MV. The dentin organic matrix - Limitations of restorative dentistry hidden on the nanometer scale. Acta Biomaterialia. 2012;8:2419–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Breschi L, Maravic T, Cunha SR, Comba A, Cadenaro M, Tjäderhane L, et al. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dental Materials. 2018;34:78–96. [DOI] [PubMed] [Google Scholar]
- [3].Eltahlah D, Lynch CD, Chadwick BL, Blum IR, Wilson NHF. An update on the reasons for placement and replacement of direct restorations. J Dent. 2018;72:1–7. [DOI] [PubMed] [Google Scholar]
- [4].Ástvaldsdóttir Á, Dagerhamn J, van Dijken JWV, Naimi-Akbar A, Sandborgh-Englund G, Tranæus S, et al. Longevity of posterior resin composite restorations in adults – A systematic review. Journal of Dentistry. 2015;43:934–54. [DOI] [PubMed] [Google Scholar]
- [5].Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–7. [DOI] [PubMed] [Google Scholar]
- [6].Bedran-Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, et al. Dentin biomodification: Strategies, renewable resources and clinical applications. Dental Materials. 2014;30:62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Le Bourvellec C, Renard CMGC. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Critical Reviews in Food Science and Nutrition. 2012;52:213–48. [DOI] [PubMed] [Google Scholar]
- [8].Liu Y, Dusevich V, Wang Y. Proanthocyanidins rapidly stabilize the demineralized dentin layer. Journal of Dental Research. 2013;92:746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vidal CM, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, et al. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014;10:3288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Y, Green A, Yao X, Liu H, Nisar S, Gorski JP, et al. Cranberry juice extract rapidly protects demineralized dentin against digestion and inhibits its gelatinolytic activity. Materials. 2021;14:3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaur H, Kaur G. A Critical Appraisal of Solubility Enhancement Techniques of Polyphenols. Journal of Pharmaceutics. 2014;2014:180845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. The Journal of nutrition. 2000;130:2073s–85s. [DOI] [PubMed] [Google Scholar]
- [13].Yi L, Yu J, Han L, Li T, Yang H, Huang C. Combination of baicalein and ethanol-wet-bonding improves dentin bonding durability. Journal of Dentistry. 2019;90:103207. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Z, Yu J, Yao C, Yang H, Huang C. New perspective to improve dentin-adhesive interface stability by using dimethyl sulfoxide wet-bonding and epigallocatechin-3-gallate. Dental Materials. 2020. [DOI] [PubMed] [Google Scholar]
- [15].Li J, Chen B, Hong N, Wu S, Li Y. Effect of Baicalein on Matrix Metalloproteinases and Durability of Resin-Dentin Bonding. Operative dentistry. 2018;43:426–36. [DOI] [PubMed] [Google Scholar]
- [16].Peng W, Yi L, Wang Z, Yang H, Huang C. Effects of resveratrol/ethanol pretreatment on dentin bonding durability. Materials science & engineering C, Materials for biological applications. 2020;114:111000. [DOI] [PubMed] [Google Scholar]
- [17].Yang H, Guo J, Deng D, Chen Z, Huang C. Effect of adjunctive application of epigallocatechin-3-gallate and ethanol-wet bonding on adhesive-dentin bonds. J Dent. 2016;44:44–9. [DOI] [PubMed] [Google Scholar]
- [18].Hass V, Li Y, Wang R, Nguyen D, Peng Z, Wang Y. Methacrylate-functionalized proanthocyanidins as novel polymerizable collagen cross-linkers – Part 1: Efficacy in dentin collagen bio-stabilization and cross-linking. Dental Materials. 2021;37:1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mazzoni A, Nascimento FD, Carrilho M, Tersariol I, Papa V, Tjäderhane L, et al. MMP activity in the hybrid layer detected with in situ zymography. J Dent Res. 2012;91:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He L, Mu C, Shi J, Zhang Q, Shi B, Lin W. Modification of collagen with a natural cross-linker, procyanidin. International journal of biological macromolecules. 2011;48:354–9. [DOI] [PubMed] [Google Scholar]
- [21].Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dental Materials. 2013;29:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miles CA, Avery NC, Rodin VV, Bailey AJ. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. Journal of molecular biology. 2005;346:551–6. [DOI] [PubMed] [Google Scholar]
- [23].Wu L, Shao H, Fang Z, Zhao Y, Cao CY, Li Q. Mechanism and Effects of Polyphenol Derivatives for Modifying Collagen. ACS Biomaterials Science & Engineering. 2019;5:4272–84. [DOI] [PubMed] [Google Scholar]
- [24].Messent AJ, Tuckwell DS, Knäuper V, Humphries MJ, Murphy G, Gavrilovic J. Effects of collagenase-cleavage of type I collagen on alpha2beta1 integrin-mediated cell adhesion. J Cell Sci. 1998;111 ( Pt 8):1127–35. [DOI] [PubMed] [Google Scholar]
- [25].Philominathan STL, Koide T, Matsushita O, Sakon J. Bacterial collagen-binding domain targets undertwisted regions of collagen. Protein Sci. 2012;21:1554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baxter NJ, Lilley TH, Haslam E, Williamson MP. Multiple Interactions between Polyphenols and a Salivary Proline-Rich Protein Repeat Result in Complexation and Precipitation. Biochemistry. 1997;36:5566–77. [DOI] [PubMed] [Google Scholar]
- [27].Vidal CM, Leme AA, Aguiar TR, Phansalkar R, Nam JW, Bisson J, et al. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir : the ACS journal of surfaces and colloids. 2014;30:14887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Y, Bai X, Li S, Liu Y, Keightley A, Wang Y. Molecular weight and galloylation affect grape seed extract constituents’ ability to cross-link dentin collagen in clinically relevant time. Dental Materials. 2015;31:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burda S, Oleszek W. Antioxidant and Antiradical Activities of Flavonoids. Journal of agricultural and food chemistry. 2001;49:2774–9. [DOI] [PubMed] [Google Scholar]
- [30].Latos-Brozio M, Masek A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chemistry & Biodiversity. 2019;16:e1900426. [DOI] [PubMed] [Google Scholar]
- [31].Shin TP, Yao X, Huenergardt R, Walker MP, Wang Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dental Materials. 2009;25:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nagpal R, Manuja N, Pandit IK. Effect of ethanol wet bonding technique on the durability of resin-dentin bond with contemporary adhesive systems. The Journal of clinical pediatric dentistry. 2015;39:133–42. [DOI] [PubMed] [Google Scholar]
- [33].Sadek FT, Braga RR, Muench A, Liu Y, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. J Dent Res. 2010;89:1499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stape THS, Seseogullari-Dirihan R, Tjäderhane L, Abuna G, Martins LRM, Tezvergil-Mutluay A. A novel dry-bonding approach to reduce collagen degradation and optimize resin-dentin interfaces. Sci Rep. 2018;8:16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tjaderhane L, Mehtala P, Scaffa P, Vidal C, Paakkonen V, Breschi L, et al. The effect of dimethyl sulfoxide (DMSO) on dentin bonding and nanoleakage of etch-and-rinse adhesives. Dental Materials. 2013;29:1055–62. [DOI] [PubMed] [Google Scholar]
- [36].Zimmerley M, McClure RA, Choi B, Potma EO. Following dimethyl sulfoxide skin optical clearing dynamics with quantitative nonlinear multimodal microscopy. Appl Opt. 2009;48:D79–D87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mancera RL, Chalaris M, Samios J. The concentration effect on the ‘hydrophobic’ and ‘hydrophilic’ behaviour around DMSO in dilute aqueous DMSO solutions. A computer simulation study. Journal of Molecular Liquids. 2004;110:147–53. [Google Scholar]
- [38].Mehtälä P, Pashley DH, Tjäderhane L. Effect of dimethyl sulfoxide on dentin collagen. Dental Materials. 2017;33:915–22. [DOI] [PubMed] [Google Scholar]
- [39].Li K, Sun Y, Tsoi JKH, Yiu CKY. The application of mussel-inspired molecule in dentin bonding. Journal of Dentistry. 2020;99. [DOI] [PubMed] [Google Scholar]
- [40].Hirshburg JM, Ravikumar KM, Hwang W, Yeh AT. Molecular basis for optical clearing of collagenous tissues. J Biomed Opt. 2010;15:055002. [DOI] [PubMed] [Google Scholar]
- [41].Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. The effects of acetone, ethanol, HEMA, and air on the stiffness of human decalcified dentin matrix. J Dent Res. 1996;75:1851–8. [DOI] [PubMed] [Google Scholar]
- [42].Gries G, Bublitz G, Lindner J. The effect of dimethyl sulfoxide on the components of connective tissue. (Clinical and experimental investigations). Ann N Y Acad Sci. 1967;141:630–7. [DOI] [PubMed] [Google Scholar]
- [43].Information NCfB. Compound Summary for CID 5280343, Quercetin. PubChem 2021.
- [44].Cadena PG, Pereira MA, Cordeiro RBS, Cavalcanti IMF, Barros Neto B, Pimentel MdCCB, et al. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2013;1828:309–16. [DOI] [PubMed] [Google Scholar]