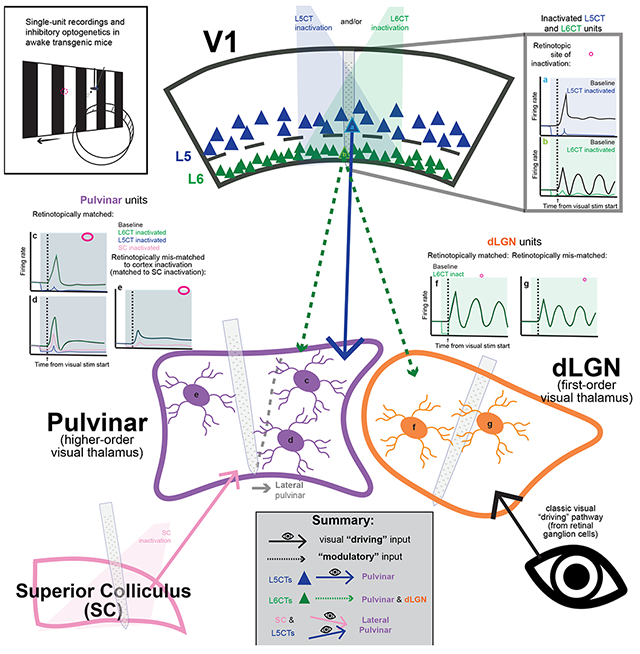
Summary
Higher-order (HO) thalamic nuclei interact extensively and reciprocally with the cerebral cortex. These corticothalamic (CT) interactions are thought to be important for sensation and perception, attention, and many other important brain functions. CT projections to HO thalamic nuclei, such as the visual pulvinar, originate from two different excitatory populations in cortical layers 5 and 6, whereas first-order nuclei (like the dorsolateral geniculate nucleus, dLGN) only receive layer 6 CT input. It has been proposed that these layer 5 and layer 6 CT pathways have different functional influences on the HO thalamus, but this has never been directly tested. By optogenetically inactivating different CT populations in the primary visual cortex (V1) and recording single-unit activity from V1, dLGN, and pulvinar of awake mice, we demonstrate that layer 5, but not layer 6, CT projections drive visual responses in the HO pulvinar, even while both pathways provide retinotopic, baseline excitation to their thalamic targets. Inactivating the superior colliculus also suppressed visual responses in the same subregion of the pulvinar, demonstrating that cortical layer 5 and subcortical inputs both contribute to HO visual thalamic activity - even at the level of putative single neurons. Altogether, these results indicate a functional division of “driver” and “modulator” CT pathways from V1 to the visual thalamus in vivo.
Graphical Abstract
Introduction
The thalamus and its interactions with the cortex are increasingly appreciated as essential for sensory-guided behaviors and complex cognition1. Yet, the nature of these interactions – how the content and manner of communication through cortico-thalamo-cortical pathways are controlled – has been difficult to decipher. This is especially true as it pertains to “higher-order” (HO) thalamic nuclei, such as the pulvinar in the visual system (also referred to as “LP” in rodents). The pulvinar has been implicated in synchronizing activity across visual cortical areas to support visual attention2,3 and in integrating sensory signals with behavioral context4,5. Still, a mechanistic understanding of the complex interactions between the cortex and HO nuclei like the pulvinar has been hindered by incomplete knowledge of the functional impact of their cortical inputs in vivo.
While critical questions remain, decades of research into the anatomy and physiology of corticothalamic (CT) circuitry across systems and species have revealed a number of common motifs. For instance, most glutamatergic synapses in the thalamus fall into two major categories, characterized by differences in synapse strength, size, number, post-synaptic receptor type, short-term plasticity, and more6,7 (Figure 1A). Their distinctions are noteworthy because these different synapse classes arise from different inputs, and at least in certain thalamic nuclei, they also serve different functions. In the dorsolateral geniculate nucleus (dLGN) - the first-order (FO) thalamic nucleus in the visual system – retinal ganglion cells make large, strong “driver” synapses, so-called because they “drive” visual activity in the dLGN8–12. In contrast, layer 6 corticothalamic neurons (L6CTs) in the primary visual cortex (V1) make small, weak, “modulator” synapses that are not responsible for the dLGN’s visual responses but instead exert more subtle (i.e., “modulatory”) influences10–13. These classes are not specific to the dLGN; FO nuclei in other sensory systems also receive peripheral “driving” and cortical L6CT “modulatory” inputs6,11,12,14.
Figure 1. The mouse pulvinar receives V1 input from two distinct corticothalamic (CT) populations that are selectively targeted by different Cre mouse lines.
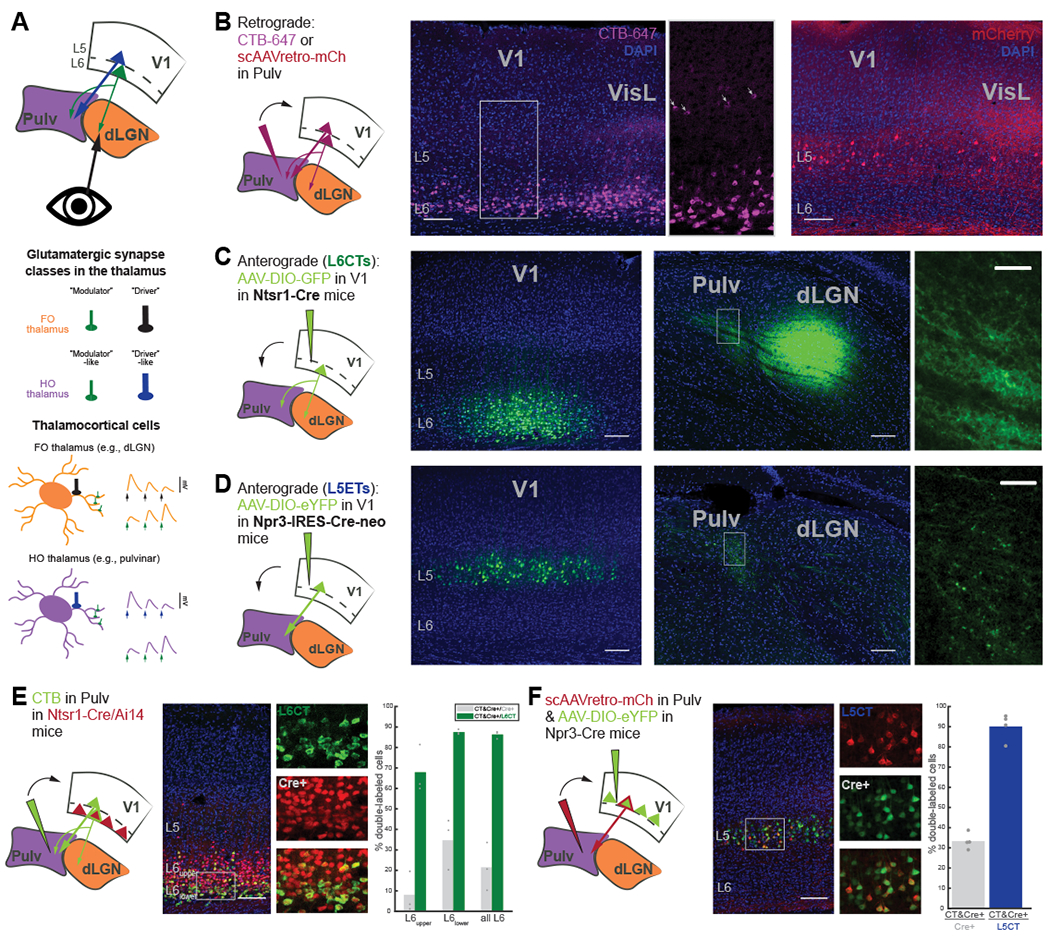
(A) Schematic of visual CT projections (top), different glutamatergic synapse classes (middle), and thalamocortical neurons in first-order (FO; e.g., dLGN) and higher-order (HO; e.g., pulvinar) thalamic nuclei (bottom) with characteristics of their different inputs (e.g., size, number and location of boutons, left; and short-term plasticity dynamics, right).
(B) Retrograde tracing from the pulvinar (left) with CTB-647 (middle) or a self-complimenting (sc)AAVretro-mCherry (right), with different tropisms for L6CTs versus L5CTs, respectively.
(C) AAV injection of Cre-dependent GFP into V1 of Ntsr1-Cre transgenic mice for L6CT labeling. Small, diffuse L6CT terminals are visible in the dLGN and pulvinar (far-right: higher magnification view of boxed region).
(D) AAV injection of Cre-dependent eYFP into V1 of Npr3-IRES-Cre-neo mice for labeling L5 extratelencephalic cells (L5ETs). L5 terminals are in the pulvinar but not the dLGN and appear larger and sparser than those from L6.
(E) Retrograde labeling (using CTB) from the pulvinar in Ntsr1-Cre/Ai 14 mice. Individual and composite channel images are from the boxed region. Right: Quantification of double-labeled cells out of all tdTomato+ cells (Cre+) and out of all CTB+ (L6CT) cells in L6upper, L6lower, and all of L6. n=3 mice.
(F) Retrograde labeling (using scAAVretro-mCherry) from the pulvinar in Npr3-Cre mice with AAV injection of Cre-dependent eYFP into V1. Right: quantification of double-labeled cells out of all eYFP+ cells (Cre+) and out of all mCherry+ cells (L5CT; n=4 mice).
All scale bars are 100μm for full images and 25μm for higher-magnification images (C, D). Bar graphs depict means across animals, and points are individual animals. See also Figure S1.
Meanwhile, the functional contributions of different inputs to HO nuclei like the pulvinar are less clear. HO nuclei (like FO) are also innervated by L6CTs with “modulator”-like synapses, but uniquely receive an additional cortical input from layer 5 neurons (L5CTs) with “driver”-like characteristics15–20 (Figure 1A). L5CTs are thus frequently referred to as “drivers” and L6CTs as “modulators” of HO nuclei6,12, but whether these parallel CT projection pathways are functionally distinct has never been directly tested7. Experiments demonstrating V1 driving influences on the pulvinar, as well as of the cortex on other HO thalamic nuclei, have relied on inferences following complete (chronic or transient) cortical inactivation21–26. However, these non-specific approaches would be expected to affect both L5 and L6 CT pathways, as well as activity in other cortical areas and subcortical structures. Thus, the functional consequences of these different classes of inputs to the HO thalamus are not known and might differ considerably from the FO thalamus27–29. Therefore, the longstanding question of whether L5 versus L6 CT projections from V1 to the pulvinar are dissociable “driving” versus “modulatory” pathways has yet to be answered.
This question is further complicated by the diversity of excitatory input sources to HO thalamic nuclei. The rodent pulvinar, for instance, receives additional L5 and L6 CT projections from extrastriate cortical areas4,5,30,31, as well as subcortical excitation from the superior colliculus (SC)32. Notably, SC projections display a mix of “driver” and “modulator” characteristics7 and have even been shown to drive activity in the caudomedial subdivision of the rodent pulvinar (cmPulv)22,23 In other pulvinar subnuclei (such as the lateral subdivision, lPulv, that receives both cortical and SC input32), it is unclear whether SC projections are still drivers or how (and if) they might interact with cortical afferents to shape visual responses. These diverse cortical and subcortical projections could suggest a role for the pulvinar as an “information hub” for intersecting pathways (feedforward and feedback, visual and more), putatively involved in multisensory and sensorimotor integration and conveying diverse signals to the cortex4,5,33–36 Yet how these different inputs – L5CT versus L6CT, cortical versus subcortical – interact in the pulvinar in vivo is not presently known.
Here, we utilize a variety of viral and transgenic approaches to selectively inactivate L5 versus L6 CT projections from V1 to the pulvinar and dLGN and assess their contributions to thalamic activity and visual responses in awake mice. We find that inactivating either L5CTs or L6CTs in V1 can reduce spontaneous activity in retinotopically aligned thalamic neurons, yet only L5CT inactivation profoundly suppresses visual activity and receptive field properties in the pulvinar. We also investigated the effects of SC inactivation combined with V1 L5CT inactivation and found a variety of neurons whose visual responses are driven by V1 L5CTs, by the SC, or even by both, as well as neurons with undetermined driving sources. Altogether, our findings demonstrate dissociable “driving” versus “modulatory” functions of distinct CT projection populations and highlight the role of the rodent HO visual thalamus in integrating inputs from both cortical and subcortical sources.
Results
Selective optogenetic inactivation of L6 vs. L5 corticothalamic pathways using different Cre transgenic mouse lines
The mouse pulvinar receives cortical input from two excitatory cell types in V1: L6CTs and L5CTs (Figure 1A). Consistent with prior studies4,5,30,31, we confirmed that injections of a retrograde tracer (CTB) into the pulvinar labeled CT neurons in V1 L6 – primarily in the lower half of L6 - and in L5, as well as in L6 and L5 of surrounding extrastriate cortical areas (Figure 1B). We also observed more prominent L5 labeling when using a self-complimenting retrograde AAV (scAAVretro) injected into the pulvinar to retrogradely express a fluorophore without infecting L6CTs (which is characteristic of AAVretro tropism37; Figure 1B).
To target each of these populations in V1 for specific optogenetic manipulations, we utilized two Cre driver lines. For L6CTs, we used the Ntsr1-Cre GN220 BAC transgenic mouse line38, which labels dLGN-projecting L6CTs with near-perfect efficiency and specificity39,40 and which we previously used to optogenetically stimulate L6CT projections to the dLGN and pulvinar41. As expected, axons were observed in both the pulvinar and dLGN following injection of a Cre-dependent AAV in V1 (Figure 1C). L6CTs in V1 that were retrogradely labeled from the pulvinar were largely restricted to lower L64,5,31 (Figures 1B and 1E) and were still a relative minority of all L6CT cells (34.71% in lower L6, 21.5% in all of L6, Figure 1E). Although these proportions are likely underestimates as a consequence of restricting our injections to avoid the dLGN, they indicate that not all V1 L6CTs project to the pulvinar. Nevertheless, nearly all (87.49% in lower L6, 86.31% in all of L6) pulvinar-projecting L6CTs were labeled (Figure 1E), thus validating the use of the Ntsr1-Cre line for reliably targeting the L6CT projection pathway for optogenetic manipulation.
To inactivate the L5 CT pathway, we characterized and used a new knock-in transgenic mouse line, Npr3-IRES-Cre-neo42, that showed considerable efficiency and specificity for L5 extratelencephalic neurons (L5ETs; Figures 1D-F and S1A-F). In addition to HO thalamic nuclei, L5ETs can also project to other subcortical structures, such as the SC, and not all L5ETs have collaterals to the thalamus (i.e., a subset of L5ETs are L5CTs; Figures S1A and S1F-H). This line is more specific than the popular Rbp4-Cre line, which also labels L5 cortico-cortical (CC) neurons43,44 (Figures S1A and S1B) that are involved in direct inter- and intra-cortical signaling and thus could confound interpretations about the L5-specific CT pathway from V1. Anterograde tracing from V1 in Npr3-Cre mice yielded axons specifically in the pulvinar, but not the dLGN, with relatively large and sparse terminals – as expected from the known anatomy of L5 CT projections18 (Figure 1D). Indeed, we found that virtually all (90.08%) L5 neurons retrogradely-labeled from the pulvinar (L5CTs) co-expressed a Cre-dependent fluorophore following AAV injection (Figure 1F) and were completely non-overlapping with retrogradely labeled CC neurons (Figures S1C-E). A third (33.33%) of these Cre+ neurons projected to the pulvinar (i.e., ~1/3 of L5ETs are L5CTs; Figure 1F), which is expected given the heterogeneity of L5ETs’ subcortical projection targets (Figures S1F-H). Therefore, the Npr3-Cre line allows for privileged access to L5ET neurons, including the putative “driving” CT pathway from V1 to the pulvinar.
Having verified the specificity of these mouse lines, we preceded to inject V1 of Ntsr1-Cre or Npr3-Cre mice with an AAV to express halorhodopsin in L6CTs or L5ETs, respectively (Figures 2A-C). We then used silicon microprobes45 to record extracellular single-unit activity from V1 in awake, headfixed mice viewing full-field drifting gratings to verify the efficacy and specificity of optogenetic inactivation. Current source density analysis was used to determine the laminar location of each recorded unit in an unbiased manner (Figure 2D). Strikingly, red light used to stimulate halorhodopsin in each of these mouse lines resulted in potent and layer-specific inactivation (Figures 2E and 2F). Because each of these lines only labels subpopulations of excitatory neurons within a layer (L6CTs or L5ETs), not all regular-spiking (i.e., excitatory) units were silenced, as expected. At least 47/223 (21.08%) and 64/108 (59.26%) regular-spiking units in layers 6 and 5, respectively, putatively expressed halorhodopsin (Figures 2G and 2H; example “inactivated” units in Figures 2I and 2J). While those in L6 were fewer than expected39, L6CTs are likely undersampled in our recordings as they are relatively small pyramidal cells with exceptionally sparse and tuned activity46, compared to L5ETs which are large cells with high (~10Hz) spontaneous firing rates and less tuning selectivity47 (Figure S2C). Consistent with prior findings39, we also observed increased activity across other layers (primarily layers 2-4; Figure S2A) upon L6CT inactivation, which provides additional evidence that our suppression was effective. Altogether, we have identified Cre-driver mouse lines, including a L5ET-specific line, which allow for layer- and cell type-specific optogenetic inactivation of distinct CT projection populations.
Figure 2. Selective inactivation of L6CTs or L5ETs in the primary visual cortex (V1) of awake mice.
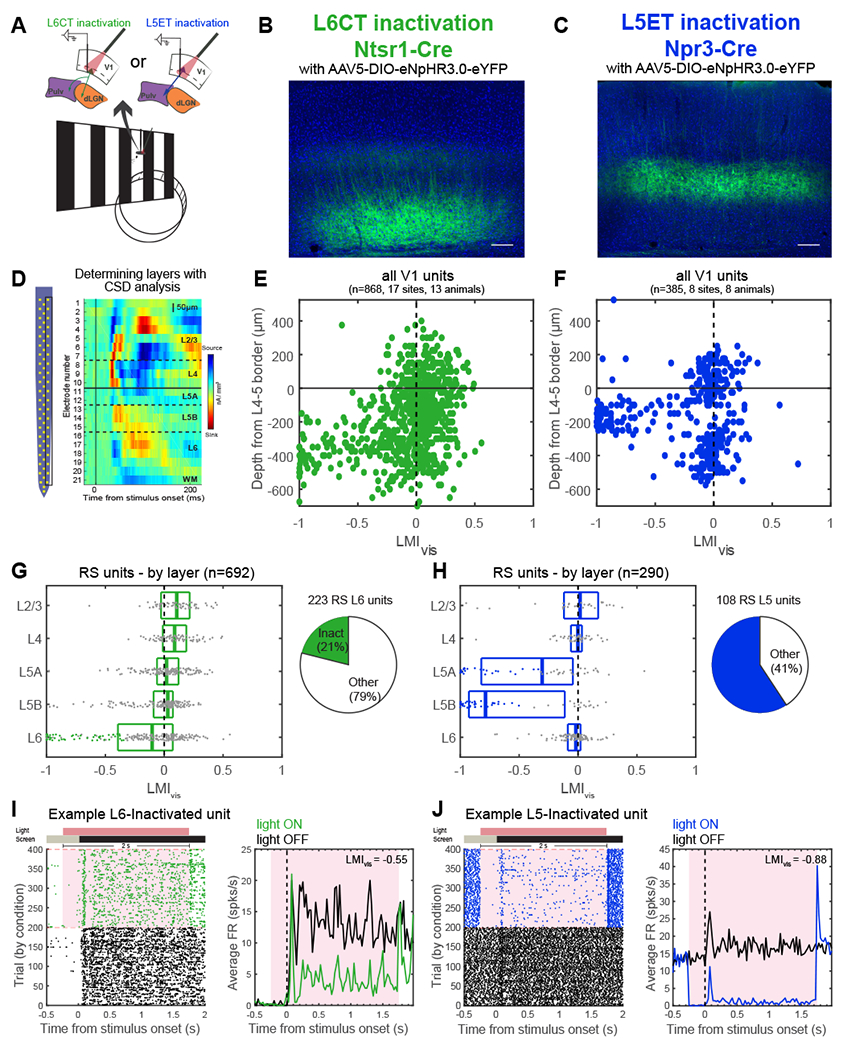
(A) Experiment schematic. Mice were awake and headfixed on a wheel and viewing full-field drifting gratings during laminar V1 recordings.
(B, C) Cre-dependent halorhodopsin (eNpHR3.0-eYFP) expression in V1 of Ntsr1-Cre (L6CTs, B) and Npr3-Cre (L5ETs, C) mice.
(D) Left: 64-channel silicon probe typically used for V1 recordings. Right: current source density plot for the right column of channels in an example recording. Layers corresponding to each channel’s location were defined by an initial current sink in L4 and a delayed sink in L5B. WM = white matter.
(E, F) Light modulation index ((FRlightON-FRlightOFF)/(FRlightON+FRlightOFF)) from visual trials (LMIvis) for all units by depth relative to the L4-5 border in L6CT (E) and L5ET (F) inactivation experiments (FR = firing rate).
(G) Left: LMIvis by layer for regular-spiking units (RS) in L6CT inactivation experiments. Boxplots display the median and quartiles, and putative inactivated units (L6 RS and LMIvis <−0.33) are colored dots. Right: proportion of all L6 RS units that were putatively inactivated (i.e., L6CTs).
(H) Same as (G) but for L5ET inactivation experiments.
(I) Example putative L6CT. Left: raster plot of all trials organized by condition (green=light ON trials for L6CT inactivation). Right: peristimulus time histogram (PSTH) of average FRs across visual light OFF (black) and light ON (green) trials. Red shading corresponds to the period of light stimulation.
(J) Same as (I) but for an example inactivated L5 unit in L5ET inactivation experiments. See also Figure S2.
L6 vs. L5 CT pathways differ in their effects on visually evoked activity in the visual thalamus
We next turned to the thalamus to determine how inactivating L6 versus L5 CT projections from V1 affected activity and visual processing in the lateral subdivision of the pulvinar (lPulv), which receives direct V1 input, as well as the dLGN in awake mice (Figures 3 and 4). In the dLGN, L6CT inactivation had disparate effects on spontaneous versus visually evoked activity. In line with another recent study in awake mice48, we observed considerable suppression of spontaneous activity in several units (Figure 3B, top) and across the whole population (Figures 3C and 3D). However, the presence of a visual stimulus greatly reduced – and in most cases eliminated – any suppression of dLGN activity induced by L6CT inactivation (Figures 3C and 3D), and even subtly increased the temporally modulated (“F1”) response (Figure 3D). Because of the distinct effects on spontaneous versus visually evoked activity, “suppressed” cells here and throughout this study (unless otherwise indicated) are those whose spontaneous activity was significantly reduced by the optogenetic manipulation (see Methods). Suppressed spontaneous and enhanced F1 responses in the dLGN were also observed when V1 was inactivated non-specifically (Figure S3D).
Figure 3. L6CT inactivation in V1 suppresses spontaneous but not visually evoked activity in the dLGN and lateral pulvinar (lPulv).
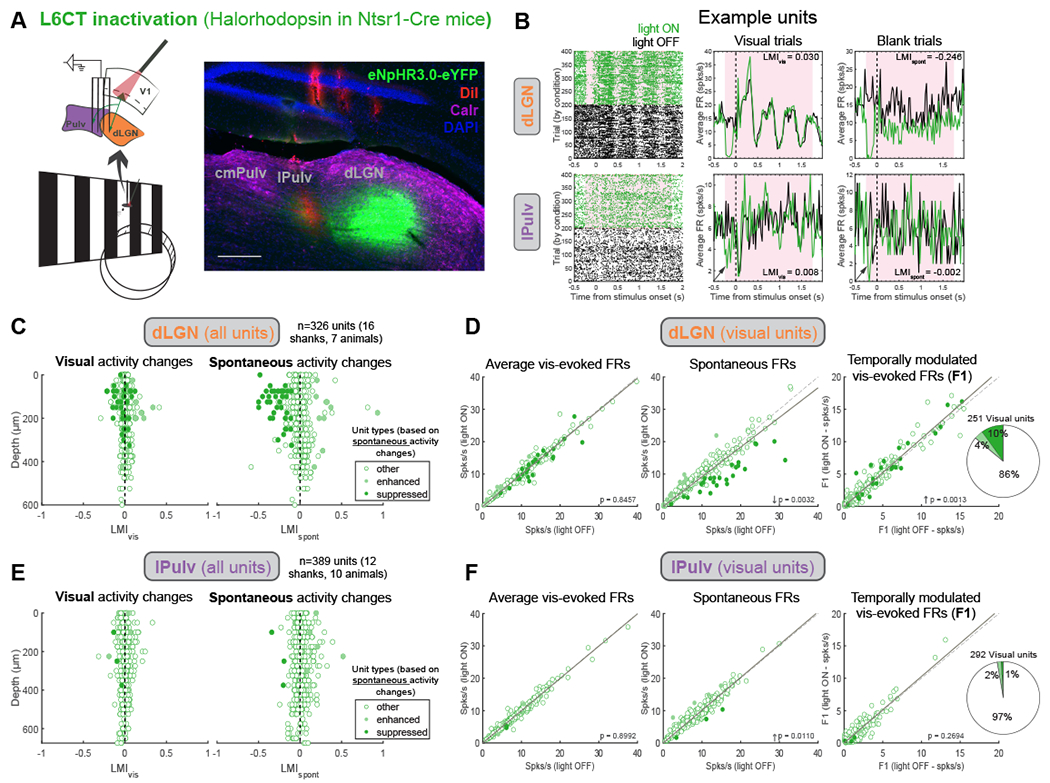
(A) Thalamus recordings with V1 L6CT inactivation. Right: histological verification of lPulv and dLGN recording locations (probe shanks coated in DiI, red). Immunohistochemical staining for calretinin (purple) delineates borders between cmPulv (Calr+ cell bodies), lPulv (Calr−), and dLGN (Calr+ axons). Scale bar = 200μm.
(B) Example dLGN (top) and lPulv (bottom) units. Raster plots (left) and PSTHs of average firing rates across all visual (middle) and blank (right) trials under different L6CT inactivation conditions. Arrows point to transient suppression at L6CT inactivation onset in the lPulv unit.
(C) LMIs of visually evoked (left) and spontaneous (right) activity from all recorded units in dLGN, by depth. Units are colored according to whether their spontaneous activity was significantly suppressed or enhanced (or unaffected, “other”) by L6CT inactivation.
(D) Effects of L6CT inactivation on dLGN units’ (left to right): mean visually evoked FRs (across visual trials); mean spontaneous firing rates (across blank trials); and temporally modulated responses (i.e., F1) to the preferred visual stimulus. Points colored as in (C). Far-right: proportion of visually responsive units suppressed, enhanced, or non-modulated (“other”). P-values from Wilcoxon signed-rank tests.
(E, F) Same as (C, D) but for lPulv units. See also Figure S3. .
Figure 4. L5ET inactivation in V1 suppresses visually evoked and spontaneous activity in the lateral pulvinar (lPulv).

(A) Thalamus recordings with V1 L5ET inactivation. Right: histological verification of lPulv recording locations. Scale bar = 200μm.
(B) Examples of suppressed lPulv units. Raster plots (left) and PSTFIs of average firing rates across all visual (middle) and blank (right) trials under different L5ET inactivation conditions.
(C) LMIs of visually evoked (left) and spontaneous (right) activity from all recorded units in lPulv, by depth. Units are colored according to whether their spontaneous activity was significantly suppressed or enhanced (or unaffected, “other”) by L5ET inactivation.
(D) Effects of L5ET inactivation on lPulv units’ (left to right): mean visually evoked FRs (across visual trials); mean spontaneous firing rates (across blank trials); and temporally modulated responses (i.e., F1) to the preferred visual stimulus. Points colored as in (C). Far-right: proportion of visually responsive units suppressed, enhanced, or non-modulated (“other”). P-values from Wilcoxon signed-rank tests.
(E, F) Median visual (E) and spontaneous (F) LMIs for suppressed and enhanced dLGN and lPulv units under L6CT vs. L5ET inactivation conditions. Visual LMIs (E): p<0.0001 for L5ET-Pulv vs. L6CT-dLGN, suppressed units, and p=0.048 for L5ET-Pulv vs. L6CT-Pulv, enhanced units. Spontaneous LMIs (F): p=0.003 for L5ET-Pulv vs. L6CT-dLGN, suppressed units. All other comparisons p≥ 0.1 (Kruskal-Wallis non-parametric test with the Dunn–Šidák post-hoc test).
(G) Average visually evoked FR changes (abs(FRvis-FRspont)) in Light-OFF vs. Light-ON trials for dLGN units in L6CT inactivation experiments (p=0.016), lPulv units in L6CT inactivation experiments (p=0.500), and lPulv units in L5ET inactivation experiments (p=0.011), Wilcoxon signed-rank tests.
(H) Distribution of visual and spontaneous LMIs for all dLGN units in L6CT inactivation experiments (orange), lPulv units in L6CT (green) and L5ET (blue) inactivation experiments, and combined lPulv and dLGN units in control experiments (grey). See also Figure S4 and S7
Similar to the dLGN, L6CT inactivation in lPulv did not suppress visually evoked activity (Figures 3E and 3F). There were also fewer instances of reduced spontaneous activity in lPulv than in the dLGN (although some units exhibited transient suppression in the first ~200ms of light onset; e.g., Figure 3B, bottom). Taken together, L6CT innervation can contribute to baseline activity, particularly in the dLGN, but is not required for visual responses in either the dLGN or pulvinar. This is consistent with a fundamentally modulatory role for L6CT projections in both FO and HO nuclei.
Inactivation of V1 L5ET projections, on the other hand, had substantial effects on both baseline activity and visual responses in lPulv neurons (Figure 4). Units whose spontaneous activity was significantly suppressed by L5ET inactivation also exhibited reduced mean activity and F1 responses in the presence of the drifting grating stimulus (Figures 4B-D). Consequently, the magnitude of optogenetic modulation of activity (visual and spontaneous light modulation index, LMIvis and LMIspont), and especially visual responses (LMIvis), was greater in L5ET-suppressed lPulv neurons compared to L6CT-suppressed neurons in either the dLGN or the few in lPulv (Figures 4E and 4F). Considered another way, L5ET inactivation reduced the effect of the visual stimulus on firing rates (magnitude of difference between visual and spontaneous firing rates, Figure 4G) in suppressed lPulv units, suggesting that some degree of visual information is conveyed to these pulvinar neurons through the L5 CT pathway. In contrast, visually induced activity changes somewhat increased in L6CT-suppressed dLGN units, as a consequence of their reduced baseline but unchanged visually evoked firing rates (Figure 4G). These differences cannot be attributed to differences in firing mode or tuning induced by L6CT versus L5ET inactivation because we observed increased bursting activity (Figure S3A) but no effects on orientation or direction tuning (Figures S3B and S3C) in both sets of experiments. We also observed suppressed visual activity during L5ET but not L6CT inactivation in another V1-recipient pulvinar subnucleus, the rostromedial pulvinar (rmPulv; Figures S3E-H); we therefore combine data from lPulv and rmPulv (collectively referred to as “pulvinar”) for all further analyses unless indicated otherwise. Altogether, we have observed a striking dissociation between the effects of L6CT versus L5ET inactivation on visual activity in the thalamus. This distinction is consistent with the predicted “driving” function of the V1 L5 CT pathway, in contrast to the “modulatory” L6 CT pathway11.
Nevertheless, it is notable that the “driving” effects of L5ET inactivation were observed in a minority of visually responsive pulvinar units (Figures 4D and 4H). Moreover, even suppressed pulvinar units were incompletely silenced by L5ET inactivation (e.g., Figure 4B). While this could suggest that V1 L5ETs are not solely responsible for driving visual activity in the pulvinar, this could also result from incomplete optogenetic inactivation of L5ETs themselves. In fact, we noticed that halorhodopsin was more effective at silencing L5ETs’ spontaneous than visually evoked activity, which could counterintuitively sharpen receptive fields in “inactivated” L5 cells (Figures S4A and S4B). We therefore conducted additional L5ET inactivation experiments in Npr3-Cre mice using the blue-light-activated chloride channel stGtACR2 (Figure S4C-K). Despite completely silencing V1 L5ETs with this opsin (Figures S4C-G), we still observed a similarly small proportion of pulvinar units that were significantly suppressed (Figure S4I). This contrasts with some expectations from proposed models of L5 “drivers” mediating a trans-thalamic feedforward pathway from primary to higher-order cortical areas10,12,14,49,50. We therefore sought to gain further insight into the functional organization of the distinct CT “driving” and “modulatory” pathways.
Retinotopic organization of both L6 and L5 excitatory CT projections to the dLGN and pulvinar
The pulvinar is topographically organized – both in its representations of visual space (i.e., retinotopy4,22,51,52) and its connections with visual cortical areas53,54. V1 projections to the pulvinar22, as well as pulvinar projections back to V14, are also coarsely retinotopic. Even though we limited our pulvinar analyses to recordings in which the recording shank passed through CT terminals from V1, nearby pulvinar neurons can have RFs separated by more than 20°22,55. Thus, pulvinar neurons that were not modulated by L5ET suppression might not have been retinotopically aligned to the cortical inactivation area, causing us to underestimate the “driving” influence of V1 L5 CT projections on the pulvinar. Additionally, given recent findings of spatially organized effects of L6CT activation48, we wondered whether retinotopy might also explain some of the effects of L6CT inactivation on spontaneous activity that we observed in the dLGN, and more modestly in the pulvinar.
To identify receptive field (RF) locations of V1 and thalamic neurons, we utilized a sparse noise stimulus protocol (Methods). This allowed us to identify both “on” and “off” subfields (in response to luminance increases and decreases, respectively; Figures S5A-D) and to coordinate L6CT or L5ET inactivation with the visual stimulation (Figure 5A). An example L6CT inactivation experiment, in which V1, dLGN and lPulv were all recorded from simultaneously, is depicted in Figure 5B; and a L5ET inactivation experiment with consecutive V1 and lPulv recordings is shown in Figure 5C. For units whose RFs could be confidently identified (see Methods for criteria), their RF information was then related to the effects of optogenetic inactivation on their responses to drifting grating stimuli (from Figures 3 and 4).
Figure 5. Corticothalamic excitation by both L6CTs and L5ETs is retinotopically organized.
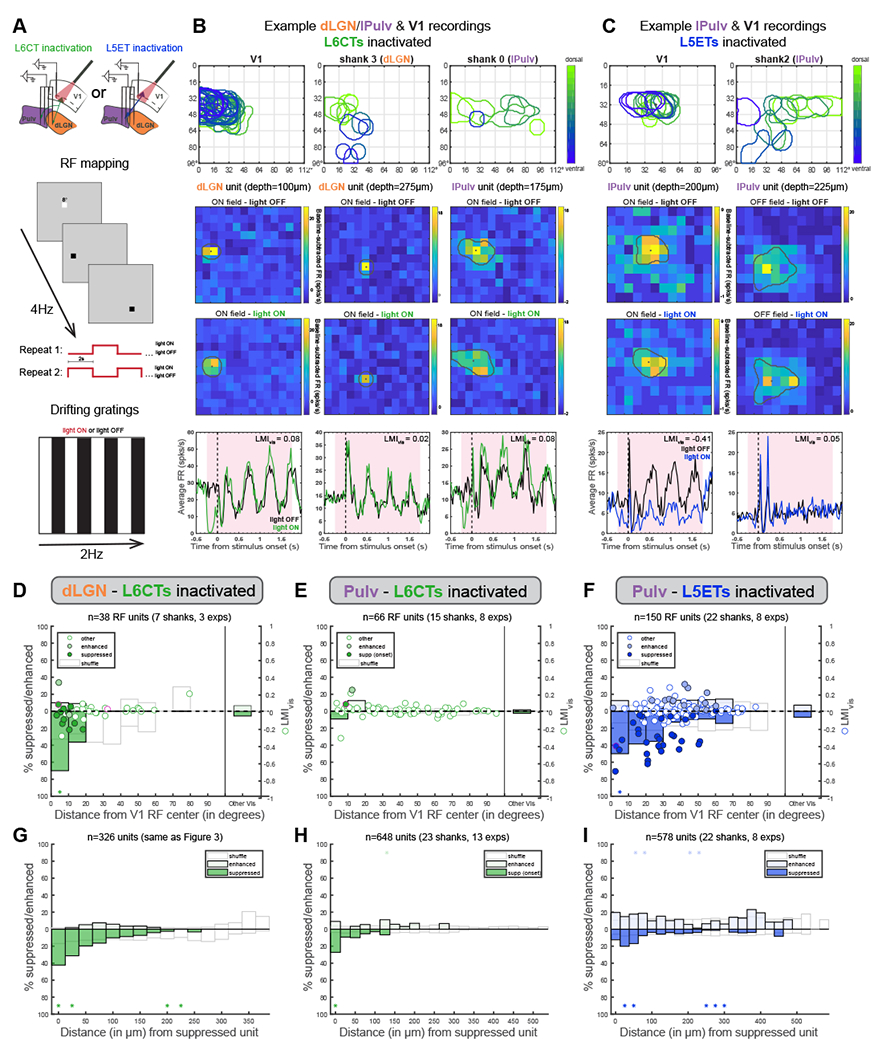
(A) Schematics of V1 and thalamus recording configurations (top) and sparse noise (for receptive field mapping, middle) and drifting grating visual stimulation protocols (bottom).
(B) An example L6CT inactivation experiment in which V1, dLGN and lPulv were recorded from simultaneously. Top: overlayed ON-field RFs (in response to luminance increases) from recording shanks in V1, dLGN and lPulv, colored according to the unit’s relative depth within its respective region. Sparse noise stimuli (8° squares) were presented in a 12x14 grid. Middle: Example dLGN (first two columns) and lPulv (third column) units’ RFs without (top) and with (bottom) L6CTs inactivated, shown as baseline-subtracted FRs at the timepoint of peak response. “Depths” are relative to the most dorsal thalamic unit. Overlayed perimeters and dots indicate estimated RF outline and centroids (see Methods). Note that the first dLGN unit’s and the lPulv unit’s RFs overlap with the retinotopic location of V1 recording; the second dLGN unit does not. Bottom: PSTHs of the same units in response to drifting grating stimuli (as in Figure 3).
(C) Same as (B) but for a L5ET-inactivation experiment in which V1 and pulvinar were recorded from consecutively (10x12 grid for V1 recording, 12x14 for pulvinar).
(D) Relationship between light modulation (in drifting grating experiments) and retinotopic displacement from V1 recording site (i.e., the retinotopic locus of L6CT inactivation) from experiments in which sparse noise stimulation was used for both dLGN and V1 recordings. Dots indicate LMIvis (right y-axis) of enhanced, suppressed, and other cells (same as Figure 3 - classified from blank trials). Pink-outlined dots are the example units from (B). Bars indicate proportion of units significantly suppressed or enhanced (left y-axis), binned by retinotopic distance (10° bins). ‘Other Vis’ are all other units from the same experiments whose RFs could not be determined. White bars reflect means of 1000 shuffled distributions (shuffled within experiment).
(E) Same as (D) but for pulvinar units defined as “suppressed” from the prestimulus period following light onset (e.g., example pulvinar unit in B).
(F) Same as (D) but for pulvinar units during L5ET inactivation experiments.
(G) Percent of all unit pairs-consisting of two units, at least one of which was significantly suppressed, recorded on the same shank in the same experiment-in which the second unit was suppressed (lower half of graph) or enhanced (upper half), binned by vertical distance between the channels from which those units were recorded. White bars reflect means of 1000 shuffled distributions (shuffled separately for each recording shank). Asterisks indicate where actual proportions fell beyond either tail (2.5%) of shuffled distributions.
(H, I) Same as (G) but for pulvinar units in L6CT inactivation experiments (H; “onset suppressed” cells as in E) and pulvinar units in L5ET inactivation experiments (I). See also Figure S5 and S7
In both L6CT and L5ET inactivation experiments, the largest suppressive effects (whether on spontaneous and/or visual activity) were observed in thalamic units whose RFs were closely aligned to those recorded in V1, while units whose RF centers were displaced 20° or more were typically unaffected (examples in Figures 5B and 5C; RF distance versus LMIvis for all units with significant RFs in Figures 5D-F). Thus, when considering thalamic units retinotopically aligned (within 10°) to our V1 recordings (and thus confirmed V1 inactivation), at least 50% of dLGN and pulvinar units were classified as “suppressed” by L6CT or L5ET inactivation, respectively, during the drifting grating experiments (Figures 5D, 5F), compared to 10% and 16% across all visually responsive dLGN and lPulv units (Figures 3D and 4D). This was similarly true of our L5ET inactivation experiments with stGtACR2 (Figure S5G). This relationship was not observed when we randomly reassigned RF distances among all units recorded within the same experiment (Figures 5D-F, “shuffle”). Because of the thalamus’ retinotopic organization, we also found that in the dLGN with L6CT inactivation and pulvinar with L5ET inactivation, units recorded from the same or nearby channels as a suppressed cell were more likely to also be suppressed (Figures 5G, 5I and S5H). This relationship was most striking in the dLGN, where cells’ physical and retinotopic distances are more closely linked than in the pulvinar22. Pulvinar cells whose onset responses (first 200ms) were significantly suppressed by L6CT inactivation (like the example lPulv unit in Figure 5B) also tended to be retinotopically aligned to the V1 inactivation site and recorded in close proximity to one another (Figures 5E and 5H). We also noticed that while relatively few (since sparse noise stimulation was not used in all L6CT inactivation experiments), most dLGN and pulvinar units with aligned RFs exhibited enhanced temporally modulated visual responses (F1) with L6CT inactivation (e.g., Figure 5B). This contrasts with the reduced visual responses in aligned pulvinar units with L5ET inactivation (e.g., Figure 5C), further emphasizing the difference in effects of L6CT versus L5ET inactivation despite similar retinotopic CT organization. Taken together, these findings show that direct CT excitation, whether from V1 L6CTs or L5ETs to dLGN or pulvinar, is retinotopically organized, even while its influence on visual versus baseline activity depends critically on the CT source (Figures 3 and 4).
Combined inactivation of different CT populations shows that individual pulvinar neurons are driven by L5CT, but not L6CT, inputs from V1
Although we have demonstrated different effects of V1 L6CT versus L5ET inactivation on activity in the pulvinar that are consistent with their hypothesized “driving” and “modulatory” roles, the degree of suppression we observed from specific L5ET inactivation – even when L5ETs were essentially completely silenced (Figure S4) - was less complete than in other studies that broadly inactivated all of V122,23 or in our own nonspecific cortical inactivation experiments (Figures S6A-F). We thus considered the possibility that while inactivating V1 L6CTs on their own had minimal effect on pulvinar activity (Figures 3E and 3F), perhaps they could exert more influence when in concert with the L5 CT pathway.
To address this possibility, we took advantage of the fact that AAVretro injected into the pulvinar infects only L5, but not L6, CT neurons37 (Figure 1B), thus allowing us to express Flp recombinase specifically in corticothalamic L5ET neurons (L5CTs). These injections were made in Ntsr1-Cre mice, where Cre is already present in L6CTs, so that a mixture of AAVs encoding Flp-dependent stGtACR2 and Cre-dependent halorhodopsin injected to V1 (Figure 6A) resulted in separate inhibitory opsins expressed in each CT population (Figure 6B). V1 recordings confirmed that blue (455nm) LED light for stGtACR2 specifically silenced units in L5 (Figure 6C), whereas red (617nm) LED light for halorhodopsin inactivated units in L6 (Figure 6D). Fewer L5 units were putatively inactivated in these experiments than when using Npr3-Cre mice, which was expected since opsin expression was restricted to pulvinar-projecting L5ETs (i.e., L5CTs). Across the full depth of V1, combined blue and red LED illumination reduced activity in cells across the infragranular layers, while each LED alone only suppressed units in its corresponding layer (Figure 6E). We confirmed in control animals expressing only one opsin that halorhodopsin was unaffected by our blue LED stimulation, nor was stGtACR2 affected by the red LED (Figures S6G-N).
Figure 6. Individual pulvinar neurons are suppressed by V1 L5CT, but not L6CT, inactivation.
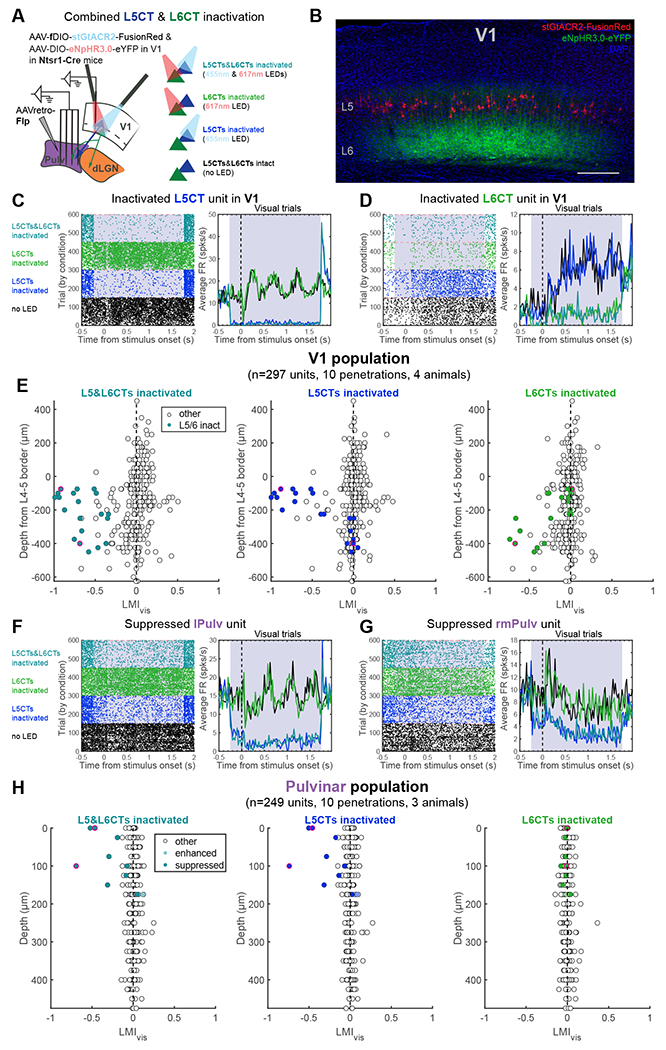
(A) Experiment schematic of injection, recording, and light stimulation sites (left) and LED stimulation conditions (right; randomly interspersed in actual experiments) for achieving L5CT and L6CT inactivation in the same mice.
(B) Confocal image (maximum intensity projection) of stGtACR2-expressing L5CTs and halorhodopsin-expressing L6CTs in V1. Scale bar = 200μm.
(C-D) Example L5CT (C) and L6CT (D) inactivated units in V1. Raster plots (left) and PSTHs of average firing rates across visual trials (right). Grey shading indicates the period of LED stimulation.
(E) LMIvis calculated from L5CT&L6CT-inactivation (left), L5CT-inactivation (middle), and L6CT-inactivation (right) trials. Colored units are those whose FRs were significantly suppressed by at least 50% in visual trials with combined L5CT&L6CT-inactivation, and dots outlined in pink are the example units in (C) and (D).
(F-G) Example lPulv (F) and rmPulv (G) units that were suppressed by L5CT, but not L6CT, inactivation.
(H) LMIvis of all pulvinar units across light stimulation conditions of significantly suppressed, enhanced and non-modulated units (classified from blank trials with combined L5CT&L6CT-inactivation). Dots outlined in pink are the example units in (F) and (G). See also Figure S6 and S7
Using this approach to inactivate L5CTs or L6CTs alone or in combination, we once again observed a subset of pulvinar units that were suppressed by L5CT inactivation (Figures 6F and 6G). Importantly, however, these units were unaffected by L6CT inactivation, and the effect of combined L5CT and L6CT inactivation was indistinguishable from that of L5CT inactivation alone (Figure 6H). While we cannot definitively prove that these same neurons receive input from L6CTs in V1, our prior demonstration of the retinotopic organization of both L5 and L6 CT projections (Figure 5) would support this inference. Thus, these experiments conclusively show that individual pulvinar neurons can be driven by specifically L5, but not L6, CT inputs from V1.
While we have demonstrated that the L5 CT pathway from V1 “drives” a subset of retinotopically aligned pulvinar cells, the full extent of visual activity observed in the pulvinar is not accounted for. When we virally ablated L5ETs (or L6CTs) in V1 to further ensure the complete inactivation of these populations, we still observed robust visual responses in the pulvinar – even in some neurons whose RFs aligned with the cortical area of ablation (Figure S7). Although chronic ablation has the potential to induce compensatory mechanisms, these results are consistent with our optogenetics experiments, where we achieved transient and virtually complete L5ET inactivation and still observed residual visual responses (Figures 4 and S4) and even some unaffected yet retinotopically aligned pulvinar cells (Figure S5E). Together, these findings suggest that V1 L5ETs contribute significantly to but are not strictly required for visual responsiveness in the pulvinar. We were therefore interested whether inputs other than from V1 might also play prominent roles in shaping pulvinar activity.
Cortical and subcortical “driving” pathways converge in the lateral pulvinar
In the lateral pulvinar (lPulv) in particular, an important source of additional input may come from the superior colliculus (SC)32. Tectopulvinar synapses exhibit a mixture of “modulator”- and “driver”-like characteristics7,56,57. Moreover, recent studies have shown that the SC, but not the cortex, drives activity in the caudomedial pulvinar (cmPulv)22,23. While our experiments with V1 CT manipulations did not concern this region because it does not receive direct V1 input32, lPulv, which we did record from, is innervated by both V1 and the ipsilateral SC. Although axons emanating from topographically aligned regions of V1 and SC are largely segregated within lPulv (Figure 7A), cortical and SC terminals have been found in close proximity at the electron microscopic level57,58. Moreover, pulvinar neurons have wide-reaching dendrites that can even stretch across pulvinar subdivisions32, and short-latency effects of both V1 and SC stimulation on single pulvinar cells have been described59. Therefore, we were interested: a) whether the visually responsive cells we recorded in lPulv that were unaffected by L5ET inactivation would be suppressed instead by SC inactivation; and b) whether individual neurons in lPulv might receive convergent L5ET and SC “driving” inputs.
Figure 7. Subsets of neurons in the lateral pulvinar (lPulv) are driven by V1 L5ETs, the superior colliculus (SC), or both.
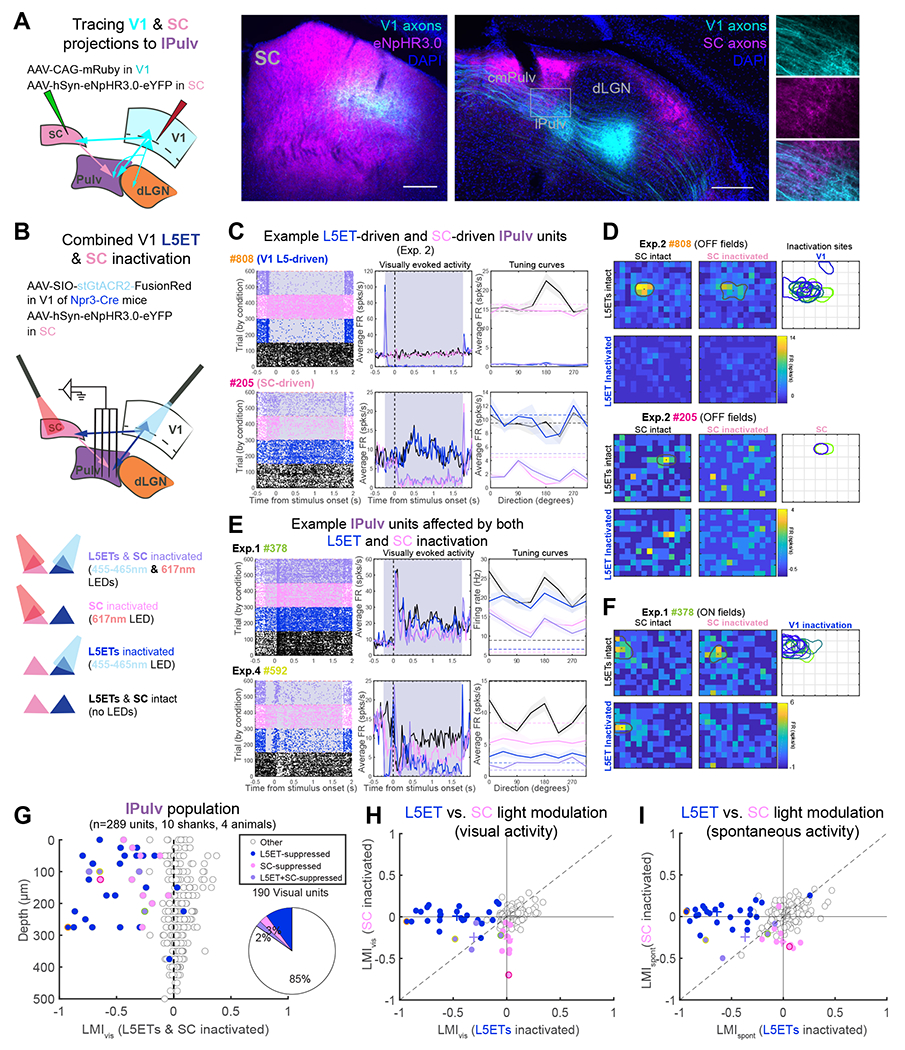
(A) Anterograde tracing of V1 and SC projections to the pulvinar. AAV injection of eNpFIR3.0-eYFP overlaps with V1 axons in the SC (middle), yet cortical and SC axons are largely segregated with minor overlap in lPulv (right, with higher magnification of boxed area in each channel at far right). Scale bars = 200μm.
(B). Experimental schematic for dual-optogenetic inactivation of V1 L5ETs and SC during pulvinar recordings. Bottom: four inactivation conditions.
(C) Two example lPulv units, recorded on the same shank in the same experiment. From left to right: raster plots with trials organized by inactivation condition; PSTFIs of average visually evoked FRs; and mean FRs in response to different drifting grating directions (shading indicates ±1 SE, dashed lines indicate spontaneous FRs).
(D) Receptive fields (RFs) of units from (C) under different inactivation conditions. RF maps depict average baseline-subtracted firing rates at the timepoint of peak response across all OFF stimulus (black square) positions. Outlines and dots depict estimated RF boundaries and centroids (see Methods) under each condition. Right: overlayed RFs of V1 (upper) and SC (lower) units recorded in the same animal.
(E) Example units that exhibit combined effects of L5ET and SC inactivation. Same plot descriptions as for (C).
(F) Unit#378’s (from E) RF under different inactivation conditions. Right: Overlayed RFs of simultaneously recorded V1 units. SC was not recorded from in this animal. No RF information from Exp. #4 (for unit #592).
(G) LMIvis from combined L5ET & SC inactivation trials, by depth. Units are classified according to whether their visual and/or spontaneous activity was significantly suppressed by SC inactivation, L5ET inactivation, or both (see Methods for details). Color-outlined dots correspond to example units in (C) and (E). Right: proportion of different suppressed unit classes out of all visually responsive units.
(H, I) LMIvis (H) and LMIspont (I) for each unit in L5ET inactivation versus SC inactivation conditions (same unit classification as in G). Crosses indicate medians of each unit class. See also Figure S8.
To address these questions, we recorded single-unit activity from lPulv in awake mice expressing stGtACR2 in L5ETs (using Npr3-Cre mice) and halorhodopsin in the SC, thereby allowing us to use different LEDs to inactivate L5ETs and/or SC (Figure 7B). We also made recordings from V1 (data in Figures S4C-G) and the SC (Figures S8E-I) to verify the efficacy of our optogenetic manipulations. Not only were there individual units in lPulv which were strongly suppressed by L5ET inactivation, but we also found units, even in the same recording penetration, which were instead suppressed specifically by SC inactivation (Figure 7C). These example units were “driven” selectively by V1 L5ETs or the SC, as their responses to sparse noise stimuli presented within their RFs were abolished by optogenetic silencing of only their cortical or subcortical inputs, respectively (Figure 7D).
Across our recorded population, we observed a wide range of effects of V1 L5ET versus SC silencing (Figure 7G). Despite the dorsal-ventral distribution of SC and V1 axons in lPulv (Figure 7A), units whose visually evoked and/or spontaneous firing rates were significantly suppressed only by L5ET inactivation or by SC inactivation (L5ET-suppressed or SC-suppressed, respectively) were interspersed (Figure 7G). While most of the modulated units were only L5ET- or SC- suppressed (i.e., units falling along either axis in Figures 7H and 7I), a handful of units were suppressed under both L5ET and SC inactivation conditions (lower left quadrants of Figures 7H and 7I).
Indeed, we found a number of examples of combinatorial effects of L5ET and SC silencing on individual units. For instance, the example L5ET-driven unit (Figure 7C, #808) was also suppressed by SC-only inactivation, but exclusively in the presence of its preferred direction stimulus. Other units’ spontaneous and/or visual activity were suppressed to a greater degree by combined SC and L5ET inactivation than by either alone (Figure 7E, #592), while other units showed distinct effects of SC versus L5ET inactivation mainly in their RFs (Figures 7E and 7F, #378). Cases such as these are suggestive of some degree of visual “drive” coming from both cortical and subcortical sources. We made similar observations of cortical and subcortical inactivation having varied and sometimes combinatorial effects on individual units in additional experiments in which V1 (rather than L5ETs specifically) were inactivated with the SC (Figures S8A-D).
We have therefore shown that even within a region of the pulvinar that receives L5 “driving” input from V1, sources of visual drive can be heterogenous and mixed. Indeed, some lPulv units are driven by SC projections, and some even receive varying degrees of visual drive from both sources. Given our previous demonstration of the retinotopic specificity of L5ET excitation (Figure 5) and the fact that L5ET and SC inactivation centers were not always retinotopically aligned (e.g., Figure 7D), we may well underestimate the extent of combined cortical and subcortical influences. Regardless, we have shown that cortical (V1 L5CT) driving projections converge with subcortical (SC) driving inputs in the lateral pulvinar to shape visual activity in the HO thalamus.
Discussion
What roles do two, seemingly parallel corticothalamic pathways from the primary visual cortex play in thalamic processing in an awake animal? The distinct characteristics of L5 versus L6 CT inputs have for decades led to the idea that these are not redundant pathways but rather distinct “driving” versus “modulatory” projections10,11,14. This idea has been highly influential to the study of corticothalamic circuit organization and function but never directly tested, largely due to technical limitations in selectively silencing specific CT pathways and assessing downstream effects on the thalamus in vivo. Utilizing cell type-specific transgenic mouse lines, optogenetics and high-density, multielectrode recordings of single-unit activity in awake mice, we find pronounced differences in how these populations influence sensory responses in the visual thalamus. While both L5 and L6 CT projections from V1 provide retinotopic excitation to their thalamic targets, the extent to which that excitation imparts visual information differs considerably. Our inactivation studies demonstrate that V1 L6CTs provide some degree of baseline drive, mainly onto retinotopically aligned cells of the dLGN, yet are not required for visual responses in either FO or HO visual thalamus. In contrast, silencing the L5CT pathway from V1 suppressed visually evoked as well as baseline activity in many retinotopically matched pulvinar neurons, some of which relied entirely on that input for their tuning and/or RF properties (e.g., Figure 7C-D). Therefore, our results affirm a longstanding hypothesis that the L5, but not the L6, CT pathway from V1 constitutes a functionally “driving” pathway that conveys visual information to the rodent pulvinar, whereas the L6 CT pathway is fundamentally “modulatory”.
While we were able to selectively inactivate L5ET versus L6CT subpopulations by using different transgenic mouse lines, there were still some limitations of our approach. For instance, although the Ntsr1-Cre line is selective for L6CTs that project to the dLGN40, not all project to the pulvinar (Figure 1E). If anything, this might have led us to overestimate the influence of V1 L6CTs in the pulvinar, yet we saw minimal impact of their inactivation. On the other hand, because L6CTs exert inhibitory gain control within V138,39 they could, in theory, have indirect effects on pulvinar activity by inhibiting L5CTs. However, the facilitatory effects of L6CT inactivation on other layers were mainly confined to the upper layers (Figure S2A), and L6CT inactivation did not facilitate putative L5CTs (Figures 6C and 6E). These findings are consistent with reports that L6CTs do not inhibit (and can even excite) layer 560 and make it unlikely that intracortical effects of L6CT inactivation can explain the consequences (or lack thereof) of L6CT inactivation on the pulvinar. Another consideration is that many L6 projections to the rodent pulvinar come from extrastriate areas4,5,30,31 (Figure 1B), which were not targeted by our injections. Our findings of fundamentally similar “modulatory” influences of V1 L6CTs in the dLGN and pulvinar (from our present inactivation and prior activation studies41) would lead us to expect that the cumulative effect of L6CT inactivation across cortical areas on the pulvinar would be similar to what we observed in this study in the dLGN (which gets the majority of its L6CT input from V161,62) – namely, suppressed spontaneous but not visual activity. Since L6CTs themselves have low spontaneous firing rates (Figure S2C), we attribute their effect on baseline thalamic activity to the high degree of convergence of many L6CTs onto single thalamic cells6,15 and to the fact that our recordings were conducted in awake animals as opposed to under anesthesia, which reduces spontaneous firing rates in the thalamus63. Additionally, our L6CT inactivation approach most likely excluded L6b cells that exclusively project to HO thalamus and exhibit “driver”-like characteristics64,65. Although we did not observe many pulvinar-projecting L6CT neurons that might fall within this category (retrogradely labeled tdT-negative cells in Ntsr1-Cre/Ai14 mice, Figure 1E), whether they would more closely resemble L5 “drivers” or other L6 “modulators” in terms of their contributions to functional response properties in the pulvinar in vivo is an open question. Altogether, our results demonstrate that V1 L6CTs do not drive visual responsiveness in the pulvinar (as in the dLGN) and thus can be considered fundamentally modulatory. However, their influence on spontaneous activity and in the contexts of attention, arousal, and other behavioral states61,66–68, require further study to clarify the precise “modulatory” functions of these projections.
Meanwhile, our primary strategy for silencing L5 CT projections was to use the Npr3-Cre mouse line to restrict inhibitory opsin expression to L5ET neurons in V1. This offers a marked improvement in specificity from other studies that have relied on non-specific cortical inactivation22–25, which would be expected to also suppress activity in other cortical areas69 and thus confound the driving influence of V1, specifically L5ETs, on the pulvinar with that of extrastriate cortex. Indeed, we observed somewhat more widespread pulvinar inactivation when we non-specifically inactivated cortex, even when that inactivation was confined to V1 through AAV injection (Figure S6A-F). Still, not all our inactivated L5ETs project to the pulvinar (Figure 1F), as these neurons can innervate any combination of multiple subcortical areas (including pons and SC, Figure S1, and striatum70). In fact, considering ours (Figure 7) and others’ demonstrations of SC driving influence on the pulvinar22,23, it is possible that some of the suppression we observed in the pulvinar from L5ET inactivation could be mediated indirectly by the SC. However, we still observed suppressed pulvinar activity when we restricted our L5 inactivation to L5CTs (Figure 6) and found largely separable effects of SC and L5ET inactivation in the lateral pulvinar (Figures 7 and S8A-D) and even within the SC itself (Figures S8E-I). Altogether, this would suggest that L5ETs do not rely on an indirect pathway through the SC to drive visual responses in the pulvinar. Another caveat to our L5ET and L5CT silencing approaches is that our area of inactivation was limited by the spread of our AAVs (Figure S5F) and likely did not encompass all of V1. However, this limitation was addressed by assessing the effects of L5ET inactivation on retinotopically aligned pulvinar neurons (Figures 5 and S5E-H). The fact that half of those cells were still unmodulated by V1 L5ET inactivation suggests that there may be other “driving” inputs to the pulvinar.
Indeed, the pulvinar is innervated by a number of other cortical and subcortical inputs whose in vivo functional influences are not yet known. First, it receives inhibitory input from areas like the ventral LGN (vLGN), the zona incerta and the anterior pretectal nucleus – all of which receive collaterals from L5ETs5,18,71–73. These inhibitory pathways might underlie some of the excitatory effects we observed in the pulvinar with L5ET inactivation (Figures 4C and 4D). Meanwhile, the rodent pulvinar also receives excitatory L5 input not just from V1, but also from extrastriate areas4,5,30,31 (Figure 1B). Based on our observations of some retinotopically aligned pulvinar units that were unaffected by L5ET inactivation (Figures 5F and S5E) or ablation (Figures S7H-J) – similar to what was observed in the cat following V1 cooling24 - we predict that L5ETs in extrastriate areas may provide additional driving inputs. Further work is needed to conclusively address this possibility and whether L5 inputs from multiple cortical areas could provide combined, functionally driving input to thalamic neurons, as was suggested by a recent anatomical study36.
Our results also show that subcortical excitatory inputs from the SC can intermingle with L5 driving inputs within the lateral pulvinar and even converge onto individual neurons. In fact, while relatively rare, we found cases of units whose firing rates, tuning and/or receptive fields were influenced by both L5ET and SC inactivation (Figures 7 and S8A-D). This would suggest that some pulvinar neurons integrate information conveyed by cortical (L5) and subcortical (SC) sources. This inference is supported by prior reports of cortical and subcortical stimulation having combined effects in the cat LP-pulvinar complex74 and in the rodent HO somatosensory thalamic nucleus, POm75 Given the role of the SC in processing object motion, eye movements, and spatial attention76, integration of various visual, motor, and other signals within the pulvinar could play a key role in sensory-guided behaviors. This idea is in line with evidence that the rodent pulvinar conveys contextual information (e.g., a mismatch between visual flow and running speed) to extrastriate areas5 and even back to V14. It is notable, however, that SC inactivation/lesion was shown to considerably impair visual responses in regions of the pulvinar homologous to the mouse lPulv52 in rabbits59, but not in macaque monkeys25. It is therefore possible that subcortical inputs might play a more prominent role in shaping pulvinar visual response properties in non-primates59.
Altogether, our results demonstrate a striking functional dissociation between L6 and L5 corticothalamic pathways. Many notable distinctions between FO and HO thalamic nuclei – such as their excitatory input sources10, relative proportions of different synapse types27–29, transcriptional profiles77, and functional properties1 – could have led “driver”-and “modulator”-resembling inputs to differently contribute to activity in the HO compared to FO thalamus. Instead, we find that the effects of inactivating V1 L5 versus L6 CT pathways on visual responses in the pulvinar of awake mice are consistent with their hypothesized “driving” versus “modulatory” functions. Given the similarities in CT synapse types14 and the consistent effects of cortical lesion/inactivation on sensory activity in the HO thalamus across systems and species21–26, we suggest that this functional distinction between L5 and L6 CT projections to HO thalamus is a common organizational principle of corticothalamic function. We also found that the superior colliculus can play important “driving” roles in the rodent pulvinar and converge with the L5 cortical driving pathway; the extent to which this, too, generalizes across species will be of considerable interest for future studies of pulvinar function. Our results thus illuminate how sensory information is routed to the HO thalamus through distinct cortical (and subcortical) channels, which has important implications for understanding how the HO thalamus supports sensation and behavior in the awake animal.
STAR Methods
Resource availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Edward Callaway (callaway@salk.edu).
Materials availability
Plasmid for Flp-dependent stGtACR2-FusionRed (pAAV-Ef1a-fDIO-stGtACR2-FusionRed) that was generated for this study will be made available upon request to the Lead Contact.
Data and code availability
Processed data and relevant code for generating the figures and analyses in this manuscript have been deposited on Mendeley Data (DOI: 10.17632/7cr8w9k5pb.1). Additional code can be found on the Callaway lab’s GitHub (https://github.com/SNLC/openephys/Megan_code_opto). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
The following transgenic mouse lines were used in this study:
Ntsr1-Cre GN22038 - L6CT inactivation and combined L5CT & L6CT inactivation experiments
Npr3-IRES-Cre-neo42 - L5ET-inactivation experiments
PV-Cre and Dlx5-Flp/CCK-IRES-Cre - V1 inactivation by activating PV+/Dlx5+ inhibitory interneurons
PV-Cre/Ai32 - unrestricted V1 inactivation by activating constitutively ChR2-expressing PV interneurons
Ntsr1-Cre/Ai14 - L6CT quantification
Cre-negative animals were also used for control physiology experiments and anatomy experiments not requiring Cre-dependent AAV expression (e.g., Figures S1F-H). Both female and male mice were used between 8-17 weeks of age (more commonly 9-14 weeks) at the time of AAV injection and <20 weeks at the time of in vivo recordings. All experimental procedures followed protocols approved by the Salk Institute Animal Care and Use Committee.
METHOD DETAILS
Surgeries
For injections, mice were anesthetized with a ketamine/xylazine cocktail (100mg/kg ketamine, 10mg/kg xylazine) via intraperitoneal injection and secured in a stereotax (David Kopf Instruments Model 940 series). A small craniotomy was made at the injection site, and a glass pipette (24-30μm tip diameter) was lowered to the desired depth and was left for 3-5 minutes before injecting. AAV or a cholera toxin subunit B-Alexa Fluor conjugate (see below) was pressure-injected with a syringe at an approximate rate of 20μl/minute and let rest for at least 5 minutes (10+ minutes for subcortical injections) before raising to prevent backflow. The following stereotactic coordinates were used for injection sites (all left hemisphere, in mm relative to bregma; A/P, M/L, D/V):
V1: −3.2, −2.65, −0.64-0.3 (2 depths)
lPulv: −1.6, −1.8, −2.45
rmPulv: −1.05, −1.2, −2.55
SC, medial site: −3.5, −0.45, −1.1-1.2; SC, lateral: −3.5, −0.8, −1.25-1.3
Pons: −3.5, −0.85, −5.75-5.9
lateral visual cortex (VisL): −2.6-2.75, −3.8, −0.45-0.3 (2 depths)
At the end of injections, the skin incision was closed with Vetbond (3M) and mice were given a subcutaneous injection of buprenex (0.5-1.0 mg/kg) and Ibuprofen in their water bottles.
At least four days and up to 1 week prior to recordings, mice underwent an acute surgery for headframe implantation. Under isoflurane anesthesia, skin was cut away and a circular headframe (7-mm inner diameter) was secured to the skull with dental cement (C&B-Metabond, Parkell). A dull pipette attached to a micromanipulator (MP-285, Sutter Instrument) was used to relocate bregma and mark positions with a waterproof pen for aiming craniotomies for thalamus recordings (coordinates relative to bregma: 1.50-2.75mm lateral, 1.8-1.9mm posterior for lPulv, 1.0-2.0mm lateral, 1.0mm posterior for rmPulv). The exposed skull was covered with a silicone elastomer (Kwik-Cast, World Precision Instruments) and mice were given a subcutaneous injection of buprenex (0.5-1.0 mg/kg), Ibuprofen in their water bottles, and at least one full day undisturbed in their cages.
Viruses
The following AAV vectors were used for optogenetics experiments in this study:
AAV5-EF1a-DIO-eNpHR3.0-eYFP (120-200nl, 3-4e12 GC/ml, UNC) - for L6CT/L5ET halorhodopsin inactivation
AAV1-hsyn1-SIO-stGtACR2-FusionRed (120-200nl, 0.9-1.8e12 GC/ml, Addgene) - for stGtACR2 inactivation
AAV5-Ef1a-fDIO-stGtACR2-FusionRed (200-240nl, 1.77e12 GC/ml, Salk Vector Core) - for L5CT inactivation
AAV5-hsyn-eNpHR3.0-eYFP-WPRE-pA (60-70nl, 5.5e12 GC/ml, UNC) - for SC inactivation
AAV1-EF1a-fDIO-hChR2(H134R)-EYFP (150nl, 7.78e11 GC/ml, Salk Vector Core) - V1 inactivation in Dlx5-Flp/CCK-IRES-Cre mice
AAV1-EF1a-DIO-hChR2(H134R)-eYFP.WPRE.hGH(Addgene20298P) (100nl, 8.4e12 GC/ml, uPenn Vector Core) - V1 inactivation in PV-Cre mice
The Cre-dependent halorhodopsin AAV was injected into V1 of Ntsr1-Cre mice and was allowed up to 6 weeks for expression (as well as for non-specific halorhodopsin expression in the SC). However, slightly smaller (120-150nl) halorhodopsin injections were made in Npr3-IRES-Cre-neo mice and restricted to 3 weeks expression time because expression in other layers and an unhealthy-looking L5 appeared at longer timepoints and/or with larger injection volumes in this mouse line. Consequently, animals in which axons were visible in areas not targeted by L5ETs (e.g., TRN) were excluded.
Viruses for anterograde tracing were:
AAV5-CAG-FLEX-GFP (120-160nl, 5.2GC/ml, Salk Vector Core)
AAV5-EF1a-DIO-eYFP (120-180nl, 2-4e12 GC/ml, UNC)
AAV8-CAG-mRuby2 (100nl, 2.4 GC/ml, Salk Vector Core)
For Flp-dependent stGtACR2 expression in L5CTs, we injected 20-40nl of AAVretro-EF1a-Flp (3.6e11, Salk Vector Core) into both lPulv and rmPulv injection sites. For retrograde tracing of L6CTs, we injected 40nl of cholera toxin subunit B (CTB) conjugated to Alexa Fluor 488 (0.5%) or 647 (0.25%; Thermo Fisher) into the pulvinar. In 2/3 of these experiments, we injected one of each of these CTBs into lPulv and rmPulv sites (remaining animal was only injected in lPulv). For retrograde tracing of L5CTs, we used a self-complimenting (sc)AAVRetro-hSyn-mCherry (1.51e12 GC/ml, Salk Vector Core) because we observed more widespread and brighter labeling of L5CTs than with CTB. 20nl were injected into both lPulv and rmPulv injection sites.
For diphtheria toxin experiments, we injected 120-170nl of a mixture of AAV8-mCherry-flex-dtA (3.7e12 GC/ml, UNC) and either of the DIO-eYFP/GFP anterograde viruses (above, at 1:1 ratio; volume split across two V1 injection sites in one Npr3-Cre animal), as well as a 1:2 dilution of the same anterograde virus at the same injection sites and volumes in the right hemisphere. Mice were recorded from 10-15 days later.
In vivo electrophysiology
Following 2-4 days of habituation to the running wheel at the recording rig (30+minute sessions), mice were anesthetized with isoflurane to make craniotomies above the desired recording sites. Mice were then head-fixed on their wheel, where they were free to run at their will and their movement was tracked with a rotary encoder. A black curtain was secured around the headframe holder to prevent the mice from seeing light from optogenetic stimulation. Individual recording sessions typically lasted 2-3 hours. In the case of multiple recording sessions from a single animal, mice were given at least 30 minutes and up to a day undisturbed in their cages with food and water between sessions.
For our recordings, we used 64- or 128-channel silicon microprobes45,78 connected to an Intan RHD2164 128-channel amplifier board. Data was acquired at 20kHz with the OpenEphys data acquisition system79. For the majority of recordings, V1/SC and thalamus recordings were conducted simultaneously using two separate 128-channel amplifier boards (thus 192-256 channels recorded simultaneously through OpenEphys). For V1 and SC recordings, we used either 64D (64 channels on one shank, 1.05mm vertical extent of electrodes) or 128AN (two shanks each with 64D configuration, separated by 300μm) probe configurations. For thalamus recordings, we always used a 4-shank probe (128DN or 128D; 32 channels per shank over 775μm depth, 150μm or 330μm separation between shanks, respectively). All probes were gold-electroplated with an Intan RHD electroplating board to reach ~0.2MΩ electrode impedances.
Probes were lowered slowly into the brain with a digital and/or manual micromanipulator (MP-285, Sutter Instrument Co; 1760-61, David Kopf Instruments also used for simultaneous V1/SC recordings, 80° orientation) to approximate depths of 1.1mm (V1), 1.5mm (SC), or 2.5mm (pulvinar). Agarose (~3.5%; A9793, Sigma-Aldrich) was then poured to fill the well of the headframe holder, thus covering the probe shank(s) and the tip of the optical fiber(s); this helped with grounding as well as recording stability. After lowering, the probes were left in place untouched for at least 30 minutes before data acquisition commenced. For all recordings, probes were coated with a 1-2.5% solution DiI or DiD (D282 or D7757, Thermo Fisher) in order to verify recording locations postmortem. For thalamus recordings, probes were typically lowered once without DiI/DiD to check their location (based on presence or lack of highly temporally modulated visual responses, which indicated placement in dLGN) before being raised and relowered with DiI/DiD (for some experiments using 128D probes, they were not raised and relowered with DiI/DiD in the same position until after data acquisition was complete).
Visual stimulation
Visual stimuli were generated through custom MATLAB code using Psychtoolbox and presented on a 24” LED monitor (GL2450-B, BenQ). The monitor screen was positioned 12cm from the mouse’s right eye. For drifting gratings experiments, full-field square-wave gratings were presented at four orientations in eight directions, 0.04 cycles/° spatial frequency, and 2Hz temporal frequency. A full trial consisted of a 0.5-second pre-stimulus period (grey screen), 2 seconds of drifting grating presentation, and 1.5-2 seconds post-stimulus period (grey screen). 20% of trials were “blank” trials, in which the screen remained grey for the full trial duration; these trials were used for assessing effects on spontaneous activity (see Analysis section below). Each unique drifting grating stimulus was presented at least 15 times under each light stimulation condition across the full experiment.
For receptive field mapping, a sparse noise stimulus protocol was used in which individual black or white 8° squares were displayed at random on a grey background anywhere within a chosen areal grid (10x12, 12x14 or 14x16). Each square was presented for 250ms. A “trial” consisted of both black and white squares being presented exactly once at all possible stimulus locations, and the same pattern of stimulus presentations was repeated in each light stimulation condition (see below). Timings of individual stimulus presentations were identified with a photodiode attached to the upper left corner of the monitor, whose luminance changed at each new stimulus presentation (i.e., every 250ms). Across the full experiment, each stimulus (black or white square) was presented at each location in the grid 12-24 times under each light stimulation condition.
For V1 recordings, an additional 2-minute run of 2-second full-field screen flashes was presented at the beginning and end of the recording session. These were used for current source density analysis to identify cortical layers (see Analysis section below).
Optogenetic stimulation
Optogenetic stimulation was controlled via an Arduino Zero microcontroller board, which interfaced with MATLAB through a serial port connection. A 12-bit DAC and/or a digital output pin was connected to an LED driver (ThorLabs T-Cube LED driver or Plexon PlexBright LD-1 single channel LED driver) or a laser module (635nm collimated diode laser, Laserglow Technologies). LEDs were used for most experiments, but the 635nm laser was used for five L6CT-halorhodopsin experiments. For other halorhodopsin stimulation experiments, we used a 617nm fiber-coupled LED module (ThorLabs), and for stGtACR2 and ChR2 experiments we used a 465nm (PlexBright, Plexon) or a 470nm (ThorLabs) fiber-coupled LED module. Alternatively, a 455nm LED (ThorLabs) was used for combined halorhodopsin and stGtACR2 CT inactivation experiments (Figure 5) to avoid the blue LED falling within halorhodopsin’s excitation range. LED/laser light was outputted through a custom optical fiber patch cord (1mm diameter, 0. 39 NA, ThorLabs; or 400μm diameter, 0.39NA, ThorLabs for dual-optogenetics experiments) and positioned as close as possible to the pial surface without hitting the recording probes. Light powers measured from the optical fiber tip (with PM100D with S121C power sensor, ThorLabs) were: 4-5.5mW from 1mm fiber or 0.75-1.0mW from 400μm fiber for halorhodopsin stimulation (617nm LED or 635nm laser); 1mW from 1mm fiber or 0.12-.16mW from 400μm fiber for L5-stGtACR2 stimulation (455nm or 465nm LEDs); 2.2mW from 1mm fiber or 0.25mW from 400μm fiber for L6CT-stGtACR2 stimulation (455nm or 465nm LEDs); 5-10mW from 1mm fiber for AAV-targeted ChR2-stimulation of interneurons for V1 inactivation (470nm LED); and 0.8-1.1mW from 1mm fiber or 0.46mW from 400μm fiber for V1 inactivation in PV-Cre/Ai32 mice (465nm or 470nm LEDs).
In drifting grating experiments, light stimulation began 250ms after the trial start and 250ms prior to the visual/blank stimulus presentation and stayed on for 2 seconds. All drifting grating stimuli (including “blank” stimuli) were presented an equal number of times under every light stimulation condition (including no light). Trials in different light stimulation conditions were randomly interleaved throughout the experiment.
In receptive field mapping experiments, light alternated between ON and OFF in 2 second intervals for the full trial duration, and each unique pattern of sparse noise stimuli was presented with all possible patterns of light stimulation (e.g., 1st repeat: light ON, light OFF…; 2nd repeat: light OFF, light ON…). For dual-optogenetic experiments, there were four possible light stimulation patterns: 1) LED#1 ON and LED#2 OFF, LED#1 OFF and LED#2 ON…; 2) LED#1 OFF and LED#2 ON, LED#1 ON and LED#2 OFF…; 3) LEDs#1&2 ON, LEDs#1&2 OFF…; 4) LEDs#1&2 OFF, LEDs#1&2 ON….
Histology
At the end of recordings, animals were given an intraperitoneal injection of euthasol (15.6mg/ml) and perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were dissected out, post-fixed in 2% PFA and 15% sucrose solution at 4°C for ~24 hours, moved to 30% sucrose at 4°C for another ~24 hours, and then frozen in sucrose and sliced on a freezing microtome. Brains were sliced coronally into 50μm sections, starting from the anterior edge of the hippocampus to the posterior end of cortex. All sections were counterstained with 10μM DAPI in PBS before being mounted and cover-slipped with Polyvinyl alcohol mounting medium containing DABCO. Additional immunohistochemistry for calretinin was performed on thalamic sections (except in cases with DiD and both red- and green-fluorescent axons in the thalamus; e.g., combined SC and L5/V1 inactivation experiments) by incubating at 4°C for 16-20 hours with rabbit anti-calretinin primary antibody (1:1000; Swant 7697) in 1% Donkey Serum/. 1% Triton-X 100/PBS, followed by donkey anti-rabbit conjugated to Alexa 647 (1:500; A-31573, Life Technologies) before DAPI counterstaining. Imaging was performed on an Olympus BX63 microscope (10x objective for most images, 20x for cortical z-stacks for cell counting) or a Zeiss LSM880 confocal microscope (20x).
QUANTIFICATION AND STATISTICAL ANALYSIS
Data Processing
We used Kilosort280 to semi-automatically spike-sort extracellularly recorded data. First, different recordings from the same recording session (drifting gratings and RF mapping experiments) were concatenated together into a single binary file. We then subtracted out the large voltage deflections caused by light onsets and offsets prior to high-pass filtering (>150Hz) in Kilosort2 because otherwise we found that spike detection within ~10ms of the light onset/offset times was compromised. Additional “spikes” were removed within 1.5ms of the light onset/offset times to ensure that no further optogenetic artifacts were included in the analyses.
Phy2 was used to validate and further curate as needed the clusters outputted by Kilosort2. In some experiments where optogenetic artifacts couldn’t be completely removed in Kilosort2, they were typically easily removed in Phy2 because they appeared as outliers in its principal component features view. Units were considered “good” single-units whose waveforms clearly deviated from the noise, had a clear refractory period in their auto-correlogram, and no evident refractory period in their cross-correlogram with other units. From there, “good” units were included in subsequent analyses which had fewer than 0.5% refractory period violations, visually evoked and/or spontaneous firing rates ≥0.25Hz, and “unit quality” (isolation distance81) greater than 16.
Unit classification
For thalamus recordings, the fluorescent traces left by the lipophilic dyes (DiI or DiD) on the probe shanks were identified in histology and used to assign all units recorded from each shank to a particular thalamic nucleus. This was aided by calretinin immunohistochemistry, which provided clear boundaries between rmPulv/cmPulv (calretinin+ cell bodies), lPulv (calretinin−), and dLGN (calretinin+ axons). While calretinin does not distinguish between rmPulv and cmPulv, the distinction was clear because V1 axons were only in rmPulv, but not cmPulv32. Thus, for pulvinar recordings, we only included units in our analyses that were recorded on shanks that passed through fluorescent V1 axons (in the absence of V1 axons, e.g., L5ET-dtA experiments, we compared our calretinin staining and the shape of the dentate gyrus to the Allen Brain Institute’s Reference Atlas and our own images with both calretinin staining and V1 axons). Because the boundaries of dLGN were perfectly clear with calretinin staining, any shanks in the dLGN, even if they did not pass through L6CT terminals, were included in our analyses. The dorsal/ventral boundaries of our thalamic recordings were determined by assessing visual responsivity (see below for criteria for being “visually responsive”); since the top channels of our recording probe were typically in the hippocampus and thus virtually silent, we considered the most dorsal channel with a visually responsive unit as the top of dLGN/pulvinar (hence, “depth” in LMI plots throughout this study are relative to the position of this first channel). Similarly, the ventral boundary of the thalamus was identified as the last channel with a visually responsive unit, and all units recorded from those and intervening channels were included in analyses.
Units were considered “visually responsive” if there was a significant difference in spike counts during either the initial (first 200ms) or sustained (250-1750ms) period following the onset of the drifting grating stimulus, compared between preferred visual stimulus trials (i.e., the direction with the biggest difference from baseline) and blank trials (using the Wilcoxon rank-sum test with p=0.025 significance threshold) in which the mouse was stationary. For non-optogenetic experiments (i.e., diphtheria toxin experiments), there were no “blank” trials and so instead the first 200ms or sustained 500ms (250-750ms) periods from visual stimulus onset were compared to the same durations at the start of the trial (pre-stimulus) with the paired Wilcoxon signed-rank test.
A similar approach was used to determine the significance of a unit’s light modulation; spike counts during the sustained (250-1750ms from visual stimulus onset) period were compared between blank (stationary) trials with and without light stimulation. We used the Benjamini-Hochburg method for false discovery rate correction to limit the estimated rate of false positives among our modulated cells to <10% (typical significance threshold ~p=0.025, no less than 0.01). These significantly modulated cells were then classified as “suppressed” or “enhanced” based on whether their spontaneous light modulation index was less than (suppressed) or greater than 0 (enhanced). In joint SC- and L5ET/V1-inactivation experiments where we sought to separately assess the effects of four different light stimulation conditions (no light, SC-inactivation, L5/V1-inactivation, SC&L5-inactivation), instead of the rank-sum test we used the Kruskal-Wallis test with the Dunn–šidák post-hoc test. We did this separately for both visual (preferred direction) and blank trials and designated units as SC-suppressed, L5/V1-suppressed, or suppressed by both if comparisons were significant for either their visual or spontaneous activity (since we noticed that unlike with L5/V1-inactivation, SC inactivation often impacted visual but not spontaneous activity59). As an additional catch for false positives, units had to be significantly (p<0.025) suppressed in their visual or spontaneous activity during the combined inactivation condition (L5/V1- and SC-inactivation) as well as in at least one other inactivation condition to be classified as SC-suppressed, L5/V1-suppressed, or suppressed by both.
For V1, the short recordings of cortical activity in response to screen flashes (see Visual Stimulation above) were used for current source density (CSD) analysis to determine recorded units’ laminar position. We used CSDPlotter82 to compute the second spatial derivative of the low-pass-filtered (<1000Hz) local field potential during the transitions between screen-off and screen-on periods. We then assigned electrode channels to cortical layers based on typical spatiotemporal patterns of current sources and sinks immediately following a screen flash (see Figure 2C), and units whose largest voltage deflection was picked up from a particular channel were assigned to that channel’s layer. Units were classified as “fast-spiking” if the time from the trough to the peak of their waveforms was less than .475ms (which typically marked a clear division in the bimodal distribution of trough-to-peak times); all others were considered “regular-spiking”. Units were considered putatively opsin-expressing (“inactivated” units) if they were regular-spiking, in the expected layer (L5 or L6 in Npr3-Cre and Ntsr1-Cre mice, respectively), their visually evoked activity was significantly light modulated (as described above), and LMIvis <−0.33 (i.e., >50% suppression of visually evoked activity).
Quantification and analysis – drifting gratings experiments
Average visually evoked and spontaneous firing rates (FRvis and FRspont) were calculated in the window 250-1750ms after stimulus onset across visual and blank trials, separately for trials in different light stimulation conditions. Only “stationary” (speed < 2cm/s) trials were used for these calculations because running has been shown to affect firing rates in V183 and the thalamus84, and our mice did not run very often (typically <10% of trials). Light modulation indices (LMIs) were then calculated as the difference divided by the sum of average firing rates (FRvis or FRspont) in no-light versus light stimulation conditions. The visually evoked FR change (Figure 4G) was calculated as the absolute value of the difference between FRvis and FRspont, separately for each light condition. F1 responses were calculated by computing the Fast Fourier Transform (FFT) of the baseline-subtracted (baseline from the same time window in blank trials for each light stimulation condition separately) average peristimulus time histogram (10ms bins) across preferred direction trials during the sustained response period (250-1750ms after visual stimulus onset). The F1 response was then the component of the resulting spike-response amplitude spectrum at the temporal frequency of the visual stimulus (2Hz). Bursting rates were calculated as the ratio of the number of spikes occurring in bursts to the total number of spikes during the sustained stimulation period in visual and stationary trials, separately for each light condition. Burst spikes were defined as those preceded by an inter-spike interval (ISI) ≥ 100ms and followed by an ISI ≤ 4ms (first spikes in a burst), as well as all subsequent spikes which were preceded by an ISI ≤ 4ms.
Quantification and analysis – RF mapping experiments
For each unit, we generated a peristimulus time histogram matrix M of average baseline-subtracted firing rates for each unique stimulus type (white or black square at each possible stimulus position under each light stimulation condition; baseline = mean of first 20ms) at each 10ms time bin following the start of the stimulus (250ms total stimulus presentation = 25bins). We then performed singular value decomposition (SVD) of this matrix M:
to extract spatial (U1,i) and temporal (V1,j) filter approximations of the spatiotemporal receptive field.
The following criteria were then used for identifying significant receptive fields:
The relative variance of the first singular value was >10%, AND the peak of the temporal filter (V1,j) exceeded the mean over the first three time bins by at least 5 standard deviations.
The “spatial SNR” of U1,i (reshaped to U1,x,y) - the ratio of the maximum variance at any x/y position to the mean variance at the furthest two x/y positions, across x and y dimensions separately - exceeded the 2.5% tails of a null distribution of spatial SNRs (from 1000 shuffles of entries in U1,x,y) in either x or y dimensions. In other words, there was a non-random spatial organization of the estimated receptive field. OR, units also passed this step if a value of U1,x,y exceeded 5 standard deviations above the mean of U1,x,y (otherwise, units with very small RFs - most common in dLGN - did not pass the spatial SNR criterion).
At least one value of U1,i was significant relative to a null distribution (from SVD of 500 shuffled M matrices), with a significance threshold corrected for <20% FDR (Benjamini-Hochburg method).
For units who passed these criteria for at least 1/4 of their computed receptive field estimates (on and off subfields, light on and light off conditions), we then used the p-values generated in step 3 as a mask for the spatial filter map (U1,x,y). This significant RF map was then bilinearly interpolated to 1° resolution, smoothed with a 2D Gaussian filter (σ=4), and binarized, from which the borders and area of the estimated RF could be determined.
For analyses of retinotopic alignment, we used the estimated receptive field OFF- and ON-fields exclusively from no-light (i.e., control) conditions. RF distance was calculated as the magnitude of the vector connecting the center of all RFs from a single V1 penetration (median x and y positions of all individual unit RF centers) to the center of each thalamic RF, separately for OFF- and ON-subfields. Whichever RF distance (between OFF- and ON-subfields) was shorter was that unit’s final RF distance used for analysis in Figure 5.
For ease of interpretation, all RFs in this manuscript are illustrated as baseline-subtracted firing rates (pre-SVD) at the peak timepoint (i.e., j at which V1,j is maximal). When the peak timepoint differed between light stimulation conditions, the same peak timepoint (from the no-light condition) was used for all conditions.
Quantification – cell counting
For quantification of L6CT cells in Ntsr1-Cre/Ai14 mice, we acquired z-stack images at 20x magnification with a Zeiss LSM880 confocal microscope. The counted region was restricted to the area of V1 (as identified by a thicker L4 in the DAPI channel and the relative lack of retrograde labeling in upper L6 relative to more lateral and medial cortical areas, as consistently described in other studies4,5,31. Within this area, L6 was then split into partitions (as in 85); cells in the upper 10-40% of L6 were counted as L6upper, cells in the lower 60-110% were counted as L6lower, and all cells throughout the full depth of L6 were included for “all L6” quantification. Only animals in which the CTB injection was confined to the pulvinar and did not spread into dLGN were included in this quantification (n=3). Two or three sections spaced 200-400μm apart were counted per animal using FIJI.
For quantification of L5ETs, we acquired z-stack images at 20x magnification with an Olympus BX63 microscope. For quantification in Npr3-Cre mice, the area of GFP expression (from AAV injection) and the mCherry+ pulvinar axons (dense in L4 of extrastriate areas but not V1) were used to set the counting boundaries in V1. Three sections spaced 50-150μm apart were counted in each of four animals. For quantification of L5ETs with different subcortical targets, cells retrogradely labeled from the SC with CTB-647 were the most spatially restricted and thus were the main determiners of counting boundaries in V1 (V1 boundaries identified by mCherry+ axons as before). Since the SC injection was also the most difficult because of its close proximity to retrosplenial cortex, animals were only included for quantification if all three injections were well targeted and yielded retrograde labeling in V1 but not in the LD thalamic nucleus (a major input to retrosplenial cortex; n=3). Five sections, each separated by 150μm, were counted for each animal. FIJI software was used for all cell counting.
Statistical analysis
For paired comparisons, we used the Wilcoxon signed-rank test. For independent comparisons with two groups, we used the Wilcoxon rank-sum test, and for more than two groups we used the Kruskal-Wallis test with the Dunn–šidák post-hoc test for multiple comparisons. Statistical significance was assessed at the 0.05 level unless otherwise specified.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-calretinin | Swant | Cat#7697 |
| Donkey anti-rabbit, Alexa Fluor 647 | Life Technologies | Cat#A-31573 |
| Bacterial and virus strains | ||
| AAV5-EF1a-DIO-eNpHR3.0-eYFP | UNC Vector Core | n/a |
| AAV1-hsyn1-SIO-stGtACR2-FusionRed | Addgene | Cat#105677-AAV1 |
| AAV5-Ef1a-fDIO-stGtACR2-FusionRed | Salk Vector Core GT3 | n/a |
| AAV5-hsyn-eNpHR3.0-eYFP-WPRE-pA | UNC Vector Core GT3 | n/a |
| AAV1-EF1a-fDIO-hChR2(H134R)-EYFP | Salk Vector Core GT3 | n/a |
| AAV1-EF1a-DIO-hChR2(H134R)-eYFP.WPRE.hGH | UPenn Vector Core/Addgene | Cat#20298-AAV1 |
| AAV5-CAG-FLEX-GFP | Salk Vector Core GT3 | n/a |
| AAV5-EF1a-DIO-eYFP | UNC Vector Core GT3 | n/a |
| AAV8-CAG-mRuby2 | Salk Vector Core GT3 | n/a |
| AAVretro-EF1a-Flp | Salk Vector Core GT3 | n/a |
| scAAVRetro-hSyn-mCherry | Salk Vector Core GT3 | n/a |
| AAV8-mCherry-flex-dtA | UNC Vector Core GT3 | n/a |
| Chemicals, peptides, and recombinant proteins | ||
| Cholera toxin subunit B – Alexa Fluor 647 conjugate | Thermo Fisher | Cat#C34778 |
| Cholera toxin subunit B – Alexa Fluor 488 conjugate | Thermo Fisher | Cat#C34775 |
| C&B Metabond Quick Adhesive Cement System | Parkell | Cat#S380 |
| DiI | Thermo Fisher | Cat#D282 |
| DiD | Thermo Fisher | Cat#D7757 |
| Agarose | Sigma-Aldrich | Cat#A9793 |
| DAPI | Thermo Fisher | Cat#D1306 |
| Triton X-100 | Thermo Fisher | Cat#AC327371000 |
| Gold plating solution | NeuraLynx | n/a |
| Deposited data | ||
| Data for figures | Mendeley Data | DOI: 10.17632/7cr8w9k5pb.1 |
| Experimental models: Organisms/strains | ||
| Mouse: Ntsr1-Cre GN220 | MMRRC | MMRRC:030648-UCD |
| Mouse: Npr3-IRES-Cre-neo | Gift from Dr. Hongkui Zeng, Allen Institute | n/a |
| Mouse: PV-Cre | JAX | B6;129P2-Pvalbtm1(cre)Arbr/J JAX Stock #008069 |
| Mouse: Dlx5/6-Flp | JAX | Tg(mI56i-flpe)39Fsh/J JAX Stock #010815 |
| Mouse: Ai14 | JAX | B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J JAX Stock #007914 |
| Mouse: Ai32 | JAX | B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J JAX Stock #012569 |
| Mouse: C57BL/6J | JAX | JAX Stock #000664 |
| Recombinant DNA | ||
| pAAV-Ef1a-fDIO-stGtACR2-FusionRed | Custom | n/a |
| Software and algorithms | ||
| Kilosort 2 | Github | https://github.com/MouseLand/Kilosort |
| Phy 2 | Github | https://github.com/cortex-lab/phy |
| CSDPlotter 0.1.1 | http://arken.umb.no/compneuro/iCSD_download.html | |
| MATLAB 2017b | Mathworks | https://www.mathworks.com/products/matlab.html |
| Zen | Zeiss | n/a |
| FIJI | NIH ImageJ Software | https://fiji.sc |
| Probe configurations | Masmanidis Lab, UCLA | https://github.com/sotmasman/Silicon-microprobes |
| Custom analysis code | M.A.K., Callaway Lab | https://github.com/SNLC/openephys/tree/master/Megan_code_opto |
| Other | ||
| BX63 Microscope | Olympus | n/a |
| Airyscan 880 | Zeiss | n/a |
| Silicon microprobes | Dr. Sotiris Masmanidis | 64D, 128AN,128DN, 128D (https://github.com/sotmasman/Silicon-microprobes) |
| Digital micromanipulator | Sutter | MP-285 |
| Manual micromanipulator | David Kopf | 1760-61 |
| 128-channel headstage | Intan Technologies | RHD2164 |
| Electroplating board | Intan Technologies | C3180 |
| OpenEphys acquisition board | OpenEphys | https://open-ephys.org/acq-board#cite |
| Arduino Zero | Arduino | Arduino Zero |
| T-Cube LED Driver | Thorlabs | LEDD1B |
| Fiber-coupled LED modules | Thorlabs | 470nm (Cat#M470F3), 455nm (Cat#M455F3), 617nm (Cat#M617F2) |
| PlexBright LED driver | Plexon | LD-1 |
| PlexBright table-top LED module | Plexon | 465nm |
| 635nm collimated diode laser | Laserglow Technologies | LRD-0635-PFR-00200-03 |
| Power meter | Thorlabs | PM100D |
| Optical fiber patch cords (0.39 NA) | Thorlabs | FT400UMT-CUSTOM, FT1000UMT-CUSTOM |
Highlights:
Corticothalamic neurons (CTs) in layers 5 or 6 of V1 were inactivated in awake mice
Layer 5 (L5), but not layer 6 (L6), CTs “drive” visual responses in the pulvinar
Both CT projection pathways to the visual thalamus are retinotopically organized
Driving inputs from L5 CTs and superior colliculus converge in the lateral pulvinar
Kirchgessner et al. find that inactivating corticothalamic (CT) neurons in layer 5, but not layer 6, of the mouse visual cortex suppresses visual responses in the pulvinar, indicating a “driving” versus “modulatory” distinction between CT populations. Layer 5 “drivers” converge with subcortical (superior colliculus) driving inputs in the pulvinar.
Acknowledgements
We thank Dr. Hongkui Zeng (Allen Institute) for informing us about and sharing the Npr3-IRES-Cre-neo line used in this study, as well as Dr. Farran Briggs and Dr. Sonja Hofer for providing feedback on the manuscript. We also thank other members of the Callaway Lab, especially Dr. Celine Vuong for making the fDIO-stGtACR2 plasmid and Rachel Cassidy for comments on the manuscript, as well as the Salk Institute’s Viral GT3 and Advanced Biophotonics Cores for additional support. This work was supported by NIH grants F31 EY028853 (M.A.K.), R01 MH063912 and R01 EY022577 (E.M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
References
- 1.Halassa MM, and Sherman SM, (2019). Thalamocortical Circuit Motifs: A General Framework. Neuron 103, 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saalmann YB, Pinsk MA, Wang L, Li X, and Kastner S, (2012). The pulvinar regulates information transmission between cortical areas based on attention demands. Science (New York, N.Y.) 337, 753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou H, Schafer R, and Desimone R, (2016). Pulvinar-Cortex Interactions in Vision and Attention. Neuron 89, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, and Hofer SB, (2016). Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature neuroscience 19, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blot A, Roth MM, Gasler I, Javadzadeh M, Imhof F, and Hofer SB, (2021). Visual intracortical and transthalamic pathways carry distinct information to cortical areas. Neuron 109, 1996–2008.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman SM, (2016). Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience 16. [DOI] [PubMed] [Google Scholar]
- 7.Bickford ME, (2015). Thalamic Circuit Diversity: Modulation of the Driver/Modulator Framework. Frontiers in neural circuits 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleland BG, Dubin MW, and Levick WR, (1971). Sustained and transient neurones in the cat’s retina and lateral geniculate nucleus. The Journal of physiology 217, 473–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usrey WM, Reppas JB, and Reid RC, (1999). Specificity and strength of retinogeniculate connections. Journal of neurophysiology 82, 3527–40. [DOI] [PubMed] [Google Scholar]
- 10.Sherman SM, and Guillery RW, (1996). Functional organization of thalamocortical relays. Journal of neurophysiology 76, 1367–95. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SM, and Guillery RW, (1998). On the actions that one nerve cell can have on another: Distinguishing “drivers” from “modulators.” Proceedings of the National Academy of Sciences 95, 7121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman SM, and Guillery RW, (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions Royal Soc Lond Ser B Biological Sci 357, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briggs F, and Usrey WM, (2008). Emerging views of corticothalamic function. Current opinion in neurobiology 18, 403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouiller EM, and Welker E, (2000). A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Research Bulletin 53, 727–41. [DOI] [PubMed] [Google Scholar]
- 15.Reichova I, and Sherman SM, (2004). Somatosensory corticothalamic projections: distinguishing drivers from modulators. Journal of neurophysiology 92, 2185–97. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Guido W, and Bickford ME, (2003). Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. Journal of neurophysiology 90, 3429–40. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Wang S, and Bickford ME, (2003). Comparison of the ultrastructure of cortical and retinal terminals in the rat dorsal lateral geniculate and lateral posterior nuclei. The Journal of comparative neurology 460, 394–409. [DOI] [PubMed] [Google Scholar]
- 18.Bourassa J, and Deschênes M, (1995). Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience 66, 253–63. [DOI] [PubMed] [Google Scholar]
- 19.Rockland KS, (1996). Two types of corticopulvinar terminations: round (type 2) and elongate (type 1). The Journal of comparative neurology 368, 57–87. [DOI] [PubMed] [Google Scholar]
- 20.Mathers LH, (1972). The synaptic organization of the cortical projection to the pulvinar of the squirrel monkey. Journal of Comparative Neurology 146, 43–59. [DOI] [PubMed] [Google Scholar]
- 21.Mease RA, Sumser A, Sakmann B, and Groh A, (2016). Cortical Dependence of Whisker Responses in Posterior Medial Thalamus In Vivo. Cerebral Cortex 26, 3534–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett C, Gale SD, Garrett ME, Newton ML, Callaway EM, Murphy GJ, and Olsen SR, (2019). Higher-Order Thalamic Circuits Channel Parallel Streams of Visual Information in Mice. Neuron 102, 477–492.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltramo R, and Scanziani M, (2019). A collicular visual cortex: Neocortical space for an ancient midbrain visual structure. Science 363, 64–69. [DOI] [PubMed] [Google Scholar]
- 24.Casanova C, Savard T, and Darveau S, (1997). Contribution of Area 17 to Cell Responses in the Striate-recipient Zone of the Cat’s Lateral Posterior-Pulvinar Complex. Eur J Neurosci 9, 1026–1036. [DOI] [PubMed] [Google Scholar]
- 25.Bender DB, (1983). Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain Research 279, 258–261. [DOI] [PubMed] [Google Scholar]
- 26.Diamond ME, Armstrong-James M, Budway MJ, and Ebner FF, (1992). Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus: Dependence on the barrel field cortex. J Comp Neurol 319, 66–84. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Eisenback MA, and Bickford ME, (2002). Relative distribution of synapses in the pulvinar nucleus of the cat: implications regarding the “driver/modulator” theory of thalamic function. The Journal of comparative neurology 454, 482–94. [DOI] [PubMed] [Google Scholar]
- 28.Horn SCV, and Sherman SM, (2007). Fewer driver synapses in higher order than in first order thalamic relays. Neuroscience 146, 463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horn SCV, Erişir A, and Sherman SM, (2000). Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. The Journal of comparative neurology 416, 509–20. [PubMed] [Google Scholar]
- 30.Scholl LR, Foik AT, and Lyon DC, (2020). Projections between visual cortex and pulvinar in the rat. J Comp Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza B.O.F. de, Frigon É, Tremblay-Laliberté R, Casanova C, and Boire D, (2020). Laminar distribution of cortical projection neurons to the pulvinar: A comparative study in cats and mice. J Comp Neurol. [DOI] [PubMed] [Google Scholar]
- 32.Zhou NA, Maire PS, Masterson SP, and Bickford ME, (2017). The mouse pulvinar nucleus: Organization of the tectorecipient zones. Visual neuroscience 34, E011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaramillo J, Mejias JF, and Wang X-J, (2019). Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 101, 321–336.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieve KL, Acuña C, and Cudeiro J, (2000). The primate pulvinar nuclei: vision and action. Trends in neurosciences 23, 35–9. [DOI] [PubMed] [Google Scholar]
- 35.Froesel M, Cappe C, and Hamed SB, (2021). A multisensory perspective onto primate pulvinar functions. Neurosci Biobehav Rev 125, 231–243. [DOI] [PubMed] [Google Scholar]
- 36.Sampathkumar V, Miller-Hansen A, Sherman SM, and Kasthuri N, (2021). Integration of signals from different cortical areas in higher order thalamic neurons. Proc National Acad Sci 118, e2104137118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tervo DG, Hwang B-YY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, et al. (2016). A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, and Gerfen CR, (2007). Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of neuroscience: the official journal of the Society for Neuroscience 27, 9817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen SR, Bortone DS, Adesnik H, and Scanziani M, (2012). Gain control by layer six in cortical circuits of vision. Nature 483, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bortone DS, Olsen SR, and Scanziani M, (2014). Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirchgessner MA, Franklin AD, and Callaway EM, (2020). Context-dependent and dynamic functional influence of corticothalamic pathways to first- and higher-order visual thalamus. Proc National Acad Sci, 202002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. (2018). A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, et al. (2018). Shared and distinct transcriptomic cell types across neocortical areas. Nature 563, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris JA, Mihalas S, Hirokawa KE, Whitesell JD, Choi H, Bernard A, Bohn P, Caldejon S, Casal L, Cho A, et al. (2019). Hierarchical organization of cortical and thalamic connectivity. Nature 575, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du J, Blanche TJ, Harrison RR, Lester HA, and Masmanidis SC, (2011). Multiplexed, high density electrophysiology with nanofabricated neural probes. PloS one 6, e26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vélez-Fort M, Rousseau CV, Niedworok CJ, Wickersham IR, Rancz EA, Brown AP, Strom M, and Margrie TW, (2014). The stimulus selectivity and connectivity of layer six principal cells reveals cortical microcircuits underlying visual processing. Neuron 83, 1431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson RS, and Polley DB, (2019). Parallel pathways for sound processing and functional connectivity among layer 5 and 6 auditory corticofugal neurons. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Born G, Erisken S, Schneider FA, Klein A, Mobarhan MH, Lao CL, Spacek MA, Einevoll GT, and Busse L, (2020). Corticothalamic feedback sculpts visual spatial integration in mouse thalamus. Biorxiv, 2020.05.19.104000. [DOI] [PubMed] [Google Scholar]
- 49.Theyel BB, Llano DA, and Sherman SM, (2010). The corticothalamocortical circuit drives higher-order cortex in the mouse. Nature neuroscience 13, 84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato N, (1990). Cortico-thalamo-cortical projection between visual cortices. Brain Res 509, 150–152. [DOI] [PubMed] [Google Scholar]
- 51.Allen AE, Procyk CA, Howarth M, Walmsley L, and Brown TM, (2016). Visual input to the mouse lateral posterior and posterior thalamic nuclei: photoreceptive origins and retinotopic order. The Journal of Physiology 594, 1911–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldwin MKLK, Balaram P, and Kaas JH, (2017). The evolution and functions of nuclei of the visual pulvinar in primates. The Journal of comparative neurology 525, 3207–3226. [DOI] [PubMed] [Google Scholar]
- 53.Juavinett AL, Kim EJ, Collins HC, and Callaway EM, (2020). A systematic topographical relationship between mouse lateral posterior thalamic neurons and their visual cortical projection targets. The Journal of comparative neurology 528, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tohmi M, Meguro R, Tsukano H, Hishida R, and Shibuki K, (2014). The extrageniculate visual pathway generates distinct response properties in the higher visual areas of mice. Current biology: CB 24, 587–97. [DOI] [PubMed] [Google Scholar]
- 55.Chalupa L, Williams R, and Hughes M, (1983). Visual response properties in the tectorecipient zone of the cat’s lateral posterior-pulvinar complex: a comparison with the superior colliculus. Journal of Neuroscience 3, 2587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masterson SP, Li J, and Bickford ME, (2010). Frequency-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. Journal of neurophysiology 104, 1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masterson SP, Li J, and Bickford ME, (2009). Synaptic organization of the tectorecipient zone of the rat lateral posterior nucleus. Journal of Comparative Neurology 515, 647–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rovó Z, Ulbert I, and Acsády L, (2012). Drivers of the Primate Thalamus. The Journal of Neuroscience 32, 17894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casanova C, and Molotchnikoff S, (1990). Influence of the superior colliculus on visual responses of cells in the rabbit’s lateral posterior nucleus. Experimental brain research 80, 387–96. [DOI] [PubMed] [Google Scholar]
- 60.Kim J, Matney CJ, Blankenship A, Hestrin S, and Brown SP, (2014). Layer 6 corticothalamic neurons activate a cortical output layer, layer 5a. The Journal of neuroscience: the official journal of the Society for Neuroscience 34, 9656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briggs F, (2020). Role of Feedback Connections in Central Visual Processing. Annu Rev Vis Sc 6, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bienkowski MS, Benavidez NL, Wu K, Gou L, Becerra M, and Dong H, (2019). Extrastriate connectivity of the mouse dorsal lateral geniculate thalamic nucleus. J Comp Neurol 527, 1419–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durand S, Iyer R, Mizuseki K, Vries S. de, Mihalas S, and Reid CR, (2016). A Comparison of Visual Response Properties in the Lateral Geniculate Nucleus and Primary Visual Cortex of Awake and Anesthetized Mice. Journal of Neuroscience 36, 12144–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ansorge J, Humanes-Valera D, Pauzin FP, Schwarz MK, and Krieger P, (2020). Cortical layer 6 control of sensory responses in higher-order thalamus. J Physiology 598. [DOI] [PubMed] [Google Scholar]
- 65.Hoerder-Suabedissen A, Hayashi S, Upton L, Nolan Z, Casas-Torremocha D, Grant E, Viswanathan S, Kanold PO, Clasca F, Kim Y, et al. (2018). Subset of Cortical Layer 6b Neurons Selectively Innervates Higher Order Thalamic Nuclei in Mice. Cerebral cortex (New York, N Y.: 1991) 28, 1882–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Augustinaite S, and Kuhn B, (2020). Complementary Ca2+ Activity of Sensory Activated and Suppressed Layer 6 Corticothalamic Neurons Reflects Behavioral State. Curr Biol. [DOI] [PubMed] [Google Scholar]
- 67.Clayton KK, Williamson RS, Hancock KE, Tasaka G, Mizrahi A, Hackett TA, and Polley DB, (2020). Auditory Corticothalamic Neurons Are Recruited by Motor Preparatory Inputs. Curr Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stoelzel CR, Bereshpolova Y, Alonso J-M, and Swadlow HA, (2017). Axonal conduction delays, brain state, and corticogeniculate communication. J Neurosci Official J Soc Neurosci 37, 6342–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leopold DA, (2012). Primary Visual Cortex: Awareness and Blindsight*. Neuroscience 35, 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim EJ, Juavinett AL, Kyubwa EM, Jacobs MW, and Callaway EM, (2015). Three Types of Cortical Layer 5 Neurons That Differ in Brain-wide Connectivity and Function. Neuron 88, 1253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halassa MM, and Acsády L, (2016). Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends in neurosciences 39, 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bokor H, Frère SG, Eyre MD, Slézia A, Ulbert I, Lüthi A, and Acsády L, (2005). Selective GABAergic control of higher-order thalamic relays. Neuron 45, 929–40. [DOI] [PubMed] [Google Scholar]
- 73.Barthó P, Freund TF, and Acsády L, (2002). Selective GABAergic innervation of thalamic nuclei from zona incerta. The European journal of neuroscience 16, 999–1014. [DOI] [PubMed] [Google Scholar]
- 74.Benedek G, Norita M, and Creutzfeldt OD, (1983). Electrophysiological and anatomical demonstration of an overlapping striate and tectal projection to the lateral posterior-pulvinar complex of the cat. Exp Brain Res 52, 157–169. [DOI] [PubMed] [Google Scholar]
- 75.Groh A, Bokor H, Mease RA, Plattner VM, Hangya B, Stroh A, Deschenes M, and Acsády L, (2014). Convergence of Cortical and Sensory Driver Inputs on Single Thalamocortical Cells. Cerebral Cortex 24, 3167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krauzlis RJ, Lovejoy LP, and Zénon A, (2013). Superior Colliculus and Visual Spatial Attention. Neuroscience 36, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phillips JW, Schulmann A, Hara E, Winnubst J, Liu C, Valakh V, Wang L, Shields BC, Korff W, Chandrashekar J, et al. (2019). A repeated molecular architecture across thalamic pathways. Nature Neuroscience 22, 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L, Lee K, Villagracia J, and Masmanidis SC, (2020). Open source silicon microprobes for high throughput neural recording. J Neural Eng 17, 016036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegle JH, López AC, Patel YA, Abramov K, Ohayon S, and Voigts J, (2017). Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J Neural Eng 14, 045003. [DOI] [PubMed] [Google Scholar]
- 80.Pachitariu M, Steinmetz N, Kadir S, Carandini M, and D. HK, (2016). Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. Biorxiv, 061481. [Google Scholar]
- 81.Schmitzer-Torbert N, Jackson J, Henze D, Harris K, and Redish AD, (2005). Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131, 1–11. [DOI] [PubMed] [Google Scholar]
- 82.Pettersen KH, Devor A, Ulbert I, Dale AM, and Einevoll GT, (2006). Current-source density estimation based on inversion of electrostatic forward solution: Effects of finite extent of neuronal activity and conductivity discontinuities. J Neurosci Meth 154, 116–133. [DOI] [PubMed] [Google Scholar]
- 83.Niell CM, and Stryker MP, (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erisken S, Vaiceliunaite A, Jurjut O, Fiorini M, Katzner S, and Busse L, (2014). Effects of locomotion extend throughout the mouse early visual system. Current biology: CB 24, 2899–907. [DOI] [PubMed] [Google Scholar]
- 85.Frandolig J, Matney C, Lee K, Kim J, Chevée M, Kim S-J, Bickert A, and Brown S, (2019). The Synaptic Organization of Layer 6 Circuits Reveals Inhibition as a Major Output of a Neocortical Sublamina. Cell Reports 28, 3131–3143.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Processed data and relevant code for generating the figures and analyses in this manuscript have been deposited on Mendeley Data (DOI: 10.17632/7cr8w9k5pb.1). Additional code can be found on the Callaway lab’s GitHub (https://github.com/SNLC/openephys/Megan_code_opto). Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


