Abstract
Background.
HIV and pregnancy may affect latent TB infection (LTBI) diagnostics. Tuberculin skin test (TST) and newer generation QuantiFERON-TB Gold Plus (QFT-Plus) evaluations in pregnant women with (WLHIV) and without HIV are lacking.
Methods.
In this cross-sectional study, pregnant women underwent TST and QFT-Plus testing during antenatal care in Kenya. We estimated LTBI prevalence and TST and QFT-Plus performance. Diagnostic agreement was assessed with kappa statistic, participant characteristics associated with LTBI and HIV with generalized linear models, and QFT-Plus quantitative responses with Mann Whitney test.
Results.
We enrolled 400 pregnant women (200 WLHIV/200 HIV-negative) at median 28 weeks gestation (Interquartile Range [IQR] 24–30). Among WLHIV (all on antiretroviral therapy), median CD4 was 464 cells/mm3 (IQR 325–654); 62.5% (125) had received isoniazid preventive therapy. LTBI prevalence was 35.8% and similar among WLHIV and HIV-negative women. QFT-Plus identified 3-fold more women with LTBI vs. TST (32% vs. 12%, p<0.0001). QFT-Plus-positivity prevalence was similar regardless of HIV status, though TB-specific antigen responses were lower in WLHIV vs. HIV-negative women with LTBI (median QFT-TB1 1.05 vs. 2.65 IU/mL, p=0.035; QFT-TB2 1.26 vs. 2.56 IU/mL, p=0.027). TST-positivity was more frequent among WLHIV than HIV-negative women (18.5% vs 4.6%; p<0.0001).
Conclusion.
QFT-Plus had higher diagnostic yield than TST for LTBI in WLHIV and HIV-negative women despite lower TB antigen-specific responses in WLHIV. Higher TST-positivity was observed in WLHIV. LTBI diagnostic performance in context of pregnancy and HIV has implications for clinical use and prevention studies which rely on these diagnostics for TB-infection entry criteria or outcomes.
Keywords: HIV, pregnancy, latent tuberculosis, Kenya, QuantiFERON-TB Gold Plus
INTRODUCTION
Tuberculosis (TB) during pregnancy is associated with increased risk of maternal morbidity and mortality and poor pregnancy and infant outcomes,1,2 especially among women living with HIV (WLHIV) despite widespread antiretroviral use.3,4 Although data regarding TB in pregnancy is not routinely reported, models estimate >200,000 active TB cases occur during pregnancy annually, with the highest burden in Africa.2 Both HIV and potentially pregnancy increase the risk of progression from M. tuberculosis infection to active TB disease,5–7 highlighting importance of latent TB infection (LTBI) detection in pregnancy in high HIV/TB settings which may allow targeted prevention efforts.
There is evidence that pregnancy8,9 and HIV10 affect LTBI diagnostic performance.11,12 Tuberculin skin tests (TST) indirectly measure M. tuberculosis infection by detecting a delayed-type hypersensitivity reaction in individuals with cell-mediated immunity to tuberculin antigens, but are subject to cross-reactivity with non-tuberculous mycobacteria and Bacillus Calmette-Guérin vaccine and have poor sensitivity in immunocompromised patients.13,14 Interferon gamma (IFN-γ)-release assays (IGRA) measure T-cell release of IFN-γ following M. tuberculosis-specific antigen stimulation; QuantiFERON-TB Gold assay (QFT-Gold) is an enzyme-linked immunosorbent assay (ELISA) that measures IFN-γ secreted primarily by CD4+ T cells, while newer generation QuantiFERON-TB Gold Plus (QFT-Plus) detects CD4+ and CD8+ T cell responses, which may increase sensitivity in populations with lower CD4 counts including people living with HIV (PLHIV).14–16
Previous studies demonstrated discordance between older generation QFT-Gold and TST in pregnancy, with QFT-Gold having higher sensitivity (detecting ~2-fold or greater of women with LTBI) compared to TST in separate cohorts of pregnant WLHIV and HIV-negative women in India,8,9 Kenya,17 and within a multinational trial of isoniazid preventive therapy (IPT) in pregnant WLHIV.18 Pregnancy also appears to affect QFT-Gold as evidenced by lower mean mitogen and TB antigen responses, and higher rates of indeterminate results (due to lower mitogen response), during pregnancy compared to early postpartum.17 A cross-sectional study in Ethiopia enrolling primarily HIV-negative women utilizing newer generation QFT-Plus (no TST performed) found more borderline TB antigen QFT responses among pregnant WLHIV than HIV-negative women, prompting the authors to suggest consideration of lowering the QFT-positive cut-off in pregnant WLHIV.11 A more recent Ethiopian cross-sectional study enrolling pregnant and non-pregnant women with and without HIV found that both pregnancy and HIV reduced LTBI detection by both TST and QFT-Gold.12
We designed a study to estimate LTBI prevalence and effect of HIV on diagnostic performance of the newest generation QFT-Plus compared to TST, including TB-specific antigen responses, in a large cross-sectional evaluation of pregnant WLHIV and without HIV in western Kenya. Additionally, we investigated correlates of LTBI-diagnostic positivity and yield of varying diagnostic cut-offs.
METHODS
Study Setting and Participants
We enrolled pregnant WLHIV and HIV-negative women at four public antenatal clinics in in Kisumu and Siaya counties in western Kenya, where HIV prevalence is 14–21%.19 Women were eligible if they were pregnant (between 20–34 weeks gestation) and ≥16 years. Participants were excluded if they were diagnosed with TB disease in the past year or were found to have TB on enrollment. Participants were enrolled consecutively until the pre-determined sample size of 400 participants (200 WLHIV, 200 HIV-negative women) was reached.
Procedures
Enrollment
Informed consent was obtained by study staff who administered standardized questionnaires regarding sociodemographic, clinical, obstetric, and HIV-related factors, prior TB exposure and history, and maternal and household member TB symptoms using WHO symptom screen (fever, cough, weight loss, and night sweats).20 Physical examination was performed including body mass index (BMI) calculation and middle upper-arm circumference (MUAC). Medical records were used to abstract data on antiretroviral therapy (ART), IPT, maternal HIV viral load, and CD4 counts. Kenyan guidelines recommend a six-month course of IPT for PLHIV including pregnant women; neither TST or IGRA are used to identify LTBI prior to IPT initiation.21 Participants continued to receive routine antenatal and HIV care. WLHIV found to have positive TST or IGRA in the study were referred for IPT evaluation if they had not already received IPT; per Kenyan guidelines at the time of the study, HIV-negative adults without known TB exposure are not routinely provided IPT.22 Data were entered into password protected tablets using Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN).
QFT-Plus and TST
QFT-Plus:
Five milliliters of blood was collected into a single lithium heparinized blood collection tube and transported to Kenya Medical Research Institute (KEMRI) Centers for Disease Control (CDC) lab and aliquoted into QFT-Plus assay collection tubes (nil, mitogen, TB antigen 1 [ESAT-6 and CFP-10 CD4 peptides], TB antigen 2 [ESAT-6, CFP-10 CD4 and CD8 peptides]) and processed per manufacturer recommendations.23 TB1 or TB2 antigen response of ≥0.35 IU/ml (minus nil, with nil <8 IU/ml and positive mitogen control) was considered positive.15
TST:
Trained study personnel injected 0.1 ml (5 international units) of tuberculin purified protein derivative (PPD) intradermally to the forearm volar surface using the Mantoux method. Induration was measured at 48–96 hours following TST placement.24,25 This reading window allowed for TST placement throughout the week and is within the allowable seven days used in International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT).23,26 TST of ≥ 5 mm was considered positive in WLHIV, and ≥10 mm among HIV-negative women per routine clinical cut-offs.27
Statistical Analysis
Baseline participant characteristics were described using proportions for categorical and medians and interquartile ranges for continuous variables. Generalized linear models with a log link and Poisson family were used to estimate relative risk ratios (RR) to assess differences in baseline characteristics by HIV status and correlates of LTBI in the overall cohort and stratified by HIV status. Median quantitative QFT-Plus responses were compared by Mann Whitney tests. Multinomial logistic regression was used to assess association between baseline correlates and QFT-positive or -indeterminate results compared to the reference QFT-negative. TST and QFT agreement, as well as QFT TB1 and TB2 antigen agreement were assessed using a kappa statistic. Correlation between TST diameter and quantitative TB1 and TB 2 antigens was assessed by Spearman’s rho.
Sensitivity analyses were performed varying cut-offs for TST (including ≥10 mm and ≥5 mm for both WLHIV and HIV-negative groups), as well as varying QFT-Plus TB1 and TB2 antigens (minus nil) cut-offs to ≥0.20 IU/ml and ≥0.70 IU/ml to represent lower and upper cut-offs described for borderline values in the literature. All estimates were reported using 95% confidence intervals: all statistical tests were two sided with alpha = 0.05. Analyses were performed using Stata 14 (StataCorp, College Station, TX).
Ethics Statement
This study was approved by University of Nairobi-Kenyatta National Hospital Ethics and Research Committee and the University of Washington Institutional Review Board.
RESULTS
Participant Characteristics Overall and by HIV status
From January 2018 to December 2019, we enrolled 400 pregnant women (200 WLHIV and 200 HIV-negative women) (Figure 1); 188 (47%) from Kisumu and 212 (53%) from Siaya counties. Median age at enrollment was 26 (interquartile range [IQR] 22–29) years, median gestational age was 28 weeks (IQR 24–30) with median BMI of 24.1 2 kg/m2 (IQR 22.3–27.5) (Table 1). Roughly half were employed (52.0% [209]), 46.0% (185) had running water in their home, and 22.5% (90) lived in a single room household. Median number of years of education was 10 (IQR 8–12). Three women (0.8%) had a history of active pulmonary TB, and 3.3% (13) had a household member with recent TB symptoms.
Figure 1. Study flow study evaluating QFT-Plus and TST among women with and without HIV.
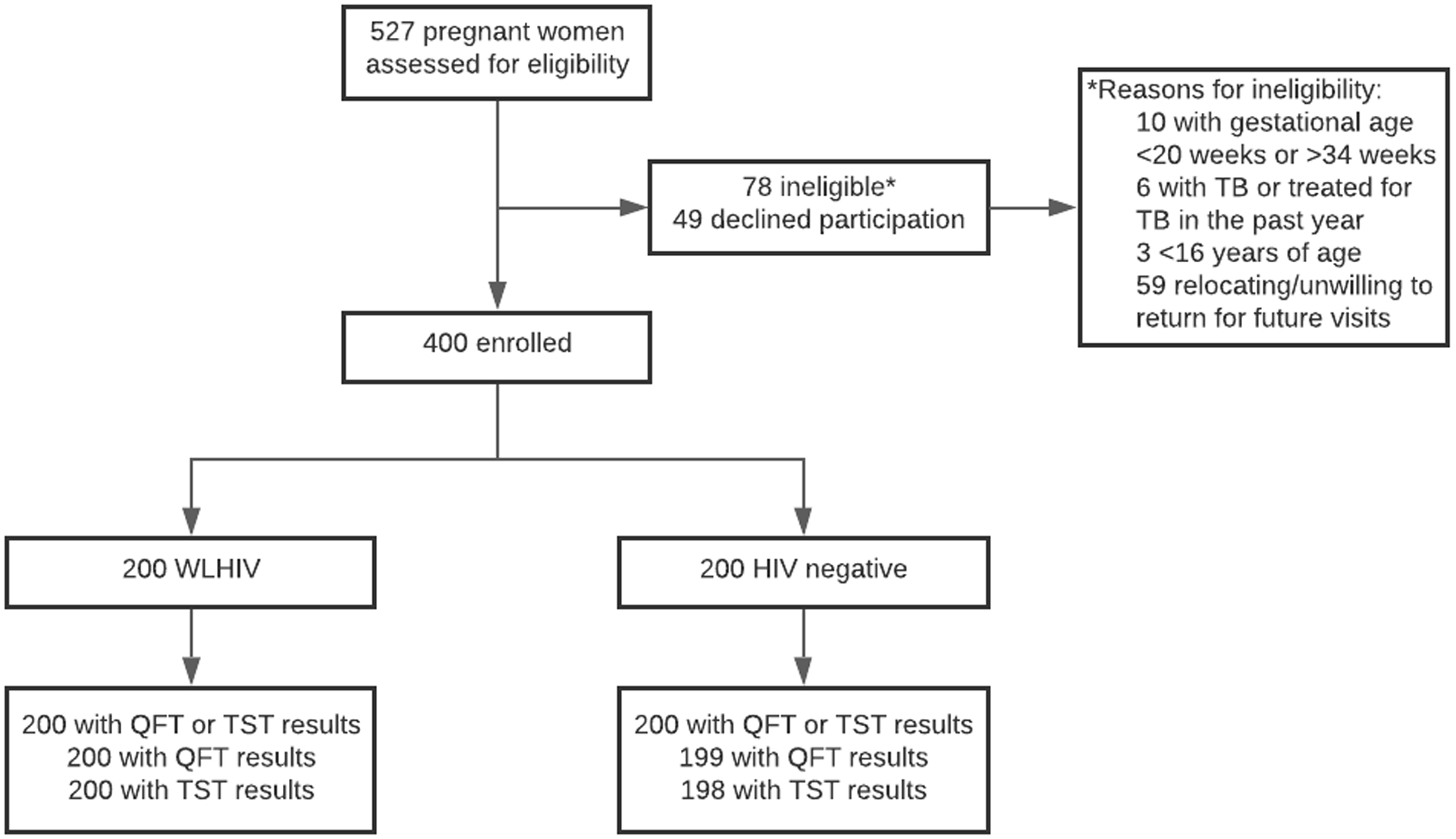
Abbreviations: WLHIV, women living with HIV; QFT, QuantiFERON-TB Gold Plus; TST, tuberculin skin test
Table 1.
Participant characteristics and results of LTBI testing by HIV status
| All participants n=400 | WLHIV n=200 | HIV negative n=200 | RR* (95% CI) | p | |
|---|---|---|---|---|---|
| n (%) or median (IQR) |
n (%) or median (IQR) |
n (%) or median (IQR) |
|||
| Sociodemographic characteristics | |||||
| Age, yrs | 26 (22–29) | 28 (24–32) | 23 (21–26) | 1.06 (1.05–1.08) | <0.001 |
| Gestational age, wks | 28 (24–30) | 26 (23–30) | 28 (25–32) | 0.96 (0.94–0.98) | <0.001 |
| BMI, kg/m2 | 24.1 (22.3–27.5) | 24.2 (22.2–27.5) | 24.0 (22.4–27.4) | 1.01 (0.99–1.03) | 0.434 |
| MUAC, cm | 26.0 (25.0–28.0) | 26.2 (25.1– 28.8) | 26.0 (24.527.2) | 1.04 (1.02–1.07) | <0.001 |
| Education, yrs | 10 (8–12) | 10 (8–12) | 10 (8–12) | 0.98 (0.95–1.00) | 0.082 |
| Employed | 209 (52.0) | 116 (58.0) | 93 (46.5) | 1.26 (1.03–1.54) | 0.023 |
| Residential characteristics | |||||
| Running water in home | 185 (46.0) | 81 (40.5) | 104 (52.0) | 0.79 (0.65–0.97) | 0.024 |
| Electricity in home | 255 (64.0) | 131 (65.5) | 124 (62.0) | 1.08 (0.88–1.33) | 0.472 |
| Pit latrine use | 377 (94.3) | 187 (93.5) | 190 (95.0) | 0.88 (0.61–1.27) | 0.493 |
| Persons in household | 4 (3–5) | 4 (3–5) | 3 (2–5) | 1.05 (0.99–1.12) | 0.077 |
| Single room household | 90 (22.5) | 36 (18.0) | 54 (27.0) | 0.76 (0.57–0.99) | 0.046 |
| TB history, symptoms, exposures | |||||
| Previous TB | 3 (0.8) | 2 (1.0) | 1 (0.5) | 1.34 (0.60–3.00) | 0.481 |
| WHO TB symptom past month † | 16 (4.0) | 10 (5.0) | 6 (3.0) | 1.26 (0.85–1.87) | 0.244 |
| Household WHO TB symptom | 13 (3.3) | 7 (3.5) | 6 (3.0) | 1.08 (0.65–1.80) | 0.770 |
| TB exposure past 2 years | 4 (1.0) | 3 (1.5) | 1 (0.5) | 1.51 (0.85–2.68) | 0.162 |
| HIV | |||||
| CD4, cells/mm3 (n=90) § | 464 (325–654) | ||||
| HIV viral load, copies/ml (n=174) § | 0 (0–54) | ||||
| HIV viral load undetectable (n=174) || | 153 (87.9) | ||||
| Time since HIV diagnosis, yrs | 3.1 (0.4–6.9) | ||||
| ART before pregnancy | 135 (67.5) | ||||
| ART on enrollment | 200 (100.0) | ||||
| Any IPT | 125 (62.5) | ||||
| IPT on enrollment (n=125) | 38 (30.1) | ||||
| LTBI test results | |||||
| LTBI+ (TST+ or QFT-Plus+) | 143 (35.8) | 74 (37.0) | 69 (34.5) | 1.06 (0.86–1.29) | 0.600 |
| TST+ (n=398) ‡ | 46 (11.6) | 37 (18.5) | 9 (4.6) | 1.74 (1.45–2.08) | <0.001 |
| QFT-Plus (n=399) | |||||
| Positive | 129 (32.3) | 63 (31.5) | 66 (33.2) | 0.96 (0.77–1.19) | 0.691 |
| Negative | 245 (61.4) | 125 (62.5) | 120 (60.3) | ref | |
| Indeterminate | 25 (6.3) | 12 (6.0) | 13 (6.6) | 0.94 (0.61–1.44) | 0.779 |
Abbreviations: IQR, interquartile range; RR, relative risk; yrs, years; wks, weeks; BMI, body mass index; MUAC, mid-upper arm circumference; TB, tuberculosis; WHO, World Health Organization; LTBI, latent tuberculosis infection; TST, tuberculin skin test; QFT-Plus, QuantiFERON-TB Gold Plus; ART, antiretroviral therapy; IPT, isoniazid preventive therapy
Relative risk (RR) estimated using a generalized linear model (GLM) with log link and Poisson family
WHO TB symptoms: fever, cough, weight loss, night sweats
TST positive defined as ≥5 mm induration if HIV positive and ≥10mm induration if HIV negative; median time of TST read was 47 hours (IQR 45–70).
CD4 and viral load data were collected from routine programmatic data; viral load was collected at a median of 55 (6–91) days prior to enrollment
Undetectable viral load ≤20 copies/ml
For 200 WLHIV, median CD4 count was 464 cells/mL (IQR 325–654), and most (87.9%; 153) were virally suppressed (viral load <20 copies/mL), based on a viral load drawn at a median of 55 days (IQR 6–91) prior to enrollment. Median length of HIV diagnosis was 3.1 years prior to enrollment (IQR 0.4–6.9); 67.5% (135) were on ART before this pregnancy, and 100% were on ART at enrollment. Approximately two thirds (62.5%; 125) of WLHIV had ever received IPT, including 30.1% (38) currently on IPT at enrollment.
WLHIV were older (median age 28 vs. 23 years), enrolled at an earlier gestational age (median 26 vs. 28 weeks), and had slightly larger MUAC (median 26.2 vs. 26.0 cm) (all p<0.001) compared to HIV-negative women. WLHIV were more likely to be employed (58.0% vs. 46.5%), but less likely to have running water in their household (40.5% vs. 52.0%) or live in a single room household (18% vs. 27%) (all p<0.05).
Prevalence of Latent Tuberculosis Infection (LTBI) including QFT-Plus and TST results
Overall, 35.8% (143) of women were LTBI-positive (by either QFT-Plus or TST) with similar proportions of LTBI among WLHIV and HIV-negative women (Table 1; Figure 2). One third (129; 32.3%) of women were QFT-positive, and 25 (6.3%) were indeterminate (majority due to low mitogen responses: 21/25; 84.0%) with similar proportions of QFT-positive and indeterminates between WLHIV and HIV-negative women, respectively (QFT positive: 31.5% vs. 33.2%; QFT indeterminate: 6.0% vs. 6.6%). In contrast, overall TST-positivity (by clinical cut-offs of ≥5mm for WLHIV and ≥10mm for HIV-negative women) prevalence was lower compared to QFT-positivity (TST 11.6% vs. QFT-Plus 32.3%; p<0.0001), though WLHIV were significantly more likely to have a positive TST than HIV-negative women (18.5% vs 4.6%; p<0.0001).
Figure 2. Prevalence of latent tuberculosis infection by TST and QFT-Plus among pregnant women by HIV status.
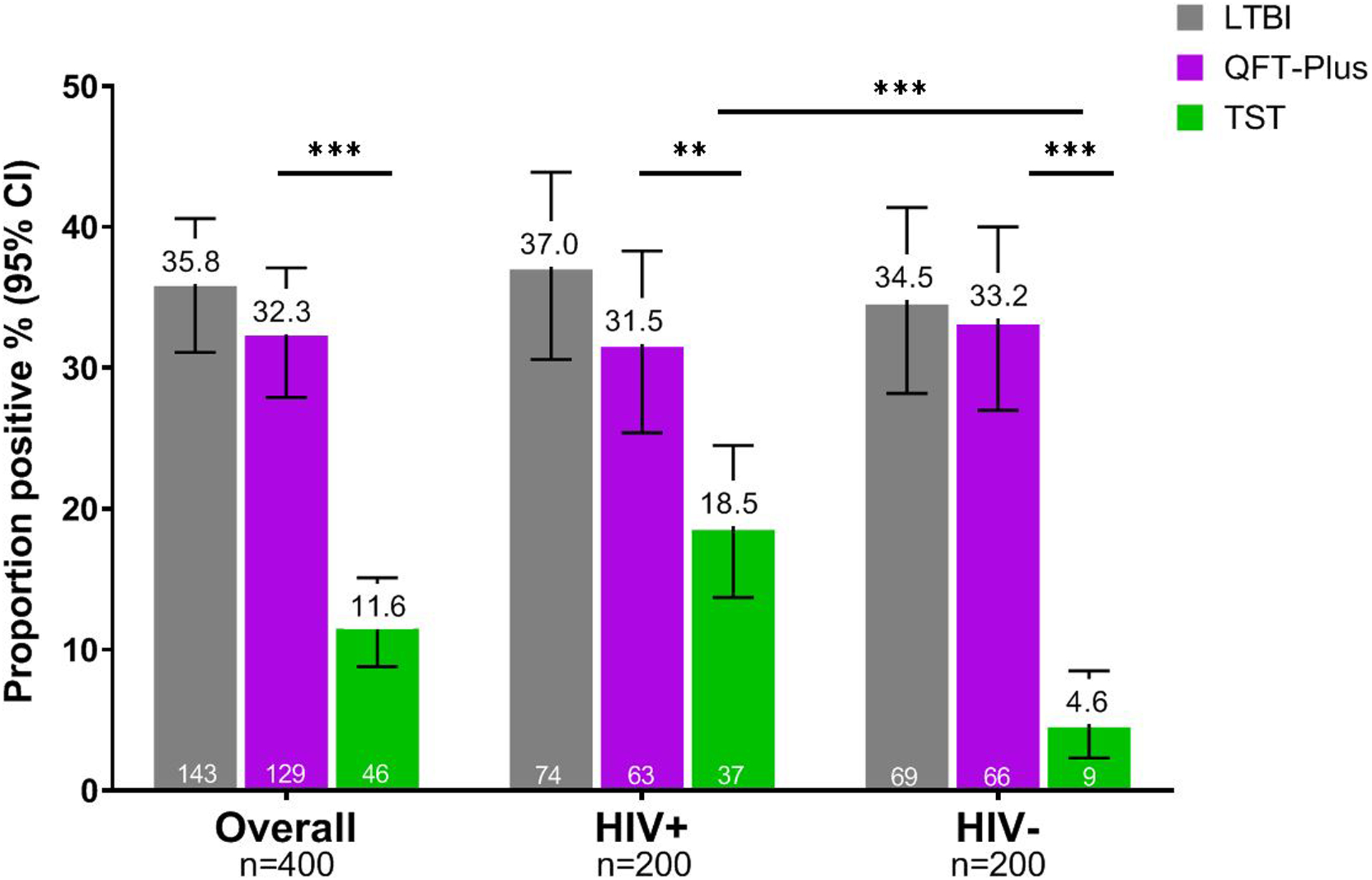
Abbreviations: LTBI, latent tuberculosis infection; QFT-Plus, QuantiFERON-TB Gold Plus; TST, tuberculin skin test
TST positive defined as ≥5 mm induration for women living with HIV (HIV+) and ≥10mm induration if HIV negative (HIV−)
*** indicates p<0.0001, ** indicates p<0.005
Correlates of LTBI, QFT-Plus, and TST-positivity Overall and by HIV status
Overall, women with LTBI had lower median years of education (median 9 vs. 10 years; p=0.006) and were more likely to report a previous history of TB (2.1% vs. 0; p<0.001) (Table 2a). There was a trend for women with LTBI to be slightly older (median 26 vs. 35 years; p=0.068). In general, QFT-positive women were similar to QFT-negative women with regards to baseline characteristics. In contrast, QFT-indeterminate women were younger (median 22 vs. 25 years; p=0.047) and had a lower BMI (median 23.2 vs. 24.2 kg/m2; p=0.012) (supplemental Table E2a). TST-positivity defined by routine clinical cut-offs was associated with higher MUAC (median 26.5 vs. 26.0 cm), HIV (80.4% vs. 46.3%), and history of TB (4.4% vs. 0.3%) (all <0.005) (supplemental Table E2b). Correlates for LTBI-positivity remained similar when adjusting for HIV status and baseline characteristics that were significantly different between LTBI-positive and -negative women (data not shown).
Table 2a.
Participant characteristics by LTBI status
| All participants (n=400) | ||||
|---|---|---|---|---|
| LTBI positive*
n=143 |
LTBI negative n=257 |
RR† (95% CI) | p | |
| n (%) or median (IQR) | n (%) or median (IQR) | |||
| Sociodemographic characteristics | ||||
| Age, yrs | 26 (22–30) | 25 (21–28) | 1.02 (1.00–1.04) | 0.068 |
| Gestational age, wks | 28 (24–30) | 28 (24–30) | 1.00 (0.97–1.03) | 0.892 |
| BMI, kg/m2 | 24.1 (22.2–27.5) | 24 (22.3–27.3) | 1.00 (0.96–1.03) | 0.721 |
| MUAC, cm | 26 (25–28) | 26 (24.8–28) | 1.00 (0.96–1.04) | 0.979 |
| Education, yrs | 9 (8–11) | 10 (8–12) | 0.95 (0.92–0.99) | 0.006 § |
| Employed | 78 (54.6) | 131 (51.0) | 1.10 (0.84–1.43) | 0.494 |
| Residential characteristics | ||||
| Running water in home | 63 (44.1) | 122 (47.5) | 0.92 (0.70–1.19) | 0.513 |
| Electricity in home | 93 (65.0) | 162 (63.0) | 1.06 (0.80–1.40) | 0.692 |
| Pit latrine use | 136 (95.1) | 241 (93.8) | 1.19 (0.63–2.23) | 0.599 |
| Persons in household | 3 (3–5) | 4 (3–5) | 1.03 (0.95–1.12) | 0.475 |
| Single room household | 28 (19.6) | 62 (24.1) | 0.84 (0.60–1.18) | 0.311 |
| TB history, symptoms, exposures | ||||
| Previous TB | 3 (2.1) | 0 | 2.84 (2.48–3.24) | <0.001 § |
| WHO TB symptom past month ‡ | 6 (4.2) | 10 (3.9) | 1.05 (0.55–2.01) | 0.880 |
| Household WHO TB symptom | 4 (2.8) | 9 (3.5) | 0.86 (0.37–1.96) | 0.714 |
| TB exposure past 2 yrs | 2 (1.4) | 2 (0.8) | 1.40 (0.52–3.78) | 0.502 |
| HIV (n=200) | 74 (51.8) | 126 (49.0) | 1.07 (0.82–1.40) | 0.603 |
Abbreviations: LTBI, latent tuberculosis infection; IQR, interquartile range; RR, relative risk; yrs, years; wks, weeks; BMI, body mass index; MUAC, mid-upper arm circumference; TB, tuberculosis; WHO, World Health Organization; ART, antiretroviral therapy; IPT, isoniazid preventive therapy
LTBI defined as TST or QFT positive, where TST positive ≥5mm for WLHIV and ≥10mm for HIV negative women
Relative risk (RR) estimated using a generalized linear model (GLM) with log link and Poisson family
WHO TB symptoms: fever, cough, weight loss, night sweats
Remained statistically significant in multivariable models adjusting for education, previous TB, and HIV status (data not shown)
For both WLHIV and HIV-negative women, LTBI was associated with previous history of TB (both <0.001). For WLHIV, both LTBI and QFT-positivity was associated with higher median CD4 counts (both <0.05), and a trend for lower likelihood of ever having received IPT (LTBI+ p=0.056, QFT-Plus+ p=0.078) (Table 2b, supplemental Table E2a). For HIV-negative women, LTBI was associated with older age and fewer years of education (both p<0.02), and TB exposure in past 2 years (p<0.001).
Table 2b.
Participant characteristics by LTBI status stratified by HIV status
| WLHIV (n=200) | HIV negative (n=200) | |||||||
|---|---|---|---|---|---|---|---|---|
| LTBI* positive n=74 n (%) or median (IQR) |
LTBI negative n=126 n (%) or median (IQR) |
RR† (95% CI) | p | LTBI* positive n=69 n (%) or median (IQR) |
LTBI negative n=131 n (%) or median (IQR) |
RR† (95% CI) | p | |
| Sociodemographic characteristics | ||||||||
| Age, yrs | 28 (23–32) | 28 (24–31) | 1.00 (0.97–1.04) | 0.700 | 24 (22–27) | 23 (20–26) | 1.04 (1.01–1.08) | 0.017 |
| Gestational age, wks | 28 (24–30) | 26 (22–30) | 1.02 (0.98–1.07) | 0.240 | 28 (24–30) | 28 (25–32) | 0.97 (0.93–1.01) | 0.190 |
| BMI, kg/m2 | 24.1 (22.1–27.4) | 24.2 (22.3–27.6) | 0.99 (0.94–1.04) | 0.658 | 24.1 (22.4–27.6) | 24.0 (22.3–27.2) | 1.00 (0.95–1.05) | 0.935 |
| MUAC, cm | 26.0 (25.5–28.8) | 26.5 (25.0–29.0) | 0.99 (0.94–1.05) | 0.760 | 26 (25–27.4) | 25.6 (24.5–27.0) | 1.01 (0.95–1.07) | 0.821 |
| Education, yrs | 8.5 (8.0–11.0) | 10 (8–12) | 0.97 (0.92–1.02) | 0.212 | 9 (8–12) | 10 (8–12) | 0.94 (0.90–0.99) | 0.014 |
| Employed | 40 (54.1) | 76 (60.3) | 0.85 (0.59–1.22) | 0.385 | 38 (55.1) | 55 (42.0) | 1.41 (0.96–2.07) | 0.080 |
| Residential characteristics | ||||||||
| Running water in home | 25 (33.8) | 56 (44.4) | 0.75 (0.51–1.11) | 0.149 | 38 (55.1) | 66 (50.4) | 1.13 (0.77–1.66) | 0.530 |
| Electricity in home | 46 (62.2) | 85 (67.5) | 0.87 (0.60–1.25) | 0.443 | 47 (68.1) | 77 (58.8) | 1.31 (0.86–1.99) | 0.208 |
| Pit latrine use | 71 (96.0) | 116 (92.1) | 1.65 (0.60–4.53) | 0.335 | 65 (94.2) | 125 (95.4) | 0.86 (0.39–1.88) | 0.697 |
| Persons in household | 4 (3–5) | 4 (3–5) | 1.08 (0.97–1.20) | 0.183 | 3 (2–5) | 3 (2–5) | 0.98 (0.86–1.11) | 0.748 |
| Single room household | 11 (14.9) | 25 (19.8) | 0.80 (0.47–1.35) | 0.398 | 17 (24.6) | 37 (28.2) | 0.88 (0.56–1.39) | 0.592 |
| TB history, symptoms, exposures | ||||||||
| Previous TB | 2 (2.7) | 0 (0.0) | 2.75 (2.29–3.31) | <0.001 | 1 (1.5) | 0 (0.0) | 2.93 (2.41–3.55) | <0.001 |
| WHO TB symptom past month ‡ | 4 (5.4) | 6 (4.8) | 1.09 (0.50–2.38) | 0.837 | 2 (2.9) | 4 (3.1) | 0.97 (0.31–3.05) | 0.952 |
| Household WHO TB symptom | 2 (2.7) | 5 (4.0) | 0.77 (0.23–2.51) | 0.660 | 2 (2.9) | 4 (3.1) | 0.97 (0.31–3.05) | 0.952 |
| TB exposure past 2 yrs | 1 (1.4) | 2 (1.6) | 0.90 (0.18–4.52) | 0.898 | 1 (1.5) | 0 (0.0) | 2.93 (2.41–3.55) | <0.001 |
| HIV | ||||||||
| CD4, cells/mm3 (N=90) § | 546 (391–725) | 448 (287–597) | 1.00 (1.00–1.00) | 0.039 | ||||
| HIV viral load, copies/ml (n=174) § | 0 (0–40) | 0 (0–65) | 1.00 (1.00–1.00) | 0.114 | ||||
| HIV viral load undetectable (n=174) || | 58 (90.6) | 95 (86.4) | 1.32 (0.65–2.69) | 0.434 | ||||
| Time since HIV diagnosis, yrs | 2.8 (0.4–7.6) | 3.3 (0.5–6.3) | 1.00 (0.96–1.05) | 0.847 | ||||
| ART before pregnancy | 50 (67.6) | 85 (67.5) | 1.00 (0.68–1.48) | 0.988 | ||||
| Any IPT | 40 (54.1) | 85 (67.5) | 0.71 (0.49–1.00) | 0.056 | ||||
| IPT on enrollment (n=125) | 12 (30.0) | 26 (30.2) | 0.99 (0.57–1.74) | 0.979 | ||||
Abbreviations: LTBI, latent tuberculosis infection; IQR, interquartile range; RR, relative risk; yrs, years; wks, weeks; BMI, body mass index; MUAC, mid-upper arm circumference; TB, tuberculosis; WHO, World Health Organization; ART, antiretroviral therapy; IPT, isoniazid preventive therapy
LTBI defined as TST or QFT positive, where TST positive ≥5mm for WLHIV and ≥10mm for HIV negative women
Relative risk (RR) estimated using a generalized linear model (GLM) with log link and Poisson family
WHO TB symptoms: fever, cough, weight loss, night sweats
CD4 and viral load data were collected from routine programmatic data
Undetectable viral load ≤20 copies/ml
QFT-Plus Quantitative Results by HIV status
Median QFT mitogen (mitogen-nil) response was similar among women with and without HIV (6.68 vs. 8.19 IU/mL; p=0.332), while median nil response was higher among HIV-negative women (WLHIV 0.08 vs. HIV-negative 0.12 IU/mL; p<0.001) (Figure 3a; supplemental Table E3). Among women with LTBI (either QFT or TST-positive, n=142), WLHIV had significantly lower median TB1 (1.05 vs. 2.65 IU/mL; p=0.035) and TB2 (1.26 vs. 2.56 IU/mL; p=0.027) antigen responses (Figure 3b; supplemental Table E3). TB1 and TB2 antigen responses remained lower in WLHIV than HIV-negative women in analyses limited to TST-positive or QFT-positive women but not significantly so (supplemental Table E3).
Figure 3. Quantitative QFT-Plus responses by HIV status among pregnant women.
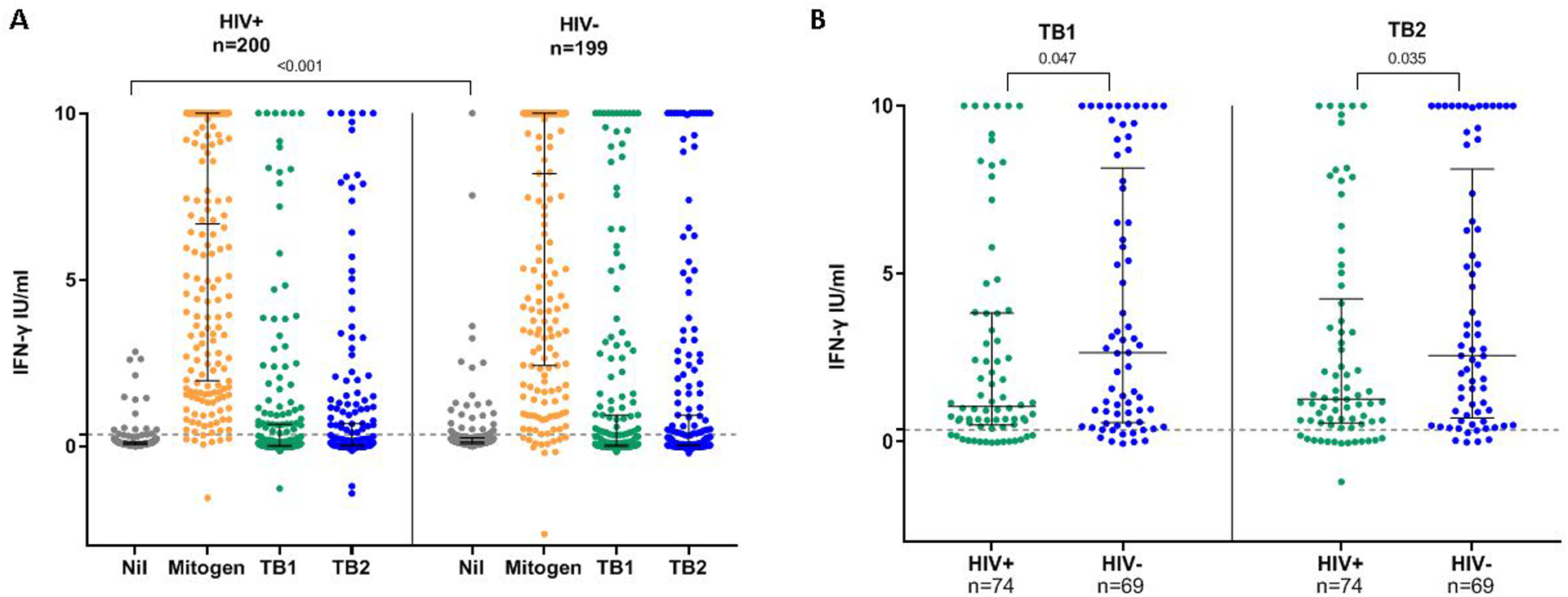
A) Quantitative QFT-Plus responses compared between women with and without HIV , and B) TB-specific antigen responses compared between women with and without HIV among LTBI+ (QFT+ or TST+) women.
Mitogen = mitogen – nil, TB1 = TB1 – nil, TB2 = TB2 – nil
QFT-Plus and TST Concordance
Among 397 participants with QFT-plus and TST (using clinical cut-offs for TST) results, agreement was 66.0% (kappa 0.19; p<0.001) (supplemental Table E4). Stratified by HIV status, WLHIV had 70.0% agreement (kappa 0.31; p<0.001) and HIV-negative women had 61.9% agreement (kappa 0.07; p=0.022). For QFT-Plus results overall, there was 95.5% agreement between TB1 and TB2 antigen positive results (kappa 0.90; p<0.001), which was similar when stratified by HIV status (supplemental Table E5a).
For WLHIV there was a positive significant correlation between TST induration (mm) and both TB1 (Spearman’s rho 0.293; p<0.001) and TB2 antigen responses (Spearman’s rho 0.387; p<0.001), but not for HIV-negative women (TST/TB1: 0.00, p=0.980; TST/TB2: 0.019, p=0.787) (supplemental Table E5b and Figure E1).
Sensitivity Analyses Varying QFT-Plus and TST Cut-offs
By increasing TST-positivity cut-off to ≥10 mm for WLHIV, 17 (8.5%) fewer WLHIV would have been considered TST positive, but differences between WLHIV and HIV-negative women remained statistically significant (WLHIV 10.0% vs. HIV-negative 4.6%; p=0.011) (supplemental Table E1), without improvement of agreement (supplemental Table E4). Lowering the TST-positivity threshold to ≥5mm for HIV-negative women would identify an additional 17 (8.5%) of HIV-negative women as TST-positive, with differences between groups no longer statistically significant (WLHIV 18.5% vs. HIV-negative 13.1%; p=0.116).
Lowering the QFT-positivity threshold to 0.20 IU/mL from 0.35 IU/mL increased the overall proportion of QFT-positivity to 38.1% (from 35.8%) by identifying 23 additional women as QFT-positive (10 WLHIV; 13 HIV-negative), but similar to the conventional cut-off, there were no significant differences in QFT-Plus positivity between WLHIV and HIV-negative women (supplemental Table E1). Data regarding proportion and correlates of LTBI-positivity of WLHIV and HIV-negative women at various LTBI, TST, and QFT-Plus cut-offs are in supplemental Tables E1, E2a, E2b.
DISCUSSION
In this large cross-sectional analysis of pregnant women evaluated for LTBI in a high HIV/TB burden setting in western Kenya, newer generation IGRA assay, QFT-Plus, identified three times as many pregnant women overall with LTBI compared to TST (32% vs. 12%), with similar prevalence of QFT-Plus-positivity in WLHIV and HIV-negative women. The finding of higher prevalence of QFT-Plus-positivity compared to TST was further amplified among HIV-negative women, even when increasing TST cut-offs to ≥10 mm for both WLHIV and HIV-negative women. Among women with LTBI, quantitative TB1 and TB2 antigen responses were significantly lower in WLHIV compared to HIV-negative women despite similar prevalence of QFT-positivity between groups.
Our finding of 30% QFT-positivity among pregnant women is similar to previous studies in Kenya17,28 and other high HIV/TB burden settings including India and Ethiopia.8,9,11 We found TST had lower rates of positivity in detecting LTBI among pregnant women irrespective of HIV-status, which has been reported in separate cohorts of WLHIV and HIV-negative women with the older generation QFT-Gold.8,9,17,18 To our knowledge this is one of the first studies to directly compare TST and QFT-positivity between pregnant women with and without HIV infection concurrently with next generation QFT-Plus. While overall TST-positivity was lower compared to QFT-Plus, our study found a larger proportion of TST-positivity in WLHIV compared to HIV-negative pregnant women, when using recommended clinical TST cut-offs based on HIV status. This higher TST-positivity prevalence among WLHIV remained even when we increased the cut-off to ≥10mm for all women in sensitivity analyses, suggesting this difference was not due to an artifact of differing routine clinical cut-offs by HIV status. While TST identified a lower proportion of pregnant women with LTBI overall, TST identified a different population as having LTBI than QFT-Plus as shown in the concordance analysis. WLHIV are potentially at higher risk of M. tuberculosis infection which the TST may have identified through broader or different antigen selection.
Our findings differ from an Ethiopian study that found a higher proportion of QFT and TST positivity among HIV-negative women than WLHIV.12 The authors suggested that these differences were due to test performance rather than differences in true LTBI positivity between groups, as the study was done in an area with over 50% LTBI prevalence.29,30 A study in Ugandan postpartum mothers found similar QFT-Gold positivity but higher TST-positivity in HIV-negative women compared to WLHIV.31 We postulate that pregnant WLHIV in our study were relatively immunocompetent as they were all on ART with relatively high CD4 counts, which may have allowed them to mount a TST response. Our results indicate HIV-negative women had slightly lower MUAC than WLHIV, which indicates that nutritional status could have contributed to false negatives. When decreasing the TST cut-off to ≥5mm for all women, the difference in TST positivity between WLHIV and HIV-negative women was no longer significant, suggesting that perhaps these two groups are more similar with regards to immune status than other studies with WLHIV who are more immunosuppressed, which could be an argument for changing the TST threshold in certain populations of WLHIV who have robust CD4 counts and are virally suppressed. Additionally, there may be some other explanations contributed to negative results among HIV-negative women. TST false negativity, or anergy, has been associated with HIV positivity, but also other immunosuppression, malnutrition, chronic kidney disease, and alcohol use.32,33
While the prevalence of QFT-positivity was similar between pregnant women with and without HIV in our study, QFT-Plus IFN-γ levels in response to TB1 and TB2 antigens were lower in WLHIV compared to HIV-negative women with LTBI. This is consistent with studies of pregnant women in Ethiopia,11 adults in Italy34 and recent unpublished data from western Kenya which all showed lower TB1 and TB2 responses in PLHIV compared to HIV-negative persons.35 CD4 cells from PLHIV have impaired IFN-γ secreting capacity,36 and IFN-γ concentration in response to TB-specific antigens (using QFT-Gold) has been correlated to CD4 cell count.37 In our study, WLHIV with LTBI had higher median CD4 counts than those with negative LTBI results, consistent with this mechanism. Higher IPT usage among LTBI-negative WLHIV may have also influenced results; although IPT decreases risk of active TB disease, it may have reduced risk of LTBI and has been associated with a loss of QFT-positivity,18 TST reversion,38 and decrease in IFN-γ concentration.39
Previous studies have suggested lowering QFT-plus cut-offs for pregnant WLHIV.11 However in our study QFT-positivity prevalence between WLHIV and HIV-negative women remained similar after adjusting the QFT-positivity threshold to ≥0.20 IU/mL or ≥0.70 IU/mL instead of the manufacturer threshold of ≥0.35 IU/mL. Similarly, changing the QFT threshold did not have an impact on differential LTBI prevalence in our cohort, suggesting WLHIV in our study (with relatively high median CD4 count and universal ART coverage) may have had robust immune responses to TB antigens in contrast to more immunosuppressed cohorts.40
We found higher TST/QFT agreement in WLHIV compared to HIV-negative women (70 vs 62%, respectively), which is lower agreement but showing a similar trend to Birku et al (98% vs. 90%).12 Other studies found similar, lower TST/QFT agreement in pregnant WLHIV in Kenya (kappa 0.20)17 and India (kappa 0.25)9, as well as at delivery in a multinational study (kappa 0.40),18 due to a similar pattern of higher QFT positivity compared to TST. It is postulated that because the TST response requires IFN-γ,TNF-α, IL-2, and other cytokines to stimulate a delayed-type hypersensitivity reaction, there may be more falsely negative TSTs in pregnant women or immunocompromised populations, which could explain the lower TST positivity compared to QFT.9 This would not, however, explain why our study showed a higher proportion of TST-positivity in WLHIV than HIV-negative women, which remained even after utilizing the higher cut-off for both groups in sensitivity analyses.
There were limitations and strengths to our study. Baseline characteristics differed between WLHIV and HIV-negative women, which may have influenced LTBI diagnostic performance. To address this, we assessed cofactors of LTBI in the entire cohort and in analyses stratified by HIV status. We relied on participant report of IPT initiation, which may have affected our estimates of IPT use. While IPT roll-out is high in western Kenya and estimates of IPT use overall among pregnant WLHIV were similar to those reported in the same setting in our previously published estimates, notably only 54% of WLHIV with evidence of LTBI in this study had received IPT.41 Given the cross-sectional nature of our study and limitations in general for LTBI diagnosis we are unable ascertain the timing of M. tuberculosis infection acquisition and previous IPT use. Of note, only 54% of WLHIV with evidence of LTBI had received IPT There is some data to suggest that LTBI diagnostics could be potentially most affected by changes occurring in the late 3rd trimester, and median gestational age in our cohort was 28 weeks.42 Our study has significant strengths in that it is one of the largest study to directly compare LTBI diagnostics including the newer generation QFT-Plus between pregnant WLHIV and HIV-negative pregnant women and in the contemporary setting of universal ART coverage and high IPT uptake among WLHIV.
In conclusion, this study adds to our knowledge regarding performance of TST and QFT-Plus diagnostics in detecting LTBI in pregnant women with and without HIV. Our data showed a pattern of overall lower TST-positivity compared to QFT in pregnancy, and while QFT-positivity was similar between WLHIV and HIV-negative women, HIV infection was associated with lower TB antigen responses among women with LTBI. Although the current Kenyan guidelines recommend IPT for all PLHIV including among those who are pregnant, our data identified WLHIV with LTBI but had not yet received IPT. Given the results of a recent large-scale randomized noninferiority study demonstrating the association of higher risk of adverse pregnancy outcomes with initiation of IPT during pregnancy, understanding how pregnancy affects the performance of LTBI diagnostics will be crucial to safely target groups with the highest risk of infection for TB prevention, including pregnant mothers with particular risk factors.43 Furthermore, the ability to detect LTBI accurately with diagnostics influences how policy makers and researchers measure LTBI prevalence and risk factors for infection, which has implications for larger public health interventions and design of vaccine and other prevention studies which rely on these diagnostics for TB-infection entry criteria or outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the MITIPS Study Clinic Staff, the Kisumu and Siaya County Directors of Health, health facility staff, University of Washington (UW)-Kenya, and Kenyatta National Hospital Research and Programs operational staff. The authors also thank Qiagen for providing discounted QFT-Plus test kits, and the Kenya Medical Research Institute (KEMRI)/Centers for Disease Control and Prevention (CDC) in Kisumu, Kenya, for performing the QFT-Plus assays. Most of all, the authors thank the women who participated in the study.
FINANCIAL SUPPORT
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Advancing Translational Sciences at National Institutes of Health (grant numbers NIH/NIAID K23AI120793 to S.M.L.; NIH/NIAID R01AI142647 to G.J.-S.; NIH/NICHD R21HD098746 to S.M.L.; and NIH UL1TR000423 for REDCap). S.R.K. was supported by the INTERSECT-Ellison Research Fellowship Grant (University of Washington), the Center for AIDS Research (CFAR) Trainee Support Grant at the University of Washington (P30 AI027757) and the IDSA ID Week Mentorship Program and travel grant.
Footnotes
Preliminary data was presented at IDWeek, Washington DC, October 2–6, 2019.
REFERENCES
- 1.Sobhy S, Babiker Z, Zamora J, Khan KS, Kunst H. Maternal and Perinatal Mortality and Morbidity Associated with Tuberculosis During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis. BJOG : an international journal of obstetrics and gynaecology 2017;124:727–33. [DOI] [PubMed] [Google Scholar]
- 2.Sugarman J, Colvin C, Moran AC, Oxlade O. Tuberculosis in Pregnancy: An Estimate of the Global Burden of Disease. Lancet Glob Health 2014;2:e710–6. [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Bates M, Mwaba P. The Neglected Global Burden of Tuberculosis in Pregnancy. The Lancet Global health 2014;2:e675–6. [DOI] [PubMed] [Google Scholar]
- 4.Salazar-Austin N, Hoffmann J, Cohn S, Mashabela F, Waja Z, Lala S, Hoffmann C, Dooley KE, Chaisson RE, Martinson N, Team TS. Poor Obstetric and Infant Outcomes in Human Immunodeficiency Virus-Infected Pregnant Women with Tuberculosis in South Africa: The Tshepiso Study. Clinical Infectious Diseases 2018;66:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathad JS, Gupta A. Tuberculosis in Pregnant and Postpartum Women: Epidemiology, Management, and Research Gaps. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012;55:1532–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Wood R, Wilkinson RJ. Changing Concepts of “Latent Tuberculosis Infection” in Patients Living with Hiv Infection. Clin Dev Immunol 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of Tuberculosis in Pregnancy: A National, Primary Care-Based Cohort and Self-Controlled Case Series Study. Am J Respir Crit Care Med 2012;185:779–84. [DOI] [PubMed] [Google Scholar]
- 8.Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, Nangude A, Chopade K, Suryavanshi N, Deshpande P, Kulkarni V, Glesby MJ, Fitzgerald D, Bharadwaj R, Sambarey P, Gupta A. Pregnancy Differentially Impacts Performance of Latent Tuberculosis Diagnostics in a High-Burden Setting. PloS one 2014;9:e92308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N, Gupte N, Joshi S, Chandanwale A, Dupnik KM, Kulkarni V, Deshpande P, Fitzgerald DW, Gupta A. Quantitative Ifn-Gamma and Il-2 Response Associated with Latent Tuberculosis Test Discordance in Hiv-Infected Pregnant Women. Am J Respir Crit Care Med 2016;193:1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Marston BJ, Huang L, Hopewell PC, Pai M. Interferon-Gamma Release Assays for the Diagnosis of Latent Tuberculosis Infection in Hiv-Infected Individuals: A Systematic Review and Meta-Analysis. Journal of acquired immune deficiency syndromes 2011;56:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konig Walles J, Tesfaye F, Jansson M, Tolera Balcha T, Winqvist N, Kefeni M, Garoma Abeya S, Belachew F, Sturegard E, Bjorkman P. Performance of Quantiferon-Tb Gold Plus for Detection of Latent Tuberculosis Infection in Pregnant Women Living in a Tuberculosis- and Hiv-Endemic Setting. PloS one 2018;13:e0193589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birku M, Desalegn G, Kassa G, Tsegaye A, Abebe M. Effect of Pregnancy and Hiv Infection on Detection of Latent Tb Infection by Tuberculin Skin Test and Quantiferon-Tb Gold in-Tube Assay among Women Living in a High Tb and Hiv Burden Setting. Int J Infect Dis 2020;101:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium Tuberculosis Infection. The New England journal of medicine 2015;372:2127–35. [DOI] [PubMed] [Google Scholar]
- 14.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. Gamma Interferon Release Assays for Detection of Mycobacterium Tuberculosis Infection. Clin Microbiol Rev 2014;27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiagen. Quantiferon®-Tb Gold Plus (Qft®-Plus) Elisa Package Insert. http://www.quantiferon.com/irm/content/PI/QFT/PLUS/2PK-Elisa/UK.pdf.
- 16.Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Ohmiti K, Goletti D, Bart PA, Hanekom W, Scriba TJ, Nicod L, Pantaleo G, Harari A. Combined Use of Mycobacterium Tuberculosis-Specific Cd4 and Cd8 T-Cell Responses Is a Powerful Diagnostic Tool of Active Tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2015;60:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, Horne DJ, John-Stewart G. Effect of Pregnancy on Interferon Gamma Release Assay and Tuberculin Skin Test Detection of Latent Tb Infection among Hiv-Infected Women in a High Burden Setting. Journal of acquired immune deficiency syndromes 2017;75:128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg A, Aaron L, Montepiedra G, Sterling TR, Browning R, Mmbaga B, Vhembo T, Naik S, Kabugho E, Masheto G, Pahwa S, Mathad JS, LaCourse SM, McCarthy K, Bradford S, Theron G, Costello D, Zimmer B, Pierre MF, Gausi K, Denti P, Haas DW, Gupta A, team IPs. Effects of Pregnancy and Isoniazid Preventive Therapy on Mycobacterium Tuberculosis Interferon Gamma Response Assays in Women with Hiv. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenya National AIDS Control Council. Kenya Hiv Estimates Report 2018: Kenya Ministry of Health; 2018. October 2018.
- 20.World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations.2013. [PubMed]
- 21.Kenyan Ministry of Health. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing Hiv in Kenya, 2018 Edition. 2018.
- 22.Kenyan Ministry of Health. Guidelines for Management of Tuberculosis and Leprosy in Kenya. July 2013. ed2013.
- 23.LaCourse SM, Richardson BA, Kinuthia J, Warr AJ, Maleche-Obimbo E, Matemo D, Cranmer LM, Mecha J, Escudero JN, Hawn TR, John-Stewart G. A Randomized Controlled Trial of Isoniazid to Prevent Mycobacterium Tuberculosis Infection in Kenyan Hiv-Exposed Uninfected Infants. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuberculin Reaction Size on Five Consecutive Days. Bull World Health Organ 1955;12:189–96. [PMC free article] [PubMed] [Google Scholar]
- 25.Cobelens F, Van Deutekom H, Draayer-Jansen I, Schepp-Beelen A, Van Gerven P, Mensen M. Tuberculin Skin Test Reactions by Time of Reading among Dutch Travellers. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2003;7:758–63. [PubMed] [Google Scholar]
- 26.Impaact P1041: A Randomized, Double Blind, Placebo Controlled Trial to Determine the Efficacy of Isoniazid (Inh) in Preventing Tuberculosis Disease and Latent Tuberculosis Infection among Infants with Perinatal Exposure to Hiv Trial Protocol [Protocol]. (Accessed 24 January 2021, at https://impaactnetwork.org/studies/P1041.asp.)
- 27.American Thoracic Society. Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection. MMWR Recomm Rep;49:1–51. [PubMed] [Google Scholar]
- 28.Jonnalagadda S, Lohman Payne B, Brown E, Wamalwa D, Maleche Obimbo E, Majiwa M, Farquhar C, Otieno P, Mbori-Ngacha D, John-Stewart G. Latent Tuberculosis Detection by Interferon Gamma Release Assay During Pregnancy Predicts Active Tuberculosis and Mortality in Human Immunodeficiency Virus Type 1-Infected Women and Their Children. The Journal of infectious diseases 2010;202:1826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teklu T, Legesse M, Medhin G, Zewude A, Chanyalew M, Zewdie M, Wondale B, Haile-Mariam M, Pieper R, Ameni G. Latent Tuberculosis Infection and Associated Risk Indicators in Pastoral Communities in Southern Ethiopia: A Community Based Cross-Sectional Study. BMC public health 2018;18:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bekele A, Ashenafi S, Aderay G, Assefa G, Aseffa A, Anderssen J, Brighenti S. Latent Tuberculosis among Adult Ethiopian Patients at Chest Clinic, Tikuranbessa Specialized Hospital, Addis Ababa, Ethiopia. Ethiop Med J 2016;54:181–8. [PubMed] [Google Scholar]
- 31.Marquez C, Chamie G, Achan J, Luetkemeyer AF, Kyohere M, Okiring J, Dorsey G, Kamya MR, Charlebois ED, Havlir DV. Tuberculosis Infection in Early Childhood and the Association with Hiv-Exposure in Hiv-Uninfected Children in Rural Uganda. The Pediatric infectious disease journal 2016;35:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almeida Santos J, Duarte R, Nunes C. Tuberculin Skin Test and Predictive Host Factors for False-Negative Results in Patients with Pulmonary and Extrapulmonary Tuberculosis. Clin Respir J 2020;14:541–8. [DOI] [PubMed] [Google Scholar]
- 33.Pelly TF, Santillan CF, Gilman RH, Cabrera LZ, Garcia E, Vidal C, Zimic MJ, Moore DA, Evans CA. Tuberculosis Skin Testing, Anergy and Protein Malnutrition in Peru. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2005;9:977–84. [PMC free article] [PubMed] [Google Scholar]
- 34.Petruccioli E, Chiacchio T, Navarra A, Vanini V, Cuzzi G, Cimaglia C, Codecasa LR, Pinnetti C, Riccardi N, Palmieri F, Antinori A, Goletti D. Effect of Hiv-Infection on Quantiferon-Plus Accuracy in Patients with Active Tuberculosis and Latent Infection. J Infect 2020;80:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zifodya JS LS, Temu TM, Attia E, Masyuko S, Nyale G, Nyabiage J, Onyango D, Kinuthia J, Page S, Farquhar C, Crothers K. Latent Tb Infection and Quantferon-Tb Gold Plus in Hiv-Infected and Uninfected Kenyan Adults. American Thoracic Society; 2020. May 15–20, 2020; Virtual. [Google Scholar]
- 36.Sutherland R, Yang H, Scriba TJ, Ondondo B, Robinson N, Conlon C, Suttill A, McShane H, Fidler S, McMichael A, Dorrell L. Impaired Ifn-Γ-Secreting Capacity in Mycobacterial Antigen-Specific Cd4 T Cells During Chronic Hiv-1 Infection Despite Long-Term Haart. Aids 2006;20:821–9. [DOI] [PubMed] [Google Scholar]
- 37.Leidl L, Mayanja-Kizza H, Sotgiu G, Baseke J, Ernst M, Hirsch C, Goletti D, Toossi Z, Lange C. Relationship of Immunodiagnostic Assays for Tuberculosis and Numbers of Circulating Cd4+ T-Cells in Hiv Infection. Eur Respir J 2010;35:619–26. [DOI] [PubMed] [Google Scholar]
- 38.Johnson DF, Malone LL, Zalwango S, Mukisa Oketcho J, Chervenak KA, Thiel B, Mayanja-Kizza H, Stein CM, Boom WH, Lancioni CL. Tuberculin Skin Test Reversion Following Isoniazid Preventive Therapy Reflects Diversity of Immune Response to Primary Mycobacterium Tuberculosis Infection. PloS one 2014;9:e96613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Shea MK, Fletcher TE, Beeching NJ, Dedicoat M, Spence D, McShane H, Cunningham AF, Wilson D. Tuberculin Skin Testing and Treatment Modulates Interferon-Gamma Release Assay Results for Latent Tuberculosis in Migrants. PloS one 2014;9:e97366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonnalagadda SR, Brown E, Lohman-Payne B, Wamalwa D, Farquhar C, Tapia K, Cranmer LM, John-Stewart GC. Consistency of Mycobacterium Tuberculosis-Specific Interferon-Gamma Responses in Hiv-1-Infected Women During Pregnancy and Postpartum. Infect Dis Obstet Gynecol 2012;2012:950650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaCourse SM, Wagner AD, Cranmer LM, Copeland A, Maleche-Obimbo E, Richardson BA, Matemo D, Kinuthia J, John-Stewart G. Brief Report: High Programmatic Isoniazid Preventive Therapy (Ipt) Use in Pregnancy among Hiv-Infected Women. Journal of acquired immune deficiency syndromes 2019;82:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha A, Escuduero J, Layouni T, Richardson B, Hou S, Mugo N, Mujugira A, Celum C, Baeten JM, Lingappa J, John-Stewart GC, LaCourse SM, Shah JA. Mycobacterium Tuberculosis-Specific T Cell Responses Are Impaired During Late Pregnancy with Elevated Biomarkers of Tuberculosis Risk Postpartum. medRxiv 2021:2021.06.11.21258789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theron G, Montepiedra G, Aaron L, McCarthy K, Nahida C, Jean-Philippe P, Zimmer B, James Loftis A, Chipato T, Nematadzira T, Nyati M, Onyango-Makumbi C, Masheto G, Ngocho J, Tongprasert F, Patil S, Lespinasse D, Weinberg A, Gupta A. Individual and Composite Adverse Pregnancy Outcomes in a Randomized Trial on Isoniazid Preventative Therapy among Women Living with Hiv. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


