Abstract
During enamel formation, the organic enamel protein matrix interacts with calcium phosphate minerals to form elongated, parallel, and bundled enamel apatite crystals of extraordinary hardness and biomechanical resilience. The enamel protein matrix consists of unique enamel proteins such as amelogenin, ameloblastin, and enamelin, which are secreted by highly specialized cells called ameloblasts. The ameloblasts also facilitate calcium and phosphate ion transport toward the enamel layer. Within ameloblasts, enamel proteins are transported as a polygonal matrix with 5nm subunits in secretory vesicles. Upon expulsion from the ameloblasts, the enamel protein matrix is re-organized into 20nm subunit compartments. Enamel matrix subunit compartment assembly and expansion coincide with C-terminal cleavage by the MMP20 enamel protease and N-terminal amelogenin self-assembly. Upon enamel crystal precipitation, the enamel protein phase is reconfigured to surround the elongating enamel crystals and facilitate their elongation in C-axis direction. At this stage of development, and upon further amelogenin cleavage, central and polyproline-rich fragments of the amelogenin molecule associate with the growing mineral crystals through a process termed “shedding”, while hexagonal apatite crystals fuse in longitudinal direction. Enamel protein sheath-coated enamel “dahlite” crystals continue to elongate until a dense bundle of parallel apatite crystals is formed, while the enamel matrix is continuously degraded by proteolytic enzymes. Together, these insights portrait enamel mineral nucleation and growth as a complex and dynamic set of interactions between enamel proteins and mineral ions that facilitate regularly seeded apatite growth and parallel enamel crystal elongation.
Ultrastructural changes in enamel matrix patterning during amelogensis
Developing tooth enamel is a highly dynamic environment consisting of proteins and minerals. In this review, we have integrated results from 3D-NMR, electron microscopic, biochemical, physical, and atomic force microscopy studies generated in our and other laboratories to present a model of the first steps involved in amelogenesis. Previous studies have demonstrated that the formative enamel environment (enamel matrix) starts out as a tissue rich in water, proteins, and minerals, which becomes increasingly mineralized due to the resorptive activity of ameloblasts and the degradation of the enamel protein matrix by enzymes (Robinson et al., 1998). On a microscopic level, the proteins and minerals of the developing enamel are secreted by ameloblasts, a layer of highly polarized cells differentiated from the inner enamel epithelium. Ameloblasts are equipped with ion channels to transport calcium into the enamel layer (Lacruz, 2017) and secretory vesicles to ferry phosphate to the enamel mineralization front (Pandya et al., 2017a). Ameloblast secretory vesicles also carry enamel proteins such as amelogenin, ameloblastin, and enamelin for enamel matrix deposition and enamel crystal growth (Diekwisch et al., 1993; Diekwisch et al., 1995; Diekwisch et al., 1997). Once secretory vesicles reach the ameloblast cell membrane, vesicle contents are expulsed into the extracellular enamel matrix (Fig. 1).
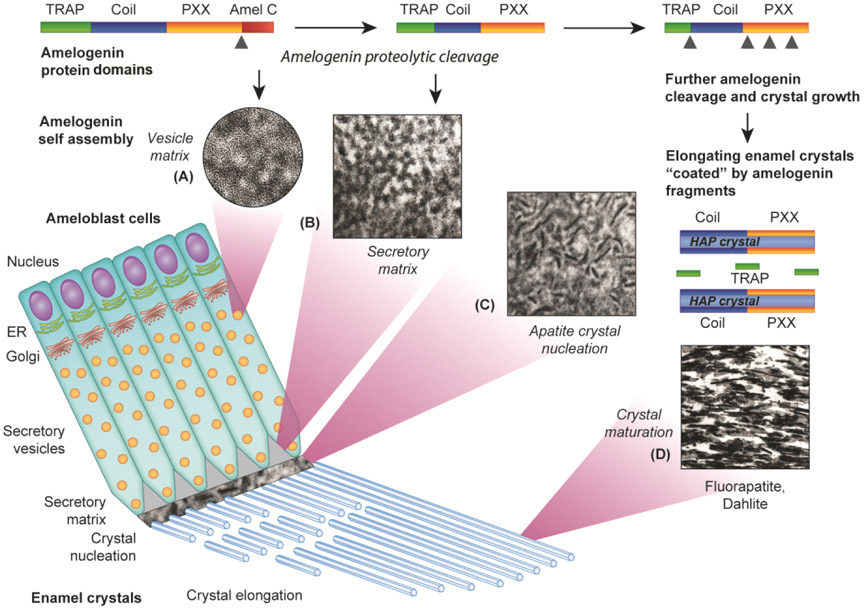
Figure 1 (Sketch). Ultrastructural changes in enamel matrix configuration during ameloblast secretory vesicle transport (A), secretory matrix assembly (B), apatite crystal nucleation (C), and enamel crystal elongation (D).
This is a composite sketch seeking to summarize cellular changes (lower left corner), changes in the processing of the major tooth enamel protein amelogenin (top panel) and resulting ultrastructural changes (A-D) during the secretory stage of amelogenesis. On a cellular level, rows of highly polarized cells (ameloblasts) generate and transport specialized enamel proteins and facilitate mineral ion diffusion toward the enamel layer (lower left aspect of the sketch). Specifically, enamel proteins such as amelogenin are synthesized in the endoplasmic reticulum (ER) and then packaged by the Golgi apparatus (Golgi) into secretory vesicles (vesicle matrix, A). Upon secretion into the enamel layer, the subunit dimensions of this matrix become four-fold enlarged (secretory matrix, B), likely due to a reorganization of the matrix following the cleavage of the hydrophilic C-terminus of the amelogenin molecule (Amel C, top panel). Accumulation of excess ions in the enamel matrix leads to the nucleation of apatite crystals (C) and a reconfiguration of the enamel matrix structure (C). The protonation resulting from the apatite crystallization process promotes a reorganization of the amelogenin matrix and a dissociation of amelogenin fragments into (i) polyproline repeat fragments lining the elongating apatite crystals and (ii) amelogenin TRAP fragments freely floating in between elongating crystals (upper right corner). Continuous elongation of these fluoridated and carbonated apatite crystals and packaging into rods of multiple crystals results in the formation of mature enamel (Dahlite). Note the cellular locations corresponding to the changes in matrix configuration (bottom left) and the phases of amelogenin proteolytic processing at each stage of matrix assembly (top).
Upon exit into the extracellular enamel space, the 3D configuration of the enamel matrix changes from the 5nm diameter intravesicular base unit pattern to a polygonal configuration with 20nm diameter subunits (Pandya et al., 2017b)(Fig. 1). This change in matrix configuration is the result of the C-terminal cleavage of the major tooth enamel matrix protein amelogenin. Upon the formation of hydroxyapatite crystals, the protons released during this process induce a dramatic change in matrix configuration, resulting in the formation of a sheath-like coat surrounding the precipitating crystallites (Diekwisch et al., 1995; Pandya et al., 2017b)(Fig. 1). The subsequent stage of apatite crystal maturation is dominated by the formation of long and parallel crystal bundles (Pandya and Diekwisch, 2019)(Fig. 1). Throughout enamel crystal elongation, enamel crystals are surrounded by a tightly wrapped coat of organic matter (Diekwisch, 1998; Diekwisch et al., 1995; Warshawsky, 1989)(Fig. 1). The thickness of this organic matrix coat gradually decreases during enamel crystal growth and maturation.
Experimental evidence suggests that the enamel mineral and protein phase are tightly linked throughout development. Ion channels facilitate uptake and release of calcium ions as they traverse the ameloblast layer, while proteins and a portion of the phosphate ions travel to the enamel matrix within coated vesicles. Calcium and phosphate ions then precipitate within the extracellular organic enamel matrix, causing apatite crystal formation and instantaneous matrix re-configuration (Diekwisch et al., 1995; Pandya et al., 2017b). Finally, enamel apatite crystal elongation and c-axis growth are promoted by the continuous coating of elongating enamel crystals by polyproline-rich amelogenin fragments (Gopinathan et al., 2014; Jin et al., 2009).
Stage-specific changes in enamel protein matrix configuration during amelogenesis
The majority of amelogenin proteins within the ameloblast cell layer occur as full-length amelogenins within secretory vesicles (Diekwisch et al., 1997; Pandya et al., 2017b; Satchell et al., 2002)(Fig. 2). Another classic enamel protein that contributes to enamel crystal growth and habit includes enamelin (Hu et al. 2014), while ameloblastin alone and in the absence of amelogenin promotes the growth of a crystalline enameloid layer with short and randomly oriented crystals (Lu et al. 2011). In addition to these classic “enamel proteins”, other proteins such as alkaline phosphatase, stress and heat shock proteins, cofilin-1, calmodulin, and peptidyl-prolyl cis-trans isomerase are involved in amelogenesis (Pandya et al., 2017c).
Figure 2 (Sketch). Amelogenin proteolytic processing and changes in protein assembly during amelogenesis.
This sketch focuses on the protein phase involved in amelogenesis from the packaging of enamel proteins into secretory vesicles (A) to the reorganization of the protein phase as part of the secretory enamel matrix (B) to the subsequent processing of the enamel proteins at the time of apatite nucleation (C) and finally to the formation of protein sheaths guiding the elongation of enamel apatite crystals (D). (A) illustrates the assembly of full-length amelogenins within secretory ameloblast vesicles to form micelles assembled at their N-termini as described by Fukae and colleagues. (B) illustrates the expansion of matrix subunits following C-terminal cleavage of the amelogenin molecule. At this stage, the amelogenin N-terminus remains at the core of the protein assemblies. (C) Calcium phosphate mineral precipitates trigger further cleavage of the amelogenin protein, matrix disassembly, and association of the amelogenin polyproline-rich domain with the elongating apatite crystals via shedding. (D) C-axis elongation and expansion of the apatite crystals are facilitated by polyproline-rich amelogenin fragment ribbons that form a sheath-like coat surrounding elongating crystals.
Inside of secretory vesicles, enamel proteins are organized into 5nm subunits (Brookes et al., 2006; Diekwisch et al., 1995; Pandya et al., 2017b)(Fig. 2). 3D-NMR, atomic force microscopy self-assembly, and nickel-labeling His-tag studies have demonstrated that amelogenin self-assembly is driven by intermolecular interactions at the amelogenin N-terminus (Bromley et al., 2011; Pandya et al., 2017b; Zhang et al., 2011), while the amelogenin C-terminus supports self-assembly through weaker interactions (Fukae et al., 2007; Zhang et al., 2011). However, the amelogenin C-terminal teleopeptide is lost upon entry into the extracellular enamel matrix, where enzymes such as MMP20 cleave the hydrophilic C-terminus, resulting in a loss of C-terminal interactions and a rapid reorganization of the organic enamel matrix structural configuration into a 20nm subunit pattern (Pandya et al., 2017b)(Fig. 2). It is likely that the expansion of the enamel matrix subunit dimensions after C-terminal cleavage is driven by an extension release of the spring-like α-helical amelogenin N-terminus (Zhang et al., 2011). Remarkably, the 20nm subunit pattern of the extracellular enamel protein matrix coincides with the 20nm spacing between initial enamel crystallites, suggesting that the enamel matrix protein pattern plays a role in the 20nm spacing between initial enamel crystals (Diekwisch et al., 1995). This separately nucleated and spaced growth of individual enamel crystals within enamel rods (prism bundles) provides the structural basis for the hard yet resilient biomechanical properties of mammalian tooth enamel (Pandya and Diekwisch, 2019).
Upon enamel crystal nucleation, the protons generated as a result of this process once more trigger a reconfiguration of the protein matrix and a deposition of disassembled amelogenin substructures onto apatite crystal surfaces (“shedding”)(Jokisaari et al., 2019; Lu et al., 2011; Pandya et al., 2017b; Tarasevich et al., 2009)(Figs. 1 and 2). This “shedding” involves most likely a continuous deposition of amelogenin polyproline-rich fragments onto the elongating aspect of the apatite crystals. Our Western blot studies that allowed for the biochemical identification of amelogenin fragments within discrete enamel organ layers suggest that only the amelogenin coil and polyproline tripeptide (PXX) domains associate with the elongating apatite surface (Gopinathan et al., 2014; Pandya et al., 2017b)(Fig. 2), while the amelogenin N-terminus freely resides within the space between apatite crystals (Pandya et al., 2017b)(Fig. 2). An earlier review related amelogenin post-secretory processing (Fincham et al., 1991) summarized similar amelogenin cleavage events, specifically (i) the cleavage of the hydrophilic C-terminus, (ii) a cleavage of the remainder of the molecule at AA 44-45 into a hydrophobic core and the TRAP molecule (Fincham et al., 1983; Sasaki and Shimokawa, 1979), (iii) further proteolytic processing of the hydrophobic polyproline rich core, and (iv) a low affinity of the remaining TRAP molecule to apatite (Aoba et al., 1989), confirming our fractionated biochemical analysis (Pandya et al., 2017b).
Several studies have linked the occurrence of elongated enamel apatite crystals with an elongated PXX domain in vertebrates, suggesting that PXX repeat elongation leads to enhanced enamel matrix subunit compaction and increased stabilization of new ion deposits onto the crystal facets (Bonass et al., 1994; Gopinathan et al., 2014; Jin et al., 2009; Lu et al., 2011). Electron microscopic studies from our laboratory reveal that enamel protein fragments line the elongating enamel crystals and form a dense protein sheath around the apatite crystal core (Diekwisch, 1998; Diekwisch et al., 1995). This protein sheath stabilizes the integration of individual apatite crystals and promotes further crystal elongation in C-axis direction (Diekwisch, 1998; Fang et al., 2011; Jokisaari et al., 2019; Le Norcy et al., 2011; Moradian-Oldak and George, 2021). During the enamel crystal maturation phase, the enamel protein coat becomes gradually degraded and removed with the aid of KLK4 and other enzymes (Bartlett and Simmer, 2014)(Fig. 2).
Enamel apatite crystal nucleation and growth during amelogenesis
Atomic resolution STEM studies have demonstrated that initial enamel crystals form through particle-attachment mechanisms and lattice-guided elongation (Jokisaari et al., 2019). Data from this study suggest that at the atomic level, physical self-assembly mechanisms of the enamel mineral phase play an essential role for apatite crystal growth (Jokisaari et al., 2019). The spacing of enamel crystal nucleation sites is controlled by the 20nm spaced subunits of the extracellular enamel matrix (Jokisaari et al., 2019), providing a patterning template for regularly spaced initial enamel crystals (Fig. 3). This aggregation of nanocrystals to a superstructure of iso-oriented nanoparticles suggests that enamel crystals form through non-classical crystallization mechanisms (Jehannin et al., 2019). Self-assembly of nanoparticles in crystallographic register by oriented aggregation occurs at the onset of amelogenesis (Jokisaari et al., 2019) and involves the formation of mesocrystals (Jehannin et al., 2019). Several studies have indicated that biological enamel mineral starts out as amorphous calcium phosphate, transitions through tri- and octacalcium phosphate intermediary states, and eventually matures into apatite, as reflected in continuously increased calcium/phosphate ratios (Diekwisch et al., 1995; Pandya et al., 2017c)(Fig. 3). Once nucleated, individual apatite crystals fuse to form elongated and parallel enamel apatite crystals that provide the basis for extremely hard yet resilient vertebrate enamel biomechanical properties (Pandya and Diekwisch, 2019)(Fig. 3).
Figure 3 (Sketch). Stages of enamel crystal growth from amorphous calcium phosphate precipitates to mature enamel crystals.
This sketch focuses on the changes in the mineral phase during amelogenesis from (A) calcium phosphate mesocrystals to (B) regularly seeded nucleation clusters to (C) rows (ribbons) of attached apatite particles to (D) mature fluoridated carbonated apatite crystals organized into enamel rods. (A) illustrates the transition from amorphous calcium phosphate precipitates over tricalcium phosphate and octacalcium phosphate intermediaries to hydroxyapatites. (B) Hydroxyapatite crystals are regularly spaced in mineralization clusters within the organic enamel matrix to prevent en bloc apatite mineralization. Instead, enamel apatite crystal seeds form within 20nm subunits to give rise to regularly spaced and elongated dahlite crystals. (C) Under the control of enamel proteins, individual apatite crystals are arranged in C-axis direction, fused into crystal ribbons and then form elongated apatite crystals. (D) Mature and parallel oriented enamel apatite crystals (dahlite).
The question whether the organic enamel protein phase or the inorganic enamel mineral phase is the driving force behind enamel crystal growth and patterning resembles the well-known “chicken or the egg” conundrum, as neither phase occurs separate from the other. Our data indicate that the self-organization properties of the enamel mineral phase exert control during the earliest stages of enamel apatite particle attachment and lattice-guided crystal growth, while the enamel protein phase plays a key role during mineral transport, spacing, and C-axis crystal growth by limiting horizontal apatite crystal expansion and favoring vertical c-axis crystal growth.
It is not clear whether amelogenins self-assemble into nanospheres, as dynamic light scattering and tapping mode atomic force micrographs suggest (Fincham et al., 1995) or whether they form multidirectional rows of protein subunits in immediate association with the enamel mineral layer (Jin et al., 2009; Pandya et al., 2017b). In vitro studies have suggested that enamel proteins provide templates for enamel mineralization (Bai et al., 2020; Carneiro et al., 2016). However, in developing enamel, protein ribbons are closely associated with elongating enamel apatite crystals, allowing enamel proteins to exert stabilizing and compressive forces onto forming calcium phosphate crystals and elongating apatite microribbons (Fang et al., 2011; Le Norcy et al., 2011; Lu et al., 2011; Shin et al., 2020). Thus, enamel crystals and prisms evolve in close association and through reciprocal interactions at all stages of development (Diekwisch et al., 1995; Gopinathan et al., 2014; Pandya et al., 2017b), resulting in the formation of this most unique biomineral: tooth enamel.
Acknowledgement
Generous funding by NIDCR grants DE13378, DE018900 and DE028869 to TGHD is gratefully acknowledged.
Tom Diekwisch reports financial support was provided by National Institute for Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit author statement
Both authors contributed equally to the writing, review and editing of the manuscript.
References
- Aoba T, Moreno EC, Kresak M, Tanabe T, 1989. Possible Roles of Partial Sequences at N- and C-termini of Amelogenin in Protein-Enamel Mineral Interaction. Journal of Dental Research 68, 1331–1336. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yu Z, Ackerman L, Zhang Y, Bonde J, Li W, Cheng Y, Habelitz S, 2020. Protein nanoribbons template enamel mineralization. Proceedings of the National Academy of Sciences 117, 19201–19208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Simmer JP, 2014. Kallikrein-related peptidase-4 (KLK4): role in enamel formation and revelations from ablated mice. Frontiers in physiology 5, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonass W, Robinson P, Kirkham J, Shore R, Robinson C, 1994. Molecular cloning and DNA sequence of rat amelogenin and a comparative analysis of mammalian amelogenin protein sequence divergence. Biochemical and biophysical research communications 198, 755–763. [DOI] [PubMed] [Google Scholar]
- Bromley KM, Kiss AS, Lokappa SB, Lakshminarayanan R, Fan D, Ndao M, Evans JS, Moradian-Oldak J, 2011. Dissecting amelogenin protein nanospheres: characterization of metastable oligomers. Journal of Biological Chemistry 286, 34643–34653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Lyngstadaas SP, Robinson C, Shore RC, Kirkham J, 2006. Intracellular nanosphere subunit assembly as revealed by amelogenin molecular cross-linking studies. European journal of oral sciences 114, 280–284. [DOI] [PubMed] [Google Scholar]
- Carneiro KM, Zhai H, Zhu L, Horst JA, Sitlin M, Nguyen M, Wagner M, Simpliciano C, Milder M, Chen C-L, 2016. Amyloid-like ribbons of amelogenins in enamel mineralization. Scientific reports 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekwisch T, David S, Bringas P, Santos V, Slavkin HC, 1993. Antisense inhibition of AMEL translation demonstrates supramolecular controls for enamel HAP crystal growth during embryonic mouse molar development. Development 117, 471–482. [DOI] [PubMed] [Google Scholar]
- Diekwisch TG, 1998. Subunit compartments of secretory stage enamel matrix. Connective tissue research 38, 101–111. [DOI] [PubMed] [Google Scholar]
- Diekwisch TG, Berman BJ, Gentner S, Slavkin HC, 1995. Initial enamel crystals are not spatially associated with mineralized dentine. Cell and tissue research 279, 149–167. [DOI] [PubMed] [Google Scholar]
- Diekwisch TG, Ware J, Fincham AG, Zeichner-David M, 1997. Immunohistochemical similarities and differences between amelogenin and tuftelin gene products during tooth development. Journal of Histochemistry & Cytochemistry 45, 859–866. [DOI] [PubMed] [Google Scholar]
- Fang P-A, Conway JF, Margolis HC, Simmer JP, Beniash E, 2011. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proceedings of the National Academy of Sciences 108, 14097–14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham A, Moradian-Oldak J, Diekwisch T, Lyaruu D, Wright J, Bringas P Jr, Slavkin H, 1995. Evidence for amelogenin" nanospheres" as functional components of secretory-stage enamel matrix. Journal of structural biology 115, 50–59. [DOI] [PubMed] [Google Scholar]
- Fincham AG, Belcourt AB, Termine JD, Butler WT, Cothran WC, 1983. Amelogenins. Sequence homologies in enamel-matrix proteins from three mammalian species. Biochem J 211, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham AG, Hu Y, Lau EC, Slavkin HC, Snead ML, 1991. Amelogenin post-secretory processing during biomineralization in the postnatal mouse molar tooth. Arch Oral Biol 36, 305–317. [DOI] [PubMed] [Google Scholar]
- Fukae M, Yamamoto R, Karakida T, Shimoda S, Tanabe T, 2007. Micelle structure of amelogenin in porcine secretory enamel. Journal of dental research 86, 758–763. [DOI] [PubMed] [Google Scholar]
- Gopinathan G, Jin T, Liu M, Li S, Atsawasuwan P, Galang M-T, Allen M, Luan X, Diekwisch TG, 2014. The expanded amelogenin polyproline region preferentially binds to apatite versus carbonate and promotes apatite crystal elongation. Frontiers in physiology 5, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehannin M, Rao A, Cölfen H, 2019. New Horizons of Nonclassical Crystallization. Journal of the American Chemical Society 141, 10120–10136. [DOI] [PubMed] [Google Scholar]
- Jin T, Ito Y, Luan X, Dangaria S, Walker C, Allen M, Kulkarni A, Gibson C, Braatz R, Liao X, 2009. Elongated polyproline motifs facilitate enamel evolution through matrix subunit compaction. PLoS biology 7, e1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisaari JR, Wang C, Qiao Q, Hu X, Reed DA, Bleher R, Luan X, Klie RF, Diekwisch TG, 2019. Particle-attachment-mediated and matrix/lattice-guided enamel apatite crystal growth. ACS nano 13, 3151–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, 2017. Enamel: molecular identity of its transepithelial ion transport system. Cell Calcium 65, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Norcy E, Kwak S-Y, Wiedemann-Bidlack F, Beniash E, Yamakoshi Y, Simmer J, Margolis H, 2011. Potential role of the amelogenin N-terminus in the regulation of calcium phosphate formation in vitro. Cells Tissues Organs 194, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ito Y, Kulkarni A, Gibson C, Luan X, Diekwisch TG, 2011. Ameloblastin-rich enamel matrix favors short and randomly oriented apatite crystals. European journal of oral sciences 119, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradian-Oldak J, George A, 2021. Biomineralization of Enamel and Dentin Mediated by Matrix Proteins. Journal of Dental Research, 00220345211018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Diekwisch TG, 2019. Enamel biomimetics—fiction or future of dentistry. International journal of oral science 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Rosene L, Farquharson C, Millán JL, Diekwisch TG, 2017a. Intravesicular phosphatase PHOSPHO1 function in enamel mineralization and prism formation. Frontiers in physiology 8, 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Lin T, Li L, Allen MJ, Jin T, Luan X, Diekwisch TG, 2017b. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Frontiers in physiology 8, 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Liu H, Dangaria SJ, Zhu W, Li LL, Pan S, Abufarwa M, Davis RG, Guggenheim S, Keiderling T, 2017c. Integrative temporo-spatial, mineralogic, spectroscopic, and proteomic analysis of postnatal enamel development in teeth with limited growth. Frontiers in physiology 8, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Brookes SJ, Shore RC, Kirkham J, 1998. The developing enamel matrix: nature and function. European journal of oral sciences 106, 282–291. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shimokawa H, 1979. Enamel Proteins: Biosynthesis and Chemistry. Journal of Dental Research 58, 765–772. [DOI] [PubMed] [Google Scholar]
- Satchell PG, Anderton X, Ryu OH, Luan X, Ortega AJ, Opamen R, Berman BJ, Witherspoon DE, Gutmann JL, Yamane A, 2002. Conservation and variation in enamel protein distribution during vertebrate tooth development. Journal of Experimental Zoology 294, 91–106. [DOI] [PubMed] [Google Scholar]
- Shin N-Y, Yamazaki H, Beniash E, Yang X, Margolis SS, Pugach MK, Simmer JP, Margolis HC, 2020. Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor. Journal of Biological Chemistry 295, 1943–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasevich BJ, Lea S, Bernt W, Engelhard M, Shaw WJ, 2009. Adsorption of amelogenin onto self-assembled and fluoroapatite surfaces. The Journal of Physical Chemistry B 113, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshawsky H, 1989. Organization of crystals in enamel. The Anatomical Record 224, 242–262. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ramirez BE, Liao X, Diekwisch TG, 2011. Amelogenin supramolecular assembly in nanospheres defined by a complex helix-coil-PPII helix 3D-structure. Plos one 6, e24952. [DOI] [PMC free article] [PubMed] [Google Scholar]