Abstract
Introduction
Rising rates of methamphetamine use among populations using opioids is an escalating public health concern. The purpose of this manuscript is to identify socioecologic factors driving increases in methamphetamine use among Appalachian Kentucky adults with a history of opioid use.
Methods
Semi-structured qualitative interviews were conducted among 20 Appalachian Kentuckians in the Social Network of Appalachian Peoples (SNAP) cohort who reported lifetime opioid use and past 30-day methamphetamine use. Interviews focused on initiation of methamphetamine use, factors that influence methamphetamine use at the individual, interpersonal, community and society levels.
Results
Participants reported using methamphetamine to self-treat underlying issues, including withdrawal from opioids, chronic pain, and emotional distress. Initiation of use was most often facilitated through their drug networks. Participants reported that methamphetamine was widely available and affordable in their community. Several participants with extensive histories of non-medical prescription opioid (NMPO) use described transitioning to methamphetamine as their drug of choice as opioids became less available in their community. Participants also reported economic distress and lack of recreational opportunities as drivers of increased methamphetamine use.
Discussion
Recent increases in methamphetamine use among those with a history of opioid use is facilitated by methamphetamine’s relative availability and affordability. Methamphetamine use was also highly influenced by societal factors such as economic deprivation and policies that decreased availability of NMPOs. Surging methamphetamine use exacerbates inequities in addiction care brought to light by the opioid epidemic. Interventions aimed at addressing the socioecological drivers of methamphetamine use among people who use opioids are warranted.
Keywords: methamphetamine, rural, Appalachia, opioid
1. Introduction
Methamphetamine use among people who use opioids is an escalating public health concern in the United States and has been coined as the “twin epidemics” (Cicero et al., 2020; Daniulaityte et al., 2020; Ellis et al., 2018; Strickland et al., 2019). Between July 2017 and June 2018, opioid overdose deaths co-occurring with methamphetamine use increased by almost 15% (Gladden et al., 2019). In addition, past-month methamphetamine use among patients with opioid use disorder (OUD) entering drug treatment programs across the U.S. increased from 18.8% in 2011 to 34.2% in 2017, with rates among rural residents as high as 45.7% in 2017 (Ellis et al., 2018).
The current methamphetamine epidemic follows methamphetamine epidemics in the 1990s and early 2000s among localized but diverse populations across the country (Anglin et al., 2000) including the rural Western U.S. among White and Native American communities (Freese et al., 2000); Hawaii (Freese et al., 2000; Goebert et al., 2008); the rural Midwest (Grant et al., 2007); Eastern metropolitan areas (Daniulaityte et al., 2007; Halkitis et al., 2005); and the South (Sexton et al., 2006). In some regions specifically, methamphetamine use emerged as an epidemic among men who have sex with men (MSM) in the 1990s and is disproportionately used among MSM populations to enhance sexual function (Halkitis et al., 2001; Maxwell et al., 2019). By 2005, Newsweek magazine named methamphetamine “America’s Most Dangerous Drug” (Jefferson et al., 2005), though it was soon supplanted by the opioid crisis.
Then and now, methamphetamine poses serious public health concerns. Nationally, over 22% of individuals who use methamphetamine inject, which puts them at risk for infection and overdose (Gladden et al., 2019; Havens et al., 2013; Jones, 2020; McCarthy et al., 2020). Among MSM, chemsex with methamphetamine is associated with increased risk of acquiring HIV and other sexually transmitted infections (STIs) (Nerlander et al., 2018; Shoptaw and Reback, 2007). Methamphetamine use is also associated with neurotoxicity that may lead to significant neurocognitive decline (Scott et al., 2007). Further, many people who use methamphetamine do not receive treatment for substance use disorders (SUD). In a recent study of admissions for treatment of SUD, it was found that although more than half of those reporting past-year use of methamphetamine met diagnostic criteria for methamphetamine use disorder (MUD), less than a third of those actually accessed treatment (Jones et al., 2020).
Interventions to address this expanding epidemic are urgently needed. Any such intervention requires integration of social and cultural factors driving methamphetamine initiation and continued use. Past qualitative research focusing on methamphetamine use reflects experiences with methamphetamine use prior to the opioid epidemic (Daniulaityte et al., 2007; Sexton et al., 2006). It is not well understood how drivers of methamphetamine use in the wake of the opioid epidemic differ from drivers of past methamphetamine epidemics, specifically in a region severely impacted by the opioid crisis like rural Appalachian Kentucky. Rural Appalachian Kentucky was an early center of the opioid epidemic (Havens et al., 2007), and it continues to suffer from stark disparities in OUD-associated morbidity and mortality (Brown et al., 2018; Meit et al., 2019). While non-medical prescription opioid (NMPO) use remains high in Appalachia (Havens et al., 2020), methamphetamine use is also increasing: in a cohort of rural Appalachian people who use NMPOs followed since 2008, almost 40% of the participants reported use of methamphetamine in the past 6 months in the most recent follow-up visit, corresponding to a 700% increase in odds of recent methamphetamine use between 2008 and 2020 (Havens et al., 2020).
This study aims to shed light on the increasing prevalence of methamphetamine use via analysis of qualitative interviews among people who use methamphetamine and opioids in Appalachian Kentucky. Specifically, a detailed examination of participants’ initiation or continuation of methamphetamine use using a socioecological framework with four levels of influence to explore individual, interpersonal, community and societal drivers of methamphetamine use was undertaken (Jalali et al., 2020; Keyes et al., 2014).
2. Methods
2.1. Data Collection
All study participants were members of the Social Networks Among Appalachian People (SNAP) cohort, which is described in detail elsewhere (Havens et al., 2013). Briefly, SNAP consists of rural residents of Appalachian Kentucky counties who are 18 years or older self-reporting past 30-day use of NMPO, methamphetamine, cocaine, or heroin for the purposes of getting high at the time of enrollment. Participants for this qualitative study were recruited both from the original cohort of 503 participants in 2008, and the newly recruited cohort of participants initiated in 2018. Eligible participants for this qualitative analysis reported lifetime use of NMPOs and past 30-day use of methamphetamine. Using data from participants’ most recent quantitative interview, full-time field office staff identified participants reporting recent methamphetamine use and provided a list to the lead author with the age and gender of participants. Participants were purposely sampled to include a wide range of ages and an equal gender distribution. A total of 20 participants were recruited and interviewed. Written informed consent was obtained from each participant, including permission to record and transcribe the interview and to publish findings using deidentified data. The study was approved by the Institutional Review Board at the University of Kentucky and participants were compensated with $50 for their time.
To provide consistency, all interviews were conducted by one individual (ERH). All interviews were conducted in-person in a private room at the storefront field office located in the center of the largest town in the county of study. The interviewer used a semi-structured interview guide to elicit candid answers to open-ended questions. Example questions focused on the experiences study participants had with methamphetamine use, including: 1) Why did you start using methamphetamine, 2) How does your use of methamphetamine affect your use of other drugs, and 3) How has methamphetamine changed recently in this community. At 20 individuals, the study reached thematic saturation.
2.2. Data Analysis
Interviews were recorded and transcribed verbatim using a transcription service. Identifying information was removed from interviews and stored on password-protected computers. Two researchers (ERH, MM) used MAXQDA and Microsoft Excel to analyze transcribed interviews. The researchers applied an a priori codebook and developed emergent codes through an iterative process. Team members reviewed and revised coding constantly and sought inter-reader reliability for all coding.
Initially, readers identified segments of text where participants discussed their reasons for methamphetamine use and their observations about methamphetamine use in their community. Next, these segments of text were sub-coded with specific, emergent codes describing themes identified through interviews. We grouped these specific codes in our socioecological model of analysis with four levels of influence to explore individual, interpersonal, community and societal influences (Jalali et al., 2020; Keyes et al., 2014).
3. Results
Of the 20 interviews completed, the median time for completion was 44 minutes (interquartile range [IQR]: 31–61 minutes). The median age of participants was 31 years, with a range of 19 to 52 years of age. Nine of the twenty participants were female. Nineteen identified as White while one identified as Black (Table 1). All participants endorsed a lifetime history of NMPO use and past 30-day methamphetamine use. Three-quarters of participants reported injecting drugs, including methamphetamine. Participants are individually described briefly in Table 2.
Table 1.
Characteristics of Interview Participants
| Characteristic | N=20 |
|---|---|
| Gender | |
| Female | 9 (45%) |
| Male | 11 (55%) |
| Age (years) | |
| <20 | 1 (5%) |
| 20–29 | 9 (45%) |
| 30–39 | 4 (20%) |
| 40–49 | 4 (20%) |
| 50+ | 2 (10%) |
| Race | |
| White | 19 (95%) |
| Black | 1 (5%) |
Table 2.
Demographics & Drug Use of Individual Participants
| Participant | Age | Gender | Race | Polysubstance Use | Current Primary Drug of Choice | Housing Status |
|---|---|---|---|---|---|---|
| P1 | 19 | M | White | Occasional heroin & pain pill use | Methamphetamine | Couch surfing with friends & family |
| P2 | 20 | M | White | Infrequent opioid use | Methamphetamine | Sleeps outside |
| P3 | 21 | M | White | Past heroin use Infrequent pain pill use | Methamphetamine | Lives with family |
| P4 | 21 | M | White | Past heroin use Infrequent pain pill use Regular hallucinogen use | Methamphetamine | Lives in own apartment |
| P5 | 22 | F | African American | Concurrent cocaine use Infrequent pain pill use | Methamphetamine | Lives with family |
| P6 | 25 | M | White | Concurrent pain pill use | Methamphetamine | Couch surfing with friends & family |
| P7 | 25 | M | White | Past pain pill use Concurrent synthetic marijuana use | Methamphetamine & synthetic marijuana | Lives with family |
| P8 | 25 | M | White | Concurrent use of benzodiazepines, synthetic marijuana, and pain pills | Benzodizepines & synthetic marijuana | Lives with family |
| P9 | 25 | F | White | Past pain pill use | Methamphetamine | Couch surfing with friends & family |
| P10 | 28 | M | White | Past pain pill use | Methampetamine | Sleeps outside |
| P11 | 34 | F | White | Past pain pill use | Methamphetamine | Lives in own home |
| P12 | 35 | F | White | Concurrent pain pill use | Methamphetamine | Sleeps outside |
| P13 | 38 | M | White | Past pain pill use | Methamphetamine | Lives with family |
| P14 | 39 | M | White | Past pain pill use | Methamphetamine | Lives with family |
| P15 | 40 | F | White | Past pain pill and benzodizepine use | Methamphetamine | Lives with family |
| P16 | 45 | M | White | Past pain pill & cocaine use | Methamphetamine | Lives with family |
| P17 | 46 | F | White | Concurrent pain pill use | Methamphetamine | Lives with family |
| P18 | 48 | F | White | Concurrent pain pill use | Methamphetamine | Lives with family |
| P19 | 52 | F | White | Concurrent pain pill & benzodiazepine use | Methamphetamine | Lives in own apartment |
| P20 | 52 | F | White | Concurrent cocaine and pain pill use | Methamphetamine | Lives with family |
Drivers of methamphetamine use follow below and are grouped by socioecological level (Figure 1) and encompass the individual, interpersonal/network, community and societal levels.
Figure 1.
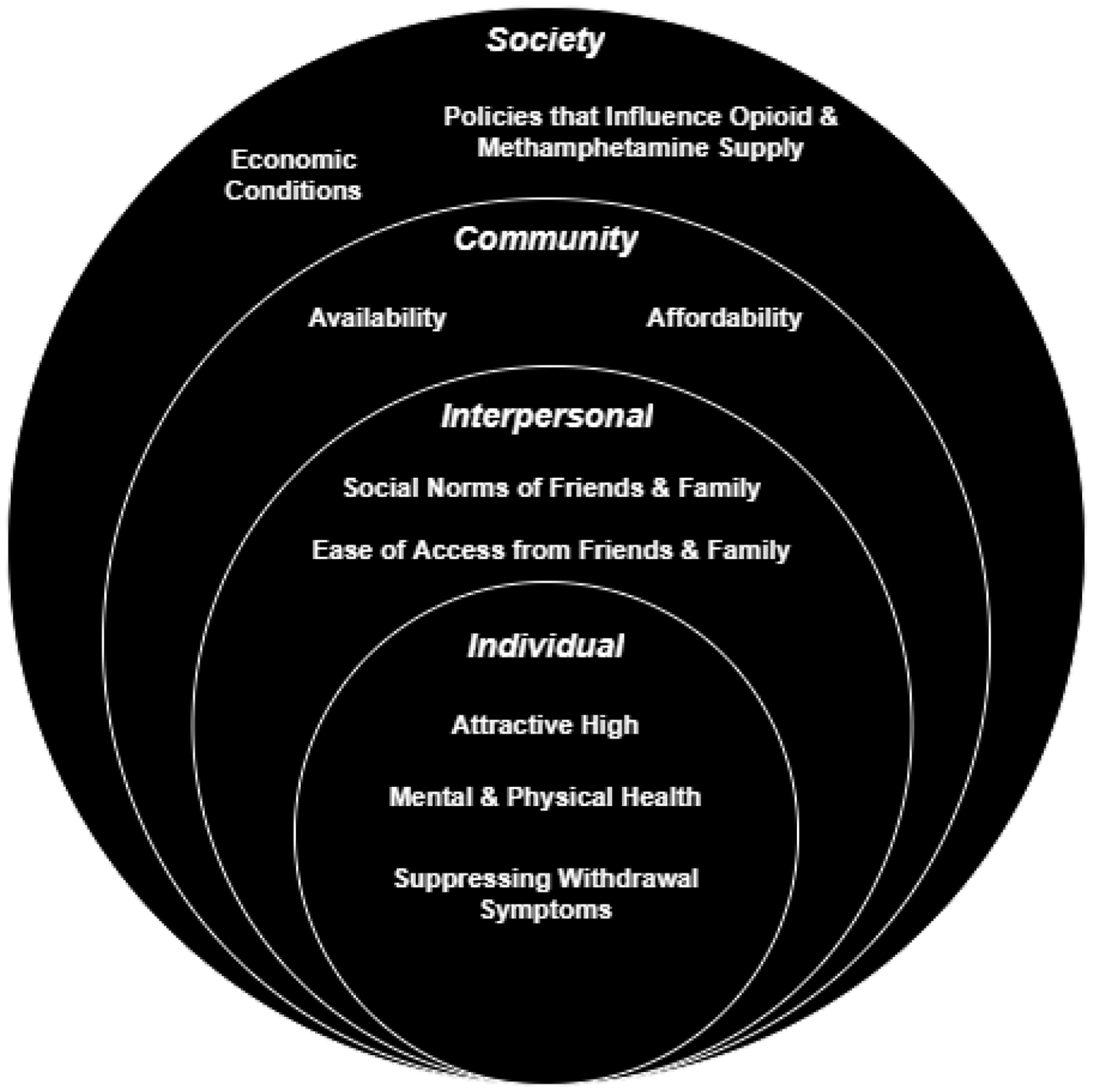
Drivers of methamphetamine use among rural opioid users using a socioecological model.
3.1. Individual Factors
Participants found the high of methamphetamine attractive for the increased energy, perception of focus, and confidence. Two male participants independently offered that methamphetamine enhanced their sexual encounters, but this theme was not heard throughout other interviews. Participants often described the focus and energy from methamphetamine desirable compared to the drowsiness caused by buprenorphine and other opioids. One nineteen-year-old man (Table 2, P1) commented:
“[Methamphetamine] gave me confidence [and] courage to do the things I wouldn’t normally do and give me the energy to do things I normally didn’t wanna do.”
Participants described using methamphetamine to treat mental health concerns including attention difficulties and mood disorders, and as a coping mechanism for emotional trauma. Trauma was often coupled with anxieties about housing security and economic strain, a lack of confidence in the ability to reach gainful employment, and a sense of boredom and perceived lack of healthy recreation options. Participants who described methamphetamine as a treatment for mental health concerns reported poor access to psychiatric care or mentioned that the medications offered by health care providers (e.g. quetiapine) did not work as effectively as methamphetamine. One 28-year-old man (P10) described:
“It was, you know, the best – to me, the best thing ever. All this energy. I didn’t have to sleep because I have nightmares. I got PTSD from my childhood, and when I sleep, I have nightmares. So, meth kept me awake, and I didn’t have them nightmares, and I was better. I thought I was better. I was numbing my pain. I still know that I’m numbing my pain, but…I didn’t think a lot about my childhood.”
A few participants also reported methamphetamine as a way in which to treat chronic pain. Specifically, it was stated that use of methamphetamine reduced pain and provided energy that allowed them to work manual labor jobs like landscaping and construction. One 39-year-old male laborer with a history of chronic pain (P14) said:
“When we’ve got a big day at work, I’ll take me a couple hits that morning, work all day, daylight to dark or whatever we have to do to get done to make ends meet.”
Participants also described how methamphetamine use helped to suppress withdrawal symptoms and cravings for opioids. Several participants prescribed buprenorphine for OUD reported being removed from treatment after testing positive for methamphetamine, which led to opioid withdrawal and increased use of methamphetamine to “treat” the withdrawal. One 52-year-old woman (P19) shared:
“Meth will stop withdrawal; did you know that? For anything. It stops it for any kind of drugs – Xanax, Neurontin, [pain pills], Suboxone. So, a lot of people have used meth to get off Suboxone.”
Despite using methamphetamine to self-treat a variety of afflictions, many participants shared concerns that their methamphetamine was contaminated with products including synthetic opioids and wasp spray. Many participants shared stories of friends or family members who had overdoses or psychotic episodes they attributed to poor-quality methamphetamine, which increased the perception of danger around methamphetamine. One 38-year old man (P13) shared:
“Sometimes [the meth] is fake bug spray, and sometimes it’s real. You never know what you’re getting….”
3.2. Interpersonal Factors
Participants’ initiation of methamphetamine use was often facilitated by members of their drug networks. Many participants indicated that they first tried meth passively or accidentally with people within their drug networks. Several younger participants described trying methamphetamine without knowing what it was. One 19-year-old man (P1)described:
“I was 15 [when] I first tried meth… I seen one of my friends that I hadn’t seen in a while and she tells me that she had “ice cream”. I didn’t know what it was. And my person that I got weed off of was out, so I gave her $20 and she told me to Google what “ice cream” was. I was looking for weed, [but] as soon as I first took my first hit of meth I was like, “Oh my god” because I fell in love.”
Participants described being surrounded by people using methamphetamine. One 45-year-old man (P16) stated:
“I live close to people that are dealing in drugs all the time…. That’s the biggest part of why I started using the most. It was just around me. All the time around me. Just daily. And then there was times, I broke down, and that I went crying … Just like, ‘I don’t know what to do. I can’t get away from it.’”
3.3. Community Factors
Participants uniformly described how methamphetamine is now the easiest illicit substance to acquire, allowing for the rapid increase in new onset in the region. One 21-year-old man (P4) described:
“[Methamphetamine] is the most easiest thing to get in Hazard right now. Like you could walk not even 100 feet and get some.”
Another 25-year old man (P8) stated how methamphetamine accessibility defined his community:
“Meth’s already took over. Meth is every corner, every street, every straight stretch you see, it’s every where you look, meth. You know, it ain’t hard to find out where it’s at either, you just gotta sit and watch people doing it.”
One 52-year-old woman (P19) shared:
“You don’t even hear about selling Lorcets or Percocets anymore… Only thing that people want now is meth, a nerve pill, Neurontin, or Suboxone. Opioids are gone except for people that actually take them for real pain.”
Participants consistently described how the ubiquity made methamphetamine affordable, especially compared to NMPOs, marijuana, and benzodiazepines. One 52-year-old woman (P20) indicated:
“It’s getting harder [to find prescription opioids] because they [community physicians] are cutting everybody off and they’re going up. [Drug dealers] are starting to charge more…. If there wasn’t no pain pills around, I might do a little bit more [methamphetamine] because I wouldn’t have my pain pills. But right now, the pain pills keep me from doing it.”
3.4. Societal Factors
Participants observed that substance use patterns changed through time. Many participants described this transition occurring very quickly. One participant shared that methamphetamine “hit like a horse race—picked up, took off.” One 52-year old woman (P19) shared:
“But everybody I know does meth. Everybody I know that does drugs does meth. I never heard of meth until last year, about a year and a half ago. Never ever seen it. Ever even heard it spoken.”
Participants described how policies designed to combat the opioid epidemic such as decreased prescribing of opioids by healthcare providers impacted availability of NMPOs. Older participants in particular often described methamphetamine initiation only after their sources of NMPOs “dried up.” When one 48-year old woman (P18) shared:
“When we couldn’t find pain pills, we’d run into people who have meth, we’ve done meth. The doctors has cracked down writing [prescriptions for pain pills]….. [Initiation of methamphetamine occurred] Probably around last year, I guess…. Just, like getting pain pills – I know they, the doctors won’t hardly write them around here anymore. They didn’t write me none. I do have a bad back, but they didn’t write me any. They wanted me to do therapy…. I have [gone to physical therapy] before, it don’t help. I mean, not having the pills there, I think if there had been more pain pills, I don’t think I would have started doing meth, I don’t think.”
Some participants also linked rising rates of methamphetamine use to poor economic conditions. A shift from higher paying jobs, such as those in the mining industry, to minimum wage employment was frequently noted, especially among older participants. One former coal industry worker (P14) shared:
“I think mainly people are just trying to figure out a way to forget about their problems. Once they shut coal down, everybody around here… it went from being a happy-go-lucky town to a ghost town… pretty much as soon as the coal shut down, [the miners] went from making good money to making about minimum wage. I don’t know how many of them I knew lost their vehicles, file for bankruptcy, … I’d say about 50–60 percent of the ones I knew at work in the mines have done meth or are still on meth because I guess they just didn’t see no other way out.”
Another 38-year old man (P13) shared feeling fatalistic about the prevalence of methamphetamine while expressing concern for poor methamphetamine quality:
“I don’t know what’d help Hazard. I believe it’s too far gone. My honest opinion. I believe it is too far gone. Because he ain’t gonna get all of it out of here….The police officer telling us one time, like if the arrest two dudes, there’d be two more step up right behind them and start selling drugs again. Yeah, it’s this endless process…. And that is the truth, I mean, it’s everywhere you go. There be nine houses, there’d be seven of them selling meth.”
4. Discussion
Our findings suggest the observed surge in methamphetamine use is driven by a variety of factors at the individual, interpersonal, community and societal levels. At the individual level, many of our participants reported that methamphetamine offered an attractive high, consistent with past studies (Baker et al., 2020; Boeri et al., 2009; Daniulaityte et al., 2007; Daniulaityte et al., 2020; Herbeck et al., 2014; Hoffmann et al., 2017; Lopez et al., 2021). Though two male participants shared that methamphetamine enhanced heterosexual sexual encounters, no participants described methamphetamine primarily for enhancing sexual function. There was some indication that motivations of use were not exclusive to get high however; pre-opioid epidemic studies documented methamphetamine was used as a “work drug” to improve function during manual labor and housekeeping responsibilities in both metropolitan and rural areas (Boeri et al., 2009; Daniulaityte et al., 2007; Sexton et al., 2006). Our study aligned with these findings and suggested that the perceived benefits of methamphetamine use may have facilitated the substitution of opioids with methamphetamine for some participants.
Interpersonal, or network factors, were especially important with regard to initiation of methamphetamine use in this cohort of rural people who use drugs. Many participants reported unintentionally using the drug for the first time while engaged with other drug network members. This finding is consistent with other research within rural and other methamphetamine using populations (Boeri et al., 2009; Herbeck et al., 2014; Sexton et al., 2006; Sun, 2007), and suggests that interventions aimed at preventing or reducing methamphetamine use should not ignore the impact of individuals’ networks on initiation and continued use.
Pre-opioid epidemic studies found that increased availability facilitated methamphetamine surges in localized communities (Daniulaityte et al., 2007; Sexton et al., 2006). In our population, this relative accessibility is facilitating a population-level transition and even some individual-level substitution behavior from NMPOs to methamphetamine. A 2007 study from one metropolitan area of Ohio found that study participants who used methamphetamine in the 1970s or 1980s turned to other substances including crack cocaine, heroin, and NMPOS, then returned to methamphetamine in the mid-late 1990s or early 2000s when they “rediscovered” methamphetamine through increased availability in their community (Daniulaityte et al., 2007). In our study, some older participants reported similar substitution behavior with methamphetamine based on the reduced availability opioids, though more participants in our study describe co-usage of methamphetamine and opioids versus complete substitution, consistent with other recent studies (Lopez et al., 2021; Palmer et al., 2020). Additionally, the participants in the Ohio study had drug use histories most commonly involving prescription stimulants and cocaine that facilitated initial methamphetamine use (Daniulaityte et al., 2007); in our study, prescription stimulant and cocaine use was relatively rare compared with the ubiquity of opioid use. In this way, the emergence of widespread methamphetamine use in the wake of the opioid crisis is a unique phenomenon.
Policies implemented to address the opioid epidemic may be playing a role pushing methamphetamine to the forefront in many rural communities. Several participants suggested that their motivation for methamphetamine initiation was that their preferred opioids were not available at the time. Methamphetamine was inexpensive and readily available. These policies, including introduction of abuse deterrent opioid formulations and enhanced diversion control (Alpert et al., 2018), played a large role in the transition from NMPO use to use of heroin and synthetic opioids by decreasing availability of NMPOs and creating an opportunity for other drugs to enter illicit drug markets (Cicero et al., 2012). Policies that aim to decrease supply of ingredients to make methamphetamine or methamphetamine-like substances may be less effective given that ingredients may be substituted, as evidenced by use of chemically altered wasp insecticide(“wasp dope”) by people seeking methamphetamine (Dobkin et al., 2014; Young et al., 2020).
At the societal level, underlying health and economic inequities contributed to methamphetamine use among our participants. The people of Central Appalachia experience physical and mental health inequities and unequitable access to high quality healthcare (Case and Deaton, 2015; Meit et al., 2019; Meit et al., 2017). Study participants reported using methamphetamine for the self-treatment of physical and mental health conditions, a perceived need exacerbated by underlying disparities in mental health conditions and chronic pain. This is consistent with one recent study in rural Oregon that found individuals who concurrent use methamphetamine and opioids often used methamphetamine to mitigate harm from heroin use(Lopez et al., 2021). In addition, consistent with previous studies in rural populations, most of the participants described poverty and housing insecurity (Das-Douglas et al., 2008; Jonas et al., 2012). In regions severely impacted by the opioid epidemic, past research demonstrated widespread poverty and systemically high rates of opioid use contribute to feelings of fatalism which further facilitated opioid use and minimized perceptions of harm while decreasing help-seeking behaviors (Boardman et al., 2001; McLean, 2016; Monnat, 2018; Thompson et al., 2020). We found that similar themes of economic distress and a sense of hopelessness in the region are facilitating methamphetamine use. This further demonstrates the need to have interventions that address both the individual as well as the structural inequities driving use of methamphetamine in rural areas.
This surge in methamphetamine use among people who use opioids both locally (Havens et al., 2020) and nationally (Ellis et al., 2018; Strickland et al., 2019) demonstrates a pressing need to address MUD in rural communities. Addiction treatments that exclusively focus on treating OUD may leave room for methamphetamine use a substitute for a high (Palmer et al., 2020). However, current interventions for MUD are limited. Unlike medications for opioid use disorder (MOUD), there are no FDA-approved pharmacotherapies for MUD and there is a paucity of research on efficacious treatments for MUD among people who use opioids (Chan et al., 2020). One recent clinical trial in the New England Journal of Medicine studied the use of bupropion and naltrexone for MUD (Trivedi et al., 2021). Although the authors found that this particular drug combination was efficacious compared to placebo, less than one in five patients with MUD had a positive response. Further, this trial excluded some participants who used opioids at the time of randomization, limiting the generalizability of the study findings (Trivedi et al., 2021). Recent research exploring use of stimulants such as lisadezamfetamine to treat MUD have yet to show conclusive results (Ezard et al., 2018). Additionally, untreated MUD complicates pharmacotherapy for OUD, as methamphetamine use is associated with decreased retention in treatment for OUD (Krawczyk et al., 2021; Tsui et al., 2020).Sustained-release dexamfetamine, a stimulant, is sometimes used for the treatment of cocaine use among individuals in treatment for heroin use (Nuijten et al., 2016), and studies examining use of agonist-based therapies are ongoing (Ezard et al., 2018) However, these therapies are currently not FDA-approved for treatment of MUD in the U.S. Additionally, any future pharmacotherapy for concurrent OUD and MUD may be limited by pre-existing barriers to addiction care. Patients seeking pharmacotherapy for OUD in rural areas often face barriers to treatment including healthcare providers perceptions of MOUD as unsatisfactory for rural patients and stigma from providers (Lister et al., 2020). Similar barriers and attitudes may further complicate future interventions for MUD.
Limitations of this study include a potential lack of generalizability and recall bias. Participants were recruited from one small community in Appalachia; therefore, findings may not be generalizable to other regions affected by the opioid epidemic. However, national studies that show high rates of methamphetamine use among individuals seeking treatment for OUD suggests our findings may not be unique to Appalachian Kentucky (Cicero et al., 2020; Jones et al., 2020). In addition, the interview guide was designed to elicit responses that were reliant on the participant’s memory around methamphetamine initiation and use and is therefore subject to recall bias. To mitigate this potential bias, only those with methamphetamine use in the past 30-days were approached for participation.
5. Conclusion
In conclusion, although motivations for use varied across participants, they were not confined to the individual and were also influenced at the network, community and societal levels. The socioecological model employed in this qualitative analysis demonstrates that a broad approach is necessary to understanding the increased use of methamphetamine that has emerged from the opioid epidemic. As such, interventions and policies designed to address the opioid epidemic cannot ignore the growing issue of methamphetamine use. Future research is necessary around how to address MUD in the absence of evidence-based treatments specific to MUD and in the context of treatment for OUD in rural areas.
Highlights:
Recent increases in methamphetamine use among rural people who use opioids are noted.
Qualitative interviews reveal socioecological drivers of methamphetamine use.
Ease of access to methamphetamine facilitates transitions from opioids.
Methamphetamine use exacerbates disparities highlighted by the opioid epidemic.
Interventions should address socioecological drivers of methamphetamine use.
Acknowledgment:
The authors wish to thank Egan Bailey, project director in Hazard, KY, for assistance with recruitment of study participants.
Role of Funding Source:
This work was supported by R01DA033862 and R01DA024598 and the University of Kentucky’s Center for Clinical and Translational Science’s Professional Student Mentored Research Fellowship program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflict declared.
References
- Alpert A, Powell D, Pacula RL, 2018. Supply-side drug policy in the presence of substitutes: Evidence from the introduction of abuse-deterrent opioids. American Economic Journal: Economic Policy 10(4), 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S, 2000. History of the methamphetamine problem. Journal of psychoactive drugs 32(2), 137–141. [DOI] [PubMed] [Google Scholar]
- Baker R, Leichtling G, Hildebran C, Pinela C, Waddell EN, Sidlow C, Leahy JM, Korthuis PT, 2020. “ Like Yin and Yang”: Perceptions of Methamphetamine Benefits and Consequences Among People Who Use Opioids in Rural Communities. Journal of Addiction Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Finch BK, Ellison CG, Williams DR, Jackson JS, 2001. Neighborhood disadvantage, stress, and drug use among adults. Journal of health and social behavior, 151–165. [PubMed] [Google Scholar]
- Boeri MW, Harbry L, Gibson D, 2009. A qualitative exploration of trajectories among suburban users of methamphetamine. Journal of ethnographic and qualitative research 3(3), 139. [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Goodin AJ, Talbert JC, 2018. Rural and Appalachian disparities in neonatal abstinence syndrome incidence and access to opioid abuse treatment. The Journal of rural health 34(1), 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Deaton A, 2015. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences 112(49), 15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Freeman M, Ayers C, Korthuis PT, Paynter R, Kondo K, Kansagara D, 2020. A systematic review and meta-analysis of medications for stimulant use disorders in patients with co-occurring opioid use disorders. Drug Alcohol Depend 216, 108193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA, 2020. Polysubstance use: A broader understanding of substance use during the opioid crisis. American journal of public health 110(2), 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, 2012. Effect of abuse-deterrent formulation of OxyContin. New England Journal of Medicine 367(2), 187–189. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R, Carlson RG, Kenne DR, 2007. Methamphetamine use in Dayton, Ohio: preliminary findings from the Ohio Substance Abuse Monitoring Network. J Psychoactive Drugs 39(3), 211–221. [DOI] [PubMed] [Google Scholar]
- Daniulaityte R, Silverstein SM, Crawford TN, Martins SS, Zule W, Zaragoza AJ, Carlson RG, 2020. Methamphetamine use and its correlates among individuals with opioid use disorder in a midwestern US city. Substance Use & Misuse, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das-Douglas M, Colfax G, Moss AR, Bangsberg DR, Hahn JA, 2008. Tripling of methamphetamine/amphetamine use among homeless and marginally housed persons, 1996–2003. Journal of Urban Health 85(2), 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C, Nicosia N, Weinberg M, 2014. Are supply-side drug control efforts effective? Evaluating OTC regulations targeting methamphetamine precursors. Journal of Public Economics 120, 48–61. [Google Scholar]
- Ellis MS, Kasper ZA, Cicero TJ, 2018. Twin epidemics: the surging rise of methamphetamine use in chronic opioid users. Drug and alcohol dependence 193, 14–20. [DOI] [PubMed] [Google Scholar]
- Ezard N, Dunlop A, Hall M, Ali R, McKetin R, Bruno R, Phung N, Carr A, White J, Clifford B, 2018. LiMA: a study protocol for a randomised, double-blind, placebo controlled trial of lisdexamfetamine for the treatment of methamphetamine dependence. BMJ open 8(7), e020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese TE, Obert J, Dickow A, Cohen J, Lord RH, 2000. Methamphetamine abuse: issues for special populations. Journal of psychoactive drugs 32(2), 177–182. [DOI] [PubMed] [Google Scholar]
- Gladden RM, O’Donnell J, Mattson CL, Seth P, 2019. Changes in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamine—25 states, July–December 2017 to January–June 2018. Morbidity and Mortality Weekly Report 68(34), 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebert D, Haning III WF, Nishimura S, Toles M, Rohr AM, 2008. Methamphetamine Use in Hawaii. Addictive Disorders & Their Treatment 7(1), 31–40. [Google Scholar]
- Grant KM, Kelley SS, Agrawal S, Meza JL, Meyer JR, Romberger DJ, 2007. Methamphetamine use in rural Midwesterners. The American Journal on Addictions 16(2), 79–84. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Green KA, Mourgues P, 2005. Longitudinal investigation of methamphetamine use among gay and bisexual men in New York City: findings from Project BUMPS. Journal of Urban Health 82(1), i18–i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Parsons JT, Stirratt MJ, 2001. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission. Journal of homosexuality 41(2), 17–35. [DOI] [PubMed] [Google Scholar]
- Havens JR, Knudsen HK, Young AM, Lofwall MR, Walsh SL, 2020. Longitudinal trends in nonmedical prescription opioid use in a cohort of rural Appalachian people who use drugs. Preventive Medicine, 106194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Lofwall MR, Frost SD, Oser CB, Leukefeld CG, Crosby RA, 2013. Individual and network factors associated with prevalent hepatitis C infection among rural Appalachian injection drug users. American journal of public health 103(1), e44–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Oser CB, Leukefeld CG, 2007. Increasing prevalence of prescription opiate misuse over time among rural probationers. Journal of opioid management 3(2), 107–111. [DOI] [PubMed] [Google Scholar]
- Herbeck DM, Brecht M-L, Christou D, Lovinger K, 2014. A qualitative study of methamphetamine users’ perspectives on barriers and facilitators of drug abstinence. Journal of psychoactive drugs 46(3), 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann L, Schumann N, Richter M, 2017. Methamphetamine use in central germany: a qualitative study on consumer groups and motives from the experts’ perspective. Psychotherapie, Psychosomatik, medizinische Psychologie 68(8), 329–336. [DOI] [PubMed] [Google Scholar]
- Jalali MS, Botticelli M, Hwang RC, Koh HK, McHugh RK, 2020. The opioid crisis: a contextual, social-ecological framework. Health research policy and systems 18(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson DJ, Shenfeld H, Murr A, Campo-Flores A, Childress S, Skipp C, 2005. America’s most dangerous drug. Newsweek 146(6), 40–48. [PubMed] [Google Scholar]
- Jonas AB, Young AM, Oser CB, Leukefeld CG, Havens JR, 2012. OxyContin® as currency: OxyContin® use and increased social capital among rural Appalachian drug users. Social science & medicine 74(10), 1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, 2020. Patterns and Characteristics of Methamphetamine Use Among Adults—United States, 2015–2018. MMWR. Morbidity and Mortality Weekly Report 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Underwood N, Compton WM, 2020. Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction 115(2), 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Cerdá M, Brady JE, Havens JR, Galea S, 2014. Understanding the rural–urban differences in nonmedical prescription opioid use and abuse in the United States. American journal of public health 104(2), e52–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N, Williams AR, Saloner B, Cerdá M, 2021. Who stays in medication treatment for opioid use disorder? A national study of outpatient specialty treatment settings. Journal of Substance Abuse Treatment 126, 108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Weaver A, Ellis JD, Himle JA, Ledgerwood DM, 2020. A systematic review of rural-specific barriers to medication treatment for opioid use disorder in the United States. The American Journal of Drug and Alcohol Abuse 46(3), 273–288. [DOI] [PubMed] [Google Scholar]
- Lopez AM, Dhatt Z, Howe M, Al-Nassir M, Billing A, Artigiani E, Wish ED, 2021. Co-use of methamphetamine and opioids among people in treatment in Oregon: A qualitative examination of interrelated structural, community, and individual-level factors. International Journal of Drug Policy 91, 103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S, Shahmanesh M, Gafos M, 2019. Chemsex behaviours among men who have sex with men: A systematic review of the literature. Int J Drug Policy 63, 74–89. [DOI] [PubMed] [Google Scholar]
- McCarthy NL, Baggs J, See I, Reddy SC, Jernigan JA, Gokhale RH, Fiore AE, 2020. Bacterial infections associated with substance use disorders, large cohort of United States hospitals, 2012–2017. Clinical Infectious Diseases 71(7), e37–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K, 2016. “There’s nothing here”: Deindustrialization as risk environment for overdose. International Journal of Drug Policy 29, 19–26. [DOI] [PubMed] [Google Scholar]
- Meit M, Heffernan M, Tanenbaum E, 2019. Investigating the impact of the diseases of despair in Appalachia. Journal of Appalachian Health 1(2), 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meit M, Heffernan M, Tanenbaum E, Hoffmann T, 2017. Appalachian diseases of despair. Appalachian Regional Commission; Washington DC. [Google Scholar]
- Monnat SM, 2018. Factors associated with county-level differences in US drug-related mortality rates. American journal of preventive medicine 54(5), 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlander LM, Hoots BE, Bradley H, Broz D, Thorson A, Paz-Bailey G, Group N., 2018. HIV infection among MSM who inject methamphetamine in 8 US cities. Drug and alcohol dependence 190, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM, 2016. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. The Lancet 387(10034), 2226–2234. [DOI] [PubMed] [Google Scholar]
- Palmer A, Scott N, Dietze P, Higgs P, 2020. Motivations for crystal methamphetamine-opioid co-injection/co-use amongst community-recruited people who inject drugs: a qualitative study. Harm reduction journal 17(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I, 2007. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology review 17(3), 275–297. [DOI] [PubMed] [Google Scholar]
- Sexton RL, Carlson RG, Leukefeld CG, Booth BM, 2006. Methamphetamine use and adverse consequences in the rural southern United States: An ethnographic overview. Journal of psychoactive drugs 38(sup3), 393–404. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, 2007. Methamphetamine use and infectious disease‐related behaviors in men who have sex with men: implications for interventions. Addiction 102, 130–135. [DOI] [PubMed] [Google Scholar]
- Strickland JC, Havens JR, Stoops WW, 2019. A nationally representative analysis of “twin epidemics”: Rising rates of methamphetamine use among persons who use opioids. Drug and alcohol dependence 204, 107592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A-P, 2007. Relapse among substance-abusing women: components and processes. Substance use & misuse 42(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Creasy SL, Mair CF, Burke JG, 2020. Drivers of opioid use in Appalachian Pennsylvania: cross-cutting social and community-level factors. International Journal of Drug Policy 78, 102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Walker R, Ling W, dela Cruz A, Sharma G, Carmody T, Ghitza UE, Wahle A, Kim M, Shores-Wilson K, 2021. Bupropion and Naltrexone in Methamphetamine Use Disorder. New England Journal of Medicine 384(2), 140–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Mayfield J, Speaker EC, Yakup S, Ries R, Funai H, Leroux BG, Merrill JO, 2020. Association between methamphetamine use and retention among patients with opioid use disorders treated with buprenorphine. J Subst Abuse Treat 109, 80–85. [DOI] [PubMed] [Google Scholar]
- Young AM, Livingston M, Vickers‐Smith R, Cooper HL, 2020. Emergence of wasp dope in rural Appalachian Kentucky. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]


