Abstract
Neurons in the organum vasculosum of the lamina terminalis (OVLT) sense extracellular NaCl and angiotensin II concentrations to regulate body fluid homeostasis and arterial blood pressure (ABP). Lesion of the anteroventral third ventricular region or OVLT attenuates multiple forms of neurogenic hypertension. However, the extent by which OVLT neurons directly regulate sympathetic nerve activity (SNA) to produce hypertension is not known. Therefore, the present study tested this hypothesis by using a multi-faceted approach including optogenetics, single-unit and multi-fiber nerve recordings, and chemogenetics. First, optogenetic activation of OVLT neurons in conscious Sprague-Dawley rats (250–400g) produced frequency-dependent increases in ABP and heart rate. These responses were not altered by the vasopressin receptor antagonist [β-mercapto-β,β-cyclopentamethylenepropionyl1,O-me-Tyr2,Arg8]-vasopressin but eliminated by the ganglionic blocker chlorisondamine. Second, optogenetic activation of OVLT neurons significantly elevated renal, splanchnic, and lumbar SNA. Third, single-unit recordings revealed optogenetic activation of the OVLT significantly increased the discharge of bulbospinal, sympathetic neurons in the rostral ventrolateral medulla. Lastly, chronic chemogenetic activation of OVLT neurons for 7 days significantly increased 24-h fluid intake and mean ABP. When the 24-h fluid intake was clamped at baseline intakes, chemogenetic activation of OVLT neurons still produced a similar increase in ABP. Neurogenic pressor activity assessed by the ganglionic blocker chlorisondamine was greater at 7 days of OVLT activation versus baseline. Collectively, these findings indicate that acute or chronic activation of OVLT neurons produces a sympathetically-mediated hypertension.
Keywords: blood pressure, optogenetic, chemogenetic, sympathetic nerve activity, hypothalamus
Graphical Abstract
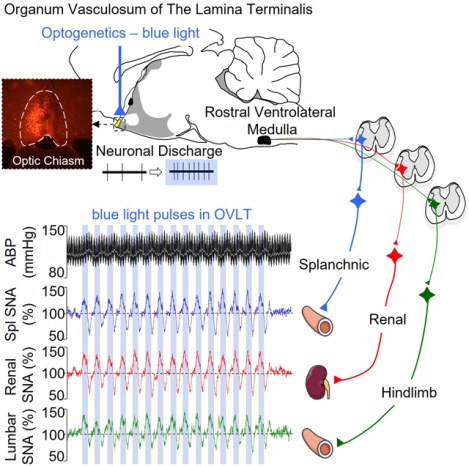
INTRODUCTION
Neurogenic mechanisms contribute to the etiology of hypertension primarily through increased sympathetic nerve activity (SNA) and total peripheral resistance1–3. Multiple sites within the central nervous system contribute to overactive SNA in hypertension including but not limited to the rostral ventrolateral medulla, hypothalamic paraventricular nucleus, and the forebrain lamina terminalis2, 3. The forebrain lamina terminalis is located along the rostral wall of the 3rd ventricle and consists of three interconnected structures including the subfornical organ, median preoptic nucleus, and organum vasculosum of the lamina terminalis (OVLT)4–6. The subfornical organ and OVLT are circumventricular organs and also lack a complete blood brain barrier thereby permitting these neurons to sense factors in the circulation and cerebrospinal fluid to regulate thirst, neuroendocrine function, and arterial blood pressure (ABP). A myriad of studies have identified multiple signaling mechanisms within SFO neurons that regulate SNA and contribute to various experimental models of hypertension7, 8. However, there is limited data regarding how OVLT neurons regulate ABP.
Activation of OVLT neurons stimulates thirst9–11, excites magnocellular vasopressin neurons12, 13, and increases ABP14, 15. OVLT neurons express a variety of receptors for circulating factors5, and in vitro and in vivo electrophysiology demonstrate a majority of OVLT neurons are responsive to elevated extracellular NaCl or angiotensin II concentrations9, 14, 16, 17. Acute injection of hypertonic NaCl elevates lumbar SNA and ABP14. Importantly, lesion of the OVLT attenuates deoxycorticosterone and angiotensin II models of hypertension18–20. These same lesion studies reported that neurogenic pressor activity assessed by the depressor responses to ganglionic blockade were reduced in lesion version sham animals19–21 thereby suggesting OVLT neurons may chronically regulate ABP through the sympathetic nervous system. Therefore, the purpose of the present study was to establish whether acute and chronic activation of OVLT produces hypertension and whether such effects are mediated by sympathetic nervous system.
MATERIALS AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals.
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh School of Medicine. Male Sprague-Dawley rats (250–400g; Charles River Laboratories) were housed in a temperature-controlled room (22±1°C) with a 12-hour dark:light cycle (lights on at 7AM), given access to deionized water, and fed standard chow (Harlan Teklad Global Diet 2018). Once AAV constructs were injected into OVLT, the diet was switched to 0.1% NaCl (D17020, Research Diets) for the remainder of experiments. An expanded Material and Methods section is available as an online supplement.
Experiment 1. Hemodynamic Responses to Optogenetic Activation of OVLT in Conscious Animals.
These animals represent a subset of the animals in which thirst responses to optogenetic stimulation of OVLT have been reported previously9. At least 3 weeks after injection of rAAV9-CaMKIIa-hChR2(H134R)-mCherry (50nL; 1×1013 vg/mL, Addgene) and implantation of optical ferrules, ABP and heart rate were recorded during 473nm light pulses (5ms width, 8–10mW confirmed posthoc at the ferrule tip, CrystaLaser CL473–075-O) applied at various frequencies (0– 20Hz, 50% duty cycle 2s on – 2s off) for a duration of 10 min. A single frequency was tested per day in randomized order. Since the ingestion of water produces a pressor response22, food and water were removed at least 1 hour before experiments began. To test whether the hemodynamic effects were mediated by vasopressin or the autonomic nervous system, animals were pretreated with saline (1mL/kg, IV), the vasopressin receptor-1 blocker [β-Mercapto-β,β-cyclopentamethylenepropionyl1,O-me-Tyr2,Arg8]-Vasopressin (10ug/kg, IV, Sigma-Aldrich), or the ganglionic blocker chlorisondamine (5mg/kg, IV, Sigma-Aldrich) or at 10 min before optogenetic stimulation.
Experiment 2. Sympathetic Nerve Recordings.
A second set of rats received an OVLT injection of rAAV9-CaMKIIa-hChR2(H134R)-mCherry and optical ferrule as described above. After a 3-week recovery, rats were anesthetized with Inactin (120mg/kg, IV) and prepared for simultaneous recordings of renal, splanchnic, and lumbar SNA as described previously14, 23, 24. ABP and SNA responses to OVLT optogenetic activation was assessed using one of three protocols: 1) Light-triggered averaging (5ms pulse duration, 1Hz, 120 sweeps), 2) continuous light pulses (5ms pulse duration, 15s train, 0– 20Hz), and 3) 50% duty cycle light trains (5ms pulse, 2s on / 2s off, 60s, 0–20Hz).
Experiment 3: RVLM Single-Unit Recordings.
A subset of animals in Experiment 2 were also prepared for RVLM single-unit recordings using a transcerebellar approach as described previously24. Once a barosensitive, spinally-projecting neuron was identified, discharge responses to optogenetic stimulation were assessed using one of two protocols: 1) continuous light pulses (5ms pulse duration, 15s train, 0– 20Hz) and 2) 50% duty cycle light trains (5ms pulse, 2s on / 2s off, 60s, 0–20Hz). Then, RVLM units were juxtacellular labelled as described elsewhere14 and anatomical location confirmed.
Experiment 4: Chronic Chemogenetic Activation of OVLT Neurons.
Rats were anesthetized with 2–3% isoflurane, instrumented with a telemetry unit TRM56SP (Kaha Sciences Ltd) into the aorta via the femoral artery, and injected with rAAV9-hSYN-HA-hM3D(Gq)-IRES-mCherry (chemogenetic experiments, 50nL, 1×1013 vg/mL, Addgene) into the OVLT. Animals were returned to home cages with access to 0.1% NaCl chow (D17020, Research Diets) and 0.9% NaCl solution instead of water for 3 weeks. A 0.9% NaCl drinking solution was used as prior studies reported that acute optogenetic activation of rat OVLT neurons produces a significant increase in water intake9. After a 4-day baseline recording, clozapine-N-oxide (CNO, APExBIO, Houston, TX) was added to the 0.9% drinking bottle for 7 days followed by a 5-day wash-out. The concentration was adjusted daily at 6PM and based on the fluid intake over the previous 24-h hours and body weight to yield ~3mg/kg/day. Ganglionic blockade was performed using hexamethonium (30 mg/kg, IV) at 1 day prior and 7 and 12 days after the start of CNO administration. Blood samples (0.2mL) were collected through a chronic indwelling jugular catheter at 4 hours after injection of hexamethonium. Blood electrolytes were measured by an EasyElectrolyte Analyzer (Medica).
Statistical Analysis.
Data are presented as mean±SEM plus individual data points when possible. Data normality was tested using a Shapiro-Wilk test. Data were analyzed using one or two-way ANOVAs with repeated measures (Systat 10.2). When significant F values were obtained, a layered Bonferroni paired or independent t tests were performed to identify differences. In two instances when normality did not pass, a nonparametric test produced the same statistical outcome. Therefore, the data analyses for ANOVAs and posthoc testing are reported for simplicity. P<0.05 was statistically significant for all comparisons. Group sizes are noted in the text and figure legends.
RESULTS
Experiment 1: Hemodynamic Responses to Optogenetic Activation of OVLT
Figure 1A illustrates a schematic diagram of OVLT injection sites (n=6) and a representative image of mCherry expression throughout the rostrocaudal extent of the OVLT. mCherry expression did not extend dorsally into the median preoptic nucleus, rarely into the diagonal band of Broca, and stopped caudally within 200um at the start of the 3rd ventricle. Three additional rats did not show mCherry expression in the OVLT but rostral in the diagonal band of Broca (n=2) or no mCherry expression (n=1). These animals are subsequently referred to as OVLT-o. In vivo single-unit recordings of OVLT neurons (n=8 neurons in 4 animals, baseline discharge: 1.2±0.3 Hz) confirmed a 5ms light (473nm) pulse duration produced an action potential phase-locked to the light stimulus across a range of frequencies (Figure 1B). The 5ms light pulses evoked a single action potential in 99±1% of stimulation events (n=8). However, a 10 or 20 ms pulse duration evoked ≥2 action potentials per light pulse in 29±4% or 68±8% of the stimulation events, respectively.
Figure 1.
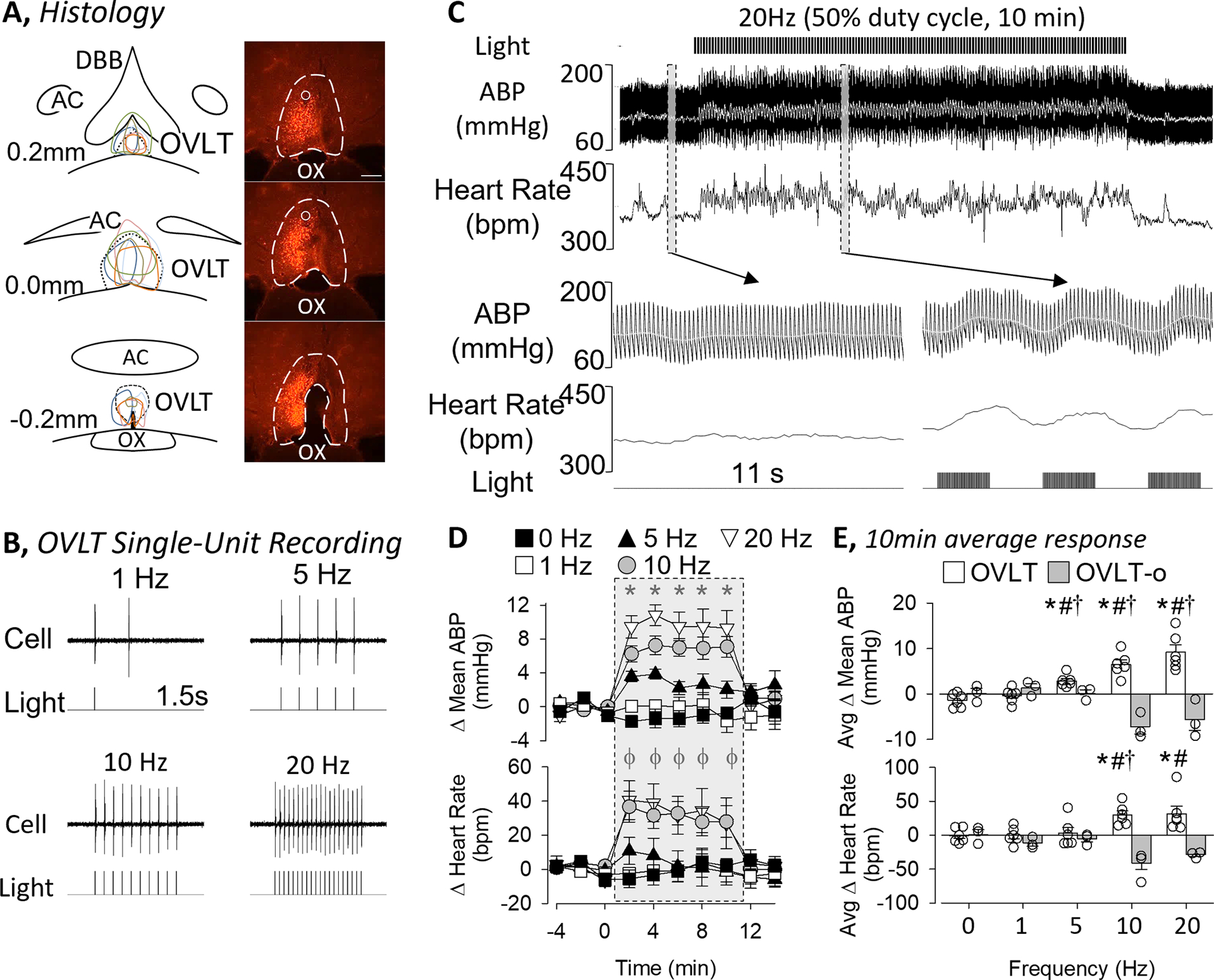
A, Schematic illustration of mCherry expression for 6 OVLT rats and digital image of representative mCherry expression and optical ferrule. Scale bar = 200μm. B, In vivo single-unit recording of an OVLT neuron demonstrates optogenetic stimulation using 473nm light pulses (5ms pulse, 1s train) evokes 1:1 light:action potential response at various frequencies. C, ABP, mean ABP (grey line), and heart rate of an unanesthetized rat before, during, and after optogenetic stimulation of OVLT (20Hz, 5 ms pulse, 50% duty cycle). Grey bars highlight a 11s example of ABP and heart rate before and during stimulation. Note, the higher ABP and heart but also the oscillations present during the laser pulses. D, Mean±SEM of mean ABP and heart rate in response to optogenetic stimulation of OVLT (0, 1, 5, 10, 20 Hz; 5ms pulse, 10 min). *P<0.05 0Hz vs 5, 10, and 20Hz; ϕ P<0.05 0Hz vs 10 and 20 Hz. E, Mean±SEM of mean ABP and heart rate averaged during the 10 min stimulation period for animals with mCherry expression within the OVLT versus outside the OVLT or no expression (OVLT-o). Data were analyzed by a 2-way ANOVA with repeated measures followed by independent or paired t-tests with a layered bonferonni correction. *P<0.05 vs 0Hz, #P<0.05 OVLT vs OVLT-o, †P<0.05 vs lower frequency
Optogenetic excitation of OVLT neurons in conscious animals produced frequency-dependent increases in ABP and heart rate (Figure 1C–E). Figure 1C illustrates ABP and heart rate promptly increased upon onset of the light pulses and remained elevated throughout the duration of the light stimulation. When the light pulses ended, both variables returned to baseline values. A closer examination of the responses revealed that ABP and heart rate oscillated in phase to the 2s train as ABP and heart rate increased immediately after initiation of a 2s train of light and declined when the light was off (see inset in Figure 1C). Optogenetic stimulation at 5, 10, and 20Hz produced a significant increase in mean ABP at each time point (Figure 1D). These hemodynamic changes were also observed in both systolic and diastolic ABP (data not shown). When the 10-min optogenetic train ended, mean ABP returned to baseline values within 2 min. In addition, optogenetic activation of OVLT neurons at 10 and 20Hz significantly elevated heart rate (Figure 1D). When the Δ mean ABP and Δ heart rate were averaged over the duration of the 10 min light stimulation, optogenetic activation of OVLT neurons significantly elevated the Δ mean ABP at ≥5Hz and Δ heart rate ≥10 Hz in OVLT. Importantly, ABP and HR did not change in OVLT-o animals. Baseline mean ABP (107±4 vs 106±1mmHg, p=0.941) and heart rate (345±11 vs 360±11 bpm, p=0.468) did not differ between OVLT and OVLT-o rats.
To assess the contribution of circulating vasopressin versus SNA to OVLT-induced pressor responses, optogenetic stimulation of the OVLT was repeated at 20Hz after pretreatment with a vasopressin receptor antagonist or the ganglionic blocker chlorisondamine (Figure 2). IV administration of the vasopressin antagonist did not significantly alter mean ABP (pre: 106±4 vs post: 102±5 mmHg, p=0.290) or heart rate (pre: 347±12 vs post: 357±13 bpm, p=0.527). Pretreatment with the ganglionic blocker significantly lowered mean ABP (64±6 mmHg, p<0.001) and heart rate (318±11 bpm, p=0.028). Optogenetic stimulation of OVLT neurons significantly increased mean ABP in rats pretreated with saline or the AVP receptor antagonist. In marked contrast, these responses were absent after pretreatment with the ganglionic blocker (Figure 2B). Furthermore, the increase in ABP during optogenetic stimulation of OVLT was not different after saline and AVP antagonist treatments. The tachycardic response to optogenetic stimulation of OVLT neurons was unaffected by pretreatment of the AVP antagonist but abolished after the ganglionic blocker (Figure 2B). Analyses of the 10-min average for the Δ mean ABP or Δ heart rate indicate vasopressin blockade did not affect these responses whereas ganglionic blockade eliminated the optogenetic response. (Figure 2C).
Figure 2.
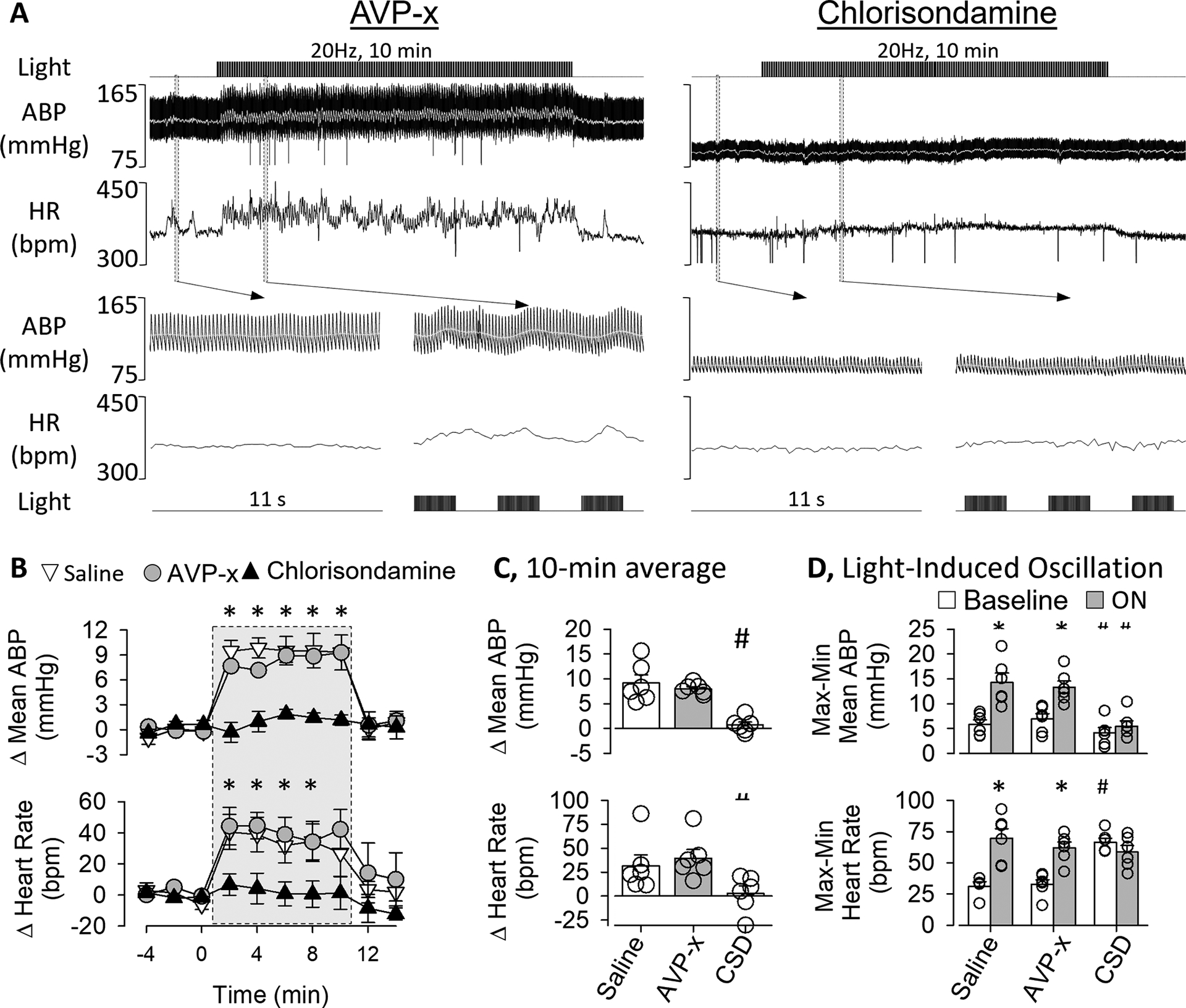
A, ABP, mean ABP (grey line), and heart rate of a rat pretreated with the AVP receptor antagonist Manning Compound (10μg/kg, IV) or ganglionic blocker chlorisondamine (5mg/kg, IV) before optogenetic activation of OVLT neurons (20Hz, 5 ms pulse, 10 min). Grey bars highlight a 11s example of ABP and heart rate before and during stimulation. Note, the laser-evoked oscillations and higher ABP and heart are present after the AVP receptor blockade but abolished after ganglionic blockade. B, Mean±SEM mean ABP and heart rate as function of time. *P<0.05 saline or AVP versus vs chlorisondamine. C, Mean±SEM of mean ABP and heart rate averaged during the 10 min stimulation period. # P<0.05 vs saline or AVP-x. D, Light-induced oscillations were calculated as the difference in maximum and minimum changes in mean ABP or heart rate for each 4s cycle. *P<0.05 vs baseline, # P<0.05 vs baseline saline or AVP. Abbrevations: AVP-x, vasopressin receptor antagonist; CSD, chlorisondamine.
As noted above, optogenetic activation of OVLT neurons using a 50% duty cycle (2s on, 2s off) produced oscillations in mean ABP and heart rate. These oscillations were quantified by calculating the difference between the minimum and maximum mean ABP (or heart rate) during each 4s cycle (2 s light on, 2 s light off) and compared to a 10-min baseline period. Figure 2D indicates optogenetic stimulation significantly increased the magnitude of these oscillations in both mean ABP and heart rate for rats pretreated with saline or the AVP-antagonist. However, these oscillations were absent in rats pretreated with the ganglionic blocker chlorisondamine (Figure 2D).
Experiment 2: SNA Responses to Optogenetic Stimulation of OVLT Neurons.
To directly test the extent by which activation of OVLT neurons increases SNA, simultaneous recordings of renal, splanchnic and lumbar SNA were performed during optogenetic stimulation of OVLT neurons. Baseline mean ABP and heart rate were 102±4 mmHg (n=5) and 419±19 bpm (n=5), respectively. Light-triggered averaging of SNA revealed a significant increase in renal, splanchnic, and lumbar SNA (Figure 3B). SNA increased across all three nerves, and the magnitude was not statistically different across nerves. However, the latency to peak SNA was significantly different across groups as the peak lumbar SNA was longer than those of renal or splanchnic SNA (Figure 3B).
Figure 3.
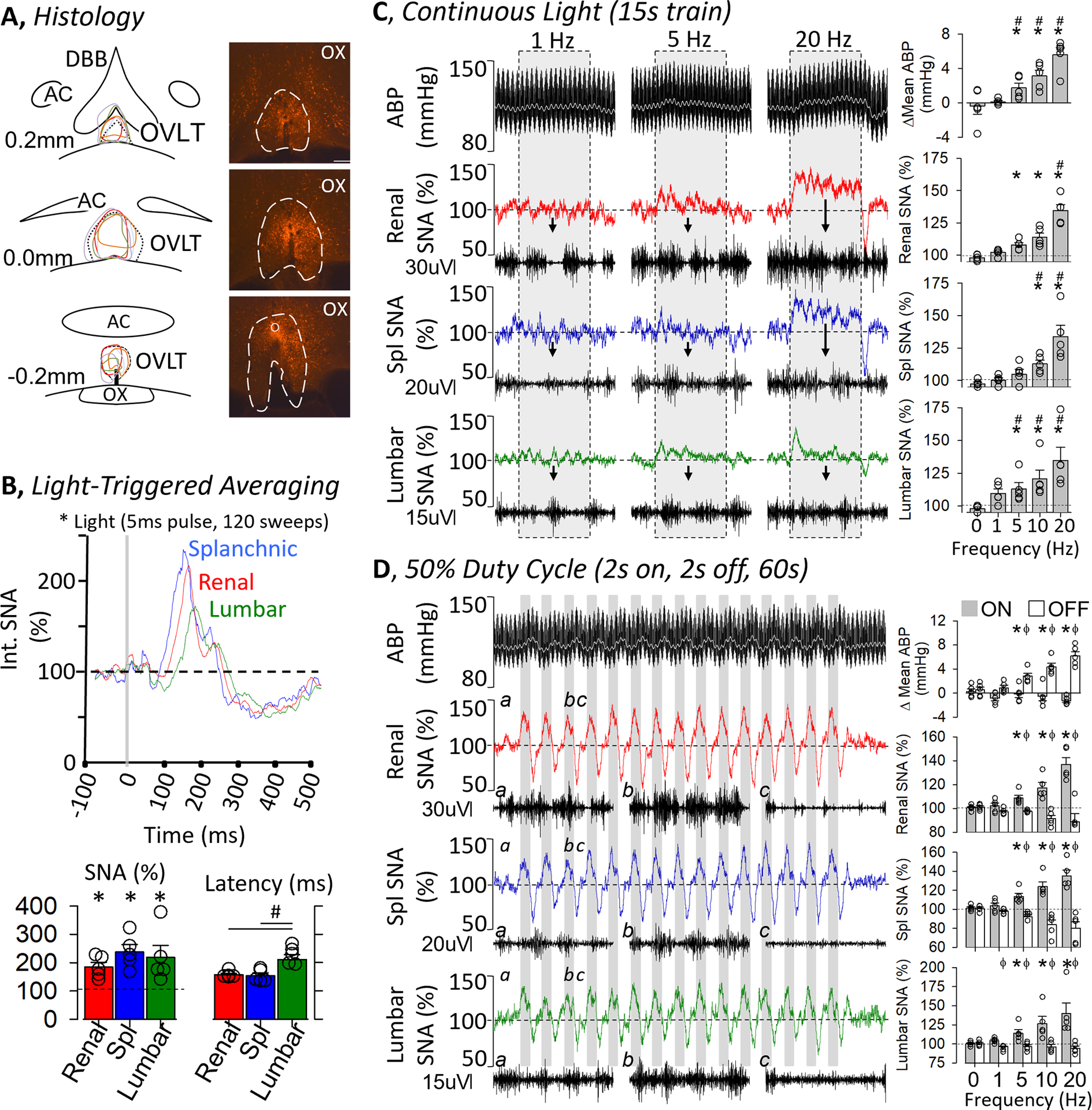
A, Schematic illustration of mCherry expression for 5 OVLT rats and digital image of representative mCherry expression and optical ferrule. Scale bar = 200μm. B (TOP), Light-triggered averaging of splanchnic, renal and lumbar SNA during optogenetic stimulation of OVLT (1 Hz, 5ms pulse, 120 sweeps). (BOTTOM) 10-s peak change in SNA (% baseline) and latency to peak (ms). C and D, ABP and mean ABP (grey line) plus rectified/integrated renal, splanchnic, and lumbar SNA during optogenetic activation of OVLT neurons. Insets underneath each nerve illustrate 0.5s trace of raw nerve activity. (C) Continuous light pulses (15 s train, 5ms pulse; 1, 5 and 20Hz) were applied through the optical ferrule. (RIGHT) Summary data (mean±SEM and data points) illustrate the average change in ABP or SNA. (D) Intermittent light pulses (50% duty cycle, 60 s train, 5ms pulse) were applied. Note the oscillations in ABP and SNA. (RIGHT) Summary data (mean±SEM plus individual data points) illustrate the average peak change in ABP and SNA when the laser was “on” versus “off”. *P<0.05 vs 0 Hz, # P<0.05 vs lower frequency, ϕ P<0.05 light on vs off
SNA responses to optogenetic stimulation of OVLT were also assessed to both continuous (15s) and intermittent (50% duty cycle: 2s on / 2s off, 60s) light trains. Continuous optogenetic stimulation of OVLT neurons produced frequency-dependent increases in renal SNA, lumbar SNA, splanchnic SNA and ABP. (Figure 3C). SNA and ABP promptly increased at the onset of light stimulation and returned to baseline levels when the light trains stopped. Post-hoc analyses indicated optogenetic stimulation of OVLT increased renal SNA, lumbar SNA, and mean ABP at ≥ 5Hz but splanchnic SNA at ≥ 10Hz (Figure 3C). Optogenetic stimulation using a 50% duty cycle also promptly increased renal, splanchnic, and lumbar SNA (Figure 3D). Interestingly, these variables oscillated as a function of the light pulses (on versus off). In addition, ABP also increased immediately and oscillated with the light pulses but peaked after SNA. Again, a frequency-dependent analyses indicated intermittent light stimulation of OVLT neurons increased renal, splanchnic, and lumbar SNA ≥ 5Hz. ABP increased significantly at ≥ 5Hz (Figure 3D). In addition, post-hoc evaluation of mCherry expression revealed a second set of animals (n=4) without mCherry expression in the OVLT (no expression or mCherry was outside of OVLT). SNA and ABP of these animals did not significantly change from baseline values in response to light-triggered averaging, continuous light trains, or intermittent (50% duty cycle) light to OVLT (data not shown).
Experiment 3: Single-Unit Recordings for Bulbospinal RVLM Neurons.
A subset of both OVLT and OVLT-x animals used in SNA recordings were also prepared for single-unit recordings of RVLM neurons. Baseline mean ABP and heart rate were 102±2 mmHg and 409±21 bpm (OVLT, n=3 rats) versus 102±3 mmHg and 421±19 bpm (OVLT-x, n=3 rats). Characteristics of identified bulbospinal, barosensitive RVLM neurons did not differ between OVLT (n=6 neurons) and OVLT-x (n=5 neurons) groups: discharge (12.9±3.1 vs 10.6±6.2 Hz, respectively; P>0.7) or conduction velocity (2.8±0.5 vs 2.4±0.9 m/s, respectively; P>0.6). In OVLT animals, 100% (6/6) of RVLM neurons displayed frequency-dependent increases in neuronal discharge during optogenetic stimulation of the OVLT (Figure 4A, B). Both RVLM discharge and SNA increased promptly at the start of the light train but abruptly returned to baseline values when the light train was terminated. Post-hoc analyses indicated optogenetic stimulation of OVLT increased RVLM discharge in the OVLT group at ≥ 5Hz (Figure 4B). In marked contrast, 0% (0/5) of RVLM neurons in OVLT-x rats were affected by optogenetic stimulation (1–20Hz). The changes in SNA and mean ABP were not statistically different from those presented in Figure 3C (summary data not shown).
Figure 4.
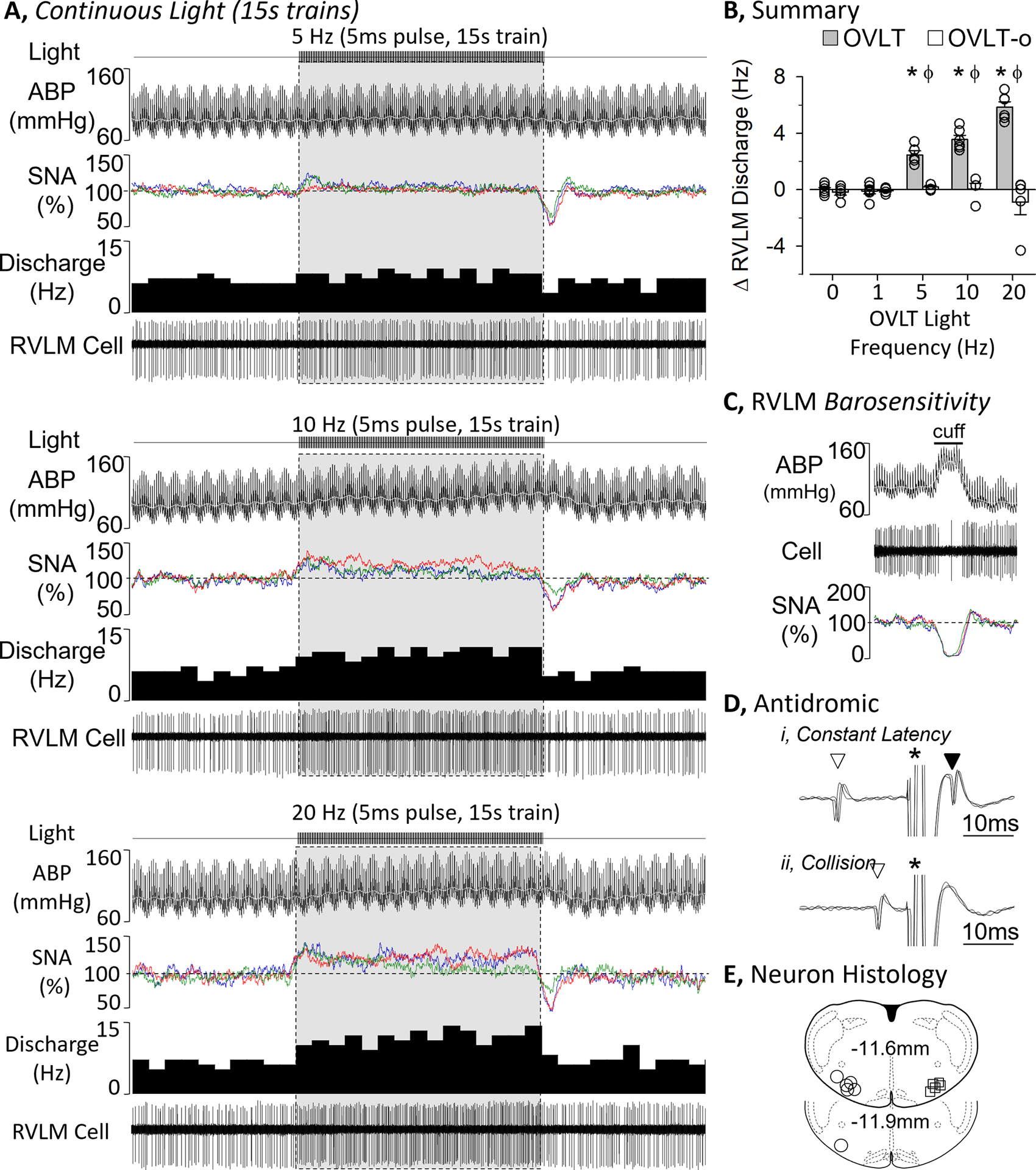
A, ABP, rectified/integrated SNA (renal – red, splanchnic - blue, lumbar - green), and RVLM single-unit discharge (rate histogram and raw cell activity) during optogenetic stimulation of OVLT neurons at 5, 10, and 20 Hz using continuous light train (5ms pulse, 15s train). B, Mean±SEM of the change in RVLM discharge and rectified/integrated renal SNA during optogenetic activation of OVLT neurons at 0, 1, 5, 10, and 20 Hz in rats with OVLT mCherry expression versus OVLT-o (without mCherry expression). C and D, Identification of RVLM sympathetic neuron via (C) barosensitive as inflation of an aortic cuff promptly increased ABP and decreased RVLM cell discharge, and (D) RVLM unit was antidromically stimulated from the T2 spinal segment with a constant onset latency (i) and collided by an orthodromic spike (ii). Antidromic examples represent 3 overlapping traces. *P<0.05 vs 0 Hz and lower frequency, ϕ P<0.05 OVLT vs OVLT-x
In addition, optogenetic stimulation of the OVLT using a 50% duty cycle also promptly increased discharge in 100% (6/6) of RVLM neurons in a frequency-dependent manner (Figure 5). Similar to SNA, RVLM discharge oscillated as a function of the light pulses (on versus off) and was phase-locked to the SNA responses. The increase in RVLM discharge was observed during light stimulation of the OVLT at frequencies ≥ 5Hz (Figure 5B). These light-induced oscillations of RVLM discharge were not observed in single-unit RVLM recordings in OVLT-x animals (n=5 neurons, data not shown). Again, the changes in SNA and mean ABP were not statistically different from those presented in Figure 3D (summary data not shown).
Figure 5.
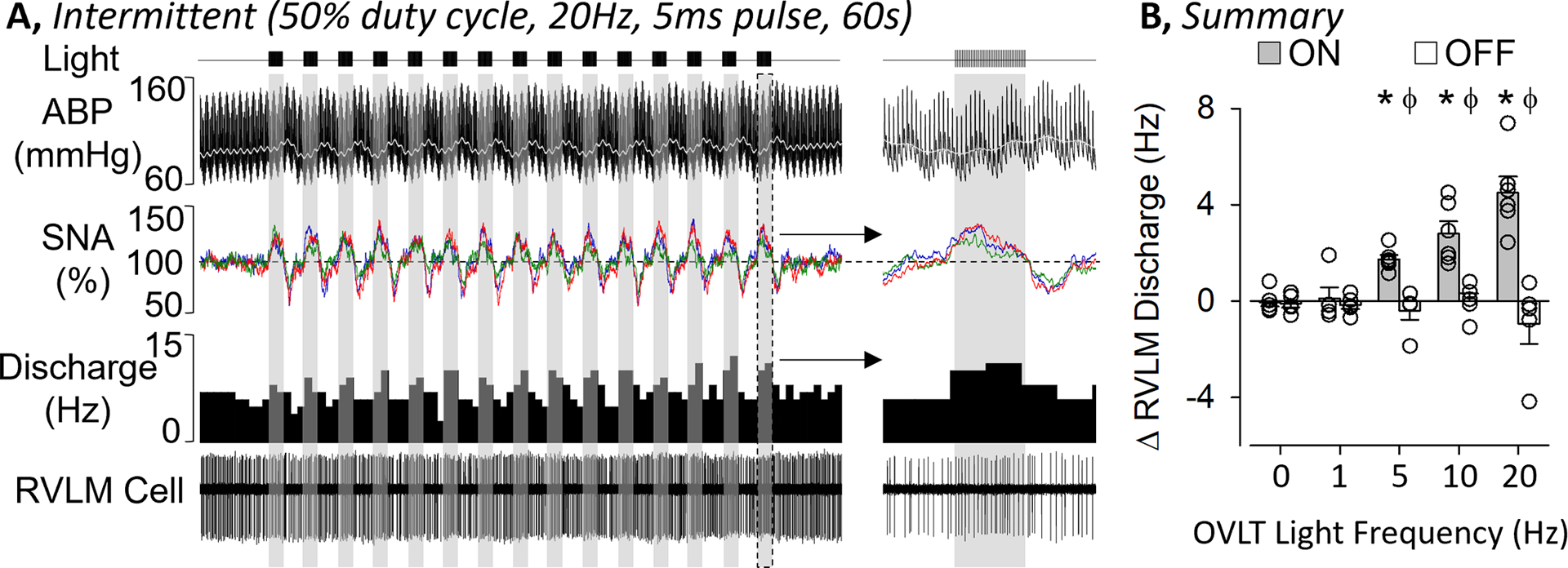
A, ABP, rectified/integrated SNA (renal – red, splanchnic - blue, lumbar - green), and RVLM single-unit discharge (rate histogram and raw cell activity) during optogenetic stimulation of OVLT neurons at 20 Hz using a 50% duty cycle light pulses (5ms pulse, 2s on / 2 s off, 60s train). B, Mean±SEM of the change in RVLM discharge during optogenetic activation of OVLT neurons at 0, 1, 5, 10, and 20 Hz in rats with OVLT mCherry expression when the laser was “on” versus “off”. *P<0.05 vs 0 Hz and lower frequency for light “on”, ϕ P<0.05 “on” versus “off”
Experiment 4: Chronic Chemogenetic Activation of OVLT Neurons.
A final set of experiments were performed to test the extent by which chronic activation of OVLT neurons increased ABP. CNO was administered through the 0.9% NaCl drinking bottle for 7 days, and the concentration adjusted daily to result in ~3.0mg/kg/day. Administration of CNO to OVLT rats (with mCherry expression localized to OVLT) significantly increased mean ABP above baseline levels at Day 3 and remained elevated until Day 10 (Figure 6A). Daily fluid intake of OVLT rats significantly increased on Day 1 and remained elevated through Day 8. Since the large increase in fluid intake may contribute to the rise in mean ABP, a second group of rats with localized injection to the OVLT were administered CNO but 0.9% NaCl intake was limited to 50mL per day (OVLT-50). Again, CNO administration to OVLT-50 rats significantly increased mean ABP on Day 3, and both variables remained elevated through Day 10. In fact, the absolute levels of mean ABP or the change in mean ABP did not differ between OVLT versus OVLT-50 rats at any time. In marked contrast, administration of CNO to rats with mCherry expression rostral to OVLT or no expression (OVLT-x) did not alter mean ABP, fluid intake, and transiently reduced heart rate (Figure 6A). In fact, mean ABP or the change in mean ABP was significantly greater in OVLT and OVLT-50 rats versus OVLT-x rats from Day 2 thru Day 7. Administration of CNO did reduce food intake in all three groups but the duration of this effect was longer in OVLT and OVLT-50 groups. Body weight did not significantly differ across groups at any time.
Figure 6.
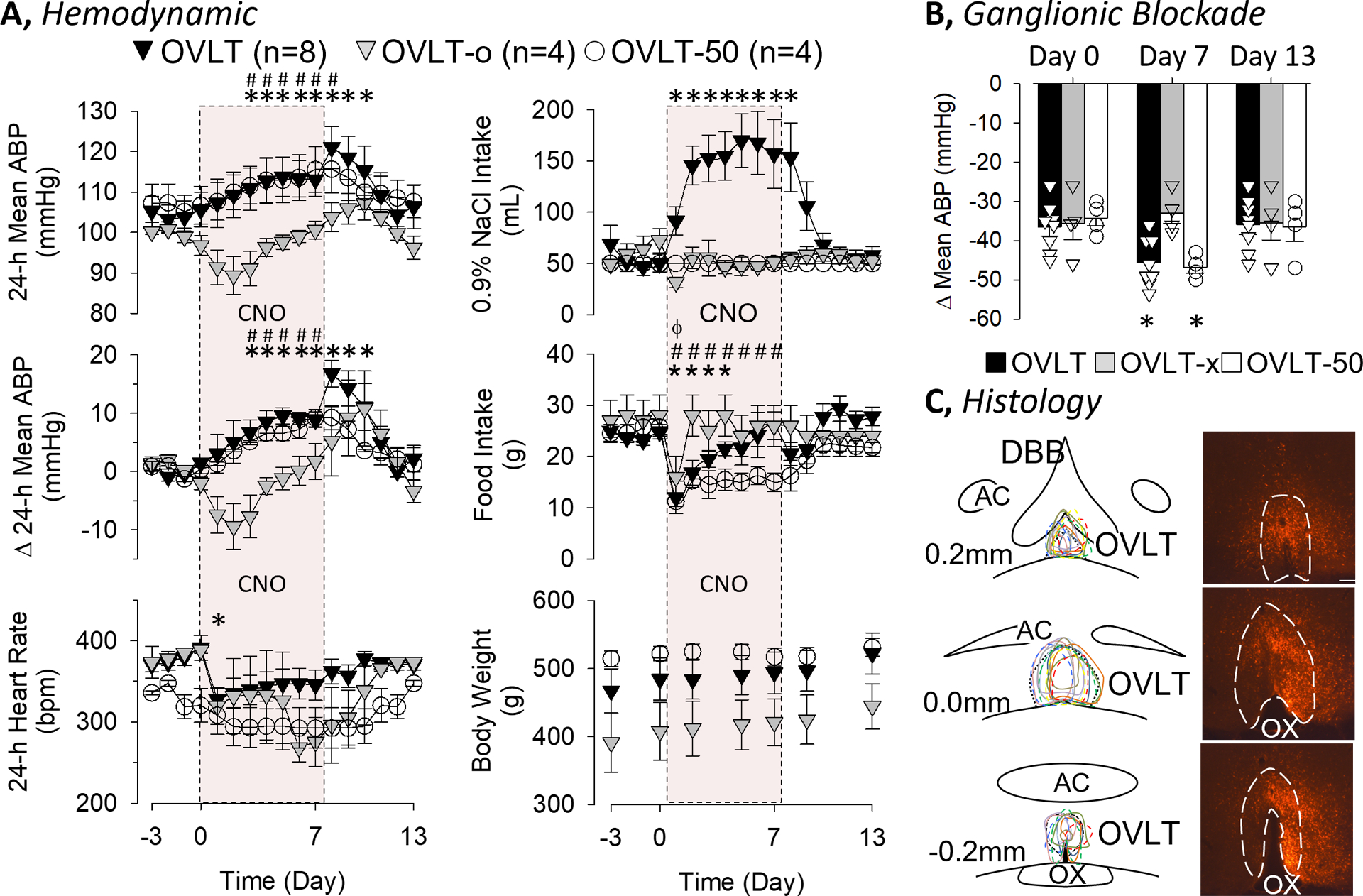
A, Mean±SEM of mean ABP, change in mean ABP, heart rate, 0.9% fluid intake, food intake, and body weight of rats with mCherry expression (marking the presence of cells transduced by the DREADD) in OVLT (OVLT) or without (OVLT-o) and free access to 0.9% NaCl drinking solution instead of water. Clozapine-N-oxide was dissolved in the drinking solution and administered Day 1–7 (red-box). A third group of rats with mCherry expression in the OVLT were restricted to 50mL of 0.9% NaCl per day but given Clozapine-N-oxide. B, Mean±SEM and individual data of the depressor response to the ganglionic blocker hexamethonium (30mg/kg, iv) on Day 0, 7, and 13. C, Schematic illustration of mCherry expression for OVLT (solid line) and OVLT-50 (dashed line) rats and digital image of representative mCherry expression. Scale bar = 200μm. *P<0.05 vs baseline for OVLT, #P<0.05 vs baseline for OVLT-50, ϕP<0.05 vs baseline for OVLT-o
To test the extent by which the differences in mean ABP were sympathetically-mediated, the depressor response to hexamethonium (30mg/kg, ip) was assessed on Days 0, 7, and 13 (Figure 6B). The depressor response was significantly greater in OVLT and OVLT-50 versus OVLT-o rats on Day 7. These differences between groups were not present on Day 0 or 13. Plasma electrolyte concentrations did not differ across groups on Day 0, 7, or 13 (see online supplement).
DISCUSSION
This study provides strong evidence that activation of OVLT neurons elevates SNA to produce hypertension in rats. Optogenetic activation of OVLT produced frequency-dependent increases in ABP that were attenuated by blockade of ganglionic neurotransmission but not vasopressin receptors. Multifiber sympathetic nerve recordings demonstrate optogenetic activation of OVLT neurons increases renal, splanchnic and lumbar SNA. Consistent with this observation, optogenetic stimulation of the OVLT increased the discharge of bulbospinal, sympathetic premotor neuron in the rostral ventrolateral medulla. Finally, chronic activation of OVLT neurons using chemogenetics increased ABP independent of fluid intake. The resultant hypertension was associated with a greater neurogenic pressor activity. Collectively, these findings provide compelling evidence that OVLT neurons regulate SNA to produce hypertension.
Selective lesion of OVLT attenuated deoxycorticosterone-salt, angiotensin II, and angiotensin II-salt models of hypertension18–20. Such lesions were also associated with a reduced neurogenic pressor activity (assessed by ganglionic blockade). These findings suggest OVLT neurons may regulate SNA, but limited data supports this notion. Electrical stimulation of OVLT increased renal and mesenteric resistance and ABP in urethane-anesthetized rats, and these responses were eliminated by ganglionic blockade15. In addition, local injection of hypertonic NaCl into the OVLT increased lumbar SNA and ABP14. Herein, optogenetic stimulation of OVLT neurons in conscious animals produced frequency-dependent increases in ABP and heart rate. Interestingly, both ABP and HR oscillated with the light pulses thereby suggesting a fast-acting neural efferent pathway was involved versus neuroendocrine mechanisms. Indeed, blockade of ganglionic neurotransmission, but not vasopressin receptors, eliminated both pressor and tachycardic responses as well as the light-induced oscillations. Together with direct recordings of renal, splanchnic and lumbar SNA, these findings provide clear support that acute activation of OVLT neurons increase SNA to regulate ABP.
OVLT neurons densely innervate magnocellular vasopressin neurons of the hypothalamic paraventricular and supraoptic nuclei6, 13, 25. Stimulation of the OVLT excited magnocellular vasopressin neurons13, and lesion of the OVLT in dogs attenuated vasopressin secretion stimulated by acute hypernatremia or infusion of angiotensin II26. Therefore, the absence of a vasopressin contribution to the pressor response observed herein was surprising. A recent paper reported optogenetic stimulation of angiotensin type 1A receptor neurons in the OVLT/median preoptic nucleus of mice increased ABP, and this response was prevented by vasopressin receptor blockade27. Although species differences may account for the discrepancies as well as the activated cell group (OVLT versus OVLT and preoptic), these experiments were conducted using isoflurane anesthesia which will robustly increase vasopressin levels. Second, baseline ABP values were not reported. Third, the optogenetic stimulation parameters (15Hz, 20ms pulse) likely resulted in much higher discharge rates due to the large pulse duration. While our study suggests vasopressin does not contribute to the OVLT-induced pressor responses, this does not exclude the possibility that vasopressin levels increase to alter renal function. Circulating vasopressin affects renal function at low levels (<8 pg/mL) whereas higher levels (>10 pg/mL) are required to induce a pressor response28, 29. Indeed, we recently reported that optogenetic stimulation of OVLT neurons stimulated thirst without any increase in urine volume for several hours9 thereby suggesting plasma vasopressin levels may increase but to levels that primarily affect kidney function in conscious animals.
Optogenetic activation of OVLT neurons increased the discharge of bulbospinal, sympathetic neurons in the RVLM. The neural circuit by which OVLT neurons increase RVLM discharge and SNA is not defined but likely involves a polysynaptic pathway. OVLT neurons do not directly innervate the RVLM but do innervate multiple hypothalamic nuclei including the median preoptic nucleus and hypothalamic paraventricular nucleus5, 6, 30. Indeed, we observed mCherry-positive terminals distributed throughout these regions but not the RVLM (data not shown). Yet, a curious finding is that optogenetic stimulation of OVLT neurons increased renal, splanchnic and lumbar SNA whereas local OVLT injection of hypertonic NaCl increased lumbar SNA14. This suggests the OVLT may house unique populations of neurons that regulate SNA to different end-organs.
A critical experiment was to assess the extent by which chronic activation of OVLT neurons produced a sustained, neurogenic hypertension. Since prior studies reported that activation of OVLT neurons increased water intake9, 10, water bottles were replaced with isotonic saline to avoid hyponatremia as a confounding variable. Chemogenetic activation of OVLT did, in fact, robustly increase fluid intake (~3 fold) and ABP. When fluid was restricted to baseline levels (~50mL/day), chronic activation of OVLT neurons still increased ABP. Interestingly, ganglionic blockade produced a greater drop in ABP thereby suggesting a larger neurogenic pressor activity and sympathetic activation. These data suggest chronic activation of OVLT neurons increase ABP, in part, through a neurogenic mechanism.
Viral-mediated transduction of OVLT neurons was conducted using general promoters, CaMKIIa (excitatory neuronal promoter) or hSYN (general neuronal promoter). Thus, the viral expression may have differed between experiments (excitatory neurons versus excitatory and inhibitory neurons, respectively). Moreover, OVLT neurons sense numerous circulating factors including extracellular NaCl and angiotensin II concentrations9 and regulate thirst stimulated by these inputs9, 11. In vitro and in vivo recordings suggest hypertonic NaCl and angiotensin II may target overlapping populations of OVLT neurons9. However, it is unclear whether OVLT injection of NaCl and angiotensin II evoke a similar sympathetic and hemodynamic response. Recent RNAseq and functional studies have identified multiple and neurochemically distinct populations of OVLT neurons in mice that regulate thirst and include angiotensin II type 1 receptor10, nNos31, Pdyn11, and Rxfp111 neurons. To what extent activation of these populations elevate SNA and produce hypertension is not yet known.
PERSPECTIVES
OVLT neurons reside outside the blood-brain-barrier and serve as a sensory interface between the circulation and central nervous system to maintain body fluid homeostasis and regulate ABP. These neurons sense changes in electrolyte and angiotensin II concentrations9, 14. Lesion of the OVLT attenuated deoxycorticosterone-salt, angiotensin II, and angiotensin II-salt models18–20. These findings demonstrate activation of OVLT neurons elevate SNA to produce hypertension. Taken together, these observations suggest that OVLT neuronal activity is elevated to neurogenic hypertension. However, this possibility has not been directly tested in experimental models or humans.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Optogenetic stimulation of OVLT neurons produced a sympathetically-mediated increase in ABP.
Optogenetic stimulation of OVLT elevated renal, splanchnic, and lumbar SNA and increased the discharge of bulbospinal, sympathetic neurons in the rostral ventrolateral medulla.
Chronic chemogenetic activation of OVLT neurons produced hypertension associated with increase neurogenic pressor activity.
What is Relevant?
Lesion of OVLT neurons attenuate multiple experimental models of hypertension. These findings provide the first direct evidence that OVLT neurons regulate ABP through the sympathetic nervous system.
Summary
Acute or chronic activity of OVLT neurons produces a sympathetically-mediated hypertension.
SOURCE of FUNDING
The research was supported by NIH Grants HL113270 (S.D.S), HL145875 (S.D.S.) and HL128388 (W.B.F. and S.D.S).
Footnotes
CONFLICT OF INTEREST/DISCLOSURES
The authors have no disclosures.
SUPPLEMENTARY MATERIALS
Expanded Material and Methods
REFERENCES
- 1.Grassi G, Mark A and Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyenet PG, Stornetta RL, Souza G, Abbott SBG and Brooks VL. Neuronal Networks in Hypertension: Recent Advances. Hypertension. 2020;76:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLalio LJ, Sved AF and Stocker SD. Sympathetic Nervous System Contributions to Hypertension: Updates and Therapeutic Relevance. Can J Cardiol. 2020;36:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinsman BJ, Nation HN and Stocker SD. Hypothalamic Signaling in Body Fluid Homeostasis and Hypertension. Curr Hypertens Rep. 2017;19:50. [DOI] [PubMed] [Google Scholar]
- 5.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A and Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:III–XII, 1–122, back cover. [DOI] [PubMed] [Google Scholar]
- 6.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–31. [DOI] [PubMed] [Google Scholar]
- 7.Cancelliere NM, Black EA and Ferguson AV. Neurohumoral Integration of Cardiovascular Function by the Lamina Terminalis. Curr Hypertens Rep. 2015;17:93. [DOI] [PubMed] [Google Scholar]
- 8.Coble JP, Grobe JL, Johnson AK and Sigmund CD. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: importance of the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2015;308:R238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsman BJ, Simmonds SS, Browning KN, Wenner MM, Farquhar WB and Stocker SD. Integration of Hypernatremia and Angiotensin II by the Organum Vasculosum of the Lamina Terminalis Regulates Thirst. J Neurosci. 2020;40:2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leib DE, Zimmerman CA, Poormoghaddam A, Huey EL, Ahn JS, Lin YC, Tan CL, Chen Y and Knight ZA. The Forebrain Thirst Circuit Drives Drinking through Negative Reinforcement. Neuron. 2017;96:1272–1281 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pool AH, Wang T, Stafford DA, Chance RK, Lee S, Ngai J and Oka Y. The cellular basis of distinct thirst modalities. Nature. 2020;588:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thrasher TN, Keil LC and Ramsay DJ. Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically-induced drinking and vasopressin secretion in the dog. Endocrinology. 1982;110:1837–9. [DOI] [PubMed] [Google Scholar]
- 13.Richard D and Bourque CW. Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro. J Physiol. 1995;489 (Pt 2):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinsman BJ, Simmonds SS, Browning KN and Stocker SD. Organum Vasculosum of the Lamina Terminalis Detects NaCl to Elevate Sympathetic Nerve Activity and Blood Pressure. Hypertension. 2017;69:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangiapane ML and Brody MJ. Vasoconstrictor and vasodilator sites within anteroventral third ventricle region. Am J Physiol. 1987;253:R827–31. [DOI] [PubMed] [Google Scholar]
- 16.Kinsman BJ, Browning KN and Stocker SD. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J Physiol. 2017;595:6187–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif Naeini R, Witty MF, Seguela P and Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–8. [DOI] [PubMed] [Google Scholar]
- 18.Collister JP, Nahey DB, Hartson R, Wiedmeyer CE, Banek CT and Osborn JW. Lesion of the OVLT markedly attenuates chronic DOCA-salt hypertension in rats. Am J Physiol Regul Integr Comp Physiol. 2018;315:R568–R575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collister JP, Olson MK, Nahey DB, Vieira AA and Osborn JW. OVLT lesion decreases basal arterial pressure and the chronic hypertensive response to AngII in rats on a high-salt diet. Physiol Rep. 2013;1:e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira AA, Nahey DB and Collister JP. Role of the organum vasculosum of the lamina terminalis for the chronic cardiovascular effects produced by endogenous and exogenous ANG II in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1564–71. [DOI] [PubMed] [Google Scholar]
- 21.Collister JP, Nahey DB, Hartson R, Wiedmeyer CE, Banek CT and Osborn JW. Lesion of the OVLT markedly attenuates chronic deoxycorticosterone acetate (DOCA) salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman WE, Phillips MI, Wilson E and Schmid PG. A pressor response associated with drinking in rats. Proc Soc Exp Biol Med. 1977;154:121–4. [DOI] [PubMed] [Google Scholar]
- 23.Simmonds SS, Lay J and Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension. 2014;64:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker SD, Lang SM, Simmonds SS, Wenner MM and Farquhar WB. Cerebrospinal Fluid Hypernatremia Elevates Sympathetic Nerve Activity and Blood Pressure via the Rostral Ventrolateral Medulla. Hypertension. 2015;66:1184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss ML and Hatton GI. Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res Bull. 1990;24:231–8. [DOI] [PubMed] [Google Scholar]
- 26.Thrasher TN and Keil LC. Regulation of drinking and vasopressin secretion: role of organum vasculosum laminae terminalis. Am J Physiol. 1987;253:R108–20. [DOI] [PubMed] [Google Scholar]
- 27.Frazier CJ, Harden SW, Alleyne AR, Mohammed M, Sheng W, Smith JA, Elsaafien K, Spector EA, Johnson DN, Scott KA, Krause EG and de Kloet AD. An Angiotensin-Responsive Connection from the Lamina Terminalis to the Paraventricular Nucleus of the Hypothalamus Evokes Vasopressin Secretion to Increase Blood Pressure in Mice. J Neurosci. 2021;41:1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Share L Role of vasopressin in cardiovascular regulation. Physiol Rev. 1988;68:1248–84. [DOI] [PubMed] [Google Scholar]
- 29.Cowley AW Jr. and Liard JF. Vasopressin and arterial pressure regulation. Special lecture. Hypertension. 1988;11:I25–32. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield BJ, Badoer E, Hards DK and McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60:255–62. [DOI] [PubMed] [Google Scholar]
- 31.Augustine V, Gokce SK, Lee S, Wang B, Davidson TJ, Reimann F, Gribble F, Deisseroth K, Lois C and Oka Y. Hierarchical neural architecture underlying thirst regulation. Nature. 2018;555:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


