Abstract
With rapid emergence of multi-drug resistant microbes, it is imperative to seek alternative means for infection control. Optical waveguides are an auspicious delivery method for precise administration of phototherapy. Studies have shown that phototherapy is promising in fighting against a myriad of infectious pathogens (i.e. viruses, bacteria, fungi, and protozoa) including biofilm-forming species and drug-resistant strains while evading treatment resistance. When administered via optical waveguides, phototherapy can treat both superficial and deep-tissue infections while minimizing off-site effects that afflict conventional phototherapy and pharmacotherapy. Despite great therapeutic potential, exact mechanisms, materials, and fabrication designs to optimize this promising treatment option are underexplored. This review outlines principles and applications of phototherapy and optical waveguides for infection control. Research advances, challenges, and outlook regarding this delivery system are rigorously discussed in a hope to inspire future developments of optical waveguide-mediated phototherapy for the management of infection and beyond.
1. Introduction
Infection refers to the process in which pathogens colonize host tissues producing a subsequent host response to respective pathogens or its toxins. Pathogens that cause infectious disease include bacteria, fungi, protozoa, helminths, and viruses among others. However, many are commonplace in the environment, and even function symbiotically with host species. For example, many symbiotic bacteria and fungi are present in gastrointestinal tract (GI), mucous membranes, and on the skin in mammals. The human body has multiple lines of physical and chemical defenses against pathogenic microbes under normal circumstances. However, if these barriers are compromised due to trauma, burns, immunosuppression, chronic illness or other comorbidities, infection can manifest in significant patient morbidity or death. Moreover, due to improper use of drug therapies, antimicrobial and antiviral treatment resistance is emerging globally with the onset of multi-drug resistant pathogens that are refractory to present pharmaceutical treatments[1]. According to the Centers for Disease control and Prevention (CDC), 2.8 million people are affected by resistant infections with an annual death toll of approximately 35,000 in the United States[2]. Furthermore, it is difficult for current systemic antimicrobials to sufficiently penetrate biofilms, a protective extracellular polymeric matrix produced by particular bacterial and fungal species, which demands high doses of medications that increase the risk of adverse effects without the promise of sufficient efficacy[3–7]. Despite government incentives, such as the Generating Antibiotic Incentives Act (GAIN), to develop novel pharmaceutical agents (i.e. antibacterial vaccines, phage therapy, immunostimulants, adjuvants, probiotics, and others), medication development is a slow and costly process relative to the emergence of resistant microbes. Further, the development of novel pharmaceutical agents still leaves the risk of resistance development to these new therapies[1, 8].
There is potential for phototherapy to be considered as an alternative or adjunct to conventional pharmaceutical therapies for infection control attributed to the low treatment resistance (with exceptions seen in photothermal and UVC therapies), and a broad spectrum of antimicrobial and antiviral activities[9–25]. Implementation of this alternative therapy into routine clinical practice can reduce the use of drug therapies and may be leveraged against existing drug-resistant strains of pathogens[9]. Most phototherapies have minimal invasiveness, and negligible systemic side effects, unlike their pharmaceutical counterparts[7, 26, 27]. Phototherapy comprises any therapeutic approach involving the use of light[9, 28–31]. Types of phototherapy can be classified according to the type of incident light and therapeutic mechanism. Categories include photodynamic, laser, photothermal, and other light-based (i.e. ultraviolet (UV) light, blue light) therapies. By illuminating an infected region with the appropriate wavelength, dose, duration, and frequency of light with or without exogenous agents (i.e. substrate, photosensitizer, or medium to propagate light), pathogens at the source of infection can be inactivated or irradicated. Amongst the widespread advantages compared to traditional therapies, there remain shortcomings of phototherapy in infection treatment, such as limited penetration depth, imperfect selectivity for pathogenic cells, lack of flexible and precise delivery, and safety issues (e.g. inflammation, healthy tissue damage, carcinogenesis) that warrant further research to reach the full therapeutic potential of phototherapy for infection treatment. Delivering phototherapy via optical waveguides is an auspicious approach to overcome some of the shortcomings of phototherapy by directing the propagation of light with minimal losses and less off-target irradiation than that of phototherapy alone to address local infections even to deep tissues.
Optical waveguides are indispensable to modern living being an integral component of telecommunications, sensors, and photonic integrated circuits to name a few applications. Optical waveguide-mediated phototherapy is a recently revived area of research being investigated for cancer ablation, biosensing, endoscopy, surgical laser delivery, and infection treatment[17, 32–36]. The latter application will be examined in this review. Optical waveguides are constructs with different geometries composed of dielectric materials that confine light propagation to a particular destination due to differences in the refractive indices of constituent materials[37–40]. Conventional optical waveguides are made of non-compliant and rigid materials (i.e. glasses and plastics), that are destructive to most body tissues[37–44]. Curvlite Sales developed an optical waveguide comprised of polymethyl methacrylate (PMMA), a polymer widely used for dental illumination in 1939. This and the emergence of interdisciplinary research fields, such as biomedical engineering and materials science and engineering, have since inspired the development of a plethora of transparent, biocompatible polymers intended for optical waveguides in medicine[45]. Increasingly, advanced, multifunctional, and/or stimuli-responsive optical waveguides possessing suitable mechanical and optical properties are being developed to perform in clinical environments[40, 46, 47]. The delivery of phototherapy with emerging optical waveguide technology has the potential to serve a critical role in local infection treatment, especially for drug-resistant microbes and viruses, deep tissue infections and biofilm-forming pathogens while eschewing off-site effects that are often inevitable in photo- and pharmacotherapies (Fig. 1). While other reviews eloquently focus on optical waveguide materials or medical applications, various types of phototherapies for infection and biomedical applications at large, as well as elucidation of components of such therapies (i.e. photosensitizers (PS), photothermal agents (PTAs)), this review uniquely compiles the basic principles, applications, and combinatory use of phototherapy and optical waveguide techniques specifically towards infection control. Pertinent research advances, current challenges, and future directions are highlighted in this work to shed light on opportunities for innovation and optimization of this promising therapeutic strategy to tackle the shortcomings of conventional treatments for infectious disease.
Fig. 1.
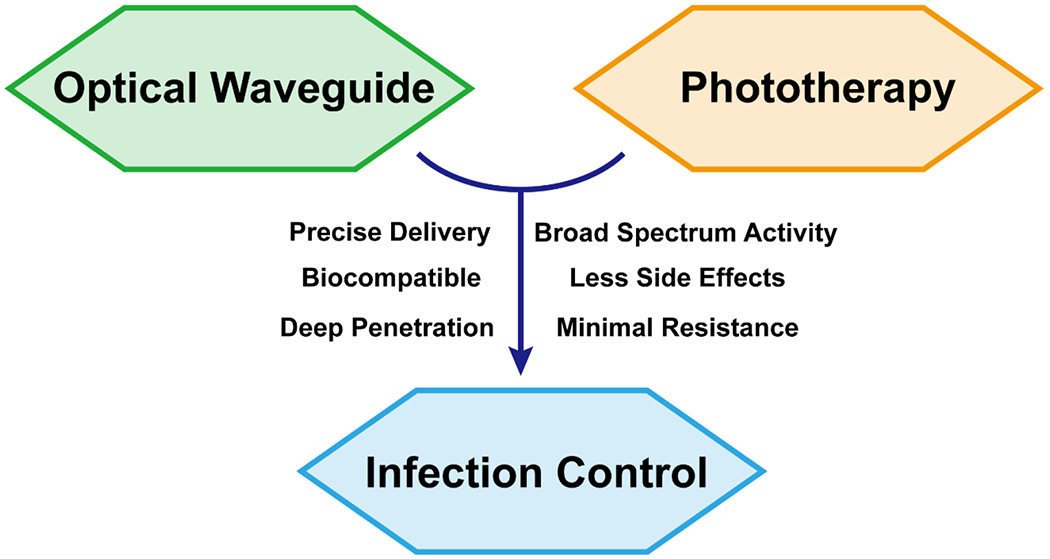
The integration of phototherapy and optical waveguide technology is an innovative and promising approach to combat pathogens and tackle the rapidly emerging threat of multi-drug resistant microbes and viruses. Optical waveguides of which are ideally biodegradable, biocompatible, and mechanically compliant with the working environment can direct incident light to the immediate site of infection minimizing off-site effects and systemic side effects that are often inevitable with traditional phototherapy and pharmaceutical therapy, respectively. The use of optical waveguides for treatment of infection harnesses the benefits of phototherapy, meanwhile overcoming the barrier of limited penetration depth expanding the use of phototherapy to deep tissue infections. Through the multiple mechanisms of action that phototherapy provides and the precise delivery by optical waveguides, there is great potential to fulfill the requirements of infection control caused by a variety of pathogens including those refractories to conventional therapies while reducing the need for drug treatment.
2. Phototherapy for infection treatment
The birth of phototherapy began with Niels Finsen, who was awarded the Nobel Prize for successfully treating smallpox and cutaneous tuberculosis using red and UV light in 1903[48, 49]. Phototherapies are effective at treating pathogenic organisms through various mechanisms all with the commonality of administering some form of light to a colonized site. This review categorizes the types of phototherapies according to pathogen inactivation mechanisms. The classifications reviewed herein include photodynamic therapy (PDT), photothermal therapy (PTT) and direct light-based phototherapies. The above classification was based on the pathogen inactivation mechanisms rather than the wavelength or the form of the light source. For example, when the laser was used to excite photosensitizers or photothermal agents to generate ROS or heat, the phototherapy should be categorized as PDT or PTT, respectively. When the laser was used directly to change the membrane potential of the pathogen, the phototherapy should be categorized as direct laser therapy. Similarly, if the visible light was used to generate ROS, the phototherapy should be categorized as PDT no matter which wavelength was used.
2.1. Photodynamic therapy
Among the types of phototherapies, photodynamic therapy is the most commonly studied technique. Photodynamic therapy (PDT) or photodynamic inactivation (PDI) is a means of phototherapy combining light, a substrate, and a photosensitizer to generate free radicals and reactive oxygen species (ROS) to eradicate undesired cells[50, 51].
2.1.1. Mechanism and principles
The underlying principles of PDT can be best illustrated by a Jablonski diagram (Fig 2a). This diagram demonstrates that after a quantum event, an electron in the singlet ground state (S0) absorbs a photon exciting it to a higher energy level (i.e. the singlet excited state (Sn)) with the same spin. Subsequently, the excited electron may return to the ground state from S1 to S0 by means of releasing a photon (fluorescence; lifetime ~10−8 s) or vibrational energy dissipated as heat by a process referred to as internal conversion (IC). The process when the excited electron changes spin state and enters the lowest excited triplet state (T1) is known as intersystem crossing (ISC). Afterwards, the electron relaxes to the S0 state in the form of photon release (phosphorescence, lifetime above 10−6 s) or as heat through IC. Based on quantum mechanics principles, the radiative decay process from T1 to S0 is forbidden. Hence, the decay time from T1 to S0 relative to S1 to S0 is longer[52]. The longer lifetime of triplet excitons results in the increased opportunity to transfer electrons (type I reaction) or energy (type II reaction) to target molecules rather than decay through internal conversion by heat. These processes are referred to as photosensitization and are necessary for PDT activity in infection treatment. The molecule which absorbs photons is known as the photosensitizer (PS), while the target molecule of photosensitization is referred to as the acceptor or substrate.
Fig. 2.
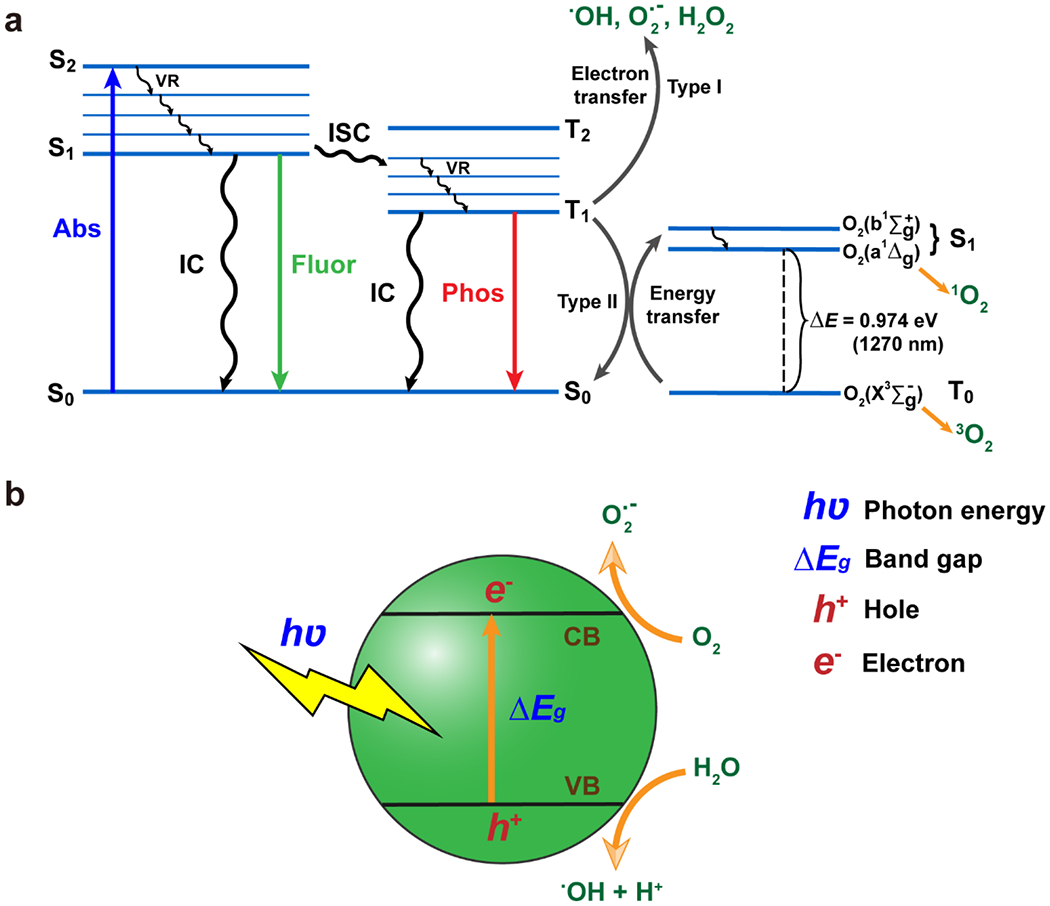
Underlying principles of PDT and PTT. (a)The Jablonski diagram demonstrates the general mechanism of reactive oxygen species and free radical generation in PDT and PTT upon photon absorption (abs) by a photosensitizer or photothermal agent, respectively. Following photon absorption, electrons are promoted from the ground state (S0) to an excited state (Sn; n≠0) then relax by emitting energy as heat (VR, IC) or light (fluorescence or phosphorescence). Type I reactions result in the generation of free radicals (O2•−, •OH) and H2O2. Type II reactions result in singlet oxygen generation (1O2). (b) Schematic of free radical generation in metal oxide nanomaterials. Valence electrons are excited from the valence band (VB) to the conductive band (CB) when exposed to light that exceeds the energy of the bandgap. The resulting charge separated state is conducive to energy transfer (type II) reactions producing free radical species, which degrade microbial and viral macromolecules.
Following a type I reaction, two radicals or radical ions (i.e. O2•− and •OH) and/or H2O2 are generated. In contrast, type II reactions result in singlet oxygen (1O2) generation following energy transfer to ground state molecular oxygen (3O2)[51, 53]. According to current evidence, both type I and II PDT result in the damage of most biomacromolecules including proteins, lipids and nucleic acids to kill cells and has roles in inflammatory processes[54, 55]. The potential differences in pathogen control between applying type I and type II PDT has yet to be elucidated. It should be noted, however, that type I and type II photosensitization generally occur concurrently in PDT where the ratio of type I to type II reactions is dependent on the PS, PS concentration, substrates, and environment[56]. The differences between the effects of type I and II reactions in addition to the downstream effects of damage to biomacromolecules inflicted by PDT can be a focus of future research. Such studies may deliver a more profound understanding of the killing mechanisms of type I and II reactions to direct novel designs of PSs and inform researchers how to protect host cells while selecting for pathogenic cells.
There is some inherent selectivity for pathogenic cells by PDT, which is believed to be attributed to: 1) the smaller cell volume and larger specific surface area of microbial cells and viral particles compared to that of mammalian cells, which results in a more probable binding and uptake of PSs by pathogenic cells; 2) the limited diffusion distances of ROSs (e.g. that of 1O2 is 100-200 nm) make it less probable for ROS to penetrate and damage enough cellular structures in larger host cells than that of the small pathogenic cells; 3) the higher rate of division and metabolic processes of pathogenic cells compared to that of host cells leads to increased sensitivity to free radicals and ROS[51, 57]. Increased selectivity for pathogenic cells may be facilitated by local application of PSs, as opposed to systemic administration, as well as through conjugating targeting moieties to PSs (e.g. cationic groups)[51].
2.1.2. PDT components and materials
The three necessary components for PDT include incident light, a photosensitizer, and a substrate. Since PDT has a broad working wavelength range, various light sources can be used, such as light emitting diodes (LEDs) and lasers. Ultrafast laser is a promising light source in PDT due to high spatial accuracy, the minimal potential for non-specific tissue damage and enhanced penetration depth[58]. As the source of excitation, light should be of the appropriate energy to bring PSs to the excited state. Near infrared (NIR) light can penetrate deeper into tissues than shorter wavelength light. Hence, NIR-activated PSs are more desirable for infection treatment. Some PSs have working wavelengths in the NIR region; however, the working wavelength of most current PSs is within the UV-visible region. For example, the absorption of riboflavin falls into UV to blue light range and blue light is commonly used for riboflavin activation in practical PDT applications[59–61]. Methylene blue (MB), toluidine blue O (TBO) and protoporphyrin IX (i.e. PPIX, active form of the prodrug, 5-aminolevulinic acid (5-ALA)) all work in the red light range in practical PDT applications[59, 62–65]. Similarly, most aggregation-induced emission luminogens that can function as PSs typically work in the visible region[66–68]. There are few reported PSs that can respond to NIR, such as indocyanine green (ICG)[59, 69]. More photosensitizers used in clinical trials and antimicrobial applications can be found in the following reviews[10, 17]. A suitable triplet state energy of the PS is also crucial for ROS generation in PDT. Photoluminescent materials with efficient ISC, high quantum yield of triplet excitons, and relatively long triplet state lifetime constitute ideal properties for PSs for PDT applications to achieve sufficient free radicals and ROS generation. If the lowest triplet state energy level (T1) of the PS is lower than the energy level of excited state of molecular oxygen (O2(b1Σg+) and O2(a1Δg)), the energy/electron transfer process is unable to occur. The rate constant of ISC (kISC) is dependent on the spin orbital coupling (SOC) constants (ξ) as well as the energy gap between the singlet and triplet states (ΔEST) according to perturbation theory and Fermi’s golden rule[70, 71]. Materials with large ξ, having a greater ability to alter the electronic spin direction, and low ΔEST will increase kISC. To achieve a large ξ, heavy atoms (i.e. atoms with atomic number (Z) greater than 30 and halogens except for fluorine) are used to exploit the heavy atom effect. Therefore, it is common to covalently conjugate halogen atoms to PS molecules to enhance ROS quantum yield in PDT materials[72]. Ruthenium (Ru), platinum (Pt), and iridium (Ir) may also be used. However, these elements are not only costly, but they are also undesirable for biomedical applications due to inherent toxicity. Another way to increase ROS quantum yield is to reduce the ΔEST. This is commonly done by incorporating a donor-acceptor (D-A) structure with a twisted conformation[73, 74]. However, this design approach is not desirable for PDT applications because the twisted conformation results in a reduced extinction coefficient and hypsochromic shift (i.e. blue shift) compared to non-twisted structures[75]. Substrates that are conducive to effective PDT should possess an appropriate redox potential and energy level to increase the proclivity to accept energy or electrons from PSs. It is also critical that substrates are abundant physiological environments. Main substrates include molecular oxygen and water.
There are several types of materials studied for PDT applications, such as organic-metal complexes and inorganic semiconducting materials[76]. A comprehensive review of PSs can be found in the review by Lan et al.[77] When metal oxides and other inorganic materials are excited by light with greater photoenergy than the band gap of the material, valence electrons can travel across the band gap to the conductive band resulting in a charge separated state, which facilitates electron transfer reactions (i.e. type I photosensitization) (Fig. 2b)[78]. Several metal oxides[79–87] and carbon nanomaterials[86, 88–97] were reported to have promise as PSs for PDT. However, metal oxides mainly absorb in the UV range limiting their penetration depth[56]. As a result, combining upconversion materials, of which can convert lower-energy NIR light to higher-energy UV emission, with metal oxide nanomaterials[98–106] and applying optical waveguides can be two feasible approaches to overcome this issue. Some other PDT related works are also listed here including novel developments involving composite organic and inorganic nanomaterials and metal peroxides[107–117].
For most organic materials, following photoexcitation electrons undergo rapid vibrational relaxation to the S1 and only a minority of electrons will transit to T1 through ISC. However, owing to biocompatibility, tunability, processability and low cost, organic materials are PSs popular for biomedical applications. Efforts to enhance ISC processes in organic materials have occurred since the 1970s when the first generation of organic PSs were developed, which included hematoporphyrin derivatives. The first-generation PSs suffered from poor selectivity and low extinction coefficients within their therapeutic window. The second-generation PSs emerged in the late 1980s. Representative second-generation PSs include tetrapyrrolic macrocycles and derivatives, transition metal coordination complexes, and cationic compounds, such as phenothiazines, xanthenes and cyanines. With the development of nanotechnology, the design of the third generation of PSs mainly focusing on improving delivery and targeting ability of PSs by nanocarriers[56]. For example, Zhang et al. developed a nanoassembly of Förster resonance energy transfer (FRET) photosensitizer pairs using FDA approved chlorin e6 (Ce6) as the donor component and 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (Dir) as the acceptor[118]. For infection control, there has been several attempts to use conventional organic PSs, such as ICG[119, 120] and polymer-based PSs[121] alone or in combination with other therapeutic components to form synergistic PDT nanosystems. Conventional organic PSs usually contain highly conjugated chromophores, which tend to self-assemble in the aqueous environments leading to photoluminescence quenching (i.e. aggregation-caused quenching (ACQ)) typically destroying the PDT performance. Opposite to ACQ, aggregation-induced emission (AIE) was identified by Benzhong Tang in 2001[122], which is attractive for PDT applications. There are many reports studying AIE-based materials and platforms in PDT[68, 123–149].
Molecular oxygen serves as an excellent substrate due to its triplet ground state (O2(X3Σg−)), which is conducive to accepting energy of triplet excitons that are the products of ISC[57, 150, 151]. Fortunately, molecular oxygen is relatively stable, inert, and abundant in the biological environment with an ability to travel far distances and permeate cell membranes and is a primary substrate of type I reactions. Water (H2O) is common substrate for type II reactions[56, 152–155].
2.1.3. Applications of photodynamic therapy for infection treatment
Many studies have demonstrated remarkable success with PDT against a wide array of pathogens including bacteria, fungi, viruses, and protozoa. Moreover, PDT is effective in biofilm producing organisms, which are difficult to treated by conventional pharmaceutical therapies and often require high doses of medication[156–167]. PDT treatment has been employed for several clinically relevant infections, such as diabetic ulcers, osteomyelitis, middle ear infection, acne, viral lesions, burns and wounds, and periodontitis in addition to being evaluated blood sterilization as detailed in other review works[9, 10, 12, 168–171].
PDT has been most often studied in bacterial and fungal models. For infections caused by bacteria, PDT has demonstrated to be effective against Gram-positive species like Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA), as well as, Gram-negative species like Acinetobacter baumannii, Pseudomonas Aeroginosa, and drug-resistant strains of Gram-negative bacteria[9, 172–216]. Lombard and colleagues have demonstrated efficacy to treat brain abscesses by topically applying hematoporphyrin intraoperatively and irradiating the infected site for five minutes[217]. Acne vulgaris, caused by the colonization of Cutibacterium acnes (formerly Propionibacterium acnes) results in skin lesions typically found on the face, back, and upper arms and has been shown to be sensitive to PDT therapy using different PSs and wavelengths of light. Examples of PDT regimens for acne vulgaris include methyl aminoevulinate (MAL) with blue light, 5-aminolevulanic acid (ALA) with 550-570 nm light, and indocyanine green (ICG) activated by NIR irradiation[218–222]. Colonization of the skin by Corynebacterium minutissimum in superficial skin layers and folds leads to an infection known as erythrasma and has been treated with red light PDT[223–225]. More recent publications are listed here[226–243].
Among fungal species, Candida is one of the most prevalent pathogenic fungi, which has attracted many in vitro and in vivo studies[63, 244–256]. Cutaneous infection commonly found in skin folds caused by Candida albicans and Trychophyton species has been treated with red light and ALA PDT. Other studies have also demonstrated PDT activity against Trichophyton rubrum[257, 258], Aspergillus fumigatus[259, 260], Metarhizium anisopliae and Aspergillus nidulans[261]. More recent antifungal PDT works are provided in the following references:[262–281].
For viral infections, study results have indicated that compared to non-enveloped viruses, enveloped viruses are more sensitive to PDT[282–287]. The difference in sensitivity may be due to increased accessibility of the phospholipid membrane, which is critical for the pathogenicity of enveloped viruses, by ROS. Herpes simplex virus (HSV), a highly contagious virus leading to lesions of the lips and genitals, was popularly treated with PDT in the late 20th century until it as found that it lacked therapeutic benefit while having adverse effects and potentially increasing risk of cancer. Human papilloma virus (HPV) infection manifests as genital warts and increases risk for cervical cancer. HPV has been treated with both been treated with PDT using a 630 nm laser with polyhematoporphyrin as well as with ALA- and MAL-PDT. ALA-PDT with activation from a helium-neon laser showed significantly less recurrence and comparable efficacy when compared to CO2 laser therapy after two treatments[288]. However, other randomized controlled trial results have demonstrated no difference in recurrence between the two treatment types[289]. Other studies using ALA and red light have similarly shown high response rates with low recurrence. Vesicular stomatitis virus (VSV), a disease that plagues many livestock animals, caused by Rhabdoviridae and Semliki forest virus transmitted by mosquitoes in Africa caused by Togaviridae infection are enveloped viruses sensitive to PDT with buckminsterfullerene (C60)[283, 290].
The coronavirus disease (COVID-19) pandemic caused by the SARS-CoV-2 virus remains a serious threat to public health. Various therapeutic strategies, such as pharmacotherapeutic therapies have been investigated[291, 292]. PDT is considering to be a promising therapeutic approach to treat SARS-CoV-2 infection[293–314]. For example, Kipshidze et al. first proposed using PDT and sonodynamic therapy (SDT) to treat COVID-19 by injecting porphyrin-based photosensitizers either systemically or locally into the lungs through the pulmonary artery using micro-catheters and in the absence of specialized photonics or in resource-limited settings, some PSs may be activated using transthoracic continuous wave ultrasound (SDT)[315, 316]. Weber et al. reported a successful reduction of SARS-CoV-2 viral load in 20 COVID-19 positive patients by PDT and verified by qPCR[317]. Besides PDT, several other biophotonic technologies and phototherapies were proposed or conducted to diagnose or treat COVID-19 or help biomodulation and rehabilitation during and after COVID-19[318–330].
Current antimicrobials are often ineffective against parasitic and protozoa infections that cause diseases like granulomatous amoebic encephalitis (GAE), amoebic keratitis, leishmaniasis, malaria, trypanosomiasis, and giardiasis due to rapidly emerging resistance[17, 331–334]. Consequently, several studies have been conducted to show activity against these refractory microbes using PDT. Sand flies from the Phlebotominae subfamily are a vector for the protozoan parasites in the genus Leishmania that can lead to a disease known as leishmaniasis that burdens people in developing nations. Clinical studies showed topical PDT is safe and effective in the treatment of cutaneous leishmaniasis boils. Some results have demonstrated significantly better efficacy compared to treatment with paromomycin and methylbenzethonium chloride. Further, PDT has been shown to be effective against protozoa that were refractory to other therapies[9, 332, 335–339]. There are several studies on the efficacy of PDT for microbial killing in vitro and in vivo[332, 340–345]. Other notable results of PDT to treat protozoa and parasitic organisms are as follows: Kassab et al. reported a complete inhibition of viability of Acanthamoeba palestinensis[346]; Chen et al. demonstrated that PDT using hypocrellins B (HB) induced complete arrest of the growth stage of protozoa and cysts in a dose-dependent manner[347]; investigation from Mito et al. showed that MB-mediated PDT reduced the respiratory activity of Acanthamoeba castellanii trophozoites (i.e. growing state of protozoa) in an MB-concentration dependent manner[348]. For Plasmodium spp., two studies demonstrated that PDT treatment can induce effective inactivation and can be used as an alternative approach to antimicrobials to control the infection[349, 350]. Other evidence showed that PDT is also capable of eliminating Trypanosoma cruzi, a protozoan parasite common to Latin America causing Chagas disease[351–356]. More recent publications are also listed here[357–373].
Another therapeutic approach to prevent the spread of infectious diseases is through treatment of pathogen vectors (i.e. insects and pests). PDT has been reported to inactivate pest populations of Anopheles, which transmits malaria, and Aedes, which mainly spreads diseases like dengue fever, yellow fever, West Nile virus, eastern equine encephalitis, and Zika virus[374–379]. More recent and related publications on PDT for mosquito control are listed here[380–390]. This preventative approach is a promising technique to reduce disease transmission.
Since therapeutic resistance to conventional antimicrobials is a leading global concern, some groups have investigated the potential for pathogens to develop resistance to PDT. To date, evidence does not demonstrate induced resistance of pathogens to PDT therapy. This is likely due to the fact that ROS have various structural and metabolic targets that harm pathogens, rather than single target like conventional antibiotics, making it more difficult to develop a viable resistance mechanism[17, 18]. Lauro et al. tested whether the photosensitizing action of porphycenes leads to the selection of photoresistant cells or a change in the spectrum of sensitivity to the action of different antibiotics and no appreciable resistance and no difference in sensitivity to antibacterial drugs were found[11]. Tavares et al. reported that PDT treatment using Tri-Py+-Me-PF as a photosensitizer presented a promising approach to efficiently destroy Vibrio fischeri and recombinant Escherichia coli after a single treatment. After treatment, the microorganisms did not recover their viability and after ten generations of partially photosensitized cells neither of the bacterial species developed resistance to PDT[19]. Giuliani et al. also demonstrated that 20 consecutive antimicrobial PDT with RLP068/Cl did not result in any resistant mutants[20].
2.1.4. Challenges for PDT materials
Although PDT is a promising therapy option for infection treatment, it is still a method that can be improved. For example, PSs that absorb long wavelengths of light often necessitate large conjugated systems, which lead to strong π-π interactions and aggregation, causing a reduction in ROS quantum yield. Additionally, current PSs with a large two-photon absorption cross section (δ) that are desirable for NIR excitation and resulting increased penetration depth possess high intramolecular charge transfer (ICT)[391–393]. However, ICT usually facilitates non-radiative decay of an excited state, which strongly competes with energy transfer from PSs to molecular oxygen, and hence harms the production of ROS[394]. Furthermore, a PS that exhibits high ICT possesses a high propensity for intermolecular charge transfer, which also promotes non-radiative decay diminishing its therapeutic activity[395–398]. Moreover, large conjugated systems also increase hydrophobicity and therefore, reduce water solubility of photosensitizers limiting biological applications. Currently, poor water solubility can be improved using nanocarriers[75, 399–407]. Although AIE can enhance the ROS quantum yield in the aggregated state, the current PDT platform still suffers from poor water solubility, complicated composition, poor reproducibility, unstable, harsh and high-cost synthesis conditions. Another challenge for PDT is patient tolerability to therapy. Due to incomplete sensitivity for pathogens, ROS production and chemicals used for PSs may lead to patient discomfort or harm. There have been reports of tissue damage in vivo and minor burning at the site of treatment[9]. Hence, increasing selectivity for bacteria over host cells is another area worth attention to minimize patient harm and optimize efficacy. This calls for a deeper investigation into underlying mechanisms to instruct ideal irradiation regimens and PS designs.
2.2. Photothermal therapy
Photothermal therapy (PTT) is comprised of: photothermal agents (PTAs) and incident light. The light sources of PTT are the same as those of PDT. However, unlike photodynamic therapy, PTT is not dependent on oxygen or other substrates for efficacy. Instead, incident light interacts with PTAs to elevate the temperature of infected sites and inactivate pathogens[408]. Hence, materials that can efficiently convert light to heat energy (vibrational dissipation through ISC) are appealing candidates for PTT. Photothermal conversion efficiency (ηPT) can be calculated by equation (1):[409–411]
| (1) |
where h is the heat transfer coefficient, A is the surface area of the system, ΔTmax is the temperature difference between the maximum steady-state temperature and ambient temperature, and I is the power of the incident light. A method to calculate heat transfer of metallic nanomaterials has also been developed and requires measurement of the extinction cross sections of the samples[23, 412–415].
2.2.1. Classification and mechanisms of PTT
PTT treatment is divided into three subtypes depending on the thermal therapeutic range as follows: diathermia (<41 °C); hyperthermia (41-46 °C); and thermal ablation (>46 °C)[23, 416–420]. Diathermia is a mild treatment used for radiation and chemotherapy that sensitizes cells through increasing blood flow[23, 416]. Hyperthermia can induce protein denaturation and aggregation, loss of cell membrane integrity, and DNA cross-linking resulting in cellular dysfunction and eventual inactivation[23, 417, 418, 421]. Studies have shown that treatment with hyperthermia PTT can sensitize pathogenic cells towards heat[419], antibiotics[422] and other therapies[420]. In thermal ablation, stress caused by heat results in coagulative necrosis and irreversible damage of cells within a few minutes. Temperatures exceeding 60 °C leads to rapid necrosis of cells will occur owing to protein denaturation and enzyme inactivation[23, 416, 423].
2.2.2. Materials used in PTT
To date, several PTA candidates have been reported to achieve thermal ablation temperatures. Inorganic materials include carbon-based nanomaterials[97, 424–426], metallic nanomaterials[427–431], phosphorus-based nanomaterials[107, 110, 115, 116, 417, 432–434], metal-organic frameworks (MOFs)[114], manganese dioxide[115, 435], metal sulfides[116, 408, 436], metal peroxides[108], organic dyes[117, 437, 438], and conjugated polymers[439–441]. Major types of organic PTAs include cyanine- [112, 442–458], diketopyrrolopyrrole-[459–462], croconaine-[463–466], porphyrin-[467–470], polymer-based agents[471–481] among others[109, 482–485]. Additionally, AIE-based materials have emerged as a novel family of PTAs[148, 149, 486–496]. Many organic PSs can be viable PTAs since they can be tailored to dissipate absorbed light through internal conversion and/or intersystem crossing generating heat with or without ROS generation. The heat generation mechanisms of inorganic materials are more complicated and diverse that that of organic ones. There is a recent review by Zheng et al. detailing the primary photothermal mechanisms of inorganic materials: 1. the localized plasmon surface resonance (LSPR) effect present mainly in metals and semiconductors, metal oxides, and quantum dots; 2. relaxation of electron-hole pairs in low electron density semiconductors; and 3. ladder-like energy level of rare earth ions[23].
2.2.3. Applications of PTT for infection control
There have been plenty of successful in vitro and in vivo studies testing vast antimicrobial applications of PTT. Chen et al. and Xu et al. highlighted recent advances of nanomaterial-based PTT and its potential in antibacterial treatment[497, 498]. Fan et al. reported a metal-organic-framework (MOF)-derived 2D carbon nanosheet platform with PTT capability for localized bacterial eradication. This work achieved nearly 100% bactericidal efficiency at low concentrations while providing rapid and safe skin wound disinfection without damaging skin tissues or yielding accumulative toxicity[499]. Liu et al. developed humic acid (HuA) encapsulated zeolitic imidazole framework-8 (HuA-ZIF-8) nanocomposites. Synergistic antimicrobial action occurred through concomitant photothermal activity and zinc (Zn2+) release demonstrating excellent bactericidal efficiency against S. aureus and E. coli (i.e. 99.59% and 99.37%, respectively) upon 20 min of NIR irradiation[500]. Wang et al. proposed a synthetic, intelligent hydrogel for S. aureus and biofilm detection and treatment. The system worked by changing in color in response to pH change caused by bacteria colonization followed by treatment by achieving local hyperthermia under irradiation with a NIR laser (808 nm) that was able to penetrate biofilms[501]. Qing et al. described a smart nanostructure, Thermo-Responsive-Inspired Drug-Delivery Nano-Transporter or TRIDENT. This system was capable of fluorescence monitoring and synergistic killing of bacteria through using the photothermal effect to facilitates increased permeation of imipenem, a broad-spectrum antibiotic, into cells. The TRIDENT system showed desirable in vitro and in vivo MRSA eradication even at low doses[502]. Yan et al. reported a pH switchable nanoplatform, which was fabricated by grafting polyaniline (PANI) and glycol chitosan (GCS) onto the surface of persistent luminescence nanoparticles (PLNPs). Through persistent luminescence imaging, PTT selectively destroyed pathogenic cells due to the higher affinity of PLNP-PANI-GCS for bacterial cells as well as provide a stronger photothermal effect in acidic environments fostered by bacteria colonization. In vivo imaging-guided PTT to bacterial infection abscess showed effective treatment[503]. Liu et al. developed an enzyme-responsive delivery antibacterial system, AA-Ru-HA-MoS2. This system used mesoporous ruthenium nanoparticles (Ru NPs) as nanocarriers and loading the prodrug of ascorbic acid (AA), an antioxidant encapsulated by hyaluronic acid (HA) to combat resistant bacterial infections by combining chemical and photothermal therapies[504]. Some other representative works may also be found in the following references[119–121, 426, 505–522]. For more information regarding disinfection of surfaces using PTT, the reader is encouraged to consult the review by Zou et al.[426].
2.3. Direct light-based therapies
Certain forms of incident light and wavelengths can damage pathogenic cells without the need for exogenous compounds. These direct light illuminations provide a simple and noninvasive means to control infection. The following sections comprise overview and evidence of direct laser therapy as well as direct UV and direct blue light illumination therapies for pathogenic inactivation.
2.3.1. Direct laser therapy
“Laser” is an acronym for “light amplification by stimulated emission of radiation”. Although laser light can be used in PDT and PTT to activate PSs or generate heat, respectively, there are laser therapies that possess different inactivation mechanisms against pathogens. Ultrafast laser therapy and dual-wavelength laser therapy are two direct laser therapies that will be discussed in this section.
Ultrafast (ultrashort) lasers administer short pulses of light to achieve peak power that can reach up to gigawatts (GW) in magnitude, which is sufficient to achieve two-photon absorption[17, 523]. For infection control pulse durations are typically on the order of femtoseconds (10−13). Femtosecond laser therapy has been reported to be effective against enveloped and non-enveloped viruses[524–532], Gram-positive and Gram-negative bacteria[529], as well as fungi[533]. Proposed mechanisms of action are specific to the type of pathogen. In viruses, hydrogen bonds, hydrophobic interactions, and some covalent bonds (i.e. disulfide bonds) can be disrupted by the mechanical agitation under femtosecond laser illumination[529, 534]. Meanwhile, bacterial and fungal DNA may be damaged through two-photon absorption of visible femtosecond lasers[529]. There is evidence that supercoiled DNA in bacteria are relaxed by femtosecond lasers inducing cell death[535]. These unique mechanisms confer selective killing of targeted pathogens while leaving healthy cells unharmed[535].
For dual-wavelength laser therapy, two different wavelengths usually in the red to NIR range of laser are applied simultaneously. There are few investigations to date studying mechanisms, safety, and efficacy of this therapy[536]. Evidence suggests the efficacy of dual-wavelength laser therapy is attributed to a decrease of the transmembrane potential of respective pathogens and an increase in ROS generation resulting from optically-mediated mechanotransduction of cellular redox pathways[537]. However, it is noted that currently available studies demonstrate successful inactivation of various pathogens, such as S. aureus, MRSA, E. coli, C. albicans, T. rubrum, as well as Pantoea agglomerans in pressure ulcer model[536–538]. Remarkably, Krespi et al. reported that 870/930 nm laser treatment can re-sensitize erythromycin-resistant bacteria. The authors postulated the change in the cellular redox state due to the light therapy suppressed the activity of drug efflux pumps, a common mechanism of antimicrobial resistance[538].
2.3.2. Direct UV therapy
UV light can be divided into three spectral regions: UVA (315-400 nm), UVB (280-315 nm), UVC (100-280 nm)[539, 540]. Among them, UVC is the most effective and commonly used wavelength range for disinfection attributed to formation pyrimidine dimers after absorption by nucleic acid strands. Moreover, UVC light may also damage proteins in aromatic amino acid residues, which have maximum absorption in the UVC region. Specifically, phenylalanine has an absorption maximum at 260 nm, tyrosine at 275 nm, and that of tryptophan is near the UVC region at 295 nm[52, 539, 541].
There are several in vitro and in vivo studies demonstrated the efficacy of UVC therapy against bacterial and fungal infections as well as in species that produce biofilms[542–547]. Clinical investigations have also been completed by Freytes et al., Nussbaum et al. and Thai et al. to treat ulcers caused by MRSA[548–550]; Thai et al. also confirmed that UVC can kill other bacteria, such as P. aeruginosa, S. aureus and MRSA present in the superficial layers of chronic wounds[551]; Shimomura et al. demonstrated that UVC has potential to eliminate multiple species of bacteria and be used in prophylaxis of catheter related infections[552]; Boker et al. successfully treated onychomycosis, a fungal infection of finger and toe nails, using UVC irradiation[553]. It should be noted that induced resistance has been reported to direct UV therapy[22]. Several publications on direct UV therapy to treat COVID-19 are listed here[554–562].
Although there are several studies which indicate that short-term, UVC therapy at appropriate fluences does not induce significant damage to human cells and tissues[544, 563–567], the safety concerns of UVC therapy need to be taken into consideration prior to use in real-world applications. Since UVC can cause damage to DNA and protein, prolonged and repeated exposure to UV light can harm host cells leading to sequelae like skin cancer and burns[568, 569]. UVC can also lead to ophthalmological problems, such as photokeratitis (i.e. snow blindless or welder’s flash), cataracts, and chronic retinopathy, limiting the applications of UVC therapy in eye infections[570–573]. Inflammation may also be induced by UVC irradiation (i.e. sun burn)[574–576]. Treatment regimens that are effective with minimal exposure and direct delivery of UVC light to an infected area can mitigate damage to healthy tissue. Although delivery of UVC light using optical waveguides is a current challenge, precise delivery of therapeutic light could be a plausible solution to these safety concerns.
2.3.3. Direct blue light therapy
Blue light is typically defined as wavelengths between 400 to 500 nm having less energy than ultraviolet light[17]. Wavelengths falling in this blue light range can be further subclassified as violet, blue and cyan. Therapy predicated on blue light has drawn increasing attention due to its higher safety profile compared to UV light with relatively less photodegradation of the molecules it irradiates. As opposed to PDT, an exogenous photosensitizer is not necessary in blue light therapy due to endogenous photosensitizers (e.g. porphyrins and flavins) and blue light receptors present in microbes. Although, the mechanism of blue light therapy is not fully elucidated yet, current evidence indicates blue light therapy works similarly to PDT in which free radicals and ROS are generated after photosensitizer excitation causing subsequent damage to macromolecules[27, 577–580].
Blue light has broad-spectrum activity against both Gram-positive and Gram-negative bacteria as well as fungi in vitro and in vivo. Wavelengths between 402-420 nm have been reported to be the most effective range to inactivate bacteria. Wavelengths between 455 nm-470 nm have shown activity against S. aureus[578, 581–593]. Recently, Lu et al. reported a combination therapy of blue light and the photochemical, carvacrol, had synergistic effects to cure acute and chronic biofilm-associated A. baumannii as well as MRSA infections in third-degree burn wounds of mice. Furthermore, survival was higher in the light treatment groups P. aeruginosa skin wound infections of mice. Excellent pathogen targeting was exhibited due to the abundance of porphyrin-like molecules in bacteria[21]. Consistent with other studies, the authors did not find evidence of induced resistance after up to 20 cycles of treatment[19–21].
2.4. Light source, penetration depth and irradiation mode
Optical penetration depth (δ) is the distance at which the light intensity reduces to of the initial light intensity[594]. Multiple factors, such as wavelength, light source, and tissue physiology can affect penetration depth of light in living tissues[595]. Endogenous fluorophores, especially hemoglobin and melanin, have a strong absorption below 600-700 nm[594–596]. Therefore, NIR light (700–2,500 nm) can penetrate biological tissues more efficiently than visible light because these tissues scatter and absorb less light at longer wavelengths[594–597]. At wavelengths longer than 950 nm, however, the absorption of water and lipids increases drastically, which would adversely affect light transmission in tissue[596, 598–603]. Therefore, the region from about 600 to 1300 nm is often called the optical window of tissue[595, 597, 604–606]. However, NIR light longer than 850 nm was found to be less effective in activating PSs in practical PDT because of the relatively fast non-radiative transition resulting from the narrow energy gap and the insufficient triplet energy level[595, 604, 607, 608]. Thus, current desirable PSs should possess strong absorption at the wavelength ranging from 600 to 850 nm. There are experimental reports of penetration depths of various lights in different tissues. Ogawa and Kobuke stated based on two publications that generally, the penetration depth ranges between 0.5 and 1.5 mm at the wavelength from 480 to 600 nm and gradually improves to 4–5 mm with increasing wavelength[606]. Kim and Darafsheh reported δ < 0.5 mm at 400–430 nm, 1 mm at 500 nm, 2–3 mm at 630 nm, and 5–6 mm at 700–800 nm in most tissues[594]. Stolik et al. reported how δ changes upon varying wavelengths of red and NIR light in human ex vivo tissues[609]. Clement et al. and Ash et al. reported that red light penetrates 4–6 mm beneath the surface of the skin, blue light penetrates around 1 mm, and ultraviolet light hardly penetrates human tissue[610]. Such limited penetration depth of phototherapies largely hinders their wide applications in deep tissue.
As for the light source, take PDT as an example, no single light source is ideal for all PDT applications, even with the same PS[604]. PS absorption spectrum, location and size of lesions, and tissue characteristics should be considered when the light source of PDT was chosen[604]. Both lasers and incandescent light sources have been used for PDT and show similar efficacies[611]. Compared to the pumped dye lasers, diode lasers are smaller, more cost-effective, have facile installation, a longer operational lifetime, and are capable of automated dosing[604]. Light-emitting diode (LED) is an alternative light source with relatively narrow spectral bandwidths and high fluence rates[612, 613]. Complex dosimetry, such as total light dose, light exposure time and light delivery mode (single, fractionated and metronomic light delivery) can affect the clinical efficacy of PDT[495] and the light fluence rate also affects PDT response[614]. Integrated systems that measure the light distribution and fluence rate interstitially or at the irradiated tissue surface can inform how to adjust the light output for effective treatment. Examples of uses of various light sources for PDT treatment include, the use of lasers can be coupled into fibers with diffusing tips to reach the lesions in the urinary bladder and the digestive tract[604, 615]. Inflatable balloons can be fit into organs with strongly scattering material on the inside to evenly disperse light are also commercially available[604, 615]. Furthermore, implanting a light source in solid organs under image guidance is feasible[604]. The proper combination of PSs, light sources, and treatment parameters is crucial for an optimal PDT efficacy[616, 617].
The most commonly applied irradiation mode in phototherapy is single light delivery. The light dose can also be administered in a fractionated manner, which means there is a dosing interval (seconds to hours) between two illumination doses[618]. In phototherapies with exogeneous compounds, such as PDT and PTT, the exogenous agents may also be administered in a fragmented manner[618]. The metronomic mode refers to low doses of exogeneous agents administered concomitantly with light over several hours[618, 619]. Several publications have reported fractionated PDT mediated pathogen inactivation and explored the antimicrobial efficacy difference between fractionated and single light delivery PDT. For example, Misba and Khan observed a 6–6.5 log10 reduction of planktonic and 3.6–4.2 log10 reduction in biofilm after irradiation with fractionated light compared to continuous light administration[620]. Sampaio et al. demonstrated that MB-mediated PDT was efficient to achieve total microbial load reduction in both fractionated and continuous modes, but in fractionated mode it was possible to use a lower light dose[621]. The metronomic mode has been tested in a preclinical brain tumor model with PDT[619] but not for antimicrobial applications to date. Both in vitro and in vivo results from the preclinical brain tumor model showed enhanced induction of apoptosis of cancer cells compared to acute, high-dose PDT (aPDT) and it worth to study the feasibility and efficacy of metronomic mode in antimicrobial phototherapy.
3. Current challenges and considerations in phototherapy
Amidst the multitude of therapeutic mechanisms and promise of phototherapy for infection control, there remains inherent limitations and considerations that can be found in Table 1. Main drawbacks common to each of the phototherapies include limited penetration depth, limited working wavelength ranges, incomplete selectivity for pathogenic cells, and off-site effects. Illumination treatment should be simple, fast and effective to facilitate patient adherence and practicality in the clinical setting.
Table 1.
Types of phototherapy and respective limitations
| Phototherapy | Description | Mechanism of Action | Limitations |
|---|---|---|---|
| PDT | Light activates a topical or injected PS in the presence of a substrate produce ROS and radical ions[50, 51] | ROS and free radicals destroy macromolecules critical to cell function[54, 55] | ROS are not cell selective[51, 57] |
| Poor biocompatibility or phototoxicity of PSs[23, 73, 74, 77, 623, 624] | |||
| Thermal effects are not cell selective[408] | |||
| PTT | Irradiation of photothermal agents (PTAs) with light | Local hyperthermia leads to denaturation of proteins, cell aggregation, loss of membrane integrity, and cross-linking of DNA[408] | Photobleaching, poor biocompatibility or toxicity of PTAs[408, 426, 437, 497, 583] |
| High temperatures, power density, or overexposure to light irradiation may damage host tissue | |||
| Induced resistance has been reported[23–25] | |||
| Direct Ultrafast (Ultrashort) Pulsed Laser | Ultrafast pulses of light with power on the order of GW. Femtosecond lasers are the most commonly used[535] | Viruses: Induced viral capsid aggregation and inhibition of viral replication and transcription[534, 535] | Requires specialty equipment and training |
| DNA damage and relaxation of supercoiled DNA in bacteria and fungi[535] | |||
| Activation of endogenous porphyrins to generate ROS[535, 637] | |||
| Increase in cell membrane permeability increasing sensitivity to drug treatment[637] | |||
| Direct Dual-Wavelength Laser | Two different wavelengths of laser are applied to infected site. Low power and red/NIR light can be used[536] | Reduced membrane potential[537] | Requires specialty equipment and training |
| ROS production by endogenous chromophores[537] | |||
| Aberrant electron transport chain processes and energy production[536] | Mechanism of action, safety, and efficacy are largely underexplored | ||
| Direct UV | Direct light (100 - 400 nm) irradiation. UVC (100 - 280 nm) is the most commonly employed[22, 539, 541] | Inhibited DNA replication due to formation of thymine dimers[539] | Limited tissue penetration depth[22] |
| Risk of carcinogenesis, burns, and retinal damage upon prolonged exposure[539] | |||
| Possible damage to aromatic amino acids in proteins[638] | Induced resistance has been reported[22] | ||
| Direct Blue Light | Direct light (400 - 500 nm) irradiation. An aerobic environment may be necessary[578] | May activate endogenous PSs (e.g. porphyrins, flavins, cytochromes) in microbes to generate ROS[27] | Poor tissue penetration[27] |
| Risk of eye damage upon prolonged exposure[27] |
PDT: photodynamic therapy; NIR: near-infrared; ROS: reactive oxygen species; PS: photosensitizer; UV: ultraviolet; GW: gigawatts; PTT: photothermal therapy; PTA: photothermal agent; DNA: deoxyribose nucleic acid
Many of the drawbacks of PTT mirror that of PDT. PDT and PTT are both non-selective techniques that may target not only pathogenic microbes, but also microbes that are symbiotic with the host. By guiding light to the exact infected site, which is feasible with optical waveguides, collateral symbiotic flora and healthy tissue damage can be minimized. There are some reports on active-targeting nanoparticle systems or platforms with conjugated binding components so that PSs and PTAs actively bind to the target cells and can be only activated by specific wavelength of light illumination, which makes PDT and PTT safer and more accurate[622]. Additionally, PSs and PTAs may have inherent chemical toxicity or poor biocompatibility, which can lead to local irritation or damage[23, 77, 408, 426, 437, 497, 623, 624]. Moreover, patients exposed to sunlight or other strong light sources after receiving PDT or PTT treatment may potentially acquire over exposure and activation of PSs or PTAs[623, 624]. In terms of resistance, some studies have reported induced microbial resistance to PTT treatment within the hyperthermia temperature range via high expression of heat shock protein, a molecular chaperone that repairs thermal damage to cells[23–25]. Therefore, when designing materials, it is important to consider the resultant treatment temperature, the possibility of induced resistance in response to PTT treatment, and to consider measures to combat the possibility of induced resistance and minimize thermal damage to host tissue.
Regardless of wavelength, power, and source of light is used, light will inevitably be attenuated due to absorption by and scattering within tissues. The epidermal, dermal, and hypodermal layers of human skin have respective thicknesses of roughly 0.1 mm, 1.5-2 mm and 7-20 mm[625–627]. Light must be able to penetrate at least this much for superficial infections and more for infections beneath the skin. However, the penetration depth of 480 to 600 nm light generally ranges between 0.5 to 1.5 mm, and even longer wavelengths of light that have a higher penetration depth can only travel a couple millimeters into mammalian tissues[595, 604, 606, 628–630]. Multi-photon excitation techniques can help increase penetration depth, but still fall short of biomedical and clinical demands[606, 631–633]. Moreover, multi-photon excitation techniques necessitate extensive training, large instrumentation and delicate handling for proper operation making implementation into the clinical environment difficult.
Access to difficult-to-reach areas of the body may also be limited without the guidance of therapeutic light. In efforts to overcome limited penetration depth and off-site effects of phototherapies, optical waveguide technology can be used to precisely deliver phototherapy to the infection site[210, 578, 634–636]. The different types of phototherapies for infection treatment are illustrated in Fig. 3.
Fig. 3.
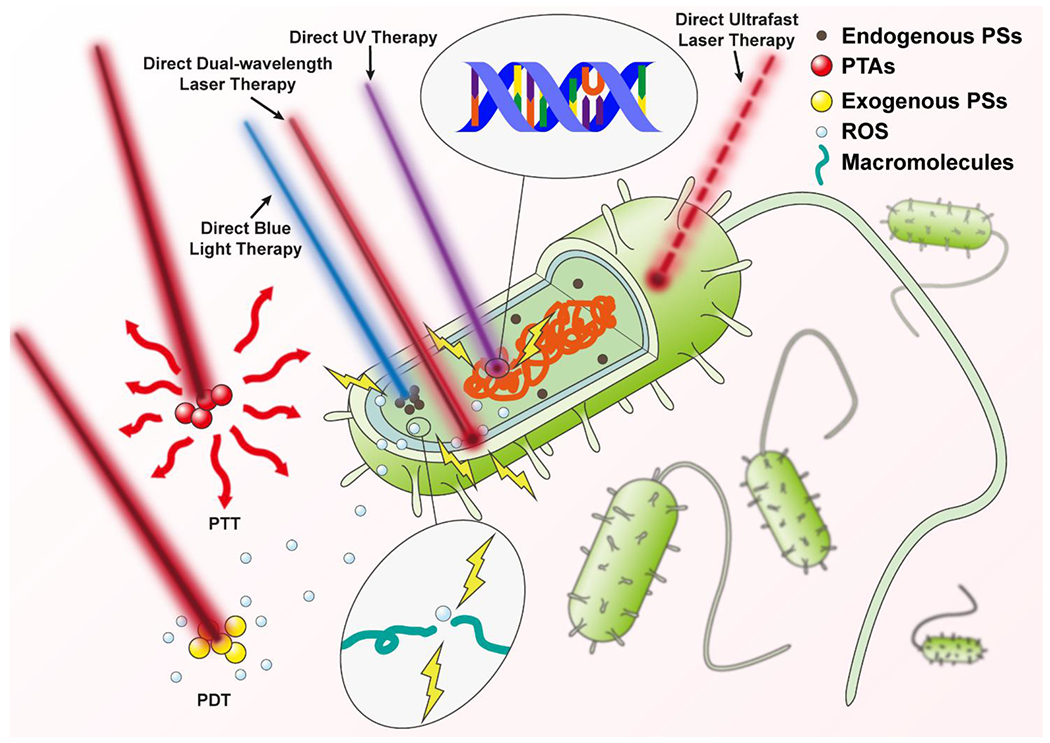
Schematic illustration of different types of phototherapies for infection treatment. From left to right: Photodynamic therapy (PDT) activates exogenous photosensitizers (PSs), which interact with substrates to produce reactive oxygen species (ROS) that damage biomacromolecules[54, 55] (the colors used in the picture do not directly reflect the actual wavelengths); Photothermal therapy (PTT) relies on the illumination of photothermal agents (PTAs) to generate heat for pathogen inactivation[408] (the colors used in the picture do not directly reflect the actual wavelengths); Direct blue light therapy is believed to activate endogenous PSs to yield similar effects to that of PDT[27]; Direct dual-wavelength laser therapy may reduce membrane potential, activate endogenous PSs, and alter cellular respiration[536]; Direct ultraviolet (UV) therapy induces dimerization of thymine nucleotides preventing DNA replication and translation[539]; Direct ultrashort laser therapy delivers rapid-pulses of light providing microbe specific damage[534, 535].
4. Optical waveguides
4.1. Basic principles and material requirements
As the name indicates, optical waveguides direct the propagation of particular wavelengths of light to a desired destination. The geometries of optical waveguides vary including fibers, films, strips, slabs, and microneedles[37–44]. Step-index optical fibers, which possess discrete differences in the refractive indices at the interface of constituent materials are the most prevalent of the optical waveguide design used in biomedical applications and infection control due to high mechanical flexibility and ability to guide light over long and variable distances without significant losses. Therefore, the following section will focus on the basic principles and applications of step-index optical fibers in conjunction with phototherapies to treat infection.
The refractive index (n) describes how fast light travels through a material relative to the speed of light in vacuum and is a material-specific property:
| (2) |
Here, n is refractive index (RI), c is the speed of light in vacuum (3.0×108 m/s), and v refers to the speed of light through a certain medium. Snell’s Law describes the behavior of incident and refracted light at an interface by the given equation (3):
| (3) |
Where n1 and n2 are the RIs of medium 1 and 2, respectively, θ1 is the angle between incidence and the normal and θ2 is the angle between refraction and the normal. If n1 > n2, then sinθ1 < sinθ2 and θ1 < θ2. As θ1 increases, θ2 will follow. Refraction will disappear once θ2 reaches 90° and the incident light will only be reflected. The corresponding angle for θ1 is known as the critical angle (θc) and this phenomenon is described by total internal reflection (TIR) (Fig. 4). The core principle of optical waveguides is TIR (apart from photonic crystal fibers (PCFs) and holey fibers (HFs) which are predicated on the photonic bandgap effect). To achieve TIR, the RI of the inner layer of the waveguide must exceed that of the outer cladding layer or medium (i.e. air)[37–39, 41–44]. The most common approach to meet the RI requirements conducive to TIR is to fabricate a concentric cylinder structure with two layers. The inner layer is where incident light traverses and is referred to as the core, while the outer layer is referred to as the cladding (Fig. 4). There are non-cladding types of optical waveguides that treat the external environment as the outer layer to the core. However, waveguide designs without cladding come at the cost of increased risk for optical loss and sensitivity to the surrounding environment compared to cladded optical waveguide designs[45, 639, 640].
Fig. 4.
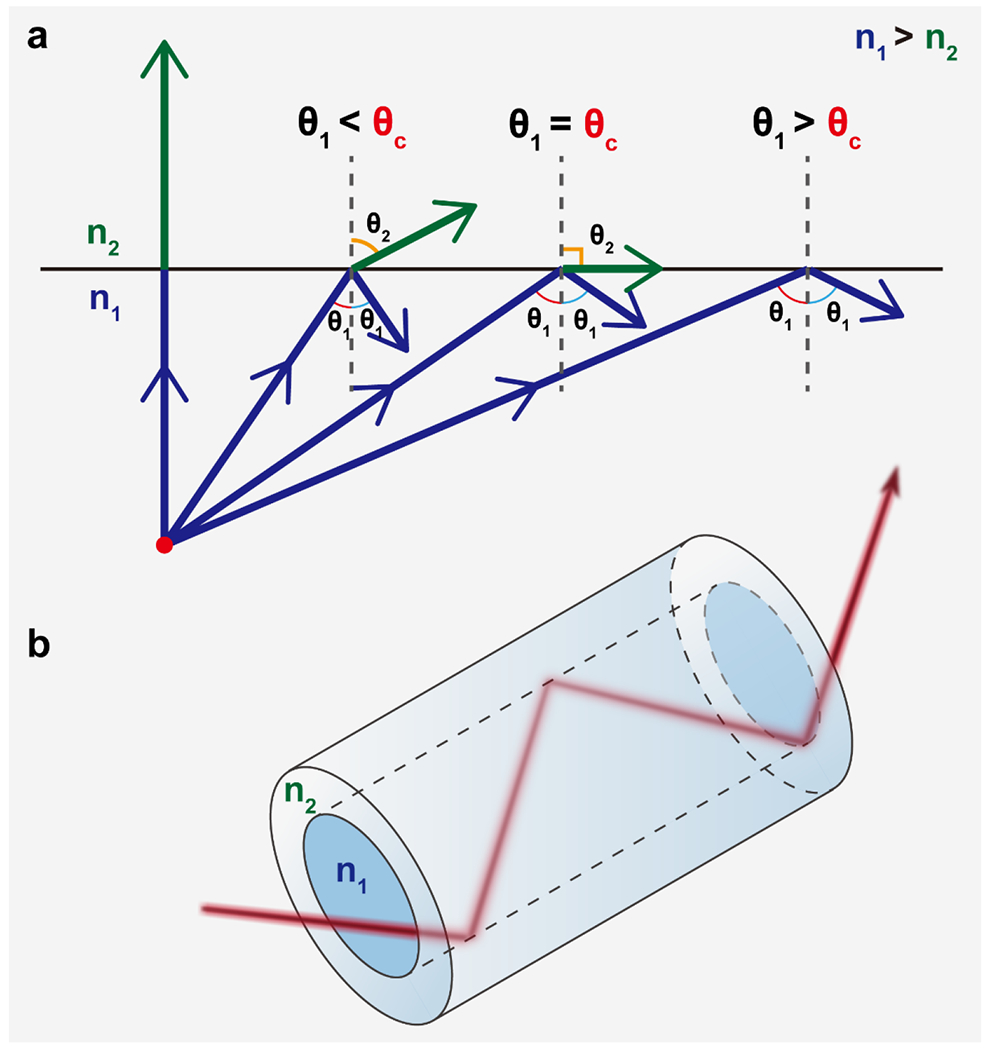
Schematic illustration of the (a) dependence on incident light angle and refractive index to achieve total internal reflection and (b) light propagation through a step-index optical fiber via total internal reflection resulting in minimal energy loss over the length of the fiber. θ1: incident angle; θ2: transmitted angle; θc: critical angle; n1 and n2: refractive indices of media 1 and 2, respectively.
4.2. Materials for optical waveguides used in biomedical applications
A viable optical waveguide should have the ability to transmit light over variable distances with low optical loss over a broad range of therapeutic wavelengths. The refractive index of the core material should always be larger than that of cladding material over the working wavelength range to keep light attenuation low. Core materials should also minimally absorb the incident light to prevent losses during propagation and it is additionally desirable for materials to have optical clarity[641–648].
Conventional optical waveguide materials include glass, plastic, crystal, semiconductors, ceramics, and metals[45, 649–651]. However, these materials suffer from poor biocompatibility, non-degradability, poor tunability of properties, and noncompliance with mechanics of most body tissues. Taken together, conventional optical waveguide materials are poor candidates for waveguides in the clinical setting[45]. Since of the first biomedically compatible waveguides composed of PMMA was developed for dental illumination in 1939 many researchers have been developing optical waveguide materials and designing optical waveguides to achieve improved biocompatibility, degradability, chemical functionality, and desirable mechanical properties, and tunability of each with sufficient light-guiding performance relative to conventional waveguides for biomedical use[45, 652–657].
Several developed optical waveguides composed of various biocompatible materials that may be used to deliver phototherapy can be found in Fig. 5. Biomaterial candidates can be categorized according to their origin (i.e. natural or synthetic) and properties (i.e. mechanical, degradability, hydrophilicity, etc.). Natural materials, such as agar[658], gelatin and agarose[659], silk and silk proteins[639, 660, 661], have been processed to perform as biocompatible optical materials. Even mammalian and bacterial cells have been used to fabricate as optical waveguide biomaterials. Muller cells were found to serve as optical fibers helping image projection through retinal tissue with less image distortion and light scattering loss[662, 663], and E.coli was reported to also form functional optical waveguides[664, 665]. Synthetic materials have reduced batch-to-batch variability and less inherent antigenicity compared to that of natural materials[666–668]. Furthermore, synthetic biopolymers have the benefits of tunable properties that can be done through adjusting composition (i.e. ratios of constituent monomers or polymer(s), compositing, chemical conjugation, etc.), polymer concentration, molecular weight, functional groups, and degree of crosslinking. Hydrogels (i.e. superabsorbent polymers that absorb as much as 90% of their weight in water), elastomers, and biodegradable or nonbiodegradable polymers may all be synthetic or natural in origin[669]. Polydimethylsiloxane (PDMS), for example, is a synthetic elastomer that is bioinert and non-toxic, but is not biodegradable. Although not used in clinical setting yet, a biodegradable optical waveguide may be beneficial as it evades the need for a secondary surgery for removal and the associated risks. Among biodegradable polymers for optical waveguides, polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic) acid (PLGA), and silk fibroin are some of the most used and successfully developed materials in tissue engineering[639, 670–674]. More recently, citrate-based polymers, a new class of synthetic biodegradable polymers exhibit great potential for optical waveguide applications. Citric acid, a Krebs cycle intermediate, is the core chemical used in the citrate-based polymer syntheses, through which various biodegradable elastomeric polymers can be synthesized by reacting citric acid with different diols and/or amino acids via a facile polycondensation reaction. Differing from the natural polymers as silk or the aforementioned traditional synthetic degradable polymers that usually have limited tunability, citrate-based polymers are advantageous due to their ultrafine tuning refractive index (~103), mechanical strengths (from tens of Pascal to mega Pascal), degradation rates (from a few days to over a year), antibacterial and anti-inflammatory effects in addition to excellent biocompatibility[666, 675–695]. Furthermore, due to the abundant carboxyl and hydroxyl groups in the polymers, citrate-based polymers are easily functionalized for drug tagging, imaging, sensing, or to bear desirable properties, such as electrical conductivity and luminescent properties. Therefore, citrate-based polymers are an ideal class of biodegradable polymer platform for the design of multifunctional devices promising for versatile biomedical applications such as tissue engineering (blood vessel, bone, nerve, skin etc.), wound healing, theranostic cancer nanomedicine, and biosensing[666, 675–702]. Among the citrate polymers, poly(octamethylene citrate) (POC) synthesized by reacting citric acid and 1,8-octanediol has been used to develop a number of FDA-cleared biodegradable interference screws and suture anchors such as Citrelock™, Citrefix™, and Citrespline™ for various orthopedic indications including knee, foot and ankle, shoulder, elbow, and wrist applications[667, 668]. To tune the refractive index of POC, another citrate polymer, poly(octamethylene maleate citrate) (POMC) was synthesized by partially replacing citric acid with maleic anhydride in synthesis. Although there is only a minor difference in the chemical structure between POC and POMC, POMC possesses a higher refractive index over a broad range of wavelengths from 300 nm to 1000 nm with an index difference of ~0.003, similar to that between the cladding and the core of silica optical fibers. Both POC and POMC have relatively low absorption (<0.13 dB/cm) at visible and near-infrared wavelengths. A flexible step-index optical waveguide was fabricated using POC as the cladding while POMC as the core that is promising for organ scale light delivery and collection[653].
Fig. 5.
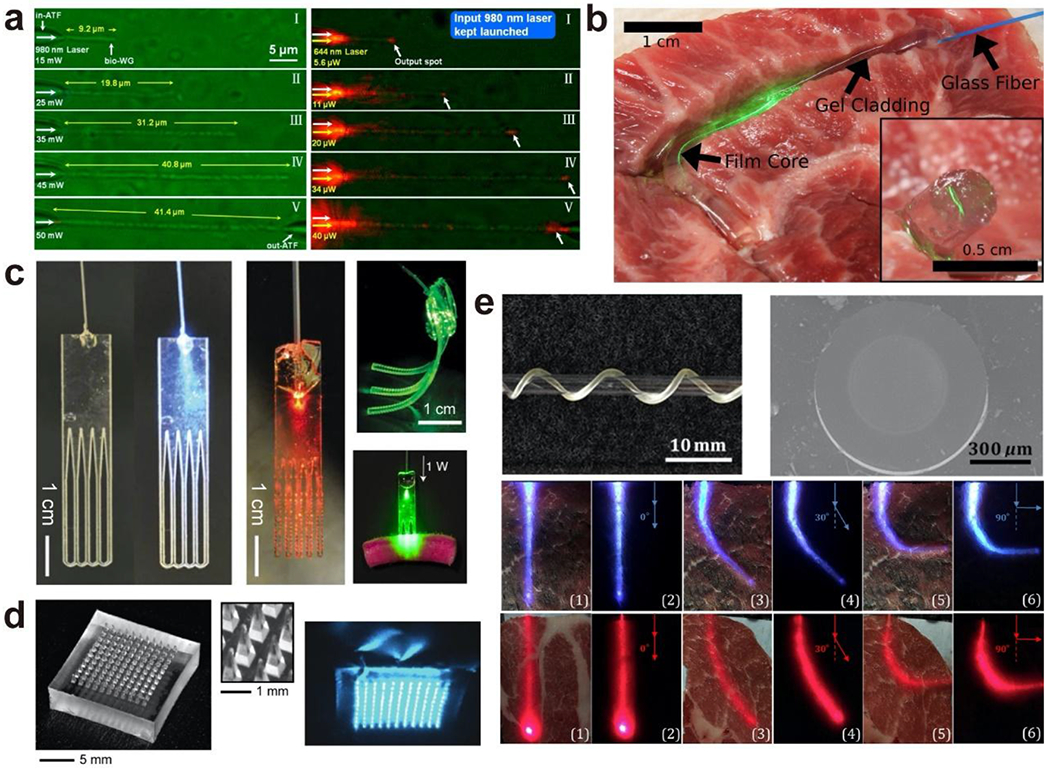
Representative biocompatible optical waveguides comprised of cells and polymeric biomaterials (a) E. coli-based biophotonic waveguide. Adapted with permission from [665]. Copyright (2013) American Chemical Society; (b) Step-index optical waveguides made from silk. Adapted with permission from [639]. Copyright (2015) Optical Society of America; (c) Bioabsorbable optical waveguides made from poly(l-lactic acid) (PLLA) or polyethylene glycol (PEG). Adapted with permission from [656]. Copyright (2016) Nature Publishing Group; (d) Optical microneedle array made from poly(lactic acid) (PLA). Adapted with permission from [655]. Copyright (2016) Optical Society of America; (e) Biodegradable step-index optical fiber made from citrate-based polymers: poly(octamethylene citrate) (POC) as cladding while poly(octamethylene maleate citrate) (POMC) as core. Adapted with permission from [653]. Copyright (2017) Elsevier.
One of the future trends will be to develop novel optical biomaterials with multi-functionality and stimuli-responsive properties to facilitate more practical and versatile clinical applications in which they can be used. For more extensive reviews on biomaterials used for optical waveguides and biophotonic applications, the reader is invited to peruse the following publications: [45, 703, 704].
4.3. Existing works on phototherapies delivered via optical waveguides to treat infection
Use of optical waveguides with phototherapy permits more freedom to vary multiple treatment factors than phototherapy alone. For example, total energy delivery, rate of energy delivery, penetration depth, and location can be tuned with optical waveguides[618]. Besides, implantable optical waveguides, integrated with sensing and/or imaging modules enables easier and more accurate post-treatment and real-time assessment. The use of optical waveguides for biomedical applications has established and investigational uses in skin imaging, scar analysis, biosensing, cancer ablation, and deep tissue photomedicine (Fig. 5)[655–657]. However, attempts to combine the optical waveguide and phototherapy for treatment of infections at present is underexplored and results implicating efficacy are mixed leaving a large unmet need for research to optimize this therapy alternative. By using optical waveguides to deliver phototherapy for infection treatment, minimally attenuated light is guided through the center of the waveguide to the target area to circumvent the physical barriers with minimum power loss and necessary penetration depth. Optical waveguides can precisely deliver phototherapy to deep tissue infections (i.e. respiratory, gastrointestinal tract) and to photosensitive regions that off-site exposure would be otherwise harmful (i.e. eye area, use of high energy light) (Fig. 6). The use optical waveguides to deliver shorter wavelengths for already available PSs and PTAs activated by UV to visible wavelengths of light could expand the optical range of PDT and PTT in treating various infections not achievable previously by PDT or PTT alone[7]. Moreover, optical waveguides that deliver phototherapy may be designed to possess multifunctionality and/or stimuli-responsive activity is an emerging area of research that provides additional therapeutic utility to standard phototherapy treatment. For example, many current research works are focused on the designs and applications of optical waveguide for pathogen sensing[634, 705–709]. The Seok Hyun Yun group demonstrated for the first time efficacy of a cell-containing optical waveguide composed of a polyethylene glycol (PEG)-based hydrogel to achieve in vivo cell-based sensing and therapy[710].
Fig. 6.
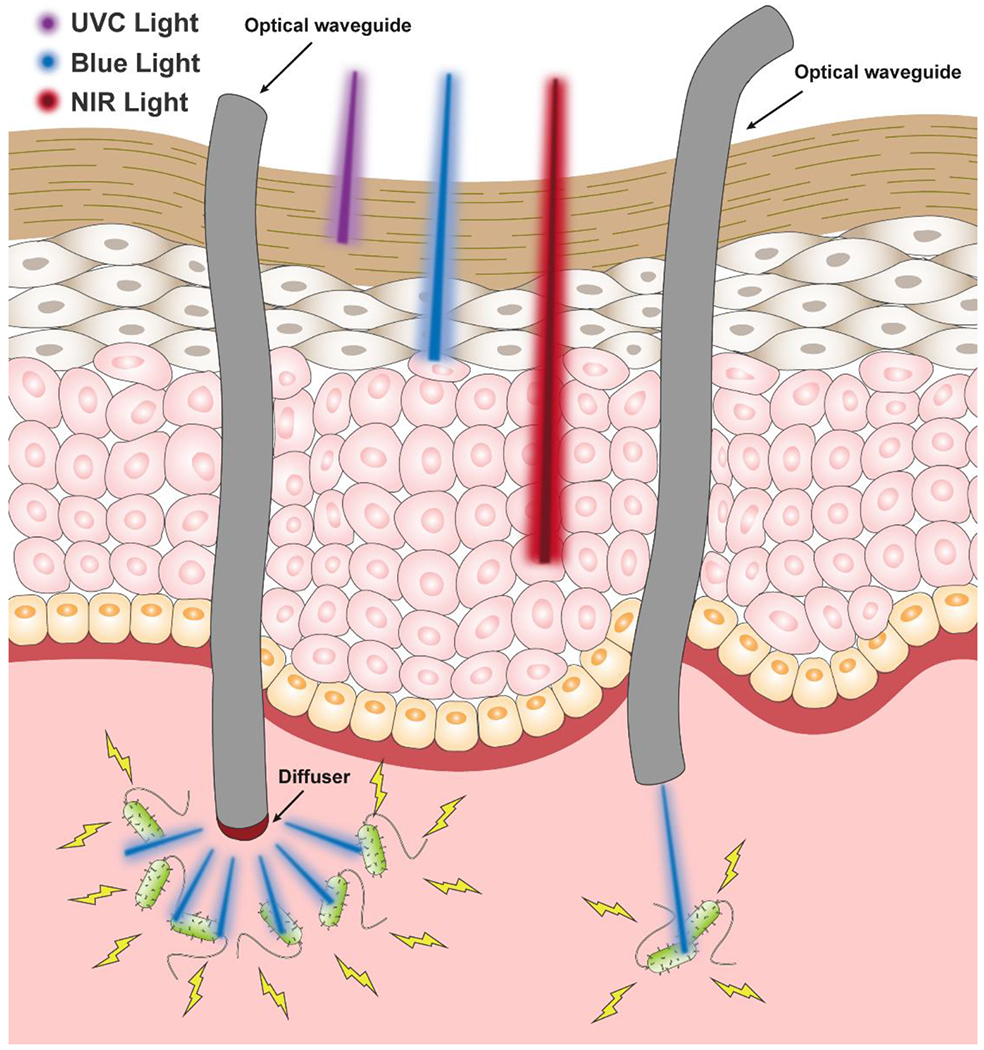
Schematic illustration of the penetration depths of various therapeutic lights (UVC, blue, and NIR) in tissues and the optical waveguide-mediated light delivery for phototherapy. Left: diffused light delivery through a diffuser/diffusing tip at a large lesion; Right: precise light delivery at a specific target site.
Representative examples delivering phototherapy by optical waveguides are detailed here and can be found in Table 2. Bisland et al. proposed PDT as an alternative treatment to antibiotics for osteomyelitis using a bioluminescent strain of biofilm-producing S. aureus grown on kirschner wires (K-wires). The S. aureus-coated K-wires were exposed to methylene blue (MB) or 5-aminolevulinic acid (ALA)-mediated PDT either in vitro or following implantation into the tibial medullary cavity of SD rats. S. aureus infections were subject to PDT for 10 days post inoculation. 300 mg kg−1 of ALA was administrated intraperitoneally followed by percutaneous light illumination (635 ± 10 nm; 75 J cm−2) 4 hours later via an optical fiber placed onto the tibia. This treatment resulted in significant inhibition of bacterial growth[711]. For treatment of dental infections, PSs can be deposited into dental pockets followed by direct irradiation with light noninvasively via optical fibers. This procedure can be usually completed in a few minutes giving optical waveguide PDT therapy a significant advantage over treatment with antiseptics and antibiotics that have difficulty reaching sufficient concentrations in the infected dental area, longer treatment times, and systemic side effects[27, 712]. Kömerik et al. reported effective inactivation of Porphyromonas gingivalis by applying up to 48 J of a 630 nm laser light via an optical fiber in the presence of toluidine blue[210]. Lee et al. used a phenothiazinium-based PS and delivered low-intensity red laser light via an optical fiber with a diffuser to access the root canal lumen to treat Gram-positive bacteria without damaging the host tissues[636]. Soukos et al. performed antimicrobial PDT with E. faecalis using methylene blue (25 μg/mL) for 5 min followed by exposure to 30 J/cm2 of 665nm light delivered by an optical fiber with multiple cylindrical diffusers that uniformly distributed light over 360° to achieve a 53% killing rate, which was then increased to 97% after increasing the power to 222 J/cm2[713].
Table 2.
Representative studies administering phototherapy via optical waveguides for infection treatment
| Infection Model | Treatment | Results | Reference |
|---|---|---|---|
| Osteomyelitis induced by S. aureus in tibia of SD rats | In vivo: Percutaneous administration of 635 ± 10 nm laser (fluence of 75 J/cm2) at 250 mW/cm2 for 4 hours post-IP injection of ALA (300 mg/kg) | Significant inhibition of bacterial growth after 24 hours | [711] |
| No significant difference after 48 hours compared to ALA treatment only | |||
| Human root canals infected with E. faecalis | Ex vivo: Methylene blue (25 μg/mL) incubation for 5 minutes followed by illumination with 665 nm light (fluence 222 J/cm2) | 97% killing after 7 days of sample incubation relative to no treatment controls. | [714] |
| In vivo and clinical H. pylori infection | In vivo: Several 15-minute treatments of 31 to 46 kJ of 408 nm light to porcine stomachs | Clinical results revealed transient decrease in infection 8 hours after treatment according to UBT | [715] |
| Clinical: 15, 30, 45, or 60-minute treatment time; UBT and bacterial counts were evaluated at time of enrollment, 5 days, and 5 weeks after treatment, respectively. | CFU reduction was highest and only significant in the antrum (97.7% killing) | ||
| The highest reduction in microbe count was observed in the 30-minute treatment group | |||
| In vitro and clinical H. pylori infection | In vitro: 2 cm2 spot size of 405 nm light (4, 8, 16, and 32 J/cm2 fluence) for 5 minutes | In vitro: 32 J/cm2 energy density caused a 5-log reduction of bacterial viability 7 days post-treatment | [585] |
| Clinical: 1 cm2 spot size of 405 nm light (40 J/cm2 fluence) for 4.5 minutes in the antrum | Clinical: CFUs/gram of tissue demonstrated significant (91%) bacterial kill relative to non-treated samples | ||
| Periodontitis induced by P. gingivalis in SD rats | 6, 12, 24, 48 J of 630 nm light for 1, 2, 4, and 8 minutes with toluidine blue (0.01, 0.1, and 1 mg/ml) for bacterial kill measurements | No detectable bacteria in 1 mg/mL toluidine blue with light treatment group and significant reductions (at least one log10) in viable count of all other treatment groups. | [210] |
| 48 J of 630 nm light was administered for 8 min using toluidine blue (0.01, 0.1, and 1 mg/ml) for bone loss measurements | Significant differences in reduction in bone loss in 0.1 and 1 mg/mL toluidine groups using 48 J of light | ||
| Clinical COVID-19 pulmonary infection | Blue and/or red light administered via endobronchial or pulmonary arterial routes proposed for antiviral, antibacterial, anti-inflammatory, and vasculoprotective effects | Proposed study design | [311] |
| Intubated patients with COVID-19 pulmonary infection and/or acute respiratory distress syndrome | Direct trachea-bronchial UVC irradiation (2.0-2.5 mW) for 6 minutes every 4 hours for 24 hours | Proposed study design | [561] |
S. aureus: Staphylococcus aureus; SD: Sprague-Dawley; IP: intraperitoneal; ALA: 5-Aminolevulinic acid hydrochloride; E. faecalis: Enterococcus faecalis; InGaN: indium gallium nitride; UBT: urea breath test; CFU: colony forming unit; P. gingivalis: Porphyromonas gingivalis
Furthermore, optical waveguide-mediated delivery of phototherapy may be a potential treatment option for Helicobacter pylori infection in the stomach, but still demands further research to optimize treatment conditions for sustained eradication. Lembo et al. were the first ones to report in patients infected with H. pylori, the whole stomach can be safely illuminated with violet light. In this study, a novel light source consisting of laser diodes connected to diffusing fibers to deliver 408 nm light to yield a significant antibacterial effect[716]. Moreover, Ganz et al. delivered violet light (405 nm, 40 J/cm2) to 1 cm diameter spot-size in the gastric antrum via an optical fiber to achieve inactivation of H. pylori[585]. Kipshidze et al. recently proposed to direct energy delivery through an endoluminal microcatheter with an illuminating balloon containing a diffusing fiber-optic array at its distal tip capable antiviral, antibacterial, anti-inflammatory, and vasculoprotective properties of the blue and red wavelengths of light[311]. Khan et al. developed an optical fiber-based photonic crystal fiber sensor for THz sensing of COVID-19 disinfecting products[323]. Stawiki proposed to treat COVID-19 by using diffusive optical fibers to deliver UVC light with repeated short-term tracheobronchial illumination[561]. Shan et al. developed the aforementioned biodegradable, citrate-based step-index optical fibers with low optical loss of 0.4 dB/cm. A proof of concept study using a 20 mW 532 nm laser in Sprague-Dawley (SD) rats was conducted to demonstrate a strong potential for active optical waveguide mediated-phototherapy for infection treatment[653].
4.4. Challenges and considerations in phototherapy delivered by optical waveguides for infection treatment
Although combining optical waveguide techniques with phototherapy shows promising efficacy for infectious diseases, there are some drawbacks to their unique advantages. Firstly, optical waveguides deliver the light locally meaning an invasive approach and incisions to the site of deep tissue infections that are not easily accessible are typically necessary[639, 656, 661]. To minimize invasiveness, microneedles have been used to penetrate skin to guide light into tissues[655, 717]. However, the penetration depth was still limited. For deep tissue light delivery, traditional non-biodegradable optical waveguides need to be removed from the body after treatment. By replacing the traditional optical waveguides with biodegradable counterparts, the removal step may be evaded. The biocompatibility of optical waveguides, its respective degradation products if degradable, and PSs and PTAs used should all be considered to minimize the risk of adverse host responses. Regarding the optical waveguide material design, it is imperative to be mindful of the desired operational wavelengths, area of treatment, and tissue mechanics for the design and fabrication of effective optical waveguides.
5. Outlook and perspectives
Since antibiotic resistance is becoming increasingly serious with the emergence of “super bugs” refractory to conventional therapies, the development of alternatives or combinational therapies is critical to combat infectious diseases. Namely, phototherapy appears to be a promising approach for the local infection treatment and prophylaxis due to its broad-spectrum antimicrobial and antiviral activities, low invasiveness, minimal systemic side effects, and no evidence of inducing treatment resistance (except direct UV therapy and PTT). Phototherapies reviewed in this paper include photodynamic (PDT), photothermal (PTT), direct laser, direct UV and direct blue light therapies. These modes of therapies have been combined with optical waveguide technologies to target a variety of pathogens through multiple mechanisms with access to deep or normally inaccessible infections and overcome the off-site effects that are experienced by phototherapies alone. It is likely that the biodegradable optical waveguides may also be loaded with drugs as a drug delivery platform. After the phototherapy treatment, as the optical waveguide degrades, the drugs may be released in a designed manner, either enhancing the therapeutic efficacy or facilitating the healing process. Moreover, the combination of optical waveguides with PDT and PTT should enable the use of PSs and PTAs that are responsive to lights at UV regions for deep tissue treatments. PSs and PTAs that are only UV-light responsive are usually smaller and less conjugated than their NIR-responsive counterparts resulting in better solubility, processability, biocompatibility and stability.
Another area at the forefront of research is stimuli-responsive and multi-functional optical waveguide-mediated phototherapies for more precise targeting and treatment. Phototherapy can be combined with drug delivery options as well as with other nonpharmacologic treatment modalities, such as sonodynamic therapy. Phototherapy combined with sensing and diagnostic techniques can potentially be developed into point-of-care theranostics that enable feedback-informed treatment through monitoring the treatment progression in real-time. The development of biodegradable optical waveguide materials with tunable refractive indexes, biomimetic mechanics, flexibility and multifunctional or self-cleaning potential is a promising direction for the design of new optical waveguide-mediated phototherapies.
Furthermore, an underexplored phenomenon in the field of optical materials that is gaining increasing attention is organic ultralong room temperature phosphorescence (URTP)[718–722]. Thus far, materials with URTP capabilities have been studied for applications, such as anticounterfeiting, data encryption and bioimaging investigations[722–728]. However, the biomedical applications of URTP materials haven not been extensively studied other than some bioimaging applications. The ultralong triplet lifetime of URTP materials confers higher probability of energy/electron transfer compared to the traditional PSs making them appealing for PDT. Some possible reasons that URTP materials have not been exploited for PDT applications include: 1. The absorption maximum of the current URTP materials is always in the UV range with few reports in the violet range thus it is not suitable for deep tissue treatment by itself; 2. Crystallization is the most common and effective approach to achieve URTP. Unfortunately, crystals that have compact structure prevents the diffusion of the quencher (e.g. molecular oxygen), which attenuates energy/electron transfer to substrates resulting in poor ROS generation efficiency. There should be a niche area for the use of URTP materials if combined with optical waveguides. Optical waveguides may allow the delivery of shorter wavelengths that activate URTP materials in deep tissues for phototherapies . With such long lifetimes and high triplet quantum yield, PDT in conjunction with URTP materials loaded in the optical waveguides has great potential to further improve the efficacy of phototherapy for infection control.
The use of phototherapies in combination with optical waveguides overcome many of the challenges and shortcomings of the conventional pharmaceutical and phototherapies. Optical waveguide-mediated phototherapies have gained significant attention as a powerful therapeutic approach or alternative to the current antimicrobial and antiviral therapies and holds great promise for the management of the infection-related diseases and beyond.
Acknowledgements
This work was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) award (AR072731), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) award (DK123949).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Dr. Jian Yang and The Pennsylvania State University have a financial interest in Acuitive Technologies, Inc. and Aleo BME, Inc. These interests have been reviewed by the University’s Institutional and Individual Conflict of Interest Committees and are currently being managed by the University.
References
- [1].Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJV, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI, Tackling antibiotic resistance, Nature Reviews Microbiology, 9 (2011) 894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].(2020, July 20). Retrieved April 13, 2021, from https://www.cdc.gov/drugresistance/index.html.
- [3].Vlamakis H, Kolter R, Biofilms, Bacterial Stress Responses, (2010) 365–373. [Google Scholar]
- [4].Hall-Stoodley L, Costerton JW, Stoodley P, Bacterial biofilms: from the natural environment to infectious diseases, Nature reviews microbiology, 2 (2004) 95–108. [DOI] [PubMed] [Google Scholar]
- [5].Stewart PS, Costerton JW, Antibiotic resistance of bacteria in biofilms, The lancet, 358 (2001) 135–138. [DOI] [PubMed] [Google Scholar]
- [6].Lewis K, Riddle of biofilm resistance, Antimicrobial agents and chemotherapy, 45 (2001) 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP, All you need is light, Virulence, 2 (2011) 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alekshun MN, Levy SB, Targeting virulence to prevent infection: to kill or not to kill?, Drug Discovery Today: Therapeutic Strategies, 1 (2004) 483–489. [Google Scholar]
- [9].Dai T, Huang Y-Y, Hamblin MR, Photodynamic therapy for localized infections—state of the art, Photodiagnosis and photodynamic therapy, 6 (2009) 170–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hamblin MR, Hasan T, Photodynamic therapy: a new antimicrobial approach to infectious disease?, Photochemical & Photobiological Sciences, 3 (2004) 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lauro FM, Pretto P, Covolo L, Jori G, Bertoloni G, Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene–polylysine conjugates, Photochemical & Photobiological Sciences, 1 (2002) 468–470. [DOI] [PubMed] [Google Scholar]
- [12].Kharkwal GB, Sharma SK, Huang YY, Dai T, Hamblin MR, Photodynamic therapy for infections: clinical applications, Lasers in surgery and medicine, 43 (2011) 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tim M, A New Strategy to Destroy Antibiotic Resistant Microorganisms: Antimicrobial Photodynamic Treatment, Mini-Reviews in Medicinal Chemistry, 9 (2009) 974–983. [DOI] [PubMed] [Google Scholar]
- [14].Wei G, Yang G, Wang Y, Jiang H, Fu Y, Yue G, Ju R, Phototherapy-based combination strategies for bacterial infection treatment, Theranostics, 10 (2020) 12241–12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Qiao Y, Liu X, Li B, Han Y, Zheng Y, Yeung KWK, Li C, Cui Z, Liang Y, Li Z, Zhu S, Wang X, Wu S, Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing, Nature Communications, 11 (2020) 4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He D, Yang T, Qian W, Qi C, Mao L, Yu X, Zhu H, Luo G, Deng J, Combined photothermal and antibiotic therapy for bacterial infection via acidity-sensitive nanocarriers with enhanced antimicrobial performance, Applied Materials Today, 12 (2018) 415–429. [Google Scholar]
- [17].Yin R, Dai T, Avci P, Jorge AES, de Melo WC, Vecchio D, Huang Y-Y, Gupta A, Hamblin MR, Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond, Current opinion in pharmacology, 13 (2013) 731–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wainwright M, ‘Safe’photoantimicrobials for skin and soft-tissue infections, International journal of antimicrobial agents, 36 (2010) 14–18. [DOI] [PubMed] [Google Scholar]
- [19].Tavares A, Carvalho CMB, Faustino MA, Neves MGPMS, Tomé JPC, Tomé AC, Cavaleiro JAS, Cunha Â, Gomes NCM, Alves E, Almeida A, Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment, Marine Drugs, 8 (2010) 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giuliani F, Martinelli M, Cocchi A, Arbia D, Fantetti L, Roncucci G, In vitro resistance selection studies of RLP068/Cl, a new Zn (II) phthalocyanine suitable for antimicrobial photodynamic therapy, Antimicrobial agents and chemotherapy, 54 (2010) 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu M, Wang S, Wang T, Hu S, Bhayana B, Ishii M, Kong Y, Cai Y, Dai T, Cui W, Bacteria-specific phototoxic reactions triggered by blue light and phytochemical carvacrol, Science Translational Medicine, 13 (2021). [DOI] [PubMed] [Google Scholar]
- [22].Dai T, Vrahas MS, Murray CK, Hamblin MR, Ultraviolet C irradiation: an alternative antimicrobial approach to localized infections?, Expert review of anti-infective therapy, 10 (2012) 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zheng Q, Liu X, Zheng Y, Yeung KW, Cui Z, Liang Y, Li Z, Zhu S, Wang X, Wu S, The recent progress on metal–organic frameworks for phototherapy, Chemical Society Reviews, (2021). [DOI] [PubMed] [Google Scholar]
- [24].Zhou J, Li M, Hou Y, Luo Z, Chen Q, Cao H, Huo R, Xue C, Sutrisno L, Hao L, Cao Y, Ran H, Lu L, Li K, Cai K, Engineering of a Nanosized Biocatalyst for Combined Tumor Starvation and Low-Temperature Photothermal Therapy, ACS Nano, 12 (2018) 2858–2872. [DOI] [PubMed] [Google Scholar]
- [25].Yang Y, Zhu W, Dong Z, Chao Y, Xu L, Chen M, Liu Z, 1D coordination polymer nanofibers for low-temperature photothermal therapy, Advanced Materials, 29 (2017) 1703588. [DOI] [PubMed] [Google Scholar]
- [26].Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, Pasechnikov AD, Atwood S, Mukherjee JS, Rich ML, Furin JJ, Nardell EA, Kim JY, Shin SS, Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study, The Lancet, 372 (2008) 1403–1409. [DOI] [PubMed] [Google Scholar]
- [27].Cabral J, Ag R, Blue light disinfection in hospital infection control: advantages, drawbacks, and pitfalls, Antibiotics, 8 (2019) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bell SG, Antibiotic resistance: is the end of an era near?, Neonatal Network, 22 (2003) 47–54. [DOI] [PubMed] [Google Scholar]
- [29].Poole MD, Are we facing the end of the antibiotic era?, Ear, Nose & Throat Journal, 72 (1993) 433–433. [PubMed] [Google Scholar]
- [30].Harrison JW, Svec TA, The beginning of the end of the antibiotic era? Part I. The problem: abuse of the” miracle drugs”, Quintessence international, 29 (1998). [PubMed] [Google Scholar]
- [31].Harrison JW, Svec TA, The beginning of the end of the antibiotic era? Part II. Proposed solutions to antibiotic abuse, Quintessence international, 29 (1998). [PubMed] [Google Scholar]
- [32].Schena E, Saccomandi P, Fong Y, Laser Ablation for Cancer: Past, Present and Future, Journal of Functional Biomaterials, 8 (2017) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chiavaioli F, Baldini F, Tombelli S, Trono C, Giannetti A, Biosensing with optical fiber gratings, Nanophotonics, 6 (2017) 663–679. [Google Scholar]
- [34].Coda S, Siersema PD, Stamp GW, Thillainayagam AV, Biophotonic endoscopy: a review of clinical research techniques for optical imaging and sensing of early gastrointestinal cancer, Endoscopy international open, 3 (2015) E380–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu Y-K, Tian G-Y, Lu R-S, Zhang H, A Review of Optical NDT Technologies, Sensors, 11 (2011) 7773–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Keiser G, Xiong F, Cui Y, Shum PP, Review of diverse optical fibers used in biomedical research and clinical practice, Journal of biomedical optics, 19 (2014) 080902. [DOI] [PubMed] [Google Scholar]
- [37].Okamoto K, Fundamentals of optical waveguides (Second Edition), Academic press, Burlington, 2006. [Google Scholar]
- [38].Kogelnik H, Theory of optical waveguides, Guided-wave optoelectronics, Springer; 1988, pp. 7–88. [Google Scholar]
- [39].Snyder AW, Love J, Optical waveguide theory, Springer Science & Business Media; 2012. [Google Scholar]
- [40].Calvo ML, Lakshminarayanan V, Optical waveguides: from theory to applied technologies, CRC Press; 2018. [Google Scholar]
- [41].Ma H, Jen AY, Dalton LR, Polymer-based optical waveguides: materials, processing, and devices, Advanced materials, 14 (2002) 1339–1365. [Google Scholar]
- [42].Tamir T, Griffel G, Bertoni HL, Guided-wave optoelectronics: device characterization, analysis, and design, Springer Science & Business Media; 2013. [Google Scholar]
- [43].Senior JM, Jamro MY, Optical fiber communications: principles and practice, Pearson Education; 2009. [Google Scholar]
- [44].Udd E, Spillman WB Jr, Fiber optic sensors: an introduction for engineers and scientists, John Wiley & Sons; 2011. [Google Scholar]
- [45].Shabahang S, Kim S, Yun SH, Light-guiding biomaterials for biomedical applications, Advanced functional materials, 28 (2018) 1706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P, Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo, Nature Biotechnology, 33 (2015) 277–284. [DOI] [PubMed] [Google Scholar]
- [47].Lu C, Park S, Richner TJ, Derry A, Brown I, Hou C, Rao S, Kang J, Moritz CT, Fink Y, Anikeeva P, Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits, Science Advances, 3 (2017) e1600955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dolmans DE, Fukumura D, Jain RK, Photodynamic therapy for cancer, Nature reviews cancer, 3 (2003) 380–387. [DOI] [PubMed] [Google Scholar]
- [49].Ni K, Lan G, Lin W, Nanoscale Metal–Organic Frameworks Generate Reactive Oxygen Species for Cancer Therapy, ACS Central Science, 6 (2020) 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Daniell M, Hill J, A history of photodynamic therapy, Australian and New Zealand Journal of Surgery, 61 (1991) 340–348. [DOI] [PubMed] [Google Scholar]
- [51].Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T, Targeted antimicrobial photochemotherapy, Antimicrobial Agents and Chemotherapy, 42 (1998) 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lakowicz JR, Principles of fluorescence spectroscopy, Springer science & business media; 2013. [Google Scholar]
- [53].Oleinick NL (2011). “Basic photosensitization.” Retrieved April 13, 2021, from http://photobiology.info/Oleinick.html.
- [54].Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M, Tumor cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies, Cancer and Metastasis Reviews, 34 (2015) 643–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hamblin MR, Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes, Current opinion in microbiology, 33 (2016) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang Y-Y, Liu Y-C, Sun H, Guo D-S, Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications, Coordination Chemistry Reviews, 395 (2019) 46–62. [Google Scholar]
- [57].Mehraban N, Freeman HS, Developments in PDT sensitizers for increased selectivity and singlet oxygen production, Materials, 8 (2015) 4421–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wachter EA, Petersen MG, Dees C, Photodynamic therapy with ultrafast lasers, Commercial and biomedical applications of ultrafast lasers, International Society for Optics and Photonics, 1999, pp. 66–74. [Google Scholar]
- [59].Taniguchi M, Lindsey JS, Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD, Photochemistry and Photobiology, 94 (2018) 290–327. [DOI] [PubMed] [Google Scholar]
- [60].Thakuri P, Joshi R, Basnet S, Pandey S, Taujale S, Mishra N, Antibacterial photodynamic therapy on Staphylococcus aureus and Pseudomonas aeruginosa in-vitro, Nepal Med Coll J, 13 (2011) 281–284. [PubMed] [Google Scholar]
- [61].Bartzatt R, Wol T, Detection and Assay of Vitamin B-2 (Riboflavin) in Alkaline Borate Buffer with UV/Visible Spectrophotometry, International Scholarly Research Notices, 2014 (2014) 453085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tanaka M, Mroz P, Dai T, Huang L, Morimoto Y, Kinoshita M, Yoshihara Y, Nemoto K, Shinomiya N, Seki S, Hamblin MR, Photodynamic Therapy Can Induce a Protective Innate Immune Response against Murine Bacterial Arthritis via Neutrophil Accumulation, PLOS ONE, 7 (2012) e39823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS, Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans, Lasers in medical science, 24 (2009) 109–112. [DOI] [PubMed] [Google Scholar]
- [64].Wong T-W, Wang Y-Y, Sheu H-M, Chuang Y-C, Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus, Antimicrobial agents and chemotherapy, 49 (2005) 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Harris F, Pierpoint L, Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial Agent, Medicinal research reviews, 32 (2012) 1292–1327. [DOI] [PubMed] [Google Scholar]
- [66].Wang D, Su H, Kwok RT, Hu X, Zou H, Luo Q, Lee MM, Xu W, Lam JW, Tang BZ, Rational design of a water-soluble NIR AIEgen, and its application in ultrafast wash-free cellular imaging and photodynamic cancer cell ablation, Chemical science, 9 (2018) 3685–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yang Z, Yin W, Zhang S, Shah I, Zhang B, Zhang S, Li Z, Lei Z, Ma H, Synthesis of AIE-Active Materials with Their Applications for Antibacterial Activity, Specific Imaging of Mitochondrion and Image-Guided Photodynamic Therapy, ACS Applied Bio Materials, 3 (2020) 1187–1196. [DOI] [PubMed] [Google Scholar]
- [68].Chen H, Li S, Wu M, Huang Z, Lee CS, Liu B, Membrane-Anchoring Photosensitizer with Aggregation-Induced Emission Characteristics for Combating Multidrug-Resistant Bacteria, Angewandte Chemie International Edition, 59 (2020) 632–636. [DOI] [PubMed] [Google Scholar]
- [69].Topaloglu N, Gulsoy M, Yuksel S, Antimicrobial photodynamic therapy of resistant bacterial strains by indocyanine green and 809-nm diode laser, Photomedicine and laser surgery, 31 (2013) 155–162. [DOI] [PubMed] [Google Scholar]
- [70].Ma H, Peng Q, An Z, Huang W, Shuai Z, Efficient and long-lived room-temperature organic phosphorescence: theoretical descriptors for molecular designs, Journal of the American Chemical Society, 141 (2018) 1010–1015. [DOI] [PubMed] [Google Scholar]
- [71].Ji S, Ge J, Escudero D, Wang Z, Zhao J, Jacquemin D, Molecular structure–intersystem crossing relationship of heavy-atom-free BODIPY triplet photosensitizers, The Journal of organic chemistry, 80 (2015) 5958–5963. [DOI] [PubMed] [Google Scholar]
- [72].McClure DS, Triplet-singlet transitions in organic molecules. lifetime measurements of the triplet state, The Journal of Chemical Physics, 17 (1949) 905–913. [Google Scholar]
- [73].Liu S, Zhang H, Li Y, Liu J, Du L, Chen M, Kwok RT, Lam JW, Phillips DL, Tang BZ, Strategies to enhance the photosensitization: polymerization and the donor–acceptor even–odd effect, Angewandte Chemie International Edition, 57 (2018) 15189–15193. [DOI] [PubMed] [Google Scholar]
- [74].Leitl MJ, Krylova VA, Djurovich PI, Thompson ME, Yersin H, Phosphorescence versus thermally activated delayed fluorescence. Controlling singlet–triplet splitting in brightly emitting and sublimable Cu (I) compounds, Journal of the American Chemical Society, 136 (2014) 16032–16038. [DOI] [PubMed] [Google Scholar]
- [75].Chen J, Wen K, Chen H, Jiang S, Wu X, Lv L, Peng A, Zhang S, Huang H, Achieving High-Performance Photothermal and Photodynamic Effects upon Combining D–A Structure and Nonplanar Conformation, Small, 16 (2020) 2000909. [DOI] [PubMed] [Google Scholar]
- [76].Luo T, Ni K, Culbert A, Lan G, Li Z, Jiang X, Kaufmann M, Lin W, Nanoscale metal–organic frameworks stabilize bacteriochlorins for type I and type II photodynamic therapy, Journal of the American Chemical Society, 142 (2020) 7334–7339. [DOI] [PubMed] [Google Scholar]
- [77].Lan M, Zhao S, Liu W, Lee C-S, Zhang W, Wang P, Photosensitizers for Photodynamic Therapy, Advanced Healthcare Materials, 8 (2019) 1900132. [DOI] [PubMed] [Google Scholar]
- [78].Dimitrijevic NM, Rozhkova E, Rajh T, Dynamics of localized charges in dopamine-modified TiO2 and their effect on the formation of reactive oxygen species, Journal of the American Chemical Society, 131 (2009) 2893–2899. [DOI] [PubMed] [Google Scholar]
- [79].Kapilashrami M, Zhang Y, Liu Y-S, Hagfeldt A, Guo J, Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications, Chemical reviews, 114 (2014) 9662–9707. [DOI] [PubMed] [Google Scholar]
- [80].Szaciłowski K, Macyk W, Drzewiecka-Matuszek A, Brindell M, Stochel G, Bioinorganic photochemistry: frontiers and mechanisms, Chemical reviews, 105 (2005) 2647–2694. [DOI] [PubMed] [Google Scholar]
- [81].Schwarz PF, Turro NJ, Bossmann SH, Braun AM, Wahab A-MAA, Duerr H, A new method to determine the generation of hydroxyl radicals in illuminated TiO2 suspensions, The Journal of Physical Chemistry B, 101 (1997) 7127–7134. [Google Scholar]
- [82].Xu P, Wang R, Ouyang J, Chen B, A new strategy for TiO 2 whiskers mediated multi-mode cancer treatment, Nanoscale research letters, 10 (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yamaguchi S, Kobayashi H, Narita T, Kanehira K, Sonezaki S, Kubota Y, Terasaka S, Iwasaki Y, Novel Photodynamic Therapy Using Water-dispersed TiO2–Polyethylene Glycol Compound: Evaluation of Antitumor Effect on Glioma Cells and Spheroids In Vitro, Photochemistry and photobiology, 86 (2010) 964–971. [DOI] [PubMed] [Google Scholar]
- [84].Zeng L, Ren W, Xiang L, Zheng J, Chen B, Wu A, Multifunctional Fe 3 O 4–TiO 2 nanocomposites for magnetic resonance imaging and potential photodynamic therapy, Nanoscale, 5 (2013) 2107–2113. [DOI] [PubMed] [Google Scholar]
- [85].Paunesku T, Rajh T, Wiederrecht G, Maser J, Vogt S, Stojićević N, Protić M, Lai B, Oryhon J, Thurnauer M, Woloschak G, Biology of TiO2–oligonucleotide nanocomposites, Nature Materials, 2 (2003) 343–346. [DOI] [PubMed] [Google Scholar]
- [86].Yamakoshi Y, Sueyoshi S, Fukuhara K, Miyata N, Masumizu T, Kohno M, ∂ OH and O2∂-Generation in Aqueous C60 and C70 Solutions by Photoirradiation: An EPR Study, Journal of the American Chemical Society, 120 (1998) 12363–12364. [Google Scholar]
- [87].Fan S, Zhang Y, Tan H, Xue C, He Y, Wei X, Zha Y, Niu J, Liu Y, Cheng Y, Cui D, Manganese/iron-based nanoprobes for photodynamic/chemotherapy combination therapy of tumor guided by multimodal imaging, Nanoscale, 13 (2021) 5383–5399. [DOI] [PubMed] [Google Scholar]
- [88].Cheng P, Li G, Zhan X, Yang Y, Next-generation organic photovoltaics based on non-fullerene acceptors, Nature Photonics, 12 (2018) 131–142. [Google Scholar]
- [89].Nakanishi W, Minami K, Shrestha LK, Ji Q, Hill JP, Ariga K, Bioactive nanocarbon assemblies: Nanoarchitectonics and applications, Nano Today, 9 (2014) 378–394. [Google Scholar]
- [90].Gupta VK, Saleh TA, Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview, Environmental science and pollution research, 20 (2013) 2828–2843. [DOI] [PubMed] [Google Scholar]
- [91].Ge J, Lan M, Zhou B, Liu W, Guo L, Wang H, Jia Q, Niu G, Huang X, Zhou H, Meng X, Wang P, Lee C-S, Zhang W, Han X, A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation, Nature Communications, 5 (2014) 4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gao W, The chemistry of graphene oxide, Graphene oxide, (2015) 61–95. [Google Scholar]
- [93].Jiang BP, Zhou B, Lin Z, Liang H, Shen XC, Recent advances in carbon nanomaterials for cancer phototherapy, Chemistry–A European Journal, 25 (2019) 3993–4004. [DOI] [PubMed] [Google Scholar]
- [94].Markovic Z, Trajkovic V, Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60), Biomaterials, 29 (2008) 3561–3573. [DOI] [PubMed] [Google Scholar]
- [95].Spesia MB, Milanesio ME, Durantini EN, Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives, European journal of medicinal chemistry, 43 (2008) 853–861. [DOI] [PubMed] [Google Scholar]
- [96].Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR, Photodynamic therapy with fullerenes, Photochemical & Photobiological Sciences, 6 (2007) 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jiang W, Wang L, Wang Q, Zhou H, Ma Y, Dong W, Xu H, Wang Y, Reversing Immunosuppression in Hypoxic and Immune-Cold Tumors with Ultrathin Oxygen Self-Supplementing Polymer Nanosheets under Near Infrared Light Irradiation, Advanced Functional Materials, 31 (2021) 2100354. [Google Scholar]
- [98].Kamkaew A, Chen F, Zhan Y, Majewski RL, Cai W, Scintillating nanoparticles as energy mediators for enhanced photodynamic therapy, ACS nano, 10 (2016) 3918–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hou Z, Zhang Y, Deng K, Chen Y, Li X, Deng X, Cheng Z, Lian H, Li C, Lin J, UV-emitting upconversion-based TiO2 photosensitizing nanoplatform: near-infrared light mediated in vivo photodynamic therapy via mitochondria-involved apoptosis pathway, ACS nano, 9 (2015) 2584–2599. [DOI] [PubMed] [Google Scholar]
- [100].Lv R, Zhong C, Li R, Yang P, He F, Gai S, Hou Z, Yang G, Lin J, Multifunctional anticancer platform for multimodal imaging and visible light driven photodynamic/photothermal therapy, Chemistry of Materials, 27 (2015) 1751–1763. [Google Scholar]
- [101].Yu Z, Sun Q, Pan W, Li N, Tang B, A near-infrared triggered nanophotosensitizer inducing domino effect on mitochondrial reactive oxygen species burst for cancer therapy, ACS nano, 9 (2015) 11064–11074. [DOI] [PubMed] [Google Scholar]
- [102].Lucky SS, Muhammad Idris N, Li Z, Huang K, Soo KC, Zhang Y, Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy, ACS nano, 9 (2015) 191–205. [DOI] [PubMed] [Google Scholar]
- [103].Idris NM, Lucky SS, Li Z, Huang K, Zhang Y, Photoactivation of core–shell titania coated upconversion nanoparticles and their effect on cell death, Journal of Materials Chemistry B, 2 (2014) 7017–7026. [DOI] [PubMed] [Google Scholar]
- [104].Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y, In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers, Nature medicine, 18 (2012) 1580. [DOI] [PubMed] [Google Scholar]
- [105].Zeng L, Pan Y, Luo S, Ren W, Gong A, Ma X, Liang H, Lu G, Wu A, Inorganic photosensitizer coupled Gd-based upconversion luminescent nanocomposites for in vivo magnetic resonance imaging and near-infrared-responsive photodynamic therapy in cancers, Biomaterials, 44 (2015) 82–90. [DOI] [PubMed] [Google Scholar]
- [106].Zeng L, Pan Y, Tian Y, Wang X, Ren W, Wang S, Lu G, Wu A, Doxorubicin-loaded NaYF4: Yb/Tm–TiO2 inorganic photosensitizers for NIR-triggered photodynamic therapy and enhanced chemotherapy in drug-resistant breast cancers, Biomaterials, 57 (2015) 93–106. [DOI] [PubMed] [Google Scholar]
- [107].Zhang X, Tang J, Li C, Lu Y, Cheng L, Liu J, A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy, Bioactive materials, 6 (2021) 472–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].He J, Fu L-H, Qi C, Lin J, Huang P, Metal peroxides for cancer treatment, Bioactive materials, 6 (2021) 2698–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Jin A, Wang Y, Lin K, Jiang L, Nanoparticles modified by polydopamine: Working as “drug” carriers, Bioactive materials, 5 (2020) 522–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Huang B, Tan L, Liu X, Li J, Wu S, A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light, Bioactive materials, 4 (2019) 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wei Z, Zou H, Liu G, Song C, Tang C, Chen S, Zhang G, Ran J, Wang Y, Yin X, Cai Y, Han W, Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma, Bioactive Materials, 6 (2021) 2144–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li Y, Zhou Y, Yue X, Dai Z, Cyanine conjugates in cancer theranostics, Bioactive Materials, 6 (2021) 794–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yang M, Li J, Gu P, Fan X, The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment, Bioactive materials, 6 (2021) 1973–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fan Z, Liu H, Xue Y, Lin J, Fu Y, Xia Z, Pan D, Zhang J, Qiao K, Zhang Z, Liao Y, Reversing cold tumors to hot: An immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy, Bioactive Materials, 6 (2021) 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Wu Q, Chen G, Gong K, Wang J, Ge X, Liu X, Guo S, Wang F, MnO2-laden black phosphorus for MRI-guided synergistic PDT, PTT, and chemotherapy, Matter, 1 (2019) 496–512. [Google Scholar]
- [116].Jana D, Jia S, Bindra AK, Xing P, Ding D, Zhao Y, Clearable black phosphorus nanoconjugate for targeted cancer phototheranostics, ACS applied materials & interfaces, 12 (2020) 18342–18351. [DOI] [PubMed] [Google Scholar]
- [117].Bian H, Ma D, Zhang X, Xin K, Yang Y, Peng X, Xiao Y, Tailored Engineering of Novel Xanthonium Polymethine Dyes for Synergetic PDT and PTT Triggered by 1064 nm Laser toward Deep-Seated Tumors, Small, (2021) 2100398. [DOI] [PubMed] [Google Scholar]
- [118].Zhang X, Xiong J, Wang K, Yu H, Sun B, Ye H, Zhao Z, Wang N, Wang Y, Zhang S, Zhao W, Zhang H, He Z, Luo C, Sun J, Erythrocyte membrane-camouflaged carrier-free nanoassembly of FRET photosensitizer pairs with high therapeutic efficiency and high security for programmed cancer synergistic phototherapy, Bioactive Materials, 6 (2021) 2291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bilici K, Atac N, Muti A, Baylam I, Dogan O, Sennaroglu A, Can F, Acar HY, Broad spectrum antibacterial photodynamic and photothermal therapy achieved with indocyanine green loaded SPIONs under near infrared irradiation, Biomaterials Science, 8 (2020) 4616–4625. [DOI] [PubMed] [Google Scholar]
- [120].Yuan Z, Lin C, He Y, Tao B, Chen M, Zhang J, Liu P, Cai K, Near-Infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination, ACS nano, 14 (2020) 3546–3562. [DOI] [PubMed] [Google Scholar]
- [121].Zhang H, Liang Y, Zhao H, Qi R, Chen Z, Yuan H, Liang H, Wang L, Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy, Macromolecular bioscience, 20 (2020) 1900301. [DOI] [PubMed] [Google Scholar]
- [122].Luo J, Xie Z, Lam JWY, Cheng L, Chen H, Qiu C, Kwok HS, Zhan X, Liu Y, Zhu D, Tang BZ, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chemical Communications, (2001) 1740–1741. [DOI] [PubMed] [Google Scholar]
- [123].Wang D, Lee MMS, Xu W, Kwok RTK, Lam JWY, Tang BZ, Theranostics based on AIEgens, Theranostics, 8 (2018) 4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wang D, Lee MM, Shan G, Kwok RT, Lam JW, Su H, Cai Y, Tang BZ, Highly efficient photosensitizers with far-red/near-infrared aggregation-induced emission for in vitro and in vivo cancer theranostics, Advanced Materials, 30 (2018) 1802105. [DOI] [PubMed] [Google Scholar]
- [125].Zheng Z, Zhang T, Liu H, Chen Y, Kwok RTK, Ma C, Zhang P, Sung HHY, Williams ID, Lam JWY, Wong KS, Tang BZ, Bright Near-Infrared Aggregation-Induced Emission Luminogens with Strong Two-Photon Absorption, Excellent Organelle Specificity, and Efficient Photodynamic Therapy Potential, ACS Nano, 12 (2018) 8145–8159. [DOI] [PubMed] [Google Scholar]
- [126].Li Y, Wu Q, Kang M, Song N, Wang D, Tang BZ, Boosting the photodynamic therapy efficiency by using stimuli-responsive and AIE-featured nanoparticles, Biomaterials, 232 (2020) 119749. [DOI] [PubMed] [Google Scholar]
- [127].Zhang Y-H, Li X, Huang L, Kim HS, An J, Lan M, Cao Q-Y, Kim JS, AIE based GSH activatable photosensitizer for imaging-guided photodynamic therapy, Chemical Communications, 56 (2020) 10317–10320. [DOI] [PubMed] [Google Scholar]
- [128].Liu J, Jin C, Yuan B, Liu X, Chen Y, Ji L, Chao H, Selectively lighting up two-photon photodynamic activity in mitochondria with AIE-active iridium (III) complexes, Chemical Communications, 53 (2017) 2052–2055. [DOI] [PubMed] [Google Scholar]
- [129].Zhao E, Chen Y, Wang H, Chen S, Lam JW, Leung CW, Hong Y, Tang BZ, Light-enhanced bacterial killing and wash-free imaging based on AIE fluorogen, ACS applied materials & interfaces, 7 (2015) 7180–7188. [DOI] [PubMed] [Google Scholar]
- [130].You X, Ma H, Wang Y, Zhang G, Peng Q, Liu L, Wang S, Zhang D, Pyridinium-Substituted TetraphenylethyleneEntailing Alkyne Moiety: Enhancement of Photosensitizing Efficiency and Antimicrobial Activity, Chemistry–An Asian Journal, 12 (2017) 1013–1019. [DOI] [PubMed] [Google Scholar]
- [131].Kang M, Kwok RTK, Wang J, Zhang H, Lam JWY, Li Y, Zhang P, Zou H, Gu X, Li F, Tang BZ, A multifunctional luminogen with aggregation-induced emission characteristics for selective imaging and photodynamic killing of both cancer cells and Gram-positive bacteria, Journal of Materials Chemistry B, 6 (2018) 3894–3903. [DOI] [PubMed] [Google Scholar]
- [132].Li Y, Zhao Z, Zhang J, Kwok RTK, Xie S, Tang R, Jia Y, Yang J, Wang L, Lam JWY, Zheng W, Jiang X, Tang BZ, A Bifunctional Aggregation-Induced Emission Luminogen for Monitoring and Killing of Multidrug-Resistant Bacteria, Advanced Functional Materials, 28 (2018) 1804632. [Google Scholar]
- [133].Gao M, Hu Q, Feng G, Tomczak N, Liu R, Xing B, Tang BZ, Liu B, A multifunctional probe with aggregation-induced emission characteristics for selective fluorescence imaging and photodynamic killing of bacteria over mammalian cells, Advanced healthcare materials, 4 (2015) 659–663. [DOI] [PubMed] [Google Scholar]
- [134].Feng G, Zhang C-J, Lu X, Liu B, Zinc (II)-tetradentate-coordinated probe with aggregation-induced emission characteristics for selective imaging and photoinactivation of bacteria, ACS omega, 2 (2017) 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Jain N, Alam P, Laskar IR, Panwar J, ‘Aggregation induced phosphorescence’active iridium (iii) complexes for integrated sensing and inhibition of bacterial growth in aqueous solution, RSC advances, 5 (2015) 61983–61988. [Google Scholar]
- [136].Li NN, Li JZ, Liu P, Pranantyo D, Luo L, Chen JC, Kang E-T, Hu XF, Li CM, Xu LQ, An antimicrobial peptide with an aggregation-induced emission (AIE) luminogen for studying bacterial membrane interactions and antibacterial actions, Chemical Communications, 53 (2017) 3315–3318. [DOI] [PubMed] [Google Scholar]
- [137].Feng G, Yuan Y, Fang H, Zhang R, Xing B, Zhang G, Zhang D, Liu B, A light-up probe with aggregation-induced emission characteristics (AIE) for selective imaging, naked-eye detection and photodynamic killing of Gram-positive bacteria, Chemical Communications, 51 (2015) 12490–12493. [DOI] [PubMed] [Google Scholar]
- [138].Li Y, Hu X, Tian S, Li Y, Zhang G, Zhang G, Liu S, Polyion complex micellar nanoparticles for integrated fluorometric detection and bacteria inhibition in aqueous media, Biomaterials, 35 (2014) 1618–1626. [DOI] [PubMed] [Google Scholar]
- [139].Li Y, Yu H, Qian Y, Hu J, Liu S, Amphiphilic Star Copolymer-Based Bimodal Fluorogenic/Magnetic Resonance Probes for Concomitant Bacteria Detection and Inhibition, Advanced Materials, 26 (2014) 6734–6741. [DOI] [PubMed] [Google Scholar]
- [140].Zhao J, Dong Z, Cui H, Jin H, Wang C, Nanoengineered peptide-grafted hyperbranched polymers for killing of bacteria monitored in real time via intrinsic aggregation-induced emission, ACS applied materials & interfaces, 10 (2018) 42058–42067. [DOI] [PubMed] [Google Scholar]
- [141].Ma H, Ma Y, Lei L, Yin W, Yang Y, Wang T, Yin P, Lei Z, Yang M, Qin Y, Zhang S, Light-Enhanced Bacterial Killing and Less Toxic Cell Imaging: Multicationic Aggregation-Induced Emission Matters, ACS Sustainable Chemistry & Engineering, 6 (2018) 15064–15071. [Google Scholar]
- [142].Zhou T, Hu R, Wang L, Qiu Y, Zhang G, Deng Q, Zhang H, Yin P, Situ B, Zhan C, Qin A, Tang BZ, An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections, Angewandte Chemie International Edition, 59 (2020) 9952–9956. [DOI] [PubMed] [Google Scholar]
- [143].Alam P, He W, Leung NL, Ma C, Kwok RT, Lam JW, Sung HH, Williams ID, Wong KS, Tang BZ, Red AIE-Active Fluorescent Probes with Tunable Organelle-Specific Targeting, Advanced Functional Materials, 30 (2020) 1909268. [Google Scholar]
- [144].Chen C, Ou H, Liu R, Ding D, Regulating the photophysical property of organic/polymer optical agents for promoted cancer phototheranostics, Advanced Materials, 32 (2020) 1806331. [DOI] [PubMed] [Google Scholar]
- [145].Du J, Liu S, Zhang P, Liu H, Li Y, He W, Li C, Chau JHC, Kwok RTK, Lam JWY, Cai L, Huang Y, Zhang W, Hou J, Tang BZ, Highly Stable and Bright NIR-II AIE Dots for Intraoperative Identification of Ureter, ACS Applied Materials & Interfaces, 12 (2020) 8040–8049. [DOI] [PubMed] [Google Scholar]
- [146].Kang M, Zhou C, Wu S, Yu B, Zhang Z, Song N, Lee MMS, Xu W, Xu F-J, Wang D, Wang L, Tang BZ, Evaluation of Structure–Function Relationships of Aggregation-Induced Emission Luminogens for Simultaneous Dual Applications of Specific Discrimination and Efficient Photodynamic Killing of Gram-Positive Bacteria, Journal of the American Chemical Society, 141 (2019) 16781–16789. [DOI] [PubMed] [Google Scholar]
- [147].He X, Yang Y, Guo Y, Lu S, Du Y, Li J-J, Zhang X, Leung NLC, Zhao Z, Niu G, Yang S, Weng Z, Kwok RTK, Lam JWY, Xie G, Tang BZ, Phage-Guided Targeting, Discriminative Imaging, and Synergistic Killing of Bacteria by AIE Bioconjugates, Journal of the American Chemical Society, 142 (2020) 3959–3969. [DOI] [PubMed] [Google Scholar]
- [148].Zhang R, Duan Y, Liu B, Recent advances of AIE dots in NIR imaging and phototherapy, Nanoscale, 11 (2019) 19241–19250. [DOI] [PubMed] [Google Scholar]
- [149].Jiang R, Dai J, Dong X, Wang O, Meng Z, Guo J, Yu Y, Wang S, Xia F, Zhao Z, Lou X, Tang BZ, Improving Image-Guided Surgical and Immunological Tumor Treatment Efficacy by Photothermal and Photodynamic Therapies Based on a Multifunctional NIR AIEgen, Advanced Materials, 33 (2021) 2101158. [DOI] [PubMed] [Google Scholar]
- [150].Sharpless CM, Blough NV, The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties, Environmental Science: Processes & Impacts, 16 (2014) 654–671. [DOI] [PubMed] [Google Scholar]
- [151].Ogilby PR, Singlet oxygen: there is indeed something new under the sun, Chemical Society Reviews, 39 (2010) 3181–3209. [DOI] [PubMed] [Google Scholar]
- [152].Lv Z, Wei H, Li Q, Su X, Liu S, Zhang KY, Lv W, Zhao Q, Li X, Huang W, Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru (II) photosensitizer through type I photochemical process, Chemical science, 9 (2018) 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kotagiri N, Sudlow GP, Akers WJ, Achilefu S, Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers, Nature nanotechnology, 10 (2015) 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Ashur I, Goldschmidt R, Pinkas I, Salomon Y, Szewczyk G, Sarna T, Scherz A, Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST11, noncovalently bound to serum albumin, The journal of physical chemistry A, 113(2009) 8027–8037. [DOI] [PubMed] [Google Scholar]
- [155].Vakrat-Haglili Y, Weiner L, Brumfeld V, Brandis A, Salomon Y, McLIroy B, Wilson BC, Pawlak A, Rozanowska M, Sarna T, Scherz A, The Microenvironment Effect on the Generation of Reactive Oxygen Species by Pd–Bacteriopheophorbide, Journal of the American Chemical Society, 127 (2005) 6487–6497. [DOI] [PubMed] [Google Scholar]
- [156].Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P, Toluidine blue-mediated photodynamic effects on staphylococcal biofilms, Antimicrobial agents and chemotherapy, 52 (2008) 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zanin ICJ, Goncalves RB, Junior AB, Hope CK, Pratten J, Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study, Journal of Antimicrobial Chemotherapy, 56 (2005) 324–330. [DOI] [PubMed] [Google Scholar]
- [158].Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, Höfling JF, Gonçalves RB, Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode, European journal of oral sciences, 114 (2006) 64–69. [DOI] [PubMed] [Google Scholar]
- [159].Pereira CA, Romeiro RL, Costa ACBP, Machado AKS, Junqueira JC, Jorge AOC, Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study, Lasers in medical science, 26 (2011) 341–348. [DOI] [PubMed] [Google Scholar]
- [160].Lin H-Y, Chen C-T, Huang C-T, Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells, Applied and environmental microbiology, 70 (2004) 6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P, The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms, Biomaterials, 30 (2009) 3158–3166. [DOI] [PubMed] [Google Scholar]
- [162].Saino E, Sbarra MS, Arciola CR, Scavone M, Bloise N, Nikolov P, Ricchelli F, Visai L, Photodynamic action of Tri-meso (N-methylpyridyl), meso (N-tetradecyl-pyridyl) porphine on Staphylococcus epidermidis biofilms grown on Ti6AI4V alloy, The International journal of artificial organs, 33 (2010) 636–645. [DOI] [PubMed] [Google Scholar]
- [163].Wood S, Metcalf D, Devine D, Robinson C, Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms, Journal of Antimicrobial Chemotherapy, 57 (2006) 680–684. [DOI] [PubMed] [Google Scholar]
- [164].de Carvalho Goulart R, Thedei G, Souza SLS, Tedesco AC, Ciancaglini P, Comparative Study of Methylene Blue and Erythrosine Dyes Employed in Photodynamic Therapy for Inactivation of Planktonic and Biofilm-Cultivated Aggregatibacter actinomycetemcomitans, Photomedicine and Laser Surgery, 28 (2010) S-85–S-90. [DOI] [PubMed] [Google Scholar]
- [165].Lee C-F, Lee C-J, Chen C-T, Huang C-T, δ-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures, Journal of Photochemistry and Photobiology B: Biology, 75 (2004) 21–25. [DOI] [PubMed] [Google Scholar]
- [166].Collins TL, Markus EA, Hassett DJ, Robinson JB, The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms, Current microbiology, 61 (2010) 411–416. [DOI] [PubMed] [Google Scholar]
- [167].Hegge AB, Bruzell E, Kristensen S, Tønnesen H, Photoinactivation of Staphylococcus epidermidis biofilms and suspensions by the hydrophobic photosensitizer curcumin–effect of selected nanocarrier: studies on curcumin and curcuminoides XLVII, European Journal of Pharmaceutical Sciences, 47 (2012) 65–74. [DOI] [PubMed] [Google Scholar]
- [168].Meimandi M, Ardakani MRT, Nejad AE, Yousefnejad P, Saebi K, Tayeed MH, The effect of photodynamic therapy in the treatment of chronic periodontitis: a review of literature, Journal of lasers in medical sciences, 8 (2017) S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Azaripour A, Dittrich S, Van Noorden CJ, Willershausen B, Efficacy of photodynamic therapy as adjunct treatment of chronic periodontitis: a systematic review and meta-analysis, Lasers in medical science, 33 (2018) 407–423. [DOI] [PubMed] [Google Scholar]
- [170].Joseph B, Janam P, Narayanan S, Anil S, Is antimicrobial photodynamic therapy effective as an adjunct to scaling and root planing in patients with chronic periodontitis? A systematic review, Biomolecules, 7 (2017) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Cheng G, Li B, Nanoparticle-based photodynamic therapy: new trends in wound healing applications, Materials Today Advances, 6 (2020) 100049. [Google Scholar]
- [172].Soncin M, Fabris C, Busetti A, Dei D, Nistri D, Roncucci G, Jori G, Approaches to selectivity in the Zn (II)–phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus, Photochemical & Photobiological Sciences, 1 (2002) 815–819. [DOI] [PubMed] [Google Scholar]
- [173].Tomé JP, Neves MG, Tomé AC, Cavaleiro JA, Soncin M, Magaraggia M, Ferro S, Jori G, Synthesis and antibacterial activity of new poly-S-lysine– Porphyrin conjugates, Journal of medicinal chemistry, 47 (2004) 6649–6652. [DOI] [PubMed] [Google Scholar]
- [174].Schastak S, Ziganshyna S, Gitter B, Wiedemann P, Claudepierre T, Efficient photodynamic therapy against gram-positive and gram-negative bacteria using TFIPTS, a cationic photosensitizer excited by infrared wavelength, PloS one, 5 (2010) e11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Schastak S, Gitter B, Flandzel R, Flermann R, Wiedemann P, Improved photoinactivation of gram-negative and gram-positive methicillin-resistant bacterial strains using a new near-infrared absorbing meso-tetrahydroporphyrin: a comparative study with a chlorine e6 photosensitizer photolon, Methods and findings in experimental and clinical pharmacology, 30 (2008) 129–133. [DOI] [PubMed] [Google Scholar]
- [176].Mantareva V, Kussovski V, Angelov I, Wöhrle D, Dimitrov R, Popova E, Dimitrov S, Non-aggregated Ga (III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms, Photochemical & Photobiological Sciences, 10 (2011) 91–102. [DOI] [PubMed] [Google Scholar]
- [177].Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR, Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 42 (2010) 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Wainwright M, Phoenix D, Gaskell M, Marshall B, Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp, Journal of antimicrobial chemotherapy, 44 (1999) 823–825. [DOI] [PubMed] [Google Scholar]
- [179].Xing B, Jiang T, Bi W, Yang Y, Li L, Ma M, Chang C-K, Xu B, Yeow EKL, Multifunctional divalent vancomycin: the fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria, Chemical Communications, 47 (2011) 1601–1603. [DOI] [PubMed] [Google Scholar]
- [180].Gad F, Zahra T, Hasan T, Hamblin MR, Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria, Antimicrobial agents and chemotherapy, 48 (2004) 2173–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Nordmann P, Naas T, Fortineau N, Poirel L, Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010, Current opinion in microbiology, 10 (2007) 436–440. [DOI] [PubMed] [Google Scholar]
- [182].Dijkshoorn L, Nemec A, Seifert H, An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii, Nature reviews microbiology, 5 (2007) 939–951. [DOI] [PubMed] [Google Scholar]
- [183].Tseng S, Teng L, Chen C, Lo T, Hung W, Chen H, Hsueh P, Tsai J, Toluidine blue O photodynamic inactivation on multidrug-resistant pseudomonas aeruginosa, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 41 (2009) 391–397. [DOI] [PubMed] [Google Scholar]
- [184].Kussovski V, Mantareva V, Angelov I, Orozova P, Wöhrle D, Schnurpfeil G, Borisova E, Avramov L, Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity, FEMS microbiology letters, 294 (2009) 133–140. [DOI] [PubMed] [Google Scholar]
- [185].Trannoy L, Terpstra F, De Korte D, Lagerberg J, Verhoeven A, Brand A, Van Engelenburg F, Differential sensitivities of pathogens in red cell concentrates to Tri-P (4)-photoinactivation, Vox sanguinis, 91 (2006) 111–118. [DOI] [PubMed] [Google Scholar]
- [186].Jassal M, Bishai WR, Extensively drug-resistant tuberculosis, The Lancet infectious diseases, 9 (2009) 19–30. [DOI] [PubMed] [Google Scholar]
- [187].O’Riordan K, Sharlin DS, Gross J, Chang S, Errabelli D, Akilov OE, Kosaka S, Nau GJ, Hasan T, Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection, Antimicrobial agents and chemotherapy, 50 (2006) 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].O’Riordan K, Akilov OE, Chang SK, Foley JW, Hasan T, Real-time fluorescence monitoring of phenothiazinium photosensitizers and their anti-mycobacterial photodynamic activity against Mycobacterium bovis BCG in in vitro and in vivo models of localized infection, Photochemical & Photobiological Sciences, 6 (2007) 1117–1123. [DOI] [PubMed] [Google Scholar]
- [189].Wiegell SR, Kongshoj B, Wulf HC, Mycobacterium marinum infection cured by photodynamic therapy, Archives of dermatology, 142 (2006) 1231–1244. [DOI] [PubMed] [Google Scholar]
- [190].Shih M-H, Huang F-C, Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis, Investigative ophthalmology & visual science, 52 (2011) 223–229. [DOI] [PubMed] [Google Scholar]
- [191].Thota S, Wang M, Jeon S, Maragani S, Hamblin MR, Chiang LY, Synthesis and characterization of positively charged pentacationic [60] fullerene monoadducts for antimicrobial photodynamic inactivation, Molecules, 17 (2012) 5225–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Shrestha A, Kishen A, Polycationic chitosan-conjugated photosensitizer for antibacterial photodynamic therapy, Photochemistry and photobiology, 88 (2012) 577–583. [DOI] [PubMed] [Google Scholar]
- [193].Yang K, Gitter B, Rüger R, Albrecht V, Wieland GD, Fahr A, Wheat germ agglutinin modified liposomes for the photodynamic inactivation of bacteria, Photochemistry and photobiology, 88 (2012) 548–556. [DOI] [PubMed] [Google Scholar]
- [194].Yang K, Gitter B, Rüger R, Wieland GD, Chen M, Liu X, Albrecht V, Fahr A, Antimicrobial peptide-modified liposomes for bacteria targeted delivery of temoporfin in photodynamic antimicrobial chemotherapy, Photochemical & Photobiological Sciences, 10 (2011) 1593–1601. [DOI] [PubMed] [Google Scholar]
- [195].Tsai T, Yang YT, Wang TH, Chien HF, Chen CT, Improved photodynamic inactivation of gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 41 (2009) 316–322. [DOI] [PubMed] [Google Scholar]
- [196].Tsai T, Chien H-F, Wang T-H, Huang C-T, Ker Y-B, Chen C-T, Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria, Antimicrobial agents and chemotherapy, 55 (2011) 1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Orlandi VT, Caruso E, Banfi S, Barbieri P, Effect of organic matter on the in vitro photoeradication of Pseudomonas aeruginosa by means of a cationic tetraaryl-porphyrin, Photochemistry and photobiology, 88 (2012) 557–564. [DOI] [PubMed] [Google Scholar]
- [198].Walther J, Bröcker MJ, Wätzlich D, Nimtz M, Rohde M, Jahn D, Moser J, Protochlorophyllide: a new photosensitizer for the photodynamic inactivation of Gram-positive and Gram-negative bacteria, FEMS microbiology letters, 290 (2009) 156–163. [DOI] [PubMed] [Google Scholar]
- [199].Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T, Optical monitoring and treatment of potentially lethal wound infections in vivo, The Journal of infectious diseases, 187 (2003) 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Hamblin MR, O’Donnell DA, Murthy N, Contag CH, Hasan T, Rapid Control of Wound Infections by Targeted Photodynamic Therapy Monitored by In Vivo Bioluminescence Imagin¶, Photochemistry and photobiology, 75 (2002) 51–57. [DOI] [PubMed] [Google Scholar]
- [201].Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR, Photodynamic therapy for Acinetobacter baumannii burn infections in mice, Antimicrobial agents and chemotherapy, 53 (2009) 3929–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML, Antibody-targeted photolysis of bacteria in vivo, Biotechnology (N Y), 12 (1994) 703–706. [DOI] [PubMed] [Google Scholar]
- [203].Hashimoto MC, Prates RA, Kato IT, Nunez SC, Courrol LC, Ribeiro MS, Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection. An in vivo study, Photochemistry and photobiology, 88 (2012) 590–595. [DOI] [PubMed] [Google Scholar]
- [204].Park J-H, Ahn M-Y, Kim Y-C, Kim S-A, Moon Y-H, Ahn S-G, Yoon J-H, In vitro and in vivo antimicrobial effect of photodynamic therapy using a highly pure chlorin e6 against Staphylococcus aureus Xen29, Biological and Pharmaceutical Bulletin, 35 (2012) 509–514. [DOI] [PubMed] [Google Scholar]
- [205].Tanaka M, Kinoshita M, Yoshihara Y, Shinomiya N, Seki S, Nemoto K, Hamblin MR, Morimoto Y, Photodynamic therapy using intra-articular photofrin for murine MRSA arthritis: Biphasic light dose response for neutrophil-mediated antibacterial effect, Lasers in surgery and medicine, 43 (2011) 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Goto B, Iriuchishima T, Horaguchi T, Tokuhashi Y, Nagai Y, Harada T, Saito A, Aizawa S, Therapeutic effect of photodynamic therapy using Na-pheophorbide a on osteomyelitis models in rats, Photomedicine and laser surgery, 29 (2011) 183–189. [DOI] [PubMed] [Google Scholar]
- [207].Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G, Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis, Clinical oral implants research, 12 (2001) 104–108. [DOI] [PubMed] [Google Scholar]
- [208].Shibli JA, Martins MC, Theodora LH, Lotufo RFM, Garcia VG, Marcantonio E Jr, Lethal photosensitization in microbiological treatment of ligature-induced peri-implantitis: a preliminary study in dogs, Journal of Oral Science, 45 (2003) 17–23. [DOI] [PubMed] [Google Scholar]
- [209].Hayek RR, Araújo NS, Gioso MA, Ferreira J, Baptista-Sobrinho CA, Yamada AM Jr, Ribeiro MS, Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs, Journal of periodontology, 76 (2005) 1275–1281. [DOI] [PubMed] [Google Scholar]
- [210].Kömerik N, Nakanishi H, MacRobert A, Henderson B, Speight P, Wilson M, In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model, Antimicrobial agents and chemotherapy, 47 (2003) 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Sigusch BW, Pfitzner A, Albrecht V, Glockmann E, Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model, Journal of periodontology, 76 (2005) 1100–1105. [DOI] [PubMed] [Google Scholar]
- [212].Bottura P, Milanezi J, Fernandes L, Caldas H, Abbud-Filho M, Garcia V, Baptista M, Nonsurgical periodontal therapy combined with laser and photodynamic therapies for periodontal disease in immunosuppressed rats, Transplantation proceedings, Elsevier, 2011, pp. 2009–2016. [DOI] [PubMed] [Google Scholar]
- [213].Fernandes LA, De Almeida JM, Theodora LH, Bosco AF, Nagata MJH, Martins TM, Okamoto T, Garcia VG, Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats, Journal of clinical periodontology, 36 (2009) 219–228. [DOI] [PubMed] [Google Scholar]
- [214].de Almeida JM, Theodora LH, Bosco AF, Nagata MJH, Bonfante S, Garcia VG, Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes, Journal of periodontology, 79 (2008) 2156–2165. [DOI] [PubMed] [Google Scholar]
- [215].de Almeida JM, Theodora LH, Bosco AF, Nagata MJH, Oshiiwa M, Garcia VG, Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats, Journal of periodontology, 78 (2007) 566–575. [DOI] [PubMed] [Google Scholar]
- [216].Xu Y, Liu X, Zheng Y, Li C, Yeung KWK, Cui Z, Liang Y, Li Z, Zhu S, Wu S, Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light, Bioactive materials, 6 (2021) 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Lombard G, Tealdi S, Lanotte MMR, The treatment of neurosurgical infections by lasers and porphyrins, Photodynamic therapy of tumors and other diseases, Libreria Progetto Editore, Italy, 1985, pp. 364–366. [Google Scholar]
- [218].Gold MH, Acne and PDT: new techniques with lasers and light sources, Lasers in medical science, 22 (2007) 67–72. [DOI] [PubMed] [Google Scholar]
- [219].Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC, Phototherapy in the treatment of acne vulgaris, American journal of clinical dermatology, 5 (2004) 211–216. [DOI] [PubMed] [Google Scholar]
- [220].Wiegell S, Wulf H, Photodynamic therapy of acne vulgaris using methyl aminolaevulinate: a blinded, randomized, controlled trial, British Journal of Dermatology, 154 (2006) 969–976. [DOI] [PubMed] [Google Scholar]
- [221].Wiegell SR, Wulf HC, Photodynamic therapy of acne vulgaris using 5-aminolevulinic acid versus methyl aminolevulinate, Journal of the American Academy of Dermatology, 54 (2006) 647–651. [DOI] [PubMed] [Google Scholar]
- [222].Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB, A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 33 (2003) 296–310. [DOI] [PubMed] [Google Scholar]
- [223].Holdiness MR, Management of cutaneous erythrasma, Drugs, 62 (2002) 1131–1141. [DOI] [PubMed] [Google Scholar]
- [224].Darras-Vercambre S, Carpentier O, Vincent P, Bonnevalle A, Thomas P, Photodynamic action of red light for treatment of erythrasma: preliminary results, Photodermatology, photoimmunology & photomedicine, 22 (2006) 153–156. [DOI] [PubMed] [Google Scholar]
- [225].Calzavara-Pinton P, Venturini M, Capezzera R, Sala R, Zane C, Photodynamic therapy of interdigital mycoses of the feet with topical application of 5-aminolevulinic acid, Photodermatology, photoimmunology & photomedicine, 20 (2004) 144–147. [DOI] [PubMed] [Google Scholar]
- [226].Liu C, Hu M, Ma D, Lei J.e., Xu J, Photodynamic inactivation of antibiotic-resistant bacteria and biofilms by hematoporphyrin monomethyl ether, Lasers in Medical Science, 31 (2016) 297–304. [DOI] [PubMed] [Google Scholar]
- [227].Ma W, Wang T, Zang L, Jiang Z, Zhang Z, Bi L, Cao W, Bactericidal effects of hematoporphyrin monomethyl ether-mediated blue-light photodynamic therapy against Staphylococcus aureus, Photochemical & Photobiological Sciences, 18 (2019) 92–97. [DOI] [PubMed] [Google Scholar]
- [228].Iluz N, Maor Y, Keller N, Malik Z, The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus, Lasers in Surgery and Medicine, 50 (2018) 535–551. [DOI] [PubMed] [Google Scholar]
- [229].Huang J, Guo M, Jin S, Wu M, Yang C, Zhang G, Wang P, Ji J, Zeng Q, Wang X, Wang H, Antibacterial photodynamic therapy mediated by 5-aminolevulinic acid on methicillin-resistant Staphylococcus aureus, Photodiagnosis and Photodynamic Therapy, 28 (2019) 330–337. [DOI] [PubMed] [Google Scholar]
- [230].Souza BMN, Pinto JG, Pereira AHC, Miñán AG, Ferreira-Strixino J, Efficiency of Antimicrobial Photodynamic Therapy with Photodithazine® on MSSA and MRSA Strains, Antibiotics, 10 (2021) 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Wong T-W, Liao S-Z, Ko W-C, Wu C-J, Wu SB, Chuang Y-C, Huang l.-H., Indocyanine Green—Mediated Photodynamic Therapy Reduces Methicillin-Resistant Staphylococcus aureus Drug Resistance, Journal of Clinical Medicine, 8 (2019) 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Yuwen L, Qiu Q, Xiu W, Yang K, Li Y, Xiao H, Yang W, Yang D, Wang L, Hyaluronidase-responsive phototheranostic nanoagents for fluorescence imaging and photothermal/photodynamic therapy of methicillin-resistant Staphylococcus aureus infections, Biomaterials Science, 9 (2021) 4484–4495. [DOI] [PubMed] [Google Scholar]
- [233].Li S, Cui S, Yin D, Zhu Q, Ma Y, Qian Z, Gu Y, Dual antibacterial activities of a chitosan-modified upconversion photodynamic therapy system against drug-resistant bacteria in deep tissue, Nanoscale, 9 (2017) 3912–3924. [DOI] [PubMed] [Google Scholar]
- [234].Sueoka K, Chikama T, Latief MA, Ko J-A, Kiuchi Y, Sakaguchi T, Obana A, Time-dependent antimicrobial effect of photodynamic therapy with TONS 504 on Pseudomonas aeruginosa, Lasers in Medical Science, 33 (2018) 1455–1460. [DOI] [PubMed] [Google Scholar]
- [235].Zhao Y, Lu Z, Dai X, Wei X, Yu Y, Chen X, Zhang X, Li C, Glycomimetic-Conjugated Photosensitizer for Specific Pseudomonas aeruginosa Recognition and Targeted Photodynamic Therapy, Bioconjugate Chemistry, 29 (2018) 3222–3230. [DOI] [PubMed] [Google Scholar]
- [236].Aspiroz C, Sevil M, Toyas C, Gilaberte Y, Photodynamic Therapy With Methylene Blue for Skin Ulcers Infected With Pseudomonas aeruginosa and Fusarium spp, Actas Dermo-Sifiliográficas (English Edition), 108 (2017) e45–e48. [DOI] [PubMed] [Google Scholar]
- [237].Gao Q, Huang D, Deng Y, Yu W, Jin Q, Ji J, Fu G, Chlorin e6 (Ce6)-loaded supramolecular polypeptide micelles with enhanced photodynamic therapy effect against Pseudomonas aeruginosa, Chemical Engineering Journal, 417 (2021) 129334. [Google Scholar]
- [238].Kim J.-w., Lim H-S, Effect of antimicrobial photodynamic therapy with Radachlorin and a 660 nm diode laser on Pseudomonas aeruginosa: An in vitro study, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101931. [DOI] [PubMed] [Google Scholar]
- [239].Liu W, Zhang Y, You W, Su J, Yu S, Dai T, Huang Y, Chen X, Song X, Chen Z, Near-infrared-excited upconversion photodynamic therapy of extensively drug-resistant Acinetobacter baumannii based on lanthanide nanoparticles, Nanoscale, 12 (2020) 13948–13957. [DOI] [PubMed] [Google Scholar]
- [240].Li J, Qin M, Liu C, Ma W, Zeng X, Ji Y, Antimicrobial photodynamic therapy against multidrug-resistant Acinetobacter baumannii clinical isolates mediated by aloe-emodin: An in vitro study, Photodiagnosis and Photodynamic Therapy, 29 (2020) 101632. [DOI] [PubMed] [Google Scholar]
- [241].Wang Y, Li J, Geng S, Wang X, Cui Z, Ma W, Yuan M, Liu C, Ji Y, Aloe-emodin-mediated antimicrobial photodynamic therapy against multidrug-resistant Acinetobacter baumannii: An in vivo study, Photodiagnosis and Photodynamic Therapy, 34 (2021) 102311. [DOI] [PubMed] [Google Scholar]
- [242].Boluki E, Pourhajibagher M, Bahador A, The combination of antimicrobial photocatalysis and antimicrobial photodynamic therapy to eradicate the extensively drug-resistant colistin resistant Acinetobacter baumannii, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101816. [DOI] [PubMed] [Google Scholar]
- [243].Marcolan De Mello M, De Barros PP, de Cassia Bernardes R, Alves SR, Ramanzini NP, Figueiredo-Godoi LMA, Prado ACC, Jorge AOC, Junqueira JC, Antimicrobial photodynamic therapy against clinical isolates of carbapenem-susceptible and carbapenem-resistant Acinetobacter baumannii, Lasers in Medical Science, 34 (2019) 1755–1761. [DOI] [PubMed] [Google Scholar]
- [244].Wilson M, Mia N, Sensitisation of Candida albicans to killing by low-power laser light, Journal of oral pathology & medicine, 22 (1993) 354–357. [DOI] [PubMed] [Google Scholar]
- [245].de Souza SC, Junqueira JC, Balducci I, Koga-lto CY, Munin E, Jorge AOC, Photosensitization of different Candida species by low power laser light, Journal of Photochemistry and Photobiology B: Biology, 83 (2006) 34–38. [DOI] [PubMed] [Google Scholar]
- [246].Junqueira JC, da Silva Martins J, Faria RL, Colombo CED, Jorge AOC, Photodynamic therapy for the treatment of buccal candidiasis in rats, Lasers in medical science, 24 (2009) 877–884. [DOI] [PubMed] [Google Scholar]
- [247].Bliss JM, Bigelow CE, Foster TH, Haidaris CG, Susceptibility of Candida species to photodynamic effects of photofrin, Antimicrobial agents and chemotherapy, 48 (2004) 2000–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Lazarova G, Effect of glutathione on rose bengal photosensitized yeast damage, Microbios, 75 (1993) 39–43. [PubMed] [Google Scholar]
- [249].Bertoloni G, Rossi F, Valduga G, Jori G, AN H, Van Lier J, Photosensitizing activity of water-and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells, Microbios, 71 (1992) 33–46. [PubMed] [Google Scholar]
- [250].Dovigo LN, Pavarina AC, de Oliveira Mima EG, Giampaolo ET, Vergani CE, Bagnato VS, Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata, Mycoses, 54 (2011) 123–130. [DOI] [PubMed] [Google Scholar]
- [251].Teichert M, Jones J, Usacheva M, Biel MA, Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 93 (2002) 155–160. [DOI] [PubMed] [Google Scholar]
- [252].Jackson Z, Meghji S, MacRobert A, Flenderson B, Wilson M, Killing of the yeast and hyphal forms of Candida albicans using a light-activated antimicrobial agent, Lasers in medical science, 14 (1999) 150–157. [DOI] [PubMed] [Google Scholar]
- [253].Chabrier-Roselló Y, Foster TH, Mitra S, Haidaris CG, Respiratory deficiency enhances the sensitivity of the pathogenic fungus Candida to photodynamic treatment, Photochemistry and photobiology, 84 (2008) 1141–1148. [DOI] [PubMed] [Google Scholar]
- [254].Dovigo LN, Carmello JC, de Souza Costa CA, Vergani CE, Brunetti IL, Bagnato VS, Pavarina AC, Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis, Sabouraudia, 51 (2013) 243–251. [DOI] [PubMed] [Google Scholar]
- [255].de Oliveira Mima EG, Pavarina AC, Dovigo LN, Vergani CE, de Souza Costa CA, Kurachi C, Bagnato VS, Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 109 (2010) 392–401. [DOI] [PubMed] [Google Scholar]
- [256].Mima E.G.d.O., Pavarina AC, Ribeiro DG, Dovigo LN, Vergani CE, Bagnato VS, Effectiveness of photodynamic therapy for the inactivation of Candida spp. on dentures: in vitro study, Photomedicine and laser surgery, 29 (2011) 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Smijs TG, Schuitmaker HJ, Photodynamic Inactivation of the Dermatophyte Trichophyton rubrum¶, Photochemistry and photobiology, 77 (2003) 556–560. [DOI] [PubMed] [Google Scholar]
- [258].Smijs TG, van der Haas RN, Lugtenburg J, Liu Y, de Jong RL, Schuitmaker HJ, Photodynamic Treatment of the Dermatophyte Trichophyton rubrum and its Microconidia with Porphyrin Photosensitize¶, Photochemistry and photobiology, 80 (2004) 197–202. [DOI] [PubMed] [Google Scholar]
- [259].Friedberg JS, Skema C, Baum ED, Burdick J, Vinogradov SA, Wilson DF, Horan AD, Nachamkin I, In vitro effects of photodynamic therapy on Aspergillus fumigatus, Journal of Antimicrobial Chemotherapy, 48 (2001) 105–107. [DOI] [PubMed] [Google Scholar]
- [260].Jackson M, Flower C, Shneerson J, Treatment of symptomatic pulmonary aspergillomas with intracavitary instillation of amphotericin B through an indwelling catheter, Thorax, 48(1993) 928–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Gonzales FP, Da Silva SH, Roberts DW, Braga GU, Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue, Photochemistry and photobiology, 86 (2010) 653–661. [DOI] [PubMed] [Google Scholar]
- [262].Sueoka K, Chikama T, Pertiwi YD, Ko J-A, Kiuchi Y, Sakaguchi T, Obana A, Antifungal efficacy of photodynamic therapy with TONS 504 for pathogenic filamentous fungi, Lasers in Medical Science, 34 (2019) 743–747. [DOI] [PubMed] [Google Scholar]
- [263].Samusenkov V, Tsarev V, Ippolitov E, Podporin M, Yumashev A, Vlasova N, Borisov V, Tsareva T, Substantiation of use of photodynamic therapy in experimental research in vitro with strains of periodontopathogenic bacteria and fungi Candida, Journal of Global Pharma Technology, 12 (2020) 178–186. [Google Scholar]
- [264].Martín Santiago MP, Gutknecht N, Martín-Carrillo N, Foronda P, Valladares B, Montero Gómez N, In vitro study of photodynamic therapy with visible laser systems applied to fungal infections, Lasers in Dental Science, 4 (2020) 103–110. [Google Scholar]
- [265].Paziani MH, Tonani L, de Menezes HD, Bachmann L, Wainwright M, Braga GÚL, von Zeska Kress MR, Antimicrobial photodynamic therapy with phenothiazinium photosensitizers in non-vertebrate model Galleria mellonella infected with Fusarium keratoplasticum and Fusarium moniliforme, Photodiagnosis and Photodynamic Therapy, 25 (2019) 197–203. [DOI] [PubMed] [Google Scholar]
- [266].Alrabiah M, Alsahhaf A, Alofi RS, Al-Aali KA, Abduljabbar T, Vohra F, Efficacy of photodynamic therapy versus local nystatin in the treatment of denture stomatitis: A randomized clinical study, Photodiagnosis and Photodynamic Therapy, 28 (2019) 98–101. [DOI] [PubMed] [Google Scholar]
- [267].Abduljabbar T, Al-Askar M, Baig MK, AlSowygh ZH, Kellesarian SV, Vohra F, Efficacy of photodynamic therapy in the inactivation of oral fungal colonization among cigarette smokers and non-smokers with denture stomatitis, Photodiagnosis and Photodynamic Therapy, 18 (2017) 50–53. [DOI] [PubMed] [Google Scholar]
- [268].Koren A, Salameh F, Sprecher E, Artzi O, Laser-assisted photodynamic therapy or laser-assisted amorolfine lacquer delivery for treatment of toenail onychomycosis: an open-label comparative study, Acta dermato-venereologica, 98 (2018) 467–468. [DOI] [PubMed] [Google Scholar]
- [269].Gilaberte Y, Robres MP, FrÍas MP, GarcÍa-Doval I, Rezusta A, Aspiroz C, Methyl aminolevulinate photodynamic therapy for onychomycosis: a multicentre, randomized, controlled clinical trial, Journal of the European Academy of Dermatology and Venereology, 31 (2017) 347–354. [DOI] [PubMed] [Google Scholar]
- [270].Oliveira G.B.d., Antonio JR, Antonio CR, Tomé FA, The association of fractional CO2 laser 10.600 nm and photodynamic therapy in the treatment of onychomycosis, Anais brasileiros de dermatologia, 90 (2015) 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Cutaneous Sporotrichosis Treated with Photodynamic Therapy: An in Vitro and in Vivo Study, Photomedicine and Laser Surgery, 32 (2014) 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Lyon JP, de Maria Pedroso e Silva Azevedo C, Moreira LM, de Lima CJ, de Resende MA, Photodynamic Antifungal Therapy Against Chromoblastomycosis, Mycopathologia, 172 (2011) 293–297. [DOI] [PubMed] [Google Scholar]
- [273].Cai Q, Yang L.-j., Chen J, Yang H, Gao Z.-q., Wang X-I, Successful Sequential Treatment with Itraconazole and ALA-PDT for Cutaneous Granuloma by Candida albicans: A Case Report and Literature Review, Mycopathologia, 183 (2018) 829–834. [DOI] [PubMed] [Google Scholar]
- [274].Shi L, Luo M, Chen WR, Hu C, Zhang G, Zhang F, Chen J, Mo X, Cai Q, Yang L, Wang X, In situ photoimmunotherapy for cutaneous granuloma caused by itraconazole-resistant Candida guilliermondii, Dermatologic Therapy, 29 (2016) 353–357. [DOI] [PubMed] [Google Scholar]
- [275].Alberdi Jerónimo E, Gómez C, 539 Successful treatment of extensive Pityriasis Versicolor by Photodynamic Therapy mediated by methylene blue, Journal of Investigative Dermatology, 139 (2019) S307. [Google Scholar]
- [276].Liu Z-H, Xia X-J, Successful sequential treatment with itraconazole and ALA-PDT for chromoblastomycosis because of Alternaria alternata, Dermatologic Therapy, 27 (2014) 357–360. [DOI] [PubMed] [Google Scholar]
- [277].Lu J, Li W, Zheng W.a., Huang R, Wu W, Successful treatment of kerion with itraconazole and ALA-PDT: A case report, Photodiagnosis and Photodynamic Therapy, 27 (2019) 385–387. [DOI] [PubMed] [Google Scholar]
- [278].Houang J, Perrone GG, Pedrinazzi C, Longo L, Mawad D, Boughton PC, Ruys AJ, Lauto A, Genetic Tolerance to Rose Bengal Photodynamic Therapy and Antifungal Clinical Application for Onychomycosis, Advanced Therapeutics, 2 (2019) 1800105. [Google Scholar]
- [279].da Silva AP, Carbinatto FM, Bagnato VS, Inada NM, A promising strategy for the treatment of onychomycosis with curcumin and photodynamic therapy, J. Pharm. Pharmacol, 3 (2015) 434–437. [Google Scholar]
- [280].Morgado LF, Trávolo ARF, Muehlmann LA, Narcizo PS, Nunes RB, Pereira PAG, Py-Daniel KR, Jiang C-S, Gu J, Azevedo RB, Longo JPF, Photodynamic Therapy treatment of onychomycosis with Aluminium-Phthalocyanine Chloride nanoemulsions: A proof of concept clinical trial, Journal of Photochemistry and Photobiology B: Biology, 173 (2017) 266–270. [DOI] [PubMed] [Google Scholar]
- [281].Alberdi E, Gómez C, Efficiency of methylene blue-mediated photodynamic therapy vs intense pulsed light in the treatment of onychomycosis in the toenails, Photodermatology, Photoimmunology & Photomedicine, 35 (2019) 69–77. [DOI] [PubMed] [Google Scholar]
- [282].Hirayama J, Abe H, Kamo N, SHINBO T, OHNISHI-YAMADA Y, KUROSAWA S, IKEBUCHI K, SEKIGUCHI S, Photoinactivation of vesicular stomatitis virus with fullerene conjugated with methoxy polyethylene glycol amine, Biological and Pharmaceutical Bulletin, 22 (1999) 1106–1109. [DOI] [PubMed] [Google Scholar]
- [283].Käsermann F, Kempf C, Photodynamic inactivation of enveloped viruses by buckminsterfullerene, Antiviral research, 34 (1997) 65–70. [DOI] [PubMed] [Google Scholar]
- [284].Käsermann F, Kempf C, Buckminsterfullerene and photodynamic inactivation of viruses, Reviews in Medical Virology, 8 (1998) 143–151. [DOI] [PubMed] [Google Scholar]
- [285].Lin Y-L, Lei H-Y, Wen Y-Y, Luh T-Y, Chou C-K, Liu H-S, Light-independent inactivation of dengue-2 virus by carboxyfullerene C3 isomer, Virology, 275 (2000) 258–262. [DOI] [PubMed] [Google Scholar]
- [286].Nims RW, Plavsic M, Polyomavirus inactivation–a review, Biologicals, 41 (2013) 63–70. [DOI] [PubMed] [Google Scholar]
- [287].Lee Y, Baron ED, Photodynamic therapy: current evidence and applications in dermatology, Seminars in cutaneous medicine and surgery, WB Saunders, 2011, pp. 199–209. [DOI] [PubMed] [Google Scholar]
- [288].Chen K, Chang B, Ju M, Zhang X, Gu H, Comparative study of photodynamic therapy vs. CO2 laser vaporization in treatment of condylomata acuminata, a randomized clinical trial, British Journal of Dermatology, 156 (2007) 516–520. [DOI] [PubMed] [Google Scholar]
- [289].SZEIMIES RM, Schleyer V, Moll I, Stocker M, Landthaler M, Karrer S, Adjuvant Photodynamic Therapy Does Not Prevent Recurrence of Condylomata Acuminata After Carbon Dioxide Laser Ablation—A Phase III, Prospective, Randomized, Bicentric, Double-Blind Study, Dermatologic surgery, 35 (2009) 757–764. [DOI] [PubMed] [Google Scholar]
- [290].Whelan SPJ, Vesicular Stomatitis Virus, in: Mahy BWJ, Van Regenmortel MHV (Eds.) Encyclopedia of Virology (Third Edition), Academic Press, Oxford, 2008, pp. 291–299. [Google Scholar]
- [291].Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M.-d., Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, Remdesivir for the Treatment of Covid-19 — Final Report, New England Journal of Medicine, 383 (2020) 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C, A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19, New England Journal of Medicine, 382 (2020) 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Conrado PCV, Sakita KM, Arita GS, Galinari CB, Gonçalves RS, Lopes LDG, Lonardoni MVC, Teixeira JJV, Bonfim-Mendonça PS, Kioshima ES, A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2, Photodiagnosis and Photodynamic Therapy, 34 (2021) 102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Dias LD, Blanco KC, Bagnato VS, COVID-19: Beyond the virus. The use of photodynamic therapy for the treatment of infections in the respiratory tract, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Moghissi K, Dixon K, Gibbins S, Does PDT have potential in the treatment of COVID 19 patients?, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Bhapkar S, Kumbhar N, Gacche R, Jagtap S, Jadhav U, Photodynamic Therapy (PDT): An Alternative Approach for Combating COVID-19, Biointerface Res. Appl. Chem, 11 (2021) 12808–12830. [Google Scholar]
- [297].Dias LD, Bagnato VS, An update on clinical photodynamic therapy for fighting respiratory tract infections: a promising tool against COVID-19 and its co-infections, Laser Physics Letters, 17 (2020) 083001. [Google Scholar]
- [298].Almeida A, Faustino MAF, Neves MGPMS, Antimicrobial Photodynamic Therapy in the Control of COVID-19, Antibiotics, 9 (2020) 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Law S, Lo C, Han J, Leung AW, Xu C, Antimicrobial photodynamic therapy with hypocrellin B against SARS-CoV-2 infection?, Photodiagnosis and Photodynamic Therapy, 34 (2021) 102297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Khorsandi K, Fekrazad S, Vahdatinia F, Farmany A, Fekrazad R, Nano antiviral photodynamic therapy: A probable biophysicochemical management modality in SARS-CoV-2, Expert Opinion on Drug Delivery, 18 (2021) 265–272. [DOI] [PubMed] [Google Scholar]
- [301].Fekrazad R, Photobiomodulation and Antiviral Photodynamic Therapy as a Possible Novel Approach in COVID-19 Management, Photobiomodulation, Photomedicine, and Laser Surgery, 38 (2020) 255–257. [DOI] [PubMed] [Google Scholar]
- [302].Queiroz GB, Foggiato AA, Neto JLT, da Silva DF, Photodynamic Therapy and possible action against Sars-Cov-2, Brazilian Journal of Development, 6 (2020) 52313–52327. [Google Scholar]
- [303].Nunes LP, Chalub LO, Strazzi-Sahyon HB, dos Santos PH, Cintra LTÂ, Sivieri-Araujo G, Photodynamic therapy as a potential oral disinfection protocol during COVID-19 outbreak, Photodiagnosis and Photodynamic Therapy, 33 (2021) 102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Law S, Lo C, Han J, Leung AW, Xu C, Photodynamic therapy with curcumin for combating SARS-CoV-2, Photodiagnosis and Photodynamic Therapy, 34 (2021) 102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Pinto M.V.d.M., Silva CHMB, Sampaio AR, Baron MV, Sancho AG, Fortuny E, Silva LFA, Picariello F, Carvalho R.r.M.a.d., Photodynamic Therapy with Phthalomethyl D: Perspectives against SARS-CoV-2, Journal of Biosciences and Medicines, Vol.08No.10 (2020) 13. [Google Scholar]
- [306].Ramires MCCH, Mattia MB, Tateno RY, Palma LF, Campos L, A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection, Photodiagnosis and Photodynamic Therapy, 33 (2021) 102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Svyatchenko VA, Nikonov SD, Mayorov AP, Gelfond ML, Loktev VB, Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin, Photodiagnosis and Photodynamic Therapy, 33 (2021) 102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Gendrot M, Andreani J, Duflot I, Boxberger M, Le Bideau M, Mosnier J, Jardot P, Fonta I, Rolland C, Bogreau H, Hutter S, La Scola B, Pradines B, Methylene blue inhibits replication of SARS-CoV-2 in vitro, International Journal of Antimicrobial Agents, 56 (2020) 106202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Pourhajibagher M, Azimi M, Haddadi-Asl V, Ahmadi H, Gholamzad M, Ghorbanpour S, Bahador A, Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model, Photodiagnosis and Photodynamic Therapy, 34 (2021) 102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Kipshidze N, White C, Kipshidze N, Reddy V, Dangas G, TCT CONNECT-218 Transcatheter Therapies For COVID-19, Journal of the American College of Cardiology, 76 (2020) B94. [Google Scholar]
- [311].Kipshidze NN, Kipshidze N, Horn JB, Transcatheter Endoluminal Phototherapy as a Possible Adjunct Treatment for Patients with COVID-19, Photobiomodulation, photomedicine, and laser surgery, 38 (2020) 579–580. [DOI] [PubMed] [Google Scholar]
- [312].Kipshidze N, Chekanov VS, Kipshidze N, Reddy VY, Dangas G, Transpulmonary electrotherapy for reduction of lung viral load of SARS-CoV-2 in patients with COVID-19, Medical Hypotheses, 143 (2020) 110071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Kipshidze N, Dangas G, White CJ, Kipshidze N, Siddiqui F, Lattimer CR, Carter CA, Fareed J, Viral Coagulopathy in Patients With COVID-19: Treatment and Care, Clinical and Applied Thrombosis/Hemostasis, 26 (2020) 1076029620936776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [314].Kipshidze N, Iversen P, Porter TR, Kipshidze N, Siddiqui F, Dangas G, Fareed J, Targeted, Site-Specific, Delivery Vehicles of Therapeutics for COVID-19 Patients. Brief Review, Clinical and Applied Thrombosis/Hemostasis, 26 (2020) 1076029620954911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kipshidze N, Yeo N, Kipshidze N, Photodynamic and sonodynamic therapy of acute hypoxemic respiratory failure in patients with COVID-19, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Kipshidze N, Yeo N, Kipshidze N, Photodynamic therapy for COVID-19, Nature Photonics, 14 (2020) 651–652. [Google Scholar]
- [317].Weber HM, Mehran YZ, Orthaber A, Saadat HH, Weber R, Wojcik M, Successful reduction of SARS-CoV-2 viral load by photodynamic therapy (PDT) verified by QPCR—a novel approach in treating patients in early infection stages, Med Clin Res, 5 (2020) 311–325. [Google Scholar]
- [318].Nogueira MS, Biophotonic telemedicine for disease diagnosis and monitoring during pandemics: overcoming COVID-19 and shaping the future of healthcare, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Nogueira MS, Biophotonics for pandemic control: large-area infection monitoring and microbial inactivation of COVID-19, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].e Silva L.F.d.C., de Carvalho MSN, Optical techniques for fast screening–Towards prevention of the coronavirus COVID-19 outbreak, Photodiagnosis and photodynamic therapy, 30 (2020) 101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Nogueira MS, Optical theranostics and treatment dosimetry for COVID-19 lung complications: towards increasing the survival rate of vulnerable populations, Photodiagnosis and photodynamic therapy, 31 (2020) 101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Nogueira MS, Ultraviolet-based biophotonic technologies for control and prevention of COVID-19, SARS and related disorders, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Khan MRH, Ali FAM, Islam MR, THz sensing of CoViD-19 disinfecting products using photonic crystal fiber, Sensing and Bio-Sensing Research, 33 (2021) 100447. [Google Scholar]
- [324].Scholtz A, Ramoji A, Silge A, Jansson JR, de Moura IG, Popp J, Sram JP, Armani AM, COVID-19 Diagnostics: Past, Present, and Future, ACS Photonics, (2021). [DOI] [PubMed] [Google Scholar]
- [325].Cognetti JS, Steiner DJ, Abedin M, Bryan MR, Shanahan C, Tokranova N, Young E, Klose AM, Zavriyev A, Judy N, Piorek B, Meinhart C, Jakubowicz R, Warren H, Cady NC, Miller BL, Disposable photonics for cost-effective clinical bioassays: application to COVID-19 antibody testing, Lab on a Chip, 21 (2021) 2913–2921. [DOI] [PubMed] [Google Scholar]
- [326].Hanna R, Dalvi S, Sălăgean T, Bordea IR, Benedicenti S, Phototherapy as a rational antioxidant treatment modality in COVID-19 management; new concept and strategic approach: critical review, Antioxidants, 9 (2020) 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Soler M, Estevez MC, Cardenosa-Rubio M, Astua A, Lechuga LM, How Nanophotonic Label-Free Biosensors Can Contribute to Rapid and Massive Diagnostics of Respiratory Virus Infections: COVID-19 Case, ACS Sensors, 5 (2020) 2663–2678. [DOI] [PubMed] [Google Scholar]
- [328].Taha BA, Al Mashhadany Y, Bachok NN, Bakar AA, Hafiz Mokhtar MH, Dzulkefly Bin Zan MS, Arsad N, Detection of COVID-19 Virus on Surfaces Using Photonics: Challenges and Perspectives, Diagnostics, 11 (2021) 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Soler M, Scholtz A, Zeto R, Armani AM, Engineering photonics solutions for COVID-19, APL Photonics, 5 (2020) 090901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Taha BA, Perspectives of Photonics Technology to Diagnosis COVID–19 Viruses: A Short Review, Journal of Applied Sciences and Nanotechnology, 1 (2021) 1–6. [Google Scholar]
- [331].Illingworth CD, Cook SD, Acanthamoeba keratitis, Survey of ophthalmology, 42 (1998) 493–508. [DOI] [PubMed] [Google Scholar]
- [332].Enk CD, Fritsch C, Jonas F, Nasereddin A, Ingber A, Jaffe CL, Ruzicka T, Treatment of cutaneous leishmaniasis with photodynamic therapy, Archives of dermatology, 139 (2003) 432–434. [DOI] [PubMed] [Google Scholar]
- [333].Reithinger R, Dujardin J-C, Louzir H, Pirmez C, Alexander B, Brooker S, Cutaneous leishmaniasis, The Lancet infectious diseases, 7 (2007) 581–596. [DOI] [PubMed] [Google Scholar]
- [334].Baptista M, Wainwright M, Photodynamic antimicrobial chemotherapy (PACT) for the treatment of malaria, leishmaniasis and trypanosomiasis, Brazilian Journal of Medical and Biological Research, 44 (2011) 1–10. [DOI] [PubMed] [Google Scholar]
- [335].Gonzalez U, Pinart M, Reveiz L, Alvar J, Interventions for Old World cutaneous leishmaniasis, Cochrane database of systematic reviews, (2008). [DOI] [PubMed] [Google Scholar]
- [336].Sohl S, Kauer F, Paasch U, Simon JC, Photodynamic treatment of cutaneous leishmaniasis, JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 5 (2007) 128–130. [DOI] [PubMed] [Google Scholar]
- [337].Gardlo K, Horska Z, Enk CD, Rauch L, Megahed M, Ruzicka T, Fritsch C, Treatment of cutaneous leishmaniasis by photodynamic therapy, Journal of the American Academy of Dermatology, 48 (2003) 893–896. [DOI] [PubMed] [Google Scholar]
- [338].Stojiljkovic I, Evavold BD, Kumar V, Antimicrobial properties of porphyrins, Expert opinion on investigational drugs, 10 (2001) 309–320. [DOI] [PubMed] [Google Scholar]
- [339].Asilian A, Davami M, Comparison between the efficacy of photodynamic therapy and topical paromomycin in the treatment of Old World cutaneous leishmaniasis: a placebo-controlled, randomized clinical trial, Clinical and Experimental Dermatology: Clinical dermatology, 31 (2006) 634–637. [DOI] [PubMed] [Google Scholar]
- [340].Song D, Lindoso JAL, Oyafuso LK, Kanashiro EHY, Cardoso JL, Uchoa AF, Tardivo JP, Baptista MS, Photodynamic therapy using methylene blue to treat cutaneous leishmaniasis, Photomedicine and laser surgery, 29 (2011) 711–715. [DOI] [PubMed] [Google Scholar]
- [341].Akilov OE, Kosaka S, O’Riordan K, Hasan T, Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation, Photochemical & Photobiological Sciences, 6 (2007) 1067–1075. [DOI] [PubMed] [Google Scholar]
- [342].Akilov OE, Kosaka S, O’Riordan K, Hasan T, Parasiticidal effect of δ-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells, Experimental dermatology, 16 (2007) 651–660. [DOI] [PubMed] [Google Scholar]
- [343].Akilov OE, Yousaf W, Lukjan SX, Verma S, Hasan T, Optimization of topical photodynamic therapy with 3, 7-bis (di-n-butylamino) phenothiazin-5-ium bromide for cutaneous leishmaniasis, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 41 (2009) 358–365. [DOI] [PubMed] [Google Scholar]
- [344].Hernández IP, Montanari J, Valdivieso W, Morilla MJ, Romero EL, Escobar P, In vitro phototoxicity of ultradeformable liposomes containing chloroaluminum phthalocyanine against New World Leishmania species, Journal of Photochemistry and Photobiology B: Biology, 117 (2012) 157–163. [DOI] [PubMed] [Google Scholar]
- [345].Taylor VM, Cedeño DL, Muñoz DL, Jones MA, Lash TD, Young AM, Constantino MH, Esposito N, Vélez ID, Robledo SM, In vitro and in vivo studies of the utility of dimethyl and diethyl carbaporphyrin ketals in treatment of cutaneous leishmaniasis, Antimicrobial agents and chemotherapy, 55 (2011) 4755–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Kassab K, Dei D, Roncucci G, Jori G, Coppellotti O, Phthalocyanine-photosensitized inactivation of a pathogenic protozoan, Acanthamoeba palestinensis, Photochemical & Photobiological Sciences, 2 (2003) 668–672. [DOI] [PubMed] [Google Scholar]
- [347].Chen Z, Xuguang S, Zhiqun W, Ran L, In vitro amoebacidal activity of photodynamic therapy on Acanthamoeba, British journal of ophthalmology, 92 (2008) 1283–1286. [DOI] [PubMed] [Google Scholar]
- [348].Mito T, Suzuki T, Kobayashi T, Zheng X, Hayashi Y, Shiraishi A, Ohashi Y, Effect of photodynamic therapy with methylene blue on Acanthamoeba in vitro, Investigative ophthalmology & visual science, 53 (2012) 6305–6313. [DOI] [PubMed] [Google Scholar]
- [349].Lustigman S, Ben-Hur E, Photosensitized inactivation of Plasmodium falciparum in human red cells by phthalocyanines, Transfusion, 36 (1996) 543–546. [DOI] [PubMed] [Google Scholar]
- [350].Smith TG, Kain KC, Inactivation of Plasmodium falciparum by photodynamic excitation of Heme-cycle intermediates derived from d-aminolevulinic acid, The Journal of infectious diseases, 190 (2004) 184–191. [DOI] [PubMed] [Google Scholar]
- [351].Wagner SJ, Skripchenko A, Salata J, O’Sullivan AM, Cardo LJ, Inactivation of Leishmania donovani infantum and Trypanosoma cruzi in red cell suspensions with thiazole orange, Transfusion, 48 (2008) 1363–1367. [DOI] [PubMed] [Google Scholar]
- [352].Docampo R, Moreno S, Gadelha F, De Souza W, Cruz F, Prevention of Chagas’ disease resulting from blood transfusion by treatment of blood: toxicity and mode of action of gentian violet, Biomedical and environmental sciences: BES, 1 (1988) 406–413. [PubMed] [Google Scholar]
- [353].Docampo R, Moreno SN, Cruz FS, Enhancement of the cytotoxicity of crystal violet against Trypanosoma cruzi in the blood by ascorbate, Molecular and biochemical parasitology, 27 (1988) 241–247. [DOI] [PubMed] [Google Scholar]
- [354].Gottlieb P, Shen LG, Chimezie E, Bahng S, Kenney ME, Horowitz B, Ben-Hur E, Inactivation of Trypanosoma cruzi trypomastigote forms in blood components by photodynamic treatment with phthalocyanines, Photochemistry and photobiology, 62 (1995) 869–874. [DOI] [PubMed] [Google Scholar]
- [355].Gottlieb P, Margolis-Nunno H, Robinson R, Shen LG, Chimezie E, Horowitz B, Ben-Hur E, Inactivation of Trypanosoma cruzi trypomastigote forms in blood components with a psoralen and ultraviolet A light, Photochemistry and photobiology, 63 (1996) 562–565. [DOI] [PubMed] [Google Scholar]
- [356].(2021, February 17). Retrieved April 13, 2021, from https://www.cdc.gov/parasites/chagas/gen_info/detailed.html#intro.
- [357].Sepúlveda AAL, Arenas Velásquez AM, Patiño Linares IA, de Almeida L, Fontana CR, Garcia C, Graminha MAS, Efficacy of photodynamic therapy using TiO2 nanoparticles doped with Zn and hypericin in the treatment of cutaneous Leishmaniasis caused by Leishmania amazonensis, Photodiagnosis and Photodynamic Therapy, 30 (2020) 101676. [DOI] [PubMed] [Google Scholar]
- [358].Ribeiro JBP, Miranda-Vilela AL, Amorim AAS, Garcia RD, Moreira JR, Gomes CM, Takano GHS, de Oliveira GMF, Lima AV, da Silva ICR, Sampaio RNR, Study of the efficacy of N-methyl glucamine antimoniate (SbV) associated with photodynamic therapy using liposomal chloroaluminium phthalocyanine in the treatment of cutaneous leishmaniasis caused by Leishmania (L.) amazonensis in C57BL6 mice, Photodiagnosis and Photodynamic Therapy, 26 (2019) 261–269. [DOI] [PubMed] [Google Scholar]
- [359].de Oliveira de Siqueira LB, da Silva Cardoso V, Rodrigues IA, Vazquez-Villa AL, dos Santos EP, da Costa Leal Ribeiro Guimarãe B, dos Santos Cerqueira Coutinho C, Vermelho AB, Junior ER, Development and evaluation of zinc phthalocyanine nanoemulsions for use in photodynamic therapy forLeishmaniaspp, Nanotechnology, 28 (2017) 065101. [DOI] [PubMed] [Google Scholar]
- [360].Cabral FV, Sabino CP, Dimmer JA, Sauter IP, Cortez MJ, Ribeiro MS, Preclinical Investigation of Methylene Blue-mediated Antimicrobial Photodynamic Therapy on Leishmania Parasites Using Real-Time Bioluminescence, Photochemistry and Photobiology, 96 (2020) 604–610. [DOI] [PubMed] [Google Scholar]
- [361].Goldin H, Kohen S, Taxy J, Libman M, Cibull T, Billick K, Leishmania tropica infection of the ear treated with photodynamic therapy, JAAD Case Reports, 6 (2020) 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Aureliano DP, Lindoso JAL, de Castro Soares SR, Takakura CFH, Pereira TM, Ribeiro MS, Cell death mechanisms in Leishmania amazonensis triggered by methylene blue-mediated antiparasitic photodynamic therapy, Photodiagnosis and Photodynamic Therapy, 23 (2018) 1–8. [DOI] [PubMed] [Google Scholar]
- [363].Lopes SC, Silva RA, Novais MV, Coelho LD, Ferreira LA, Souza PE, Tedesco A, Azevedo RB, Aguiar MG, Oliveira MC, Topical photodynamic therapy with chloroaluminum phthalocyanine liposomes is as effective as systemic pentavalent antimony in the treatment of experimental cutaneous leishmaniasis, Photodiagnosis and Photodynamic Therapy, 28 (2019) 210–215. [DOI] [PubMed] [Google Scholar]
- [364].Navasconi TR, dos Reis VN, Freitas CF, Pereira PCS, Caetano W, Hioka N, Lonardoni MVC, Aristides SMA, Silveira TGV, Photodynamic Therapy With Bengal Rose and Derivatives Against Leishmania amazonensis, Journal of Lasers in Medical Sciences, 8 (2017) 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Johansen MB, Jemec GBE, Fabricius S, Effective treatment with photodynamic therapy of cutaneous leishmaniasis: A case report, Dermatologic Therapy, 32 (2019) e13022. [DOI] [PubMed] [Google Scholar]
- [366].Miranda N, Gerola AP, Novello CR, Ueda-Nakamura T, de Oliveira Silva S, Dias-Filho BP, Hioka N, de Mello JCP, Nakamura CV, Pheophorbide a, a compound isolated from the leaves of Arrabidaea chica, induces photodynamic inactivation of Trypanosoma cruzi, Photodiagnosis and Photodynamic Therapy, 19 (2017) 256–265. [DOI] [PubMed] [Google Scholar]
- [367].de Morais FAP, Enumo A, Gonçalves RS, Cesar GB, Miranda N, Vilsinski BH, Combuca da Silva R, Vataru Nakamura C, Hioka N, Caetano W, Hypericin photodynamic activity. Part III: in vitro evaluation in different nanocarriers against trypomastigotes of Trypanosoma cruzi, Photochemical & Photobiological Sciences, 18 (2019) 487–494. [DOI] [PubMed] [Google Scholar]
- [368].Barbosa AFS, Santos IP, Santos GMP, Bastos TM, Rocha VPC, Meira CS, Soares MBP, Pitta IR, Pinheiro ALB, Anti–Trypanosoma cruzi effect of the photodynamic antiparasitic chemotherapy using phenothiazine derivatives as photosensitizers, Lasers in Medical Science, 35 (2020) 79–85. [DOI] [PubMed] [Google Scholar]
- [369].Wainwright M, Dyes, flies, and sunny skies: photodynamic therapy and neglected tropical diseases, Coloration Technology, 133 (2017) 3–14. [Google Scholar]
- [370].Lechuga GC, Pereira MCS, Bourguignon SC, Heme metabolism as a therapeutic target against protozoan parasites, Journal of Drug Targeting, 27 (2019) 767–779. [DOI] [PubMed] [Google Scholar]
- [371].Chidi AE, Use of photodynamic therapy for the treatment of localised infections of acanthamoeba keratitis, University of Sunderland, 2020. [Google Scholar]
- [372].Silva Fonseca TH, Alacoque M, Silva Oliveira FM, Soares BM, Leite HV, Caliari MV, Gomes MA, Busatti H, Photodynamic therapy as a new approach to Trichomonas vaginalis inactivation, Photodiagnosis and Photodynamic Therapy, 22 (2018) 91–95. [DOI] [PubMed] [Google Scholar]
- [373].Machado SM, Pacheco-Soares C, Marciano FR, Lobo AO, da Silva NS, Photodynamic therapy in the cattle protozoan Tritrichomonas foetus cultivated on superhydrophilic carbon nanotube, Materials Science and Engineering: C, 36 (2014) 180–186. [DOI] [PubMed] [Google Scholar]
- [374].Amor TB, Jori G, Sunlight-activated insecticides: historical background and mechanisms of phototoxic activity, Insect Biochemistry and Molecular Biology, 30 (2000) 915–925. [DOI] [PubMed] [Google Scholar]
- [375].Amor TB, Bortolotto L, Jori G, Porphyrins and related compounds as photoactivatable insecticides. 3. Laboratory and field studies, Photochemistry and Photobiology, 71 (2000) 124–128. [DOI] [PubMed] [Google Scholar]
- [376].Lucantoni L, Magaraggia M, Lupidi G, Ouedraogo RK, Coppellotti O, Esposito F, Fabris C, Jori G, Habluetzel A, Novel, meso-substituted cationic porphyrin molecule for photo-mediated larval control of the dengue vector Aedes aegypti, PLoS Negl Trap Dis, 5 (2011) e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [377].Shao G, Jiang D-X, Xu H-H, Zeng W, Yu H-J, Tian Y-Q, Synthesis and photoactivated insecticidal activity of tetraethynylsilanes, Journal of Photochemistry and Photobiology B: Biology, 98 (2010) 52–56. [DOI] [PubMed] [Google Scholar]
- [378].Dondji B, Duchon S, Diabate A, Herve JP, Corbel V, Hougard J-M, Santus R, Schrevel J, Assessment of Laboratory and Field Assays of Sunlight-Induced Killing of Mosquito Larvae by Photosensitizers, Journal of Medical Entomology, 42 (2005) 652–656. [DOI] [PubMed] [Google Scholar]
- [379].Fabris C, Ouédraogo RK, Coppellotti O, Dabiré RK, Diabaté A, Di Martino P, Guidolin L, Jori G, Lucantoni L, Lupidi G, Martena V, Sawadogo SP, Soncin M, Habluetzel A, Efficacy of sunlight-activatable porphyrin formulates on larvae of Anopheles gambiae M and S molecular forms and An. arabiensis: A potential novel biolarvicide for integrated malaria vector control, Acta Tropica, 123 (2012) 239–243. [DOI] [PubMed] [Google Scholar]
- [380].Araújo BP, Silva EA, Rosa LP, Inada NM, Iermak I, Romano RA, Mezzacappo NF, Melo FF, Silva FC, Rocha MP, Silva RAA, Galantini MPL, Silveira EA, Garbuio M, Morpho-molecular mechanisms study of photodynamic therapy and curcumin larvicide action on wild mosquitoes larvae of genus <em>Aedes</em>, bioRxiv, (2020) 2020.2007.2013.200295. [Google Scholar]
- [381].De Souza L, Pratavieira S, Inada NM, Kurachi C, Corbi J, Guimarães FEG, Bagnato VS, Efficacy of photodynamic therapy against larvae of Aedes aegypti: confocal microscopy and fluorescence-lifetime imaging, Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII, International Society for Optics and Photonics, 2014, pp. 89472D. [Google Scholar]
- [382].Shiao S-H, Weng S-C, Luan L, Vicente M.d.G.H., Jiang X-J, Ng DK, Kolli BK, Chang KP, Novel phthalocyanines activated by dim light for mosquito larva-and cell-inactivation with inference for their potential as broad-spectrum photodynamic insecticides, Plos one, 14 (2019) e0217355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].de Souza LM, Venturini FP, Inada NM, Iermak I, Garbuio M, Mezzacappo NF, de Oliveira KT, Bagnato VS, Curcumin in formulations against Aedes aegypti: Mode of action, photolarvicidal and ovicidal activity, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101840. [DOI] [PubMed] [Google Scholar]
- [384].de Souza LM, Inada NM, Venturini FP, Carmona-Vargas CC, Pratavieira S, de Oliveira KT, Kurachi C, Bagnato VS, Photolarvicidal effect of curcuminoids from Curcuma longa Linn. against Aedes aegypti larvae, Journal of Asia-Pacific Entomology, 22 (2019) 151–158. [Google Scholar]
- [385].Lucantoni L, Magaraggia M, Lupidi G, Ouedraogo RK, Coppellotti O, Esposito F, Fabris C, Jori G, Habluetzel A, Novel, meso-substituted cationic porphyrin molecule for photo-mediated larval control of the dengue vector Aedes aegypti, PLoS neglected tropical diseases, 5 (2011) e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [386].Abdel-Kader MH, Eltayeb TA, Photodynamic Control of Malaria Vector, Noxious Insects and Parasites, Photodynamic Therapy: From Theory to Application, Springer, Berlin Heidelberg: 2014, pp. 269–291. [Google Scholar]
- [387].Mahmoud A, Tarek E-T, Chlorophyll derivative activated with sunlight for malaria vector control: a small-scale field study, Research Square, (2021). [Google Scholar]
- [388].Preuß A, Pfitzner M, Röder B, Mosquito larvae control by photodynamic inactivation of their intestinal flora – a proof of principal study on Chaoborus sp, Photochemical & Photobiological Sciences, 18 (2019) 2374–2380. [DOI] [PubMed] [Google Scholar]
- [389].Deda DK, Iglesias BA, Alves E, Araki K, Garcia CRS, Porphyrin Derivative Nanoformulations for Therapy and Antiparasitic Agents, Molecules, 25 (2020) 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Younis M, Hussein A, Aboelela H, Rashed G, The Potential Role of Photosensitizers in Fight against Mosquitoes: Phototoxicity of Rose Bengal against Culex Pipiens Larvae, Benha Medical Journal, 38 (2021) 1–10. [Google Scholar]
- [391].Terenziani F, Katan C, Badaeva E, Tretiak S, Blanchard-Desce M, Enhanced Two-Photon Absorption of Organic Chromophores: Theoretical and Experimental Assessments, Advanced Materials, 20 (2008) 4641–4678. [Google Scholar]
- [392].Pawlicki M, Collins HA, Denning RG, Anderson HL, Two-Photon Absorption and the Design of Two-Photon Dyes, Angewandte Chemie International Edition, 48 (2009) 3244–3266. [DOI] [PubMed] [Google Scholar]
- [393].He GS, Tan L-S, Zheng O, Prasad PN, Multiphoton Absorbing Materials: Molecular Designs, Characterizations, and Applications, Chemical Reviews, 108 (2008) 1245–1330. [DOI] [PubMed] [Google Scholar]
- [394].Flors C, Ogilby PR, Luis JG, Grillo TA, Izquierdo LR, Gentili P-L, Bussotti L, Nonell S, Phototoxic Phytoalexins. Processes that Compete with the Photosensitized Production of Singlet Oxygen by 9-Phenylphenalenones†, Photochemistry and Photobiology, 82 (2006) 95–103. [DOI] [PubMed] [Google Scholar]
- [395].Jensen P-G, Arnbjerg J, Tolbod LP, Toftegaard R, Ogilby PR, Influence of an Intermolecular Charge-Transfer State on Excited-State Relaxation Dynamics: Solvent Effect on the Methylnaphthalene–Oxygen System and its Significance for Singlet Oxygen Production, The Journal of Physical Chemistry A, 113 (2009) 9965–9973. [DOI] [PubMed] [Google Scholar]
- [396].Schweitzer C, Schmidt R, Physical Mechanisms of Generation and Deactivation of Singlet Oxygen, Chemical Reviews, 103 (2003) 1685–1758. [DOI] [PubMed] [Google Scholar]
- [397].Kristiansen M, Scurlock RD, lu KK, Ogilby PR, Charge-transfer state and singlet oxygen (1. DELTA, g O2) production in photoexcited organic molecule-molecular oxygen complexes, The Journal of Physical Chemistry, 95 (1991) 5190–5197. [Google Scholar]
- [398].McGarvey DJ, Wilkinson F, Worrall DR, Hobley J, Shaikh W, Picosecond absorption studies on the role of charge transfer interactions in the mechanism of quenching of triplet states by molecular oxygen, Chemical Physics Letters, 202 (1993) 528–534. [Google Scholar]
- [399].Yang X, Wang N, Zhang L, Dai L, Shao H, Jiang X, Organic nanostructure-based probes for two-photon imaging of mitochondria and microbes with emission between 430 nm and 640 nm, Nanoscale, 9 (2017) 4770–4776. [DOI] [PubMed] [Google Scholar]
- [400].Xie S, Manuguri S, Proietti G, Romson J, Fu Y, Inge AK, Wu B, Zhang Y, Häll D, Ramström O, Yan M, Design and synthesis of theranostic antibiotic nanodrugs that display enhanced antibacterial activity and luminescence, Proceedings of the National Academy of Sciences, 114(2017) 8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Li M, Gao Y, Yuan Y, Wu Y, Song Z, Tang BZ, Liu B, Zheng OC, One-step formulation of targeted aggregation-induced emission dots for image-guided photodynamic therapy of cholangiocarcinoma, ACS nano, 11 (2017) 3922–3932. [DOI] [PubMed] [Google Scholar]
- [402].Fan W, Yung B, Huang P, Chen X, Nanotechnology for multimodal synergistic cancer therapy, Chemical reviews, 117 (2017) 13566–13638. [DOI] [PubMed] [Google Scholar]
- [403].Lim C, Moon J, Sim T, Won WR, Lee ES, Youn YS, Oh KT, A nano-complex system to overcome antagonistic photo-chemo combination cancer therapy, Journal of Controlled Release, 295 (2019) 164–173. [DOI] [PubMed] [Google Scholar]
- [404].Li Y, Deng Y, Tian X, Ke H, Guo M, Zhu A, Yang T, Guo Z, Ge Z, Yang X, Chen H, Multipronged Design of Light-Triggered Nanoparticles To Overcome Cisplatin Resistance for Efficient Ablation of Resistant Tumor, ACS Nano, 9 (2015) 9626–9637. [DOI] [PubMed] [Google Scholar]
- [405].Wang Q, Dai Y, Xu J, Cai J, Niu X, Zhang L, Chen R, Shen Q, Huang W, Fan Q, All-in-one phototheranostics: single laser triggers NIR-II fluorescence/photoacoustic imaging guided photothermal/photodynamic/chemo combination therapy, Advanced Functional Materials, 29 (2019) 1901480. [Google Scholar]
- [406].Li L, Shao C, Liu T, Chao Z, Chen H, Xiao F, He H, Wei Z, Zhu Y, Wang H, Zhang X, Wen Y, Yang B, He F, Tian L, An NIR-II-Emissive Photosensitizer for Hypoxia-Tolerant Photodynamic Theranostics, Advanced Materials, 32 (2020) 2003471. [DOI] [PubMed] [Google Scholar]
- [407].Zhang Z, Xu W, Kang M, Wen H, Guo H, Zhang P, Xi L, Li K, Wang L, Wang D, Tang BZ, An All-Round Athlete on the Track of Phototheranostics: Subtly Regulating the Balance between Radiative and Nonradiative Decays for Multimodal Imaging-Guided Synergistic Therapy, Advanced Materials, 32 (2020) 2003210. [DOI] [PubMed] [Google Scholar]
- [408].Shi J, Li J, Wang Y, Cheng J, Zhang CY, Recent advances in MoS 2-based photothermal therapy for cancer and infectious disease treatment, Journal of materials chemistry B, 8 (2020) 5793–5807. [DOI] [PubMed] [Google Scholar]
- [409].Cao Y, Dou J-H, Zhao N.-j., Zhang S, Zheng Y-Q, Zhang J-P, Wang J-Y, Pei J, Wang Y, Highly efficient NIR-II photothermal conversion based on an organic conjugated polymer, Chemistry of Materials, 29 (2017) 718–725. [Google Scholar]
- [410].Roper DK, Ahn W, Hoepfner M, Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles, The Journal of Physical Chemistry C, 111 (2007) 3636–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Cao Y, Wang Z, Liao S, Wang J, Wang Y, A Light-Activated Microheater for the Remote Control of Enzymatic Catalysis, Chemistry–A European Journal, 22 (2016) 1152–1158. [DOI] [PubMed] [Google Scholar]
- [412].Long R, Li Y, Song L, Xiong Y, Coupling solar energy into reactions: materials design for surface plasmon-mediated catalysis, Small, 11 (2015) 3873–3889. [DOI] [PubMed] [Google Scholar]
- [413].Chen H, Shao L, Ming T, Sun Z, Zhao C, Yang B, Wang J, Understanding the photothermal conversion efficiency of gold nanocrystals, small, 6 (2010) 2272–2280. [DOI] [PubMed] [Google Scholar]
- [414].Baffou G, Quidant R, Thermo-plasmonics: using metallic nanostructures as nano-sources of heat, Laser & Photonics Reviews, 7 (2013) 171–187. [Google Scholar]
- [415].Jain PK, Lee KS, El-Sayed IH, El-Sayed MA, Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine, The journal of physical chemistry B, 110 (2006) 7238–7248. [DOI] [PubMed] [Google Scholar]
- [416].Jaque D, Maestro LM, Del Rosal B, Haro-Gonzalez P, Benayas A, Plaza J, Rodriguez EM, Sole JG, Nanoparticles for photothermal therapies, nanoscale, 6 (2014) 9494–9530. [DOI] [PubMed] [Google Scholar]
- [417].Kumar CS, Mohammad F, Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery, Advanced drug delivery reviews, 63 (2011) 789–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Beik J, Abed Z, Ghoreishi FS, Hosseini-Nami S, Mehrzadi S, Shakeri-Zadeh A, Kamrava SK, Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications, Journal of Controlled Release, 235 (2016) 205–221. [DOI] [PubMed] [Google Scholar]
- [419].Hervault A, Thanh NTK, Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer, Nanoscale, 6 (2014) 11553–11573. [DOI] [PubMed] [Google Scholar]
- [420].Datta NR, Bodis S, Hyperthermia with radiotherapy reduces tumour alpha/beta: Insights from trials of thermoradiotherapy vs radiotherapy alone, Radiotherapy and Oncology, 138 (2019) 1–8. [DOI] [PubMed] [Google Scholar]
- [421].Huang X, Jain PK, El-Sayed IH, El-Sayed MA, Plasmonic photothermal therapy (PPTT) using gold nanoparticles, Lasers in medical science, 23 (2008) 217–228. [DOI] [PubMed] [Google Scholar]
- [422].Luo Y, Li J, Liu X, Tan L, Cui Z, Feng X, Yang X, Liang Y, Li Z, Zhu S, Zheng Y, Yeung KWK, Yang C, Wang X, Wu S, Dual Metal–Organic Framework Heterointerface, ACS Central Science, 5 (2019) 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [423].Chu KF, Dupuy DE, Thermal ablation of tumours: biological mechanisms and advances in therapy, Nature Reviews Cancer, 14 (2014) 199–208. [DOI] [PubMed] [Google Scholar]
- [424].Mao HY, Laurent S, Chen W, Akhavan O, Imani M, Ashkarran AA, Mahmoudi M, Graphene: promises, facts, opportunities, and challenges in nanomedicine, Chemical reviews, 113 (2013) 3407–3424. [DOI] [PubMed] [Google Scholar]
- [425].Feng L, Wu L, Qu X, New horizons for diagnostics and therapeutic applications of graphene and graphene oxide, Advanced Materials, 25 (2013) 168–186. [DOI] [PubMed] [Google Scholar]
- [426].Zou Y, Zhang Y, Yu Q, Chen H, Photothermal bactericidal surfaces: killing bacteria using light instead of biocides, Biomaterials Science, (2020). [DOI] [PubMed] [Google Scholar]
- [427].Li B, Tan L, Liu X, Li Z, Cui Z, Liang Y, Zhu S, Yang X, Yeung KWK, Wu S, Superimposed surface plasma resonance effect enhanced the near-infrared photocatalytic activity of Au@ Bi2WO6 coating for rapid bacterial killing, Journal of hazardous materials, 380 (2019) 120818. [DOI] [PubMed] [Google Scholar]
- [428].Wang Y, Wei T, Qu Y, Zhou Y, Zheng Y, Huang C, Zhang Y, Yu Q, Chen H, Smart, photothermally activated, antibacterial surfaces with thermally triggered bacteria-releasing properties, ACS applied materials & interfaces, 12 (2019) 21283–21291. [DOI] [PubMed] [Google Scholar]
- [429].Qu Y, Wei T, Zhao J, Jiang S, Yang P, Yu Q, Chen H, Regenerable smart antibacterial surfaces: full removal of killed bacteria via a sequential degradable layer, Journal of Materials Chemistry B, 6 (2018) 3946–3955. [DOI] [PubMed] [Google Scholar]
- [430].Liao J, Shi K, Jia Y, Wu Y, Qian Z, Gold nanorods and nanohydroxyapatite hybrid hydrogel for preventing bone tumor recurrence via postoperative photothermal therapy and bone regeneration promotion, Bioactive materials, 6 (2021) 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [431].Chen J, Li X, Zhao X, Wu Q, Zhu H, Mao Z, Gao C, Doxorubicin-conjugated pH-responsive gold nanorods for combined photothermal therapy and chemotherapy of cancer, Bioactive materials, 3 (2018) 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].Cheng L, Cai Z, Zhao J, Wang F, Lu M, Deng L, Cui W, Black phosphorus-based 2D materials for bone therapy, Bioactive Materials, 5 (2020) 1026–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [433].Shao J, Ruan C, Xie H, Li Z, Wang H, Chu PK, Yu XF, Black-Phosphorus-Incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer, Advanced Science, 5 (2018) 1700848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Shao J, Xie H, Huang H, Li Z, Sun Z, Xu Y, Xiao Q, Yu X-F, Zhao Y, Zhang H, Wang H, Chu PK, Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy, Nature Communications, 7 (2016) 12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Wang X, Su K, Tan L, Liu X, Cui Z, Jing D, Yang X, Liang Y, Li Z, Zhu S, Yeung KWK, Zheng D, Wu S, Rapid and Highly Effective Noninvasive Disinfection by Hybrid Ag/CS@MnO2 Nanosheets Using Near-Infrared Light, ACS Applied Materials & Interfaces, 11 (2019) 15014–15027. [DOI] [PubMed] [Google Scholar]
- [436].Ouyang R, Cao P, Jia P, Wang H, Zong T, Dai C, Yuan J, Li Y, Sun D, Guo N, Miao Y, Zhou S, Bistratal Au@Bi2S3 nanobones for excellent NIR-triggered/multimodal imaging-guided synergistic therapy for liver cancer, Bioactive Materials, 6 (2021) 386–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [437].Jung HS, Verwilst P, Sharma A, Shin J, Sessler JL, Kim JS, Organic molecule-based photothermal agents: an expanding photothermal therapy universe, Chemical Society Reviews, 47 (2018) 2280–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [438].Wei T, Yu Q, Chen H, Antibacterial Coatings: Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way (Adv. Healthcare Mater. 3/2019), Advanced Healthcare Materials, 8 (2019) 1970007. [DOI] [PubMed] [Google Scholar]
- [439].Mao C, Xiang Y, Liu X, Zheng Y, Yeung KWK, Cui Z, Yang X, Li Z, Liang Y, Zhu S, Wu S, Local Photothermal/Photodynamic Synergistic Therapy by Disrupting Bacterial Membrane To Accelerate Reactive Oxygen Species Permeation and Protein Leakage, ACS Applied Materials & Interfaces, 11 (2019) 17902–17914. [DOI] [PubMed] [Google Scholar]
- [440].Chen X, Zhang M, Li S, Li L, Zhang L, Wang T, Yu M, Mou Z, Wang C, Facile synthesis of polypyrrole@ metal–organic framework core–shell nanocomposites for dual-mode imaging and synergistic chemo-photothermal therapy of cancer cells, Journal of Materials Chemistry B, 5 (2017) 1772–1778. [DOI] [PubMed] [Google Scholar]
- [441].Shu G, Chen M, Song J, Xu X, Lu C, Du Y, Xu M, Zhao Z, Zhu M, Fan K, Fan X, Fang S, Tang B, Dai Y, Du Y, Ji J, Sialic acid-engineered mesoporous polydopamine nanoparticles loaded with SPIO and Fe3+ as a novel theranostic agent for T1/T2 dual-mode MRI-guided combined chemo-photothermal treatment of hepatic cancer, Bioactive Materials, 6 (2021) 1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [442].Jon S, Biomedical Nanomaterials. Edited by Yuliang Zhao and Youqing Shen, ChemMedChem, 13 (2018) 122–123. [Google Scholar]
- [443].Yoon H-J, Lee H-S, Lim J-Y, Park J-H, Liposomal indocyanine green for enhanced photothermal therapy, ACS applied materials & interfaces, 9 (2017) 5683–5691. [DOI] [PubMed] [Google Scholar]
- [444].Zheng X, Xing D, Zhou F, Wu B, Chen WR, Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy, Molecular pharmaceutics, 8 (2011) 447–456. [DOI] [PubMed] [Google Scholar]
- [445].Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P, Gao G, Pan H, Liu L, Ma A, Cui H, Ma Y, Cai L, Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy, ACS Nano, 10 (2016) 10049–10057. [DOI] [PubMed] [Google Scholar]
- [446].Zheng M, Yue C, Ma Y, Gong P, Zhao P, Zheng C, Sheng Z, Zhang P, Wang Z, Cai L, Single-step assembly of DOX/ICG loaded lipid–polymer nanoparticles for highly effective chemo-photothermal combination therapy, ACS nano, 7 (2013) 2056–2067. [DOI] [PubMed] [Google Scholar]
- [447].Cheng L, He W, Gong H, Wang C, Chen Q, Cheng Z, Liu Z, PEGylated micelle nanoparticles encapsulating a non-fluorescent near-infrared organic dye as a safe and highly-effective photothermal agent for in vivo cancer therapy, Advanced Functional Materials, 23 (2013) 5893–5902. [Google Scholar]
- [448].Yue C, Liu P, Zheng M, Zhao P, Wang Y, Ma Y, Cai L, IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy, Biomaterials, 34 (2013) 6853–6861. [DOI] [PubMed] [Google Scholar]
- [449].Luo S, Tan X, Fang S, Wang Y, Liu T, Wang X, Yuan Y, Sun H, Qi Q, Shi C, Mitochondria-targeted small-molecule fluorophores for dual modal cancer phototherapy, Advanced Functional Materials, 26 (2016) 2826–2835. [Google Scholar]
- [450].Zhang J, Liu Z, Lian P, Qian J, Li X, Wang L, Fu W, Chen L, Wei X, Li C, Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects, Chemical science, 7 (2016) 5995–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [451].Gordon PF, Gregory P, Organic chemistry in colour, Springer Science & Business Media; 2012. [Google Scholar]
- [452].Camerin M, Rello-Varona S, Villanueva A, Rodgers MA, Jori G, Metallo-naphthalocyanines as photothermal sensitisers for experimental tumours: In Vitro and in vivo studies, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 41 (2009) 665–673. [DOI] [PubMed] [Google Scholar]
- [453].Mathew S, Murakami T, Nakatsuji H, Okamoto H, Morone N, Heuser JE, Hashida M, Imahori H, Exclusive photothermal heat generation by a gadolinium bis (naphthalocyanine) complex and inclusion into modified high-density lipoprotein nanocarriers for therapeutic applications, Acs Nano, 7 (2013) 8908–8916. [DOI] [PubMed] [Google Scholar]
- [454].Lim C-K, Shin J, Lee Y-D, Kim J, Oh KS, Yuk SH, Jeong SY, Kwon IC, Kim S, Phthalocyanine-aggregated polymeric nanoparticles as tumor-homing near-infrared absorbers for photothermal therapy of cancer, Theranostics, 2 (2012) 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [455].Wei J-P, Chen X-L, Wang X-Y, Li J-C, Shi S-G, Liu G, Zheng N-F, Polyethylene glycol phospholipids encapsulated silicon 2, 3-naphthalocyanine dihydroxide nanoparticles (SiNcOH-DSPE-PEG (NH2) NPs) for single NIR laser induced cancer combination therapy, Chinese Chemical Letters, 28 (2017) 1290–1299. [Google Scholar]
- [456].Taratula O, Schumann C, Duong T, Taylor KL, Taratula O, Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy, Nanoscale, 7 (2015) 3888–3902. [DOI] [PubMed] [Google Scholar]
- [457].Zhou B, Li Y, Niu G, Lan M, Jia Q, Liang Q, Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer, ACS applied materials & interfaces, 8 (2016) 29899–29905. [DOI] [PubMed] [Google Scholar]
- [458].Jung HS, Lee J-H, Kim K, Koo S, Verwilst P, Sessler JL, Kang C, Kim JS, A mitochondria-targeted cryptocyanine-based photothermogenic photosensitizer, Journal of the American Chemical Society, 139 (2017) 9972–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [459].Cai Y, Liang P, Tang Q, Yang X, Si W, Huang W, Zhang Q, Dong X, Diketopyrrolopyrrole–triphenylamine organic nanoparticles as multifunctional reagents for photoacoustic imaging-guided photodynamic/photothermal synergistic tumor therapy, ACS nano, 11 (2017) 1054–1063. [DOI] [PubMed] [Google Scholar]
- [460].Cai Y, Si W, Tang Q, Liang P, Zhang C, Chen P, Zhang Q, Huang W, Dong X, Small-molecule diketopyrrolopyrrole-based therapeutic nanoparticles for photoacoustic imaging-guided photothermal therapy, Nano Research, 10 (2017) 794–801. [Google Scholar]
- [461].Pu K, Mei J, Jokerst JV, Hong G, Antaris AL, Chattopadhyay N, Shuhendler AJ, Kurosawa T, Zhou Y, Gambhir SS, Bao Z, Rao J, Diketopyrrolopyrrole-Based Semiconducting Polymer Nanoparticles for In Vivo Photoacoustic Imaging, Advanced Materials, 27 (2015) 5184–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [462].Liu H, Wang K, Yang C, Huang S, Wang M, Multifunctional polymeric micelles loaded with doxorubicin and poly (dithienyl-diketopyrrolopyrrole) for near-infrared light-controlled chemo-phototherapy of cancer cells, Colloids and Surfaces B: Biointerfaces, 157 (2017) 398–406. [DOI] [PubMed] [Google Scholar]
- [463].Spence GT, Hartland GV, Smith BD, Activated photothermal heating using croconaine dyes, Chemical Science, 4 (2013) 4240–4244. [Google Scholar]
- [464].Spence GT, Lo SS, Ke C, Destecroix H, Davis AP, Hartland GV, Smith BD, Near-infrared croconaine rotaxanes and doped nanoparticles for enhanced aqueous photothermal heating, Chemistry–A European Journal, 20 (2014) 12628–12635. [DOI] [PubMed] [Google Scholar]
- [465].Guha S, Shaw SK, Spence GT, Roland FM, Smith BD, Clean photothermal heating and controlled release from near-infrared dye doped nanoparticles without oxygen photosensitization, Langmuir, 31 (2015) 7826–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [466].Guha S, Shaw GK, Mitcham TM, Bouchard RR, Smith BD, Croconaine rotaxane for acid activated photothermal heating and ratiometric photoacoustic imaging of acidic pH, Chemical Communications, 52 (2016) 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [467].Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, Chan WC, Cao W, Wang LV, Zheng G, Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents, Nature materials, 10 (2011) 324–332. [DOI] [PubMed] [Google Scholar]
- [468].Jin CS, Lovell JF, Chen J, Zheng G, Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly, ACS nano, 7 (2013) 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [469].Zou Q, Abbas M, Zhao L, Li S, Shen G, Yan X, Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy, Journal of the American Chemical Society, 139 (2017) 1921–1927. [DOI] [PubMed] [Google Scholar]
- [470].Guo B, Feng G, Manghnani PN, Cai X, Liu J, Wu W, Xu S, Cheng X, Teh C, Liu B, A porphyrin-based conjugated polymer for highly efficient in vitro and in vivo photothermal therapy, Small, 12 (2016) 6243–6254. [DOI] [PubMed] [Google Scholar]
- [471].Ban Q, Bai T, Duan X, Kong J, Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers, Biomaterials science, 5 (2017) 190–210. [DOI] [PubMed] [Google Scholar]
- [472].Yang J, Choi J, Bang D, Kim E, Lim E-K, Park H, Suh J-S, Lee K, Yoo K-H, Kim E-K, Huh Y-M, Haam S, Convertible Organic Nanoparticles for Near-Infrared Photothermal Ablation of Cancer Cells, Angewandte Chemie International Edition, 50 (2011) 441–444. [DOI] [PubMed] [Google Scholar]
- [473].Cheng L, Yang K, Chen Q, Liu Z, Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer, ACS nano, 6 (2012) 5605–5613. [DOI] [PubMed] [Google Scholar]
- [474].Yang K, Xu H, Cheng L, Sun C, Wang J, Liu Z, In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles, Advanced materials, 24 (2012) 5586–5592. [DOI] [PubMed] [Google Scholar]
- [475].Wang C, Xu H, Liang C, Liu Y, Li Z, Yang G, Cheng L, Li Y, Liu Z, Iron oxide@ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect, ACS nano, 7 (2013) 6782–6795. [DOI] [PubMed] [Google Scholar]
- [476].Zha Z, Yue X, Ren Q, Dai Z, Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells, Advanced materials, 25 (2013) 777–782. [DOI] [PubMed] [Google Scholar]
- [477].Tian Q, Wang Q, Yao KX, Teng B, Zhang J, Yang S, Han Y, Multifunctional Polypyrrole@ Fe3O4 Nanoparticles for Dual-Modal Imaging and In Vivo Photothermal Cancer Therapy, Small, 10 (2014) 1063–1068. [DOI] [PubMed] [Google Scholar]
- [478].Song X, Gong H, Yin S, Cheng L, Wang C, Li Z, Li Y, Wang X, Liu G, Liu Z, Ultra-small iron oxide doped polypyrrole nanoparticles for in vivo multimodal imaging guided photothermal therapy, Advanced Functional Materials, 24 (2014) 1194–1201. [Google Scholar]
- [479].Zhu Y-D, Chen S-P, Zhao H, Yang Y, Chen X-Q, Sun J, Fan H-S, Zhang X-D, PPy@ MIL-100 nanoparticles as a pH-and near-IR-irradiation-responsive drug carrier for simultaneous photothermal therapy and chemotherapy of cancer cells, ACS applied materials & interfaces, 8 (2016) 34209–34217. [DOI] [PubMed] [Google Scholar]
- [480].Zhang H, Xiong L, Liao X, Huang K, Controlled-Release System of Small Molecules Triggered by the Photothermal Effect of Polypyrrole, Macromolecular rapid communications, 37 (2016) 149–154. [DOI] [PubMed] [Google Scholar]
- [481].Yang T, Liu L, Deng Y, Guo Z, Zhang G, Ge Z, Ke H, Chen H, Ultrastable near-infrared conjugated-polymer nanoparticles for dually photoactive tumor inhibition, Advanced Materials, 29 (2017) 1700487. [DOI] [PubMed] [Google Scholar]
- [482].Liu Y, Ai K, Liu J, Deng M, He Y, Lu L, Dopamine-melanin colloidal nanospheres: an efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy, Advanced materials, 25 (2013) 1353–1359. [DOI] [PubMed] [Google Scholar]
- [483].Gao F-P, Lin Y-X, Li L-L, Liu Y, Mayerhöffer U, Spenst P, Su J-G, Li J-Y, Würthner F, Wang H, Supramolecular adducts of squaraine and protein for noninvasive tumor imaging and photothermal therapy in vivo, Biomaterials, 35 (2014) 1004–1014. [DOI] [PubMed] [Google Scholar]
- [484].Huang S, Kannadorai RK, Chen Y, Liu Q, Wang M, A narrow-bandgap benzobisthiadiazole derivative with high near-infrared photothermal conversion efficiency and robust photostability for cancer therapy, Chemical Communications, 51 (2015) 4223–4226. [DOI] [PubMed] [Google Scholar]
- [485].Jiao Y, Liu K, Wang G, Wang Y, Zhang X, Supramolecular free radicals: near-infrared organic materials with enhanced photothermal conversion, Chemical science, 6(2015) 3975–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [486].Chen Y, Ai W, Guo X, Li Y, Ma Y, Chen L, Zhang H, Wang T, Zhang X, Wang Z, Mitochondria-Targeted Polydopamine Nanocomposite with AIE Photosensitizer for Image-Guided Photodynamic and Photothermal Tumor Ablation, Small, 15 (2019) 1902352. [DOI] [PubMed] [Google Scholar]
- [487].Wang J, Xu M, Wang K, Chen Z, Stable mesoporous silica nanoparticles incorporated with MoS2 and AIE for targeted fluorescence imaging and photothermal therapy of cancer cells, Colloids and Surfaces B: Biointerfaces, 174 (2019) 324–332. [DOI] [PubMed] [Google Scholar]
- [488].Wang K, Zhuang J, Chen L, Xu D, Zhang X, Chen Z, Wei Y, Zhang Y, One-pot synthesis of AIE based bismuth sulfide nanotheranostics for fluorescence imaging and photothermal therapy, Colloids and Surfaces B: Biointerfaces, 160 (2017) 297–304. [DOI] [PubMed] [Google Scholar]
- [489].Fan Z, Ren L, Zhang W, Li D, Zhao G, Yu J, AIE luminogen-functionalised mesoporous silica nanoparticles as nanotheranostic agents for imaging guided synergetic chemo-/photothermal therapy, Inorganic Chemistry Frontiers, 4 (2017) 833–839. [Google Scholar]
- [490].Zhao X, Fan Z, Qiao Y, Chen Y, Wang S, Yue X, Shen T, Liu W, Yang J, Gao H, Zhan X, Shang L, Yin Y, Zhao W, Ding D, Xi R, Meng M, AIEgens Conjugation Improves the Photothermal Efficacy and Near-Infrared Imaging of Heptamethine Cyanine IR-780, ACS Applied Materials & Interfaces, 12 (2020) 16114–16124. [DOI] [PubMed] [Google Scholar]
- [491].Wang K, Fan X, Zhao L, Zhang X, Zhang X, Li Z, Yuan Q, Zhang Q, Huang Z, Xie W, Zhang Y, Wei Y, Aggregation Induced Emission Fluorogens Based Nanotheranostics for Targeted and Imaging-Guided Chemo-Photothermal Combination Therapy, Small, 12 (2016) 6568–6575. [DOI] [PubMed] [Google Scholar]
- [492].Alifu N, Zebibula A, Qi J, Zhang H, Sun C, Yu X, Xue D, Lam JWY, Li G, Qian J, Tang BZ, Single-Molecular Near-Infrared-II Theranostic Systems: Ultrastable Aggregation-Induced Emission Nanoparticles for Long-Term Tracing and Efficient Photothermal Therapy, ACS Nano, 12 (2018) 11282–11293. [DOI] [PubMed] [Google Scholar]
- [493].Liu S, Zhou X, Zhang H, Ou H, Lam JW, Liu Y, Shi L, Ding D, Tang BZ, Molecular motion in aggregates: manipulating TICT for boosting photothermal theranostics, Journal of the American Chemical Society, 141 (2019) 5359–5368. [DOI] [PubMed] [Google Scholar]
- [494].Cai X, Liu J, Liew WH, Duan Y, Geng J, Thakor N, Yao K, Liao L-D, Liu B, Organic molecules with propeller structures for efficient photoacoustic imaging and photothermal ablation of cancer cells, Materials Chemistry Frontiers, 1 (2017) 1556–1562. [Google Scholar]
- [495].Zhao Z, Chen C, Wu W, Wang F, Du L, Zhang X, Xiong Y, He X, Cai Y, Kwok RTK, Lam JWY, Gao X, Sun P, Phillips DL, Ding D, Tang BZ, Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles, Nature Communications, 10 (2019) 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [496].Tian S, Bai H, Li S, Xiao Y, Cui X, Li X, Tan J, Huang Z, Shen D, Liu W, Wang P, Tang BZ, Lee C-S, Water-Soluble Organic Nanoparticles with Programable Intermolecular Charge Transfer for NIR-II Photothermal Anti-Bacterial Therapy, Angewandte Chemie International Edition, 60 (2021) 11758–11762. [DOI] [PubMed] [Google Scholar]
- [497].Chen Y, Gao Y, Chen Y, Liu L, Mo A, Peng O, Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment, Journal of Controlled Release, (2020). [DOI] [PubMed] [Google Scholar]
- [498].Xu J-W, Yao K, Xu Z-K, Nanomaterials with a photothermal effect for antibacterial activities: an overview, Nanoscale, 11 (2019) 8680–8691. [DOI] [PubMed] [Google Scholar]
- [499].Fan X, Yang F, Huang J, Yang Y, Nie C, Zhao W, Ma L, Cheng C, Zhao C, Haag R, Metal–organic-framework-derived 2D carbon nanosheets for localized multiple bacterial eradication and augmented anti-infective therapy, Nano letters, 19 (2019) 5885–5896. [DOI] [PubMed] [Google Scholar]
- [500].Liu Z, Tan L, Liu X, Liang Y, Zheng Y, Yeung KWK, Cui Z, Zhu S, Li Z, Wu S, Zn2+-assisted photothermal therapy for rapid bacteria-killing using biodegradable humic acid encapsulated MOFs, Colloids and Surfaces B: Biointerfaces, 188 (2020) 110781. [DOI] [PubMed] [Google Scholar]
- [501].Wang H, Zhou S, Guo L, Wang Y, Feng L, Intelligent Hybrid Hydrogels for Rapid in Situ Detection and Photothermal Therapy of Bacterial Infection, ACS Applied Materials & Interfaces, 12 (2020) 39685–39694. [DOI] [PubMed] [Google Scholar]
- [502].Oing G, Zhao X, Gong N, Chen J, Li X, Gan Y, Wang Y, Zhang Z, Zhang Y, Guo W, Luo Y, Liang X-J, Thermo-responsive triple-function nanotransporter for efficient chemo-photothermal therapy of multidrug-resistant bacterial infection, Nature Communications, 10 (2019) 4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [503].Yan LX, Chen LJ, Zhao X, Yan XP, pH switchable nanoplatform for in vivo persistent luminescence imaging and precise photothermal therapy of bacterial infection, Advanced Functional Materials, 30 (2020) 1909042. [Google Scholar]
- [504].Liu Y, Lin A, Liu J, Chen X, Zhu X, Gong Y, Yuan G, Chen L, Liu J, Enzyme-responsive mesoporous ruthenium for combined chemo-photothermal therapy of drug-resistant bacteria, ACS applied materials & interfaces, 11 (2019) 26590–26606. [DOI] [PubMed] [Google Scholar]
- [505].Wang H, Zhao B, Dong W, Zhong Y, Zhang X, Gong Y, Zhan R, Xing M, Zhang J, Luo G, Qian W, A dual-targeted platform based on graphene for synergistic chemo-photothermal therapy against multidrug-resistant Gram-negative bacteria and their biofilms, Chemical Engineering Journal, 393 (2020) 124595. [Google Scholar]
- [506].Fekrazad R, Khoei F, Hakimiha N, Bahador A, Photoelimination of Streptococcus mutans with two methods of photodynamic and photothermal therapy, Photodiagnosis and photodynamic therapy, 10 (2013) 626–631. [DOI] [PubMed] [Google Scholar]
- [507].Liu M, He D, Yang T, Liu W, Mao L, Zhu Y, Wu J, Luo G, Deng J, An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy, Journal of nanobiotechnology, 16 (2018) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [508].Zhang M, Zhang H, Feng J, Zhou Y, Wang B, Synergistic chemotherapy, physiotherapy and photothermal therapy against bacterial and biofilms infections through construction of chiral glutamic acid functionalized gold nanobipyramids, Chemical Engineering Journal, 393 (2020) 124778. [Google Scholar]
- [509].Younis MR, An RB, Yin Y-C, Wang S, Ye D, Xia X-H, Plasmonic nanohybrid with high photothermal conversion efficiency for simultaneously effective antibacterial/anticancer photothermal therapy, ACS Applied Bio Materials, 2 (2019) 3942–3953. [DOI] [PubMed] [Google Scholar]
- [510].Cui T, Wu S, Sun Y, Ren J, Qu X, Self-Propelled Active Photothermal Nanoswimmer for Deep-Layered Elimination of Biofilm In Vivo, Nano Letters, 20 (2020) 7350–7358. [DOI] [PubMed] [Google Scholar]
- [511].Bermúdez-Jiménez C, Niño-Martínez N, Patiño-Marín N, Martínez-Gutiérrez F, Ruiz F, Bach H, Martínez-Castañón G, Effective control of biofilms by photothermal therapy using a gold nanorod hydrogel, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 108 (2020) 333–342. [DOI] [PubMed] [Google Scholar]
- [512].Zhang Y, Xiu W, Gan S, Shan J, Ren S, Yuwen L, Weng L, Teng Z, Wang L, Antibody-functionalized mos2 nanosheets for targeted photothermal therapy of staphylococcus aureus focal infection, Frontiers in bioengineering and biotechnology, 7 (2019) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [513].He J, Shi M, Liang Y, Guo B, Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds, Chemical Engineering Journal, 394 (2020) 124888. [Google Scholar]
- [514].Wei T, Yu Q, Chen H, Responsive and synergistic antibacterial coatings: fighting against bacteria in a smart and effective way, Advanced healthcare materials, 8 (2019) 1801381. [DOI] [PubMed] [Google Scholar]
- [515].Anisimov AP, Amoako KK, Treatment of plague: promising alternatives to antibiotics, Journal of medical microbiology, 55 (2006) 1461–1475. [DOI] [PubMed] [Google Scholar]
- [516].Yang X, Chen Z, Fang J, Yang Q, Zhao W, Qian X, Zhou D, Liu C, Chen M, Freestanding 3D MoS2 nanosheets/graphene aerogel heterostructure as a recyclable photocatalyst for efficiently degrading antibiotic residues, Materials Letters, 252 (2019) 5–7. [Google Scholar]
- [517].Pal A, Jana TK, Roy T, Pradhan A, Maiti R, Choudhury SM, Chatterjee K, MoS2-TiO2 nanocomposite with excellent adsorption performance and high antibacterial activity, ChemistrySelect, 3 (2018) 81–90. [Google Scholar]
- [518].Yang X, Li J, Liang T, Ma C, Zhang Y, Chen H, Hanagata N, Su H, Xu M, Antibacterial activity of two-dimensional MoS 2 sheets, Nanoscale, 6 (2014) 10126–10133. [DOI] [PubMed] [Google Scholar]
- [519].Gao Q, Zhang X, Yin W, Ma D, Xie C, Zheng L, Dong X, Mei L, Yu J, Wang C, Gu Z, Zhao Y, Functionalized MoS2 Nanovehicle with Near-Infrared Laser-Mediated Nitric Oxide Release and Photothermal Activities for Advanced Bacteria-Infected Wound Therapy, Small, 14(2018) 1802290. [DOI] [PubMed] [Google Scholar]
- [520].Zhu M, Liu X, Tan L, Cui Z, Liang Y, Li Z, Yeung KWK, Wu S, Photo-responsive chitosan/Ag/MoS2 for rapid bacteria-killing, Journal of hazardous materials, 383 (2020) 121122. [DOI] [PubMed] [Google Scholar]
- [521].Alimohammadi F, Sharifian Gh M, Attanayake NH, Thenuwara AC, Gogotsi Y, Anasori B, Strongin DR, Antimicrobial properties of 2D MnO2 and MoS2 nanomaterials vertically aligned on graphene materials and Ti3C2 MXene, Langmuir, 34 (2018) 7192–7200. [DOI] [PubMed] [Google Scholar]
- [522].Moudgil A, Singh S, Mishra N, Mishra P, Das S, MoS2/TiO2 Hybrid Nanostructure-Based Field-Effect Transistor for Highly Sensitive, Selective, and Rapid Detection of Gram-Positive Bacteria, Advanced Materials Technologies, 5 (2020) 1900615. [Google Scholar]
- [523].Shen N, Photodisruption in biological tissues using femtosecond laser pulses, 2003. [Google Scholar]
- [524].Tsen K-T, Tsen S-WD, Chang C-L, Hung C-F, Wu T, Kiang JG, Inactivation of viruses with a very low power visible femtosecond laser, Journal of Physics: Condensed Matter, 19 (2007) 322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [525].Tsen K-T, Tsen S-WD, Chang C-L, Hung C-F, Wu T, Kiang JG, Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process, Journal of biomedical optics, 12 (2007) 064030. [DOI] [PubMed] [Google Scholar]
- [526].Tsen KT, Tsen S-WD, Sankey OF, Kiang JG, Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses, Journal of Physics: Condensed Matter, 19 (2007) 472201. [Google Scholar]
- [527].Tsen K-T, Tsen S-WD, Chang C-L, Hung C-F, Wu T-C, Ramakrishna B, Mossman K, Kiang JG, Inactivation of viruses with a femtosecond laser via impulsive stimulated Raman scattering, Optical Interactions with Tissue and Cells XIX, International Society for Optics and Photonics, 2008, pp. 68540N. [Google Scholar]
- [528].Tsen S-WD, Tsen Y-SD, Tsen K-T, Wu T, Selective inactivation of viruses with femtosecond laser pulses and its potential use for in vitro therapy, Journal of Healthcare Engineering, 1 (2010) 185–196. [Google Scholar]
- [529].Tsen K-T, Cope S, Vaiana S, Tsen S-WD, Fu Q, Lindsay SM, Li Z, Kiang JG, Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation mechanisms, Journal of biomedical optics, 16 (2011) 078003. [DOI] [PubMed] [Google Scholar]
- [530].Tsen S-WD, Chapa T, Beatty W, Tsen K-T, Yu D, Achilefu S, Inactivation of enveloped virus by laser-driven protein aggregation, Journal of biomedical optics, 17 (2012) 128002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [531].Tsen K-T, Tsen S-W, Fu Q, Lindsay S, Kibler K, Jacobs B, Wu TC, Karanam B, Jagu S, Roden R, Hung C-F, Sankey O, Ramakrishna B, Kiang J, Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser, Journal of Biomedical Optics, 14 (2009) 064042. [DOI] [PubMed] [Google Scholar]
- [532].Tsen K-T, Tsen S-WD, Chang C-L, Hung C-F, Wu T, Kiang JG, Inactivation of viruses by coherent excitations with a low power visible femtosecond laser, Virology journal, 4(2007) 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [533].Manevitch Z, Lev D, Hochberg M, Palhan M, Lewis A, Enk CD, Direct antifungal effect of femtosecond laser on Trichophyton rubrum onychomycosis, Photochemistry and photobiology, 86 (2010) 476–479. [DOI] [PubMed] [Google Scholar]
- [534].Tsen S-WD, Chapa T, Beatty W, Xu B, Tsen K-T, Achilefu S, Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus, Antiviral research, 110 (2014) 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [535].Tsen S-WD, Wu TC, Kiang JG, Tsen K-T, Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation, Journal of biomedical science, 19 (2012) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [536].Thomé Lima AMC, da Silva Sergio LP, da Silva Neto Trajano LA, de Souza BP, da Motta Mendes JP, Cardoso AFR, Figueira CP, dos Anjos Tavares B, Figueira DS, Mencalha AL, Trajano ETL, de Souza da Fonseca A, Photobiomodulation by dual-wavelength low-power laser effects on infected pressure ulcers, Lasers in Medical Science, 35 (2020) 651–660. [DOI] [PubMed] [Google Scholar]
- [537].Bornstein E, Hermans W, Gridley S, Manni J, Near-infrared photoinactivation of bacteria and fungi at physiologic temperatures, Photochemistry and photobiology, 85 (2009) 1364–1374. [DOI] [PubMed] [Google Scholar]
- [538].Krespi YP, Kizhner V, Laser-assisted nasal decolonization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, American journal of otolaryngology, 33 (2012) 572–575. [DOI] [PubMed] [Google Scholar]
- [539].Reed NG, The history of ultraviolet germicidal irradiation for air disinfection, Public health reports, 125 (2010) 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [540].(2016, March 9). “Radiation: Ultraviolet (UV) radiation.” Retrieved April 13, 2021, from https://www.who.int/news-room/q-a-detail/radiation-ultraviolet-(uv).
- [541].Gurzadyan GG, Görner H, Schulte-Frohlinde D, Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies, Radiation research, 141 (1995) 244–251. [PubMed] [Google Scholar]
- [542].Conner-Kerr T, Sullivan P, Gaillard J, Franklin M, Jones R, The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro, Ostomy/wound management, 44 (1998) 50–56. [PubMed] [Google Scholar]
- [543].Su Y, Sun J, Rao S, Cai Y, Yang Y, Photodynamic antimicrobial activity of hypocrellin A, Journal of Photochemistry and Photobiology B: Biology, 103 (2011) 29–34. [DOI] [PubMed] [Google Scholar]
- [544].Dean SJ, Petty A, Swift S, McGhee JJ, Sharma A, Shah S, Craig JP, Efficacy and safety assessment of a novel ultraviolet C device for treating corneal bacterial infections, Clinical & experimental ophthalmology, 39 (2011) 156–163. [DOI] [PubMed] [Google Scholar]
- [545].Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A, Dose requirements for UVC disinfection of catheter biofilms, Biofouling, 25 (2009) 289–296. [DOI] [PubMed] [Google Scholar]
- [546].Dai T, Tegos G, Rolz-Cruz G, Cumbie W, Hamblin M, Ultraviolet C inactivation of dermatophytes: implications for treatment of onychomycosis, British Journal of Dermatology, 158 (2008) 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [547].Sullivan P, Conner-Kerr T, A comparative study of the effects of UVC irradiation on select procaryotic and eucaryotic wound pathogens, Ostomy/wound management, 46 (2000) 28–34. [PubMed] [Google Scholar]
- [548].Freytes HA, Fernandez B, Fleming WC, Ultraviolet light in the treatment of indolent ulcers, Southern medical journal, 58 (1965) 223–226. [DOI] [PubMed] [Google Scholar]
- [549].Nussbaum EL, Biemann I, Mustard B, Comparison of ultrasound/ultraviolet-C and laser for treatment of pressure ulcers in patients with spinal cord injury, Physical therapy, 74 (1994) 812–823. [DOI] [PubMed] [Google Scholar]
- [550].Thai TP, Houghton PE, Keast DH, Campbell KE, Woodbury MG, ULTRAVIOLET LIGHT Cin THE TREATMENT OF CHRONIC WOUNDS WITH MRSA: ACase STUDY, Ostomy/wound management, 48 (2002) 52–60. [PubMed] [Google Scholar]
- [551].Thai TP, Keast DH, Campbell KE, Woodbury MG, Houghton PE, Effect of ultraviolet light C on bacterial colonization in chronic wounds, Ostomy/wound management, 51 (2005) 32–45. [PubMed] [Google Scholar]
- [552].Shimomura A, Tahara D, Tominaga M, Uchigiri S, Yamaguchi Y, Ishioka H, Nakahata A, The effect of ultraviolet rays on the prevention of exit-site infections, Adv Perit Dial, 11 (1995) 152–156. [PubMed] [Google Scholar]
- [553].Boker A, Rolz-Cruz G, Cumbie B, Kimball A, A single-center, prospective, open-label, pilot study of the safety, local tolerability, and efficacy of ultraviolet-C (UVC) phototherapy for the treatment of great toenail onychomycosis, Journal of the American Academy of Dermatology, 58 (2008) AB82. [Google Scholar]
- [554].Kitagawa H, Nomura T, Nazmul T, Omori K, Shigemoto N, Sakaguchi T, Ohge H, Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination, American Journal of Infection Control, 49 (2021) 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [555].Minamikawa T, Koma T, Suzuki A, Mizuno T, Nagamatsu K, Arimochi H, Tsuchiya K, Matsuoka K, Yasui T, Yasutomo K, Nomaguchi M, Quantitative evaluation of SARS-CoV-2 inactivation using a deep ultraviolet light-emitting diode, Scientific Reports, 11 (2021) 5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [556].Storm N, McKay LGA, Downs SN, Johnson RI, Birru D, de Samber M, Willaert W, Cennini G, Griffiths A, Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation, Scientific Reports, 10 (2020) 22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [557].Heilingloh CS, Aufderhorst UW, Schipper L, Dittmer U, Witzke O, Yang D, Zheng X, Sutter K, Trilling M, Alt M, Steinmann E, Krawczyk A, Susceptibility of SARS-CoV-2 to UV irradiation, American Journal of Infection Control, 48 (2020) 1273–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [558].Sabino CP, Sellera FP, Sales-Medina DF, Machado RRG, Durigon EL, Freitas-Junior LH, Ribeiro MS, UV-C (254 nm) lethal doses for SARS-CoV-2, Photodiagnosis and Photodynamic Therapy, 32 (2020) 101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [559].Türsen Ü, Türsen B, Lotti T, Ultraviolet and COVID-19 pandemic, Journal of Cosmetic Dermatology, 19 (2020) 2162–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [560].Yang H, Hu J, Li P, Zhang C, Ultraviolet germicidal irradiation for filtering facepiece respirators disinfection to facilitate reuse during COVID-19 pandemic: A review, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [561].Stawicki SP, Could tracheo-bronchial ultraviolet C irradiation be a valuable adjunct in the management of severe COVID-19 pulmonary infections?, International Journal of Academic Medicine, 6 (2020) 156. [Google Scholar]
- [562].Chiappa F, Frascella B, Vigezzi G, Moro M, Diamanti L, Gentile L, Lago P, Clementi N, Signorelli C, Mancini N, The efficacy of ultraviolet light-emitting technology against coronaviruses: a systematic review, Journal of Hospital Infection, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [563].Sosnin EA, Stoffels E, Erofeev MV, Kieft IE, Kunts SE, The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: a comparative approach, IEEE Transactions on Plasma Science, 32 (2004) 1544–1550. [Google Scholar]
- [564].Dai T, Tegos GP, Denis TG, Anderson D, Sinofsky E, Hamblin MR, Ultraviolet-C irradiation for prevention of central venous catheter-related infections: An in vitro study, Photochemistry and photobiology, 87 (2011) 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [565].Dai T, Kharkwal GB, Zhao J, Denis TGS, Wu Q, Xia Y, Huang L, Sharma SK, d’Enfert C, Hamblin MR, Ultraviolet-C light for treatment of Candida albicans burn infection in mice, Photochemistry and photobiology, 87 (2011) 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [566].Mohr H, Steil L, Gravemann U, Thiele T, Hammer E, Greinacher A, Müller TH, Völker U, BLOOD COMPONENTS: A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light, Transfusion, 49 (2009) 2612–2624. [DOI] [PubMed] [Google Scholar]
- [567].Sterenborg H, Van Der Putte S, Van Der Leun J, The dose-response relationship of tumorigenesis by ultraviolet radiation of 254 nm, Photochemistry and photobiology, 47 (1988) 245–253. [DOI] [PubMed] [Google Scholar]
- [568].Trevisan A, Piovesan S, Leonardi A, Bertocco M, Nicolosi P, Pelizzo MG, Angelini A, Unusual high exposure to ultraviolet-C radiation, Photochemistry and photobiology, 82 (2006) 1077–1079. [DOI] [PubMed] [Google Scholar]
- [569].Ullrich SE, Photoimmune suppression and photocarcinogenesis, Front Biosci, 7 (2002) 684–703. [DOI] [PubMed] [Google Scholar]
- [570].Young AR, Acute effects of UVR on human eyes and skin, Progress in biophysics and molecular biology, 92 (2006) 80–85. [DOI] [PubMed] [Google Scholar]
- [571].Sliney DH, Photoprotection of the eye–UV radiation and sunglasses, Journal of Photochemistry and Photobiology B: Biology, 64 (2001) 166–175. [DOI] [PubMed] [Google Scholar]
- [572].Roberts JE, Ocular phototoxicity, Journal of Photochemistry and Photobiology B: Biology, 64 (2001) 136–143. [DOI] [PubMed] [Google Scholar]
- [573].Johnson G, The environment and the eye, Eye, 18 (2004) 1235–1250. [DOI] [PubMed] [Google Scholar]
- [574].Griffiths C, Dearman R, Cumberbatch M, Kimber I, Cytokines and Langerhans cell mobilisation in mouse and man, Cytokine, 32 (2005) 67–70. [DOI] [PubMed] [Google Scholar]
- [575].Barr RM, Walker SL, Tsang W, Harrison GI, Ettehadi P, Greaves MW, Young AR, Suppressed alloantigen presentation, increased TNF-α, IL-1, IL-1Ra, IL-10, and modulation of TNF-R in UV-irradiated human skin, Journal of investigative dermatology, 112 (1999) 692–698. [DOI] [PubMed] [Google Scholar]
- [576].Brink N, Szamel M, Young A, Wittern K, Bergemann J, Comparative quantification of IL-1 β, IL-10, IL-10r, TNF α and IL-7 mRNA levels in UV-irradiated human skin in vivo, Inflammation Research, 49 (2000) 290–296. [DOI] [PubMed] [Google Scholar]
- [577].Oriel S, Nitzan Y, Photoinactivation of Candida albicans by its own endogenous porphyrins, Current microbiology, 60 (2010) 117–123. [DOI] [PubMed] [Google Scholar]
- [578].Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR, Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond?, Drug Resistance Updates, 15 (2012) 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [579].Feuerstein O, Ginsburg I, Dayan E, Veler D, Weiss E.l., Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum, Photochemistry and Photobiology, 81 (2005) 1186–1189. [DOI] [PubMed] [Google Scholar]
- [580].Takahama U, Shimizu-Takahama M, Egashira T, Reduction of exogenous cytochrome c by Neurospora crassa conidia: effects of superoxide dismutase and blue light, Journal of bacteriology, 152 (1982) 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [581].De Lucca AJ, Carter-Wientjes C, Williams KA, Bhatnagar D, Blue light (470 nm) effectively inhibits bacterial and fungal growth, Letters in applied microbiology, 55 (2012) 460–466. [DOI] [PubMed] [Google Scholar]
- [582].Guffey JS, Wilborn J, In vitro bactericidal effects of 405-nm and 470-nm blue light, Photomedicine and laser therapy, 24 (2006) 684–688. [DOI] [PubMed] [Google Scholar]
- [583].Maclean M, MacGregor SJ, Anderson JG, Woolsey G, Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array, Applied and environmental microbiology, 75 (2009) 1932–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [584].Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG, Bactericidal Effects of 405 nm Light Exposure Demonstrated by Inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in Liquid Suspensions and on Exposed Surfaces, The Scientific World Journal, 2012 (2012) 137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [585].Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR, Helicobacter pylori in patients can be killed by visible light, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 36 (2005) 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [586].Ashkenazi H, Malik Z, Harth Y, Nitzan Y, Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light, FEMS Immunology & Medical Microbiology, 35 (2003) 17–24. [DOI] [PubMed] [Google Scholar]
- [587].Fukui M, Yoshioka M, Satomura K, Nakanishi H, Nagayama M, Specific-wavelength visible light irradiation inhibits bacterial growth of Porphyromonas gingivalis, Journal of periodontal research, 43 (2008) 174–178. [DOI] [PubMed] [Google Scholar]
- [588].Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM, Phototargeting oral black-pigmented bacteria, Antimicrobial agents and chemotherapy, 49 (2005) 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [589].Nitzan Y, Salmon-Divon M, Shporen E, Malik Z, ALA induced photodynamic effects on gram positive and negative bacteria, Photochemical & Photobiological Sciences, 3 (2004) 430–435. [DOI] [PubMed] [Google Scholar]
- [590].Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK, Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 40 (2008) 734–737. [DOI] [PubMed] [Google Scholar]
- [591].Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D, Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro, Photomedicine and laser surgery, 27 (2009) 221–226. [DOI] [PubMed] [Google Scholar]
- [592].Kawada A, Aragane Y, Kameyama H, Sangen Y, Tezuka T, Acne phototherapy with a high-intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation, Journal of dermatological science, 30 (2002) 129–135. [DOI] [PubMed] [Google Scholar]
- [593].Maclean M, Macgregor S, Anderson J, Woolsey G, The role of oxygen in the visible-light inactivation of Staphylococcus aureus, Journal of photochemistry and photobiology B: Biology, 92 (2008) 180–184. [DOI] [PubMed] [Google Scholar]
- [594].Kim MM, Darafsheh A, Light Sources and Dosimetry Techniques for Photodynamic Therapy, Photochemistry and Photobiology, 96 (2020) 280–294. [DOI] [PubMed] [Google Scholar]
- [595].Zhou Z, Song J, Nie L, Chen X, Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy, Chemical Society Reviews, 45 (2016) 6597–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [596].Hu J, Tang Y.a., Elmenoufy AH, Xu H, Cheng Z, Yang X, Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment, Small, 11 (2015) 5860–5887. [DOI] [PubMed] [Google Scholar]
- [597].Smith AM, Mancini MC, Nie S, Second window for in vivo imaging, Nature Nanotechnology, 4 (2009) 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [598].Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR, The Nuts and Bolts of Low-level Laser (Light) Therapy, Annals of Biomedical Engineering, 40 (2012) 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [599].Dasa MK, Markos C, Maria M, Petersen CR, Moselund PM, Bang O, High-pulse energy supercontinuum laser for high-resolution spectroscopic photoacoustic imaging of lipids in the 1650-1850 nm region, Biomedical Optics Express, 9 (2018) 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [600].Hui J, Li R, Phillips EH, Goergen CJ, Sturek M, Cheng J-X, Bond-selective photoacoustic imaging by converting molecular vibration into acoustic waves, Photoacoustics, 4 (2016) 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [601].Hale GM, Querry MR, Optical Constants of Water in the 200-nm to 200-μm Wavelength Region, Appl. Opt, 12 (1973) 555–563. [DOI] [PubMed] [Google Scholar]
- [602].Anderson RR, Farinelli W, Laubach H, Manstein D, Yaroslavsky AN, Gubeli J III, Jordan K, Neil GR, Shinn M, Chandler W, Williams GP, Benson SV, Douglas DR, Dylla HF, Selective photothermolysis of lipid-rich tissues: A free electron laser study, Lasers in Surgery and Medicine, 38 (2006) 913–919. [DOI] [PubMed] [Google Scholar]
- [603].Van Veen R, Sterenborg HJ, Pifferi A, Torricelli A, Chikoidze E, Cubeddu R, Determination of visible near-IR absorption coefficients of mammalian fat using time-and spatially resolved diffuse reflectance and transmission spectroscopy, Journal of biomedical optics, 10(2005) 054004. [DOI] [PubMed] [Google Scholar]
- [604].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Photodynamic therapy of cancer: an update, CA: a cancer journal for clinicians, 61 (2011) 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [605].Ashley JW, Gemert M.J.C.v., Chapter 24 - Lasers in medicine, Electro-Optics Handbook, Second Edition, McGraw-Hill Education, New York, 2000. [Google Scholar]
- [606].Ogawa K, Kobuke Y, Recent advances in two-photon photodynamic therapy, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 8 (2008) 269–279. [DOI] [PubMed] [Google Scholar]
- [607].Wilson BC, Photodynamic Therapy for Cancer: Principles, Canadian Journal of Gastroenterology, 16 (2002) 743109. [DOI] [PubMed] [Google Scholar]
- [608].Luo S, Zhang E, Su Y, Cheng T, Shi C, A review of NIR dyes in cancer targeting and imaging, Biomaterials, 32 (2011) 7127–7138. [DOI] [PubMed] [Google Scholar]
- [609].Stolik S, Delgado JA, Pérez A, Anasagasti L, Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues, Journal of Photochemistry and Photobiology B: Biology, 57 (2000) 90–93. [DOI] [PubMed] [Google Scholar]
- [610].Clement M, Daniel G, Trelles M, Optimising the design of a broad-band light source for the treatment of skin, Journal of Cosmetic and Laser Therapy, 7 (2005) 177–189. [DOI] [PubMed] [Google Scholar]
- [611].Brancaleon L, Moseley H, Laser and Non-laser Light Sources for Photodynamic Therapy, Lasers in Medical Science, 17 (2002) 173–186. [DOI] [PubMed] [Google Scholar]
- [612].Juzeniene A, Juzenas P, Ma L-W, lani V, Moan J, Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy, Lasers in Medical Science, 19 (2004) 139–149. [DOI] [PubMed] [Google Scholar]
- [613].Szeimies R, Morton CA, Sidoroff A, Braathen LR, Photodynamic therapy for non-melanoma skin cancer, ACTA DERMATOVENEREOLOGICA-STOCKHOLM-, 85 (2005) 483. [DOI] [PubMed] [Google Scholar]
- [614].Henderson BW, Busch TM, Snyder JW, Fluence rate as a modulator of PDT mechanisms, Lasers in Surgery and Medicine, 38 (2006) 489–493. [DOI] [PubMed] [Google Scholar]
- [615].Beyer W, Systems for light application and dosimetry in photodynamic therapy, Journal of Photochemistry and Photobiology B: Biology, 36 (1996) 153–156. [DOI] [PubMed] [Google Scholar]
- [616].Wilson BC, Patterson MS, The physics, biophysics and technology of photodynamic therapy, Physics in Medicine and Biology, 53 (2008) R61–R109. [DOI] [PubMed] [Google Scholar]
- [617].Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T, Photophysics and photochemistry of photodynamic therapy: fundamental aspects, Lasers in Medical Science, 24 (2009) 259–268. [DOI] [PubMed] [Google Scholar]
- [618].Moore CM, Pendse D, Emberton M, Photodynamic therapy for prostate cancer—a review of current status and future promise, Nature Clinical Practice Urology, 6 (2009) 18–30. [DOI] [PubMed] [Google Scholar]
- [619].Powers SK, Cush SS, Walstad DL, Kwock L, Stereotactic Intratumoral Photodynamic Therapy for Recurrent Malignant Brain Tumors, Neurosurgery, 29 (1991) 688–696. [DOI] [PubMed] [Google Scholar]
- [620].Misba L, Khan AU, Enhanced photodynamic therapy using light fractionation against Streptococcus mutans biofilm: type I and type II mechanism, Future Microbiology, 13 (2018) 437–454. [DOI] [PubMed] [Google Scholar]
- [621].Sampaio LS, de Annunzio SR, de Freitas LM, Dantas LO, de Boni L, Donatoni MC, de Oliveira KT, Fontana CR, Influence of light intensity and irradiation mode on methylene blue, chlorin-e6 and curcumin-mediated photodynamic therapy against Enterococcus faecalis, Photodiagnosis and Photodynamic Therapy, 31 (2020) 101925. [DOI] [PubMed] [Google Scholar]
- [622].Demidova T, Hamblin M, Photodynamic therapy targeted to pathogens, International journal of immunopathology and pharmacology, 17 (2004) 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [623].(2020, March 2). “Getting Photodynamic Therapy.” Retrieved April 13, 2021, from https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation/photodynamic-therapy.html.
- [624].Petersen B, Wiegell S, Wulf H, Light protection of the skin after photodynamic therapy reduces inflammation: an unblinded randomized controlled study, British Journal of Dermatology, 171 (2014) 175–178. [DOI] [PubMed] [Google Scholar]
- [625].Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, Hennino A, Laurent PE, Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines, Vaccine, 25 (2007) 6423–6430. [DOI] [PubMed] [Google Scholar]
- [626].MA G, Arce CH. Byron KJ. Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations, Curr Med Res Opin, 26 (2010) 1519–1530. [DOI] [PubMed] [Google Scholar]
- [627].Derraik JG, Rademaker M, Cutfield WS, Pinto TE, Tregurtha S, Faherty A, Peart JM, Drury PL, Hofman PL, Effects of age, gender, BMI, and anatomical site on skin thickness in children and adults with diabetes, PLoS One, 9 (2014) e86637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [628].Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Through-skull fluorescence imaging of the brain in a new near-infrared window, Nature photonics, 8 (2014) 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [629].Salehpour F, Cassano P, Rouhi N, Hamblin MR, De Taboada L, Farajdokht F, Mahmoudi J, Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: a review of literature, Photobiomodulation, photomedicine, and laser surgery, 37 (2019) 581–595. [DOI] [PubMed] [Google Scholar]
- [630].Bashkatov A, Genina E, Kochubey V, Tuchin V, Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm, Journal of Physics D: Applied Physics, 38 (2005) 2543. [Google Scholar]
- [631].Helmchen F, Denk W, Deep tissue two-photon microscopy, Nature methods, 2 (2005) 932–940. [DOI] [PubMed] [Google Scholar]
- [632].May M (2019). “Shedding light on deep tissue: Multiphoton microscopy.” Retrieved April 13, 2021, from https://www.sciencemag.org/features/2019/03/shedding-light-deep-tissue-multiphoton-microscopy.
- [633].Zakir Hossain S, Golam Azam S, Enayetul Babar S, Cancer treatment using multiphoton photodynamic therapy, Molecular & Cellular Toxicology, 2 (2006) 1–6. [Google Scholar]
- [634].Maldonado J, González-Guerrero AB, Domínguez C, Lechuga LM, Label-free bimodal waveguide immunosensor for rapid diagnosis of bacterial infections in cirrhotic patients, Biosensors and Bioelectronics, 85 (2016) 310–316. [DOI] [PubMed] [Google Scholar]
- [635].Garcez AS, Ribeiro MS, Tegos GP, Núñez SC, Jorge AO, Hamblin MR, Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 39 (2007) 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [636].Lee MT, Bird PS, Walsh LJ, Photo-activated disinfection of the root canal: a new role for lasers in endodontics, Australian Endodontic Journal, 30 (2004) 93–98. [DOI] [PubMed] [Google Scholar]
- [637].Banavath HN, Allam SR, Valathati SS, Sharan A, Rajasekaran B, Femtosecond laser pulse assisted photoporation for drug delivery in Chronic myelogenous leukemia cells, Journal of Photochemistry and Photobiology B: Biology, 187 (2018) 35–40. [DOI] [PubMed] [Google Scholar]
- [638].Li R, Dhankhar D, Chen J, Cesario TC, Rentzepis PM, A tryptophan synchronous and normal fluorescence study on bacteria inactivation mechanism, Proceedings of the National Academy of Sciences, 116 (2019) 18822–18826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [639].Applegate MB, Perotto G, Kaplan DL, Omenetto FG, Biocompatible silk step-index optical waveguides, Biomedical optics express, 6 (2015) 4221–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [640].Jacques SL, Optical properties of biological tissues: a review, Physics in Medicine & Biology, 58 (2013) R37. [DOI] [PubMed] [Google Scholar]
- [641].Martincek I, Pudis D, Chalupova M, Technology for the preparation of PDMS optical fibers and some fiber structures, IEEE Photonics technology letters, 26 (2014) 1446–1449. [Google Scholar]
- [642].Missinne J, Kalathimekkad S, Van Hoe B, Bosman E, Vanfleteren J, Van Steenberge G, Stretchable optical waveguides, Optics Express, 22 (2014) 4168–4179. [DOI] [PubMed] [Google Scholar]
- [643].To C, Hellebrekers TL, Park Y-L, Highly stretchable optical sensors for pressure, strain, and curvature measurement, 2015 IEEE/RSJ international conference on intelligent robots and systems (IROS), IEEE, 2015, pp. 5898–5903. [Google Scholar]
- [644].Kwok SJ, Kim M, Lin HH, Seiler TG, Beck E, Shao P, Kochevar IE, Seiler T, Yun S-H, Flexible optical waveguides for uniform periscleral cross-linking, Investigative ophthalmology & visual science, 58 (2017) 2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [645].Guo J, Niu M, Yang C, Highly flexible and stretchable optical strain sensing for human motion detection, Optica, 4 (2017) 1285–1288. [Google Scholar]
- [646].Kolle M, Lethbridge A, Kreysing M, Baumberg JJ, Aizenberg J, Vukusic P, Bio-inspired band-gap tunable elastic optical multilayer fibers, Advanced materials, 25 (2013) 2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [647].Martincek I, Pudis D, Gaso P, Fabrication and optical characterization of strain variable PDMS biconical optical fiber taper, IEEE Photonics technology letters, 25 (2013) 2066–2069. [Google Scholar]
- [648].Paek J, Cho I, Kim J, Microrobotic tentacles with spiral bending capability based on shape-engineered elastomeric microtubes, Scientific reports, 5 (2015) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [649].Kaufman JJ, Tao G, Shabahang S, Deng DS, Fink Y, Abouraddy AF, Thermal drawing of high-density macroscopic arrays of well-ordered sub-5-nm-diameter nanowires, Nano letters, 11 (2011) 4768–4773. [DOI] [PubMed] [Google Scholar]
- [650].Shabahang S, Tao G, Kaufman JJ, Qiao Y, Wei L, Bouchenot T, Gordon AP, Fink Y, Bai Y, Hoy RS, Abouraddy AF, Controlled fragmentation of multimaterial fibres and films via polymer cold-drawing, Nature, 534 (2016) 529–533. [DOI] [PubMed] [Google Scholar]
- [651].Tao G, Kaufman JJ, Shabahang S, Naraghi RR, Sukhov SV, Joannopoulos JD, Fink Y, Dogariu A, Abouraddy AF, Digital design of multimaterial photonic particles, Proceedings of the National Academy of Sciences, 113 (2016) 6839–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [652].Meikle JL, American plastic: a cultural history, Rutgers University Press; 1995. [Google Scholar]
- [653].Shan D, Zhang C, Kalaba S, Mehta N, Kim GB, Liu Z, Yang J, Flexible biodegradable citrate-based polymeric step-index optical fiber, Biomaterials, 143 (2017) 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [654].Kunthadong P, Molloy R, Worajittiphon P, Leejarkpai T, Kaabbuathong N, Punyodom W, Biodegradable plasticized blends of poly (L-lactide) and cellulose acetate butyrate: from blend preparation to biodegradability in real composting conditions, Journal of Polymers and the Environment, 23 (2015) 107–113. [Google Scholar]
- [655].Kim M, An J, Kim KS, Choi M, Humar M, Kwok SJ, Dai T, Yun SH, Optical lens-microneedle array for percutaneous light delivery, Biomedical optics express, 7 (2016) 4220–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [656].Nizamoglu S, Gather MC, Humar M, Choi M, Kim S, Kim KS, Hahn SK, Scarcelli G, Randolph M, Redmond RW, Yun SH, Bioabsorbable polymer optical waveguides for deep-tissue photomedicine, Nature Communications, 7 (2016) 10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [657].Choi WJ, Reif R, Yousefi S, Wang RK, Improved microcirculation imaging of human skin in vivo using optical microangiography with a correlation mapping mask, Journal of biomedical optics, 19 (2014) 036010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [658].Jain A, Yang AH, Erickson D, Gel-based optical waveguides with live cell encapsulation and integrated microfluidics, Optics letters, 37 (2012) 1472–1474. [DOI] [PubMed] [Google Scholar]
- [659].Manocchi AK, Domachuk P, Omenetto FG, Yi H, Facile fabrication of gelatin-based biopolymeric optical waveguides, Biotechnology and bioengineering, 103 (2009) 725–732. [DOI] [PubMed] [Google Scholar]
- [660].Parker ST, Domachuk P, Amsden J, Bressner J, Lewis JA, Kaplan DL, Omenetto FG, Biocompatible silk printed optical waveguides, Advanced Materials, 21 (2009) 2411–2415. [Google Scholar]
- [661].Qiao X, Qian Z, Li J, Sun H, Han Y, Xia X, Zhou J, Wang C, Wang Y, Wang C, Synthetic engineering of spider silk fiber as implantable optical waveguides for low-loss light guiding, ACS applied materials & interfaces, 9 (2017) 14665–14676. [DOI] [PubMed] [Google Scholar]
- [662].Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J, Müller cells are living optical fibers in the vertebrate retina, Proceedings of the National Academy of Sciences, 104 (2007) 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [663].Agte S, Junek S, Matthias S, Ulbricht E, Erdmann I, Wurm A, Schild D, Käs JA, Reichenbach A, Müller glial cell-provided cellular light guidance through the vital guinea-pig retina, Biophysical journal, 101 (2011) 2611–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [664].Bezryadina A, Hansson T, Gautam R, Wetzel B, Siggins G, Kalmbach A, Lamstein J, Gallardo D, Carpenter EJ, Ichimura A, Morandotti R, Chen Z, Nonlinear Self-Action of Light through Biological Suspensions, Physical Review Letters, 119 (2017) 058101. [DOI] [PubMed] [Google Scholar]
- [665].Xin H, Li Y, Liu X, Li B, Escherichia coli-based biophotonic waveguides, Nano letters, 13 (2013) 3408–3413. [DOI] [PubMed] [Google Scholar]
- [666].Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, Mason RP, Serrano CA, Schug KA, Tang L, Development of aliphatic biodegradable photoluminescent polymers, Proceedings of the National Academy of Sciences, 106 (2009) 10086–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [667].Yang J, Webb AR, Ameer GA, Novel citric acid-based biodegradable elastomers for tissue engineering, Advanced Materials, 16 (2004) 511–516. [Google Scholar]
- [668].Yang J, Webb AR, Pickerill SJ, Hageman G, Ameer GA, Synthesis and evaluation of poly (diol citrate) biodegradable elastomers, Biomaterials, 27 (2006) 1889–1898. [DOI] [PubMed] [Google Scholar]
- [669].Bahram M, Mohseni N, Moghtader M, An Introduction to Hydrogels and Some Recent Applications, in: Majee SB (Ed.) Emerging Concepts in Analysis and Applications of Hydrogels, Intechopen: 2016, pp. 9–38. [Google Scholar]
- [670].Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP, Preparation and characterization of poly (L-lactic acid) foams, Polymer, 35 (1994) 1068–1077. [Google Scholar]
- [671].Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, Vacanti JP, Langer R, Mikos AG, In vitro degradation of porous poly (L-lactic acid) foams, Biomaterials, 21 (2000) 1595–1605. [DOI] [PubMed] [Google Scholar]
- [672].Lu L, Peter SJ, Lyman MD, Lai H-L, Leite SM, Tamada JA, Uyama S, Vacanti JP, Langer R, Mikos AG, In vitro and in vivo degradation of porous poly (DL-lactic-co-glycolic acid) foams, Biomaterials, 21 (2000) 1837–1845. [DOI] [PubMed] [Google Scholar]
- [673].Gentile P, Chiono V, Carmagnola I, Hatton PV, An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering, International journal of molecular sciences, 15 (2014) 3640–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [674].Kujala S, Mannila A, Karvonen L, Kieu K, Sun Z, Natural silk as a photonics component: A study on its light guiding and nonlinear optical properties, Scientific reports, 6 (2016) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [675].Jiao Y, Gyawali D, Stark JM, Akcora P, Nair P, Tran RT, Yang J, A rheological study of biodegradable injectable PEGMC/HA composite scaffolds, Soft Matter, 8 (2012) 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [676].Su L-C, Xie Z, Zhang Y, Nguyen KT, Yang J, Study on the antimicrobial properties of citrate-based biodegradable polymers, Frontiers in bioengineering and biotechnology, 2 (2014) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [677].Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J, Citric-acid-derived photo-cross-linked biodegradable elastomers, Journal of Biomaterials Science, Polymer Edition, 21 (2010) 1761–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [678].Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J, Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure, Biomaterials, 33 (2012) 7972–7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [679].Qiu H, Yang J, Kodali P, Koh J, Ameer GA, A citric acid-based hydroxyapatite composite for orthopedic implants, Biomaterials, 27 (2006) 5845–5854. [DOI] [PubMed] [Google Scholar]
- [680].Kang Y, Yang J, Khan S, Anissian L, Ameer GA, A new biodegradable polyester elastomer for cartilage tissue engineering, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 77 (2006) 331–339. [DOI] [PubMed] [Google Scholar]
- [681].Yang J, Motlagh D, Webb AR, Ameer GA, Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering, Tissue engineering, 11 (2005) 1876–1886. [DOI] [PubMed] [Google Scholar]
- [682].Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L, Yang J, Development of biodegradable crosslinked urethane-doped polyester elastomers, Biomaterials, 29 (2008) 4637–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [683].Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HK, Yang J, Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery, Biomaterials, 31 (2010) 9092–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [684].Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, Kapadia M, Carroll TJ, Ameer GA, Modulating expanded polytetrafluoroethylene vascular graft host response via citric acid-based biodegradable elastomers, Advanced Materials, 18 (2006) 1493–1498. [Google Scholar]
- [685].Guo J, Wang W, Hu J, Xie D, Gerhard E, Nisic M, Shan D, Qian G, Zheng S, Yang J, Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives, Biomaterials, 85 (2016) 204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [686].Sun D, Chen Y, Tran RT, Xu S, Xie D, Jia C, Wang Y, Guo Y, Zhang Z, Guo J, Yang J, Jin D, Bai X, Citric Acid-based Hydroxyapatite Composite Scaffolds Enhance Calvarial Regeneration, Scientific Reports, 4 (2014) 6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [687].Zhang Y, Tran RT, Qattan IS, Tsai Y-T, Tang L, Liu C, Yang J, Fluorescence imaging enabled urethane-doped citrate-based biodegradable elastomers, Biomaterials, 34 (2013) 4048–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [688].Gyawali D, Nair P, Kim HK, Yang J, Citrate-based biodegradable injectable hydrogel composites for orthopedic applications, Biomaterials science, 1 (2013) 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [689].Tran RT, Wang L, Zhang C, Huang M, Tang W, Zhang C, Zhang Z, Jin D, Banik B, Brown JL, Xie Z, Bai X, Yang J, Synthesis and characterization of biomimetic citrate-based biodegradable composites, Journal of Biomedical Materials Research Part A, 102 (2014) 2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [690].Xie D, Guo J, Mehdizadeh MR, Tran RT, Chen R, Sun D, Qian G, Jin D, Bai X, Yang J, Development of injectable citrate-based bioadhesive bone implants, Journal of Materials Chemistry B, 3 (2015) 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [691].Tran RT, Palmer M, Tang S-J, Abell TL, Yang J, Injectable drug-eluting elastomeric polymer: a novel submucosal injection material, Gastrointestinal endoscopy, 75 (2012) 1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [692].Kibbe MR, Martinez J, Popowich DA, Kapadia MR, Ahanchi SS, Aalami OO, Jiang Q, Webb AR, Yang J, Carroll T, Ameer GA, Citric acid-based elastomers provide a biocompatible interface for vascular grafts, Journal of Biomedical Materials Research Part A, 93A (2010) 314–324. [DOI] [PubMed] [Google Scholar]
- [693].Guo Y, Tran RT, Xie D, Wang Y, Nguyen DY, Gerhard E, Guo J, Tang J, Zhang Z, Bai X, Yang J, Citrate-based biphasic scaffolds for the repair of large segmental bone defects, Journal of Biomedical Materials Research Part A, 103 (2015) 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [694].Tang J, Guo J, Li Z, Yang C, Xie D, Chen J, Li S, Li S, Kim GB, Bai X, Zhang Z, Yang J, A fast degradable citrate-based bone scaffold promotes spinal fusion, Journal of Materials Chemistry B, 3 (2015) 5569–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [695].Shan D, Kothapalli S-R, Ravnic DJ, Gerhard E, Kim JP, Guo J, Ma C, Guo J, Gui L, Sun L, Lu D, Yang J, Development of Citrate-Based Dual-Imaging Enabled Biodegradable Electroactive Polymers, Advanced Functional Materials, 28 (2018) 1801787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [696].Wadajkar AS, Kadapure T, Zhang Y, Cui W, Nguyen KT, Yang J, Dual-imaging enabled cancer-targeting nanoparticles, Advanced healthcare materials, 1 (2012) 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [697].Nair A, Thevenot P, Dey J, Shen J, Sun M-W, Yang J, Tang L, Novel polymeric scaffolds using protein microbubbles as porogen and growth factor carriers, Tissue Engineering Part C: Methods, 16 (2010) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [698].Kim JP, Xie Z, Creer M, Liu Z, Yang J, Citrate-based fluorescent materials for low-cost chloride sensing in the diagnosis of cystic fibrosis, Chemical science, 8 (2017) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [699].Xie Z, Su Y, Kim GB, Selvi E, Ma C, Aragon-Sanabria V, Hsieh JT, Dong C, Yang J, Immune Cell-Mediated Biodegradable Theranostic Nanoparticles for Melanoma Targeting and Drug Delivery, Small, 13 (2017) 1603121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [700].Ma C, Tian X, Kim JP, Xie D, Ao X, Shan D, Lin C, Hudock MR, Bai X, Yang J, Citrate-based materials fuel human stem cells by metabonegenic regulation, Proceedings of the National Academy of Sciences, 115 (2018) E11741–E11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [701].Xie Z, Kim JP, Cai Q, Zhang Y, Guo J, Dhami RS, Li L, Kong B, Su Y, Schug KA, Yang J, Synthesis and characterization of citrate-based fluorescent small molecules and biodegradable polymers, Acta Biomaterialia, 50 (2017) 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [702].Ma C, Gerhard E, Lu D, Yang J, Citrate chemistry and biology for biomaterials design, Biomaterials, 178 (2018) 383–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [703].Wang J, Dong J, Optical waveguides and integrated optical devices for medical diagnosis, health monitoring and light therapies, Sensors, 20 (2020) 3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [704].Shan D, Gerhard E, Zhang C, Tierney JW, Xie D, Liu Z, Yang J, Polymeric biomaterials for biophotonic applications, Bioactive materials, 3 (2018) 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [705].Zinoviev KE, González-Guerrero AB, Domínguez C, Lechuga LM, Integrated bimodal waveguide interferometric biosensor for label-free analysis, Journal of lightwave technology, 29 (2011) 1926–1930. [Google Scholar]
- [706].Mukundan H, Kumar S, Price DN, Ray SM, Lee Y-J, Min S, Eum S, Kubicek-Sutherland J, Resnick JM, Grace WK, Anderson AS, Hwang SH, Cho SN, Via LE, Barry C, Sakamuri R, Swanson B.l., Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a waveguide-based optical biosensor, Tuberculosis, 92 (2012) 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [707].Bechinger C, Giebel K-F, Schnell M, Leiderer P, Deising HB, Bastmeyer M, Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus, Science, 285 (1999) 1896–1899. [DOI] [PubMed] [Google Scholar]
- [708].Kamil YM, Bakar MA, Mustapa M, Yaacob M, Abidin N, Syahir A, Lee H, Mahdi M, Label-free Dengue E protein detection using a functionalized tapered optical fiber sensor, Sensors and Actuators B: Chemical, 257 (2018) 820–828. [Google Scholar]
- [709].Zhang H, Huang J, Li T, Svanberg S, Svanberg K, Optical detection of middle ear infection using spectroscopic techniques: phantom experiments, Journal of biomedical optics, 20 (2015) 057001. [DOI] [PubMed] [Google Scholar]
- [710].Choi M, Choi JW, Kim S, Nizamoglu S, Hahn SK, Yun SH, Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo, Nature photonics, 7 (2013) 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [711].Bisland SK, Chien C, Wilson BC, Burch S, Pre-clinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis, Photochemical & Photobiological Sciences, 5 (2006) 31–38. [DOI] [PubMed] [Google Scholar]
- [712].Jori G, Fabris C, Soncin M, Ferro S, Coppellotti O, Dei D, Fantetti L, Chiti G, Roncucci G, Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 38 (2006) 468–481. [DOI] [PubMed] [Google Scholar]
- [713].Soukos NS, Chen PS-Y, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Luschke Bammann L, Fontana CR, Doukas AG, Stashenko PP, Photodynamic Therapy for Endodontic Disinfection, Journal of Endodontics, 32 (2006) 979–984. [DOI] [PubMed] [Google Scholar]
- [714].Soukos NS, Chen PS-Y, Morris JT, Ruggiero K, Abernethy AD, Som S, Foschi F, Doucette S, Bammann LL, Fontana CR, Photodynamic therapy for endodontic disinfection, Journal of endodontics, 32 (2006) 979–984. [DOI] [PubMed] [Google Scholar]
- [715].Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC III, Lindmark WR, McGrath JR, Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: A pilot clinical trial, Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery, 41 (2009) 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [716].Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC III, Lindmark WR, McGrath JR, Hamblin MR, Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: A pilot clinical trial, Lasers in Surgery and Medicine, 41 (2009) 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [717].Abaya TVF, Implantable light delivery interfaces for optical neural stimulation, Ph. D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2013. [Google Scholar]
- [718].An Z, Zheng C, Tao Y, Chen R, Shi H, Chen T, Wang Z, Li H, Deng R, Liu X, Huang W, Stabilizing triplet excited states for ultralong organic phosphorescence, Nature Materials, 14 (2015) 685–690. [DOI] [PubMed] [Google Scholar]
- [719].Xue P, Sun J, Chen P, Wang P, Yao B, Gong P, Zhang Z, Lu R, Luminescence switching of a persistent room-temperature phosphorescent pure organic molecule in response to external stimuli, Chemical Communications, 51 (2015) 10381–10384. [DOI] [PubMed] [Google Scholar]
- [720].Zhang G, Chen J, Payne SJ, Kooi SE, Demas JN, Fraser CL, Multi-Emissive Difluoroboron Dibenzoylmethane Polylactide Exhibiting Intense Fluorescence and Oxygen-Sensitive Room-Temperature Phosphorescence, Journal of the American Chemical Society, 129 (2007) 8942–8943. [DOI] [PubMed] [Google Scholar]
- [721].Yuan WZ, Shen XY, Zhao H, Lam JWY, Tang L, Lu P, Wang C, Liu Y, Wang Z, Zheng Q, Sun JZ, Ma Y, Tang BZ, Crystallization-Induced Phosphorescence of Pure Organic Luminogens at Room Temperature, The Journal of Physical Chemistry C, 114 (2010) 6090–6099. [Google Scholar]
- [722].Yang Z, Mao Z, Zhang X, Ou D, Mu Y, Zhang Y, Zhao C, Liu S, Chi Z, Xu J, Wu Y-C, Lu P-Y, Lien A, Bryce MR, Intermolecular Electronic Coupling of Organic Units for Efficient Persistent Room-Temperature Phosphorescence, Angewandte Chemie International Edition, 55 (2016) 2181–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [723].Xue P, Wang P, Chen P, Yao B, Gong P, Sun J, Zhang Z, Lu R, Bright persistent luminescence from pure organic molecules through a moderate intermolecular heavy atom effect, Chemical Science, 8 (2017) 6060–6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [724].Jiang K, Zhang L, Lu J, Xu C, Cai C, Lin H, Triple-Mode Emission of Carbon Dots: Applications for Advanced Anti-Counterfeiting, Angewandte Chemie International Edition, 55 (2016) 7231–7235. [DOI] [PubMed] [Google Scholar]
- [725].Palner M, Pu K, Shao S, Rao J, Semiconducting Polymer Nanoparticles with Persistent Near-Infrared Luminescence for In Vivo Optical Imaging, Angewandte Chemie International Edition, 54 (2015) 11477–11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [726].Xu S, Chen R, Zheng C, Huang W, Excited State Modulation for Organic Afterglow: Materials and Applications, Advanced Materials, 28 (2016) 9920–9940. [DOI] [PubMed] [Google Scholar]
- [727].Yang J, Zhen X, Wang B, Gao X, Ren Z, Wang J, Xie Y, Li J, Peng Q, Pu K, Li Z, The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens, Nature Communications, 9 (2018) 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [728].Zhen X, Tao Y, An Z, Chen P, Xu C, Chen R, Huang W, Pu K, Ultralong Phosphorescence of Water-Soluble Organic Nanoparticles for In Vivo Afterglow Imaging, Advanced Materials, 29 (2017) 1606665. [DOI] [PubMed] [Google Scholar]


