Highlights
-
•
Myrrh mouthwash (MM) is used against an oral health microbe that causes gingivitis.
-
•
Adding silver nanoparticles (SN) may increase antimicrobial activity (AA).
-
•
Our laboratory analysis compared AA between MM with and without SN.
-
•
After 48 h, AA between MM with and without SN was similar.
-
•
Adding SN to MM does not increase antimicrobial activity.
Keywords: Metal nanoparticles, OxyR protein, P.Gingivalis, Commiphora, oral health, Anti-infective agents
Abstract
Introduction
Gingivitis is an oral condition characterized by inflammation and bleeding of the gingiva (gums), largely caused by Porphyromonas gingivalis. Oral hygiene options for controlling P. gingivalis include mouthwash containing Commiphora myrrha (myrrh), which has been shown to be effective against the microbe. Silver nanoparticles (SN) have been studied for their antibacterial effect in different oral health applications, including mouthwash. This was an in vitro laboratory study of the anti-microbial actions of myrrh and SN against P. gingivalis.
Methods
We compared the anti-microbial properties against P. gingivalis of four solutions: a) placebo solution, b) myrrh solution (MS), c) MS mixed with silver nanoparticles (MSN), and d) SN suspension alone. Sixteen agar plates were divided into four groups of four plates, and each group was treated with one of the solutions/suspensions. The solution/suspension was administered on the agar disc diffusion method, and inhibition zones (IZs) were measured after 24 (time 1), 48 (time 2), and 72 h (time 3). To characterize MSN and SN, Fourier-transform infrared spectroscopy (FT-IR) was used. UV–Vis spectroscopy and energy dispersive X-ray (EDX) were used to further characterize MSN.
Results
After 24 h, the median IZ for the MS plates was 16 mm, and the median IZ for MSN plates was 15 mm. At time 2, the MS median IZ was 15 mm, but the MSN median IZ increased to 18 mm, and the interquartile ranges (IQRs) did not overlap. At time 3, the median IZs was similar again, with MSN and MS having IZs of 16 mm and 15 mm, respectively. SN alone showed no anti-microbial activity.
Conclusions
Our findings show that MSN displayed superior anti-microbial activity against P. gingivalis compared to MS and SN after 48 h of incubation, but not after 24 h. Also, the increased anti-microbial activity had ceased by 72 h.
1. Introduction
Gingivitis is an oral condition characterized by inflammation and bleeding of the gingiva (gums) (Zahid and Alblowi, 2019). Gingivitis is induced by bacteria that accumulates into bacteria-laden plaques, and is reversible, as the removal of plaques reduces the inflammation (Aminu et al., 2020, Zahid and Alblowi, 2019). Although several bacteria are associated with the occurrence of gingivitis, plaque-associated gingivitis is often caused by Porphyromonas gingivalis (How et al., 2016). In gingivitis patients, there is a buildup of P. gingivalis and other bacteria, which might accumulate in plaque and cause recurrence of disease (Arunachalam et al., 2017). Patients are therefore encouraged to develop a habit of regular oral health maintenance to prevent this buildup (Arunachalam et al., 2017).
Patients susceptible to gingivitis are encouraged to adopt oral hygiene practices used to control plaque which typically include an anti-microbial mouthwash (Huang et al., 2016). Chlorhexidine (CX) has been the most researched anti-microbial agent put in mouthwash and found to be effective in treating and preventing gingivitis. Yet, due to some of its less desirable features, natural alternatives have also been researched (Arunachalam et al., 2017, Zahid and Alblowi, 2019). One of the natural alternatives researched as a potential alternative to CX mouthwash has been Commiphora myrrha (myrrh) (Zahid and Alblowi, 2019). Myrrh has been studied for its anti-microbial activity and potential application as a natural mouthwash to promote oral health, with generally positive results (Bassiouny and Al Barrak, 2014, Zahid and Alblowi, 2019). Myrrh has also been reported to promote oral healing (Laugisch et al., 2016) and prevent gingivitis as part of an anti-microbial toothpaste (Kariuki et al., 2017). These findings are especially important to patients with dental implants, who have a higher risk of gingivitis as well as other peri-implant diseases (Padial-Molina et al., 2016).
There is a need to prevent P. gingivalis in patients with dental implants by developing approaches to add anti-microbial activity to the implants (Song and Ge, 2019). Hence, not unlike myrrh, silver nanoparticles (SNs) have been studied for their antibacterial effect in different oral health applications, including mouthwash, being added to the dental resin, in the repair of tooth defects, and in toothpaste (Aminu et al., 2017). One study compared SNs that were biosynthesized using the endophytic fungus Fusarium semitectum with two dilutions of CX and saline (placebo); the SN condition performed as well as both CX dilution conditions against P. gingivalis, and all three conditions greatly outperformed the placebo (Halkai et al., 2018). Other in vitro studies of anti-microbial efficacy of SN against oral pathogens have either used generalized measures of the anti-microbial activity or studied pathogens other than P. gingivalis, and therefore, it is difficult to pinpoint SN’s specific action against P. gingivalis (Panpaliya et al., 2019).
Our specific aim was to compare the anti-microbial activity of myrrh mixed with SN (MSN) against P. gingivalis and to also determine the anti-microbial activity of myrrh solution (MS) and SN in their separate forms.
2. Materials and methods
2.1. Materials
Solutions/suspensions were made using double-distilled water (DDW). Dried myrrh was obtained from a local store in the Kingdom of Saudi Arabia (KSA). Silver nitrate powder (SNP, AgNO3 > 99.9%) was from Sigma Aldrich (Hamburg, Germany). The average particle size of AgNO3 was <150 nm as specified by the manufacturer. Fourier-transform infrared spectroscopy (FT-IR) was done using Spectrum BX spectrometer (PerkinElmer, Waltham, MA, USA), UV–Vis spectrum was analyzed using UV 2450 Spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Dispersive X-ray spectroscopy (EDX, Oxford Instruments, UK) was done using a JEM-2100F transmission electron microscope (JEM-2100F 200 kV, Joel Ltd, Japan).
2.2. Solution preparation
2.2.1. Placebo solution
DDW water was used for the placebo solution.
2.2.2. Myrrh solution
To develop a myrrh solution (MS), 5 g of myrrh was immersed in 100 ml of boiling DDW (ratio 1:20) and soaked overnight. The next day, the solution was then centrifuged for 10 min at 5000 rpm. The supernatant was stored at 4 °C until use.
2.2.3. Suspension with silver nanoparticles (AgNPs) using myrrh extract
To create a suspension of AgNPs using myrrh extract (myrrh + AgNP suspension, MSN), 5 ml from the aqueous myrrh extract was mixed with 50 ml of AgNO3 (1.0 mM). This suspension alone is the AgNP suspension (SN). This suspension was then placed on an electric heater and magnetic stirrer at 60° C until a yellowish color was observed, indicating the formation of silver nanoparticles (AlMasoud et al., 2020v, Alomar et al., 2020). This suspension was stored in a conical glass flask covered with foil at room temperature until use, as studies show the suspension remains stable under these conditions (AlMasoud et al., 2020v, Alomar et al., 2020).
2.2.4. Characterization of synthesized silver nanoparticles
Both the MS and MSN solutions/suspensions were characterized using FT-IR (Faghihzadeh et al., 2016). The samples were analyzed within the wavelength range between 4000 and 400 cm−1. Two additional tests were done to characterize the MSN. The first was ultraviolet–visible spectroscopy, which measures the intensity of light passing through the sample as an estimation of purity and/or quality of the sample (Abbas, 2019). The UV–visible spectrophotometric analysis was conducted on the MSN to characterize the relative components of the suspension of the MS and the AgNP suspension. The second was EDX analysis conducted with a JEM-2100F transmission electron microscope. This is an elemental analysis done to confirm the presence of silver in the suspension.
2.3. Agar disc diffusion method
The agar disc diffusion assay was used in this study to compare the placebo, MS, SN, and MSN’s anti-bacterial effect against P. gingivalis. The diffusion method involves the use of agar plates that have been inoculated with the test microorganism (Balouiri et al., 2016). Paper discs containing the test compound with a diameter of about 6 mm are placed on the agar surface (Balouiri et al., 2016). The agar plates with the discs are incubated for 24 h before the inoculation, and the diameters of the zone in which growth is inhibited are measured (Balouiri et al., 2016). In this way, larger zones indicate larger areas of inhibition, called inhibitions zones (IZ), and a greater antimicrobic capacity (Balouiri et al., 2016) (Fig. 1).
Fig. 1.
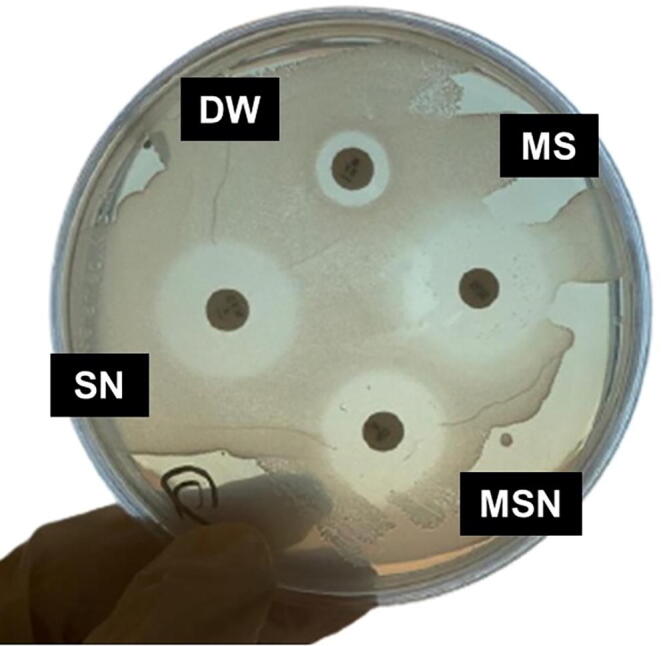
Agar disc diffusion assay. Paper discs containing the test compound with a diameter of about 6 mm are placed on the agar surface. The inoculum is labeled as follows: DW (distilled water, placebo), myrrh solution (MS), silver nanoparticle suspension (SN), and a myrrh solution mixed with silver nanoparticle (MSN). They are incubated for 24 h, and the diameters of the zone in which growth is inhibited, called “inhibition zones” (IZs), are measured.
The test microorganism was P. gingivalis (ATCC® 33277TM), which was suspended in 0.85% saline corresponding to No. 0.5 McFarland turbidity standard. This culture was incubated at 37 °C for 18 h, then diluted to 1/10 concentration to yield a culture density of approximately 1.5 × 108 CFU/mL. These subcultures were incubated at 37 °C for 24 h.
2.4. Statistical analysis
FT-IR, UV–Vis spectrum, and EDX descriptive analyses were conducted. To determine if MSN has a superior extended pattern of anti-microbial activity against P. gingivalis compared to MS and SN alone, first, the median and interquartile range (IQR) for IZs were calculated for each set of four plates in each condition at each time interval. Next, these medians were plotted with the IQR over time intervals for each group. Because only four plates were included in each condition at each time period, the data were insufficient to perform any statistical tests.
3. Results
Fig. 1 shows the agar disc diffusion method, and Fig. 2 compares FT-IR results between MSN and MS.
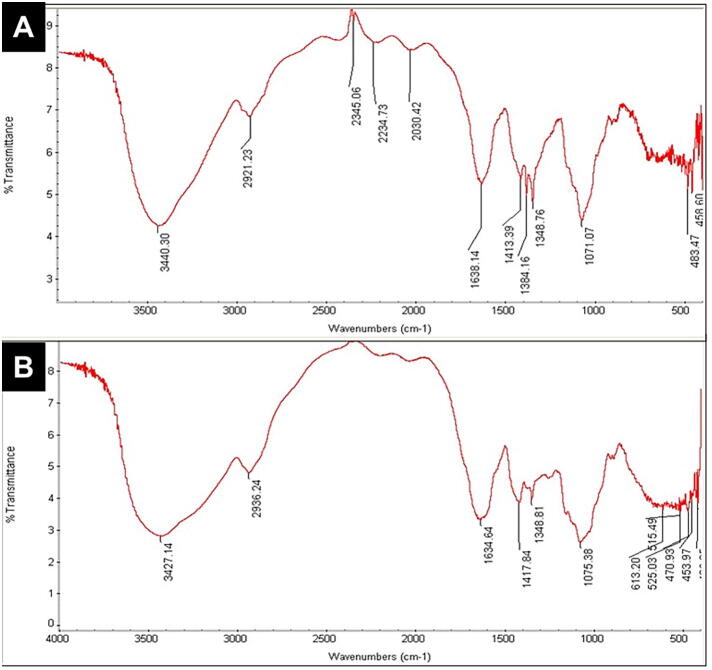
Fig. 2.
Fourier-transform infrared spectroscopy (FT-IR) results. FT-IR spectra of (A) myrrh solution mixed with silver nanoparticles (MSN), and (B) myrrh solution alone.
The FT-IR spectra of MS with and without SN showed similar peaks with a slight shift (Fig. 2). The OH-stretching group or NH group of amines or amides absorption band was at 3,427.14 cm−1 for the MS spectrum, while the MSN band is at 3,440.30 cm−1 (El-Sherbiny et al., 2013). The bands at 2,936.24 cm−1 for the MS spectrum and at 2,921.23 cm−1 for the MSN spectrum are designated to CH-stretching. The bands related to the CN group in the MS spectrum are at 1,634.64 cm−1, but in MSN, they are shifted to 1,638.14 cm−1. The bands at 1,634.64 cm−1, 1,384.81 cm−1, and 1,417.84 cm−1 were considered to be related to the C O group of carboxylic acids, and the C C group of alkenes. The slight shift in the MS spectrum can also be seen at 1,638.14 cm−1, 1,413.39 cm−1, and 1,348.76 cm−1. The C C group of alkenes in the MS spectrum peaked at 1,417.84 cm−1, while on the contrary, it peaked at 1,413.39 cm−1 in the MSN spectrum.
The UV–Vis spectrum results for MSN are given in Fig. 3, and the EDX results for MSN are in Fig. 4.
Fig. 3.
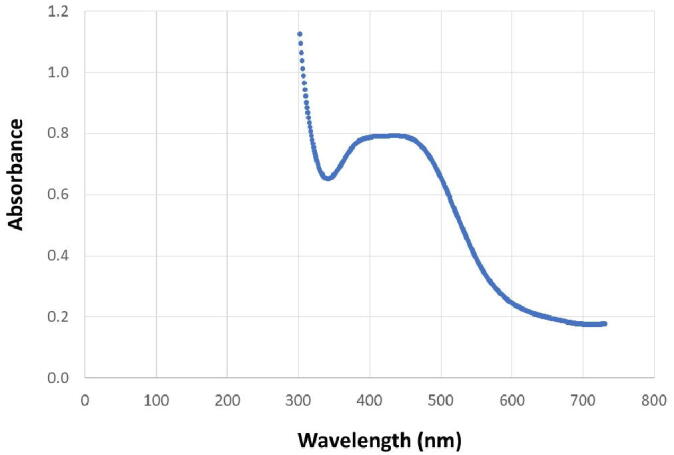
UV–vis spectroscopy results. The UV–vis spectroscopy results for myrrh solution mixed with silver nanoparticles (MSN). UV–visible spectral analysis was recorded from 300 to 800 nm, and the maximum absorption wavelength in the UV–vis absorption spectrum was 439 nm.
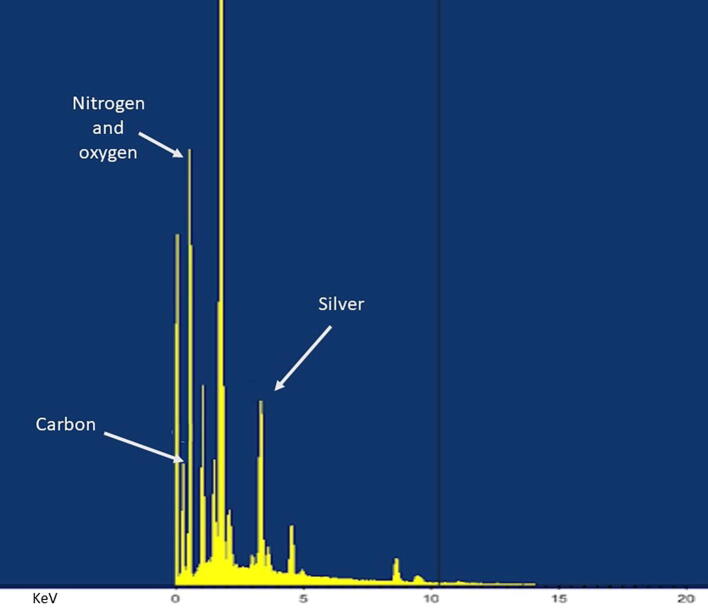
Fig. 4.
Energy dispersive X-ray (EDX) results. The EDX results for myrrh solution mixed with silver nanoparticles (MSN). Silver can be seen to be present at round 4 KeV. The pattern also illustrates some peaks correlating with the binding energies of carbon, nitrogen, and oxygen.
As shown in Fig. 3, the UV–visible spectrophotometric analysis was recorded from 300 to 800 nm, and the maximum absorption wavelength in UV–vis spectroscopy absorption spectrum was 439 nm. Silver can be seen to be present at around 4 KeV (Fig. 4). The pattern also illustrates some peaks correlating with the binding energies of carbon, nitrogen, and oxygen, which might represent contaminants that were introduced during the drying of the samples.
Fig. 5 shows the results of the agar disc diffusion method.
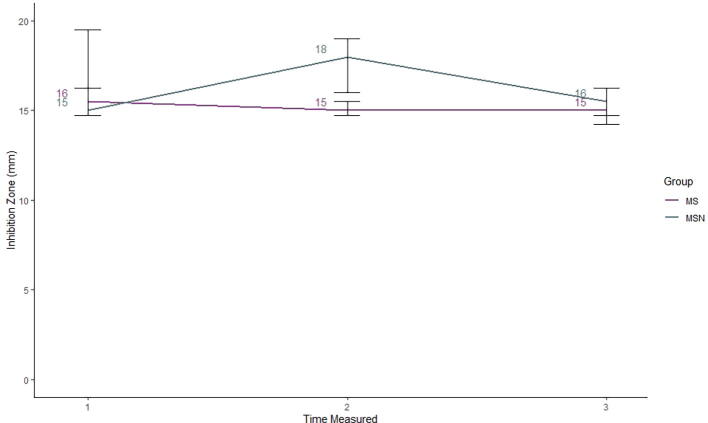
Fig. 5.
Agar disc diffusion results. Four conditions were tested: placebo (distilled water), myrrh solution (MS), silver nanoparticle suspension (SN), and a myrrh solution mixed with silver nanoparticle (MSN). Four plates were assigned to each condition, and the inhibition zone (IZ) was measured in mm after three 24-h increments. Both placebo and SN had IZs of zero at all time points and, therefore, are not represented on the plot.
At time 1, after 24 h of incubation, both the MS and MSN conditions had a median IZs, which were close, with MS at 16 mm and MSN at 15 mm (Fig. 5). After another 24 h, at time 2, the MS median was 15 mm, but the median IZ for MSN had increased to 18 mm, and its IQR did not overlap the IQR of MS. This increased IZ did not persist after another 24 h at time 3, when MSN returned to a median IZ of 16 mm compared to MS’s time 3 median of 15 mm.
4. Discussion
Here we report that MSN indeed displayed superior anti-microbial activity against P. gingivalis compared to MS and SN after 48 h of incubation, but not after 24 h. Also, the increased anti-microbial activity had ceased by 72 h. SN alone showed no anti-microbial activity.
These findings confirm the anti-microbial activity of myrrh alone against P. gingivalis as documented in the literature (Bassiouny and Al Barrak, 2014, Zahid and Alblowi, 2019). They also show that when myrrh is mixed with SN, the anti-microbial activity can be increased compared to myrrh alone. With MSN, anti-microbial activity increased to a peak after 48 h, then returns to baseline. The AgNPs, which are negatively charged, may have deteriorated by 72 h, which would explain this phenomenon. This behavior suggests that when using SN to improve myrrh’s natural anti-microbial action, this strategy will only work if the oral environment has at least 48 h of exposure to the MSN.
These findings have implications for how SN could be used to enhance oral health. As described earlier, patients with dental implants are at higher risk for oral health infections, and P. gingivalis is the primary pathogen implicated. We speculate that adding myrrh mixed with SN to the surface of dental implants could theoretically inhibit microbes, but having a constant delivery would be challenging, and obstacles would need to be addressed. A recent systematic review and meta-analysis reviewed 61 articles focused on examining the success of osseointegration and bone regeneration around dental implants with local drug or chemical compound delivery feature experimentally placed in animals (Alenezi et al., 2018). The two main methods used for local drug delivery were through the implant surface, and through the local applications of drugs at the implant site using carriers (Alenezi et al., 2018). Given our specific findings, it seems that the second method would be more appropriate, because doses could be timed such that the peak inhibition we saw after 48 h in our data could be leveraged. The meta-analysis found that overall, bone-to-implant contact was improved through the use of local drug and chemical compound delivery through implants, but the authors cautioned that there were no clear implications for patients in the clinical setting (Alenezi et al., 2018).
This study has several limitations. Because this study was largely exploratory, it was underpowered, so statistical tests could not be performed. Nevertheless, the descriptive analysis performed suggested that the research aims have been met. This study only looked at anti-microbial activity against P. gingivalis; many other oral pathogens exist, and the findings for MS, SN, and MSN and anti-microbial activity may be different for them. The main limitation of this study is that it is not clear how the findings could be applied clinically. The use of nanoparticles in clinical care raises serious questions of safety and practical application. Therefore, more work will need to be done on how nanoparticles can be safely in patients before planning a line of research toward any practical application.
5. Conclusions
In conclusion, our study of the anti-microbial effect of MS alone and mixed with SN against P. gingivalis showcased myrrh’s well-documented action against P. gingivalis while providing insight into myrrh’s enhanced action in the presence of SN. Studies of the potential use of nanoparticles in oral health maintenance can be useful to provide insight into the possibility of new approaches to the prevention and treatment of oral health issues, including gingivitis and peri-implantitis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank Monika Wahi for her help with editing and scientific review. The authors would like to thank the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding through the Fast-track Research Funding Program.
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas Q. Understanding the UV-Vis spectroscopy for nanoparticles. J. Nanomater. Mol. Nanotechnol. 2019;8:1–3. doi: 10.4172/2324-8777.1000268. [DOI] [Google Scholar]
- Alenezi A., Chrcanovic B., Wennerberg A. Effects of local drug and chemical compound delivery on bone regeneration around dental implants in animal models: a systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants. 2018;33:e1–e18. doi: 10.11607/jomi.6333. [DOI] [PubMed] [Google Scholar]
- AlMasoud N., Alomar T.S., Awad M.A., El-Tohamy M.F., Soliman D.A. Multifunctional green silver nanoparticles in pharmaceutical and biomedical applications. Green Chem. Lett. Rev. 2020;13:316–327. doi: 10.1080/17518253.2020.1839572. [DOI] [Google Scholar]
- Alomar T.S., AlMasoud N., Awad M.A., El-Tohamy M.F., Soliman D.A. An eco-friendly plant-mediated synthesis of silver nanoparticles: characterization, pharmaceutical and biomedical applications. Mater. Chem. Phys. 2020;249:123007. doi: 10.1016/j.matchemphys.2020.123007. [DOI] [Google Scholar]
- Aminu N., Chan S.-Y., Toh S.-M. Roles of Nanotechnological approaches in periodontal disease therapy. J.. App. Pharm. Sci. 2017;7:234–242. doi: 10.7324/JAPS.2017.70735. [DOI] [Google Scholar]
- Aminu N., Yam M.-F., Chan S.-Y., Bello I., Umar N.M., Nuhu T., Toh S.-M. The evaluation of healing effect of triclosan and flurbiprofen-loaded nanogels in experimental periodontitis in rats by morphometric analysis. Saudi Dental J. (in press) 2020 doi: 10.1016/j.sdentj.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam L.T., Sudhakar U., Vasanth J., Khumukchum S., Selvam V.V. Comparison of anti-plaque and anti-gingivitis effect of curcumin and chlorhexidine mouth rinse in the treatment of gingivitis: a clinical and biochemical study. J. Indian Soc. Periodontol. 2017;21:478–483. doi: 10.4103/jisp.jisp_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating anti-microbial activity: a review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiouny G., Al Barrak H. The anti-plaque effect of miswak and myrrh mouthwashes versus chlorhexidine in the treatment of chronic gingivitis: a comparative clinical trial. Med. Sci. 2014;9:32–37. [Google Scholar]
- El-Sherbiny I.M., Salih E., Reicha F.M. Green synthesis of densely dispersed and stable silver nanoparticles using myrrh extract and evaluation of their antibacterial activity. J. Nanostruct. Chem. 2013;3:8. doi: 10.1186/2193-8865-3-8. [DOI] [Google Scholar]
- Faghihzadeh F., Anaya N.M., Schifman L.A., Oyanedel-Craver V. Fourier transform infrared spectroscopy to assess molecular-level changes in microorganisms exposed to nanoparticles. Nanotechnol. Environ. Eng. 2016;1:1. doi: 10.1007/s41204-016-0001-8. [DOI] [Google Scholar]
- Halkai K.R., Halkai R., Mudda J.A., Shivanna V., Rathod V. Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: an in vitro study. J. Conserv. Dent. 2018;21:662. doi: 10.4103/JCD.JCD_203_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How K.Y., Song K.P., Chan K.G. Porphyromonas gingivalis: an Overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Li Z., He T., Bo C., Chang J., Li L., He Y., Liu J., Charbonneau D., Li R., Xu J. Microbiota-based signature of gingivitis treatments: a randomized study. Sci. Rep. 2016;6:24705. doi: 10.1038/srep24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki H.M., Alumera H., Wagaiyu E.G. Effect of a natural extract toothpaste on the bacteria colonies of initial dental plaque colonizers. IOSR J. Dental Med. Sci. 2017;16:13–21. [Google Scholar]
- Laugisch O., Ramseier C.A., Salvi G.E., Hägi T.T., Bürgin W., Eick S., Sculean A. Effects of two different post-surgical protocols including either 0.05 % chlorhexidine herbal extract or 0.1 % chlorhexidine on post-surgical plaque control, early wound healing and patient acceptance following standard periodontal surgery and implant placement. Clin. Oral Investig. 2016;20:2175–2183. doi: 10.1007/s00784-016-1713-7. [DOI] [PubMed] [Google Scholar]
- Padial-Molina M., López-Martínez J., O’Valle F., Galindo-Moreno P. Microbial profiles and detection techniques in peri-implant diseases: a systematic review. J. Oral Maxillofac. Res. 2016;7 doi: 10.5037/jomr.2016.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panpaliya N.P., Dahake P.T., Kale Y.J., Dadpe M.V., Kendre S.B., Siddiqi A.G., Maggavi U.R. In vitro evaluation of anti-microbial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dental J. 2019;31:76–83. doi: 10.1016/j.sdentj.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Ge S. Application of antimicrobial nanoparticles in dentistry. Molecules. 2019;24:1–15. doi: 10.3390/molecules24061033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid T.M., Alblowi J.A. Anti-Inflammatory and Anti-plaque effects of commiphora myrrh mouthwash: a preliminary pilot clinical study. Open Dent. J. 2019;13:1–5. doi: 10.2174/1874210601913010001. [DOI] [Google Scholar]