Abstract
Introduction
Periodontitis, a complex infectious disease that may lead to irreversible loss of periodontium, is considered a predisposing agent for developing insulin resistance due to the release of inflammatory mediators, showing a bilateral relationship with diabetes mellitus. The investigation of periodontal disease requires a clinical approach and complete intraoral radiographs, even with increasing concerns about radiation exposure. Thus, this study assesses pixel linear analysis accuracy using digital radiography via Digora® in detecting alveolar bone destruction in diabetic rats with periodontal disease.
Methodology
40 rats were divided into groups (n = 10): control (C), rats with periodontal disease (PD), experimental diabetic rats (ED), experimental diabetic rats with periodontal disease (ED-PD). Diabetes was induced by streptozotocin and periodontal disease by periodontal ligature. After 30 days, maxillae bone destruction was obtained by linear analysis of vertical bone loss using digital radiography and then assessed by micro-CT and histology. Data were analyzed by ANOVA and Tukey’s test (p < 0.05).
Results
Radiographic, micro-CT and histological analysis presented accurate and similar results. PD and ED-PD groups showed higher bone destruction than C and ED groups (p < 0.05). Moreover, the ED-PD group had higher bone loss than the PD group (p < 0.05).
Conclusion
The pixel linear analysis via digital radiography was an accurate, low-cost alternative in detecting alveolar bone loss in this rat model. Micro-CT and histological analysis may also be used to obtain linear measures to assess and compare periodontal bone destruction in diabetic rats.
Keywords: Experimental diabetes mellitus, Histology, Periodontal disease, Radiography, Emission-computed tomography
1. Introduction
Periodontitis is considered a complex infectious disease with several etiologic factors(Meyle and Chapple, 2015), resulted from bacterial-induced chronic inflammation that may lead to irreversible loss of periodontium supporting tissue (Nazir, 2017). Previous reports evidenced an association between periodontitis and systemic disorders, including diabetes (Genco et al., 2005).
Diabetes Mellitus is one of the most common endocrine disorders, characterized by chronic hyperglycemia resulting from depletion of insulin secretion, insulin action or both, estimated to affect nearly 439 million people by 2030 (Shaw et al., 2010, American Diabetes Association, 2014). Diagnosis of two main types of diabetes may be observed: type 1 diabetes (autoimmune destruction of the insulin-producing pancreatic beta cells) and type 2 diabetes (insulin resistance and failure of the beta cell to compensate), commonly observed in middle-aged adults (King, 2012).
The beginning of obesity in type 2 diabetes is mainly triggered by inflammation of the adipose tissue, which leads to increased lipolysis and contributes to the synthesis of cytokines and adipocytokines. During a low-intensity chronic inflammation of the adipose tissue, immunoinflammatory mediators are released, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (Gomes and Accardo, 2019). The constant release of inflammatory mediators in a chronic situation as bacterial or viral infections increases insulin resistance, even in people without diabetes (Genco et al., 2005, Mealey and Oates, 2006).
Periodontal diseases like periodontitis may act as a predisposing condition for the development of insulin resistance because the same inflammatory cytokines are released continuously, increasing the possibility of worsening the glycemia (Mealey and Oates, 2006).
Previous studies showed evidence between these two pathological conditions as a bilateral relationship (Cintra et al., 2014a), especially regarding prevalence, severity and progression of periodontal disease in diabetic individuals (Cintra et al., 2014a, Duarte et al., 2014). The bilateral relationship reports, on the one hand, that periodontal treatment improves diabetes outcomes, acting on glycemic control by reducing glycated hemoglobin in diabetic individuals (Teshome and Yitayeh, 2016). On the other, diabetes influences periodontitis progression by altering the subgingival bacterial community, offering a favorable environment for pathogens’ development (Sakallıoğlu et al., 2008), besides the increased periodontal destruction by reducing the proliferating capability and activating osteoclastic resorptive signals due to local acidosis (Duarte et al., 2014).
The influence of diabetes in alveolar bone destruction is related to an unbalance of osteoclast and osteoblasts by increasing the expression of inflammatory cytokines and RANKL/osteoprotegerin (OPG) ratios, which results in a reduction of osteoblast formation and an increase of osteoclast formation in inflamed areas, enhancing bone resorption. Hence, those bone markers and other inflammatory cytokines, such as TNF, are critical mediators of the increased bone resorption in people with diabetes with periodontal disease (Pacios et al., 2012, Lappin et al., 2009, Wu et al., 2015).
The assessment of periodontal disease diagnoses, stage and progression is provided by the association between clinical examination and imaging techniques, as intraoral and/or panoramic radiographs, to determine bone loss (Lang and Bartold, 2018, Machado et al., 2020). Even with the possibility of using superior imaging technique as micro-tomography, radiography is still considered a reliable tool for periodontitis detection (Machado et al., 2020).
Linear measurements between the cementoenamel junction and the alveolar crest are used to analyze bone defects to determine possible therapy outcomes and periodontal risk assessment (Mol, 2004), with a positive correlation between clinical bone sounding and bone height via analysis of dental radiographs (Zybutz et al., 2000). Moreover, automated systems such as computer software could allow more accurate assessments of periodontal bone loss on dental radiographs (Krois et al., 2019, Machado et al., 2020). However, the inconsistency in maintaining nearly identical alignment in successive radiographic images may reduce reliability (Rawlinson et al., 2005, Vijay et al., 2013).
Micro-computed tomography (micro-CT) is another digital technology that assesses bone morphometry characteristics as an alternative to conventional histological analyses when considering tridimensional volume over a two-dimensional histologic section (Irie et al., 2018). The wide use in dentistry is due to the possibility of in vivo imaging, making it possible to assess bone tissue repair in human or even in animal samples (Balto et al., 2000, Irie et al., 2018).
Thus, this study aimed to assess the accuracy of a linear pixel analysis using digital radiography (via Digora® histogram pixel computer software) in detecting alveolar bone loss in diabetic rats with periodontal disease, compared with micro-CT and histologic analysis. The null hypothesis was that there would be no difference between the three methodologies used to assess bone loss.
2. Material and methods
Forty male Wistar rats weighing 250–280 g and aged three months were used in the study. The animals were housed in temperature-controlled rooms (25 ± 2 °C, 70% humidity) and kept under a 12-/12-hour light/dark cycle, with food and water available ad libitum for 48 h before the beginning of the experiment, for an acclimation period. The general health condition of the animals was observed throughout the experimental period. The experimental protocol was approved by and conducted under the guidelines of the institutional ethics committee (protocols CEUA #00582 and #00357). The rats were divided into four groups (n = 10): control (C), rats with periodontal disease (PD), experimental diabetic rats (ED), and experimental diabetic rats with periodontal disease (ED-PD).
2.1. Sample size
The sample size estimates were based on a previous study (Cintra et al., 2014a), supported by statistical software (SigmaPlot V.12.0, Systat Software, Inc. CA, USA), which calculated the power sample with the following parameters: adopting an alpha error of 0.05, and a 90% power for a significant difference when using four groups, a minimum of 8 animals/group was indicated. Due to possible animal death, two more animals were added to each group.
2.2. Diabetes induction
Diabetes was induced according to previous protocols (Cintra et al., 2013, Cintra et al., 2014b). The rats were fasted overnight (Day 0), and tail-tip blood was used to measure the fasting blood glucose levels (Day 1) with an automatic blood glucose monitoring system (Accu-Check® Performa; Roche Diagnostics Corporation, Indianapolis, IN, USA). Subsequently, the rats were intramuscularly anesthetized with ketamine (87 mg/kg; Francotar; Virbac do Brasil Ind. e Com. Ltda., Roseira, Brazil) and xylazine (13 mg/kg; Rompun; Bayer S. A., São Paulo, Brazil).
Then, animals were randomly assigned into groups and endovenously injected in the penile vein with citrate buffer solution 0.01 mol/L with a pH of 4.5 (C and PD) for normoglycemic rats or streptozotocin (Sigma Aldrich Corp., St. Louis, MO, USA) dissolved in citrate buffer solution at 35 mg/kg body weight for experimental induction of diabetes groups (ED and ED-PD) (Cintra et al., 2013, Cintra et al., 2014a, Cintra et al., 2014b).
Three days after diabetes was induced, blood samples were collected from each animal to determine their blood glucose levels. The rats with blood glucose levels over 200 mg/dL were used in the Diabetes groups (Cintra et al., 2013, Cintra et al., 2014b), totaling 20 rats considered diabetics and 20 rats as normoglycemic (not diabetic). Blood glucose concentration was measured again at day 33 (end of experimental period).
2.3. Periodontal disease induction
After blood confirmation of hyperglycemia on day 3, animals were anesthetized to induce periodontal disease with the anesthetic protocol previously described. The periodontal disease was induced in rats from groups PD and ED-PD with sterile 4/0 silk ligatures (Ethicon Johnson & Johnson, São Paulo, SP, Brazil), tied around the maxillary left second molar (Cintra et al., 2013, Cintra et al., 2014a, Cintra et al., 2014b).
The placement of ligatures in the gingival sulcus around the molar teeth acts by increasing biofilm accumulation and disrupting the gingival epithelium, leading to osteoclastogenesis and bone loss (Cai et al., 2008).
2.4. Digital radiographic analysis
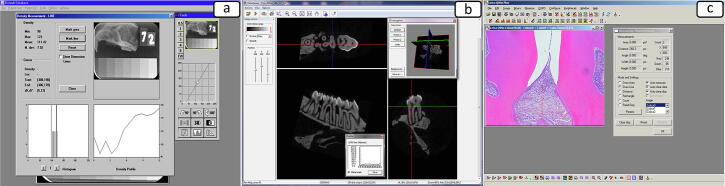
After 30 days of periodontal disease induction, the animals were killed with an overdose of Thiopental anesthetic (240 mg/kg; Thiopentax, Cristália – Produtos Químicos Farmacêuticos Ltda, Itapira, SP, Brazil). The maxillae were surgically removed, separated, dissected and fixed in a solution of 4% buffered formaldehyde for 24 h. Images were evaluated to assess bone loss using the Digora® (Soredex, Orion Corporation, Helsinki, Finland) digital radiographic system (Fig. 1a).
Fig. 1.
a) Histogram and bone density evaluation using the digital radiographic analysis (Digora® software); (b) Linear bone loss measure using the Micro-CT analysis with Data Viewer software; (c) Linear bone distance measure using histologic analysis with Leica Qwin V3 software.
The radiographs were taken by placing the specimen on the sensor (phosphorous board) of 30 mm × 40 mm (size and active area), similar to a periapical radiographic film. Its matrix (image size) measures 416 × 560 pixels, with a pixel size of 71 × 71 µm and a 6.1p/mm resolution. The exposition was performed as follows: 65 KV and 10 mA, with a source-to-film distance set at 50 cm and 0.2 s exposure time, as instructed by the manufacturer for digital radiography of phosphor plates.
The hemi-maxillas of all groups were radiographed using X-ray apparatus GE (General Electric Company, Milwaukee, Wisconsin, USA), model GE 1000, and analyzed using Digora® (Soredex, Orion Corporation, Helsinki, Finland), totaling 40 digital radiographic images. The capture was obtained employing activated phosphorus plates with an incorporated aluminum step wedge with variable thickness (ranging from 1 to 8 mm, in 1-mm increments), serving as a radiographic density reference. The same phosphor plate was used for all exposures to avoid possible differences between plates, reducing bias.
Radiographic evaluation was performed in a blind manner regarding the groups by a single calibrated operator and conducted according to Teixeira et al. (2011): to quantify the bone loss area in pixel, a region of interest (ROI/252 pixels) was opened in the lateral periodontium of every tooth, at the site corresponding to the height loss of alveolar bone of each sample. Pixel values were calibrated on the aluminum step wedge to enable normalization of the ROI pixel measurements. This standard image technique is essential for controlling of day-to-day variations in the sensitivity of the detector array. The pixel values of the ROI were established using the histogram tool. The normalized pixel values of the images (NPI) were obtained using the following equation: NPI = PI/CR, where PI is the ROI mean pixel value and CR is the ROI pixel value of the standard aluminum step wedge. The pixel value of zero corresponded to black and the pixel value of 255 to white.
2.5. Micro-CT analysis
The maxillae were scanned using the micro-CT 1174 (SkyScan- Kontich, Belgium). The source of x-ray was operated at 50 kV, 180-degree rotation with 0.8-degree angular increment, producing three-dimensional images on a voxel size of 16 µm and size of 1024 pixels. The images were analyzed by Data Viewer software (SkyScan- Kontich, Belgium).
A single calibrated operator blinded to the groups obtained linear measurements (in micrometer) from the cementoenamel junction (CEJ) to the alveolar crest (AC) in the interdental region between the first and second molars and the second and third molars to determine the vertical bone loss. All images were reoriented, so the CEJ and the root apex appeared on the micro-CT slice while analyzed. Measurements of root lengths (µm) from the apex to the CEJ were also tabulated to assess the remaining vertical bone (Fig. 1b).
2.6. Histologic analysis
After radiographic and tomographic analysis, the maxillae were decalcified in buffered (pH 8.0) 17% EDTA (Sigma Chemical Co, St Louis, MO). After the decalcification period, samples were histologically processed for paraffin embedding. Serial sections (6 µm) were cut and stained with hematoxylin and eosin for analysis under an optical microscope (DM 4000B, Leica, Wetzlar, Germany).
For each sample, the central histologic section containing the CEJ, periodontal tissues, and the alveolar bone were histometrically measured by a single calibrated histologist, blinded to the groups, using an image processing system, which consisted of a light microscope (DM 4000B, Leica, Wetzlar, Germany), a color camera (DFC 500, Leica), a color image processor (Leica Qwin V3 software, Leica) and a personal computer (Intel Corel i7, 3.4 GHz, Windows 7 Ultimate). The alveolar bone loss was assessed by measuring the linear distance between the CEJ and the buccal alveolar bone crest, in the central histologic sections from the side of the maxilla (Fig. 1c).
2.7. Statistical analysis
The obtained values were tabulated for each experimental group, and a single calibrated operator analyzed the data in a blinded manner concerning the different groups. After the Shapiro-Wilk normality test, one-way analysis of variance (ANOVA) followed by Tukey test were performed for parametric data with a significance level of 5%.
3. Results
We observed the health conditions of the animals during the experimental period. From our vivarium experience, diabetic rats demonstrated intense thirst, polyuria and apathy, in accordance with their systemic condition. No animal loss occurred during the experiment.
3.1. Diabetes monitoring: blood glucose levels
The animals’ blood glucose levels were measured on the first (Day 1), third (Day 3) and last day of the experiment (Day 33). The results showed that diabetic rats (groups ED and ED-PD) have higher blood glucose levels at third and last day after diabetes induction than normoglycemic rats (groups N and PD), as shown in Table 1.
Table 1.
Mean and standard deviation (SD) of blood glucose levels (mg / dL), according to each group.
| Blood glucose levels (mg/dL) ± SD* |
||||
|---|---|---|---|---|
| Group | Day1 | Day 3 | Day 33 | N |
| N | 75.2 ± 11.2a | 104.8 ± 9.9a | 101.3 ± 10.1a | 10 |
| N-PD | 76.2 ± 9.2a | 103.5 ± 12.1a | 94.5 ± 7.9a | 10 |
| D | 77.7 ± 8.8a | 328.2 ± 37.8b | 361.8 ± 42.9b | 10 |
| D-PD | 78.4 ± 7.6a | 316.9 ± 38.4b | 376.5 ± 44.7b | 10 |
Same superscript letters indicate no statistical difference (p > 0.05).
3.2. Radiographic analysis
The radiographic analysis showed that rats with periodontal disease (PD and ED-PD) presented lower bone density when compared to rats without periodontal disease (N and ED) (p < 0.05). Besides, diabetic rats with periodontal disease (ED-PD) have lower bone density when compared to normoglycemic rats with periodontal disease (PD) (p < 0.05). No statistical difference was found among the groups without periodontal disease (N and ED) (p > 0.05) (Table 2, Fig. 2).
Table 2.
Mean and standard deviation (SD) of bone loss in radiographic, tomographic, and histologic analysis, according to each group.
| Group | Bone loss measurement |
n | ||
|---|---|---|---|---|
| Radiographic (pixel values) | Tomographic (µm) | Histologic (µm) | ||
| N | 143.4 ± 8,4a | 225,2 ± 23,6a | 151,1 ± 56,1a | 10 |
| N-PD | 123,2 ± 7,0b | 1019,9 ± 248,6b | 1012,5 ± 151.8b | 10 |
| ED | 138,4 ± 20,1a | 242,0 ± 24,4a | 200,2 ± 60,3a | 10 |
| ED-PD | 101,5 ± 20,1c | 1414,0 ± 443,2c | 1186,9 ± 138.6c | 10 |
*Different superscript letters represent significant difference (p < 0.05).
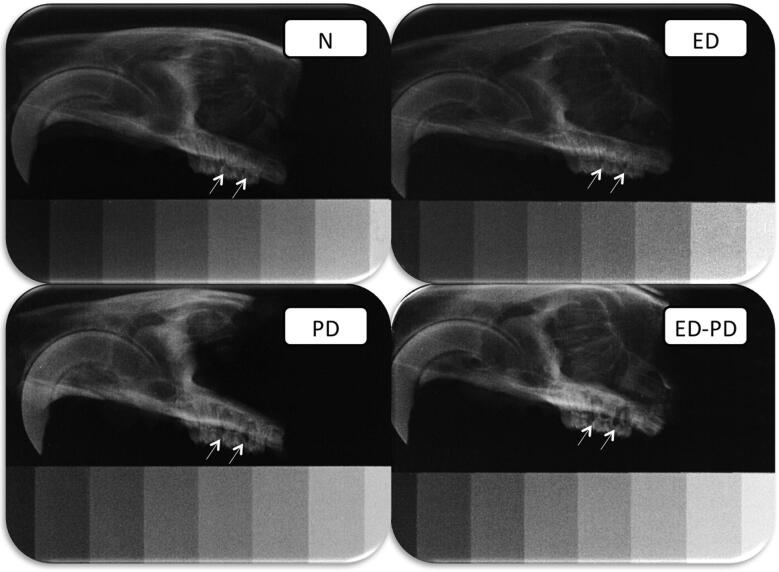
Fig. 2.
Radiographs of representative images of N, PD, ED and ED-PD groups. N and ED: radiographic images of the maxillae showing a normal aspect and the area of the bone density measurement (arrows). PD: radiographic image of the maxillae showing a radiolucent area in the periodontal region (arrows). ED-PD: radiographic image showing an extensive radiolucent area in the periodontal region (arrows).
3.3. Tomographic analysis
The tomographic analysis also evidenced that rats with periodontal disease (PD and ED-PD) have higher bone loss than rats without periodontal disease (N and ED) (p < 0.05). We also verified greater bone loss (p < 0.05) in diabetic rats with periodontal disease group (ED-PD) in comparison with normoglycemic rats with periodontal disease (PD) via tomographic evaluation.
We found no statistical difference between the groups without periodontal disease (N and ED) (p > 0.05) (Table 2, Fig. 3).
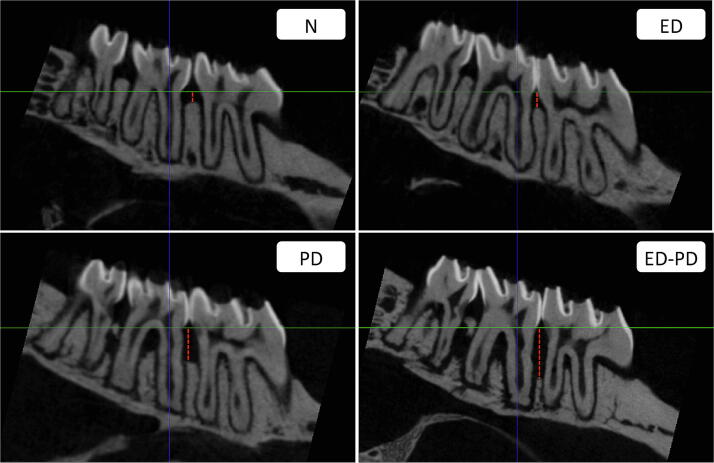
Fig. 3.
Tomographic of representative images of N, PD, ED, and ED-PD groups. N and ED: tomographic images of the maxillae showing a normal aspect and the area of the linear measurement (red dotted trace). PD: tomographic image of the maxillae showing a linear bone loss (red dotted trace). ED-PD: tomographic image showing an extensive linear bone loss (red dotted trace).
3.4. Histologic analysis
The histologic evaluation also exhibited the higher bone loss of rats with periodontal disease (PD and ED-PD) when compared to rats without periodontal disease (N and ED) (p < 0.05). Diabetic rats with periodontal disease (ED-PD) have a higher bone loss when compared to normoglycemic rats with periodontal disease (PD) (p < 0.05).
No statistical difference was found between the groups without periodontal disease (N and ED) (p > 0.05) (Table 2, Fig. 4).
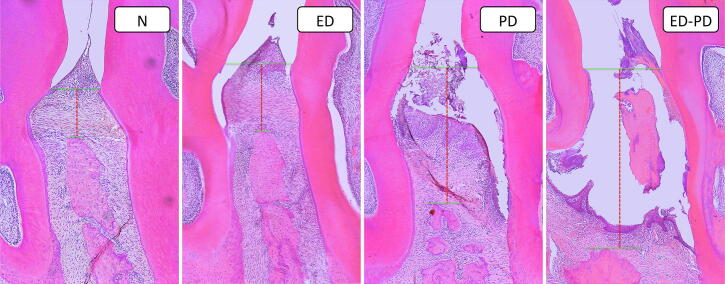
Fig. 4.
Histologic of representative images of N, PD, ED, and ED-PD groups. N and ED: histologic images of the periodontal region showing a normal aspect and the area of the histometric measurement (red dotted trace). PD: histologic image of the periodontal region showing a linear bone loss (red dotted trace). ED-PD: histologic image of the periodontal region showing an extensive linear bone loss (red dotted trace).
4. Discussion
This study investigated the accuracy of linear pixel analysis regarding quantification of alveolar bone loss, whether in normal or diabetic rats with periodontal disease, compared with micro-CT images and histologic analysis. According to our results, the three methods allowed us to observe the same differences in bone loss between groups, confirming that a linear pixel analysis via digital radiographic is a reliable technique to assess the bone loss progression. Thus, the null hypothesis was accepted.
The use of animal species is a common practice in dental research, with a predominance of rodents, mainly due to physiological characteristics that may mimic human behavior. Because of the often superiority to in vitro research, a high-quality designed animal model supports future clinical trials in humans (Bernardino et al., 2014, Nokhbatolfoghahaei et al., 2020).
Studies in rats have been previously used in different models to assess tissue response in subcutaneous (Bueno et al., 2016, Bueno et al., 2019), apical periodontitis (Barcelos et al., 2020, Cosme‐Silva et al., 2020Cosme‐Silva et al., 2020Cosme‐Silva et al., 2020), dental alveolus (Bueno et al., 2017), or periodontal (Cintra et al., 2013), whether in normal or under systemic conditions, such as diabetic (Cintra et al., 2013, Cintra et al., 2014b), hypertensive (Martins et al., 2016) or even under alcohol consumption (Dal‐Fabbro et al., 2019).
The choice of using normoglycemic and diabetic rats with periodontal disease was mainly because bone loss would be promptly observed and distinguished, as expected in the ED-PD group, and would validate or discard the pixel analysis when the following micro-CT and histologic sections were analyzed.
The diabetes model used was streptozotocin induction, as conducted by previous studies (Cintra et al., 2013, Cintra et al., 2014a, Cintra et al., 2014b), which resembles type 1 diabetes, and, therefore, our results may not accurately be applied to other forms of diabetes. The glucose levels in diabetic rats were approximately four times higher than those observed in normoglycemic rats. The blood glucose levels were higher in the rats of diabetic groups during all experimental periods, indicating that the method of diabetic induction was effective, corroborating results of a previous blood profile study in diabetic rats with periodontal disease, conducted by Cintra et al. (2014b).
On day 1, all rats had similar glycemic levels (p > 0.05). Three days after induction of Diabetes Mellitus, blood glucose levels were higher than they were in rats without diabetes (p < 0.05). At the end of the experimental period, we measured the glycemic levels again, showing similar glycemic alterations to day 3, evidencing the hyperglycemic condition (Table 1).
The process of periodontal disease induction by placing a ligature was extensively used in animal studies (Cintra et al., 2013, Cintra et al., 2014a, Cintra et al., 2014b, Colombo et al., 2012). The presence of ligature around teeth favors the formation of bacterial plaques and induces an inflammatory response, reproducing periodontal diseases in humans (Colombo et al., 2012). Diabetic rats with periodontal disease showed higher bone loss in all analyzed methodologies than nondiabetic rats with periodontal disease. This result is consistent with previous findings (Cintra et al., 2014b, Duarte et al., 2014) because diabetes enhances periodontal destruction by increasing osteoclastic activity, due to an intense inflammatory response observed in hyperglycemic condition.
As stated, the release of inflammatory mediators, such as TNF- α when affected by periodontal disease, may lead to insulin resistance (Mealey and Oates, 2006): this chemical mediator decreases tyrosine phosphorylation of the insulin receptor substrate (IRS-1) on serine, altering the insulin signal transduction and making this molecule inhibitory to the insulin receptor signaling ability (Kroder et al., 1996).
The bilateral relationship between DM and periodontitis stems from the link between the degree of hyperglycemia and the severity of periodontitis. Diabetes directly affects osteoblast (reducing differentiation and turnover rate) and osteoclast (elevating its formation in inflamed areas), potentiating the severity of periodontitis and accelerating bone resorption by increasing the expression of inflammatory mediators and receptor activator of NF-κB ligand (RANKL)/osteoprotegerin (OPG) ratios. After inflammation initiates, inflammatory cytokines and activate cells related to bone resorption start alveolar bone demineralization. Active osteoclasts (tartrate-resistant acid phosphatase – TRAP) stimulate bone resorption through an imbalance in the RANK/RANKL/OPG equilibrium. In bone, RANKL stimulates osteoclastic differentiation, while OPG protects the bone tissue from excessive resorption by binding to RANKL, thus preventing it from binding to RANK (Dal‐Fabbro et al., 2019Dal‐Fabbro et al., 2019Dal‐Fabbro et al., 2019, Cosme‐Silva et al., 2020Cosme‐Silva et al., 2020Cosme‐Silva et al., 2020).
Diabetic patients with severe periodontitis have six times more insufficient glycemic control (hyperglycemia) than patients with healthy periodontium, leading to exaggerated immune-inflammatory responses induced by periodontal pathogens. However, improved glycemic control reduces the severity of periodontal disease (Badiger et al., 2019, Wu et al., 2015).
Concerning the use of imaging techniques to assess bone defects in periodontal disease, besides the advantages of three-dimensional (3D) imaging information provided by micro-CT over two-dimensional (2D) histological sections, the micro-CT allows non-destructive imaging easy to perform. Another advantage of the micro-CT is that the same sample can later be prepared for histologic analysis, enabling complementary information of the same tissue from different areas, unrestricted to the sections provided by histology images (Bouxsein et al., 2010). However, the routine use of 3D images for periodontal diagnosis does not appear to be justified due to radiation exposure and cost-effectiveness (Kim and Bassir, 2017).
Digital measurement techniques have been proposed to assess bone density by determining shades of gray, measured in pixels, and generating values (Shrout et al., 2003). The use of Digora® system to assess radiopacity of dental materials by pixel gray values associated with an aluminum step wedge has been previously reported (Borges et al., 2011, Teixeira et al., 2011). When using digital radiographs to analyze bone density, Nackaerts et al. (2007) stated that the assessment of bone density was far more accurate when associating an aluminum step wedge consisting of at least three steps as a reference. The study by Teixeira et al. (2011) also used the radiographic pixel analysis to confirm the presence and intensity of bone resorption in rat’s mandible, observed in a histological section, via histogram tool by establishing pixel values of the ROIs, overcoming a conventional visual analysis. Also, results by Shrout et al., 2003, Jett et al., 2004, Bozzo et al., 2004, and Goes et al. (2010) also confirmed the possibility of bone loss detection by analyzing pixel values or density, even if it was not yet visible radiographically, corroborating our results.
Regarding the methodologies used to evaluate bone loss, there are no studies in the literature comparing the images obtained by digital radiography with micro-CT and histological findings. The present research highlighted this comparison, showing that low-cost imaging methodologies may still be a valid and reliable technique to linearly quantify bone loss, with a valuable benefit-cost and a lower time required to obtain the results. Although histology remains the most indicated method to evaluate the inflammatory infiltrate, histologic analysis is laborious, time-consuming, costly, and sample destructive, besides the unfeasibility of performing in clinical routine (Balto et al., 2000, Von Knebel Doeberitz and Wentzensen, 2008).
However, Machado et al. (2020) highlighted limitations of radiographic assessment, such as the inability to reproduce the bone structure three-dimensionally, which may decrease accuracy. Also, lack of standardization in radiography may show milder bone destruction than observed (Vijay and Raghavan, 2013).
Although intraoral radiographs may be less accurate than other techniques, recent studies focus on computer-assisted radiography to overcome human visual limitations in detecting bone loss. Recent studies suggested that automated systems could allow more reliable and accurate assessments of periodontal bone loss on panoramic dental radiographs, and the development of automated prediction systems could enhance periodontitis surveillance (Krois et al., 2019, Machado et al., 2020).
According to the results, due to the low cost and easy practicability in clinical routine, the use of this radiographic pixel analysis could be extrapolated to clinical research with digital radiographs aided by computer tools, after initial periodontics investigation with a periodontal probe in pocket depths and before a costlier exam as the micro-CT. Further tests with human digital radiographs may validate this as a preliminary exam to verify the presence/progression of bone loss in periodontal disease.
5. Conclusion
In conclusion, based on the compared methodologies, although micro-CT and histological analysis are reliable/gold standard methods to assess bone loss, the presented radiographic technique using pixel analysis showed to be an accurate, low-cost, and easy-access alternative to detect alveolar bone loss in diabetic rats with periodontal disease.
Nevertheless, all techniques showed that diabetes provides increased bone loss in rats with periodontal disease.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Before any procedure, the project was approved by the institutional Ethics Committee on the Use of Animals at Araçatuba School of dentistry/UNESP and conducted in accordance with relevant guidelines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by a grant (2011/11337-0 and 2010/16999-9) from the Sao Paulo Research Foundation (FAPESP), Sao Paulo, SP, Brazil and by a grant (311650/2018-0) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Footnotes
Peer review under responsibility of King Saud University.
References
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- Badiger A.B., Gowda T.M., Chandra K., Mehta D.S. Bilateral interrelationship of diabetes and periodontium. Curr. Diabetes Rev. 2019;15(5):357–362. doi: 10.2174/1573399815666190115144534. [DOI] [PubMed] [Google Scholar]
- Balto K., Muller R., Carrington D.C., Dobeck J., Stashenko P. Quantification of periapical bone destruction in mice by micro-computed tomography. J. Dent. Res. 2000;79(1):35–40. doi: 10.1177/00220345000790010401. [DOI] [PubMed] [Google Scholar]
- Barcelos R.C.S., Rosa H.Z., Roversi K., Tibúrcio-Machado C.D.S., Inchaki P.T., Burger M.E., Bier C.A.d.S. Apical periodontitis induces changes on oxidative stress parameters and increases Na+/K+-ATPase activity in adult rats. Arch. Oral Biol. 2020;118:104849. doi: 10.1016/j.archoralbio.2020.104849. [DOI] [PubMed] [Google Scholar]
- Bernardino Í.d.M., Farias Í.d.L., Cardoso A.M.R., Xavier A.F.C., Cavalcanti A.L. Use of animal models in experimental research in dentistry in Brazil. Pesq. Bras. Odontoped. Clin. Integr. 2014;14(1):17–21. [Google Scholar]
- Borges A.H., Pedro F.L.M., Semanoff-Segundo A., Miranda C.E.S., Pécora J.D., Cruz Filho A.M. Radiopacity evaluation of Portland and MTA-based cements by digital radiographic system. J. Appl. Oral Sci. 2011;19(3):228–232. doi: 10.1590/S1678-77572011000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone. Miner. Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Bozzo R.d.O., Rocha R.G., Haiter Neto F., Paganini G.A., Cavalcanti M.G.P. Linear density analysis of bone repair in rats using digital direct radiograph. J. Appl. Oral Sci. 2004;12(4):317–321. doi: 10.1590/s1678-77572004000400012. [DOI] [PubMed] [Google Scholar]
- Bueno C.R.E., Lopes G.A., Valentim D., Marques V.A.S., Vasques A.M.V., Cury M.T., Sivieri-Araujo G., Jacinto R.D.C., Cintra L.T.A., Dezan-Junior E. Mixing failures of endodontic sealers: an in vivo biocompatibility study. Braz. Dent. Sci. 2017;20(4):85–92. [Google Scholar]
- Bueno C.R.E., Valentim D., Marques V.A.S., Gomes-Filho J.E., Cintra L.T.A., Jacinto R.D.C., Dezan-Junior E. Biocompatibility and biomineralization assessment of bioceramic-, epoxy-, and calcium hydroxide-based sealers. Braz. Oral Res. 2016;30(1) doi: 10.1590/1807-3107BOR-2016.vol30.0081. [DOI] [PubMed] [Google Scholar]
- Bueno C.R.E., Vasques A.M.V., Cury M.T.S., Sivieri-Araújo G., Jacinto R.C., Gomes-Filho J.E., Cintra L.T.A., Dezan-Júnior E. Biocompatibility and biomineralization assessment of mineral trioxide aggregate flow. Clin. Oral Investig. 2019;23(1):169–177. doi: 10.1007/s00784-018-2423-0. [DOI] [PubMed] [Google Scholar]
- Cai X., Li C., Du G., Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodont. Res. 2008;43(1):14–21. doi: 10.1111/j.1600-0765.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- Cintra L.T.A., da Silva Facundo A.C., Azuma M.M., Sumida D.H., Astolphi R.D., Bomfim S.R.M., Narciso L.G., Gomes-Filho J.E. Pulpal and periodontal diseases increase triglyceride levels in diabetic rats. Clin. Oral Investig. 2013;17(6):1595–1599. doi: 10.1007/s00784-012-0853-7. [DOI] [PubMed] [Google Scholar]
- Cintra L.T.A., da Silva Facundo A.C., Prieto A.K.C., Sumida D.H., Narciso L.G., Mogami Bomfim S.R., e Silva C.O., Dezan-Júnior E., Gomes-Filho J.E. Blood profile and histology in oral infections associated with diabetes. J. Endod. 2014;40(8):1139–1144. doi: 10.1016/j.joen.2014.01.034. [DOI] [PubMed] [Google Scholar]
- Cintra L.T.A., Samuel R.O., Azuma M.M., Ribeiro C.P., Narciso L.G., de Lima V.M.F., Sumida D.H., Coclete G.A., Dezan-Júnior E., Gomes-Filho J.E. Apical periodontitis and periodontal disease increase serum IL-17 levels in normoglycemic and diabetic rats. Clin. Oral. Investig. 2014;18(9):2123–2128. doi: 10.1007/s00784-014-1192-7. [DOI] [PubMed] [Google Scholar]
- Colombo N.H., Shirakashi D.J., Chiba F.Y., Sara de Lima Coutinho M., Ervolino E., Saliba Garbin C.A., Machado U.F., Sumida D.H. Periodontal disease decreases insulin sensitivity and insulin signaling. J. Periodontol. 2012;83(7):864–870. doi: 10.1902/jop.2011.110349. [DOI] [PubMed] [Google Scholar]
- Cosme‐Silva L., Dal‐Fabbro R., Cintra L.T.A., Ervolino E., Plazza F., Mogami Bomfim S., Duarte P.C.T., Junior V.E.D.S., Gomes‐Filho J.E. Reduced bone resorption and inflammation in apical periodontitis evoked by dietary supplementation with probiotics in rats. Int. Endod. J. 2020;53(8):1084–1092. doi: 10.1111/iej.13311. [DOI] [PubMed] [Google Scholar]
- Dal‐Fabbro R., Marques‐de‐Almeida M., Cosme‐Silva L., Ervolino E., Cintra L.T.A., Gomes‐Filho J.E. Chronic alcohol consumption increases inflammation and osteoclastogenesis in apical periodontitis. Int. Endod. J. 2019;52(3):329–336. doi: 10.1111/iej.13014. [DOI] [PubMed] [Google Scholar]
- Duarte P.M., Bezerra J.P., Miranda T.S., Feres M., Chambrone L., Shaddox L.M. Local levels of inflammatory mediators in uncontrolled type 2 diabetic subjects with chronic periodontitis. J. Clin. Periodontol. 2014;41(1):11–18. doi: 10.1111/jcpe.12179. [DOI] [PubMed] [Google Scholar]
- Genco R.J., Grossi S.G., Ho A., Nishimura F., Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J. Periodontol. 2005;76(11-s):2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- Goes P., Lima A.P.S., Melo I.M., Rêgo R.O.C.C., Lima V. Effect of Atorvastatin in radiographic density on alveolar bone loss in wistar rats. Braz. Dent. J. 2010;21(3):193–198. doi: 10.1590/s0103-64402010000300003. [DOI] [PubMed] [Google Scholar]
- Gomes B.F., Accardo C.M. Immunoinflammatory mediators in the pathogenesis of diabetes mellitus. Einstein (São Paulo) 2019;17(1):eRB4596. doi: 10.31744/einstein_journal/2019RB4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M.S., Rabelo G.D., Spin-Neto R., Dechichi P., Borges J.S., Soares P.B.F. Use of micro-computed tomography for bone evaluation in dentistry. Braz. Dent. J. 2018;29(3):227–238. doi: 10.1590/0103-6440201801979. [DOI] [PubMed] [Google Scholar]
- Jett S., Shrout M., Mailhot J., Potter B., Borke J. An evaluation of the origin of trabecular bone patterns using visual and digital image analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;98(5):598–604. doi: 10.1016/S1079210404005244. [DOI] [PubMed] [Google Scholar]
- Kim D.M., Bassir S.H. When is cone-beam computed tomography imaging appropriate for diagnostic inquiry in the management of inflammatory periodontitis? An American academy of periodontology best evidence review. J. Periodontol. 2017;88(10):978–998. doi: 10.1902/jop.2017.160505. [DOI] [PubMed] [Google Scholar]
- King A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012;166(3):877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroder G., Bossenmaier B., Kellerer M., Capp E., Stoyanov B., Mühlhöfer A., Berti L., Horikoshi H., Ullrich A., Häring H. Tumor necrosis factor-alpha- and hyperglycemia-induced insulin resistance. Evidence for different mechanisms and different effects on insulin signaling. J. Clin. Invest. 1996;97(6):1471–1477. doi: 10.1172/JCI118569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krois J., Ekert T., Meinhold L., Golla T., Kharbot B., Wittemeier A., Dorfer C., Schwendicke F. Deep learning for the radiographic detection of periodontal bone loss. Sci. Rep. 2019;9:8495. doi: 10.1038/s41598-019-44839-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N.P., Bartold P.M. Periodontal health. J. Periodontol. 2018;89(Suppl 1):S9–S16. doi: 10.1002/JPER.16-0517. [DOI] [PubMed] [Google Scholar]
- Lappin D.F., Eapen B., Robertson D., Young J., Hodge P.J. Markers of bone destruction and formation and periodontitis in type 1 diabetes mellitus. J. Clin. Periodontol. 2009;36(8):634–641. doi: 10.1111/j.1600-051X.2009.01440.x. [DOI] [PubMed] [Google Scholar]
- Machado V., Proença L., Morgado M., Mendes J.J., Botelho J. Accuracy of panoramic radiograph for diagnosing periodontitis comparing to clinical examination. J. Clin. Med. 2020;9(7):2313. doi: 10.3390/jcm9072313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C.M., Gomes-Filho J.E., de Azevedo Queiroz Í.O., Ervolino E., Cintra L.T.A. Hypertension undermines mineralization inducing capacity of and tissue response to mineral trioxide aggregate endodontic cement. J. Endod. 2016;42(4):604–609. doi: 10.1016/j.joen.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Mealey B.L., Oates T.W. American academy of periodontology. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006;77(8):1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- Meyle J., Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2015;69(1):7–17. doi: 10.1111/prd.12104. [DOI] [PubMed] [Google Scholar]
- Mol A. Imaging methods in periodontology. Periodontol. 2004;2000(34):34–48. doi: 10.1046/j.0906-6713.2003.003423.x. [DOI] [PubMed] [Google Scholar]
- Nackaerts O., Jacobs R., Horner K., Zhao F., Lindh C., Karayianni K., van der Stelt P., Pavitt S., Devlin H. Bone density measurements in intra-oral radiographs. Clin. Oral Investig. 2007;11(3):225–229. doi: 10.1007/s00784-007-0107-2. [DOI] [PubMed] [Google Scholar]
- Nazir M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. (Qassim) 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- Nokhbatolfoghahaei H., Paknejad Z., Bohlouli M., Rezai Rad M., Khojasteh A. Applications of Biomedical Engineering in Dentistry. Springer International Publishing; Cham: 2020. pp. 377–442. [DOI] [Google Scholar]
- Pacios S., Kang J., Galicia J., Gluck K., Patel H., Ovaydi‐Mandel A., Petrov S., Alawi F., Graves D.T. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26(4):1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson A., Elcock C., Cheung A., Al-Buhairi A., Khanna S., Walsh T.F., Ellwood R.P. An in-vitro and in-vivo methodology study of alveolar bone measurement using extra-oral radiographic alignment apparatus, Image Pro-Plus software and a subtraction programme. J. Dent. 2005;33(9):781–788. doi: 10.1016/j.jdent.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Sakallıoğlu E.E., Lütfioğlu M., Sakallıoğlu U., Dıraman E., Keskiner İ. Fluid dynamics of gingiva in diabetic and systemically healthy periodontitis patients. Arch. Oral Biol. 2008;53(7):646–651. doi: 10.1016/j.archoralbio.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shrout M.K., Jett S., Mailhot J.M., Potter B.J., Borke J.L., Hildebolt C.F. Digital Image analyses of cadaver mandibular trabecular bone patterns. J. Periodontol. 2003;74(9):1342–1347. doi: 10.1902/jop.2003.74.9.1342. [DOI] [PubMed] [Google Scholar]
- Teixeira R.C., Rubira C.M.F., Assis G.F., Lauris J.R.P., Cestari T.M., Rubira-Bullen I.R.F. Radiological and histopathological evaluation of experimentally-induced periapical lesion in rats. J. Appl. Oral Sci. 2011;19(5):500–504. doi: 10.1590/S1678-77572011005000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshome A., Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health. 2016;17:31. doi: 10.1186/s12903-016-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay G., Raghavan V., Kailasam S. Radiology in periodontics. J. Indian. Acad. Oral. Med. Radiol. 2013;25:24–29. [Google Scholar]
- Von Knebel Doeberitz M., Wentzensen N. In: Comprehensive Cytopathology. third ed. Bibbo M., Wilbur D., editors. Saunders Publisher; 2008. The cell: basic structure and function; pp. 3–22. [Google Scholar]
- Wu Y.-Y., Xiao E., Graves D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral. Sci. 2015;7(2):63–72. doi: 10.1038/ijos.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybutz M., Rapoport D., Laurell L., Persson G.R. Comparison of clinical and radiographic measurements of interproximal vertical defects before and 1 year after surgical treatments. J. Clin. Periodontol. 2000;27:179–186. doi: 10.1034/j.1600-051x.2000.027003179.x. [DOI] [PubMed] [Google Scholar]