Abstract
PCRs were developed to detect 11 Escherichia coli virulence genes. Primers amplified the respective genes without cross-reaction with other genes. Specificity was maintained in multiplex reactions; excellent amplification of target genes was possible with a minimum of four multiplex reactions. These reactions successfully identified genes in E. coli from the feces of four dogs.
The virulence mechanisms that characterize Escherichia coli are genetically coded for by chromosomal, plasmid, and bacteriophage DNAs and include heat-labile (LTI, LTIIa, and LTIIb) and heat-stable (STI and STII) toxins, verotoxin types 1, 2, and 2e (VT1, VT2, and VT2e, respectively), cytotoxic necrotizing factors (CNF1 and CNF2), attaching and effacing mechanisms (eaeA), enteroaggregative mechanisms (Eagg), and enteroinvasive mechanisms (Einv). With the advent of PCR, it has become possible to identify these genes in bacterial isolates, offering the possibility of rapid diagnosis of the mechanisms operating in specific E. coli infections. PCR methods using single primer sets have been reported (1, 2, 3, 4, 5, 6, 7, 8, 10, 11, 12, 13, 14, 15), but screening of bacterial isolates for the myriad of virulence genes requires a large number of individual PCRs if single primer sets are used in separate reactions. To reduce the number of tests needed for screening E. coli for virulence factor genes, we have developed multiplex PCR systems that can identify 11 genes.
Primers for amplifying segments of the VT1, VT2, VT2e, CNF1, CNF2, LTI, STI, STII, eaeA, Einv, and Eagg genes were designed so that the PCR products from the genes were sufficiently different in size to be distinguishable by agarose gel electrophoresis (Table 1). Comparisons with gene sequences in the EMBL gene bank ensured that the genes for the PCR products lacked homology with genes other than the target genes.
TABLE 1.
Sequences of PCR primers, product sizes, and accession numbers in the EMBL gene bank from which the sequences were obtained
| Gene | Primersa | Product size (nucleotides) | EMBL accession no. |
|---|---|---|---|
| VT1 | fp: 5′-ACGTTACAGCGTGTTGCRGGGATC-3′ | 121 | L04539 and Z36901 |
| bp: 5′-TTGCCACAGACTGCGTCAGTRAGG-3′ | |||
| VT2 | fp: 5′TGTGGCTGGGTTCGTTAATACGGC-3′ | 102 | L11078 |
| bp: 5′-TCCGTTGTCATGGAAACCGTTGTC-3′ | |||
| VT2e | fp: 5′-CCAGAATGTCAGATAACTGGCGAC-3′ | 322 | M21534 |
| bp: 5′-GCTGAGCACTTTGTAACAATGGCTG-3′ | |||
| eaeA | fp: 5′-TGAGCGGCTGGCATGAGTCATAC-3′ | 241 | X60439 |
| bp: 5′-TCGATCCCCATCGTCACCAGAGG-3′ | |||
| CNF1 | fp: 5′-GGCGACAAATGCAGTATTGCTTGG-3′ | 552 | U42629 |
| bp: 5′-GACGTTGGTTGCGGTAATTTTGGG-3′ | |||
| CNF2 | fp: 5′GTGAGGCTCAACGAGATTATGCACTG-3′ | 839 | U01097 |
| bp: 5′-CCACGCTTCTTCTTCAGTTGTTCCTC-3′ | |||
| LTI | fp: 5′-TGGATTCATCATGCACCACAAGG-3′ | 360 | S60731 |
| bp: 5′-CCATTTCTCTTTTGCCTGCCATC-3′ | |||
| STI | fp: 5′-TTTCCCCTCTTTTAGTCAGTCAACTG-3′ | 160 | M25607 |
| bp: 5′-GGCAGGATTACAACAAAGTTCACAG-3′ | |||
| STII | fp: 5′-CCCCCTCTCTTTTGCACTTCTTTCC-3′ | 423 | M35586 |
| bp: 5′-TGCTCCAGCAGTACCATCTCTAACCC-3′ | |||
| Einv | fp: 5′-TGGAAAAACTCAGTGCCTCTGCGG-3′ | 140 | M32063 |
| bp: 5′-TTCTGATGCCTGATGGACCAGGAG-3′ | |||
| Eagg | fp: 5′-AGACTCTGGCGAAAGACTGTATC-3′ | 194 | X81423 |
| bp: 5′-ATGGCTGTCTGTAATAGATGAGAAC-3′ |
fp, forward primer; bp, backward primer; R = A and G.
DNA standards were extracted from bacteria known to contain the relevant genes. Bacteria containing LTI and STII were O147:K89:K88ac and O147:KT:K88 isolates, respectively, obtained from the Central Veterinary Laboratory, Ministry of Agriculture, Fisheries and Food, Addlestone, United Kingdom, and the bacterium containing VT2e was an O141:H9 isolate obtained from the Public Health Laboratory Service, London, United Kingdom. Genes in other isolates had been identified by DNA hybridization in the laboratory of one of us (R.M.B.) or in laboratories of colleagues. The bacteria were grown in Luria broth or on MacConkey agar plates at 37°C. Fecal swabs from dogs were cultured on MacConkey agar plates, and up to 100 colonies from the plates were combined for PCR analysis.
The cell resuspension solution, cell lysis solution, neutralization solution, and minicolumns for DNA isolation were purchased as a Wizard Miniprep DNA Purification System from Promega. DNA was extracted from centrifuged bacterial pellets or bacterial colonies after suspension in 200 μl of resuspension solution. Cell lysis solution (200 μl) was added, and the sample was vortexed for 20 to 30 s. The sample was vortexed for 10 s after the addition of 200 μl neutralization solution and then centrifuged at 12,000 × g for 5 min. The supernatant was decanted into 1 ml of resin suspension containing 1.5 g diatomaceous earth in 100 ml of 4 M guanidine isothiocyanate, 10 mM Tris (pH 7.6), and 1 mM EDTA (pH 8). The resin suspension was applied to a minicolumn and washed with 3 ml of a column wash solution containing 200 mM NaCl, 29 mM Tris-HCl (pH 7.5), and 2 mM EDTA in 55% ethanol. The filter was dried by centrifugation at 12,000 × g for 2 min, and the DNA was eluted into 100 μl of water that had been preheated to 65°C. For direct PCR of bacteria, individual colonies from culture plates were suspended in 50 μl of water.
PCR was performed with 0.5-ml Eppendorf tubes on a Techne PHC-3 thermal cycler with a reaction volume of 25.25 or 50.5 μl overlaid with 15 or 30 μl of paraffin oil, respectively. The DNA template (5 μl containing 90 to 200 pg of DNA) or 5 μl of bacterial suspension was added to a 50-μl reaction mixture containing 0.1 mM each dATP, dCTP, dGTP, and dUTP (Bioline UK Ltd.); a buffer solution consisting of 16 mM (NH4)2SO4, 67 mM Tris-HCl (pH 8.8 at 25°C), and 0.01% Tween 20; 3 mM MgCl2; and the PCR primers (PE-Applied Biosystems, Warrington, United Kingdom). The concentrations of primers that gave satisfactory amplification of genes are given in Table 2. Taq polymerase (2.5 U in 0.5 μl; Bioline UK Ltd.) was added. The PCR program was 95°C for 30 s followed by 72°C for 1 min for 5 cycles; 95°C for 30 s followed by 63°C for 30 s and then 72°C for 30 s for 20 cycles; and 72°C for 5 min when isolated DNA was amplified. The PCR program was preceded by 5 min at 95°C to lyse the bacteria when bacterial suspensions were examined. Reaction products were separated by agarose gel electrophoresis by adding 5 μl of loading dye containing 0.25% bromophenol blue in 40% sucrose to a 50-μl reaction mixture and loading 5 μl onto a 3% agarose gel (NuSeive 3:1; FMC). The buffer in the electrophoresis chamber and in the agarose gel was 0.5× Tris-borate-EDTA (9) and contained ethidium bromide (1 μg/ml). One hundred volts and 25 mA were applied across the gel. DNA in the gel was visualized by exposing the gel to UV light and was photographed on Polaroid film. The figures were produced with a computer graphics program after computer scanning of the Polaroid photographs.
TABLE 2.
Concentrations of primers in reaction mixtures
| Gene | Primer concn (pmol/μl)a in the following primer set:
|
|
|---|---|---|
| Single | Multiplex | |
| VT1 | 0.5 | 0.1 |
| VT2 | 0.5 | 0.1 |
| VT2e | 0.5 | 0.1 |
| eaeA | 0.1 | 0.05 |
| CNF1 | 0.2 | 0.2 |
| CNF2 | 0.2 | 0.03 |
| LTI | 0.2 | 0.03 |
| STI | 0.2 | 0.05 |
| STII | 0.2 | 0.1 |
| Einv | 0.2 | 0.2 |
| Eagg | 0.2 | 0.1 |
The concentration applies to each of the forward and reverse primers.
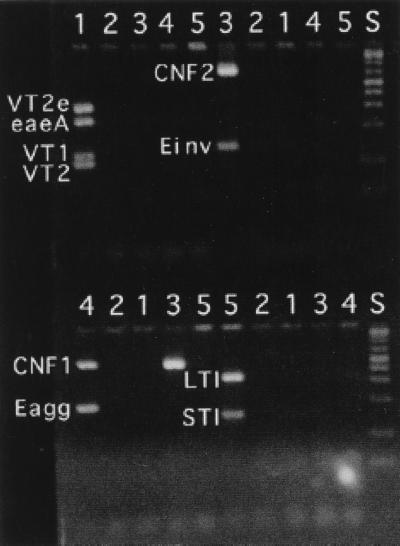
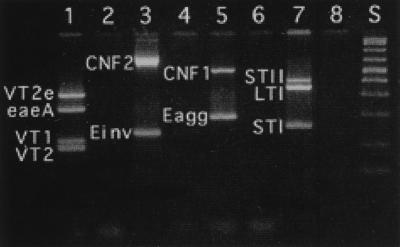
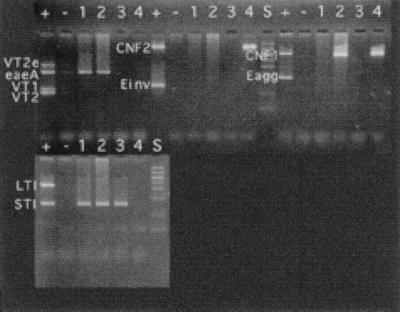
PCR primers amplified fragments of DNA of the predicted size, and amplification was specific for the gene in question (Fig. 1 and 2). When primer pairs were combined in various multiplex combinations, some primers interfered with the amplification of other genes. For instance, CNF1 and CNF2 primer pairs could not be used in combination, as spurious products that interfered with the interpretation of the electrophoretic results were produced. The following four combinations of primers gave adequate amplification of their respective target genes: VT1, VT2, VT2e, and eaeA; CNF1 and Eagg; CNF2 and Einv; and LTI, STI, and STII (Fig. 3). Only the target genes were amplified (Fig. 2). These combinations were used successfully to amplify genes in E. coli cultured from feces of four dogs (Fig. 4). It was possible to use combinations other than those listed above to amplify more genes in a single reaction (Fig. 5). However, there was a tendency to lose the amplification of some genes, particularly if all of the genes corresponding to the primer pairs were present in the reaction. Nevertheless, it is unlikely that more than three or four genes would be present in any one clinical sample. Therefore, more complex combinations would be satisfactory, particularly if several positive control samples were included in the assay, each with a limited number of genes present.
FIG. 1.
Agarose gel electrophoresis of the PCR products of E. coli virulence genes. Lanes: +, DNA template with the gene; −, control DNA without the gene; S, DNA standards.
FIG. 2.
Agarose gel electrophoresis of mixtures of DNA templates showing the specificity of the multiplex reactions. DNA templates 1 to 5 contained the following genes: template 1 contained genes for VT1, VT2, VT2e, and eaeA; template 2 did not contain any target gene; template 3 contained genes for CNF2 and Einv; template 4 contained genes for CNF1 and Eagg; and template 5 contained genes for LTI and STI. The reactions used to generate products in the first five lanes in the top half of the figure contained primers for VT1, VT2, VT2e, and eaeA; the next 5 lanes were for CNF2 and Einv. The reactions used to generate products in the first five lanes in the bottom half of the figure contained primers for CNF1 and Eagg; the next five lanes were for LTI and STI. Lane S, DNA standards. Note that for template 3, the CNF2-positive DNA also contained the gene for CNF1.
FIG. 3.
Agarose gel electrophoresis of the PCR products of E. coli virulence genes from multiplex PCRs. Lanes 1, 3, 5, and 7 are from reactions containing the respective genes, and lanes 2, 4, 6, and 8 are from reactions with control DNA and no target genes. Lane S, DNA standards.
FIG. 4.
Agarose gel electrophoresis of products from multiplex PCRs with DNA from E. coli isolated from the feces of four dogs. Lanes: +, positive control DNA; −, negative control DNA; 1 to 4 DNA isolated from E. coli cultured from dog feces. E. coli from dog 1 was positive for eaeA and STI; that from dog 2 was positive for eaeA, CNF1, and STI; that from dog 3 was positive for STI; and that from dog 4 was positive for CNF1 and CNF2. Lane S, DNA standards.
FIG. 5.
Agarose gel electrophoresis of multiplex PCRs with DNAs containing several virulence genes (+) and DNAs without the genes (−). The genes and PCR primers in each lane, from left to right, were VT1, VT2, VT2e, and eaeA in the 1st lane; VT1, VT2, VT2e, eaeA, CNF2, and Einv in the 2nd and 3rd lanes; VT1, VT2, VT2e, eaeA, CNF1, and Eagg in the 4th and 5th lanes; VT1, VT2, VT2e, eaeA, LTI, and STI in the 6th and 7th lanes; VT1, VT2, VT2e, eaeA, CNF2, Einv, LTI, and STI in the 8th and 9th lanes; and VT1, VT2, VT2e, eaeA, CNF1, Eagg, LTI, and STI in the 10th and 11th lanes. Lane S, DNA standards.
Amplification of the target gene segment occurred when either extracted DNA or whole bacteria were used as the DNA template. It was necessary to heat the sample for 5 min at 95°C to release the DNA for the PCR when intact bacteria were used as the source of DNA. This extra step did not interfere with PCR amplification. This result indicates that these analyses could be used for bacteria isolated directly from biological materials.
The present results show that it is possible to detect 11 of the major virulence genes of E. coli with four multiplex PCRs. This method offers a practical possibility for mass screening of samples or for rapid identification of likely pathogenic mechanisms operating in active infections.
Acknowledgments
We thank staff at the Royal Veterinary College, who collected samples for analyses.
REFERENCES
- 1.Candrian U, Furrer B, Hofelein C, Myer R, Jermini M, Luthy J. Detection of Escherichia coli and identification of enterotoxigenic strains by primer-directed enzymatic amplification of specific DNA sequences. Int J Food Microbiol. 1991;12:339–352. doi: 10.1016/0168-1605(91)90148-i. [DOI] [PubMed] [Google Scholar]
- 2.Fratamico P M, Sackitey S K, Weidmann M, Deng M Y. Detection of Escherichia coli O157:H7 by multiplex PCR. J Clin Microbiol. 1995;33:2188–2191. doi: 10.1128/jcm.33.8.2188-2191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson M P. Identification of Shiga-like toxin type II producing Escherichia coli using the polymerase chain reaction and a digoxigenin labelled DNA probe. Mol Cell Probes. 1992;6:209–214. doi: 10.1016/0890-8508(92)90018-s. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Kurazono H, Yamasaki S, Takeda Y. Detection of various variant verotoxin genes in Escherichia coli by polymerase chain reaction. Microbiol Immunol. 1993;37:543–548. doi: 10.1111/j.1348-0421.1993.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 5.Louie M, de Azavedo J, Clarke R, Borczyk A, Lior H, Richter M, Brunton J. Sequence heterogeneity of the eae gene and detection of verotoxin-producing Escherichia coli using serotype-specific primers. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mainil J G, Jacquemin E, Herault F, Oswald E. Presence of pap-, sfa-, and afa-related sequences in necrotoxigenic Escherichia coli isolates from cattle: evidence for new variants of the AFA family. Can J Vet Res. 1997;61:193–199. [PMC free article] [PubMed] [Google Scholar]
- 7.Olive D M. Detection of enterotoxigenic Escherichia coli after polymerase chain reaction amplification with a thermostable DNA polymerase. J Clin Microbiol. 1989;27:261–265. doi: 10.1128/jcm.27.2.261-265.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Rapid and specific detection of verotoxin genes in Escherichia coli by polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 10.Saulnier P, Chachaty E, Hilali F, Andremont A. Single-step polymerase chain reaction for combined gene detection and epidemiological typing in three bacterial models. FEMS Microbiol Lett. 1997;150:311–316. doi: 10.1111/j.1574-6968.1997.tb10386.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt H, Knop C, Franke S, Aleksic S, Heesemann J, Karch H. Development of PCR for screening enteroaggregative Escherichia coli. J Clin Microbiol. 1995;33:701–705. doi: 10.1128/jcm.33.3.701-705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethabutr O, Echeverria P, Hoge C W, Bodhidatta L, Pitarangsi C. Detection of Shigella and enteroinvasive Escherichia coli by PCR in the stools of patients with dysentery in Thailand. J Diarrhoeal Dis Res. 1994;12:265–269. [PubMed] [Google Scholar]
- 13.Thomas A, Jiggle B, Smith H R, Rowe B. The detection of Vero cytotoxin-producing Escherichia coli and Shigella dysenteriae type 1 in faecal specimens using polymerase chain reaction gene amplification. Lett Appl Microbiol. 1994;19:406–409. doi: 10.1111/j.1472-765x.1994.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas A, Cheasty T, Chart H, Rowe B. Isolation of vero cytotoxin-producing Escherichia coli serotypes O9ab:H− and O101:H− carrying VT2 variant gene sequences from a patient with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis. 1994;13:1074–1076. doi: 10.1007/BF02111832. [DOI] [PubMed] [Google Scholar]
- 15.Tornieporth N G, John J, Salgado K, de Jesus P, Latham E, Melo M C, Gunzburg S T, Riley L W. Differentiation of pathogenic Escherichia coli strains in Brazilian children by PCR. J Clin Microbiol. 1995;33:1371–1374. doi: 10.1128/jcm.33.5.1371-1374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]