Abstract
Seventy-two Angus-crossbred steers (411 ± 16 kg) were assigned to a 2 × 3 factorial arrangement of treatments to examine the effects of blended Zn source supplementation on performance, carcass characteristics, and trace mineral parameters of steers administered no implant or a two-implant program. Factors included implant (IMP) strategies and Zn supplementation. During the 126-d study, steers were either not implanted (NoIMP) or implanted (IS/200; Elanco, Greenfield, IN) on days 0 (Component TE-IS; 80 mg trenbolone acetate + 16 mg estradiol) and 57 (Component TE-200; 200 mg trenbolone acetate + 20 mg estradiol). All steers were fed 70 mg Zn/kg on a dry matter (DM) basis from ZnSO4 + 30 mg Zn/kg DM from either basic ZnCl (Vistore Zn, Phibro Animal Health, Teaneck, NJ), Zn glycinate (Gemstone Zn, Phibro Animal Health), or ZnSO4 (ZnB, ZnG, or ZnS, respectively). Steers were blocked by weight into pens of 6 and fed a dry rolled corn-based diet via GrowSafe bunks (GrowSafe Systems Ltd.; Airdrie, AB, Canada). Data were analyzed using the Mixed Procedure of SAS, with fixed effects of Zn, IMP, and the interaction. Steer was the experimental unit (n = 12 steers/treatment). Liver and muscle collected on days −5, 14, 71, and 120 were analyzed for Zn concentration, and data were analyzed as repeated measures (repeated effect = Day). An IMP × Zn tendency (P = 0.07) was observed for day 126 body weight with no effects of Zn within NoIMP, whereas ZnS tended to be heavier than ZnB with ZnG intermediate within IS/200. Carcass-adjusted overall feed efficiency (G:F) was greatest for ZnS (Zn; P = 0.02). Implanted cattle had greater DM intake, G:F, and carcass-adjusted performance (P ≤ 0.01). Liver Zn concentrations were greater for IS/200 by day 120 (IMP × Day; P = 0.02). Within IS/200, ZnG tended to have greater muscle Zn than ZnS, whereas ZnB was intermediate (Zn × IMP; P = 0.09). No Zn or IMP × Zn (P ≥ 0.12) effects were observed for carcass data. However, IS/200 had greater hot carcass weight, dressing percentage, and ribeye area than NoIMP (P ≤ 0.001). These data suggest that implants improve growth and influence Zn metabolism. Future work should examine Zn sources and supplementation alongside implant strategies.
Keywords: anabolic implant, cattle, chelated zinc, growth, hydroxy zinc
INTRODUCTION
Widespread use of anabolic implants in feedlot cattle (NAHMS, 2013) stems from considerable improvements in performance of implanted cattle (Duckett and Pratt, 2014). Furthermore, supplementation of Zn, Cu, Mn, Co, and Se at concentrations 2 to 3 times greater than NASEM (2016) recommendations improved carcass-adjusted average daily gain (ADG) by 13.3% compared to non-supplemented steers (Niedermayer et al., 2018). In particular, Zn supports protein synthesis (Oberleas and Prasad, 1969; Suttle, 2010) and N retention (Carmichael et al., 2018), suggesting increased dietary Zn may be needed in growing cattle.
Inorganic ZnSO4 is commonly fed to feedlot cattle, but blends of inorganic and organic trace minerals are also fed (Samuelson et al., 2016). Zinc glycinate, an organic amino acid chelate, is considered more bioavailable than ZnSO4 in rats, chickens, and cattle (Spears et al., 2004; Schlegel and Windisch, 2006; Sridhar et al., 2015). Limited research on basic ZnCl, a hydroxy source, suggests similar bioavailability for basic ZnCl as ZnSO4 (Cao et al., 2000; Batal et al., 2001), though Shaeffer et al. (2017) found basic ZnCl to be more bioavailable than ZnSO4 in cattle. To the best of our knowledge, a direct comparison between Zn glycinate and basic ZnCl has not been reported.
Although nutritional Zn requirements can be met with any Zn source, those with greater bioavailability may be optimal during periods of high Zn demand such as implant-induced growth and may effectively decrease the amount of supplemental Zn needed to meet these demands. Messersmith (2018) found implanted steers experiencing 29% greater ADG than non-implanted steers 14 d after implant administration had a 14% decrease in plasma Zn concentrations. This suggests that implanted cattle have a demand for Zn to support rapid growth, drawing down circulating concentrations. Therefore, this study’s objective was to determine the effects of feeding 100% ZnSO4, or 70% ZnSO4 with 30% of either basic ZnCl or Zn glycinate on performance, carcass characteristics, and trace mineral parameters of feedlot steers not implanted or implanted with a moderately aggressive two implant strategy. It was hypothesized that implanted cattle would better utilize the more available Zn sources towards muscle accretion.
MATERIALS AND METHODS
Care and Use of Animals
All procedures and protocols utilized in this trial were approved by the Iowa State University Institutional Animal Care and Use Committee (log number: IACUC-18-344).
Animals and Experimental Design
Seventy-two Angus-cross steers (initial body weight = 411 ± 16 kg) were utilized in a 2 × 3 factorial arrangement of treatments. The effects of implant strategy and Zn source on performance and carcass characteristics in a 126-d study were examined using a randomized complete block design. Steers were housed in partially covered concrete pens (n = 6 steers/pen) equipped with automatic waterers and GrowSafe bunks (GrowSafe Systems Ltd.; Airdrie, AB, Canada). Individual steer’s radio frequency tags were linked to GrowSafe software to record individual steer feed disappearance. As-fed feed disappearance was corrected for dry matter (DM) and used to calculate individual steer dry matter intake (DMI). Implant strategies included either no implant (NoIMP) or a Component TE-IS (80 mg trenbolone acetate + 16 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0 followed by a Component TE-200 (200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health; IS/200) on day 57. Additionally, all cattle received 70 mg Zn/kg DM from ZnSO4 + 30 mg Zn/kg DM from one of three sources: basic ZnCl (Vistore Zn, Phibro Animal Health, Teaneck, NJ), Zn glycinate (Gemstone Zn, Phibro Animal Health), and ZnSO4 (ZnB, ZnG, and ZnS, respectively). Steers were assigned to block (light or heavy) by body weight (BW) and randomly assigned to treatment and pen; all treatments were equally represented within each weight block. On day 127, steers were harvested at a commercial abattoir (National Beef, Tama, IA) via industry standard captive bolt and hot carcass weight (HCW) data were obtained. Following a 48-h chill, ribeye area (REA), 12th rib fat (BF), percent kidney, pelvic, and heart fat (KPH), and marbling score data were collected by trained University personnel and yield grade (YG) was calculated.
Sample Collection and Analysis
Steers were fed a dry rolled corn-based diet (Table 1) once daily (0800 h) with weekly total mixed ration (TMR) samples dried in a forced air oven for 48 h at 70 °C to determine diet DM. A grinder fitted with a 2-mm screen (Retsch Zm100 grinder; Glen Mills Inc., Clifton, NJ) was utilized to grind dried TMR. Ground samples were composited by month for nutrient analysis by Dairyland Laboratories (Arcadia, WI). Body weights were collected approximately every 28 d with consecutive d BW to begin the study (days −1 and 0), at re-implant (days 56 and 57), and before harvest (days 125 and 126). Pre-feeding blood was collected via jugular venipuncture in trace mineral grade EDTA and heparin-containing vacuum capped tubes (Becton Dickenson, Rutherford, NJ) for TM and plasma urea nitrogen (PUN) analysis, respectively, on days −1, 14, 56, 71 or 72, and 125 for all steers. Blood was centrifuged at 1,000 × g for 20 min to separate plasma for storage at −20 °C until analysis. A Urea Nitrogen Reagent (Colorimetric Method, Teco Diagnostics, Anaheim, CA) was used to determine PUN. Inter-assay and intra-assay CV were 7.33% and 3.02%, respectively. Liver and muscle biopsies were conducted on all steers over two d for each time point (days −5/−4, 14/15, 71/72, and 120/121) with half of the steers biopsied on each day utilizing the methods described by Engle and Spears (2000) for liver and adapted from Pampusch et al. (2008) for muscle. Muscle biopsies were collected from the longissimus thoracis between the 10th and 13th ribs. Rib spacing and side were alternated between each muscle biopsy timepoint. Liver and muscle samples were stored at −20 °C prior to trace mineral analyses. Composited TMR, liver, muscle, and plasma samples were analyzed for trace mineral concentrations via inductively coupled plasma optical emission spectrometry (ICP Optima 7000 DV, Perkin Elmer, Waltham, MA) as described by Pogge and Hansen (2013) and Richter et al. (2012). Each run utilized a standard to ensure instrument accuracy (plasma: Trace Elements Serum Control #66816; UTAK Laboratories Inc., Valencia, CA; liver, muscle and TMR: Bovine Liver #1577c; National Institute of Standards and Technology, Gaithersburg, MD).
Table 1.
Diet composition
| Ingredient | % of diet DM |
|---|---|
| Dry rolled corn | 57.0 |
| Sweet brana | 20.0 |
| Bromegrass hay | 8.0 |
| DDGSb | 13.03 |
| Limestone | 1.5 |
| Salt | 0.31 |
| Vitamin and mineral premixc,d,e | 0.1441 |
| Rumensin | 0.0135 |
| Analyzed composition | |
| Crude proteinf | 13.9 |
| Neutral detergent fiberf | 23.9 |
| Ether extractf | 4.2 |
| Cu, mg/kg DMg | 13 |
| Fe, mg/kg DMg | 134 |
| Mn, mg/kg DMg | 37 |
| Zn, mg/kg DMg | 130 |
| Calculated compositionh | |
| NEm | 2.05 |
| NEg | 1.39 |
a Branded wet corn gluten feed (Cargill Corn Milling, Blair, NE).
b Dried distillers grains with solubles.
c Premix provided 2,200 IU vitamin A and 25 IU vitamin E/kg diet.
d Wtih the exception of Zn, trace minerals were supplemented at NASEM (2016) recommendations for Co, Cu, I, Mn, and Se from inorganic sources.
e All diets included supplemental Zn at 70 mg Zn/kg DM provided as ZnSO4. Additional Zn (30 mg Zn/kg DM) was provided for respective treatments from ZnSO4 (ZnS), basic ZnCl (ZnB; Phibro Animal Health) or Zn glycinate (ZnG; Gemstone Zn; Phibro Animal Health), to achieve a total of 100 mg supplemental Zn/kg DM.
f Analysis of ZnS TMR conducted by Dairyland Laboratories (Arcadia, WI).
g Analyzed values for trace minerals represent the average of ZnB, ZnG, and ZnS treatments measured by inductively coupled plasma optical emission spectrometry (ICP Optima 7000 DV, Perkin Elmer, Waltham, MA).
h Calculations for net energy of maintenance (NEm) and net energy of gain (NEg) were conducted using NASEM (2016) nutrient values for diet ingredients.
Statistical Analysis
Data were analyzed as a randomized complete block design with a 2 × 3 factorial arrangement of treatments for performance, blood, tissue, and carcass characteristics using the Mixed Procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC). Fixed effects included implant (IMP), Zn, and IMP × Zn. The experimental unit was steer (n = 11 or 12/treatment) and initial BW was used as a covariate in performance and carcass analyses. Plasma trace mineral, PUN, and liver trace mineral data were analyzed as repeated measures using the compound symmetry covariance structure, whereas muscle Zn concentration data utilized repeated measures with the heterogeneous compound symmetry covariance structure determined by the lowest corrected Akaike’s information criterion. The repeated effect was Day. Normality was assessed using Shapiro–Wilks test and outliers were evaluated using Cook’s D, with no outliers being found. One steer was removed from all analysis due to health concerns unrelated to treatment (NoIMP-ZnG) and another removed from carcass data analysis due to unaccepted hide coloration at the commercial abattoir (IS/200-ZnB). Data are reported as least squares means with the SEM. Statistical significance was determined at P ≤ 0.05, with tendencies defined as 0.05 < P ≤ 0.10.
RESULTS AND DISCUSSION
Although anabolic implants are extensively used in the feedlot industry (NAHMS, 2013; Samuelson et al., 2016), implanted cattle’s micronutrient requirements are poorly understood. Incorporation of trace minerals into the diet at greater than NASEM (2016) recommendations has been found to increase HCW (Niedermayer et al., 2018). However, little research has been conducted to determine if a single trace mineral is responsible for this growth response in cattle. A multitude of enzymes require Zn for catalytic or structural roles (Suttle, 2010) and Zn is required for protein synthesis (Oberleas and Prasad, 1969). Therefore, Zn is a promising candidate for strategic supplementation aimed to improve growth in high growth potential animals such as those utilizing anabolic implants. Furthermore, Zn source, and ultimately bioavailability, must be considered to determine a strategic trace mineral program targeting optimal performance. Therefore, we hypothesized that steers supplemented a more bioavailable Zn source would have improved performance over steers receiving less bioavailable Zn sources, with greater benefits within implanted steers.
Organic Zn-glycinate has been observed to be more bioavailable than inorganic ZnSO4 in both rats and broiler chickens (Schlegel and Windisch, 2006; Sridhar et al., 2015). Although a direct comparison between Zn-glycinate and basic ZnCl has not been reported, research in chickens and cattle has indicated that basic ZnCl has a similar bioavailability as ZnSO4 (Cao et al., 2000; Batal et al., 2001), whereas others suggest that basic ZnCl is more bioavailable than ZnSO4 in cattle (Shaeffer et al., 2017). Although not definitive, these data suggest Zn-glycinate may be more bioavailable than the hydroxy source, basic ZnCl. However, basic ZnCl may be considered advantageous over ZnSO4 due to its insoluble properties (Cao et al., 2000; Shaeffer, 2006), protecting this source of Zn from dietary antagonists in the rumen.
Assessment of performance measures (Table 2) in the current study revealed no differences in body weights taken on days 28, 57, 84, 112, and 126 due to Zn source (P ≥ 0.22), nor were IMP × Zn effects observed for day 28 or 57 BW. However, by day 28, IS/200 steers tended to be heavier than NoIMP (P = 0.08), leading to a 14 kg advantage in BW for IS/200 steers compared to NoIMP on d 57 (P = 0.001). Interestingly, a tendency for an IMP × Zn (P ≤ 0.10) effect for days 84, 112, and 126 BW was observed, where Zn source did not affect BW of NoIMP steers, whereas within IS/200 treatment, ZnS tended to have greater BW than ZnB, with ZnG being intermediate. A tendency for an IMP × Zn effect (P = 0.08) was observed for days 0–56 ADG in which ZnS was numerically less than ZnB and ZnG within NoIMP and ZnB was numerically less than ZnG and ZnS. However, a tendency for an IMP × Zn (P = 0.07) effect in overall ADG found no differences in overall ADG between Zn sources within NoIMP, whereas ZnS was greater than ZnB and ZnG was intermediate within IS/200. Furthermore, implanted cattle had greater d 57-126 ADG (P < 0.0001), though no Zn or IMP × Zn effects (P ≥ 0.12) were observed for ADG during this period.
Table 2.
The effects of implanta and zinc sourceb on performance measures in finishing feedlot steers
| Implant treatments | NoIMP | IS/200 | SEM | P-values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zinc treatments | ZnB | ZnG | ZnS | ZnB | ZnG | ZnS | IMP | Zn | IMP × Zn | |
| Steer (n)c | 12 | 11 | 12 | 12 | 12 | 12 | ||||
| Body weight, kg | ||||||||||
| d 0 | 411 | 411 | 409 | 410 | 412 | 412 | 3.1 | 0.70 | 0.96 | 0.80 |
| d 28 | 462 | 461 | 452 | 459 | 469 | 465 | 4.0 | 0.08 | 0.22 | 0.11 |
| d 57 | 508 | 506 | 497 | 511 | 520 | 522 | 5.1 | <0.001 | 0.74 | 0.11 |
| d 84 | 542yz | 538yz | 536z | 555xy | 567wx | 575w | 6.3 | <0.001 | 0.48 | 0.10 |
| d 112 | 586z | 579z | 581z | 611y | 622xy | 637x | 7.3 | <0.001 | 0.30 | 0.10 |
| d 126 | 603z | 594z | 591z | 630y | 639xy | 654x | 7.8 | <0.001 | 0.62 | 0.07 |
| Average daily gain, kg | ||||||||||
| d 0–56 | 1.70yz | 1.65yz | 1.55z | 1.77xy | 1.90x | 1.91x | 0.069 | <0.001 | 0.75 | 0.08 |
| d 57–126 | 1.38 | 1.28 | 1.37 | 1.72 | 1.72 | 1.91 | 0.070 | <0.001 | 0.12 | 0.32 |
| Dry matter intake, kg | ||||||||||
| d 0–56 | 10.5 | 10.6 | 9.8 | 10.5 | 10.8 | 10.4 | 0.28 | 0.23 | 0.08 | 0.55 |
| d 57–126 | 10.4 | 10.5 | 10.3 | 10.9 | 11.0 | 11.5 | 0.36 | 0.01 | 0.84 | 0.45 |
| Zn intake, mg/d | ||||||||||
| d 0–56 | 1367 | 1295 | 1310 | 1367 | 1321 | 1398 | 37.2 | 0.20 | 0.24 | 0.45 |
| d 57–126 | 1361 | 1294 | 1383 | 1419 | 1353 | 1549 | 46.9 | 0.01 | 0.01 | 0.40 |
| G:Fd | ||||||||||
| d 0–56 | 0.161 | 0.147 | 0.174 | 0.200 | 0.194 | 0.228 | 0.0093 | <0.001 | 0.01 | 0.70 |
| d 57–126 | 0.132 | 0.121 | 0.133 | 0.160 | 0.156 | 0.169 | 0.0072 | <0.001 | 0.19 | 0.83 |
| Overall performance | ||||||||||
| ADGe, kg | 1.49z | 1.41z | 1.38z | 1.69y | 1.74xy | 1.84x | 0.060 | <0.0001 | 0.53 | 0.07 |
| DMI, kg | 10.5 | 10.6 | 10.1 | 10.7 | 10.9 | 11.0 | 0.30 | 0.03 | 0.74 | 0.45 |
| G:F | 0.15 | 0.14 | 0.15 | 0.16 | 0.17 | 0.18 | 0.005 | <0.0001 | 0.13 | 0.36 |
| Carcass-adjusted performancef | ||||||||||
| Final BW, kg | 598 | 590 | 590 | 635 | 637 | 660 | 8.7 | <0.0001 | 0.33 | 0.12 |
| ADG, kg | 1.48 | 1.42 | 1.44 | 1.79 | 1.79 | 1.98 | 0.062 | <0.0001 | 0.21 | 0.12 |
| G:F | 0.14 | 0.13 | 0.14 | 0.17 | 0.16 | 0.18 | 0.005 | <0.0001 | 0.02 | 0.49 |
a Implant strategies included no implant (NoIMP) or a Component TE-IS (IS/200; 80 mg trenbolone acetate + 16 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0 followed by a Component TE-200 (200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health) on day 57.
b Cattle received 70 mg Zn/kg DM from ZnSO4 + 30 mg Zn/kg DM from basic ZnCl, Zn glycinate, or ZnSO4 (ZnB, ZnG, or ZnS, respectively). Total Zn supplementation was targeted at 100 mg Zn/kg DM to meet industry consultant recommendations (Samuelson et al., 2016).
c Unlike superscripts represent tendencies for differences between treatment means (0.05 < P ≤ 0.10).
d Feed efficiency.
e Average daily gain was analyzed as repeated measures with the repeated effect of Day. Zn × Day P = 0.01; IMP × Day P = 0.02; Day P < 0.0001; Zn × IMP × Day P = 0.86.
f Carcass-adjusted performance was calculated using the average dressing percent: 64.35%.
Although ADG was influenced by IMP × Zn, no effects of the interaction were observed for d 0-56 DMI, daily Zn intake, or feed efficiency (G:F), d 57-126 DMI, daily Zn intake, or G:F, overall DMI or G:F, carcass-adjusted final BW, and carcass-adjusted overall ADG or G:F (P ≥ 0.12). Implanted cattle had greater days 0–56 G:F, days 57–126 DMI, daily Zn intake, and G:F, overall DMI and G:F, carcass-adjusted final BW, carcass-adjusted overall ADG, and G:F (P ≤ 0.01). Daily Zn intake, days 0–56 G:F, and carcass-adjusted overall G:F were greater for ZnS than ZnB and ZnG (P ≤ 0.02). These effects were likely driven by greater ADG throughout most of the trial and a tendency (P = 0.08) for lesser DMI during the days 0–56 initial implant period for ZnS. Numerical differences in overall intakes between Zn sources may also be driving the overall ADG IMP × Zn response in which steers that ate more had greater gains. No other effects of IMP or Zn on performance measures were observed (P ≥ 0.13).
Although performance was hypothesized to improve with greater bioavailability of Zn, neither days 0–56 or 57–126 ADG were influenced by Zn treatment. The numerical BW advantage of ZnG steers on day 28 may have been exacerbated by a modest response to the initial implant by other Zn treatments and aided by the availability of ZnG in the diet. Broilers fed 30 mg Zn/kg DM from Zn-glycinate had improved feed efficiency over broilers fed at the requirement (40 mg Zn/kg DM) from ZnSO4 (Sridhar et al., 2014), indicating a more bioavailable source can outperform a less bioavailable source, particularly when supplemented at a low concentration in the diet. In the present study, the benefits of a more bioavailable source may have been outweighed by the large concentration of supplemental Zn, regardless of source. In salmon, low concentrations of Mn-glycinate were more bioavailable than MnSO4, whereas high concentrations of Mn-glycinate downregulated Mn absorption (Prabhu et al., 2019). Similarly, in the current study, the supplementation of 100 mg Zn/kg DM may have led to the downregulation of Zn absorption. However, the bioavailability of Zn was not directly measured in this study.
As reported by others, differences in animal performance due to dietary Zn supplementation have been variable. Cao et al. (2000) and Gunter et al. (2001) detected no differences in chick and steer performance, respectively, due to Zn source (ZnSO4, basic ZnCl, ZnO, Zn polysaccharide, or Zn amino acid complex). However, Genther-Schroeder et al. (2016) found increasing supplemental Zn (Zn amino acid chelate and ZnSO4 blend supplemented up to 150 mg Zn/kg DM) linearly increased final BW and ADG of steers that received ractopamine hydrochloride. Spears and Kegley (2002) found steers supplemented Zn-proteinate had greater HCW than steers receiving the control diet or ZnO. Although the current study supplemented the same concentration of Zn between each Zn treatment, more bioavailable sources of Zn would be hypothesized to improve cattle performance if Zn had been limiting growth. The high inclusion of supplemental Zn across Zn treatments may have limited the detection of individual Zn source differences in the present study.
It is common in the feedlot industry to supplement blends of trace mineral sources (Samuelson et al., 2016); therefore, we examined varying Zn sources at industry relevant blends of 70 mg Zn/kg DM from inorganic ZnSO4 and 30 mg Zn/kg DM from the other Zn source (basic ZnCl, Zn glycinate, or ZnSO4). Interestingly, Shaeffer (2006) found basic ZnCl fed steers had poorer performance than ZnSO4 supplemented steers when supplemented at 30 mg Zn/kg DM. However, at higher concentrations, similar to this study (90 mg Zn/kg DM), no differences in performance were observed between these two inorganic sources (Shaeffer, 2006). Perhaps, a greater inclusion of supplemental Zn is adequate to meet the Zn requirements of the animal regardless of source; however, this does not support why basic ZnCl did not perform similarly to ZnSO4 in the current study.
No differences in carcass characteristics were noted in this study (Table 3; P ≥ 0.12). Previous work examining different concentrations of Zn in the diet of heifers has revealed interim performance improvements but no differences in carcass characteristics due to Zn concentrations similar to the present study (100 mg Zn/kg DM from ZnSO4) or national recommendations for cattle (30 mg Zn/kg DM from ZnSO4; Messersmith et al., 2021). However, others have noted positive effects of Zn on carcass characteristics when supplemental Zn was added to a Zn deficient basal diet (Spears and Kegley, 2002). Such differences in carcass characteristics may not have been observed in the current study because all treatments were supplemented 100 mg Zn/kg DM regardless of source. The portion of Zn supplemented from basic ZnCl or Zn glycinate may not have been enough to impact carcass characteristics regardless of bioavailability differences between sources. However, implanted steers had greater HCW, DP, and REA than NoIMP (P ≤ 0.001) as expected with anabolic implant use (Duckett and Pratt, 2014). No effects of IMP were observed for BF, KPH, marbling, or yield grade (P ≥ 0.23).
Table 3.
The effects of implanta and zinc sourceb on carcass characteristics and carcass-adjusted performance in finishing feedlot steers
| Implant treatments | NoIMP | IS/200 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zinc treatments | ZnB | ZnG | ZnS | ZnB | ZnG | ZnS | SEM | IMP | Zn | IMP × Zn |
| Steer (n) | 12 | 11 | 12 | 11 | 11 | 9 | ||||
| Hot carcass weight, kg | 385 | 380 | 380 | 408 | 410 | 424 | 5.6 | <0.001 | 0.33 | 0.12 |
| Dress, % | 63.8 | 63.9 | 64.2 | 64.6 | 64.8 | 64.8 | 0.37 | 0.01 | 0.72 | 0.85 |
| Ribeye area, cm2 | 80.0 | 81.2 | 80.2 | 86.8 | 83.4 | 83.9 | 2.23 | 0.01 | 0.76 | 0.51 |
| Rib fat, cm | 1.69 | 1.71 | 1.80 | 1.80 | 1.87 | 1.81 | 0.125 | 0.31 | 0.86 | 0.79 |
| KPHc, % | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.7 | 0.09 | 0.63 | 0.77 | 0.96 |
| Marblingd | 530 | 511 | 572 | 546 | 517 | 515 | 35.7 | 0.66 | 0.63 | 0.50 |
| Yield grade | 3.94 | 3.85 | 4.00 | 3.90 | 4.17 | 4.21 | 0.181 | 0.23 | 0.53 | 0.55 |
a Cattle were either non-implanted (NoIMP) or received a Component TE-IS (IS/200; 80 mg trenbolone acetate + 16 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0 followed by a Component TE-200 (200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health) on day 57.
b Cattle received 70 mg Zn/kg DM from ZnSO4 + 30 mg Zn/kg DM from basic ZnCl, Zn glycinate, or ZnSO4 (ZnB, ZnG, or ZnS, respectively). Total Zn supplementation was targeted at 100 mg Zn/kg DM to meet industry consultant recommendations (Samuelson et al., 2016).
c Kidney, pelvic, and heart fat.
d Marbling scores: slight = 300, small = 400, modest = 500, moderate = 600, slightly abundant = 700, moderately abundant = 800.
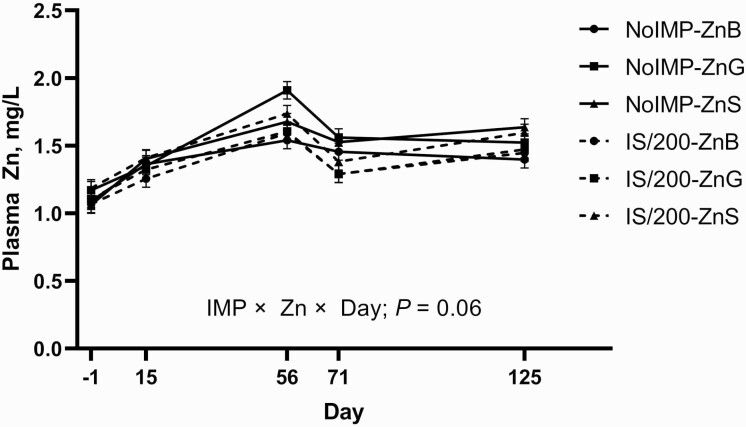
The evaluation of plasma and tissue trace mineral concentrations of these steers provides a more in-depth understanding of mineral metabolism in implanted cattle. An IMP × Day effect (P = 0.003) and a tendency for a Zn × Day effect (P = 0.10) were observed for plasma Zn concentrations. Furthermore, a tendency for an IMP × Zn × Day effect (Figure 1; P = 0.06) was observed for plasma Zn in which differences between the full factorial of treatments began to emerge by day 56. Assessing differences in LSM of treatments revealed that plasma Zn of IS/200 steers was less than NoIMP steers on day 71, with a more substantial drop in plasma Zn for IS/200 steers from days 56 to 71 than NoIMP. Decreases in plasma Zn concentrations following a terminal implant have previously been reported within 14 to 18 d of implant administration (Messersmith, 2018; Messersmith and Hansen, 2021), indicating that Zn metabolism is influenced during the period of peak hormonal release of implants (first 40 d after implant administration; Johnson et al., 1996). Zinc is heavily involved in growth processes through protein synthesis (Oberleas and Prasad, 1969). Therefore, plasma Zn may be decreasing as tissues take up increased amounts of Zn to support growth in high performing animals. However, it is unclear why plasma Zn for NoIMP-ZnG decreased between days 56 and 71.
Figure 1.
Plasma Zn tends to respond to implant and Zn treatments throughout study. Steers received either no hormonal implant (NoIMP) or were implanted with Component TE-IS on day 0 followed by Component TE-200 on day 57 (IS/200; Elanco) and fed 70 mg Zn/kg DM from ZnSO4 + 30 mg Zn/kg DM from basic ZnCl, Zn glycinate, or ZnSO4 (ZnB, ZnG, and ZnS, respectively). Blood was collected on days −1, 15, 56, 71/72, and 125 for plasma trace mineral analysis. Data were analyzed using repeated measures of the mixed procedure of SAS.
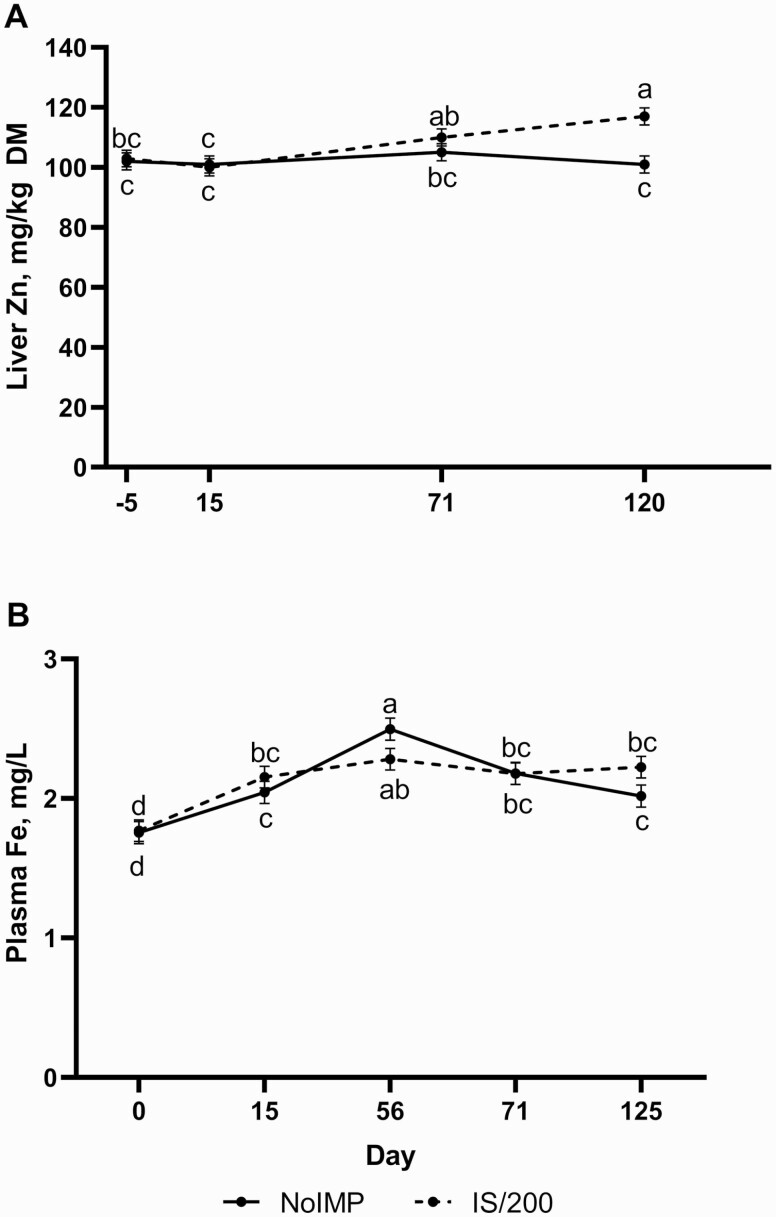
Liver Zn, a poor biomarker of Zn status due to the steady nature of tissue Zn concentrations (Suttle, 2010), was not influenced by Zn or Zn × Day (P ≥ 0.62). However, an IMP × Day effect in liver Zn revealed greater liver Zn concentrations in IS/200 steers by day 120 than NoIMP (Figure 2A; P = 0.02). This increase in liver Zn concentrations may be linked to greater Zn retention of implanted animals as observed in lambs (Hufstedler and Greene, 1995), although both immediate and persistent effects of implant administration on Zn absorption and retention have not been examined in cattle. Perhaps, the slight increase in liver Zn concentrations of implanted steers is reflective of greater DMI and subsequently greater Zn intake in IS/200 steers. However, this is in contrast to the decrease in liver Zn concentrations observed by Messersmith (2018) within 14 d of implant administration when 61 mg Zn/kg DM was fed in the diet. Considering dietary Zn in the current study was much higher than this (130 mg Zn/kg DM), feeding high concentrations of Zn in the diet may have prevented a decline in liver Zn concentrations of implanted cattle (Table 4).
Figure 2.
Steers were implanted with either no hormonal implant (NoIMP) or a Component TE-IS on day 0 followed by a Component TE-200 on day 57 (IS/200; Elanco). Liver biopsies were collected on days −5/−4, 14/15, 71/72, and 120/121. Blood was collected on days 0, 15, 56, 71/72, and 125 for plasma Fe analysis. Data were analyzed using repeated measures of the mixed procedure of SAS. Unlike superscripts indicate differences across all time points (P ≤ 0.05). (A) Liver Zn concentrations influenced by implant throughout the study (IMP × Day P = 0.02). (B) Plasma Fe concentrations were influenced by implant treatment throughout trial (IMP × Day P = 0.01).
Table 4.
| Implant treatments | NoIMP | IS/200 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zinc treatments | ZnB | ZnG | ZnS | ZnB | ZnG | ZnS | SEM | IMP | Zn | IMP × Zn |
| Steer (n)a | 12 | 11 | 12 | 11 | 11 | 9 | ||||
| Liver, mg/kg DMe | ||||||||||
| Znf | 103 | 102 | 101 | 105 | 106 | 112 | 2.9 | 0.02 | 0.62 | 0.21 |
| Cu | 303a | 314a | 240b | 298a | 278ab | 301a | 18.3 | 0.62 | 0.19 | 0.02 |
| Mne | 9.0 | 8.5 | 9.1 | 8.5 | 8.3 | 8.8 | 0.30 | 0.19 | 0.19 | 0.86 |
| Fe | 167 | 153 | 156 | 164 | 147 | 155 | 6.3 | 0.53 | 0.04 | 0.92 |
| Muscle, mg/kg DMe,g | ||||||||||
| Zn | 118y | 122y | 123xy | 124xy | 133x | 119y | 3.7 | 0.16 | 0.14 | 0.09 |
| Plasma, mg/Le | ||||||||||
| Cu | 0.99 | 1.05 | 1.02 | 1.00 | 1.07 | 1.03 | 0.046 | 0.76 | 0.34 | 0.99 |
| Fef | 1.92 | 2.24 | 2.13 | 1.97 | 2.25 | 2.15 | 0.107 | 0.79 | 0.02 | 0.98 |
| PUN, mg/dLe,f | 8.20 | 9.02 | 9.12 | 7.96 | 7.83 | 7.37 | 0.485 | 0.01 | 0.78 | 0.30 |
a Implant strategies included no implant (NoIMP) or a Component TE-IS (IS/200; 80 mg trenbolone acetate + 16 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0 followed by a Component TE-200 (200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health) on day 57.
b Cattle received 70 mg Zn/kg DM from ZnSO4 + 30 mg Zn/kg DM from basic ZnCl, Zn glycinate, or ZnSO4 (ZnB, ZnG, or ZnS, respectively). Total Zn supplementation was targeted at 100 mg Zn/kg DM to meet industry consultant recommendations (Samuelson et al., 2016).
c Liver and muscle samples were taken on days −5/−4, 14/15, 71/72, and 120/121, whereas blood for plasma trace mineral and PUN analysis was collected on days −1, 14, 56, 71/72, and 125.
d Unlike superscripts indicate differences between treatment means; a,b,c represent P ≤ 0.05 and x,y,z represent 0.05 < P ≤ 0.10.
e Data were analyzed using repeated measures of the mixed procedure of SAS and represent treatment means. No IMP × Zn × Day or Zn × Day interactions were observed (P ≥ 0.15) for all measurements except plasma Zn (IMP × Zn × Day; P = 0.06; Figure 1).
f An IMP × Day effect (P ≤ 0.01) for liver Zn (Figure 2A), plasma Fe (Figure 2B), liver Mn (Figure 3A), and plasma urea nitrogen (PUN; Figure 3B). No other IMP × Day effects were observed (P ≥ 0.27).
g A Day effect was observed (P < 0.0001; Figure 3).
Zinc effects were observed on liver and plasma Fe concentrations (P ≤ 0.04) where ZnB increased liver Fe concentrations while decreasing plasma Fe concentrations relative to ZnG. However, ZnS was not different from either Zn treatment in both liver and plasma analyses of Fe. Greater bioavailability of Zn source may have contributed to competition for absorption between Zn and Fe leading to lesser plasma Fe for ZnG but this does not explain the increase in liver Fe observed for ZnB. Additionally, an IMP × Day (Figure 2B; P = 0.01) effect was observed on plasma Fe in which NoIMP plasma Fe increased from days 15 to 56 before decreasing to concentrations similar to day 15 for the remainder of the trial, whereas IS/200 plasma Fe remained similar during this time point with no further differences in plasma Fe on days 0, 15, 71, or 125. Steers within ZnS had lesser liver Cu within NoIMP, and no differences in liver Cu concentrations due to Zn source were observed within IS/200 (IMP × Zn; P = 0.02). No further effects of IMP × Day, Zn × Day, or IMP × Zn × Day were observed for plasma or liver Fe and Cu (P ≥ 0.17).
Albeit its relatively slow Zn metabolism compared to other Zn stores, skeletal muscle comprises a large percentage of Zn storage in the body making it a potential indicator of Zn nutrition (Gumpper and Ma, 2019). A tendency for an IMP × Zn effect for muscle Zn concentration was observed (P = 0.09) in which IS/200-ZnG steers had greater muscle Zn than IS/200-ZnS, NoIMP-ZnB, and NoIMP-ZnG steers while not different from IS/200-ZnB or NoIMP-ZnS. We hypothesized that muscle Zn concentrations would increase with greater Zn source bioavailability and that implanted cattle would experience additional increases in muscle Zn concentrations due to the demands of Zn in growth processes such as DNA synthesis. However, muscle Zn responses were variable. Additionally, muscle Zn was increased by day 120, though days 15 and 71 muscle Zn concentrations were not different from day −5 (120, 119, 121, and 132 mg Zn/kg DM SEM: 2.0 for days −5, 15, 71, and 120, respectively; P < 0.0001). No IMP × Day, Zn × Day, or IMP × Zn × Day effects (P ≥ 0.44) were observed for muscle Zn concentrations.
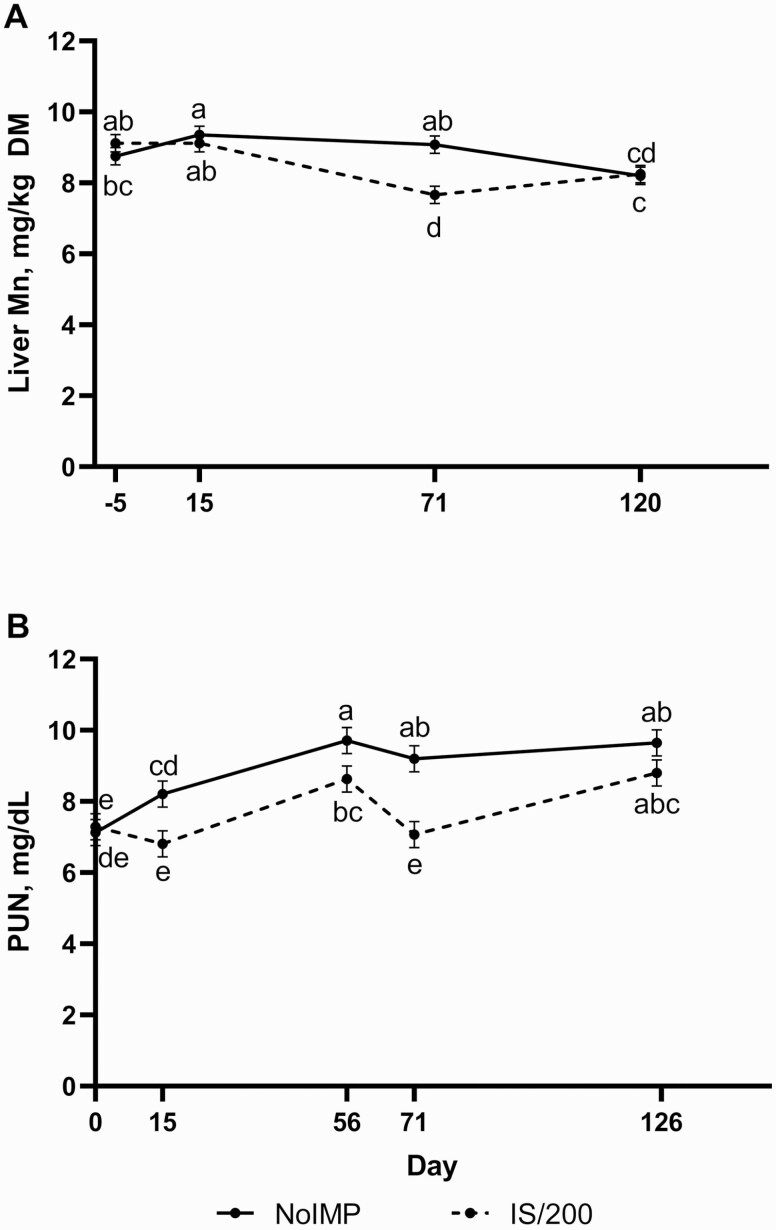
Interestingly, liver Mn concentrations of IS/200 steers on day 71 were less than NoIMP, but liver Mn concentrations were not affected by implant on any other sampling d (Figure 3A; IMP × Day; P = 0.001). Various implant studies conducted in our laboratory have revealed that implants decreased liver Mn concentrations by day 14 post-implant (Messersmith, 2018; Niedermayer et al., 2018). Although no differences in liver Mn were observed on day 14 of the present trial, day 71 corresponds to 14 d following the terminal implant matching the implant potency of these previous trials. This liver Mn response may be linked to increased growth from potent implants leading to greater protein synthesis and lesser protein degradation. A decrease in protein degradation would cause lesser need for the urea cycle to detoxify ammonia. Arginase, the urea cycle’s terminal enzyme, is a Mn-dependent enzyme (Bond et al., 1983; Watts, 1990). Therefore, a decrease in protein degradation could indicate a lesser need for arginase, and thus Mn, resulting in the observed decrease in liver Mn concentrations. Considering arginase is thought be a main driver of Mn requirements, implant-induced decreases in liver Mn concentrations may be linked to increased biliary excretion of Mn and lesser reabsorption of Mn from the bile, a primary mechanism in Mn metabolism in cattle (Suttle, 2010). The decrease in liver Mn in the present study pairs with the observed lesser PUN concentrations of implanted vs. non-implanted steers. Across the trial, PUN were less for IS/200 on days 15, 56, and 71, whereas no difference was observed by day 125 (Figure 3B; P = 0.001). In general, PUN increased in NoIMP until day 56, then held steady for the remainder of the feeding period, whereas IS/200 increased from days 15 to 56, dropped again after re-implant, and then increased to similar concentrations as NoIMP at the end of the trial. No Zn × Day or IMP × Zn × Day effects were observed for liver Mn or PUN (P ≥ 0.34). As expected, these data appear inversely related to the ADG response observed consistent with periods of increased growth and lesser protein degradation as reflected by decreased PUN. However, this relationship between liver Mn concentrations and implant use has not been further evaluated. No further effects of IMP, Zn, or the interaction were observed for trace mineral or blood parameters (P ≥ 0.14).
Figure 3.
Steers were implanted with either no hormonal implant (NoIMP) or a Component TE-IS on day 0 followed by a Component TE-200 on day 57 (IS/200; Elanco). Liver biopsies were conducted on days −5/−4, 14/15, 71/72, and 120/121 for analysis of liver Mn concentrations. Blood was collected on days 0, 15, 56, 71/72, and 125 for plasma urea nitrogen (PUN) analysis. Data were analyzed using repeated measures of the mixed procedure of SAS. Unlike superscripts indicate differences across time points (P ≤ 0.05). (A) Liver Mn concentrations are influenced by implant treatment throughout study (IMP × Day P = 0.001). (B) An IMP × Day effect (P = 0.001) was observed for PUN.
CONCLUSION
Collectively, these data revealed interim performance differences due to feeding 100% ZnSO4, or 70% ZnSO4 with 30% of either basic ZnCl or Zn glycinate within both implanted and non-implanted steers and effects on steer trace mineral concentrations in plasma and liver. Zinc source did not impact carcass characteristics in this study. Further work should investigate titration of lesser concentrations of dietary Zn from highly bioavailable sources to determine source differences in performance of implanted cattle, as the dietary consumption of Zn may have been high enough in all treatments in the present study to mask potential differences due to source.
ACKNOWLEDGMENTS
This work was funded by Phibro Animal Health, Teaneck, NJ, USA.
Conflict of interest statement. The authors declare no conflicts of interest.
LITERATURE CITED
- Batal, A. B., Parr T. M., and Baker D. H.. . 2001. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult. Sci. 80:87–90. doi: 10.1093/ps/80.1.87. [DOI] [PubMed] [Google Scholar]
- Bond, J. S., Failla M. L., and Unger D. F.. . 1983. Elevated manganese concentration and arginase activity in livers of streptozotocin-induced diabetic rats. J. Biol. Chem. 258:8004–8009. [PubMed] [Google Scholar]
- Cao, J., Henry P. R., Ammerman C. B., Miles R. D., and Littell R. C.. . 2000. Relative bioavailability of basic zinc sulfate and basic zinc chloride for chicks. J. Appl. Poult. Res. 9:513–517. doi: 10.1093/japr/9.4.513. [DOI] [Google Scholar]
- Carmichael, R. N., Genther-Schroeder O. N., Blank C. P., Deters E. L., Hartman S. J., Niedermayer E. K., and Hansen S. L.. . 2018. The influence of supplemental zinc and ractopamine hydrochloride on trace mineral and nitrogen retention of beef steers 1. J. Anim. Sci. 96:2939–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett, S. K., and Pratt S. L.. . 2014. Meat Science and Muscle Biology Symposium—anabolic implants and meat quality. J. Anim. Sci. 92:3–9. doi: 10.2527/jas.2013-7088. [DOI] [PubMed] [Google Scholar]
- Engle, T. E., and Spears J. W.. . 2000. Effects of dietary copper concentration and source on performance and copper status of growing and finishing steers. J. Anim. Sci. 78:2446–2451. doi: 10.2527/2000.7892446x. [DOI] [PubMed] [Google Scholar]
- Genther-Schroeder, O. N., Branine M. E., and Hansen S. L.. . 2016. The effects of increasing supplementation of zinc-amino acid complex on growth performance, carcass characteristics, and inflammatory response of beef cattle fed ractopamine hydrochloride. J. Anim. Sci. 94:3389–3398. doi: 10.2527/jas.2015-0209. [DOI] [PubMed] [Google Scholar]
- Gumpper, K., and Ma J.. . 2019. Zinc signaling in skeletal muscle. In: Fukada T. and Kambe T., editors. Zinc signaling. 2nd ed. Springer, Singapore. p. 123–137. [Google Scholar]
- Gunter, S. A., Malcolm-Callis K. J., Duff G. C., and Kegley E. B.. . 2001. Performance of steers supplemented with zinc during grazing and receiving at the feedlot. Prof. Anim. Sci. 17:280–286. doi: 10.15232/S1080-7446(15)31641-7. [DOI] [Google Scholar]
- Hufstedler, G. D., and Greene L. W.. . 1995. Mineral and nitrogen balance in lambs implanted with zeranol. J. Anim. Sci. 73:3785–3788. doi: 10.2527/1995.73123785x. [DOI] [PubMed] [Google Scholar]
- Johnson, B. J., Anderson P. T., Meiske J. C., and Dayton W. R.. . 1996. Effect of a combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J. Anim. Sci. 74:363–371. doi: 10.2527/1996.742363x. [DOI] [PubMed] [Google Scholar]
- Messersmith, E. M. 2018. The effect of copper supplementation on performance and carcass characteristics of cattle utilizing growth promoting technologies. Graduate Theses and Dissertations. 16857. https://lib.dr.iastate.edu/etd/16857. [Google Scholar]
- Messersmith, E. M., and Hansen S. L.. . 2021. 162 Increasing concentrations of supplemental zinc influence performance, carcass characteristics, and trace mineral status of non-implanted and implanted steers. J. Anim. Sci. 99:123–123. doi: 10.1093/jas/skab054.204. [DOI] [Google Scholar]
- Messersmith, E. M., Niedermayer E. K., Thornton K. J., Crawford G. I., and Hansen S. L.. . 2021. Zinc supplementation strategies in feedlot heifers receiving an extended-release implant or an aggressive re-implant program. J. Anim. Sci. 99:1–8. doi: 10.1093/JAS/SKAB212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAHMS (National Animal Health Monitoring System). 2013. USDA Part I: Management Practices on U.S. Feedlots with a Capacity of 1,000 or More Head. Accessed June 26, 2019. https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartI_1.pdf.
- NASEM. 2016. Nutrient Requirements of Beef Cattle. 8th edn. Washington, DC: National Academy Press. [Google Scholar]
- Niedermayer, E. K., Genther-Schroeder O. N., Loy D. D., and Hansen S. L.. . 2018. Effect of varying trace mineral supplementation of steers with or without hormone implants on growth and carcass characteristics. J. Anim. Sci. 96:1159–1170. doi: 10.1093/jas/skx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleas, D., and Prasad A. S.. . 1969. Growth as affected by zinc protein nutrition. Am. J. Clin. Nutr. 22:1304–1314. [DOI] [PubMed] [Google Scholar]
- Pampusch, M. S., White M. E., Hathaway M. R., Baxa T. J., Chung K. Y., Parr S. L., Johnson B. J., Weber W. J., and Dayton W. R.. . 2008. Effects of implants of trenbolone acetate, estradiol, or both, on muscle insulin-like growth factor-I, insulin-like growth factor-I receptor, estrogen receptor-α, and androgen receptor messenger ribonucleic acid levels in feedlot steers. J. Anim. Sci. 86:3418–3423. doi: 10.2527/jas.2008-1085. [DOI] [PubMed] [Google Scholar]
- Pogge, D. J., and Hansen S. L.. . 2013. Supplemental vitamin C improves marbling in feedlot cattle consuming high sulfur diets. J. Anim. Sci. 91:4303–4314. doi: 10.2527/jas.2012-5638. [DOI] [PubMed] [Google Scholar]
- Prabhu, P. A. J., Silva M. S., Kröeckel S., Holme M. H., Ørnsrud R., Amlund H., Lock E. J., and Waagbø R.. . 2019. Effect of levels and sources of dietary manganese on growth and mineral composition of post-smolt Atlantic salmon fed low fish meal, plant-based ingredient diets. Aquaculture. 512:734287. doi: 10.1016/j.aquaculture.2019.734287. [DOI] [Google Scholar]
- Richter, E. L., Drewnoski M. E., and Hansen S. L.. . 2012. Effects of increased dietary sulfur on beef steer mineral status, performance, and meat fatty acid composition. J. Anim. Sci. 90:3945– 3953. doi: 10.2527/jas.2011-4512. [DOI] [PubMed] [Google Scholar]
- Samuelson, K. L., Hubbert M. E., Galyean M. L., and Löest C. A.. . 2016. Nutritional recommendations of feedlot consulting nutritionists: The 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas.2016-0282. [DOI] [PubMed] [Google Scholar]
- Schlegel, P., and Windisch W.. . 2006. Bioavailability of zinc glycinate in comparison with zinc sulphate in the presence of dietary phytate in an animal model with 65Zn labelled rats. J. Anim. Physiol. Anim. Nutr. (Berl). 90:216–222. doi: 10.1111/j.1439-0396.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- Shaeffer, G. L. 2006. Evaluation of Basic Zinc Chloride as a Zinc Source for Cattle. NC State Thesis and Dissertations.http://www.lib.ncsu.edu/resolver/1840.16/1835. [Google Scholar]
- Shaeffer, G. L., Lloyd K. E., and Spears J. W.. . 2017. Bioavailability of zinc hydroxychloride relative to zinc sulfate in growing cattle fed a corn-cottonseed hull-based diet. Anim. Feed Sci. Technol. 232:1–5. doi: 10.1016/j.anifeedsci.2017.07.013. [DOI] [Google Scholar]
- Spears, J. W., and Kegley E. B.. . 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers1,2. J. Anim. Sci. 80:2747–2752. doi: 10.1093/ansci/80.10.2747. [DOI] [PubMed] [Google Scholar]
- Spears, J. W., Schlegel P., Seal M. C., and Lloyd K. E.. . 2004. Bioavailability of zinc from zinc sulfate and different organic zinc sources and their effects on ruminal volatile fatty acid proportions. Livest. Prod. Sci. 90:211–217. doi: 10.1016/j.livprodsci.2004.05.001. [DOI] [Google Scholar]
- Sridhar, K., Nagalakshmi D., and Rama Rae S. V.. . 2015. Effect of graded concentration of organic zinc (Zn-glycinate) on skin quality, haematological and serum biochemical constituents in broiler chicks Effect of graded concentration of organic zinc (zinc glycinate) on skin quality, hematological and ser. Indian J. Anim. Sci. 85:643–648. [Google Scholar]
- Sridhar, K., Nagalakshimi D., Srinivasa Roa D., and Rama Roa S. V.. . 2014. Effect of dietary supplementation of organic acids in combination on performance and carcass traits of broiler chicken. Anim. Nutr. Feed Technol. 17:181–187. doi: 10.5958/0974-181X.2017.00019.1. [DOI] [Google Scholar]
- Suttle, N. F. 2010. Mineral Nutrition of Livestock. 4th edn. Wallingford, UK: CABI Publishing. [Google Scholar]
- Watts, D. L. 1990. The nutritional relationships of manganese. J. Orthomol. Med. 5:219–222. [Google Scholar]