Abstract
Background:
People with HIV (PWH) have a higher hospitalization rate than the general population. The Veterans Aging Cohort Study (VACS) Index at study entry well predicts hospitalization in PWH, but it is unknown if the time-updated parameter improves hospitalization prediction. We assessed the association of parameterizations of the VACS Index 2.0 with the 5-year risk of hospitalization.
Setting:
PWH ≥ 30 years old with at least 12 months of antiretroviral therapy (ART) use, and contributing hospitalization data from 2000 to 2016 in North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) were included. Three parameterizations of the VACS Index 2.0 were assessed and categorized by quartile: 1) “baseline” measurement at study entry, 2) time-updated measurements, and 3) cumulative scores calculated using the trapezoidal rule.
Methods:
Discrete-time proportional hazard models estimated the crude and adjusted associations (and 95% confidence intervals [CI]) of the VACS Index parameterizations and all-cause hospitalizations. The Akaike information criterion (AIC) assessed the model fit with each of the VACS Index parameters.
Results:
Among 7,289 patients, 1,537 were hospitalized. Time-updated VACS Index fitted hospitalization best with a more distinct dose-response relationship (score <43: reference; score 43–55: aHR=1.93 [95% CI 1.66, 2.23], score 55–68: aHR=3.63 [95% CI 3.12, 4.23], score ≥68: aHR=9.98 [95% CI: 8.52, 11.69]) than study entry and cumulative VACS Index after adjusting for known risk factors.
Conclusions:
Time-updated VACS Index 2.0 had the strongest association with hospitalization and best fit to the data. Healthcare providers should consider using it when assessing hospitalization risk among PWH.
Keywords: HIV, VACS Index, hospitalization
INTRODUCTION
The use of modern combination antiretroviral therapy (ART) has lengthened life expectancies among people with HIV (PWH) over the past two decades, although disparities in outcome by race and ethnicity, sex, and HIV acquisition risk group still persist1. Consequently, the cumulative incidence and mortality due to non-AIDS defining conditions such as cardiovascular disease, chronic kidney or liver disease, and non-AIDS defining cancer have increased2,3. While ART reduces the HIV-related hospitalization risk in the short-term4, patients still face hospitalizations due to aging, treatment side effects or failures, and non-AIDS defining conditions5–8. Non-AIDS-defining hospitalizations are more common now than AIDS-defining hospitalizations9. Hospitalizations for AIDS-defining illnesses still occur, however, and the length of stay could be over 10 days5. Hospitalization is a marker of moderate to severe morbidity, and PWH want to avoid hospitalization due to the fear of treatment, high cost, and concurrent lowered quality of life and frailty6,10. Identifying patients with high risk of hospitalization could be clinically important. Having an accurate understanding of the future risk of hospitalization may improve resource allocation in clinical practice (e.g. employing case managers for high-risk patients)6,11.
The Veterans Aging Cohort Study Risk Index (VACS Index) 2.0 predicts 5-year mortality based on age, sex, race, routinely collected clinical biomarkers including CD4 count, HIV-1 RNA, and laboratory measurements of hemoglobin, aspartate and alanine transaminase (AST, ALT), platelets, creatinine and hepatitis C virus (HCV) status12. The VACS Index 1.0 has been shown to predict all-cause mortality13, cardiovascular disease14,15, neurocognitive dysfunction16,17, inflammation18, and frailty fracture19,20. Further, the VACS Index has been shown to have good predictive accuracy among sub-groups including women, men, Black, white, people with HIV-1 RNA <500 copies/ml, HIV-1 RNA >500 copies/ml, and young people13,21. VACS Index 2.0 was shown to provide a more comprehensive means of tracking disease burden than an earlier restricted version of the VACS Index (1.0) that included only HIV-related risk factors and age13. VACS Index 2.0, which included continuous variables for albumin, white blood cell count (WBC), and body mass index (BMI), has improved discrimination for predicting 5-year mortality22.
Previous studies found strong association between VACS Index 1.0 at study entry and hospitalization within 2 years among veterans with HIV6,10 and women23; the VACS Index 2.0 has not yet been shown to predict hospitalizations. Additionally, the parameterization of the VACS Index 2.0 may be important to predictive accuracy. Salinas and colleagues14 found that time-updated VACS Index models fitted hospitalization data better than the study entry and cumulative VACS Index 1.0 models with the composite outcome of myocardial infarction and mortality. The study entry VACS Index only reflects health status at the study-defined baseline. A cumulative measurement depends on the duration of time in a particular health status. A time-updated measurement reflects the latest state, and it might be more clinically valuable. Finally, the study by Salinas and colleagues14 was nested in the VACS study population, and results may be different in a more diverse non-veteran population.
Without a doubt, medical providers need a more reliable tool that captures the risk of hospitalization more precisely. Rather than predictions based on baseline parameters6,10, a tool such as VACS Index 2.0 could capture the dynamic nature of beneficial effects of ART and PWH compliance with it and the longitudinal evolution of other comorbidities and aging that occur in PWH. Therefore, we aimed to estimate the associations of study entry, time-updated, and cumulative VACS Index 2.0 with 5-year hospitalization in a non-veteran population. Our results and parameterization estimates could guide medical providers and inform health care policy decision-makers on how best to prioritize resources for PWH at high risk of hospitalization.
METHODS
Study population
The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) is a region of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) initiative that includes PWH enrolled in interval and clinical cohorts in the United States and Canada24. Our source population were people with HIV in 5 clinical cohorts that provided hospitalization data and clinical measurements that are required for the VACS Index calculation25. According to the pre-defined clinically significant range of age in the VACS Index 2.022, we included PWH who were 30 to 75 years old in the NA-ACCORD. Subjects initiated ART for at least 1 year who had not been previously hospitalized, had at least one visit between 1/1/2000 and 12/31/2016, and had all variables required to calculate VACS Index 2.0 were under observation24,25 for up to five years or first hospitalization. We excluded the first 12 months of ART use due to the variability found in the VACS Index measurement in the first year after ART initiation22. We also excluded patients with indicators that were outside their clinically meaningful ranges22. Patients were censored if death occurs, and they were lost to follow-up if the date of the last VACS Index measurement preceded a gap of > 2 years. Patients without a VACS Index measurement at study entry (defined as within the window of 6 months before 6 months after study entry) and patients with hospitalization before study entry were excluded. We excluded patients that first hospitalized before the observation period because of health behavioral change26 (e.g. smoking cessation) among patients after first-time hospitalization and different covariates set when the outcome was re-admission27,28. Patients provided informed consent to their local cohorts that were reviewed and approved by their institutional review boards. The NA-ACCORD collaborative study was approved by the institutional review board of the Johns Hopkins School of Medicine.
Outcome
Our primary outcome was first-time all-cause hospitalization occurring within 5 years after study entry. The hospitalization data was from the electronic health records in each cohort’s medical system. We excluded hospitalizations with length of stay of less than one day, because we could not distinguish one-day hospitalizations from outpatient procedures (e.g. endoscopy)29. Patients with a hospitalization record missing complete admission and discharge dates were excluded from analyses.
VACS Index
The VACS Index 1.0 predicts 5-year all-cause mortality by summing the pre-assigned points for age, traditional HIV indicators (HIV-1 RNA and CD4 count), organ system injury indicators (e.g. hemoglobin, FIB-4, eGFR), and HCV infection8,18. Higher VACS Index scores indicate an increased risk of mortality. The VACS Index 2.0, which added albumin, WBC, and BMI, has better discrimination and external validation than the VACS Index 1.022.
VACS Index 2.0 was our exposure of interest. We investigated three parameterizations of the VACS Index: 1) measured at our defined study entry; 2) time-updated measurements based on receipt of healthcare; and 3) cumulative VACS Index using the time-updated measurements. VACS Index at study entry was assessed as closest to the study entry date as possible within the window of 6 months before to 6 months after the study entry date. Time-updated VACS Index was measured every time new laboratory data were available; this parameterization treated VACS Index as a time-varying variable with no lag14. Cumulative VACS Index was calculated as the area under the VACS Index curve to estimate the VACS Index score-years, similar to viremia copy-years (in copy-years/mL)30,31.
Covariates
Sex (male and female), race/ethnicity (Hispanic, Non-Hispanic white, Non-Hispanic Black, Asian, Indigenous and Other/Unknown), HIV acquisition risk factor (history of injected drug use [IDU], men having sex with men (MSM), heterosexual, and other/unknown) were measured at the time of parent cohort enrollment, and time-fixed smoking (ever, never, and unknown) was measured based on recorded clinical diagnoses and/or questionnaire responses32,33. Time-updated variables, including depression diagnosis (defined as depression diagnosis and no diagnosis of bipolar disorder) and calendar year (2000–2012 and 2012–2017)34, were included in multivariable models of hospitalization and VACS Index. Selection of covariates was based on existing literature and scientific knowledge5,6,10,23,35–38.
Statistical analyses
We compared demographic characteristics, HIV-1 RNA, CD4, and depression diagnosis at study entry by outcome status during follow-up. Continuous data were characterized by medians and interquartile ranges (IQR) and differences were tested with the Wilcoxon rank-sum test. Categorical variables were described by frequencies and percentages and differences were tested using χ2 tests.
We used a discrete time-to-event approach for hospitalizations with month as the width of the discrete time interval. If more than one measurement per month was made for any variable needed to estimate the VACS Index 2.0, we used the monthly mean value with the exception of HIV-1 RNA (the maximum value in the month was used). The time-updated and cumulative VACS Index scores were carried forward for up to 12 months. BMI, a covariate of interest in our models, was carried forward for up to 24 months and the maximum value was used if more than one measurement was made in a month.
For each parameterization of the VACS Index, quartiles of the distribution of risk scores among those who were hospitalized were used as the cut-offs for categories of risk scores to ensure similar number of outcomes within each category. We plotted the hazard by the categories of the VACS Index to visualize the changes in hazard over time and test the proportional hazard assumption for each parameterization. Univariate and multivariable discrete time-to-event proportional hazard models were used to assess the association between different parameterizations of VACS Index and first-time all-cause hospitalization. We used complementary log-log regression models to estimate the hazard ratio and 95% confidence intervals. Univariate models included each parameterization of the VACS Index as the only predictor. Covariates in the multivariable models included sex, race/ethnicity, smoking, HIV acquisition risk factor, depression, and calendar year. We used Akaike information criterion (AIC) to evaluate model fitness; models with lower AIC values had better fitness14. We used the magnitude and precision of the point estimates for the VACS Index quartiles to determine which parameterization best predicted hospitalizations within 5 years.
To examine whether time-updated VACS Index best predict hospitalization in all sex and race/ethnicity groups, we conducted sub-group analyses via stratified analyses. In sensitivity analyses, we expanded our study population to included patients with indicators that were outside their clinically meaningful ranges (i.e. 18–29-year-olds and those >75 years), substituted with the upper or lower boundaries of the ranges for the value (as previously demonstrated by Tate and colleagues22). We lagged the VACS Index by one month in additional sensitivity analyses to determine the robustness of our findings to the timing of the measurement prior to hospitalization in our month-level discrete time-to-event approach. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina). Interpretation of statistical significance was guided by a p-value<0.05.
RESULTS
Of the 38,600 PWH in the 5 NA-ACCORD clinical cohorts with hospitalization data, 34,360 (89%) initiated ART and were on ART for at least 12 months and 7,289 (19%) met the additional inclusion criteria for our study (see Figure 1, Supplemental Digital Content 1, which demonstrates the selection of study population). A total of 246 participants were excluded due to missing year of hospitalization. The VACS Index input parameter that was missing among the greatest proportion of individuals was albumin 22%. The distributions of sex, race/ethnicity, and year of birth were similar between the source (n=38,600) and the study population (n=7,289) except for a slightly higher proportion of males (84% vs. 82%, p<0.01) and white (49% vs. 42%, p<0.01) in the study population (see Figure 1, Supplemental Digital Content 1, which demonstrates the selection of study population). The 7,289 study participants contributed 21,548 person-years and 1,537 hospitalizations. Only 5 died prior to hospitalization, loss to follow-up or administrative censoring. 61.6% of hospitalizations happened within two years after study entry; the median (IQR) of time from study entry to hospitalization was 1.5 (0.5 – 2.8) years and 3.7 (1.4 – 5.0) years for those who were censored. Hospitalized PWH were more likely to be female, Black, smokers, had injection drug use as their HIV acquisition risk factor, had a lower CD4 count, a higher viral load, and were more likely to be HCV co-infected. Patients with hospitalization had higher study entry VACS Index (Table 1).
Table 1.
| Characteristicsc | No Hospitalization n=5752 |
Any Hospitalization n=1537 |
|---|---|---|
| Age in year, median (IQR) | 43 (36–49) | 44 (38−-51) |
| Male sex | 4888 (85) | 1221 (79) |
| Race/ethnicity | ||
| Black | 1650 (29) | 669 (44) |
| White | 2890 (50) | 645 (42) |
| Hispanic | 818 (14) | 168 (11) |
| Asian | 172 (3) | 26 (2) |
| Indigenous | 47 (1) | 4 (0) |
| Other/unknown | 175 (3) | 25 (2) |
| BMId, kg/m3 | 25.3(23.0–28.2) | 25.2(22.6–28.2) |
| Smoking | ||
| Ever | 3177 (55) | 1074 (70) |
| Never | 1053 (18) | 214 (14) |
| Unknown | 1522 (26) | 249 (16) |
| HIV acquisition risk factor | ||
| Injected drug use | 527 (9) | 317 (21) |
| Men having sex with men | 3715 (65) | 785 (51) |
| Heterosexual | 1253 (22) | 355 (23) |
| Other/Unknown | 257 (4) | 80 (5) |
| Quartile of study entry VACS Index scoree | ||
| <42 | 2805 (49) | 374 (24) |
| 42–53 | 1613 (28) | 394 (26) |
| 53–64 | 873 (15) | 373 (24) |
| ≥64 | 461 (8) | 396 (26) |
| Log10 HIV-1 RNA in copies/mL, median (IQR) | 1.7(1.6–2.6) | 2.0(1.7–3.9) |
| CD4 count in cells/mm3, median (IQR) | 456 (288–632) | 349 (188–543) |
| Hemoglobin in g/dL, median (IQR) | 14.4(13.4–15.2) | 13.7(12.4–14.8) |
| FIB-4 value, median (IQR) | 1.0(0.7–1.4) | 1.2(0.8–1.7) |
| eGFRd in mL/min/1.73 m2, median (IQR) | 101 (86–112) | 101.3(86–114) |
| Albumin in g/dL median (IQR) | 4.3(4.0–4.5) | 4.1(3.8–4.4) |
| White blood cell countd in 103/uL median (IQR) | 5.3(4.3–6.5) | 5.2(4.1–6.5) |
| Hepatitis C diagnosis | 1092 (19) | 550 (36) |
| Depression diagnosisd | ||
| Yes | 1448 (25) | 354 (23) |
| No | 4118 (72) | 1130 (74) |
| Unknown | 186 (3) | 53 (3) |
| Median follow-up time (IQR) in yearsf | 1.5 (0.5–2.8) | 5.0 (3.2–5.0) |
Abbreviations:
IQR=interquartile range
Statistics given in median (interquartile range) or n (%).
Study entry defined as defined as 12 months after ART initiation, the cohort open date, the cohort-specific hospitalization observation start date, 30 years of age, or 1 January 2000, whichever came last.
Hospitalization defined as first-time all-cause hospitalization occurring within 5 years after study entry. One-day discharges were excluded.
Tested for significance with χ2 and Wilcoxon rank-sum tests.
P-value not statistically significant.
Study entry VACS Index score measured 6 months before to 6 months after study entry, selecting the measurement closest to study entry within the window.
For hospitalized patients, the median follow-up time in years after removing hospitalization as a reason for study exit was the same as those who did were not hospitalized (median follow-up=5.0 (3.2, 5.0) years).
The discrete-time hazard showed no visual evidence of non-proportionality by quartile of VACS Index measured at study entry, time-updated, or cumulative risk score (see Figure 2, Supplemental Digital Content 2, which demonstrates the observed hazard of hospitalization). Kaplan-Meier plots showed distinct differences in time to first hospitalization by quartiles of study entry VACS Index (log rank p-value <0.001, see Figure 3, Supplemental Digital Content 3, which shows the hospitalization-free survival within five years).
Increasing VACS Index scores at study entry, time-updated, and cumulative were significantly associated with increasing risk of hospitalization in unadjusted analyses (Table 2). After adjusting for sex, race and ethnicity, smoking, HIV acquisition risk factor, depression, and calendar year, all three parameterizations of the VACS Index scores were attenuated but remained statistically significantly associated with hospitalization. In multivariable models, the time-updated VACS Index had the strongest association with hospitalization in the highest quartile. Based on AIC measures (Table 2) of unadjusted and adjusted models, the time-updated VACS Index models fitted hospitalization data better than the study entry and cumulative VACS Index models.
Table 2.
Crude and adjusted hazard ratios and 95% confidence intervals comparing time to first hospitalization by VACS Index score quartilesa, and Akaike Information Criterionb values for crude and adjusted complementary log-log regression models, among 7289 persons with HIV in NA-ACCORD, 2000–2016
| Quartiles of VACS Index score | Person-years | Hospitalizations | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)c |
|---|---|---|---|---|
| Study entry VACS Index score | ||||
| <42 | 9583 | 374 | 1 | 1 |
| 42–53 | 6209 | 394 | 1.63 (1.42, 1.88) | 1.53 (1.33, 1.77) |
| 53–64 | 3762 | 373 | 2.56 (2.21, 2.95) | 2.25 (1.94, 2.62) |
| ≥64 | 1995 | 396 | 5.00 (4.34, 5.76) | 4.02 (3.44, 4.70) |
| AIC values for the study entry VACS Index models | 18281 | 18229 | ||
| Time-updated VACS Index score | ||||
| <43 | 11327 | 349 | 1 | 1 |
| 43–55 | 5740 | 400 | 1.98 (1.72, 2.29) | 1.93 (1.66, 2.23) |
| 55–68 | 2980 | 395 | 3.84 (3.32, 4.43) | 3.63 (3.12, 4.23) |
| ≥68 | 1502 | 393 | 10.80 (9.34, 12.48) | 9.98 (8.52, 11.69) |
| AIC values for the time-updated VACS Index models | 17771 | 17752 | ||
| Cumulative VACS Index scored | ||||
| <78 | 1018 | 377 | 1 | 1 |
| 78–124 | 2137 | 390 | 2.99 (2.50, 3.57) | 2.52 (2.10, 3.02) |
| 124–187 | 4660 | 384 | 6.94 (5.51, 8.75) | 5.09 (4.01, 6.47) |
| ≥187 | 13733 | 386 | 13.86 (10.55, 18.21) | 8.68 (6.49, 11.60) |
| AIC values for the cumulative VACS Index models | 18404 | 18312 | ||
VACS Index scores include age, CD4 count, viral load, hemoglobin, FIB-4, eGFR, HCV, albumin, WBC, and BMI. All parameterizations of the VACS Index scores are categorized into quartiles to ensure similar hospitalizations within each category.
AIC = −2(log-likelihood) + 2K, K is the number of model parameters (the number of variables in the model plus the intercept). Log-likelihood is a measure of model fit. The lower the AIC value, the better the fit.
Adjusted variables include sex, race/ethnicity, smoking, HIV acquisition risk factor, depression, and calendar year.
Cumulative VACS Index scores were calculated as VACS Index score-years.
In sub-group analyses, all parameterizations of increasing VACS Index were associated with increasing hospitalization risk among males, females, Black, white, and Hispanic (Figures 1 & 2). Similar to the primary analyses, the model with the time-updated VACS Index fitted the data best among these subgroups.
Figure 1.
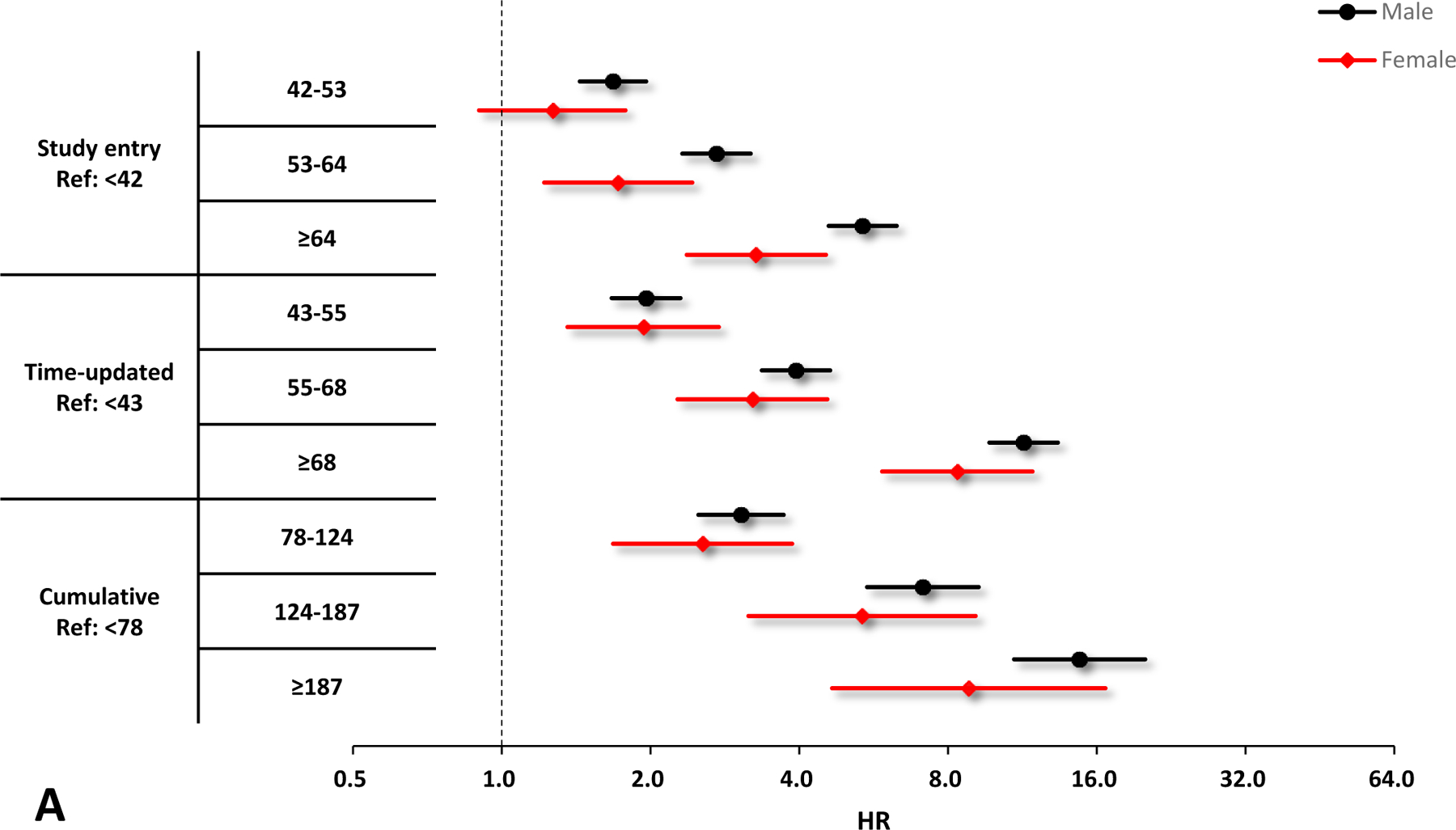
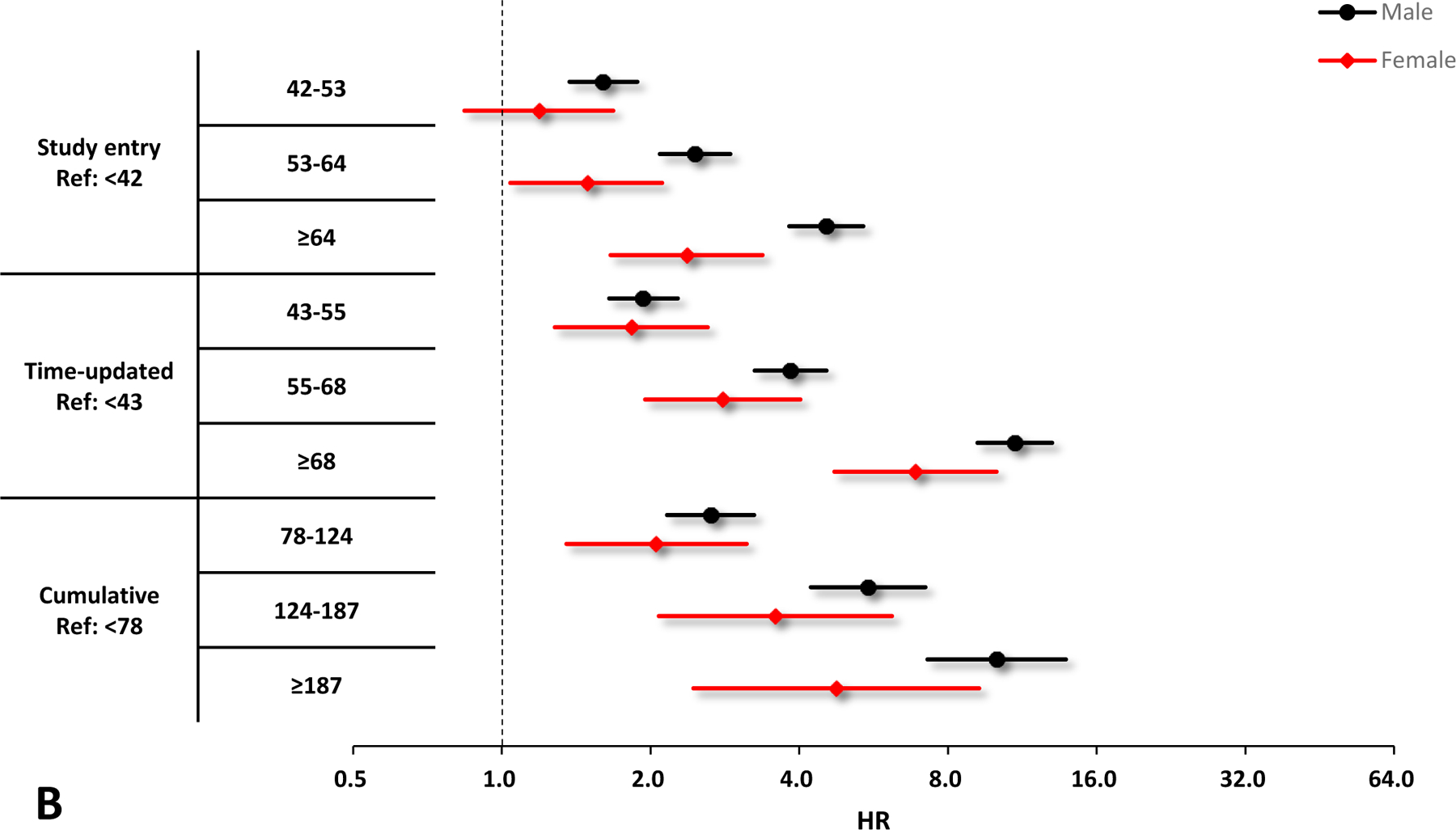
Crude and adjusted hazard ratios (HRs) and 95% confidence interval (CIs) of VACS Index scores for the risk of hospitalization by sex. A. Unadjusted results by sex, black circles denote male and red diamonds denote female. B. Adjusted results by sex, black circles denote male and red diamonds denote female. Adjusting variables include race/ethnicity, smoking, HIV acquisition risk factor, depression, and calendar year. All the HRs and 95% CIs are presented on a log2 scale.
Figure 2.
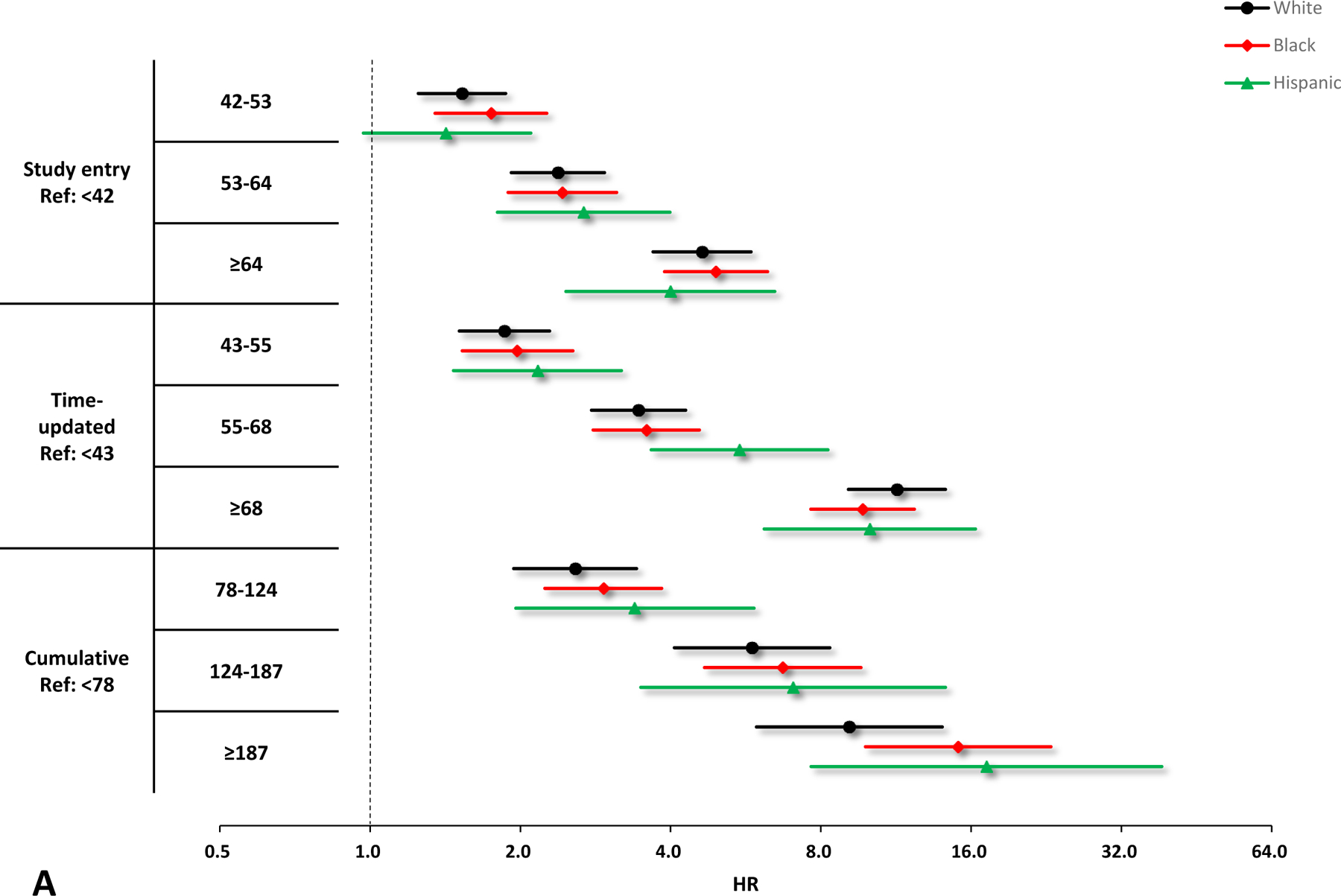
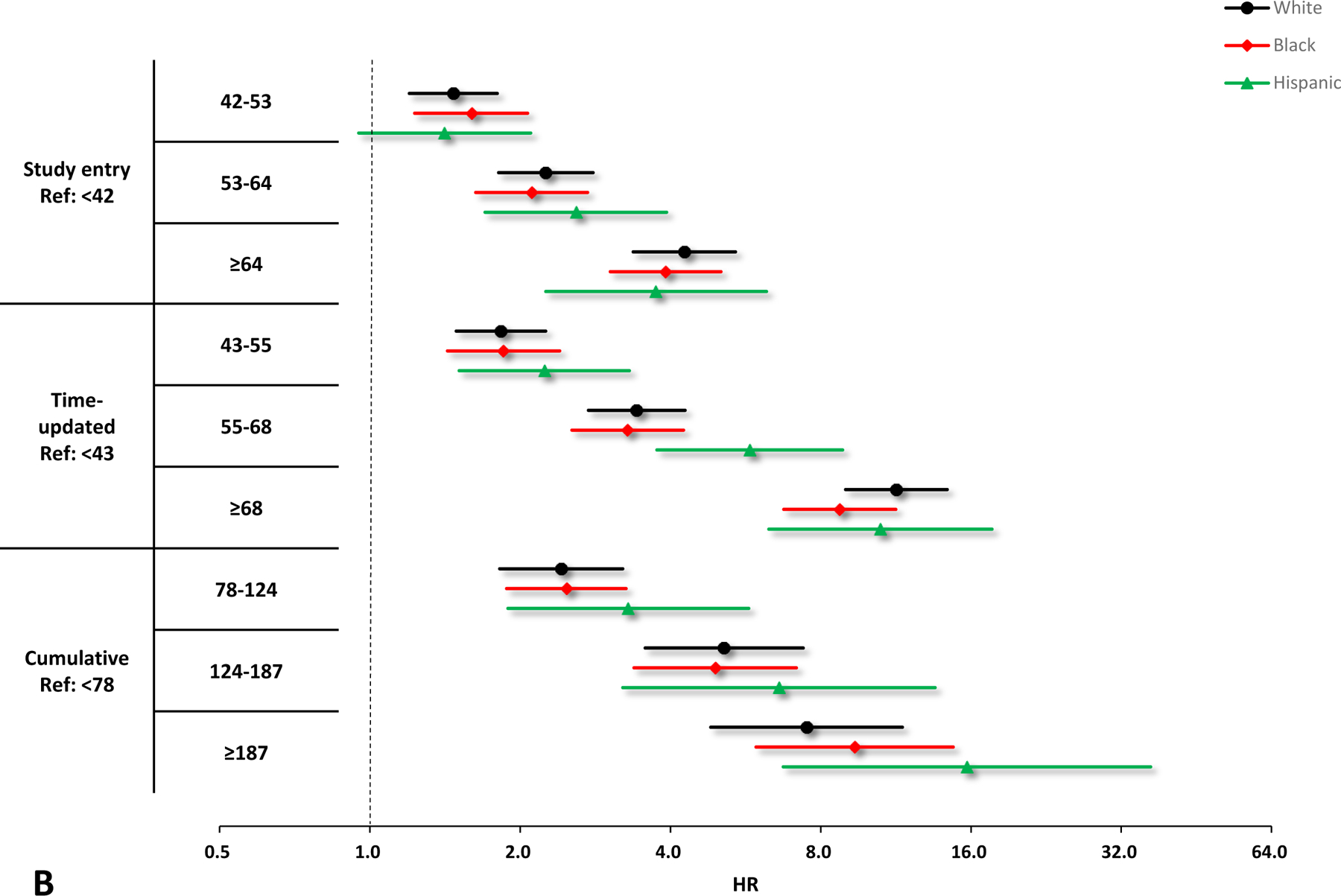
Crude and adjusted hazard ratios (HRs) and 95% confidence interval (CIs) of VACS Index scores for the risk of hospitalization by race/ethnicity. A. Unadjusted results by race/ethnicity, black circles denote white, red diamonds denote Black, and green triangles denote Hispanic. B. Adjusted results by race/ethnicity, black circles denote white, red diamonds denote Black, and green triangles denote Hispanic. Adjusting variables include sex, smoking, HIV acquisition risk factor, depression, and calendar year. All the HRs and 95% CIs are presented on a log2 scale.
In sensitivity analyses, we used boundary values of pre-defined ranges of the indicators22 to substitute out-of-range values. The magnitude of the hazard ratio estimates for time-updated VACS Index were strengthened after including patients without plausible VACS Index indicators (n=1,108). The AIC measures of unadjusted and adjusted models indicated that the time-updated VACS Index provided more information regarding the risk of hospitalization than the study entry and cumulative VACS Index.
We did not lag VACS Index scores, which mimics the timing of the clinician’s access to the score, but also may measure the VACS Index in the same month as hospitalization (removing the temporality of the VACS Index and hospitalization). The proportion of participants that had a different time-updated VACS Index score category in the month prior to hospitalization was 18% (276/1537). This proportion for cumulative VACS Index score was 6% (87/1537). The results were robust to sensitivity analyses lagging the time-updated and cumulative VACS Index by one month.
DISCUSSION
Consistent with prior studies of the VACS Index and numerous chronic disease outcomes6,10,14,23, we found the risk of hospitalization increased with increasing VACS Index 2.0. The time-updated VACS Index 2.0 best predicted hospitalizations with a 10-fold increase in the risk of hospitalization for a score of ≥68 after adjusting for demographic characteristics and other risk factors including depression and calendar year.
Assessment closer to the time of event is more predictive. Although nadir HIV markers such as CD4 and HIV viral load are essential to consider, prognosis of the patients changes with ART and immune reconstitution. The presence of prevalent comorbidities after HIV diagnosis and aging play a clinically significant role in modifying the health status of an individual and the risk of hospitalization. Therefore, clinicians need to consider a tool that accounts for these factors. Previously, Salinas and colleagues14 found that the time-updated VACS Index provided better information about the risk of myocardial infarction and mortality than the study entry and cumulative VACS Index. The cumulative score highly depended on the duration of a health status, and the generalizability was limited14. Our study adds to accumulating evidence that demonstrates the utility of the VACS Index in the clinical setting. In addition to the ability of the risk score to predict another outcome (namely, hospitalizations), it also shows a clear dose-response relationship of increasing risk of hospitalization with increasing VACS Index. Salinas and colleagues did not find an association between the VACS Index at study entry and myocardial infarction, whereas we found a significant, dose-response relationship with hospitalizations for all three parameterizations of the VACS Index. This suggests the VACS Index is apt to predict hospitalizations. Previous studies by Akgun6,10 and Hotton23 and colleagues found VACS Index (as a continuous variable) measured at study entry had a positive association with hospitalizations with increasing VACS Index. Our study builds on these findings to suggest time-updated VACS Index parameterization is a better predictor of hospitalizations than measurement only at study entry or a cumulative measurement of VACS Index.
The time-updated VACS Index fitted hospitalizations best of the three parameterizations examined, and this was consistent across sex and race/ethnicity subgroups. Women had a higher proportion of hospitalizations than men, but the estimates were more extreme among men (see Table 1 and Figure 1). Sex disparities in HIV disease and hospitalization risk continue5,9,39–41, and prior researches showed that women with HIV had higher rates of hospitalizations for opportunistic infection, lower access to treatment, and lower ART adherence than men39,42. Cohen and colleagues43 found that the VACS Index accurately predicted mortality among females but still had space for improvement in predictive accuracy (e.g. incorporating depression and transactional sex in the VACS Index). Our findings showed adjustment for additional characteristics including HIV acquisition risk factor and depression improved the fit of the model to predicting hospitalization by time-updated VACS Index among women. A prior study showed Black/African American PWH had a 42% higher hospitalization rate, and Hispanic PWH had an 18% lower rate than white PWH39, and similar results were found in HIV elite controllers40. Other studies5,9,41 found higher hospitalization rate in both Black and Hispanic than white. In the current study, the hospitalization rate among Black was higher than white, and Hispanic had a lower rate than white.
The time-updated VACS Index has great public health significance and prospective clinical application. First, it helps clinicians identify individuals at high-risk for hospitalization. Previous research shows that women, older patients, Blacks, and people who inject drugs have higher hospitalization rates than their reference populations38,39. Future studies might investigate the applicability of building it into electronic health records (EHRs) and triggering more intensive diseases management including further follow-ups among patients with high risk of hospitalization. Healthcare providers might better distribute medical resources based on the time-updated VACS Index scores, including employing case managers for high-risk patients6. Second, the time-updated VACS Index is a comprehensive score that reflects earlier subtle organ injuries6. It can reflect the risk of conditions due to non-AIDS defining illness given that the patients have suppressed viral load and normal CD4 count. For instance, men are not considered anemic unless their hemoglobin is <1344. However, the VACS Index would be 10 points higher and indicate higher risk of mortality when hemoglobin is <146. Finally, demonstrations of the predictive accuracy of the VACS Index for numerous outcomes (including hospitalization) strengthens the use of the tool to balance study groups and control for potential confounders in observational studies13.
Our study has limitations. First, we only included patients ≥30 who survived after one year of ART and had never been hospitalized, and the limitation of generalizability to young adults still exists. We only included patients who have not previously been hospitalized and potential selection bias could lead to overestimating the performance of the VACS Index. Second, the measurements of the VACS Index are generated by HIV care visits and therefore the time between measurements can be different among individuals, creating time periods of missing data45. We carried forward the input measurements and the VACS Index itself for varying lengths of time based on scientific knowledge about the speed at which the true input measurement may change. If the mechanisms driving missed appointments are also driving poorer health status than at the last clinical visit, carrying values forward will not capture the decline in health status. We categorized the VACS Index parameters based on quartiles to assess the dose-response relationships, and differences in association may be not only due to better predictive accuracy. Third, there was a limited sample size in Asians and an insufficient number of Indigenous individuals to conduct the analyses within this group. Fourth, the reasons for hospitalization were not considered and could influence the association between the VACS Index and cause-specific hospitalization. Fifth, we only adjusted behavioral factors (e.g. smoking, IDU) in our models, and future studies should assess whether incorporating these factors in the VACS Index improves its capability in assessing the risk of hospitalization. To our knowledge, the current study is the first study to compare the association between different parameterizations of VACS Index and hospitalization. The time-updated score incorporates changes in the health status of patients and has more value in predicting future hospitalization among PWH than VACS Index scores measured at study entry or cumulatively. All three parameterizations of the VACS Index demonstrated a dose-response relationship with hospitalization when the parameter was categorized into quartiles. We examined the three parameterizations of VACS Index in sex and race/ethnicity sub-groups to identify potential disparities.
In conclusion, the time-updated score best assessed the risk of hospitalization in the NA-ACCORD. Healthcare providers should consider using the time-updated VACS Index when assessing the risk of hospitalization among PWH. Further research may address the use of the VACS Index to assess the risk of re-admission at hospitalization discharge.
Supplementary Material
Supplemental Digital Content 1. Figure that demonstrates the selection of study population.pdf
Supplemental Digital Content 2. Figure that demonstrates the observed hazard of hospitalization.pdf
Supplemental Digital Content 3. Figure that shows the hospitalization-free survival within five years.pdf
Supplemental Digital Content 4. Table that shows the ranges of plausible values associated with VACS Index 2.0 score.pdf
Supplemental Digital Content 5. Table that shows the AIC values for models among men and women.pdf
Supplemental Digital Content 6. Table that shows the AIC values for models among white, Black, and Hispanic.pdf
ACKNOWLEDGMENTS
NA-ACCORD Collaborating Cohorts and Representatives:
AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch
AIDS Link to the IntraVenous Experience: Gregory D. Kirk
Emory-Grady HIV Clinical Cohort: Vincent C. Marconi and Jonathan A. Colasanti
Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Viviane Lima, P. Richard Harrigan, Julio SG Montaner, Benita Yip, Julia Zhu, and Kate Salters
HIV Outpatient Study: Kate Buchacz and Jun Li
HIV Research Network: Kelly A. Gebo and Richard D. Moore
Johns Hopkins HIV Clinical Cohort: Richard D. Moore
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Jeffrey M. Jacobson
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg
Kaiser Permanente Northern California: Michael J. Silverberg
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne
MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza
Maple Leaf Medical Clinic: Graham Smith, Mona Loutfy, and Meenakshi Gupta
The McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein
Multicenter Hemophilia Cohort Study–II: Charles Rabkin
Ontario HIV Treatment Network Cohort Study: Abigail E. Kroch, Ann N, Burchell, Adrian Betts, and Joanne Lindsay
Parkland/UT Southwestern Cohort: Ank E. Nijhawan
Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico: Angel M. Mayor
Southern Alberta Clinic Cohort: M. John Gill
Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James H. Willig
University of California at San Diego: Laura P. Bamford and Maile Y. Karris
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner
Veterans Aging Cohort Study: Kathleen A. McGinnis and Amy C. Justice
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman
Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober
Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Raynell Lang, Brenna Hogan, Bin You, Elizabeth Humes, Lucas Gerace, Cameron Stewart, and Sally Coburn
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or those of the Veterans Affairs.
Source of Funding:
This work was supported by National Institutes of Health grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA036297, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176; contracts CDC-200–2006–18797 and CDC-200–2015–63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Additional support was provided by the National Institute Of Allergy And Infectious Diseases (NIAID), National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Althoff KN, Chandran A, Zhang J, et al. Life-expectancy disparities among adults with HIV in the United States and Canada: the impact of a reduction in drug- and alcohol-related deaths using the lives saved simulation model. Am J Epidemiol. 2019;188(12):2097–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. The Lancet. 2014;384(9939):241–248. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry SA, Manabe YC, Moore RD, et al. Hospitalization risk following initiation of highly active antiretroviral therapy. HIV Med. 2010;11(5):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry SA, Fleishman JA, Moore RD, et al. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. 2012;59(4):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akgun KM, Gordon K, Pisani M, et al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected Veterans. J Acquir Immune Defic Syndr. 2013;62(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7(2):69–76. [DOI] [PubMed] [Google Scholar]
- 8.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazar R, Kersanske L, Xia Q, et al. Hospitalization rates among people with HIV/AIDS in New York City, 2013. Clin Infect Dis. 2017;65(3):469–476. [DOI] [PubMed] [Google Scholar]
- 10.Akgun KM, Tate JP, Crothers K, et al. An adapted frailty-related phenotype and the VACS Index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr. 2014;67(4):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijhawan AE, Higashi RT, Marks EG, et al. Patient and provider perspectives on 30-day readmissions, preventability, and strategies for improving transitions of care for patients with HIV at a safety net hospital. J Int Assoc Provid AIDS Care. 2019;18:2325958219827615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study Index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas JL, Rentsch C, Marconi VC, et al. Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis. 2016;63(11):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow D, Thomas BS, Liang C, et al. Role of the Veterans Aging Cohort Study Index in assessing total atherosclerotic burden. Clin Infect Dis. 2012;55(5):750–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquine MJ, Umlauf A, Rooney AS, et al. The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquine MJ, Montoya JL, Umlauf A, et al. The Veterans Aging Cohort Study (VACS) Index and neurocognitive change: a longitudinal study. Clin Infect Dis. 2016;63(5):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Womack JA, Goulet JL, Gibert C, et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis. 2013;56(10):1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin MT, Shiau S, Rimland D, et al. Fracture prediction with modified-FRAX in older HIV-infected and uninfected men. J Acquir Immune Defic Syndr. 2016;72(5):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bebu I, Tate J, Rimland D, et al. The VACS Index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65(2):226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tate JP, Sterne JAC, Justice AC, et al. Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS. 2019;33(5):903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotton AL, Weber KM, Hershow RC, et al. Prevalence and predictors of hospitalizations among HIV-infected and at-risk HIV-uninfected women. J Acquir Immune Defic Syndr. 2017;75(2):e27–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Althoff KN, Wong C, Hogan B, et al. Mind the gap: observation windows to define periods of event ascertainment as a quality control method for longitudinal electronic health record data. Ann Epidemiol. 2019;33:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rigotti NA, Chang Y, Tindle HA, et al. Association of E-Cigarette use with smoking cessation among smokers who plan to quit after a hospitalization: a prospective study. Ann Intern Med. 2018;168(9):613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davy-Mendez T, Napravnik S, Hogan BC, et al. Hospitalization rates and causes among persons with HIV in the US and Canada, 2005–2015. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 30.Mugavero MJ, Napravnik S, Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53(9):927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole SR, Napravnik S, Mugavero MJ, et al. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong C, Gange SJ, Moore RD, et al. Multimorbidity among persons living with human immunodeficiency virus in the United States. Clin Infect Dis. 2018;66(8):1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elion RA, Althoff KN, Zhang J, et al. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr. 2018;78(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, March 27, 2012. Available at: http://aidsinfo.nih.gov/Guidelines/HTML/1/adult-and-adolescent-treatment-guidelines/0.
- 35.Gardner LI, Klein RS, Szczech LA, et al. Rates and risk factors for condition-specific hospitalizations in HIV-infected and uninfected women. J Acquir Immune Defic Syndr. 2003;34(3):320–330. [DOI] [PubMed] [Google Scholar]
- 36.Rentsch C, Tate JP, Akgun KM, et al. Alcohol-related diagnoses and all-cause hospitalization among HIV-infected and uninfected patients: a longitudinal analysis of United States Veterans from 1997 to 2011. AIDS Behav. 2016;20(3):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crum-Cianflone NF, Grandits G, Echols S, et al. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr. 2010;54(3):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yehia BR, Fleishman JA, Hicks PL, et al. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr. 2010;53(3):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachhuber MA, Southern WN. Hospitalization rates of people living with HIV in the United States, 2009. Public Health Rep. 2014;129(2):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowell TA, Gebo KA, Blankson JN, et al. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211(11):1692–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleming J, Berry SA, Moore RD, et al. U.S. Hospitalization rates and reasons stratified by age among persons with HIV 2014–15. AIDS Care. 2020;32(11):1353–1362. [DOI] [PubMed] [Google Scholar]
- 42.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis. 2012;55(9):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen MH, Hotton AL, Hershow RC, et al. Gender-related risk factors improve mortality predictive ability of VACS Index among HIV-infected women. J Acquir Immune Defic Syndr. 2015;70(5):538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stucchi M, Cantoni S, Piccinelli E, et al. Anemia and acute coronary syndrome: current perspectives. Vasc Health Risk Manag. 2018;14:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesko CR, Jacobson LP, Althoff KN, et al. Collaborative, pooled and harmonized study designs for epidemiologic research: challenges and opportunities. Int J Epidemiol. 2018;47(2):654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure that demonstrates the selection of study population.pdf
Supplemental Digital Content 2. Figure that demonstrates the observed hazard of hospitalization.pdf
Supplemental Digital Content 3. Figure that shows the hospitalization-free survival within five years.pdf
Supplemental Digital Content 4. Table that shows the ranges of plausible values associated with VACS Index 2.0 score.pdf
Supplemental Digital Content 5. Table that shows the AIC values for models among men and women.pdf
Supplemental Digital Content 6. Table that shows the AIC values for models among white, Black, and Hispanic.pdf


