Abstract
Human respiratory coronaviruses (HCoVs), including the recently emerged SARS-CoV-2, the causative agent of the coronavirus disease 2019 (COVID-19) pandemic, potentially cause severe lung infections and multiple organ damages, emphasizing the urgent need for antiviral therapeutics and vaccines against HCoVs. Small animal models, especially mice, are ideal tools for deciphering the pathogenesis of HCoV infections as well as virus-induced immune responses, which is critical for antiviral drug development and vaccine design. In this review, we focus on the antiviral innate immune response, antibody response and T cell response in HCoV infected mouse models, and discuss the potential implications for understanding the anti-HCoV immunity and fighting the COVID-19 pandemic.
Current Opinion in Virology 2022, 52:102–111
This review comes from a themed issue on Viral immunology
Edited by Antonio Bertoletti and Matteo Iannacone
For complete overview about the section, refer Viral Immunology (2022)
Available online 11th December 2021
https://doi.org/10.1016/j.coviro.2021.11.015
1879-6257/© 2021 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
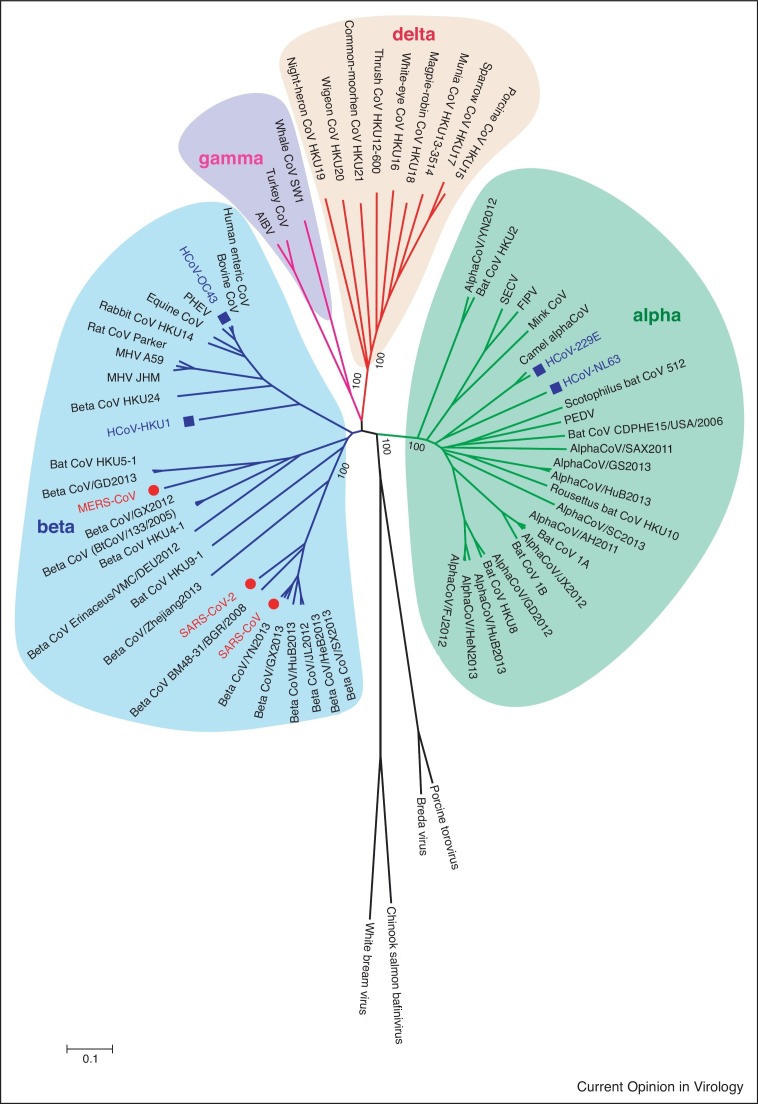
The human respiratory coronaviruses (HCoVs) belong to the Coronaviridae family, the Coronavirinae subfamily, and the Coronavirus genera, with a ∼30 kb long positive-sense RNA genome, encoding four structural proteins (spike (S), envelope (E), membrane (M) and nucleocapsid (N) protein) and several accessary proteins. There are seven HCoVs including three highly pathogenic CoVs and four low pathogenic CoVs (Figure 1 ), causing a wide spectrum of symptoms and diseases from a flu-like to severe acute respiratory infection in humans. The severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are highly pathogenic HCoVs that can cause severe pneumonia, multiple organ damages and result in global pandemics. Low pathogenic HCoVs (HCoV-229E, HCoV-OC43, HCoV-NL63 and HCoV-HKU1) are believed to cause only mild and self-limiting respiratory infections in humans. The SARS-CoV outbreak started in the winter of 2002 in China, which infected 8098 patients with 774 total deaths (9.6%) [1•,2], MERS-CoV was first identified in 2012 in Saudi Arabia and caused more than two thousand cases with around 35% fatality, which is still circulating in Middle East countries and perhaps in Africa [3,4,5••], SARS-CoV-2 was first discovered in December 2019 in China, and it is the causative agent for the current coronavirus disease 2019 (COVID-19) pandemic. As of October 29, 2021, more than 245 million confirmed cases with near 5 million deaths have been reported to WHO [6•,7]. HCoV-229E and HCoV-OC43 were isolated in the 1960s from nasopharyngeal samples of individuals experiencing common colds [8,9]. HCoV-NL63 and HCoV-HKU1 were discovered right after the SARS-CoV epidemic [10,11]. Low pathogenic HCoVs represent nearly 15–30% of the common cold respiratory tract infections in humans each year, and more than 90% of adults are serologically positive against these low pathogenic HCoVs [12]. Occasionally, in the elderly, child and immunocompromised patients, these four HCoVs cause life-threatening pneumonia and bronchiolitis as well as fetal encephalitis [13, 14, 15, 16, 17].
Figure 1.
Phylogenetic analyses of Coronaviridae family.
All available complete genomes of Coronaviridae family from GenBank were collected and used for the evolutional analysis using MEGA 7.0. Three highly pathogenic (red) and four low pathogenic (blue) human coronaviruses are highlighted in the phylogenetic tree.
The emergence of SARS-CoV, MERS-CoV and SARS-CoV-2, three strains of animal coronaviruses that crossed the species barrier to infect human and caused severe respiratory infections in humans within the last 20 years, have emphasized that coronaviruses represent a major public health threat that are not restrained by international borders. There is an urgent need to understand the pathogenesis of HCoV infection and the regulatory mechanisms for anti-HCoV immune responses, which are critical for vaccine design and drug development. However, antiviral immune responses are also a double-edge sword. It is of great importance to distinguish protective versus pathogenic immune response and restore the balance of immune regulation. These studies require animal models, especially mice which are cost-effective, easily available and handling and provide multiple approaches to validate therapies and vaccines in vivo. In this review, we focus on antiviral innate immune responses, antibody responses and T cell responses in HCoV-infected mouse models, and discuss the potential implications for understanding the anti-HCoV immunity and fighting the COVID-19 pandemic.
Application of mouse models in HCOV research
Animal models are essential tools in infectious disease research, including HCoV infections. Several animals have therefore been evaluated as models for HCoV studies, including mice, hamsters, ferrets, and non-human primates (NHPs). Promising animal models that can reproduce manifestations of clinical patients have been broadly applied not only for elucidating disease pathogenesis and host immune response of infections but also for the evaluation of antiviral therapies and vaccine candidates. However, each model has its limitations. For example, as the gold standard animal model, non-human primates (NHPs) are closely related to humans, but their application is limited due to the cost and availability. In SARS-CoV, MERS-CoV and SARS-CoV-2 infections, NHPs only reproduce mild diseases observed in human [2]. On the contrary, mice are ideal small animals because they are cost-effective, easily available and handling and of clear genetic background. However, laboratory mice also have some disadvantages in certain infections. For examples, they do not support the infections by several HCoVs at the receptor level, including MERS-CoV, some variants of SARS-CoV-2, 229E, NL63 and HKU1 [18•,19•,20, 21, 22, 23]. To solve this problem, several strategies have been developed, such as application of HCoV receptor transgenic or knock-in mice [24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38], in vivo transduction of replication-defective vectors expressing HCoV receptors [39••,40, 41, 42], modifying viruses by serial passages in the respiratory tract of mice for generation of mouse-adapted (MA) strains or by reverse genetics to remodel the interaction between the S proteins and the receptors [43, 44, 45, 46, 47]. However, there is still no available mouse model for HCoV-HKU1 and HCoV-NL63. Table 1 summarizes most of the available mouse models for HCoV infections. These mouse models can permit HCoV entry, but they have pros and cons in various aspects. Some transgenic mouse models permit efficient viral entry but may cause severe diseases and even death due to brain infection which is not ideal for the study of respiratory tract infection. The mouse models generated by exogenous receptor transduction can successfully mimic human lung infection and be used in the study of host immune responses. However, these models often induce mild disease and are hard to recapitulate severe pneumonia. Mouse-adapted viruses generated by serial passaging the virus in the mouse respiratory tract can efficiently replicate in the mouse lungs and induce severe pneumonia, which are used to study the immune responses and pathogenesis. However, these viruses are different from the parental virus and containing distinct adaptive mutations. Mouse hepatitis coronavirus (MHV) also has served as models for dissecting the viral and immunologic determinants of coronavirus disease [48, 49, 50]. Distinct MHV strains exhibit differences in tropism and virulence. For example, MHV-1, but not the other strains of MHV infected mice produces clinical and pathological SARS-like disease with high mortality, which also could be used as a model for human respiratory coronavirus infections [51]. Researchers need to select proper mouse models based on their study objectives.
Table 1.
Rceptors and mouse models of HCoVs
| HCoVs | Receptor | Receptor transgenic mice | Receptor knock-in mice | Delivery receptor by exogenous vector | Genetically adapted virus |
|---|---|---|---|---|---|
| SARS-CoV | hACE2 [18•] | mAce2-hACE2 ICR mice [25]; K18-hACE2 C57BL/6 mice [24] | N/A | N/A | MA15 in young BALB/c mice [43]; v2163 in young BALB/c mice [46] |
| MERS-CoV | hDPP4 [19•] | CAG-hCD26 C57BL/6 J and/or B6C3F1/J mice [27]; codon-optimized CAG-hDPP4 C57BL/6 mice [31]; hDPP4 Tg C57BL/6 mice [32] | hDPP4 whole humanized using the VelociGene technology [33]; mDPP4 (A288 L/T330R) combined with MERS-15 [28]; hDPP4 KI combined with MERSMA [26]; CRISPR/Cas9 KI [34]; | Ad5-hDPP4 [39••] | MERS-15 in CRISPR–Cas9 genetically engineered C57BL/6 mice [28]; MERSMA30 in hDPP4-KI C57BL/6 mice [26] |
| SARS-CoV-2 | hACE2 [20] | mAce2 hACE2 ICR mice [29]; HFH4-hACE2 C3B6 mice [30]; K18-hACE2 [35, 36, 37] | CRISPR/Cas9 KI [38] | Ad5-hACE2 [40]; AAV-hACE2 [41]; VEEV-VRP-hACE2 [42] | SARS-CoV-2 MA10 in BALB/c mice [47]; SARS-CoV-2 MA in BALB/c mice [44]; MASCp6 in aged BALB/c mice [45] |
| HCoV-HKU1 | 9-O-acetylatedsialic acids [23] | N/A | N/A | N/A | N/A |
| HCoV-OC43 | 9-O-acetylatedsialic acids [23] | Mice are susceptible to OC43 | |||
| HCoV-NL63 | hACE2 [22] | N/A | N/A | N/A | N/A |
| HCoV-229E | hAPN [21] | hAPN+/+ Stat1−/− ICR mice [84] | N/A | N/A | N/A |
Innate immune response
The innate immune system acts as the first line of defense against pathogens, including HCoVs. The innate immune system recognizes invaded pathogens by sensing their pathogen-associated molecular patterns (PAMPs) with various pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), the nucleotide-binding oligomerization domain (NOD)-like receptor family proteins (NLRs), and so on [52]. Following downstream signaling pathway activation, cells produce interferon (IFN) and inflammatory molecules and then promote the body to form an ‘antiviral state’ against virus infections by producing hundreds of interferon-stimulated genes (ISGs) [53••]. However, activation of the innate immune system must be tightly regulated, because excessive activation may lead to systemic inflammation and tissue damage, which are detrimental to the host [54•].
The innate immune responses to SARS-CoV, MERS-CoV and SARS-CoV-2 are frequently characterized by high-level production of pro-inflammatory chemokines and cytokines in patients, animal models as well as in tissue cultures in vitro [40,55, 56, 57, 58, 59, 60]. Innate viral recognition leads to both nuclear factor-κB (NF-κB)-mediated induction of pro-inflammatory cytokines and interferon regulatory factor 3 (IRF3) and IRF7-mediated induction of type I and type III IFNs. TLR7 is a membrane-bound PRR on endosomes, where it can recognize ssRNA motifs from invading SARS-CoV, MERS-CoV, and MHV, then TLR7 dimerizes and recruits the myeloid differentiation primary response 88 (MyD88) adaptor protein triggering IRF7 and NF-κB, which stimulates the production of type I IFNs and proinflammatory cytokines, respectively, for host defense [61••,62]. Melanoma differentiation-associated gene 5 (MDA5) is a cytosolic PRRs in the RLR family that can sense double-stranded long RNAs in MHV infection and then lead to the production of type I IFNs and proinflammatory cytokines via the activation and nuclear translocation of IRF3 and NF-κB [63,64]. RIG-I is another cytosolic RLR mediating type I IFN response in MHV and influenza virus infections [63].
Regulated type I IFN (IFN-I) production is protective in SARS-CoV, MERS-CoV, SARS-CoV-2, and mouse hepatitis coronavirus (MHV) infections by enhancing viral clearance [54•,61••,65••,66,67]. Dysregulation of the innate immune response are pathogenic in SARS-CoV-infected and MERS-CoV-infected mice. Channappanavar et al. found that early treatment with recombinant IFN-β or poly(I:C) to induce IFN-I production in the lung resulted in complete protection from lethal infection. However, administration of IFN-β at the peak of SARS-CoV replication led to delayed viral clearance and enhanced lethality rather than protection [65••]. This delayed-IFN-I enhanced disease is characterized by apoptosis of T cells and elevated inflammatory monocyte and macrophage (IMMs) accumulation in the lungs. Antibody-mediated depletion of IMMs is fully protective. These data suggest that regulated IFN-I production is crucial for viral clearance as well as reduced immunopathology induced by inflammation [61••,68].
Despite this innate host antiviral strategy, some HCoVs remain highly pathogenic, at least in part due to the various viral mechanisms to evade and suppress the IFN response. HCoV immune escape strategies include encoding viral proteins dedicated to evading innate recognition by PRRs [69, 70, 71, 72, 73, 74], inhibiting IFN-I and IFN-III production [75, 76, 77, 78, 79, 80], blocking IFNAR and IFNAR signaling [77,81,82], and directly suppressing ISG effector functions [83].
Comparing with SARS-CoV, MERS-CoV and SARS-CoV-2, innate immune responses against human common cold coronaviruses remain largely unclear. Caroline Lassnig et al. has generated double transgenic hAPN+/+ STAT1−/− mice that are susceptible to HCoV-229E infection indicating the protective role of IFN-I and STAT1 pathway [84]. Intranasal administration of recombinant IFN-α before infection protected against experimental HCoV-229E infection, resulting in reduced viral loads, diminished incidence and severity of symptoms in volunteers [85]. Structural (M and N) and accessory (NS2a and NS5a) proteins of HCoV-OC43 are able to inhibit antiviral response elements (ISRE, IFN-β promoter, and NF-κB-RE) to block the activation of IFN-I and NF-κB signaling pathways in vitro [86]. However, another study showed that OC43 N protein potentiates NF-κB activation following cytokine or TLR ligand stimulation by binding RNA, specifically microRNA 9 [87].
Innate immune cells recognize coronaviruses by cytosolic and endosomal RNA sensors and produce pro-inflammatory cytokines, chemokines and IFNs to fight infections. However, overwhelming chemokines and cytokines or dysregulated innate response could increase lung pathology, and contribute to the lethal SARS-CoV and MERS-CoV infections. All of these evidences suggest that tightly regulated host innate immunity is crucial to protect the infected mice.
Humoral immune response
Humoral immune responses to HCoVs are mediated by antibodies that are directly against viral structural proteins, mainly the spike glycoprotein and the nucleocapsid protein. A large amount of relevant information on antibody responses to HCoVs focused on serological tests in patients, few studies were performed in mouse models [88, 89, 90, 91, 92, 93]. Seroconversion in SARS were within 10–16 days post onset of disease (d.p.o.) in patients [88,89], while 2–3 weeks after MERS-CoV and SARS-CoV-2 infections [90, 91, 92, 93]. A low level of neutralizing antibodies could be detected in the mouse models infected with SARS-CoV-2 by 10 d.p.i. and wane afterwards [30,40]. However, in MHV infected mice, the T cell-dependent humoral immunity remained stable for at least 60 days. Further, B cells or IgM deficient mice experience viral recrudescence following initial viral clearance indicating humoral immunity mediates protective immunity in MHV infection [94, 95, 96]. Passive transfer of neutralizing antibodies was found to protect SARS-CoV, MERS-CoV and SARS-CoV-2 infections in mice [40,97, 98, 99, 100]. Antibody responses to SARS-CoV also waned over time in SARS convalescents at 2–3 years after the symptom onset [101•]. Large serological surveys of natural infections with HCoV-229E and HCoV-OC43 has revealed similar patterns of antibody waning that were associated with reinfections [102,103]. One study found a two-way cross-reactive binding but not neutralizing antibody against spike protein between SARS-CoV-2 and HCoV-OC43. However, high HCoV-OC43 S-IgG levels were found to relate with systemic inflammatory responses in COVID-19 patients, which might be a risk factor for clinical outcomes of COVID-19 [104•].
Humoral immunity is required for controlling CoV infections. Several studies have indicated that convalescent plasma transfusion could elicit protective role in infected patients and mice [40,105,106]. However, antibody-dependent enhancement (ADE) is still of concern and require further studies.
T cell response
Humoral and T cell immune responses are the major components of the adaptive immune system. In contrast to the innate immune response, the adaptive immune response has an immunological memory that are highly specific against reinfections. While antibody response is not long lasting, memory T cells were reported to have significant longer longevity in HCoV patients [100,107••].
T cell responses against HCoV infections also have been characterized in mice [39••,40,108•,109, 110, 111, 112]. Depletion of CD4+ T cells delayed virus clearance and further enhanced interstitial pneumonitis in 12–14 month-old BALB/c mice infected with SARS-CoV intranasally (i.n.) [109]. MERS-CoV could be cleared in mice lacking B cells, while there was a delayed viral clearance in mice lacking T cells, indicating that T cells are required to clear MERS-CoV [39••]. However, another study showed that depletion of CD8+ T cells in a sublethal MERS mouse model resulted in diminished lung pathology and clinical disease without impacting the viral titers, suggesting that these cells may also play a role in immunopathogenesis [110]. Both CD4+ and CD8+ T cells contributed to SARS-CoV-2 clearance in C57BL/6 and BALB/c mice [40,108•]. The role of CD4+ and CD8+ T cells in low pathogenic HCoV infections have not yet been addressed.
In the above-mentioned studies, the role of bulk CD4+ or CD8+ T cell populations were studied. However, only viral antigen-specific T cells can respond efficiently against a viral infection. These T cells recognize virus-derived peptides (epitopes) presented by MHC class I or class II molecules on virus-infected or antigen presenting cells. Usually, HCoV-specific T cell epitopes in the model mouse will be mapped and then the function of specific T cells can be further investigated [39••,108•,111,113, 114, 115, 116]. Several studies showed that virus-specific CD4+ and CD8+ T cells contributed to rapid viral clearance and amelioration of the clinical disease in HCoV infected mice, demonstrated by adoptive transfer of virus-specific CD4+ and CD8+ T cells or immunization of mice with Venezuelan equine encephalitis replicon particles (VRPs) encoding a single dominant T cell epitope [108•,112,117••]. A study focusing on COVID-19 convalescent donors and vaccinees showed that SARS-CoV-2 variants of concern (VOC) partially escaped from humoral but not T cell response [118•], indicating that T cell response is more conserved and could mediate cross protection against virus containing homologous epitopes. Coronaviruses are relatively conservative in structural proteins and cross-reactive T cell epitopes among different CoVs have been reported. One of HCoV-OC43-specific CD4+ T cell epitopes located in the membrane (M) protein (M133-147) is identical with a MHV-specific CD4+ T cell epitope (M133-147) in C57BL/6 mice [119]. However, the OC43-specific CD8+ T cell response and other low pathogenic HCoV-specific T cell responses are still unknown. Immunization of mice with a VRP encoding a CD4+ T cell epitope from SARS-CoV N protein resulted in reduced viral load of MERS-CoV, proved that SARS-CoV-specific CD4+ T cells are cross-protective against MERS-CoV in some degree [117••]. Another research showed that there are cross-reactive T cell responses between SARS-CoV and SARS-CoV-2 in BALB/c mice [108•]. The role of virus-specific and cross-reactive T cell responses against HCoVs need to be further studied in mice.
Optimal virus-specific T cell responses play an essential role in viral clearance. However, SARS-CoV specific T cell responses can be impaired by the defects in respiratory dendritic cells (rDC) and alveolar macrophages (AM). Depletion of AM or pretreatment of mice with poly I:C could correct these defects and improve T cell responses in SARS-CoV infected mice [120•]. Zhao et al. also found that suboptimal T cell responses resulted from an impairment of rDC migration from the lungs to the lymph nodes, which was governed by elevated prostaglandin D2 (PGD2) and phospholipase (PLA2G2D) expression in aged mice after SARS-CoV infection [121•]. In addition, Zhuang et al. showed that IFN-I signaling was required for optimal SARS-CoV-2–specific T cell development and functionality.
These studies indicate that both virus-specific CD4+ and CD8+ T cells are protective in coronavirus infections, and restoring impaired T cell response may improve the outcome in some patients. In addition, T cell response is required for optimal protective effect for a CoV vaccine.
Perspective for COVID-19 immunity from other HCoV infections
Today, it is well-known that HCoVs are undergoing rapid evolution due to high nucleotide substitution and recombination rate and linked with major outbreaks worldwide of human fatal pneumonia since the beginning of the 21st century [122, 123, 124]. Here, we review the immune responses against HCoVs in mice and discuss key scientific issues that need attention in SARS-CoV-2 infection immunity.
Identifying factors that are protective versus pathogenic in patients, especially in severe disease, could inform the selection of therapeutic options. Delayed and dysregulated IFN responses contribute to the pathogenesis of SARS-CoV and MERS-CoV rather than protection. It suggests that early and timely treatment of type I interferon may help prevent from severe disease.
Vaccine immunization is the best expedient path to herd immunity. Whether anti-SARS-CoV-2 neutralizing antibodies or specific T cells alone can effectively prevent SARS-CoV-2 pandemic remain unclear. How much antibodies or T cell response are sufficient? How to avoid ADE? What are the roles of pre-existing cross-reactive immune responses in the SARS-CoV-2 infection? All of these questions need to be answered. Neutralizing antibodies against HCoVs wane following infection or immunization, potentially allowing for SARS-CoV-2 reinfection which is similar to the common cold coronaviruses. Therefore, vaccine design and immunization strategies need to be improved. Introduction of T cell epitopes in the vaccines probably could prolong the immunization durability, and intranasal immunization may enhance the generation of protective mucosal immunity [117••,125,126].
Author contributions
All authors contributed to writing and critical revision of the manuscript. All authors approved the final version.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Dr Jingxian Zhao for critical review of the manuscript and Dr Yanqun Wang for helping in Phylogenetic analyses of Coronaviridae family. This work was supported by the grants from The National Key Research and Development Program of China (2018YFC1200100, 2018ZX10301403, 2020YFC0842400), National Natural Science Foundation of China (82025001), Ministries of Science and Technology, Education of Guangdong province (2020B1111330001, 2020A111128008, 2020B1111320003, 2020A0505100063, 2020KZDZX1158, B195001248, 2020A1515010911, 2019TX05Y120), National Key Technology R&D Program (2018YFC1311900), Guangdong Science and Technology Foundation (2019B030316028), Guangzhou Institute of Respiratory Health Open Project (Funds provided by China Evergrande Group, 2020GIRHHMS07 and 2020GIRHHMS24).
References
- 1•.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]; This study identifies a novel coronavirus caused SARS and named the first isolate as the Urbani strain of SARS-associated coronavirus.
- 2.Singh A., Singh R.S., Sarma P., Batra G., Joshi R., Kaur H., Sharma A.R., Prakash A., Medhi B. A comprehensive review of animal models for coronaviruses: SARS-CoV-2, SARS-CoV, and MERS-CoV. Virol Sin. 2020;35:290–304. doi: 10.1007/s12250-020-00252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) 2019. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Situation Update.https://applications.emro.who.int/docs/EMROPub-MERS-SEP-2019-EN.pdf?ua=1&ua=1 [Google Scholar]
- 5••.Mok C.K.P., Zhu A., Zhao J., Lau E.H.Y., Wang J., Chen Z., Zhuang Z., Wang Y., Alshukairi A.N., Baharoon S.A., et al. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: an observational cohort study. Lancet Infect Dis. 2021;21:385–395. doi: 10.1016/S1473-3099(20)30599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights that detection of virus-specific T-cell responses is a more sensitive method for detecting past infection compared with the serological tests being used hitherto, and suggests that the incidence of MERS infections taking place in Africa is underestimated.
- 6•.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a novel coronavirus SARS-CoV-2 caused COVID-19 and confirms that SARS-CoV-2 uses the same cell entry receptor-angiotensin converting enzyme II (ACE2)-as SARS-CoV.
- 7.(WHO) WHO . 2021. Coronavirus Disease 2019 (COVID-19) Situation Report.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- 8.McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc Natl Acad Sci U S A. 1967;57:933–940. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyrrell D.A., Bynoe M.L. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–1470. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouchier R.A., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H., Poon R.W., Cai J.J., Luk W.K., et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorse G.J., Patel G.B., Vitale J.N., O’Connor T.Z. Prevalence of antibodies to four human coronaviruses is lower in nasal secretions than in serum. Clin Vaccine Immunol. 2010;17:1875–1880. doi: 10.1128/CVI.00278-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vassilara F., Spyridaki A., Pothitos G., Deliveliotou A., Papadopoulos A. A rare case of human coronavirus 229E associated with acute respiratory distress syndrome in a healthy adult. Case Rep Infect Dis. 2018;2018 doi: 10.1155/2018/6796839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Li X., Liu W., Gan M., Zhang L., Wang J., Zhang Z., Zhu A., Li F., Sun J., et al. Discovery of a subgenotype of human coronavirus NL63 associated with severe lower respiratory tract infection in China, 2018. Emerg Microbes Infect. 2020;9:246–255. doi: 10.1080/22221751.2020.1717999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pene F., Merlat A., Vabret A., Rozenberg F., Buzyn A., Dreyfus F., Cariou A., Freymuth F., Lebon P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. 2003;37:929–932. doi: 10.1086/377612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson A., Edner N., Albert J., Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond) 2020;52:419–422. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 17.Morfopoulou S., Brown J.R., Davies E.G., Anderson G., Virasami A., Qasim W., Chong W.K., Hubank M., Plagnol V., Desforges M., et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 18•.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies angiotensin-converting enzyme 2 (ACE2), isolated from SARS coronavirus (SARS-CoV)-permissive Vero E6 cells, which is the receptor of the SARS-CoV.
- 19•.Lu G., Hu Y., Wang Q., Qi J., Gao F., Li Y., Zhang Y., Zhang W., Yuan Y., Bao J., et al. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the molecular basis of the interaction between the MERS-CoV spike protein and DPP4 is delineated from crystal structures of the receptor binding domain of the MERS-CoV spike protein free and complexed with DPP4.
- 20.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–28292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulswit R.J.G., Lang Y., Bakkers M.J.G., Li W., Li Z., Schouten A., Ophorst B., van Kuppeveld F.J.M., Boons G.J., Bosch B.J., et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci U S A. 2019;116:2681–2690. doi: 10.1073/pnas.1809667116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–459. [PubMed] [Google Scholar]
- 26.Li K., Wohlford-Lenane C.L., Channappanavar R., Park J.E., Earnest J.T., Bair T.B., Bates A.M., Brogden K.A., Flaherty H.A., Gallagher T., et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc Natl Acad Sci U S A. 2017;114:E3119–e3128. doi: 10.1073/pnas.1619109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agrawal A.S., Garron T., Tao X., Peng B.H., Wakamiya M., Chan T.S., Couch R.B., Tseng C.T. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cockrell A.S., Yount B.L., Scobey T., Jensen K., Douglas M., Beall A., Tang X.C., Marasco W.A., Heise M.T., Baric R.S. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol. 2016;2 doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 30.Jiang R.D., Liu M.Q., Chen Y., Shan C., Zhou Y.W., Shen X.R., Li Q., Zhang L., Zhu Y., Si H.R., et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182:50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G., Jiang Y., Qiu H., Gao T., Zeng Y., Guo Y., Yu H., Li J., Kou Z., Du L., et al. Multi-organ damage in human dipeptidyl peptidase 4 transgenic mice infected with middle east respiratory syndrome-coronavirus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Kotani O., Sato H., Sekimukai H., Fukushi S., Suzuki T., Sato Y., Takeda M., et al. Acute respiratory infection in human dipeptidyl peptidase 4-transgenic mice infected with Middle East respiratory syndrome coronavirus. J Virol. 2019;93 doi: 10.1128/JVI.01818-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascal K.E., Coleman C.M., Mujica A.O., Kamat V., Badithe A., Fairhurst J., Hunt C., Strein J., Berrebi A., Sisk J.M., et al. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2015;112:8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan C., Wu X., Liu Q., Li Q., Liu S., Lu J., Yang Y., Cao Y., Huang W., Liang C., et al. A human DPP4-knockin mouse’s susceptibility to infection by authentic and pseudotyped MERS-CoV. Viruses. 2018;10 doi: 10.3390/v10090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler E.S., Bailey A.L., Kafai N.M., Nair S., McCune B.T., Yu J., Fox J.M., Chen R.E., Earnest J.T., Keeler S.P., et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21:1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden J.W., Cline C.R., Zeng X., Garrison A.R., Carey B.D., Mucker E.M., White L.E., Shamblin J.D., Brocato R.L., Liu J., et al. Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.142032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathnasinghe R., Strohmeier S., Amanat F., Gillespie V.L., Krammer F., García-Sastre A., Coughlan L., Schotsaert M., Uccellini M.B. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg Microbes Infect. 2020;9:2433–2445. doi: 10.1080/22221751.2020.1838955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun S.H., Chen Q., Gu H.J., Yang G., Wang Y.X., Huang X.Y., Liu S.S., Zhang N.N., Li X.F., Xiong R., et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28:124–133.e4. doi: 10.1016/j.chom.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Zhao J., Li K., Wohlford-Lenane C., Agnihothram S.S., Fett C., Zhao J., Gale M.J., Jr., Baric R.S., Enjuanes L., Gallagher T., et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study transduces replication defective vectors in vivo for generation of mouse model of MERS-CoV for the first time, which also provides a information for the rapid development of animal model in the outbreak of emerging infectious diseases in the future.
- 40.Sun J., Zhuang Z., Zheng J., Li K., Wong R.L., Liu D., Huang J., He J., Zhu A., Zhao J., et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182:734–743.e5. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Israelow B., Song E., Mao T., Lu P., Meir A., Liu F., Alfajaro M.M., Wei J., Dong H., Homer R.J., et al. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217 doi: 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y.N., Li X.D., Zhang Z.R., Zhang H.Q., Li N., Liu J., Li J.Q., Zhang H.J., Wang Z.J., Shen S., et al. A mouse model for SARS-CoV-2 infection by exogenous delivery of hACE2 using alphavirus replicon particles. Cell Res. 2020;30:1046–1048. doi: 10.1038/s41422-020-00405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L., et al. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3 doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinnon K.H., 3rd, Leist S.R., Schäfer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Jr., Hou Y.J., Adams L.E., et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586:560–566. doi: 10.1038/s41586-020-2708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu H., Chen Q., Yang G., He L., Fan H., Deng Y.Q., Wang Y., Teng Y., Zhao Z., Cui Y., et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day C.W., Baric R., Cai S.X., Frieman M., Kumaki Y., Morrey J.D., Smee D.F., Barnard D.L. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395:210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leist S.R., Dinnon K.H., 3rd, Schäfer A., Tse L.V., Okuda K., Hou Y.J., West A., Edwards C.E., Sanders W., Fritch E.J., et al. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell. 2020;183:1070–1085.e12. doi: 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucchiari M.A., Martin J.P., Modolell M., Pereira C.A. Acquired immunity of A/J mice to mouse hepatitis virus 3 infection: dependence on interferon-gamma synthesis and macrophage sensitivity to interferon-gamma. J Gen Virol. 1991;72:1317–1322. doi: 10.1099/0022-1317-72-6-1317. [DOI] [PubMed] [Google Scholar]
- 49.Pope M., Chung S.W., Mosmann T., Leibowitz J.L., Gorczynski R.M., Levy G.A. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2, response, whereas pre-existing antibody partially protects against primary infection. J Immunol. 1996;156:3342–3349. [PubMed] [Google Scholar]
- 50.Williamson J.S., Sykes K.C., Stohlman S.A. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J Neuroimmunol. 1991;32:199–207. doi: 10.1016/0165-5728(91)90189-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Albuquerque N., Baig E., Ma X., Zhang J., He W., Rowe A., Habal M., Liu M., Shalev I., Downey G.P., et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol. 2006;80:10382–10394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 53••.Lee S., Channappanavar R., Kanneganti T.D. Coronaviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Trends Immunol. 2020;41:1083–1099. doi: 10.1016/j.it.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review systematically summarizes the innate immune response, inflammatory body activation, inflammatory cell death pathway and cytokine secretion during SARS-CoV, MERS-CoV and SARS-CoV-2 infection.
- 54•.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that low levels of type I and III interferons juxtaposed to elevated chemokines and high expression of IL-6 are the defining and driving features of COVID-19.
- 55.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J., et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., Ye L., Xu S., Sun R., Wang Y., et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y., Na S.H., Kim M., Song K.H., Bang J.H., et al. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min C.K., Cheon S., Ha N.Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.Y., Inn K.S., Kim J.H., et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6 doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., Sompallae R., McCray P.B., Jr., Meyerholz D.K., Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that relative timing of the IFN-I response and maximal virus replication is key in determining outcomes, at least in infected mice.
- 62.Cervantes-Barragan L., Züst R., Weber F., Spiegel M., Lang K.S., Akira S., Thiel V., Ludewig B. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth-Cross J.K., Bender S.J., Weiss S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zalinger Z.B., Elliott R., Rose K.M., Weiss S.R. MDA5 is critical to host defense during infection with murine coronavirus. J Virol. 2015;89:12330–12340. doi: 10.1128/JVI.01470-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that dysregulated type I Interferon and Inflammatory Monocyte-Macrophage (IMMs) promote lethal SARS-CoV infection and identify IFN-I and IMMs as potential therapeutic targets in patients infected with pathogenic coronavirus and perhaps other respiratory viruses.
- 66.Frieman M.B., Chen J., Morrison T.E., Whitmore A., Funkhouser W., Ward J.M., Lamirande E.W., Roberts A., Heise M., Subbarao K., et al. SARS-CoV pathogenesis is regulated by a STAT1 dependent but a type I, II and III interferon receptor independent mechanism. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahlakõiv T., Ritz D., Mordstein M., DeDiego M.L., Enjuanes L., Müller M.A., Drosten C., Staeheli P. Combined action of type I and type III interferon restricts initial replication of severe acute respiratory syndrome coronavirus in the lung but fails to inhibit systemic virus spread. J Gen Virol. 2012;93:2601–2605. doi: 10.1099/vir.0.046284-0. [DOI] [PubMed] [Google Scholar]
- 68.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L., et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. 2013;19:1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menachery V.D., Yount B.L., Jr., Josset L., Gralinski L.E., Scobey T., Agnihothram S., Katze M.G., Baric R.S. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2’-o-methyltransferase activity. J Virol. 2014;88:4251–4264. doi: 10.1128/JVI.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stertz S., Reichelt M., Spiegel M., Kuri T., Martínez-Sobrido L., García-Sastre A., Weber F., Kochs G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology. 2007;361:304–315. doi: 10.1016/j.virol.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y., Cai H., Pan J., Xiang N., Tien P., Ahola T., Guo D. Functional screen reveals SARS coronavirus nonstructural protein nsp14 as a novel cap N7 methyltransferase. Proc Natl Acad Sci U S A. 2009;106:3484–3489. doi: 10.1073/pnas.0808790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menachery V.D., Gralinski L.E., Mitchell H.D., Dinnon K.H., 3rd, Leist S.R., Yount B.L., Jr., Graham R.L., McAnarney E.T., Stratton K.G., Cockrell A.S., et al. Middle East respiratory syndrome coronavirus nonstructural protein 16 is necessary for interferon resistance and viral pathogenesis. mSphere. 2017;2 doi: 10.1128/mSphere.00346-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng Y., Zhuang M.W., Han L., Zhang J., Nan M.L., Zhan P., Kang D., Liu X., Gao C., Wang P.H. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct Target Ther. 2020;5 doi: 10.1038/s41392-020-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W., Zhou Z., Xiao X., Tian Z., Dong X., Wang C., Li L., Ren L., Lei X., Xiang Z., et al. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell Mol Immunol. 2021;18:945–953. doi: 10.1038/s41423-020-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siu K.L., Kok K.H., Ng M.J., Poon V.K.M., Yuen K.Y., Zheng B.J., Jin D.Y. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J Biol Chem. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y., Zhang L., Geng H., Deng Y., Huang B., Guo Y., Zhao Z., Tan W. The structural and accessory proteins M, ORF 4a, ORF 4b, and ORF 5 of Middle East respiratory syndrome coronavirus (MERS-CoV) are potent interferon antagonists. Protein Cell. 2013;4:951–961. doi: 10.1007/s13238-013-3096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu X., Pan J., Tao J., Guo D. SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes. 2011;42:37–45. doi: 10.1007/s11262-010-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu Y., Ma L., Zhuang Z., Cai S., Zhao Z., Zhou L., Zhang J., Wang P.H., Zhao J., Cui J. Main protease of SARS-CoV-2 serves as a bifunctional molecule in restricting type I interferon antiviral signaling. Signal Transduct Target Ther. 2020;5 doi: 10.1038/s41392-020-00332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vazquez C., Swanson S.E., Negatu S.G., Dittmar M., Miller J., Ramage H.R., Cherry S., Jurado K.A. SARS-CoV-2 viral proteins NSP1 and NSP13 inhibit interferon activation through distinct mechanisms. PLoS One. 2021;16 doi: 10.1371/journal.pone.0253089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wathelet M.G., Orr M., Frieman M.B., Baric R.S. Severe acute respiratory syndrome coronavirus evades antiviral signaling: role of nsp1 and rational design of an attenuated strain. J Virol. 2007;81:11620–11633. doi: 10.1128/JVI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frieman M., Yount B., Heise M., Kopecky-Bromberg S.A., Palese P., Baric R.S. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol. 2007;81:9812–9824. doi: 10.1128/JVI.01012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thornbrough J.M., Jha B.K., Yount B., Goldstein S.A., Li Y., Elliott R., Sims A.C., Baric R.S., Silverman R.H., Weiss S.R. Middle East respiratory syndrome coronavirus NS4b protein inhibits host RNase L activation. mBio. 2016;7 doi: 10.1128/mBio.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lassnig C., Sanchez C.M., Egerbacher M., Walter I., Majer S., Kolbe T., Pallares P., Enjuanes L., Müller M. Development of a transgenic mouse model susceptible to human coronavirus 229E. Proc Natl Acad Sci U S A. 2005;102:8275–8280. doi: 10.1073/pnas.0408589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Higgins P.G., Phillpotts R.J., Scott G.M., Wallace J., Bernhardt L.L., Tyrrell D.A. Intranasal interferon as protection against experimental respiratory coronavirus infection in volunteers. Antimicrob Agents Chemother. 1983;24:713–715. doi: 10.1128/aac.24.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beidas M., Chehadeh W. Effect of human coronavirus OC43 structural and accessory proteins on the transcriptional activation of antiviral response elements. Intervirology. 2018;61:30–35. doi: 10.1159/000490566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai F.W., Stephenson K.B., Mahony J., Lichty B.D. Human coronavirus OC43 nucleocapsid protein binds microRNA 9 and potentiates NF-κB activation. J Virol. 2014;88:54–65. doi: 10.1128/JVI.02678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., Yang P.C. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 2004;10:1062–1066. doi: 10.1111/j.1469-0691.2004.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee N., Chan P.K., Ip M., Wong E., Ho J., Ho C., Cockram C.S., Hui D.S. Anti-SARS-CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J Clin Virol. 2006;35:179–184. doi: 10.1016/j.jcv.2005.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corman V.M., Albarrak A.M., Omrani A.S., Albarrak M.M., Farah M.E., Almasri M., Muth D., Sieberg A., Meyer B., Assiri A.M., et al. Viral shedding and antibody response in 37 patients with middle east respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park W.B., Perera R.A., Choe P.G., Lau E.H., Choi S.J., Chun J.Y., Oh H.S., Song K.H., Bang J.H., Kim E.S., et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21:2186–2189. doi: 10.3201/eid2112.151421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y., Zhang L., Sang L., Ye F., Ruan S., Zhong B., Song T., Alshukairi A.N., Chen R., Zhang Z., et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130:5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 94.Gil-Cruz C., Perez-Shibayama C., Firner S., Waisman A., Bechmann I., Thiel V., Cervantes-Barragan L., Ludewig B. T helper cell- and CD40-dependent germline IgM prevents chronic virus-induced demyelinating disease. Proc Natl Acad Sci U S A. 2012;109:1233–1238. doi: 10.1073/pnas.1115154109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramakrishna C., Stohlman S.A., Atkinson R.D., Shlomchik M.J., Bergmann C.C. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol. 2002;168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- 96.Matthews A.E., Weiss S.R., Shlomchik M.J., Hannum L.G., Gombold J.L., Paterson Y. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J Immunol. 2001;167:5254–5263. doi: 10.4049/jimmunol.167.9.5254. [DOI] [PubMed] [Google Scholar]
- 97.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R., Subbarao K., Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K., Packard M., Shieh W.J., Zaki S., Murphy B. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corti D., Zhao J., Pedotti M., Simonelli L., Agnihothram S., Fett C., Fernandez-Rodriguez B., Foglierini M., Agatic G., Vanzetta F., et al. Prophylactic and postexposure efficacy of a potent human monoclonal antibody against MERS coronavirus. Proc Natl Acad Sci U S A. 2015;112:10473–10478. doi: 10.1073/pnas.1510199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao J., Alshukairi A.N., Baharoon S.A., Ahmed W.A., Bokhari A.A., Nehdi A.M., Layqah L.A., Alghamdi M.G., Al Gethamy M.M., Dada A.M., et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aan5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101•.Wu L.P., Wang N.C., Chang Y.H., Tian X.Y., Na D.Y., Zhang L.Y., Zheng L., Lan T., Wang L.F., Liang G.D. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that SARS-specific antibodies are maintained for an average of 2 years, and significant reduction of immunoglobulin G-positive percentage and titers occurred in the third year.
- 102.Monto A.S., Lim S.K. The Tecumseh study of respiratory illness. VI. Frequency of and relationship between outbreaks of coronavirus infection. J Infect Dis. 1974;129:271–276. doi: 10.1093/infdis/129.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmidt O.W., Allan I.D., Cooney M.K., Foy H.M., Fox J.P. Rises in titers of antibody to human coronaviruses OC43 and 229E in Seattle families during 1975-1979. Am J Epidemiol. 1986;123:862–868. doi: 10.1093/oxfordjournals.aje.a114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104•.Guo L., Wang Y., Kang L., Hu Y., Wang L., Zhong J., Chen H., Ren L., Gu X., Wang G., et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect. 2021;10:664–676. doi: 10.1080/22221751.2021.1905488. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research identifies a two-way cross-reactive antibodies between SARS-CoV-2 and HCoV-OC43, and indicates that there is a correlation between cross-reactive antibody against HCoV-OC43 spike protein and disease severity in COVID-19 patients.
- 105.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yeh K.M., Chiueh T.S., Siu L.K., Lin J.C., Chan P.K., Peng M.Y., Wan H.L., Chen J.H., Hu B.S., Perng C.L., et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107••.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]; This study shows that patients who recovered from SARS possess long-lasting memory T cells that are reactive to the N protein of SARS-CoV 17 years after the outbreak of SARS in 2003, and these T cells displayed robust cross-reactivity to the N protein of SARS-CoV-2.
- 108•.Zhuang Z., Lai X., Sun J., Chen Z., Zhang Z., Dai J., Liu D., Li Y., Li F., Wang Y., et al. Mapping and role of T cell response in SARS-CoV-2-infected mice. J Exp Med. 2021;218 doi: 10.1084/jem.20202187. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that a single dominant epitope-specific memory T cells can protect mouse from SARS-CoV-2 infection.
- 109.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coleman C.M., Sisk J.M., Halasz G., Zhong J., Beck S.E., Matthews K.L., Venkataraman T., Rajagopalan S., Kyratsous C.A., Frieman M.B. CD8+ T cells and macrophages regulate pathogenesis in a mouse model of middle east respiratory syndrome. J Virol. 2017;91 doi: 10.1128/JVI.01825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J., Huang Q., Wang W., Zhang Y., Lv P., Gao X.M. Identification and characterization of dominant helper T-cell epitopes in the nucleocapsid protein of severe acute respiratory syndrome coronavirus. J Virol. 2007;81:6079–6088. doi: 10.1128/JVI.02568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao J., Zhao J., Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu M.S., Pan Y., Chen H.Q., Shen Y., Wang X.C., Sun Y.J., Tao K.H. Induction of SARS-nucleoprotein-specific immune response by use of DNA vaccine. Immunol Lett. 2004;92:237–243. doi: 10.1016/j.imlet.2004.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin H., Xiao C., Chen Z., Kang Y., Ma Y., Zhu K., Xie Q., Tu Y., Yu Y., Wang B. Induction of Th1 type response by DNA vaccinations with N, M, and E genes against SARS-CoV in mice. Biochem Biophys Res Commun. 2005;328:979–986. doi: 10.1016/j.bbrc.2005.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhi Y., Kobinger G.P., Jordan H., Suchma K., Weiss S.R., Shen H., Schumer G., Gao G., Boyer J.L., Crystal R.G., et al. Identification of murine CD8 T cell epitopes in codon-optimized SARS-associated coronavirus spike protein. Virology. 2005;335:34–45. doi: 10.1016/j.virol.2005.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117••.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K., Agnihothram S., Baric R.S., David C.S., Perlman S. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research shows that intranasal but not subcutaneous vaccination induced airway memory CD4+ T cells are protective in SARS-CoV and MERS-CoV infection, and cross-reactive T cell responses induced by conserved epitope in SARS-CoV and MERS-CoV protect mice from infection.
- 118•.Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that some variants can partially escape humoral immunity induced by SARS-CoV-2 infection or BNT162b2 vaccination, but S-specific CD4+ T-cell activation is not affected by the mutations in the B.1.1.7 and B.1.351 variants.
- 119.Butler N., Pewe L., Trandem K., Perlman S. Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology. 2006;347:410–421. doi: 10.1016/j.virol.2005.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120•.Zhao J., Zhao J., Van Rooijen N., Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that lethal disease in mice infected with SARS-CoV is correlated with a lack of activation of alveolar macrophages and respiratory dendritic cells that induce poor T cell response.
- 121•.Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that diminished respiratory dendritic cell migration associated with virus-specific defects in T cell responses in aging mice is not a result of cell-intrinsic defect, rather it reflected the observed age-dependent increases in PGD(2) expression.
- 122.Vijgen L., Keyaerts E., Moës E., Maes P., Duson G., Van Ranst M. Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. J Clin Microbiol. 2005;43:5452–5456. doi: 10.1128/JCM.43.11.5452-5456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baric R.S. Emergence of a highly fit SARS-CoV-2 variant. N Engl J Med. 2020;383:2684–2686. doi: 10.1056/NEJMcibr2032888. [DOI] [PubMed] [Google Scholar]
- 124.Makoni M. South Africa responds to new SARS-CoV-2 variant. Lancet. 2021;397 doi: 10.1016/S0140-6736(21)00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jia W., Channappanavar R., Zhang C., Li M., Zhou H., Zhang S., Zhou P., Xu J., Shan S., Shi X., et al. Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection. Emerg Microbes Infect. 2019;8:760–772. doi: 10.1080/22221751.2019.1620083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim M.H., Kim H.J., Chang J. Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220196. [DOI] [PMC free article] [PubMed] [Google Scholar]