Abstract
Metastatic tumors account for 5-10% of all ovarian malignancies. They are usually bilateral tumors with a multinodular surface and extensive extra ovarian spread. Lung cancer is a rare source (0.3% of metastatic ovarian tumors). Among synchronous primary cancers, ovarian cancer is most frequently associated with endometrial cancer. The differential diagnosis between a primary ovarian carcinoma, synchronous primary cancers, and metastatic ovarian carcinoma is very important, as the treatment and prognosis are markedly different.
We report the case of a 25-year-old woman who had been diagnosed and treated for stage IIIB small cell lung carcinoma (SCLC). Imaging undertaken for abdominal pain revealed a unilateral 8.5 cm ovarian tumor for which adnexectomy was performed. Histology and immunohistochemistry led to the diagnosis of ovarian metastasis from SCLC, a high-grade neuroendocrine lung tumor. This patient’s particular features, all infrequent in a metastatic tumor, are the lesion’s unilaterality (atypical for ovarian metastases in other cancers, but often observed in SCLC), the smooth ovarian surface with intact capsule, and the absence of intra-abdominal dissemination. The patient developed liver and vertebral metastases.
This report focuses on the differential diagnosis between primary and metastatic ovarian neoplasms. We performed an extensive search of the literature on SCLC and ovarian metastases. Immunohistochemistry is essential for diagnosis when imaging and the pathological evaluation of the ovarian tumor cannot make the differential diagnosis.
Keywords: ovarian metastasis, small cell lung carcinoma, neuroendocrine tumor
INTRODUCTION
Worldwide, breast and colorectal cancers represent the first and second most common cancers in women, followed by lung cancer (8.4% of the total new cancer cases in 2018) (1). Small cell lung cancer (SCLC) accounts for 10-15% of all lung malignancies (2). Many patients with lung cancer have advanced-stage cancer at clinical presentation due to the aggressive biology and the absence of symptoms until the locally advanced or metastatic disease is present. Extrathoracic metastases of lung cancer can be found in any part of the body, most frequently in the liver, adrenal glands, bones, and brain (3,4). The ovary is an uncommon site of lung cancer metastasis, accounting for 2-4% of all metastatic ovarian masses (2,5). Ovarian metastases primary originate from the digestive system (colon cancer, gastric cancer or appendix cancer) or from the breast (6).
Neuroendocrine tumors (NETs) are a group of neoplasms that arise from the neuroendocrine system cells found in most human tissues (7). Lung NETs are classified into four subtypes characterized by increasing biological aggressiveness: typical carcinoids (TCs), atypical carcinoids (ACs), large-cell neuroendocrine carcinomas (LCNECs), and small-cell lung cancers (SCLCs). LCNECs and SCLCs are high-grade NETs (3,8,9). Lung NETs account for approximately 20–30% of all NETs and represent about 25% of lung cancers. Of these, SCLC represents 15–20% and LCNEC 3–5% (4).
The imaging techniques and the ovarian tumor’s pathologic analysis can often be unreliable for the differential diagnosis between primary or metastatic cancer. In such cases, the use of appropriate immunohistochemical markers can provide additional evidence to differentiate primary from metastatic neoplasms (2,5).
An extensive search was carried out using the following keywords: “small cell lung carcinoma,” “neuroendocrine lung tumors,” and “ovarian metastasis.” In the selection process, we have focused on clinical trials (CT), meta-analyses (MA), randomized clinical trials (RCT), and systematic reviews (SR). We analyzed the type of lesion, investigations, type of treatment regimens, histological and immunohistochemical findings, complications, and prognosis.
The search of the literature published in the last 20 years in the PubMed® database retrieved 102 results filtered to 15 reports and in the Web of Science database retrieved 169 results filtered to 5 reports refined to “human” (Fig. 1).
Figure 1.
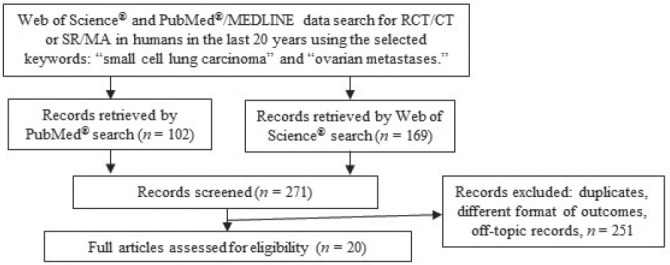
Flow diagram - search of databases and study selection process.
We present the diagnosis difficulties in a young patient with unilateral ovarian metastasis from SCLC, with a survival duration of less than 3 years.
CASE PRESENTATION
A 25 years old woman presented in 2015 to the Department of Pneumology for persistent cough and was diagnosed with a lung tumor. A thoracic CT scan made in August 2015 revealed the following features:
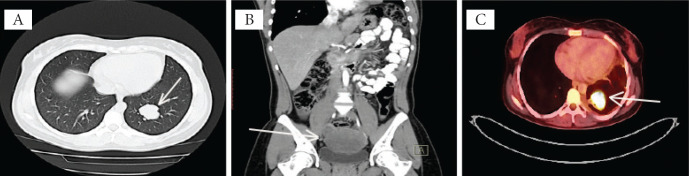
- a hyperdense iodophilic thoracic mass in the posterior basal segment of the left lung’s inferior lobe, measuring 35/36 mm, with a regular shape and well-defined borders (Fig. 2 A);
Figure 2.
Chest plain computed tomography revealed (A) a hyperdense iodophilic thoracic mass in the posterior basal segment of the inferior lobe of the left lung and (B) a hypodense, low enhancing mass in the 7th segment of the right lobe of the liver, with a regular shape and well-defined borders; and a hypodense homogeneous periuterine mass. (C) Fluorodeoxyglucose (FDG)-positron emission tomography revealed high FDG uptake in the left lung in the posterior basal segment of the inferior lobe and in the superior mediastinum.
- a large mediastinal mass, measuring 67/45 mm, with irregular shape and relatively well-defined borders suggestive of a mediastinal adenopathy block. The mass had a compressive effect over the left pulmonary artery and vein and left bronchus.
- a fibrotic-like lesion of 44/33 mm in the anterior apical segment of the left lung’s superior lobe.
The whole-body PET/CT scan made in September 2015 confirmed the presence of a left lung mass in the posterior basal segment of the inferior lobe and a superior mediastinal mass (Fig. 2 C), both with a high uptake of fluorodeoxyglucose - FDG (SUV max of 15.7 and 12.8, respectively).
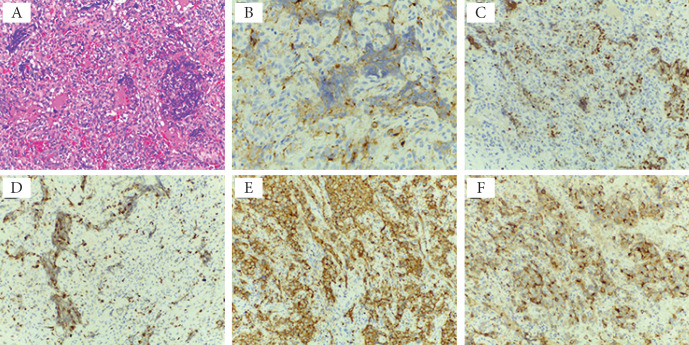
In September 2015, the patient had a biopsy of the mediastinal adenopathic block. The pathology of tissue fragments revealed a malignant cell proliferation composed of round, monomorphic, eosinophilic cells, with poor cytoplasm, intense vascular proliferation of small vessels, and large areas tumor necrosis. The cells had a high mitotic index (Fig. 3 A). The immunohistochemical staining was performed with the following results (Fig. 3 B-F):
Figure 3.
Histological and immunohistochemical findings of the mediastinal adenopathic block. A Biopsy tissue of the mediastinal adenopathy block: malignant cell proliferation composed of round, monomorphic, eosinophilic cells, with poor cytoplasm, the intense vascular proliferation of small vessels, and large areas of tumor necrosis. The cells have a high mitotic index (H&E staining, ×20). Immunohistochemistry findings - immunoreactivity of tumor cells with antibodies to chromogranin (B immunostain, ×40), TTF-1 (C immunostain, ×20), Ki67 (D immunostain, ×20), synaptophysin (E immunostain, ×20), and EMA (F immunostain, ×20).
- Cytokeratin AE1/AE3 was slightly positive; Chromogranin A, thyroid transcription factor 1 (TTF1), epithelial membrane antigen (EMA) positive; Synaptophysin intensely positive; caudal type homeobox 2 protein (CDX2), calcitonin, carcinoembryonic antigen (CEA) negative; 35% of the tumor cells expressed Ki67;
- CD34, CD31, factor VIII-related antigen (FVIII) were negative in the tumor cells and positive in the vessel cells.
The pathology and immunohistochemical findings were suggestive of SCLC, a high-grade neuroendocrine lung tumor. The initial assessment was suggestive of a stage IIIB SCLC.
The patient was treated with 6 cycles of chemotherapy (etoposide and cisplatin) and with intensity-modulated radiation therapy, using a total dose of 60 Gy divided into 30 fractions. The treatment was well tolerated and the patient was apparently well until September 2016.
The surveillance following the initial treatment consisted of physical examination and repeated imaging studies. The cerebral, chest and abdominal computed tomography (CT) scan made in September 2016 revealed the presence of new pulmonary lesions: a 5 mm thoracic mass in the 7th segment of the inferior lobe of the left lung and a 7 mm iodophilic thoracic mass in the apical segment of the inferior lobe of the right lung, surrounded by a pseudonodular area measuring 20/16 mm (i.e. the presence of the halo sign). The lesion was suggestive of metastatic disease.
A second course of chemotherapy was started in September 2016, with Epirubicin, 5-Fluorouracyl and Dacarbazine, for 3 months.
In December 2016 the patient was investigated in the Department of Gynecology of “Filantropia” Clinical Hospital for moderate diffuse abdominal pain, persistent in the preceding month. The physical examination revealed a palpable, mobile, slightly painful left adnexal mass and the presence of a thoracic scar after a mediastinal biopsy, with no signs of carcinoid syndrome or paraneoplastic syndromes (inappropriate secretion of vasopressin or ectopic adrenocorticotropic hormone production). The patient had no risk factors for ovarian or lung cancer, such as a family history of ovarian or lung cancer, smoking, infertility, endometriosis, obesity, or polycystic ovarian syndrome (5,10).
Abdominal and pelvic ultrasonography made in December 2016 revealed:
- a hypoechoic lesion in the 7th segment of the liver’s right lobe, measuring 23/17 mm, surrounded by an anechoic halo; the lesion had a weak Doppler signal; the aspect was suggestive of a new metastatic nodule;
- a left adnexal hypoechoic mass, measuring 85/67 mm, with intense Doppler flow in the outer part of the tumor and mild Doppler flow in the central part of the tumor.
The patient declared that a previous ultrasonography done 3 months before (unavailable images) showed no pelvic mass, thus suggesting a fast-growing tumor.
A chest and abdominal plain CT scan performed in December 2016 showed a hypodense homogeneous periuterine mass, measuring 84/87 mm, and a newly occurred hypodense, low enhancing mass in the 7th segment of the right lobe of the liver, measuring 1.8 cm, with a regular shape and well-defined borders (interpreted as liver metastasis, although the CT features were not specific for malignancy, in discordance with the ultrasound description); (Fig. 2 B); there were no changes of the initial lesions.
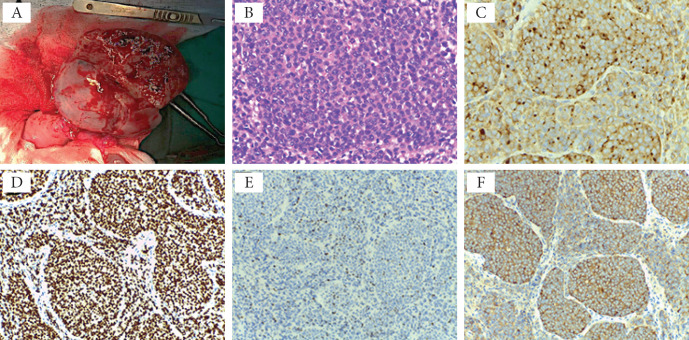
Based on the patient’s medical history and imaging results, the decision was to perform an exploratory laparotomy. In December 2016, we found a left ovarian tumor during the surgical intervention, measuring 9/8 cm with a friable consistency and a regular surface (Fig. 4 A). Pelvic and abdominal exploration was carried out, assessing the organs’ status. No lesions were observed on the uterus, fallopian tubes, right ovary, nor in the peritoneal cavity. Peritoneal washings for cytology were collected. A unilateral left adnexectomy was performed, and the specimen was sent for pathologic examination. The patient had a good postoperative evolution.
Figure 4.
Histological and immunohistochemical findings of the left ovarian metastasis. Surgical sampling: small cell proliferation with the invasive proliferation of heterozygous cells with a high N/C ratio is observed in the region. The nuclear chromatin is in the form of fine granules, and nucleoli are not noticeable (A) H&E staining, ×40). Immunohistochemistry findings of the left ovarian tumor - immunoreactivity of tumor cells with antibodies to B) chromogranin A (×40), C) TTF-1 (×20), D) Ki67 (×20), and E) synaptophysin (×20); F) specimen of surgical sampling.
Intraoperative histopathologic exam of the frozen sections indicated a poorly differentiated small cell proliferation (Fig. 4 B) and could not make the differential diagnosis between a primary and a metastatic ovarian tumor. The final histopathologic evaluation revealed a solid, well encapsulated, soft, 8 cm tumor with a grey surface. There was no evidence of peritoneal dissemination at the cytologic evaluation of the peritoneal fluid.
The immunohistochemical staining of the tumor tissue revealed (Fig. 4 C-F):
- 30% of the tumor cells expressed Ki67;
- Chromogranin A, TTF1, Synaptophysin positive;
- Estrogen receptor (ER) negative;
- Wilms tumor protein 1 (WT1), cytokeratin Ck7 positive in a small number of tumor cells.
The immunohistochemical findings were suggestive for an ovarian metastasis from the SCLC.
During the follow-up period after the surgical intervention, the patient presented sudden posterior cervical pain and consulted the Neurology department. In January 2017, a spine MRI scan showed two right paravertebral masses, one at level C6-C7, measuring 16/13/18 mm, and the other at level C7-T1, measuring 17/13/16 mm. Serum biomarkers showed elevated CEA 7.62 ng/mL (normal value for smokers < 4.9 ng/mL and for nonsmokers <2.5 ng/mL) and neuron-specific enolase (NSE) 72.61 μg/L (normal value < 13 μg/L), normal level of CA125. A diagnosis of vertebral metastases from lung cancer was made. The patient resumed chemotherapy with Epirubicin, 5-Fluorouracyl and Dacarbazine for 6 more cycles in April 2017, and zoledronic acid was added. In November 2017 she was treated with palliative intensity-modulated radiation therapy, using a dose of 30 Gy administered in 10 fractions. There was clinical and imaging evidence of disease progression until January 2018, when the patient deceased.
DISCUSSION
Ovarian complex masses are generally primary carcinomas and less frequently metastases from extra-gynecological tumors, such as the stomach, colon, breast, pancreas, kidney adenocarcinomas (11). Small cell carcinoma (SCC) of the female genital tract is a rare condition, accounting for less than 2% of all gynecological malignancies (12). It occurs most frequently in the cervix but can also occur in the endometrium, ovary, fallopian tube, vagina, and vulva. Most of them are represented by secondary gastrointestinal tumors. Ovarian metastasis from lung cancer represents only 2-4% of all metastatic ovarian masses (5).
As treatment and prognosis are different, distinguishing between a primary and metastatic ovarian neoplasm is crucial for an adequate therapeutic strategy. The microscopic elements of SCC of the genital tract are not distinguishable from SCC of the lungs. Differential diagnosis is based on the expression of neuroendocrine markers and histological growth patterns (13).
In primary ovarian cancers, there is a high frequency of unilateral ovarian involvement. In contrast, bilaterality in approximately three-fourths of the cases and multinodularity are cardinal features of ovarian metastases, in general (2). The particular features in the reported case are the lesion’s unilaterality, the smooth ovarian surface with intact capsule, and no intra-abdominal dissemination. These characteristics are all infrequent for a metastatic ovarian tumor (2). However, it should be noted that in SCLC the ovarian metastases appear to be mostly unilateral, as seen in the literature case reports summarized in Table 1 and in other case series (14).
Table 1.
A synopsis of literature case reports on lung cancer with ovarian metastasis
| Study | N | Lesion | Investigations | Treatment | HP+IHC | Complications |
| Phadke22 1999 |
1 | Abdominal pelvic mass from the pelvis to the xiphoid process |
|
|
|
|
| Bing20 2005 |
Right ovarian mass 20cm |
|
|
|
|
|
| Garcia21 2010 |
1 |
|
|
|
|
|
| Losito5 2013 |
1 | Fast growing pelvic mass |
|
|
|
|
| Oneda17 2020 |
1 | 6/4cm mass in the left pelvic region |
|
|
|
|
Legend: SCLC (small cell lung cancer); CT (computed tomography); MRI (magnetic resonance imaging); Histopathology (HP); Immunohistochemistry (IHC); SCOPT - small cell carcinoma of the ovary of pulmonary type.
Metastatic tumors of the ovary account for approximately 5-10% of all ovarian malignancies (9,11). In some cases, the metastatic tumor can be the initial manifestation of a patient’s cancer, causing difficulties in the differential diagnosis with a primary ovarian neoplasm, even after microscopic examination.
An adnexal mass may be symptomatic or incidentally discovered on pelvic or imaging examination. The diagnosis is challenging in a young and non-smoker patient, without any risk factors for SCLC, because SCLC’s incidence is lower in non-smokers. In women of reproductive age, the differential diagnosis of an adnexal mass is usually difficult. Moreover, women with ovarian cancer often have one or more nonspecific symptoms, such as lower abdominal pain or pressure, bloating, constipation, vaginal bleeding or discharge, urinary frequency, or dyspareunia. The advanced stages of the disease can be associated with abdominal distention, nausea, anorexia, or early satiety due to the presence of ascites and abdominal metastases (15). In our patient, a moderate abdominal pain was the only symptom related to her ovarian tumor.
Small-cell lung cancer (SCLC) accounts for 15% of lung cancers, and unfortunately, two-third of patients are diagnosed at extensive-stage (13). SCLC is an aggressive form of malignancy derived from the precursors of neuroendocrine cells. It is characterized by rapid progression with early metastases and resistance to treatment (15).
Lung cancer is a rare origin of ovarian metastases. The analysis of the few reported cases of ovarian metastasis from lung cancer revealed that the primary lung tumor was small cell carcinoma (in 45% of cases), adenocarcinoma (in 32.5%), and large cell carcinoma (in 12.5%) (16). In more than half of the cited cases, lung cancer was detected before the ovarian tumor (as in our patient); in one-third of the cases, the lung and ovarian tumors were seen simultaneously. In the remaining cases, the cancer was first detected in the ovary and then in the lung (2,16,17).
Imaging studies of the ovary cannot distinguish between a primary malignant tumor and a metastatic tumor. Several sonographic findings may predict malignancy in an adnexal mass: an irregular multilocular solid tumor with the largest diameter ≥100 mm, the presence of ascites, strong blood flow at Doppler examination, the presence of at least four papillary structures. A Sassone scoring system was developed to differentiate benign and malignant adnexal masses. The score considers the inner wall structure, wall thickness, septa presence, and the tumor’s echogenicity. A score ≥ 9 indicates a malignant tumor (18), as it was the case in our patient, in whom the Sassone score was 9.
For the diagnosis of ovarian tumors, PET/CT is superior to pelvis US, abdominopelvic CT, and pelvic MRI and is valuable in revealing ovarian metastasis (7). In ovarian cancer, PET/CT can detect lymph node and distant metastasis staging with high accuracy. Furthermore, PET/CT evaluates treatment response, prognostic rate, pre-operative conditions and detects recurrent disease. Therefore, PET CT/MRI may be a powerful tool for tumor staging (19).
In the presence of synchronous tumors, imaging techniques (ultrasound, computed tomography, magnetic resonance), and even conventional morphology are often inadequate for a reliable diagnosis. In these circumstances, the use of appropriate immunohistochemical markers can provide additional evidence to differentiate primary from metastatic neoplasms (20).
A variety of ovarian tumors may have a pattern of small cells. These include granulosa cell tumor, primary small cell carcinoma (variably positive for AE1/AE3, EMA, CD10, calretinin, WT1, and p53), metastatic small cell carcinoma (positive for TTF-1), dysgerminoma (positive for placental alkaline phosphatase PLAP and octamer-binding transcription factor 4 OCT4), lymphoma (positive for CD45), and immature teratoma (positive for neurofilament protein, synaptophysin, NSE) (20-22). Immunohistochemical studies can establish a definitive diagnosis when the ovarian tumor’s pathologic evaluation cannot make the differential diagnosis (8,23).
Squamous cell carcinomas and large cell lung carcinomas are not frequently positive for TTF-1. Small cell carcinomas (of any primary site) are usually positive. The high rate of TTF-1 expression in extrapulmonary small cell carcinomas can make it difficult to distinguish between primary and metastatic small cell malignancies, especially when the site of the primary tumor is not known (10,24).
Synaptophysin and chromogranin A act as markers for neuroendocrine tumors (23,16). Also, in SCLCs neuroendocrine cells, these markers are expressed from the lung epithelium (25). Chromogranin A levels depend on the tumor size and increase as the tumor becomes larger. Approximately 40% of primary ovarian carcinomas are estrogen receptor (ER) and/or progesterone receptor (PR) positive (16). WT1 and CA125 are associated with ovarian carcinoma, but their expression varies with different histologic subtypes. WT1 is expressed in most primary ovarian serous adenocarcinomas but is negative in other histological subtypes (23,20).
Small cell tumors with neuroendocrine differentiation, such as lung cancer, neuroblastoma, and neuroendocrine tumors, produce NSE (neuron-specific enolase) (26,27). While Ki-67 has primarily been used in gastroenteropancreatic NETs for assisting therapy decisions (it was shown to be highly predictive), the same marker does not play an established role in the diagnosis, grading, and prognosis of lung NETs. Available data from studies suggest that Ki-67 may have a prognostic role in lung NETs, although more information is needed to determine its ideal use in this tumor setting (19,28).
In our case, immunohistochemical studies revealed positive staining for chromogranin A, TTF1, synaptophysin. WT1, Ck7 were positive in a small number of tumor cells. In serum, both CEA and NSE were elevated. Considering the results of the immunohistochemical and pathologic evaluation of the ovarian tumor, the definitive diagnosis was ovarian metastasis from SCLC, a high-grade aggressive neuroendocrine tumor as suggested by the high Ki-67 immunostaining. Another study related that Ki-67 expression level decreased in patients with extensive-stage SCLC with a major impact on overall survival (16). Available data from studies suggest that Ki-67 may have a prognostic role in lung NETs, although more information is needed to determine its ideal use in this tumor setting (28,29).
Cancer antigen CA125 has a low overall specificity and sensitivity. Elevation of serum CA125 is associated with conditions other than ovarian cancer in reproductive-age women. In our patient, CA125 was within the normal range. Serum CEA levels may be elevated in the ovary’s mucinous cystadenoma and are negative in all serous adenocarcinomas (7,11).
SCLC is a major clinical problem associated with an aggressive course with a poor prognosis, a decreased disease-free duration, and a short overall survival rate (30). Our patient’s overall survival rate was unusually high (about 3 years) since an extensive stage of SCLC with metastasis to the liver alone or in combination with other organs seems to have worse outcomes (31). A recent study by Liu reported a median overall survival (OS) of 12.3 months in patients treated with atezolizumab plus carboplatin plus etoposide (CP/ET), compared with 10.3 months treated with placebo plus CP/ET (32).
One study revealed that limited-stage SCLC patients benefit from the standard therapy (cisplatin-etoposide plus radiotherapy), while in extensive-stage SCLC the addition of radiotherapy to chemotherapy is not recommended as standard practice (33). Another study, in extensive-stage, recommended chemotherapy with platinum-etoposide plus thoracic radiotherapy followed by prophylactic cranial irradiation in patients without progression (34). The addition of immunotherapy to chemotherapy increased the overall survival (35,36). Further research is needed because the 5-year OS is still low despite the new treatment strategies in SCLC.
In conclusion, the diagnosis of metastatic ovarian tumors can be difficult, especially when the metastasis has similar imagistic and pathologic features with a primary ovarian tumor. The diagnosis of ovarian metastasis is even more complicated when the primary malignancy site is unknown. In the case of lung cancer, the ovary is an uncommon site of metastasis. The differential diagnosis between primary and secondary ovarian tumors is essential, as the treatment and prognosis are different. The immunohistochemical studies represent the most reliable way of making a definitive diagnosis in such cases.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol. 2017;12(3):425–436. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 3.Lee KA, Lee JS, Min JK, Kim HJ, Kim WS, Lee KY. Bilateral Ovarian Metastases from ALK Rearranged Non-Small Cell Lung Cancer. Tuberc Respir Dis (Seoul) 2014;77(6):258–261. doi: 10.4046/trd.2014.77.6.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young RH, Scully RE. Ovarian metastases from cancer of the lung: problems in interpretation--a report of seven cases. Gynecol Oncol. 1985;21(3):337–350. doi: 10.1016/0090-8258(85)90272-0. [DOI] [PubMed] [Google Scholar]
- 5.Losito NS, Scaffa C, Cantile M, Botti G, Costanzo R, Manna A, Franco R, Greggi S. Lung cancer diagnosis on ovary mass: a case report. J Ovarian Res. 2013;6(1):34. doi: 10.1186/1757-2215-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung YE, Lee JW, Kim BG, Bae DS. Ovarian metastasis from pulmonary adenocarcinoma. Obstet Gynecol Sci. 2013;56(5):341–344. doi: 10.5468/ogs.2013.56.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancers (Basel) 2012;4(3):777–798. doi: 10.3390/cancers4030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol. 2012;25(Suppl 1):S18–30. doi: 10.1038/modpathol.2011.150. [DOI] [PubMed] [Google Scholar]
- 9.Münstedt K, Estel R, Dreyer T, Kurata A, Benz A. Small Cell Ovarian Carcinomas - Characterisation of Two Rare Tumor Entities. Geburtshilfe Frauenheilkd. 2013;73(7):698–704. doi: 10.1055/s-0032-1328658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13(3):238–242. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]
- 11.Cengiz H, Yıldız S, Kaya C, Senyürek E, Ekin M, Yasar L. Ovarian metastasis from lung cancer: a rare entity. Case Rep Obstet Gynecol. 2013;2013 doi: 10.1155/2013/378438. 378438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowder S, Tuller E. Small cell carcinoma of the female genital tract. Semin Oncol. 2007;34(1):57–63. doi: 10.1053/j.seminoncol.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Hochberg L, Hoffman M. Levine D, Goff B, editors. Differential Diagnosis of the Adnexal Mass. Ed. UptoDate®, http://www.uptodate.com, downloaded Oct 2020.
- 14.Irving JA, Young RH. Lung carcinoma metastatic to the ovary: a clinicopathologic study of 32 cases emphasizing their morphologic spectrum and problems in differential diagnosis. Am J Surg Pathol. 2005;29(8):997–1006. [PubMed] [Google Scholar]
- 15.Basumallik N, Agarwal M. Small Cell Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island (FL) 2021 [PubMed] [Google Scholar]
- 16.Kriplani D, Patel MM. Immunohistochemistry: A diagnostic aid in differentiating primary epithelial ovarian tumors and tumors metastatic to the ovary. South Asian J Cancer. 2013;2(4):254–258. doi: 10.4103/2278-330X.119888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oneda E, Zorzi F, Gorio A, Quaglia F, Abeni C, Rota L, Zaniboni A. Differential Diagnosis of Small Cell Carcinoma of the Ovary or Ovarian Metastases of Small Cell Carcinoma of the Lung: A Case Report and Review of the Literature. Case Rep Oncol. 2020;13(2):822–828. doi: 10.1159/000507978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shende V, Kamat A, Raut S, Jagtap P, Bobade P, Kuthe S. Sassone Scoring System in Differentiating Benign and Malignant Adnexal Masses. Indian Journal of Applied Research 6. 2016 [Google Scholar]
- 19.Berek JS, Kehoe ST, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2018;143(Suppl 2):59–78. doi: 10.1002/ijgo.12614. [DOI] [PubMed] [Google Scholar]
- 20.Bing Z, Adegboyega PA. Metastasis of small cell carcinoma of lung into an ovarian mucinous neoplasm: immunohistochemistry as a useful ancillary technique for diagnosis and classification of rare tumors. Appl Immunohistochem Mol Morphol. 2005;13(1):104–107. doi: 10.1097/00129039-200503000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Garcia V, Velasco A, Gatius S, Gonzalez FJ, Matias-Guiu X. Pulmonary small cell carcinoma metastatic to the ovary: a clinicopathologic study of one case with emphasis on the importance of p53 analysis in diagnosis. Gynecol Obstet Invest. 2010;70(2):87–90. doi: 10.1159/000292911. [DOI] [PubMed] [Google Scholar]
- 22.Phadke DM, Weisenberg E, Engel G, Rhone DP. Malignant Sertoli cell tumor of the ovary metastatic to the lung mimicking neuroendocrine carcinoma: report of a case. Ann Diagn Pathol. 1999;3(4):213–219. doi: 10.1016/s1092-9134(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 23.Mittal K, Soslow R, McCluggage WG. Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med. 2008;132(3):402–423. doi: 10.5858/2008-132-402-AOITGP. [DOI] [PubMed] [Google Scholar]
- 24.Lee-May C, Jonathan SB. Epithelial Carcinoma of the Ovary, Fallopian Tube, and Peritoneum: Epidemiology and Risk Factors in UpToDate. In: Chakrabarti A, editor. UpToDate. Waltham: MA. (Accessed on May 04, 2021) 2019. [Google Scholar]
- 25.Park KS, Liang MC, Raiser DM, Zamponi R, Roach RR, Curtis SJ, Walton Z, Schaffer BE, Roake CM, Zmoos AF, Kriegel C, Wong KK, Sage J, Kim CF. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10(16):2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fizazi K, Farhat F, Theodore C, Rixe O, Le Cesne A, Comoy E, Le Chevalier T. Ca125 and neuron-specific enolase (NSE) as tumour markers for intra-abdominal desmoplastic small round-cell tumours. Br J Cancer. 75(1):76–78. doi: 10.1038/bjc.1997.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo) 2018;73(suppl 1) doi: 10.6061/clinics/2018/e490s. e490s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang HY, Li ZW, Sun W, Yang X, Zhou LX, Huang XZ, Jia L, Lin DM. Automated quantification of Ki-67 index associates with pathologic grade of pulmonary neuroendocrine tumors. Chin Med J (Engl) 2019;132(5):551–561. doi: 10.1097/CM9.0000000000000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pei R, Zhang L, Xie C, Lu Z, Wang G, Yang Z. Prognostic value of Ki-67 expression in patients with extensive-stage small cell lung cancer. Future Oncol. 2017;13(14):1247–1252. doi: 10.2217/fon-2016-0542. [DOI] [PubMed] [Google Scholar]
- 30.Motas N, Motas C, Davidescu M, Achim D, Rus O, Jianu E, Horvat T. Neuroendocrine tumors of the lung with surgical resection and lymph node dissection in a tertiary thoracic surgery center. Acta Endocrinol (Buchar) 2018;14(2):219–226. doi: 10.4183/aeb.2018.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H, Wang H, Li Z, Lin J, Yu J. The prognostic analysis of different metastatic patterns in extensive-stage small-cell lung cancer patients: a large population-based study. Future Oncol. 2018;14(14):1397–1407. doi: 10.2217/fon-2017-0706. [DOI] [PubMed] [Google Scholar]
- 32.Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133) J Clin Oncol. 2021;39(6):619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun A, Durocher-Allen LD, Ellis PM, Ung YC, Goffin JR, Ramchandar K, Darling G. Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol. 2019;26(3):e372–e384. doi: 10.3747/co.26.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dómine M, Moran T, Isla D, Martí JL, Sullivan I, Provencio M, Olmedo ME, Ponce S, Blasco A, Cobo M. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019) Clin Transl Oncol. 2020;22(2):245–255. doi: 10.1007/s12094-020-02295-w. [DOI] [PubMed] [Google Scholar]
- 35.Tsiouprou I, Zaharias A, Spyratos D. The Role of Immunotherapy in Extensive Stage Small-Cell Lung Cancer: A Review of the Literature. Can Respir J. 2019;2019 doi: 10.1155/2019/6860432. 6860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konala VM, Madhira BR, Ashraf S, Graziano S. Use of Immunotherapy in Extensive-Stage Small Cell Lung Cancer. Oncology. 2020;98(11):749–754. doi: 10.1159/000508516. [DOI] [PubMed] [Google Scholar]