Abstract
Over the past two decades, biodegradable metals (BMs) have emerged as promising materials to fabricate temporary biomedical devices, with the purpose of avoiding potential side effects of permanent implants. In this review, we first surveyed the current status of BMs in neuroscience, and briefly summarized the representative stents for treating vascular stenosis. Then, inspired by the convincing clinical evidence on the in vivo safety of Mg alloys as cardiovascular stents, we analyzed the possibility of producing biodegradable cerebrovascular Mg alloy stents for treating ischemic stroke. For these novel applications, some key factors should also be considered in designing BM brain stents, including the anatomic features of the cerebral vasculature, hemodynamic influences, neuro-cytocompatibility and selection of alloying elements. This work may provide insights into the future design and fabrication of BM neurological devices, especially for brain stents.
Keywords: Biodegradable metals, Neuroscience, Mg alloys, Brain stents, Ischemic stroke
Graphical abstract
Mg-based alloys have been used in neuroscience as filaments within nerve conduits to accelerate nerve regeneration, nerve electrode and devices for neural recording and monitoring, and stents for carotid artery stenosis and aneurysm treatment. Inspired by the convincing clinical evidence on the in vivo safety of Mg alloys as cardiovascular stents, we analyzed the possibility of producing biodegradable cerebrovascular Mg alloy stents for ischemic stroke treatment.
Highlights
-
•
The current status of the application of biodegradable metals (BM) in neuroscience was presented.
-
•
We analyzed the possibility of producing biodegradable cerebrovascular Mg alloy stents for ischemic stroke treatment.
-
•
Key factors in designing BM brain stents were discussed.
-
•
This work may provide insights into the future design and fabrication of BM neurological devices, especially for brain stents.
1. Introduction
Biodegradable metals (BMs), represented by magnesium-, zinc-, and iron-based alloys, are expected to degrade or corrode gradually in vivo after performing their supportive assisting functions during tissue healing or disease diagnosis, under the influence of appropriate host responses. Compared with their polymeric counterparts, BMs possess higher mechanical strength and show better performance as cardiovascular stents and bone implants [[1], [2], [3]]. Several reviews have presented the state-of-the-art technologies in developing BMs-based degradable biomedical implants [[4], [5], [6], [7]]. However, their application in neuroscience is still in its infancy.
Cerebrovascular disease is the leading cause of death in China and the 2nd leading cause of death globally, exerting a heavy burden on public health [8]. Their primary pathologies include the obstruction or rupture of brain blood vessels, which leads to ischemic or hemorrhagic stroke, respectively. In addition to traditional drug therapies, the neuro-interventional approach for cerebrovascular disease treatment is much more effective with improved outcomes [9]. Existing endovascular devices include coils, stents, flow diverters, and stent retrievers [10], which are mainly made of NiTi alloys, Co alloys or 316 L stainless steel, with good biomechanical compatibility for brain vessels and cytocompatibility for nerve cells. However, the constant presence of an inherent non-degradable metallic stent can impair vasomotor response and induce a prolonged inflammatory reaction and thrombosis [11]. In addition, the required dual anti-platelet therapy may increase intracranial bleeding risks. In the scenario of cardiovascular stenosis, the biodegradable stent is developed to tackle those side effects, which is considered to be the fourth revolution in interventional cardiology [12]. When it comes to cerebrovascular disease treatment, is it possible to make biodegradable metallic endovascular devices? Before answering this question, an overview of the use of BMs in neuroscience as well as their application in cardiology is critical.
Currently, with the development of biodegradable materials, novel biodegradable brain implants are fabricated, such as intracranial electronic sensors [13], wireless drug delivery device for brain tumors [14], optical sensors for pressure and temperature monitoring [15] and electrode array for electrophysiological recording [16]. Early efforts on making these bioresorbable devices were focused on biodegradable polymers and subsequently on silicon-based semiconductors [17,18]. The application of BMs in neuroscience is still in its infancy, and some of the BMs have only been exploited as device substrates for brain implants in the form of thin foils [19].
In this review, we first surveyed the current research of BMs in neuroscience, and briefly summarized the representative stents for vascular stenosis treatment. Then we explored the possibility to produce biodegradable cerebrovascular Mg alloy stents for ischemic stroke treatment and discussed some key factors that should also be considered in designing BMs brain stents, including anatomic features of the cerebral vasculature, hemodynamic influences, neuro-cytocompatibility and selection of alloying elements.
2. Application of BMs in neuroscience
Biodegradable medical devices are limited mostly to orthopedic/cardiovascular implants or surgical sutures. Representative Mg-based, Fe-based, and Zn-based BMs have been thoroughly reviewed with emphases on their physiochemical and corrosion properties, in vitro and in vivo biocompatibilities, as well as various techniques to manipulate the biodegradable behaviors [[20], [21], [22], [23], [24]]. These translational research on BM have shown their potential application in neuroscience.
2.1. Magnesium alloys
Since magnesium (Mg) and its alloys have tunable mechanical strength, and proper biodegradability, which can be further regulated by surface coating techniques [25], they are considered a promising alternative to permanent biomedical materials [26].
Besides, Mg ion can be used as a neuroprotective agent, which has been verified in several animal/clinical studies of cerebral ischemia treatment [[27], [28], [29], [30], [31]]. However, there is little research on the interactions between Mg alloys and brain tissue. Only several reports are available regarding their applications in neuroscience and neurosurgery (Fig. 1).
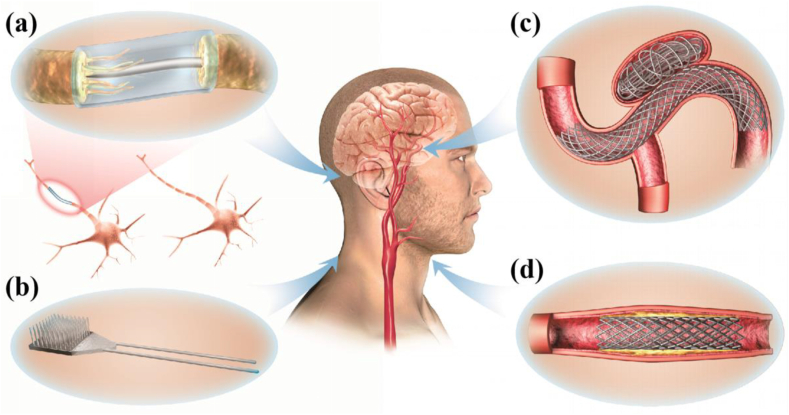
Fig. 1.
The application of Mg-based alloys in neuroscience. Mg alloys can be fabricated as filaments within: (a) nerve conduits to accelerate nerve regeneration [32,33], (b) nerve electrode and devices for neural recording and monitoring [[34], [35], [36]], (c) coiling-assisted stents for cerebrovascular aneurysms [37], and (d) expanded endovascular stents for the occlusion common carotid artery (CCA) [38].
2.1.1. Nerve repair and regeneration
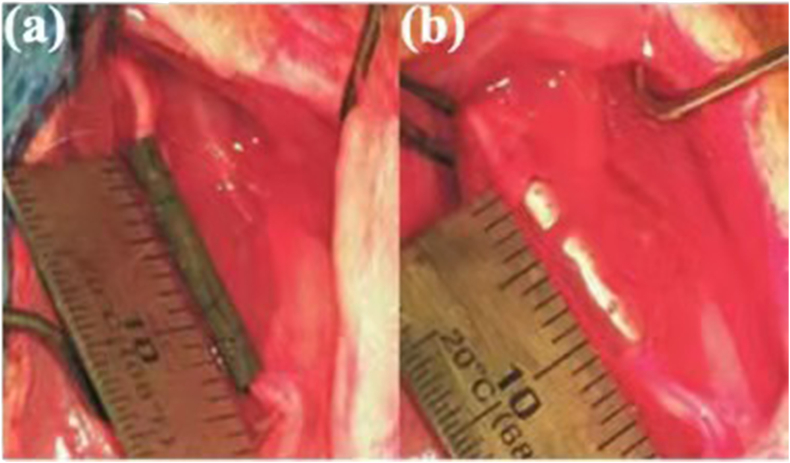
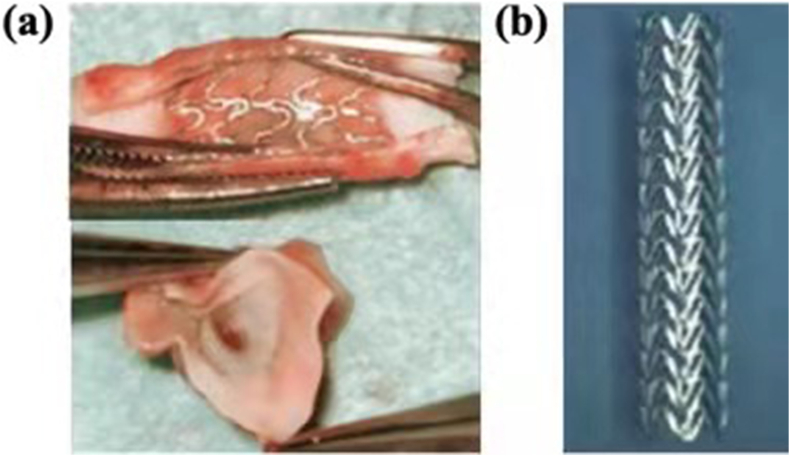
Nerve repair and regeneration is a big clinical challenge, especially for nerve defects longer than 2–4 cm [39]. Currently, drugs or growth factors are decorated onto or incorporated into various lipids, polymers, metals and carbon materials to support nerve function recovery. Compared with polymers, the use of biodegradable magnesium wires in nerve conduits will accelerate peripheral nerve regeneration across the injury gap [40], and the Mg filament implants degraded completely without evidence of scarring [41]. The Mg-based metallic glasses (Mg70Zn26Ca4) are also studied and proposed to be a promising material to make implantable nervous prosthetic devices [39]. Moreover, the Mg-based alloy (Mg–2Zn–Nd and Mg–10Li) extracts showed no neural toxicity compared to control groups [42]. Typical nerve conduits implantation photos are shown in Fig. 2. HA-WE43 nerve conduit were in implanted over the sciatic nerve gap and showed controlled degradation and absence of gas formation around the regenerated neural tissue after 12 weeks post-surgery [43].
Fig. 2.
Photos of nerve conduits implantation: (a) HA-WE43 (hydroxyapatite-coated Mg alloy) nerve conduit and (b) silicone nerve conduit [43].
2.1.2. Nerve electrode
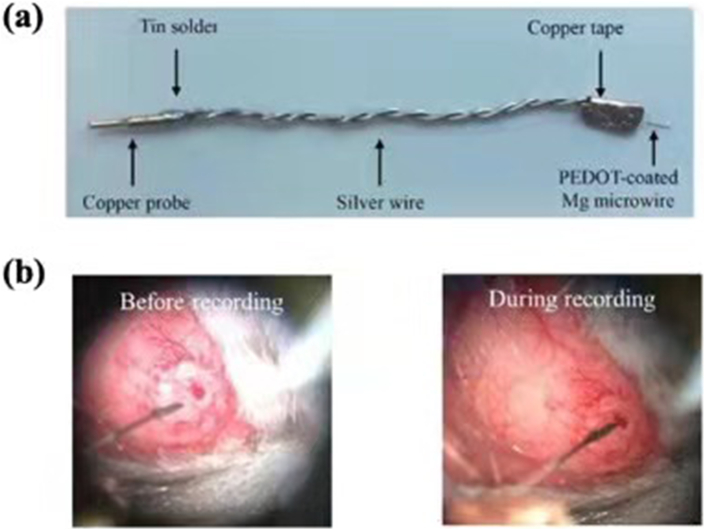
The electrical properties and signal pathways of neurons can provide significant insights into the discoveries of new treatment methods for neurological diseases [44,45]. However, the permanent presence of the rigid electrode can induce a mechanical mismatch with soft brain tissues, which is an obstacle to successful long-term implantation [44]. As an alternative, biodegradable polymers, such as silk fibroin, polyvinyl alcohol (PVA) and polylactic acid-glycolic acid (PLGA), are exploited to make biodegradable electronic devices, but they performed poor mechanical properties [46,47]. As shown in Fig. 3, implantable Mg-based electrode was firstly fabricated by Liu et al. for neural recording and stimulation and this micro-electrode was coated by poly-3,4-ethylenedioxythiophene (PEDOT) using electrochemical deposition technique [48,49] with excellent neural-recording capability and in vivo stability in the auditory cortex of a mouse [49].
Fig. 3.
(a) Mg microwire as working electrode with PEDOT coating and being attached to silver wire by copper tape; (b) Microscopic image of surface-modified Mg microwire before recording and during recording [48,49].
2.1.3. Vascular stents
Commercially available cerebrovascular stents are made of non-degradable metals [9], and their long-term existence may lead to acute thrombosis formation or late in-stent restenosis [50,51]. To address those problems, biodegradable stents are proposed and designed to disappear after fulfilling their mission. An ideal degradable stent needs to meet both the requirements of mechanical and degradable properties, and a low degradation rate within the first 6–12 months after implantation in order to ensure the success of blood vessel remodeling. Then, the stent should degrade at an appropriate corrosion rate within 12–24 months without causing any side effects.
At present, BMs cerebral vascular stents have been explored in the treatment of carotid artery stenosis and aneurysm (Fig. 4). The ruptured intracranial aneurysm can lead to a high mortality rate and poor neurological outcomes in patients. Intracranial stents are introduced together with endovascular coils to tackle this problem. Mg stent was implanted into a rat model for the treatment of saccular aneurysms (Fig. 4a) [37]. In addition, Wang et al. evaluated the biological outcomes of polytetrafluoroethylene membrane coated Mg-Nd-Zn-Zr alloy stent for the treatment of occlusion of a lateral aneurysm model in the rabbit common carotid artery (CCA). Their study suggested that the mean diameter and mean vessel area of CCAs at 6 months and one year after stents implantation were significantly greater than those treated with Co–Cr stents [38]. Also, Grüter et al. implanted Mg alloy stent into a sidewall aneurysm model and found no formation of late in-stent stenosis within 6 months and no negative interaction between the platinum coil and Mg alloy stent [52]. For carotid artery stenosis, Zhang et al. implanted a bare Mg-Nd-Zn-Zr (JDBM) stent into the CCA of New Zealand rabbits. Completed re-endothelialization of the stents was observed within 28 days and most of the JDBM stent struts were replaced by degradation products in situ within 4 months (Fig. 4b) [53].
Fig. 4.
(a) BMs stents assisted coiling for aneurysm [37]; (b) BMs carotid artery stents forvascular occlusion and stenosis [53].
2.2. Iron alloys
Iron is an important cofactor in metabolism and is featured as the most abundant transition metal element in the brain [54]. The elevated iron level in cerebrospinal fluid is associated with Alzheimer's disease pathogenesis [55]. And iron oxide nanoparticles have been used in the diagnosis and treatment of neurodegenerative diseases as imaging agents and drug delivery vehicles [56]. Biodegradable iron-based alloys have been investigated as bone replacement material [57], urinary implant material [58] and cardiovascular stent [59].
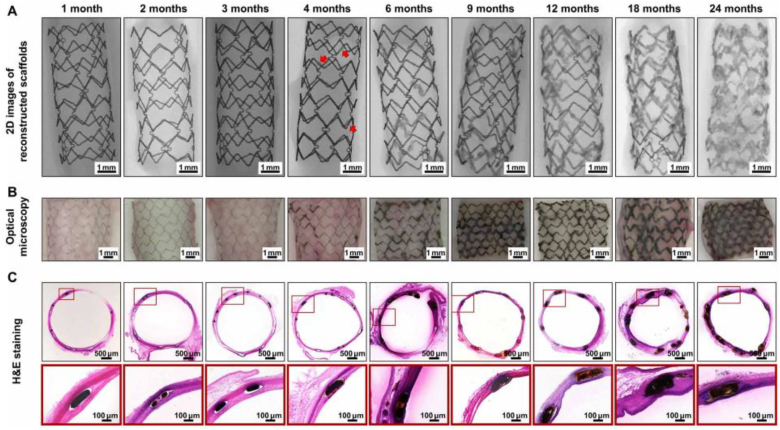
The in vivo safety of Iron stents (>99.8% iron) was initially verified in 2001 by implantation into rabbit's aortas [60], and most of the research thereafter are mainly focused on animal studies [61]. In a preclinical investigation, the first ultrathin (∼70 mm) sirolimus-eluting iron stent (Lifetech Scientific, Shenzhen, Guangdong, China) was implanted in porcine coronary arteries and exhibited comparable results with the cobalt-chromium everolimus-eluting stent (XIENCE Prime stent, Abbott Vascular, USA) [62]. The corrosion rate of the iron substrate can be increased by polylactide (PLA) coating due to its acidic degradation product [63], and the stent could provide effective local drug delivery to the target area. The preclinical result showed no significant difference in the area of stenosis compared to XIENCE stent up to 6 months while stent thrombosis was observed during the study [62,64]. A recent in vivo study in both rabbit abdominal aortas and human coronary arteries suggested that the PDLLA-Zn-nitrided Fe stents degraded completely after 24 months post implantation. Their biodegradation process is shown in Fig. 5. The surface-modified Fe stents remained intact within the early two months implantation; some corrosion products were observed after 4 months implantation [65].
Fig. 5.
In vivo degradation behavior of the PDLLA-Zn-nitrided Fe BRS in rabbit abdominal aortas at different time points after implantation [65].
Although iron-based biodegradable stent is a competitive and promising candidate as a temporary scaffold, a literature review from Dr. Scarcello [61] claims that the biocompatibility of iron alloys has not been well understood and it is not ready to classify iron or iron-based alloys as biocompatible materials. Recently, a fully biodegradable device for nerve regeneration was produced by using iron-manganese alloy as water-soluble electrodes [66]. The application of iron-based alloys in neuroscience is rarely reported. This may be due to the local toxicity on nerve cells, which has not been studied in depth, especially regarding endothelial dysfunction, oxidative stress, carcinogenicity or genotoxicity [61].
2.3. Zinc alloys
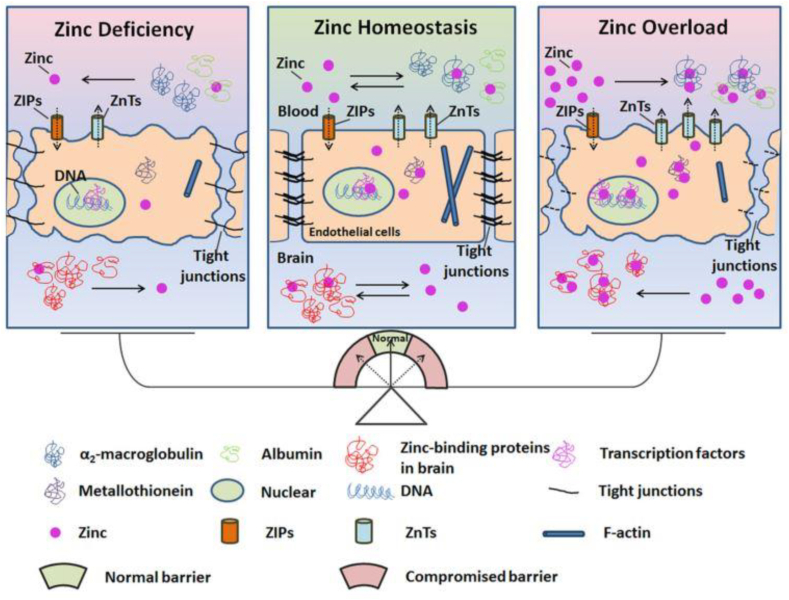
Zinc ions play significant roles in neurogenesis and synaptic activities [67] and serve as important cofactors in all six enzyme classes [68]. High zinc levels in vivo can induce focal neuronal pathology and zinc deficiency can give rise to mental lethargy [69]. Interrupting the zinc/blood-brain barrier (BBB) system will damage the microenvironment in the brain, leading to pathological diseases. The serum zinc level maybe an independent risk factor for ischemic stroke patients [70]. As it is illustrated in Fig. 6, zinc may serve as a potential target for protecting the BBB in stroke patients, and reducing hemorrhage transformation, inflammation and edema [67].
Fig. 6.
Schematic diagram of the role of zinc homeostasis in the normal function of the blood-brain barrier [67].
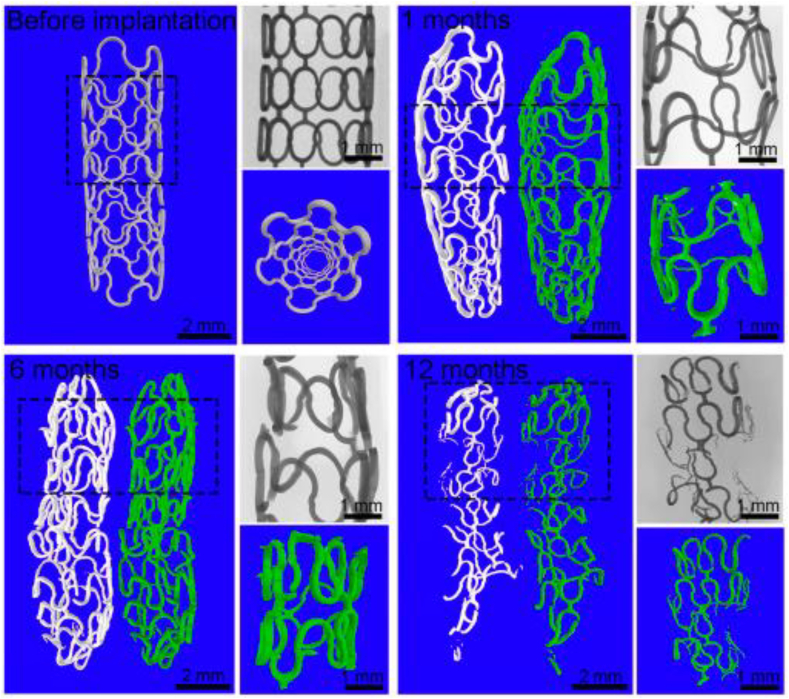
In terms of zinc-based alloys, their acceptable biodegradability and reasonable biocompatibility have made them a promising BM for both vascular and orthopedic applications [[71], [72], [73]]. In 2017, Zheng et al. implanted pure zinc stents into the abdominal aorta of rabbits for 12 months and these stents maintained mechanical integrity for 6 months with 41.75 ± 29.72% of stent volume degraded after 12 months implantation without severe inflammation, platelet aggregation, thrombosis formation or obvious intimal hyperplasia (Fig. 7) [74]. In addition, a novel biodegradable Zn-0.8Cu stent was fabricated and implanted into porcine coronary arteries for up to 24 months and the result showed that it provided sufficient structural support and exhibited an appropriate degradation rate without degradation product accumulation, thrombosis, or inflammatory responses [3]. In addition, the Zn–Cu stent exhibited an antibacterial effect against S. aureus [75].
Fig. 7.
The 2D and 3D micro-CT images of zinc stents after 0, 1, 6 and 12 months implantation in the aorta of rabbits from Ref. [74].
Zn-based alloys as the third generation stent material could be a promising candidate for stent applications due to their reliable and tunable strength and ductility, and acceptable biocompatibility [76,77]. The stents from Zn alloys can present thinner struts and better reinflation ability, with lower strength and creep resistance than that of Fe alloys [22]. The corrosion current density of Zn is higher than that of Fe, but lower than that of Mg [78]. Besides, compared with Zn alloys, the degradation of Mg alloys is accompanied by H2 evolution leading to high risk of gas embolism [79]. And the functional performance of these biomedical alloys can be improved by alloying proper elements and surface modifications. However, the research on zinc alloy cardiovascular degradable stent is still in its infancy. Besides, there are few studies about the application of zinc alloys in neuroscience, which may be due to the sensitivity of nerve cells to high concentrations of zinc ions [67].
2.4. Tungsten
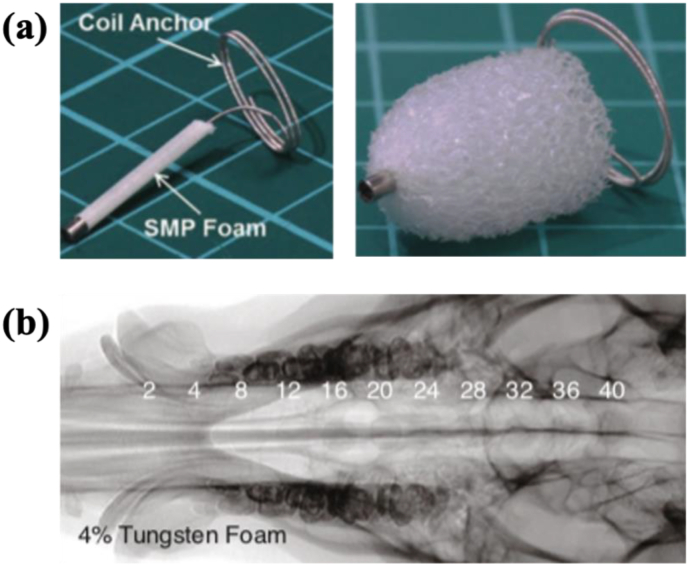
Tungsten (W) has been investigated as embolization coils in intracranial aneurysms with in vivo degradability [80]. Shape-memory polymers (SMPs) foams can be rendered radiopaque with doped tantalum or tungsten for a simulated aneurysm model (Fig. 8) [81]. The implantation of W coils (SPI, Balt, France) into the subclavian artery of New Zealand white rabbits led to a significant increase in serum W levels four months after the surgery [82]. This versatile metal was also exploited to make microwire electrodes for neural activity recording [83]. Patrick et al. reported that the corrosion rate of W microwires in 0.9% phosphate buffered saline was 300–70 μm/yr [83].
Fig. 8.
(a) Shape-memory polymers (SMPs) device before and after expansion [80]. (b) The 4% tungsten-doped SMP foam device with increasing thicknesses from 2 to 40 mm imaged via fluoroscopy with a pig's skull thickness [81].
2.5. Molybdenum
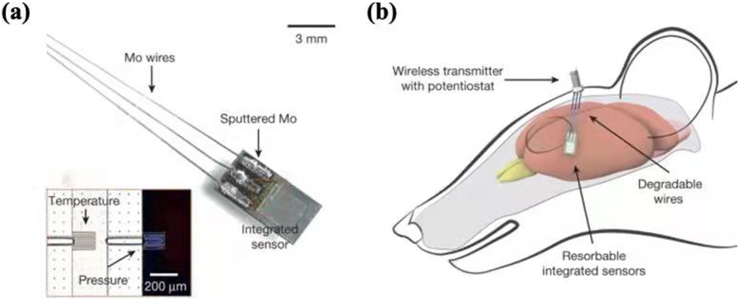
Molybdenum (Mo) was usually selected as an alloying element to make Ti–Mo alloys and Co–Cr–Mo alloys for manufacturing surgical implants [84]. In 2020, Redlich et al. explored the corrosion behavior of commercially high-purity molybdenum in simulated physiological salt solutions and stated that molybdenum could be a potential novel biodegradable material for stent fabrication [85]. Besides, Kang et al. invented a bioresorbable silicon electronic cerebral sensor by using Mo wires as an interface to the wireless module and depositing Mo foils as interconnects (Fig. 9) [13]. Combined with poly(l-lactide) and polycaprolactone composite, Mo was also used by Zhao et al. to fabricate a bioresorbable electrode array for brain monitoring [86].
Fig. 9.
Image of bioresorbable pressure and temperature sensors integrated with dissolvable metal interconnects (sputtered Mo, 2 μm thick) (a) and wires (Mo, 10 μm thick) (b) [13].
To summarize, the applications of BMs in the neuroscience are mainly divided into two categories. One of the applications was based on the mechanical properties of metal to develop vascular stents, and the other can be as core components of electronic devices for neural implantations and stimulations due to the conductive properties of metal. Molybdenum and tungsten are refractory and rare metals, which have the disadvantage of great difficulty in processing. Due to the uncertainty of the biocompatibility of iron and zinc ions in nerve cells, further studies of Fe-based and Zn-based alloys in neuroscience are limited. Compared with other metals, Mg-based alloys have a stable degradation rate and good biocompatibility, which shows a promising application in neuroscience [37,38].
3. From BM cardiovascular stents to cerebrovascular stents
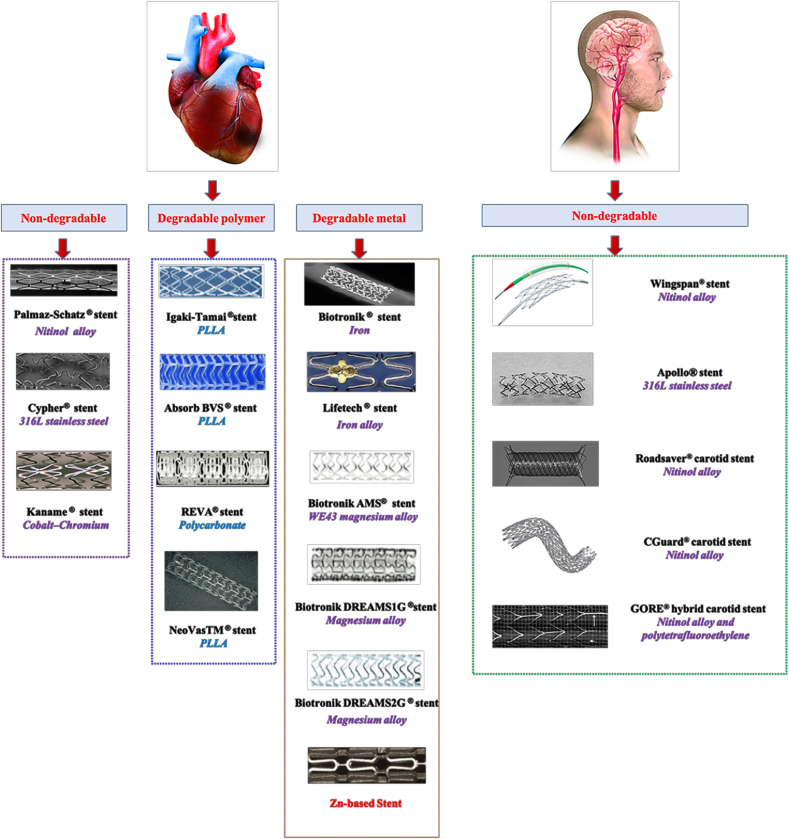
Cardiovascular stents have been studied for nearly 30 years, from the initial non-degradable bare-metal stents to the drug-eluting stents, and then to biodegradable stents. Representative and commercially available stents for cardiovascular and cerebral vascular stenosis treatment are shown in Fig. 10.
Fig. 10.
The representative and commercially available stents for cardiovascular/cerebrovascular stenosis treatment [[87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98]]. The application of Zn-based stent is still in research [99].
The biodegradable cardiovascular polymer stents are promising [100], but have the drawbacks of weak mechanical strength and thick stent strut thickness [101,102]. The elastic modulus of polymers (such as poly-l-lactic acid, polyethylene terepthalate, and poly-l-glycolic acid PLGA [103]) ranges from 1 to 5 GPa [104], which cannot confer ideal mechanical properties for stent fabrication and result in early recoil of the stent [105]. Besides, the increased strut thickness and high crossing profile can disturb the laminar blood flow and lead to difficulties in stent deliverability, especially in the use of tortuous cerebral vasculature [105].
In recent years, the research focus of biodegradable cardiovascular stents is gradually turning to the ones made of BMs, such as iron alloys, magnesium alloys, zinc alloys [106,107]. Among them, Mg alloy bioresorbable devices are much more widely studied [23] because of their superior biocompatibility and biodegradability [108]. As shown in Table 1, compared with Mg alloys, the in vivo studies of iron and zinc alloys are mainly focused on in vivo animal studies (including rat, rabbit, porcine and sheep models), and clinical investigations on their safety as cardiovascular stents are still in early stage. And their applications in neurological areas as biomedical devices are few. In Fig. 10, most of the brain stents are made of NiTi alloys without degradable properties.
Table 1.
The in vivo studies of the Mg-based, Fe-based and Zn-based BM vascular stents.
| BM stent | In vivo study | Mg Alloy | Fe Alloy | Zn Alloy | Reference | |
|---|---|---|---|---|---|---|
| Cardiovascular | Animal | Rat | ✓ | ✓ | ✓ | Mg [[109], [110], [111], [112]]: Fe [113]: Zn [114,115]: |
| Rabbit | ✓ | ✓ | ✓ | Mg [[116], [117], [118]]: Fe [63,64,[119], [120], [121], [122]]: Zn [74]: |
||
| Porcine | ✓ | ✓ | ✓ | Mg [[123], [124], [125], [126], [127], [128], [129], [130]]: Fe [62,131]: Zn [3,132,133]: |
||
| Sheep | ✓ | Mg [134]: | ||||
| Clinical | Single-center | Mg [135]: | ||||
| Multi-center | ✓ | Mg [[136], [137], [138]]: | ||||
| Cerebrovascular | Animal | Rat | ✓ | Mg [37,52]: | ||
| Rabbit | ✓ | Mg [38,53,139]: | ||||
| Porcine | ||||||
| Sheep | ||||||
| Clinical | Single-center | |||||
| Multi-center | ||||||
In 2013, the first clinical trial (BIOSOLVE-1) of the drug-eluting Mg alloy stents (DREAMS 1G) was conducted [137] and after that, in 2016, another human trial of Mg-based stent (DREAMS 2G) was reported [138]. Compared with DREAMS 1G, the DREAMS 2G displayed a more stable corrosion rate and better clinical performances. The related stent information is shown in Table 2.
Table 2.
Detailed information on degradable magnesium alloy stents.
Except for their usage as heart stents, Mg alloys can also be manufactured into devices for intraluminal tracheal stenosis treatment [140], bone repair and regeneration [140], vascular clamps and cervical spine interbody fusion [141].
Learning from the idea of BM cardiovascular stents and based on the thorough research of Mg alloys as heart stents and their application in neuroscience, we will explore the possibility of producing biodegradable cerebrovascular Mg alloy stents for ischemic cerebrovascular stenosis treatment.
4. Prospective BM cerebrovascular stents: Mg alloy stents
Ischemic strokes account for around 85% of total stroke patients [142]. Except for standard pharmacological and mechanical thrombolysis approaches, endovascular revascularization with the use of stents has been increasingly accepted for ischemic cerebrovascular disease treatment [143]. Mg alloys are chosen as a potential candidate to make brain stents and some key factors should be considered in designing it.
4.1. Cerebral vasculature
Compared with cardiovascular vessels, the cerebral vasculature is very tortuous, and the diameter of some intracranial arteries is only several millimeters. The intracranial arteries do not have an external elastic lamina and lack supporting perivascular tissue with reduced wall thickness (lumen diameter ratio), which makes the cerebral arteries susceptible to rupture under hemodynamic loads [144,145]. When a stent is implanted, it can induce alterations in the mechanical stimuli on the vascular walls, resulting in the formation and progression of in-stent restenosis [146]. Moreover, the level of restenosis is related to the level of implantation-induced vascular injury [147]. Therefore, flexibility is the first consideration when making a new cerebral stent [9]. The bending behavior of BM cerebrovascular stents as the primary factor due to the cerebrovascular curvature should be investigated to avoid the high-stress zone of the bridge as well as the inner-stent protrusion phenomenon in the bending state [148]. And it is of significant importance to ensure the biomechanical compatibility between a stent and an artery by optimizing the stent geometries by many methods, for example, by changing the location of the connection point between rings and links of stents to achiever tunable Poisson's ratio [149] and by using multi-objective optimization based on finite element analysis to improve stent flexibility [150].
4.2. Hemodynamics
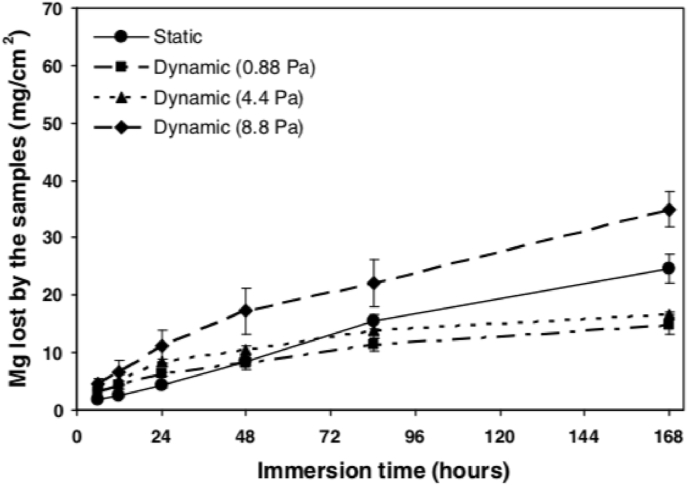
It is well acknowledged that, due to the pulsatile nature of blood flow, the vessel cells are constantly subject to mechanical forces in the form of stretch, cyclic strain and shear stress [151]. Except for their effects on restenosis and thrombosis [152], the local hemodynamic forces after stenting also have a great influence on the corrosion behaviors of BMs. Mg alloys were more susceptible to corrosion in the flowing solution under shear stress [153,154]. Taking the carotid artery as an example, the cerebral blood flow rate varies greatly between a healthy person (400 ml/min) and an ischemic stroke patient (250 ml/min) [155]. In one study, Mg–Zn–Y-Nd alloys fabricated by sub-rapid solidification processing provide excellent corrosion resistance in dynamic SBF, which opened a new window for design of biomedical materials, especially for vascular stent application [156]. A research showed that the degradation behavior of BMs is different when tested under static and dynamic conditions [157]. Also, the amplitude of shear stress has a strong influence on the corrosion process, with higher shear stress resulting in faster corrosion rate of Mg alloys (Fig. 11) [152]. In addition, coating technology can be used to regulate the corrosion behavior of the substrate in the fluid or blood environment, so that it can play a better temporary support role [158].
Fig. 11.
Mean magnesium loss as a function of test duration. It was measured by atomic absorption spectrometer for 168 h at a shear stress of 0, 0.88 Pa, 4.4 Pa and 8.8 Pa with different hemodynamics conditions [159].
4.3. Neuro-cytocompatibility
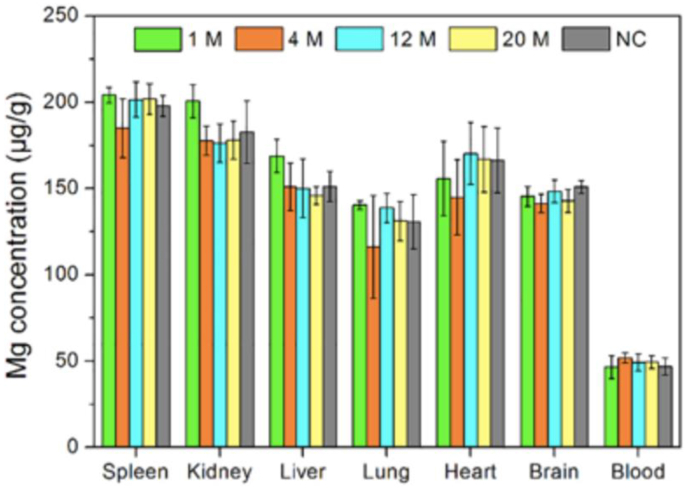
Magnesium is an essential trace element in the human body [160]. Magnesium sulfate has been used as a promising neuroprotective agent, which has been verified in several animal and single-center studies of cerebral ischemia treatment [161]. Mg ion is involved in a series of physiological functions such as nucleic acid metabolism, signal transduction, gene expression, apoptosis regulation and endocrine regulation. For ischemic stroke patients, the occluded blood vessels can lead to ATP depletion, ischemic depolarization and abnormal calcium influx, which can cause neural cell necrosis or apoptosis [162]. Mg ion can inhibit the calcium influx and the excitatory amino acids release into neurons, reduce the calcium-induced mitochondrial dysfunction and preserve cellular energy metabolism. Besides, the potential neuroprotective function of magnesium can also be reflected in expanding vessels to promote blood circulation, inhibiting platelet aggregation and increasing red blood cell deformability [163]. In the case of Mg-based alloys, Mg2+ is mainly derived from their degradation products. In one study, bare Mg-Nd-Zn-Zr stent was implanted in rabbit CCA [164], and one month later, the outer part of the stent was in situ replaced by the degradation products, which mainly consisted of Ca, Mg, P and O. Unbehau et al. evaluated the influences of brain cell activity on magnesium degradation, including glioblastoma multiforme cells, microglia cells and primary astrocytes, and their study suggested that the degradation behavior of Mg was cell type/density-dependent [165]. The concentration of Mg ions in heart, brain and blood stayed relatively stable, showing no statistically significant difference to that of the negative control (Fig. 12).
Fig. 12.
The concentration of Mg in the spleen, liver, lung, kidney, heart, brain and blood of rabbits after 1 month (n = 3), 4 month (n = 3), 12 month (n = 2), 20 month (n = 2) implantation of BMs and NC (non-specific control) [53].
4.4. Alloying elements selection
Due to the complexity of cerebrovascular vessels, it is necessary to select stent materials with higher elastic modulus and better elasticity. The biodegradable behaviors and mechanical properties of Mg alloys are tunable by suitable alloying elements [166]. Metals with low hydrogen overvoltage, including Ni, Fe, and Cu, can cause severe galvanic corrosion of magnesium alloys, while others like Al, Zn, Cd and Sn can induce a much lower corrosion rate [167]. The incorporation of Nd and Zn can improve the mechanical properties of Mg alloys through solid solution strengthening and precipitation strengthening techniques. Due to the small potential difference between the α -Mg matrix and the second phase Mg12Nd, no severe local corrosion was observed in vitro [168]. Zr is a commonly used grain refiner in Mg alloys, which can improve mechanical properties and corrosion resistance [169]. A study reported that Mg–1Mn and Mg–1Zn alloys showed low current densities in both SBF and Hank solutions, which provided evidence that Mn and Zn could improve the corrosion resistance of Mg alloys [166].
The influences of the added elements on the metabolic process should also be considered [170]. The normal concentration of Mg in the blood is around 14,130 ± 649.2 μmol/L [171]. As listed in Table 3, some trace elements may have positive effects on cerebrovascular diseases and maintain the healthy state of cerebral blood vessels.
Table 3.
The effects of some potential alloying elements on cerebrovascular disease.
| Element | Normal concentration in blood or cerebrospinal fluid | Effects on cerebrovascular disease |
|---|---|---|
| Mg | 2.74 ± 0.10 mg/dL [172] |
|
| Ca | 4.95 ± 0.70 mg/dL [172] |
|
| Zn | 17.40 ± 9.50 μg/dL [172] |
|
| Cu | 15.70 ± 13.50 μg/dL [172] |
|
| Fe | 13.10 ± 3.60 μg/dL [172] |
|
| Mn | 2.50 ± 0.70 μg/dL [172] |
|
| Ni | ∼5.5 mg/L [177,178] | |
| Al | 4–10 μg/L [181] | |
| Se | – |
|
In addition to the biocompatibility of element X in the nervous system, Mg-X alloying and the effects of X on the mechanical properties, corrosion properties, blood compatibility and nerve cells of alloys should also be considered. At the same time, it is necessary to explore whether the corrosion products and dissolved ions can still play the nerve function.
5. Conclusion and future work
With the development of material science, BMs have been fabricated into biodegradable medical devices to circumvent potential side effects from permanent implants. The application of BMs in neuroscience is still in its infancy. Compared with Fe-based and Zn-based alloys, as well as Molybdenum and Tungsten, Mg-based alloys show good neuro-cytocompatibility and have proper and tunable corrosion rate and mechanical properties. Several pioneering works have been conducted on Mg alloys for their application in neuroscience, such as filament within nerve conduits to accelerate nerve regeneration, nerve electrode for neural recording, and stents for carotid artery stenosis and aneurysm treatment. Besides, Mg ions can be used as neuroprotective agent. Based on the novel research and inspired by the promising clinical results from cardiovascular Mg alloy stents, we propose that Mg alloys could be highly promising BMs to make cerebral vascular stent for ischemic stroke treatment. Future work should be done to design novel Mg-based alloys and optimize the stent geometry to ensure their biomechanical compatibility with brain blood vessels and a mild and safe in vivo degradation.
Declaration of competing interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (82027802, 82102220, 82071468), Beijing Municipal Natural Science Foundation (721220, 61975017) and General Projects of Scientific and Technological Plan of Beijing Municipal Education Commission (KM202010025023).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yufeng Zheng, Email: yfzheng@pku.edu.cn.
Xunming Ji, Email: jixm@ccmu.edu.cn.
References
- 1.Han H.S., Jun I., Seok H.K., Lee K.S., Lee K., Witte F., Mantovani D., Kim Y.C., Glyn-Jones S., Edwards J.R. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Advanced science. 2020;7(15) doi: 10.1002/advs.202000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M.M., Deen K.M., Shabib I., Asselin E., Haider W. Controlling the dissolution of iron through the development of nanostructured Fe-Mg for biomedical applications. Acta Biomater. 2020;113:660–676. doi: 10.1016/j.actbio.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C., Li H.F., Yin Y.X., Shi Z.Z., Li T., Feng X.Y., Zhang J.W., Song C.X., Cui X.S., Xu K.L., Zhao Y.W., Hou W.B., Lu S.T., Liu G., Li M.Q., Ma J.Y., Toft E., Volinsky A.A., Wan M., Yao X.J., Wang C.B., Yao K., Xu S.K., Lu H., Chang S.F., Ge J.B., Wang L.N., Zhang H.J. Long-term in vivo study of biodegradable Zn-Cu stent: a 2-year implantation evaluation in porcine coronary artery. Acta Biomater. 2019;97:657–670. doi: 10.1016/j.actbio.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Cai A.Y., Zhu Y.J., Qi C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Advanced Materials Interfaces. 2020;7(20):2000819. [Google Scholar]
- 5.Kabir H., Munir K., Wen C., Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioactive materials. 2021;6(3):836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Jahr H., Zhou J., Zadpoor A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020;115:29–50. doi: 10.1016/j.actbio.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Wei S., Ma J.X., Xu L., Gu X.S., Ma X.L. Biodegradable materials for bone defect repair. Military Medical Research. 2020;7(1):54. doi: 10.1186/s40779-020-00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donkor E.S. 2018. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life, Stroke Research and Treatment 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Zhang H., Zhang Y., Wu H., Wei L., Zhou G., Zhang Y., Deng L., Cheng Y., Li M., Santos H.A., Cui W. Endovascular metal devices for the treatment of cerebrovascular diseases. Adv. Mater. 2019;31(8) doi: 10.1002/adma.201805452. [DOI] [PubMed] [Google Scholar]
- 10.Nahab F.B., Lynn M.J., Kasner S.E., Alexander M.J., Klucznik R.P., Zaidat O.O., Chaloupka J., Lutsep H.L., Barnwell S.L., Mawad M.E. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72(23):2014–2019. doi: 10.1212/01.wnl.0b013e3181a1863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White A.R. A greater focus on metals in biomedicine and neuroscience is needed. BMC pharmacology & toxicology. 2016;17(1):53. doi: 10.1186/s40360-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tingzhang Chun, Yang Song, Lin Qingsong, Guixue Wang. Biodegradable stents for coronary artery disease treatment: recent advances and future perspectives, Materials science & engineering. C, Materials for biological applications. 2018;91(1):163–178. doi: 10.1016/j.msec.2018.04.100. [DOI] [PubMed] [Google Scholar]
- 13.Kang S.K., Murphy R.K., Hwang S.W., Lee S.M., Harburg D.V., Krueger N.A., Shin J., Gamble P., Cheng H., Yu S., Liu Z., McCall J.G., Stephen M., Ying H., Kim J., Park G., Webb R.C., Lee C.H., Chung S., Wie D.S., Gujar A.D., Vemulapalli B., Kim A.H., Lee K.M., Cheng J., Huang Y., Lee S.H., Braun P.V., Ray W.Z., Rogers J.A. Bioresorbable silicon electronic sensors for the brain. Nature. 2016;530(7588):71–76. doi: 10.1038/nature16492. [DOI] [PubMed] [Google Scholar]
- 14.Lee J., Cho H.R., Cha G.D., Seo H., Kim D.H. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019;10(1):5205. doi: 10.1038/s41467-019-13198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin J., Liu Z., Bai W., Liu Y., Rogers J.A. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Science Advances. 2019;5(7):1899. doi: 10.1126/sciadv.aaw1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K., Li S., Dong S., Zhang S., Pan G., Wang G., Shi L., Guo W., Yu C., Luo J. Bioresorbable electrode array for electrophysiological and pressure signal recording in the brain. Advanced Healthcare Materials. 2019;8(15) doi: 10.1002/adhm.201801649. [DOI] [PubMed] [Google Scholar]
- 17.Kang S.K., Koo J., Lee Y.K., Rogers J.A. Advanced materials and devices for bioresorbable electronics. Acc. Chem. Res. 2018;51(5):988–998. doi: 10.1021/acs.accounts.7b00548. [DOI] [PubMed] [Google Scholar]
- 18.Li R., Wang L., Kong D., Yin L. Recent progress on biodegradable materials and transient electronics. Bioactive Materials. 2018;3(3):322–333. doi: 10.1016/j.bioactmat.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin L., Cheng H., Mao S., Haasch R., Liu Y., Xie X., Hwang S.-W., Jain H., Kang S.-K., Su Y., Li R., Huang Y., Rogers J.A. Dissolvable metals for transient electronics. Adv. Funct. Mater. 2014;24(5):645–658. [Google Scholar]
- 20.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. [Google Scholar]
- 21.Toong D.W.Y., Ng J.C.K., Huang Y., Wong P.E.H., Leo H.L., Venkatraman S.S., Ang H.Y. Bioresorbable metals in cardiovascular stents: material insights and progress. Materialia. 2020;12 [Google Scholar]
- 22.Li G., Yang H., Zheng Y., Chen X.H., Yang J.A., Zhu D., Ruan L., Takashima K. Challenges in the use of zinc and its alloys as biodegradable metals: perspective from biomechanical compatibility. Acta Biomater. 2019;97:23–45. doi: 10.1016/j.actbio.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Han H.-S., Loffredo S., Jun I., Edwards J., Kim Y.-C., Seok H.-K., Witte F., Mantovani D., Glyn-Jones S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today. 2019;23:57–71. [Google Scholar]
- 24.Xia D., Yang F., Zheng Y., Liu Y., Zhou Y. Research status of biodegradable metals designed for oral and maxillofacial applications: a review. Bioactive materials. 2021;6(11):4186–4208. doi: 10.1016/j.bioactmat.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riaz U., Shabib I., Haider W. The current trends of Mg alloys in biomedical applications—a review. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(6):1970–1996. doi: 10.1002/jbm.b.34290. [DOI] [PubMed] [Google Scholar]
- 26.Li L.Y., Cui L.Y., Zeng R.C., Li S.Q., Chen X.B., Zheng Y., Kannan M.B. Advances in functionalized polymer coatings on biodegradable magnesium alloys - a review. Acta Biomater. 2018;79:23–36. doi: 10.1016/j.actbio.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Muir K.W., Lees K.R. Dose optimization of intravenous magnesium sulfate after acute stroke. Stroke. 1998;29(5):918–923. doi: 10.1161/01.str.29.5.918. [DOI] [PubMed] [Google Scholar]
- 28.Sirin B.H., Coşkun E., Yilik L., Ortaç R., Sirin H., Tetik C. Neuroprotective effects of preischemia subcutaneous magnesium sulfate in transient cerebral ischemia. Eur. J. Cardio. Thorac. Surg. 1998;14(1):82–88. doi: 10.1016/s1010-7940(98)00140-7. [DOI] [PubMed] [Google Scholar]
- 29.Spandou E., Soubasi V., Papoutsopoulou S., Augoustides-Savvopoulou P., Loizidis T., Pazaiti A., Karkavelas G., Guiba-Tziampiri O. Neuroprotective effect of long-term MgSO4 administration after cerebral hypoxia-ischemia in newborn rats is related to the severity of brain damage. Reprod. Sci. 2007;14(7):667–677. doi: 10.1177/1933719107305864. [DOI] [PubMed] [Google Scholar]
- 30.Westermaier T., Stetter C., Kunze E., Willner N., Raslan F., Vince G.H., Ernestus R.I. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Experimental traumatic brain injury. 2013;5(1):6. doi: 10.1186/2040-7378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H., Meloni B.P., Moore S.R., Majda B.T., Knuckey N.W. Intravenous administration of magnesium is only neuroprotective following transient global ischemia when present with post-ischemic mild hypothermia. Brain Res. 2004;1014(1–2):53–60. doi: 10.1016/j.brainres.2004.03.073. [DOI] [PubMed] [Google Scholar]
- 32.Daly W., Yao L., Zeugolis D., Windebank A., Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface. 2012;9(67):202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riaz U., Shabib I., Haider W. The current trends of Mg alloys in biomedical applications-A review. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(6):1970–1996. doi: 10.1002/jbm.b.34290. [DOI] [PubMed] [Google Scholar]
- 34.Fan J.Z., Lopez-Rivera V., Sheth S.A. Over the horizon: the present and future of endovascular neural recording and stimulation. Front. Neurosci. 2020;14:432. doi: 10.3389/fnins.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehoe S., Zhang X.F., Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee S., Saxena M., Padmanabhan D., Jayachandra M., Pandya H.J. Futuristic medical implants using bioresorbable materials and devices. Biosens. Bioelectron. 2019;142 doi: 10.1016/j.bios.2019.111489. [DOI] [PubMed] [Google Scholar]
- 37.Grüter B.E., Täschler D., Strange F., Rey J., von Gunten M., Grandgirard D., Leib S.L., Remonda L., Widmer H.R., Nevzati E., Fandino J., Marbacher S., Coluccia D. Testing bioresorbable stent feasibility in a rat aneurysm model. J. Neurointerventional Surg. 2019;11(10):1050. doi: 10.1136/neurintsurg-2018-014697. [DOI] [PubMed] [Google Scholar]
- 38.Wang W., Wang Y.L., Chen M., Chen L., Zhang J., Li Y.D., Li M.H., Yuan G.Y. Magnesium alloy covered stent for treatment of a lateral aneurysm model in rabbit common carotid artery: an in vivo study. Sci. Rep. 2016;6:37401. doi: 10.1038/srep37401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monfared A., Ghaee A., Ebrahimibarough S. Fabrication of tannic acid/poly(N-vinylpyrrolidone) layer-by-layer coating on Mg-based metallic glass for nerve tissue regeneration application. Colloids Surf. B Biointerfaces. 2018;170:617–626. doi: 10.1016/j.colsurfb.2018.06.060. [DOI] [PubMed] [Google Scholar]
- 40.Vennemeyer J.J., Hopkins T., Hershcovitch M., Little K.D., Hagen M.C., Minteer D., Hom D.B., Marra K., Pixley S.K. Initial observations on using magnesium metal in peripheral nerve repair. J. Biomater. Appl. 2015;29(8):1145–1154. doi: 10.1177/0885328214553135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins T., Little K.J., Vennemeyer J., Triozzi J.L., Turgeon M.K., Heilman A.M., Minteer D.M., Marra K.G., Hom D.B., Pixley S.K. Short and long gap peripheral nerve repair with magnesium metal filaments. J. Biomed. Mater. Res. 2017;105(11):3148–3158. doi: 10.1002/jbm.a.36176. [DOI] [PubMed] [Google Scholar]
- 42.Fei J., Wen X., Lin X., Saijilafu, Wang W., Ren O., Chen X., Tan L., Yang K., Yang H., Yang L. Biocompatibility and neurotoxicity of magnesium alloys potentially used for neural repairs, Materials science & engineering. C, Materials for biological applications. 2017;78:1155–1163. doi: 10.1016/j.msec.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 43.Almansoori A.A., Ju K.W., Kim B., Kim S.M., Lee S.-M., Lee J.-H. Hydroxyapatite coated magnesium alloy for peripheral nerve regeneration. Oral Biology Research. 2018;42(3):105–113. [Google Scholar]
- 44.Shi Y., Liu R., He L., Feng H., Li Y., Li Z. Recent development of implantable and flexible nerve electrodes. Smart Materials in Medicine. 2020;1:131–147. [Google Scholar]
- 45.Tang L.J., Wang M.H., Tian H.C., Kang X.Y., Hong W., Liu J.Q. Progress in research of flexible MEMS microelectrodes for neural interface. Micromachines. 2017;8(9) doi: 10.3390/mi8090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ceyssens F., Bovet Carmona M., Kil D., Deprez M., Tooten E., Nuttin B., Takeoka A., Balschun D., Kraft M., Puers R. Chronic neural recording with probes of subcellular cross-section using 0.06 mm2 dissolving microneedles as insertion device. Sensor. Actuator. B Chem. 2019;284:369–376. [Google Scholar]
- 47.Zhou H., Li G., Sun X., Zhu Z., Xu B., Jin Q., Zhao J., Ren Q.-S. A new process for fabricating tip-shaped polymer microstructure array with patterned metallic coatings. Sensor Actuator Phys. 2009;150(2):296–301. [Google Scholar]
- 48.Zhang C., Driver N., Tian Q., Jiang W., Liu H. Electrochemical deposition of conductive polymers onto magnesium microwires for neural electrode applications. J. Biomed. Mater. Res. 2018;106(7):1887–1895. doi: 10.1002/jbm.a.36385. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C., Wen T.H., Razak K.A., Lin J., Villafana E., Jimenez H., Liu H. Fabrication and characterization of biodegradable metal based microelectrodes for in vivo neural recording. MRS Advances. 2019;4(46–47):2471–2477. [Google Scholar]
- 50.Brown S.C., Falcone G.J., Hebert R.M., Yaghi S., Mac Grory B., Stretz C. Stenting for acute carotid artery dissection. Stroke. 2020;51(1):e3–e6. doi: 10.1161/STROKEAHA.119.027827. [DOI] [PubMed] [Google Scholar]
- 51.Muller M.D., Gregson J., McCabe D.J.H., Nederkoorn P.J., van der Worp H.B., de Borst G.J., Cleveland T., Wolff T., Engelter S.T., Lyrer P.A., Brown M.M., Bonati L.H. Stent design, restenosis and recurrent stroke after carotid artery stenting in the international carotid stenting study. Stroke. 2019;50(11):3013–3020. doi: 10.1161/STROKEAHA.118.024076. [DOI] [PubMed] [Google Scholar]
- 52.Nevzati E., Rey J., Coluccia D., D'Alonzo D., Gruter B., Remonda L., Fandino J., Marbacher S. Biodegradable magnesium stent treatment of saccular aneurysms in a rat model - introduction of the surgical technique. Jove-Journal of Visualized Experiments. 2017;128 doi: 10.3791/56359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Li H., Wang W., Huang H., Pei J., Qu H., Yuan G., Li Y. The degradation and transport mechanism of a Mg-Nd-Zn-Zr stent in rabbit common carotid artery: a 20-month study. Acta Biomater. 2018;69:372–384. doi: 10.1016/j.actbio.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Lane D.J.R., Ayton S., Bush A.I. Iron and alzheimer's disease: an update on emerging mechanisms. J. Alzheim. Dis.: JAD. 2018;64(s1):S379–S395. doi: 10.3233/JAD-179944. [DOI] [PubMed] [Google Scholar]
- 55.Ayton S., Faux N.G., Bush A.I., Alzheimer's Disease Neuroimaging I. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat. Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dadfar S.M., Roemhild K., Drude N.I., von Stillfried S., Knuchel R., Kiessling F., Lammers T. Iron oxide nanoparticles: diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019;138:302–325. doi: 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monika H.á., Miriam K., Miroslav D.U. Fe and Fe-P foam for biodegradable bone replacement material: morphology, corrosion behaviour, and mechanical properties. Advances in Materials Science and Engineering 2016. 2016:1–9. [Google Scholar]
- 58.Zheng Ma, Ming Gao, Di Na, Yangde Li. Lili, Study on a biodegradable antibacterial Fe-Mn-C-Cu alloy as urinary implant material. Mater. Sci. Eng. C. 2019;103:109718. doi: 10.1016/j.msec.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Liu B., Zheng Y.F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011;7(3):1407–1420. doi: 10.1016/j.actbio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Peuster M., Wohlsein P., Brügmann M., Ehlerding M., Seidler K., Fink C., Brauer H., Fischer A., Hausdorf G. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal—results 6–18 months after implantation into New Zealand white rabbits. Heart. 2001;86(5):563–569. doi: 10.1136/heart.86.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scarcello E., Lison D. Are Fe-based stenting materials biocompatible? A critical review of in vitro and in vivo studies. J. Funct. Biomater. 2019;11(1):2. doi: 10.3390/jfb11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng J.F., Qiu H., Tian Y., Hu X.Y., Luo T., Wu C., Tian Y., Tang Y., Song L.F., Li L., Xu L., Xu B., Gao R.L. Preclinical evaluation of a novel sirolimus-eluting iron bioresorbable coronary scaffold in porcine coronary artery at 6 months. JACC Cardiovasc. Interv. 2019;12(3):245–255. doi: 10.1016/j.jcin.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Qi Y., Qi H., He Y., Lin W., Li P., Qin L., Hu Y., Chen L., Liu Q., Sun H., Liu Q., Zhang G., Cui S., Hu J., Yu L., Zhang D., Ding J. Strategy of metal-polymer composite stent to accelerate biodegradation of iron-based biomaterials. ACS Appl. Mater. Interfaces. 2018;10(1):182–192. doi: 10.1021/acsami.7b15206. [DOI] [PubMed] [Google Scholar]
- 64.Lin W., Qin L., Qi H., Zhang D., Zhang G., Gao R., Qiu H., Xia Y., Cao P., Wang X., Zheng W. Long-term in vivo corrosion behavior, biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta Biomater. 2017;54:454–468. doi: 10.1016/j.actbio.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Shen D., Qi H., Lin W., Zhang W., Bian D., Shi X., Qin L., Zhang G., Fu W., Dou K., Xu B., Yin Z., Rao J., Alwi M., Wang S., Zheng Y., Zhang D., Gao R. PDLLA-Zn-nitrided Fe bioresorbable scaffold with 53-μm-thick metallic struts and tunable multistage biodegradation function. Science Advances. 2021;7(23) doi: 10.1126/sciadv.abf0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L., Lu C., Yang S., Sun P., Wang Y., Guan Y., Liu S., Cheng D., Meng H., Wang Q., He J., Hou H., Li H., Lu W., Zhao Y., Wang J., Zhu Y., Li Y., Luo D., Li T., Chen H., Wang S., Sheng X., Xiong W., Wang X., Peng J., Yin L. A fully biodegradable and self-electrified device for neuroregenerative medicine. Science Advances. 2020;6(50) doi: 10.1126/sciadv.abc6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi Z., Liu K.J. The interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019;364:114–119. doi: 10.1016/j.taap.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 69.Bartzatt R. Neurological impact of zinc excess and deficiency in vivo. European Journal of Nutrition & Food Safety. 2017;7(3):155–160. [Google Scholar]
- 70.Munshi A., Babu S., Kaul S., Shafi G., Rajeshwar K., Alladi S., Jyothy A. Depletion of serum zinc in ischemic stroke patients. Methods Find Exp. Clin. Pharmacol. 2010;32(6):433–436. doi: 10.1358/mf.2010.32.6.1487084. [DOI] [PubMed] [Google Scholar]
- 71.Chen X., Tan B., Wang S., Tang R., Bao Z., Chen G., Chen S., Tang W., Wang Z., Long C., Lu W.W., Yang D., Bian L., Peng S. Rationally designed protein cross-linked hydrogel for bone regeneration via synergistic release of magnesium and zinc ions. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120895. [DOI] [PubMed] [Google Scholar]
- 72.Jia B., Yang H., Zhang Z., Qu X., Jia X., Wu Q., Han Y., Zheng Y., Dai K. Biodegradable Zn-Sr alloy for bone regeneration in rat femoral condyle defect model: in vitro and in vivo studies. Bioactive materials. 2021;6(6):1588–1604. doi: 10.1016/j.bioactmat.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen X., Zhang Y., Ma P., Sutrisno L., Luo Z., Hu Y., Yu Y., Tao B., Li C., Cai K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials. 2019;212:1–16. doi: 10.1016/j.biomaterials.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Yang H., Wang C., Liu C., Chen H., Wu Y., Han J., Jia Z., Lin W., Zhang D., Li W., Yuan W., Guo H., Li H., Yang G., Kong D., Zhu D., Takashima K., Ruan L., Nie J., Li X., Zheng Y. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials. 2017;145:92–105. doi: 10.1016/j.biomaterials.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Mintegui C., Cordoba L.C., Buxadera-Palomero J., Marquina A., Jimenez-Pique E., Ginebra M.P., Cortina J.L., Pegueroles M. Zn-Mg and Zn-Cu alloys for stenting applications: from nanoscale mechanical characterization to in vitro degradation and biocompatibility. Bioactive materials. 2021;6(12):4430–4446. doi: 10.1016/j.bioactmat.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mostaed E., Sikora-Jasinska M., Drelich J.W., Vedani M. Zinc-based alloys for degradable vascular stent applications. Acta Biomater. 2018;71:1–23. doi: 10.1016/j.actbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu J., Su Y., Qin Y.X., Zheng Y., Wang Y., Zhu D. Evolution of metallic cardiovascular stent materials: a comparative study among stainless steel, magnesium and zinc. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong H., Lin F., Boccaccini A.R., Virtanen S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: comparison of Mg, Zn and Fe. Corrosion Sci. 2021;182 [Google Scholar]
- 79.Aghion E. Biodegradable Metals, Metals. 2018;8(10) [Google Scholar]
- 80.Hu J., Albadawi H., Chong B.W., Deipolyi A.R., Sheth R.A., Khademhosseini A., Oklu R. Advances in biomaterials and technologies for vascular embolization. Adv. Mater. 2019;31(33) doi: 10.1002/adma.201901071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodriguez J.N., Yu Y.J., Miller M.W., Wilson T.S., Hartman J., Clubb F.J., Gentry B., Maitland D.J. Opacification of shape memory polymer foam designed for treatment of intracranial aneurysms. Ann. Biomed. Eng. 2012;40(4):883–897. doi: 10.1007/s10439-011-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peuster M., Fink C., Wohlsein P., Bruegmann M., Günther A., Kaese V., Niemeyer M., Haferkamp H., Schnakenburg C.v. Degradation of tungsten coils implanted into the subclavian artery of New Zealand white rabbits is not associated with local or systemic toxicity. Biomaterials. 2003;24(3):393–399. doi: 10.1016/s0142-9612(02)00352-6. [DOI] [PubMed] [Google Scholar]
- 83.Patrick E., Orazem M.E., Sanchez J.C., Nishida T. Corrosion of tungsten microelectrodes used in neural recording applications. J. Neurosci. Methods. 2011;198(2):158–171. doi: 10.1016/j.jneumeth.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ribeiro A.M., Flores-Sahagun T.H.S., Paredes R.C. A perspective on molybdenum biocompatibility and antimicrobial activity for applications in implants. J. Mater. Sci. 2016;51(6):2806–2816. [Google Scholar]
- 85.Redlich C., Quadbeck P., Thieme M., Kieback B. Molybdenum – a biodegradable implant material for structural applications? Acta Biomater. 2020;104 doi: 10.1016/j.actbio.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 86.Seitz J.M., Durisin M., Goldman J., Drelich J.W. Recent advances in biodegradable metals for medical sutures: a critical review. Advanced Healthcare Materials. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 87.Ang H.Y., Bulluck H., Wong P., Venkatraman S.S., Huang Y., Foin N. Bioresorbable stents: current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017;228:931–939. doi: 10.1016/j.ijcard.2016.11.258. [DOI] [PubMed] [Google Scholar]
- 88.Basalus M.W., Tandjung K., Sen H., Apeldoorn A.V., Grijpma D.W., Birgelen C.V. Recent insights from scanning electron microscopic assessment of durable polymer-coated drug-eluting stents. Intervent Cardiol. 2012;4(6):661–674. [Google Scholar]
- 89.Carrie D., Schachinger V., Danzi G.B., Macaya C., Zeymer U., Putnikovic B., Iniguez A., Moreno R., Mehmedbegovic Z., Beleslin B., Kare I. Cobalt-chromium KAname coRonary stEnt system in the treatment of patients with coronary artery disease (KARE study) J. Intervent. Cardiol. 2014;27(5):491–499. doi: 10.1111/joic.12144. [DOI] [PubMed] [Google Scholar]
- 90.Casana R., Tolva V., Odero A., Jr., Malloggi C., Paolucci A., Triulzi F., Silani V. Safety and efficacy of the new micromesh-covered stent CGuard in patients undergoing carotid artery stenting: early experience from a single centre. Eur. J. Vasc. Endovasc. Surg. 2017;54(6):681–687. doi: 10.1016/j.ejvs.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Iqbal J., Onuma Y., Ormiston J., Abizaid A., Waksman R., Serruys P. Bioresorbable scaffolds: rationale, current status, challenges, and future. Eur. Heart J. 2014;35(12):765–776. doi: 10.1093/eurheartj/eht542. [DOI] [PubMed] [Google Scholar]
- 92.Jiang W.J., Xu X.T., Jin M., Du B., Dong K.H., Dai J.P. Apollo stent for symptomatic atherosclerotic intracranial stenosis: study results. AJNR. American Journal of Neuroradiology. 2007;28(5):830–834. [PMC free article] [PubMed] [Google Scholar]
- 93.Moravej M., Mantovani D. Biodegradable metals for cardiovascular stent application: interests and new opportunities. Int. J. Mol. Sci. 2011;12(7):4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ozturk A.K., Bulsara K.R. The evolving paradigm in the management of intracranial atherosclerotic disease. International Journal of Vascular Medicine 2012. 2012 doi: 10.1155/2012/289852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schatz R.A., Baim D.S., Leon M., Ellis S.G., Goldberg S., Hirshfeld J.W., Cleman M.W., Cabin H.S., Walker C., Stagg J., et al. Clinical experience with the Palmaz-Schatz coronary stent. Initial results of a multicenter study. Circulation. 1991;83(1):148–161. doi: 10.1161/01.cir.83.1.148. [DOI] [PubMed] [Google Scholar]
- 96.Schonholz C., Yamada R., Montgomery W., Brothers T., Guimaraes M. First-in-man implantation of a new hybrid carotid stent to prevent periprocedural neurological events during carotid artery stenting. J. Endovasc. Ther. 2014;21(4):601–604. doi: 10.1583/14-4735R.1. [DOI] [PubMed] [Google Scholar]
- 97.Yilmaz U., Korner H., Muhl-Benninghaus R., Simgen A., Kraus C., Walter S., Behnke S., Fassbender K., Reith W., Unger M.M. Acute occlusions of dual-layer carotid stents after endovascular emergency treatment of tandem lesions. Stroke. 2017;48(8):2171–2175. doi: 10.1161/STROKEAHA.116.015965. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y.J., Wang X.Z., Fu G., Jing Q.M., Wang G., Jin C.Y., Xie L.H., Cai J.Z., Xu B., Han Y.L. Clinical and multimodality imaging results at 6 months of a bioresorbable sirolimus-eluting scaffold for patients with single de novo coronary artery lesions: the NeoVas first-in-man trial. EuroIntervention. 2016;12(10):1279–1287. doi: 10.4244/EIJV12I10A209. [DOI] [PubMed] [Google Scholar]
- 99.Scafa Udriste A., Niculescu A.G., Grumezescu A.M., Badila E. Cardiovascular stents: a review of past, current, and emerging devices. Materials. 2021;14(10) doi: 10.3390/ma14102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishio S., Kosuga K., Igaki K., Okada M., Kyo E., Tsuji T., Takeuchi E., Inuzuka Y., Takeda S., Hata T., Takeuchi Y., Kawada Y., Harita T., Seki J., Akamatsu S., Hasegawa S., Bruining N., Brugaletta S., de Winter S., Muramatsu T., Onuma Y., Serruys P.W., Ikeguchi S. Long-Term (>10 Years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: igaki-Tamai stents. Circulation. 2012;125(19):2343–2353. doi: 10.1161/CIRCULATIONAHA.110.000901. [DOI] [PubMed] [Google Scholar]
- 101.Ellis S.G., Kereiakes D.J., Metzger D.C., Caputo R.P., Rizik D.G., Teirstein P.S., Litt M.R., Kini A., Kabour A., Marx S.O., Popma J.J., McGreevy R., Zhang Z., Simonton C., Stone G.W., Investigators A.I. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N. Engl. J. Med. 2015;373(20):1905–1915. doi: 10.1056/NEJMoa1509038. [DOI] [PubMed] [Google Scholar]
- 102.Stone G.W., Gao R., Kimura T., Kereiakes D.J., Ellis S.G., Onuma Y., Cheong W.-F., Jones-McMeans J., Su X., Zhang Z., Serruys P.W. 1-year outcomes with the Absorb bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. 2016;387(10025):1277–1289. doi: 10.1016/S0140-6736(15)01039-9. [DOI] [PubMed] [Google Scholar]
- 103.Mani G., Feldman M.D., Patel D., Agrawal C.M. Coronary stents: a materials perspective. Biomaterials. 2007;28(9):1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 104.B.R. Biomaterials Science: an Introduction to Materials in Medicine/-second ed..
- 105.McMahon S., Bertollo N., Cearbhaill E.D.O., Salber J., Pierucci L., Duffy P., Dürig T., Bi V., Wang W. Bio-resorbable polymer stents: a review of material progress and prospects. Prog. Polym. Sci. 2018;83:79–96. [Google Scholar]
- 106.Scarcello E., Lobysheva I., Bouzin C., Jacques P.J., Lison D., Dessy C. Endothelial dysfunction induced by hydroxyl radicals - the hidden face of biodegradable Fe-based materials for coronary stents, Materials science & engineering. C, Materials for biological applications. 2020;112:110938. doi: 10.1016/j.msec.2020.110938. [DOI] [PubMed] [Google Scholar]
- 107.Zhou C., Feng X., Shi Z., Song C., Cui X., Zhang J., Li T., Toft E.S., Ge J., Wang L., Zhang H. Research on elastic recoil and restoration of vessel pulsatility of Zn-Cu biodegradable coronary stents, Biomedizinische Technik. Biomed. Eng. 2020;65(2):219–227. doi: 10.1515/bmt-2019-0025. [DOI] [PubMed] [Google Scholar]
- 108.Lafont A., Yang Y. Magnesium stent scaffolds: DREAMS become reality. Lancet. 2016;387(10013):3–4. doi: 10.1016/S0140-6736(15)00804-1. [DOI] [PubMed] [Google Scholar]
- 109.Li H., Peng F., Wang D., Qiao Y., Xu D., Liu X. Layered double hydroxide/poly-dopamine composite coating with surface heparinization on Mg alloys: improved anticorrosion, endothelialization and hemocompatibility. Biomaterials Science. 2018;6(7):1846–1858. doi: 10.1039/c8bm00298c. [DOI] [PubMed] [Google Scholar]
- 110.Wang P., Liu J., Shen S., Li Q., Luo X., Xiong P., Gao S., Yan J., Cheng Y., Xi T. In vitro and in vivo studies on two-step alkali-fluoride-treated Mg-Zn-Y-Nd alloy for vascular stent application: enhancement in corrosion resistance and biocompatibility. ACS Biomater. Sci. Eng. 2019;5(7):3279–3292. doi: 10.1021/acsbiomaterials.9b00140. [DOI] [PubMed] [Google Scholar]
- 111.Wu J., Zhao D., Lee B., Roy A., Yao R., Chen S., Dong Z., Heineman W.R., Kumta P.N. Effect of lithium and aluminum on the mechanical properties, in vivo and in vitro degradation, and toxicity of multiphase ultrahigh ductility Mg-Li-Al-Zn quaternary alloys for vascular stent application. ACS Biomater. Sci. Eng. 2020;6(4):1950–1964. doi: 10.1021/acsbiomaterials.9b01591. [DOI] [PubMed] [Google Scholar]
- 112.Ye C., Wang J., Zhao A., He D., Maitz M.F., Zhou N., Huang N. Atorvastatin eluting coating for magnesium‐based stents: control of degradation and endothelialization in a microfluidic assay and in vivo. Advanced Materials Technologies. 2020;5(4) [Google Scholar]
- 113.Shi J., Miao X., Fu H., Jiang A., Liu Y., Shi X., Zhang D., Wang Z. In vivo biological safety evaluation of an iron-based bioresorbable drug-eluting stent. Biometals. 2020;33(4–5):217–228. doi: 10.1007/s10534-020-00244-2. [DOI] [PubMed] [Google Scholar]
- 114.Drelich A.J., Zhao S., Guillory R.J., 2nd, Drelich J.W., Goldman J. Long-term surveillance of zinc implant in murine artery: surprisingly steady biocorrosion rate. Acta Biomater. 2017;58:539–549. doi: 10.1016/j.actbio.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guillory R.J., 2nd, Bowen P.K., Hopkins S.P., Shearier E.R., Earley E.J., Gillette A.A., Aghion E., Bocks M., Drelich J.W., Goldman J. Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants. ACS Biomater. Sci. Eng. 2016;2(12):2355–2364. doi: 10.1021/acsbiomaterials.6b00591. [DOI] [PubMed] [Google Scholar]
- 116.Bennett J., Vanhaverbeke M., Vanden Driessche N., Hiltrop N., Adriaenssens T., Desmet W., Sinnaeve P., Dubois C. The drug-eluting resorbable magnesium vascular scaffold in complex coronary bifurcations: insights from an in vivo multimodality imaging study. EuroIntervention. 2018;13(17):2036–2046. doi: 10.4244/EIJ-D-17-00248. [DOI] [PubMed] [Google Scholar]
- 117.Wang Z., Zheng Q., Guan S., Sun Z., Liu S., Zhang B., Duan T., Xu K. In vitro and in vivo assessment of the biocompatibility of a paclitaxel-eluting poly-l-lactide-coated Mg-Zn-Y-Nd alloy stent in the intestine, Materials science & engineering. C, Materials for biological applications. 2019;105 doi: 10.1016/j.msec.2019.110087. [DOI] [PubMed] [Google Scholar]
- 118.Wu J., Mady L.J., Roy A., Aral A.M., Lee B., Zheng F., Catalin T., Chun Y., Wagner W.R., Yang K., Trejo Bittar H.E., Chi D., Kumta P.N. In-vivo efficacy of biodegradable ultrahigh ductility Mg-Li-Zn alloy tracheal stents for pediatric airway obstruction. Communications Biology. 2020;3(1):787. doi: 10.1038/s42003-020-01400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bian D., Qin L., Lin W., Shen D., Qi H., Shi X., Zhang G., Liu H., Yang H., Wang J., Zhang D., Zheng Y. Magnetic resonance (MR) safety and compatibility of a novel iron bioresorbable scaffold. Bioactive materials. 2020;5(2):260–274. doi: 10.1016/j.bioactmat.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin W., Zhang H., Zhang W., Qi H., Zhang G., Qian J., Li X., Qin L., Li H., Wang X., Qiu H., Shi X., Zheng W., Zhang D., Gao R., Ding J. In vivo degradation and endothelialization of an iron bioresorbable scaffold. Bioactive materials. 2021;6(4):1028–1039. doi: 10.1016/j.bioactmat.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin W.-J., Zhang D.-Y., Zhang G., Sun H.-T., Qi H.-P., Chen L.-P., Liu Z.-Q., Gao R.-L., Zheng W. Design and characterization of a novel biocorrodible iron-based drug-eluting coronary scaffold. Mater. Des. 2016;91:72–79. [Google Scholar]
- 122.Mandal S., Viraj, Nandi S.K., Roy M. Effects of multiscale porosity and pore interconnectivity on in vitro and in vivo degradation and biocompatibility of Fe-Mn-Cu scaffolds. J. Mater. Chem. B. 2021;9(21):4340–4354. doi: 10.1039/d1tb00641j. [DOI] [PubMed] [Google Scholar]
- 123.Coyan G.N., D'Amore A., Matsumura Y., Pedersen D.D., Luketich S.K., Shanov V., Katz W.E., David T.E., Wagner W.R., Badhwar V. In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve. J. Thorac. Cardiovasc. Surg. 2019;157(5):1809–1816. doi: 10.1016/j.jtcvs.2018.09.128. [DOI] [PubMed] [Google Scholar]
- 124.Kandala B., Zhang G., C L.C., Paquin M., Chagnon M., Begun D., Shanov V. Preliminary study on modelling, fabrication by photo-chemical etching and in vivo testing of biodegradable magnesium AZ31 stents. Bioactive materials. 2021;6(6):1663–1675. doi: 10.1016/j.bioactmat.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koo Y., Tiasha T., Shanov V.N., Yun Y. Expandable Mg-based helical stent assessment using static, dynamic, and porcine ex vivo models. Sci. Rep. 2017;7(1):1173. doi: 10.1038/s41598-017-01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lipinski M.J., Acampado E., Cheng Q., Adams L., Torii S., Gai J., Torguson R., Hellinga D.G., Joner M., Harder C., Zumstein P., Finn A.V., Kolodgie F.D., Virmani R., Waksman R. Comparison of acute thrombogenicity for magnesium versus stainless steel stents in a porcine arteriovenous shunt model. EuroIntervention. 2019;14(13):1420–1427. doi: 10.4244/EIJ-D-17-00958. [DOI] [PubMed] [Google Scholar]
- 127.Liu X.N., Qu C.J., Zhang Y.B., Fang J., Teng L.Q., Zhang X.Y., Shen C.Y. A novel sirolimus-eluting biodegradable magnesium-based alloy scaffold: six-month results in porcine peripheral arteries. Clin. Investigative Med. 2021;44(1):E28–E37. doi: 10.25011/cim.v44i1.35292. [DOI] [PubMed] [Google Scholar]
- 128.Menze R., Wittchow E. In vitro and in vivo evaluation of a novel bioresorbable magnesium scaffold with different surface modifications. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(9):1292–1302. doi: 10.1002/jbm.b.34790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi Y., Zhang L., Chen J., Zhang J., Yuan F., Shen L., Chen C., Pei J., Li Z., Tan J., Yuan G. In vitro and in vivo degradation of rapamycin-eluting Mg-Nd-Zn-Zr alloy stents in porcine coronary arteries, Materials science & engineering. C, Materials for biological applications. 2017;80:1–6. doi: 10.1016/j.msec.2017.05.124. [DOI] [PubMed] [Google Scholar]
- 130.Wittchow E., Adden N., Riedmüller J., Savard C., Waksman R., Braune M. Bioresorbable drug-eluting magnesium-alloy scaffold: design and feasibility in a porcine coronary model. EuroIntervention. 2013;8(12):1441–1450. doi: 10.4244/EIJV8I12A218. [DOI] [PubMed] [Google Scholar]
- 131.Li X., Zhang W., Lin W., Qiu H., Qi Y., Ma X., Qi H., He Y., Zhang H., Qian J., Zhang G., Gao R., Zhang D., Ding J. Long-term efficacy of biodegradable metal-polymer composite stents after the first and the second implantations into porcine coronary arteries. ACS Appl. Mater. Interfaces. 2020;12(13):15703–15715. doi: 10.1021/acsami.0c00971. [DOI] [PubMed] [Google Scholar]
- 132.Hehrlein C., Schorch B., Haberstroh J., Bode C., Mey L., Schwarzbach H., Kinscherf R., Meckel S., Schiestel S., Kovacs A., Fischer H., Nennig E. Bioresorbable zinc stent with ultra-thin center struts attenuates stent jail in porcine femoral artery bifurcations. Minim Invasive Ther. Allied Technol. 2020:1–8. doi: 10.1080/13645706.2020.1770797. [DOI] [PubMed] [Google Scholar]
- 133.Hehrlein C., Schorch B., Kress N., Arab A., von Zur Muhlen C., Bode C., Epting T., Haberstroh J., Mey L., Schwarzbach H., Kinscherf R., Stachniss V., Schiestel S., Kovacs A., Fischer H., Nennig E. Zn-alloy provides a novel platform for mechanically stable bioresorbable vascular stents. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bartosch M., Peters H., Koerner A., Schmitt B., Berger F., Hort N., Witte F. New methods for in vivo degradation testing of future stent materials. Mater. Corros. 2018;69(2):156–166. [Google Scholar]
- 135.Waksman R., Erbel R., Di Mario C., Bartunek J., de Bruyne B., Eberli F.R., Erne P., Haude M., Horrigan M., Ilsley C., Bose D., Bonnier H., Koolen J., Luscher T.F., Weissman N.J., Investigators P.-A. Early- and long-term intravascular ultrasound and angiographic findings after bioabsorbable magnesium stent implantation in human coronary arteries. JACC Cardiovasc. Interv. 2009;2(4):312–320. doi: 10.1016/j.jcin.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 136.Erbel R., Di Mario C., Bartunek J., Bonnier J., de Bruyne B., Eberli F.R., Erne P., Haude M., Heublein B., Horrigan M., Ilsley C., Böse D., Koolen J., Lüscher T.F., Weissman N., Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369(9576):1869–1875. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]
- 137.Haude M., Erbel R., Erne P., Verheye S., Degen H., Böse D., Vermeersch P., Wijnbergen I., Weissman N., Prati F., Waksman R., Koolen J. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet. 2013;381(9869):836–844. doi: 10.1016/S0140-6736(12)61765-6. [DOI] [PubMed] [Google Scholar]
- 138.Haude M., Ince H., Abizaid A., Toelg R., Lemos P.A., von Birgelen C., Christiansen E.H., Wijns W., Neumann F.-J., Kaiser C., Eeckhout E., Lim S.T., Escaned J., Garcia-Garcia H.M., Waksman R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387(10013):31–39. doi: 10.1016/S0140-6736(15)00447-X. [DOI] [PubMed] [Google Scholar]
- 139.Jamshidi M., Rajabian M., Avery M.B., Sundararaj U., Ronsky J., Belanger B., Wong J.H., Mitha A.P. A novel self-expanding primarily bioabsorbable braided flow-diverting stent for aneurysms: initial safety results. J. Neurointerventional Surg. 2020;12(7):700–705. doi: 10.1136/neurintsurg-2019-015555. [DOI] [PubMed] [Google Scholar]
- 140.Luffy S.A., Wu J., Kumta P.N., Gilbert T.W. Evaluation of magnesium alloys for use as an intraluminal tracheal for pediatric applications in a rat tracheal bypass model. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(6):1844–1853. doi: 10.1002/jbm.b.34277. [DOI] [PubMed] [Google Scholar]
- 141.Daentzer D., Willbold E., Kalla K., Bartsch I., Welke B. Bioabsorbable interbody magnesium-polymer cage: degradation kinetics, biomechanical stiffness, and histological findings from an ovine cervical spine fusion model. Spine. 2014;39(20):1220–1227. doi: 10.1097/BRS.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 142.Wu S., Wu B., Liu M., Chen Z., Wang W., Anderson C.S., Sandercock P., Wang Y., Huang Y., Cui L., Pu C., Jia J., Zhang T., Liu X., Zhang S., Xie P., Fan D., Ji X., Wong K.-S.L., Wang L., Wu S., Wu B., Liu M., Chen Z., Wang W., Anderson C.S., Sandercock P., Wang Y., Huang Y., Cui L., Pu C., Jia J., Zhang T., Liu X., Zhang S., Xie P., Fan D., Ji X., Wong K.-S.L., Wang L., Wei C., Wang Y., Cheng Y., Liu Y., Li X., Dong Q., Zeng J., Peng B., Xu Y., Yang Y., Wang Y., Zhao G., Wang W., Xu Y., Yang Q., He Z., Wang S., You C., Gao Y., Zhou D., He L., Li Z., Yang J., Lei C., Zhao Y., Liu J., Zhang S., Tao W., Hao Z., Wang D., Zhang S. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 143.Campbell B.C.V., Khatri P. Stroke, The Lancet. 2020;396(10244):129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- 144.Inci S., Spetzler R.F. Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surg. Neurol. 2000;53(6):530–542. doi: 10.1016/s0090-3019(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 145.Lindberg M.R., Lamps L.W. In: Diagnostic Pathology: Normal Histology. second ed. Lindberg M.R., Lamps L.W., editors. Elsevier; 2018. Arteries; pp. 94–97. [Google Scholar]
- 146.Jinxuan W., Xuepu J., Yuhua H., Xiaolin R., Desha L., Dongchuan Y., Dongyu J., Kang Z., Jianhua T., Xiaoyan D. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regenerative Biomaterials. 2018;5(3):177–187. doi: 10.1093/rb/rby006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.A C.L., B F.D., A P.J.P. Cardiovascular stent design and vessel stresses: a finite element analysis. J. Biomech. 2005;38(8):1574–1581. doi: 10.1016/j.jbiomech.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 148.Shi W., Li H., Zhu T., Jin Y., Wang H., Yang J., Zhao D. Study on the bending behavior of biodegradable metal cerebral vascular stents using finite element analysis. J. Biomech. 2020;108:109856. doi: 10.1016/j.jbiomech.2020.109856. [DOI] [PubMed] [Google Scholar]
- 149.Han Y., Lu W. Optimizing the deformation behavior of stent with nonuniform Poisson's ratio distribution for curved artery. Journal of the mechanical behavior of biomedical materials. 2018;88:442–452. doi: 10.1016/j.jmbbm.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Shen X., Zhu H., Jiang J., Deng Y., Ji S. Multi-objective optimization design of balloon-expandable coronary stent. Cardiovascular Engineering and Technology. 2019;10(1):10–21. doi: 10.1007/s13239-019-00401-w. [DOI] [PubMed] [Google Scholar]
- 151.Lehoux S., Tedgui A. Cellular mechanics and gene expression in blood vessels. J. Biomech. 2003;36(5):631–643. doi: 10.1016/s0021-9290(02)00441-4. [DOI] [PubMed] [Google Scholar]
- 152.Ng J., Bourantas C.V., Torii R., Ang H.Y., Tenekecioglu E., Serruys P.W., Foin N. Local hemodynamic forces after stenting: implications on restenosis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017;37(12):2231–2242. doi: 10.1161/ATVBAHA.117.309728. [DOI] [PubMed] [Google Scholar]
- 153.Gu X.N., Lu Y., Wang F., Lin W., Li P., Fan Y. The effect of tensile and fluid shear stress on the in vitro degradation of magnesium alloy for stent applications. Bioactive materials. 2018;3(4):448–454. doi: 10.1016/j.bioactmat.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li N., Guo C., Wu Y.H., Zheng Y.F., Ruan L.Q. Comparative study on corrosion behaviour of pure Mg and WE43 alloy in static, stirring and flowing Hank's solution, Corrosion Engineering. Sci. Technol. 2013;47(5):346–351. [Google Scholar]
- 155.Cattaneo G., Schumacher M., Wolfertz J., Jost T., Meckel S. Combined selective cerebral hypothermia and mechanical artery recanalization in acute ischemic stroke: in vitro study of cooling performance. AJNR. American Journal of Neuroradiology. 2015;36(11):2114–2120. doi: 10.3174/ajnr.A4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J., Wang L., Guan S., Zhu S., Ren C., Hou S. Microstructure and corrosion properties of as sub-rapid solidification Mg-Zn-Y-Nd alloy in dynamic simulated body fluid for vascular stent application. J. Mater. Sci. Mater. Med. 2010;21(7):2001–2008. doi: 10.1007/s10856-010-4063-z. [DOI] [PubMed] [Google Scholar]
- 157.Witte F., Hort N., Vogt C., Cohen S., Kainer K.U., Willumeit R., Feyerabend F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008;12(5):63–72. [Google Scholar]
- 158.Li J.A., Chen L., Zhang X.Q., Guan S.K. Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials, Materials science & engineering. C, Materials for biological applications. 2020;109:110607. doi: 10.1016/j.msec.2019.110607. [DOI] [PubMed] [Google Scholar]
- 159.Boland E.L., Shine R., Kelly N., Sweeney C.A., McHugh P.E. A review of material degradation modelling for the analysis and design of bioabsorbable stents. Ann. Biomed. Eng. 2016;44(2):341–356. doi: 10.1007/s10439-015-1413-5. [DOI] [PubMed] [Google Scholar]
- 160.Li Y., Yan J., Zhou W., Xiong P., Wang P., Yuan W., Zheng Y., Cheng Y. In vitro degradation and biocompatibility evaluation of typical biodegradable metals (Mg/Zn/Fe) for the application of tracheobronchial stenosis. Bioactive materials. 2019;4:114–119. doi: 10.1016/j.bioactmat.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang J.J., Mack W.J., Saver J.L., Sanossian N. Magnesium: potential roles in neurovascular disease. Front. Neurol. 2014;5 doi: 10.3389/fneur.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Westermaier T., Stetter C., Kunze E., Willner N., Raslan F., Vince G.H., Ernestus R.I. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Exp. Transl. Stroke Med. 2013;5(1) doi: 10.1186/2040-7378-5-6. 6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Slutsky I., Abumaria N., Wu L.J., Huang C., Zhang L., Li B., Zhao X., Govindarajan A., Zhao M.G., Zhuo M., Tonegawa S., Liu G. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65(2):165–177. doi: 10.1016/j.neuron.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 164.Wang J., Wang L., Guan S., Zhu S., Ren C., Hou S. Microstructure and corrosion properties of as sub-rapid solidification Mg–Zn–Y–Nd alloy in dynamic simulated body fluid for vascular stent application. J. Mater. Sci. Mater. Med. 2010;21(7):2001–2008. doi: 10.1007/s10856-010-4063-z. [DOI] [PubMed] [Google Scholar]
- 165.Unbehau R., Luthringer-Feyerabend B.J.C., Willumeit-Romer R. The impact of brain cell metabolism and extracellular matrix on magnesium degradation. Acta Biomater. 2020;116:426–437. doi: 10.1016/j.actbio.2020.08.043. [DOI] [PubMed] [Google Scholar]
- 166.Gu X., Zheng Y., Cheng Y., Zhong S., Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30(4):484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 167.Song G., Atrens A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999;1(1):11–33. [Google Scholar]
- 168.Mao L., Shen L., Chen J., Zhang X., Kwak M., Wu Y., Fan R., Zhang L., Pei J., Yuan G., Song C., Ge J., Ding W. A promising biodegradable magnesium alloy suitable for clinical vascular stent application. Sci. Rep. 2017;7:46343. doi: 10.1038/srep46343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Coy A.E., Viejo F., Skeldon P., Thompson G.E. Susceptibility of rare-earth-magnesium alloys to micro-galvanic corrosion. Corrosion Sci. 2010;52(12):3896–3906. [Google Scholar]
- 170.White A.R. A greater focus on metals in biomedicine and neuroscience is needed. BMC pharmacology & toxicology. 2016;17(1):53. doi: 10.1186/s40360-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bayir A., Ak A., Kara H., Sahin T.K. Serum and cerebrospinal fluid magnesium levels, Glasgow Coma Scores, and in-hospital mortality in patients with acute stroke. Biol. Trace Elem. Res. 2009;130(1):7–12. doi: 10.1007/s12011-009-8318-9. [DOI] [PubMed] [Google Scholar]
- 172.Romaris E.M., Cervantes, Lopez J.M., Marcen J.F. Concentration of calcium and magnesium and trace elements (zinc, copper, iron and manganese) in cerebrospinal fluid: a try of a pathophysiological classification. J. Trace Elem. Med. Biol. 2011;25(Suppl 1):S45–S49. doi: 10.1016/j.jtemb.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 173.Horn J., Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001;32(2):570–576. doi: 10.1161/01.str.32.2.570. [DOI] [PubMed] [Google Scholar]
- 174.Lai M., Wang D., Lin Z., Zhang Y. Small molecule copper and its relative metabolites in serum of cerebral ischemic stroke patients. J. Stroke Cerebrovasc. Dis.: the official journal of National Stroke Association. 2016;25(1):214–219. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 175.Gill D., Monori G., Tzoulaki I., Dehghan A. Iron status and risk of stroke. Stroke. 2018;49(12):2815–2821. doi: 10.1161/STROKEAHA.118.022701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aschner M., Guilarte T.R., Schneider J.S., Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007;221(2):131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alimonti A., Bocca B., Pino A., Ruggieri F., Forte G., Sancesario G. Elemental profile of cerebrospinal fluid in patients with Parkinson's disease. J. Trace Elem. Med. Biol. 2007;21(4):234–241. doi: 10.1016/j.jtemb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 178.Gellein K., Roos P.M., Evje L., Vesterberg O., Flaten T.P., Nordberg M., Syversen T. Separation of proteins including metallothionein in cerebrospinal fluid by size exclusion HPLC and determination of trace elements by HR-ICP-MS. Brain Res. 2007;1174:136–142. doi: 10.1016/j.brainres.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 179.Gavazzo P., Mazzolini M., Tedesco M., Marchetti C. Nickel differentially affects NMDA receptor channels in developing cultured rat neurons. Brain Res. 2006;1078(1):71–79. doi: 10.1016/j.brainres.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 180.Gavazzo P., Tedesco M., Chiappalone M., Zanardi I., Marchetti C. Nickel modulates the electrical activity of cultured cortical neurons through a specific effect on N-methyl-D-aspartate receptor channels. Neuroscience. 2011;177:43–55. doi: 10.1016/j.neuroscience.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 181.Bogdanovic M., Bulat P. Biliary function in workers occupationally exposed to aluminium dust and fumes. Arh. Hig. Rada. Toksikol. 2008;59(2):135–139. doi: 10.2478/10004-1254-59-2008-1860. [DOI] [PubMed] [Google Scholar]
- 182.Carmen A., José Luis A., Eduardo S.-m., Ma Jesús O.G., Ma G. Pilar, added after anoxia-reoxigenation stress, genistein rescues from death the rat embryo cortical neurons. Neurosci. Med. 2010;1(2):50–59. [Google Scholar]
- 183.Kawahara M., Kato-Negishi M. Link between aluminum and the pathogenesis of alzheimer's disease: the integration of the aluminum and amyloid cascade hypotheses. Journal of Alzheimer's Disease 2011. 2011:276393. doi: 10.4061/2011/276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.al-Deeb S., al-Moutaery K., Bruyn G.W., Tariq M. Neuroprotective effect of selenium on iminodipropionitrile-induced toxicity. J. Psychiatr. Neurosci. 1995;20(3):189–192. [PMC free article] [PubMed] [Google Scholar]