Abstract
Astrocyte heterogeneity is an emerging concept in which astrocytes within or between brain regions show variable morphological and/or gene expression profiles that presumably reflect different functional roles. Recent evidence indicates that retrotrapezoid nucleus (RTN) astrocytes sense changes in tissue CO2/H+ to regulate respiratory activity; however, mechanism(s) by which they do so remain unclear. Alterations in inward K+ currents represent a potential mechanism by which CO2/H+ signals may be conveyed to neurons. Here, we use slice electrophysiology in rats of either sex to show that RTN astrocytes intrinsically respond to CO2/H+ by inhibition of an inward rectifying potassium (Kir) conductance and depolarization of the membrane, while cortical astrocytes do not exhibit such CO2/H+-sensitive properties. Application of Ba2+ mimics the effect of CO2/H+ on RTN astrocytes as measured by reductions in astrocyte Kir-like currents and increased RTN neuronal firing. These CO2/H+-sensitive currents increase developmentally, in parallel to an increased expression in Kir4.1 and Kir5.1 in the brainstem. Finally, the involvement of Kir5.1 in the CO2/H+-sensitive current was verified using a Kir5.1 KO rat. These data suggest that Kir inhibition by CO2/H+ may govern the degree to which astrocytes mediate downstream chemoreceptive signaling events through cell-autonomous mechanisms. These results identify Kir channels as potentially important regional CO2/H+ sensors early in development, thus expanding our understanding of how astrocyte heterogeneity may uniquely support specific neural circuits and behaviors.
Keywords: chemoreception, retrotrapezoid nucleus, astrocyte physiology, slice electrophysiology, respiratory regulation
Introduction
Astrocytes play essential roles in brain physiology, from maintaining extracellular ion and transmitter concentrations to directly communicating with neurons to regulate their activity. An emerging concept in the research of astrocytes is their heterogeneity: while once considered a homogenous cell type across the CNS, recent research indicates that astrocytes vary in their morphology (Matyash and Kettenmann 2010), gene and protein expression profile (Chai et al. 2017; Morel et al. 2017; Rusnakova et al. 2013), ion channel and receptor expression (Bordey and Sontheimer 2000; Hoft et al. 2014; Zhou and Kimelberg 2000), physiology (Verkhratsky and Nedergaard 2018), response to stimulus (Cholewinski and Wilkin 1988; el-Etr et al. 1989), and interaction with neurons (Ben Haim and Rowitch 2017; Oberheim et al. 2012; Xin and Bonci 2018) across different brain regions and developmental stages (Chaboub and Deneen 2012; Farmer and Murai 2017; Zhang and Barres 2010). Thus, understanding how astrocytes differ between brain areas may provide insight regarding the difference in their roles in CNS physiology from region to region.
We are particularly interested in contributions of astrocytes to control of breathing. A brainstem region known as the retrotrapezoid nucleus (RTN) regulates depth and frequency of breathing in response to changes in CO2/H+ (Guyenet and Bayliss 2015), process known as central respiratory chemoreception. Chemosensitive RTN neurons intrinsically respond to reduced pH by increasing their firing rate to drive respiration and correct changes in blood gases. Disruption of RTN neurons genetically (Dubreuil et al. 2008), by targeted lesion (Takakura et al. 2008), or selective ablation of their pH sensing mechanisms (Kumar et al. 2015) results in a dramatically reduced ventilatory response to CO2 or respiratory acidosis. This evidence that RTN neurons are intrinsically pH sensitive does not preclude the possibility that CO2/H+-dependent output of this region is also subject to modulation by surrounding cells. Glia in the region have been proposed as functional pH detectors since their depolarization in response to acidification was first recognized over 40 years ago (Fukuda et al. 1978). Indeed, pharmacologic depolarization (Erlichman et al. 1998; Holleran et al. 2001; Sobrinho et al. 2017) or channel rhodopsin-mediated stimulation (Gourine et al. 2010) of astrocytes is sufficient to activate RTN neurons in vitro and increase respiration in vivo. These results establish RTN astrocytes as elements in RTN chemotransduction. However, specific upstream mechanism(s) by which CO2/H+ “activates” astrocytes and leads to altered respiratory drive appear varied and remain unclear.
Recent evidence suggests that RTN astrocytes may sense lowered pH by inhibition of inwardly rectifying potassium currents (Kir) (Wenker et al. 2010), which are produced by channels of the Kir family (for review, see (Hibino et al. 2010)). These currents are characterized by larger inward than outward currents (inward rectification). Kir channels are tetramers that can be composed of four identical subunits (homotetrameric, e.g. Kir4.1) or pairs of different subunits (heterotetrameric, e.g. Kir4.1/5.1). The most abundant glial Kir channel is Kir4.1 (Tang et al. 2009), and as such, is a major determinant of astrocyte function (for review, see, Nwaobi et al. 2016). Some Kir channels are intrinsically H+ sensitive, including Kir4.1 channels, which possess an intracellular H+ sensor, albeit in a non-physiological range (pKa=6.7) (Casamassima et al. 2003; Xu et al. 2000). Interestingly, when heteromerized with Kir5.1, Kir4.1/5.1 channels are H+-sensitive in the physiological range (pKa=7.45). Thus, differentially expressed Kir channels and/or different combination of Kir channel subunits may contribute to regional differences in astrocyte CO2/H+ sensing. Additionally, robust developmental upregulation of Kir4.1 (Nwaobi et al. 2014) coincides with enhancement of the ventilatory response to CO2/H+ from the time of birth to adulthood (Davis et al. 2006; Putnam et al. 2005). However, to date, no differences in Kir currents have been shown to contribute to developmental or regional specificity of astrocyte chemosensitivity.
In the present study, we used slice-patch electrophysiology to characterize functional heterogeneity between RTN and cortical astrocytes based on Kir CO2/H+-sensitive currents. We found that RTN but not cortical astrocytes sense changes in CO2/H+ by inhibition of Kir-like currents beginning at postnatal day 2–3 (PND2–3; the earliest time point studied), and that this CO2/H+-sensitive conductance increases over postnatal development. We also identified a requisite role of Kir5.1 in RTN astrocyte CO2/H+-sensitivity. These collective findings suggest that Kir inhibition by CO2/H+ is another form of astrocyte heterogeneity, which may have implications for the development of resilience to respiratory failure throughout early life.
Results
While a limited number of studies have evaluated the function of astrocytes in any brainstem region, none have comprehensively characterized their developmental electrophysiological properties as has been done in the forebrain (Zhong et al. 2016; Zhou et al. 2006). Only one previous study evaluated the current-voltage (I-V) relationships in a small number of RTN astrocytes in response to CO2/H+ (Wenker et al. 2010). This limited number of electrophysiological studies is in contrast to a larger cohort of calcium imaging studies (Forsberg et al. 2017; Gourine et al. 2010; Kasymov et al. 2013; Sheikhbahaei et al. 2018), which show that cortical and brainstem astrocytes differ in their responses to pH changes. To gain mechanistic insight into the basis of differential astrocyte CO2/H+ sensitivity, we characterized properties of CO2/H+ sensitive currents in cortical and RTN astrocytes during the first two weeks of life.
Whole-cell current-clamp recordings from astrocytes in the RTN and in deep layer 1 and layer 2/3 of the motor cortex were made under control conditions (95% O2, 5% CO2, pH=7.30) and hypercapnic conditions (90% O2, 10% CO2, pH=7.00) in slices from Sprague Dawley (SD) rat pups at three time points: PND2–3, PND4–7, and PND9–13. Because we were interested in the intrinsic, cell-autonomous properties of astrocytes in these regions, all electrophysiological experiments were performed in tetrodotoxin (TTX) unless otherwise noted. Additionally, we utilized a reporter SD rat model that expresses eGFP under the promoter of the gene encoding S100β, a predominantly astrocytic Ca2+-binding protein, to assist in identifying RTN astrocytes. S100β has long been considered an astrocyte marker and co-expression with glial fibrillary acidic protein (GFAP) is considered a reliable means of distinguishing astrocytes from other glial cell populations, including NG2 cells (Grosche et al. 2013; Nishiyama et al. 1996; Ogata and Kosaka 2002; Raponi et al. 2007; Zhu et al. 2008). Our previously published work in cortex (Nwaobi et al. 2014) and data obtained from RTN (Supplementary Figure 1) indicate eGFP+ cells at the ventral surface of the RTN (S100β-eGFP) also have emanating processes that are GFAP+, suggesting the majority of eGFP+ cells are astrocytes. Thus, astrocytes were identified by eGFP expression, relatively round somas, no predominant outward rectification, the resting membrane potential was more negative than −75mV, and the cell recording remained stable through the experiment. While we recognize that NG2 cells and astrocytes display some similar electrophysiological characteristics, we excluded cells that had the “typical” appearance of NG2 cells (predominantly outwardly rectifying, and Na+ channel activation in experiments without TTX). However, we cannot exclude the possibility we inadvertently evaluated some fraction of NG2 cells, however, based on our exclusion criteria- NG2 cells would represent a small fraction of the cells we recorded from. Although we were easily able to detect cells using the above criteria that remained stable upon whole-cell breakthrough at PND2–3 in the RTN, we were unable to find and record from such cells in the cortex at this early time point.
Electrical properties of cortical and RTN Astrocytes
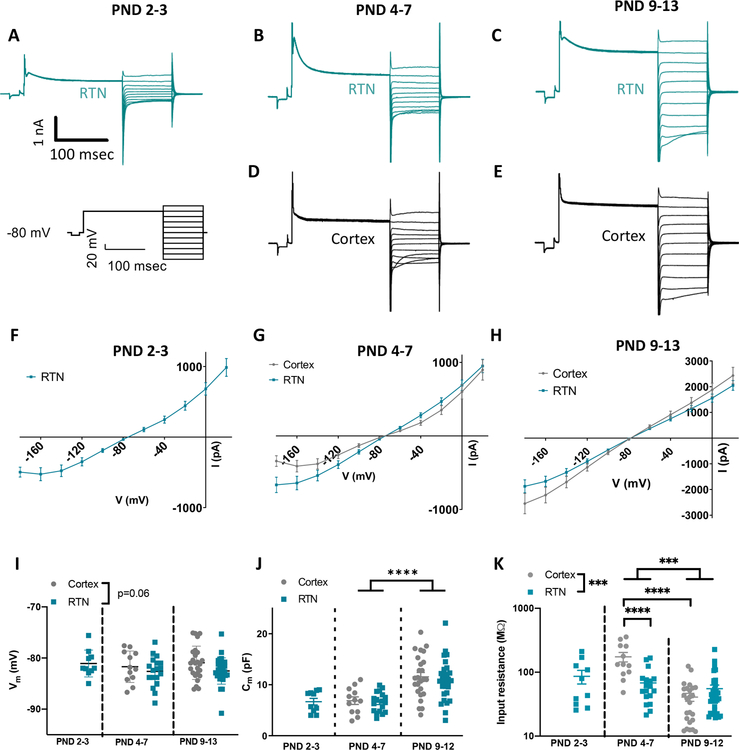
Astrocyte Kir conductances were evaluated using a voltage step protocol that included a 0 mV pre-pulse to inactivate transient outward rectifying K+ channels (Figure 1A–E representative traces, protocol below Figure 1A) (Olsen 2012). Current responses to voltage steps from −180 mV to 120 mV were recorded; for our analysis, we focused on currents measured from −180 mV to −80 mV to avoid symmetry around ~−80 mV that obscures the main effect. Current density did not significantly differ between regions both at PND4–7 (Figure 1G main effect of region: F(1,28)=3.8, p=0.06; RTN: n=18; cortex: n=12) and at PND9–13 (Figure 1H main effect of region: F(1,54)=2.1, p=0.15; RTN: n=31; cortex: n=25). As noted above, we were unable to identify and record from astrocytes at PND2–3 in cortex.
Figure 1. Cortical and RTN astrocytes display similar electrophysiological phenotypes under control conditions.
A-E. Representative traces of baseline voltage step in the RTN (A-C) and cortex (D-E) across three developmental time points (2 for cortex). Voltage step protocol utilized to elicit currents is shown on the left.
F-H. Currents elicited in cortical and RTN astrocytes do not differ from one another under control conditions (RTN: PND2–3 n=10, PND4–7 n=18, PND 9–13 n=31; cortex: PND 4–7 n=12, PND 9–13 n=25). As noted in the text, we were unable to identify or record from astrocytes at PND2–3 in cortex.
I. Plot of resting membrane potential of RTN and cortical astrocytes as a function of age, obtained in current clamp ~1 minute after whole-cell breakthrough. Developmental but not regional differences were observed throughout early postnatal development (RTN: PND2–3 n=10, PND4–7 n=18, PND 9–13 n=31; cortex: PND 4–7 n=12, PND 9–13 n=25).
J. Developmental but not regional differences were observed in whole cell capacitance (RTN: PND2–3 n=10, PND4–7 n=18, PND 9–13 n=30; cortex: PND 4–7 n=12, PND 9–13 n=25).
K. Input resistance decreases throughout early postnatal development in cortical astrocytes (PND 4–7 n=12, PND 9–13 n=25) but not RTN astrocytes (PND2–3 n=10, PND4–7 n=18, PND 9–13 n=31).
The RTN astrocyte resting membrane potential (Vm) was slighly hyperpolarized compaired to cortical astrocytes at similar developmental time points (RTN: PND2–3 Vm=−81.1±0.8 mV, n=10; PND4–7 Vm=−82.6±0.7 mV, n=18; PND9–13 Vm=−82.5±0.5 mV, n=31; cortex: PND4–7 Vm=−81.2±0.9 mV, n=12; PND9–13 Vm=−80.9±0.7 mV, n=25; 2-way ANOVA performed only on PND4–7 and PND9–13; main effect of age: F(1,82)=0.41, p=0.52; main effect of region: F(1,82)=3.5, p=0.06; interaction: F(1,82)=0.26, p=0.61; Figure 1I). Whole cell capacitance (Cm), a measure of cellular membrane area, increased in both regions across development, as expected with presumably increasing morphologic complexity paralleling maturation (RTN: PND2–3 Cm=6.7±0.7 pF, n=10; PND4–7 Cm=6.6±0.5 pF, n=18; PND9–13 Cm=10.9±0.7 pF, n=30; cortex: PND4–7 Cm=6.9±0.7 pF, n=12, PND9–13 Cm=11.6±0.8 pF, n=25; 2-way ANOVA performed only on PND4–7 and PND9–13; main effect of age: F(1,81)=31.2, p<0.0001; main effect of region: F(1,81)=0.3, p=0.56; interaction: F(1,81)=0.6, p=0.83; Figure 1J). We also found that astrocytes showed an age dependent decrease in input resistance specifically in the cortex (2-way ANOVA performed only on PND4–7 and PND9–13; main effect of age: F(1,82)=37.4, p<0.0001; interaction: F(1,92)=24.6, p<0.0001; for RTN: t(82)=0.9, p=0.94; for cortex: t(82)=7.2, p<0.0001), and lower input resistance in the RTN than in cortex (main effect of region: F(1,82)=14.9; p=0.0002), specifically at PND4–7 (t(82)=5.4, p<0.0001) but not in PND9–13 (t(82)=0.9, p=0.92) (cortical astrocyte input resistance: PND4–7, 174.0±29.4 MΩ; PND9–13, 40.9±5.8 MΩ; RTN astrocyte input resistance: PND2–3, 86.3±20.8 MΩ; PND4–7, 67.9±10.0 MΩ; PND9–13, 54.0±8.0 MΩ; n same as Vm; Figure 1K). Interestingly, there also appears to be regional heterogeneity in resting membrane potential and input resistance of RTN astrocytes at the early PND2–3 time point relative to neonatal hippocampal astrocytes per prior reports (Zhong et al. 2016).
RTN astrocytes display intrinsic CO2/H+-sensitive current; cortical astrocytes do not
Based on previous work that showed by two weeks of age, RTN astrocytes sense CO2/H+ by inhibition of a Kir-like conductance (Wenker et al. 2010), therefore, we next wanted to determine whether astrocytes in the cortex and RTN show different regional sensitivity to CO2/H+ prior to and including this developmental window. Whole-cell currents were recorded under control conditions (pHo ~7.30) and after an exposure to 10% CO2 (pHo ~7.00). Membrane conductance was monitored using a single voltage step to −120 mV every 20 seconds. We found that exposure to 10% CO2 (5–10 min, selected recordings from one PND9–13) minimally effected whole cell conductance in cortical astrocytes (Figure 2A). Conversely, RTN astrocytes showed a strong reversible decrease in conductance in response to this same level of CO2/H+ (Figure 2B, selected recordings from one PND9–13). This is also reflected in selected current tracings from one PND9–13 from each region in Figure 2C. As expected, bath application of Ba2+ at 100 μM, a concentration which inhibits astrocytic but not neuronal Kir currents (Cui et al. 2018; Olsen and Sontheimer 2008; Ransom and Sontheimer 1995), decreased conductance in both cortical and RTN astrocytes (Figure 2A–B). Furthermore, the CO2/H+-sensitive current (ICO2/H+), which was determined by subtracting I-V relationships recorded under each condition, underlying observed changes in conductance in RTN astrocytes also increased over the developmental window studied. We also found that RTN astrocytes demonstrate a ICO2/H+ at PND2–3 (Figure 2D). The currents were larger than cortical ICO2/H+ at both PND 4–7 (main effect of region: F(1,29)=7.9, p=0.009; RTN: n=18; cortex: n=13; Figure 2E) and PND9–13 (main effect of region: F(1,55)=6.2, p=0.0157; RTN: n=32; cortex: n=25; Figure 2F). Analysis of the development of the CO2/H+-sensitive current in the RTN reveals developmental increase of these currents (main effect of age: F(2,57)=9. 1, p=0.0003; Supplementary Figure 2).
Figure 2. CO2-sensitive current amplitude is greater in the RTN than the cortex.
A-B. Conductance of a representative cortical astrocyte (A, measured by a single −120 mV voltage step every 20 seconds) is inhibited by 100 μM Ba2+, and not by 10 % CO2. Conductance of a representative RTN astrocyte is inhibited by both (B).
C. Selected recordings from one PND9–13 RTN (top) and cortical (bottom) astrocyte demonstrating CO2-sensitive currents (right) yielded from subtraction of currents measured in 10% CO2 (middle) from currents measured in 5% CO2 (left).
D-F. CO2-sensitive current in RTN astrocytes is present as early as PND2–3 (D, n=10) and is significantly greater than that observed in cortical astrocytes at PND4–7 (E, cortex n=12, RTN n=28) and PND9–13 (F, cortex n=27, RTN n=32).
We next tested whether non-cell autonomous mechanisms may play a role in potentiating the astrocyte response to hypercapnia in either the cortex or the RTN. Thus, in a different group of PND9–13 cells, we performed identical experiments to those outlined thus far in the absence of TTX. We found that the I-V relationship of ICO2/H+ was similar under both experimental conditions in RTN astrocytes (main treatment effect: F(1,41)=0.2, p=0.670, TTX n=32, no TTX n=11; Supplementary Figure 3A). However, the I-V relationship of ICO2/H+ in cortical astrocytes changed when TTX was omitted from the bath solution (main treatment effect: F(1,38)=4.1, p=0.05, TTX n=25, no TTX n=15; Supplementary Figure 3B). This result suggests that yet unknown neuronal mechanisms may enhance ICO2/H+ in cortical astrocytes.
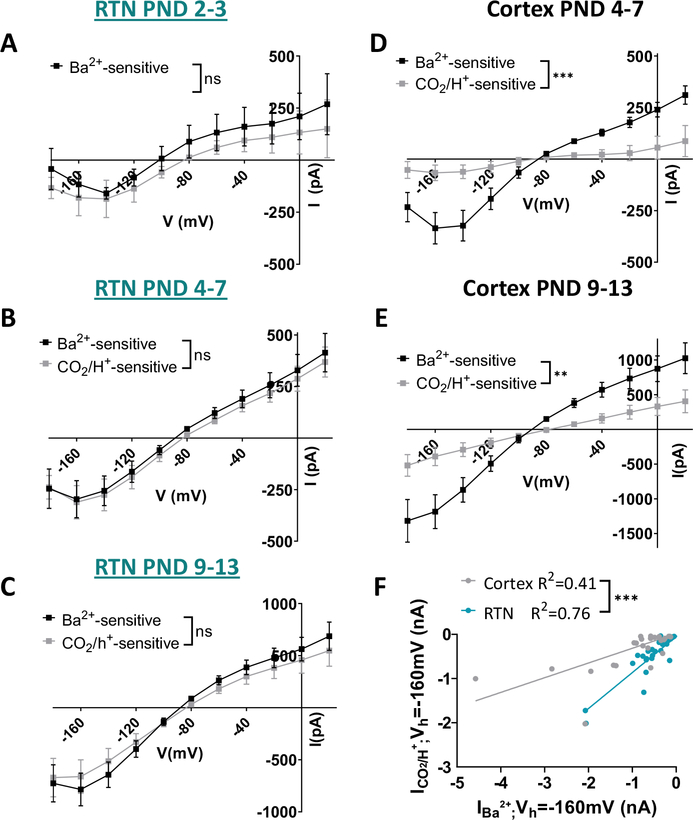
The above evidence suggests RTN astrocytes are intrinsically CO2/H+ sensitive by a mechanism involving inhibition of Kir channels. To further explore this possibility, we compared the density and voltage dependence of the CO2/H+-sensitive current to the Ba2+-sensitive current (measured in 5% CO2) in the same cells. If Kir channels mediate CO2/H+-sensitivity of RTN astrocytes, we expect CO2/H+- and Ba2+-sensitive currents to exhibit similar voltage-dependent properties. Consistent with this, we found that the I-V relationship of the CO2/H+- and Ba2+ sensitive currents were similar across all time points (main effect of treatment: PND2–3 F(1,5)=0.4, p=0.578, n=6; PND4–7 F(1,10)=0.2, p=0.628, n=11; PND9–13 F(1,13)=1.2, p=0.301, n=14; Figure 3A–C). Furthermore, amplitude of the Ba2+-sensitive current is positively correlated with amplitude of the CO2/H+-sensitive current in all RTN astrocytes (Figure 3F). In striking contrast to the RTN, we found that the CO2/H+-sensitive and Ba2+-sensitive I-V plots in cortical astrocytes were dissimilar at all time points considered. For example, CO2/H+-sensitive current densities are smaller and the I-V relationship more linear as compared to the Ba2+ sensitive current (main effect of treatment: PND4–7 F(1,8)=26.6, p=0.0009, n=9; PND9–13 F(1,18)=11.0, p=0.004, n=19; Figure 3D–E). The voltage-independent profile of the cortical CO2/H+-sensitive current is suggestive of a pH-sensitive leak-type K+ conductance reminiscent of two-pore domain K+ channel such as TASK1 (for review (Ryoo and Park 2016)), or TREK-1, which is expressed highly in cortical astrocytes (Zhang et al. 2014).
Figure 3. CO2-sensitive currents resemble Ba2+-sensitive currents in the RTN.
A-C. RTN astrocyte currents in response to 10% CO2 application (lighter squares) resemble those elicited by 100μM Ba2+ application (darker squares) in the same cells throughout early postnatal development (A, PND2–3 n=6; B, PND4–7 n=11; C, PND9–13, n=14).
D-E. Cortical astrocyte currents in response to 10% CO2 application (lighter circles) do not closely resemble those elicited by 100uM Ba2+ application in the same cells (darker circles) (D, PND4–7, n=9; E, PND9–13, n=19).
F. The correlation between the Ba2+-sensitive current (IBarium) and the CO2-sensitive current (ICO2) taken at a single voltage step (Vh=−160 mV) is significantly stronger in the RTN than in the cortex.
One of the possible mechanisms for inhibition of IKir is via hypercapnia-induced changes in extracellular K+ rather than a direct inhibition of the currents, as Kir4.1 channels decrease conductance with lower extracellular potassium, [K+]o, in a pH-independent manner (Edvinsson et al. 2011). However, this modulation generally does not occur under physiologic conditions in the brain. In order to evaluate this hypothesis, we analyzed the changes in the reversal potential (EK) of RTN astrocytes after perfusion of CO2, (Supplementary Figure 4) and found that the positive shifts in EK correspond to an increase, not decrease, in [K+]o per the Nernst equation. Thus, modulation of IKir by [K+]o cannot explain the observed decrease in Kir currents.
To further explore the possibility that CO2/H+ and Ba2+ are inhibiting the same Kir conductance in RTN astrocytes, we next wanted to determine whether Ba2+ could occlude ICO2/H+. Consistent with this possibility, in voltage clamp we found that in the continued presence of Ba2+ (100 uM) subsequent exposure to 10% CO2 minimally affected whole cell conductance (Figure 4A) and the amplitude of currents (Figure 4B). These data collectively suggest that Kir channels in RTN astrocytes are largely intrinsically CO2/H+-sensitive and that Kir constitutes the majority of CO2/H+-sensitive currents in these cells.
Figure 4. Application of 10% CO2 in the presence of 100 μM Ba2+ does not inhibit currents, but depolarizes RTN astrocytes.
A-B. Representative example (A) and summary (B, n=7) of application of 5% CO2 (A, black) 10% CO2 alone (A, grey), Ba2+ alone (A, red) or 10% CO2 in the presence of Ba2+ (A, blue). Ba2+ significantly diminishes the CO2-sensitive current (A-B).
C-D. Representative example of RTN astrocyte membrane voltage (C) and summary of both RTN and cortical astrocytes (D) shows that both RTN and cortical astrocytes are depolarized by Ba2+. However, only RTN astrocytes depolarize in response to 10% CO2, both with and without Ba2+. Letter code next to data refers to significance in a Sidak post-hoc comparisons after 2Way ANOVA, relative to other groups: a – RTN control, b – RTN 10% CO2, c – RTN Ba2+, d – RTN 10% CO2 + Ba2+, e – cortex control, f – cortex 10% CO2, g – cortex Ba2+, h – cortex 10% CO2 + Ba2+. Capital letter denotes inter-regional difference, and small letter denote effect of solution within region.
RTN astrocytes display intrinsic hypercapnia-induced depolarization
Previous studies suggest RTN astrocytes undergo intrinsic H+ or hypercapnia-induced depolarization (Fukuda et al. 1978; Ritucci et al. 2005; Sobrinho et al. 2017; Wenker et al. 2012), and that this process may lead to numerous downstream effects, including the further acidification of the extracellular space, Ca2+ influx, and the release of signaling molecules that further propagate the chemosensory message (Mulkey and Wenker 2011). Given the importance of Kir in setting the resting membrane potential of astrocytes, its inhibition by intracellular H+ has been suggested as a candidate mechanism of this depolarization (Wenker et al. 2010). To further explore this possibility, we wanted to determine whether a more modest CO2 stimulus (10% CO2) than utilized in previous in vitro studies can depolarize RTN or cortical astrocytes. In a subset of experiments separate from those described previously, we patched RTN and cortical astrocytes at PND9–13 and, in whole-cell current clamp mode, measured changes in resting membrane potential in response to 10% CO2 or Ba2+ alone and in combination (Figure 4C–D). We found that bath application of Ba2+ (100 μM) consistently depolarized cortical and RTN astrocytes (RTN: Δ8.3±1.3 mV, t(15)=6.3, p<.0001; cortex: Δ15.2±2.7 mV, t(6)=5.649, p=.008). However, only RTN astrocytes showed a depolarizing voltage response to 10% CO2 alone (RTN: Δ3.5±1.0 mV, t(21)=3.5, p=0.014; cortex: Δ0.3±0.5 mV, t(6)=0.6, p=0.998)). As above (Figure 4C–D), exposure to 10% CO2 also depolarized RTN astrocyte membrane potential in the presence of Ba2+ (Δ4.8±0.7 mV, t(14)=7.0, p<0.0001). To rule out the possibility of incomplete Ba2+ block at the concentration utilized we performed a dose response in HEK cells for two combinations of glial inward rectifiers – Kir4.1 and Kir4.1/5.1. We confirmed that Ba2+ blocks Kir4.1 and Kir4.1/5.1 with an IC50 of 30.14 μM and 25.5 μM, respectively (Supplementary Figure 5). These results suggest that mechanisms in addition to Kir channels contribute to RTN astrocyte CO2/H+ sensitivity with regard to depolarization. Nevertheless, astrocytes in the cortex do not depolarize in response to CO2/H+, even in the presence of Ba2+ (Δ1.7±0.9 mV, t(6)=1.931, p=.4746).
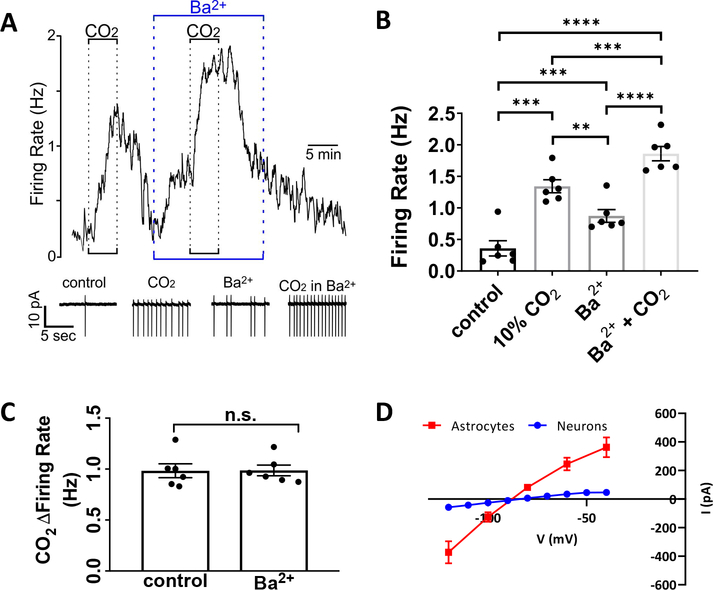
The relevance of depolarization in astrocytes is generally underappreciated since these cells do not generate action potentials and so are considered electrically non-excitable. Despite this, previous studies have shown that fluorocitrate-induced depolarization of RTN astrocytes is sufficient to increase RTN neuronal firing rate (Wenker et al. 2010) and ventilatory output in vivo (Sobrinho et al. 2017). However, since fluorocitrate is a metabolic poison with potential non-specific effects, we wanted to determine if 100 μM Ba2+, which strongly depolarizes RTN astrocytes (Figure 4D), is sufficient to activate chemosensitive RTN neurons. In cell-attached voltage-clamp mode, chemosensitive RTN neurons were identified by their characteristic firing response to CO2/H+; exposure to 10% CO2 increased activity by 0.98±0.06 Hz (q(5)=20.52, p=0.0001, Figure 5A–B). After returning to control conditions, bath application of Ba2+ alone mimicked the effects of CO2/H+ by increasing neural activity (0.51±.04 Hz, q(5)=16.27, p=.0003, Figure 5A–B). In the continued presence of Ba2+ subsequent exposure to 10% CO2 increased neural firing by 0.98±.05 Hz (q(5)=26.67, p<0.0001). This CO2/H+-mediated change was similar to the CO2/H+ firing response in the absence of Ba2+ (t(5)=0.08, p=.9393, Figure 5C). In order to verify that 100 μM Ba2+ does not inhibit neuronal K+ channels in the RTN as reported in the lateral habenula (Cui et al. 2018), we repeated the same protocol utilized by Cui et al. to measure Kir currents in RTN neurons in response to 100 μM Ba2+ under TTX block. While neurons exhibited some IKir, it is minute in comparison to astrocytic IKir (Figure 5D). These results suggest astrocytic IKir inhibition augments the intrinsic neuronal CO2/H+ response. These results are consistent with the possibility that RTN neurons are intrinsically CO2/H+-sensitive (Guyenet and Bayliss 2015; Kumar et al. 2015; Wang et al. 2013) and add evidence that astrocyte depolarization by CO2/H+- or Ba2+-induced IKir inhibition may contribute to downstream mechanisms activating RTN neurons.
Figure 5. RTN neuron firing rate is increased by both application of Ba2+ and CO2.
A-B. RTN neurons increase their firing rate in response to hypercapnia and Ba2+ alone, and fire most rapidly under application of both 10% CO2 and Ba2+ together (A, representative example, spikes are truncated; B, summary; n=6).
C. Change in firing rate with the application of 10% CO2 is not significantly different between control and Ba2+ (n=6).
D. I-V curve (−120 mV to −40 mV, 10 mV steps) from whole-cell patch clamped RTN neurons from PND9–13 rats (in blue). Superimposed, the Ba2+-sensitive current measured in astrocytes at the same age from Figure 3C (in red).
Distinct developmental changes in Kir proteins in the brainstem and cortex
Given these results, we evaluated regional expression of the most abundant Kir subunit in glia cells, Kir4.1, during development. Previous studies have found a developmental increase in Kir4.1 protein as well as an elevated expression in the brainstem relative to cortex (Nwaobi et al. 2014). Here we show by Western blot that expression of Kir4.1 protein is detectable early in development and continues to increase through the first month of life, with higher expression in the brainstem relative to cortex (main effect of brain region - F(1,36)=78.03, p<.0001; main effect of age - F(5,36)=16.18, p<.0001; Figure 6A–B). However, the measured baseline electrophysiological currents do not differ as robustly as might be expected based on the difference in Kir4.1 expression between these regions. There remains a possibility that Kir4.1 channel are differentially sequestered in these regions, contributing to downstream functional heterogeneity. Furthermore, expression of Kir4.1 in other glial cell populations may differ between cortex and RTN.
Figure 6: Kir4.1 and Kir5.1 are upregulated during development with more Kir4.1 and Kir5.1 in brainstem than in cortex.
A-C. Kir4.1 and Kir5.1 (A, representative Western blot; B-C, summary of n=4 animals) protein expression increases during development, with higher expression of both proteins in brainstem than cortex (C, D). *middle panel in A, indicates non-specific binding as it shows up in the negative control or HEK cell lane.
As Kir4.1 can form functional heteromeric channels with Kir5.1 that are pH-sensitive in the physiological range (Pessia et al. 2001), we next evaluated Kir5.1 expression in brainstem and cortex. Similar to the Kir4.1 expression profile, we found that Kir5.1 protein is more abundantly expressed in the brainstem relative to the cortex beginning early in development, and is developmentally upregulated in both regions (Figure 6A, C; main effect of brain region - F(1,36)=39.97, p<.0001; main effect of age - F(5,36)=3.99, p=0.0056). Sex has no effect on these results (Supplementary Figure 6). Kir5.1 cannot form a functional homomers but readily forms functional pH sensitive heteromers with Kir4.1 (Hibino et al. 2004; Konstas et al. 2003; Tanemoto et al. 2002). These data suggest that an increase in pH sensitive Kir4.1/5.1 heteromers in the brainstem relative to the cortex could underlie RTN astrocyte chemosensitivity.
Kir5.1 deficiency causes attenuation of IKir and chemosensitivity in RTN astrocytes
Again using heterologous expression of either Kir4.1 alone or Kir4.1/5.1 in HEK cells as described above we confirmed that Kir4.1 channels do not demonstrate a CO2/H+ sensitive inhibition when exchanging the bathing solution from 5% to 10% CO2/H+ (Supplementary Figure 7A), while Kir4.1/5.1 show robust CO2/H+ sensitivity (Supplementary Figure 7B). Additionally, the slow time course of the CO2/H+ block of RTN astrocyte currents was recapitulated when heterologous pH-sensitive channel Kir4.1 and 5.1 channels were expressed in HEK cells (Supplementary Figure 7C).
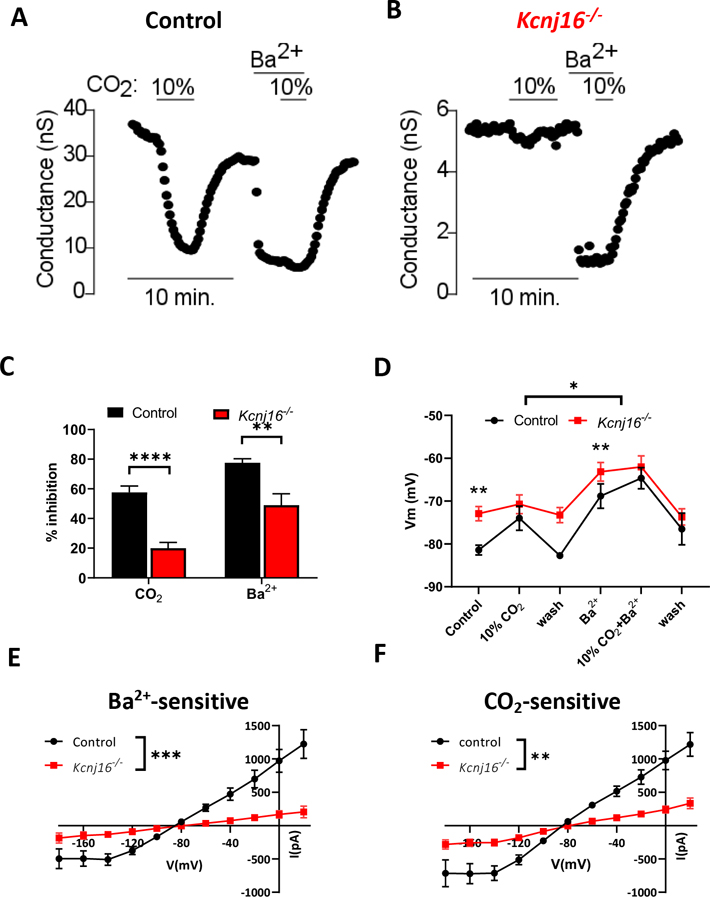
To further support the possibility that heteromeric Kir4.1/5.1 channels confer CO2/H+ sensitivity to RTN astrocytes, we also characterized ICO2/H+ in RTN astrocytes in slices from Kcnj16 (the gene encoding Kir5.1) knockout rats (Dahl/salt-sensitive background) recently developed by Palygin et al. (Palygin et al. 2017). Consistent with the possibility that Kir4.1/5.1 channels contribute to RTN astrocyte chemoreception, Kcnj16−/− rats show a reduced ventilatory response to CO2 (Puissant et al. 2019). Since this model uses a different genetic background than the SD rats used throughout this manuscript, we first characterized ICO2/H+ in RTN astrocytes in slices from Dahl/SS control rat PND8–13 pups as described above. We found that exposure to either 10% CO2 or Ba2+ decreased whole cell conductance in RTN astrocytes from control Dahl/SS rats by 58±4 % and 78±3 %, respectively (Figure 7A, C). Both treatments minimally affected conductance in RTN astrocytes in slices from PND8–13 Kcnj16−/− rats (CO2: 20±4 %, comparison to WT: t(24)=6.46, p<.0001; Ba2+: 50±8 %, comparison to WT: t(24)=3.27, p=.0033; Figure 7B–C). Likewise, the ICO2/H+ was smaller in RTN astrocytes from Kcnj16−/− rats compared to control (main effect of genotype: F(1,24)=10.38, p=.0036, Figure 7E) and reduced Ba2+-sensitive currents (main effect of genotype: F(1,24)=11.44, p=.0025, Figure 7F). Note that Ba2+ alone caused a reversible decrease in whole cell conductance in RTN astrocytes from both genotypes (Figure 7A–B), suggesting astrocytes in slices from Kcnj16−/− rats are healthy and express other pH-insensitive Kir channels. These results provide strong evidence suggesting Kir4.1/5.1 channels mediate ICO2/H+ in RTN astrocytes.
Figure 7: Genetic deletion of Kcnj16 causes attenuation of IKir and chemosensitivity in RTN astrocytes.
A-C. Representative example (A, B) and summary of inhibition (C, WT n=12, Kcnj16−/− n=14) of membrane conductance of WT and Kcnj16−/− RTN astrocytes. In WT astrocytes (A), conductance is markedly inhibited by 10% CO2 and 100 μM Ba2+. The inhibition by both CO2 and Ba2+ inhibition is markedly decreased in Kcnj16−/− astrocytes (B, C).
D. Membrane potential measurements concur with voltage clamp measurements, as Kcnj16−/− RTN astrocytes are significantly depolarized compared to controls animals under control conditions (right after seal break, before application of 10% CO2 and in wash after 10% CO2). However, when inhibiting control astrocytes with 10% CO2, Ba2+, or both, the two groups have the average membrane potentials that do not significantly differ (n varies from 4 to 14 at each time point).
E-F. I-V plots reveal a significant effect of genotype on both Ba2+-sensitive currents (E) and CO2 sensitive currents (F, WT n=12, Kcnj16−/− n=14 for both graphs).
In current clamp, RTN astrocytes in slices from Kcnj16−/− rats showed a more depolarized resting membrane potential under control conditions as compared to RTN astrocytes from Dahl/SS control rats (−81.4±1.4 mV Dahl/SS control vs −72.9±1.7 mV Kcnj16−/− rats; t(22.24)=4.20, p=.0025). Furthermore, exposure to 10% CO2 or Ba2+ depolarized membrane potential of RTN astrocytes in slices of control rats (in 10% CO2: Vm=−74.0±2.8 mV, in Ba2+: Vm=−68.8±2.8 mV) by an amount similar to RTN astrocytes in slices from SD rats (Figure 7D). Conversely, RTN astrocytes in slices from Kcnj16−/− rats did not show a voltage response to 10% CO2 or Ba2+ (in 10% CO2: Vm=−70.7±2.2 mV, t(21.59)=.917, p=.9602 compared to control; in Ba2+: Vm=−63.2±2.7 mV, t(21.52)=1.58, p=.6191 compared to control; Figure 7D).
Discussion
Regional Astrocyte Heterogeneity
Astrocyte heterogeneity is the subject of extensive recent interest. Understanding the difference in the physiology of astrocytes in different neuronal circuits aids our understanding of their role within the circuit (Zhang and Barres 2010). Astrocytes exhibit heterogeneous gene expression profiles, giving rise to the notion that astrocytes as a population are comprised of functionally discrete subtypes (Oberheim et al. 2012; Schitine et al. 2015); however, few studies have correlated unique gene or protein expression with function. Here, we show that astrocytes in the RTN but not cortex intrinsically sense CO2/H+ by inhibition of Kir-like currents, establishing Kir channels as key molecular determinants of this astrocyte population. These mechanisms may define populations of chemosensitive astrocytes more broadly.
Collectively, these findings shed light on possible mechanisms underlying functional heterogeneity of astrocytes in distinct brain regions. Potentiation of the CO2/H+ signal and subsequent excitatory neural output is part of a critical regulatory feedback loop in the RTN. Astrocyte CO2/H+-induced Kir inhibition and NBC-mediated extracellular acidification (Erlichman et al. 1998; Holleran et al. 2001), transmitter release (Gourine et al. 2010; Kasymov et al. 2013; Sobrinho et al. 2017; Wenker et al. 2010; Wenker et al. 2012), and accumulating K+ (Largo et al. 1997) may favor an increase in RTN neural firing rate to correct respiratory acidosis. In contrast, astrocytes in the cortex and other areas unassociated with central chemoreception may express lower levels of CO2/H+-sensitive Kir and primarily serve homeostatic roles by dampening CO2-mediated acidification through NBC-mediated extracellular alkalization (Theparambil et al. 2017).
Astrocyte heterogeneity across development
Consequent to the overall limited number of electrophysiological studies in RTN astrocytes, the CO2/H+-sensitivity of these cells during the first postnatal week of life has also not previously been determined. This is of significant importance because the ventilatory response to CO2 in rodents is present at birth and changes dramatically during the first week of life before maturing to a high level in adulthood (Davis et al. 2006; Putnam et al. 2005; Stunden et al. 2001; Wickstrom et al. 2002). The initially low CO2-sensitivity is thought to coincide with a period of respiratory vulnerability in fetal and early postnatal human life (Darnall 2010). Therefore, to determine whether CO2/H+-sensitivity of RTN astrocytes parallels upregulation of the drive to breathe, we characterized amplitude, kinetics, and voltage-dependent properties of CO2/H+-sensitive currents in RTN astrocytes throughout the first two weeks of postnatal life. For direct comparison, and to evaluate if CO2/H+-sensitivity is ubiquitous, we also characterized properties of CO2/H+-sensitive currents in cortical astrocytes over the same period. Our results show that RTN astrocytes exhibit CO2/H+-sensitive IKir (K+ current) as early as PND2–3 and that this current increases during early development. These electrophysiological results mimic the behavioral change in the hypercapnic response previously reported in the literature.
The purinergic component of the rodent hypercapnic chemoreceptive response reported by others seems to be absent at PND0–4 and relatively stable from PND7–12 through adulthood (Onimaru et al. 2012). Based on hippocampal astrocytes that do not possess mature IKir at that time point, it was suggested that intrinsic neuronal hypercapnic sensitivity may be responsible for in vivo ventilatory drive up until the point at which astrocytes appear in their mature form (PND4–7 at the earliest) (Wenker et al. 2012). In contrast, our data indicate some CO2/H+-sensitive IKir in RTN astrocytes as early as PND2–3. This supports the possibility that RTN astrocytes may contribute to in vivo ventilatory drive at or near the time of birth, and heightens the need to consider temporal heterogeneity when investigating the roles of astrocytes in physiological processes.
The increasing chemoreceptor function during the first weeks of postnatal life suggests that upregulation of astrocyte chemosensitivity contributes to the maturation of respiratory control. Since reduced chemoreception during the early neonatal period is associated with life-threatening respiratory failure, these results identify astrocyte Kir channels as potential therapeutic targets to improve respiratory drive and reduce vulnerability. However, these data do not rule out other pH-sensitive mechanisms in astrocytes.
Molecular underpinning of the CO2/H+-sensitive current
The slow time course of the CO2/H+ dependent block is significant, as the effect of pH on channel block is fast (Pessia et al. 2001). However, we used standard internal solution with HEPES, which may slow the kinetics of intracellular acidification. Indeed, when evaluated in HEK cells with the same conditions, CO2/H+-dependent block of IKir is similarly slow. There also exists possible biological explanations for this finding, as previous study work has demonstrated that astrocyte intracellular acidification in response to CO2/H+ occurs at the same rate observed here, and is dependent on bicarbonate transporters (Theparambil et al. 2017).
Ion channel expression by astrocytes varies across different CNS regions (Bordey and Sontheimer 2000), including heterogeneity in expression of the most common Kir channel, Kir4.1 (Poopalasundaram et al. 2000). Kir4.1 is more highly expressed in the brainstem than in the cortex and is developmentally upregulated (Nwaobi et al. 2014), suggesting a potential role in various brainstem functions including chemosensitivity. However, Kir4.1 is not pH-sensitive in the physiological range (pKa=5.99 (Pessia et al. 2001)). The correlation between the expression of Kir4.1, Kir5.1, and CO2/H+-sensitive current in the RTN suggests that Kir4.1/5.1 heteromers are involved in astrocytic chemosensation. The pH sensitivity of this channel is in the physiological range (pKa=7.35 (Pessia et al. 2001)), which together with its high expression in the brainstem makes it a prime candidate pH-sensor in chemosensitive astrocytes. Our research supports this notion, as Kcnj16−/− rats have diminished CO2/H+-sensitive currents compared to astrocytes from control tissue. Of note, according to the pH dose-response curve published in Pessia et al. 2001, the pH changes we observe here (pH=7.3 at 5% CO2, pH=7.0 at 10%CO2) should cause only a small (~10%) inhibition. However, there are several published dose-response curves for Kir4.1/5.1, and a different one published by the same group (Tucker et al. 2000) shows a steeper curve, that under our conditions inhibit ~80% of IKir.
There are several other possible inward rectifying channels that may be involved in pH chemosensation: Kir5.1 homomers, which can be expressed as a functional channel in the presence of PSD95 (Tanemoto et al. 2002) (though whether this form is pH-sensitive is not known), Kir4.2 homomers (pKa=7.1), or Kir4.2/5.1 heteromers (pKa=7.6 (Pessia et al. 2001)). However, RNA sequencing data indicates Kcnj15/Kir4.2 is not expressed in brainstem and shows little to no expression in the rest of the CNS (Human Protein Atlas, Uhlén, M. et al., Science 2015). Single cell RNA sequencing in RTN astrocytes and animals with astrocyte-specific conditional knockouts of various combinations are needed to pinpoint the exact molecular determinants of the CO2/H+-sensitive current.
Another possibility is that pH has a separate and distinct sensor on the membrane, while a secondary messenger produced by the sensor affects IKir. An additional pH sensor may be an unknown ancillary subunit of Kir4.1 that modulates its pH sensitivity, similar to how the minK subunit modulates Kv7.1 pH sensitivity (Heitzmann et al. 2007) or the effect of β2A on the pH sensitivity of the voltage gated calcium channel, Cav2.1 (Schuhmann et al. 1997).
Of note, there also appears to be a Kir-independent effect on RTN astrocyte membrane voltage, as depolarization occurs under CO2/H+ exposure when Kir channels are already blocked by Ba2+. NBCe1A, the most common astrocytic bicarbonate transporter (Zhang et al. 2014), represents one possibility for this non-Kir effect. Consistent with this, inhibition of the NBC decreased amplitude of the CO2/H+-sensitive current in RTN astrocytes (Wenker et al. 2010), suggesting that the NBC contributes to astrocyte chemoreception. The NBC current in RTN astrocytes can be expected to produce a hyperpolarizing outward current (bicarbonate influx) at resting membrane potential. However, exposure to high CO2 may transiently increase intracellular bicarbonate relative to extracellular bicarbonate due to the activity of intracellular carbonic anhydrase, and cause the NBC instead to extrude bicarbonate (inward depolarizing current). More research is needed to confirm this, and many other possibilities are viable, including other pH-sensitive sensitive cationic channels (like ASIC or TRP), or direct binding of Ba2+ to astrocytic proteins.
Physiological Importance
Astrocytic Kir channels have been long implicated in maintenance of [K+]o homeostasis; after neuronal firing, K+ ions are extruded into a relatively small extracellular volume, resulting in large increases in [K+]o that are efficiently dissipated by astrocytic IKir (Kofuji and Newman 2004). IKir has also proved important for maintaining baseline levels of [K+]o in the hippocampus (D’Ambrosio et al. 2002), and a decrease in this current in murine models of Rett Syndrome and Huntington disease leads to altered potassium dynamics (Kahanovitch et al. 2018; Tong et al. 2014). Thus, we propose that under normal circumstances, IKir in RTN astrocytes helps maintain a low level of [K+]o. However, inhibition of Kir4.1/5.1 channels during exposure to high CO2/H+ will conceivably diminish astrocyte K+ buffering capacity and potentially enhance activity of nearby RTN neurons to increase respiratory drive. To support the importance of Kir5.1-dependent IKir on CO2 homeostasis, rats that do not express Kir5.1 show a reduction in in the ventilatory response to normoxic hypercapnia (Puissant et al. 2019; Trapp et al. 2011). However, this respiratory deficit may involve peripheral chemoreceptors since Kir5.1−/− mice showed a normal ventilatory response to CO2 when peripheral chemoreceptor drive was minimized under hyperoxic conditions or in the reduced brainstem-spinal cord preparation (Trapp et al. 2011).
Future Experimental Approaches and Translational Implications
In this study, room temperature was utilized, as higher physiologic temperatures resulted in frequent destabilization of the chamber pH likely due to spontaneous conversion of bicarbonate to CO2, which has been previously reported (Theparambil et al. 2014). Since IKir amplitude and inhibition by CO2/H+ and Ba2+ might have a temperature-sensitive component, a future experiment is needed to confirm the CO2/H+ block of these currents in physiological temperatures. Such an experiment would have to stabilize the pH at these temperatures, e.g. by using pressurized chamber to prevent the bicarbonate to CO2 conversion.
In the current study we utilized a rat reporter line which expresses eGFP under the S100β promoter to aid in the identification of cortical and RTN astrocytes for electrophysiological recordings. This decision was based on previous studies indicating S100β expression is a reliable marker for astrocytes (Grosche et al. 2013; Nishiyama et al. 1996; Ogata and Kosaka 2002; Raponi et al. 2007; Zhu et al. 2008). However, S100β has been shown to be expressed in some populations of NG2 cells in mice (Karram et al. 2008). Confounding this, there is overlap in the electrical properties of NG2 cells and immature astrocytes, the former being more likely to display voltage gated currents and have higher input resistances (Zhong et al., 2016) when compared to mature astrocytes and similar to what has been reported for NG2 cells (Larson et al. 2016, Chittajallu et al. 2004). For instance, using BAC-ALDH1L1-eGFP transgenic mice, Zhong et al., report the vast majority of eGFP+ astrocytes (also double labeled with SR101) display variably rectifying currents and have significantly higher input resistances than mature astrocytes. With this in mind, we cannot exclude the possibility that some fraction of cells in our study are S100+ NG2 glia, rather than immature astrocytes. Future studies using these animals will employ a double labeling approach, either using SR101 to positively identify eGFP+/SR101+ astrocytes (Zhong et al., 2016), or post-hoc immunohistochemistry using astrocyte markers in eGFP+ biocytin labeled cells.
These results broadly have interesting implications for neurodevelopmental disorders such as Rett Syndrome, in which genes encoding Kir channels may be abnormally epigenetically regulated during key periods of brainstem respiratory network maturation. Indeed, in Rett Syndrome, Kcnj10/Kcnj16 gene expression levels have been found to be reduced relative to wild-type mice (Kahanovitch et al. 2018; Pacheco et al. 2017). Not only do Rett animals demonstrate abnormal respiratory response to hypercapnia (Bissonnette et al. 2014; Garg et al. 2015; Ren et al. 2012; Voituron et al. 2009), evidence also suggests that astrocytes from Rett animals fail to respond to hypercapnic challenge with appropriate Ca2+ influx (Turovsky et al. 2015). Thus, Kir channels represent exciting molecular targets for further investigation of the ways in which astrocyte ion channels influence both normal and abnormal development of respiratory networks.
Materials and Methods
Subjects
All experimental protocols were in accordance with the NIH guidelines and were carried out with approval from the Animal Care and Use Committee of the University of Alabama at Birmingham (Animal Permit Number: 09409) and of Virginia Tech (IACUC: 16–241). Every effort was made to minimize pain and discomfort. Animals used in all experiments were littermates bred in our animal facility by crosses of Sprague Dawley females from Jackson Laboratories to Wistar:Crlj S100b eGFP male rats obtained from the National Bioresource Project Rat (Japan) (Itakura et al. 2007) and subsequently bred out to a Sprague Dawley background. Kcnj16−/− generation was described before (Palygin et al. 2017). Dahl/SS control rats were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed with water and food available ad libitum on a 12/12 light/dark cycle and all experiments were performed during the animals’ wake (dark) cycle.
Brainstem and Cortical Slices
Mixed gender neonatal rats (PND2-PND13) were rapidly decapitated, brain removed and sliced in half and embedded in 4% agarose in PBS. Coronal brainstem and cortical slices (250μm) were cut using a microtome in ice cold ACSF containing (in mM): 121 NaCl, 3 KCl, 5 MgCl2, 1 CaCl2, 26.2 NaHCO3, 11.1 glucose. Slices were incubated for 30 min at 37°C and subsequently at room temperature in ACSF containing (in mM): 121 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 26.2 NaHCO3, 11.1 glucose. All solutions were bubbled with 95% O2–5% CO2 throughout. Coronal sections containing motor cortex analogous to those between approximately +2.52 and −1.32mm relative to bregma in the adult rat were harvested. RTN slices analogous to those located between approximately −10.80mm and −11.88mm relative to bregma in the adult rat were harvested (i.e. at the caudal end of the facial nucleus and the pyramids).
Whole-Cell and Cell-Attached Recordings
Slices containing the motor cortex or RTN were transferred to a recording chamber mounted on a fixed-stage microscope (Zeiss Axio Examiner .D1, Zeiss, Oberkochen, Germany) and perfused (~3–4mL/min−1) with ACSF at room temperature via a pinch-valve syringe delivery system (VC-6, Warner Instruments, Hamden, CT). Room temperature was utilized, as higher physiologic temperatures resulted in frequent destabilization of the chamber pH likely due to spontaneous conversion of bicarbonate to CO2, which has been previously reported (Theparambil et al. 2014). Solutions were bubbled with 95% O2–5% CO2 (bath pH=7.3) or 90% O2–10% CO2 (bath pH=7.0) as indicated throughout experiments.
Whole-cell, voltage-clamp and current-clamp recordings were made from astrocytes in deep layer 1 and 2/3 of the motor cortex and from RTN astrocytes within 5 cell bodies or ~50μm of the pial surface. All recordings were made with an Axopatch 200B patch-clamp amplifier, digitized with Axon Digidata 1550B, and recorded using pCLAMP 10.6 (Molecular Devices, San Jose, CA). Electrodes were pulled from premium thin wall borosilicate glass (Warner Instruments) to a resistance of 4–6 MΩ when filled with internal solution containing the following (in mM): 127mM KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 Mg-ATP, and 0.3 Na-GTP, .05 Alexa Fluor 568 dye (Thermo Fisher). Resting membrane potentials were measured directly from the amplifier ~1 minute after whole cell access was obtained. Whole cell capacitance and series resistance were measured directly from the amplifier and ~70% compensated to minimize voltage errors. Astrocytes were held at −80mV and series resistance and input resistance were monitored during voltage clamp experiments with a 5 or 10 mV hyperpolarizing voltage step. Voltage step protocol is described in Figure 1A. Full protocol includes voltage steps from −180 mV to 120 mV. Due to brevity, all voltages above 0 mV are not shown, and statistical analysis was performed from −180 mV to −80 mV (see Statistical Analysis section). Changes in extracellular pH were monitored throughout all recordings utilizing a combination pH electrode for micro wells (Warner Instruments).
Activity of RTN neurons was measured in the cell-attached voltage-clamp mode (Vholding=−60 mV) at room temperature (~22°C) with electrodes filled with the same pipette internal solution as above. Action potential discharge was integrated in 10-second bins to construct firing rate histograms using Spike 5.0 software (Cambridge Electronic Design, CED, Cambridge, U.K.). Whole cell voltage clamp recording in neurons (Figure 5D) was performed according to a protocol by (Cui et al. 2018). Neurons were kept at current clamp between voltage clamp protocols that consisted holding at −60 mV, and then 2.5 sec 10 mV voltage steps from −120 mV to −40 mV.
Immunohistochemistry
P12 wildtype males were anesthetized with an intraperitoneal injection of ketamine and xylazine (90 mg/kg and 10 mg/kg, respectively) and perfused with ice cold PBS for 5 minutes followed by 4% paraformaldehyde for 30 minutes. The brain was removed and stored in 4% paraformaldehyde for at least 3 days prior to slicing. After rinsing in PBS, 50 μM coronal slices were cut (Vibratome 3000 sectioning system). Sections were incubated in blocking buffer containing 10% goat serum and .3% Triton-X in PBS for 1 hour shaking at room temperature. Following blocking, slices were incubated shaking overnight at 4°C in the following primary antibodies made up in diluted in blocking buffer (1:3 solution with PBS): 1:1000 mouse anti-GFAP (MAB360, Millipore); 1:200 anti-S100β (Z0311, Dako). Slices were then washed 3×15 minutes in diluted blocking buffer and incubated in 1:750 goat polyclonal to rabbit Alexa Fluor 546 and to mouse Alexa Fluor 488 secondary antibody (Thermo Fisher) for 1 hour shaking at room temperature. Slices were then washed for 5 minutes in PBS and mounted on slides using ProLong diamond antifade mountant (Thermo Fisher). Confocal z-stack images were obtained on a confocal microscope (Zeiss LSM510) utilizing 543 nm and 488 nm lasers.
Protein Analysis by Western Blotting
Mixed sex rats PND3–28 were rapidly decapitated. Brains were removed and cortex and brainstem were dissected from whole tissue in ice cold PBS and sonicated for 14 s in lysis buffer containing 1M Tris pH 7.5, 10% SDS, and 1:10 protease and phosphatase inhibitors. Tissue homogenates were centrifuged for 5 min at 12,000 g at 4°C. Following BCA protein assay (Thermo Fisher), equal amounts of protein (10ug) were heated to 60°C for 15 min in an equal volume of 2X sample buffer (100 mM Tris, pH 6.8, 4% SDS, in Laemmli-sodium dodecyl sulfate, 600 mM B-mercaptoethanol, 200 mM Dithiothreitol (DTT), and 20% glycerol) and electrophoresed on 4–20% Criterion TGX precast gels (BioRad, Hercules, CA) for 1.5 hours at 150V at room temperature. Protein was transferred onto nitrocellulose membranes (Licor, Lincoln, NE) at 10V overnight spinning at 4° C using two layers of sponges and cold packs. Membranes were blocked in 1:1 TBS and TBS blocking buffer (Licor) shaking for 1 hour at room temperature. Membranes were incubated shaking, in abovementioned blocking buffer with .2% Tween added, with the following primary antibodies: chicken polyclonal to alpha tubulin at .25ug/mL (Abcam, Cambridge, UK) for 40 minutes at room temperature, 1:1000 rabbit polyclonal to Kir4.1 (Alomone, Jerusalem, Israel) for 1 hour at room temperature, 1:1000 rabbit polyclonal to Kir5.1 (Abcam) for 3 hours at room temperature. Membranes were then washed 3×5 min in TBST and incubated shaking with secondary antibodies, prepared in the same solution as primary antibodies: 1:20,000 goat anti rabbit IR 800 and donkey anti chicken IR 680 (Licor) for 40 minutes at room temperature. Membranes were then washed 3×5 minutes in TBST and for 5 minutes in TBS before being imaged with an Odyssey scanner. Bands were quantified using Licor ImageStudio Version 4.0 and normalized to alpha tubulin. To confirm that protein expression did not differ between sexes, sets of blots in which both male and female samples were run together were also analyzed and demonstrated no sex-dependent difference in Kir4.1 or Kir5.1 protein expression in brainstem or cortex (data not shown).
Drugs
All experiments included TTX (0.1μM, Tocris Bioscience, Bristol, UK) in the recording solution except where indicated (Supplementary Figure 4). Barium chloride (100μM Thermo Fisher) was also used where indicated. Drugs were stored in frozen stock solution and dissolved in recording solution prior to each experiment.
Statistical Analysis
All raw data were graphed and statistically analyzed in GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla California USA). Statistical tests used are detailed in Supplementary Table 1. Voltage step protocol was analyzed for the range −180 to −80 mV, to avoid symmetry around resting Vm that would dampen differences between regions/genotype. We chose this method over analyzing current at a single voltage step, as to not influence analysis with that choice. We also preferred not to analyze slope, as the I-V curve is linear only in some of the voltage steps. All data are reported as mean±standard error of the mean within the text, with results of statistical tests and p values. n, number of cells (electrophysiology) or animals (Western blotting) are indicated in figure legends. * indicates p<.05, ** indicates p<.01, *** indicates p<.001, **** indicates p<.0001.
Supplementary Material
Main Points:
Astrocytes in the RTN but not cortex intrinsically sense CO2/H+ by inhibition of a Kir conductance
Inhibition of astrocyte Kir is sufficient to increase RTN neuronal firing
Chemosensitivity of RTN astrocytes is dependent on Kir4.1/5.1 channels
Acknowledgements
This work was supported by funds from the National Institutes of Health Grants R01 NS075062 (MLO), R01 HL104101 (MLO and DKM), R01 HL137094 (DKM), and R35 HL135749 (AS). We would like to thank the National Bioresource Project (NBRP) for the Rat (Kyoto, Japan for generously providing S100β-eGFP+ rats.
Abbreviations:
- Ba2+
barium
- Ca2+
calcium
- Cm
cell capacitance
- CO2
carbon dioxide
- DTT
Dithiothreitol
- eGFP
enhanced green fluorescent protein
- EK
potassium equilibrium/reversal potential
- GFAP
glial fibrillary acidic protein
- H+
hydrogen ions
- I-V
current-voltage
- ICO2/H+
CO2/H+-sensitive current
- IKir
Kir channel current
- Kir
inward rectifying potassium
- [K+]o
extracellular potassium
- Kcnj10
gene encoding Kir4.1 protein
- Kcnj16
gene encoding Kir5.1 protein
- NG2
polydendrocyte cell
- PND
postnatal day
- RTN
retrotrapezoid nucleus
- S100β
S100 calcium binding protein beta
- SD
Sprague Dawley
- TTX
tetrodotoxin
- Vm
resting membrane potential
- wt
wild-type
References
- Ben Haim L, Rowitch DH. 2017. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18:31–41. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Schaevitz LR, Knopp SJ, Zhou Z. 2014. Respiratory phenotypes are distinctly affected in mice with common Rett syndrome mutations MeCP2 T158A and R168X. Neuroscience 267:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. 2000. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia 30:27–38. [DOI] [PubMed] [Google Scholar]
- Casamassima M, D’Adamo MC, Pessia M, Tucker SJ. 2003. Identification of a heteromeric interaction that influences the rectification, gating, and pH sensitivity of Kir4.1/Kir5.1 potassium channels. J Biol Chem 278:43533–40. [DOI] [PubMed] [Google Scholar]
- Chaboub LS, Deneen B. 2012. Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci 34:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP and others. 2017. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95:531–549.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. 2004. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol 561:109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewinski AJ, Wilkin GP. 1988. Astrocytes from forebrain, cerebellum, and spinal cord differ in their responses to vasoactive intestinal peptide. J Neurochem 51:1626–33. [DOI] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y and others. 2018. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554:323–327. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Gordon DS, Winn HR. 2002. Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J Neurophysiol 87:87–102. [DOI] [PubMed] [Google Scholar]
- Darnall RA. 2010. The role of CO2 and central chemoreception in the control of breathing in the fetus and the neonate. Respir Physiol Neurobiol 173:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. 2006. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol (1985) 101:1097–103. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. 2008. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci U S A 105:1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson JM, Shah AJ, Palmer LG. 2011. Kir4.1 K+ channels are regulated by external cations. Channels (Austin) 5:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Etr M, Cordier J, Torrens Y, Glowinski J, Premont J. 1989. Pharmacological and functional heterogeneity of astrocytes: regional differences in phospholipase C stimulation by neuromediators. J Neurochem 52:981–4. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. 1998. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol (1985) 85:1599–604. [DOI] [PubMed] [Google Scholar]
- Farmer WT, Murai K. 2017. Resolving Astrocyte Heterogeneity in the CNS. Front Cell Neurosci 11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg D, Ringstedt T, Herlenius E. 2017. Astrocytes release prostaglandin E2 to modify respiratory network activity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. 1978. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflugers Arch 376:229–35. [DOI] [PubMed] [Google Scholar]
- Garg SK, Lioy DT, Knopp SJ, Bissonnette JM. 2015. Conditional depletion of methyl-CpG-binding protein 2 in astrocytes depresses the hypercapnic ventilatory response in mice. J Appl Physiol (1985) 119:670–6. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. 2010. Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche A, Grosche J, Tackenberg M, Scheller D, Gerstner G, Gumprecht A, Pannicke T, Hirrlinger PG, Wilhelmsson U, Hüttmann Kand others. 2013. Versatile and simple approach to determine astrocyte territories in mouse neocortex and hippocampus. PLoS One 8:e69143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA. 2015. Neural Control of Breathing and CO2 Homeostasis. Neuron 87:946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D, Koren V, Wagner M, Sterner C, Reichold M, Tegtmeier I, Volk T, Warth R. 2007. KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cell Physiol Biochem 19:21–32. [DOI] [PubMed] [Google Scholar]
- Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. 2004. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J Biol Chem 279:44065–44073. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. 2010. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90:291–366. [DOI] [PubMed] [Google Scholar]
- Hoft S, Griemsmann S, Seifert G, Steinhauser C. 2014. Heterogeneity in expression of functional ionotropic glutamate and GABA receptors in astrocytes across brain regions: insights from the thalamus. Philos Trans R Soc Lond B Biol Sci 369:20130602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran J, Babbie M, Erlichman JS. 2001. Ventilatory effects of impaired glial function in a brain stem chemoreceptor region in the conscious rat. J Appl Physiol (1985) 90:1539–47. [DOI] [PubMed] [Google Scholar]
- Itakura E, Odaira K, Yokoyama K, Osuna M, Hara T, Inoue K. 2007. Generation of transgenic rats expressing green fluorescent protein in S-100beta-producing pituitary folliculo-stellate cells and brain astrocytes. Endocrinology 148:1518–1523. [DOI] [PubMed] [Google Scholar]
- Kahanovitch U, Cuddapah VA, Pacheco NL, Holt LM, Mulkey DK, Percy AK, Olsen ML. 2018. MeCP2 Deficiency Leads to Loss of Glial Kir4.1. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K, Goebbels S, Schwab M, Jennissen K, Seifert G, Steinhäuser C, Nave KA, Trotter J. 2008. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis 46:743–57. [DOI] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. 2013. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. 2004. Potassium buffering in the central nervous system. Neuroscience 129:1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas AA, Korbmacher C, Tucker SJ. 2003. Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels. Am J Physiol Cell Physiol 284:C910–7. [DOI] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM and others. 2015. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Ibarz JM, Herreras O. 1997. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78:295–307. [DOI] [PubMed] [Google Scholar]
- Larson VA, Zhang Y, Bergles DE. 2016. Electrophysiological properties of NG2(+) cells: Matching physiological studies with gene expression profiles. Brain Res 1638:138–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. 2010. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev 63:2–10. [DOI] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J, Tai A, Yang Y. 2017. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci 37:8706–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Wenker IC. 2011. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol 96:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. 1996. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43:299–314. [DOI] [PubMed] [Google Scholar]
- Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. 2016. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaobi SE, Lin E, Peramsetty SR, Olsen ML. 2014. DNA methylation functions as a critical regulator of Kir4.1 expression during CNS development. Glia 62:411–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M. 2012. Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. 2002. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113:221–33. [DOI] [PubMed] [Google Scholar]
- Olsen M 2012. Examining potassium channel function in astrocytes. Methods Mol Biol 814:265–281. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. 2008. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. 2012. Postsynaptic mechanisms of CO2 responses in parafacial respiratory neurons of newborn rats. J Physiol 590:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco NL, Heaven MR, Holt LM, Crossman DK, Boggio KJ, Shaffer SA, Flint DL, Olsen ML. 2017. RNA sequencing and proteomics approaches reveal novel deficits in the cortex of Mecp2-deficient mice, a model for Rett syndrome. Mol Autism 8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. 2017. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Imbrici P, D’Adamo MC, Salvatore L, Tucker SJ. 2001. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol 532:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, Gallo V, Wilkin GP. 2000. Glial heterogeneity in expression of the inwardly rectifying K+ channel, Kir4.1, in adult rat CNS. Glia 30:362–372. [DOI] [PubMed] [Google Scholar]
- Puissant MM, Muere C, Levchenko V, Manis AD, Martino P, Forster HV, Palygin O, Staruschenko A, Hodges MR. 2019. Genetic mutation of Kcnj16 identifies Kir5.1-containing channels as key regulators of acute and chronic pH homeostasis. FASEB J 33:5067–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. 2005. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol 149:165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom CB, Sontheimer H. 1995. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. Journal of Neurophysiology 73:333–346. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. 2007. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 55:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ. 2012. Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome. J Neurosci 32:17230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. 2005. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289:R851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnakova V, Honsa P, Dzamba D, Stahlberg A, Kubista M, Anderova M. 2013. Heterogeneity of astrocytes: from development to injury - single cell gene expression. PLoS One 8:e69734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo K, Park JY. 2016. Two-pore Domain Potassium Channels in Astrocytes. Exp Neurobiol 25:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schitine C, Nogaroli L, Costa MR, Hedin-Pereira C. 2015. Astrocyte heterogeneity in the brain: from development to disease. Front Cell Neurosci 9:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann K, Voelker C, Hofer GF, Pflugelmeier H, Klugbauer N, Hofmann F, Romanin C, Groschner K. 1997. Essential role of the beta subunit in modulation of C-class L-type Ca2+ channels by intracellular pH. FEBS Lett 408:75–80. [DOI] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC and others. 2018. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho CR, Goncalves CM, Takakura AC, Mulkey DK, Moreira TS. 2017. Fluorocitrate-mediated depolarization of astrocytes in the retrotrapezoid nucleus stimulates breathing. J Neurophysiol 118:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. 2001. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127:135–55. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. 2008. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586:2975–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemoto M, Fujita A, Higashi K, Kurachi Y. 2002. PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron 34:387–97. [DOI] [PubMed] [Google Scholar]
- Tang X, Taniguchi K, Kofuji P. 2009. Heterogeneity of Kir4.1 channel expression in glia revealed by mouse transgenesis. Glia 57:1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theparambil SM, Naoshin Z, Defren S, Schmaelzle J, Weber T, Schneider HP, Deitmer JW. 2017. Bicarbonate sensing in mouse cortical astrocytes during extracellular acid/base disturbances. J Physiol 595:2569–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theparambil SM, Ruminot I, Schneider HP, Shull GE, Deitmer JW. 2014. The electrogenic sodium bicarbonate cotransporter NBCe1 is a high-affinity bicarbonate carrier in cortical astrocytes. J Neurosci 34:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV and others. 2014. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci 17:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Gourine AV. 2011. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16). Exp Physiol 96:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Imbrici P, Salvatore L, D’Adamo MC, Pessia M. 2000. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem 275:16404–7. [DOI] [PubMed] [Google Scholar]
- Turovsky E, Karagiannis A, Abdala AP, Gourine AV. 2015. Impaired CO2 sensitivity of astrocytes in a mouse model of Rett syndrome. J Physiol 593:3159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M. 2018. Physiology of Astroglia. Physiol Rev 98:239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. 2009. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol 168:109–18. [DOI] [PubMed] [Google Scholar]
- Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA. 2013. Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33:7756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. 2010. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104:3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK. 2012. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol 590:2137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom R, Hokfelt T, Lagercrantz H. 2002. Development of CO2-response in the early newborn period in rat. Respir Physiol Neurobiol 132:145–58. [DOI] [PubMed] [Google Scholar]
- Xin W, Bonci A. 2018. Functional Astrocyte Heterogeneity and Implications for Their Role in Shaping Neurotransmission. Front Cell Neurosci 12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. 2000. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol 524 Pt 3:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. 2010. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20:588–94. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N and others. 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Du Y, Kiyoshi CM, Ma B, Alford CC, Wang Q, Yang Y, Liu X, Zhou M. 2016. Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol Brain 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. 2000. Freshly isolated astrocytes from rat hippocampus show two distinct current patterns and different [K(+)](o) uptake capabilities. J Neurophysiol 84:2746–2757. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. 2006. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95:134–143. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. 2008. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135:145–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.