Abstract
Delta-Like Non-Canonical Notch Ligand 1 (DLK1) is one of the key genes involved in the development of muscle, liver, pancreas, and lung cells; adipocytes production; and the improvement of digestion, growth performance, and meat quality. It has been documented that fennel is effective on increasing the DLK1 gene (DLK1) expression in the testis, liver, and muscle tissues, which may consequently have important implications for sheep production. Hence, the aim of the current investigation was to evaluate the fennel seed powder's effect on DLK1 expression in testis, liver, and humeral muscle tissues in growing lambs. For the purpose of this study, 30 male Kermani sheep were fed with three different group of diets (number of animals in each group was 10), including control (without any fennel seed powder), treatment 1 (with 10 g/kg of dry matter (DM) fennel seed powder), and treatment 2 (with 20 g/kg of DM fennel seed powder) during a 3-month period. Thereafter, total RNA was extracted, cDNA was synthesized, and Real-Time PCR was performed. The addition of fennel seed powder (in the treatment 1 and treatment 2 groups) in the growing lambs diets consequently resulted in greater expression of DLK1 in both the liver and humeral muscle tissues compared to the testis tissue (P < 0.05). Furthermore, the increased DLK1 expression was higher in the tissue of humeral muscle (P < 0.05) in comparison to the other two tissues. As well, the concentration of blood testosterone was greater (P < 0.05) for the animals fed with fennel powder compared to growing lambs fed with the control diet. However, the concentrations of blood liver enzymes, including serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), decreased by the addition of 10 g/kg DM fennel to diets of lambs compared to the control diet (no fennel). Therefore, it can be concluded that using fennel seed powder in the diet of growing lamb by affecting the expression of DLK1, can improve the concentrations of blood testosterone, SGOT, SGPT, and muscle structure (increased mass of muscle and size of muscle fiber).
Keywords: DLK1, Hepatic enzymes, Lamb, Meat production, Testosterone
DLK1; Hepatic enzymes; Lamb; Meat production; Testosterone.
1. Introduction
The Kermani sheep breed is one of the 27 breeds and ecotypes living in Iran, and animal management plays a key role in achieving the optimal genetic and phenotypic characteristics of this breed (Masoudzadeh et al., 2020). The use phytobiotics and bioactive plants with antimicrobial growth promoters in animal feeding has been suggested to improve zootechnical output variables and to prevent the development of some types of diseases (Aćimović et al., 2016). These plants have several biological properties such as antioxidants, hypocholesterolemics, antimicrobial activity, stimulants of immune system, stress reduction (Lee et al., 2015), and the nutrient digestion improvement (Aćimović et al., 2016). Furthermore, in comparison with other feed additives, another advantage of these plants is their low cost. Hence, the use of these plants in stimulating digestive enzymes and improving liver functions can be considered as a promising step in sheep industry. Carcass yield, feed conversion ratio, and feed consumption have also been reported to be enhanced in response to the addition of bioactive plants to animals’ diets (Aćimović et al., 2016). One of the most important plants with bioactive compounds is fennel (Foeniculum vulgare), which belongs to the Apiaceae family that has been used by humans since the ancient era. It has been demonstrated that using diets containing fennel for sheep could improve their growth outputs and performance of muscle tissue, thereby resulting in better feed conversion rates (Hajalizadeh et al., 2019).
Delta-Like Non-Canonical Notch Ligand 1 gene (DLK1) is also known as a preadipocyte factor 1 (Pref-1) or fetal antigen 1 (FA1). Accordingly, this gene is expressed in fetal cells, adult adrenal cortex, adrenal gland tumours, and endocrine tissues, and hardly in testicular adrenal rest tumors, fetal leydig cells, and in a subset of adult leydig cells (Lottrup et al., 2015). According to the study by Waddell et al. (2010), DLK1 has a regulative function in different tissues, which is related to terminal differentiation and cell-fate decision of progenitor cells. Moreover, the DLK1 has dissimilar expression patterns in leydig cells during their development and its patterns suggest that this gene acts as a marker of progenitor or immature leydig cells (Lottrup et al., 2015). In another study, Tanimizu et al. (2003) have demonstrated that DLK1, which consists of two transmembrane and soluble forms, has a significant task in extension of liver. In addition, Rocha et al. (2007) in their research have observed DLK1 expression in the liver with lower levels from the maternal allele. Correspondingly, similar DLK1 expression patterns in the liver and muscle tissues were reported by in the study by Oczkowicz et al. (2010). Moreover, Yevtodiyenko and Schmidt (2006) have shown that DLK1 is highly expressed in the hepatocytes of the fetal liver on embryonic day 12.5 and down-regulates on embryonic day 16.5. Besides, this gene is expressed in the connective tissue capsule of the liver, but not in the hematopoietic cells of liver, even though these cells constitute more than 50% of liver.
One of the most important phenotypes in sheep is muscle hypertrophy of the hindquarters, also known as “callipyge (CLPG) phenotype”, which does not follow Mendel's rules. Accordingly, its inheritance is one type of polar overdominance. In this regard, when the offspring inherits the mutated allele from father and the wild type allele from mother, it shows the CLPG phenotype. Previously, DLK1 has been widely studied in sheep for the diagnosis of the Single Nucleotide Polymorphism (SNP) for the CLPG phenotype (Cockett et al., 1996). Smit et al. (2003) have studied DLK1 expression in the CLPG phenotype and as a result, they have shown that the expression level of DLK1 enhanced. Due to the important role of DLK1 in muscle and its association with the CLPG phenotype in sheep, it is an important candidate gene in marker-assisted selection. However, the mutation in CLPG, which is breed-specific, is only seen in callipyge herds (Smit et al., 2003). In a previous study, Kim et al. (2004) have studied genetic diversity of DLK1 and reported that a correlation exists among its polymorphisms and its polar over-dominant inheritance, animal development, obesity, and composition of body. According to the study by Amiri Roudbar et al. (2018), when a sheep genotype is heterozygous for the DLK1-MEG3 domain and inherits the mutation from father, it perfectly produces a muscular hypertrophy phenotype different from the normal phenotype. Additionally, the DLK1 has been shown to play important roles in the preservation of hypertrophy in fully differentiated myofibers and in the commitment and/or proliferation of fetal myoblasts (White et al., 2008). In another study, Georges et al. (2003) have demonstrated that the CLPG mutation is defined as a physiological interaction in trans between a maternally expressed inhibitor and its paternally expressed growth-improving target and they have also concluded that the DLK1 is the best candidate of growth promoter.
In a previous study (Masoudzadeh et al., 2020), we have also investigated the effects of various amounts of fennel on growth characteristics and on the expression of DLK1 in some tissues, including rumen muscle, femur muscle, adipose, and brain tissues in the Kermani sheep breed. Accordingly, the results of this study showed that enhancing the consumption of fennel in the diet causes a positive effect on the expression of DLK1 in both the rumen and femur muscle tissues. Thus, DLK1 can be considered as an interesting candidate gene in farm animals for marker-assisted selection (Yevtodiyenko and Schmidt 2006).
According to previous studies, the effect of fennel and the role of DLK1 expression on testis, liver, and humeral muscle tissues of livestock, especially sheep, have not been reported in any study up to now. Therefore, in continuous with the previous research (Masoudzadeh et al., 2020) and in order to develop and complete its findings, the current investigation was conducted to investigate the associations among the expression of DLK1 in humeral muscle, liver, and testis tissues, growth and selected biochemical traits, and fennel feeding in Kermani lamb.
2. Materials and methods
2.1. Ethics approval and consent to participate
All the ethical guidelines and native standards were applied for performing the current study in terms of the Iranian Council of Animal Care Guide and Use of Experimental Animals (IACUC Protocol #IR2018011).
2.2. Selection of lambs, rations, and experimental design
Since the current study was conducted to complete and develop the findings of our previous study, the same animals, diets, and experimental design were used (for more information please see the study by Masoudzadeh et al., 2020). After the adaptation period, before the morning feeding, the animals were weighed during a 14-day interval to compute the feed conversion (FC) by dividing total dry matter intake by total weight gain for the interval. Thereafter, the mean of daily live weight gain for the animals was calculated by subtracting the initial weight from the final weight during the performance phase period (80 days) as explained in our previous investigation (Masoudzadeh et al., 2020). Different treatments, including zero, 10, and 20 g/kg DM of fennel were used in the diet of sheep for a 90-day duration (Table 1) with 10 animals included in each group. In the present study, the combination of the used basal (control) diet (dry matter basis in %) was as follows: wheat straw, chopped (10%); alfalfa hay, chopped (30%); corn grain, ground (9%); barley grain, ground (28%); wheat bran (13%); soybean meal (8%); vitamin D, E, and A premix (0.6%) (includes 5,000,000 IU of Vitamin D, 500,000 IU of Vitamin E and 5,000,000 IU of Vitamin A per kg); trace-mineralized salt (0.6%) (Containing 20.5% Dynamad, 75.15% NaCl, 3.046% Mn, 0.253% Znsulphate, 1.025% Cu-sulphate, 0.011% Na-selenide, and 0.015% EDDI-80); sodium bicarbonate (0.5%); and limestone (0.3%) (As described in the study by Masoudzadeh et al., 2020).
Table 1.
Chemical analysis and components of fennel (DM basis).
| Ingredients | amounts |
|---|---|
| Dry matter | 91% |
| Organic matter | 87.03% |
| Crude protein | 15% |
| Ether extract | 9.76% |
| Metabolizable energy | 12.12 mega joules per kilo gram |
2.3. Measurements and analytical methods
Blood samples were collected in 10-mL tubes containing anticoagulant (EDTA) by passing 3 h from the morning feeding on the day before slaughter. Subsequently, the samples were centrifuged at 6000×rpm for 10 min. For further analyses, these obtained samples were frozen at −20 °C. In order to calculate the concentration of serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), an automatic analyzer (Technicon RA 1000; Bayer Co., NY, USA) was used via proper testing kits (Parsazmun Laboratory, Tehran, Iran). ELISA kit (Stat Fax) and testosterone set (Patangostar-e-Eisar Co, Iran) were used for calculating testosterone concentration. All the studied animals were sacrificed immediately after the end of the experiment and tissue sampling was performed from liver, testis, and humeral muscle tissues (270 samples, including 10 animals × 3 groups × 3 tissues × 3 repeats for each tissue) along with recording weights for the testis, gallbladder, before slaughter, back muscle (loin), warm carcass, lean meat, femur (leg) muscle, and eye muscle area (as described in the study by Masoudzadeh et al., 2020).
2.4. Analysis of RNA expression results
For each tissue sample, a real time polymerase chain reaction (RT-PCR) was done as defined in our previous investigation (Masoudzadeh et al., 2020). Some specific primers were used for DLK1 and beta actin (ACTB) was applied as the reference gene (internal control) for RT-PCR technique (Table 2) using Rotor-Gene Q MDx device (QIAGEN Hilden, Germany).
Table 2.
Oligonucleotide primers used for RT-PCR analysis of DLK1 (target gene) and ACTB (reference gene) in Kermani lamb tissues.
| Studied genes | Sequence of used primer (5′-3′) | GenBank No. | Tm (°C) | Size of amplicon (bp) | Efficiency (%) |
|---|---|---|---|---|---|
| DLK1 (Target gene) | F: CGTCTTCCTCAACAAGTGCGA | NM-174037 | 57 | 102 | 98 |
| R: TCCTCCCCGCTGTTGTAGTG | |||||
| beta actin (ACTB) (reference gene) |
F: CCTGGCACCCAGCACAAT | NM_001101.3 | 57 | 144 | 99 |
| R: GGGCCGGACTCGTCATAC |
Standard methods of AOAC (2000) were used for analyzing nitrogen (method 976.05; Kjeldahl Vap50 Gerhardt, Germany), DM (dry matter), ash (method 942.05; Shimifan F-47, Tehran, Iran) and ether extract (method 920.39; Soxhlet Model 2000 Automatic Gerhardt, Germany). The method of Van Soest et al. (1991) was used to determine the NDFom and ADFom.
The final volume of each RT-PCR reaction was 15 μL, which contained 1.5 μL of template cDNA, 7.5 μL from 2X SYBR Green PCR Master Mix (Fermentase Co., Tehran, Iran), 1 μL from 10 μM forward and reverse primers, 0.3 μL of ROX, and 4.7 μL of ddH2O. As well, PCR conditions included an initial denaturation for 3 min at 94 °C, followed by 35 cycles of denaturation for 60 s at 94 °C s, annealing for 60 s at 57 °C, and synthesis for 60 s at 72 °C s followed by a melt curve at a temperature that gradually increased from 55 °C to 95 °C with increments of 0.5 °C in every 5 s. Thereafter, single sharp peaks were seen in the amplification and melting curves of both the ACTB and DLK1 PCR products. The best annealing temperature was chosen as 57 °C. The PCR of DLK1 (target) and ACTB (internal control) were obtained as 102 bp and 144 bp, respectively. For evaluating RT-PCR data, the Pfaffl method (Pfaffl et al., 2002) was employed as described in our previous investigation (Masoudzadeh et al., 2020).
2.5. Statistical analysis
The MIXED procedure of SAS was applied for evaluating the data in the format of the complete randomized design (SAS 2005). Next, in order to examine the normal pattern of the data distribution, the Pair Wise Fixed Reallocation Randomisation Test© was applied (REST, 2009). The LSD test was also performed for comparing the means (P < 0.05). Finally, the results of real time PCR were analyzed via Pfaffl formula and using REST software (Pfaffl et al., 2002).
For assessing the main effect of the level of fennel seed powder and the tissue effect with the fennel × tissue interaction, the following statistical model was used:
| X ijm = μ + α i + β j + αβ ij + ε m (ij) |
Where α i shows the main effect of tissue at level i, μ shows the mean, β j shows the main effect of fennel at level j, ε m (ij) shows the effect of all other extraneous variables on subject m in the treatment group ij, αβ ij is the interaction effect of tissue at level i and fennel at level j, and X ijm shows the dependent variable score for subject m in the treatment group ij (as defined in our previous investigation; Masoudzadeh et al., 2020).
3. Results
The finest feed conversion (FC) was established in the studied animals fed with 20 g/kg DM fennel powder (P < 0.05). The weights of the testis and gallbladder, and body weight before slaughter were greater for the animals fed with 20 g/kg DM fennel powder compared to those lambs fed with the control diet (0 g/kg DM fennel powder). However, the liver weight was lower in the animals fed with 20 g/kg DM fennel powder compared to the lambs fed with the control diet (P < 0.05) (Table 3). The results of blood liver enzymes of SGOT, SGPT, and Testosterone (Table 4) showed that blood testosterone concentration was greater in the animals fed with 10 and 20 g/kg DM fennel powder compared to those fed with the control diet (3.50 and 4.40 vs. 1.70 ng/dl, P < 0.05). It is noteworthy that concentrations of blood liver enzymes SGOT and SGPT only decreased by adding 10 g/kg DM fennel powder to the diet compared to the control animals (P < 0.05). However, no significant difference was found between the animals fed with 20 g/kg DM fennel powder and the lambs fed with 0 g/kg DM fennel powder (control). The results of two-way ANOVA analysis in terms of the comparison of the means among different tissues and different levels of fennel seed powder feeding for DLK1 expression based on LSD test are shown in Tables 5 and 6.
Table 3.
The fennel seed powder feeding effect on some parameters relating to testis, liver and humeral muscle tissues of Kermani lambs (for additional information, refer to Table 1 of our previous study Masoudzadeh et al., 2020).
| Assessed variables | Level of fennel (g/Kg DM) |
SEM | P value | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | |||
| Initial weight (kg) | 27.0 | 27.9 | 27.7 | 0.49 | 0.638 |
| Feed conversion (kg DMI/kg gain)∗ | 6.18b | 6.27a | 6.09c | 0.02 | 0.039 |
| Weight before slaughter (kg) | 44.7b | 45.8a | 46.5a | 0.23 | 0.024 |
| Weight of testis (kg) | 0.29b | 0.35a | 0.36a | 0.02 | 0.040 |
| Weight of gallbladder (kg) | 0.027b | 0.031ab | 0.041a | 0.004 | 0.049 |
| Weight of liver (kg) | 0.73a | 0.62b | 0.64b | 0.02 | 0.013 |
| Weight of humeral muscle (kg) | 2.96 | 3.10 | 3.02 | 0.10 | 0.506 |
a,b,cValues within a row with different superscripts differ significantly at P < 0.05.
Feed conversion was calculated as total dry matter intake (DMI) divided by total weight gain.
Table 4.
The effect of feeding fennel powder on blood liver enzymes of serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT) and testosterone in Kermani sheep.
| Assessed variables | Fennel consumption (g/Kg DM) |
P value | SEM | ||
|---|---|---|---|---|---|
| 0 | 10 | 20 | |||
| Testosterone (ng/dl) | 1.70b | 3.50a | 4.40a | 0.025 | 0.23 |
| Serum glutamic pyruvic transaminase (U/L) | 30.40a | 24.40b | 29.10a | 0.040 | 1.08 |
| Serum glutamic oxaloacetic transaminase (U/L) | 112.60a | 78.60b | 105.20a | 0.001 | 2.85 |
a,bValues within a row with different superscripts differ significantly at P < 0.05.
Table 5.
Two-way ANOVA analysis for expression of DLK1 in Kermani lambs in different tissues at different levels of fennel feeding.
| Source of variation | df | Mean square |
|---|---|---|
| Tissue | 2 | 1.1233∗∗ |
| Fennel | 2 | 16.8133∗∗ |
| Tissue∗ Fennel | 4 | 0.8733∗∗ |
| Coeff Var | 3.46 |
Table 6.
Comparison of means between different tissues and different levels of fennel feeding for expression of DLK1 in Kermani lambs based on LSD test.
| Tissue | Level of fennel (g/Kg DM) |
|||
|---|---|---|---|---|
| 0 | 10 | 20 | Fennel mean | |
| Humeral muscle | 1.00g | 2.40e | 4.80a | 2.73a |
| Liver | 1.00g | 2.60d | 3.30b | 2.30b |
| Testis | 1.00g | 2.00f | 3.10c | 2.03c |
| Tissue mean | 1.00c | 2.33b | 3.73a | |
Treatments that have at least one letter in common do not differ significantly. Fennel mean column numbers are compared with each other (a, b and c), tissue mean row numbers are compared with each other (a, b and c) and other numbers that show the interaction between tissue and Level of fennel (g/Kg DM) are compared with each other (a, b, c, d, e, f and g).
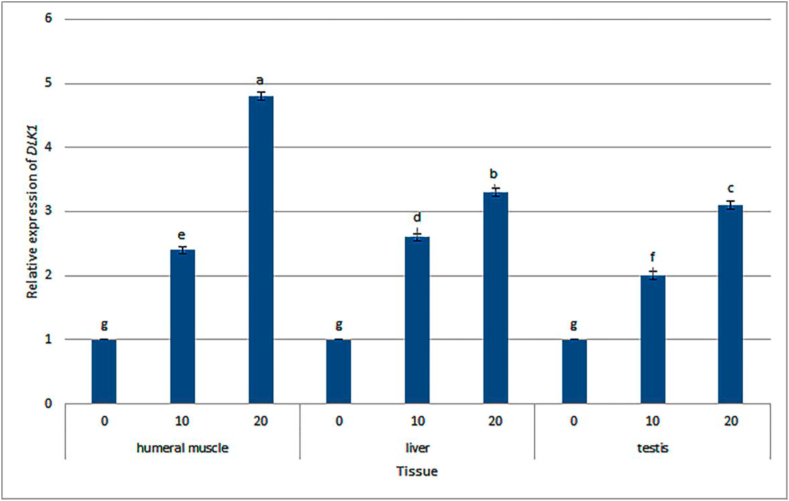
The comparison of means among different tissues and fennel seed powder consumption (with different levels) for of DLK1 expression based on LSD test is given in Figure 1. When the lambs were fed with the diets with high fennel level, the expression level of DLK1 in the studied tissues (including testis, liver, and humeral muscle) enhanced (P < 0.05). The highest rate of expression for DLK1 was observed in the lambs fed with the diets with 20 g/kg DM fennel powder. For the addition of fennel seed powder to diet at 10 g/kg DM level, the testis, humeral muscle, and liver tissues showed greater DLK1 expression in humeral muscle and liver tissues compared to testis tissues (P < 0.05). Moreover, at 20 g/kg DM level of fennel powder feeding, DLK1 was more expressed in the humeral muscle and liver tissues compared to the testis tissue, which was the most in the liver tissue (P < 0.05). Of note, fennel consumption and tissue interaction effects were also found to be significant.
Figure 1.
Means comparison between different tissues and between different levels of fennel feeding for expression of DLK1 based on LSD test. a,b,c, …,gDifferent values on the top of columns differ significantly at P < 0.05.
4. Discussion
By investigating the DLK1 expression, it was demonstrated that this gene was expressed in all humeral muscle, liver, and testis tissues of the studied animals. Accordingly, the humeral muscle tissue had the highest DLK1 gene expression in comparison to other studied tissues. These results are consistent with those of various studies that have previously shown the DLK1 expression in muscle of mouse (Yevtodiyenko and Schmidt, 2006) and pigs (Oczkowicz et al., 2010), testis of human (Lottrup et al., 2015) and mice (Yuan et al., 2018), and liver of mice (Tanimizu et al., 2003) and pigs (Oczkowicz et al., 2010).
The improved FC (indicated by a lower value) was observed in the diet containing 20 g/kg DM fennel powder, which may be related to higher daily weight gain of the animals. FC for Iranian meat sheep breeds varies in the range of 5–7. As well, according to the study by SoltaniNezhad et al. (2016), it is affected by various factors such as age, diet, breed, and weight. In another study, Benchaar et al. (2006) have added herbal flavors up to 2% to the diet of fattening cows and demonstrated that it could improve FC. Moreover, in a study by Saeedi et al. (2017), fennel seed powder (FSP) was added to the dairy cattle starter diets, and it was observed that it improved FC. Therefore, they have concluded that this increase was due to the addition of fennel. The results of the current study are in line with the results of some other researchers who have shown that fennel could improve FC (Benchaar et al., 2007; Meyer et al., 2007; Soltan, 2009; Vakili et al., 2013). Flavoring herbs have been found to contain chemicals with the property to modulate bacterial fermentation in the rumen and to reduce methane production, thereby improving FC (Evan and Martin, 2000). Zolfaghari Moheb et al. (2015) in their research have used diets containing 9% fennel to feed lambs, and as a result, they have reported that FC improved. Considering that lambs fed with diets containing 20 g/kg DM fennel powder had better performance and this was confirmed by better FC, it can be concluded that the use of medicinal plants to stimulate and improve growth can lead to some positive economic effects on production industry, especially meat production.
Increasing the amount of fennel powder in diets of the studied animals was found to be associated with higher expression of DLK1 in the studied tissues (including testis, liver, and humeral muscle). In another study, Yuan et al. (2018) showed that the expression of DLK1 is upregulated in testis. Additionally, Lottrup et al. (2015) in their study have investigated the potentials of leydig cells developmental markers and adrenal steroidogenic markers in the differential diagnosis of testicular adrenal rest tumors and malignant leydig cells tumors. According to their findings, testicular steroidogenic enzymes was expressed in all specimens and DLK1 was highly expressed in testicular adrenal rest tumors. Malignant leydig cells tumours seem to have lost DLK1 expression and did not resemble immature leydig cells. In regard to the stimulating effect of fennel powder on the expression of DLK1 in the testis as well as the role of this gene in preventing cancer in testis (Lottrup et al., 2015), it can be concluded that using fennel powder may possibly play a role in the prevention of cancer in the testis tissue, which can be investigated. Although in confirmation of this suggestion, it is necessary to study the role of fennel powder in the expression of DLK1 in both healthy and cancer testis tissues more precisely. The addition of fennel powder to the diets of the studies sheep consequently increased the weight of testis and concentration of testosterone. In this regard, Ghorbani-Ranjbari et al. (2014) have added nettle plant to the diet of rats and reported that blood level of testosterone and weight of testis decreased in response to the phyto-estrogenic characteristics of nettle plant. As well, it has been shown that hydro-alcoholic compounds of chamomile plant can enhance the number of spermatozoa, weight of testis, and concentration of blood testosterone in rat, which can be attributed to the presence of antioxidants. Plants with bioactive compounds have a dose-dependent effect on spermatogenesis. Correspondingly, in the study by Heydari et al. (2008), the production of sperm reduced in the high dose of rosemary, but its low dose had no effect on sperm production. In regards to our results and results of the above-mentioned investigations, it can be concluded that one of the ways to increase testosterone concentration is the use of fennel powder in the diet of lambs.
The present study showed that DLK1 is expressed in the liver and the fennel powder could significantly enhance DLK1 expression in this organ. In agreement with our results, Tanimizu et al. (2003) have also shown that transmembrane and soluble forms of DLK1 affect liver development. Based on the report by Rocha et al. (2007), DLK1 is expressed at low levels in the liver from the maternal allele. The same patterns for the expression of DLK1 have also presented in the liver and muscle tissues (Oczkowicz et al., 2010).
In the current study, the addition of 10 g/kg DM fennel powder to the diet of lambs led to the decreased concentration of blood liver enzymes SGOT and SGPT, so that the lowest concentrations were observed in this level of fennel. This may possibly be due to the active components of fennel. From an animal production point of view, lower levels of liver enzymes SGOT and SGPT are desirable, so adding fennel to the diet can have beneficial effects in this regard. Based on the report by Chang et al. (2013), there are various polyphenolic compounds such as anethole, D-limonene, and β-myrcene with antioxidant functions and fennel performs its protective functions via reducing the concentrations of SGPT and SGOT blood liver enzymes. On the other hand, the addition of 20 g/kg DM fennel powder to diet increased the concentration of blood liver enzymes SGOT and SGPT compared to 10 g/kg DM fennel powder. While this concentration decreased in comparison to 0 g/kg DM fennel powder, which may be due to superior quantity of anethole in the diet containing 20 g/kg DM fennel seed powder. Based on the report by Bazmi et al. (2015), several combinations such as transanethole, cumarin, ethyl acetate, and their extractions from hogweed plant enhanced SGOT and SGPT levels. In another study, Eman et al. (2011) have reported that some components of fennel seed such as β-myrcene and D-limonene have an efficient hepatoprotective function. Membrane lipids peroxidation is created by free radicals and this peroxidation consequently enhances enzymes of liver, in order to increase defense system of the body. The increased dry matter intake increases the weight of visceral organs, including the liver. Because the amount of dry matter consumption in the treatment 3 (20 g/kg DM fennel) was slightly higher than the treatment 2 (10 g/kg DM fennel), this slight difference can cause a non-significant difference in liver weights between these two treatments. In addition, the increased dry matter intake in the treatment 3 (20 g/kg DM fennel) probably increased the passage rate in the gastrointestinal tract and then led diet 3 (20 g/kg DM fennel) to play minor role in reducing the liver enzymes SGOT and SGPT, compared to the treatment 2 (10 g/kg DM fennel).
In the present study, an increase was observed in humeral muscle weight and eye muscle area along with an associated increase in the DLK1 expression. The DLK1 expression in the muscle has been previously described in the studies by Su et al. (2014), Yevtodiyenko and Schmidt (2006), Oczkowicz et al. (2010), and Davis et al. (2004). Moreover, the DLK1 expression has been described in the muscle of sheep (Yevtodiyenko and Schmidt 2006), and it was concluded that DLK1 plays a significant role in muscle growth. The considerable, positive association among the expression of IGF1 and DLK1, fiber shear stress, and fiber diameter of muscle has been reported as well (Su et al., 2014). In another study, Davis et al. (2005) have shown that over-expressing DLK1 in the transgenic mice skeletal muscle significantly improves mass and fiber size of muscle, which is known as the cause of muscle hypertrophy in callipyge sheep (Kim et al., 2004). As well, Waddell et al. (2010) have reported that hypertrophy of muscle and distinction of neighbouring satellite cells are enhanced by the DLK1 expression via aborning or regenerating myofibers. Additionally, Davis et al. (2005) have demonstrated that fiber size and mass of muscle are significantly increased by DLK1. In our previous study, it was found that enriching the diet of lambs with fennel seed powder can consequently result in the increased DLK1 expression in the femur muscle because the addition of 20 g/kg DM fennel to the diet significantly increased the expression of DLK1 in this tissue compared to the control group (0 g/kg DM fennel) (Masoudzadeh et al., 2020).
In another study, Yu et al. (2018) have identified genes directly responding to DLK1 signaling in Callipyge sheep and indicated that DLK1 expression was significantly increased in hypertrophied muscles, but not in non-hypertrophied muscles. They have concluded that retrotransposon-like 1 (RTL1) alone is insufficient to induce muscle hypertrophy and DLK1 is the likely primary inducer of the hypertrophy phenotype. Based on our results, DLK1 expression can be increased in different tissues of Kermani lambs by fennel powder consumption, but more studies are needed to take into account various genetic, epigenetic, and physiological statuses to achieve a more decisive result in this regard.
5. Conclusion
In this study, we reported that increasing DLK1 expression is associated with the consumption of fennel powder, along with causing some positive effects on FC, physiological, and growth characteristics. Our results show that using fennel powder in the diets of Kermani lamb can be effective on the expression of DLK1 in three important tissues, including the testis, liver, and muscle. The increased concentrations of testosterone and blood liver enzymes SGOT and SGPT along with the increased humeral muscle weight and area were also reported, which presumably are the affirmative consequences of DLK1 expression. As fennel feeding was found to be associated with the enhanced the level of DLK1 expression in several tissues (including testis, liver, and muscle), diet supplementation with this herb should be remarked for improving the growth of animals and mass of muscle, which is of great importance in the farm animal breeding, especially meat production. For obtaining a final and accurate conclusion on the benefits of using fennel as a feed supplement used to improve sheep production traits, in the future studies, physiological and epigenetic aspects should be considered in addition to genetic aspects. This research highlighted the need for more extensive research in this area.
Declarations
Author contribution statement
Mohammadreza Mohammadabadi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Seyed Hojat Masoudzadeh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Amin Khezri; Valentyna Petrivna Oleshko: Conceived and designed the experiments; Wrote the paper.
Oleksandr Kalashnyk; Ruslana Volodymyrivna Stavetska: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nataliia Ihorivna Klopenko: Analyzed and interpreted the data; Wrote the paper.
Serhii Vasyliovych Tkachenko: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by Shahid Bahonar University of Kerman, the Vice Chancellor for Research and Technology (Grant number: G-311/8718).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the animal science department of our universities to provide animal breeding units as well as equipment needed in laboratories.
References
- Aćimović M.G., Kostadinović L.M., Puvača N.M., Popović S.J., Urošević M.I. Phytochemical constituents of selected plants from apiaceae family and their biological effects in poultry. Food Feed Res. 2016;43:35–41. [Google Scholar]
- Amiri Roudbar M., Abdollahi-Arpanahi R., Ayatollahi Mehrgardi A., Mohammadabadi M., Taheri Yeganeh A., Rosa G.J.M. Estimation of the variance due to parent-of-origin effects for productive and reproductive traits in Lori-Bakhtiari sheep. Small Rumin. Res. 2018;160:95–102. [Google Scholar]
- AOAC . seventeenth ed. Association of Official Analytical Chemists; Arlington, VA, USA: 2000. Official Methods of Analysis. [Google Scholar]
- Bazmi F., Mokhtari M., Khatamsaz S. Effect of hydro-alcoholic extract of Heracleum persicum during pregnancy on liver enzymes (AST-ALT-ALP) and biochemical factors (albumin and protein) of infant male rats. Pars J. Med. Sci. 2015;13:7–13. [Google Scholar]
- Benchaar C., Petie H.V., Berthiaume R., Ouellet D.R., Chiquette J., Chouinard P.Y. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage on corn silage. J. Dairy Sci. 2007;90:886–897. doi: 10.3168/jds.S0022-0302(07)71572-2. [DOI] [PubMed] [Google Scholar]
- Benchaar C., Petit H.V., Berthianume R., Whyte T.D., Chouinard P.Y. Effects of addition of essential oils and monensin premix on digestion, ruminal fermentation, milk production and milk composition in dairy cows. J. Dairy Sci. 2006;89:4352–4365. doi: 10.3168/jds.S0022-0302(06)72482-1. [DOI] [PubMed] [Google Scholar]
- Chang S.H., Bassiri A., Jalali H. Evaluation of antioxidant activity of fennel (Foeniculum vulgare) seed extract on oxidative stability of olive oil. J. Chem. Health Risk. 2013;3:53–61. [Google Scholar]
- Cockett N.E., Jackson S.P., Shay T.L., Farnir F., Berghmans S., Snowder G.D., Nielsen D.M., Georges M. Polar overdominance at the ovine callipyge locus. Science. 1996;273:236–238. doi: 10.1126/science.273.5272.236. [DOI] [PubMed] [Google Scholar]
- Davis E., Caiment F., Tordoir X., Cavaille J., Ferguson-Smith A., Cockett N., Georges M., Charlier C. RNAi-mediated allelic trans-Interaction at the imprinted Rtl1/Peg11 locus. Curr. Biol. 2005;15:743–749. doi: 10.1016/j.cub.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Davis E., Jensen C.H., Schroder H.D., Farnir F., Shay-Hadfield T., Kliem A., Cockett N., Georges M., Charlier C. Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype. Curr. Biol. 2004;14:1858–1862. doi: 10.1016/j.cub.2004.09.079. [DOI] [PubMed] [Google Scholar]
- Eman G.E., Fatma Ahmed E., Amira M. Effect of fennel (Foeniculum vulgare) on hyperlipidemic rats. Egypt J. Hospital Med. 2011;43:212–225. [Google Scholar]
- Evan J.D., Martin S.A. Effects of thymol on ruminal microorganisms. Curr. Microbiol. 2000;41:336–340. doi: 10.1007/s002840010145. [DOI] [PubMed] [Google Scholar]
- Georges M., Charlier C., Cockett N. The callipyge locus: evidence for the trans interaction of reciprocally imprinted genes. Trends Genet. 2003;19:248–252. doi: 10.1016/S0168-9525(03)00082-9. [DOI] [PubMed] [Google Scholar]
- Ghorbani Ranjbari A., Ghorbani Ranjbari N., Ghorbani Ranjbari Z., Ghorbani Ranjbari S. Study of nettle hydro alcoholic extraction (Urtica dioica) influence on spermatogenesis and testosterone, spermatogenesis hormones, changes in rats. New Cell Mol. Biotechnol. 2014;4:31–39. [Google Scholar]
- Hajalizadeh Z., Dayani O., Khezri A., Tahmasbi R., Abadi M.R.M. The effect of adding fennel (Foeniculum vulgare) seed powder to the diet of fattening lambs on performance, carcass characteristics and liver enzymes. Small Rumin. Res. 2019;175:72–77. [Google Scholar]
- Heydari M.A.F., Ghaffari-Novin M., Vaezi G.H., Keramati K., Rajaei F. Antiandrogenic effects of Rosmarinus officinalis extract on the reproductive tract of male rats. Tehran Univ. Med. J. 2008;65:26–31. [Google Scholar]
- Kim K.S., Kim J.J., Dekkers J.C., Rothschild M.F. Polar overdominant inheritance of a DLK1 polymorphism is associated with growth and fatness in pigs. Mamm. Genome. 2004;15:552–559. doi: 10.1007/s00335-004-2341-0. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim D.H., Guan L.L., Ahn S.K., Cho K.W., Lee S.S. Effect of medicinal plant by-products supplementation to total mixed ration on growth performance, carcass characteristics and economic efficacy in the late fattening period of hanwoo steers. Asian Austr. J. Anim. Sci. 2015;28:1729–1735. doi: 10.5713/ajas.15.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottrup G., Nielsen J.E., Skakkebæk N.E., Juul A., Meyts E.R. Abundance of DLK1, differential expression of CYP11B1, CYP21A2 and MC2R, and lack of INSL3 distinguish testicular adrenal rest tumours from leydig cell tumours. Euro. J. Endocrinolol. 2015;172:491–499. doi: 10.1530/EJE-14-0810. [DOI] [PubMed] [Google Scholar]
- Masoudzadeh S.H., Mohammadabadi M., Khezri A., Stavetska R.V., Oleshko V.P., Babenko O.I., Yemets Z., Kalashnik O.M. Effects of diets with different levels of fennel (Foeniculum vulgare) seed powder on DLK1 gene expression in brain, adipose tissue, femur muscle and rumen of Kermani lambs. Small Rumin. Res. 2020;193 [Google Scholar]
- Meyer N.F., Erickson G.E., Klopfenstein T.J., Greenquist M.A., Williams P., Losa R. 2007. Effect of Crina Ruminants AF, a Mixture of Essential Oil Compounds on Finishing Beef Steer Performance Nebraska Beef Cattle Reports.http://digitalcommons.unl.edu/animalsci nbcr/80 [Google Scholar]
- Oczkowicz M., Piestrzyska-Kajtoch A., Piórkowska K., Rejduch B., Rózycki M. Expression of DLK1 and MEG3 genes in porcine tissues during postnatal development. Genet. Mol. Biol. 2010;33:790–794. doi: 10.1590/s1415-47572010000400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W., Horgan G.W., Dempfle L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in Real-Time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S.T., Tevendale M., Knowles E., Takada Sh., Watkins M., Ferguson-Smith A.C. Restricted co-expression of DLK1 and the reciprocally imprinted non-coding RNA, Gtl2: implications for cis-acting control. Dev. Biol. 2007;306:810–823. doi: 10.1016/j.ydbio.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Saeedi S., Dayani O., Tahmasbi R., Khezri A. Effect of supplementation of calf starter with fennel powder on performance, weaning age and fermentation characteristics in Holstein dairy calves. J. Anim. Physiol. Anim. Nutr. 2017;101:81–87. doi: 10.1111/jpn.12511. [DOI] [PubMed] [Google Scholar]
- Smit M., Segers K., Carrascosa L.G., Shay T., Baraldi F., Gyapay G. Mosaicism of solid gold supports the causality of a non coding A-to-G transition in the determinism of the callipyge phenotype. Genetics. 2003;163:453–456. doi: 10.1093/genetics/163.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan M.A. Effect of essential oils supplementation on growth performance, nutrient digestibility, health condition of Holstein male calves during pre- and postweaning periods. Pakistan J. Nutr. 2009;8:642–652. [Google Scholar]
- SoltaniNezhad B., Dayani O., Khezri A., Tahmasbi R. Performance and carcass characteristics in fattening lambs feed diets with different levels of pistachio byproducts silage with wasted date. Small Rumin. Res. 2016;137:177–182. [Google Scholar]
- Su R., Sun W., Li D., Wang Q.Z., Lv X.Y., Musa H.H., Chen L., Zhang Y.F., Wu W.Z. Association between DLK1 and IGF-I gene expression and meat quality in sheep. Genet. Mol. Res. 2014;13:10308–10319. doi: 10.4238/2014.December.4.26. [DOI] [PubMed] [Google Scholar]
- Tanimizu N., Nishikawa M., Saito H., Tsujimura T., Miyajima A. Isolation of hepatoblasts based on the expression of dlk/pref-1. J. Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- Vakili A.R., Khorrami B., Danesh Mesgaran M., Parand E. Effect of thyme and cinnamon essential oils on performance, rumen fermentation and blood metabolites in Holstein calves consuming high concentrate diet. Asian-Austr. J. Anim. Sci. 2013;26:935–944. doi: 10.5713/ajas.2012.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Waddell J.N., Zhang P., Wen Y., Gupta S.K., Yevtodiyenko A., Schmidt J.V., Bidwell C.A., Kumar A., Kuang S. DLK1 is necessary for proper skeletal muscle development and regeneration. PLoS One. 2010;5:1–12. doi: 10.1371/journal.pone.0015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.D., Vuocolo T., McDonagh M., Grounds M.D., Harper G.S., Cockett N.E., Tellam R. Analysis of the callipyge phenotype through skeletal muscle development; association of DLK1 with muscle precursor cells. Differentiation. 2008;76:283–298. doi: 10.1111/j.1432-0436.2007.00208.x. [DOI] [PubMed] [Google Scholar]
- Yevtodiyenko A., Schmidt J.V. DLK1 expression marks developing endothelium and sites of branching morphogenesis in the mouse embryo and placenta. Develop. Dyn. 2006;235:1115–1123. doi: 10.1002/dvdy.20705. [DOI] [PubMed] [Google Scholar]
- Yuan B., Zhang H., Wang X., Pan Y., Jiang J. Effect of nano-SiO2 on expression and aberrant methylation of imprinted genes in lung and testis. Nanoscale Res. Lett. 2018;13:266–271. doi: 10.1186/s11671-018-2673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Waddell J.N., Kuang S., Tellam R.L., Cockett N.E., Bidwell C.A. Identification of genes directly responding to DLK1 signaling in Callipyge sheep. BMC Genom. 2018;19:1–16. doi: 10.1186/s12864-018-4682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari Moheb S., Fatahnia F., Alipour D. Effect of fennel by-product on performance of growing lambs and gas production parameters of their diets. Iran. J. Anim. Sci. 2015;46:201–210. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.