Abstract
Background
Nitric oxide (NO) plays multiple roles regulating the central nervous, cardiovascular, and immune systems.
Objective
Our aim was to investigate the role of NO in the efficacy of hypertonic saline (7.5% sodium chloride [NaCl]) adenosine, lidocaine, and magnesium (ALM) to improve mean arterial pressure (MAP) and heart rate following hemorrhagic shock.
Methods
One hundred one male Sprague-Dawley rats (mean [SD] weight = 425 [6] g) were randomly assigned to 20 groups (groups of 4–8 rats each). Hemorrhagic shock (MAP < 40 mm Hg) was induced by 20-minute pressure-controlled bleeding (∼40% blood volume), and the animal was left in shock (MAP = 35-40 mm Hg) for 60 minutes. The NO synthase (NOS) inhibitor L-NAME was administered with a 0.3-mL bolus of different combinations of 7.5% NaCl ALM active ingredients and hemodynamic parameters were monitored for 60 minutes. A number of specific NOS and NO inhibitors were tested.
Results
We found that 7.5% NaCl ALM corrected MAP after hemorrhagic shock. In contrast, the addition of L-NAME to 7.5% NaCl ALM led to a rapid fall in MAP, sustained ventricular arrhythmias, and 100% mortality. Saline controls receiving 7.5% NaCl with NG-nitro-l-arginine methyl ester (L-NAME) showed improved MAP with no deaths. None of the specific NOS and NO inhibitors mimicked L-NAME's effect on ALM. The addition of inducible NOS inhibitor 1400W to 7.5% NaCl ALM failed to resuscitate, whereas the NO scavenger PTIO and the PI3K inhibitor wortmannin reduced MAP recovery during 60-minute resuscitation.
Conclusions
The ability of 7.5% NaCl ALM to resuscitate appears to be linked to 1 or more NO-producing pathways. Nonspecific NOS inhibition with L-NAME blocked ALM resuscitation and led to cardiovascular collapse. More studies are required to examine NO site-specific contributions to ALM resuscitation. (Curr Ther Res Clin Exp. 2022; 82:XXX–XXX)
Key words: endothelium, hemorrhagic shock, nitric oxide, nitric oxide synthase, resuscitation
Introduction
Hemorrhage is responsible for 30% to 40% of early trauma mortalities in the civilian population and up to 50% of deaths on the battlefield.1 Over the past 3 decades, resuscitating bleeding patients with large fluid volumes of crystalloids/colloids has led to poor outcomes.2 The current resuscitation guidelines for hemorrhage without suspected brain injury suggest the use of smaller fluid volumes and a target mean arterial blood pressure (MAP) of 60 to 65 mm Hg (permissive hypotension) to prevent rebleeding and secondary complications.1 We have been developing a small-volume fluid therapy for prehospital use comprising hypertonic saline with adenosine, lidocaine, and magnesium (ALM).3,4 Hypertonic saline assists ALM to increase blood pressure into the permissive hypotensive range, and the combination has been shown to increase survival after hemorrhagic shock by improving cardiac function, reducing inflammation, correcting coagulopathy, and preventing ischemia-reperfusion injury.1,3,5, 6, 7, 8, 9, 10, 11 We have also shown that the ALM combination is key to permissive hypotensive resuscitation and protection; not the individual active ingredients adenosine, lidocaine, and magnesium or other combinations.1,3,12
Nitric oxide (NO) is a ubiquitous signalling messenger molecule involved in diverse pathophysiologic processes such as neurotransmission, inflammatory and immune responses, and regulation of cardiovascular function.13 The regulation of myocardial function by NO is important for the maintenance of myocardial calcium ion homeostasis, relaxation and distensibility, and protection from arrhythmias and the sympathetic-induced stress response.13, 14, 15 Thus, the fine NO balance between production and synthesis maintains cardiac function, vasculature patency, arterial blood pressure, endothelial function, and tissue perfusion.16 Because NO is pivotal in regulating the cardiovascular, central nervous and immune systems,14,15,17 and hypertonic saline at 7.5% sodium chloride (NaCl) ALM has been shown to modulate and protect these systems, our aim was to investigate the role of NO in the efficacy of 7.5% NaCl ALM resuscitation following severe hemorrhagic shock in a rat model. Our hypothesis is that addition of the nonspecific NOS inhibitor NG-nitro-l-arginine methyl ester (L-NAME), to ALM therapy will influence its resuscitation potential. L-NAME has been shown to resuscitate and increase MAP in multiple preclinical animal models of hemorrhage via its arteriolar vasoconstrictive effects.15,18,19 We will examine the role of specific NO inhibitors to further understand the underlying mechanism of ALM resuscitation.
Methods
Animals and ethics
Male Sprague Dawley rats (weight = 425 [6] g) were obtained from our institutional breeding colony, and housed in a 14/10 hour light/dark cycle with free access to food and water ad libitum. Animals were heparinized with 2,500 IU Heparin Sodium (Hospira, Lake Forest, Illinois) and anesthetized intraperitoneally with 100 mg/kg sodium thiopentone (Thiobarb, Rutherford, Australia). Anesthetic was administered as required throughout the protocol. The study was approved by our institutional Animal Ethics Committee (No. A1148) and conforms to the Australian Code for the Care and Use of Animals for Scientific Purposes (8th edition; 2013) and the Guide for Care and Use of Laboratory Animals (8th edition; 2011). As per the Australian code, the 3Rs (ie, replacement, reduction, and refinement) have been applied in this study. Hemorrhage results in the activation of multiple body systems that cannot be accurately mimicked using an alternative in vitro method (ie, replacement). Power analysis was conducted to determine the smallest number of animals necessary to achieve the study's aims (see Statistical Analysis) (ie, reduction), and depth of anesthesia was continuously monitored to ensure animal well-being (ie, refinement).
Surgical protocol
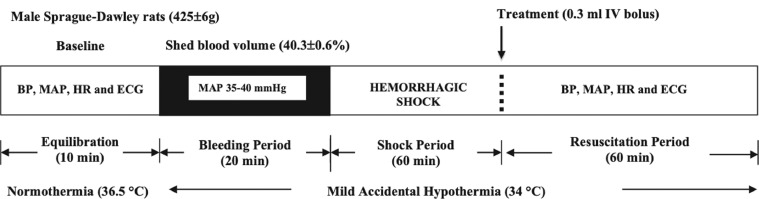
The surgical procedure has been described previously.1,12 Following anesthesia, a tracheotomy was performed, and animals were ventilated on humidified room air at 90 strokes/minute with positive end expiratory pressure of 1 cm, and tidal volume of 5 mL/kg (Harvard Small Animal Ventilator, Holliston, Massachusetts, USA). Temperature was monitored throughout with a rectal probe. Temperature was left to drift, with no thermal support provided during surgery, bleeding, or resuscitation. The left femoral vein and artery were cannulated using PE-50 tubing for drug infusions and hemodynamic monitoring (Powerlab; ADInstruments, Bella Vista, New South Wales, Australia), and the right femoral artery was cannulated for blood-letting. All cannulae contained heparinized saline (1000 U/mL saline). Lead II electrocardiogram was attached for BioAmp recording (ADInstruments). Following surgical instrumentation, anesthetized animals had a 10-minute baseline equilibration period (Figure 1). Animals were excluded from study if they were difficult to anesthetize; experienced complex arrhythmias during preparation, stabilization, or within the shock period; or were hemodynamically unstable before phlebotomy.
Figure 1.
A schematic of the pressure controlled in vivo rat protocol of hemorrhagic shock. Shed blood volume was taken over a 20-minute period to maintain mean arterial blood pressure of 35 to 40 mm Hg (40.3% [0.6%] blood loss), and the rat remained in shock for a period of 60 minutes before resuscitation (0.3 mL intravenous fluid bolus). See Methods for treatment group details.
Experimental design
Rats (n = 101) were randomly assigned to 1 of 20 groups (see the Table 1). 7.5% NaCl was used as the vehicle for all groups. Study groups 1 through 12 examined the effect of the nonspecific NO synthase (NOS) inhibitor L-NAME on 7.5% NaCl ± ALM resuscitation. Doses were 30 mg/kg (40 mg/mL) L-NAME, 1 mM (0.26 mg/mL) adenosine, 3 mM (0.8 mg/mL) lidocaine, and 2.5 mM (0.3 mg/mL) magnesium sulfate, as per previous studies.1,9,18,20 Study groups 13 through 20 examined the effect of specific NOS inhibitors and modulators, including wortmannin (1 mg/kg),21 oxadiazolo[4,3-a] quinoxalin-1-one (ODQ) (2 mg/kg),22,23 aminoguanidine hydrochloride (AMG) (20 mg/kg),24 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) (10 mg/kg),25 N-[3-aminomethyl]benzyl] acetamidine (1400W) (10 mg/kg),26 S-methyl-l-thiocitrulline (SMTC) (1 mg/kg),27 ARL17477 dihydrochloride hydrate (ARL17477) (1 mg/kg),28 and 1-(2-trifluoromethylphenyl) imidazole (TRIM) (1 mg/kg)29 (see the Table 1 for group sizes and specifics).
Table 1.
Experimental groups and doses.
| Group | Name | Abbreviation | Size | Dose (mg/mL) | Description |
|---|---|---|---|---|---|
| 1 | Saline control | 7.5% NaCl | n = 7 | 0.075 | Vehicle for all groups |
| 2 | Adenosine + lidocaine + magnesium | ALM | n = 8 | A: 0.26; L: 0.8; M: 0.3 | Novel resuscitation fluid therapy |
| 3 | Adenosine + lidocaine | AL | n = 8 | A: 0.26; L: 0.8 | ALM resuscitation therapy individual actives |
| 4 | Magnesium | M | n = 8 | 0.3 | |
| 5 | NG-nitro-l-arginine methyl ester | L-NAME | n = 4 | 40 | Non-specific NO synthase inhibitor45 |
| 6 | Adenosine + lidocaine + magnesium + NG-nitro-l-arginine methyl ester |
ALM + L-NAME | n = 8 | A: 0.26; L: 0.8; M: 0.3; L-NAME: 40 | Effect of nonspecific NO synthase inhibitor on ALM resuscitation |
| 7 | Adenosine + NG-nitro-l-arginine methyl ester | A + L-NAME | n = 4 | A: 0.26; L-NAME: 40 | Effect of nonspecific NO synthase inhibition on individual actives and combinations of ALM fluid therapy |
| 8 | Lidocaine + NG-nitro-l-arginine methyl ester | L + L-NAME | n = 4 | L: 0.8; L-NAME: 40 | |
| 9 | Magnesium + NG-nitro-l-arginine methyl ester | M + L-NAME | n = 4 | M: 0.3; L-NAME: 40 | |
| 10 | Adenosine + lidocaine + NG-nitro-l-arginine methyl ester | A + L + L-NAME | n = 4 | A: 0.26; L: 0.8; L-NAME: 40 | |
| 11 | Adenosine + magnesium + NG-nitro-l-arginine methyl ester | A + M + L-NAME | n = 4 | A: 0.26; M: 0.3; L-NAME: 40 | |
| 12 | Lidocaine + magnesium + NG-nitro-l-arginine methyl ester | L + M + L-NAME | n = 4 | L: 0.8; M: 0.3; L-NAME: 40 | |
| 13 | Adenosine + lidocaine + magnesium + wortmannin |
ALM + Wortmannin | n = 4 | A: 0.26; L: 0.8; M: 0.3; wortmannin: 1.33 | Effect of phosphatidylinositol-3-kinase inhibitor on ALM resuscitation. PI-3-kinase activation and protein kinase B/Akt signaling increases eNOS activity21 |
| 14 | Adenosine + lidocaine + magnesium + 1H-[1,2,4] oxadiazolo[4,3-a] quinoxalin-1-one |
ALM + ODQ | n = 4 | A: 0.26; L: 0.8; M: 0.3; ODQ: 2.67 | Effect of selective inhibition of NO-sensitive guanylyl cyclase which mediates cardiovascular and platelet actions of NO22,23 on ALM resuscitation |
| 15 | Adenosine + lidocaine + magnesium + aminoguanidine hydrochloride |
ALM + AMG | n = 4 | A: 0.26; L: 0.8; M: 0.3; AMG: 26.7 | Effect of iNOS selective inhibitor24 on ALM resuscitation |
| 16 | Adenosine + lidocaine + magnesium + 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide |
ALM + PTIO | n = 4 | A: 0.26; L: 0.8; M: 0.3; PTIO: 13.3 | Effect of NO scavenger25 on ALM resuscitation |
| 17 | Adenosine + lidocaine + magnesium + N-[3-aminomethyl]benzyl] acetamidine |
ALM + 1400W | n = 4 | A: 0.26; L: 0.8; M: 0.3; 1400W: 13.3 | Effect of iNOS selective inhibitor26 on ALM resuscitation |
| 18 | Adenosine + lidocaine + magnesium + S-methyl-l-thiocitrulline |
ALM + SMTC | n = 4 | A: 0.26; L: 0.8; M: 0.3; SMTC: 1.33 | Effect of nNOS selective inhibitor27 on ALM resuscitation |
| 19 | Adenosine + lidocaine + magnesium + ARL17477 Dihydrochloride hydrate |
ALM + ARL17477 | n = 4 | A: 0.26; L: 0.8; M: 0.3; ARL17477: 1.33 | Effect of nNOS selective inhibitor28 on ALM resuscitation |
| 20 | Adenosine + lidocaine + magnesium + 1-(2-trifluoromethylphenyl) imidazole |
ALM + TRIM | n = 4 | A: 0.26; L: 0.8; M: 0.3; TRIM: 1.33 | Effect of nNOS and iNOS selective inhibitor29 on ALM resuscitation |
NO = nitric oxide; eNOS = endothelial nitric oxide synthase; iNOS = inducible nitric oxide synthase; nNOS = neuronal nitric oxide synthase.
Shock protocol
Hemorrhagic shock was induced by withdrawing arterial blood to MAP of 35 to 40 mm Hg. Blood-letting started at ∼1 mL/min before decreasing to ∼0.4 mL/min. Phlebotomy was continued for 20 minutes and then rats were left in shock for 60 minutes with blood withdrawal or infusion to ensure MAP remained between 35 to 40 mm Hg (Figure 1). The average shed volume was 10.6 (0.2 mL) and represented an average blood loss of 40.3% (0.6%) (calculated from [(0.06 × body weight [g]) + 0.77]30), with no difference between groups. At the end of shock, rats were injected with 0.3 mL treatment bolus (∼3%–4% of shed volume) into the femoral vein over a 10-second period, and were monitored for a further 60 minutes. Hemodynamic parameters were monitored throughout the study, including heart rate (HR), systolic pressure, diastolic pressure, and MAP. Death time was defined as the point of last detectable electrical activity on lead II electrocardiogram. Ventricular arrhythmias, including premature ventricular contractions, and episodes of bigeminy, salvos, and ventricular tachycardia, were identified by an investigator blinded to treatment groups using the Lambeth convention as previously outlined in Canyon and Dobson.31
Statistical Analysis
SPSS Statistical Package 24 was used for all statistical analysis (IBM; St Leonards, New South Wales, Australia). All values are expressed as mean (SEM). Data normality was assessed numerically with Shapiro-Wilk test. ANOVA was used to evaluate parametric data with Tukey honestly significant difference or Dunnett post-hoc test dependent on Levene's homogeneity of variance. Survival was assessed using the Kaplan-Meier method with a log-rank test for comparison between treatment groups. Statistical significance was defined as P < 0.05. A priori power analysis was conducted using G*power3 program (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) to determine sample size to minimize Type 1 errors (MAP = 15 minutes resuscitation; n = 4; Cohen's d = 3; Critical t = 2.45; α error probability = 0.05; Power (1-β err prob) = 0.94).
Results
Survival
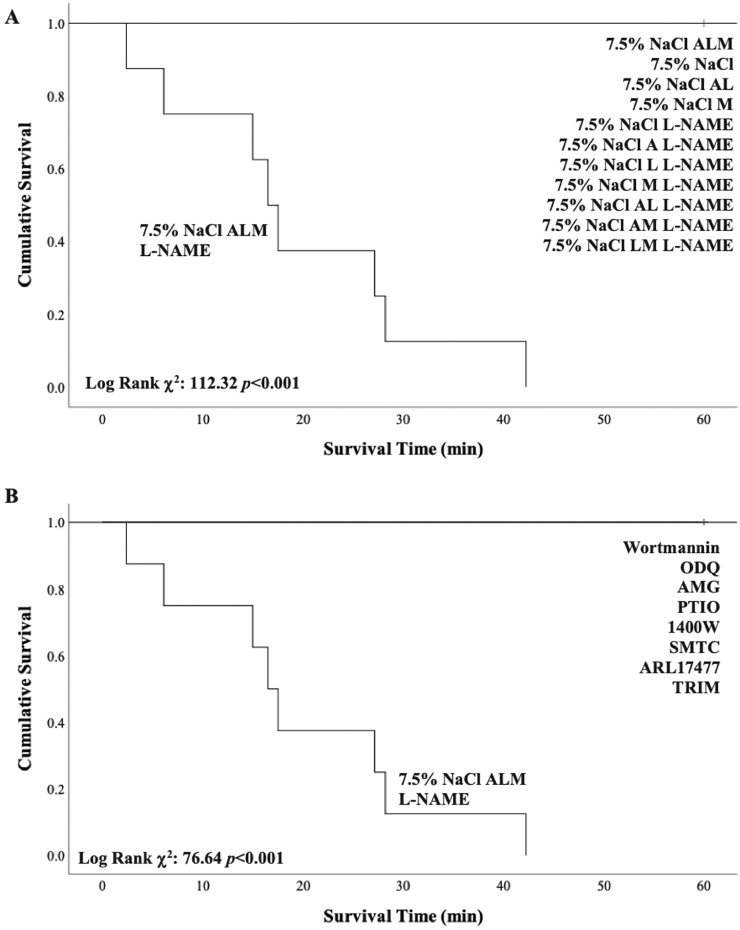
The addition of L-NAME to 7.5% NaCl ALM led to 100% mortality (mean [SD] survival time = 19.4 [4.5] minutes; P < 0.001) (Figure 2). All other groups survived the 60-minute resuscitation period following 20-minute blood loss and 60-minute shock (Figures 2A and 2B). The early cardiovascular collapse in 7.5% NaCl ALM + L-NAME animals was associated with extensive ventricular arrhythmias (65.5 [1.5] arrhythmic episodes) compared with no ventricular arrhythmias detected in 7.5% NaCl ALM alone-treated animals.
Figure 2.
Kaplan-Meier survival curves for (A) NG-nitro-l-arginine methyl ester (L-NAME) study and (B) nitric oxide/nitric oxide synthase (NO/NOS) inhibitor study. One hundred percent of 7.5% sodium chloride (NaCl) adenosine, lidocaine, and magnesium (ALM) + L-NAME-treated animals died during the 60-minute resuscitation period (mean survival time = 19.4 [4.5] minutes; P < 0.001). A = adenosine; AL = adenosine and lidocaine; AM = adenosine and magnesium; AMG = aminoguanidine; L = lidocaine; LM = lidocaine and magnesium; M = magnesium; ODQ = 1H-[1,2,4] Oxadiazolo[4,3-a] quinoxalin-1-one; PTIO = 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; 1400W = N-[3-aminomethyl]benzyl] acetamidine; SMTC = S-methyl-l-thiocitrulline; ARL17477 = ARL17477 Dihydrochloride hydrate; TRIM = 1-(2-trifluoromethylphenyl) imidazole.
Effect of L-NAME on MAP and HR in ALM and controls
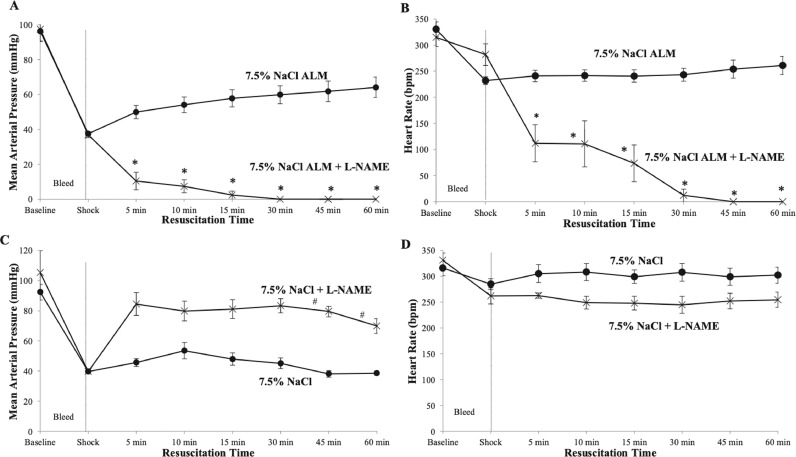
Small-volume 7.5% NaCl ALM improved MAP to the permissive hypotensive range following hemorrhagic shock from 38 to 64 mm Hg at 60 minutes (67% baseline) (Figure 3A), whereas HR remained at a constant 240 to 260 bpm (73%–79% of baseline) (Figure 3B). In contrast, in the presence of L-NAME, MAP decreased toward baseline at 5 minutes (10 mm Hg) and HR decreased to 112 bpm. MAP and HR approached 0 at 30 minutes (Figures 3A and 3B). The effect of L-NAME to cause cardiovascular collapse did not occur if it was added after 60 minutes of 7.5% NaCl ALM resuscitation. There was no change in MAP (66 mm Hg 15 minutes after 30 mg/kg L-NAME IV administration), no arrhythmias, and no mortality (n = 3, data not shown), indicating lethality only occurs when ALM and L-NAME are administered together after shock.
Figure 3.
Mean arterial pressure (MAP) and heart rate (HR) at baseline, after 60-minutes of shock, and during 60-minute resuscitation period for 7.5% sodium chloride (NaCl) adenosine, lidocaine, and magnesium (ALM) and 7.5% NaCl ALM + NG-nitro-l-arginine methyl ester (L-NAME) groups (A and B) and 7.5% NaCl and 7.5% NaCl + L-NAME groups (C and D). Values are presented as mean (SEM). *P < 0.05 compared with 7.5% NaCl ALM; #P < 0.05 compared with 7.5% NaCl.
In contrast to 7.5% NaCl ALM, the saline control (7.5% NaCl alone) failed to resuscitate, with MAP only increasing to 53 mm Hg at 10 minutes before falling below 40 mm Hg at 60 minutes (Figure 3C). When L-NAME was added to 7.5% NaCl alone, MAP increased from shock values by up to 1.7-fold over the 60 minutes, and HR decreased by ∼17% (Figures 3C and 3D).
Effect of L-NAME on 7.5% NaCl with different combinations of ALM active ingredients
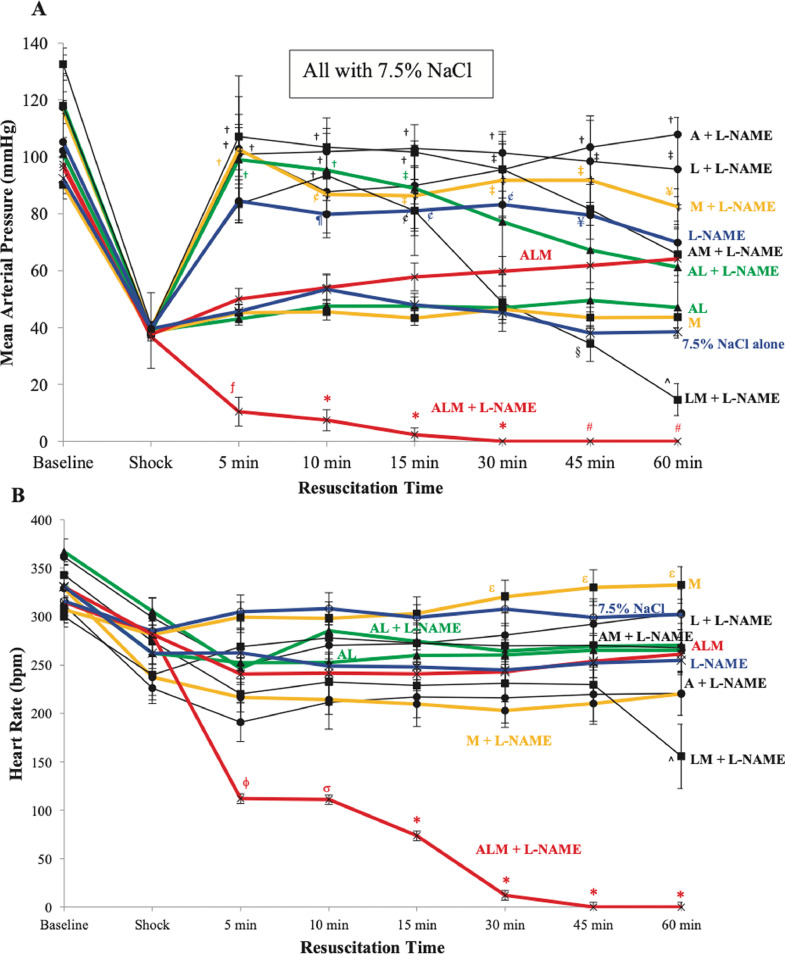
Control animals (7.5% NaCl) receiving adenosine, lidocaine, or magnesium alone with L-NAME increased MAP during resuscitation (Figure 4A). When controls received the combinations of AM, AL, or LM with L-NAME, MAP was corrected early at 5 minutes before slowly declining at different rates over 45 minutes, with LM dropping MAP to 15 mm Hg at 60 minutes (Figure 4A). In contrast, 7.5% NaCl AL, 7.5% NaCl magnesium, or 7.5% NaCl alone (no L-NAME) did not resuscitate. HR was defended across all groups relative to their shock values with the exception of 7.5% NaCl ALM + L-NAME (after 5 minutes) and 7.5% NaCl LM + L-NAME (after 45 minutes) (Figure 4B).
Figure 4.
(A) Mean arterial pressure (MAP) and (B) heart rate (HR) at baseline, after 60-minutes of shock, and during 60-minute resuscitation period for 7.5% sodium chloride (NaCl) alone; 7.5% NaCl with adenosine, lidocaine, and magnesium (ALM), adenosine and lidocaine (AL), and magnesium (M); 7.5% NaCl + NG-nitro-l-arginine methyl ester (L-NAME); and 7.5% NaCl + L-NAME with adenosine (A), lidocaine (L), M, AL, adenosine and magnesium (AM), lidocaine and magnesium (LM), and ALM. Values are presented as mean (SEM). fP < 0.05 compared with all groups except 7.5% NaCl alone and 7.5% NaCl AL; *P < 0.05 compared with all groups; #P < 0.05 compared with all groups except 7.5% NaCl LM + L-NAME; †P < 0.05 compared with 7.5% NaCl, 7.5% NaCl AL, 7.5% NaCl M, and 7.5% NaCl ALM groups; ¶P < 0.05 compared with 7.5% NaCl M; ¥P < 0.05 compared with 7.5% NaCl; ¢P < 0.05 with 7.5% NaCl M and 7.5% NaCl AL; ‡P < 0.05 compared with 7.5% NaCl, 7.5% NaCl AL, and 7.5% NaCl M; §P < 0.05 compared with 7.5% NaCl + L-NAME, 7.5% NaCl A + L-NAME, 7.5% NaCl L + L-NAME, and 7.5% NaCl M + L-NAME; ^P < 0.05 compared with 7.5% NaCl + L-NAME, 7.5% NaCl A + L-NAME, 7.5% NaCl L + L-NAME, 7.5% NaCl M + L-NAME, and 7.5% NaCl ALM; ϕP < 0.05 compared with all groups except 7.5% NaCl A + L-NAME and 7.5% NaCl M + L-NAME; σP < 0.05 compared with 7.5% NaCl L-NAME, 7.5% NaCl L + L-NAME, 7.5% NaCl AL + L-NAME, 7.5% NaCl AM + L-NAME, and 7.5% NaCl LM + L-NAME; εP < 0.05 compared with 7.5% NaCl A + L-NAME, 7.5% NaCl M + L-NAME, and 7.5% NaCl LM + L-NAME.
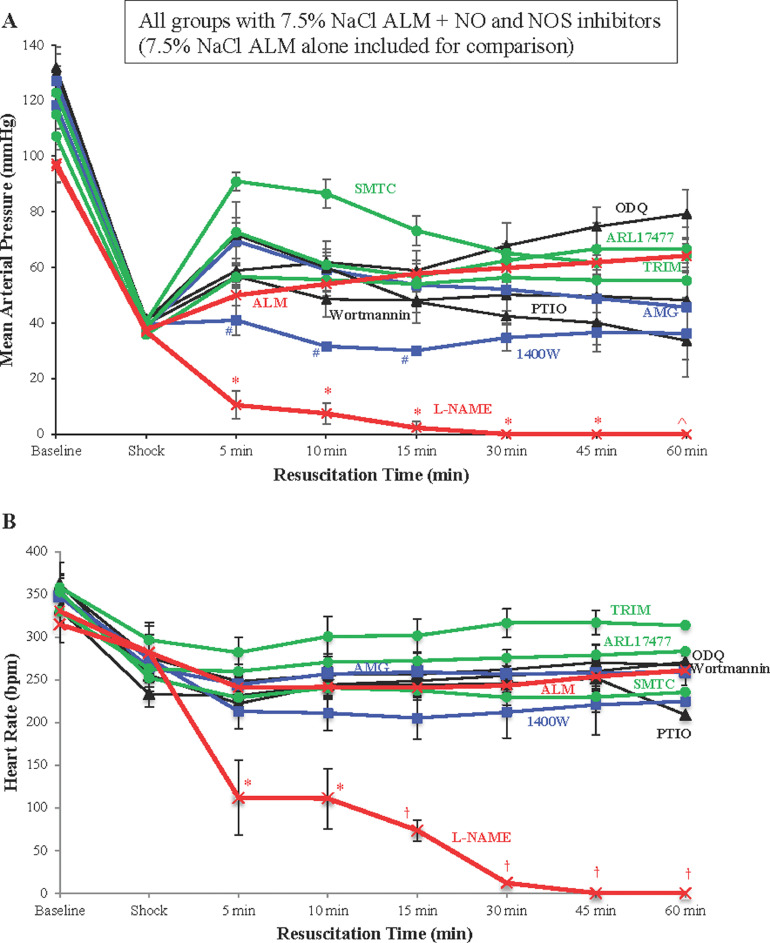
Effect of specific NOS inhibitors on MAP resuscitation with 7.5% NaCl ALM
In contrast to L-NAME, which led to cardiovascular collapse, the addition of other NOS or NO inhibitors to 7.5% NaCl ALM did not have this effect (Figure 5). At 5 minutes of resuscitation, the presence of NOS inhibitors SMTC, ARL17477, and AMG, and NO scavenger PTIO increased MAP by 1.4- to 1.8-fold compared with 7.5% NaCl ALM alone (Figure 5A). In contrast, ODQ, TRIM, and wortmannin had little effect on MAP recovery with 7.5% NaCl ALM, whereas the addition of the inducible NOS inhibitor 1400W failed to resuscitate from shock (Figure 5A). After the initial MAP increase, the AMG and PTIO groups fell toward or below shock values (Figure 5A). After 5 minutes, 7.5% NaCl ALM with wortmannin failed to improve MAP.
Figure 5.
(A) Mean arterial pressure (MAP) and (B) heart rate (HR) at baseline, after 60-minutes of shock, and during 60-minute resuscitation period for 7.5% sodium chloride (NaCl) adenosine, lidocaine, and magnesium (ALM) alone, and 7.5% NaCl ALM with NG-nitro-l-arginine methyl ester (L-NAME), wortmannin, 1H-[1,2,4] Oxadiazolo[4,3-a] quinoxalin-1-one (ODQ), aminoguanidine (AMG), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), N-[3-aminomethyl]benzyl] acetamidine (1400W), S-methyl-l-thiocitrulline (SMTC), ARL17477 dihydrochloride hydrate (ARL17477), and 1-(2-trifluoromethylphenyl) imidazole (TRIM). Values are presented as mean (SEM). Inducible nitric oxide synthase (iNOS) selective inhibitors (1400W and AMG) are highlighted in blue, whereas neuronal nitric oxide synthase (nNOS) selective inhibitors (SMTC, ARL17477, and TRIM) are highlighted in green. *P < 0.05 compared with all groups except 1400W; ^P < 0.05 compared with all groups except 1400W and PTIO; #P < 0.05 compared with SMTC; †P < 0.05 compared with all groups.
None of the specific NO inhibitors reduced HR similar to 7.5% NaCl ALM + L-NAME, which produced significantly lower heart rates from 15- to 60-minute resuscitation compared with all other groups (Figure 5B). All groups maintained a relatively constant HR across 60-minute resuscitation, except for the NO scavenger PTIO, which fell by 17% from 252 bpm at 45 minutes to 209 bpm at 60 minutes. The neuronal NOS inhibitors TRIM and ARL17477 produced the highest HRs (15%–23% and 8%–12% higher than 7.5% NaCl ALM alone, respectively) (Figure 5B). Similar to its failure to improve MAP, the addition of 1400W to 7.5% NaCl ALM reduced HR by ∼15% over the course of resuscitation, compared with 7.5% NaCl ALM alone.
Discussion
We report that the efficacy of 7.5% NaCl ALM to resuscitate into the protective permissive hypotensive range and prevent ventricular arrhythmias after severe hemorrhagic shock is completely abrogated in the presence of the nonselective inhibitor of NOS L-NAME. The addition of L-NAME to 7.5% NaCl ALM led to 100% mortality, whereas controls receiving 7.5% NaCl (no ALM) with L-NAME corrected MAP with no deaths. It appears that the resuscitation efficacy of 7.5% NaCl ALM is linked to a mechanism involving 1 or more NO-producing pathways. Using a variety of specific NOS and NO inhibitors, we report that no inhibitor mimicked 7.5% NaCl ALM L-NAME's effect to elicit cardiovascular collapse.
7.5% NaCl ALM resuscitation appears to be NO-dependent
Similar to previous studies, and in contrast to 7.5% NaCl alone or in combination with individual adenosine, lidocaine, and magnesium actives, 7.5% NaCl ALM was the only treatment to resuscitate and maintain MAP in the permissive hypotensive range over 60-minute monitoring (Figures 3 and 4).1 A key finding of the present study was that NO inhibition with L-NAME in the presence of 7.5% NaCl ALM led to a rapid and lethal hypotensive state following hemorrhagic shock (Figures 2, 3, and 5). This was surprising because 7.5% NaCl L-NAME (no ALM) significantly increased MAP (Figure 3C), and L-NAME has been shown to be a powerful resuscitative agent in a number of preclinical models.15,18,19 We further showed that L-NAME with other combinations (adenosine, lidocaine, magnesium, AM, and AL) resuscitated with 100% survival (Figures 2 and 4). However, in terms of our development of a new ALM fluid therapy, we have previously shown that the individual actives or AM, LM, and AL combinations are not optimal,1,4,12,32 and despite L-NAME's well-known ability to increase MAP, it has been shown to have multiple adverse effects in animals and human beings on liver and kidney function.18,33, 34, 35
The underlying mechanisms of why inhibiting NOS with L-NAME abolishes ALM's resuscitative effect in our model is not currently known. When we examined single and dual combinations of ALM active ingredients, we found that after 15 minutes, LM with L-NAME combination contributed to 7.5% NaCl ALM + L-NAME's effect, but it did not explain the precipitous fall in MAP at 5 minutes (Figures 4 and 5). It was only when the 3 components—adenosine, lidocaine, and magnesium—were present that MAP plummets to zero in the presence of L-NAME, which suggests a role for NO for 7.5% NaCl ALM resuscitative actions. The possible NO mechanisms for cardiovascular collapse include loss of vascular tone, loss of cardiac function, and/or a major defect in the central nervous system (eg, nucleus tractus solitaries [NTS]) controlling cardiovascular function.
We believe a direct effect of NO on vascular tone and cardiac function are unlikely candidates for the cardiovascular collapse because removing the effect of NO by blocking its synthesis with L-NAME would result in constriction (higher MAP) and have a positive inotropic effect,18,36 not the opposite as we found (Figure 3A). Similarly, specifically blocking NO actions through cyclic guanosine monophosphate (cGMP) with ODQ would increase MAP because elevation of cGMP in cardiomyocytes or intact heart is associated with a negative inotropic effect.37,38 Blocking cGMP-dependent NO functions using ODQ added to 7.5% NaCl ALM improved MAP; however, substituting ODQ with L-NAME dropped MAP implying cardiovascular collapse mechanisms are not directly leveled at vascular tone or cardiac function. The finding that HR remained steady or dropped slightly despite a decrease in blood pressure during the bleed period is typical of rats, and has been reported by us, and others, in hemorrhagic shock models.1,9,11 The maintenance of lower HRs in some groups during 60-minute resuscitation compared with baseline (Figure 4B), may be due to the differential bradycardic effects of adenosine, lidocaine, and magnesium1,39; however, the rapid and profound bradycardic effect observed with 7.5% NaCl ALM L-NAME was likely due to a central nervous system effect leveled at the sinatrial node as part of irreversible shock (MAP < 20 mm Hg). This question requires further study.
A likely candidate for the cardiovascular collapse from 7.5% NaCl ALM with L-NAME is an effect in the NTS located in the medulla. L-NAME is known to cross the blood-brain barrier to the NTS, where both endothelial and neuronal forms of NOS are expressed.40,41 The medullary NTS integrates convergent information from the body's organs and regulates sympathetic and parasympathetic outflows, and itself is the site of substantial modulation by NO,14,42,43 adenosine,44, 45, 46, 47 and sodium ion fast-channel modulating drugs, such as lidocaine.48 For example, activation of adenosine receptors and NO pathways in the NTS has been shown to differentially inhibit or reset the baroreflex control of MAP, HR, and renal sympathetic nerve activity.44,47,49 Bilateral microinjections of lidocaine (and γ-Aminobutyric acid type A (GABA-A) receptor agonists) into the NTS have also been shown to increase MAP in alpha-chloralose-anesthetized control rats.50 Similarly, Wang et al48 have also shown that the tonic blockade of cardiac sympathetic afferent reflex by epicardial lidocaine in chronic heart failure experiments can reduce the activity of the NTS chemoreceptive neurons, and alter sympathetic outflows to the heart, and possibly other organs. We conclude that the cardiovascular collapse after administration of 7.5% NaCl ALM + L-NAME appears to be linked to a complex interaction between NO and ALM in the NTS, a proposal that requires further investigation.
Contribution of different NOS isoforms to the observed protective effect of ALM
Our study also found that MAP increased at 5 minutes of resuscitation when specific neuronal NOS inhibitors SMTC or ARL17477 were added to 7.5% NaCl ALM, which was opposite to 7.5% NaCl ALM + L-NAME (Figure 5A). These data support Copp et al's51 earlier studies showing that SMTC increases peripheral vasoconstriction in animal models. Similarly, as previously mentioned, MAP increased in the presence of the selective guanylyl cyclase inhibitor ODQ, which is consistent with the study of Olson et al29 who showed that it reversed NO-induced vasorelaxation.29 MAP was also corrected to different degrees with neuronal and inducible NOS inhibitor TRIM,52 and inducible NOS inhibitor AMG (Figure 5A). Following an initial increase, MAP fell 48% with NO scavenger PTIO, and 25% when endothelial NOS activity was blocked through phosphatidylinositol 3-kinase inhibition with wortmannin, suggesting continued NO availability is required for ALM resuscitation. This is consistent with previous studies demonstrating a beneficial effect of NO synthesis after hemorrhagic shock.34 MAP did not increase from shock values when 7.5% NaCl ALM was combined with inducible NOS inhibitor, 1400W, which was different from the large increases in a rat model reported in the study of Kan et al18 (MAP = 104 mm Hg), and with L-NAME alone (108 mm Hg).
We therefore conclude that the cardiovascular collapse after administration of the nonselective NOS inhibitor L-NAME with 7.5% NaCl ALM does not appear to involve NO produced by neuronal NOS pathways but may have involved a partial contribution from inducible NOS (Figure 5). However, no NO or NOS inhibitor mimicked the rapid fall in MAP or mortality found with 7.5% NaCl ALM with L-NAME. On the basis of our data it is possible that the cardiovascular collapse involved endothelial NOS inhibition because it has been reported that L-NAME has 2000 times more selectivity for endothelial NOS than inducible NOS.53 Unfortunately, there are no specific endothelial NOS inhibitors at present that can be solubilized for safe animal administration to test this hypothesis. The use of another nonspecific NOS inhibitor, N5-(1-Iminoethyl)-L-ornithine (L-NIO) dihydrochloride, was considered to support the L-NAME findings; however, the dose for effective inhibition in a rat (20 mg/kg) would require 26.67 mg/mL, which exceeds the solubility limit of 24.61 mg/mL. Another potential limitation of the present study was the variation in selectivity of the NOS inhibitors tested, especially in the in vivo environment where anesthesia, artificial ventilation, and heparinization may affect drug pharmacokinetics. Further experiments are required to tease apart the underlying NO-specific mechanisms to understand the nature of the L-NAME effect in the presence of hypertonic ALM resuscitation fluid, and loss of whole-body protection. This includes measurement of circulating NO and levels in heart muscle and vascular tissue (eg, NOS protein expression) before, during, and following shock.
Conclusions
The resuscitation efficacy of 7.5% NaCl ALM appears to be linked to 1 or more NO-producing pathways. Using a variety of NOS and NO inhibitors, we report that nonspecific NOS inhibition with L-NAME blocked ALM resuscitation and led to cardiovascular collapse. Future work will examine NO site-specific contributions and actions to support cardiovascular function, and possible involvement of the central nervous system.
CRediT authorship contribution statement
Hayley L. Letson: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Writing – review & editing. Geoffrey P. Dobson: Conceptualization, Supervision, Methodology, Resources, Visualization, Writing – original draft.
Conflicts of Interest
G. Dobson is the inventor of the ALM concept for cardiac surgery, trauma, and sepsis. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- 1.Letson HL, Dobson GP. Ultra-small intravenous bolus of 7.5% NaCl/Mg2+ with adenosine and lidocaine improves early resuscitation outcome in the rat after severe hemorrhagic shock in vivo. J Trauma. 2011;71:708–719. doi: 10.1097/TA.0b013e3181fa27c7. [DOI] [PubMed] [Google Scholar]
- 2.Ramesh GH, Uma JC, Farhath S. Fluid resuscitation in trauma: what are the best strategies and fluids? Int J Emerg Med. 2019;12:38. doi: 10.1186/s12245-019-0253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobson GP, Letson HL. Adenosine, Lidocaine and Mg2+ (ALM): From Cardiac Surgery to Combat Casualty Care: Teaching Old Drugs New Tricks. J Trauma Acute Care Surg. 2016;80:135–145. doi: 10.1097/TA.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 4.Dobson GP, Letson HL. Far Forward Gaps in Hemorrhagic Shock and Prolonged Field Care: An Update of ALM Fluid Therapy for Field Use. J Spec Oper Med. 2020;20:78–84. doi: 10.55460/06VT-9IH4. [DOI] [PubMed] [Google Scholar]
- 5.Letson HL, Morris JL, Biros E, Dobson GP. ALM fluid therapy leads to 72 hr survival after hemorrhagic shock: a model for studying differential gene expression and extending biological time. J Trauma Acute Care Surg. 2019;87(3):606–613. doi: 10.1097/TA.0000000000002397. [DOI] [PubMed] [Google Scholar]
- 6.Letson HL, Granfeldt A, Jensen TH, Mattson TH, Dobson GP. Vol. 253. 2020. Adenosine, Lidocaine, and Magnesium Support a High Flow, Hypotensive, Vasodilatory State With Improved Oxygen Delivery and Cerebral Protection in a Pig Model of Noncompressible Hemorrhage; pp. 127–138. (J Surg Res). [DOI] [PubMed] [Google Scholar]
- 7.Granfeldt A, Letson HL, Hyldebrandt JA, et al. Small-volume 7.5% NaCl adenosine, lidocaine, and Mg2+ has multiple benefits during hypotensive and blood resuscitation in the pig following severe blood loss: rat to pig translation. Crit Care Med. 2014;42:e329–e344. doi: 10.1097/CCM.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 8.Letson H, Dobson G. Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage. PloS one. 2017;12 doi: 10.1371/journal.pone.0188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Letson HL, Dobson GP. Unexpected 100% survival following 60% blood loss using small-volume 7.5% NaCl with adenocaine and Mg(2+) in the rat model of extreme hemorrhagic shock. Shock. 2011;36:586–594. doi: 10.1097/SHK.0b013e318237eb0c. [DOI] [PubMed] [Google Scholar]
- 10.Letson HL, Dobson GP. Correction of acute traumatic coagulopathy with small-volume 7.5% NaCl adenosine, lidocaine, and Mg2+ occurs within 5 minutes: a ROTEM analysis. J Trauma Acute Care Surg. 2015;78:773–783. doi: 10.1097/TA.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 11.Letson HL, Dobson GP. 3% NaCl adenosine, lidocaine, Mg2+ (ALM) bolus and 4 hours “drip” infusion reduces noncompressible hemorrhage by 60% in a rat model. J Trauma Acute Care Surg. 2017;82:1063–1072. doi: 10.1097/TA.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 12.Letson HL, Pecheniuk NM, Mhango LP, Dobson GP. Reversal of acute coagulopathy during hypotensive resuscitation using small-volume 7.5% NaCl adenocaine and Mg2+ in the rat model of severe hemorrhagic shock. Crit Care Med. 2012;40:2417–2422. doi: 10.1097/CCM.0b013e31825334c3. [DOI] [PubMed] [Google Scholar]
- 13.Wang L. Role of nitric oxide in regulating cardiac electrophysiology. Exp Clin Cardiol. 2001;6:167–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence AJ. Nitric oxide as a modulator of medullary pathways. Clin Exp Pharm Physiol. 1997;24:760–763. doi: 10.1111/j.1440-1681.1997.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C, Billiar TR. Novel roles of nitric oxide in hemorrhagic shock. Shock. 1999;12:1–9. doi: 10.1097/00024382-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–712. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013;65:1655–1662. doi: 10.1016/s1734-1140(13)71527-5. [DOI] [PubMed] [Google Scholar]
- 18.Kan WH, Hsu JT, Schwacha MG, et al. Selective inhibition of iNOS attenuates trauma-hemorrhage/resuscitation-induced hepatic injury. J Appl Physiol. 2008;105:1076–1082. doi: 10.1152/japplphysiol.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krausz MM, Amstislavsky T, Bitterman H. The effect of nitric oxide synthase inhibition on hypertonic saline treatment of controlled hemorrhagic shock. Shock. 1997;8:422–426. [PubMed] [Google Scholar]
- 20.Fitch RM, Vergona R, Sullivan ME, Wang Y-X. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res. 2001;51:351–358. doi: 10.1016/s0008-6363(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen JX, Meyrick B. Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab Invest. 2004;84:182–190. doi: 10.1038/labinvest.3700027. [DOI] [PubMed] [Google Scholar]
- 22.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 23.Moro MA, Russel RJ, Cellek S, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams JA, Wu D, Bassuk J, et al. Nitric oxide synthase isoform inhibition before whole body ischemia reperfusion in pigs: vital or protective? Resuscitation. 2007;74:516–525. doi: 10.1016/j.resuscitation.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Robin E, Derichard A, Vallet B, Hassoun SM, Neviere R. Nitric oxide scavenging modulates mitochondrial dysfunction induced by hypoxia/reoxygenation. Pharmacol Rep. 2011;63:1189–1194. doi: 10.1016/s1734-1140(11)70638-7. [DOI] [PubMed] [Google Scholar]
- 26.Garvey EP, Oplinger JA, Furfine ES, et al. 1400W is a Slow Tight Binding and Highly Selective Inhibitor of Inducible Nitric Oxide Synthase In Vitro and In Vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YH, Jin CZ, Jang JH, Wang Y. Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology. J Physiol. 2014;592:3189–3200. doi: 10.1113/jphysiol.2013.270306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babu BR, Griffith OW. Design of isoform-selective inhibitors of nitric oxide synthase. Curr Opin Chem Biol. 1998;2:491–500. doi: 10.1016/s1367-5931(98)80125-7. [DOI] [PubMed] [Google Scholar]
- 29.Olson LJ, Knych ET, Herzig TC, Drewett JG. Selective Guanylyl Cyclase Inhibitor Reverses Nitric Oxide-Induced Vasorelaxation. Hypertension. 1997;29:254–261. doi: 10.1161/01.hyp.29.1.254. [DOI] [PubMed] [Google Scholar]
- 30.Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 31.Canyon SJ, Dobson GP. Protection against ventricular arrhythmias and cardiac death using adenosine and lidocaine during regional ischemia in the in vivo rat. Am J Physiol Heart Circ Physiol. 2004;287:H1286–H1295. doi: 10.1152/ajpheart.00273.2004. [DOI] [PubMed] [Google Scholar]
- 32.Dobson GP, Letson HL. Adenosine, lidocaine, and Mg2+ (ALM): From cardiac surgery to combat casualty care-Teaching old drugs new tricks. J Trauma Acute Care Surg. 2016;80:135–145. doi: 10.1097/TA.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 33.López A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 34.Harbrecht BG, Wu B, Watkins SC, Marshall HP, Jr., Peitzman AB, Billiar TR. Inhibition of nitric oxide synthase during hemorrhagic shock increases hepatic injury. Shock. 1995;4(5):332–337. doi: 10.1097/00024382-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Atkins JL, Day BW, Handrigan MT, Zhang Z, Pamnani MB, Gorbunov NV. Brisk production of nitric oxide and associated formation of S-nitrosothiols in early hemorrhage. J Appl Physiol. 2006;100:1267–1277. doi: 10.1152/japplphysiol.01059.2005. [DOI] [PubMed] [Google Scholar]
- 36.Cotter G, Kaluski E, Milo O, et al. LINCS: L-NAME (a NO synthase inhibitor) in the treatment of refractory cardiogenic shock: a prospective randomized study. Eur Heart J. 2003;24:1287–1295. doi: 10.1016/s0195-668x(03)00193-3. [DOI] [PubMed] [Google Scholar]
- 37.Francis SH. The Role of cGMP-dependent Protein Kinase in Controlling Cardiomyocyte cGMP. Circ Res. 2010;107:1164–1166. doi: 10.1161/CIRCRESAHA.110.233239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koesling D, Russwurm M, Mergia E. Regulation and Physiological Functions of NO-Sensitive Guanylyl Cyclase. Nitric Oxide. 2017:107–116. [Google Scholar]
- 39.Singh RB, Singh VP, Jha VK, Katiyar BC. Magnesium and the heart. Acta Cardiol. 1976;31:401–409. [PubMed] [Google Scholar]
- 40.Soszynski D, Daniluk M, Galazka M, Dmitruk K. Blockade of nitric oxide formation in the rat brain does not disturb development of endotoxin tolerance. J Physiol Pharmacol. 2013;64:779–788. [PubMed] [Google Scholar]
- 41.Lin LH, Nitschke Dragon D, Jin J, et al. Decreased expression of neuronal nitric oxide synthase in the nucleus tractus solitarii inhibits sympathetically mediated baroreflex responses in rat. J Physiol. 2012;590:3545–3559. doi: 10.1113/jphysiol.2012.237966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owlya R, Vollenweider L, Trueb L, et al. Cardiovascular and Sympathetic Effects of Nitric Oxide Inhibition at Rest and During Static Exercise in Humans. Circulation. 1997;96:3897–3903. doi: 10.1161/01.cir.96.11.3897. [DOI] [PubMed] [Google Scholar]
- 43.Machado NL, Silva FC, Chianca DAJ, de Menezes RC. Nitric oxide modulates blood pressure through NMDA receptors in the rostral ventrolateral medulla of conscious rats. Brain Res. 2016;1643:159–167. doi: 10.1016/j.brainres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Scislo TJ, Ichinose TK, O'Leary DS. Stimulation of NTS A1 adenosine receptors differentially resets baroreflex control of regional sympathetic outputs. Am J Physiol Heart Circ Physiol. 2008;294:H172–H182. doi: 10.1152/ajpheart.01099.2007. [DOI] [PubMed] [Google Scholar]
- 45.Scislo TJ, O'Leary DS. Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am J Physiol Heart Circ Physiol. 2006;291:H2453–H2461. doi: 10.1152/ajpheart.00158.2006. [DOI] [PubMed] [Google Scholar]
- 46.Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2009;296:H1058–H1068. doi: 10.1152/ajpheart.00906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nassar NN, Abdel-Rahman AA. Brain stem adenosine receptors modulate centrally mediated hypotensive responses in conscious rats: A review. J Adv Res. 2015;6:331–340. doi: 10.1016/j.jare.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H1216–H1226. doi: 10.1152/ajpheart.00557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scislo TJ, Tan N, O'Leary DS. Differential role of nitric oxide in regional sympathetic responses to stimulation of NTS A2a adenosine receptors. Am J Physiol Heart Circ Physiol. 2005;288:H638–H649. doi: 10.1152/ajpheart.00857.2004. [DOI] [PubMed] [Google Scholar]
- 50.Schreihofer AM, Sved AF. Nucleus tractus solitarius and control of blood pressure in chronic sinoaortic denervated rats. Am J Physiol. 1992;263:R258–R266. doi: 10.1152/ajpregu.1992.263.2.R258. [DOI] [PubMed] [Google Scholar]
- 51.Copp SW, Hirai DM, Sims GE, et al. Neuronal nitric oxide synthase inhibition and regional sympathetic nerve discharge: implications for peripheral vascular control. Respir Physiol Neurobiol. 2013;186:285–289. doi: 10.1016/j.resp.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salgado MC, Justo SV, Joaquim LF, Fazan RJ, Salgado HC. Role of nitric oxide and prostanoids in attenuation of rapid baroreceptor resetting. Am J Physiol Heart Circ Physiol. 2006;290:H1059–H1063. doi: 10.1152/ajpheart.00219.2005. [DOI] [PubMed] [Google Scholar]
- 53.Tulic MK, Wale JL, Holt PG, Sly PD. Differential effects of nitric oxide synthase inhibitors in an in vivo allergic rat model. Eur Resp J. 2000;15:870–877. doi: 10.1034/j.1399-3003.2000.15e10.x. [DOI] [PubMed] [Google Scholar]