Abstract
Background
Foot ulcers are a major complication of diabetes mellitus, often leading to amputation. Growth factors derived from blood platelets, endothelium, or macrophages could potentially be an important treatment for these wounds but they may also confer risks.
Objectives
To assess the benefits and harms of growth factors for foot ulcers in patients with type 1 or type 2 diabetes mellitus.
Search methods
In March 2015 we searched the Cochrane Wounds Group Specialised Register, The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), Ovid MEDLINE, Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, Ovid EMBASE and EBSCO CINAHL. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
Randomised clinical trials in any setting, recruiting people with type 1 or type 2 diabetes mellitus diagnosed with a foot ulcer. Trials were eligible for inclusion if they compared a growth factor plus standard care (e.g., antibiotic therapy, debridement, wound dressings) versus placebo or no growth factor plus standard care, or compared different growth factors against each other. We considered lower limb amputation (minimum of one toe), complete healing of the foot ulcer, and time to complete healing of the diabetic foot ulcer as the primary outcomes.
Data collection and analysis
Independently, we selected randomised clinical trials, assessed risk of bias, and extracted data in duplicate. We estimated risk ratios (RR) for dichotomous outcomes. We measured statistical heterogeneity using the I2 statistic. We subjected our analyses to both fixed‐effect and random‐effects model analyses.
Main results
We identified 28 randomised clinical trials involving 2365 participants. The cause of foot ulcer (neurologic, vascular, or combined) was poorly defined in all trials. The trials were conducted in ten countries. The trials assessed 11 growth factors in 30 comparisons: platelet‐derived wound healing formula, autologous growth factor, allogeneic platelet‐derived growth factor, transforming growth factor β2, arginine‐glycine‐aspartic acid peptide matrix, recombinant human platelet‐derived growth factor (becaplermin), recombinant human epidermal growth factor, recombinant human basic fibroblast growth factor, recombinant human vascular endothelial growth factor, recombinant human lactoferrin, and recombinant human acidic fibroblast growth factor. Topical intervention was the most frequent route of administration. All the trials were underpowered and had a high risk of bias. Pharmaceutical industry sponsored 50% of the trials.
Any growth factor compared with placebo or no growth factor increased the number of participants with complete wound healing (345/657 (52.51%) versus 167/482 (34.64%); RR 1.51, 95% CI 1.31 to 1.73; I2 = 51%, 12 trials; low quality evidence). The result is mainly based on platelet‐derived wound healing formula (36/56 (64.28%) versus 7/27 (25.92%); RR 2.45, 95% 1.27 to 4.74; I2 = 0%, two trials), and recombinant human platelet‐derived growth factor (becaplermin) (205/428 (47.89%) versus 109/335 (32.53%); RR 1.47, 95% CI 1.23 to 1.76, I2= 74%, five trials).
In terms of lower limb amputation (minimum of one toe), there was no clear evidence of a difference between any growth factor and placebo or no growth factor (19/150 (12.66%) versus 12/69 (17.39%); RR 0.74, 95% CI 0.39 to 1.39; I2 = 0%, two trials; very low quality evidence). One trial involving 55 participants showed no clear evidence of a difference between recombinant human vascular endothelial growth factor and placebo in terms of ulcer‐free days following treatment for diabetic foot ulcers (RR 0.64, 95% CI 0.14 to 2.94; P value 0.56, low quality of evidence)
Although 11 trials reported time to complete healing of the foot ulcers in people with diabetes , meta‐analysis was not possible for this outcome due to the unique comparisons within each trial, failure to report data, and high number of withdrawals. Data on quality of life were not reported. Growth factors showed an increasing risk of overall adverse event rate compared with compared with placebo or no growth factor (255/498 (51.20%) versus 169/332 (50.90%); RR 0.83; 95% CI 0.72 to 0.96; I2 = 48%; eight trials; low quality evidence). Overall, safety data were poorly reported and adverse events may have been underestimated.
Authors' conclusions
This Cochrane systematic review analysed a heterogeneous group of trials that assessed 11 different growth factors for diabetic foot ulcers. We found evidence suggesting that growth factors may increase the likelihood that people will have complete healing of foot ulcers in people with diabetes. However, this conclusion is based on randomised clinical trials with high risk of systematic errors (bias). Assessment of the quality of the available evidence (GRADE) showed that further trials investigating the effect of growth factors are needed before firm conclusions can be drawn. The safety profiles of the growth factors are unclear. Future trials should be conducted according to SPIRIT statement and reported according to the CONSORT statement by independent investigators and using the Foundation of Patient‐Centered Outcomes Research recommendations.
Keywords: Humans; Amputation, Surgical; Diabetes Mellitus, Type 1; Diabetes Mellitus, Type 1/complications; Diabetes Mellitus, Type 2; Diabetes Mellitus, Type 2/complications; Diabetic Foot; Diabetic Foot/therapy; Growth Substances; Growth Substances/adverse effects; Growth Substances/therapeutic use; Randomized Controlled Trials as Topic; Wound Healing
Plain language summary
Growth factors for treating diabetic foot ulcers
What are diabetic foot ulcers?
People who suffer from diabetes mellitus (usually referred to as ‘diabetes’) can develop wounds (ulcers) on their feet and ankles. These diabetic foot ulcers can take a long time to heal, and affect quality of life for people with diabetes. In some people, failure of these ulcers to heal can contribute to the need for some level of amputation on the foot. Any treatments that encourage diabetic foot ulcers to heal will be valuable.
What are growth factors?
Growth factors are substances that occur naturally in the body. They promote growth of new cells and healing of wounds. Treatment of diabetic foot ulcers with growth factors may improve the healing of ulcers.
The purpose of this review
This Cochrane review tried to identify the benefits and harms of treating diabetic foot ulcers with growth factors in addition to providing standard care (i.e. pressure relief, removal of dead tissue from the wound, infection control and application of dressings).
Findings of this review
The review authors searched the medical literature up to 3 March 2015, and identified 28 relevant medical trials, with a total of 2365 participants. The trials were performed in ten different countries, generally in out‐patient settings. All the trials had low numbers of participants, which makes potential overestimation of benefits and underestimation of harms more likely. Half of the trials were sponsored by the pharmaceutical industry that produces these growth factors.
The trials tested 11 different types of growth factor, usually by applying them to the ulcer surface. Growth factors had no effect on the risk of having one toe or more amputated when compared with either another growth factor, or placebo (inactive fake medicine), or standard care alone (evidence from four trials). However, when compared with placebo or no growth factor, growth factors seemed to make complete healing of ulcers (wound closure) more likely to occur (evidence from 12 trials).
Shortcomings of the trials included in this review
None of the trials reported data on participants’ quality of life. Harms caused by treatments were poorly reported, so the safety profile of growth factors remains unclear.
It is clear that more trials are required to assess the benefits and harms of growth factors in the treatment of diabetic foot ulcers. These trials should be well‐designed, conducted by independent researchers (not industry‐sponsored), and have large numbers of participants. They should report outcomes that are of interest to patients, such as: how many of the participants’ ulcers healed, and how long the healing took; any level of amputation in the foot; quality of life; ulcer‐free days following treatment; and harms caused by treatment, including whether there are any potential cancer risks.
Summary of findings
Summary of findings for the main comparison. Any growth factor compared with placebo or no growth factor for diabetic foot ulcer.
| Any growth factor compared with placebo or no growth factor for diabetic foot ulcer | ||||||
| Patient or population: foot ulcers in people with diabetes Settings: outpatient Intervention: any growth factor Comparison: placebo or no growth factor | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Any growth factor | |||||
| Complete wound closure Follow‐up: 4 to 24 weeks | 346 per 10001 | 523 per 1000 (454 to 599) | RR 1.51 (1.31 to 1.73) | 1316 (12 studies) | ⊕⊕⊝⊝ low2,3 | 1.‐ Growth factors investigated included autologous growth factor (1 trial); platelet‐derived wound healing formula (2 trials); recombinant human platelet‐derived growth factor (becaplermin) (5 trials), recombinant human basic fibroblast growth factor (2 trials), recombinant human epidermal growth factor (1 trial), and transforming growth factor (1 trial). 2.‐ Trials differed in quality. |
| Lower limb amputation (minimum of one toe) Follow‐up: 8 to 20 weeks | 174 per 10001 | 123 per 1000 (64 to 235) | RR 0.74 (0.39 to 1.39) | 219 (2 studies) | ⊕⊝⊝⊝ very low4,5 | Platelet‐derived wound healing formula (1 trial), and recombinant human epidermal growth factor (1 trial) |
| Ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence) Follow‐up: 12 weeks | See comment | See comment | Not estimable | 55 (1 study) | See comment | Trial authors reported recurrence of ulcer in 27% (4/15) of participants receiving growth factor (recombinant human vascular endothelial growth factor) versus 33% (3/9) in placebo group. Hazard ratio was calculated using data transformation. |
| Time to complete healing of the diabetic foot ulcer | See comment | See comment | Not estimable | 0 (0) | See comment | Meta‐analysis was not possible due to the unique comparisons within each trial, failure to report data, with or without a high rate of withdrawals. |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | None of the trials assessed this outcome. |
| Adverse events (non‐serious and serious) Follow‐up: 5 to 20 weeks | 412 per 10001 | 404 per 1000 (325 to 502) | RR 0.98 (0.79 to 1.22) | 385 (4 studies) | ⊕⊕⊝⊝ low4,6 | Recombinant human epidermal growth factor (1 trial), recombinant human platelet‐derived growth factor (1 trial), recombinant human vascular endothelial growth factor (1 trial), thrombin‐induced, platelet‐released platelet‐derived wound healing formula (1 trial). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed risk is based on the risks for the control group in the meta‐analysis. 2 Downgraded one level for limitations in design and execution: Eleven out thirteen trials assessing this outcome have high risk for selection bias. And outcome assessment was performed in unclear fashion. 3 Downgraded one level for inconsistency (I2: 51%). 4 Downgraded one level for limitations in design and execution. 5 Downgraded two levels for imprecision: small sample size and very low rate of events conducting to wide confidence intervals. 6 Downgraded one level for imprecision: Low rate of adverse events resulting in wide confidence intervals.
Background
See Appendix 1 for medical and epidemiological terms.
Description of the condition
It is estimated that in 2011, approximately 366 million people had diabetes, that is 7.0% of the world’s population (Bakker 2012a). Around 80% of these people live in low‐ or middle‐income countries. By 2030, the global estimate is expected to rise to 552 million – that is 8.3% of the adult population (Bakker 2012a). The development of foot ulcers is a major complication of diabetes mellitus (Boulton 2005; Lipsky 2004; Rathur 2007; Richard 2008; Sibbald 2008). The International Working Group on the Diabetic Foot defines a foot ulcer as a full thickness wound involving the foot or ankle (Lavery 2008), that is, "a wound penetrating through the dermis" (Schaper 2004). A wound is a break in the epithelial integrity of the skin and may be accompanied by disruption of the structure and function of underlying normal tissue (Enoch 2008).
Epidemiology of the foot ulcer in people with diabetes
The proportion of diabetic foot ulcers among people with diabetes mellitus varies across studies, ranging from 5% to 43% (Appendix 2). There are four classification systems for diabetic foot ulcers that are summarised in Appendix 3 (Ince 2008; Lavery 1996; Schaper 2004; Wagner 1981). Outcomes for diabetic foot ulcers are predicted by ulcer area, presence of peripheral arterial disease, duration of diabetes, and presence of osteomyelitis (infection of bone) (Ince 2007; Lavery 2009; Oyibo 2001).
There is a close relationship between the presence of a diabetic foot ulcer and the amputation of a toe or a lower limb (Boulton 2008; Bakker 2012b; Younes 2004). Indeed, Boulton 2008 and Bakker 2012a reported that more than 85% of such amputations were preceded by an active foot ulcer. Amputation is a major complication for people with a diabetic foot ulcer (Bartus 2004; Schaper 2012a), and is a risk factor for increased mortality (Izumi 2009). The incidence of amputations is higher in people with diabetes (range 0.64 to 5.25 per 1000 person‐years) than in people without diabetes (0.03 to 0.24 per 1000 person‐years) (Schaper 2012a). The reported annual incidence of major amputation in industrialised countries ranges from 0.06 to 3.83 per 1000 diabetic people (Jeffcoate 2005). The incidence varies between countries, races, and communities (Jeffcoate 2005), however, there is concern about the methods used to calculate incidence and prevalence of amputation in people with diabetes (Van Houtum 2008). The incidence of reamputation in diabetic people with history of amputation within two years is almost 50% (Kanade 2007). Reamputation could be due to poor selection of the original amputation level through efforts to save as much of the lower extremity as possible (Skoutas 2009).
Diabetic foot ulcer pathways
The commonest causes of foot ulcers in people with diabetes are peripheral neuropathy (nerve damage), foot deformity, external trauma, peripheral vascular disease, and peripheral oedema (Boulton 2008; Figueroa‐Romero 2008; Quattrini 2008; Schaper 2012b; Szabo 2009). Other significant risk factors include being over 75 years of age, use of insulin, poor psychosocial status, hyperkeratosis (thickening of the outermost layer of skin), macrovascular and microvascular complications, and duration of diabetes (Chao 2009; Iversen 2008; Leymarie 2005).
Description of the intervention
Many studies have experimented with biological agents, aiming to modify the pathophysiology of diabetic foot ulcers. Growth factors are examples of these biological agents, and are considered to be a potentially important technological advance in the area of wound healing (Papanas 2007). Growth factors are platelet‐derived, endothelium‐derived, or macrophage‐derived, and include granulocyte colony‐stimulating factor, platelet‐derived growth factor, epidermal growth factor, transforming growth factor, fibroblast growth factor, vascular endothelial growth factor, insulin‐like growth factor, and keratinocyte growth factor (Amery 2005; Barrientos 2008; Bennet 2003; Blair 2009; Cruciani 2009; Foster 2009; Galkoswka 2006; Grazul‐Bilska 2003; Rozman 2007; Smyth 2009). Growth factors are administered topically (on the surface) (Afshari 2005; Agrawal 2009; Bhansali 2009; Chen 2004; d'Hemecourt 1998; Driver 2006; Hanft 2008; Hardikar 2005; Holloway 1993; Jaiswal 2010; Kakagia 2007; Landsman 2010; Lyons 2007; Niezgoda 2005; Richard 1995; Robson 2002; Saldalamacchia 2004; Steed 1992; Steed 1995a; Steed 1995b; Steed 1996; Tan 2008; Tsang 2003; Uchi 2009; Viswanathan 2006; Wieman 1998a), or intra lesionally (within the wound) (Fernández‐Montequin 2007; Fernández‐Montequin 2009).
How the intervention might work
Normal wound healing has four phases: coagulation, inflammation, migration/proliferation, and remodelling (Papanas 2008). Sheehan 2006 observed that a 53% or greater reduction in the area of a foot ulcer area after four weeks of observation was a robust predictor of healing at 12 weeks. Since chronic wound healing may be limited by a lack of the necessary growth factors, healing may be speeded up by replacing or stimulating these growth factors, so enhancing the formation of granulation tissue, that precedes healing, within the wounds (Amery 2005; Barrientos 2008; Bennet 2003; Galkoswka 2006; Grazul‐Bilska 2003; Köveker 2000; Pradhan 2009; Viswanathan 2006). See Appendix 4 for wound‐healing and tissue‐forming ability of growth factors.
Why it is important to do this review
Diabetic foot ulcers represent a pervasive and important problem for people suffering from diabetes mellitus. Foot‐related problems are responsible for up to 50% of diabetes‐related hospital admissions (Albert 2002; Boulton 2001; Boulton 2005). Foot ulcers cause a low quality of life and often lead to lower extremity amputation (Armstrong 2008; Boutoille 2008; Goodridge 2006; Herber 2007; Kinmond 2003; Meatherall 2005; Price 2004; Ribu 2008; Schaper 2012a; Valensi 2007). Amputation causes prolonged hospitalisation, rehabilitation, and an increased need for home care and social services (Ali 2008; Ashry 1998; Girod 2003; Habib 2010; Lantis 2009; Redekop 2004; Siriwardana 2007; Van Acker 2000; Viswanathan 2005; Willrich 2005). Management of the diabetic foot has major economic consequences for patients, their families and society (Jeffcoate 2003; Jeffcoate 2004; Milman 2001; Rathur 2007; Smith 2004), and quality of life for caregivers is also unsatisfactory (Nabuurs‐Frassen 2005).
Several randomised clinical trials (RCTs) have assessed the benefits and harms of growth factors for treating diabetic foot ulcers, and they need a critical appraisal for risk of systematic errors that can cause bias (that is, could cause overestimation of benefits and underestimation of harms) and risk of random errors (that is, play of chance). Several narrative reviews and meta‐analyses have assessed the use of growth factors for treating diabetic foot ulcers, but these have been prone to errors (that is, lack of rigorous assessment of bias risks; no or insufficient evaluation of the risks of random errors; no evaluation of statistical heterogeneity; poor reporting of search methods; and potential conflicts of interest as authors of the reviews were also trialists of the included trials) (Hinchliffe 2008; Papanas 2008).
A systematic review of the most up to date evidence, including a rigorous assessment of the quality of that evidence, may help clinicians and clinical researchers make informed decisions about the use of growth factors for treating diabetic foot ulcers.
Objectives
To assess the benefits and harms of growth factors for diabetic foot ulcers in patients with type 1 or type 2 diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in any setting.
Types of participants
Adults (>18 years of age) with a diabetic foot ulcer of any aetiology.
Types of interventions
See Appendix 1.
Experimental interventions
Platelet‐derived wound healing formula
Autologous growth factor
Allogeneic platelet‐derived growth factor
Transforming growth factor β2
Arginine‐glycine‐aspartic acid (RGD) peptide matrix
Recombinant human platelet‐derived growth factor (becaplermin)
Recombinant human epidermal growth factor
Recombinant human basic fibroblast growth factor
Recombinant human vascular endothelial growth factor (telbermin)
Recombinant human lactoferrin
Recombinant human acidic fibroblast growth factor
In addition to receiving the experimental intervention (growth factors) participants also received standard care (see below).
Trials of granulocyte‐colony stimulating factors were excluded as they are the focus of another Cochrane review (Cruciani 2009).
Control interventions
Standard care (for example, antibiotic therapy, debridement, wound dressings) alone or plus placebo.
We noted whether the standard care was delivered similarly to intervention groups and noted any differences between intervention groups.
Types of outcome measures
Primary outcomes
Complete wound healing (defined as 100% epithelialisation or skin closure without drainage).
Lower limb amputation (minimum of one toe).
Time to complete healing of the diabetic foot ulcer.
Secondary outcomes
Ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence).
Quality of life (as measured by a validated scale).
Adverse events: number and type of adverse events defined as any untoward medical occurrence ‐ not necessarily having a causal relationship with the treatment. We reported separately on adverse events that led, and did not lead, to treatment discontinuation. We defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines as any event that at any dose results in death, is life‐threatening, requires in‐patient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability, or is a congenital anomaly/birth defect, and any important medical event that may have jeopardised the patient or requires intervention to prevent it (ICH‐GCP 1997). All other adverse events were considered non‐serious.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched to identify reports of relevant randomised clinical trials:
The Cochrane Wounds Group Specialised Register (searched 03 March 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 2);
Ovid MEDLINE (1946 to March 2, 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (March 2, 2015);
Ovid EMBASE (1974 to March 2, 2015);
EBSCO CINAHL (1982 to March 3, 2015).
We used the following search strategy in The Cochrane Central Register of Controlled Trials (CENTRAL):
MeSH descriptor Foot Ulcer explode all trees
MeSH descriptor Diabetic Foot explode all trees
diabet* NEAR/3 ulcer*:ti,ab,kw
diabet* NEAR/3 (foot or feet):ti,ab,kw
diabet* NEAR/3 wound*:ti,ab,kw
(#1 OR #2 OR #3 OR #4 OR #5)
MeSH descriptor Intercellular Signaling Peptides and Proteins explode all trees
MeSH descriptor Insulin‐Like Growth Factor Binding Proteins explode all trees
growth NEXT factor*:ti,ab,kw
EGF or FGF or PDGF:ti,ab,kw
plermin or regranex or becaplermin:ti,ab,kw
(#7 OR #8 OR #9 OR #10 OR #11)
(#6 AND #12)
This strategy was adapted to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL (please see Appendix 5). The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefevbre 2011). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010). There were no restrictions with respect to language, date of publication or study setting.
Searching other resources
The following web sites were also searched:
Food and Drug Administration (http://www.fda.gov/);
European Medicines Agency (http://www.emea.europa.eu);
International Working Group on the Diabetic Foot (http://iwgdf.org/);
MedWatch The FDA Safety Information and Adverse Event Reporting Program (http://www.fda.gov/Safety/MedWatch/default.htm);
Medicines and Healthcare products Regulatory Agency (http://www.mhra.gov.uk/index.htm);
Scirus (www.scirus.com);
CenterWatch (http://www.centerwatch.com);
Evidence in Health and Social Care (http://www.evidence.nhs.uk/);
Dailymed (http://dailymed.nlm.nih.gov/dailymed/about.cfm).
WHO International Clinical Trials Registry Platform Search Portal (http://apps.who.int/trialsearch/).
We also checked the reference lists of all the potentially relevant trials identified by the above methods.
Data collection and analysis
We summarised data using standard Cochrane Collaboration methodologies (Higgins 2011).
Selection of studies
Two review authors (AJM‐C, SN) independently assessed each reference identified by the search against the inclusion criteria. We resolved disagreements that arose through discussion. Those references that appeared to meet the inclusion criteria were retrieved in full for further independent assessment by two review authors.
Data extraction and management
One review author independently extracted data (SN) from the included trials using a spreadsheet data extraction form and two review authors (AJM‐C, DSR) checked the data entered. We extracted the following data: eligibility criteria, demographics (age, sex, country), characteristic of the ulcers (anatomic site, size, number of ulcers, presence of infection, duration of ulceration), type of diabetes mellitus, duration of diabetes mellitus, ulcer treatments, and outcomes assessed. We discussed any discrepancies between review authors in order to achieve a final consensus.
Assessment of risk of bias in included studies
Independently, three review authors (AJM‐C, SN, DSR) assessed the risk of bias of each included trial using the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). See, Appendix 6 for details.
Three review authors (LR, PO, JCT) checked these assessments. The review authors discussed discrepancies and achieved consensus.
Overall risk of bias
We made explicit judgements about whether the RCTs were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias as being high if any of the above domains was assessed as being at unclear or high risk of bias.
Trials that had adequate generation of allocation sequence, allocation concealment, blinding, handling of incomplete outcome data, and no selective outcome reporting, and that were without other risks of bias were considered to be trials with a low risk of bias. We explored the impact of the risk of bias through undertaking subgroup analyses.
Measures of treatment effect
For binary outcomes, such as incidence of complete wound healing, amputation, and adverse events, we calculated the risk ratio (RR) with 95% confidence intervals (CI) for each. For ulcer‐free days following treatment, a time‐to‐event outcome, we calculated the hazard ratio (HR) with 95% CI (Zavala 2007).
Dealing with missing data
We assessed the percentage of dropouts for each included trial, and for each intervention group, and evaluated whether an intention‐to‐treat (ITT) analysis had been performed or could have been performed from the available published information. We contacted authors to resolve some queries on this issue.
In order to undertake an ITT analysis, we sought data from the trial authors on the number of participants in treatment groups, irrespective of compliance and whether or not participants were later thought to be ineligible, or otherwise excluded from treatment or lost to follow‐up. If this information was not forthcoming, we undertook a complete patient analysis, knowing that it might be biased.
We included patients with incomplete or missing data in sensitivity analyses by imputing them according to the following scenarios (Hollis 1999).
Extreme case analysis favouring the experimental intervention ('best‐worse' case scenario): none of the drop‐outs/participants lost from the experimental arm, but all of the drop‐outs/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator.
Extreme case analysis favouring the control ('worst‐best' case scenario): all drop‐outs/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator.
Assessment of heterogeneity
We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error (Higgins 2003). We considered statistical heterogeneity to be present if I2 was greater than 50% (Higgins 2011). When significant heterogeneity was detected (i.e. when I2 exceeded 50%), we attempted to identify the possible causes of heterogeneity.
Assessment of reporting biases
We assessed publication bias and other bias by a funnel‐plot (Sterne 2011). We calculated Egger's test with Comprehensive Meta‐analysis software (CMA 2011).
Data synthesis
We calculated pooled estimates and 95% confidence intervals (CI) using fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We had anticipated clinical heterogeneity in the effect of the intervention and we planned to conduct the following sub‐group analyses had the data had been available. Furthermore, subgroup analysis would be performed only for complete wound healing (primary outcome).
We could not perform preplanned analyses for clinical subgroups (insulin‐using compared to non insulin‐using participants, severity and depth of wound, and use or not of antibiotics (Appendix 7; Appendix 8)) due to a lack of available data. We conducted the following preplanned subgroup analyses.
Duration of follow‐up: trials with less 20 weeks of follow‐up compared to trials with 20 weeks or more of follow‐up.
Type of growth factor.
The subgroup analyses were only performed for the outcome of complete wound closure.
Sensitivity analysis
We performed the following sensitivity analysis in order to explore the influence of these factors on the intervention effect size.
Repeating the analysis taking attrition bias into consideration: 'Best‐worst case' scenario versus 'Worst‐best case' scenario
'Summary of findings' tables
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes where possible (complete wound closure, lower limb amputation, ulcer‐free day following treatment for diabetic foot ulcers, time to complete healing of the diabetic foot ulcer, quality of life, and adverse events) (Guyatt 2011f). We constructed 'Summary of findings' tables using the GRADE software. The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2011i; Guyatt 2012).
Results
Description of studies
Results of the search
We identified 424 references using our search strategies. Twenty‐eight trials (35 references) involving 2365 participants met our inclusion criteria (Afshari 2005; Agrawal 2009; Bhansali 2009; Chen 2004; d'Hemecourt 1998; Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008; Hardikar 2005; Holloway 1993; Jaiswal 2010; Kakagia 2007; Landsman 2010; Lyons 2007; Niezgoda 2005; Richard 1995; Robson 2002; Saldalamacchia 2004; Steed 1992; Steed 1995a; Steed 1995b; Steed 1996; Tan 2008; Tsang 2003; Uchi 2009; Viswanathan 2006; Wieman 1998a). See Figure 1 for details of the flow of studies.
1.

Study flow diagram.
Included studies
Tables of Characteristics of included studies show a detailed description of the studies.
Growth factors and populations assessed in the trials
The 28 RCTs reported 11 different growth factors compared with several different control interventions.
The experimental interventions included both non‐recombinant and recombinant growth factors. The non‐recombinant growth factors investigated were: platelet‐derived wound healing formula (Holloway 1993; Steed 1992); autologous growth factors (Driver 2006; Kakagia 2007; Saldalamacchia 2004); allogeneic platelet‐derived growth factor (Steed 1996); transforming growth factor β2 (Robson 2002); arginine‐glycine‐aspartic acid (RGD) peptide matrix (Steed 1995b). The recombinant growth factors were recombinant human platelet‐derived growth factor (Agrawal 2009; Bhansali 2009; d'Hemecourt 1998; Hardikar 2005; Jaiswal 2010; Landsman 2010; Niezgoda 2005; Steed 1995a; Wieman 1998a); recombinant human epidermal growth factors (Afshari 2005; Chen 2004; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Tsang 2003; Viswanathan 2006); recombinant human basic fibroblast growth factors (Richard 1995; Tan 2008; Uchi 2009); recombinant human vascular endothelial growth factor (Hanft 2008); recombinant human lactoferrin (Lyons 2007); and recombinant human acidic fibroblast growth factor (Tan 2008).
Twenty trials compared growth factors against no growth factor or against placebo (without or with co‐interventions). The comparisons were: no growth factor (d'Hemecourt 1998; Fernández‐Montequin 2009; Jaiswal 2010; Saldalamacchia 2004); saline solution (Bhansali 2009; Driver 2006; Holloway 1993; Richard 1995; Steed 1992; Steed 1995b; Steed 1996); or placebo (Agrawal 2009; Hanft 2008; Hardikar 2005; Lyons 2007; Robson 2002; Steed 1995a; Uchi 2009; Viswanathan 2006; Wieman 1998a). The characteristics of the placebo were not sufficiently described in Agrawal 2009, Hardikar 2005, Steed 1995a, Uchi 2009, or Wieman 1998a. Accordingly, only four trials used an appropriate placebo (Hanft 2008; Lyons 2007; Robson 2002; Viswanathan 2006).
Two trials compared one growth factor versus another growth factor, or different doses of the same growth factor (with or without co‐interventions). Tan 2008 compared recombinant human acidic fibroblast growth factor versus recombinant human basic fibroblast growth factor. Fernández‐Montequin 2007 compared two doses of recombinant human epidermal growth factor, 75 µg and 25 µg.
Six trials compared growth factors versus other interventions (with or without co‐interventions): silver sulphadiazine (Afshari 2005); insulin (Chen 2004); oxidized regenerated cellulose/collagen biomaterial (Kakagia 2007); moisture‐regulating dressing (Landsman 2010); oasis wound matrix (Niezgoda 2005); and actovegin (Tsang 2003).
The co‐interventions used most frequently in both the experimental and the control groups were: wound debridement (Afshari 2005; Agrawal 2009; Bhansali 2009; d'Hemecourt 1998; Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008; Hardikar 2005; Jaiswal 2010; Kakagia 2007; Lyons 2007;Robson 2002; Steed 1992;Steed 1995a; Steed 1995b; Tsang 2003; Uchi 2009; Wieman 1998a); wound dressing (Afshari 2005; Agrawal 2009; d'Hemecourt 1998; Hardikar 2005, Kakagia 2007; Landsman 2010; Lyons 2007;Robson 2002;Steed 1992;Steed 1995a; Tan 2008); antibiotics ‐ topical (Chen 2004), and systemic (Afshari 2005; d'Hemecourt 1998; Fernández‐Montequin 2007; Hardikar 2005; Lyons 2007;Viswanathan 2006); glycaemic control (Agrawal 2009; Chen 2004; Fernández‐Montequin 2007; Hardikar 2005;Richard 1995;Viswanathan 2006); and offloading of local pressure on the foot ulcer (Bhansali 2009; d'Hemecourt 1998; Driver 2006; Hanft 2008; Hardikar 2005; Jaiswal 2010; Landsman 2010; Lyons 2007; Niezgoda 2005; Richard 1995;Robson 2002;Steed 1992;Steed 1995a; Steed 1995b; Steed 1996). Two trials did not report the use of any co‐intervention (Holloway 1993; Saldalamacchia 2004).
Twenty‐six trials administered the intervention topically; two trials involving recombinant human epidermal growth factor used intralesional administration (Fernández‐Montequin 2007; Fernández‐Montequin 2009).
The intervention was administered: once daily in 12 trials (Afshari 2005; Agrawal 2009; Bhansali 2009; d'Hemecourt 1998; Hardikar 2005; Jaiswal 2010; Landsman 2010; Steed 1992; Steed 1995a; Steed 1996; Tan 2008; Uchi 2009); daily during the six weeks for which participants were inpatients, then twice a week for 12 weeks in one trial (Richard 1995); twice daily in three trials (Lyons 2007; Viswanathan 2006; Wieman 1998a); once a week in one trial (Niezgoda 2005); twice a week in two trials (Robson 2002; Steed 1995b); or three times a week on alternate days in three trials (Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008). Six trials did not report on the frequency of administration (Chen 2004; Driver 2006; Holloway 1993; Kakagia 2007; Saldalamacchia 2004; Tsang 2003).
The mean age of participants was 59.1 years (standard deviation (SD) ± 4.16), and most were male (66.6% (SD ± 16.1%)). Ten trials included participants with type 1 and type 2 diabetes mellitus (Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008; Hardikar 2005; Jaiswal 2010; Landsman 2010; Niezgoda 2005; Viswanathan 2006; Wieman 1998a). Eighteen trials did not report the type of diabetes mellitus explicitly (Afshari 2005; Agrawal 2009; Bhansali 2009; Chen 2004; d'Hemecourt 1998; Hardikar 2005; Kakagia 2007; Lyons 2007; Richard 1995; Robson 2002; Saldalamacchia 2004; Steed 1992; Steed 1995a; Steed 1995b; Steed 1996; Tan 2008; Tsang 2003; Uchi 2009). Trials included participants with target foot ulcers at nine different sites (fore‐foot, mid‐foot, hind‐foot, internal and external edge, sole, plantar surface, ankle). Six trials included participants with neuropathic ulcers (Hardikar 2005; Lyons 2007; Richard 1995; Robson 2002; Steed 1992; Steed 1996). The remaining 22 trials did not report the cause of the foot ulcers. Generally, the trials were conducted in the out‐patient (ambulatory) setting.
Location of trials
The trials were conducted in ten countries: three in China (Chen 2004; Tan 2008; Tsang 2003); two in Cuba (Fernández‐Montequin 2007; Fernández‐Montequin 2009); one in Greece (Kakagia 2007); five in India (Agrawal 2009; Bhansali 2009; Hardikar 2005; Jaiswal 2010; Viswanathan 2006); one in Iran (Afshari 2005); one in Italy (Saldalamacchia 2004); one in Japan (Uchi 2009); and eleven in the USA (d'Hemecourt 1998; Driver 2006; Hanft 2008; Holloway 1993; Landsman 2010; Robson 2002; Steed 1992; Steed 1995a; Steed 1995b; Steed 1996; Wieman 1998a). One trial was conducted in both Canada and the USA (Niezgoda 2005), and another in both France and Italy (Richard 1995).
Trial methods
All trials were conducted using the parallel group trial design. Eighty‐two per cent of the trials (23/28) were conducted without reporting an a priori estimation of sample size. Trials were small with sample sizes ranging from 13 to 382 participants, with a median sample size of 60 and a mean of 87 (± SD 76). Fourteen trials had follow‐up periods of less than 20 weeks (range five to 18 weeks) (Afshari 2005; Agrawal 2009; Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008; Jaiswal 2010; Kakagia 2007; Richard 1995; Saldalamacchia 2004; Steed 1995b; Tan 2008; Uchi 2009; Viswanathan 2006). Thirteen trials had a follow‐up of 20 weeks or more (range 20 weeks to 26 weeks) (Bhansali 2009; d'Hemecourt 1998; Hardikar 2005; Holloway 1993; Landsman 2010; Lyons 2007; Niezgoda 2005; Robson 2002; Steed 1992; Steed 1995a; Steed 1996; Tsang 2003; Wieman 1998a). One trial did not report length of follow‐up (Chen 2004). In the trials, the units of randomisation and analysis were the participants. In terms of assessing the stage of the ulcer ‐ trials variously used Wagner's classification, the University of Texas Diabetic classification, or the International Association Enterostomal Therapy classification for (Appendix 3). Appendix 9 shows the methods for assessing ulcer dimension.
Excluded studies
We excluded nine studies for the following reasons: case reports (Acosta 2006; Miller 1999; Tuyet 2009); non‐RCTs (Aminian 2000; Saad Setta 2011); case series (Embil 2000; Hong 2006); and phase IV study (post‐marketing surveillance study) (Mohan 2007; Yera‐Alos 2013). See the Characteristics of excluded studies table.
Ongoing trials
We identified six ongoing trials (NCT00521937; NCT00709514; NCT00915486; NCT00926068; NCT01060670; NCT01098357). Full details are shown in the table of Characteristics of ongoing studies.
Studies awaiting classification
Four citations are 'Awaiting classification' (Gomez‐Villa 2014; Morimoto 2013; Singla 2014; Young 1992; see Characteristics of studies awaiting classification for details).
Risk of bias in included studies
The risk of bias in the included trials is summarised in Figure 2 and Figure 3, and detailed in the Characteristics of included studies table.
2.
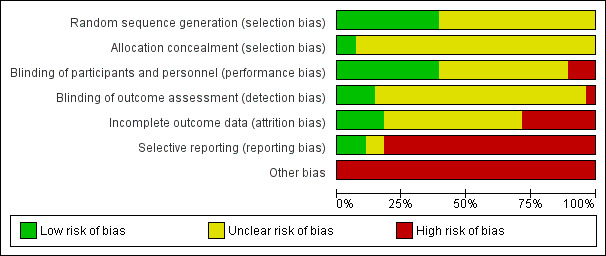
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
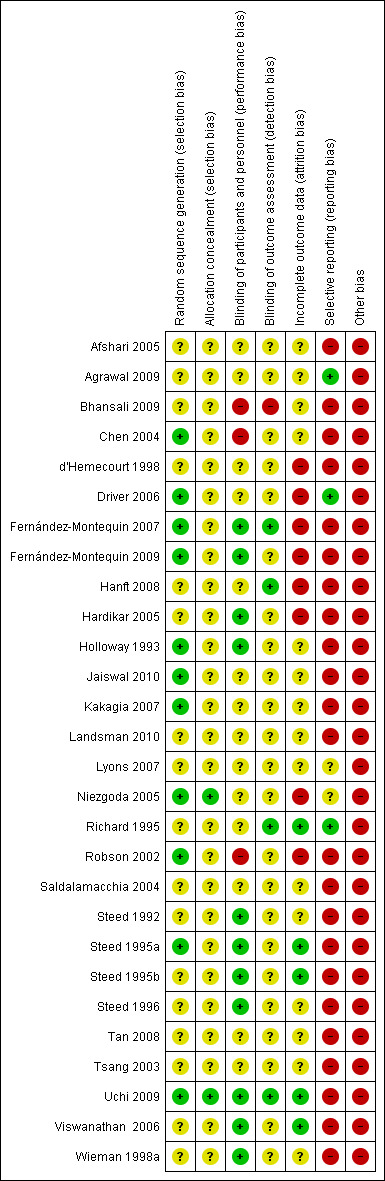
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The risk of bias arising from the method of generation of the allocation sequence was considered to be low in eleven trials (Chen 2004; Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Holloway 1993; Jaiswal 2010; Kakagia 2007; Niezgoda 2005; Robson 2002; Steed 1995a; Uchi 2009). The remaining 17 trials had unclear risk of bias for this domain.
Allocation concealment
The risk of bias arising from the method of allocation concealment was considered to be low in two trials (Niezgoda 2005; Uchi 2009). The remaining 26 trials had an unclear risk for this domain.
Blinding
The risk of bias due to lack of blinding of participants and personnel was rated as low in 11 trials (Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hardikar 2005; Holloway 1993; Steed 1992; Steed 1995a; Steed 1995b; Steed 1996; Uchi 2009; Viswanathan 2006; Wieman 1998a). The risk of bias of performance bias was high in the remaining 17 trials.
In four trials outcome assessment was clearly reported as blinded, and detection bias was considered to be low (Fernández‐Montequin 2007; Hanft 2008; Richard 1995; Uchi 2009). Blinding of outcome assessors was unclear or not performed in the remaining 24 trials, so the risk of detection bias was considered to be high.
Incomplete outcome data
Risk of attrition bias was rated as low in six trials (Richard 1995; Robson 2002; Steed 1995a; Steed 1995b; Uchi 2009; Viswanathan 2006), but high in the remaining 22 trials.
Selective reporting
Risk of selective outcome reporting bias was rated as low in three trials (Agrawal 2009; Driver 2006; Richard 1995), two trials was rated as having unclear risk (Lyons 2007; Niezgoda 2005), and rated as high in the remaining 23 trials. It was mainly due to these trials neither measured nor reported complete wound closure or safety data.
Other potential sources of bias
Risk of other bias was rated as high in all 28 trials due to bias in the presentation of data or design bias. Accordingly, all trials were considered to have an overall high risk of bias.
Effects of interventions
See: Table 1
Primary outcomes
Complete wound closure (defined as 100% epithelialisation or skin closure without drainage)
Any growth factor versus placebo or no growth factor
Meta‐analysis of 12 trials showed that growth factors, when considered as a group, increased the incidence of complete wound healing compared with placebo or no growth factor (345/657 (52.51%) versus 167/482 (34.64%); RR fixed‐effect model 1.51 95% CI 1.31 to 1.73; I2 = 51%, low quality evidence due to limitation in design, execution or both, and inconsistency) (d'Hemecourt 1998; Hanft 2008; Hardikar 2005; Holloway 1993; Jaiswal 2010; Richard 1995; Robson 2002; Saldalamacchia 2004; Steed 1992; Steed 1995a; Uchi 2009; Viswanathan 2006; Wieman 1998a). See Analysis 1.1. Figure 4 shows a funnel‐plot of this meta‐analysis.
1.1. Analysis.

Comparison 1 Any growth factor versus placebo or no growth factor, Outcome 1 Complete wound closure.
4.
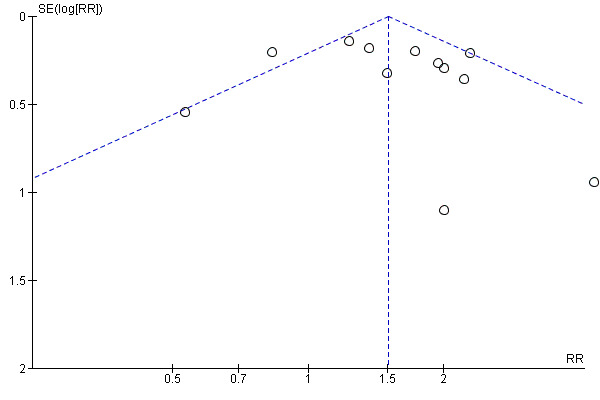
Funnel plot for comparison effect of any growth factor versus placebo or no growth factor on 100% complete wound closure.
P‐value (two tailed) for Egger's test = 0.43
Subgroup analysis of trials with follow‐up of less than 20 weeks compared to trials with follow‐up of 20 weeks or longer
Meta‐analysis of five trials with follow‐up of less than 20 weeks shows uncertainty due to imprecision (small sample size and low rate of event) in the proportion of complete wound healing comparing any growth factor versus placebo or no growth factor (102/167 (61.07%) versus 60/119 (50.42%); RR 1.24, 95% CI 1.00 to 1.55; I2 = 57%; P value 0.05) (Jaiswal 2010; Richard 1995; Saldalamacchia 2004; Uchi 2009; Viswanathan 2006). Meta‐analysis of seven trials with a follow‐up of 20 weeks or longer showed an increase in the incidence of complete wound healing comparing any growth factor versus placebo or no growth factor (243/490 (49.59%) versus 107/363 (29.47%); RR 1.65, 95% CI 1.38 to 1.98; I2 = 34%) (d'Hemecourt 1998; Hanft 2008; Hardikar 2005; Holloway 1993; Steed 1992; Steed 1995a; Wieman 1998a). The subgroup test showed high inconsistency between the two groups (I2 = 73.5%, P value 0.05). See Analysis 2.1.
2.1. Analysis.

Comparison 2 Any growth factor versus placebo or no growth factor (subgroup analysis of trials with follow‐up < 20 weeks versus follow‐up ≥ 20 weeks), Outcome 1 Participants with complete wound closure.
Subgroup analysis by type of growth factor
One trial comparing autologous growth factor versus placebo or no growth factor showed inconclusive results regarding complete wound closure due to high imprecision (2/7 (28.57%) versus 1/7 (14.28%); RR 2.0, 95% CI 0.23 to 17.34; P value 0.53) (Saldalamacchia 2004). Meta‐analysis of two trials comparing platelet‐derived wound healing formula versus placebo showed a significant increase in the likelihood of participants with complete wound healing receiving growth factor (36/56 (64.28%) versus 7/27 (25.92%); RR 2.45, 95% CI 1.27 to 4.74, I2 = 0%) (Holloway 1993; Steed 1992). Meta‐analysis of five trials showed that recombinant human platelet‐derived growth factor (becaplermin) increased the proportion of the participants with complete wound healing compared with placebo (205/428 (47.89%) versus 109/335 (32.53%); RR 1.47, 95% CI 1.23 to 1.76, I2 = 74%) (d'Hemecourt 1998; Hardikar 2005; Jaiswal 2010; Steed 1995a; Wieman 1998a). Meta‐analysis of two trials comparing recombinant human basic fibroblast growth factor versus placebo or no growth factor showed inconclusive results regarding proportion of participants with complete wound healing (60/106 (56.60%) versus 27/59 (45.76%); RR 1.23, 95% CI 0.88 to 1.72, I2 = 62% P value 0.22) (Richard 1995; Uchi 2009). One trial comparing recombinant human epidermal growth factor versus placebo showed an increase in the incidence of complete wound healing using growth factor (25/29 (86.28%) versus 14/28 (50%); RR 1.72, 95% CI 1.16 to 2.57) (Viswanathan 2006). One trial comparing recombinant human vascular endothelial growth factor versus placebo showed no clear evidence of a difference regarding complete wound closure (15/29 (51.72%) versus 9/26 (34.61%); RR 1.49, 95% CI 0.79 to 2.82; P value 0.21) (Hanft 2008). The subgroup test showed no significant difference between the two groups (I2 = 0%, P value 0.55). However, the quality of the evidence showed in this subgroup analysis should be considered either low or very low. It is due to severe imprecision (wide confidence intervals) based on small sample size and low number of event (complete wound closure), inconsistency and limitations of design and execution of these trials. See Analysis 3.1.
3.1. Analysis.

Comparison 3 Any growth factor versus placebo or no growth factor (subgroup analysis by type of growth factor), Outcome 1 Complete wound closure.
Sensitivity analysis taking attrition into consideration
Eight of the 12 trials combined for this outcome reported the exact number of participants with missing data in the intervention and the control groups. These trials involved 1043 participants (d'Hemecourt 1998; Hanft 2008; Hardikar 2005; Holloway 1993; Steed 1995a; Uchi 2009; Viswanathan 2006; Wieman 1998a).
'Best‐worst case' scenario
In a best‐worst case scenario analysis where none of the drop‐outs/participants were lost from the experimental arm, but all of the drop‐outs/participants lost from the control arm experienced the outcome, including all randomised participants in the denominator, meta‐analysis of eight trials showed a higher likelihood of complete wound healing in the participants receiving any growth factor compared with those exposed to placebo or no growth factor (417/607 (68.69%) versus 142/436 (32.56%); RR 2.09, 95% CI 1.81 to 2.41; I2 = 57%; P value 0.00001).
'Worst‐best case' scenario
In a worst‐best case scenario analysis (all drop‐outs/participants lost from the experimental arm, but none from the control arm experienced the outcome, including all randomised participants in the denominator) we did not find clear evidence of a difference in the proportion of participants assigned to any growth factor with complete wound healing compared with placebo or no growth factor (318/607 (52.38%) versus 218/436 (50%); RR 1.05, 95% CI 0.93 to 1.19; I2 = 60%; P value 0.43).
A test for subgroup differences showed a significant difference (I² = 98.2%; P value 0.0001). See Analysis 4.1.
4.1. Analysis.

Comparison 4 Any growth factor versus placebo or no growth factor (sensitivity analyses considering attrition), Outcome 1 Complete wound closure.
Individual growth factor versus active control
There is inconclusive evidence of a difference between autologous growth factor and oxidized regenerate cellulose/collagen biomaterial regarding complete wound healing (4/34 (11.76%) versus 2/17 (11.76%); RR 1.00, 95% CI 0.20 to 14.93; P value 1.00) (Kakagia 2007). One trial reported inconclusive effects when recombinant human platelet‐derived growth factor (becaplermin) was compared with OASIS Wound Matrix for achieving complete wound healing (10/36 (27.77%) versus 18/37 (48.64%); RR 0.57, 95% CI 0.31 to 1.06; P value 0.08) (Niezgoda 2005). There is not conclusive results when recombinant human epidermal growth factor was compared with silver sulphadiazine for reaching complete wound healing (7/30 (23.33%) versus 2/20 (10%); RR 2.33, 95% CI 0.54 to 10.11; P value 0.26) (Afshari 2005). There was a higher proportion of complete wound healing in participants allocated to recombinant human epidermal growth factor than those receiving actovegin (32/42 (76.19%) versus 8/19 (42.10%); RR 1.81, 95% CI 1.04 to 3.15; P value 0.04) (Tsang 2003).
Lower limb amputation (minimum of one toe)
No trials described the extent of the amputation (Fernández‐Montequin 2007; Fernández‐Montequin 2009; Holloway 1993; Tsang 2003).
Any growth factor versus placebo or no growth factor
Meta‐analysis of two trials showed no clear difference in number of lower limb amputations for growth factors, considered as a group, compared with placebo or no growth factor (19/150 (12.66%) versus 12/69 (17.39%); RR fixed‐effects model 0.74, 95% CI 0.39 to 1.39; I2 = 0%; P value 0.34, low quality evidence due to limitation in design, execution or both, and imprecision) (Fernández‐Montequin 2009; Holloway 1993). See Analysis 1.2.
1.2. Analysis.

Comparison 1 Any growth factor versus placebo or no growth factor, Outcome 2 Lower limb amputation (minimum of one toe).
Individual growth factor versus active control
One trial comparing recombinant human epidermal growth factor versus actovegin showed no clear evidence of a difference regarding the incidence of lower limb amputation (2/42 (4.76%) versus 2/19 (10.52%); RR 0.45, 95% CI 0.07 to 2.98; P value 0.41) (Tsang 2003). Meta‐analysis of two trials comparing two doses of recombinant human epidermal growth factor, 75 μg and 25 μg, showed no clear difference regarding lower limb amputation (15/76 (19.73%) versus 16/66 (24.24%); RR 0.82, 95% CI 0.44 to 1.52; I2 = 0%; P value 0.52) (Fernández‐Montequin 2007; Fernández‐Montequin 2009).
Time to complete healing of the diabetic foot ulcer
Fifteen trials assessed time to complete healing of the diabetic foot ulcer. However, no trial reported hazard ratios or information that would allow us to calculate it. Most trials did not state explicitly that all participants achieved complete healing (Bhansali 2009; Chen 2004; d'Hemecourt 1998; Driver 2006; Fernández‐Montequin 2007; Fernández‐Montequin 2009; Hanft 2008; Hardikar 2005; Holloway 1993; Niezgoda 2005; Robson 2002; Steed 1995a; Steed 1995b; Viswanathan 2006; Wieman 1998a), see Appendix 10 for details.
Secondary outcomes
Ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence)
One trial comparing recombinant human vascular endothelial growth factor (29 participants) versus placebo (26 participants) showed inconclusive difference in terms of ulcers‐free days following treatment (HR 0.64, 95% CI 0.14 to 2.94 P value 0.56) (Hanft 2008).
Quality of life
None of the included trials addressed quality of life.
Adverse events
Any growth factor versus placebo or no growth factor
Meta‐analysis of four trials reporting number of participants with events showed no clear evidence of a difference between all growth factors when considered as a group compared with placebo or no growth factor in terms of adverse events (non‐serious and serious) (109/232 (46.98%) versus 63/153 (41.17%); RR 0.98, 95% CI 0.79 to 1.22; I2 = 0%; P value 0.85, low quality evidence) (Fernández‐Montequin 2009; Hanft 2008; Hardikar 2005; Holloway 1993). See Analysis 1.4.
1.4. Analysis.

Comparison 1 Any growth factor versus placebo or no growth factor, Outcome 4 Adverse events (non‐serious and serious).
Individual growth factor versus placebo or no growth factor
One trial comparing arginine‐glycine‐aspartic acid peptide matrix with placebo reported adverse events as follows: "0.65 events per patient (N = 26) in arginine‐glycine‐aspartic acid peptide matrix compared with 1.16 (N = 29) in the placebo group" (Steed 1995b). One trial showed no clear difference in overall adverse events when recombinant human platelet‐derived growth factor (becaplermin) was compared with placebo (31/61 (50.81%) versus 34/57 (59.64%); RR 0.85, 95% CI 0.61 to 1.18; P value 0.34) (Steed 1995a).
One trial comparing recombinant human platelet‐derived growth factor versus placebo reported an incidence of serious adverse events similar across comparison groups (25%, 30% and 24% either recombinant human platelet‐derived growth factor 30 μg/g or 100 μg/g, and placebo, respectively (Wieman 1998a).
Meta‐analysis of two trials comparing recombinant human platelet‐derived growth factor (becaplermin) with placebo showed no clear difference between treatment groups in terms of: infection (35/95 (36.84%) versus 28/127 (22.04%); RR 1.57, 95% CI 0.37 to 6.71, I2 = 88%; P value 0.54); cellulitis (11/165 (6.66%) versus 17/127 (13.38%); RR 0.49, 95% CI 0.24 to 1.02, I2 = 0%; P value 0.06); peripheral oedema (9/165 (5.45%) versus 16/127 (12.59%); RR 0.44, 95% CI 0.20 to 0.96, I2 = 0%; P value 0.04); pain (17/165 (10.30%) versus 16/125 (12.8%); RR 0.78, 95% CI 0.41 to 1.49, I2 = 0%; P value 0.45); or skin ulceration (14/165 (8.48%) versus 10/127 (7.87%); RR 1.08, 95% CI 0.49 to 2.37, I2 = 0%; P value 0.85) (d'Hemecourt 1998; Steed 1995a). See Analysis 6.2 to Analysis 6.6.
6.2. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 2 Adverse event: infection.
6.6. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 6 Adverse event: skin ulceration.
Meta‐analysis of two trials comparing recombinant human basic fibroblast growth factor with placebo did not find a difference in terms of infection (3/106 (2.83%) versus 3/59 (5.08%); RR 0.77, 95% CI 0.18 to 3.29; I2 = 0%; P value 0.72) (Richard 1995; Uchi 2009). Analysis 7.2.
7.2. Analysis.

Comparison 7 Recombinant human basic fibroblast growth factor (rHubFBGF) versus placebo, Outcome 2 Adverse event: infection.
One trial showed no clear evidence of a difference between recombinant human basic fibroblast growth factor versus placebo in terms of adverse events (4/97 (4.12%) versus 3/51 (5.88%); RR 0.26, 95% CI 0.02 to 2.83; P value 0.27) (Uchi 2009). One trial showed no clear difference between recombinant human epidermal growth factor group and placebo in terms of any adverse event (65/101 (64.35%) versus 31/48 (64.58%); RR 1.00, 95% CI 0.77 to 1.29; P value 0.98), or any severe adverse event (8/101 (7.92%) versus 2/48 (4.16%); RR 1.90, 95% CI 0.42 to 8.61; P value 0.40) (Fernández‐Montequin 2009). One trial comparing recombinant human vascular endothelial growth factor with placebo showed inconclusive results in the incidence of adverse events during the six‐week treatment period (14/29 (48.27%) versus 13/26 (50%); RR 0.97, 95% CI 0.56 to 1.65; P value 0.90) or the 12‐week observation period (5/26 (19.23%) versus (6/23 (26.08%); RR 0.74, 95% CI 0.26 to 2.10; P value 0.57). This trial also did not show a conclusive difference in terms of serious adverse events during the six‐week treatment period (2/29 (6.89%) versus 3/26 (11.53%); RR 0.60, 95% CI 0.11 to 3.30; P value 0.56) or 12‐week observation period (3/26 (11.53%) versus 3/26 (11.53%); RR 1.00, 95% CI 0.22 to 4.50; P value 1.00) (Hanft 2008).
Individual growth factor versus active control
One trial comparing recombinant human platelet‐derived growth factor (becaplermin) with OASIS Wound Matrix did not find clear evidence of a difference in terms of treatment related events (10/36 (27.77%) versus 17/37 (45.94%); RR 0.60, 95% CI 0.32 to 1.14; P value 0.12) (Niezgoda 2005). One trial comparing different doses of recombinant human epidermal growth factor, 75 μg versus 25 μg, showed no difference in terms of burning sensation (5/23 (21.73%) versus 2/18 (11.11%); RR 1.96, 95% CI 0.43 to 8.94 P value 0.39; 41 participants), or local pain (4/23 (17.39%) versus 3/18 (16.66%); RR 1.04, 95% CI 0.27 to 4.08; P value 0.95; 41 participants) (Fernández‐Montequin 2007). One trial showed evidence of a difference in reduction of local wound pain in participants who received recombinant human acidic fibroblast growth factor compared with those who received recombinant human basic fibroblast growth factor (2/104 (1.92%) versus 6/35 (17.14%); RR 0.11, 95% CI 0.02 to 0.53; 9 P value 0.006) (Tan 2008).
Discussion
Summary of main results
This Cochrane systematic review of growth factors for treating foot ulcers in people with diabetes included 28 randomised clinical trials that incorporated 2365 participants. Trials evaluated 11 different experimental growth factors compared with several different control interventions. Overall, the trials had a high risk of bias and were underpowered. Most of the trials, 82% (23/28), did not report an a priori sample size estimation. Drug companies sponsored at least 14 of the trials. The trials were conducted in 10 countries (Canada, China, Cuba, France, Greece, India, Iran, Italy, Japan, and the USA). In general the trials were conducted in the out‐patient (ambulatory) setting. The reporting of trial participants characteristics was ill‐defined with regard to their type of diabetes mellitus and etiology of their diabetic foot ulcer.
Meta‐analysis was possible only on six types of experimental growth factors: platelet‐derived growth factor, autologous growth factor; platelet‐derived wound healing formula, recombinant human platelet‐derived growth factor, recombinant human basic fibroblast growth factor, and human epidermal growth factor.
We were able to meta‐analyse data on trial participants with complete wound healing. Meta‐analysis of 12 trials showed that all growth factors, when considered as a group, seemed to increase the proportion of participants with complete wound healing significantly compared with placebo or no growth factor. The quality of the estimate was qualified as low due to limitations in design and execution of included trials, and inconsistency (Table 1).
We were able to meta‐analyse data on lower limb amputation (minimum of one toe). One meta‐analysis of two trials showed no clear evidence that growth factors, when as considered as a group, reduced the risk of lower limb amputation compared with placebo or no growth factor. Evidence was downgraded to very low due to pitfalls in design and execution of included trials, and a very small sample size and very low number of events (Table 1). Another meta‐analysis of two trials that compared two doses of recombinant human epidermal growth factor, 75 μg and 25 μg, did not show a significant difference between the two doses.
Eleven trials reported time to complete healing of the diabetic foot ulcer, however, meta‐analysis was not possible due to the unique comparisons within each trial, failure to report data, with or without a high rate of withdrawals. One trial comparing recombinant human vascular endothelial growth factor versus placebo showed inconclusive result on ulcer‐free days following treatment. Trials did not report data on quality of life. Growth factors compared against placebo or no growth factor showed no difference in terms of any adverse event. However, overall, safety data were poorly reported and adverse events may have been underestimated. Evidence was considered as low due to limitations in design and execution, and low number of event (Table 1).
Trials with a 20‐week or longer follow‐up seemed to be more effective in increasing the number of participants with complete wound closure than trials with a follow‐up of less than 20 weeks. However, there was no an conclusive difference between these groups.
We conducted a subgroup analysis of trials by type of growth factor. There was an inconclusive difference between growth factor versus placebo or no growth factor in terms of the number of participants with complete wound closure. This is could be due to small sample size and low number of events.
In terms of complete wound closure we found a clear difference between the 'best‐worse case' scenario and the 'worst‐best case' scenario in sensitivity analyses that took attrition into consideration. It should be interpreted as inconsistency due to missing data.
Overall completeness and applicability of evidence
This Cochrane review found evidence suggesting that growth factors might be useful for increasing complete wound closure of foot ulcers in people with diabetes , though this conclusion is based on randomised clinical trials with a high risk of bias due to pitfalls in design and execution of the included trials. Therefore, and based on GRADE findings, future research are a need to know with a better certainty the clinical benefits of growth factors for treating diabetic foot ulcers. Furthermore, the safety profile of all the growth factors is unclear.
The results in this review are based on data from trials that included a broad range of participants with different co‐morbidities, who received different treatment approaches. That heterogeneity downgraded the quality of evidence. We cannot rule out that the calculations of the potential effects have been overestimated due to poor methodological quality (bias risks, design, analysis and the small information size. Therefore, these three variables, i.e., high heterogeneity, pitfalls in methodology, and small sample size and low number of events, even after meta‐analysis, depleted the quality of evidence. Futhermore, we cannot exclude an underestimation of harms. A caveat concerning the safety of recombinant human platelet‐derived growth factor (becaplermin) has been outlined recently; this relates to the risk of cancer in people who use three tubes or more compared to that in non‐users (FDA 2008; Papanas 2010). However, an observational study reported that this growth factor does not appear to increase the risk of cancer or cancer mortality (Ziyadeh 2011), though further high‐quality data are clearly needed.
Quality of the evidence
GRADE assessments were conducted on outcomes of both meta‐analysis and non‐pooled trials. None of the trials was graded as providing strong evidence, primarily because of small sample sizes (even after meta‐analysis) which generate wide confidence intervals with low precision of estimate of the intervention effects, and the high risk of bias due to a lack of adequate randomisation methods, lack of blinding, high attrition, and unclear reporting of outcomes.
Quality of evidence also had to be downgraded due to inconsistency. We can't reject a potential detection bias ‐wound healing is a fairly subjective outcome‐ where is there is no blinded outcome assessment or unclear for this, even though due to clear definition of complete wound closure.
See Table 1 for complete assessment and rationale for ratings.
This review assessed the impact of missing data on the effect of intervention in increasing the proportion of participants with complete wound closure (Analysis 4.1) using best/worst and /best case scenarios. If the amount of missing data is large, the conclusion on the difference between the comparison groups is not valid (Hollis 1999). This Cochrane review found a significant subgroup difference comparing all trials, best/worst and /best case scenarios (Analysis 4.1).
This Cochrane review has identified the following issues that should be considered when planning future trials: inconsistent information concerning the healing percentage of wound closure definition provided by trial reports, differences in definitions of outcomes, and inconsistency of reported outcomes need to be avoided. Trials should adopt an agreed set of core outcomes for each medical condition (Clarke 2007). This approach may reduce the impact of outcome reporting bias (Kirkham 2010).
The impact of outcome reporting bias may be reduced by adopting the recommendations of The Patient‐Centered Outcomes Research Institute (PCORI) (PCORI 2012). This organisation was established by United States Congress as an independent, non‐profit organisation, created to conduct research to provide information about the best available evidence to help patients and their healthcare providers make more informed decisions. PCORI’s research is intended to give patients a better understanding of the prevention, treatment and care options available, and the science that supports those options (Gabriel 2012; Basch 2012; PCORI 2012; Selby 2012).
Potential biases in the review process
There is a group of biases called significance‐chasing biases (Ioannidis 2010), which includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias. Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. However, this Cochrane review has a low risk of publication bias due to the meticulous trial search that was performed, and the fact that we emailed the main authors of a number of the trials identified. Selective outcome reporting bias operates through suppression of information about specific outcomes and has similarities to publication bias of whole studies or trials, in that ‘negative’ results remain unpublished (Ioannidis 2010). We were surprised to find how few times amputations or mortality were reported in the trials. This Cochrane review found that 75% of the included randomised clinical trials had high risks of selective outcome reporting. For example, adverse events were not reported (Afshari 2005; Bhansali 2009; Chen 2004, Jaiswal 2010; Kakagia 2007; Landsman 2010; Saldalamacchia 2004; Steed 1992; Steed 1996; Tan 2008; Tsang 2003), or were poorly reported in a total of 16 trials (Hardikar 2005; Holloway 1993; Lyons 2007; Viswanathan 2006; Wieman 1998a). These 16 trials incorporated 55% of the randomised participants (1289/2365) included in this review. This review found no evidence of asymmetry of the funnel plot for complete wound closure (Figure 4).
Agreements and disagreements with other studies or reviews
The results of our review are similar to the findings from other systematic reviews (Buchberger 2010; O'Meara 2000) and a narrative review of complete closure of diabetic foot ulcer using growth factors (Wieman 1998b).
Authors' conclusions
Implications for practice.
There is insufficient evidence from RCTs to recommend or refute the use of growth factors in treating diabetic foot ulcers. The results are based on the results of 28 RCTs with a high risk of bias. There is a paucity of information for other main clinical outcomes such as lower limb amputation, time to complete healing of the diabetic foot ulcer and ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence). There is an absence of information on mortality, and quality of life. In addition, adverse events data remain unclear. Therefore, prescription of growth factors for treating people with diabetic foot ulcers can not be supported or rejected until new evidence from a large, high‐quality trial becomes available and alters this conclusion.
Implications for research.
This systematic review has identified the need for well‐designed, adequately powered RCTs to assess the benefits and harms of growth factors with complete wound closure, lower limb amputation, and adverse events as the primary outcomes. Since epidemiological studies have connected to the recombinant human platelet‐derived growth factor (becaplermin) to a five‐fold increase in cancer mortality in people who used more than three tubes of it compared to non‐users, potential risk needs to be ruled out in large randomised clinical trials before wider use can be recommended. The trials should be designed according to the SPIRIT statement (Chan 2013), and reported according to the CONSORT statement for improving the quality of reporting of efficacy; the trials should also provide better reports of harms/adverse events encountered during their conduct (Ioannidis 2004; Moher 2010; Schulz 2010). Future trials should be planned following the Foundation of Patient‐Centered Outcomes Research recommendations (Basch 2012; Gabriel 2012; McKinney 2012). Potential trials should also include clinical outcomes such as, incidence of lower limb amputation (minimum of one toe, with the extent of amputation being specified, and data the incidence of different extents of amputation also reported separately), time to complete healing of the diabetic foot ulcer, quality of life, ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence), and adverse events.
Acknowledgements
We want to express our gratitude to Sally EM Bell‐Syer and Nicky Cullum for the suggestions to enhance the quality of this review. In addition we acknowledge the comments made by the peer referees; Julie Bruce, Jane Burch, Gill Worthy, Anita Raspovic, Mark Rodgers and Stephanie Wu. The protocol and review were copy‐edited by Elizabeth Royle. Our thanks go to Li Xun for translating Chen 2004 from the original Chinese.
We want to express our thanks to María Ximena Rojas who was a review author on the protocol.
Appendices
Appendix 1. Glossary of medical and epidemiological terms
| Terms | Definition | Reference |
| Ankle Brachial Index | Comparison of the blood pressure between the brachial artery and the posterior tibial artery. It is a predictor of peripheral arterial disease. | http://www.ncbi.nlm.nih.gov/mesh |
| Actovegin | A biological drug ‐ a calf blood haemodialysate ‐ manufactured from a natural source | Buchmayer 2011 |
| Amputation | The removal of a limb or other appendage or outgrowth of the body | http://www.ncbi.nlm.nih.gov/mesh |
| Arginine‐glycine–aspartic acid (RGD) peptide matrix (Argidene Gel®, formerly Telio‐Derm Gel®, Telios Pharmaceuticals, San Diego, CA, USA) |
This peptide matrix contains the arginine‐glycine‐aspartic acid amino acid sequence, through which cells in vivo become attached to macromolecules of extracellular matrix via surface integrin receptors. The matrix (intervention) is a sterile non‐preserved clear viscous gel, formulated in phosphate‐buffered saline and dispensed from a single‐use syringe. The functional ingredient of RGD peptide matrix is a complex formed by the combination of a synthetic 18‐amino acid peptide and sodium hyaluronate. It also contains added unconjugated sodium hyaluronate as a viscosity‐increasing agent, and, therefore, does not need to be prepared from patient's samples | O'Meara 2000 |
| Attrition bias | A type of selection bias due to systematic differences between the study groups in the quantitative and qualitative characteristics of the process of loss of their members during study conduct, i.e., due to attrition among subjects in the study. | Porta 2008 |
| Autologous platelet gel | See 'platelet‐rich plasma' | Lacci 2010 |
| Basic fibroblast growth factor (bFGF) (Farmitalia Carlo Erba, Milan, Italy) |
A heparin‐building, single‐chain peptide of 146 amino acids, ubiquitously distributed in mesoderm‐ and neuroectoderm‐derived tissues. This is a potent mitogen for all cell types involved in the healing process. It is highly angiogenic and chemotactic for fibroblasts and endothelial cells. bFGF is produced by recombinant DNA technology using Escherichia coli type b | O'Meara 2000 |
| Bias in the presentation of data | Error due to irregularities produced by digit preference, incomplete data, poor techniques of measurement, technically poor laboratory procedures, or an intentional attempt to mislead | Porta 2008 |
| Burning sensation | An abnormal feeling of burning in the absence of heat | http://www.healthline.com/hlc/burning‐sensation |
| Callus |
A hard, thickened area of skin occurring in parts of the body that are subjected to pressure or friction, particularly the soles of the feet and the palms of the hands | O'Meara 2000 |
| Cellulitis | An acute, diffuse, and suppurative inflammation of loose connective tissue, particularly the deep subcutaneous tissues, and sometimes muscle, most commonly seen as a result of infection of a wound, ulcer, or other skin lesion | http://www.ncbi.nlm.nih.gov/mesh |
| Co‐intervention | In a randomised controlled trial, the application of additional diagnostic or therapeutic procedures to members of either, some or all of the experimental and control groups | Porta 2008 |
| Connective tissue disease | A heterogeneous group of disorders, some hereditary, others acquired, characterised by abnormal structure or function of one or more of the elements of connective tissue, i.e. collagen, elastin, or the mucopolysaccharides | http://www.ncbi.nlm.nih.gov/mesh |
| CT‐102 activated platelet supernatant (APST) (Curative Technologies, Setauket, NY, USA) (synonym: platelet‐derived wound‐healing formula (PDWHF)) |
A combination of growth factors released from ρ‐granules of human platelets by thrombin | O'Meara 2000 |
| Debridement |
The removal of foreign material and dead or contaminated tissue from, or adjacent to, a wound until the surrounding healthy tissue is exposed | O'Meara 2000 |
| Design bias | The difference between a true value and that obtained through the faulty design of a study. Examples include uncontrolled studies where the effects of two or more processes cannot be separated because of lack of measurement of key causes of the exposure or outcome (confounding); also studies performed on poorly‐defined populations or with unsuitable control groups | Porta 2008 |
| Diabetes Mellitus, type 1 | A subtype of diabetes mellitus that is characterized by insulin deficiency. It is manifested by the sudden onset of severe hyperglycemia, rapid progression to diabetic ketoacidosis, and death unless treated with insulin. The disease may occur at any age, but is most common in childhood or adolescence. | http://www.ncbi.nlm.nih.gov/mesh |
| Diabetes Mellitus, type 2 | A subclass of diabetes mellitus that is not insulin‐responsive or dependent (NIDDM). It is characterized initially by insulin resistance and hyperinsulinemia; and eventually by glucose intolerance; hyperglycemia; and overt diabetes. Type II diabetes mellitus is no longer considered a disease exclusively found in adults. Patients seldom develop ketosis but often exhibit obesity. | http://www.ncbi.nlm.nih.gov/mesh |
| Diabetic coma | A state of unconsciousness that is a complication of diabetes mellitus. It occurs in cases of extreme hyperglycaemia or hypoglycaemia that may occur as a complication of insulin therapy | http://www.ncbi.nlm.nih.gov/mesh |
| Diabetic ketoacidosis | A life‐threatening complication of diabetes mellitus (primarily of type 1) exacerbated by severe insulin deficiency and extreme hyperglycaemia. It is characterised by the metabolism of fatty acids (ketosis); dehydration; and depressed consciousness leading to coma | http://www.ncbi.nlm.nih.gov/mesh |
| Epidermal growth factor (EGF) | This growth factor stimulates keratinocyte proliferation and locomotion, and inhibits fibroblast proliferation. It is a chemoattractant for mesodermal and epidermal cells | O'Meara 2000 |
| Gangrene |
The death and decay of part of the body due to a deficiency in, or the cessation of, the blood supply. Causes can include disease, injury, atheroma in major blood vessels, frostbite and severe burns. Dry gangrene is the death and withering of tissues caused by the cessation of the local blood circulation; moist gangrene is the death and putrefactive decay of tissue due to bacterial infection | O'Meara 2000 |
| Growth factors | A group of multifunctional peptides thought to promote cellular proliferation, migration and protein synthesis. They may be derived from platelets, endothelial cells, monocytes, tissue macrophages, fibroblasts or epidermal cells | O'Meara 2000 |
| Hypersensitivity | Heightened reactivity to an antigen, which can result in pathologic reactions to subsequent exposure to that particular antigen | http://www.ncbi.nlm.nih.gov/mesh |
| Hypotension | Abnormally low blood pressure that can cause inadequate blood flow to the brain and other vital organs. A common symptom is dizziness, but greater negative impacts occur when there is prolonged depravation of oxygen and nutrients | http://www.ncbi.nlm.nih.gov/mesh |
| Information bias | A flaw in measuring exposure, covariate, or outcome variables that results in different quality (accuracy) of information between comparison groups. The information bias may not be independent of selection biases. Bias in an estimate arising from measurement errors | Porta 2008 |
| International Association Enterostomal Therapy classification for assessing the stage of ulcers |
Stage I An observable pressure‐related alteration of intact skin, which, when compared to adjacent skin or an opposite area on the body, may include changes in one or more of the following: skin temperature (warmth or coolness), tissue consistency (firm or boggy feel), and/or sensation (pain, itching). In lightly‐pigmented skin, an ulcer appears as a defined area of persistent redness, while in darker skin, an ulcer may appear with persistent red, blue or purple hues Stage II Partial‐thickness skin loss involving epidermis or dermis, or both. The ulcer is superficial and presents as an abrasion, blister, or shallow crater Stage III Full‐thickness skin loss involving damage to or necrosis of subcutaneous tissue that may extend down to, but not through, underlying fascia. The ulcer presents clinically as a deep crater with, or without, undermining of adjacent tissue Stage IV Full‐thickness skin loss with extensive destruction, tissue necrosis, or damage to muscle, bone or supporting structures (e.g. tendon, joint capsule). Undermining and sinus tracts also may be associated with stage IV pressure ulcers |
http://www.wocn.org/pdfs/WOCN_Library/Position_Statements/staging.pdf |
| Ischaemia | A deficiency of blood in a body part due to functional constriction, or actual obstruction, of a blood vessel | O'Meara 2000 |
| Impaired glucose tolerance | A pathological state in which the blood glucose level is less than approximately 140 mg/100 ml of plasma at fasting, and above approximately 200 mg/100 ml plasma at 30‐, 60‐, or 90‐minutes during a glucose tolerance test. This condition is seen frequently in diabetes mellitus, but also occurs with other diseases and malnutrition | http://www.ncbi.nlm.nih.gov/mesh |
| Neuropathic ulcer | An ulcer that usually occurs on the plantar surface of the foot. These ulcers are often associated with sensory neuropathy and, therefore, are often painless. They are typically surrounded by callus tissue, as they occur at sites of high mechanical pressure | O'Meara 2000 |
| Oedematous (oedema) | Abnormal fluid accumulation in tissues or body cavities. Most cases of oedema are present under the skin in subcutaneous tissue | http://www.ncbi.nlm.nih.gov/mesh |
| Peripheral vascular disease | A general or unspecified disease of the blood vessels outside the heart | O'Meara 2000 |
| Platelet‐derived growth factors | Mitogenic peptide growth hormone carried in the alpha‐granules of platelets released when platelets adhere to traumatised tissues. Connective tissue cells near the traumatised region respond by initiating the process of replication | http://www.ncbi.nlm.nih.gov/mesh |
| Platelet‐enriched plasma | See platelet‐rich plasma | Lacci 2010 |
| Platelet releasate | See platelet‐rich plasma | Lacci 2010 |
| Platelet‐rich concentrate | See platelet‐rich plasma | Lacci 2010 |
| Platelet‐rich plasma | Portion of the plasma fraction of autologous blood having a platelet concentration above baseline | Lacci 2010 |
| Recombinant human form of platelet‐derived growth factor (rhPDGF‐BB), homodimer (Chiron Corp, Emeryville, CA, USA) | Growth factor produced from genetically engineered yeast cells into which the gene for the β‐chain of PDGF has been inserted | O'Meara 2000 |
| Skin ulceration (ulcer) | A lesion on the surface of the skin, or a mucous surface, produced by the sloughing of inflammatory necrotic tissue | http://www.ncbi.nlm.nih.gov/mesh |
| Toe‐brachial index (TBI) | Ratio of the brachial and big toe systolic pressures: the brachial pressure is obtained by Doppler and the toe pressure by photo plethysmography | http://www.deh‐inc.com/index.cfm?fuseaction=productdetail&productid=56 |
| Transforming growth factor | Hormonally‐active polypeptides that can induce the transformed phenotype when added to normal, non‐transformed cells | http://www.ncbi.nlm.nih.gov/mesh |
| University of Texas diabetic classification of ulcers | Stages
A: No infection or ischaemia
B: Infection present
C: Ischaemia present
D: Infection and ischaemia present Grading 0: Epithelialised wound 1: Superficial wound 2: Wound penetrates to tendon or capsule 3: Wound penetrates to bone or joint |
http://www.fpnotebook.com/Surgery/Exam/UnvrstyOfTxsDbtcWndClsfctn.htm |
| Wagner's classification of foot ulcers | Grade 1: Superficial diabetic ulcer Grade 2: Ulcer extension a) Involves ligament, tendon, joint capsule or fascia b) No abscess or osteomyelitis Grade 3: Deep ulcer with abscess or osteomyelitis Grade 4: Gangrene to portion of forefoot Grade 5: Extensive gangrene of foot |
http://www.fpnotebook.com/surgery/Exam/WgnrUlcrClsfctn.htm |
Appendix 2. Frequency of foot ulcers in people with diabetes
| Study/year/country | Frequency of foot ulcers | Frequency of amputation | Study design and follow‐up time | Participants (setting and sample size) | Notes |
| Rith‐Najarian 1992 USA | 41/358: 11.4% | 14/358: 3.9% 14/41: 34.1% | Prospective cohort, 32 months | N = 406 (results came from 358) diabetic American‐Indian people. Community setting | |
| Veves 1992 UK | 35% | Not available | Prospective observational study, 30 months | N = 86 (mean age: 53.3 years; range: 17.7 to 77 years) | |
| Young 1994 UK | Cumulative incidence: VPT < 15 V: 2.9% VPT > 25 V: 19.8% | Not available | Prospective observational study | N not available | |
| Humphrey 1996 Australia | Not available | Study cohort: 8.1 per 1000 person‐years Nationally: 7.6 per 1000 person‐years | Population‐based survey, 12 years | Nauruans (N = 1564; age: ≥ 20 years) | NIDDM |
| Lee 1993 USA | Not available | 18.0/1000 person‐years | Prospective observational study, 9.9 ± 4.3 years | Oklahoma Indians with NIDDM. (N = 1012) Data based on: 875 participants | NIDDM |
| Lehto 1996 Finland | Not available | Male: 5.6% Female: 5.3% | Prospective observational study, 7 years | N = 1044 NIDDM Gender: 571 male, 473 female Age: 45 to 64 years | NIDDM |
| Moss 1996 USA | Not available | Cumulative incidence: younger‐onset: 5.4%; older‐onset: 7.3% | Prospective observational study, 10 years | Primary care setting | |
| Nelson 1988 USA | Not available | Amputation performed in 84 participants NIDDM participants: 95% (80/84) | Prospective observational study, 12 years | Pima Indians of the Gila River Indian Community in Arizona N = 4399 | NIDDM |
| Abbott 2002 UK | 2% per year | Not available | Prospective observational study, 2 years | Community healthcare setting N = 9710 (diabetic patients) | |
| Winkley 2007 UK | 43.2% (recurrences) | 15.5% | Prospective population‐based cohort study, 18 months | N = 253 | |
| Ramsey 1999 USA | 5.8% | 15.6% | Retrospective cohort, 3 years | N = 8905 | |
|
Abbreviations NIDDM = non insulin‐dependent diabetes mellitus UK = United Kingdom USA = United States of America VPT = vibration perception threshold | |||||
Appendix 3. Clasification systems for diabetic foot ulcers in people with diabetes mellitus
|
Wagner classification (Wagner 1981) Components:
|
University of Texas Wound Classification System (Lavery 1996) Components:
|
The SINBAD system (Ince 2008) Components:
|
PEDIS systemSchaper 2004 Components:
|
|
Grade 0: pre‐ or post‐ulcerative lesion Grade 1: partial/full thickness ulcer Grade 2: probing to tendon or capsule Grade 3: deep with osteitis Grade 4: partial foot gangrene Grade 5: whole foot gangrene |
Grade 0: pre‐ or post‐ulcerative site that has healed Grade 1: superficial wound not involving tendon, capsule, or bone Grade 2: wound penetrating to tendon or capsule Grade 3: wound penetrating bone or joint Each wound grade has 4 stages: Stage A: clean wounds Stage B: non ischaemic infected wounds Stage C: ischaemic non‐infected wounds Stage D: ischaemic infected wounds |
Ulcer site: forefoot (distal to tarso‐metatarsal joint = 0; midfoot/hindfoot = 1) Ischaemia: blood flow relatively intact (at least one pulse palpable on the affected foot = 0; evidence of ischaemia i.e. neither pulse palpable with signs of reduced tissue perfusion with, or without, gangrene = 1) Neuropathy: absent = 0; present = 1 (based on a routine examination using either Neurotips or 10 g nylon monofilaments) Bacterial infection: absent = 0; present = 1 Area: <1 cm2 = 0; ≥1 cm2 = 1 Depth: superficial = 0; deep (tendon, periosteum, joint capsule, or bone) = 1 |
GRADE 1 No symptoms or signs of PAD in the affected foot, in combination with palpable dorsal pedal and posterior tibial artery, or
GRADE 2 Symptoms or signs of PAD, but not of CLI:
GRADE 3 CLI, as defined by:
|
|
Abbreviations CLI = critical limb ischaemia PAD = peripheral arterial disease TcPO2 = transcutaneous oxygen pressure | |||
Appendix 4. Sources of growth factors
| Growth factor | Sources | Wound‐healing and tissue‐forming ability | Biologic activities | Source |
| Epidermal growth factor (EGF) | Blood vessel cells, outer skin cells Fibroblasts, and many other cell types |
|
Cell proliferation |
Foster 2009; Rozman 2007 |
| Platelet‐derived growth factor (PDGF) | Fibroblasts, smooth muscle cells, chondrocytes, osteoblasts, mesenchymal stem cells |
|
Chemoattraction, cell proliferation | Foster 2009; Rozman 2007 |
| Transforming growth factor alpha (TGF‐α) | Blood vessel cells, outer skin cells |
|
Cell proliferation | Rozman 2007 |
| Transforming growth factor beta (TGF‐ß1) | Blood vessel tissue, outer skin cells Fibroblasts, monocytes Osteoblasts—highest levels of TGF‐βr |
|
Promotes matrix synthesis | Foster 2009; Rozman 2007 |
| Fibroblast growth factor; acidic. (aFGF or FGF‐1) | Blood vessels Fibroblasts, other cell types |
|
Angiogenesis, fibroblast proliferation | Foster 2009; Rozman 2007 |
| Fibroblast growth factor; basic (bFGF or FGF‐2) | Blood vessels, smooth muscle, skin fibroblasts, other cell types |
|
Angiogenesis, fibroblast proliferation | Foster 2009; Rozman 2007 |
| Vascular endothelial growth factor (VEGF/ VEP) | Blood vessel cells |
|
Angiogenesis | Foster 2009; Rozman 2007 |
| Endothelial cell growth factor (ECGF) | Blood vessel cells |
|
Endothelial cell proliferation, angiogenesis | Foster 2009; Rozman 2007 |
| Lactoferrin | Polymorphonuclear leukocytes |
|
A glycoprotein with antibiotic, anti‐inflammatory and immunomodulatory activity |
Weinberg 2003; Andersen 2004 http://www.ncbi.nlm.nih.gov/mesh |
Appendix 5. Search strategies
MEDLINE
1 exp Foot Ulcer/ 2 exp Diabetic Foot/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 or/1‐5 7 exp "Intercellular Signaling Peptides and Proteins"/ 8 exp Insulin‐Like Growth Factor Binding Proteins/ 9 growth factor*.tw. 10 (EGF or FGF or PDGF).tw. 11 (plermin or regranex or becaplermin).tw. 12 or/7‐11 13 6 and 12 14 randomized controlled trial.pt. 15 controlled clinical trial.pt. 16 randomized.ab. 17 placebo.ab. 18 clinical trials as topic.sh. 19 randomly.ab. 20 trial.ti. 21 or/14‐20 22 (animals not (humans and animals)).sh. 23 21 not 22 24 13 and 23
EMBASE
1 exp Foot Ulcer/ 2 exp Diabetic Foot/ 3 (diabet* adj3 ulcer*).tw. 4 (diabet* adj3 (foot or feet)).tw. 5 (diabet* adj3 wound*).tw. 6 or/1‐5 7 exp Growth Factor/ 8 growth factor*.tw. 9 (EGF or FGF or PDGF).tw. 10 (plermin or regranex or becaplermin).tw. 11 or/7‐10 12 6 and 11 13 Clinical trial/ 14 Randomized controlled trials/ 15 Random Allocation/ 16 Single‐Blind Method/ 17 Double‐Blind Method/ 18 Cross‐Over Studies/ 19 Placebos/ 20 Randomi?ed controlled trial$.tw. 21 RCT.tw. 22 Random allocation.tw. 23 Randomly allocated.tw. 24 Allocated randomly.tw. 25 (allocated adj2 random).tw. 26 Single blind$.tw. 27 Double blind$.tw. 28 ((treble or triple) adj blind$).tw. 29 Placebo$.tw. 30 Prospective Studies/ 31 or/13‐30 32 Case study/ 33 Case report.tw. 34 Abstract report/ or letter/ 35 or/32‐34 36 31 not 35 37 animal/ 38 human/ 39 37 not 38 40 36 not 39 41 12 and 40
CINAHL
S20 S15 and S19 S19 S16 or S17 or S18 S18 lower extremity N3 ulcer* or AB lower extremity N3 ulcer* S17 TI (varicose ulcer* or venous ulcer* or leg ulcer* or foot ulcer* or (feet N1 ulcer*) or stasis ulcer* or crural ulcer*) or AB (varicose ulcer* or venous ulcer* or leg ulcer* or foot ulcer* or (feet N1 ulcer*) or stasis ulcer* or crural ulcer*) S16 (MH "Leg Ulcer+") S15 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 S14 TI ( diatherm* or microwave* ) or AB ( diatherm* or microwave* ) S13 (MH "Microwaves") S12 (MH "Diathermy+") S11 TI ( monophasic or galvanic ) or AB ( monophasic or galvanic ) S10 TI interferential therap* or AB interferential therap* S9 TI ( TENS or NMES ) or AB ( TENS or NMES ) S8 TI high voltage or AB high voltage S7 TI ( low intensity or low frequency ) or AB ( low intensity or low frequency ) S6 TI ( direct current or pulsed current or alternating current ) or AB ( direct current or pulsed current or alternating current ) S5 TI electric* current or AB electric* current S4 TI electric* stimulation or AB electric* stimulation S3 TI ( electromagnetic* or electrotherap* ) or AB ( electromagnetic* or electrotherap* ) S2 (MH "Electric Stimulation+") S1 (MH "Electromagnetics+")
Appendix 6. Assessment of risk of bias in included studies
Generation of allocation sequence (checking for possible selection bias)
Low risk (any truly random process, e.g. random number table, computer random number generator, that is likely to produce comparable groups).
High risk (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number, that is unlikely to produce comparable groups).
Unclear risk, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Allocation concealment (checking for possible selection bias)
Low risk (e.g. telephone or central randomisation or consecutively‐numbered, sealed, opaque envelopes; allocation is unlikely to be foreseen in advance or become known later).
High risk (open random allocation or unsealed or non‐opaque envelopes, alternation, date of birth; allocation could be foreseen in advance or become known later).
Unclear risk, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Blinding or masking (checking for possible performance and detection bias)
We assessed the adequacy of blinding separately for participants, carers/personnel and outcome assessors, and also for different outcomes or classes of outcomes.
Low risk: participants, carers/personnel and/or outcome assessors blinded regarding the intervention participants received, or lack of blinding could not have affected the results.
High risk: participants, carers/personnel and/or outcome assessors were not blinded regarding the intervention participants received and this could have affected the results.
Unclear risk: blinding of participants, carers/personnel and outcome assessors was not reported.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
-
Low risk (any one of the following):
no missing outcome data;
reasons for missing outcome data were unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size;
missing data have been imputed using appropriate methods.
-
High risk (any one of the following):
reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
-
Unclear risk (either of the following):
insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ to be made (e.g. number randomised not stated, no reasons for missing data provided);
the study did not address this outcome.
Selective outcome reporting bias
-
Low risk (either of the following):
the study protocol is available and all the pre‐specified (primary and secondary) outcomes were reported in the final report;
the study protocol was not available but it was clear that the published reports included all expected outcomes.
-
High risk (any one of the following):
not all of the study’s pre‐specified primary outcomes have been reported;
one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified;
one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis;
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk: insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ to be made.
Other biases
We described for each included study any important concerns we had about other possible sources of bias (academic bias, bias in presentation data, etc.)
Low risk of bias, the trial appears to be free of other components that could put it at risk of bias.
Unclear risk, the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias, there are other factors in the trial that could put it at risk of bias.
Appendix 7. Wound‐based severity grade for diabetic foot ulcers, according to the damaged anatomic sites
|
Grade (Beckert 2006) |
Anatomic sites |
| 1 | Dermis |
| 2 | Subcutaneous |
| 3 | Fascia |
| 4 | Muscle |
| 5 | Bone |
Appendix 8. Diabetic ulcer severity score for foot ulcers in people with diabetes
|
Severity (Beckert 2006) |
Score |
| Absent pedal pulses | Yes = 1 No = 0 |
| Bone involvement | Yes = 1 No = 0 |
| Site of ulceration | Toe = 0 Foot = 1 |
| Number of ulcers | Multiple = 1 Single = 0 |
Appendix 9. Methods used in included trials for evaluating skin wound evolution
| Study | METHOD |
| d'Hemecourt 1998 | “ . . . The area of the target ulcer was also measured (length by width).” (p 71) |
| Driver 2006 | “Wounds were assessed and measured (length, width, and depth using a metric tape measure at each visit. The measurements and other wound variables including undermining or tunnelling, characteristics of wound exudates (i.e., presence, colour, amount, and odour), necrotic tissue, and granulation tissue were documented.” (p 71) |
| Fernández‐Montequin 2007 | “…a standardized photograph was taken to permit further audit of result.” (p 335) |
| Fernández‐Montequin 2009 | “Ulcer areas and percent granulation were measured by planimetry from a manual tracing on a transparent grid sheet.”(p 434) |
| Hanft 2008 | “Weekly 35 mm photographs documented the physical features of the ulcers, while the surface area was measured using quantitative planimetric tracing of the ulcer margin.” (p 31) |
| Hardikar 2005 | “ . . . greatest length by greatest width . . .” (p 142) |
| Holloway 1993 | “. . . each scheduled visit the wounds were evaluated for length, width, depth and granulation tissue.” (p 200) |
| Jaiswal 2010 | “The ulcer area was calculated, by obtaining the impression of the ulcer floor on a sheet of cellophane paper and transferring on to a graph paper.” (p 32) |
| Kakagia 2007 | “All wounds were photographed digitally at initial debridement and then once weekly with a reference marker of scale in three dimensions. Computarized planimetry was used . . . to compare the progression of wound healing in the three groups.” (p 389) |
| Lyons 2007 | “Ulcer area was determined by tracing the debridement target ulcer onto an acetate medium at screening, . . . An image of the acetate was obtained by scanning a photocopy of the acetate containing the metering device affixed to the photocopy. For the analysis of healing, the area of the target ulcer was determined by planimetry of the image of the acetate . . . ” (p 51) |
| Niezgoda 2005 | “. . . photo planimetry . . . ” (p 260) |
| Richard 1995 | “ . . . the length and width of the ulcer were measured and photograph was taken . . . ” (p 67) |
| Robson 2002 | “Specific ulcer evaluation included photography, size and depth measurements . . . The areas of unclosed ulcers were obtained using digitised image analysis . . . of ulcer outline tracings made on double thickness plastic sheets . . . ” (p 134) |
| Saldalamacchia 2004 | “ . . . the wounds area was estimated by considering the wound like an ellipses whose diameters were the largest and shortest dimensions of the wound.” (p 395) |
| Steed 1992 | “ . . . length, width, depth were measured and the ulcers were photographed at each visit.” (p 1599) |
| Steed 1995a | “([area at baseline‐current area]/area at baseline)*100.” (p 73) |
| Steed 1995b | “Ulcer area was determined by manually tracing the ulcer outline on the acetate and calculating the area of the tracing using computerized planimetry.” (p 40) |
| Tan 2008 | " . . . were photographed weekly with a . . . camera with MD 50 nm lens and range flash" (p 434) |
| Tsang 2003 | “Throughout the study, ulcerate areas were overlaid with grid paper for size reference in photography . . . ” (p 1858) |
| Uchi 2009 | “ . . . photographed target ulcers during each visit . . . digital camera . . . ” (p 462) |
| Viswanathan 2006 | “ . . . Wound measurements were divided into 3 major groups: ruler based assessment schemes, transparent tracings, and optical methods . . . ” (p 188) |
| Wieman 1998a | “At each visit, the area of the target ulcer was measured (length multiplied by width)." (p 824) |
Appendix 10. Time to complete healing: RCTs not included in the meta‐analysis
| Study | Results | Reasons |
| Bhansali 2009 | Intervention group: 90 days Control group: 120 days |
Reported incomplete information for calculating hazard ratio |
| d'Hemecourt 1998 | Intervention group I: 98 days Intervention group II: 85 days Control group: 141 days |
Reported incomplete information for calculating hazard ratio |
| Driver 2006 | Intervention group: 45 days Control group: 85 days |
Reported incomplete information for calculating hazard ratio |
| Fernández‐Montequin 2007 | Intervention group: 144.2 days Control group: 136.5 days |
Reported incomplete information for calculating hazard ratio |
| Fernández‐Montequin 2009 | Intervention group I: 98 days Intervention group II: 84 days Control group: 140 days |
Reported incomplete information for calculating hazard ratio |
| Hanft 2008 | Treatment group: 58 days Control group: could not be estimated |
Reported incomplete information for calculating hazard ratio |
| Hardikar 2005 | Intervention group: 57 days (at week 20) Control group: 96 days (at week 20) |
Reported incomplete information for calculating hazard ratio |
| Holloway 1993 | Intervention group: 140 days Control group: could not be determined |
Reported incomplete information for calculating hazard ratio |
| Niezgoda 2005 | Intervention group: 73 days Control group: 60 days |
Reported incomplete information for calculating hazard ratio |
| Robson 2002 | Intervention group I: 16 weeks Intervention group: 12 weeks Intervention group: 13 weeks Control group I: 9 weeks Control group II: could not be determined |
Reported incomplete information for calculating hazard ratio |
| Steed 1995a | Intervention group: 30 days Control group: 40 days |
Reported incomplete information for calculating hazard ratio. |
| Steed 1995b | Intervention group: 4 weeks Control group: 8 weeks |
Reported incomplete information for calculating hazard ratio. |
| Wieman 1998a | Intervention group: 86 days Control group: 127 days |
Reported incomplete information for calculating hazard ratio. |
| Viswanathan 2006 | Intervention group: 8.5 weeks Control group: 9.8 weeks |
Reported incomplete information for calculating hazard ratio. |
Data and analyses
Comparison 1. Any growth factor versus placebo or no growth factor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 12 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.31, 1.73] |
| 2 Lower limb amputation (minimum of one toe) | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.39, 1.39] |
| 3 Ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence) | 1 | Hazard Ratio (Fixed, 95% CI) | 0.64 [0.14, 2.94] | |
| 4 Adverse events (non‐serious and serious) | 4 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
1.3. Analysis.

Comparison 1 Any growth factor versus placebo or no growth factor, Outcome 3 Ulcer‐free days following treatment for diabetic foot ulcers (free from any recurrence).
Comparison 2. Any growth factor versus placebo or no growth factor (subgroup analysis of trials with follow‐up < 20 weeks versus follow‐up ≥ 20 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with complete wound closure | 12 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.31, 1.73] |
| 1.1 Trials with length of follow‐up < 20 weeks | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.00, 1.55] |
| 1.2 Trials with length of follow up ≥ 20 weeks | 7 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.38, 1.98] |
Comparison 3. Any growth factor versus placebo or no growth factor (subgroup analysis by type of growth factor).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 12 | 1137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.30, 1.73] |
| 1.1 Autologous growth factor (AGF) | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.23, 17.34] |
| 1.2 Platelet‐derived wound‐healing formula (PDWHF) | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.27, 4.74] |
| 1.3 Recombinant human platelet‐derived growth factor (rHuPDGF) | 5 | 763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.23, 1.76] |
| 1.4 Recombinant human basic fibroblast growth factor (rHubFBGF) | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.88, 1.72] |
| 1.5 Recombinant human epidermal growth factor (rHuEGF) | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.16, 2.57] |
| 1.6 Recombinant human vascular endothelial growth factor (rHuVEGF) | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.79, 2.82] |
Comparison 4. Any growth factor versus placebo or no growth factor (sensitivity analyses considering attrition).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All trials | 12 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.31, 1.73] |
| 1.2 Best‐worst case scenario | 8 | 1049 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.79, 2.38] |
| 1.3 Worst‐best case scenario | 8 | 1043 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.19] |
Comparison 5. Platelet derived wound healing formula (PDWHF) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [1.27, 4.74] |
| 2 Lower limb amputation (minimum of one toe) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.11, 43.95] |
5.1. Analysis.

Comparison 5 Platelet derived wound healing formula (PDWHF) versus control, Outcome 1 Complete wound closure.
5.2. Analysis.

Comparison 5 Platelet derived wound healing formula (PDWHF) versus control, Outcome 2 Lower limb amputation (minimum of one toe).
Comparison 6. Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 5 | 753 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.21, 1.73] |
| 2 Adverse event: infection | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.41, 2.97] |
| 3 Adverse event: cellulitis | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.01] |
| 4 Adverse event: peripheral oedema | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.20, 0.96] |
| 5 Adverse event: pain | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.48] |
| 6 Adverse event: skin ulceration | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.49, 2.37] |
6.1. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 1 Complete wound closure.
6.3. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 3 Adverse event: cellulitis.
6.4. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 4 Adverse event: peripheral oedema.
6.5. Analysis.

Comparison 6 Recombinant human platelet‐derived growth factor (rHuPDGF) versus placebo, Outcome 5 Adverse event: pain.
Comparison 7. Recombinant human basic fibroblast growth factor (rHubFBGF) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete wound closure | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.11] |
| 2 Adverse event: infection | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.20] |
7.1. Analysis.

Comparison 7 Recombinant human basic fibroblast growth factor (rHubFBGF) versus placebo, Outcome 1 Complete wound closure.
Comparison 8. Recombinant human epidermal growth factor versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lower limb amputation (minimum of one toe) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Recombinant human epidermal growth factor versus actovegin | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.07, 2.98] |
| 1.2 Recombinant human epidermal growth factor 75 μg dose versus 25 μg dose | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.43, 1.47] |
8.1. Analysis.

Comparison 8 Recombinant human epidermal growth factor versus active control, Outcome 1 Lower limb amputation (minimum of one toe).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afshari 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: both groups received wound debridement and irrigation with normal saline solution, systemic antibiotic therapy and daily wound dressing |
|
| Outcomes | Primary: complete wound closure | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were entered to the study randomly until a total of 50 patients" (p 760) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: " . . . The placebo formulation contained . . . in the same hydrophilic base . . . " (p 760) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided to permit judgment of 'low risk' or 'high risk' Withdrawals were not reported |
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk |
|
Agrawal 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: both groups received debridement, dressing, pressure relief and glycaemic control |
|
| Outcomes | Healing: participants categorised as complete responders, partial responders, non‐complete responders (p 84). | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were randomised to receive either . . . " (p 81) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imbalance between groups for participants lost after randomisation (18% (5/28) in total) Control group: 36% (5/14) during the final week (week 12), reasons not reported Experimental group: none |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published report included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk |
|
Bhansali 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: debridement at baseline, offloading |
|
| Outcomes | Incidence of complete wound closure, duration of complete healing, rate of healing and safety | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Patients were randomized . . " (p e14) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label (p e14) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label (p e14) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (e.g. number randomized not stated, no reasons for missing data provided) |
| Selective reporting (reporting bias) | High risk | Safety data were not reported |
| Other bias | High risk | Bias in the presentation of data and design bias, see Appendix 1 |
Chen 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: 3% hydrogen peroxide, normal saline, ethacridine lactate (rivanol), gentamicin, diabetic diet, blood sugar control |
|
| Outcomes | Unclear This trial reported data for time‐to complete healing of diabetic foot ulcers |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Difference between intervention and control treatments easy to identify |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Only one outcome reported; it was not pre‐specified Author reported 'time‐to complete healing of the diabetic foot ulcer' as a continuous measure |
| Other bias | High risk | Failure to report inclusion/exclusion criteria Bias of presentation of the data, see Appendix 1 Design bias, see Appendix 1 |
d'Hemecourt 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: all groups received good wound care consisting of initial sharp debridement of ulcers to remove nonviable tissue, daily moist saline dressing changes, offloading of pressure, and systemic control of infection |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "patients were randomly assigned in a 2:2:1 ratio to one of three treatment groups" (p 71) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "were conducted in double‐blind fashion" (p 71) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall lost: 24% (41/172 participants)
Reasons: main reasons for withdrawals were
|
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis This trial did not report time to complete wound closure |
| Other bias | High risk | Bias in the presentation of data and design bias, see Appendix 1 |
Driver 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: cleaning and interim wound assessment of vital signs, offloading orthosis walker |
|
| Outcomes | Primary: proportion of participants with a healed wound | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "was electronically generated, blocked per investigational center" (p 70) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote " . . . to maintain blinding of the investigators, investigative sites staff, patients, sponsor, and CRO staff, and monitor" (p 70) This trial did not report the approach used to guarantee adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote ". . . to maintain blinding of the investigators, investigative sites staff, patients, sponsor, and CRO staff, and monitor" (p 70) This trial did not report the approach used to guarantee adequate blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall lost: 44% (32/72 participants) Reasons: failure to complete treatment (25%; 8/32) and protocol violations (75%; 24/32) |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | Bias in the presentation of data and design bias, see Appendix 1 |
Fernández‐Montequin 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standardised wound care regimen, ulcers were sharply debrided, gangrenous and necrotic tissue removed whenever necessary, and broad‐spectrum antibiotics and metabolic control as required |
|
| Outcomes | Primary: response at 5 to 8 weeks (percentage of ulcer area covered by granulation tissue).
Secondary efficacy endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "computer‐generated random list" (p 335) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "both vials were indistinguishable . . . " (p 335) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "both vials were indistinguishable . . . " (p 335) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of participants: 36.5% (15/41)
|
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered into a meta‐analysis |
| Other bias | High risk | Bias in the presentation of data and design bias, see Appendix 1 |
Fernández‐Montequin 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐intervention: standard wound care |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " Randomisation was simple, central and stratified by investigation sites" (p 434) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "both vials were indistinguishable . . . " (p 434) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Placebo, characteristics of nature and schedules were not given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of participants: 27% (40/149)
Quote "Nine patients in the lower EGF dose and placebo groups switched treatment at week 2 and are defined as non healers in further analysis. This design could have some impact on outcome regarding granulation rates at the week 8 visit and closure rates at 1 year follow‐up" (p 437) It is likely that the principle of ITT analysis was violated |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis Time‐to‐complete response was reported using odds ratio (p 435) |
| Other bias | High risk |
|
Hanft 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standard good wound care including periodic sharp debridement at the clinician's discretion, also offloading |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote " . . . a masked, third‐party, reading centre . . performed the quantitative measurements" (p 31) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost participants: total loss = 20% (11/55): Intervention group 24% (7/29); Control group 15% (4/26) Reasons:
|
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so they could not be entered in a meta‐analysis Quote "Median time to complete healing was 58 days for telbermin‐treated subjects. This could not be estimated for placebo‐treated subjects because fewer than 50% healed completely during the 12‐week study period." (p 35) |
| Other bias | High risk | Bias in the presentation of data and design bias, see Appendix 1 Funding bias Many trial authors are or have been employed by Genentech or own Genentech stock |
Hardikar 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standard wound care regimen consisting of appropriate sharp surgical debridement; daily ulcer cleaning and dressing; and offloading (e.g. crutches or wheelchair), or, where possible, complete bed rest. During this 1‐week period, before the baseline visit (visit 2), a regimen of daily wound cleaning and dressing with appropriate non‐weight bearing was followed. The use of antidiabetic medication and appropriate use of systemic antibiotics was advised during the treatment period |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote " the authors are not privy to the differences in the formulation available commercially" (p 2/11) Comment: both comparisons probably had similar appearances |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up: total = 19% (21/113)
Intervention group: 13% (7/55) and placebo group 24% (14/58) Imbalance between comparison groups: 11%. Reasons:
Comment: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups |
| Selective reporting (reporting bias) | High risk | Safety data were poorly reported, and, therefore, could not be entered in a meta‐analysis This trial did not report hazard ratios for assessing time to complete wound closure |
| Other bias | High risk | Design bias, bias of presentation bias, see Appendix 1 Funding bias |
Holloway 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary: healed (functional assessment points 3 & 4 in original paper): "3: 100% epithelized; maturing skin with a small amount of drainage; requires protective dressing only. 4: 100% epithelized; maturing skin with a small amount of drainage; no dressing required" (p 200) |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " . . . computer generated list of random numbers . . . " (p 200) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "the appearance of the double‐blind medication and the packaging were identical for drug and placebo to prevent unblinding" (p 200) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost 16% (16/97) post randomisation because failed to meet inclusion criteria ‐ no details provided regarding loss according to group Loss of randomised and treated participants (total = 81) by comparison group
Quote "eleven patients with 14 wounds were excluded from the efficacy analysis due to noncompliance with protocol" (p 201) Reasons:
Imbalance between comparison groups: unclear Quote "four patients were lost to follow up after 12 weeks and 5 patients missed the last or second‐to‐last visit" (p 201) Authors did not report whether these losses were in addition to those specified above and did not supply information about the timing of the losses |
| Selective reporting (reporting bias) | High risk | One outcome (complete wound closure) was reported incompletely This trial did not report safety data |
| Other bias | High risk | Bias of the presentation data and information bias, see Appendix 1 Quote " . . . The first 14 patients were given either 0.01 dilution of CT‐102 or a placebo consisting of physiologic saline solution. The study was later revised to include two additional dilutions of CT‐102 at 0.1 and 0.033. At that time a new randomization scheme was generated. A limited analysis conducted on the first 14 patients to ensure that there were no problems with the protocol. The placebo solution was at that time changed to an isotonic platelet buffer containing N1‐2‐hydroxiethiyl piperazine‐N‐2‐ethanesulfonic acid (HEPES), glucose sodium chloride and potassium chloride (pH=6.6) . . . " (p 200) Funding bias |
Jaiswal 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standardised regimen of good wound care; pressure offloading for participants with plantar ulcers |
|
| Outcomes | Primary outcome: healing or percent reduction in size of the wound | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "on the basis of computer generated numbers" (p 32) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | Information on time to healed and safety was not provided |
| Other bias | High risk | Bias of presentation data, see Appendix 1 |
Kakagia 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: all wounds were sharply debrided prior to the first application of dressings and were assessed weekly for 8 weeks |
|
| Outcomes | Change in ulcer dimensions within the 8‐week follow‐up | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " . . . random number generator" (p 388) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk | Bias of presentation data, see Appendix 1 Author did not supply information on wound area at the end of the trial |
Landsman 2010.
| Methods | Randomized, controlled trial, parallel design (2 arms)
Country study: USA
Intention to treat: unclear
Follow up period: 20 weeks Unit of randomization: unclear Unit of analysis: ulcers |
|
| Participants | Randomised: 32, no further details provided Age (years) Intervention group (becaplermin plus TheraGauze): 58.1 Control group (TheraGauze): 56.2 Gender (male): not reported Inclusion criteria
Exclusion criteria
Withdrawal: not reported |
|
| Interventions |
Co‐interventions: standard wound debridement as needed, offloading with a fixed ankle walker (Royce Medical Diabetic Boot; Ossur Medical, Camarillo, California), and dressing changes every other day |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "Professional, independent monitoring and centralized randomization" (p 156) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | One outcome (Time to closure (full epithelialization) was reported incompletely so that it could not be included in the meta‐analysis. This trial did not report safety data |
| Other bias | High risk | Design bias and bias of presentation data, see Appendix 1 Funding bias |
Lyons 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standard care consisted of:
|
|
| Outcomes | Primary endpoint of phase 2: percentage of participants achieving ≥ 75% closure of the target ulcer | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " . . . randomization was central . . . " (p 50) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote " . . . and none of the personnel of any of the site were informed of the blinding code before completion of the study" (p 50) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available, but it is clear that the published reports included all expected outcomes, including those that were pre‐specified However, this trial failed to provide separate data for each study arm |
| Other bias | High risk | Bias of presentation data, see Appendix 1 |
Niezgoda 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standard wound care, pressure‐relief shoes (DH Pressure Relief Shoe; Royce Medical Co, Camarillo, CA) |
|
| Outcomes | Primary outcome: incidence of complete wound healing by 12 weeks | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " were assigned to a treatment group using a centralized computer system" (p 259) |
| Allocation concealment (selection bias) | Low risk | Quote " individual investigators were blinded to the size of the block" (p 259) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost post randomisation (treated): total = 26% (25/98)
Reasons for loss:
|
| Selective reporting (reporting bias) | Unclear risk | One or more outcomes of interest in the review were reported incompletely so that they could not be included in a meta‐analysis |
| Other bias | High risk | Bias of presentation data, see Appendix 1 Funding bias |
Richard 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions:
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'Yes'or 'No |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote " . . . ulcer perimeter and area were measured by one of us (J.P.D), unaware of the patient's identity, data of photographs, and nature of the treatment" (p 65) Comment: J.P.D is Jean‐Pierre Daures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | High risk | Design bias, see Appendix 1 Funding bias |
Robson 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary
Secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " . . . computer‐generation patients numbers" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote "Double blinding for all 4 groups receiving collagen sponge was maintaining by code labelling for all collagen‐sponge packaging materials . . . the standardized care group could not be blinded since these patients did not receive a collagen sponge like the other four treatment groups" (p 2/10) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost post randomisation: 15% (27/177)
Lost after treatment: 7% (11/150) Total lost: 21% (38/177) Reasons: not reported Losses by intervention group: not reported Potentially inappropriate application of simple imputation |
| Selective reporting (reporting bias) | High risk | Safety was reported incompletely so the data could not be entered in a meta‐analysis |
| Other bias | High risk | Bias of presentation data, see Appendix 1 |
Saldalamacchia 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Reduction rate (%) Complete healing or reduction of at least 50% |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote ". . . were randomly . . . " (p 395) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "observers were blind with respect to treatment assignments" (p 395) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote "observers were blind with respect to treatment assignments" (p 395) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk | Bias of presentation data, see Appendix 1 |
Steed 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: normal cotton gauze applied to the wound for the next 12 hours; participants were supplied with a half‐shoe (IPOS North American Niagara Fakks, NY) that transferred the weight to their heel and could be used for balance, they also had access to wheel‐chairs, crutches, or walkers to avoid weight‐bearing; debridements |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote " . . . were identical in appearance. Neither the investigators nor the patients were able to distinguish between the two products" (p 1599) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk | Design bias, see Appendix 1 Funding bias Imbalance in wound area (mm2) and initial volume (mm3) at baseline |
Steed 1995a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: a saline‐moistened gauze dressing was placed over the target ulcer and gel, and the foot wrapped with a roll of gauze. Pressure relief for the target ulcer achieved by means of crutches, wheelchairs, orthotic shoes, or other methods. Sharp debridement could be performed at each office visit if the investigator thought it was necessary |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " . . . computer generation . . . " (p 73) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote " . . . were no differences of colour, consistency, or odour between the placebo and rhPDGF‐BB gel" (p 72) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost post randomisation: total = 27% (32/118)
Intervention group = 23% (14/61); Control group = 32% (18/57) Imbalance between group: 9% Reasons
|
| Selective reporting (reporting bias) | High risk | One outcome of interest (time to complete wound closure) in the review was reported incompletely so the data could not be entered in a meta‐analysis |
| Other bias | High risk | Bias of presentation data and design bias, see Appendix 1 This trial did not report hazard ratio for time to complete wound closure Funding bias |
Steed 1995b.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: debridements as required; shoes designed to relieve pressure on the study ulcer |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "A member of the study support staff other than the investigators applied the treatment . . . identical syringes were used to administer RGD peptide matrix and saline placebo" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost post randomisation: total = 22% (14/65)
Intervention group = 20% (8/40); Control group = 24% (6/25) Imbalance between group: 4% Reasons
|
| Selective reporting (reporting bias) | High risk | The safety profile was incompletely reported |
| Other bias | High risk | Design bias and bias of presentation of data, see Appendix 1 Funding bias |
Steed 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: half‐shoe to redistribute weight, also used crutches, wheelchairs, or walkers for offloading |
|
| Outcomes | Recurrence rate of the diabetic neurotrophic ulcer | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "patients were randomized to receive . . ." (p 231) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "identical in appearance to the placebo" (p 231) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | High risk | Safety data were not reported |
| Other bias | High risk | Design bias and Bias of presentation data, see Appendix 1 |
Tan 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: sterile cotton dressing without antibiotics |
|
| Outcomes | Outcomes were not described explicitly as primary end points Quote "after 6‐week treatment, the wounds were divided into four categories: complete healing, significant healing if more than 50% of the wound are had healed; effective healing, if 20‐50% of the wound area had healed; ineffective healing, if less than 20% of the wound had healed" (p 434) | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote " . . . prospective study by randomly . . ." (p 433) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis. This trial did not report safety data |
| Other bias | High risk |
|
Tsang 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: standard wound care consisted of debridement of necrotic tissue and reduction of callus |
|
| Outcomes | Complete healing defined as full epithelialisation of the wound with an absence of discharge | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "randomization was performed by drawing envelopes" (p 1857) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote " . . . patients and physicians were blind to the hEGF concentrations" (p 1858) This trial did not reported how blinding was performed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit a judgement of ‘low risk’ or ‘high risk’ (i.e. no reasons provided for missing data) |
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk | Design bias, see Appendix 1
Funding bias This was the first trial by this clinical group that assessed recombinant human epidermal growth factor, however, it did not investigate safety |
Uchi 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: appropriate treatments to control blood glucose levels |
|
| Outcomes | Primary outcomes:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote " . . . computer‐generated randomisation program" (p 462) |
| Allocation concealment (selection bias) | Low risk | Quote " . . . assigned to groups by telephone or fax at the KCB‐1 Registration Center (ADJUST Co., LTD., Kokkaido, Japan)" (p 462) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote " . . . participants in the blinded trial included physicians, evaluators, patients, and monitor." (p 462) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote " . . . participants in the blinded trial included physicians, evaluators, patients, and monitors." (p 462) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost post randomisation (withdrew within 4 weeks): total = 6% (9/150) Reasons
|
| Selective reporting (reporting bias) | High risk | This trial did not report safety data |
| Other bias | High risk | Bias in presentation of data. see Appendix 1 Funding bias |
Viswanathan 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐interventions: normal dose of insulin prescribed; oral and intravenous antibiotics for prevention of infection |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "the tubes containing either rhEGF or placebo were similar" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost: 5% (3/60): Intervention group = 1; Control group = 2 |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis Quote "The recorded adverse events were 1 case of rash, 3 cases of pain, and 2 cases of skin irritation” (p 4/14) This trial did not report adverse event data according to comparison group |
| Other bias | High risk | Design bias and bias of presentation bias, see Appendix 1 Funding bias: main author was paid an investigator's fee for conducting the study, another author is a consultant for the company that provided the study supplies and that supported study financially |
Wieman 1998a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Co‐intervention: standardised regimen of good wound care |
|
| Outcomes | Primary
Secondary: time required to achieve complete healing |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "patients were randomized to one of three . . . " (p 823) Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "the placebo gel was identical to the vehicle component of the gel formulation containing the active drug" (p 823) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to permit a judgment of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost post randomisation: total = 19% (73/382)
Reasons
|
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so they could not be entered in a meta‐analysis |
| Other bias | High risk | Quote "the time to complete healing, defined as the number of days until the patients achieved a functional assessment score of 1, was analyzed using Cox's proportional hazards model" (p 824) Design bias and bias of data presentation, see Appendix 1 |
Abbreviations
ABPI = ankle‐brachial pressure index CT‐102 = thrombin‐induced platelet‐released platelet‐derived wound healing formula Hb = haemoglobin HbA1c = glycated haemoglobin IAET = The International Association of Enterostomal Therapists (now known as the Wound, Ostomy, Continence Nurses' Society (WOCN)) ITT = intention to treat (analysis) IV = intravascular rhaFGF = recombinant human acidic fibroblast growth factor rhbFGF = recombinant human basic fibroblast growth factor rhEGF = recombinant human epidermal growth factor TcPO2 = transcutaneous oxygen tension
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acosta 2006 | Case report |
| Aminian 2000 | Not an randomised clinical trial |
| Embil 2000 | Case series |
| Hong 2006 | Case series |
| Miller 1999 | Case report |
| Mohan 2007 | Phase IV (post‐marketing surveillance study) |
| Saad Setta 2011 | Not an randomised clinical trial |
| Tuyet 2009 | Case report |
| Yera‐Alos 2013 | Phase IV (post‐marketing surveillance study) |
Characteristics of studies awaiting assessment [ordered by study ID]
Gomez‐Villa 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Morimoto 2013.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Singla 2014.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Young 1992.
| Methods |
**Neither the title nor abstract of this study indicated whether it was randomised. We have not been able to find the address of the authors, enquiries are ongoing. |
| Participants |
|
| Interventions |
Co‐interventions: appropriate pressure relief with orthoses or casts and weekly chiropody |
| Outcomes | Not stated, so it reported complete healing, safety |
| Notes |
|
Abbreviation
PDGF = platelet‐derived growth factor
Characteristics of ongoing studies [ordered by study ID]
NCT00521937.
| Trial name or title | A prospective, randomised, multi‐centre, blind‐observer, controlled, parallel‐group study comparing the efficacy and safety of DERMAGEN® versus conventional treatment in the treatment of diabetic neuropathic foot ulcer |
| Methods |
|
| Participants |
|
| Interventions | Intervention: Dermagen® Control: Conventional treatment |
| Outcomes |
|
| Starting date | January 2009 (date of first enrolment) |
| Contact information | Name: Olivier Chosidow, MD, PhD Address: not reported Telephone: not reported Email: not reported Affiliation: Hôpital Tenon, Paris |
| Notes | * Pedis is a classification system for diabetic foot ulcers in people with diabetes mellitus (Schaper 2004). See Appendix 3 for details. Target sample size: 388 Register: ClinicalTrials.gov Last refreshed on: 21 December 2010 Main ID: NCT00521937 Date of registration: 27 August 2007 Primary sponsor: Laboratoires Genévrier Recruitment status: active, not recruiting URL: NCT00521937 Source(s) of monetary support: Laboratoires Genévrier |
NCT00709514.
| Trial name or title | A phase II, double‐blind, placebo‐controlled clinical evaluation of DCB‐WH1 in healing of chronic diabetic foot ulcers |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcome measure: incidence of complete ulcer closure: time‐frame = 12 weeks; designated as a safety issue |
| Starting date | May 2008 (date of first enrolment) |
| Contact information | Name: David Yeh, Director Address: not reported Telephone: +886 2 26558098 Email: davidyeh@microbio.com.tw Affiliation: not reported |
| Notes | PEDIS is Target sample size: 50 Register: ClinicalTrials.gov Last refreshed on: 8 March 2011 Main ID: NCT00709514 Date of registration: 27 June 2008 Primary sponsor: Oneness Biotech Co, Ltd Recruitment status: completed URL: http://clinicaltrials.gov/show/NCT00709514 Source(s) of monetary support: Oneness Biotech Co, Ltd |
NCT00915486.
| Trial name or title | A randomized, multi‐center, controlled, parallel group, dose finding study of the efficacy and safety of topically applied I‐020201 as an adjunct to good standard‐of‐care versus good standard‐of‐care alone in patients with chronic diabetic foot ulcers |
| Methods |
|
| Participants | Enrolled: 210
|
| Interventions |
|
| Outcomes |
|
| Starting date | May 2009 (date of first enrolment) |
| Contact information | Name: Mitra Safari Address: not reported Telephone: +41 44 200 5600 Email: mitra.safari@kuros.ch Affiliation: not reported |
| Notes |
|
NCT00926068.
| Trial name or title | Safety and efficacy of HO/03/03 10 µg in the treatment of plantar neuropathic diabetic foot ulcers (Truheal) |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | February 2010 (date of first enrolment) |
| Contact information | Name: Talma Gotteiner, MPharm Address: not reported Telephone: +97289407188 Email: talma@healor.com Affiliation: not reported |
| Notes |
|
NCT01060670.
| Trial name or title | A multi‐center, randomized, controlled clinical trial to evaluate the safety and effectiveness of Integra® Dermal Regeneration Template for the treatment of neuropathic diabetic foot ulcers |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes |
|
| Starting date | April 2010 (date of first enrolment) |
| Contact information | Name: Nicola Fenty‐Stewart, PhD Address: not reported Telephone: 1‐609‐275‐0500 (http://www.integralife.com/index.aspx?redir=contact) Accessed on 3 September 2014 Email: nicolafs@amarexcro.com Affiliation: not reported |
| Notes |
|
NCT01098357.
| Trial name or title | A phase I/II, multicentre, randomised, controlled, and open‐label trial comparing the efficacy and safety of three dose regimens of BioChaperone PDGF‐BB to becaplermin gel for the treatment of diabetic foot ulcer |
| Methods |
|
| Participants |
|
| Interventions | After the screening visit, the eligible participant population randomly receive 1 of the following:
Experimental BioChaperone PDGF‐BB is a new formulation of the B isoform dimer of recombinant human platelet‐derived growth factor (rhPDGF‐BB) containing the new excipient Biochaperone, a dextran modified polymer. The finished product is administered as a sterile spray Regranex: active comparator becaplermin gel (Regranex® Gel 0.01%, Systagenix, formerly and Johnson & Johnson) is a topical gel of rhPDGF‐BB contained in a gel tube All 4 groups to be assessed weekly till the 8th week (visit 10); then once every 2 weeks (14 day duration) thereafter till the end of study. The maximum number of visits expected is 16. The study data will be presented at the end of 20 weeks |
| Outcomes |
|
| Starting date | June 2010 (date of first enrolment) |
| Contact information | Not reported |
| Notes | Target sample size: 192 Register: ClinicalTrials.gov Last refreshed on: 2 November 2010 Main ID: NCT01098357 Date of registration: 1 April 2010 Primary sponsor: Virchow Group Recruitment status: completed URL: http://clinicaltrials.gov/show/NCT01098357 Source(s) of monetary support: Virchow Group, and Adocia Secondary sponsor(s): Adocia |
Abbreviations
ABPI = ankle‐brachial pressure index AE = adverse event ALT = alanine transaminase AST = aspartate transaminase CABG = coronary artery bypass graft eGFR = epidermal growth factor receptor Hb = haemoglobin HbA1c = glycated haemoglobin HBV = Hepatitis B virus HCV = Hepatitis C virus HIV = human immunodeficiency virus PDGF = platelet‐derived growth factor PDGF‐AB = platelet‐derived growth factor (specific form) rhPEGF‐BB = recombinant human platelet‐derived growth factor min = minute(s) NSAID = non‐steroidal anti‐inflammatory SAE = serious adverse event SPI = systolic pressure index TcPO2 = transcutaneous oxygen tension TG‐PDGF.AB = transglutaminase‐ platelet‐derived growth factor AB
Differences between protocol and review
Based on Consolidated Standards of Reporting Trials (CONSORT) statement, we changed the term 'safety' into 'adverse events'. The term 'safety' may be considered to imply substantive evidence of an absence of harm. The term is often misused when there is simply absence of evidence of harm (Ioannidis 2004).
Contributions of authors
Arturo J Martì‐Carvajal: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; undertook quality assessment; analysed and interpreted data; checked quality assessment; performed part of data analysis and interpretation; performed statistical analysis; checked the quality of the statistical analysis; completed the first draft of the review; performed part of writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission; advised on the review; secured funding; wrote to study author/experts/companies; provided data and is a guarantor of the review.
Christian Gluud: checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; performed part of data analysis and interpretation; checked the quality of the statistical analysis; performed part of writing and editing the review; made an intellectual contribution to the review; approved the final review prior to submission and advised on the review..
Susana Nicola: extracted data; checked the quality of data extraction; checked quality assessment; made an intellectual contribution to the review; approved the final review prior to submission and wrote to study author/experts/companies.
Daniel Simancas‐Racines: checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; made an intellectual contribution to the review; and approved the final review prior to submission.
Ludovic Reveiz: checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; made an intellectual contribution to the review; approved the final review prior to submission and advised on the review.
Patricio Oliva: checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; made an intellectual contribution to the review and approved the final review prior to submission.
Jorge Cedeño‐Taborda: checked the quality of data extraction; undertook and checked quality assessment; analysed or interpreted data; made an intellectual contribution to the review; approved the final review prior to submission and advised on the review.
Contributions of editorial base
Nicky Cullum: edited the protocol; advised on methodology, interpretation and protocol content. Approved the final protocol prior to submission.
Jo Dumville: edited the review, advised on methodology, interpretation and review content. Approved the final review prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited and copy‐edited the protocol and review.
Rachel Richardson: edited the review.
Ruth Foxlee: designed the search strategy and edited the search methods section.
Sources of support
Internal sources
-
Facultad de Ciencias de la Salud Eugenio Espejo. Universidad Tecnológica Equinoccial, Quito, Ecuador.
Partly funded
External sources
-
National Institute of Health Research, UK.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
-
Copenhagen Trial Unit, Denmark.
Academic.
-
Cochrane Hepato‐Biliary Group, Denmark.
Academic.
-
Iberoamerican Cochrane Network, Spain.
Academic.
Declarations of interest
Arturo Martí‐Carvajal: was employed in 2004 by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. He was employed in 2007 by Merck to run a four‐hour workshop 'How to critically appraise clinical trials and how to teach this'. These activities are not related to his work with The Cochrane Collaboration or any Cochrane review.
Jorge A Cedeño‐Taborda: was employed as Medical Director for Organon Venezolana SA from 2002 to 2007. In 2007 he was employed by Novartis de Venezuela as an outsourced medical adviser for the development of scientific material to support the marketing of Vildagliptine. None of these activities is related to the subject of this Cochrane review.
Christian Gluud: None known.
Susana Nicola: None known.
Daniel Simancas‐Racines: None known.
Ludovic Reveiz: None known.
Patricio Oliva: None known.
New
References
References to studies included in this review
Afshari 2005 {published data only}
- Afshari M, Larijani B, Fadayee M, Ghahary A, Pajouhi M, Bastanhagh M, et al. Efficacy of topical epidermal growth factor in healing diabetic foot ulcers. Therapy 2005;2:759‐65. [Google Scholar]
Agrawal 2009 {published data only}
- Agrawal RP, Jhajharia A, Motha N, Dogra R, Chaudhari V, Nayak KC. Use of a platelet‐derived growth factor gel in chronic diabetic foot ulcers. Diabetic Foot Journal 2009;12(2):80‐8. [Google Scholar]
Bhansali 2009 {published data only}
- Bhansali A, Venkatesh S, Dutta P, Dhillon MS, Das S, Agrawal A. Which is the better option: recombinant human PDGF‐BB 0.01% gel or standard wound care, in diabetic neuropathic large plantar ulcers off‐loaded by a customized contact cast?. Diabetes Research and Clinical Practice 2009;83(1):e13‐6. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen A, Long XH, Huang C. Analysis of the effect of recombined human epidermal growth factor derivate applied externally in treating diabetic foot. Modern Nursing 2004;10(3):274‐5. [Google Scholar]
d'Hemecourt 1998 {published data only}
- d'Hemecourt PA, Smeill JM, Hugill JV, Prigoff MM. The effect of topically applied sodium carboxymethylcellulose gel in nonhealing, lower extremity ulcers in patients with diabetes. European Tissue Repair Society. Koln, Germany, 23‐27 August 1997:Abstracts.
- d'Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethyl cellulose aqueous‐based gel vs becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds 1998;10(3):69‐75. [Google Scholar]
Driver 2006 {published data only}
- Driver VR. A prospective, randomized, controlled, blinded, multicenter pivotal trial of a platelet rich plasma gel versus control when added to the standard of care in the treatment of non‐healing diabetic foot ulcers. Diabetes 2006;55(Suppl 1):A25. [Google Scholar]
- Driver VR, Hanft J, Fylling CP, Beriou JM, AutoGel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy/Wound Management 2006;52(6):68‐87. [PubMed] [Google Scholar]
Fernández‐Montequin 2007 {published data only}
- Fernández‐Montequin JI, Infante‐Cristia E, Valenzuela‐Silva C, Franco‐Perez N, Savigne‐Gutierrez W, Artaza‐Sanz H, et al. Intralesional injections of Citoprot‐P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. International Wound Journal 2007;4:333‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández‐Montequin 2009 {published data only}
- Fernández‐Montequín JI, Betancourt BY, Leyva‐Gonzalez G, Mola EL, Galán‐Naranjo K, Ramírez‐Navas M, et al. Intralesional administration of epidermal growth factor‐based formulation (Heberprot‐P) in chronic diabetic foot ulcer: treatment up to complete wound closure. International Wound Journal 2009;6:67‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Montequín JI, Valenzuela‐Silva CM, Díaz OG, Savigne W, Sancho‐Soutelo N, Rivero‐Fernández F, Cuban Diabetic Foot Study Group. Intra‐lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo‐controlled, double‐blind study. International Wound Journal 2009;6:432‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanft 2008 {published data only}
- Hanft JR, Pollak RA, Barbul A, Gils C, Kwon PS, Gray SM, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. Journal of Wound Care 2008;17:30‐2, 34‐7. [DOI] [PubMed] [Google Scholar]
- Kwon P, Breen TJ, Gray S, Lynch CJ, Semba CP, Hanft JR, et al. Results of a phase I, randomized, double‐blind, placebo‐controlled trial of topical rhVEGF (telbermin) for the treatment of diabetic foot ulcers. Wound Management 2006;54:102. [Google Scholar]
Hardikar 2005 {published data only}
- Hardikar JV, Reddy YC, Bung DD, Varma N, Shilotri PP, Prasad ED, et al. Efficacy of recombinant human platelet‐derived growth factor (rhPDGF) based gel in diabetic foot ulcers: a randomized, multicenter, double‐blind, placebo‐controlled study in India. Wounds 2005;17:141‐52. [Google Scholar]
Holloway 1993 {published data only}
- Holloway GA, Steed DL, DeMarco MJ, Matsumoto T, Moosa HH, Webster MW, et al. A randomised controlled dose response trial of activated platelet supernatant, topical CT‐102 in chronic, non healing diabetic wounds. Wounds 1993;5:198–206. [Google Scholar]
Jaiswal 2010 {published data only}
- Jaiswal SS, Gambhir RPS, Harish AA. Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers. Indian Journal of Surgery 2010;72:31‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakagia 2007 {published data only}
- Kakagia DD, Kazakos KJ, Xarchas KC, Karanikas M, Georgiadis GS, Tripsiannis G, et al. Synergistic action of protease‐modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. Journal of Diabetes and its Complications 2007;21:387‐91. [DOI] [PubMed] [Google Scholar]
Landsman 2010 {published data only}
- Landsman A, Agnew P, Parish L, Joseph R, Galiano RD. Diabetic foot ulcers treated with becaplermin and TheraGauze, a moisture‐controlling smart dressing: a randomized, multicenter, prospective analysis. Journal of the American Podiatric Medical Association 2010;100(3):155‐60. [DOI] [PubMed] [Google Scholar]
- Parish L, Routh H, Parish J. Diabetic foot ulcers: a randomized multicenter study comparing a moisture‐controlling dressing with a topical growth factor. Journal of the American Academy of Dermatology 2009;60(Suppl):AB202. [Google Scholar]
Lyons 2007 {published data only}
- Lyons TE, Miller MS, Serena T, Sheehan P, Lavery L, Kirsner RS, et al. Talactoferrin alfa, a recombinant human lactoferrin promotes healing of diabetic neuropathic ulcers: a phase 1/2 clinical study. American Journal of Surgery 2007;193(1):49‐54. [DOI] [PubMed] [Google Scholar]
Niezgoda 2005 {published data only}
- Niezgoda JA, Gils CC, Frykberg RG, Hodde JP. Randomized clinical trial comparing OASIS wound matrix to Regranex gel for diabetic ulcers. Advances in Skin and Wound Care 2005;18:258‐66. [DOI] [PubMed] [Google Scholar]
Richard 1995 {published data only}
- Richard JL, Parer‐Richard C, Daures JP, Clouet S, Vannereau D, Bringer J, et al. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot: A pilot, randomized, double‐blind, placebo‐controlled study. Diabetes Care 1995;18:64‐9. [DOI] [PubMed] [Google Scholar]
Robson 2002 {published data only}
- Robson MC, Steed DL, McPherson JM, Pratt BM. Effects of transforming growth factor +ƒ2 on wound healing in diabetic foot ulcers: a randomized controlled safety and dose‐ranging trial. Journal of Applied Research 2002;2:133‐45. [Google Scholar]
Saldalamacchia 2004 {published data only}
- Saldalamacchia G, Lapice E, Cuomo V, Feo E, D'Agostino E, Rivellese AA, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutrition Metabolism and Cardiovascular Diseases 2004;14:395‐6. [DOI] [PubMed] [Google Scholar]
- Saldalamacchia G, Lapice E, Cuomo V, Feo ME, D'Agostino E, Rivellese AA, et al. Use of autologous platelet gel for the treatment of diabetic foot ulcers. Giornale Italiano di Diabetologia e Metabolismo 2004;24:103‐5. [DOI] [PubMed] [Google Scholar]
Steed 1992 {published data only}
- Steed D, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomised prospective double blind trial in healing chronic diabetic foot ulcers: CT‐102 activated platelet supernatant, topical versus placebo. Diabetes Care 1992;15:1598–604. [DOI] [PubMed] [Google Scholar]
Steed 1995a {published data only}
- Steed DL. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity ulcers. Plastic and Reconstructive Surgery 2006;117 (Suppl7):143‐9. [DOI] [PubMed] [Google Scholar]
- Steed DL, Webster MW, Ricotta JJ, Luterman A, Brown S, Comerota AJ, et al. Clinical evaluation of recombinant human platelet‐derived growth factor for the treatment of lower extremity diabetic ulcers. Journal of Vascular Surgery 1995;21:71‐81. [DOI] [PubMed] [Google Scholar]
Steed 1995b {published data only}
- Steed D, Ricotta JJ, Prendergast JJ, Kaplan RJ, Webster MW, McGill JB, et al. Promotion and acceleration of diabetic ulcer healing by arginine glycine‐aspartic acid (RGD) peptide matrix. Diabetes Care 1995;18:39–46. [DOI] [PubMed] [Google Scholar]
Steed 1996 {published data only}
- Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair and Regeneration 1996;4:230‐3. [DOI] [PubMed] [Google Scholar]
Tan 2008 {published data only}
- Tan Y, Xiao J, Huang Z, Xiao Y, Lin S, Jin L, et al. Comparison of the therapeutic effects of recombinant human acidic and basic fibroblast growth factors in wound healing in diabetic patients. Journal of Health Science 2008;54:432‐40. [Google Scholar]
Tsang 2003 {published data only}
- Tsang MW, Wong WK, Hung CS, Lai KM, Tang W, Cheung EY, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care 2003;26:1856‐61. [DOI] [PubMed] [Google Scholar]
Uchi 2009 {published data only}
- Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, et al. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. European Journal of Dermatology 2009;19:461‐8. [DOI] [PubMed] [Google Scholar]
Viswanathan 2006 {published data only}
- Viswanathan V, Pendsey S, Sekar N, Murthy GSR. A phase III study to evaluate the safety and efficacy of recombinant human epidermal growth factor (REGEN‐D 150) in healing diabetic foot ulcers. Wounds 2006;18:186‐96. [Google Scholar]
Wieman 1998a {published data only}
- Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet‐derived growth factor‐BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo‐controlled double‐blind study. Diabetes Care 1998;21(5):822‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acosta 2006 {published data only}
- Acosta JB, Savigne W, Valdez C, Franco N, Alba JS, Rio A, et al. Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. International Wound Journal 2006;3(3):232‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aminian 2000 {published data only}
- Aminian B, Shams M, Soveyd M, Omrani GhR. Topical autologous platelet‐derived growth factors in the treatment of chronic diabetes ulcers. Archives of Iranian Medicine 2000; Vol. 3, issue 2:55‐9.
Embil 2000 {published data only}
- Embil JM, Papp K, Sibbald G, Tousignant J, Smiell JM, Wong B, The Canadian Becaplermin Study Group. Recombinant human platelet‐derived growth factor‐BB (becaplermin) for healing chronic lower extremity diabetic ulcers: an open‐label clinical evaluation of efficacy. Wound Repair and Regeneration 2000;8:162‐8. [DOI] [PubMed] [Google Scholar]
Hong 2006 {published data only}
- Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Annals of Plastic Surgery 2006;56:394‐8. [DOI] [PubMed] [Google Scholar]
Miller 1999 {published data only}
- Miller MS. Use of topical recombinant human platelet‐derived growth factor‐BB (becaplermin) in healing of chronic mixed arteriovenous lower extremity diabetic ulcers. The Journal of Foot and Ankle Surgery 1999;38:227‐31. [DOI] [PubMed] [Google Scholar]
Mohan 2007 {published data only}
- Mohan VK. Recombinant human epidermal growth factor (REGEN‐D 150): effect on healing of diabetic foot ulcers. Diabetes Research and Clinical Practice 2007;78:405‐11. [DOI] [PubMed] [Google Scholar]
Saad Setta 2011 {published data only}
- Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet‐rich plasma versus platelet‐poor plasma in the management of chronic diabetic foot ulcers: a comparative study. International Wound Journal 2011;8(3):307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuyet 2009 {published data only}
- Tuyet HL, Quynh TTN, Minh HVH, Bich DNT, Dinh TD, Tan DL, et al. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: the preliminary results. International Wound Journal 2009;6:159‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yera‐Alos 2013 {published data only}
- Yera‐Alos IB, Alonso‐Carbonell L, Valenzuela‐Silva CM, Tuero‐Iglesias AD, Moreira‐Martinez M, Marrero‐Rodriguez I, et al. Active post‐marketing surveillance of the intralesional administration of human recombinant epidermal growth factor in diabetic foot ulcers. BMC Pharmacology and Toxicology 2013;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gomez‐Villa 2014 {published data only}
- Gomez‐Villa R, Aguilar‐Rebolledo F, Lozano‐Platonoff A, Teran‐Soto JM, Fabian‐Victoriano MR, Kresch‐Tronik NS, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double‐blinded controlled trial. Wound Repair and Regeneration 2014;22(4):497‐503. [DOI] [PubMed] [Google Scholar]
Morimoto 2013 {published data only}
- Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue engineering 2013 Part A;19(17‐18):1931‐40. [DOI] [PubMed] [Google Scholar]
Singla 2014 {published data only}
- Singla S, Garg R, Kumar A, Gill C. Efficacy of topical application of beta urogastrone (recombinant human epidermal growth factor) in Wagner's Grade 1 and 2 diabetic foot ulcers: Comparative analysis of 50 patients. Journal of Natural Science, Biology and Medicine 2014;5(2):273‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 1992 {published data only}
- Young MJ, Larsen J, Knowles A, Parnell L, Ward JD. The treatment of diabetic neuropathic foot ulcers with biosynthetic platelet derived growth factor. Diabetic Medicine 1992, (Suppl 2):S42. [Google Scholar]
References to ongoing studies
NCT00521937 {published data only}
- NCT00521937. Efficacy and Safety Study of DERMAGEN® vs Conventional Treatment to Treat Diabetic Neuropathic Foot Ulcer. https://clinicaltrials.gov/ct2/show/NCT00521937.
NCT00709514 {published data only}
- NCT00709514. Clinical Evaluation of DCB‐WH1 in Healing of Chronic Diabetic Foot Ulcers. https://clinicaltrials.gov/ct2/show/NCT00709514.
NCT00915486 {published data only}
- NCT00915486. A Dose Finding Study of Topically Applied I‐020201 as an Adjunct to Good Standard‐of‐care in Patients With Chronic Diabetic Foot Ulcers (DFU). https://clinicaltrials.gov/ct2/show/NCT00915486.
NCT00926068 {published data only}
- NCT00926068. Safety and Efficacy of HO/03/03 10μg in the Treatment of Plantar Neuropathic Diabetic Foot Ulcers (Truheal). https://clinicaltrials.gov/ct2/show/NCT00926068.
NCT01060670 {published data only}
- NCT01060670. A Safety and Efficacy Study of INTEGRA® Dermal Regeneration Template for the Treatment of Diabetic Foot Ulcers. https://clinicaltrials.gov/ct2/show/NCT01060670.
NCT01098357 {published data only}
- NCT01098357. Comparative Study of 3 Dose Regimens of BioChaperone to Becaplermin Gel for the Treatment of Diabetic Foot Ulcer. https://clinicaltrials.gov/ct2/show/NCT01098357.
Additional references
Abbott 2002
- Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. North‐West Diabetes Foot Care Study. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetes Medicine 2002;19:377‐84. [DOI] [PubMed] [Google Scholar]
Albert 2002
- Albert S. Cost‐effective management of recalcitrant diabetic foot ulcers. Clinics in Podiatric Medicine and Surgery 2002;19:483‐91. [DOI] [PubMed] [Google Scholar]
Ali 2008
Amery 2005
- Amery CM. Growth factors and the management of the diabetic foot. Diabetic Medicine 2005;22 Suppl 1:12‐4. [DOI] [PubMed] [Google Scholar]
Andersen 2004
- Andersen JH. Technology evaluation: rh lactoferrin, Agennix. Current Opinion in Molecular Therapeutics 2004;6(3):344‐9. [PubMed] [Google Scholar]
Armstrong 2008
- Armstrong DG, Lavery LA, Wrobel JS, Vileikyte L. Quality of life in healing diabetic wounds: does the end justify the means?. Journal of Foot and Ankle Surgery 2008;47:278‐82. [DOI] [PubMed] [Google Scholar]
Ashry 1998
- Ashry HR, Lavery LA, Armstrong DG, Lavery DC, Houtum WH. Cost of diabetes‐related amputations in minorities. The Journal of Foot and Ankle Surgery 1998;37:186‐90. [DOI] [PubMed] [Google Scholar]
Bakker 2012a
- Bakker K, Schaper NC. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):116‐8. [DOI] [PubMed] [Google Scholar]
Bakker 2012b
- Bakker K, Apelqvist J, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):225‐31. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Barrientos 2008
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair and Regeneration 2008;16:585‐601. [DOI] [PubMed] [Google Scholar]
Bartus 2004
- Bartus CL, Margolis DJ. Reducing the incidence of foot ulceration and amputation in diabetes. Current Diabetes Reports 2004;4:413‐8. [DOI] [PubMed] [Google Scholar]
Basch 2012
- Basch E, Aronson N, Berg A, Flum D, Gabriel S, Goodman SN, et al. Methodological standards and patient‐centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636‐40. [DOI] [PubMed] [Google Scholar]
Beckert 2006
- Beckert S, Witte M, Wicke C, Königsrainer A, Coerper S. A new wound‐based severity score for diabetic foot ulcers: a prospective analysis of 1,000 patients. Diabetes Care 2006;29:988‐92. [DOI] [PubMed] [Google Scholar]
Bennet 2003
- Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. The British Journal of Surgery 2003;90:133‐46. [DOI] [PubMed] [Google Scholar]
Blair 2009
- Blair P, Flaumenhaft R. Platelet alpha‐granules: basic biology and clinical correlates. Blood Reviews 2009;23(4):177‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulton 2005
- Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719‐24. [DOI] [PubMed] [Google Scholar]
Boulton 2008
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes/Metabolism Research and Reviews 2008;24 (Suppl 1):3‐6. [DOI] [PubMed] [Google Scholar]
Boutoille 2008
- Boutoille D, Féraille A, Maulaz D, Krempf M. Quality of life with diabetes‐associated foot complications: comparison between lower‐limb amputation and chronic foot ulceration. Foot and Ankle International 2008;29:1074‐8. [DOI] [PubMed] [Google Scholar]
Buchberger 2010
- Buchberger B, Follmann M, Freyer D, Huppertz H, Ehm A, Wasem J. The importance of growth factors for the treatment of chronic wounds in the case of diabetic foot ulcers. GMS Health Technology Assessment 2010;6:Doc12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buchmayer 2011
- Buchmayer F, Pleiner J, Elmlinger MW, Lauer G, Nell G, Sitte HH. Actovegin(R): a biological drug for more than 5 decades. Wiener Medizinische Wochenschrift 2011;161(3‐4):80‐8. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chao 2009
- Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes/Metabolism Research and Reviews 2009;25:604‐14. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
CMA 2011 [Computer program]
- Comprehensive Meta‐Analysis team. Comprehensive Meta‐Analysis Sofware. Version 2.2.064. Englewood, NJ: Biostat, Inc, 2011.
Cruciani 2009
- Cruciani M, Lipsky BA, Mengoli C, Lalla F. Granulocyte‐colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006810] [DOI] [PubMed] [Google Scholar]
Enoch 2008
- Enoch S, Leaper DJ. Basic science of wound healing. Surgery 2008;26(2):31‐7. [Google Scholar]
FDA 2008
- Food, Drug Administration. Regranex (becaplermin) Gel. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094968.htm (accessed 6 July 2011).
Figueroa‐Romero 2008
- Figueroa‐Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Reviews in Endocrine & Metabolic Disorders 2008;9:301‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foster 2009
- Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet‐rich plasma: from basic science to clinical applications. The American Journal of Sports Medicine 2009;37(11):2259‐72. [DOI] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel SE, Normand SL. Getting the methods right ‐ the foundation of patient‐centered outcomes research. The New England Journal of Medicine 2012;367(9):787‐90. [DOI] [PubMed] [Google Scholar]
Galkoswka 2006
- Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair and Regeneration 2006;14:558‐65. [DOI] [PubMed] [Google Scholar]
Girod 2003
- Girod I, Valensi P, Laforêt C, Moreau‐Defarges T, Guillon P, Baron F. An economic evaluation of the cost of diabetic foot ulcers: results of a retrospective study on 239 patients. Diabetes and Metabolism 2003;29:269‐77. [DOI] [PubMed] [Google Scholar]
Goodridge 2006
- Goodridge D, Trepman E, Sloan J, Guse L, Strain LA, McIntyre J, et al. Quality of life of adults with unhealed and healed diabetic foot ulcers. Foot and Ankle International 2006;27:274‐80. [DOI] [PubMed] [Google Scholar]
Grazul‐Bilska 2003
- Grazul‐Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, et al. Wound healing: the role of growth factors. Drugs of Today 2003;39:787‐800. [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011i
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Guyatt 2012
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI] [PubMed] [Google Scholar]
Habib 2010
- Habib SH, Biswas KB, Akter S, Saha S, Ali L. Cost‐effectiveness analysis of medical intervention in patients with early detection of diabetic foot in a tertiary care hospital in Bangladesh. Journal of Diabetes and its Complications 2010;24(4):259‐64. [DOI] [PubMed] [Google Scholar]
Herber 2007
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;25:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org.
Hinchliffe 2008
- Hinchliffe RJ, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes/Metabolism Research and Reviews 2008;24(Suppl 1):119–44. [DOI] [PubMed] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 1996
- Humphrey AR, Dowse GK, Thoma K, Zimmet PZ. Diabetes and nontraumatic lower extremity amputations. Incidence, risk factors, and prevention ‐ a 12‐year follow‐up study in Nauru. Diabetes Care 1996;19:710‐4. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Ince 2007
- Ince P, Kendrick D, Game F, Jeffcoate W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabetic Medicine 2007;24:977‐81. [DOI] [PubMed] [Google Scholar]
Ince 2008
- Ince P, Abbas ZG, Lutale JK, Basit A, Ali SM, Chohan F, et al. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care 2008;31:964‐7. [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. Meta‐research: the art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [DOI] [PubMed] [Google Scholar]
Iversen 2008
- Iversen MM, Midthjell K, Østbye T, Tell GS, Clipp E, Sloane R, et al. History of and factors associated with diabetic foot ulcers in Norway: the Nord‐Trøndelag Health Study. Scandinavian Journal of Public Health 2008;36:62‐8. [DOI] [PubMed] [Google Scholar]
Izumi 2009
- Izumi Y, Satterfield K, Lee S, Harkless LB, Lavery LA. Mortality of first‐time amputees in diabetics: a 10‐year observation. Diabetes Research and Clinical Practice 2009;83(1):126‐31. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2003
- Jeffcoate WJ, Harding KG. Diabetic Foot Ulcers. Lancet 2003;361:1545‐51. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2004
- Jeffcoate WJ, Houtum WH. Amputation as a marker of the quality of foot care in diabetes. Diabetologia 2004;47:2051‐8. [DOI] [PubMed] [Google Scholar]
Jeffcoate 2005
- Jeffcoate WJ. The incidence of amputation in diabetes. Acta Chirurgica Belgica 2005;105:140‐2. [PubMed] [Google Scholar]
Kanade 2007
- Kanade R, Deursen R, Burton J, Davies V, Harding K, Price P. Re‐amputation occurrence in the diabetic population in South Wales, UK. International Wound Journal 2007;4:344‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinmond 2003
- Kinmond K, McGee P, Gough S, Ashford R. 'Loss of self': a psychosocial study of the quality of life of adults with diabetic foot ulceration. Journal of Tisssue Viability 2003;13:6‐8, 10, 12. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Köveker 2000
- Köveker GB. Growth factors in clinical practice. International Journal of Clinical Practice 2000;54:590‐3. [PubMed] [Google Scholar]
Lacci 2010
- Lacci KM, Dardik A. Platelet‐rich plasma: support for its use in wound healing. The Yale Journal of Biology and Medicine 2010;83(1):1‐9. [PMC free article] [PubMed] [Google Scholar]
Lantis 2009
- Lantis JC 2nd, Boone D, Gendics C, Todd G. Analysis of patient cost for recombinant human platelet‐derived growth factor therapy as the first‐line treatment of the insured patient with a diabetic foot ulcer. Advances in Skin and Wound Care 2009;22:167‐71. [DOI] [PubMed] [Google Scholar]
Lavery 1996
- Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. The Journal of Foot and Ankle Surgery 1996;35:528‐31. [DOI] [PubMed] [Google Scholar]
Lavery 2008
- Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC, International Working Group on the Diabetic Foot. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care 2008;31:154‐6. [DOI] [PubMed] [Google Scholar]
Lavery 2009
- Lavery LA, Peters EJ, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. Diabetes Research and Clinical Practice 2009;83:347‐52. [DOI] [PubMed] [Google Scholar]
Lee 1993
- Lee JS, Lu M, Lee VS, Russell D, Bahr C, Lee ET. Lower‐extremity amputation. Incidence, risk factors, and mortality in the Oklahoma Indian Diabetes Study. Diabetes 1993;42:876‐82. [DOI] [PubMed] [Google Scholar]
Lefevbre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lehto 1996
- Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Risk factors predicting lower extremity amputations in patients with NIDDM. Diabetes Care 1996;19:607‐12. [DOI] [PubMed] [Google Scholar]
Leymarie 2005
- Leymarie F, Richard JL, Malgrange D. Factors associated with diabetic patients at high risk for foot ulceration. Diabetes and Metabolism 2005;31:603‐5. [DOI] [PubMed] [Google Scholar]
Lipsky 2004
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clinical Infectious Disease 2004;39:885‐910. [DOI] [PubMed] [Google Scholar]
McKinney 2012
- McKinney M. 'Time is of the essence'. PCORI moves to implement comparative effectiveness research, funding. Modern Healthcare 2012; Vol. 42, issue 5:12‐3. [PubMed]
Meatherall 2005
- Meatherall BL, Garrett MR, Kaufert J, Martin BD, Fricke MW, Arneja AS, et al. Disability and quality of life in Canadian aboriginal and non‐aboriginal diabetic lower‐extremity amputees. Archives of Physical Medicine and Rehabilitation 2005;86:1594‐602. [DOI] [PubMed] [Google Scholar]
Milman 2001
- Milman MHSA, Leme CBM, Borelli DT, Kater FR, Baccili ECDC, Rocha RCM, et al. Diabetic foot: evaluation of evolution and hospital cost of hospitalized patients in Conjunto Hospitalar de Sorocaba. Arquivos Brasileiros de Endocrinologia e Metabologia 2001;45:447‐51. [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moss 1996
- Moss SE, Klein R, Klein BE. Long‐term incidence of lower‐extremity amputations in a diabetic population. Archives of Family Medicine 1996;5:391‐8. [DOI] [PubMed] [Google Scholar]
Nabuurs‐Frassen 2005
- Nabuurs‐Franssen MH, Huijberts MS, Nieuwenhuijzen Kruseman AC, Willems J, Schaper NC. Health‐related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005;48:1906‐10. [DOI] [PubMed] [Google Scholar]
Nelson 1988
- Nelson RG, Gohdes DM, Everhart JE, Hartner JA, Zwemer FL, Pettitt DJ, et al. Lower‐extremity amputations in NIDDM. 12‐year follow‐up study in Pima Indians. Diabetes Care 1988;11:8‐16. [DOI] [PubMed] [Google Scholar]
O'Meara 2000
- O'Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technology Assessment 2000;4(21):1‐237. [PubMed] [Google Scholar]
Oyibo 2001
- Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Armstrong DG, Harkless LB. The effects of ulcer size and site, patient’s age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabetic Medicine 2001;18:133‐8. [DOI] [PubMed] [Google Scholar]
Papanas 2007
- Papanas N, Maltezos E. Growth factors in the treatment of diabetic foot ulcers: new technologies, any promises?. The International Journal of Lower Extremity Wounds 2007;6:37‐53. [DOI] [PubMed] [Google Scholar]
Papanas 2008
- Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clinical Interventions in Aging 2008;3:233‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papanas 2010
- Papanas N, Maltezos E. Benefit‐risk assessment of becaplermin in the treatment of diabetic foot ulcers. Drug Safety 2010;33(6):455‐61. [DOI] [PubMed] [Google Scholar]
PCORI 2012
- Patient‐Centered Outcomes Research Institute (PCORI). Preliminary draft methodology report: 'Our questions, our decisions: Standards for patient‐centered outcomes research'. http://www.pcori.org/assets/Preliminary‐Draft‐Methodology‐Report.pdf (accessed 2 August 2012).
Porta 2008
- Porta M. A Dictionary of Epidemiology. Fifth. New York: Oxford University Press, 2008. [Google Scholar]
Pradhan 2009
- Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Reviews in Molecular Medicine 2009;11:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2004
- Price P. The diabetic foot: quality of life. Clinical Infectious Diseases 2004;39(Suppl 2):129‐31. [DOI] [PubMed] [Google Scholar]
Quattrini 2008
- Quattrini C, Jeziorska M, Boulton AJ, Malik RA. Reduced vascular endothelial growth factor expression and intra‐epidermal nerve fiber loss in human diabetic neuropathy. Diabetes Care 2008;31:140‐5. [DOI] [PubMed] [Google Scholar]
Ramsey 1999
- Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382‐7. [DOI] [PubMed] [Google Scholar]
Rathur 2007
- Rathur HM, Boulton AJ. The diabetic foot. Clinics in Dermatology 2007;25:109‐20. [DOI] [PubMed] [Google Scholar]
Redekop 2004
- Redekop WK, Stolk EA, Kok E, Lovas K, Kalo Z, Busschbach JJ. Diabetic foot ulcers and amputations: estimates of health utility for use in cost‐effectiveness analyses of new treatments. Diabetes and Metabolism 2004;30:549‐56. [DOI] [PubMed] [Google Scholar]
Ribu 2008
- Ribu L, Birkeland K, Hanestad BR, Moum T, Rustoen T. A longitudinal study of patients with diabetes and foot ulcers and their health‐related quality of life: wound healing and quality‐of‐life changes. Journal of Diabetes Complications 2008;22:400‐7. [DOI] [PubMed] [Google Scholar]
Richard 2008
- Richard JL, Schuldiner S. Epidemiology of diabetic foot problems [Épidémiologie du pied diabétique]. La Revue Médicine Interne 2008;29(Suppl 2):222‐30. [DOI] [PubMed] [Google Scholar]
Rith‐Najarian 1992
- Rith‐Najarian SJ, Stolusky T, Gohdes DM. Identifying diabetic patients at high risk for lower‐extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care 1992;15:1386‐9. [DOI] [PubMed] [Google Scholar]
Rozman 2007
- Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft‐tissue injuries. Acta Dermatovenerologica Alpina, Panonica, et Adriatica 2007;16(4):156‐65. [PubMed] [Google Scholar]
Schaper 2004
- Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes/Metabolism Research and Reviews 2004;20(Suppl 1):90‐5. [DOI] [PubMed] [Google Scholar]
Schaper 2012a
- Schaper NC, Apelqvist J, Bakker K. Reducing lower leg amputations in diabetes: a challenge for patients, healthcare providers and the healthcare system. Diabetologia 2012;55(7):1869‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaper 2012b
- Schaper NC, Andros G, Apelqvist J, Bakker K, Lammer J, Lepantalo M, et al. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic Foot. Diabetes/Metabolism Research and Reviews 2012;28(Suppl 1):218‐24. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [DOI] [PubMed] [Google Scholar]
Selby 2012
- Selby JV, Beal AC, Frank L. The Patient‐Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA 2012;307(15):1583‐4. [DOI] [PubMed] [Google Scholar]
Sheehan 2006
- Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4‐week period is a robust predictor of complete healing in a 12‐week prospective trial. Plastic and Reconstructive Surgery 2006;117(7 Suppl):239‐44. [DOI] [PubMed] [Google Scholar]
Sibbald 2008
- Sibbald RG, Woo KY. The biology of chronic foot ulcers in persons with diabetes. Diabetes/Metabolism Research and Reviews 2008;24(Suppl 1):25‐30. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 12 March 2010).
Siriwardana 2007
- Siriwardana HD, Weerasekera D. The cost of diabetic foot conditions. The Ceylon Medical Journal 2007;52:89‐91. [DOI] [PubMed] [Google Scholar]
Skoutas 2009
- Skoutas D, Papanas N, Georgiadis GS, Zervas V, Manes C, Maltezos E, et al. Risk factors for ipsilateral reamputation in patients with diabetic foot lesions. The International Journal of Lower Extremity Wounds 2009;8:69‐74. [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith D, Cullen MJ, Nolan JJ. The cost of managing diabetic foot ulceration in an Irish hospital. Irish Journal of Medical Science 2004;173:89‐92. [DOI] [PubMed] [Google Scholar]
Smyth 2009
- Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, et al. Platelet functions beyond hemostasis. Journal of Thrombosis and Haemotasis 2009;7(11):1759‐66. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Szabo 2009
- Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. British Journal of Pharmacology 2009;156:713‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valensi 2007
- Valensi P, Girod I, Baron F, Moreau‐Defarges T, Guillon P. Quality of life and clinical correlates in patients with diabetic foot ulcers. Diabetes and Metabolism 2005;31(3 Pt 1):263‐71. [DOI] [PubMed] [Google Scholar]
Van Acker 2000
- Acker K, Oleen‐Burkey M, Decker L, Vanmaele R, Schil P, Matricali G, et al. Cost and resource utilization for prevention and treatment of foot lesions in a diabetic foot clinic in Belgium. Diabetes Research and Clinical Practice 2000;50:87‐95. [DOI] [PubMed] [Google Scholar]
Van Houtum 2008
- Houtum WH. Amputations and ulceration; pitfalls in assessing incidence. Diabetes/Metabolism Research and Reviews 2008;24(Suppl 1):14‐8. [DOI] [PubMed] [Google Scholar]
Veves 1992
- Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia 1992;35:660‐3. [DOI] [PubMed] [Google Scholar]
Viswanathan 2005
- Viswanathan V, Thomas N, Tandon N, Asirvatham A, Rajasekar S, Ramachandran A, et al. Profile of diabetic foot complications and its associated complications ‐ a multicentric study from India. The Journal of Association Physicians of India 2005;53:933‐6. [PubMed] [Google Scholar]
Viswanathan 2006
- Viswanathan V, Pendsey S. A phase III study to evaluate the safety and efficacy of recombinant human epidermal growth factor (REGEN‐DTM 150) in healing diabetic foot ulcers. Wounds 2006;18:186‐96. [Google Scholar]
Wagner 1981
- Wagner FW. The dysvascular foot: a system of diagnosis and treatment. Foot and Ankle International 1981;2:64‐122. [DOI] [PubMed] [Google Scholar]
Weinberg 2003
- Weinberg ED. The therapeutic potential of lactoferrin. Expert Opinion on Investigational Drugs 2003;12(5):841‐51. [DOI] [PubMed] [Google Scholar]
Wieman 1998b
- Wieman TJ. Clinical efficacy of becaplermin (rhPDGF‐BB) gel. Becaplermin Gel Studies Group. American Journal of Surgery 1998;176(2A Suppl):74S‐9S. [PUBMED: 9777976] [DOI] [PubMed] [Google Scholar]
Willrich 2005
- Willrich A, Pinzur M, McNeil M, Juknelis D, Lavery L. Health related quality of life, cognitive function, and depression in diabetic patients with foot ulcer or amputation. A preliminary study. Foot and Ankle International 2005;26:128‐34. [DOI] [PubMed] [Google Scholar]
Winkley 2007
- Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population‐based prospective cohort study of people with their first diabetic foot ulcer. Journal of Diabetes and its Complications 2007;21:341‐9. [DOI] [PubMed] [Google Scholar]
Younes 2004
- Younes NA, Albsoul AM, Awad H. Diabetic heel ulcers: a major risk factor for lower extremity amputation. Ostomy/Wound Management 2004;50:50‐60. [PubMed] [Google Scholar]
Young 1994
- Young MJ, Breddy JL, Veves A, Boulton AJ. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care 1994;17:557‐60. [DOI] [PubMed] [Google Scholar]
Zavala 2007 [Computer program]
- Zavala D, Martí‐Carvajal A, Peña‐Marti G, Comunián‐Carrasco G. Software for data transformation for time‐to‐event outcomes (alfa version). Valencia: Universidad de Carabobo, 2007.
Ziyadeh 2011
- Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD. A matched cohort study of the risk of cancer in users of becaplermin. Advances in Skin and Wound Care 2011;24(1):31‐9. [DOI] [PubMed] [Google Scholar]


