Abstract
Objective
Whether to administer adjuvant treatment is a matter of great debate for oral cavity cancer harboring a single positive node without extranodal extension and positive margin (defined as low/intermediate risk pN1new in this study).
Methods
A total of 243 low/intermediate risk pN1new patients with oral cavity cancer who received curative surgery were included. Overall survival (OS), local recurrence‐free survival (LRFS), regional recurrence‐free survival (RRFS), and distant metastasis‐free survival (DMFS) were compared between patients receiving adjuvant treatment and observation alone.
Results
For patients receiving adjuvant therapy vs observation, the differences in outcomes were not statistically significant in terms of 5‐year OS, LRFS, RRFS, and DMFS. For subgroup analysis, in low/intermediate pN1new patients with one or more minor risk factors, adjuvant therapy was not significantly associated with OS, LRFS, RRFS, or DMFS in pN1new patients.
Conclusion
For low/intermediate risk pN1new patients with oral cavity cancer, adjuvant therapy might be omitted.
Level of Evidence
4.
Keywords: adjuvant radiotherapy, oral cancer, risk factor, single node, surgery
1. INTRODUCTION
Whether to administer adjuvant treatment to patients with oral cavity cancer is a matter of great debate. This is especially true for cases defined in the American Joint Committee on Cancer (AJCC) sixth/seventh editions pN1 without major risk factors, including extranodal extension (ENE) and positive margin.
In addition, a drastic change between AJCC sixth/seventh and AJCC eighth oral cavity cancer staging was noted. In AJCC sixth/seventh, pN1 was defined as a single ipsilateral lymph node of ≤3 cm in its greatest dimension, whereas N2a was defined as a single ipsilateral lymph node 3 to 6 cm in its greatest dimension. ENE was not considered in the staging system. However, in AJCC eighth, the definition of pN1 changed to a single ipsilateral lymph node of ≤3 cm that is ENE negative. Likewise, pN2a in AJCC eighth was redefined as an ENE‐negative single ipsilateral node of 3 to 6 cm or an ENE‐positive single ipsilateral node of ≤3 cm. All other patients with ENE were defined as being pN3b. Furthermore, some studies have demonstrated that a novel staging system incorporating the number of positive lymph nodes and ENE exhibited greater concordance than did the AJCC eighth system for oral cavity cancer. 1 , 2 , 3 In the novel neck staging system, pN1 was newly defined as “single positive lymph node and ENE (−).” Thus, cancers with a single positive node without ENE (referred to as pN1new in this present study) were a unique entity in both AJCC eighth and the potential novel staging system. pN1new encompassed AJCC eighth pN1 and part of pN2a patients.
In addition, studies investigating whether adjuvant treatment improves outcomes for low/intermediate risk pN1new (single positive node without ENE and positive margin) patients have been sparse and limited by a mixture of patients harboring the high‐risk factors of positive ENE or positive margin and incomplete pathological information within relevant databases. In this study, we conducted a two‐center retrospective study with complete pathological information to investigate whether adjuvant treatment improves outcomes for low/intermediate risk pN1new patients.
2. MATERIALS AND METHODS
2.1. Patients and treatment
This multicenter study included patients from two tertiary referral medical centers. This study was approved by Institutional Review Board of National Taiwan University Hospital (202002130RINB) and Chang Gung Memorial Hospital (201800383B0D001). During 2004 to 2015, a total of 243 patients with oral cavity cancer who received curative surgery with pathological single positive node metastasis, no ENE, and no positive margin were included. Staging work‐up included head and neck computed tomography or magnetic resonance imaging, chest radiographs, bone scan, and abdominal echo or positron emission tomography. All patients were restaged as per AJCC eighth edition. We included low/intermediate risk pN1new patients by reviewing pathology and selected patients who had a single positive lymph node without ENE and without positive margin.
Curative surgery included tumor wide excision and neck dissection. Radical neck dissection or modified neck dissection was performed in patients with clinically positive lymph nodes, whereas supraomohyoid neck dissection was performed in patients with clinically negative lymph nodes. Bilateral neck dissection was considered in patients with clinically positive bilateral neck lymph nodes or tumors located in or across the midline.
Adjuvant radiation therapy (RT) was given at the discretion of treating physicians, usually to patients with one or more risk factors, including advanced tumor (T3/T4), lymphovascular invasion (LVI), perineural invasion (PNI), poorly differentiated tumor, or close margin (<5 mm). Treating physicians sometimes opted to omit adjuvant therapy for certain older patients (>65 years old) who only had one risk factor, and some patients refused adjuvant therapy. Some patients with two or more risk factors received concurrent chemoradiaiton (CCRT) with weekly cisplatin 30 to 40 mg/m2 at the discretion of treating physicians. During treatment, a radiation field covered the primary tumor bed and regional neck lymphatics with conventional fractionation of 1.8 or 2 Gy at one fraction per day, 5 days a week, using 6‐MV photons. The median dose was 66 Gy (range, 60‐72 Gy). Of the 243 patients, 168 (69.1%) patients received adjuvant RT. Among 168 patients who received adjuvant RT, 21 (12.5%) received therapy using the two‐dimensional (2D) or three‐dimensional (3D) technique, 23 (13.7%) a mixed 2D/3D and intensity‐modulated radiotherapy (IMRT)/volumetric modulated arc therapy (VMAT), 123 (73.2%) with IMRT/VMAT, and 1 (0.4%) with proton therapy.
2.2. Statistical analysis
Overall survival (OS), local recurrence‐free survival (LRFS), regional recurrence‐free survival (RRFS), and distant metastasis‐free survival (DMFS) were calculated from the date of surgery. Categorical variables were analyzed using the chi‐square test. Survival outcomes were analyzed using the Kaplan‐Meier method and compared with the log‐rank test in univariate analysis. The Cox regression model was used to perform multivariate analysis. A two‐sided P value < .05 was considered to be statistically significant.
3. RESULTS
Patient characteristics are summarized in Table 1. The median age for all patients was 50 years old (range, 27‐85). Of the 243 patients, 214 (88.1%) were men. The most common tumor subsites were the buccal mucosa (41.6%), followed by the tongue (37%), gingiva (9.9%), floor of the mouth (4.1%), retromolar trigone (4.1%), lip (2.1%), and hard palate (1.2%). Sixty‐four (26.3%), 155 (63.8%), and 24 (9.9%) patients had well, moderately, and poorly differentiated tumors, respectively. Most patients had T3 (86, 35.3%) and T4 (57, 23.5%) tumors. LVI‐ and PNI‐positive rates were 10.3% and 46.1%, respectively. Clear surgical margins were achieved in 129 (53.1%) patients.
TABLE 1.
Patient characteristics
| All (%) | Without adjuvant therapy (N = 75) (percentage of 75 patients) | With adjuvant therapy (N = 168) (percentage of 168 patients) | P value | |
|---|---|---|---|---|
| Age (years old), median (range) | 50 (27‐85) | 52 (27‐85) | 49 (31‐72) | .413 |
| Gender | ||||
| Male | 214 (88.1) | 67 (89.3) | 147 (87.5) | .684 |
| Female | 29 (11.9) | 8 (10.7) | 21 (12.5) | |
| Primary site | ||||
| Buccal | 101 (41.6) | 38 (50.7) | 63 (37.5) | .000 |
| Tongue | 90 (37) | 17 (22.7) | 73 (43.5) | |
| Mouth floor | 10 (4.1) | 5 (6.6) | 5 (3.0) | |
| Retromolar trigone | 10 (4.1) | 4 (5.3) | 6 (3.6) | |
| Gingiva | 24 (9.9) | 4 (5.3) | 20 (11.9) | |
| Lip | 5 (2.1) | 4 (5.3) | 1 (0.6) | |
| Hard palate | 3 (1.2) | 3 (4.0) | 0 | |
| Differentiation | ||||
| Well | 64 (26.3) | 22 (29.3) | 42 (25) | .262 |
| Moderate | 155 (63.8) | 49 (65.3) | 106 (63.1) | |
| Poor | 24 (9.9) | 4 (5.3) | 20 (11.9) | |
| pT | ||||
| T1 | 17 (7.0) | 9 (12.0) | 8 (4.8) | .000 |
| T2 | 83 (34.2) | 43 (57.3) | 40 (23.8) | |
| T3 | 86 (35.3) | 14 (18.7) | 72 (42.9) | |
| T4a | 51 (21) | 9 (12.0) | 42 (25.0) | |
| T4b | 6 (2.5) | 0 | 6 (3.6) | |
| LVI | ||||
| Positive | 25 (10.3) | 7 (9.3) | 18 (10.7) | .743 |
| Negative | 218 (89.7) | 68 (80.7) | 150 (89.3) | |
| PNI | ||||
| Positive | 112 (46.1) | 22 (29.3) | 90 (53.6) | .000 |
| Negative | 131 (53.9) | 53 (70.7) | 78 (46.4) | |
| Margin | ||||
| Clear | 129 (53.1) | 46 (61.3) | 83 (49.4) | .085 |
| Close | 114 (46.9) | 29 (38.7) | 85 (50.6) |
Abbreviations: LVI, lymphovascular invasion; PNI, perineural invasion.
Of the 243 patients, 168 (69.1%) received adjuvant therapy. Among the 168 patients who received adjuvant therapy, 78 (46.4%) received adjuvant radiation alone and 90 (53.6%) received adjuvant CCRT. Patients who received adjuvant therapy were associated with more tongue and gingival subsites (P < .001), advanced T3/T4 tumors (P = .000), and positive PNI (P < .001).
Median follow‐up time for all patients was 4.6 years (range, 0.5 months‐13 years). Table 2 shows the results of univariate analysis for survival in all single‐node patients. Among all single‐node patients, differentiation status and PNI were significant prognostic factors for OS, LRFS, RRFS, and DMFS. Patients with T1/T2 tumors had higher 5‐year OS (74.2% vs 63.8%, P = .009) and DMFS (74.3% vs 62.7%, P = .005). However, the addition of adjuvant therapy was not associated with higher OS (72.1% vs 66.3%, P = .253; Figure 1A), LRFS (65.9% vs 62.8%, P = .671; Figure 1B), RRFS (64.7% vs 64.3%, P = .859; Figure 1C), or DMFS (72.1% vs 65.4%, P = .175; Figure 1D). Treatment intensification to adjuvant CCRT also did not improve the outcomes of OS, LRFS, RRFS, and DMFS. For patients with no adjuvant therapy, adjuvant RT, or adjuvant CCRT, the 5‐year OS was 72.1% vs 73.0% vs 60.5% (P = .270), LRFS was 65.9% vs 69.1% vs 57.2% (P = .525), RRFS was 64.7% vs 70.4% vs 58.9% (P = .600), and DMFS was 72.1% vs 71.7% vs 59.8% (P = .213). For T1/T2 only patients, the addition of adjuvant therapy was not associated with higher 5‐year OS (79.4% vs 69.5%, P = .270), LRFS (72.7% vs 61.2%, P = .348), RRFS (69.7% vs 67.7%, P = .793), or DMFS (79.4% vs 69.5%, P = .239) (Figure S1).
TABLE 2.
Univariate analysis for survival in all single node patients
| Five‐year (%) | OS | P value | LRFS | P value | RRFS | P value | DMFS | P value |
|---|---|---|---|---|---|---|---|---|
| Age (y) | ||||||||
| <65 | 69.1 | .315 | 64.0 | .796 | 64.9 | .386 | 68.7 | .245 |
| ≥65 | 59.6 | 59.6 | 61.3 | 57.4 | ||||
| Gender | ||||||||
| Male | 68.5 | .498 | 63.8 | .580 | 64.5 | .575 | 67.8 | .573 |
| Female | 66.1 | 63.9 | 64.2 | 66.4 | ||||
| Primary site | ||||||||
| Buccal | 69.6 | .329 | 63.2 | .277 | 65.5 | .390 | 68.8 | .344 |
| Tongue | 61.6 | 56.9 | 57.1 | 60.9 | ||||
| Mouth floor | 100 | 100 | 90.0 | 100 | ||||
| Retromolar trigone | 80.0 | 80.0 | 80.0 | 80.0 | ||||
| Gingiva | 70.8 | 70.8 | 70.8 | 70.8 | ||||
| Lip | 50.0 | 50.0 | 60.0 | 50.0 | ||||
| Hard palate | 66.7 | 66.7 | 66.7 | 66.7 | ||||
| Differentiation | ||||||||
| Well/moderate | 71.2 | .001 | 66.3 | .019 | 67.0 | .021 | 70.5 | .003 |
| Poor | 40.9 | 40.9 | 40.9 | 41.3 | ||||
| pT | ||||||||
| T1/2 | 74.2 | .009 | 66.8 | .095 | 68.3 | .081 | 74.3 | .005 |
| T3/4 | 63.8 | 61.4 | 61.7 | 62.7 | ||||
| LVI | ||||||||
| Positive | 68.5 | .741 | 53.8 | .109 | 53.6 | .118 | 68.5 | .816 |
| Negative | 68.1 | 64.8 | 65.6 | 67.4 | ||||
| PNI | ||||||||
| Positive | 60.7 | .019 | 56.3 | .021 | 56.7 | .019 | 59.4 | .009 |
| Negative | 74.6 | 70.2 | 71.2 | 74.6 | ||||
| Margin | ||||||||
| Clear | 69.5 | .293 | 65.5 | .231 | 65.8 | .323 | 68.9 | .271 |
| Close | 66.4 | 61.4 | 62.7 | 65.9 | ||||
| Adjuvant therapy | ||||||||
| No | 72.1 | .253 | 65.9 | .671 | 64.7 | .869 | 72.1 | .175 |
| Yes | 66.3 | 62.8 | 64.3 | 65.4 | ||||
| Adjuvant therapy | ||||||||
| No | 72.1 | .270 | 65.9 | .525 | 64.7 | .600 | 72.1 | .213 |
| RT | 73.0 | 69.1 | 70.4 | 71.7 | ||||
| CCRT | 60.5 | 57.2 | 58.9 | 59.8 |
Abbreviations: CCRT, concurrent chemoradiaiton; DMFS, distant metastasis‐free survival; LRFS, local recurrence‐free survival; LVI, lymphovascular invasion; OS, overall survival; PNI, perineural invasion; RRFS, regional recurrence‐free survival; RT, radiation therapy.
FIGURE 1.
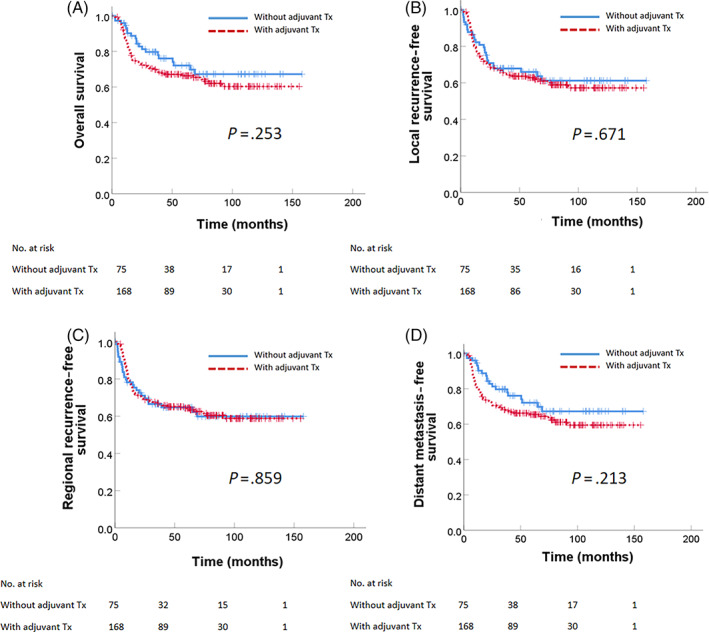
Survival outcome for all oral cavity cancer patients with a single positive node without extranodal extension and positive margin (low/intermediate pN1new). (A) Overall survival, (B) local recurrence‐free survival, (C) regional recurrence‐free survival, and (D) distant metastasis‐free survival
Multivariate analysis results are summarized in Table 3. Poor differentiation status remained a significant factor for inferior OS (P = .002, hazard ratio (HR) = 2.575, confidence interval (CI) = 1.431‐4.635), LRFS (P = .020, HR = 1.995, CI = 1.118‐3.561), RRFS (P = .018, HR = 2.020, CI = 1.131‐3.610), and DMFS (P = .004, HR = 2.387, CI = 1.330‐4.286). Patients with advanced T3/T4 tumors had poor 5‐year OS (P = .016, HR = 1.888, CI = 1.127‐3.163) and DMFS (P = .014, HR = 1.907, CI = 1.142‐3.186). However, adjuvant therapy was not significantly associated with OS (P = .754, HR = 0.918, CI = 0.537‐1.568), LRFS (P = .464, HR = 0.835, CI = 0.515‐1.354), RRFS (P = .183, HR = 0.724, CI = 0.450‐1.165), and DMFS (P = .887, HR = 0.962, CI = 0.565‐1.640).
TABLE 3.
Multivariate analysis for survival in all single node patients
| Hazard ratio, 95% confidence interval | OS | P value | LRFS | P value | RRFS | P value | DMFS | P value |
|---|---|---|---|---|---|---|---|---|
| Differentiation | ||||||||
| Well/moderate | 1 | .002 | 1 | .020 | 1 | .018 | 1 | .004 |
| Poor | 2.575 (1.431‐4.635) | 1.995 (1.118‐3.561) | 2.020 (1.131‐3.610) | 2.387 (1.330‐4.286) | ||||
| pT | ||||||||
| T1/2 | 1 | .016 | 1 | .117 | 1 | .068 | 1 | .014 |
| T3/4 | 1.888 (1.127‐3.163) | 1.448 (0.911‐2.301) | 1.546 (0.969‐2.465) | 1.907 (1.142‐3.186) | ||||
| PNI | ||||||||
| Negative | 1 | .109 | 1 | .062 | 1 | .051 | 1 | .067 |
| Positive | 1.447 (0.921‐2.274) | 1.498 (0.980‐2.289) | 1.530 (0.999‐2.345) | 1.518 (0.971‐2.374) | ||||
| Adjuvant therapy | ||||||||
| No | 1 | .754 | 1 | .464 | 1 | .183 | 1 | .887 |
| Yes | 0.918 (0.537‐1.568) | 0.835 (0.515‐1.354) | 0.724 (0.450‐1.165) | 0.962 (0.565‐1.640) |
Abbreviations: DMFS, distant metastasis‐free survival; LRFS, local recurrence‐free survival; OS, overall survival; RRFS, regional recurrence‐free survival.
As for isolated neck recurrence rate, only 7 (4.2%) isolated neck recurrences were observed in the adjuvant therapy group and 7 isolated neck recurrences (9.3%) in the observation group (P = .110).
For subgroup analysis, in low/intermediate pN1new patients with one or more minor risk factors, adjuvant therapy was not significantly associated with OS, LRFS, RRFS, or DMFS. These minor risk factors consisted of poor differentiation, T3/T4, LVI positive, PNI positive, close margin, T3/4 and PNI positive, T3/4 and close margin, and PNI positive and close margin (Figure 2). The only significant differences between outcomes and adjuvant therapy were observed in the tongue cancer subgroup. For patients with tongue cancer, although adjuvant therapy was not associated with OS or DMFS, the 5‐year LRFS (34.3% vs 62.1%, P = .042) and RRFS (33.6% vs 62.4%, P = .015) were significantly higher with adjuvant therapy (Figure 3).
FIGURE 2.
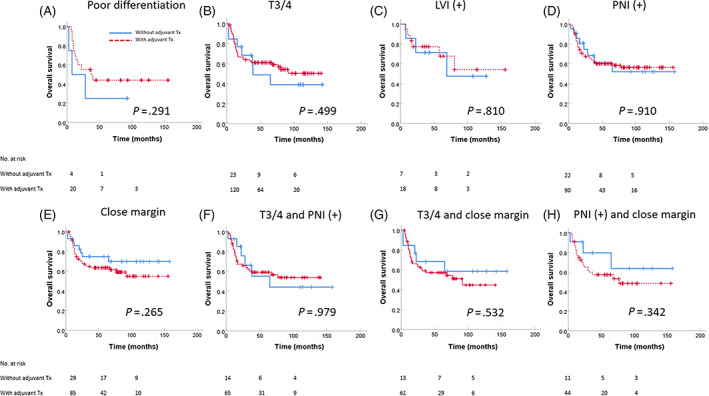
Overall survival for low/intermediate pN1new in various risk group: (A) poor differentiation, (B) T3/T4, (C) LVI positive, (D) PNI positive, (E) close margin, (F) T3/4 and PNI positive, (G) T3/4 and close margin, and (H) PNI positive and close margin. LVI, lymphovascular invasion; PNI, perineural invasion
FIGURE 3.
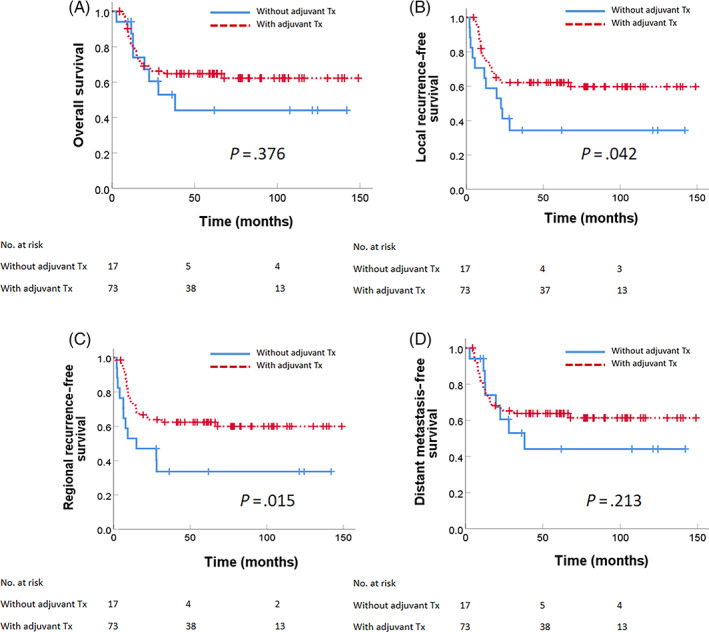
Survival outcome for low/intermediate pN1new tongue cancer patients: (A) Overall survival, (B) local recurrence‐free survival, (C) regional recurrence‐free survival, and (D) distant metastasis‐free survival
4. DISCUSSION
Decisions regarding oral cavity cancer adjuvant therapy depend on pathological risk factors. Previous research has shown that the presence of ENE and positive margin are the two major risk factors that require adjuvant CCRT. 4 Other risk factors that may indicate adjuvant RT or CCRT include LVI, PNI, close margin, T3/T4, N2/N3, and positive lymph nodes at levels 4 to 5 among patients with oropharynx and oral cavity cancer. 5 The National Comprehensive Cancer Network guidelines (2019 version 3) suggest that, for N1 patients without adverse factors, adjuvant RT may be considered (category 2A suggestion). Adjuvant CCRT may be considered for N1 patients with one or more risk factors (category 2A suggestion). The American Society of Clinical Oncology Clinical Practice Guideline 2019 suggests that adjuvant neck radiotherapy should not be administered to patients with a single pathologically positive node without ENE after high‐quality neck dissection unless indicated by the primary tumor characteristics. 6 However, the expert panel acknowledged that there were still controversies and data were limited for single‐node patients.
In previous studies, the neck recurrence rate for oral cavity pN1 (AJCC sixth/seventh) patients without adjuvant treatment was found to range widely from less than 10% 7 , 8 , 9 to 20% to 35%. 10 , 11 However, these retrospective series included patients with other major risk factors, including ENE or positive margin. For pN1 (AJCC seventh) patients without ENE, Jäckel et al 9 showed only a 10% rate of isolated nodal failures without adjuvant treatment. In our study, we showed that, in low/intermediate risk pN1new (single node without ENE or positive margin) patients, the isolated neck recurrence rate was 9.3% among patients who did not receive adjuvant therapy.
Shrime et al 12 used SEER data of 1539 T1‐2N1 (AJCC sixth/seventh) oral cavity cancer and demonstrated that adjuvant RT improved survival in patients with T2 tongue and floor of the mouth disease (52.3% vs 37.9% [P = .002] and 39.9% vs 17.7% [P = .003], respectively). Kao et al 13 used the SEER Database to analyze 2451 pN1 (AJCC sixth/seventh) patients with head and neck cancer and found that adjuvant RT was associated with significantly improved survival for pN1 (HR = 0.78; 95% CI = 0.67‐0.90; P = .001). However, the major limitation of the SEER study was that detailed pathologic information, such as ENE, margin status, LVI, and PNI, was not available. Therefore, high‐risk patients may have been included in their analysis. Chen et al 14 analyzed 1467 pT1‐2N1 (AJCC sixth/seventh) patients with oral cavity cancer by using the National Cancer Database (NCDB). They found that adjuvant RT was associated with improved OS (HR = 0.76; 95% CI = 0.63‐0.92). This association persisted for patients younger than 70 years (HR = 0.77; 95% CI = 0.61‐0.97) and those with pT2 disease (HR = 0.64; 95% CI = 0.43‐0.96). However, the major limitation in this study was that the major risk factor of ENE was incompletely coded in the NCDB.
Jäckel et al 9 analyzed 118 pN1 (AJCC sixth/seventh) head and neck cancer patients without ENE. They demonstrated that the respective 3‐year neck recurrence rates amounted to 11.2% and 2.9% (P = 0.09). Moreover, no survival benefit of receiving RT was demonstrated. Chen el al 10 analyzed 39 pathologic T1‐2/N1 (AJCC sixth) patients with tongue cancer without ENE, positive margins, LVI, or PNI. They found that the 5‐year OS rate was 92.3% for the patients receiving adjuvant RT and 54.9% for the patients not receiving adjuvant RT (P = .015). Our data revealed that only the subgroup of low/intermediate risk pN1new patients with tongue cancer benefited from adjuvant therapy for RRFS and LRFS.
In our study, we also found that among low/intermediate risk pN1new patients with one or more minor risk factors, adjuvant therapy was not significantly associated with OS, LRFS, RRFS, and DMFS in pN1new. Furthermore, tumor differentiation status, PNI, and advanced primary tumor were associated with poorer outcomes, which is consistent with the results of many studies. However, treatment intensification with the addition of adjuvant therapy in low/intermediate pN1new harboring one or more minor risk factors did not translate into more optimal outcomes. Our study provided this key clinically relevant data, as previous database studies were limited by a lack of detailed pathological information.
5. CONCLUSION
In conclusion, our study showed that in low/intermediate pN1new (single node without ENE or positive margin) patients with oral cavity cancer, adjuvant therapy was not associated with improved outcomes, even in patients with one or more minor risk factors. Only the tongue cancer site subgroup benefited in terms of RRFS and LRFS after the addition of adjuvant treatment.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Supplementary Figure S1 Survival outcome for T1/T2 oral cavity cancer patients with a single positive node without extranodal extension and positive margin (low/intermediate pN1new). (a) Overall survival (b) local recurrence‐free survival (c) regional recurrence‐free survival and (d) distant metastasis‐free survival.
Chen W‐Y, Fang K‐H, Wang C‐W, et al. Adjuvant therapy may be omitted for oral cavity cancer with only one positive lymph node. Laryngoscope Investigative Otolaryngology. 2021;6(6):1339‐1346. doi: 10.1002/lio2.679
REFERENCES
- 1. Ho AS, Kim S, Tighiouart M, et al. Metastatic lymph node burden and survival in Oral cavity cancer. J Clin Oncol. 2017;35:3601‐3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee H, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Number of positive lymph nodes better predicts survival for oral cavity cancer. J Surg Oncol. 2019;119:675‐682. [DOI] [PubMed] [Google Scholar]
- 3. Liao CT, Lee LY, Hsueh C, et al. Pathological risk factors stratification in pN3b oral cavity squamous cell carcinoma: focus on the number of positive nodes and extranodal extension. Oral Oncol. 2018;86:188‐194. [DOI] [PubMed] [Google Scholar]
- 4. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843‐850. [DOI] [PubMed] [Google Scholar]
- 5. Bernier J, Vermorken JB, Koch WM. Adjuvant therapy in patients with resected poor‐risk head and neck cancer. J Clin Oncol. 2006;24:2629‐2635. [DOI] [PubMed] [Google Scholar]
- 6. Koyfman SA, Ismaila N, Crook D, et al. Management of the Neck in squamous cell carcinoma of the Oral cavity and oropharynx: ASCO clinical practice guideline. J Clin Oncol. 2019;37:1753‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambrosch P, Kron M, Pradier O, Steiner W. Efficacy of selective neck dissection: a review of 503 cases of elective and therapeutic treatment of the neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Head Neck Surg. 2001;124:180‐187. [DOI] [PubMed] [Google Scholar]
- 8. Schiff BA, Roberts DB, El‐Naggar A, Garden AS, Myers JN. Selective vs modified radical neck dissection and postoperative radiotherapy vs observation in the treatment of squamous cell carcinoma of the oral tongue. Arch Otolaryngol Head Neck Surg. 2005;131:874‐878. [DOI] [PubMed] [Google Scholar]
- 9. Jackel MC, Ambrosch P, Christiansen H, Martin A, Steiner W. Value of postoperative radiotherapy in patients with pathologic N1 neck disease. Head Neck. 2008;30:875‐882. [DOI] [PubMed] [Google Scholar]
- 10. Chen TC, Wang CT, Ko JY, et al. Postoperative radiotherapy for primary early oral tongue cancer with pathologic N1 neck. Head Neck. 2010;32:555‐561. [DOI] [PubMed] [Google Scholar]
- 11. Byers RM, Clayman GL, D MG, et al. Selective neck dissections for squamous carcinoma of the upper aerodigestive tract: patterns of regional failure. Head Neck. 1999;21:499‐505. [DOI] [PubMed] [Google Scholar]
- 12. Shrime MG, Gullane PJ, Dawson L, et al. The impact of adjuvant radiotherapy on survival in T1‐2N1 squamous cell carcinoma of the oral cavity. Arch Otolaryngol Head Neck Surg. 2010;136:225‐228. [DOI] [PubMed] [Google Scholar]
- 13. Kao J, Lavaf A, Teng MS, Huang D, Genden EM. Adjuvant radiotherapy and survival for patients with node‐positive head and neck cancer: an analysis by primary site and nodal stage. Int J Radiat Oncol Biol Phys. 2008;71:362‐370. [DOI] [PubMed] [Google Scholar]
- 14. Chen MM, Harris JP, Hara W, Sirjani D, Divi V. Association of Postoperative Radiotherapy with Survival in patients with N1 Oral cavity and Oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:1224‐1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Survival outcome for T1/T2 oral cavity cancer patients with a single positive node without extranodal extension and positive margin (low/intermediate pN1new). (a) Overall survival (b) local recurrence‐free survival (c) regional recurrence‐free survival and (d) distant metastasis‐free survival.


