Abstract
Objective:
Prevalence of sacroiliitis in Crohn’s disease (CD) is variable depending on defining criteria. This study utilized standardized sacroiliac joint (SIJ) MRI to identify sacroiliitis in CD patients and its association with clinical and serological markers.
Methods:
In this cross-sectional study, consecutive adult subjects with CD were prospectively enrolled from an inflammatory bowel disease clinic. All subjects underwent SIJ MRI. Data collected included CD duration, history of joint/back pain, HLA-B27 status, Bath Ankylosing Spondylitis Metrology Index (BASMI), Bath Ankylosing Spondylitis Disease Activity Index, Harvey-Bradshaw Index (HBI) for activity of CD, Ankylosing Spondylitis Disease Activity Score, C-reactive protein, and various serologic markers of inflammation. Three blinded readers reviewed MRIs for active and/or structural lesions according to the Spondyloarthritis Research Consortium of Canada modules.
Results:
33 CD patients were enrolled: 76% female, 80% white, median age 36.4 years (IQR 27.2–49.0), moderate CD activity (mean HBI 8.8±SD 4.5). 19 subjects (58%) reported any back pain, 13 of whom met ASAS inflammatory back pain criteria. 4 subjects (12%) showed sacroiliitis using global approach and 6 (18%) met ASAS imaging criteria of sacroiliitis. Older age (mean 51.2 ± SD 12.5 vs 37.2 ± 14; p=0.04), history of dactylitis (50.0% vs 3.4%, p=0.03) and worse BASMI (4.1 ± 0.7 vs 2.4 ± 0.8, p= <0.001) were associated with MRI sacroiliitis; no serologic measure was associated.
Conclusion:
12–18% of CD patients had MRI evidence of sacroiliitis, which was not associated with back pain, CD activity or serologic measures. This cross-sectional study in a data-deficient field of sacroiliitis supports the hypothesis, that MRI could be a useful modality to identify subclinical axial spondyloarthritis in CD patients.
Keywords: Crohn’s Disease, Sacroiliitis, Magnetic Resonance Imaging, back pain, cytokines
Introduction
Inflammatory back pain (IBP) affects 5–6% of the United States (US) population(1) and it is the most common clinical manifestation of axial spondyloarthritis (SpA). However, prevalence of axial SpA in the US is estimated to be much lower than that of IBP (~1%) (2), suggesting IBP lacks specificity for axial SpA. It is crucial to differentiate inflammatory back pain due to axial SpA from other causes of chronic low back pain as they have different treatments and prognosis. However, diagnosis of axial SpA can be fraught with challenges (2) and can be particularly difficult in the setting of a co-existing systemic inflammatory disease such as Crohn’s disease (CD), which affects 0.2% of US adults (3). In a systematic literature review, the clinical arm of Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axial SpA showed high specificity (94%) but low sensitivity (23%) (4) and sensitivity can be lower, particularly in HLA-B27 negative Crohn’s patients (6). Standard radiographs similarly have low sensitivity, and lead to delayed diagnosis, since the transition from non-radiographic axial SpA (nr-axSpA) to radiographic axial SpA (r-axSpA) can take up to 10 years (7, 8).
Disconnect between sacroiliac joint (SIJ) pathology and musculoskeletal complaints in patients with CD is underscored by the fact that radiographic evidence of sacroiliitis is reported in up to 27% of CD patients without current symptoms of back pain (9, 10). These structural changes on x-ray suggest a history of undiagnosed inflammation, which may have been amenable to intervention. Information regarding rate of progression from nr-axial SpA to radiographic SpA in CD specifically is sparse. Since CD often affects younger patients, undertreating SIJ inflammation, and missing the opportunity to mitigate disease progression could have a significant impact on health-related quality of life (HR-QoL) and disability during prime wage-earning and child rearing years (11).
Incorporation of magnetic resonance imaging (MRI) to assess axial SpA allows early recognition of axial SpA in CD, especially when patients do not present with classic inflammatory back pain symptoms or are HLA-B27 negative (12). However, the ASAS classification criteria of active sacroiliitis are mainly based on bone marrow edema (BME), which puts less weight on structural lesions occurring when BME is absent (5). This is relevant as structural lesions can be present on MRI in the absence of changes on SIJ radiographs (13), and in the absence of BME, and may reflect damage from previous inflammatory SIJ disease. There is limited information in the literature evaluating the association of SIJ abnormalities on MRI with back pain in patients with CD, and little data on associations between MRI evidence of SIJ disease and bowel disease activity and biomarkers of systemic inflammation.
Therefore, the primary aims for this cross-sectional study in a data-deficient area were to 1) explore the prevalence of MRI evidence of sacroiliitis in CD patients 2) evaluate the relationship of MRI features of sacroiliitis in CD patients with and without back pain; 3) determine if clinical features of sacroiliitis are associated with MRI evidence of sacroiliitis in CD patients; 4) determine if clinical biomarkers in CD are associated with MRI evidence of sacroiliitis; and 5) explore association of MRI evidence of SIJ inflammation with peripheral blood cytokines.
Material and Methods
Study subjects
This is a cross-sectional study of consecutive subjects prospectively identified and enrolled from an outpatient clinic of Jill Roberts Center of inflammatory bowel disease (IBD) at a tertiary care academic medical center. Subjects between 18 and 65 were enrolled from April 2016 through May 2017 (Figure 1). All subjects met clinical, pathological or radiological criteria of CD (14–16). Patients with ulcerative colitis, indeterminate colitis, other inflammatory arthritis (e.g. rheumatoid arthritis, systemic lupus erythematosus, psoriatic or reactive arthritis), co-existent autoimmune diseases (e.g. celiac disease, Behcet’s disease) or skin psoriasis were excluded. All subjects were either biologic naïve or had been off systemic biologics >6 months prior to enrollment and could remain on non-biologic CD therapy (e.g. methotrexate, sulfasalazine, azathioprine or 6-mercaptopurine). Patients could also be on vedolizumab, an antagonist of α4β7 integrin in the intestinal epithelium which has no established efficacy in extra-intestinal manifestations of CD. Other exclusions included malignancy less than 5 years in remission (except for non-melanomatous skin cancer) or having a contraindication to MRI.
Figure 1:

Subject enrollment
Ethics Committee
Study was conducted according to Good Clinical Practice (GCP) guideline and was approved by Institutional review boards of Hospital for Special Surgery and Weill Cornell Medicine.
Clinical and serologic assessment
After obtaining informed consent, a detailed history was elicited. Subjects reporting back pain were further classified as having IBP if they met ASAS criteria (17), i.e. they fulfilled 4 out of following 5 back pain parameters: onset of symptoms <40 years of age, insidious onset of pain, nocturnal pain, improvement with exercise and no improvement with rest. Subjects with IBP were further classified as having axial SpA according to European Spondylarthropathy Study Group criteria (ESSG) (18) based on IBP plus the underlying diagnosis CD. One investigator (FM) performed a 66–68 joint count, entheses exam (lateral epicondyles of humerus, medial condyles of femur and Achilles tendons), obtained Bath Ankylosing Spondylitis Metrology Index (BASMI), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Index (ASDAS), and a Harvey Bradshaw index (HBI), a validated measure of CD activity (19). Peripheral blood was collected for measurement of C-reactive protein (CRP) and cytokine analysis (IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12/23, IL-17A, IL-17F, IL-21, IL-22, γ-IFN and TNF-α). CRP value was used to calculate ASDAS-CRP (Ankylosing Spondylitis Disease Activity Index-CRP), a validated measure of axial disease activity (20). Cytokine concentrations in serum were determined using a Legend-plex human Th cytokine 13-plex panel kit (BioLegend) according to the manufacturer’s instructions. Data was acquired with a BD LSRFortessa flow cytometer (BD Biosciences) and analyzed using the BioLegend’s LEGENDplex Data Analysis Software.
MRI protocol
MRI was performed with 1.5 Tesla clinical imaging units (GE Healthcare, Waukesha, Wisconsin) using phased-array coils. Sequences were acquired in a semi-coronal plane tilted parallel to the long axis of the SIJ with 3-mm section thickness and 34 slices acquired. Sequences were as follows: T1-weighted spin echo (T1; time to recovery [TR] 500–600 msec, time to echo [TE] 12 msec) and short tau inversion recovery (STIR) fast spin echo (TR 4000–5000 msec, time to inversion 150 msec, effective TE 15–20 msec). All subjects underwent T1 and STIR sequence MRI of SIJ.
Evaluation of SIJ MRI
The semicoronal images were independently read and scored by 2 expert rheumatologists (SJP, UW) and 1 newly trained rheumatologist reader (GK), blinded to any clinical information. SIJ MRIs were evaluated and scored for presence of bone marrow edema (BME) and structural lesions (erosion, fat metaplasia, backfill and ankylosis) using a validated scoring method originally derived from the Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ module (21, 22). SIJ MRI was considered “positive” for presence of sacroiliitis if it met global evaluation, based on reader’s overall evaluation of presence or absence of sacroiliitis by taking into account the contextual signature of both active and structural SIJ lesions (23). In addition, we tested whether the following 3 MRI criteria for sacroiliitis were met: 1) ASAS definition of active sacroiliitis (24) 2) SPondyloarthritis Caught Early (SPACE) proposal (25), based on presence of erosions and fat metaplasia and 3) Morpho proposal (26), based on presence of BME and/or erosion. For analysis, MRI positivity for sacroiliitis was defined based on majority-of-readers agreement (≥2 out of 3 readers).
Standardized lesion definitions on SIJ MRI
Standardized lesion definitions illustrated by a set of annotated reference images were applied (21, 27). SIJ lesions were scored binarily as being present or absent per joint quadrant for BME, fat metaplasia and erosion (range 0–8 lesions per MRI slice), or per joint half for backfill and ankylosis (range 0–4 lesions per MRI slice). SI joint quadrants were generated by virtual lines subdividing each SIJ into an upper and lower half on the iliac and sacral side. BME was defined as an increase in bone marrow signal in the subchondral bone on STIR images, fat metaplasia as a focal increased signal in bone marrow on T1SE images. For both lesion types, the center of the sacrum at the same craniocaudal level was used as the primary reference for normal bone marrow signal. Erosion was determined as full-thickness loss of dark appearance of either iliac or sacral cortical bone of the SIJ and change in normal bright appearance of adjacent bone marrow on T1SE images. Normal iliac or sacral marrow on the same slice at the same craniocaudal level served as reference signal. Backfill was defined as bright signal on T1SE sequence within an erosion cavity, demarcated from adjacent bone marrow by an irregular band of dark signal reflecting sclerosis at the border of the original erosion. Ankylosis was defined as bright signal on T1SE images extending across the SIJ.
Statistical analysis
Descriptive analyses were conducted for all baseline variables and are presented as means or medians for continuous variables and percentages for categorical variables. Differences between groups were quantified using the independent t-test (normally distributed data), Mann-Whitney test (non-normally distributed data) and chi-square test when appropriate. Mean (SD) and median (IQR) of SIJ quadrants/halves affected by a given lesion were computed over 3 readers pooled. The frequency of affected SIJ quadrants/halves on subject level and the frequency of MRI evidence for sacroiliitis according to four pre-defined MRI classification criteria were described as concordantly reported by the majority (≥2/3) of readers to enhance specificity. We also calculated the proportion of subjects where all 3 readers agreed that a given SIJ lesion is absent. Agreement between readers for granular SIJ lesions (i.e. BME, erosion, ankyloses, fat metaplasia and backfill) was calculated by intra-class correlation coefficient (ICC), two-way random effects, single measure, absolute agreement definition, for all readers together and for the 3 possible reader pairs separately (28). ICC values of <0.50, <0.75, ≤0.90, and >0.90 were considered to reflect poor, moderate, good, and excellent reproducibility, respectively. (29) Furthermore, kappa (κ) statistics was utilized to determine agreement between readers for dichotomous outcomes of 4 definitions of MRI positivity (30). Kappa agreement was categorized according to Landis and Koch (31): <0 = no agreement, 0.00–0.20 = slight, 0.21 – 0.40 = fair, 0.41 – 0.60 = moderate, 0.61 – 0.80 = substantial and 0.81–1.00 = perfect.. SIJ MRI of 71 patients from an unrelated axial SpA inception cohort (23) (24 subjects with active ankylosing spondylitis, 23 subjects with both IBD and ankylosing spondylitis and 24 subjects with non-specific back pain) served to determine reader agreement in a calibration sample with a relatively high frequency of the 5 SIJ lesions under consideration. SPSS Statistics, Version 25.0 (Armonk, NY: IBM) was used to perform statistical analysis.
Results
33 subjects with CD were enrolled. Subjects were 76% female, 80% white, had a median age of 36.4 years (IQR 27.2 – 49.0) with moderate CD activity (mean HBI 8.8 ± SD 4.5). 55% were noted to have peripheral arthritis on exam at the time of MRI. Nineteen subjects (58%) had back pain, 13 (39%) of which met ASAS criteria for inflammatory back pain. Only 1 subject (3%) was HLA-B-27 positive. Subjects with and without any back pain were similar in terms of duration and activity of CD (Table 1). However, more subjects with any back pain were noted to have evidence of peripheral SpA at the time of assessment compared to subjects without back pain (74% vs 29%; p= 0.015). Subjects with any back pain also had worse BASMI scores (2.94 ± 0.86 vs 2.11 ± 0.90; p=0.01), worse BASDAI scores and (5.33 ± 1.83 vs 2.39 ± 1.62, p <0.001) and worse ASDAS-CRP (3.15± 0.97 vs 1.66 ± 0.78, p <0.01; table 1).
Table 1.
Clinical characteristics and SIJ MRI finding in CD subjects stratified by presence of back pain
| Variable | Any Back Pain (N = 19, 57.6%) |
No Back Pain (N = 14, 42.4%) |
p-value |
|---|---|---|---|
| Patient | |||
| Age | 42.4 ± 13.3 | 34.1 ± 12.6 | 0.080 |
| Female gender | 13 / 19 (68.4%) | 12 / 14 (85.7%) | 0.416 |
| Tobacco use | 5 / 18 (27.8%) | 1 / 14 (7.1%) | 0.196 |
| Axial SpA (ESSG)* | 13 / 19 (68%) | - | - |
| Peripheral arthritis (current) | 14 / 19 (73.7%) | 4 / 14 (28.6%) | 0.015 |
| Peripheral arthritis (history) | 16 / 19 (84.2%) | 6 / 14 (42.9%) | 0.024 |
| BASDAI | 5.3 ± 1.8 | 2.4 ± 1.6 | <0.001 |
| BASMI | 2.9 ± 0.9 | 2.1 ± 0.9 | 0.011 |
| ASDAS-CRP | 3.1 ± 0.9 | 1.6 ± 0.8 | <0.001 |
| CRP | 1.6 ± 2.0 | 1.7± 3.3 | 0.930 |
| Crohn’s Disease | |||
| Duration of disease | 11.9 ± 6.7 | 14.2 ± 10.5 | 0.476 |
| Vedolizumab use | 3 / 19 (15.8%) | 4 / 13 (30.8%) | 0.401 |
| Past history of Biologic use | 11 / 19 (57.9%) | 8 / 13 (61.5%) | 0.837 |
| Surgery related to CD | 10 / 18 (55.6%) | 9 / 13 (69.2%) | 0.484 |
| CD activity (HBI Score) | 9.1 ± 4.9 | 6.9 ± 3.7 | 0.183 |
| MRI Positivity | |||
| Global assessment positive | 4 / 19 (21.1%) (6.1% – 45.6%)# |
0 / 14 (0%) (0.0% – 23.1%)# |
0.119 |
| Alternative MRI definitions of positivity | |||
| ASAS positive | 4 / 19 (21.1%) (6.1% – 45.6%)# |
2 / 14 (14.3%) (1.8% – 42.8%)# |
0.999 |
| SPACE positive | 0 / 19 (0%) (0.0% – 17.6%)# |
0 / 14 (0%) (0.0% – 23.1%)# |
N/A |
| 5 / 19 (26.3%) (9.1% – 51.2%)# |
0.209 |
modified ESSG: ASAS criteria was utilized to define IBP. Original ESSG Axial SpA (17) utilized Calin criteria (41).
95% CI
ASAS=Assessment of SpondyloArthritis international Society, ASDAS-CRP= Ankylosing Spondylitis Disease Activity Score C-reactive protein, BASDAI = Bath Ankylosing Spondylitis Activity Index, BASMI = Bath Ankylosing Spondylitis Metrology Index, CD= Crohn’s Disease, CRP= C-reactive protein, ESSG=European Spondyloarthropathy Study Group, HBI= Harvey Bradshaw Index, SpA= Spondyloarthritis, SPACE= SPondyloArthritis Caught Early
12% (4/33) of all CD subjects showed evidence of sacroiliitis on MRI based on global assessment (Figure 2 and 3). All 4 of these subjects reported back pain at the time of MRI and constitute 21% of CD subjects reporting back pain (4/19). None of the subjects without any back pain showed MRI sacroiliitis according to global assessment (21% vs 0%, p =0.12) (table 1). Among the CD subjects with any back pain, 21% (4/19) showed BME meeting ASAS definition of active sacroiliitis and 26% (5/19) met Morpho proposal (presence of ≥3 quadrants of BME and/or ≥2 erosions). Of subjects without back pain, 14% (2/14) and 7% (1/14) met ASAS and Morpho criteria respectively. None of the patients in either group met SPACE proposal for MRI positivity (Table 1). IBP was present in 100% (4/4) of those with global MRI positivity and in 52% (15/29) of those without global MRI positivity (p = 0.12) (Table 2).
Figure 2: Active erosive sacroiliitis.

36-year old (HLA-B27 not available) female with CD for 12 years, moderate CD activity, remote history of peripheral arthritis (absent at the time of MRI) and inflammatory back pain. The T1SE sequence (left panel) shows a sacral erosion (arrow) with perifocal fat metaplasia (arrowheads) in the right distal sacroiliac joint. The structural lesions are surrounded by bone marrow edema (broken arrows) of both sacrum and ilium (STIR scan, right panel).
Figure 3: Incipient ankylosis.
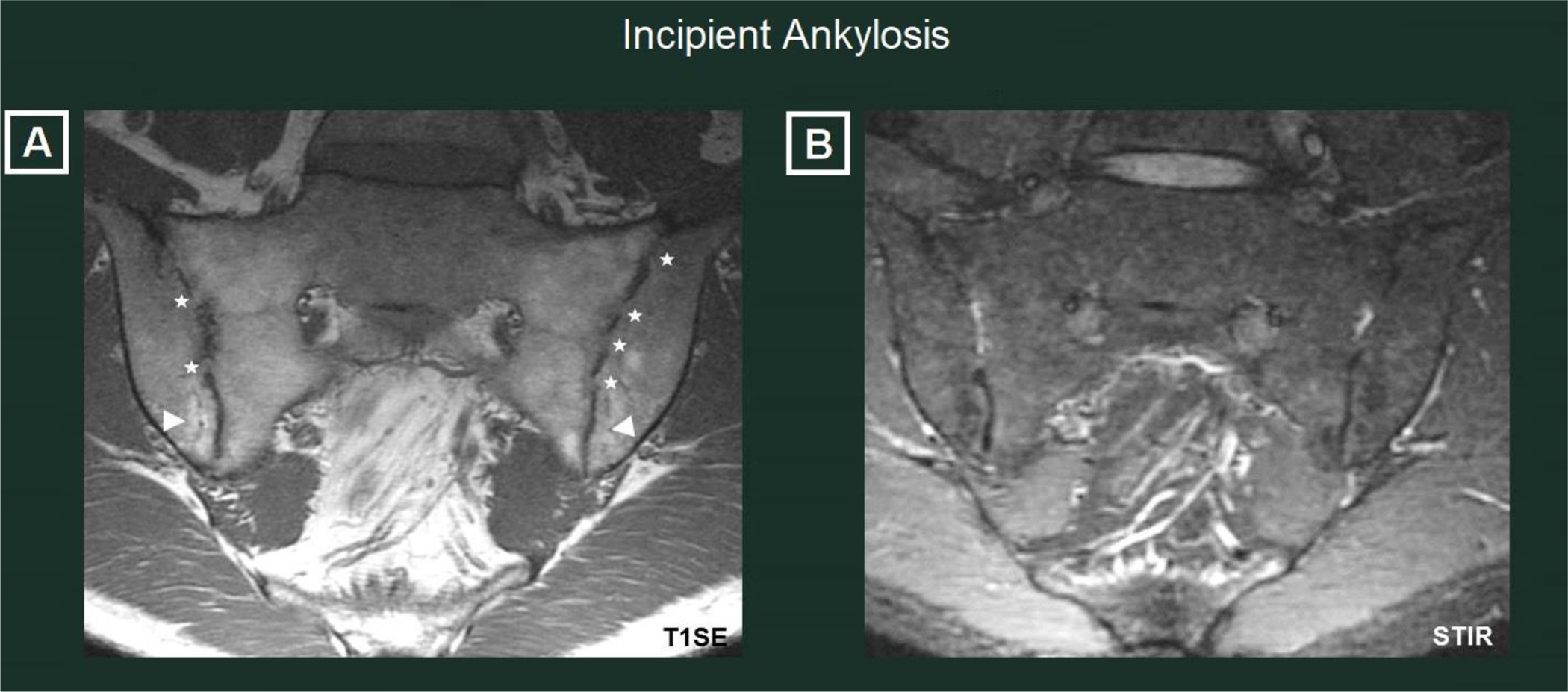
36-year old HLA-B27 negative male with CD for 13 years, moderate CD activity, peripheral arthritis on exam and inflammatory back pain. Multiple small bony bridges (asterisks) are visible in both SIJ on the T1SE sequence (left panel) together with fat metaplasia (arrowheads) in the distal ilium bilaterally. Bone marrow edema is absent on STIR slices (right panel).
Table 2.
Clinical characteristics of CD subjects stratified by global MRI positivity
| Variable | Global MRI positive (N = 4, 12.1%) |
Global MRI negative (N = 29, 87.9%) |
p-value |
|---|---|---|---|
| Demographics | |||
| Age | 51.2 ± 12.5 | 37.2 ± 12.9 | 0.04 |
| Female gender | 2 / 4 (50.0%) | 23 / 29 (79.3%) | 0.24 |
| Tobacco use | 2 / 4 (50.0%) | 4 / 28 (14.3%) | 0.15 |
| ASAS Clinical Arm Variables | |||
| Inflammatory back pain | 4 / 4 (100.0%) | 15 / 29 (51.7%) | 0.12 |
| Peripheral arthritis (current) | 3 / 4 (75.0%) | 15 / 29 (51.7%) | 0.60 |
| Peripheral arthritis (history) | 4 / 4 (100.0%) | 18 / 29 (62.1%) | 0.28 |
| Current enthesitis | 2 / 8 (25.0%) | 8 / 29 (27.6%) | 0.99 |
| History of uveitis | 1 / 4 (25.0%) | 3 / 29 (10.3%) | 0.42 |
| History of dactylitis | 2 / 4 (50.0%) | 1 / 29 (3.4%) | 0.03 |
| HLA-B27** | 0 / 4 (0.0%) | 1 / 29 (3.4%) | 0.99 |
| CRP | 2.6 ± 3.0 | 1.5 ± 2.6 | 0.43 |
| SpA Related Variables | |||
| BASDAI | 4.3 ± 1.7 | 4.2 ± 2.4 | 0.92 |
| BASMI | 4.1 ± 0.7 | 2.4 ± 0.8 | <0.001 |
| ASDAS-CRP | 3.3 ± 0.4 | 2.5 ± 1.2 | 0.19 |
| Crohn’s Disease Variables | |||
| Duration of disease | 17.9 ± 3.8 | 12.2 ± 8.7 | 0.20 |
| HBI Score | 7.8 ± 4.9 | 8.3 ± 4.5 | 0.83 |
| Vedolizumab use | 0 / 4 (0.0%) | 7 / 28 (25.0%) | 0.55 |
| Prior history of biologic use | 3 / 4 (75.0%) | 16 / 28 (57.1%) | 0.63 |
| Surgery related to CD | 3 / 4 (75.0%) | 16 / 28 (57.1%) | 0.99 |
HLA-B27 missing in 2 subjects
ASAS=Assessment of SpondyloArthritis international Society, ASDAS-CRP= Ankylosing Spondylitis Disease Activity Score C-reactive protein, BASDAI = Bath Ankylosing Spondylitis Activity Index, BASMI = Bath Ankylosing Spondylitis Metrology Index, CD= Crohn’s disease, CRP= C-reactive protein, HBI= Harvey Bradshaw Index, HLA-B27= Human Leukocyte Antigen B-27
CD subjects with global MRI positivity were older (51.2 ± 12.5 vs 37.2 ± 12.9; p= 0.04), even after controlling for CD duration (data not shown). These Crohn’s subjects also had worse BASMI scores (4.1 ± 0.7 vs 2.4 ± 0.8; p=<0.001) and reported history of dactylitis (50% vs 3.4%, p= 0.03). Presence of HLA-B27, peripheral arthritis (either current or previous history), duration of CD, CD activity, history of biologic use in the past, current treatment of CD with vedolizumab were not associated with MRI positivity (table 2). In addition, there were no statistically significant associations of any serum cytokines and MRI evidence of sacroiliitis (table 3).
Table 3.
Cytokine levels of CD subjects stratified by ASAS MRI positivity
| Variable | ASAS MRI positive (N = 6, 18%) |
ASAS MRI negative (N = 25, 82%) |
p-value |
|---|---|---|---|
| Cytokine | |||
| Interleukin 2 | 4.7 ± 2.0 | 5.6 ± 7.8 | 0.79 |
| Interleukin 4 | 3.5 ± 1.6 | 5.7 ± 4.6 | 0.33 |
| Interleukin 5 | 3.7 ± 0.0 | 3.8 ± 0.1 | 0.25 |
| Interleukin 6 | 6.2 ± 7.2 | 5.1 ± 6.9 | 0.71 |
| Interleukin 9 | 2.4 ± 1.5 | 1.9 ± 0.9 | 0.29 |
| Interleukin 10 | 31.0 ± 59.3 | 6.5 ± 1.8 | 0.36 |
| Interleukin 13 | 8.9 ± 6.3 | 8.9 ± 4.4 | 0.93 |
| Interleukin 12–23 | 214.0 ± 159.5 | 259.1 ± 227.7 | 0.65 |
| Interleukin 17A | 25.3 ± 13.1 | 21.1 ± 10.5 | 0.40 |
| Interleukin 17F | 4.8 ± 2.2 | 3.8 ± 3.5 | 0.49 |
| Interleukin 21 | 19.8 ± 36.2 | 40.0 ± 43.2 | 0.30 |
| Interleukin 22 | 15.2 ± 7.1 | 16.7 ± 11.2 | 0.75 |
| Interferon-γ | 75.7 ± 31.0 | 79.0 ± 28.8 | 0.80 |
| TNF-α | 19.5 ± 15.2 | 35.4 ± 94.1 | 0.68 |
ASAS=Assessment of SpondyloArthritis international Society, CD=Crohn’s disease, TNF= tumor necrosis factor
BME was the most frequently observed MRI lesion seen in all CD subjects with mean ± SD number of SIJ quadrants affected being 0.86 ±1.45 (Figure 1B). Up to 30% subjects showed BME in at least 1 quadrant with 21% showing BME in ≥2 SIJ quadrants while 3% had ≥6 SIJ quadrants with BME. Ankylosis was the next most frequent MRI lesion with a mean ± SD number of affected SIJ halves as 1.02 ± 3.45 (Figure 2A). Fat metaplasia was noted in up to 6% subjects affecting a mean of 0.98 ± 3.55 SIJ quadrants (Figure 1A and 2A). Erosion and backfill were least frequent (3% of subjects) (table 4). The proportion of subjects where all 3 readers agreed on absence of a given lesion type on SIJ MRI, was 58%, 88%, 97%, 88% and 91% for BME, erosion, backfill, fat metaplasia and ankylosis respectively.
Table 4:
Frequency of 5 MRI lesion types based on affected sacroiliac joint quadrants
| SIJ quadrants affected | BME | Erosion | Fat metaplasia | Backfill | Ankylosis |
|---|---|---|---|---|---|
| Mean ± SD | 0.86 ±1.45 | 0.05 ±0.15 | 0.98 ±3.55 | 0.04±0.23 | 1.02 ±3.45 |
| Median (IQR) | 0 (0–1.3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| n (%) with ≥ 1 quadrants | 10 (30) | 1 (3) | 2 (6) | 1 (3) | 2 (6) |
| n (%) with ≥ 2 quadrants | 7 (21) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| n (%) with ≥ 3 quadrants | 5 (15) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| n (%) with ≥ 4 quadrants | 2 (6) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| n (%) with ≥ 5 quadrants | 2 (6) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| n (%) with ≥ 6 quadrants | 1 (3) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
| n (%) with ≥ 7 quadrants | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 2 (6) |
Mean (SD) and median (IQR) of SIJ quadrants: pooled over 3 readers. n (%) with ≥1 to ≥7 SIJ quadrants: number of subjects (percentage) having ≥1 to ≥7 SIJ quadrants affected by a given lesion type as concordantly reported by ≥2/3 readers.
BME=Bone marrow edema
In a comparison cohort of patients with incipient axial SpA, which showed abundant SIJ lesion frequency, agreement for granular SIJ lesions (mean ICC, 95% CI) between all readers was consistent with previous reports: excellent for ankylosis (0.94, 0.91–96), good for BME (0.89, 0.85–0.92) and erosion (0.85, 0.79–0.90) and moderate for backfill (0.69, 0.57–0.78) and fat metaplasia (0.62, 0.44–0.75) (Supplementary table 1). There was a remarkably consistent concordance between the 2 experienced (R1 and R2) and the newly trained reader (R3) (supplementary table 1). In this cohort, agreement for dichotomous MRI outcomes, mean κ was substantial with an average κ of 0.72 for global, 0.64 for ASAS, 0.80 Morpho and 0.72 for SPACE. Concordance between the 2 experienced (R1 and R2) and newly trained reader (R3) remained consistent and κ values remained substantial for global (R3 and R1: 0.66, and R3 and R2: 0.77), Morpho (R3 and R1: 0.79 and R3 and R2 0.80) and SPACE (R3 and R1: 0.78 and R3 and R2: 0.66) while it was moderate to substantial for ASAS (R3 and R1: 0.53 and R3 and R2: 0.69) in the axial SpA inception cohort.. In the 33 Crohn’s subjects with low lesion frequency as outlined above, agreement (mean ICC, 95% CI) was poor for identification of BME (0.43, 0.21–0.63), erosion (0.17, −0.03–0.41) and backfill (0.30, 0.09–0.53) and moderate for fat metaplasia (0.51, 0.30–0.70) and ankylosis (0.65, 0.47–0.79). Agreement (κ) was moderate in detection of MRI positivity in all criteria (0.42 for Morpho, 0.45 for ASAS, 0.49 for SPACE and 0.51 for global) (Supplementary table 2)
Discussion
This cross-sectional study in a poorly understood subtype of SpA investigated the prevalence of sacroiliitis using four different MRI proposals in a sample of prospectively enrolled subjects with CD. We also evaluated the relationship of MRI changes with clinical and serological markers of disease activity. Our major findings were as follows: 1) 12% of patients with CD had MRI evidence of sacroiliitis, based on global assessment and 18% met ASAS definition. 2) Patient-reported overall and inflammatory back pain had no statistically significant relationship with MRI evidence of sacroiliitis in these subjects with CD. 3) Older age, and poor spinal mobility on physical exam and prior history of dactylitis were associated with MRI evidence of sacroiliitis. 4) Presence of peripheral arthritis, Crohn’s disease activity or serum cytokine levels were not associated with MRI evidence of sacroiliitis. Importantly, whether or not subjects reported inflammatory back pain had no statistically significant association with sacroiliitis on MRI. Given that about 1 out of 6 of unselected Crohn’s patients had MRI evidence of sacroiliitis, these findings suggest that MRI could be an important tool for identifying sacroiliitis in Crohn’s patients, regardless of type of back pain if present.
How best to define sacroiliitis on MRI remains controversial, and which types of MRI lesions are most relevant in different populations is unclear. We explored a number of different imaging proposals to score the MRIs for sacroiliitis (24–26). One prior study from the UK reported much higher prevalence of MRI defined sacroiliitis (39%) in CD patients, which could be in part explained by higher prevalence of HLA-B27 positivity in their cohort, as well as differences in MRI methodology and interpretation (32). Moreover, CD patients in our cohort were either biologic naïve or off systemic biologics at least 6 months. These data suggest that MRI may be a useful tool to identify sacroiliitis in CD patients, currently not on systemic biologics and may benefit from the addition of therapies with proven efficacy in treatment of axial SpA.
We did not observe a statistically significant association between patient-reported back pain and MRI evidence of sacroiliitis. In addition, we found that 7% and 14% of CD patients without any back pain had MRI evidence of sacroiliitis based on ASAS and Morpho proposals respectively. Both these criteria rely on presence of BME, which has been reported to be present in 25–33% of healthy asymptomatic subjects, challenging specificity of this finding (33). In our cohort, CD subjects without any back pain at the time of MRI did not have MRI evidence of sacroiliitis when assessed globally. Global evaluation takes into account the contextual information by concomitant presence of active and structural SIJ lesions (26). This approach mirrors strategies utilized by radiologists to determine presence of sacroiliitis in clinical practice. Our inability to detect a statistically significant association with back pain and global MRI positivity is most likely due to small sample size.
Our study showed older age was associated with MRI positivity (meeting any 1 of 4 MRI proposals as above) and this relationship was not influenced by the duration of CD. Subjects with MRI positivity also had higher BASMI scores, reflecting worse spinal mobility on physical exam. This could serve as an easy clinical exam in CD patients prior to obtaining MRI. Prior history of dactylitis was also associated with MRI positivity but having history of other SpA features in clinical arm of ASAS classification criteria (i.e. presence of IBP, peripheral arthritis, uveitis, enthesitis, HLA-B27) was also not associated with MRI positivity in our cohort. Furthermore, our findings confirm previous studies which found clinical factors related to CD, such as CD activity measures, duration of disease or surgical history have no association with sacroiliitis on imaging (34, 35). This lack of correlation with clinical disease activity and MRI evidence of SIJ inflammation underscores the potential importance of utilizing MRI for early identification of patients at risk for sacroiliitis, irrespective of type of back pain.
The IL-23/IL-17 axis plays a key role in pathogenesis of axial SpA and could explain the gut-joint axis in Crohn’s associated axial SpA as there is overexpression of IL-23 in the terminal ileum of CD patients (36), association of IL-23R single nucleotide polymorphism with both AS (37) and IBD (38) and clinical efficacy of blocking IL-12/23p40 subunit in CD (38, 39). TNF-α is another major cytokine in pathogenesis of both CD and SpA. Our results are similar to previous studies which found no difference in serum cytokine levels between subjects with and without MRI evidence of sacroiliitis (40). However, we recognize that this study is underpowered to show how tissue specific differences regulate inflammatory disease.
Our study has several limitations. Our sample size is small, hence lack of association with clinical parameters will need to be examined in a larger cohort. However, our sample size is comparable to a previously published study which included 44 subjects (32). We also examined MRI evidence of sacroiliitis in a group of CD patients with varying level and duration of disease activity, which may have also hampered our ability to identify associations. The cross-sectional design precluded evaluating the longitudinal effect of BME on disease progression. Longitudinal follow up with SIJ MRI at intervals will be important to better understand the prospective effect of subclinical sacroiliitis in CD patients.
Our agreement analysis for linear MRI lesions and various criteria of SIJ MRI positivity were excellent to moderate and substantial respectively in the external cohort, enriched for all types of SIJ lesions which showed major reader concordance consistent with the literature, yet low agreement in the Crohn’s patients, who displayed a very low frequency across all lesion types. This gap in MRI reader concordance is most likely explained by low lesion frequency and variability (41), replicating an observation from a previous report in healthy athletes (33).
The SIJ MRI findings in our study provide useful preliminary insights into a poorly understood subset of SpA patients. In conclusion, we found that 12–18% of CD subjects had MRI evidence of sacroiliitis and there was an association with older age, history of dactylitis and higher BASMI scores. Other clinical and serological markers of CD and SpA were not associated with MRI evidence of sacroiliitis, which emphasizes the potential role of SIJ MRI in identifying CD patients with subclinical sacroiliitis. This is clinically important, as early institution of axial SpA therapy might modify radiographic progression (42). Lack of concordance between CD disease activity and SpA symptoms suggests different therapeutic approaches are warranted for different clinical presentation of CD associated SpA, especially since a number of therapeutic options approved for management of axial SpA, such as NSAIDs and IL-17 blockers, can actually cause CD to flare (43). Therefore, given the prevalence of MRI evidence of sacroiliitis in CD, careful choice of therapy will be paramount, as well as the need for development of new targeted therapies, which could simultaneously target pathways responsible for both the bowel and axial manifestations of CD.
Supplementary Material
Key Messages:
12–18% of prospectively enrolled Crohn’s Disease patients had evidence of sacroiliitis on contemporary standardized MRI evaluation of sacroiliac joints
Sacroiliitis in Crohn’s Disease had no correlation with inflammatory back pain and other clinical or serological markers of Crohn’s Disease
MRI is a useful tool to recognize axial spondyloarthritis in CD patients, presence of which may impact therapeutic decision making in these patients.
Acknowledgement:
Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY
New York University School of Medicine Clinical and Translational Science Institute (NIH grant # UL1TR00144
Funding:
This work was supported by Inflammatory Arthritis Center of Excellence Grant of Hospital for Special Surgery (awarded to FM) and Clinical and Translational Science Center Seed Grant (awarded to LM), Weill Cornell Medicine (NIH/NCATS Grant # UL1TR002384), NIH/NIDDK R01 114252 (RSL).
Grant support:
Clinical and Translational Science Center seed grant (NIH/NCATS Grant # UL1TR002384) and Hospital For Special Surgery Inflammatory Arthritis Center of Excellence grant.
Footnotes
Disclosure Statement:
The authors in this manuscript declare no relevant financial conflict of interest.
References
- 1.Weisman MH. Inflammatory back pain: the United States perspective. Rheumatic diseases clinics of North America. 2012;38(3):501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reveille JD, Witter JP, Weisman MH. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res (Hoboken). 2012;64(6):905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424–9. [DOI] [PubMed] [Google Scholar]
- 4.Sepriano A, Rubio R, Ramiro S, Landewe R, van der Heijde D. Performance of the ASAS classification criteria for axial and peripheral spondyloarthritis: a systematic literature review and meta-analysis. Annals of the rheumatic diseases. 2017;76(5):886–90. [DOI] [PubMed] [Google Scholar]
- 5.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Annals of the rheumatic diseases. 2009;68(6):777–83. [DOI] [PubMed] [Google Scholar]
- 6.Deodhar A, Mease PJ, Reveille JD, Curtis JR, Chen S, Malhotra K, et al. Frequency of Axial Spondyloarthritis Diagnosis Among Patients Seen by US Rheumatologists for Evaluation of Chronic Back Pain. Arthritis & rheumatology (Hoboken, NJ). 2016;68(7):1669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mau W, Zeidler H, Mau R, Majewski A, Freyschmidt J, Stangel W, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. The Journal of rheumatology. 1988;15(7):1109–14. [PubMed] [Google Scholar]
- 8.Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis and rheumatism. 2009;60(3):717–27. [DOI] [PubMed] [Google Scholar]
- 9.Queiro R, Maiz O, Intxausti J, de Dios JR, Belzunegui J, Gonzalez C, et al. Subclinical sacroiliitis in inflammatory bowel disease: a clinical and follow-up study. Clinical rheumatology. 2000;19(6):445–9. [DOI] [PubMed] [Google Scholar]
- 10.Bandinelli F, Terenzi R, Giovannini L, Milla M, Genise S, Bagnoli S, et al. Occult radiological sacroiliac abnormalities in patients with inflammatory bowel disease who do not present signs or symptoms of axial spondylitis. Clinical and experimental rheumatology. 2014;32(6):949–52. [PubMed] [Google Scholar]
- 11.Strand V, Singh JA. Patient Burden of Axial Spondyloarthritis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2017;23(7):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber U, Lambert RG, Ostergaard M, Hodler J, Pedersen SJ, Maksymowych WP. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62(10):3048–58. [DOI] [PubMed] [Google Scholar]
- 13.Lukas C, Cyteval C, Dougados M, Weber U. MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations ‘Not everything that glisters is gold (standard)’. RMD open. 2018;4(1):e000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. Journal of Crohn’s and Colitis. 2010;4(1):7–27. [DOI] [PubMed] [Google Scholar]
- 15.Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn’s disease. Autoimmunity reviews. 2014;13(4–5):467–71. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. The American journal of gastroenterology. 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]
- 17.Sieper J, van der Heijde D, Landewe R, Brandt J, Burgos-Vagas R, Collantes-Estevez E, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Annals of the rheumatic diseases. 2009;68(6):784–8. [DOI] [PubMed] [Google Scholar]
- 18.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis and rheumatism. 1991;34(10):1218–27. [DOI] [PubMed] [Google Scholar]
- 19.Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation Between the Crohn’s Disease Activity and Harvey–Bradshaw Indices in Assessing Crohn’s Disease Severity. Clinical Gastroenterology and Hepatology. 2010;8(4):357–63. [DOI] [PubMed] [Google Scholar]
- 20.Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68 Suppl 2:ii1–44. [DOI] [PubMed] [Google Scholar]
- 21.Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, et al. Spondyloarthritis research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis and rheumatism. 2005;53(5):703–9. [DOI] [PubMed] [Google Scholar]
- 22.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. The Journal of rheumatology. 2015;42(1):79–86. [DOI] [PubMed] [Google Scholar]
- 23.Weber U, Zubler V, Pedersen SJ, Rufibach K, Lambert RG, Chan SM, et al. Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis care & research. 2013;65(6):977–85. [DOI] [PubMed] [Google Scholar]
- 24.Rudwaleit M, Landewe R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009;68(6):770–6. [DOI] [PubMed] [Google Scholar]
- 25.de Hooge M, van den Berg R, Navarro-Compan V, Reijnierse M, van Gaalen F, Fagerli K, et al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis. 2016;75(7):1308–14. [DOI] [PubMed] [Google Scholar]
- 26.Weber U, Ostergaard M, Lambert RG, Pedersen SJ, Chan SM, Zubler V, et al. Candidate lesion-based criteria for defining a positive sacroiliac joint MRI in two cohorts of patients with axial spondyloarthritis. Ann Rheum Dis. 2015;74(11):1976–82. [DOI] [PubMed] [Google Scholar]
- 27.Maksymowych WP, Lambert RG, Ostergaard M, Pedersen SJ, Machado PM, Weber U, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Annals of the rheumatic diseases. 2019;78(11):1550–8. [DOI] [PubMed] [Google Scholar]
- 28.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin. 1979;86(2):420–8. [DOI] [PubMed] [Google Scholar]
- 29.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of chiropractic medicine. 2016;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen JJE, measurement p. A coefficient of agreement for nominal scales. 1960;20(1):37–46. [Google Scholar]
- 31.Landis JR, Koch GGJb. The measurement of observer agreement for categorical data. 1977:159–74. [PubMed] [Google Scholar]
- 32.Orchard TR, Holt H, Bradbury L, Hammersma J, McNally E, Jewell DP, et al. The prevalence, clinical features and association of HLA-B27 in sacroiliitis associated with established Crohn’s disease. Aliment Pharmacol Ther. 2009;29(2):193–7. [DOI] [PubMed] [Google Scholar]
- 33.Weber U, Jurik AG, Zejden A, Larsen E, Jorgensen SH, Rufibach K, et al. Frequency and Anatomic Distribution of Magnetic Resonance Imaging Features in the Sacroiliac Joints of Young Athletes: Exploring “Background Noise” Toward a Data-Driven Definition of Sacroiliitis in Early Spondyloarthritis. Arthritis & rheumatology (Hoboken, NJ). 2018;70(5):736–45. [DOI] [PubMed] [Google Scholar]
- 34.Baeten D, De Keyser F, Mielants H, Veys EM. Ankylosing spondylitis and bowel disease. Best practice & research Clinical rheumatology. 2002;16(4):537–49. [DOI] [PubMed] [Google Scholar]
- 35.Baeten D, De Keyser F, Mielants H, Veys EM. Immune linkages between inflammatory bowel disease and spondyloarthropathies. Current opinion in rheumatology. 2002;14(4):342–7. [DOI] [PubMed] [Google Scholar]
- 36.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. The Journal of clinical investigation. 2003;112(5):693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature genetics. 2007;39(11):1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science (New York, NY). 2006;314(5804):1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtta V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14(9):1175–84. [DOI] [PubMed] [Google Scholar]
- 40.Andersen T, Rasmussen TK, Hvid M, Holm CK, Madsen KJ, Jurik AG, et al. Increased plasma levels of IL-21 and IL-23 in spondyloarthritis are not associated with clinical and MRI findings. Rheumatol Int. 2012;32(2):387–93. [DOI] [PubMed] [Google Scholar]
- 41.David Liljequist BE, Kirsti Skavberg Roaldsen. Intraclass correlation – A discussion and demonstration of basic features PLoS One 2019. [Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0219854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Annals of the rheumatic diseases. 2018;77(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


